Abstract
During chemical carcinogenesis in the liver, a population of abnormal cells in lesions referred to as altered foci precedes the appearance of neoplasms. Most altered foci do not develop further, but a small fraction progress to formation of neoplasms. Liver tumor promoters increase the fraction that progress.
The mechanisms for this action of promoters may involve an effect on the cell membrane. Cells in vivo and in vitro exchange molecules through specialized membrane organelles known as gap junctions. Intercellular transfer of growth and/or differentiation regulating factors could be involved in suppressing the growth of initiated cells in the altered foci. Several liver tumor promoters have been found to inhibit intercellular communication in an in vitro liver culture system. This effect on the cell membrane could, thus, be the basis for the release of cells in foci for further growth into neoplasms. Such an epigenetic action would account for the requirement for high doses and prolonged exposure for certain liver tumor promoters. In addition, it implies a distinct type of health risk analysis for chemicals of this type.
Several chemicals, particularly halogenated hydrocarbons, produce primarily or exclusively an increase in liver tumors in rodent strains that are characterized by a substantial background incidence of such tumors. These chemicals have not been demonstrated to have the DNA damaging capability of genotoxic carcinogens and several enhance the hepatocarcinogenicity of previously administered liver carcinogens. Moreover, they exert an inhibition of intercellular communication. Thus, carcinogens of this type may be epigenetic carcinogens functioning as liver tumor promoters. Accordingly, the health risk analysis for these chemicals is different from that for genotoxic carcinogens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DeCosse J. J., Gossens C., Kuzma J. F., Unsworth B. R. Embryonic inductive tissues that cause histologic differentiation of murine mammary carcinoma in vitro. J Natl Cancer Inst. 1975 Apr;54(4):913–922. [PubMed] [Google Scholar]
- Deleers M., Castagna M., Malaisse W. J. Phorbol esters parallel effects on tumor promotion, insulin release and calcium ionophoresis. Cancer Lett. 1981 Nov;14(2):109–114. doi: 10.1016/0304-3835(81)90119-1. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Tumor promoters: effects on proliferation and differentiation of cells in culture. Life Sci. 1978 Nov 13;23(20):1979–1988. doi: 10.1016/0024-3205(78)90229-1. [DOI] [PubMed] [Google Scholar]
- Driedger P. E., Blumberg P. M. The effect of phorbol diesters on chicken embryo fibroblasts. Cancer Res. 1977 Sep;37(9):3257–3265. [PubMed] [Google Scholar]
- Ellison M. L., Ambrose E. J., Easty G. C. Differentiation in a transplantable rat tumour maintained in organ culture. Exp Cell Res. 1969 May;55(2):198–204. doi: 10.1016/0014-4827(69)90481-9. [DOI] [PubMed] [Google Scholar]
- Emerit I., Cerutti P. A. Tumour promoter phorbol-12-myristate-13-acetate induces chromosomal damage via indirect action. Nature. 1981 Sep 10;293(5828):144–146. doi: 10.1038/293144a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. J., Murray A. W. Inhibition of intercellular communication by tumor-promoting phorbol esters. Cancer Res. 1980 Aug;40(8 Pt 1):2935–2937. [PubMed] [Google Scholar]
- Flagg-Newton J. L. The permeability of the cell-to-cell membrane channel and its regulation in mammalian cell junctions. In Vitro. 1980 Dec;16(12):1043–1048. doi: 10.1007/BF02619254. [DOI] [PubMed] [Google Scholar]
- Fujiki H., Mori M., Nakayasu M., Terada M., Sugimura T. A possible naturally occurring tumor promoter, teleocidin B from Streptomyces. Biochem Biophys Res Commun. 1979 Oct 12;90(3):976–983. doi: 10.1016/0006-291x(79)91923-5. [DOI] [PubMed] [Google Scholar]
- Goodman D. G., Ward J. M., Squire R. A., Paxton M. B., Reichardt W. D., Chu K. C., Linhart M. S. Neoplastic and nonneoplastic lesions in aging Osborne-Mendel rats. Toxicol Appl Pharmacol. 1980 Sep 30;55(3):433–447. doi: 10.1016/0041-008x(80)90045-9. [DOI] [PubMed] [Google Scholar]
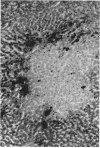
- Hirota N., Williams G. M. The sensitivity and heterogeneity of histochemical markers for altered foci involved in liver carcinogenesis. Am J Pathol. 1979 May;95(2):317–328. [PMC free article] [PubMed] [Google Scholar]
- Kimbrough R. D., Squire R. A., Linder R. E., Strandberg J. D., Montalli R. J., Burse V. W. Induction of liver tumor in Sherman strain female rats by polychlorinated biphenyl aroclor 1260. J Natl Cancer Inst. 1975 Dec;55(6):1453–1459. doi: 10.1093/jnci/55.6.1453. [DOI] [PubMed] [Google Scholar]
- King T. J., DiBerardino M. A. Transplantation of nuclei from the frog renal adenocarcinoma. I. Development of tumor nuclear-transplant embryos. Ann N Y Acad Sci. 1965 Aug 10;126(1):115–126. doi: 10.1111/j.1749-6632.1965.tb14271.x. [DOI] [PubMed] [Google Scholar]
- Kinsella A. R., Radman M. Tumor promoter induces sister chromatid exchanges: relevance to mechanisms of carcinogenesis. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6149–6153. doi: 10.1073/pnas.75.12.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa T., Sugano H. Enhancing effect of phenobarbital on the development of enzyme-altered islands and hepatocellular carcinomas initiated by 3'-methyl-4-(dimethylamino) azobenzene or diethylnitrosamine. Gan. 1978 Oct;69(5):679–687. [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Fitzgerald D. J. Tumor promoters inhibit metabolic cooperation in cocultures of epidermal and 3T3 cells. Biochem Biophys Res Commun. 1979 Nov 28;91(2):395–401. doi: 10.1016/0006-291x(79)91535-3. [DOI] [PubMed] [Google Scholar]
- O'Brien T. G. The induction of ornithine decarboxylase as an early, possibly obligatory, event in mouse skin carcinogenesis. Cancer Res. 1976 Jul;36(7 Pt 2):2644–2653. [PubMed] [Google Scholar]
- Papaioannou V. E., McBurney M. W., Gardner R. L., Evans M. J. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975 Nov 6;258(5530):70–73. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Barsness L., Goldsworthy T., Kitagawa T. Biochemical characterisation of stages of hepatocarcinogenesis after a single dose of diethylnitrosamine. Nature. 1978 Feb 2;271(5644):456–458. doi: 10.1038/271456a0. [DOI] [PubMed] [Google Scholar]
- Pitts J. D. The role of junctional communication in animal tissues. In Vitro. 1980 Dec;16(12):1049–1056. doi: 10.1007/BF02619255. [DOI] [PubMed] [Google Scholar]
- Pollard M., Luckert P. H. Spontaneous liver tumors in aged germfree Wistar rats. Lab Anim Sci. 1979 Feb;29(1):74–77. [PubMed] [Google Scholar]
- Ratanasavanh D., Tazi A., Galteau M. M., Siest G. Localization of gamma-glutamyltransferase in subcellular fractions of rat and rabbit liver: effect of phenobarbital. Biochem Pharmacol. 1979 Apr 15;28(8):1363–1365. doi: 10.1016/0006-2952(79)90438-6. [DOI] [PubMed] [Google Scholar]
- Revel J. P., Yancey S. B., Meyer D. J., Nicholson B. Cell junctions and intercellular communication. In Vitro. 1980 Dec;16(12):1010–1017. doi: 10.1007/BF02619251. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L. R., O'Brien D. H., Boutwell R. K. The stimulation of phospholipid metabolism in mouse skin following phorbol ester treatment. Biochim Biophys Acta. 1972 Sep 7;280(1):57–70. doi: 10.1016/0005-2760(72)90212-3. [DOI] [PubMed] [Google Scholar]
- Rose B., Simpson I., Loewenstein W. R. Calcium ion produces graded changes in permeability of membrane channels in cell junction. Nature. 1977 Jun 16;267(5612):625–627. doi: 10.1038/267625a0. [DOI] [PubMed] [Google Scholar]
- Rowlatt C., Chesterman F. C., Sheriff M. U. Lifespan, age changes and tumour incidence in an ageing C57BL mouse colony. Lab Anim. 1976 Oct;10(10):419–442. doi: 10.1258/002367776780956917. [DOI] [PubMed] [Google Scholar]
- Schimmel S. D., Hallam T. Rapid alteration in Ca++ content and fluxes in phorbol 12-myristate 13-acetate treated myoblasts. Biochem Biophys Res Commun. 1980 Jan 29;92(2):624–630. doi: 10.1016/0006-291x(80)90379-4. [DOI] [PubMed] [Google Scholar]
- Sheldon W. G., Greenman D. L. Spontaneous lesions in control BALB/C female mice. J Environ Pathol Toxicol. 1980;3(3 Spec No):155–167. [PubMed] [Google Scholar]
- Sivak A. Induction of cell division: role of cell membrane sites. J Cell Physiol. 1972 Oct;80(2):167–173. doi: 10.1002/jcp.1040800203. [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Dawson B., Yotti L. P., Chang C. C. Saccharin may act as a tumour promoter by inhibiting metabolic cooperation between cells. Nature. 1980 May 8;285(5760):109–110. doi: 10.1038/285109a0. [DOI] [PubMed] [Google Scholar]
- Ulland B. M., Page N. P., Squire R. A., Weisburger E. K., Cypher R. L. A carcinogenicity assay of Mirex in Charles River CD rats. J Natl Cancer Inst. 1977 Jan;58(1):133–140. doi: 10.1093/jnci/58.1.133. [DOI] [PubMed] [Google Scholar]
- Umeda M., Noda K., Ono T. Inhibition of metabolic cooperation in Chinese hamster cells by various chemical including tumor promoters. Gan. 1980 Oct;71(5):614–620. [PubMed] [Google Scholar]
- Van Duuren B. L., Sivak A., Katz C., Seidman I., Melchionne S. The effect of aging and interval between primary and secondary treatment in two-stage carcinogenesis on mouse skin. Cancer Res. 1975 Mar;35(3):502–505. [PubMed] [Google Scholar]
- Watanabe K., Williams G. M. Enhancement of rat hepatocellular-altered foci by the liver tumor promoter phenobarbital: evidence that foci are precursors of neoplasms and that the promoter acts on carcinogen-induced lesions. J Natl Cancer Inst. 1978 Nov;61(5):1311–1314. [PubMed] [Google Scholar]
- White S. J., McLean A. E., Howland C. Anticonvulsant drugs and cancer. A cohort study in patients with severe epilepsy. Lancet. 1979 Sep 1;2(8140):458–461. doi: 10.1016/s0140-6736(79)91505-8. [DOI] [PubMed] [Google Scholar]
- Williams G. M. Classification of genotoxic and epigenetic hepatocarcinogens using liver culture assays. Ann N Y Acad Sci. 1980;349:273–282. doi: 10.1111/j.1749-6632.1980.tb29532.x. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Katayama S., Ohmori T. Enhancement of hepatocarcinogenesis by sequential administration of chemicals: summation versus promotion effects. Carcinogenesis. 1981;2(11):1111–1117. doi: 10.1093/carcin/2.11.1111. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Ohmori T., Katayama S., Rice J. M. Alteration by phenobarbital of membrane-associated enzymes including gamma glutamyl transpeptidase in mouse liver neoplasms. Carcinogenesis. 1980;1(10):813–818. doi: 10.1093/carcin/1.10.813. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Telang S., Tong C. Inhibition of intercellular communication between liver cells by the liver tumor promoter 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane. Cancer Lett. 1981 Feb;11(4):339–344. doi: 10.1016/0304-3835(81)90100-2. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Watanabe K. Quantitative kinetics of development of N-2-fluorenylacetamide-induced, altered (hyperplastic) hepatocellular foci resistant to iron accumulation and of their reversion or persistence following removal of carcinogen. J Natl Cancer Inst. 1978 Jul;61(1):113–121. doi: 10.1093/jnci/61.1.113. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotti L. P., Chang C. C., Trosko J. E. Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science. 1979 Nov 30;206(4422):1089–1091. doi: 10.1126/science.493994. [DOI] [PubMed] [Google Scholar]