Abstract
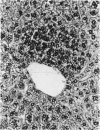
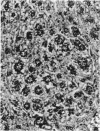
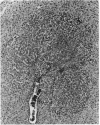
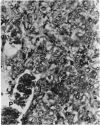
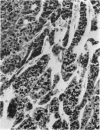
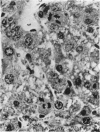
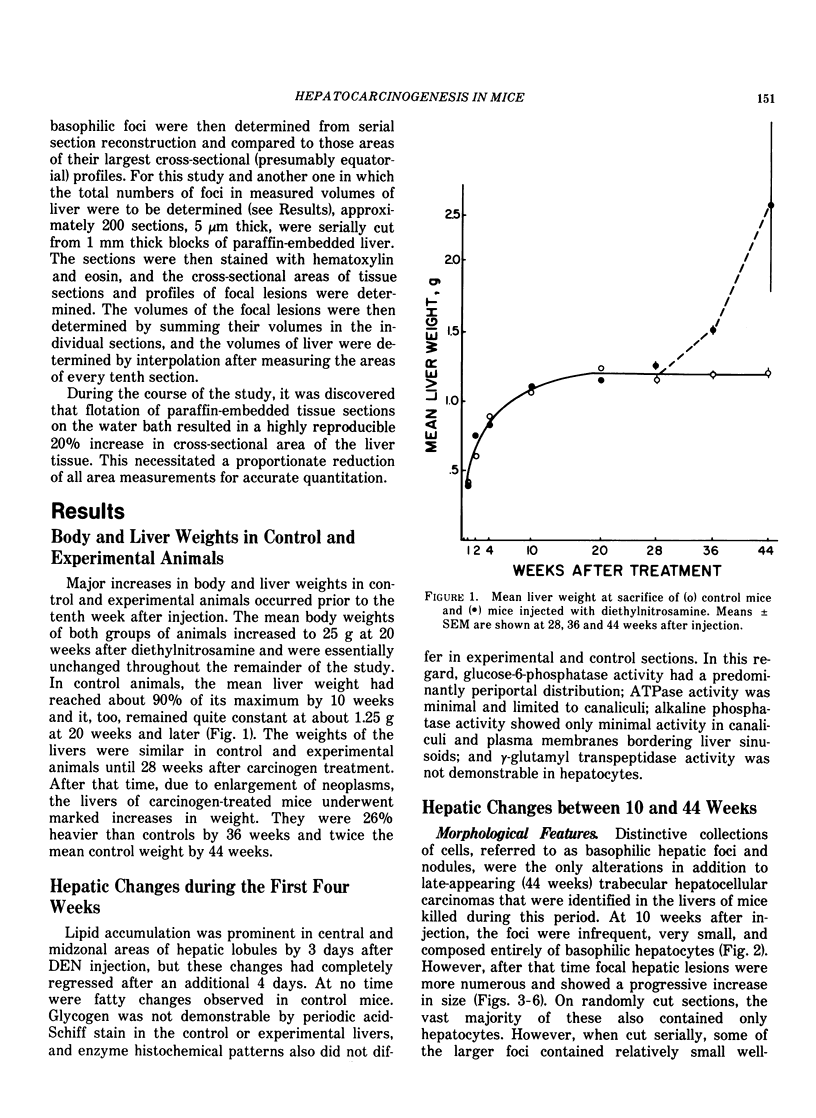
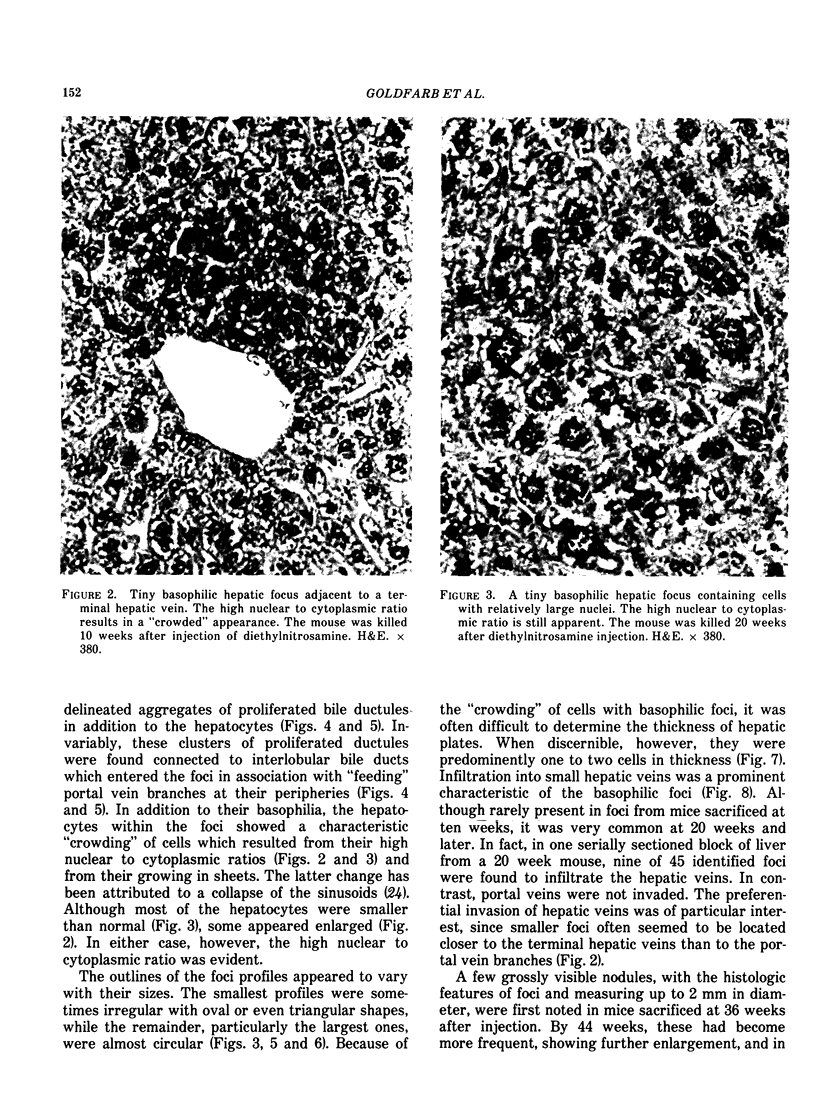
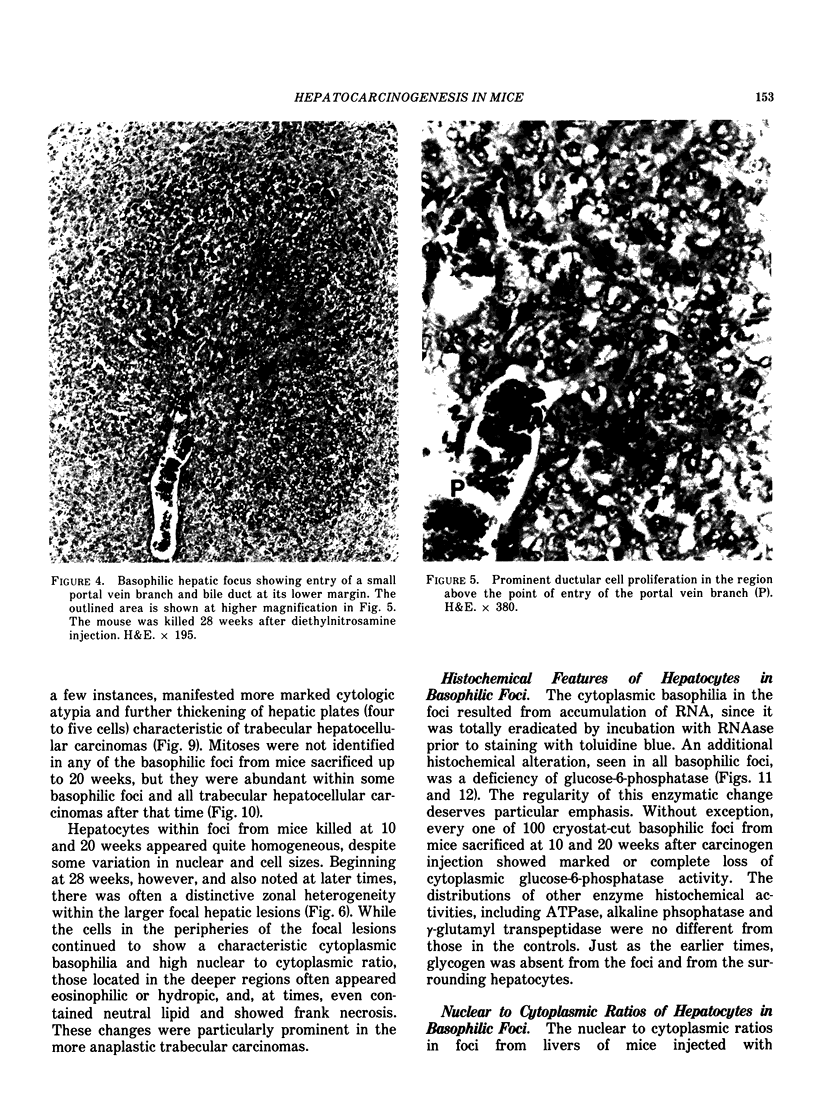
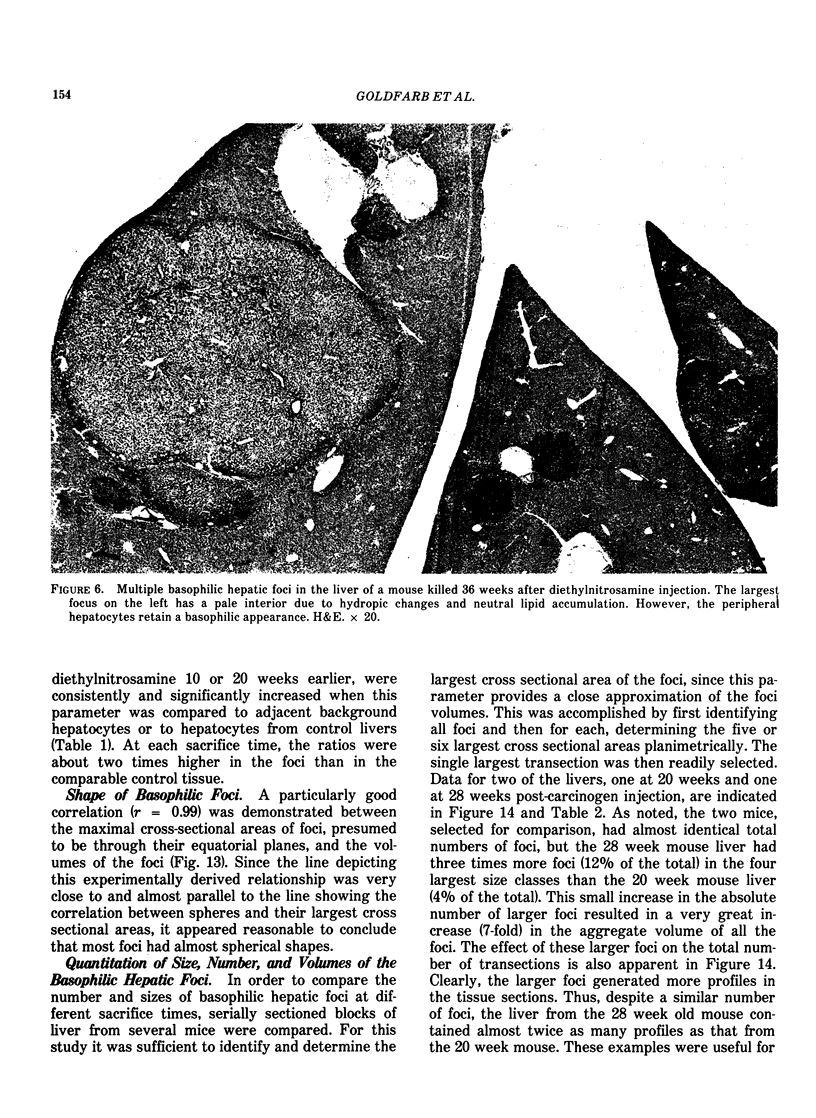
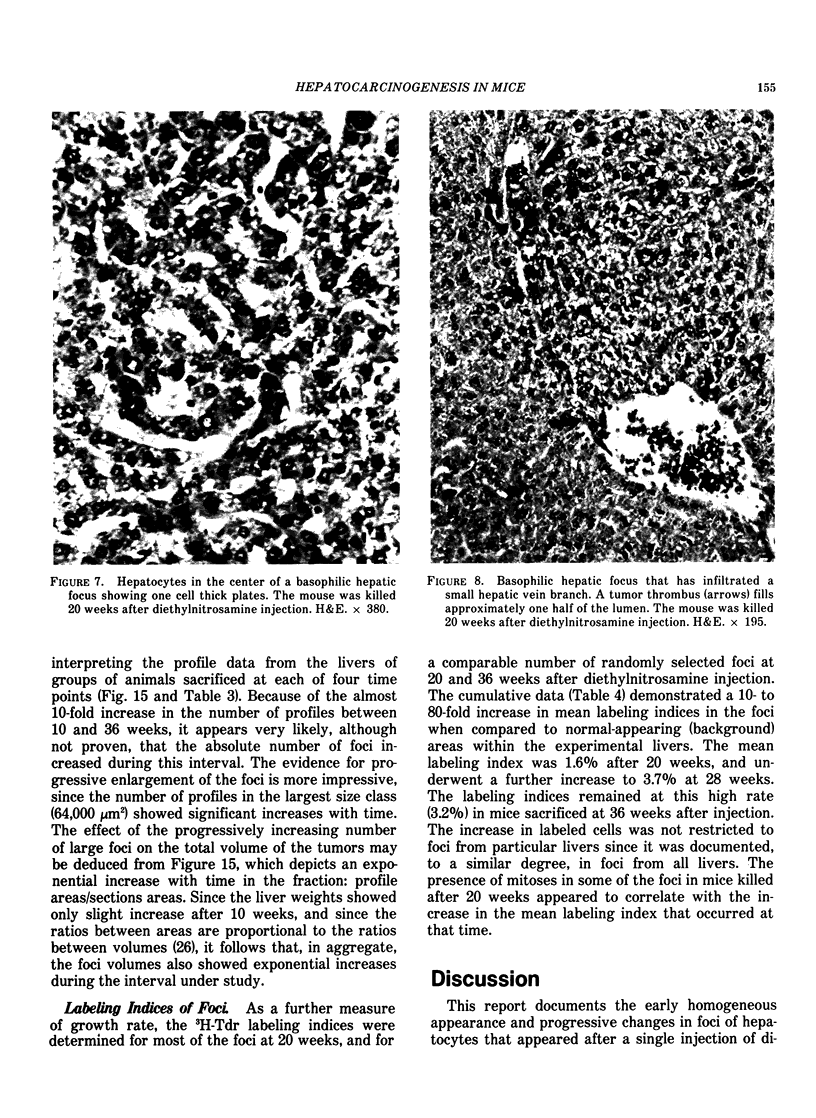
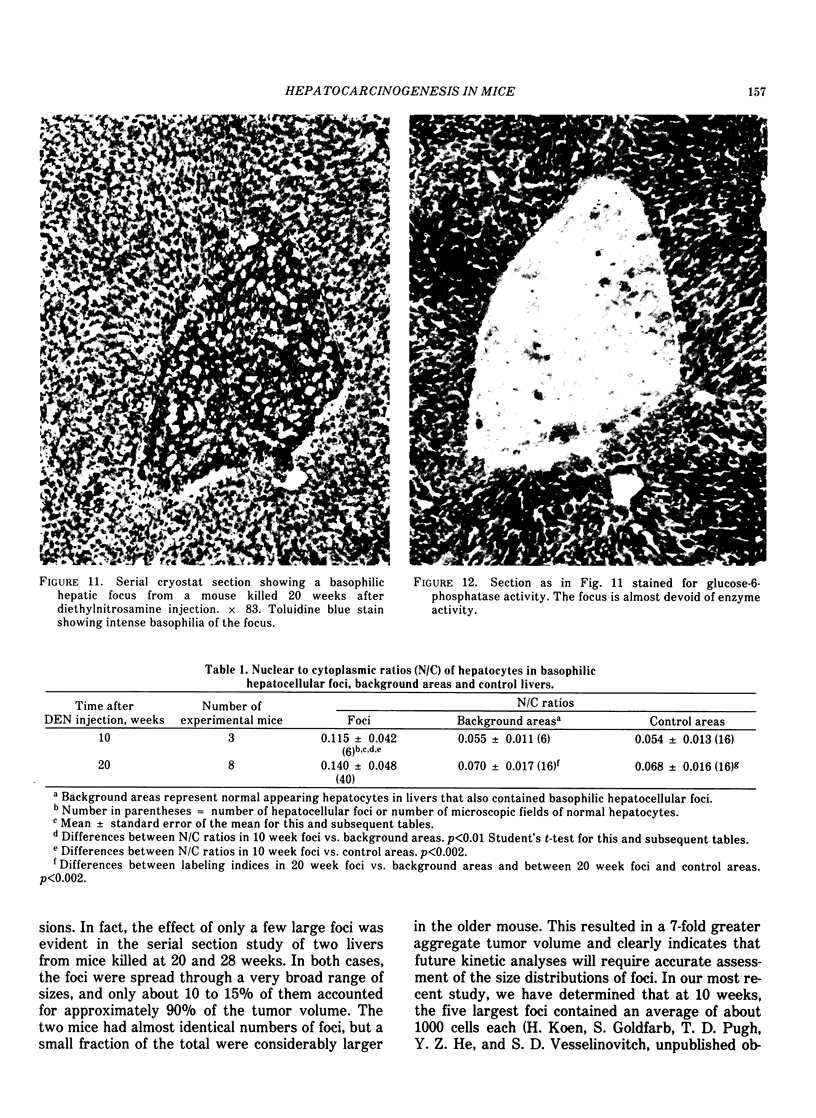
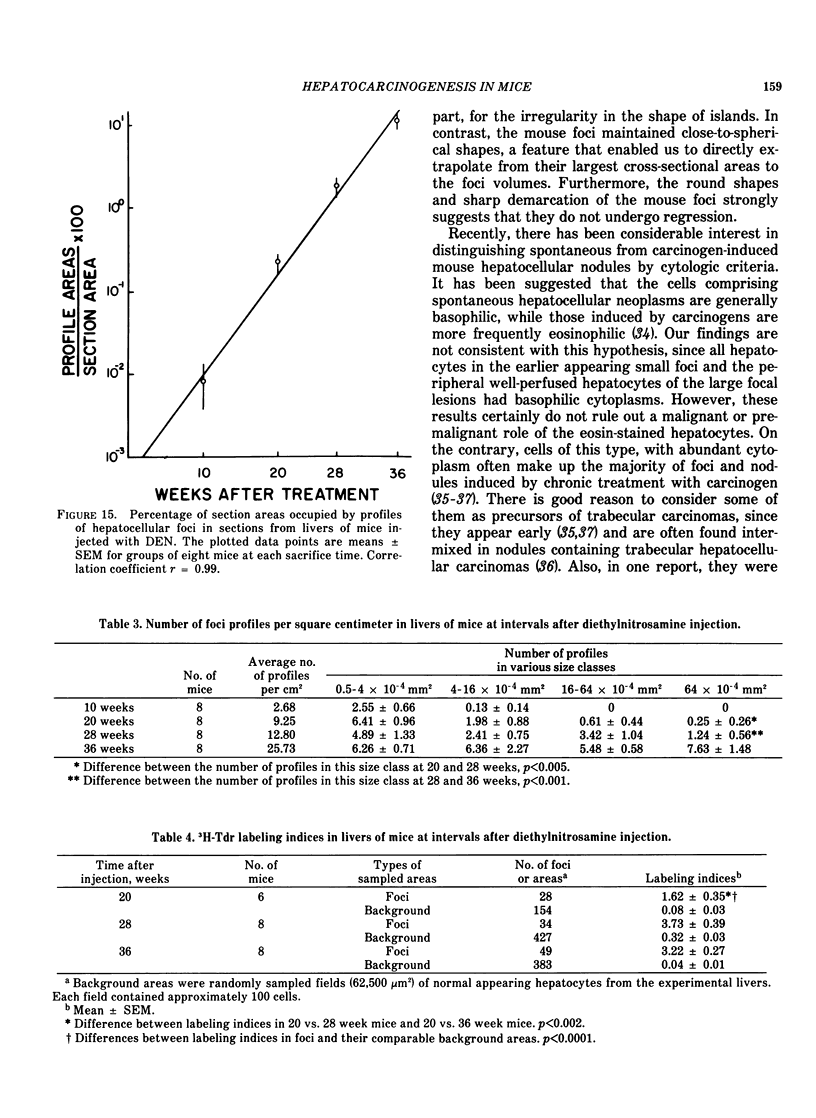
Basophilic hepatic foci, nodules, and trabecular hepatocellular carcinomas, collectively referred to as focal hepatic lesions, were induced by single injections of 5.0 micrograms of diethylnitrosamine (DEN) per gram body weight in 15-day-old C57BL/6J X C3HeB/FeJ F1 (B6C3 F1) mice. Groups of eight experimental and eight control mice were killed at 3 days and at 1, 2, 4, 10, 20, 28, 36 and 41 weeks after injection. The only observable acute hepatic toxic effect of DEN, a mild steatosis, was noted at 3 days, but this had disappeared by 7 days following injection. Basophilic foci, composed entirely of altered hepatocytes, were first noted, when very small, at 10 weeks. At later times, some of the foci also contained small collections of proliferated ductules, apparently a result of secondary ingrowth from nearby interlobular bile ducts. The hepatocytes within basophilic foci were characterized by their abundant cytoplasmic RNA, a high nuclear to cytoplasmic ratio (two times greater than normal), which gave them a "crowded appearance," and decreased glucose-6-phosphatase activity. During the course of the study, basophilic foci appeared to increase in size and number. Cytologic anaplasia also became more evident, ultimately culminating in the development of typical trabecular hepatocellular carcinomas by 44 weeks. Invasion of hepatic veins by basophilic foci, first noted at 10 weeks, was prominent by 20 weeks and indicated that many of the lesions manifested this characteristic of malignancy well in advance of the anaplastic features that are also diagnostic of hepatocellular carcinoma. The high growth rates of basophilic foci were confirmed by their greatly increased 3H-thymidine labeling indices, which were 20 times greater than background hepatocytes at 20 weeks following DEN injection. Tumor progression during the course of the study was also suggested by a doubling of labeling indices of hepatocytes in the basophilic foci between 20 to 28 weeks. (The term tumor progression is used in a broad biological sense to encompass any or all of the qualitative and quantitative changes describing the stepwise development of initiated cells to highly malignant neoplasms. This definition differs from the more clinical usage which restricts the process to qualitative changes during the late stages in the development of fully autonomous neoplasms.) An analysis of the number and size of transections through basophilic foci and in some cases, actual reconstructions of the foci from serial sections, indicated that, in aggregate, they grew exponentially between 10 to 36 weeks, with a volume doubling time of 2.5 weeks. The combined morphologic and kinetic data support the view that trabecular hepatocellular carcinomas develop from basophilic foci. Because of their ease of quantitation on conventional H&E stained sections, their rather uniformly spherical shapes, and the high probability of their clonal origin, the induced focal hepatic lesions should provide a useful model for studying tumor growth kinetics during carcinogenesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elias H., Hennig A., Schwartz D. E. Stereology: applications to biomedicalresearch. Physiol Rev. 1971 Jan;51(1):158–200. doi: 10.1152/physrev.1971.51.1.158. [DOI] [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- GOLDFARB S., ZAK F. G. Role of injury and hyperplasia in the induction of hepatocellular carcinoma. JAMA. 1961 Nov 18;178:729–731. doi: 10.1001/jama.1961.73040460007007b. [DOI] [PubMed] [Google Scholar]
- Goldfarb S. A morphological and histochemical study of carcinogenesis of the liver in rats fed 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1973 May;33(5):1119–1128. [PubMed] [Google Scholar]
- Goldfarb S., Pugh T. D., Cripps D. J. Increased alkaline phosphatase activity--a positive histochemical marker for griseofulvin-induced mouse hepatocellular nodules. J Natl Cancer Inst. 1980 Jun;64(6):1427–1433. doi: 10.1093/jnci/64.6.1427. [DOI] [PubMed] [Google Scholar]
- Hennig A., Elias H. A rapid method for the visual determination of size distribution of spheres from the size distribution of their sections. J Microsc. 1971 Apr;93(2):101–107. doi: 10.1111/j.1365-2818.1971.tb02271.x. [DOI] [PubMed] [Google Scholar]
- Hoover K. L., Ward J. M., Stinson S. F. Histopathologic differences between liver tumors in untreated (C57BL/6 X C3H)F1 (B6C3F1) mice and Nitrofen-fed mice. J Natl Cancer Inst. 1980 Nov;65(5):937–948. [PubMed] [Google Scholar]
- Kalengayi M. M., Ronchi G., Desmet V. J. Histochemistry of gamma-glutamyl transpeptidase in rat liver during aflatoxin B1-induced carcinogenesis. J Natl Cancer Inst. 1975 Sep;55(3):579–588. doi: 10.1093/jnci/55.3.579. [DOI] [PubMed] [Google Scholar]
- Kyriazis A. P., Koka M., Vesselinovitch S. D. Metastatic rate of liver tumors induced by diethylnitrosamine in mice. Cancer Res. 1974 Nov;34(11):2881–2886. [PubMed] [Google Scholar]
- Maskens A. P. Confirmation of the two-step nature of chemical carcinogenesis in the rat colon adenocarcinoma model. Cancer Res. 1981 Mar;41(3):1240–1245. [PubMed] [Google Scholar]
- Moore M. R., Drinkwater N. R., Miller E. C., Miller J. A., Pitot H. C. Quantitative analysis of the time-dependent development of glucose-6-phosphatase-deficient foci in the livers of mice treated neonatally with diethylnitrosamine. Cancer Res. 1981 May;41(5):1585–1593. [PubMed] [Google Scholar]
- Nomura T. Timing of chemically induced neoplasia in mice revealed by the antineoplastic action of caffeine. Cancer Res. 1980 Apr;40(4):1332–1340. [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Solt D. B., Farber E. Phenotypic diversity as an early property of putative preneoplastic hepatocyte populations in liver carcinogenesis. Cancer Res. 1980 Mar;40(3):725–733. [PubMed] [Google Scholar]
- PREHN R. T. A CLONAL SELECTION THEORY OF CHEMICAL CARCINOGENESIS. J Natl Cancer Inst. 1964 Jan;32:1–17. [PubMed] [Google Scholar]
- Pitot H. C., Goldsworthy T., Campbell H. A., Poland A. Quantitative evaluation of the promotion by 2,3,7,8-tetrachlorodibenzo-p-dioxin of hepatocarcinogenesis from diethylnitrosamine. Cancer Res. 1980 Oct;40(10):3616–3620. [PubMed] [Google Scholar]
- Pugh T. D., Goldfarb S. Quantitative histochemical and autoradiographic studies of hepatocarcinogenesis in rats fed 2-acetylaminofluorene followed by phenobarbital. Cancer Res. 1978 Dec;38(12):4450–4457. [PubMed] [Google Scholar]
- Reuber M. D. Histogenesis of hyperplasia and carcinomas of the liver arising around central veins in mice ingesting chlorinated hydrocarbons. Pathol Microbiol (Basel) 1975;43(4):287–298. doi: 10.1159/000162841. [DOI] [PubMed] [Google Scholar]
- SHIMKIN M. B., POLISSAR M. J. Some quantitative observations on the induction and growth of primary pulmonary tumors in strain A mice receiving urethan. J Natl Cancer Inst. 1955 Aug;16(1):75–97. [PubMed] [Google Scholar]
- Schauer A., Kunze E. Enzymhistochemische und autoradiographische Untersuchungen während der Cancerisierung der Rattenleber mit Diäthylnitrosamin. Z Krebsforsch. 1968;70(3):252–266. [PubMed] [Google Scholar]
- Scherer E., Emmelot P. Kinetics of induction and growth of precancerous liver-cell foci, and liver tumour formation by diethylnitrosamine in the rat. Eur J Cancer. 1975 Oct;11(10):689–696. doi: 10.1016/0014-2964(75)90042-0. [DOI] [PubMed] [Google Scholar]
- Shinozuka H., Lombardi B., Sell S., Iammarino R. M. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res. 1978 Apr;38(4):1092–1098. [PubMed] [Google Scholar]
- Teebor G. W., Becker F. F. Regression and persistence of hyperplastic hepatic nodules induced by N-2-Fluorenylacetamide and their relationship to hepatocarcinogenesis. Cancer Res. 1971 Jan;31(1):1–3. [PubMed] [Google Scholar]
- Vesselinovitch S. D., Mihailovich N., Rao K. V. Morphology and metastatic nature of induced hepatic nodular lesions in C57BL x C3H F1 mice. Cancer Res. 1978 Jul;38(7):2003–2010. [PubMed] [Google Scholar]
- Ward J. M., Bernal E., Buratto B., Goodman D. G., Strandberg J. D., Schueler R. Histopathology of neoplastic and nonneoplastic hepatic lesions in mice fed diets containing tetrachlorvinphos. J Natl Cancer Inst. 1979 Jul;63(1):111–118. [PubMed] [Google Scholar]