Abstract
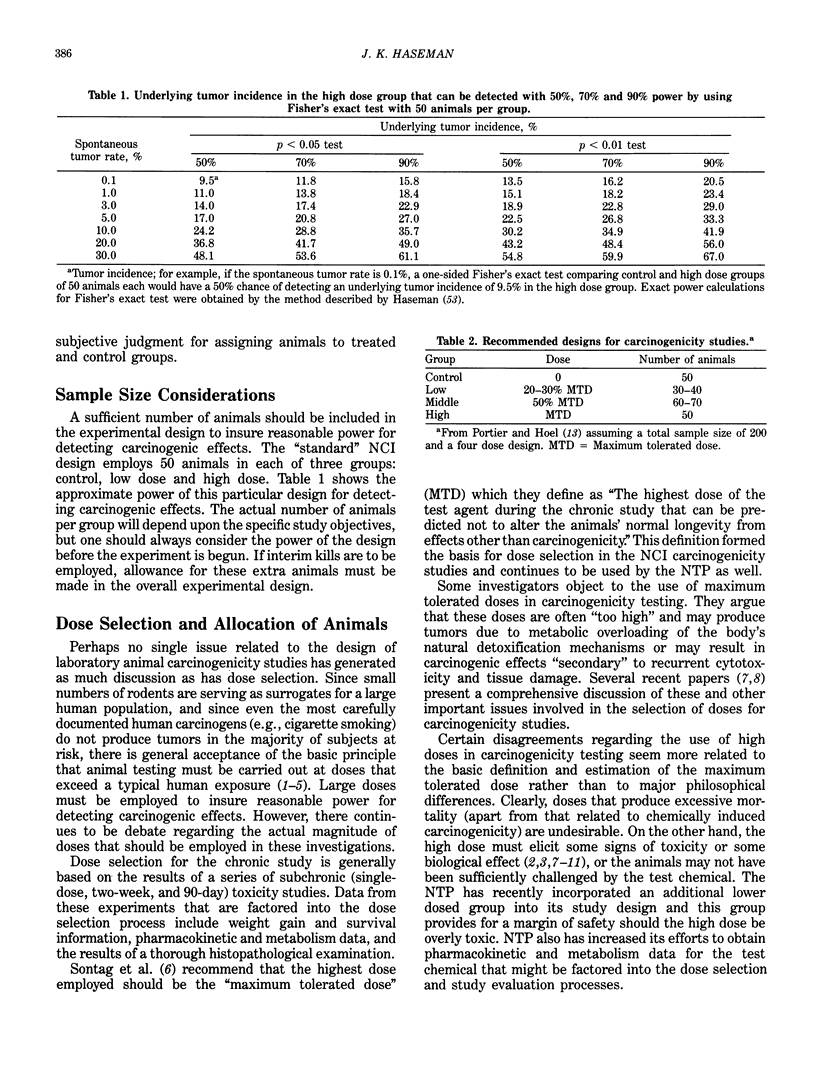
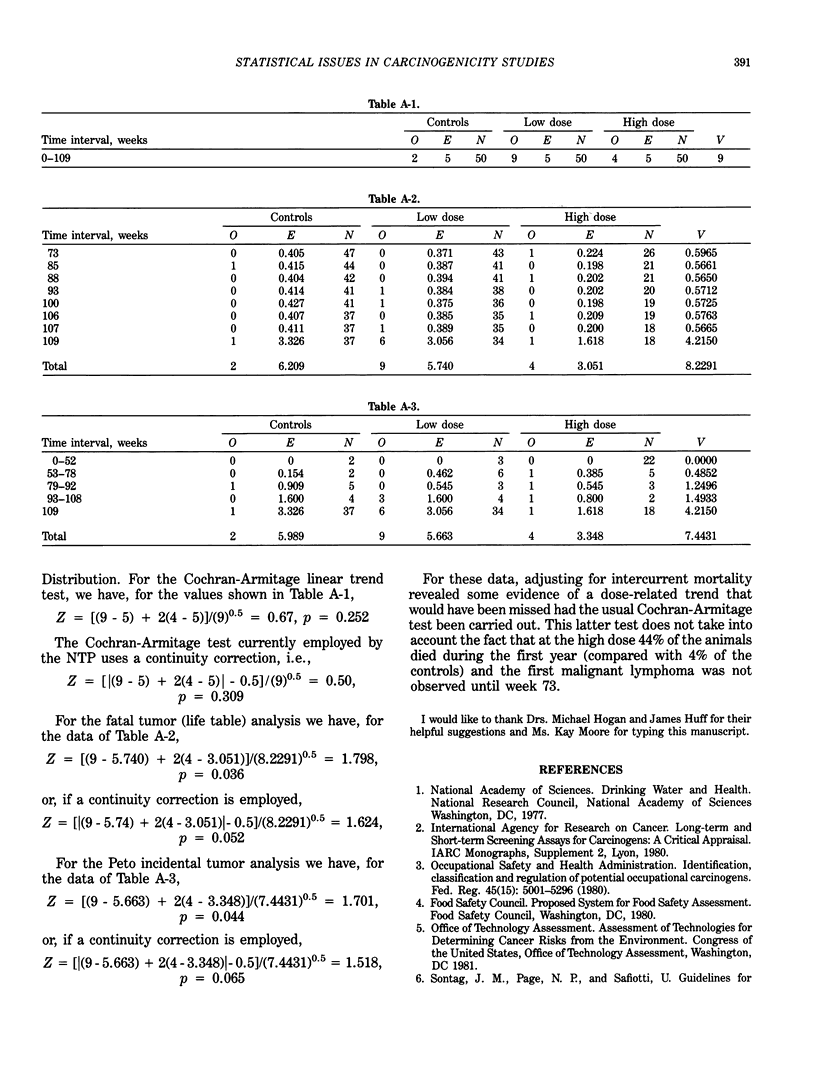
Statistical issues in the design, analysis and interpretation of animal carcinogenicity studies are discussed. In the area of experimental design, issues that must be considered include randomization of animals, sample size considerations, dose selection and allocation of animals to experimental groups, and control of potentially confounding factors. In the analysis of tumor incidence data, survival differences among groups should be taken into account. It is important to try to distinguish between tumors that contribute to the death of the animal and "incidental" tumors discovered at autopsy in an animal dying of an unrelated cause. Life table analyses (appropriate for lethal tumors) and incidental tumor tests (appropriate for nonfatal tumors) are described, and the utilization of these procedures by the National Toxicology Program is discussed. Despite the fact that past interpretations of carcinogenicity data have tended to focus on pairwise comparisons in general and high-dose effects in particular, the importance of trend tests should not be overlooked, since these procedures are more sensitive than pairwise comparisons to the detection of carcinogenic effects. No rigid statistical "decision rule" should be employed in the interpretation of carcinogenicity data. Although the statistical significance of an observed tumor increase is perhaps the single most important piece of evidence used in the evaluation process, a number of biological factors must also be taken into account. The use of historical control data, the false-positive issue and the interpretation of negative trends are also discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breslow N. E., Day N. E., Tomatis L., Turusov V. S. Associations between tumor types in a large-scale carcinogenesis study of CF-1 mice. J Natl Cancer Inst. 1974 Jan;52(1):233–239. doi: 10.1093/jnci/52.1.233. [DOI] [PubMed] [Google Scholar]
- Chu K. C., Cueto C., Jr, Ward J. M. Factors in the evaluation of 200 National Cancer Institute carcinogen bioassays. J Toxicol Environ Health. 1981 Jul-Aug;8(1-2):251–280. doi: 10.1080/15287398109530068. [DOI] [PubMed] [Google Scholar]
- Conybeare G. Effect of quality and quantity of diet on survival and tumour incidence in outbred Swiss mice. Food Cosmet Toxicol. 1980 Feb;18(1):65–75. doi: 10.1016/0015-6264(80)90013-9. [DOI] [PubMed] [Google Scholar]
- Fears T. R., Tarone R. E., Chu K. C. False-positive and false-negative rates for carcinogenicity screens. Cancer Res. 1977 Jul;37(7 Pt 1):1941–1945. [PubMed] [Google Scholar]
- Gart J. J., Chu K. C., Tarone R. E. Statistical issues in interpretation of chronic bioassay tests for carcinogenicity. J Natl Cancer Inst. 1979 Apr;62(4):957–974. [PubMed] [Google Scholar]
- Haseman J. K. A reexamination of false-positive rates for carcinogenesis studies. Fundam Appl Toxicol. 1983 Jul-Aug;3(4):334–339. doi: 10.1016/s0272-0590(83)80148-1. [DOI] [PubMed] [Google Scholar]
- Haseman J. K. Patterns of tumor incidence in two-year cancer bioassay feeding studies in Fischer 344 rats. Fundam Appl Toxicol. 1983 Jan-Feb;3(1):1–9. doi: 10.1016/s0272-0590(83)80165-1. [DOI] [PubMed] [Google Scholar]
- Haseman J. K. Response to "Use of Statistics when Examining Lifetime Studies in Rodents to Detect Carcinogenicity". J Toxicol Environ Health. 1977 Nov;3(4):633–636. doi: 10.1080/15287397709529597. [DOI] [PubMed] [Google Scholar]
- Haseman J. K. Statistical support of the proposed National Toxicology Program protocol. Toxicol Pathol. 1983;11(1):77–82. doi: 10.1177/019262338301100113. [DOI] [PubMed] [Google Scholar]
- Hoel D. G., Walburg H. E., Jr Statistical analysis of survival experiments. J Natl Cancer Inst. 1972 Aug;49(2):361–372. [PubMed] [Google Scholar]
- Kodell R. L., Farmer J. H., Gaylor D. W., Cameron A. M. Influence of cause-of-death assignment on time-to-tumor analyses in animal carcinogenesis studies. J Natl Cancer Inst. 1982 Sep;69(3):659–664. [PubMed] [Google Scholar]
- Kodell R. L., Shaw G. W., Johnson A. M. Nonparametric joint estimators for disease resistance and survival functions in survival/sacrifice experiments. Biometrics. 1982 Mar;38(1):43–58. [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- Mantel N. Assessing laboratory evidence for neoplastic activity. Biometrics. 1980 Sep;36(3):381–399. [PubMed] [Google Scholar]
- Mantel N., Bohidar N. R., Ciminera J. L. Mantel-Haenszel analyses of litter-matched time-to-response data, with modifications for recovery of interlitter information. Cancer Res. 1977 Nov;37(11):3863–3868. [PubMed] [Google Scholar]
- Mantel N., Ciminera J. L. Use of logrank scores in the analysis of litter-matched data on time to tumor appearance. Cancer Res. 1979 Nov;39(11):4308–4315. [PubMed] [Google Scholar]
- Peto R. Editorial: Guidelines on the analysis of tumour rates and death rates in experimental animals. Br J Cancer. 1974 Feb;29(2):101–105. doi: 10.1038/bjc.1974.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portier C., Hoel D. Optimal design of the chronic animal bioassay. J Toxicol Environ Health. 1983 Jul;12(1):1–19. doi: 10.1080/15287398309530403. [DOI] [PubMed] [Google Scholar]
- Ross M. H., Bras G. Lasting influence of early caloric restriction on prevalence of neoplasms in the rat. J Natl Cancer Inst. 1971 Nov;47(5):1095–1113. [PubMed] [Google Scholar]
- Salsburg D. S. Use of statistics when examining lifetime studies in rodents to detect carcinogenicity. J Toxicol Environ Health. 1977 Nov;3(4):611–628. doi: 10.1080/15287397709529595. [DOI] [PubMed] [Google Scholar]
- Salsburg D. The lifetime feeding study in mice and rats--an examination of its validity as a bioassay for human carcinogens. Fundam Appl Toxicol. 1983 Jan-Feb;3(1):63–67. doi: 10.1016/s0272-0590(83)80174-2. [DOI] [PubMed] [Google Scholar]
- Tucker M. J. The effect of long-term food restriction on tumours in rodents. Int J Cancer. 1979 Jun 15;23(6):803–807. doi: 10.1002/ijc.2910230611. [DOI] [PubMed] [Google Scholar]
- Turnbull B. W., Mitchell T. J. Exploratory analysis of disease prevalence data from survival/sacrifice experiments. Biometrics. 1978 Dec;34(4):555–570. [PubMed] [Google Scholar]
- Wahrendorf J. Simultaneous analysis of different tumor types in a long-term carcinogenicity study with scheduled sacrifices. J Natl Cancer Inst. 1983 May;70(5):915–921. [PubMed] [Google Scholar]


