Abstract
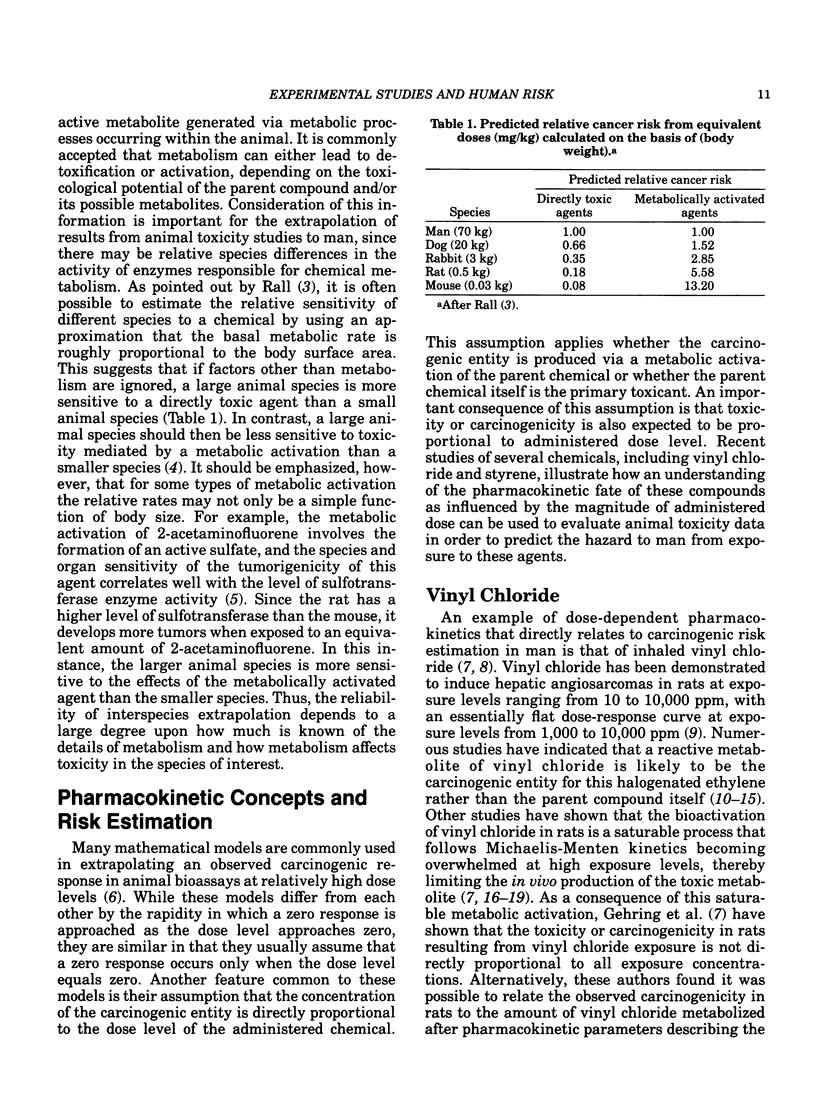
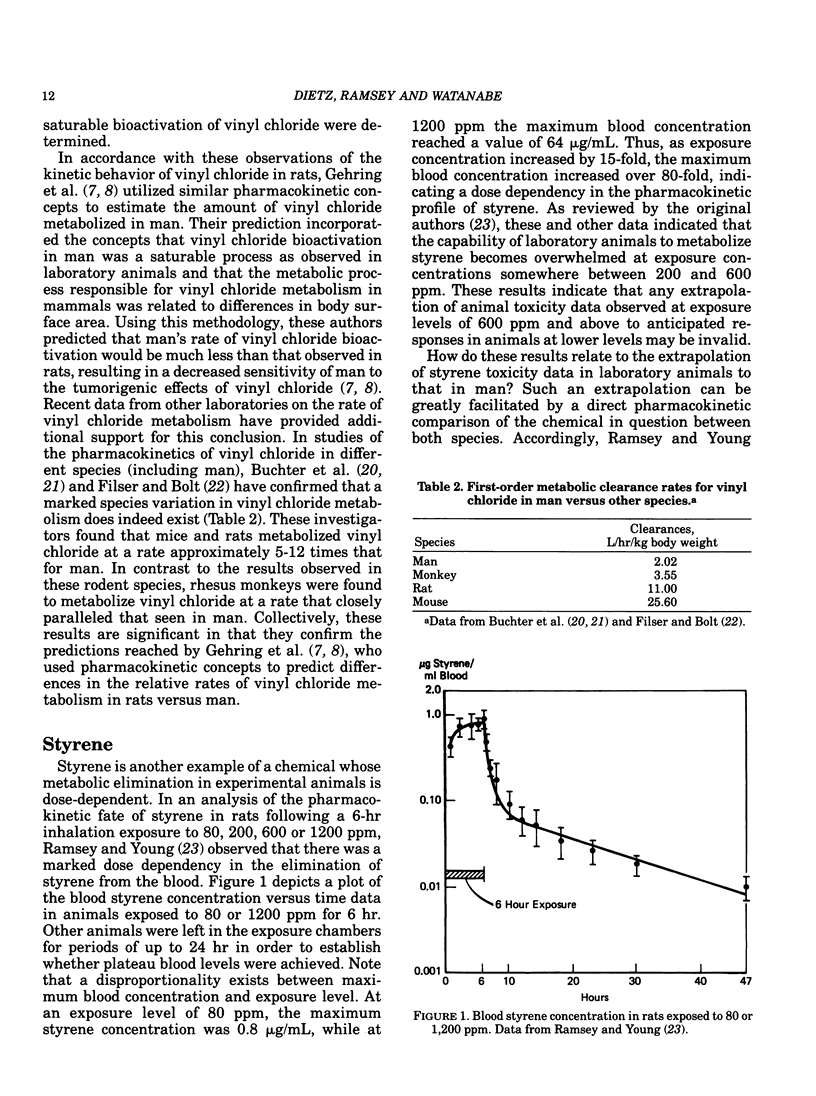
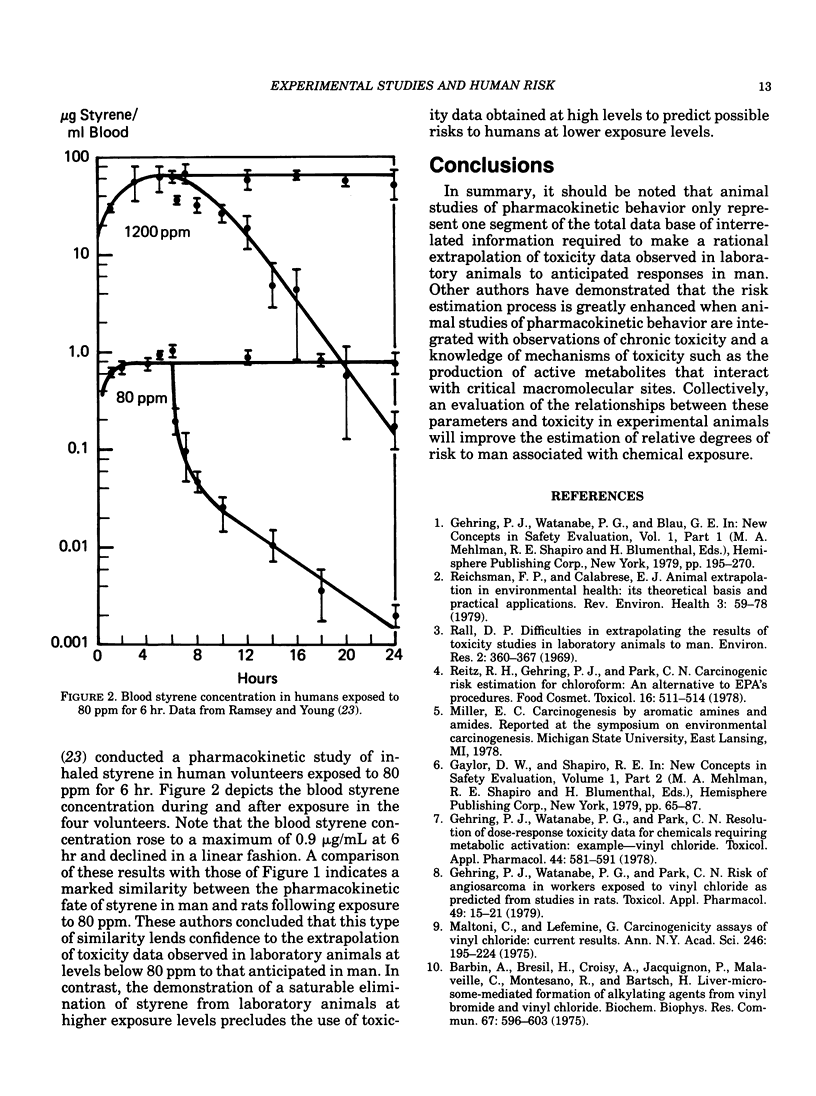
Confidence in the extrapolation of animal toxicity data to humans can be enhanced by the application of pharmacokinetic concepts integrated with chronic toxicity data and knowledge of a chemical's mechanism(s) of toxicity. Basic pharmacokinetic concepts (including dose-dependent or Michaelis-Menten kinetics) and their relationship to the risk estimation process are discussed using vinyl chloride and styrene as specific examples. Species differences in metabolic rates must be considered in order to arrive at realistic estimates of human risk to vinyl chloride-induced liver angiosarcomas utilizing vinyl chloride toxicity data observed in rats. Because small animal species generally metabolize chemicals more rapidly than larger species on a body surface area basis, small animals should be more sensitive to chemicals (such as vinyl chloride) that exert their toxicities via the metabolic formation of toxic products. Inhaled styrene is a chemical whose clearance from the blood at low exposure levels in both rats and humans follows first-order kinetics. However, at higher exposure levels, the pharmacokinetic fate of styrene in rats is dose-dependent, suggesting a saturation of styrene metabolism. These data indicate that any extrapolation of observable toxicity at elevated exposure levels in rats to anticipated responses at lower levels in either rats or humans may be invalid. An integration of the foregoing concepts provides a sound scientific basis for the use of experimental animal data to predict the risk to humans from chemical exposure.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbin A., Brésil H., Croisy A., Jacquignon P., Malaveille C., Montesano R., Bartsch H. Liver-microsome-mediated formation of alkylating agents from vinyl bromide and vinyl chloride. Biochem Biophys Res Commun. 1975 Nov 17;67(2):596–603. doi: 10.1016/0006-291x(75)90854-2. [DOI] [PubMed] [Google Scholar]
- Bartsch H., Malaveille C., Montesano R. Human, rat and mouse liver-mediated mutagenicity of vinyl chloride in S. typhimurium strains. Int J Cancer. 1975 Mar 15;15(3):429–437. doi: 10.1002/ijc.2910150309. [DOI] [PubMed] [Google Scholar]
- Bolt H. M., Kappus H., Buchter A., Bolt W. Disposition of (1,2-14C) vinyl chloride in the rat. Arch Toxicol. 1976 Jun 8;35(3):153–162. doi: 10.1007/BF00293562. [DOI] [PubMed] [Google Scholar]
- Buchter A., Filser J. G., Peter H., Bolt H. M. Pharmacokinetics of vinyl chloride in the Rhesus monkey. Toxicol Lett. 1980 Jun;6(1):33–36. doi: 10.1016/0378-4274(80)90099-5. [DOI] [PubMed] [Google Scholar]
- Filser J. G., Bolt H. M. Pharmacokinetics of halogenated ethylenes in rats. Arch Toxicol. 1979 Jun 8;42(2):123–136. doi: 10.1007/BF00316492. [DOI] [PubMed] [Google Scholar]
- Gehring P. J., Watanabe P. G., Park C. N. Resolution of dose-response toxicity data for chemicals requiring metabolic activation: example--vinyl chloride. Toxicol Appl Pharmacol. 1978 Jun;44(3):581–591. doi: 10.1016/0041-008x(78)90266-1. [DOI] [PubMed] [Google Scholar]
- Gehring P. J., Watanabe P. G., Park C. N. Risk of angiosarcoma in workers exposed to vinyl chloride as predicted from studies in rats. Toxicol Appl Pharmacol. 1979 Jun 15;49(1):15–21. doi: 10.1016/0041-008x(79)90271-0. [DOI] [PubMed] [Google Scholar]
- Kappus H., Bolt H. M., Buchter A., Bolt W. Liver microsomal uptake of (14C)vinyl chloride and transformation to protein alkylating metabolites in vitro. Toxicol Appl Pharmacol. 1976 Sep;37(3):461–471. doi: 10.1016/0041-008x(76)90208-8. [DOI] [PubMed] [Google Scholar]
- Malaveille C., Bartsch H., Barbin A., Camus A. M., Montesano R., Croisy A., Jacquignon P. Mutagenicity of vinyl chloride, chloroethyleneoxide, chloroacetaldehyde and chloroethanol. Biochem Biophys Res Commun. 1975 Mar 17;63(2):363–370. doi: 10.1016/0006-291x(75)90697-x. [DOI] [PubMed] [Google Scholar]
- Maltoni C., Lefemine G. Carcinogenicity bioassays of vinyl chloride: current results. Ann N Y Acad Sci. 1975 Jan 31;246:195–218. doi: 10.1111/j.1749-6632.1975.tb51094.x. [DOI] [PubMed] [Google Scholar]
- Rall D. P. Difficulties in extrapolating the results of toxicity studies in laboratory animals to man. Environ Res. 1969 Oct;2(5):360–367. doi: 10.1016/0013-9351(69)90006-1. [DOI] [PubMed] [Google Scholar]
- Ramsey J. C., Young J. D. Pharmacokinetics of inhaled styrene in rats and humans. Scand J Work Environ Health. 1978;4 (Suppl 2):84–91. [PubMed] [Google Scholar]
- Reichsman F. P., Calabrese E. J. Animal extrapolation in environmental health: its theoretical basis and practical applications. Rev Environ Health. 1979;3(1):59–78. [PubMed] [Google Scholar]
- Reitz R. H., Gehring P. J., Park C. N. Carcinogenic risk estimation for chloroform: an alternative to EPA's procedures. Food Cosmet Toxicol. 1978 Oct;16(5):511–514. doi: 10.1016/s0015-6264(78)80315-0. [DOI] [PubMed] [Google Scholar]
- Watanabe P. G., McGowan G. R., Gehring P. J. Fate of (14C)vinyl chloride after single oral administration in rats. Toxicol Appl Pharmacol. 1976 May;36(2):339–352. doi: 10.1016/0041-008x(76)90013-2. [DOI] [PubMed] [Google Scholar]
- Watanabe P. G., McGowan G. R., Madrid E. O., Gehring P. J. Fate of [14C]vinyl chloride following inhalation exposure in rats. Toxicol Appl Pharmacol. 1976 Jul;37(1):49–59. doi: 10.1016/s0041-008x(76)80007-5. [DOI] [PubMed] [Google Scholar]
- Watanabe P. G., Zempel J. A., Pegg D. G., Gehring P. J. Hepatic macromolecular binding following exposure to vinyl chloride. Toxicol Appl Pharmacol. 1978 Jun;44(3):571–579. doi: 10.1016/0041-008x(78)90265-x. [DOI] [PubMed] [Google Scholar]


