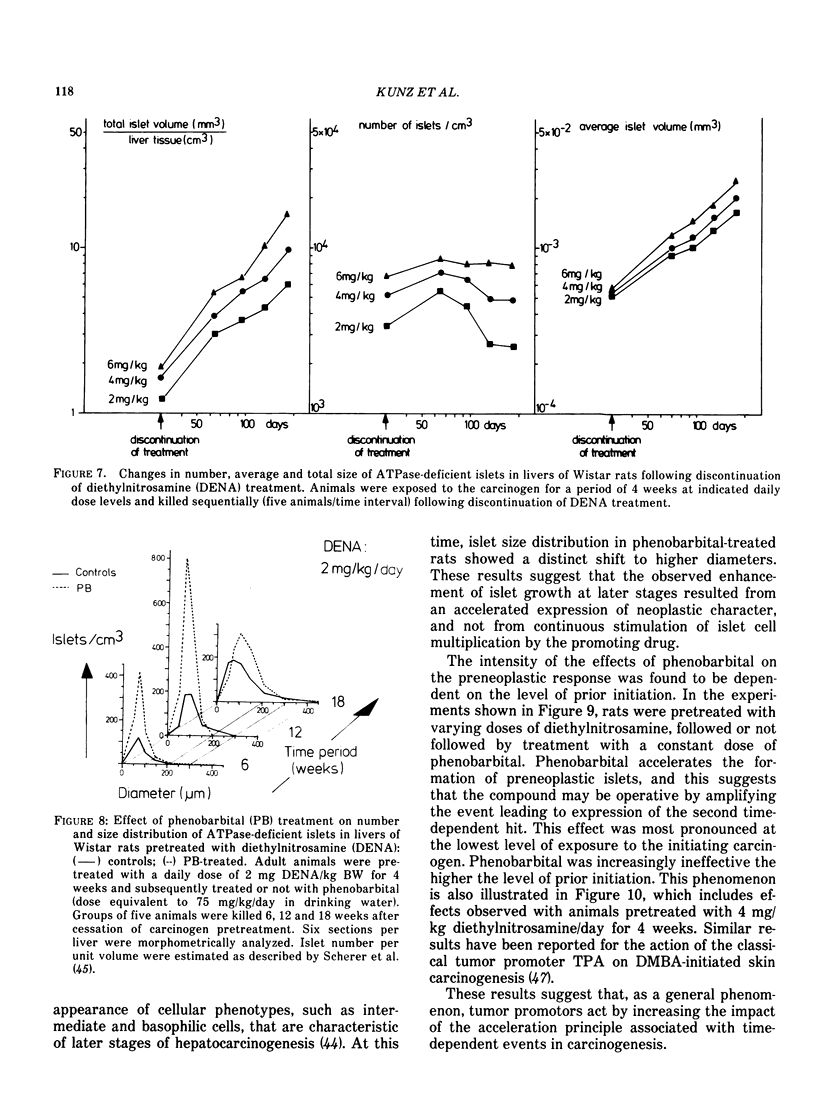
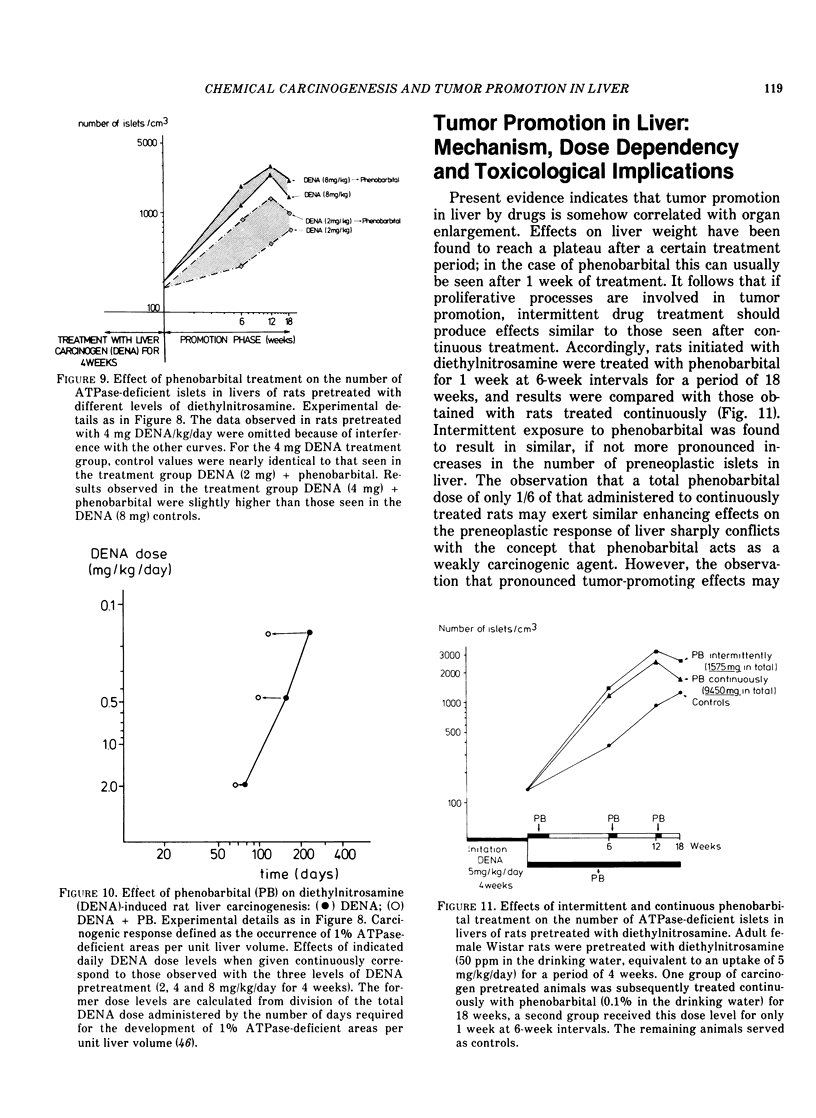
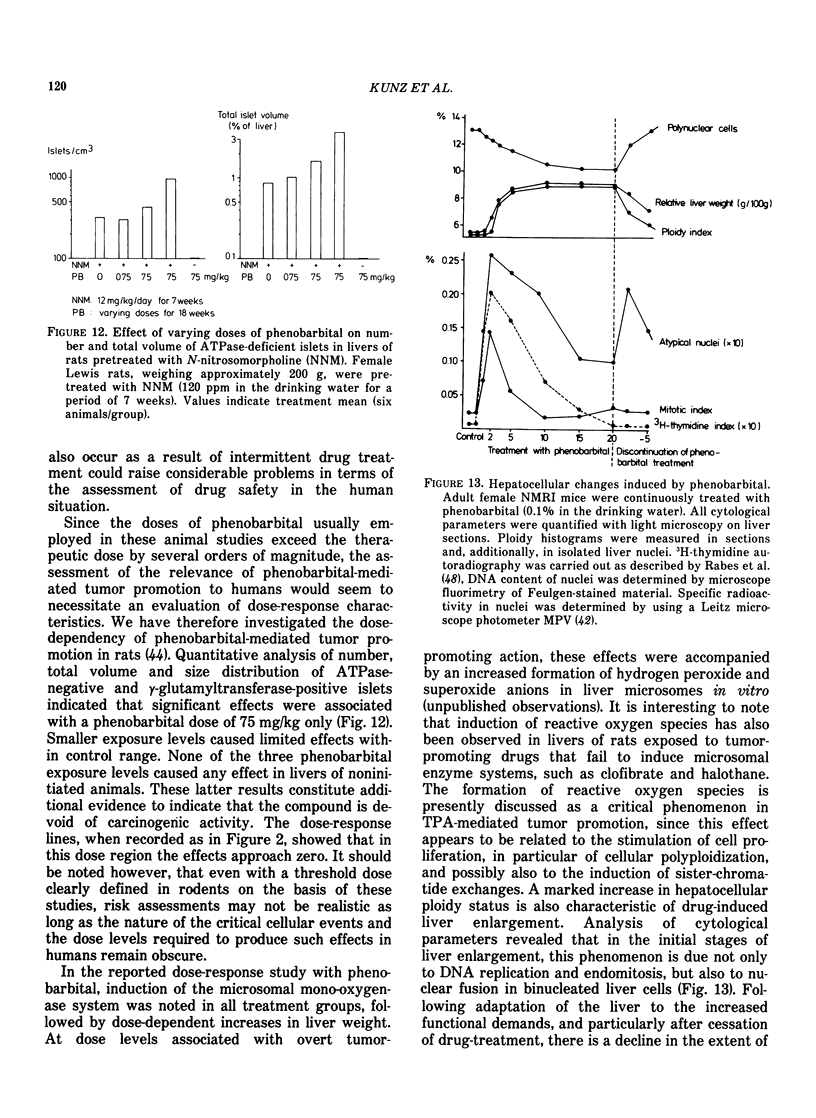
Abstract
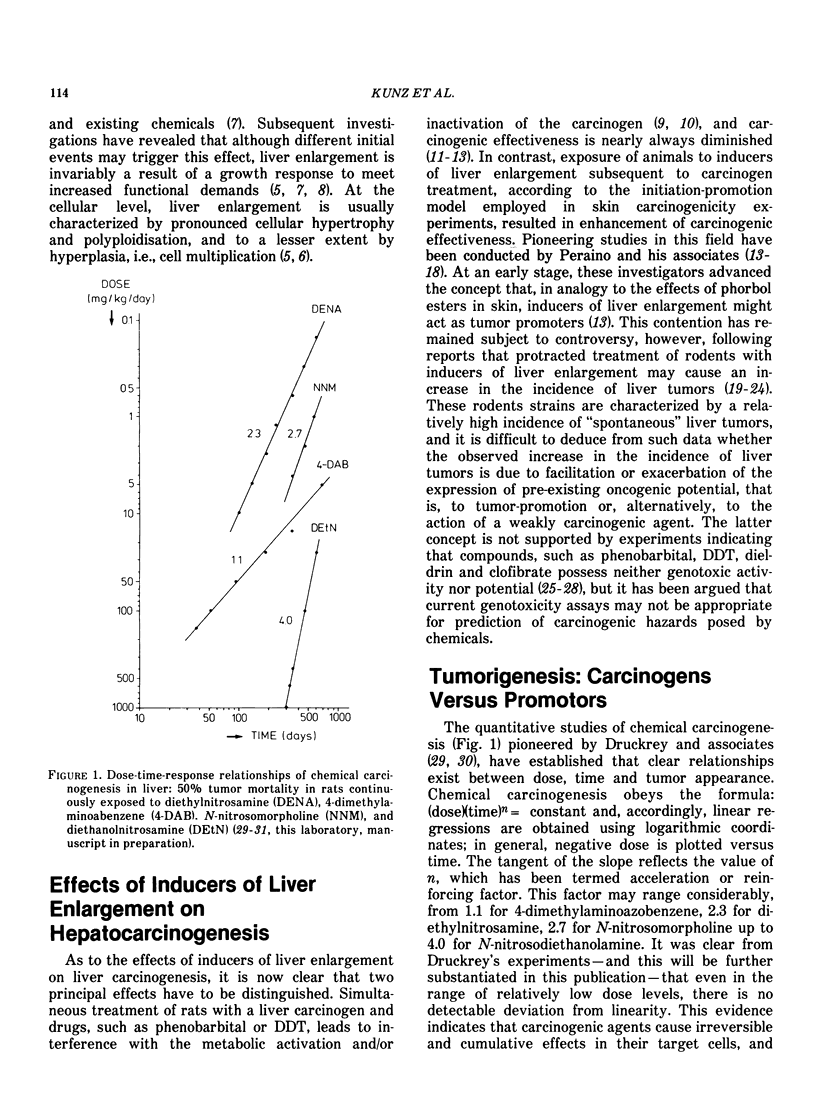
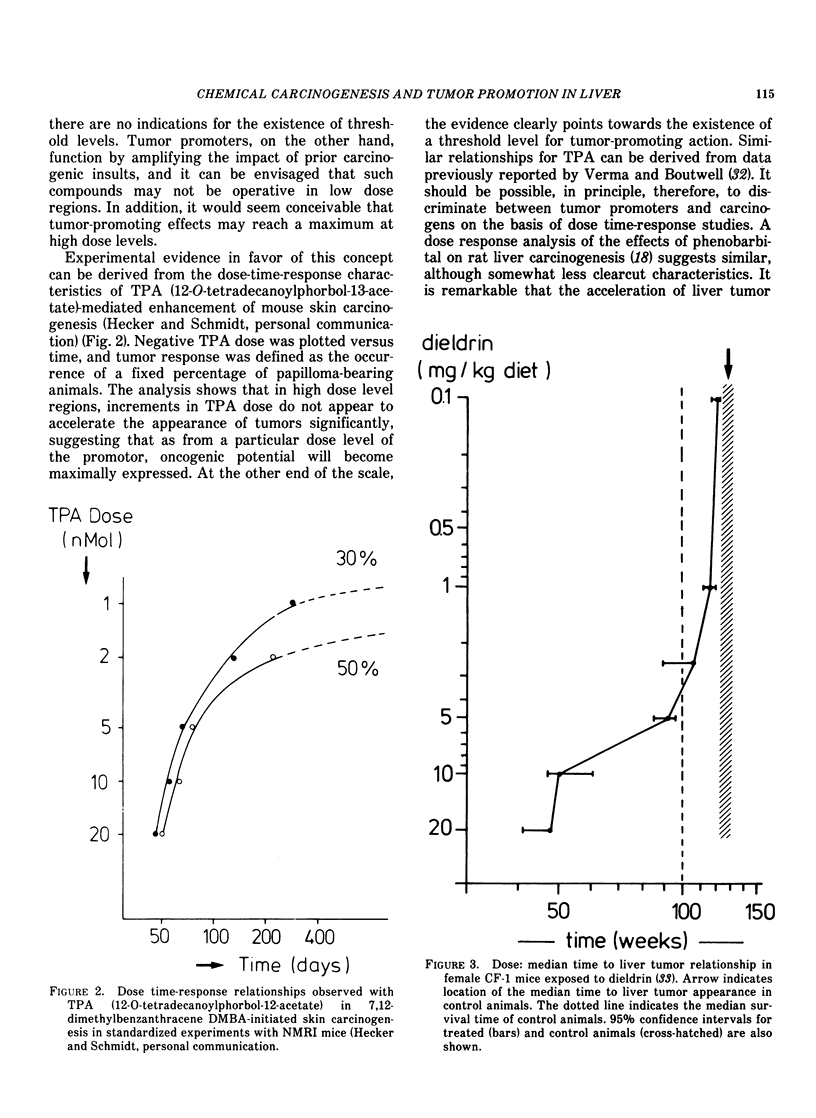
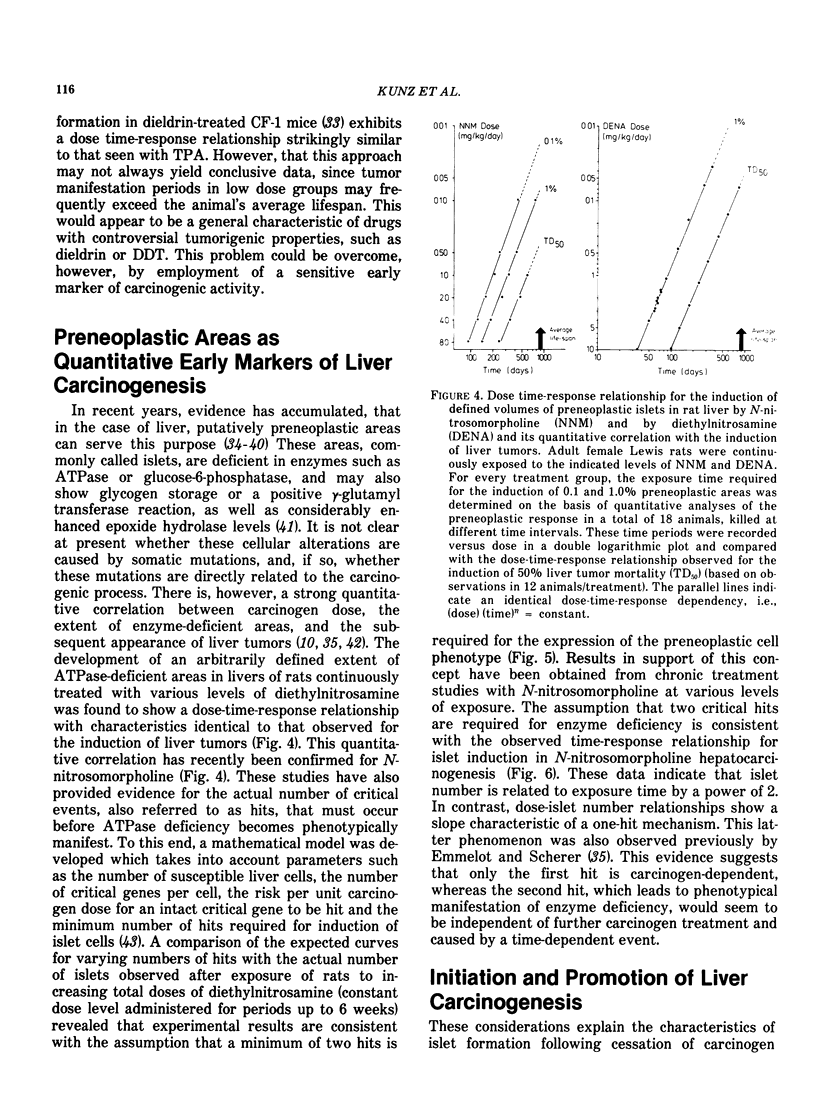
Chronic exposure of rodents to high dose levels of drugs, food additives and environmental chemicals frequently results in liver enlargement. Several of these compounds have been found to enhance the incidence of liver tumors in animals briefly exposed previously to hepatocarcinogens. Accordingly, it has been advanced that these agents act as tumor promoters. This contention has remained subject of controversy following reports that these substances may also cause liver tumors in noncarcinogen-treated rodents, particularly in those characterized by a relatively high incidence of "spontaneous" liver tumors. Since many of these chemicals are in common use, a crucial question would seem to be whether such effects are due to facilitation of the expression of pre-existing oncogenic potential, i.e., to tumor promotion, or to the synergistic action of weakly carcinogenic agents. As a result of mechanistic differences tumor promotion and syn-carcinogenesis must exhibit different dose-time-response characteristics, and, accordingly, it should be possible, in principle, to discriminate between these phenomena. However, since tumor manifestation periods in low-dose groups frequently exceed the animals average lifespan, this approach may not always yield conclusive data, unless a sensitive early marker of carcinogenic activity can be employed. There is evidence that enzyme-deficient preneoplastic areas in liver can be used for this purpose. A strong quantitative correlation between carcinogen dose, the extent of ATPase deficient areas, and the subsequent appearance of tumors has now been established for a number of hepatocarcinogens. Experimental data are consistent with the concept that two critical events (hits) are required for induction of ATPase deficiency in hepatocytes. The first hit is carcinogen-dependent, whereas the second hit would seem to be due to time-dependent event(s). Tumor-promoters, such as phenobarbital, were found to accelerate and increase formation of preneoplastic islets. This evidence, together with data indicating that the compound is devoid of carcinogenic potential, suggests that phenobarbital may be operative at relatively early stages of hepatocarcinogenesis by increasing the probability of the occurrence of the time-dependent second hit. Such effects are dose-dependent and appear to be related to the induction of liver enlargement. The changes in hepatocellular ploidy status and atypical nuclear figures observed during phenobarbital treatment and cessation thereof, suggest that this compound might induce abnormal redistributions of genetic material. It is postulated that these cytological changes may result in phenotypical manifestation of recessive oncogenic information.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel K. E., Schwarz M., Rickart R., Kunz W. Influences of inducers and inhibitors of the microsomal monooxygenase system on the alkylating intensity of dimethylnitrosamine in mice. J Cancer Res Clin Oncol. 1979 May 14;94(1):47–61. doi: 10.1007/BF00405349. [DOI] [PubMed] [Google Scholar]
- BARICH L. L., SCHWARZ J., BARICH D. Oral griseofulvin: a cocarcinogenic agent to methylcholanthrene induced cutaneous tumors. Cancer Res. 1962 Jan;22:53–55. [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Clemmesen J. Letter: Phenobarbitone, liver tumours, and thorotrast. Lancet. 1975 Jan 4;1(7897):37–38. doi: 10.1016/s0140-6736(75)92396-x. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Druckrey H., Preussmann R., Ivankovic S., Schmähl D. Organotrope carcinogene Wirkungen bei 65 verschiedenen N-Nitroso-Verbindungen an BD-Ratten. Z Krebsforsch. 1967;69(2):103–201. [PubMed] [Google Scholar]
- Emmelot P., Scherer E. The first relevant cell stage in rat liver carcinogenesis. A quantitative approach. Biochim Biophys Acta. 1980 May 6;605(2):247–304. doi: 10.1016/0304-419x(80)90006-2. [DOI] [PubMed] [Google Scholar]
- Friedrich-Freksa H., Gössner W., Börner P. Histochemische Untersuchungen der Cancerogenese in der Rattenleber nach Dauergaben von Diäthylnitrosamin. Z Krebsforsch. 1969;72(3):226–239. [PubMed] [Google Scholar]
- Friedrich-Freksa H., Papadopulu G., Gössner W. Histochemische Untersuchungen der Cancerogenese in der Rattenleber nach zeitlich begrenzter Verabfolgung von Diäthylnitrosamin. Z Krebsforsch. 1969;72(3):240–253. [PubMed] [Google Scholar]
- Hoch-Ligeti C., Argus M. F., Arcos J. C. Combined carcinogenic effects of dimethylnitrosamine and 3-methylcholanthrene in the rat. J Natl Cancer Inst. 1968 Mar;40(3):535–549. [PubMed] [Google Scholar]
- Kuhlmann W. D., Krischan R., Kunz W., Guenthner T. M., Oesch F. Focal elevation of liver microsomal epoxide hydrolase in early preneoplastic stages and its behaviour in the further course of hepatocarcinogenesis. Biochem Biophys Res Commun. 1981 Jan 30;98(2):417–423. doi: 10.1016/0006-291x(81)90856-1. [DOI] [PubMed] [Google Scholar]
- Kunz W., Schaude G., Schwarz M., Tennekes H. Quantitative aspects of drug-mediated tumour promotion in liver and its toxicological implications. Carcinog Compr Surv. 1982;7:111–125. [PubMed] [Google Scholar]
- Kunz W., Schaude G., Thomas C. Die Beeinflussung der Nitrosamincarcinogenese durch Phenobarbital und Halogenkohlenwasserstoffe. Z Krebsforsch. 1969;72(3):291–301. [PubMed] [Google Scholar]
- Peraino C., Fry R. J., Staffeldt E. Brief communication: Enhancement of spontaneous hepatic tumorigenesis in C3H mice by dietary phenobarbital. J Natl Cancer Inst. 1973 Oct;51(4):1349–1350. doi: 10.1093/jnci/51.4.1349. [DOI] [PubMed] [Google Scholar]
- Peraino C., Fry R. J., Staffeldt E., Christopher J. P. Comparative enhancing effects of phenobarbital, amobarbital, diphenylhydantoin, and dichlorodiphenyltrichloroethane on 2-acetylaminofluorene-induced hepatic tumorigenesis in the rat. Cancer Res. 1975 Oct;35(10):2884–2890. [PubMed] [Google Scholar]
- Peraino C., Fry R. J., Staffeldt E. Effects of varying the onset and duration of exposure to phenobarbital on its enhancement of 2-acetylaminofluorene-induced hepatic tumorigenesis. Cancer Res. 1977 Oct;37(10):3623–3627. [PubMed] [Google Scholar]
- Peraino C., Fry R. J., Staffeldt E., Kisieleski W. E. Effects of varying the exposure to phenobarbital on its enhancement of 2-acetylaminofluorene-induced hepatic tumorigenesis in the rat. Cancer Res. 1973 Nov;33(11):2701–2705. [PubMed] [Google Scholar]
- Peraino C., Fry R. J., Staffeldt E. Reduction and enhancement by phenobarbital of hepatocarcinogenesis induced in the rat by 2-acetylaminofluorene. Cancer Res. 1971 Oct;31(10):1506–1512. [PubMed] [Google Scholar]
- Peraino C., Staffeldt E. F., Haugen D. A., Lombard L. S., Stevens F. J., Fry R. J. Effects of varying the dietary concentration of phenobarbital on its enhancement of 2-acetylaminofluorene-induced hepatic tumorigenesis. Cancer Res. 1980 Sep;40(9):3268–3273. [PubMed] [Google Scholar]
- Pitot H. C., Barsness L., Goldsworthy T., Kitagawa T. Biochemical characterisation of stages of hepatocarcinogenesis after a single dose of diethylnitrosamine. Nature. 1978 Feb 2;271(5644):456–458. doi: 10.1038/271456a0. [DOI] [PubMed] [Google Scholar]
- Rabes H. M., Scholze P., Jantsch B. Growth kinetics of diethylnitrosamine-induced, enzyme-deficient "preneoplastic" liver cell populations in vivo and in vitro. Cancer Res. 1972 Nov;32(11):2577–2586. [PubMed] [Google Scholar]
- Rajewsky M. F., Dauber W., Frankenberg H. Liver carcinogenesis by diethylnitrosamine in the rat. Science. 1966 Apr 1;152(3718):83–85. doi: 10.1126/science.152.3718.83. [DOI] [PubMed] [Google Scholar]
- Schauer A., Kunze E. Enzymhistochemische und autoradiographische Untersuchungen während der Cancerisierung der Rattenleber mit Diäthylnitrosamin. Z Krebsforsch. 1968;70(3):252–266. [PubMed] [Google Scholar]
- Scherer E., Hoffmann M., Emmelot P., Friedrich-Freksa M. Quantitative study on foci of altered liver cells induced in the rat by a single dose of diethylnitrosamine and partial hepatectomy. J Natl Cancer Inst. 1972 Jul;49(1):93–106. [PubMed] [Google Scholar]
- Schulte-Hermann R. Induction of liver growth by xenobiotic compounds and other stimuli. CRC Crit Rev Toxicol. 1974 Sep;3(1):97–158. doi: 10.3109/10408447409079856. [DOI] [PubMed] [Google Scholar]
- Stenbäck F., Peto R., Shubik P. Initiation and promotion at different ages and doses in 2200 mice. III. Linear extrapolation from high doses may underestimate low-dose tumour risks. Br J Cancer. 1981 Jul;44(1):24–34. doi: 10.1038/bjc.1981.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenberg J. A., Petzold G. L., Harbach P. R. In vitro DNA damage/alkaline elution assay for predicting carcinogenic potential. Biochem Biophys Res Commun. 1976 Sep 20;72(2):732–738. doi: 10.1016/s0006-291x(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Tennekes H. A., Edler L., Kunz H. W. Dose-response analysis of the enhancement of liver tumour formation in CF-1 mice by dieldrin. Carcinogenesis. 1982;3(8):941–945. doi: 10.1093/carcin/3.8.941. [DOI] [PubMed] [Google Scholar]
- Tennekes H. A., Wright A. S., Dix K. M., Koeman J. H. Effects of dieldrin, diet, and bedding on enzyme function and tumor incidence in livers of male CF-1 mice. Cancer Res. 1981 Sep;41(9 Pt 1):3615–3620. [PubMed] [Google Scholar]
- Thorpe E., Walker A. I. The toxicology of dieldrin (HEOD). II. Comparative long-term oral toxicity studies in mice with dieldrin, DDT, phenobarbitone, -BHC and -BHC. Food Cosmet Toxicol. 1973 Jun;11(3):433–442. doi: 10.1016/0015-6264(73)90008-4. [DOI] [PubMed] [Google Scholar]
- Tomatis L., Turusov V., Day N., Charles R. T. The effect of long-term exposure to DDT on CF-1 MICE. Int J Cancer. 1972 Nov;10(3):489–506. doi: 10.1002/ijc.2910100308. [DOI] [PubMed] [Google Scholar]
- Walker A. I., Thorpe E., Stevenson D. E. The toxicology of dieldrin (HEOD). I. Long-term oral toxicity studies in mice. Food Cosmet Toxicol. 1973 Jun;11(3):415–432. doi: 10.1016/0015-6264(73)90007-2. [DOI] [PubMed] [Google Scholar]
- Wright A. S., Akintonwa D. A., Wooder M. F. Studies on the interactions of dieldrin with mammalian liver cells at the subcellular level. Ecotoxicol Environ Saf. 1977 Jun;1(1):7–16. doi: 10.1016/0147-6513(77)90013-6. [DOI] [PubMed] [Google Scholar]


