Abstract
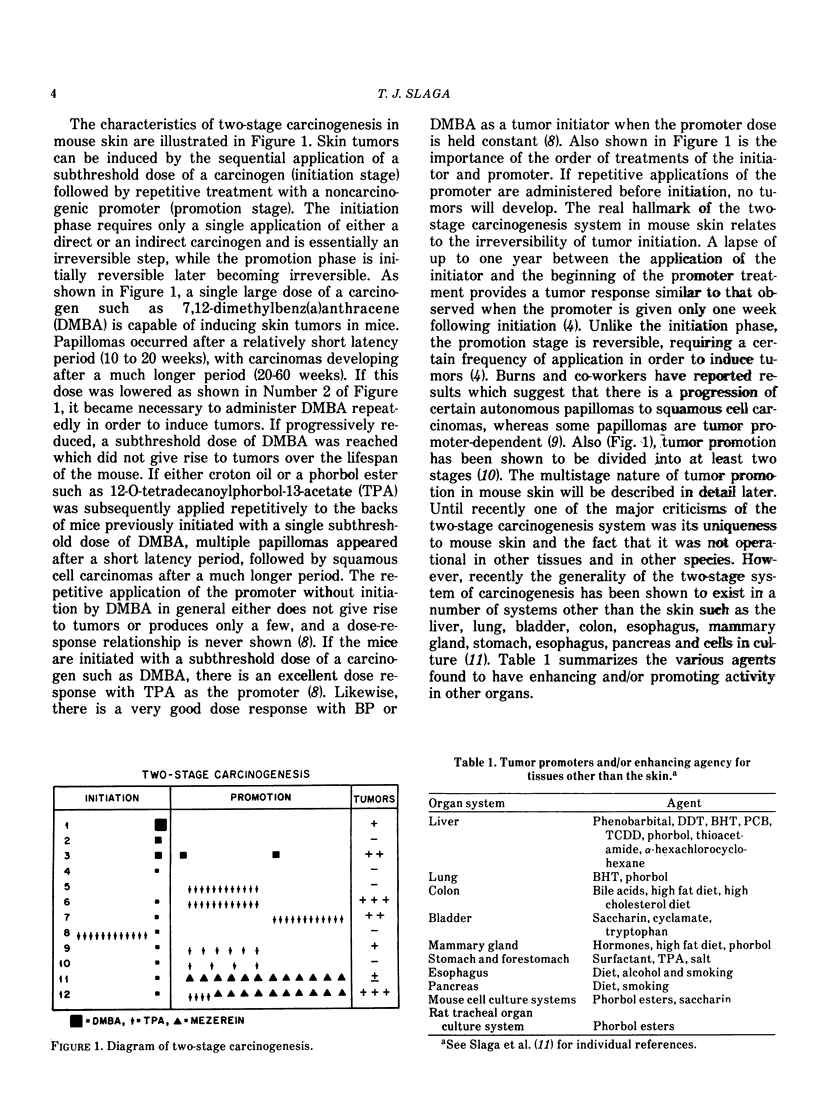
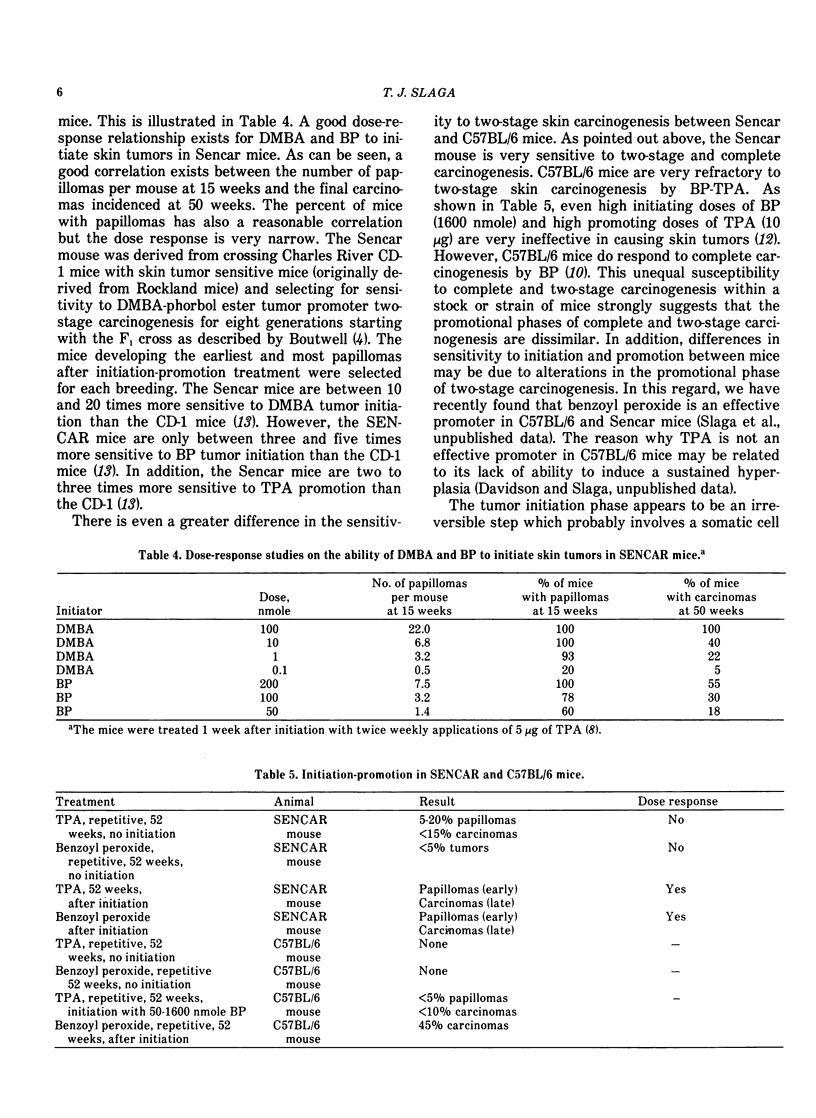
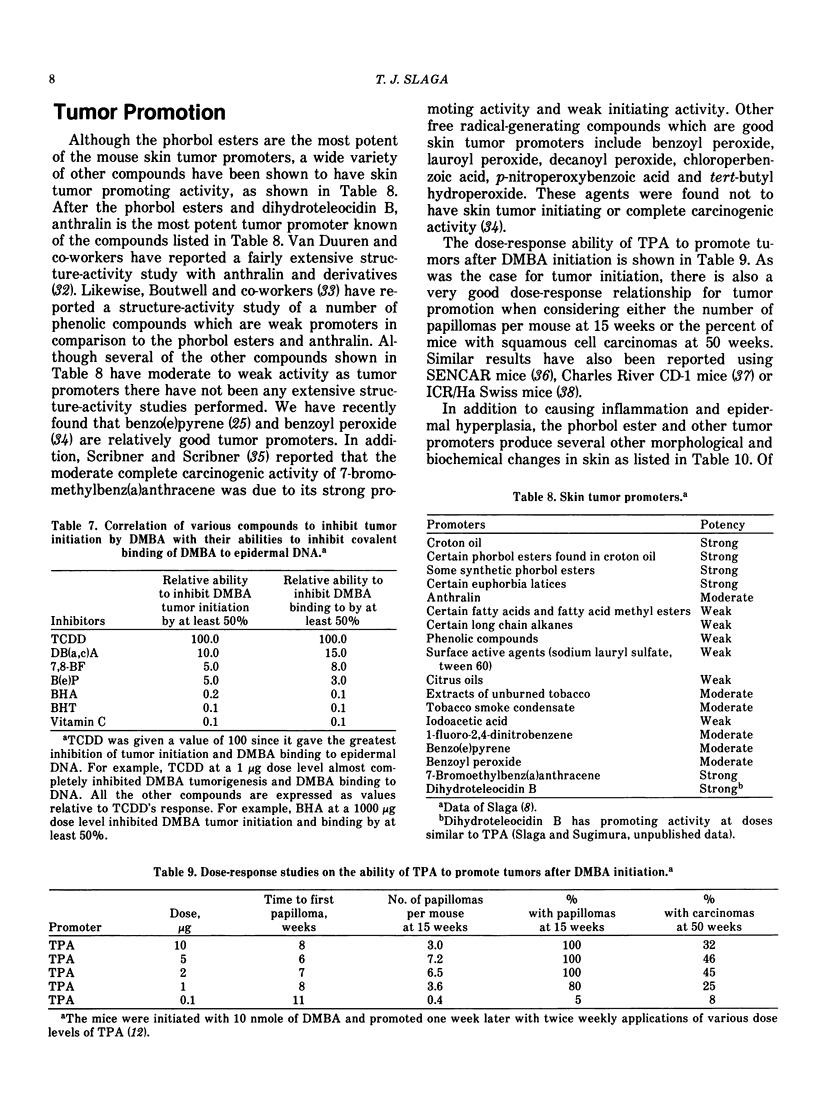
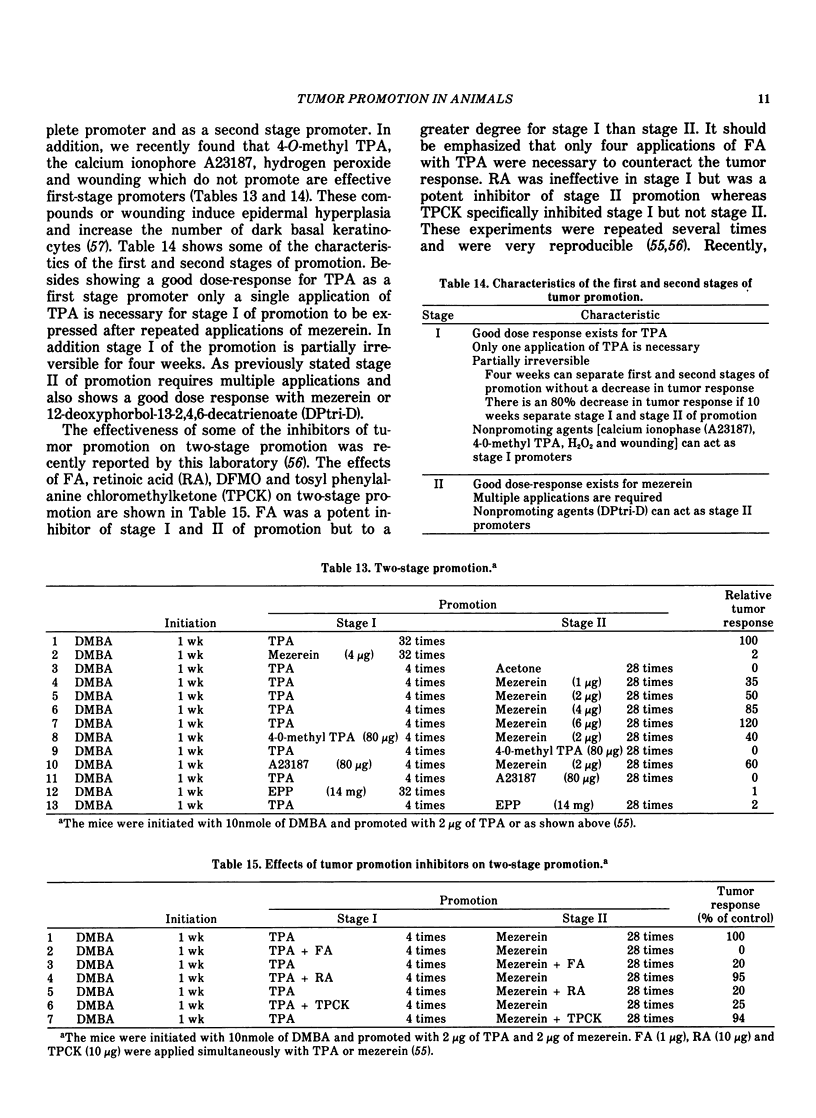
Our present understanding of two-stage carcinogenesis encompasses almost four decades of research. Evidence for chemical promotion or cocarcinogenesis was first provided by Berenblum, who reported that a regimen of croton oil (weak or noncarcinogenic) applied alternately with small doses of benzo(a)pyrene (BP) to mouse skin induced a larger number of tumors than BP alone. Subsequently, Moltram found that a single subcarcinogenic dose of BP followed by multiple applications of croton oil could induce a large number of skin tumors. These investigations as well as a number of others, such as Boutwell, Van Duuren and Hecker, were responsible in defining many important aspects of the initiation and promotion of two-stage carcinogenesis. The initiation stage in mouse skin requires only a single application of either a direct-acting carcinogen or a procarcinogen and is essentially an irreversible step which as data suggests probably involves a somatic cell mutation. The promotion stage in mouse skin can be accomplished by a wide variety of weak or noncarcinogenic agents and is initially reversible later becoming irreversible. Current information suggests that skin tumor promoters are not mutagenic but bring about a number of important epigenetic changes, such as epidermal hyperplasia, and an increase in polyamines, prostaglandins and dark basal keratinocytes as well as other embryonic conditions. Recently, tumor promotion in mouse skin was shown to consist of at least two stages, in which each stage can be accomplished by either a known promoter or a weak or nonpromoting agent. Some of the important characteristics of the first stage of promotion are: (1) only one application of a first-stage promoter, such as phorbol ester tumor promoters, calcium ionophore A23187, hydrogen peroxide and wounding is needed; (2) the action is partially irreversible; (3) an increase in dark basal keratinocytes and prostaglandins is important; and (4) such an increase can be inhibited by antiinflammatory steroids and protease inhibitors. The second stage of promotion is initially reversible but later becomes irreversible. Polyamines and epidermal cell proliferation are important events in the second stage of promotion. A number of weak or nonpromoting agents, such as mezerein, are effective second-stage promoters which can be counteracted by retinoic acid, antiinflammatory steroids and polyamine synthesis inhibitors. Although skin tumor promotion has been extensively studied in mice, not all strains and stocks of mice are susceptible to phorbol ester tumor promoters. In this regard, the C57BL/6 mice appear to be fairly resistant to phorbol ester tumor promoters. In addition, not all species are equally susceptible to phorbol ester tumor promotion.
Recently the generality of the two-stage system of inducing tumors has been shown to exist in a number of experimental carcinogenesis systems, such as the liver, bladder, lung, colon, esophagus, stomach, mammary gland, pancreas and cells in culture. In these systems, a wide variety of promoting agents such as diet, bile acids, hormones, saccharin, tryptophan, phenobarbital, polychlorinated biphenyls, polybrominated biphenyls and butylated hydroxytoluene have been used to accomplish the tumor promotion stage. It is not presently known if other experimental carcinogenesis systems and the induction of human cancer involves a series of stages similar to that in the mouse skin.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOUTWELL R. K., BOSCH D. K. The tumor-promoting action of phenol and related compounds for mouse skin. Cancer Res. 1959 May;19(4):413–424. [PubMed] [Google Scholar]
- BOUTWELL R. K. SOME BIOLOGICAL ASPECTS OF SKIN CARCINOGENISIS. Prog Exp Tumor Res. 1964;4:207–250. doi: 10.1159/000385978. [DOI] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. EVIDENCE FOR THE BINDING OF POLYNUCLEAR AROMATIC HYDROCARBONS TO THE NUCLEIC ACIDS OF MOUSE SKIN: RELATION BETWEEN CARCINOGENIC POWER OF HYDROCARBONS AND THEIR BINDING TO DEOXYRIBONUCLEIC ACID. Nature. 1964 May 23;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- Berry D. L., Slaga T. J., DiGiovanni J., Juchau M. R. Studies with chlorinated dibenzo-p-dioxins, polybrominated biphenyls, and polychlorinated biphenyls in a two-stage system of mouse skin tumorigenesis: potent anticarcinogenic effects. Ann N Y Acad Sci. 1979 May 31;320:405–414. doi: 10.1111/j.1749-6632.1979.tb56621.x. [DOI] [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Cohen G. M., Bracken W. M., Iyer R. P., Berry D. L., Selkirk J. K., Slaga T. J. Anticarcinogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene tumor initiation and its relationship to DNA binding. Cancer Res. 1979 Oct;39(10):4027–4033. [PubMed] [Google Scholar]
- De Young L. M., Mufson R. A., Boutwell R. K. An apparent inactivation of initiated cells by the potent inhibitor of two-stage mouse skin tumorigenesis, bis(2-chloroethyl)sulfide. Cancer Res. 1977 Dec;37(12):4590–4594. [PubMed] [Google Scholar]
- DiGiovanni J., Berry D. L., Juchau M. R., Slaga T. J. 2,3,7,8-Tetrachlorodibenzo-P-dioxin: potent anticarcinogenic activity in CD-1 mice. Biochem Biophys Res Commun. 1979 Feb 14;86(3):577–584. doi: 10.1016/0006-291x(79)91752-2. [DOI] [PubMed] [Google Scholar]
- DiGiovanni J., Slaga T. J., Boutwell R. K. Comparison of the tumor-initiating activity of 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene in female SENCAR and CS-1 mice. Carcinogenesis. 1980 May;1(5):381–389. doi: 10.1093/carcin/1.5.381. [DOI] [PubMed] [Google Scholar]
- Gelboin H. V., Levy H. B. Polyinosinic-polycytidylic acid inhibits chemically induced tumorigenesis in mouse skin. Science. 1970 Jan 9;167(3915):205–207. doi: 10.1126/science.167.3915.205. [DOI] [PubMed] [Google Scholar]
- Huberman E. Mutagenesis and cell transformation of mammalian cells in culture by chemical carcinogens. J Environ Pathol Toxicol. 1978 Sep-Oct;2(1):29–42. [PubMed] [Google Scholar]
- Klein-Szanto A. J., Major S. K., Slaga T. J. Induction of dark keratinocytes by 12-O-tetradecanoylphorbol-13-acetate and mezerein as an indicator of tumor-promoting efficiency. Carcinogenesis. 1980 May;1(5):399–406. doi: 10.1093/carcin/1.5.399. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto A. J., Slaga T. J. Numerical variation of dark cells in normal and chemically induced hyperplastic epidermis with age of animal and efficiency of tumor promoter. Cancer Res. 1981 Nov;41(11 Pt 1):4437–4440. [PubMed] [Google Scholar]
- McCann J., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proc Natl Acad Sci U S A. 1976 Mar;73(3):950–954. doi: 10.1073/pnas.73.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson R. A., Fischer S. M., Verma A. K., Gleason G. L., Slaga T. J., Boutwell R. K. Effects of 12-O-tetradecanoylphorbol-13-acetate and mezerein on epidermal ornithine decarboxylase activity, isoproterenol-stimulated levels of cyclic adenosine 3':5'-monophosphate, and induction of mouse skin tumors in vivo. Cancer Res. 1979 Dec;39(12):4791–4795. [PubMed] [Google Scholar]
- O'Brien T. G., Simsiman R. C., Boutwell R. K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975 Jul;35(7):1662–1670. [PubMed] [Google Scholar]
- Phillips D. H., Grover P. L., Sims P. A quantitative determination of the covalent binding of a series of polycylic hydrocarbons to DNA in mouse skin. Int J Cancer. 1979 Feb;23(2):201–208. doi: 10.1002/ijc.2910230211. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Grover P. L., Sims P. The covalent binding of polycyclic hydrocarbons to DNA in the skin of mice of different strains. Int J Cancer. 1978 Oct 15;22(4):487–494. doi: 10.1002/ijc.2910220419. [DOI] [PubMed] [Google Scholar]
- Raick A. N., Burdzy K. Ultrastructural and biochemical changes induced in mouse epidermis by a hyperplastic agent, ethylphenylpropiolate. Cancer Res. 1973 Oct;33(10):2221–2230. [PubMed] [Google Scholar]
- Raick A. N. Cell differentiation and tumor-promoting action in skin carcinogenesis. Cancer Res. 1974 Nov;34(11):2915–2925. [PubMed] [Google Scholar]
- Raick A. N. Cell proliferation and promoting action in skin carcinogenesis. Cancer Res. 1974 May;34(5):920–926. [PubMed] [Google Scholar]
- Raick A. N. Ultrastructural, histological, and biochemical alterations produced by 12-O-tetradecanoyl-phorbol-13-acetate on mouse epidermis and their relevance to skin tumor promotion. Cancer Res. 1973 Feb;33(2):269–286. [PubMed] [Google Scholar]
- Schinitsky M. R., Hyman L. R., Blazkovec A. A., Burkholder P. M. Bacillus Calmette-Guérin vaccination and skin tumor promotion with croton oil in mice. Cancer Res. 1973 Apr;33(4):659–663. [PubMed] [Google Scholar]
- Schwarz J. A., Viaje A., Slaga T. J. Fluocinolone acetonide: a potent inhibitor of mouse skin tumor promotion and epidermal DNA synthesis. Chem Biol Interact. 1977 Jun;17(3):331–347. doi: 10.1016/0009-2797(77)90096-5. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Boutwell R. K. Inhibition of the tumor-initiating ability of the potent carcinogen 7,12-dimethylbenz(a)anthracene by the weak tumor initiator 1,2,3,4-dibenzanthracene. Cancer Res. 1977 Jan;37(1):128–133. [PubMed] [Google Scholar]
- Slaga T. J., Bowden G. T., Boutwell R. K. Acetic acid, a potent stimulator of mouse epidermal macromolecular synthesis and hyperplasia but with weak tumor-promoting ability. J Natl Cancer Inst. 1975 Oct;55(4):983–987. doi: 10.1093/jnci/55.4.983. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Bracken W. M. The effects of antioxidants on skin tumor initiation and aryl hydrocarbon hydroxylase. Cancer Res. 1977 Jun;37(6):1631–1635. [PubMed] [Google Scholar]
- Slaga T. J., Buty S. G., Thompson S., Bracken W. M., Viaje A. A kinetic study on the in vitro covalent binding of polycyclic hydrocarbons to nucleic acids using epidermal homogenates as the activating system. Cancer Res. 1977 Sep;37(9):3126–3131. [PubMed] [Google Scholar]
- Slaga T. J., Fischer S. M., Nelson K., Gleason G. L. Studies on the mechanism of skin tumor promotion: evidence for several stages in promotion. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3659–3663. doi: 10.1073/pnas.77.6.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Jecker L., Bracken W. M., Weeks C. E. The effects of weak or non-carcinogenic polycyclic hydrocarbons on 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene skin tumor-initiation. Cancer Lett. 1979 Jun;7(1):51–59. doi: 10.1016/s0304-3835(79)80076-2. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Fischer S. M., Weeks C. E., Nelson K., Major S. Studies on mechanism of action of anti-tumor-promoting agents: their specificity in two-stage promotion. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2251–2254. doi: 10.1073/pnas.77.4.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Triplett L. L., Yotti L. P., Trosko K. E. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981 Aug 28;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Scribner J. D., Rice J. M., Das S. B., Thompson S. Inhibition by dexamethasone of intracellular binding of phorbol esters to mouse skin. J Natl Cancer Inst. 1974 May;52(5):1611–1618. doi: 10.1093/jnci/52.5.1611. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Thompson S., Berry D. L., Digiovanni J., Juchau M. R., Viaje A. The effects of benzoflavones on polycyclic hydrocarbon metabolism and skin tumor initiation. Chem Biol Interact. 1977 Jun;17(3):297–312. doi: 10.1016/0009-2797(77)90093-x. [DOI] [PubMed] [Google Scholar]
- Van Duuren B. L., Sivak A., Segal A., Seidman I., Katz C. Dose-response studies with a pure tumor-promoting agent, phorbol myristate acetate. Cancer Res. 1973 Sep;33(9):2166–2172. [PubMed] [Google Scholar]
- Van Duuren B. L. Tumor-promoting agents in two-stage carcinogenesis. Prog Exp Tumor Res. 1969;11:31–68. doi: 10.1159/000391388. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Rice H. M., Shapas B. G., Boutwell R. K. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced ornithine decarboxylase activity in mouse epidermis by vitamin A analogs (retinoids). Cancer Res. 1978 Mar;38(3):793–801. [PubMed] [Google Scholar]
- Wattenberg L. W. Inhibition of chemical carcinogenesis. J Natl Cancer Inst. 1978 Jan;60(1):11–18. doi: 10.1093/jnci/60.1.11. [DOI] [PubMed] [Google Scholar]
- Weeks C. E., Slaga T. J., Hennings H., Gleason G. L., Bracken W. M. Inhibition of phorbol ester-induced tumor promotion in mice by vitamin A analog and anti-inflammatory steroid. J Natl Cancer Inst. 1979 Aug;63(2):401–406. [PubMed] [Google Scholar]



