Abstract
The Hypogonadism in Males study estimated the prevalence of hypogonadism [total testosterone (TT) <300 ng/dl] in men aged ≥45 years visiting primary care practices in the United States. A blood sample was obtained between 8 am and noon and assayed for TT, free testosterone (FT) and bioavailable testosterone (BAT). Common symptoms of hypogonadism, comorbid conditions, demographics and reason for visit were recorded. Of 2162 patients, 836 were hypogonadal, with 80 receiving testosterone. Crude prevalence rate of hypogonadism was 38.7%. Similar trends were observed for FT and BAT. Among men not receiving testosterone, 756 (36.3%) were hypogonadal; odds ratios for having hypogonadism were significantly higher in men with hypertension (1.84), hyperlipidaemia (1.47), diabetes (2.09), obesity (2.38), prostate disease (1.29) and asthma or chronic obstructive pulmonary disease (1.40) than in men without these conditions. The prevalence of hypogonadism was 38.7% in men aged ≥45 years presenting to primary care offices.
Keywords: Epidemiology, testosterone, hypogonadism, hyperlipidaemia, hypertension, diabetes
Introduction
Hypogonadism in men, characterised by a reduced concentration of serum testosterone, causes a constellation of signs and symptoms that may include decreased libido, erectile dysfunction, decreased volume of ejaculate, loss of body and facial hair, weakness, decreased bone density, decreased lean body mass, increased body fat, fatigue and anaemia (1,2). Hypogonadism in adult men is often overlooked, even in the presence of associated symptoms, because hypogonadal men often ignore their symptoms or attribute them to alternate causes, including ageing (1).
Unlike female menopause, which is a universal and abrupt process associated with aging, not all men become testosterone deficient with ageing. A significant number of men remain eugonadal even with advanced age (3). However, as men age, they become increasingly likely to have conditions, such as cardiovascular disease, depression, osteoporosis and diabetes that occur concomitantly with decreased testosterone levels (1).
The impact of hypogonadism on morbidity is largely unknown because there are few data from large cross-sectional studies that address this aspect. However, small epidemiological studies point towards an association of hypogonadism with morbidity resulting from low testosterone states in ageing men (4–6). For example, a higher prevalence of depression (4,5), osteoporosis, fracture and frailty (6) have been linked to testosterone deficiency. There are no well-defined criteria for defining hypogonadism based on total testosterone (TT) concentrations (1), and the clinical criteria for testosterone deficiency remain ambiguous (7–10). However, Vermeulen has defined hypogonadism as 2.5 standard deviations (SD) below the mean normal TT value in young adults (627 ng/dl with 1 SD equal to 123 ng/dl), or 319 ng/dl (9). Hypogonadism defined as TT <300 ng/dl has been shown to be related to decreased bone mineral density (1). Other investigators, using similar criteria (2.5th percentile), but different data sets, have defined hypogonadism as TT <325 ng/dl (10).
The goal of this study was to estimate the prevalence of hypogonadism in men aged at least 45 years presenting (for any reason) to primary care practices in the United States. A second objective was to correlate the presence of hypogonadism with select comorbid conditions and symptoms. Primary care practices (inclusive of general medicine and internal medicine) were selected as appropriate settings to estimate the prevalence of hypogonadism because those practices are usually the initial site for health maintenance in most adult men. Prevalence rate estimates for hypogonadal and eugonadal men along with corresponding odds ratios and confidence intervals (CIs) for age, race, current symptoms and comorbid conditions were also calculated.
Methods
Clinicians from a random sample of 2650 primary care practices throughout the United States were contacted and 130 practices agreed to participate. All men aged 45 years and older who were seen in a participating doctor's office between 8 am and noon during a 2-week period, regardless of the reason for their visit, were invited to participate in the survey. To be eligible to participate in this study, men had to meet the following inclusion criteria: age 45 years or older; ability to provide a blood sample; and willingness to answer a brief set of questions related to medical history, social history, concomitant medications, and hypogonadal signs and symptoms. The patients had to be able to read, speak and understand English. The only specified exclusion was inability or unwillingness to sign the informed consent form.
The study protocol and the written patient informed consent form were approved by a central Institutional Review Board (IRB) that complied with the IRB regulations (21 CFR Part 56). Written approval of the study was obtained from the IRB before the study was implemented. Each patient read and signed the informed consent form before their participation in the study.
All eligible patients underwent a serum testosterone assessment by a single morning blood draw (between 8 am and noon) to test for concentrations of TT, free testosterone (FT), bioavailable testosterone (BAT) and sex hormone-binding globulin (SHBG). All serum samples were analysed by Esoterix Endocrinology (Calabasas Hills, CA, USA). SHBG was evaluated using radioimmunoassay (RIA). TT was determined by RIA after extraction of testosterone from human serum. FT was determined by equilibrium dialysis and scintillation counting through the use of a radiolabelled tracer to determine the percentage of testosterone in the free form. BAT was determined through ammonium sulphate precipitation of the SHBG-bound fraction of testosterone, followed by scintillation counting of a radioactive tracer to determine the percentage of TT in bioavailable form. The FT percentage and BAT percentage values are then multiplied by the TT to derive the FT and BAT concentrations respectively.
For the purpose of this study, hypogonadism was defined as TT <300 ng/dl. This value is the middle of the TT range (200–400 ng/dl) defined as potentially hypogonadal by the American Association of Clinical Endocrinologists (1) and is slightly lower than the normal reference range of 350–1030 ng/dl for the laboratory assay used in the study. The threshold TT concentration that triggers hypogonadal symptoms varies considerably between individuals, ranging from very low to above the lower limit of the normal reference range (2). Given the lack of a widely accepted single threshold value of TT to define hypogonadism, <300 ng/dl, which has been used in clinical studies of hypogonadal men, seemed a reasonable choice. TT concentrations were compared to determine whether there were differences between early morning (8–10 am) and later morning (10 am–noon) measurements. For all patients, demographic characteristics, medical history, social history, comorbid conditions and concomitant medications were recorded by the doctor on case report forms. In addition, all patients were interviewed to determine whether they were experiencing the symptoms associated with hypogonadism, including decline in general feeling of well-being, decrease in muscular strength, feeling of weakness, physical exhaustion, lacking vitality, decrease in sexual desire or libido, decrease in ability to perform sexually and depressed mood. All doctors recorded these symptoms using a standardised case report form.
Target enrolment was 2000 patients. A patient was considered to be hypogonadal if he had a TT level of <300 ng/dl, or if he was previously diagnosed as hypogonadal and was receiving androgen treatment, regardless of his measured testosterone concentration.
The primary statistical analyses focused on descriptive statistics and prevalence estimation. Hypogonadal prevalence estimates (with 95% CIs) were obtained for subgroups of patients derived from demographic variables and other potential underlying risk factors. The prevalence CIs were obtained using SAS™ (SAS Institute Inc., Cary, NC, USA) PROC FREQ with the BINOMIAL options.
Secondary, exploratory analyses were conducted to assess the impact of demographic variables and identify potential risk factors for the prevalence of hypogonadism. Odds ratios and the corresponding 95% CIs were constructed for these purposes. The odds ratio reflected the increased risk each evaluated comorbidity carried for hypogonadal sex steroid concentration results. Odds ratios were obtained using SAS™ PROC LOGISTIC software. The CIs were calculated using the profile likelihood method (CLODDS = PL option). These factors were further analysed as multiple factors in SAS™ PROC LOGISTIC using a stepwise inclusion and elimination procedure. This procedure was used to examine correlations among the factors. For each factor remaining in the analysis, the odds ratio and 95% confidence limit were tabulated. The Hosmer–Lemeshow goodness-of-fit test was run on the final model to check the model's adequacy for the data.
Current hypogonadal symptoms were tabulated, and a chi-square test was conducted comparing hypogonadal with nonhypogonadal patients. Current symptoms were also tabulated by TT levels and by TT levels in currently treated patients. Frequency counts were tabulated for categorical variables; means, SDs, medians and ranges were tabulated for continuous variables.
Results
Of the 2650 primary care practices throughout the United States contacted to participate, 95 practices distributed over 25 states enrolled patients. The participating practices were primarily family medicine (51%) and internal medicine (42%). Of 2498 men who were solicited to participate, 2165 enrolled in the study (87% acceptance rate) during the recruitment period from November 2003 to February 2004. The majority of enrolled patients were white (82.2%; Table 1). The prevalence of hypogonadism was similar for both white and black participants. The mean age for all patients was 60.5 years (range, 45–96 years). Most men (61.6%) were visiting their doctors for routine care (Table 2).
Table 1.
Baseline characteristics of enrolled patients
| Characteristic | Hypogonadal patients (n =836) | Eugonadal patients (n = 1326) | p-value | Total patients* (n = 2165) |
|---|---|---|---|---|
| Race, n (%) | 0.154† | |||
| White | 700 (83.7) | 1077 (81.2) | 1780 (82.2) | |
| Black | 114 (13.6) | 180 (13.6) | 294 (13.6) | |
| Hispanic | 15 (1.8) | 42 (3.2) | 57 (2.6) | |
| Asian | 2 (0.2) | 11 (0.8) | 13 (0.6) | |
| Other | 5 (0.6) | 16 (1.2) | 21 (1.0) | |
| Mean age, years (SD) | 61.6 (10.57) | 59.9 (10.11) | 0.0003‡ | 60.5 (10.33) |
| Mean BMI, kg/m2§ (SD) | 31.5 (6.06) | 28.5 (5.04) | <0.0001‡ | 29.7 (5.64) |
BMI, body mass index; SD, standard deviation.
Evaluable total testosterone values were not available for three patients.
Hypogonadal vs. eugonadal, chi-square test.
Hypogonadal vs. eugonadal, t-test.
BMI was not reported in 61 hypogonadal and 80 eugonadal patients.
Table 2.
Reason for doctor office visit for enrolled patients
| Reason for visit | Patients (n = 2098)* |
|---|---|
| General check-up | 1293 (61.6) |
| Cardiovascular | 249 (12.0) |
| Respiratory | 163 (8.0) |
| Skeletal | 137 (6.5) |
| Other | 256 (12.1) |
Values are expressed as n (%).
Total number of enrolled patients was 2165; however, the reason for the visit was not recorded for 67 patients.
A higher proportion of hypogonadal patients than eugonadal patients reported a history of hypertension, hyperlipidaemia, diabetes and obesity (p < 0.001 for all of the conditions; Table 3). All other medical conditions were reported by <20% of hypogonadal patients and eugonadal patients.
Table 3.
Medical history of enrolled patients with evaluable total testosterone
| Condition | Hypogonadal patients (n = 836) | Eugonadal patients (n = 1326) | p-value* |
|---|---|---|---|
| Hypertension | 547 (65.4) | 678 (51.1) | <0.001 |
| Hyperlipidaemia | 506 (60.5) | 670 (50.5) | <0.001 |
| Diabetes | 258 (30.9) | 237 (17.9) | <0.001 |
| Obesity | 270 (32.3) | 225 (17.0) | <0.001 |
| Prostatic disease/disorder | 165 (19.7) | 226 (17.0) | 0.121 |
| Chronic pain | 155 (18.5) | 211 (16.0) | 0.113 |
| Insomnia/sleep disturbance | 129 (15.4) | 185 (14.0) | 0.342 |
| Asthma/COPD | 102 (12.2) | 118 (8.9) | 0.013 |
| Headaches (within the last 2 weeks) | 70 (8.4) | 125 (9.4) | 0.405 |
| Rheumatoid arthritis | 28 (3.3) | 29 (2.2) | 0.101 |
| Osteoporosis | 15 (1.8) | 15 (1.1) | 0.199 |
| Not reported | 0 (0.0) | 4 (0.3) | nr |
Values are expressed as n (%). COPD, chronic obstructive pulmonary disease; nr, statistical test not conducted.
p-values obtained from chi-square test of hypogonadal vs. eugonadal patients.
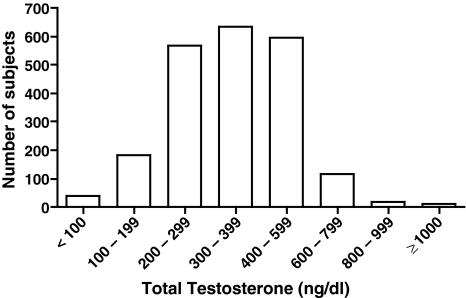
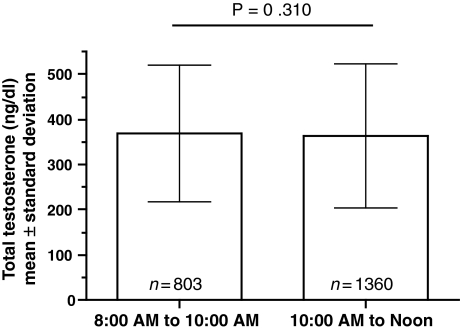
Figure 1 shows the study population distribution of TT in 100 ng/dl increments, excluding three patients who did not have evaluable TT values. The observed range of TT varied from 50 to 1573 ng/dl. The mean TT concentration in all patients was 364.8 ng/dl. The mean value of TT was 245.6 ng/dl in hypogonadal patients and 439.9 ng/dl in eugonadal patients. Consistent with the comparison of TT between groups, when BAT, FT and SHBG values were stratified by hypogonadal status, significant differences were observed between groups (p < 0.001, Table 4). There were no significant differences between sampling times for TT concentration (Figure 2).
Figure 1.
Total testosterone concentrations
Table 4.
Testosterone levels stratified by hypogonadal status
| Laboratory test (mean ± SEM) | Hypogonadal (TT <300) | Eugonadal (TT ≥300) | p-value |
|---|---|---|---|
| Total testosterone (ng/dl) | 245.6 ± 4.12 (n = 836) | 439.9 ± 3.52 (n = 1326) | N/A |
| Bioavailable testosterone (ng/dl) | 86.1 ± 2.4 (n = 821) | 108.8 ± 1.3 (n = 1317) | <0.001 |
| Free testosterone (pg/ml) | 47.9 ± 1.03 (n = 834) | 63.9 ± 0.53 (n = 1325) | <0.001 |
| SHBG (nmol/l) | 43.7 ± 0.74 (n = 836) | 68.3 ± 0.87 (n = 1326) | <0.001 |
SEM, standard error of the mean; SHBG, sex hormone-binding globulin; TT, total testosterone; N/A, not applicable.
Figure 2.
Sampling time and total testosterone concentrations for enrolled patients
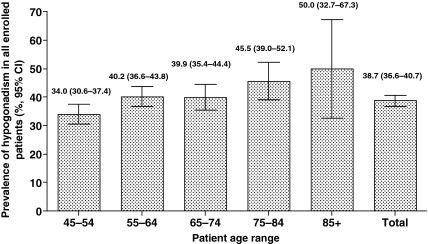
The crude prevalence rate of hypogonadism as defined in this study (TT <300 ng/dl or current androgen treatment) was 38.7% (95% CI, 36.6%–40.7%; Figure 3). For every 10-year increase in age, a patient's risk of hypogonadism increased by 17% (95% CI, 1.08–1.27). Evaluations of the prevalence of hypogonadism using values below the reported low reference level for FT and BAT were also evaluated. Based on FT levels, approximately 40% of men were hypogonadal (FT <52 pg/ml or current androgen treatment). Based on BAT levels, 45% of men were hypogonadal (BAT <95 ng/dl for men <70 years of age and <60 ng/dl for men 70 and older). The difference in the occurrence of four of the six common symptoms of hypogonadism (decrease in ability to perform sexually, decrease in sexual desire or libido, physical exhaustion or lacking vitality, and decline in general feeling of well-being) was greater in hypogonadal vs. eugonadal patients (p < 0.05). The presence of one or more symptoms occurred in 66% of patients deemed hypogonadal based on TT levels.
Figure 3.
Age-specific prevalence of hypogonadism for enrolled patients
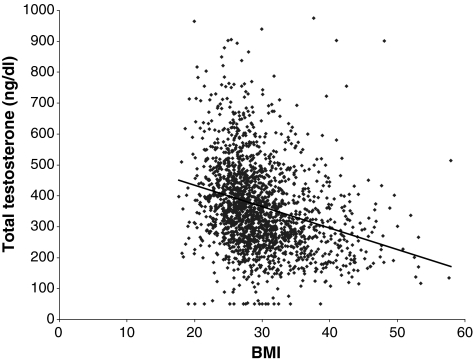
Eighty of the 2165 patients enrolled (3.7% of total) were being treated for hypogonadism. Analysis of the data excluding these 80 patients did not significantly alter the study results for the overall population. In patients not currently being treated for hypogonadism (n = 2085, 96.3% of total), the prevalence rate was 36.3% (95% CI, 34.2–38.4%). When patient characteristics were examined in those untreated men (i.e. who were not being treated for hypogonadism), the relative association of hypogonadism increased the most with increasing body mass index (BMI) (odds ratio 1.65 per 5-unit increase) (Figure 4). The odds ratio of having hypogonadism was 2.74 (95% CI, 2.07–3.07) in untreated patients with a BMI ≥25 kg/m2 vs. patients with a BMI <25 kg/m2. Men aged ≥65 years were 1.26 times (95% CI, 1.08–1.28) more likely to be hypogonadal than men aged 45–64 years. Stepwise regression analysis showed the 10-year increase in the odds ratio was 1.21 (95% CI, 1.10–1.34). In patients not already being treated for hypogonadism with at least one symptom, the 10-year increase in the odds ratio was 1.33 (95% CI, 1.11–1.61).
Figure 4.
Body mass index vs. total testosterone for enrolled patients
Table 5 shows the prevalence rate of hypogonadism and the odds ratios with specific medical conditions in the untreated patients. The odds of hypogonadism were significantly higher in patients with hypertension, hyperlipidaemia, diabetes, obesity, prostate disease, and asthma or chronic obstructive pulmonary disease (COPD) than in patients without these conditions.
Table 5.
Prevalence rates and odds ratios for selected risk factors in enrolled untreated hypogonadal patients
| Risk factor/condition | Hypogonadism prevalence rate (95% CI) | Odds ratio (95% CI) |
|---|---|---|
| Obesity | 52.4 (47.9–56.9) | 2.38 (1.93–2.93) |
| Diabetes | 50.0 (45.5–54.5) | 2.09 (1.70–2.58) |
| Hypertension | 42.4 (39.6–45.2) | 1.84 (1.53–2.22) |
| Rheumatoid arthritis | 47.3 (34.1–60.5) | 1.59 (0.92–2.72) |
| Hyperlipidaemia | 40.4 (37.6–43.3) | 1.47 (1.23–1.76) |
| Osteoporosis | 44.4 (25.5–64.7) | 1.41 (0.64–3.01) |
| Asthma/COPD | 43.5 (36.8–50.3) | 1.40 (1.04–1.86) |
| Prostatic disease/disorder | 41.3 (36.4–46.2) | 1.29 (1.03–1.62) |
| Chronic pain | 38.8 (33.7–44.0) | 1.13 (0.89–1.44) |
| Headaches (within last 2 weeks) | 32.1 (25.3–38.8) | 0.81 (0.58–1.11) |
CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Discussion
Because maintaining normal physiological concentrations of testosterone may be important for the overall health of men (1), identifying men with testosterone deficiency is important. Identifying men at risk for complications related to testosterone deficiency may also be important. Hypogonadism can have a negative impact on the health and quality of life of men because the decreases in testosterone may increase the risk of sexual dysfunction, mood disturbances, changes in bone mineral density and body composition, and decline in feeling of general well-being (1,2).
In this study, based on TT <300 ng/dl, the prevalence of hypogonadism among men aged 45 years or older was estimated to be 38.7%. Data from the Centers for Disease Control and Prevention National Health Interview Survey indicate that 74% of adult men visit a doctor's office annually (11); United States census data estimate that in 2003, 48.4 million men were aged at least 45 years (12). Using the prevalence rate observed in this study to extrapolate to current United States census data, it was estimated that 13.8 million men aged ≥45 years visiting a primary care doctor in the United States may be hypogonadal (Table 6).
Table 6.
Estimates of hypogonadism in men aged at least 45 years visiting a doctor's office relative to US census data
| Prevalence (%) | Men with hypogonadism (TT <300 ng/dl) |
|---|---|
| All men (38.7) | 13.8 million |
| Treated men (3.7) | 1.3 million |
| Untreated men (34.9) | 12.5 million |
In an effort to determine the prevalence of hypogonadism in clinical practice, several other studies have attempted to identify statistical and/or clinical working definitions of hypogonadism (7–10). A recent report from the Massachusetts Male Aging Study (MMAS) estimated the prevalence rate of androgen deficiency to be less than that found in the Hypogonadism in Males (HIM) study (8). However, the MMAS used a different patient population and different criteria for diagnosing androgen deficiency. The MMAS was an observational cohort study of health in a population-based random sample of men from the Boston, MA, area who were aged 40–70 years. Participants were studied over 7–10 years. In the MMAS, androgen deficiency was characterised by at least three signs or symptoms and TT <200 or 200–400 ng/dl with FT <8.9 ng/dl. In the MMAS, the prevalence rate of androgen deficiency at study entry, without consideration of signs or symptoms and with a cut-off TT of <400 ng/dl was estimated to be 25.3%; at follow-up, the prevalence rate was 39.3%. Considering the presence of at least three signs or symptoms and TT, the prevalence rates were 6% at baseline and 12% at follow-up. The differences observed between the present study and the MMAS may be related to the differences in populations studied, age range, and/or criteria used to define the end point. Despite these differences, the prevalence rate in MMAS based on TT was close to the prevalence rate observed in this study.
Consistent with other studies, the prevalence of hypogonadism in the HIM study increased with advancing age; men aged ≥65 years were 1.2 times more likely to demonstrate hypogonadism than men aged <65 years. The odds ratio of hypogonadism was greater with each 10-year increase in age. Longitudinal studies in specific geographical areas of the United States and some small cross-sectional studies have demonstrated a decline in testosterone occurring as early as age 30, but usually testosterone levels remain within normal limits until men reach age 60 (8,13–15).
An interesting study finding was the similar prevalence of hypogonadism between white and black participants. These prevalence results are consistent with previous longitudinal research in younger men that does not support differences in testosterone levels between black and white men after adjusting for BMI and waist circumference (16). It is well established that the incidence of prostate cancer is higher in black men. However, contrary to what one might expect, black men did not have a lower prevalence rate of hypogonadism (i.e. higher serum testosterone concentrations) in the current or previous analyses (17,18).
In the present study, the most significant differentiating factor between hypogonadal men and eugonadal men was BMI. Diagnosis of hypogonadism in obese men aged ≥45 years should involve a number of factors. Ageing has been shown to increase SHBG (15), thus decreasing BAT and increasing levels of inactive testosterone (i.e. bound to SHBG). In contrast, obesity lowers SHBG and hence TT, but not BAT or FT (19,20). Therefore, it would be anticipated that hypogonadal prevalence rates calculated using FT or BAT criteria would be lower than rates yielded by TT (given that the SHBG is lower in the individuals with higher BMI). However, that was not the case in this study. Ideally, the diagnosis of hypogonadism should include components TT and SHBG (or BAT) to determine whether the bioactive fraction of testosterone is diminished. A measurement of TT may be sufficient to identify low testosterone in the majority of patients, except in those with high BMI (who tend to have low SHBG) or the elderly (who tend to have elevated SHBG).
Advancing age (in 10-year increments) and increasing BMI (in 5-unit increments) were associated with greater prevalence rates of hypogonadism. In addition, the odds of hypogonadism were higher in patients with a history of hypertension, hyperlipidaemia, diabetes, and asthma or COPD.
One strength of this study was that we enrolled a broad-based population of men aged ≥45 years that was relatively representative of the US population based on age, with the caveat that minority groups (Hispanics and Asians) were underrepresented as expected in usual care conditions (21). In this study, the majority of patients presented to the docotor's office for a general examination, not a specific new problem. There were limitations that should be noted in interpreting these data. Collecting and analysing serial serum samples eliminates variability resulting from episodic secretion of hormones (22). This study only evaluated single samples. Therefore, it is reasonable to assume that some patients with borderline values may be transiently suppressed by acute conditions or stress and would not be considered hypogonadal upon repeat testing. However, establishing the lower threshold at 300 ng/dl vs. the reference laboratory's lower limit of normal (350 ng/dl) may offset some limitations surrounding persistently low TT levels. On the other hand, the use of a single threshold TT value for diagnosing hypogonadism may itself be a limitation; there is wide variation in the individual threshold that triggers hypogonadal symptoms (2), and some hypogonadal patients may have been missed. Another limitation may have been introduced because the symptoms of hypogonadism were not evaluated using a questionnaire validated for the assessment of hypogonadism in men, but were recorded by the doctor on a standardised case report form. Moreover, not all possible signs or symptoms of hypogonadism were considered in developing the case report form used in the study. For example, because changes in cognition and sleep disturbances – symptoms often associated with hypogonadism – were not determined, the prevalence of low TT with at least one symptom may have been underestimated.
This prevalence study also captured the comorbid conditions that may occur with hypogonadism in men who present at a primary care doctor's office seeking treatment for any reason, ranging from a general check-up to hypertension management. One limitation of the study is that the checklist of comorbid conditions on the case report form listed general conditions such as ‘prostatic disorder/disease’ rather than specific diagnoses. Nonetheless, highlighting the diseases that are most likely to occur in hypogonadal vs. eugonadal men may raise awareness among doctors that hypogonadism may be an underlying comorbid condition.
Conclusions
Based on TT concentration, the prevalence of hypogonadism in men reporting to primary care offices was estimated to be 38.7%. The medical conditions that occurred significantly more frequently among hypogonadal men than eugonadal men included increased BMI, hypertension, hyperlipidaemia, diabetes, and asthma or COPD. As men age, they are susceptible to conditions that share many of the same symptoms similar to hypogonadism. The presence of these conditions may, in effect, mask underlying hypogonadism and negatively impact quality of life.
A prevalence of this magnitude warrants consideration by the primary care doctor as the main provider of health care; however, controversy exists about the risks associated with the long-term safety of testosterone replacement therapy, particularly in older men. Therefore, men with low testosterone who present to the primary care office with medical conditions that often occur concomitantly with low testosterone should be evaluated and, if symptomatic and without contraindications, possibly considered for treatment.
Conflict of Interest
Dr Thomas Mulligan has received funding from, and been a consultant for, Solvay Pharmaceuticals, Inc. He also does research for the Department of Veteran Affairs and Ascend Therapeutics and has been a consultant for GTx.
Myra Frick is an employee of Covance Periapproval Services, Inc. Covance conducted the study on behalf of Solvay Pharmaceuticals, Inc.
Dr Qing Zuraw is an employee of Covance Periapproval Services, Inc. Covance conducted the study on behalf of Solvay Pharmaceuticals, Inc.
Annette Stemhagen was formerly an employee of Covance Periapproval Services, Inc. Covance conducted the study on behalf of Solvay Pharmaceuticals, Inc.
Cecilia McWhirter is an employee of Solvay Pharmaceuticals, Inc.
Acknowledgments
This study was supported by Solvay Pharmaceuticals, Inc.
References
- 1.American Association of Clinical Endocrinologists. Medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients – 2002 update. Endocr Pract. 2002;8:440–56. [PubMed] [Google Scholar]
- 2.Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab. 2004;89:3813–7. doi: 10.1210/jc.2004-0143. [DOI] [PubMed] [Google Scholar]
- 3.Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–98. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 4.Delhez M, Hansenne M, Legros JJ. Andropause and psychopathology: minor symptoms rather than pathological ones. Psychoneuroendocrinology. 2003;28:863–74. doi: 10.1016/s0306-4530(02)00102-6. [DOI] [PubMed] [Google Scholar]
- 5.Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry. 2004;61:162–7. doi: 10.1001/archpsyc.61.2.162. [DOI] [PubMed] [Google Scholar]
- 6.Szulc P, Claustrat B, Marchand F, et al. Increased risk of falls and increased bone resorption in elderly men with partial androgen deficiency: the MINOS study. J Clin Endocrinol Metab. 2003;88:5240–7. doi: 10.1210/jc.2003-030200. [DOI] [PubMed] [Google Scholar]
- 7.McLachlan RI, Allan CA. Defining the prevalence and incidence of androgen deficiency in aging men: where are the goal posts. J Clin Endocrinol Metab. 2004;89:5916–9. doi: 10.1210/jc.2004-2035. [DOI] [PubMed] [Google Scholar]
- 8.Araujo AB, O'Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89:5920–6. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen A. Androgen replacement therapy in the aging male – a critical evaluation. J Clin Endocrinol Metab. 2001;86:2380–90. doi: 10.1210/jcem.86.6.7630. [DOI] [PubMed] [Google Scholar]
- 10.Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 11.Lethbridge-Cejku M, Schiller J, Berndel L. Summary Health Statistics for U.S. Adults: National Health Interview Survey, 2002. Vital Health Stat 10. 2004;222:1–151. [PubMed] [Google Scholar]
- 12.Population Division US Census Bureau. Table 2: Annual estimates of the population by sex and selected age groups for the United States: April 1, 2000 to July 1, 2003 (NC-EST2003–02) 2005. [16 December 2005]. http://www.census.gov/popest/national/asrh/NC-EST2003/NC-EST2003-02.pdf.
- 13.Belanger A, Candas B, Dupont A, et al. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J Clin Endocrinol Metab. 1994;79:1086–90. doi: 10.1210/jcem.79.4.7962278. [DOI] [PubMed] [Google Scholar]
- 14.Gray A, Feldman HA, McKinlay JB, et al. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–25. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 15.Morley JE, Kaiser FE, Perry HM, 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46:410–3. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 16.Gapstur SM, Gann PH, Kopp P, et al. Serum androgen concentrations in young men: a longitudinal analysis of associations with age, obesity, and race. The CARDIA male hormone study. Cancer Epidemiol Biomarkers Prev. 2002;11:1041–7. [PubMed] [Google Scholar]
- 17.Kubricht WS, 3rd, Williams BJ, Whatley T, et al. Serum testosterone levels in African-American and white men undergoing prostate biopsy. Urology. 1999;54:1035–8. doi: 10.1016/s0090-4295(99)00290-3. [DOI] [PubMed] [Google Scholar]
- 18.Asbell SO, Raimane KC, Montesano AT, et al. Prostate-specific antigen and androgens in African-American and white normal subjects and prostate cancer patients. J Natl Med Assoc. 2000;92:445–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Amatruda JM, Harman SM, Pourmotabbed G, et al. Depressed plasma testosterone and fractional binding of testosterone in obese males. J Clin Endocrinol Metab. 1978;47:268–71. doi: 10.1210/jcem-47-2-268. [DOI] [PubMed] [Google Scholar]
- 20.Zumoff B, Strain GW, Miller LK, et al. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab. 1990;71:929–31. doi: 10.1210/jcem-71-4-929. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control. About minority health. 2005. [16 December 2005]. http://www.cdc.gov/omh/AMH/AMH.htm.
- 22.Brambilla DJ, McKinlay SM, McKinlay JB, et al. Does collecting repeated blood samples from each subject improve the precision of estimated steroid hormone levels. J Clin Epidemiol. 1996;49:345–50. doi: 10.1016/0895-4356(95)00569-2. [DOI] [PubMed] [Google Scholar]