Abstract
Dynein is a minus-end-directed microtubule motor with critical roles in mitosis, membrane transport and intracellular transport. Several proteins regulate dynein activity, including dynactin1, LIS1 (refs 2, 3) and NudEL (NudE-like)2,4–8. Here, we identify a NUDEL homologue in budding yeast and name it Ndl1. The ndl1Δ null mutant shows decreased targeting of dynein to microtubule plus ends, an essential element of the model for dynein function. We find that Ndl1 regulates dynein targeting through LIS1, with which it interacts biochemically, but not through CLIP170, another plus-end protein involved in dynein targeting9. Ndl1 is found at far fewer microtubule ends than are LIS1 and dynein. However, when Ndl1 is present at a plus end, the molar amount of Ndl1 approaches that of LIS1 and dynein. We propose a model in which Ndl1 binds transiently to the plus end to promote targeting of LIS1, which cooperatively recruits dynein.
LIS1 is required for nuclear migration in neurons during development of the cerebral cortex7,8. Genetic interactions between LIS1 and dynein have been found in studies of LIS1 homologues in Saccharomyces cerevisiae (Pac1)9,10 and Aspergillus nidulans (NudF)11. NUDE was first identified as a multi-copy suppressor of a nudF conditional mutation in A. nidulans12. Mammals have two NudE homologues, called NudE13 and NudEL4,5,14, which interact with LIS1. In mice, both NudE and NudEL are critical for brain development15,16, and both localize to microtubule organization centres4,5,13. The molecular and cellular mechanism of how NudEL and LIS1 regulate dynein is poorly understood. We choose to study this question in budding yeast, in which dynein has a single function — to position the mitotic spindle.
To identify new genes that are involved in the dynein pathway in S. cerevisiae, we screened the Research Genetics collection of viable haploid null mutants in the cold (to enhance the need for dynein), for increased levels of binucleate cells17. The screen uncovered a previously uncharacterized gene, YLR254C, which is predicted to encode a protein of 189 amino acids. BLAST searches revealed similarity between Ylr254c and zebrafish NudEL. Ylr254c has a NUDE_C (NudE carboxy-terminal) motif within residues 92–110 and an amino-terminal coiled-coil domain, which is characteristic of NudEL family proteins (see Supplementary Information, Fig. S1a, b). A BLAST search of budding yeast using the mouse NudEL coiled-coil region as a query identified Ylr254c, and a BLAST search of the Neurospora crassa proteome using Ylr254c as a query identified the NudEL homologue RO11. These results indicate that Ylr254c is probably a homologue of NudEL despite low sequence similarity.
Figure S1.
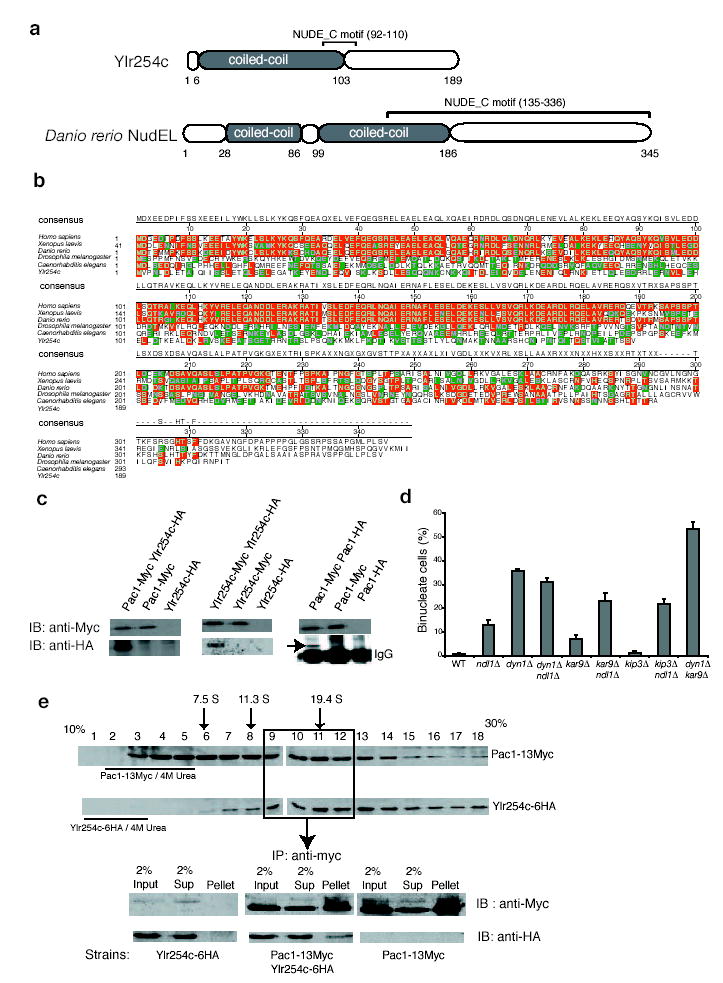
Ylr254c is the NudEL homolog in budding yeast. (a) Comparison of Ylr254c and zebra fish domain structures (24% identity, 46% similarity). Coiled-coil regions (colored grey) and the NUDE_C motif are indicated. (b) Sequence alignment of NudEL and Ylr254c. Residues identical to the consensus are red, and similar ones are green. (c) Ylr254c and Pac1 were tagged with 6HA and 13Myc in haploid strains (Ylr254c-6HA, Pac1-13Myc, and Ylr254c-6HA Pac1-13Myc) or diploid strains (Ylr254c-6HA Ylr254c- 13Myc and Pac1-6HA Pac1-13Myc). Cell lysates were immunoprecipitated with rabbit anti-Myc followed by immunoblot with indicated antibodies. The position of Pac1-HA above the IgG heavy chain is indicated. (d) The nuclear segregation defect of ndl1Δ is enhanced by mutations in the Kar9 pathway components kar9Δ and kip3Δ. A dyn1Δ kar9Δ double mutant served as a positive control. Error bars represent Standard Error of Proportion (see methods). (e) Distribution of Pac1-13Myc and Ylr254c-6HA after velocity sedimentation in a sucrose gradient. Positions of aldolase (7.5S), catalase (11.3S) and thyroglobulin (19.4S) are indicated. The distribution of Pac1- 13Myc and Ylr254c-6HA under denaturing conditions is also indicated. Fractions 9 -12 were then pooled and immunoprecipitated with anti-Myc agarose beads, followed by immunoblot with indicated antibodies. Haploid strains expressing Ylr254c-6HA or Pac1-13Myc were used as controls.
We tested whether Ylr254c was functionally related to NudEL. Purified human NudEL and LIS1 exist as stable homodimers and heterotetramers in vitro18. We tagged the endogenous copies of Ylr254c and Pac1 with haemagglutinin (HA) and myc at the C termini. The tagged proteins are functional. Ylr254c–6HA co-immunoprecipitated with Pac1–13Myc from the double-tagged strain, but not from control strains (see Supplementary Information, Fig. S1c). In addition, Ndl1–6HA co-immunoprecipitated with Pac1–13Myc throughout the cell cycle (data not shown). Both Ylr254c and Pac1 also interacted with themselves, by co-immunoprecipitation from double-tagged strains (Supplementary Information, Fig. S1c). We then asked whether Ylr254c and Pac1 associate into complexes. Sucrose gradient sedimentation of extracts from Pac1–13Myc Ylr254c–6HA cells revealed Pac1–13Myc to be distributed widely throughout the gradient, with S values from <7.5S to >19.4S. Fractions re-run on a second gradient sedimented to the same positions, and urea-denatured Pac1–13Myc sedimented at a low S value (see Supplementary Information, Fig. S1e). Thus, Pac1 participates in stable complexes. Ylr254c–6HA sedimented as a wide peak centred at 20S, overlapping in part with Pac1. Urea-denatured Ndl1–6HA sedimented at low S values (see Supplementary Information, Fig. S1e). The overlapping but different distributions of Pac1–13Myc and Ndl1–6HA could result from the existence of one complex with both proteins, or from two different complexes. We asked whether the two proteins would co-immunoprecipitate from overlapping fractions of the sucrose gradient. We found that Ylr254c–6HA was co-immunoprecipitated with Pac1–13Myc (see Supplementary Information, Fig. S1e). Therefore, some portions of the two proteins are present in the same complex. On the basis of these structural and biochemical properties, Ylr254c is homologous to NudEL, and we name Ylr254c as Ndl1.
To investigate the function of Ndl1 in the dynein pathway, we tested synthetic effects of ndl1Δ with components of the Kar9 and dynein pathways. Budding yeast need one of the two pathways to grow. For the Kar9 pathway, we tested bim1Δ and kar9Δ. ndl1Δbim1Δ double mutants (8 of 11) grew poorly at 30 °C, whereas ndl1Δkar9Δ double mutants (10 of 10) grew normally at 30 °C and had only a slight growth defect at 37 °C. All double mutants lacking Ndl1 and a dynein pathway component grew as well as single-null mutants did (data not shown).
Next, we examined the nuclear segregation phenotype of these double mutants. An asynchronous population of vegetatively growing wildtype cells, in the cold, had <1% binucleate cells. Among single mutants, dyn1Δ had 36% binucleate cells, ndl1Δ had 13%, kar9Δ 7% and kip3Δ 1% (see Supplementary Information, Fig. S1d). Among double mutants, ndl1Δkar9Δ and ndl1Δkip3Δ showed 25% and 23% binucleate cells, respectively, higher than either single-null strain, and ndl1Δdyn1Δ had a level of binucleate cells similar to that of dyn1Δ. As a positive control, dyn1Δkar9Δ had 53% binucleate cells (see Supplementary Information, Fig. S1d). Taken together, the growth and nuclear segregation assays show that NDL1 has an important but not essential role in the dynein pathway.
To examine dynein function more directly, we evaluated how Ndl1 affects the position and movement of the mitotic spindle. Wild-type cells had no late-anaphase spindles in the mother (n = 122), dyn1Δ cells had 25% (n = 115) and ndl1Δ cells had 5% (n = 227). Again, ndl1Δ had a partial dynein phenotype. The binucleate assays above were performed in the cold to enhance the need for dynein, which can account for the higher percentage of binucleate cells for the dyn1Δ and ndl1Δ mutant in the binucleate assay than in the spindle position assay. Late-anaphase spindles in ndl1Δ cells often had long cytoplasmic microtubules in the bud, another characteristic of dynein mutants19.
During mitosis, dynein moves the spindle into the neck by powering sliding of cytoplasmic microtubules along the bud cortex19. Accumulation of late-anaphase spindles in the mother cells of the ndl1Δ mutant implies a delay of spindle movement into the neck. We tested for this, using video microscopy, by measuring the time required for the spindle to move into the neck. The time from initiation of spindle elongation to entry into the neck was 3.2 ± 1.4 min (n = 15) for wild-type cells. In ndl1Δ cells, 15 of 29 spindles elongated and moved into the neck normally (4.5 ± 1.4 min), but 14 of 29 spindles experienced extended delays before entering the neck, varying from 10 to >70 min. A dyn1Δ mutant had a similar but more severe phenotype. Half of the spindles moved into the neck normally (7 of 15), but the other half remained in the mother as late-anaphase spindles for >60 min. We also looked for microtubule sliding along the bud cortex using movies. An ndl1Δ mutant showed only one sliding event in 11 instances of the spindle moving into the neck, whereas control wild-type cells showed 13 out of 25.
In our model for dynein function, dynein is targeted to microtubule plus ends and then offloaded to the cortex17. To investigate how Ndl1 regulates dynein, we determined the localization of dynein in ndl1Δ cells, using Dyn1–3GFP (green fluorescent protein). In wild-type cells, dynein is found at the plus ends of cytoplasmic microtubules, as stationary foci at the cell cortex and at the spindle pole body (SPB)9,10. In ndl1Δ cells, the frequency with which dynein was observed at the plus end and the cortex decreased by ~10-fold (Fig. 1a, b). However, the fluorescence intensity of a plus end or cortical spot that contained Dyn1–3GFP was decreased by only 50% (549 ± 79 arbitrary units (a.u.) versus 1,038 ± 76 a.u.; Fig. 1c), suggesting cooperativity in the loss of dynein targeting and offloading. The decreased dynein targeting to plus ends was not secondary to a change in microtubule dynamics, because we did not find significant loss in cytoplasmic microtubule number or length in an ndl1Δ mutant compared with wild type (see Supplementary Information, Table S2).
Figure 1.
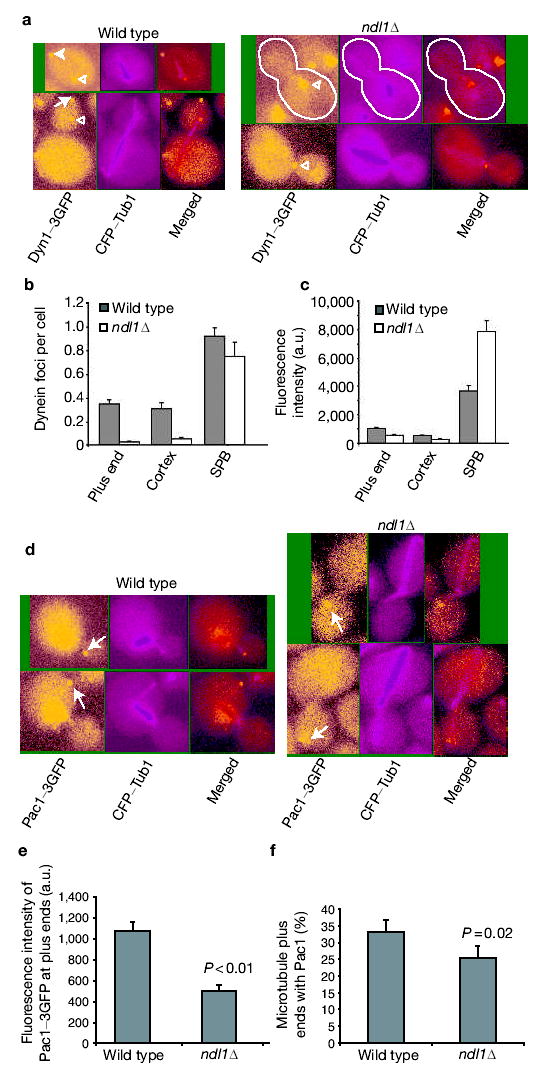
Ndl1 is required for efficient Dyn1 and Pac1 targeting to the plus ends. (a) Colocalization of Dyn1–3GFP and CFP–Tub1. Dyn1–3GFP appears at the microtubule plus end (arrow), the cortex (solid arrowhead) and the SPB (open arrowhead) in wild-type cells. In an ndl1Δ mutant, most Dyn1– 3GFP is present at the SPB. Cell shape is outlined in white. (b) Frequency of Dyn1–3GFP foci at plus ends, cortex and SPB in wild-type and ndl1Δ cells based on dual-colour images of Dyn1–3GFP and CFP–Tub1. Error bars represent standard error (n > 300). (c) Intensity of Dyn1–3GFP fluorescence intensity at the plus end, cortex and SPB in wild-type and ndl1Δ cells. Error bars represent standard error (n = 15). (d) Pac1–3GFP is present at the plus end of microtubules (arrow) and also in the nucleus. In an ndl1Δ mutant, Pac1–3GFP appears at microtubule plus ends without accumulation in the nucleus. (e) Intensity of Pac1–3GFP fluorescence at plus ends of microtubules. Error bars represent standard error (n = 15). (f) Percentage of cytoplasmic microtubules with Pac1–3GFP at the plus end (based on dualcolour images of Pac1–3GFP and CFP–Tub1) in wild-type and ndl1Δ cells. Error bars represent standard error (n > 50).
Table S2.
Number and length of cytoplasmic microtubules in wild type and ndl1Δ cells (Values are mean±S.E.M. for 48 microtubules in each case)
| MT per SPB | MT length (μm) | |||
|---|---|---|---|---|
| Cell Morphology | WT | ndl1Δ | WT | ndl1Δ |
| Unbudded (no spindle) | 2.58 ± 0.09 | 2.71 ± 0.10 | 3.20 ± 0.22 | 3.29 ± 0.18 |
| small bud (short spindle) | 1.13 ± 0.05 | 1.21 ± 0.06 | 2.13 ± 0.12 | 2.53 ± 0.14 |
| medium bud (elongating spindle) | 1.10 ± 0.04 | 1.31 ± 0.07 | 3.11 ± 0.08 | 4.69 ± 0.31 |
| large bud (long spindle) | 1.44 ± 0.09 | 1.46 ± 0.09 | 2.56 ± 0.17 | 2.48 ± 0.17 |
LIS1/Pac1 and CLIP170/Bik1 contribute to dynein targeting to the plus end. In yeast, both proteins localize to plus ends, and loss of either protein decreases targeting of dynein almost completely9,10. In the absence of Ndl1, the fluorescence intensity of Pac1–3GFP at the plus ends was decreased by ~ 50% (500 ± 55 a.u. versus 1,076 ± 86 a.u.; Fig. 1d, e). In addition, the frequency of finding Pac1–3GFP at the plus ends was decreased by a modest, statistically significant amount (Fig. 1f). In contrast, Bik1–GFP localization was not affected by loss of Ndl1. The fluorescence intensity of Bik1–GFP at the plus end was the same in ndl1Δ (516 ± 42 a.u.) and wild-type cells (517 ± 39 a.u.). In addition, the localization of Kip2–GFP, a kinesin that transports Bik1 to the plus end20, was not changed in the ndl1Δ mutant (data not shown). Neither Bik1 nor Kip2 localization was affected in a pac1Δ mutant (data not shown). Thus, Ndl1 is required for the efficient targeting of LIS1/Pac1 but not CLIP170/Bik1 to the microtubule plus ends.
To understand how Ndl1 affects LIS1/Pac1 targeting, we examined the localization of Ndl1. Localization of Ndl1–3GFP, a functional fusion, with cyan fluorescent protein (CFP)–Tub1 showed Ndl1 at the plus end of microtubules (Fig. 2a), but at a much lower frequency than is observed for Pac1 or Dyn1 (Fig. 2b). This was true at all stages of the cell cycle. To test whether Ndl1 coexists with Pac1 or dynein at the plus ends, we localized the dynein light intermediate chain, Dyn3, which is found only at the plus end and not at the cortex17. All Ndl1–3GFP foci at plus ends contained Dyn3–3CFP, and all Dyn3–3CFP foci at plus ends contained Pac1–3GFP (Fig. 2c). Thus, Ndl1 is present simultaneously with both Pac1 and dynein at plus ends.
Figure 2.
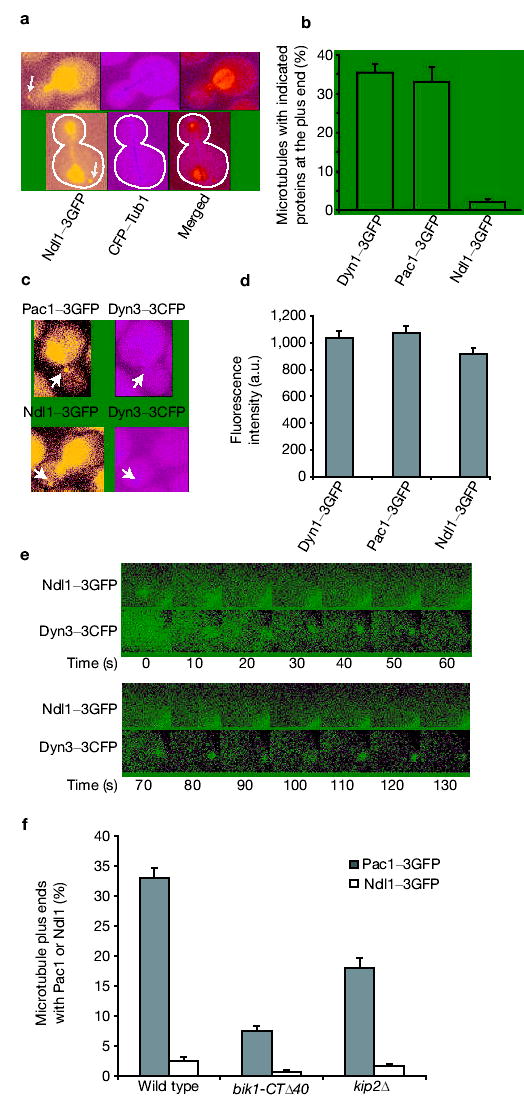
Ndl1 transiently localizes to microtubule plus ends. (a) Dualcolour images of Ndl1–3GFP and CFP–Tub1. Ndl1 localizes to the plus end of microtubules (arrow). Ndl1 is also highly concentrated in the nucleus. Cell shape is outlined in white. (b) Frequencies of finding Dyn1–3GFP, Pac1–3GFP and Ndl1–3GFP at microtubule plus ends, based on dualcolour images. Error bars represent standard error (n > 300). (c) Dual-colour images of Pac1–3GFP and Dyn3–3CFP, Ndl1–3GFP and Dyn3–3CFP. Note the colocalization of these proteins at the plus end (arrow). (d) Ratio of fluorescence intensity of Dyn1–3GFP, Pac1–3GFP and Ndl1–3GFP at the microtubule plus ends in wild-type cells, measured from a time-lapse movie. Error bars represent standard error (n = 15). (e) Frames from two-colour time-lapse movies of the Ndl1–3GFP Dyn3–3CFP strain. Only the plus-end region is shown for clarity. The bright signal at the right bottom corner is from nuclear Ndl1–3GFP. (f) Percentage of cells containing Ndl1 or Pac1 at the plus end in wild type, and bik1-CTΔ40 and kip2Δ mutants. Error bars represent standard error of proportion (n > 200).
To investigate the Ndl1 mechanism, we quantified the fluorescence intensity of the foci at the plus ends of microtubules and found a 1.0:1.0:0.9 ratio for Dyn1–3GFP: Pac1–3GFP: Ndl1–3GFP (Fig. 2d). Therefore, even though Ndl1 only appeared at a small fraction of plus ends, when Ndl1 was present its abundance was similar to that of Dyn1 and Pac1. To investigate further, we performed a two-colour time-lapse analysis of Ndl1–3GFP with Dyn3–3CFP. Ndl1 was initially colocalized with Dyn3, then Ndl1 disappeared whereas Dyn3 persisted (Fig. 2e and see Supplementary Information, Movie S1a, b). This transient association of Ndl1 with the plus end probably accounts for the low frequency of detection of Ndl1 at the plus end in populations. Efficient Pac1 targeting to the plus end requires Ndl1, as described above. We asked whether Ndl1 targeting depended on Pac1. No foci of Ndl1–3GFP were found at microtubule plus ends in pac1Δ cells (>1,000 cells; data not shown).
Because Kip2 and Bik1 contribute to dynein targeting at the plus end, we asked whether they affected Ndl1 or Pac1 plus-end targeting. Cytoplasmic microtubules are extremely short in bik1Δ cells, so we used a bik1-CTΔ40 mutant, which has a full dynein mutant phenotype but cytoplasmic microtubules of normal length9. The plus-end localization of Pac1 and Ndl1 was decreased significantly, but not completely, in kip2Δ and bik1-CTΔ40 mutants compared with wild type. The defect in the bik1-CTΔ40 mutant was more severe (Fig. 2f). The kip2Δ mutant still has some Bik1 at the plus end20, which may account for the milder defect in the kip2Δ mutant. Because the C-terminal 40 amino acids of Bik1 are required for the interaction of Bik1 with Pac1 in a yeast two-hybrid assay9, the Bik1–Pac1 interaction may be required to stabilize Pac1 at the plus end, which then recruits Ndl1.
Observing partial interdependence between Ndl1 and Pac1 for plus-end targeting, we asked whether overexpression of one protein would compensate for loss of the other. In an ndl1Δ mutant, glutathione S-transferase (GST)–Pac1 overexpression from GAL1 decreased the number of binucleate cells from 7.9 to 2.8% (P < 0.001) (Table 1), which is similar to a result in A. nidulans21. Pac1 overexpression decreased the number of binucleate cells in a pac1Δ mutant from 25 to 7%, an incomplete level of suppression that was perhaps due to the GST. Pac1 overexpression in a wild-type strain had almost no effect, as did GST alone (Table 1). We asked whether Ndl1 overexpression would suppress a pac1Δ mutant. The GAL1 promoter was integrated at the NDL1 locus, which caused the strain to behave as an ndl1Δ mutant in glucose. Galactose induction of Ndl1 overexpression in the pac1Δ mutant did not reduce the number of binucleate cells (45.5 versus 45.3%), although the ndl1Δ phenotype was suppressed (Table 2). In summary, Pac1 overexpression suppressed an ndl1Δ mutant, but not vice versa, suggesting that Pac1 may function downstream of Ndl1.
Table 1.
Overexpression of Pac1 suppresses the nuclear segregation defect in an ndl1Δ mutant: overexpression of Pac1 from a high-copy plasmid (2-μm plasmid)
| GAL1-GST | GAL1-GST-PAC1 | P value | |
|---|---|---|---|
| Wild type | 0.9 ± 0.5 | 1.3 ± 0.7 | 0.42 |
| pac1Δ | 25.6 ± 2.5 | 6.6 ± 1.4 | <10−6 |
| ndl1Δ | 7.9 ± 1.6 | 2.8± 1.0 | <10−3 |
Values shown are percentage of binucleated cells ± s.e.p. (standard error of the proportion). P values are calculated based on binomial probabilities. n > 300 for each experiment.
Table 2.
Overexpression of Pac1 suppresses the nuclear segregation defect in an ndl1Δ mutant: overexpression of Ndl1 from the endogenous locus
| Wild type | pac1Δ | |||||
|---|---|---|---|---|---|---|
| NDL1 | GAL-NDL1 | P value | NDL1 | GAL-NDL1 | P value | |
| Glucose | 0.3 ± 0.3 | 14.9 ± 2.1 | <10−6 | 41.3 ± 2.8 | 39.8 ± 2.8 | 0.38 |
| Galactose | 0.3 ± 0.3 | 0.9 ± 0.5 | 0.06 | 45.3 ± 2.9 | 45.5 ± 2.9 | 0.43 |
Values shown are percentage of binucleated cells ± s.e.p. (standard error of the proportion). P values are calculated based on binomial probabilities. n > 300 for each experiment.
In this study, we discovered the NudEL homologue of budding yeast, named it Ndl1, and placed it in the dynein pathway with functional assays. We found that Ndl1 is important for targeting of dynein to the plus ends of microtubules, working via LIS1/Pac1 but not CLIP170/Bik1. Biochemically, Ndl1 and LIS1/Pac1 were found to participate in large complexes, at least some of which contain both proteins.
In budding yeast, we propose that dynein is targeted to the plus end, then offloaded to the cortex, where it is anchored and activated to power the sliding of microtubules. On the basis of our results and previous findings, we propose a cooperative recruiting model for dynein targeting to the plus end (Fig. 3). Four microtubule plus-end proteins — LIS1/Pac1, Ndl1, CLIP170/Bik1 and Kip2 — are required for dynein targeting at the plus end. Ndl1 seems to act transiently to stabilize or activate LIS1/Pac1 at the plus end, and Kip2 appears to transport CLIP170/Bik1 along the microtubule to the plus end. The fact that Bik1 localization is independent of Pac1 and Ndl1, and that Pac1 and Ndl1 localization depend only partially on Bik1/Kip2, indicates that the two targeting pathways are largely independent. Inactivation of either pathway leads to a marked decrease of dynein targeting, indicating that the two pathways are not redundant and may function cooperatively to recruit dynein.
Figure 3.
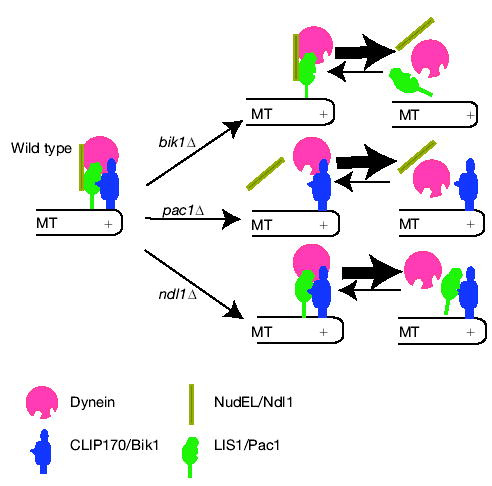
Model for dynein targeting at the plus end of microtubules. Both NudEL–LIS1 and Kip2–CLIP170 are required for the efficient targeting of dynein at microtubule plus ends. These two pathways function cooperatively, in that inactivation of either one causes a marked reduction of dynein targeting at the plus end. Ndl1 may function to secure the interaction between LIS1/Pac1 and dynein. In the absence of CLIP170/Bik1, both LIS1/Pac1 and NudEL/Ndl1 show a significant reduction at the plus end, which leads to the failure of dynein recruitment. In the absence of LIS1/Pac1, both Ndl1 and dynein targeting at the plus end is markedly reduced, although CLIP170/Bik1 localization is normal. In the absence of Ndl1, CLIP170/Bik1 is unaffected, whereas LIS1/Pac1 association with the plus end is less than in wild type, which leads to inefficient targeting of dynein at the plus ends. The complementary geometric shapes indicate interactions between two proteins.
The ndl1Δ mutant has only a partial loss of dynein function, despite a substantial decrease in the amount of dynein at the plus end and the cortex. This interesting discrepancy suggests that wild-type cells have an excess of dynein at microtubule plus ends, more than is required in laboratory conditions.
The function of dynein in yeast is simpler than its role in metazoans, but the molecular mechanism is likely to be similar. The molecular components are conserved, and metazoans control nuclear position with microtubule–cortex interactions mediated by dynein22. Our results on the mechanism of NudEL function in yeast should stimulate ideas for the function of NudEL in other systems.
METHODS
Strains and yeast genetics
General yeast manipulation, media and transformation were performed by standard methods23. Strains, plasmids and oligonucleotides are listed (see Supplementary Information, Table S1a–c). To generate double-null mutants, an ndl1Δ mutant with the Research Genetics strain background (YJC3586) was crossed with null mutant strains from the Research Genetics collection (dyn1Δ, arp1Δ, pac11Δ, kar9Δ, kip3Δ and bim1Δ) followed by tetrad dissection. Other gene deletion and epitope-tagged strains were constructed by PCR product-mediated transformation24.
Table S1a.
Yeast strain list
| YJC | Genome Type | Source | |
|---|---|---|---|
| 3411 | MATa | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 GFP-TUB1::URA3 | Castillon GA, et. al. 2003 |
| 3586 | MATa | his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ylrs254cΔ::HIS3 | This study |
| 3630 | MATα | his3 leu2 ura3 lys2Δ1 trp1-Δ63 CFP-TUB1::URA3 NDL1-YFP::HIS3 | This study |
| 3746 | MATα | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 PAC1-3GFP::TRP1 ndl1Δ::HISMX | This study |
| 3749 | MATα | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 DYN1-3GFP::TRP1 ndl1Δ::HISMX | This study |
| 3760 | MATα | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 DYN1-3GFP::TRP1 ndl1Δ::HISMX CFP-TUB1::URA3 | This study |
| 3762 | MATα | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 PAC1-3GFP::TRP1 ndl1Δ::HISMX TUB1-CFP::URA3 | This study |
| 3781 | MATα | ndl1Δ::HISMX lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52 GFP-TUB1::URA3 | This study |
| 3817 | MATα | ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL PAC1-13MYC::KAMR | This study |
| 3822 | MATα | ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL NDL1-6HA::TRP1 | This study |
| 3871 | MATα | dyn1Δ::HIS3 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52::URA3-GFP-TUB1 | This study |
| 3875 | MATa/α | ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL PAC1-6HA::TRP1 PAC1-13MYC::TRP1 | This study |
| 3876 | MATa/α | ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL NDL1-6HA::TRP1 NDL1-13MYC::TRP1 | This study |
| 3882 | MATα | ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL NDL1-6HA::TRP1 PAC1-13MYC::KAMR | This study |
| 3898 | MATa | lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52::URA3-CFP-TUB1 NDL1-3YFP::TRP1 | This study |
| 3907 | MATα | DYN1-3GFP::TRP1 pac1Δ::HIS3 CFP-TUB1-URA3::ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 | Lee W-L, et. al. 2003 |
| 3910 | MATα | ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL pac1Δ::TRP1 | This study |
| 3913 | MATα | ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL ndl1Δ::TRP1 | This study |
| 3914 | MATα | YMR299C-TAP::TRP1 ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL ndl1Δ::URA3 | This study |
| 3931 | MATα | PAC1-13MYC::KAMR ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL ndl1Δ::URA3 | This study |
| 3985 | MATα | ura3-52 trp1 lys2-801 leu2Δ1 his3Δ200 pep4::HIS3 prb1Δ1.6R can1 GAL NDL1-6HA::TRP1 pac1Δ::KAMR | This study |
| 3988 | MATa | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 NDL1-3GFP::TRP1 | This study |
| 3989 | MATa | lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52::URA3-CFP-TUB1 NDL1-3GFP::TRP1 | This study |
| 3992 | MATa | lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52::URA3-CFP-TUB1 BIK1-GFP::HixMX | This study |
| 3994 | MATa | lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52::URA3-CFP-TUB1 BIK1-GFP::HixMX ylr254Δ::TRP1 | This study |
| 4005 | MATa | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 NDL1-3GFP::TRP1 pac1Δ::KAMMX | This study |
| 4007 | MATa | lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52::URA3-CFP-TUB1 NDL1-3GFP::TRP1 pac1Δ::KAMMX | This study |
| 4008 | MATa | his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 KIP2-GFP::HISMX | Invitrogen |
| 4010 | MATα | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 PAC1-3GFP-NLS::KAMMX TRP1 ndl1Δ::HISMX | This study |
| 4012 | MATα | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 PAC1-3GFP-NLS::KAMMX TRP1 ndl1Δ::HISMX CFP-TUB1 | This study |
| 4013 | MATa | his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 NDL1-GFP-NES::KAMMX HIS3 | This study |
| 4016 | MATa | his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 NDL1-YFP-NES::KAMMX HIS3 CFP-TUB1 | This study |
| 4019 | MATa | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 pac1::HIS3 ndl1Δ::TRP1 | This study |
| 4021 | MATa | bik1Δ::HIS3 ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ndl1Δ::TRP1 | This study |
| 4022 | MATa | bik1Δ::HIS3 ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 pac1Δ::TRP1 | This study |
| 4027 | MATa | his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 KIP2-GFP::HISMX ndl1Δ::URA3MX | This study |
| 4029 | MATa | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ndl1Δ::TRP1 | This study |
| 4059 | MATα | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 ndl1Δ::HIS3 kar9Δ::KAMMX | This study |
| 4061 | MATa | his3Δ1 leu2Δ0 ura3Δ0 ndl1Δ::HIS3 dyn1Δ::KAMMX | This study |
| 4063 | MATα | his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 ndl1Δ::HIS3 kip3Δ::KAMMX | This study |
| 4065 | MATa | his3Δ1 leu2Δ0 lys2Δ0 met15Δ0 ura3Δ0 ndl1Δ::HIS3 bim1Δ::KAMMX | This study |
| 4067 | MATa | his3Δ1 leu2Δ0 lys2Δ0 met15Δ0 ura3Δ0 ndl1Δ::HIS3 arp1Δ::KAMMX | This study |
| 4069 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 ndl1Δ::HIS3 pac11Δ::KAMMX | This study |
| 4071 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 pac1Δ::KAMMX | Res. Gen. Collection |
| 4075 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 [pGAL-GST-PAC1::URA3] | This study |
| 4076 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 ndl1Δ::HIS3 [pGAL-GST-PAC1::URA3] | This study |
| 4077 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 pac1Δ::KAMMX [pGAL-GST-PAC1::URA3] | This study |
| 4078 | MATa | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 dyn1Δ::TRP1 | This study |
| 4080 | MATa | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 pac1Δ::TRP1 | This study |
| 4082 | MATa | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 bik11Δ::TRP1 | This study |
| 4084 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 [pGAL-GST::URA3] | This study |
| 4085 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 ndl1Δ::HIS3 [pGAL-GST::URA3] | This study |
| 4086 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 pac1Δ::KAMMX [pGAL-GST::URA3] | This study |
| 4124 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 pGAL-NDL1::HisMX | This study |
| 4126 | MATa | his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 pac1Δ::KamMX pGAL-NDL1::HisMX | This study |
| 4129 | MATα | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 PAC1-3GFP::TRP1 DYN3-3CFP::URA3 | This study |
| 4131 | MATa | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 NDL1-3GFP::TRP1 DYN3-3CFP::URA3 | This study |
| 4204 | MATa | lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52::URA3-CFP-TUB1 BIK1-GFP::HISMX pac1Δ::KamMX | This study |
| 4205 | MATα | ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 PAC1-3GFP::TRP1 CFP-TUB1::URA3 kip2Δ::HISMX | This study |
| 4207 | MATa | lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52::URA3-CFP-TUB1 NDL1-3GFP::TRP1 Kip2Δ::HISMX | This study |
| 4211 | MATα | PAC1-3GFP::TRP1 BIK1CTΔ40::HisMX ura3-52 lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 CFP-TUB1::URA3 | This study |
| 4213 | MATa | lys2-801 leu2-Δ1 his3-Δ200 trp1-Δ63 ura3-52::URA3-CFP-TUB1 NDL1-3GFP::TRP1 BIK1CTΔ40::HISMX | This study |
| 4218 | MATa | his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 KIP2-GFP::HISMX pac1Δ::KamMX | This study |
Table S1c.
Oligonucleotide List
| Number | Sequence |
|---|---|
| 2044 | ATGGCAACCGCAAGAGCCTTG |
| 2367 | TGTTGGTATACACCTGTGCGTGCGTAAGTGTAGAAAAAAATAGAAGGCCTCCTCTAGTACACTC |
| 2368 | GGCAACGGTTAGCTAATG |
| 2369 | TAAAAAGAGTCAAAAAAAAGGGAAAAAGAAATAGGAAAGCCAAACGCGCGCCTCGTTCAGAATG |
| 2674 | TGTATGGGCTAAATGTACG |
| 3073 | GCAGTTGGACGATATCAATGCC |
| 3248 | GGTTCGTCGTCCGGTTGTTGGTATACACCTGTGCGTGCGTAAGTGTAGAAAAAAATAGAAGAATTCGAGCTCGTTTAAAC |
| 3249 | TTTATCGAGTTCATTTTTCTGTGTATCTGTTAAAGGAAGCTGTTGCTGCCAGTTAGTCATGCACTGAGCAGCGTAATCTG |
| 3354 | ACGATGGGTCATAATACAGCAGAATGCCCCCATCACAATCCTGACAACCAGCAGTTCTTCGGAGATGGAGATGGAGGTCG |
| 3357 | GGTTTAGCAATTAATTAACTAATTTACCGGAGTCACTATTAGAGTCAGTTCGACTGCCTAGAATTCGAGCTCGTTTAAAC |
| 3829 | AAA TGA GAC TCA GCC TCT GAG ATT A |
| 4235 | CTGCAGCGAGGAGCCGTAAT |
| 4286 | ACTATCAAAGTCTCGACCACATCTT |
| 4287 | TTGAAGACTAACGACCTAACCAGAC |
| 4299 | TGTAATATTCCAAATACCCAAATAACACAATCCACTGTGATAGCAACAACGTCTTCCGTTCGGATCCCCGGGTTAATTAA |
| 4300 | GCGTTCTTTCTACTTGTAAATATATACGTAAAATTATATATAACAATTTTATATGTGTCAGAATTCGAGCTCGTTTAAAC |
| 4310 | CCTCGCAAATTTAATTGGAATATCCAAGGGACATATACGTGCAACAATTGCGACTTTCCTCGGATCCCCGGGTTAATTAA |
| 4330 | GTATCAACCAATGCTTAAAAAGAGTCAAAAAAAAGGGAAAAAGAAATAGGAAAGCCAAACGAATTCGAGCTCGTTTAAAC |
| 4340 | TGTAATATTCCAAATACCCAAATAACACAATCCACTGTGATAGCAACAACGTCTTCCGTTCGTACGCTGCAGGTCGAC |
| 4341 | GCGTTCTTTCTACTTGTAAATATATACGTAAAATTATATATAACAATTTTATATGTGTCATCGATGAATTCGAGCTCG |
| 4343 | GTATCAACCAATGCTTAAAAAGAGTCAAAAAAAAGGGAAAAAGAAATAGGAAAGCCAAACATCGATGAATTCGAGCTCG |
| 4400 | CGCGGATCCGGAAACAGCTATACAGATAATC |
| 4401 | CGCGGATCCAACGGAAGACGTTGTTGCTATC |
| 4408 | GGTTCGTCGTCCGGTTGTTGGTATACACCTGTGCGTGCGTAAGTGTAGAAAAAAATAGAACAGCTGAAGCTTCGTACGC |
| 4432 | GTTCTTTCTACTTGTAAATATATACGTAAAATTATATATAACAATTTTATATGTGTCAGCATAGGCCACTAGTGGATCTG |
| 4441 | ACCCCAAGGCAAATGATGGTCACTGGTGGATTGGATTGCAAGTCTAACGTTTTCATGAGAGCTGCTGCTGCTGCTGCTGC
TGCTGCTCGGATCCCCGGGTTAATTAA |
| 4442 | ACCCCAAGGCAAATGATGGTCACTGGTGGATTGGATTGCAAGTCTAACGTTTTCATGAGAGCTGCTGCTGCTGCTGCTG
CTGCTGCTCGTACGCTGCAGGTCGAC |
| 4480 | GTCTTGTTAGAATTTGTTACTGCTGCTGGTATTACCCATGGTATGGATGAATTGTACAAAGCTGGTGCTGGTGCTGGTGC |
| 4481 | ATAAATTGATATATATATAGTGAATGATCGTTCCGCGGCCGCTCTAGAACTAGTGGATCTGAATTCGAGCTCGTTTAAAC |
| 4483 | CTTCTTGAGTTTGTAACAGCTGCTGGGATTACACATGGCATGGATGAACTATACAAAGCTGGTGCTGGTGCTGGTGCTGG |
| 4484 | CTTATTTAATAATAAAAATCATAAATCATAAGAAATTCGCTTATTTAGAAGTGGCGCGCCGAATTCGAGCTCGTTTAAAC |
| 4514 | AGGGCAATAATACTCGTTCAGAGCTTAAATTGGAAAGTACGTCAAAACGTTTTTTAGGCACGGATCCCCGGGTTAATTAA |
| 4515 | CAGAATAGGCACGTCCACTGAGAAAACCGCGGACAAGCAAGACACGCGTACCTGAAAAGGGAATTCGAGCTCGTTTAAAC |
| 4516 | ATCAAAGGACAAGGTAAAAGGCGTTGAGCTGTGGCTGGCTGTGTATGCGTTTGAAATACCCGGATCCCCGGGTTAATTAA |
| 4520 | CCTCGCAAATTTAATTGGAATATCCAAGGGACATATACGTGCAACAATTGCGACTTTCCTGAATTCGAGCTCGTTTAAAC |
| 4521 | CAACTGGGTCTCCAGTGAGGAGATTATCTGTATAGCTGTTTCCAAATCCAAATTCGGCACCATTTTGAGATCCGGGTTTT |
| 4550 | CAGTCTACAGAACAATCCGCACAAGGAAAAAAGCACCCGA GATCTGGGACCCTCACCTAAGAATTCGAGCTCGTTTAAAC |
| 4551 | CCAGGGATCCGATAATTGTAATGCCTGGGAC |
| 4552 | CCAGGGATCCAGCACCAGCACCAGCACCAATCCTCGAATTTTCCCTATA |
| 4593 | ATTTGAAATCAAGGCAAACATAGAACACTTGATAAAATTCTTACCATAATACCACCATTGCAGCTGAAGCTTCGTACGC |
| 4631 | AAACAGTTGGAACAGGCTCAAGCACAAACAGCCGTGGAATCGTTGCCAATTTTCGACTAGCGGATCCCCGGGTTAATTAA |
Nuclear segregation cold assay
Binucleate cold assays were performed as described10. Cells with two or more nuclei (DAPI-stained bodies) in the mother cell and no nucleus in the daughter were defined as binucleate cells. The percentage of binucleate cells was calculated with the number of total cells as the denominator. For statistical analysis, we calculated the standard error of proportion defined as [P(100−P)/n]1/2 where P is the percentage of binucleate cells and n is the number of total cells. n was >300 in each experiment.
Overexpression of GST–Pac1 and Ndl1
YJC 2588 (wild type), YJC 3586 (ndl1Δ) and YJC 4071 (pac1Δ) were transformed with 2-μm plasmids containing GAL1-GST-PAC1 (pBJ1560) and GAL1-GST (pBJ383). Asynchronous cultures were grown overnight at 30 °C in glucose-containing synthetic medium lacking uracil, diluted 1: 50, grown for 6 h at 30 °C, and washed into synthetic medium with galactose lacking uracil. Cells were grown for 17 h at 30 °C, then shifted to 12 °C. After 48 h, nuclear segregation assays were performed. For Ndl1 overexpression, YJC 2588 (wild type), YJC 3586 (ndl1Δ), YJC 4071 (pac1Δ), YJC 4124 (GAL1-NDL1) and YJC 4126 (GAL1-NDL1 pac1Δ) were incubated either in glucose-containing synthetic medium or galactosecontaining synthetic medium for the same growth conditions as mentioned above.
Construction of Ndl1–3GFP and Dyn3–3CFP strains
A fragment of the NDL1 coding region (amino acids 21–567; PCR with oligonucleotides 4400 and 4401) was cloned into the BamH1 site of pBJ 1376 (pBS-3GFP-TRP1) and verified by sequencing. The resulting plasmid, Ndl1–3GFP (pBJ 1545), was linearized with NarI and transformed into a haploid strain to integrate at the endogenous NDL1 locus. Stable Trp+ transformants with green fluorescence were selected. The resulting strain did not have a nuclear segregation defect, indicating that the fusion protein is functional. To tag Dyn3 with 3CFP, a plasmid containing three tandemly fused CFP genes (Yeast Resource Center, University of Washington) and the URA3 marker, pBJ1523 (pBS-3CFP-URA3), was first constructed. A fragment of DYN3 (amino acids 2–312; PCR with oligonucleotides 4551 and 4552) was cloned into the BamHI site in pBJ1523, separated by a triple Gly-Ala linker. Cells were transformed with the resulting plasmid, which was linearized with BglII in the middle of the DYN3 sequence. Stable Ura+ transformants with CFP fluorescence were selected.
Fluorescence microscopy
Images of living cells were collected on an Olympus BX70 fluorescence microscope with a ×100 N.A. 1.35 oil objective lens and a CoolSnap HQ camera (Roper Scientific, Duluth, GA), using QED software (Media Cybernetics, San Diego, CA). Microtubules were visualized with CFP–Tub1 or GFP–Tub1 expressed from the TUB1 promoter by a plasmid (pBJ 1343 or pBJ 1333) integrated at the URA3 locus. Dual fluorescence images were collected with an 86002bs v1 beamsplitter cube (Chroma, Rockingham, VT) to capture yellow fluorescent protein (YFP) (2-s exposure for Dyn1–3GFP, Pac1–3GFP and Ndl1–3GFP) and CFP (1-s exposure for CFP–Tub1) signals sequentially. The same cube was also used for Ndl1–3GFP Dyn3–3CFP two-colour time-lapse movies (2-s exposure for YFP and 1 s exposure for CFP), with a 10-s interval. Microtubule sliding assays were performed as described10, except that GFP–Tub1 images were collected at a rate of one frame per second, with a 500-ms exposure. For Dyn1–3GFP, Pac1–3GFP and Ndl1–3GFP movies, images were collected at a 10-s interval with a 2.5-s exposure time. The fluorescence intensity of foci was measured with ImageJ and corrected for background fluorescence with an adjacent region. To measure the number and length of cytoplasmic microtubules at different cell stages, images of GFP–Tub1 cells were collected at 500-ms exposure. The length of cytoplasmic microtubules was measured with ImageJ.
Immunoprecipitation and immunoblot
Tagged proteins were demonstrated to be functional by the lack of binucleate cells seen in the cold. For synchronization, fresh cells at A600 = 1.0 were induced with 2 M hydroxyurea for 3 h, washed in medium twice, and samples were removed every 15 min. For immunoprecipitation, cells from a 5 ml overnight culture were suspended in 1 ml pre-chilled lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.2% Tween-20 and 0.5 mM DTT) with 5 μl yeast protease inhibitor cocktail (Sigma, St Louis, MO), and 1 ml acid-washed glass beads. Cells were lysed in a minibeadbeater (Cole-Parmer, Vernon Hills, IL) for 2 × 1 min, resulting in >95% lysis by phase contrast microscopy. Lysates were spun in a microcentrifuge at 16,100g for 15 min at 4 °C, and the supernatant was incubated with 30 μl rabbit anti-Myc agarose beads (Sigma) for 1 h at 4 °C. Beads were washed 4 × 1 ml lysis buffer. Bound proteins were eluted with 30 μl 0.1 M glycine-HCl pH 2.5, and neutralized immediately with 1 μl 1 M Tris base. For immunoblot, half of the eluate was separated in a 10% SDS-polyacrylamide gel, electrophoresed and transferred to a nitrocellulose membrane. The membrane was blocked with TTBS (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.05% Tween-20) and 5% non-fat milk and probed with 1: 2,000 anti-Myc (9E10; Covance, Berkeley, CA) and 1: 10,000 anti-mouse HRP-conjugated antibody (Jackson Immunology, Bar Harbor, ME) or 1: 5,000 anti-HA (polyclonal; Sigma) and 1: 10,000 anti-rabbit HRP-conjugated antibody (Biosource, Camarillo, CA).
Velocity sedimentation in sucrose gradients
Cells from a 50 ml overnight culture were suspended in 1 ml pre-chilled RIPA buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 2% Triton X-100, 0.4% SDS and 0.5 mM DTT) to increase the solubility of Ndl1 and Pac1. Cells were lysed in the presence of 1 ml acid-washed glass beads and 50 μl yeast protease inhibitor (Sigma) with a minibeadbeater (Cole-Parmer) for 4 × 1 min. Lysates were spun at 16,100g for 15 min at 4 °C, and the supernatant was spun at 191,986g in a Beckman (Fullerton, CA) TLA100.3 rotor for 1 h at 4 °C. The resulting supernatant (400 μl) was loaded onto a 3.6 ml linear gradient of 10–30% sucrose in lysis buffer (50 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.2% Tween-20 and 0.5 mM DTT). Thyroglobulin (19.4S), catalase (11.3S) and aldolase (7.5S; Amersham Biosciences, Little Chalfont, Buckinghamshire, England) were used as standards. The gradient was spun at 191,986g for 5 h at 4 °C in an SW55 rotor. Fractions of ~220 μl were collected and analysed by immunoblot. In one experiment, fractions 9–12 were pooled and used for immunoprecipitation.
Accession Numbers
NudEL sequences from Homo sapiens (AAF97497), Xenopus laevis (AAC60121), Danio rerio (AAH44386), Drosophila melanogaster (NP_648373) and Caenorhabditis elegans (NP_492172) were used in the alignment (see Supplementary Information, Fig. S1b).
BIND identifiers
Three BIND identifiers (www.bind.ca) are associated with this manuscript: 296783, 296784 and 296785.
Supplementary Material
Table S1b.
Plasmids list
| pBJ number | Plasmid name | Source |
|---|---|---|
| 383 | Gal-GST-426 | Christianson, et. al., 1992 |
| 1121 | pYM3 | Knop M, et. al., 1999 |
| 1153 | pFA6a-kanMX6 | Longtine M, et. al., 1999 |
| 1154 | pFA6a-TRP1 | Longtine M, et. al., 1999 |
| 1155 | pFA6a-HIS3MX6 | Longtine M, et. al., 2000 |
| 1158 | pFA6a-GFP(S65T)-HIS3MX6 | Longtine M, et. al., 2001 |
| 1163 | pFA6a-13Myc-TRP1 | Longtine M, et. al., 2002 |
| 1172 | pFA6a-TRP1-PGAL1-3HA | Longtine M, et. al., 2003 |
| 1333 | pAFS125 | Straight AF, et. al., 1997 |
| 1375 | pAFS125-CFP | Straight AF, et. al., 1998 |
| 1376 | 3GFP-TRP1 | Lee W-L, et. al., 2003 |
| 1519 | pAG60 | Goldstein, AL, et. al., 1999 |
| 1523 | 3CFP-Ura3 | This Study |
| 1545 | NDL1-3GFP | This Study |
| 1560 | pGal1-GST-Pac1 | Zhu H, et. al., 2001 |
| 1565 | Dyn3-3CFP | This Study |
Acknowledgments
We are grateful to R. Heil-Chapdelaine and S. Nelson for their advice and assistance. This work was supported by the American Heart Association Fellowship AHA40390 to J.L., Damon Runyon Cancer Research Foundation Fellowship DRG -1671 to W.-L.L., and NIH GM47337 to J.A.C.
Footnotes
Note: Supplementary Information is available on the Nature Cell Biology website.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
References
- 1.Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 2.Faulkner NE, et al. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nature Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- 3.Vallee RB, Tai C, Faulkner NE. LIS1: cellular function of a disease-causing gene. Trends Cell Biol. 2001;11:155–160. doi: 10.1016/s0962-8924(01)01956-0. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki S, et al. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–696. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- 5.Niethammer M, et al. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 6.Sapir T, Elbaum M, Reiner O. Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit. EMBO J. 1997;16:6977–6984. doi: 10.1093/emboj/16.23.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiner O, et al. Isolation of a Miller–Dieker lissencephaly gene containing G protein β-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 8.Pilz DT, et al. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum Mol Genet. 1998;7:2029–2037. doi: 10.1093/hmg/7.13.2029. [DOI] [PubMed] [Google Scholar]
- 9.Sheeman B, et al. Determinants of S. cerevisiae dynein localization and activation: implications for the mechanism of spindle positioning. Curr Biol. 2003;13:364–372. doi: 10.1016/s0960-9822(03)00013-7. [DOI] [PubMed] [Google Scholar]
- 10.Lee WL, Oberle JR, Cooper JA. The role of the lissencephaly protein Pac1 during nuclear migration in budding yeast. J Cell Biol. 2003;160:355–364. doi: 10.1083/jcb.200209022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang X, Osmani AH, Osmani SA, Xin M, Morris NR. NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol Biol Cell. 1995;6:297–310. doi: 10.1091/mbc.6.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efimov VP, Morris NR. The LIS1-related NUDF protein of Aspergillus nidulans interacts with the coiled-coil domain of the NUDE/RO11 protein. J Cell Biol. 2000;150:681–688. doi: 10.1083/jcb.150.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Y, et al. LIS1 regulates CNS lamination by interacting with mNudE, a central component of the centrosome. Neuron. 2000;28:665–679. doi: 10.1016/s0896-6273(00)00145-8. [DOI] [PubMed] [Google Scholar]
- 14.Sweeney KJ, Prokscha A, Eichele G. NudE-L, a novel Lis1-interacting protein, belongs to a family of vertebrate coiled-coil proteins. Mech Dev. 2001;101:21–33. doi: 10.1016/s0925-4773(00)00543-8. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Walsh CA. Mitotic spindle regulation by Nde1 controls cerebral cortical size. Neuron. 2004;44:279–293. doi: 10.1016/j.neuron.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Shu T, et al. Ndel1 operates in a common pathway with LIS1 and cytoplasmic dynein to regulate cortical neuronal positioning. Neuron. 2004;44:263–277. doi: 10.1016/j.neuron.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Lee WL, Kaiser M, Cooper JA. The offloading model for dynein function: differential function of motor subunits. J Cell Biol. 2005;168:201–207. doi: 10.1083/jcb.200407036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarricone C, et al. Coupling PAF signaling to dynein regulation; structure of LIS1 in complex with PAF-acetylhydrolase. Neuron. 2004;44:809–821. doi: 10.1016/j.neuron.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Adames NR, Cooper JA. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae. J Cell Biol. 2000;149:863–874. doi: 10.1083/jcb.149.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carvalho P, Gupta ML, Jr, Hoyt MA, Pellman D. Cell cycle control of kinesinmediated transport of Bik1 (CLIP-170) regulates microtubule stability and dynein activation. Dev Cell. 2004;6:815–829. doi: 10.1016/j.devcel.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Efimov VP. Roles of NUDE and NUDF proteins of Aspergillus nidulans: insights from intracellular localization and overexpression effects. Mol Biol Cell. 2003;14:871–888. doi: 10.1091/mbc.E02-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser, C., Michaelis, S. & Mitchell, A. Methods in Yeast Genetics (Cold Spring Harbor Laboratory Press, Plainview, 1994).
- 24.Baudin A, Ozierkalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


