Abstract
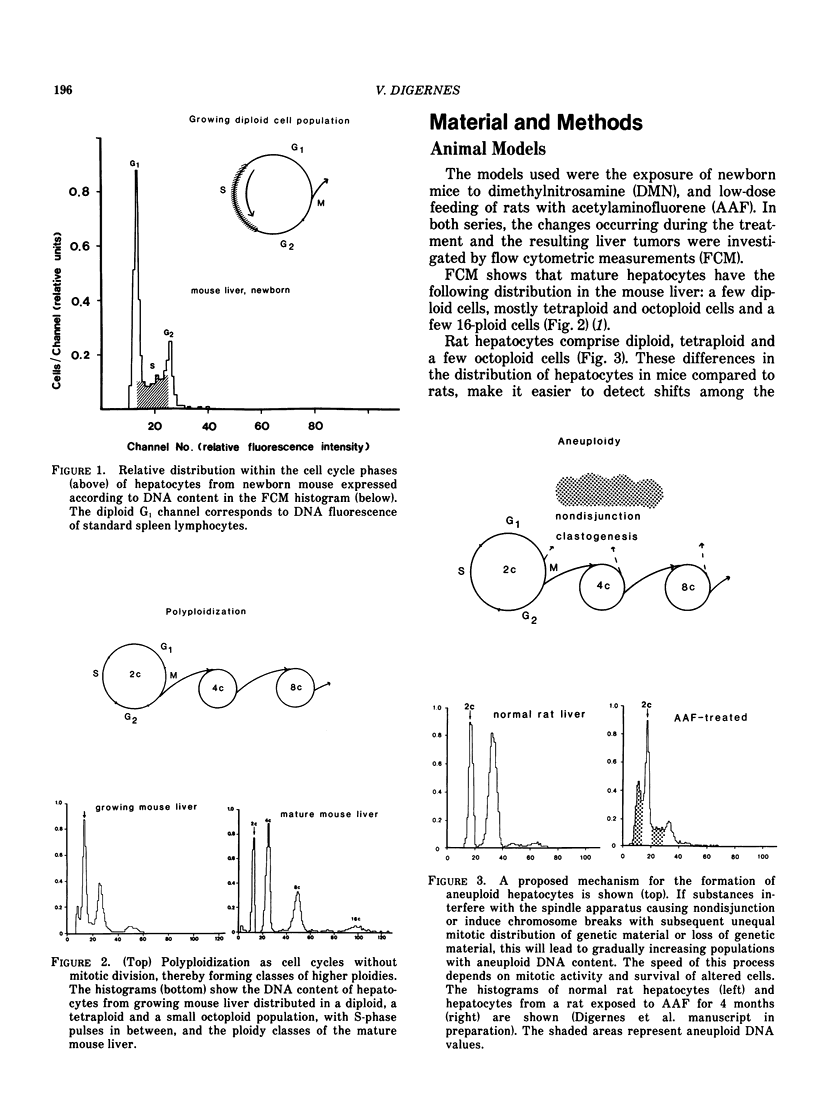
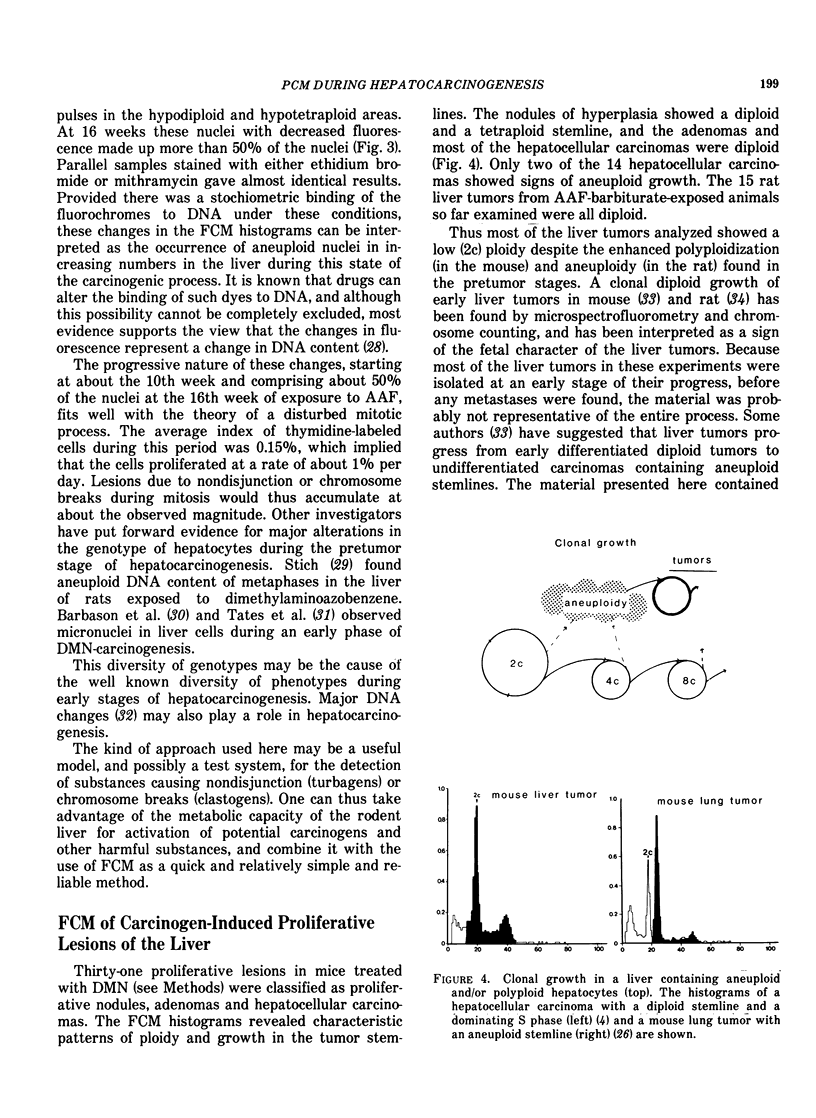
The carcinogenic process in the liver was monitored from early nonneoplastic changes to the development of tumors in two well-known models; IP administration of dimethylnitrosamine (DMN) to newborn mice, and feeding of rats with acetylaminofluorene (AAF). Liver parenchyma cells and liver tumor cells were isolated by collagenase treatment and prepared for flow cytometric (FCM) measurements of nuclear DNA content. The changes observed were correlated to morphology and 3H-thymidine incorporation. The early preneoplastic changes in hepatocytes from DMN-treated mice were shifts in the DNA content to classes of higher nuclear ploidies. AAF-feeding of rats caused gradual distortion of the DNA content of nuclei from parenchymal cells. After 16 weeks of exposure, before any tumors were seen, a majority of the nuclei displayed an aneuploid DNA content. The significance of these changes in the carcinogenic process is discussed, and a possible use of these models for detection of hepatotropic agents and agents causing aneuploidy (clastogens and turbagens) is proposed. The liver neoplasias (adenomas and hepatocellular carcinomas) induced by these models contained tumor stemlines with diploid DNA content, and some hepatocellular carcinomas showed aneuploidy. Nodules of hyperplasia contained diploid and tetraploid stemline.
Full text
PDF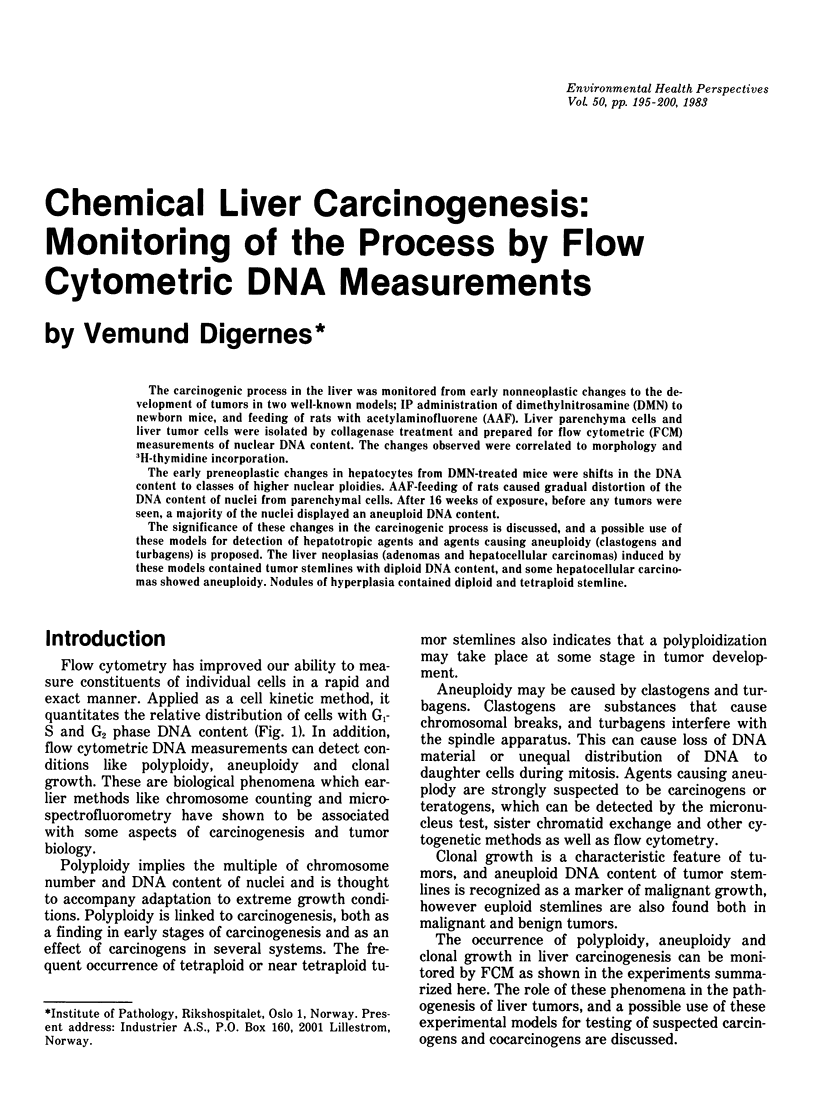



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony P. P., Vogel C. L., Barker L. F. Liver cell dysplasia: a premalignant condition. J Clin Pathol. 1973 Mar;26(3):217–223. [PMC free article] [PubMed] [Google Scholar]
- Barbason H., Fridman-Manduzio A., Betz E. H. Long term effects of a single dose of dimethylnitrosamine on the rat liver. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1975 Oct 27;84(2):135–142. doi: 10.1007/BF00304039. [DOI] [PubMed] [Google Scholar]
- Becker F. F., Fox R. A., Klein K. M., Wolman S. R. Chromosome patterns in rat hepatocytes during N-2-fluorenylacetamide carcinogenesis. J Natl Cancer Inst. 1971 Jun;46(6):1261–1269. [PubMed] [Google Scholar]
- Butler W. H., Hempsall V., Stewart M. G. Histochemical studies on the early proliferative lesion induced in the rat liver by aflatoxin. J Pathol. 1981 Apr;133(4):325–340. doi: 10.1002/path.1711330405. [DOI] [PubMed] [Google Scholar]
- Böhm N., Noltemeyer N. Excessive reversible phenobarbital induced nuclear DNA-polyploidization in the growing mouse liver. Histochemistry. 1981;72(1):63–74. doi: 10.1007/BF00496780. [DOI] [PubMed] [Google Scholar]
- CHRISTIE G. S., LE PAGE R. N. Enlargement of liver cell nuclei: effect of dimethylnitrosamine on size and desoxyribosenucleic acid content. Lab Invest. 1961 Jul-Aug;10:729–743. [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Cowell J. K., Wigley C. B. Changes in DNA content during in vitro transformation of mouse salivary gland epithelium. J Natl Cancer Inst. 1980 Jun;64(6):1443–1449. doi: 10.1093/jnci/64.6.1443. [DOI] [PubMed] [Google Scholar]
- Digernes V., Bolund L. The ploidy classes of adult mouse liver cells. A methodological study with flow cytometry and cell sorting. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979 Dec;32(1):1–10. [PubMed] [Google Scholar]
- Digernes V., Brønstad G., Sand T. E., Christoffersen T. The proliferation response of rat liver parenchymal cells after partial hepatectomy. A methodological study comparing flow cytometry of nuclear DNA content and in vivo and in vitro uptake of thymidine. Cell Tissue Kinet. 1982 Sep;15(5):521–528. doi: 10.1111/j.1365-2184.1982.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Digernes V. The cellular DNA content and profile of dimethyl nitrosamine induced marine lung tumors in relation to their histopathology. Carcinogenesis. 1981;2(3):195–198. doi: 10.1093/carcin/2.3.195. [DOI] [PubMed] [Google Scholar]
- Faktor V. M., Uryvaeva I. V. Poliploidizatsiia gepatotsitov myshi pri mnogokratnykh vozdeistviiakh chetyrekhkhloristym uglerodom. Biull Eksp Biol Med. 1980 Nov;90(11):614–616. [PubMed] [Google Scholar]
- Grimes P., Von Sallmann L. Interference with cell proliferation and induction of polyploidy in rat lens epithelium during prolonged myleran treatment. Exp Cell Res. 1966 May;42(2):265–273. doi: 10.1016/0014-4827(66)90289-8. [DOI] [PubMed] [Google Scholar]
- Hoover K. L., Ward J. M., Stinson S. F. Histopathologic differences between liver tumors in untreated (C57BL/6 X C3H)F1 (B6C3F1) mice and Nitrofen-fed mice. J Natl Cancer Inst. 1980 Nov;65(5):937–948. [PubMed] [Google Scholar]
- Johnsen A. S., Digernes V., Rugstad H. E. Effects of PUVA on a human skin epithelial cell line. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(1):61–69. doi: 10.1007/BF02892555. [DOI] [PubMed] [Google Scholar]
- Mustafin A. G. O sutochnykh ritmakh kletochnoi proliferatsii na rannikh stadiiakh gepatokantserogeneza myshei, indutsirovannogo ortoaminoazotoluolom. Biull Eksp Biol Med. 1976 Jul;82(7):856–858. [PubMed] [Google Scholar]
- Peereboom-Stegeman J. H., Morselt A. F. Increase in liver cell nuclear size after chronic cadmium treatment. Arch Toxicol. 1981 Sep;48(2-3):209–211. doi: 10.1007/BF00310490. [DOI] [PubMed] [Google Scholar]
- Pohl J., Christophers E. Photoinactivation and recovery in skin fibroblasts after formation of mono- and bifunctional adducts by furocoumarins-plus-UVA. J Invest Dermatol. 1980 Oct;75(4):306–310. doi: 10.1111/1523-1747.ep12530921. [DOI] [PubMed] [Google Scholar]
- STICH H. F. The DNA content of tumor cells. II. Alterations during the formation of hepatomas in rats. J Natl Cancer Inst. 1960 Jun;24:1283–1297. [PubMed] [Google Scholar]
- Schoental R. Toxicology and carcinogenic action of pyrrolizidine alkaloids. Cancer Res. 1968 Nov;28(11):2237–2246. [PubMed] [Google Scholar]
- Schulte-Hermann R. Adaptive liver growth induced by xenobiotic compounds: its nature and mechanism. Arch Toxicol Suppl. 1979;(2):113–124. doi: 10.1007/978-3-642-67265-1_9. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. I. Effect of Ca 2+ on enzymatic dispersion of isolated, perfused liver. Exp Cell Res. 1972 Oct;74(2):450–454. doi: 10.1016/0014-4827(72)90400-4. [DOI] [PubMed] [Google Scholar]
- Sincock A., Seabright M. Induction of chromosome changes in Chinese hamster cells by exposure to asbestos fibres. Nature. 1975 Sep 4;257(5521):56–58. doi: 10.1038/257056a0. [DOI] [PubMed] [Google Scholar]
- Stern R. S., Thibodeau L. A., Kleinerman R. A., Parrish J. A., Fitzpatrick T. B. Risk of cutaneous carcinoma in patients treated with oral methoxsalen photochemotherapy for psoriasis. N Engl J Med. 1979 Apr 12;300(15):809–813. doi: 10.1056/NEJM197904123001501. [DOI] [PubMed] [Google Scholar]
- Taper H. S., Bannasch P. Histochemical differences between so-called megalocytosis and neoplastic or preneoplastic liver lesions induced by N-nitrosomorpholine. Eur J Cancer. 1979 Feb;15(2):189–196. doi: 10.1016/0014-2964(79)90059-8. [DOI] [PubMed] [Google Scholar]
- Tates A. D., Neuteboom I., Hofker M. A micronucleus technique for detecting clastogenic effects of mutagens/carcinogens (DEN, DMN) in hepatocytes of rat liver in vivo. Mutat Res. 1980 Feb;74(1):11–20. doi: 10.1016/0165-1161(80)90187-9. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch S. D. The sex-dependent difference in the development of liver tumors in mice administered dimethylnitrosamine. Cancer Res. 1969 May;29(5):1024–1027. [PubMed] [Google Scholar]
- Wanson J. C., Bernaert D., Penasse W., Mosselmans R., Bannasch P. Separation in distinct subpopulations by elutriation of liver cells following exposure of rats to N-nitrosomorpholine. Cancer Res. 1980 Feb;40(2):459–471. [PubMed] [Google Scholar]
- Zedeck M. S., Sternberg S. S., Poynter R. W., McGowan J. Biochemical and pathological effects of methylazoxymethanol acetate, a potent carcinogen. Cancer Res. 1970 Mar;30(3):801–812. [PubMed] [Google Scholar]


