Abstract
Conservative estimates suggest that 50–90% of the existing insect species on Earth have still to be discovered, yet the named insects alone comprise more than half of all known species of organism. With such poor baseline knowledge, monitoring change in insect diversity poses a formidable challenge to scientists and most attempts to generalize involve large extrapolations from a few well-studied taxa. Butterflies are often the only group for which accurate measures of change can be obtained. Four schemes, used successfully to assess change in British butterflies, that are increasingly being applied across the world are described: Red Data Books (RDB) list the best judgements of experts of the conservation status of species in their field of expertise; mapping schemes plot the changing distributions of species at scales of 1–100 km2; transect monitoring schemes generate time series of changes in abundance in sample populations of species on fixed sites across the UK; and occasional surveys measure the number, boundaries and size of all populations of a (usually RDB) species at intervals of 10–30 years. All schemes describe consistent patterns of change, but if they are to be more generally useful, it is important to understand how well butterflies are representative of other taxa. Comparisons with similarly measured changes in native bird and plant species suggest that butterflies have declined more rapidly that these other groups in Britain; it should soon be possible to test whether this pattern exists elsewhere. It is also demonstrated that extinction rates in British butterflies are similar to those in a range of other insect groups over 100 years once recording bias is accounted for, although probably lower than in aquatic or parasitic taxa. It is concluded that butterflies represent adequate indicators of change for many terrestrial insect groups, but recommended that similar schemes be extended to other popular groups, especially dragonflies, bumblebees, hoverflies and ants. Given institutional backing, similar projects could be employed internationally and standardized. Finally, a range of schemes designed to monitor change in communities of aquatic macro-invertebrates is described. Although designed to use invertebrates as a bio-indicator of water quality for human use, these programmes could be extended to monitor the 2010 biodiversity targets of the World Summit on Sustainable Development.
Keywords: biodiversity, extinction, habitat loss, climate change, 2010 World Summit on Sustainable Development
1. Introduction
Insects pose an obstacle to the development of a scientifically rigorous process for reporting against the World Summit on Sustainable Development's 2010 target to strive for ‘a significant reduction in the current rate of loss of biological diversity’. As at an earlier Royal Society Discussion Meeting, Estimating Extinction Rates (May et al. 1995), entomological knowledge lags far behind that achieved for assessments of change in vertebrates and plants, or for major ecosystems such as coral reefs and rain forests (Bibby 1994; Coope 1994; Ehrlich 1994; Mace 1994; Thomas & Morris 1994, 1995; May et al. 1995; Pimm et al. 1994). Nevertheless, information about the scale and patterns of insect decline has advanced substantially during the past decade, particularly in developed countries, owing to a series of recording and monitoring schemes which, although mainly developed 20–30 years ago (Thomas 1984; Master et al. 2000; Wright et al. 2000), have only recently yielded decisive results. A parallel advance in empirical knowledge about the processes driving change in insect population dynamics (e.g. Dempster & Pollard 1981; Dempster & McLean 1998; Godfray & Műller 1998; Hanski 1998, 1999; Hassell 1998a; Murdoch et al. 1998; Thomas et al. 1999a,b, 2001a,b; Roy et al. 2001; Warren et al. 2001; Bourn & Thomas 2002) plus assessments of the extent to which well-studied groups of insect are representative of obscure ones (Thomas & Morris 1995; Hambler & Speight 1996,2004; McKinney 1999; Thomas & Clarke 2004), complement many theories (May 1974; Godfray & Hassell 1987,1992; Gaston 1991; Lawton 1995; Hassell 1998a,b) and today represent an additional, indirect method of measuring change.
Here I seek to describe and evaluate the main tools currently available to entomologists to assess change in insects and other terrestrial and freshwater invertebrates; I also analyse the extent to which change in butterflies (which are often the most—or only—practical insect group to study across the world) are representative of other terrestrial invertebrates, and suggest how current monitoring and mapping schemes might be improved to meet 2010 targets.
Insects are difficult to study because they represent the most species-rich, yet one of the least known, of all taxa of living organisms, a problem that is compounded by a dearth of skilled entomologists. Although the number of described insect species is uncertain due to synonyms and the lack of a global list, most authorities recognize 900 000–1 000 000 named morpho-species, representing 56% of all species known on Earth (Groombridge 1992; Anon. 2003). Sensible estimates of the number of insects yet to be discovered range from another 1 million to 30 million species (Erwin 1982, 1991), although most predict around 2–8 million more species (May 1990; Gaston 1991; Stork 1997; Ødegaard 2000). Thus, insects lie in the zone of maximum species richness when biodiversity is related to body size (May 1988; Gaston & Lawton 1988; Finlay 2004): larger species are fewer due to their comparatively wide niches, the greater resources they need and the active mobility of many of them, whereas free-living organisms smaller than 1 mm3 experience such rapid passive transportation and mixing by wind and currents that speciation through geographical (as opposed to niche) isolation is prevented (Finlay 2002, 2004).
Apart from the few trans-continental migrants, the majority of studied insect species form small, localized populations, typically occupying narrow specialized niches in patches less than 2 ha in size and so sedentary that distances of 1 m–1 km of ‘non-habitat’ may pose a major barrier to dispersal (Thomas 1991; Thomas et al. 2005). It follows that many meta-populations of insects are so geographically isolated within each species' range that regional adaptations to, and coevolution with, their local communities is commonplace, resulting in regional sets of functionally distinct subspecific forms (Thompson 1994), including, many believe, local evolution into separate sibling species over short distances, especially among insects with parasitic life-styles (Elmes et al. 1999; Schönrogge et al. 2002; Thomas et al. in press). Too little is known about the incidence of cryptic species or subspecific variation in insects for this to be considered for the 2010 target, and hereafter I describe the monitoring of described, morphologically distinct species (morpho-species). This is challenging enough: for example, most known morpho-species of insect are tropical Coleoptera and, of those named to date, about 40% are known from a single site and many from a single specimen (Anon. 2003). This, with the fact that probably 30–90% of the world's insect species have yet to be discovered, means that monitoring efforts inevitably depend much on extrapolations from subsets of the better known groups, especially butterflies, and typically from regions such as Europe, North America and Japan, where entomological interest is high.
Four complementary approaches (Red Data Books (RDBs), atlases, time-series and absolute population censuses) have been developed to assess change in butterflies, most originating in the UK. Although some have been applied to other insect taxa, the usefulness of each has been tested most thoroughly on butterflies, especially the British fauna, on which the next four sections focus. In addition, a separate approach of sampling freshwater invertebrates has been developed across Europe (Wright 1994; Alba-Tercedor & Pujante 2000; Johnson & Goedkoop 2000; Verdonschot & Nijboer 2000), Australia (Davies 2000; Simpson & Norris 2000), Canada (Rosenberg et al. 2000) and the USA (Reynoldson et al. 2000). It employs community richness, usually at the family level, as a predictive technique for evaluating the ecological status of freshwater rivers and lakes. Although primarily designed to assess annual variation in water quality for human use (Wright et al. 2000 and papers therein), it provides an infrastructure that could be extended to monitor changes in biodiversity per se (Wright et al. 1993).
2. Insect Red Data Books
Compiling a RDB is usually the first, and often only, assessment of change in insects for most regions or groups. However, it is important to realize that the many RDBs for invertebrates (e.g. IUCN 1983; Shirt 1987) contain major uncertainties compared with those for vertebrates and plants and cannot reliably be used for the sophisticated analyses of change employed for reptiles, birds and mammals (e.g. Mace 1995) without major correction for bias and recorder effort (McKinney 1999; Thomas & Clarke 2004). For example, the insect fauna of the UK are better studied than any other comparable area in the world (Bratton 1991; Collins & Thomas 1991; Ehrlich 1994) and the British RDB-insects was exceptionally thorough, being based on the recommendations of 95 experts who, over a 5 year period, reviewed their specialist insect groups (apart from Microlepidoptera, Hymenoptera Parasitica and some Diptera) using the then IUCN classifications and guidelines. Their recommendations were submitted to a small RDB selection panel, which aimed for consistency across taxa and, with the agreement of each expert, certain species were reclassified. Nevertheless, as a member of that group, I and colleagues (Thomas & Morris 1995) consider that several of the subsequent analyses that were made using the published list are invalid due to major differences in knowledge between the groups (e.g. §5).
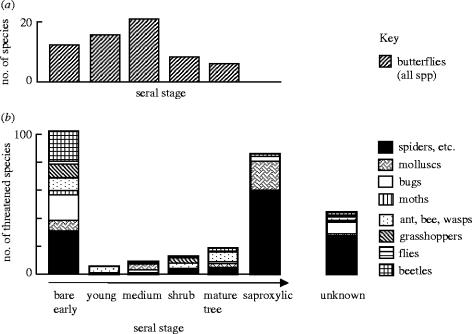
Despite their shortcomings, RDBs of insects may be useful in identifying the types of ecosystem—and types of habitat within ecosystems—that most support threatened insects (see figure 8), and they often provide an invaluable foundation for setting conservation priorities. They fall well short of providing a scientifically rigorous process for reporting against the 2010 target and, ideally, should represent the end product of other monitoring schemes rather than their foundation.
Figure 8.
The distribution of taxa in different seral stages of British terrestrial ecosystems. (a) All butterfly species (from Thomas 1986), which show a similar distribution to most insect groups. (b) Distribution of invertebrates listed as Endangered or Vulnerable in the British Red Data Book (from Thomas & Morris 1994).
3. Atlases
(a) UK mapping schemes
For some insect taxa in certain nations, the mapping of species' changing distributions has advanced usefully from the days when maps represented little more than recorder distribution. The most comprehensive examples provide sensitive measures not only of changing distributions and range sizes (Parmesan et al. 1999) but can be used as surrogates for population censuses (Asher et al. 2001; Warren et al. 2001; Thomas et al. 2004). Again, the UK system, overseen by the Biological Records Centre (BRC), is the most complete and longest-running in the world and its methodologies—which were derived from the successful plant (Perring & Walters 1962; Preston et al. 2002) and bird projects (Sharrock 1976; Gibbons et al. 1993)—have been widely adopted in other nations.
Reviews of the history of biological recording in Britain are given by Harding (1991a,b, 1995) and by Burnett et al. (1995). Details of the 39 current UK recording schemes designed to map the changing distributions of invertebrate groups are found on the BRC website (www.brc.ac.uk): they include annelids, ticks, arachnids, woodlice, non-marine molluscs, centipedes and millipedes, flatworms, beetles (16 groups), flies (18 groups), mayflies, bugs, hymenoptera (aculeates and sawflies only), lacewings and allies, dragonflies, grasshoppers and allies, fleas, caddis flies and butterflies and moths (table 1). Practical information on each scheme includes contacts of local organizers for different taxa, instructions for recording, standardized recording forms and procedures for quality control and the transfer and storage of data, including the use of different software programmes.
Table 1.
Summary of recorder effort (total records received and mean number of records per species) of UK mapping schemes for birds, plants and 39 groups of terrestrial or freshwater invertebrate.
| group | total records | records per species |
|---|---|---|
| bird | 1 252 734 | 6023 |
| plant | 9 100 000 | 5971 |
| butterfly (2001) | 1 857 400 | 26 534 |
| butterfly (1984) | 281 424 | 4020 |
| spider | 451 975 | 1773 |
| macro-moth | 378 549 | 490 |
| hoverfly | 374 784 | 1409 |
| slug, snail | 201 240 | 887 |
| ground-beetle | 141 700 | 400 |
| dragonfly | 109 117 | 1881 |
| grasshopper | 46 346 | 786 |
| milli-/centipede | 34 330 | 350 |
| soldier beetle | 30 412 | 422 |
| woodlice | 27 054 | 731 |
| brachycera fly | 20 980 | 141 |
| lacewing | 18 508 | 226 |
| bumblebee | 18 505 | 685 |
| longhorn beetle | 12 867 | 195 |
| harvestmen | 11 834 | 473 |
| atomariine beetle | 10 814 | 226 |
| water-flea | 9559 | 111 |
| ladybird | 9340 | 212 |
| rove beetle | 8644 | 103 |
| sepsid fly | 6083 | 225 |
| burnet moth | 5520 | 131 |
| picture-winged fly | 4921 | 76 |
| leech | 4414 | 276 |
| crayfish | 3896 | 649 |
| muscid fly | 2259 | 282 |
| bee, wasp, ant | 2054 | 93 |
| flea | 1939 | 52 |
| cranefly | 1894 | 271 |
| midge | 1463 | 105 |
| other beetles | 1030 | 103 |
| ciid beetles | 736 | 147 |
| well/land shrimp | 582 | 83 |
| water-slater | 582 | 194 |
| dolly/emphid fly | 582 | 29 |
| flatworm | 496 | 50 |
| parasitic wasps | 431 | 144 |
| tick (I. ricinus) | 249 | 249 |
| fairy shrimp | 163 | 83 |
(b) Case study of two surveys of British butterfly distributions
The two completed British butterfly mapping projects differ from other schemes only in their intensity of recording and illustrate the usefulness both of mapping species' distributions to fine detail and of repeating this exercise after an interval, in this case, of two decades. The methodologies and organization of each scheme are described by Heath et al. (1984) and by Asher et al. (2001) and are but briefly summarized here. Both projects aimed to achieve complete recording of the presence or absence of every butterfly species in each of the 2861 ten kilometre (10 km×10 km) grid squares of Britain. Field data were collected in 1970–1982 and 1995–1999: the large majority of records were submitted by volunteer amateur recorders on a standardized record card for each site visited. The first survey was organized by BRC's John Heath, who mobilized entomologists throughout the nation to submit records centrally or via regional organizers and local societies: the second was organized by the Butterfly Conservation society (BC) through its network of regional branches. After solving incompatibilities in software, they also caught data from the numerous local mapping schemes established in the 1980s and 1990s at the scale of individual counties (Harding & Sheail 1992). In both surveys, there was strict quality control in the accuracy of identification and recording of species and localities, involving about 70 expert (mainly amateur) regional coordinators across the country. Recording was initially undirected, but under-recorded ‘squares’ were targeted in the second half of each survey.
Volunteers were encouraged to record at the scale of the individual 1 km×1 km (1 km) squares of the national grid or smaller, but for most northern species, useful distribution maps were achieved only at the scale of 10 km squares (figure 1a). In this respect, the 10 000 recorders of the second survey achieved greater success than the ca 1500 recorders of the first survey, with 93% of records reported at 1 km2 or finer resolutions compared with 51% submitted in 1970–1982; the number of sightings of each species per visit was also recorded in 1995–1999 (Asher et al. 2001). Altogether, the volunteer recorders submitted 65 826 and 437 690 separate record cards (representing ca 2.1 million species records) in the two surveys, and sampled 98.0% and 99.6% of British 10 km grid squares: more than 90% of the missing 2% of squares in the first survey were in biodiversity ‘cold-spots’ of Scotland within the ranges of just six species (Thomas et al. 2004).
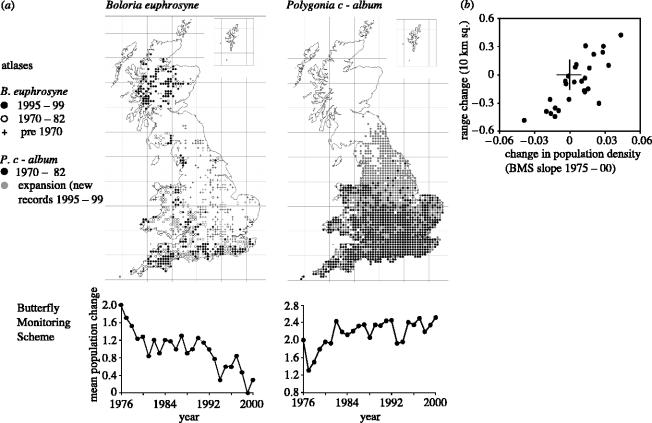
Figure 1.
Monitoring change in the distribution and abundance of British butterflies. (a) Maps comparing results from the 1970–1982 (Heath et al. 1984) and 1995–1999 (Asher et al. 2001) distributions of Boloria euphrosyne, which showed the sixth (10-percentile) greatest loss of 10 km squares for a species and Polygonia c-album which experienced the fourth (7-percentile) greatest increase between surveys. Time series of mean population size of each species on sites monitored over the same period by the BMS are shown below. (b) The correlation between 20 year trends in the population indexes of the 27 butterfly species most reliably monitored by the BMS and changes in their ranges expressed as the difference in total occupancy of 10 km squares by each species between the two mapping surveys (from Warren et al. 2001).
The first Atlas of Butterflies in Britain and Ireland (Heath et al. 1984) achieved greater cover than had previously been thought possible for any taxon of insects at the scale of a (228 073 km2) nation (Ehrlich 1994). Although the older historical records were very patchy compared with the near-complete 1970–1982 field survey, the maps suggested that about 70% of UK butterfly species had experienced losses as measured by their occupancy of 10 km squares during the twentieth century (Thomas 1984, 1991), even though several species had—in some cases simultaneously—experienced substantial northwards expansions of their historical range boundaries in recent decades (Pollard & Yates 1993). The role of climate change as one of the drivers of species' range changes was suggested by Parmesan et al. (1999), who analysed mapping schemes from the UK, Sweden, Finland, Estonia, southeast France, Catalonia, Algeria, Tunisia and Morocco. They showed that, in a sample of 35 non-migratory species, 63% had ranges that had shifted 33–240 km northwards along their northern and/or southern boundaries, coinciding with warmer summers, whereas only one species had shifted south.
The second ‘Millenium’ atlas of British butterflies (Asher et al. 2001) allowed the first quantitative comparisons of change to be made between the results of repeat surveys for an insect. Although the second (1995–1999) survey achieved a 6.6-fold higher level of recorders and recording than in the first (1970–1982), the number of record cards submitted per recorder was the same (43.8 and 43.9 cards per recorder, respectively) and accumulation curves generated by subsampling the second survey's dataset revealed substantial redundancy (for analyses assuming 10 km square occupancy) in the number of records submitted for most regions in 1995–1999 (table 1). To achieve the most accurate comparisons between the two datasets, Warren et al. (2001) employed a subsampling routine to correct for the difference in recorder effort: the 1995–1999 data were sampled 30 times by randomly selecting the 1970–1982 number of record cards from the same 1995–1999 data, subsampling separately for each 100 km Ordnance Survey grid square to retain the broad geographical distribution pattern of the 1970–1982 recording effort. Results were robust when reanalysed using different subsampling regimes, including ratios of record cards per 10 km square, the number of records rather than of cards submitted, and when distribution change was measured as the square root in area (in square kilometres) rather than as proportional change. For 43 (74% of) species, subsampling results were further validated by comparison with independent measures of species' population changes over this period (Warren et al. 2001).
The existence of a comparable pair of datasets of species' distributions after a 20 year interval suggests that atlases can be used to monitor change in certain insects for the 2010 target. For example, Warren et al. (2001) used differences in the number of 10 km squares occupied by each species between the two surveys to measure the comparative loss or gain of status by different types of species. Although Cowley et al. (1999) showed that this coarse-grained (10 km scale) sampling may underemphasize more local changes in status, we used the time series of population change generated by the UK Butterfly Monitoring Scheme (§4) for the same species over the same interval to show that range changes expressed at the 10 km scale were correlated with trends in the mean size of individual populations of each butterfly (figure 1b from Warren et al. 2001). This relationship is predicted in theory (Brown 1984; Gaston & Lawton 1988; Lawton 1995) but the closeness of the correlation between mapping and monitoring scheme results is reassuring, and allows range changes (figure 1a) to be considered as a surrogate for abundance, making each survey effectively a population census (Thomas et al. 2004).
A side effect of mapping and monitoring schemes is that a growing number of volunteers grow to enjoy recording in the field (Pollard & Yates 1993); indeed, in one mapping project, the largest number of records received arrived unsolicited for the year after the project had ended (Thomas et al. 1998b)! Given central institutional support and funding to scrutinize and process the data (ca £150 000 is required a year to run UK insect mapping schemes), it is thus a comparatively simple task to organize repeat surveys: the challenge is in launching the first one. Thus, Butterfly Conservation has embarked upon repeat surveys of similar cover to that achieved in 1995–1999 (Asher et al. 2001) for 2000–2004 and 2005–2009 (Anon. 2004; R. Fox, personal communication). If successful, the sequence of four atlases should provide the rigorous process required for British butterflies for reporting against the 2010 target. However, already the two completed measurements of species' distributions, when combined with other datasets, provide insights about the drivers and sensitivity of insects to environmental change. Two examples will suffice:
Warren et al. (2001) evaluated change in the distribution and abundances of 46 butterfly species that reach their northern climatic range margins in Britain, where climate change and habitat degradation are opposing forces. Although these insects might be expected to have responded positively to climate warming over the last 30 years (Roy et al. 2001), three-quarters of them declined: negative responses to habitat loss outweighed positive responses to climate warming. Only half of those species (e.g. Polygonia c-album, figure 1a) that were both mobile and habitat generalists increased their distribution sizes over this period, consistent with a climate explanation; whereas the other less mobile generalists and 89% of the habitat specialists (e.g. Boloria euphrosyne, figure 1a) declined in distribution size, consistent with habitat limitation. Warren et al. (2001) conclude that, in future, the dual forces of habitat modification and climate change are likely to cause specialists to decline, leaving biological communities increasingly depauperate and dominated by mobile and widespread habitat generalists.
At the first Royal Society Discussion Meeting on Estimating Extinction Rates (Lawton & May 1995), the key entomological debate was whether ‘recent’ extinction rates among insect species matched the well-recorded losses of other groups. On the one hand, the reported extinction rates of both historical and sub-fossil (Quaternary) species of insect were 2–3 orders of magnitude lower than those known for vertebrates and certain plant taxa (Coope 1994; May et al. 1995); on the other hand, historical losses of butterflies and dragonflies, recorded at the scale of populations and meta-populations, were significantly higher than those of vascular plants, birds, amphibia, reptiles and mammals in the same sites and landscapes (Thomas & Morris 1994). One hypothesis was that insects had experienced comparable rates of extinction to higher taxa but, due to low levels of early recording, the rarer species, which were also the most extinction-prone, had been overlooked and were missing from the base-line lists (May et al. 1995; Thomas & Clarke 2004). The alternative theory was that insect populations were more dynamic than vertebrates at the local level, and underwent rapid extinctions and colonizations of individual sites and landscapes while persisting as species at a larger scale (Coope 1994).
The publication of twin atlases not only of butterflies but also of changing bird and plant distributions, surveyed to the same 10 km scale over a similar period in Britain, permitted the first direct comparison to be made of regional extinctions in these three groups across a (national) landmass that was, in this case, about four orders of magnitude greater than the area occupied by a typical meta-population of a British butterfly (Thomas 1995; Thomas et al. 2004). We found that butterflies experienced greater net losses than plants or birds at this scale, disappearing on average from 13% of their previously occupied 10 km squares (figure 2). We concluded that past insect losses had been underestimated because the most threatened (rare) species had been overlooked in baseline lists (Thomas & Clarke 2004; §5d), and, applying Pimm et al.'s (1995) ‘cookie-cutter model’, we tentatively suggested that, if insects elsewhere in the world had similar levels of sensitivity to the British butterflies, the known global extinction rates of vertebrate and plant species have an unrecorded parallel among the invertebrates, strengthening the hypothesis that the natural world may be approaching the sixth major extinction event in its history (Thomas et al. 2004).
Figure 2.
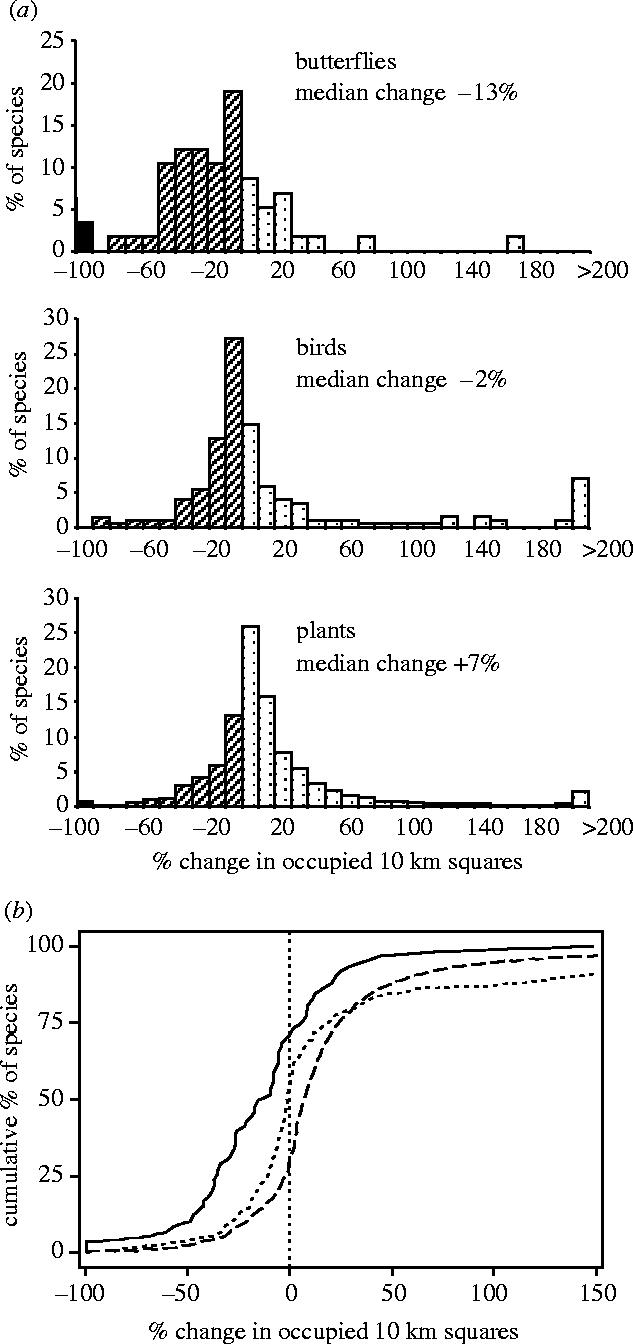
Changes in the number of 10 km squares in Britain occupied by native butterfly, bird and plant species between the two censuses of each taxon (from Thomas et al. 2004). (a) Frequency distributions: black=extinct species, hatched=declining species, dots=increasing species. Median butterfly species<median bird species (p<0.001)<median plant native species (p<0.001). (b) Cumulative frequency distributions: solid line, butterflies; dotted line, birds; dashed line, plants. Butterfly declines>bird declines>plant declines (p<0.001).
(c) Other insect mapping schemes
Many schemes for butterflies similar to that described for Britain have been initiated elsewhere, especially in Europe, where several national atlases have been published (van Swaay & Warren 2003), some matching the recording effort and cover achieved by the first British atlas. Good examples include atlases for Switzerland (Geiger 1987), the Netherlands (Tax 1989), Denmark (Stoltze 1996), the Czech Republic (Beneš & Konvička 2002) and Flanders (Maes & Van Dyck 1999). However, since none has achieved a comparable repeat survey of distributions, these are unlikely to achieve the objective of rigorously measuring change by 2010.
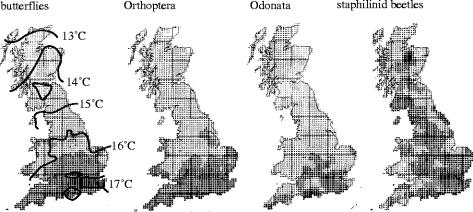
Mapping of other invertebrates in Britain also lags too far behind that of butterflies to fulfil the 2010 target. Nevertheless, realistic baseline maps are possible for several groups (see figure 9), and the level of recording in some suggests that the required cover for useful comparison will eventually be achieved through volunteer efforts (table 1). If UK entomologists continue to follow the lead of botanists, of whom only 1500 recorders made the 9 million records (ca 6000 per species) submitted for the recent atlas of 1254 native species of plant (Preston et al. 2002), recording levels adequate for quantitative analysis are surely attainable for the smaller, more popular groups (table 1); for example, more than 1500 recorders have already submitted records of Odonata (H. Arnold, personal communication). No precise analysis has been made of the intensity of recording needed to make direct comparisons between sequential mapping surveys of the same group, but the cover achieved for birds and plants of roughly twice as many records per species as the number of 10 km squares being surveyed (2861 in Britain)—or six times the occupancy of squares by the species of median range-size in each taxon—was adequate (see ‘Supplementary Information’ in Thomas et al. 2004) and is perhaps a useful guide (table 1). By these criteria, the first survey of butterflies fell short by about 25%, the second butterfly survey captured five times more records than were required (for 10 km scale analyses) and those of dragonflies, spiders and hoverflies need about two to three times their current cover (table 1).
Figure 9.
Summer isotherms and the distributions of butterflies, Orthoptera, Odonata and staphilinid beetles using smoothed species-richness data from BRC mapping schemes (from Loder 1990; Thomas 1995).
4. Time-series and absolute population estimates
(a) Time-series
A more sensitive approach than mapping distributions is to monitor trends in population sizes of species over time. At least three schemes exist in the UK that have sampled annually at fixed sites for more than 25 years, measuring aphids, macro-moths and butterflies. The first, involving standard 12.2 m high suction traps run by the Biotechnology & Biological Sciences Research Council's Rothamsted Insect Survey since 1965, is primarily designed to monitor pest species of aphid: its usefulness to measure change in rarer species for biodiversity conservation is constrained by the small number of sampling points, 16 in Britain and a further 57 in 18 other European counties (Denholm et al. 2001).
The Rothamsted moth survey began in 1965 and annually samples about 100 sites across Britain using a standard Rothamsted Light Trap (Denholm et al. 2001). Staffed mainly by volunteers, it collates daily catches of macro-moths. The assumption that variation in the numbers trapped measures change in absolute population size has been confirmed for only a few species (Raimondo et al. 2003) but is expected to be valid. This scheme is currently used mainly to measure other aspects of Lepidoptera ecology, such as phenological responses to climate change, for which it is sensitive (Sparks et al. 2001), although it has recently been extended to estimate rates of decline in some widespread species (Conrad et al. in press). Similar schemes are run in Ireland and France and clearly have the potential to provide a rigorous process for reporting against the 2010 summit.
The third scheme, which monitors butterfly abundance, has been thoroughly tested and has been successfully applied to monitor other day-flying insect groups. It is therefore described in greater detail here. The method depends on standardized transect counts of adult butterflies (Pollard et al. 1975; Pollard & Yates 1993) and is summarized in figure 3 in the simplified form used to instruct volunteer recorders. First, a representative biotope (Brereton et al. 2003) is selected for long-term monitoring and a fixed transect route is chosen, typically stratified into up to 15 sections to subsample major variations in habitat or management. This route is walked at least once a week from 1 April to 29 September (in the UK) under defined conditions of weather, time of day, etc., when adult butterflies are active, and every sighting of each species made in an imaginary 5 m×5 m×5 m box is counted in each section. At the end of the year, the mean weekly counts are summed for each species to provide an index of abundance for each generation. Similar data from other sites are collated centrally to produce a regional or national index of abundance for each species, and these in turn generate a time-series of population change when counts are repeated on the same sites in successive years (figures 1a, 5 and 6).
Figure 3.
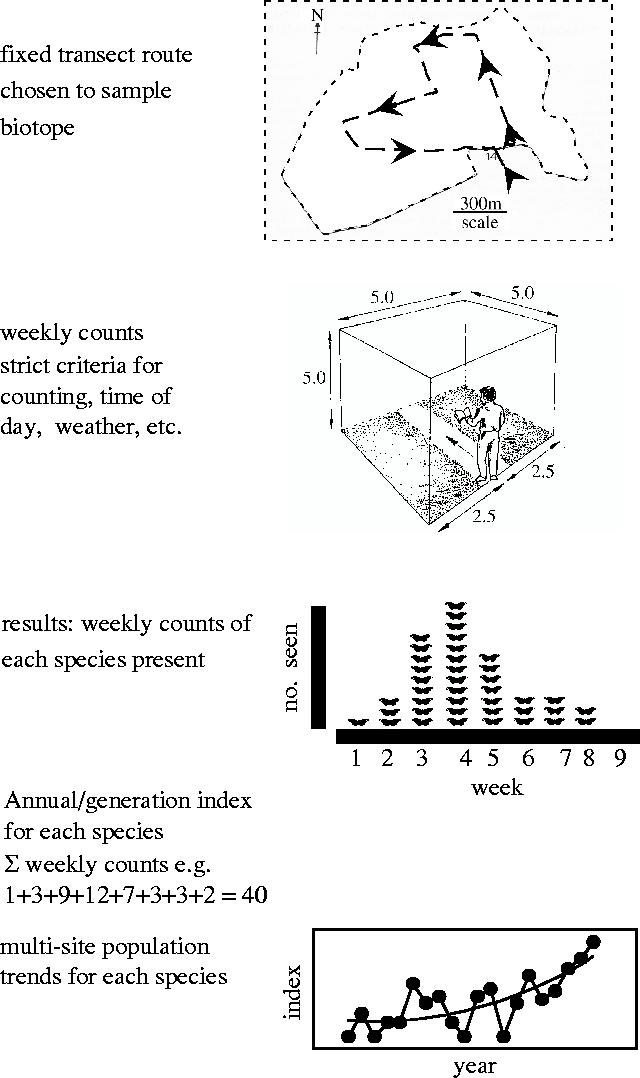
Diagram of five steps in national schemes for using transect counts to generate time-series of butterfly population changes (from T. Brereton, personal communication); see text for details.
Figure 6.
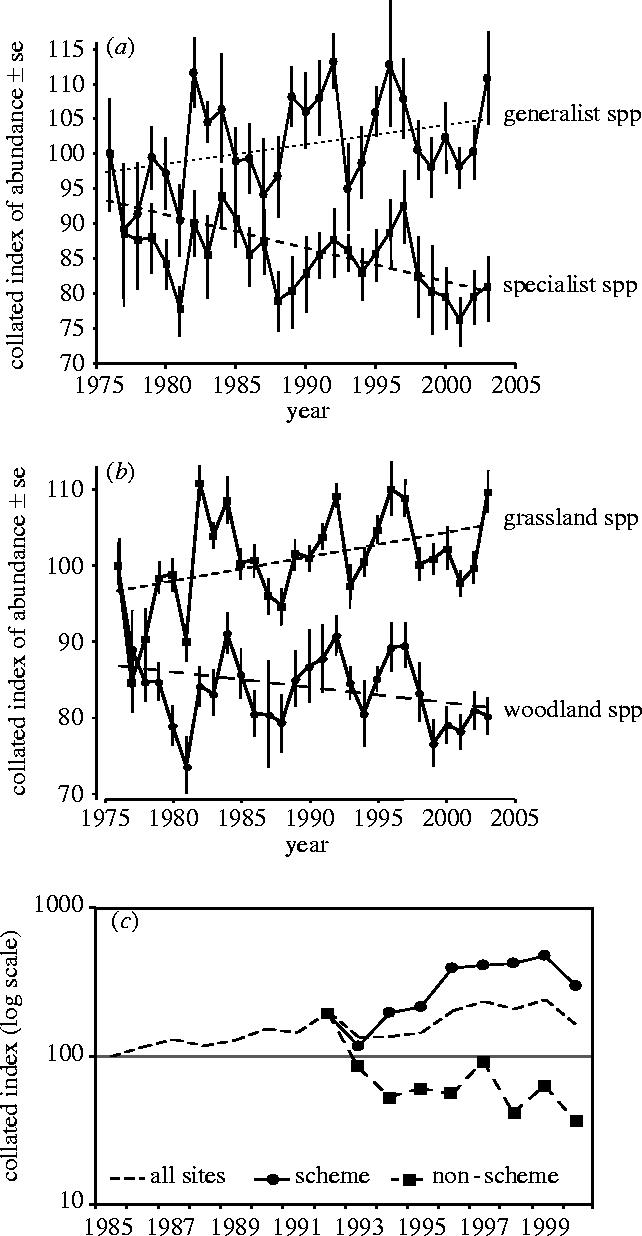
Application of trends in butterfly time series as a measure of 2010 criteria. (a) Variation in trends of generalist (b=+0.30, r2=14%, p<0.05) and specialist species (b=−0.34, r2=27%, p<0.005) measured over 28 years (=28 or 56 generations; see Pollard & Eversham 1995; Warren et al. 2001 for definitions). (b) Variation in grassland (b=+0.32, r2=18%, p<0.03) and woodland species (b=−2.0, r2=9%, ns). (c) Trends in Lysandra coridon on 68 sites managed under agri-environmental schemes and 67 sites not in the scheme (managed>non-managed, p<0.001). (a),(b) from CEH's BMS; (c) from the Butterfly Conservation scheme (T. Brereton, personal communication).
Pollard et al.'s (1975) method was not the first attempt to use transect counts to monitor abundance in butterflies (e.g. Moore 1975), but it differed in that strict standardized criteria were defined for the conditions under which recording was permitted and because these and other assumptions were tested in the field. Full details of methodology, the launch of a national Butterfly Monitoring Scheme (BMS), the treatment of data from sites with very large or very small numbers of a species to avoid bias in the collated index, the incorporation of new sites or those that drop out of the scheme, the detection of significant trends in species abundance and many of the tests used to validate the method are described in detail by Pollard et al. (1975), Pollard (1977, 1979), Thomas (1983a), Pollard & Yates (1993), Pollard et al. (1995) and Moss & Pollard (1993). Only a few key assumptions are considered here (figure 4). For example, we found that recorder effects were negligible (e.g. figure 4a, Pollard et al. 1975) in comparison to the size of differences being compared within species, which typically fluctuated by up to one order of magnitude from generation to generation on the same site due to weather variation (Thomas et al. 1998a) and which varied in density by up to three orders of magnitude between sites or on the same site over the longer term, reflecting differences or change in the quality of source habitat from optimal to suboptimal sites (e.g. figure 4b; Thomas 1983a,b, 1984). Crucially, we found that simultaneous estimates of the absolute size of populations measured by (time-consuming) MRR techniques and transect counts were closely correlated for the wide range of species tested: the example shown in figure 4b tests an application of the technique to standardize counts from different sites during surveys (§4b; Thomas 1983a) but the same principle applies. Figure 4b also tests the assumption that individuals of a species are equally visible in poor and optimum habitat: four sites are shown supporting M. cinxia populations that vary in density by 20 to 30-fold on the peak day, representing the full range in quality of source habitat for this species in Britain; but two sites were also sampled four times during the emergence, when the habitat may be considered constant but the density of adults was very different. In this and other species, the correlation between absolute population size and transect index is no closer for within-site comparisons than it is between sites.
Figure 4.
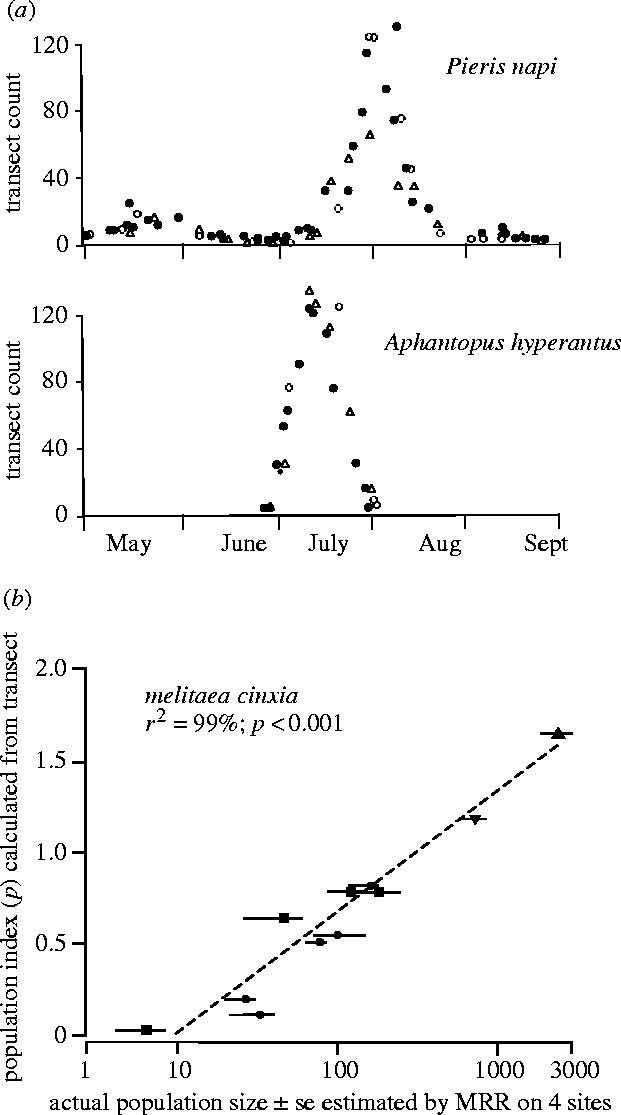
Testing assumptions in transect recording of butterflies. (a) Recorder effect: results of three different recorders (symbols) making repeated counts of two species along the same transect route during the season (from Pollard et al. 1975). (b) Comparison of transect counts with absolute population estimates of one species made on the same days on four sites (symbols) at different times in the emergence (from Thomas 1983a).
Pollard et al.'s (1975) method measures relative changes in population sizes within an individual species and is applicable to all the British butterfly species apart from the 2–3 inhabitants of woodland canopies. It is important to note that the absolute index values for different species on the same site are not directly comparable without correction for variation in inter-specific differences in behaviour and hence visibility. In addition, each sample (transect) differs from the better known bird monitoring schemes in two respects. (i) Due to the relative immobility and small patch size of about 75% of butterfly species (Thomas 1984), each transect samples one (or more) entire ‘closed’ populations of most species in the biotope, rather than a small part of an extended or open population. (ii) British butterflies are short-lived with one or two emergences a year; in most species, annual indexes reflect changes in numbers between discrete generations.
A national BMS was launched in 1976, organized by Ernie Pollard at ITE's (now CEH) Monks Wood laboratory. Twenty-eight years later (=28 or 56 generations of each butterfly species), it coordinates the records of 134 long-term sites distributed quite evenly across the UK, but with a small bias to the south where butterfly species-richness is greatest (figure 9). As already noted, butterfly monitoring became so popular—and the results so clearly useful—that many independent recorders began their own ‘Pollard walks’ (as they are affectionately known), sampling sites of local interest. The surplus data were beyond the resources of the BMS data management and unnecessary to achieve that scheme's aims, but a separate booklet, Instructions for Independent Recorders, was written to achieve the same rigour of recording as in the BMS. In the 1990s, the burgeoning number of independent recorders was coordinated by BC and extended into an equally professional parallel monitoring scheme that sampled ca 450 sites in 2003 (www.butterfly-conservation.org). Like the BMS, BC generate annual indexes of abundance and established a network of local coordinators to ensure quality control; BC also developed Transect Walker, a user-friendly software package that can be downloaded free from the BC website, which enables individual recorders to generate indices and graphs for their own sites.
The BC scheme has shorter time-series than the BMS (useful indexes start from ca 1985: figure 6c), samples four times the number of populations and is stratified to sample a higher proportion of southern sites: it therefore generates more reliable time-series for certain rare species due to the larger number of populations sampled. However, apart from the woodland sites, both schemes are currently biased in that about a third of transects sample biotopes that have some form of environmental protection, such as land in agri-environmental schemes, nature reserves, Sites of Special Scientific Interest (SSSIs) and national parks. It is important that ‘protected’ sites remain well recorded, for they support all the surviving populations of certain UK butterfly species and the majority of populations of several others (Thomas 1984, 1991). In addition, BC is now establishing additional transects on more intensive ‘ordinary’ farmland in order better to monitor success in meeting 2010 targets in the UK as a whole (Brereton et al. 2003). However, solving the current bias is less straightforward than was the case for breeding birds, for two reasons. (i) Because most butterflies live in small populations restricted to discrete patches of suitable habitat, intensive farmland is today almost bereft of butterflies to an extent that does not apply to birds, and the few species that are seen there are common and well recorded elsewhere (although the trends may differ), or those with open populations. (ii) It is less easy to persuade volunteers to make weekly transects, year after year, on land where they see a few individuals of a few common species.
Despite these problems, both monitoring schemes already contribute strongly to a scientifically rigorous process for reporting against the 2010 target, especially for the scarcer species that are largely confined to protected land. They also provide insights about the drivers of population change, ranging from weather effects and climate change (e.g. Pollard et al. 1997; Roy et al. 2001) to habitat degradation (e.g. Thomas 1983b). Many other examples are summarized by Pollard & Yates (1993). More relevant here is how easily can trends be detected in species or groups of species using transect indices? First, it should be noted that the populations of most butterfly species fluctuate considerably between generations, often synchronized over short to medium distances (Pollard 1991), in response to variation in weather (Pollard 1988; Roy et al. 2001). As theory predicts, fluctuations are amplified towards the climatic edges of species' ranges, making time series of longer than 15 years essential if underlying trends are to be detected (figure 5, Thomas et al. 1994; Pollard et al. 1995). The 28 year and 19 year runs of the BMS and BC schemes are thus adequate. For example, the collated trends in specialist and generalist species of butterfly (see Pollard & Eversham 1995; Warren et al. 2001 for definitions) from the BMS show a marked divergence since 1976 (figure 6a), confirming a key result obtained from comparisons of the two butterfly atlases (Warren et al. 2001). In addition, grassland species showed an increasing population trend over 28 years, whereas woodland species declined (figure 6b), although note that most grassland sites were semi-natural farmed biotopes such as unimproved calcareous downland. Although warmer weather has played a role (Thomas, Bodsworth et al. 2001; Warren et al. 2001), for many grassland species this encouraging increase results from targeted conservation management following knowledge gained of the ecological requirements of many species in the 1970s and 1980s (reviews: Thomas 1983a,b, 1991). Some extensive agri-environmental schemes have been particularly successful: the increase of Lysandra coridon, an indicator of high quality calcareous grassland, on targeted compared with other sites demonstrates the usefulness of the BC scheme for monitoring change (figure 6c).
Figure 5.
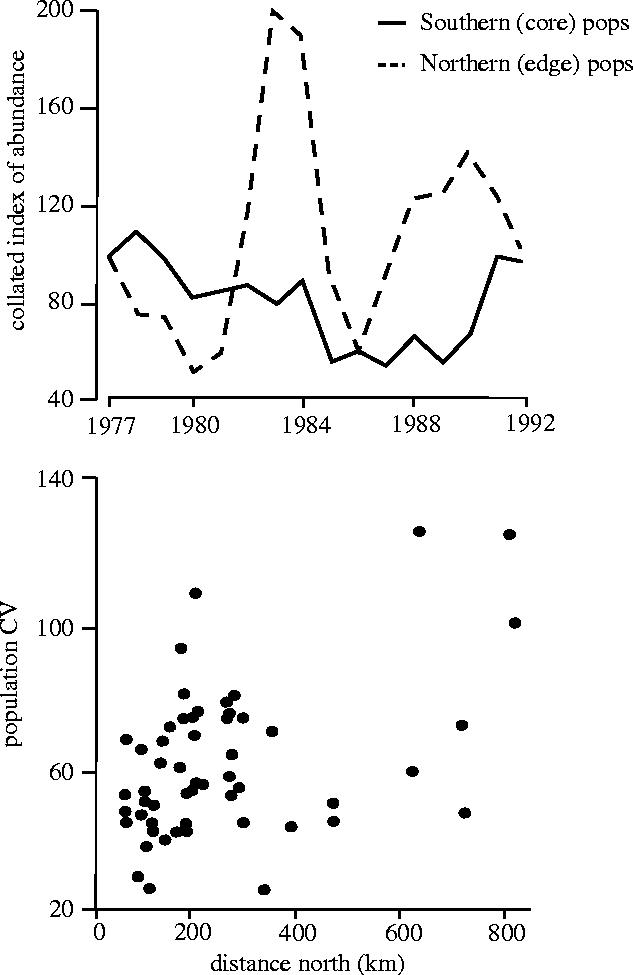
Fluctuations in the size of monitored populations of butterflies at different points in their ranges. (a) The five most northern (edge of range) sites of Maniola jurtina compared with the five most southerly (core) populations. (b) CVs of all monitored populations of M. jurtina plotted against latitude (from Thomas et al. 1994) .
In 2005, the two UK monitoring schemes will be integrated (subject to funding), then merged, and that part run by BC will expand to better monitor success in meeting government targets concerning the UK Biodiversity Action Plan (BAP), PSA (SSSI), agri-environmental schemes and sustainable development (T. Brereton, personal communication). Each scheme represents outstanding value thanks to the ca 15 000 h free labour provided annually by ca 1500 skilled volunteer recorders; however, professional coordination is essential and the full cost of developing and running both schemes to meet future targets over the next 3 years is ca £200 000 a year (D. Roy & T. Brereton, personal communication).
Successful BMSs using the same methodology have been established in the Netherlands (van Swaay et al. 2002), Flanders, Finland and Catalonia, and uncoordinated annual transect recording is practised on many sites outside Europe, for example in Japan and the USA. Small-scale attempts to extend the method to bumblebees, hoverflies, dragonflies (R. Cox, personal communication) and parasitic Hymenoptera (Thomas, unpublished) appear promising but, apart from the Hymenoptera, have yet to be tested against independent measures of population size.
(b) Surveys of absolute population size
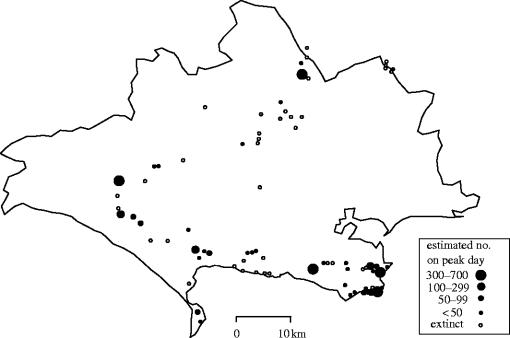
The butterfly transect method, which records changing densities of species over time, was adapted (Thomas 1983a) to measure the absolute sizes of populations on different sites in a single year. The aim was to provide a simple method of identifying the total number, boundaries and size of every population of a scarce (especially BAP) species in the UK, as a whole or over a substantial part of its range, from a one-off survey in which most sites were visited only once (e.g. figure 7). The method (Thomas 1983a) differs from BMS transects in four ways: the transect route takes a stratified sample of each population being measured, account is taken of the length of transect and the size of patch occupied by each population, and the new population size indexes for each species are calibrated from absolute measures of numbers made using MRR techniques on a few reference sites (figure 4b). I also suggested a formula for adjusting for the dates in the emergence when different sites were sampled, again using reference sites. This later proved unreliable and all surveys are now restricted to the peak two weeks of a species' emergence. Consequently, the results from each site can be assigned to only one of 4–5 broad classes of population sizes (figure 7), a level of precision that is nevertheless adequate to satisfy most conservation and scientific needs. This method is less intrusive that traditional MRR techniques of estimating absolute numbers and takes about one-fifth the amount of time per population (Thomas 1983a).
Figure 7.
Example of using a modification of transect recording to measure the relative size of every population of a species (Lysandra bellargus in its UK stronghold of Dorset) in 1 year's base-line survey (from Thomas 1983b).
Surveys encompassing all or the majority of a species' range have been made using this technique on more than 25% of the UK butterfly species to date, with repeat surveys being made at intervals of 10–20 years for six species. In future, BC aims to repeat full surveys of key declining (BAP) species every 3–5 years (N. A. D. Bourn, personal communication). In addition to providing a near-complete description of a species' national status in a particular year, the results provided insights into the population structures of different species and showed that a single biotope island may support several demographically distinct populations of the same species (Thomas 1983b, 1991; Thomas et al. 1999a,b). This, in turn, enabled the distribution of individual populations within several meta-population to be identified with greater precision than previously, and was useful in assessing the importance of meta-population dynamics as a driver of observed species' changes (Thomas et al. 1992; Thomas et al. 2001b).
5. How representative are butterflies of other insects?
(a) Introduction
Despite the success of Brown's (1991, 1996a,b) pioneering use of a wider range of groups as indicators of biodiversity-richness and change (§5d), butterflies are currently the only insect taxon that it is practical to monitor with precision in many parts of the world (e.g. Brown & Brown 1992; Ehrlich 1994). Since, for the foreseeable future, they are likely to stay elevated to this role of ‘miner's canary’ (Attenborough 2001) or ‘honorary birds’ (May et al. 1995) vis à vis insect extinction rates, it is important to examine how well butterflies represent change in other insects.
Most experts conclude that butterflies are reasonable, albeit imperfect, representatives of other invertebrates (Ehrlich 1994; Master et al. 2000). In contrast, Hambler & Speight (1996, 2004) use the listed extinctions of all taxa in the British RDB (Shirt 1987) to argue that butterflies have experienced amplified losses compared with other insects and that their use as indicators of general change is inappropriate, because ‘butterflies (being mostly warmth-loving and herbivorous) are atypical invertebrates that are relatively sensitive to climatic fluctuations and thus give a potentially misleading guide to extinction rates and human impacts’. In §5d, I suggest that the first argument is flawed due to an artefact of past recording levels. First, I consider whether British butterflies are more sensitive than other invertebrates to environmental change due to their life-styles or to thermophily.
(b) Butterfly lifestyles
Being terrestrial in all stages of their life-cycle, butterflies are clearly inappropriate indicators of freshwater species, whose declines are believed to be substantially greater than those of terrestrial ones (e.g. Shirt 1987; Master et al. 2000). They do, however, inhabit all the main terrestrial ecosystems, at least of Britain (Thomas 1984, 1986). Within these biotopes, their larvae collectively occupy niches in all the main seral stages apart from ancient rotting (saproxylic) trees (figure 8). Although the majority of species in most insect taxa inhabit the mid-successional stages of seres (e.g. Morris & Plant 1983), these are currently the least threatened types of natural habitat, and hence species, in Britain and much of the developed world (Thomas & Morris 1994). Instead, disproportionately high losses are reported from each extreme of the seral spectrum—the very early and very late successional stages (figure 8). Apart from saproxylic species, which butterflies do not represent at all, their distribution matches that of most insect taxa, with the highest species-richness in the mid successional stages of woods, heath, fen and grassland, but with adequate representation in the threatened earliest stages (figure 8).
Within these narrow niches, all but the socially parasitic butterfly species are herbivores, although perhaps a third of species are also facultative or obligate myrmecophiles, whose persistence requires a certain density—and in some cases genus—of ants to coexist with their food plants. In theory, species with more specialized traits are more sensitive to environmental change (Pimm & Lawton 1977; Pimm 1991; Lawton 1995), and indeed both world and regional RDB list a disproportionately high number of myrmecophiles, both within the lists of butterflies (Pierce et al. 2002) and among insects as a whole (Thomas & Morris 1995). Thus, butterflies are good representatives of other myrmecophiles, which account for at least 10% of the world's insect species (Elmes 1996). In addition, about half the British butterfly list can be classed as generalists and about half (including all but one of the myrmecophiles) are more specialized herbivores (Pollard & Eversham 1995).
In theory, phytophagous insects are less sensitive to environmental change than specialists, such as exploiters of rotting wood, many carnivores and (especially) insects with parasitic life-styles (Pimm 1991). Parasitoids, which may eventually account for 40% of all insect species, are potentially the most vulnerable of all insects to change (Hochberg & Ives 2000), but are too poorly described to assess critically. However, the social parasites of ants (comprising perhaps 20 000 morpho-species; Thomas et al. in press) dominate insect RDB lists (Thomas & Morris 1995; Pierce et al. 2002). Only 2–3% of the global butterfly species are social parasites: the (phytophagous) majority, judged by life-style, are more likely to under-represent losses in non-herbivorous terrestrial insects than to exaggerate them. It is perhaps telling that the two other well-recorded insect groups, each of which has a more complex lifestyle than a typical butterfly, have higher recorded extinction rates in Britain: carnivorous aquatic (dragonflies) and social terrestrial (bumblebees) (§5d, see figure 10c).
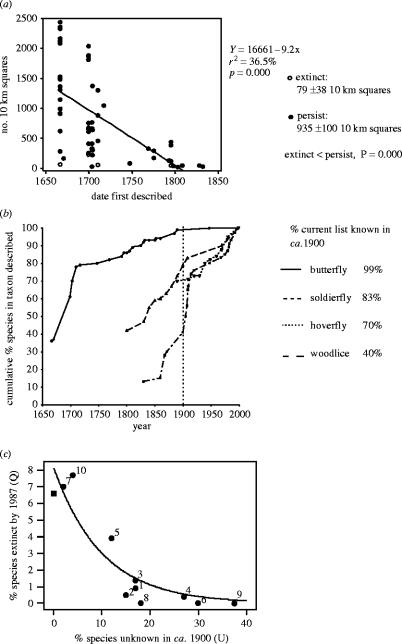
Figure 10.
Completeness of recording and of apparent extinction rates in UK invertebrates. (a) Dates of discovery of UK butterfly species (from Ford 1945; Emmett & Heath 1990; R. L. H. Dennis, personal communication) compared with range size measured as total occupancy of different 10 km squares since discovery. One cryptic species that was overlooked until the 1880s is omitted. (b) Accumulation curves of the proportion of the current species-lists of four invertebrate groups in Britain that were discovered between the seventeenth and twenty-first centuries (from Ford 1945; Emmett & Heath 1990; Thomas & Morris 1995; Stubbs & Drake 2001; Stubbs & Falk 2002; A. E. Stubbs, personal communication). (c) Percentage (Q) of insect (+spider) groups considered to have become extinct in ca 1900–1987 in relation to the percentage (U) of native species in current British lists that were unknown in ca 1900. Least squares fitted line: Q=8.13×10−0.0996U, r2=0.92, p<0.001. (Filled square) butterflies; (filled circle) other groups: (1) other Macrolepidoptera, (2) spider, (3) weevil, (4) hoverfly, (5) macro-Brachyra, (6) ant, (7) dragonfly, (8) grasshopper–cricket, (9) mosquito, (10) bumblebee (from Thomas & Clarke 2004).
(c) Are British butterflies unusually sensitive to climate change?
Most British butterfly species reach their northern climatic limit of range at some point along the 4 °C summer gradient between the warmest and coolest available latitudes and altitudes (Dennis 1993; Thomas 1993; figure 9). Although several species are also confined to warm micro-habitats near these limits, British butterflies, as a group, are not atypically thermophilous. It is the immature, not adult, stages that define climatic constraints on insects (Thomas 1991; Dennis 1993) and distribution maps show that slightly higher proportions of aculeate Hymenoptera and Orthoptera species than butterflies are restricted to the warmest regions of Britain; moths and dragonflies are similar to butterflies, whilst staphilinid beetles and woodlice are less confined to warm spots (figure 9 from Loder 1990; Thomas 1995). Furthermore, owing to recent climate warming, those butterfly species that are thermophilous experienced population increases rather than losses in Britain which frequently mitigated the effect of habitat degradation (Parmesan et al. 1999; Roy et al. 2001; Warren et al. 2001). Only four of the ten most rapidly declining butterfly species could be classed as thermophilous: the majority include alpine species (Thomas et al. 2004; Thomas & Clarke 2004). I therefore reject the argument that butterflies show exaggerated declines because they are ‘mostly warmth-loving’ (Hambler & Speight 2004).
(d) Recording artefacts and butterfly extinction rates
The fact that butterflies show higher rates of extinction in RDB lists than those reported for more obscure taxa (Hamber & Speight 2004) can be attributed to an artefact of recording. It is widely accepted that comparisons between the proportion of species believed to have become extinct in different taxonomic groups will be biased if the groups being compared experienced different levels of past recording (May et al. 1995). This occurs because the early species' lists for under-sampled groups contain a disproportionately high representation of common widespread species (Gaston et al. 1995) and it is the rare and local species in a taxon—which tended not to have been recorded in the first place—that are especially prone to extinction (May et al. 1995). McKinney (1999) quantified this artefact between six groups (mammals, birds, molluscs, crustacean, insects and marine invertebrates) and obtained a strong correlation between the proportion of species recorded as being globally extinct against the proportion of species that was estimated to have been discovered (r2=0.82).
We can now test this argument on various groups of British insect, using the RDB and other data sources (figure 10). First, as Gaston et al. (1995) found in North America, the dates of discovery of individual British butterfly species are strongly correlated with their range sizes (and hence abundance, figure 1b), with the common widespread species being found first (figure 10a). In addition, despite a small sample size, the five species that later became extinct in Britain were all among the rarest species (figure 10a: mean range size of extinct species=79±38 occupied 10 km squares cf. 935±100 occupied 10 km squares for persistent species; p=0.000). Thus, if the main discovery period of British butterflies had occurred 200 years later, we would be unaware today that three of the five extinct species had ever been lost to the British fauna, because they would never have been recorded as indigenous species before their extinction occurred.
The history of the recording of other British invertebrates (apart from dragonflies and bumblebees: Thomas & Morris 1995) shows that typical discovery rates did indeed lag at least 200 years behind that of the butterflies, as demonstrated by the accumulation curves for four taxa in figure 10b. We can therefore apply McKinney's (1999) theoretical comparisons of the relationship between the incidence of recorded extinctions and the level of discovery in a taxon by plotting the proportion of the British list considered to have become extinct in each of 11 insect groups by the late twentieth century against the proportion of species in recent lists that had not been discovered by around 1900. Figure 10c, which represents change in 9.2% of all known British insect species plus spiders, shows a similar relationship to that of McKinney (1999), indicating that for groups in which ‘only’ 90% of species had been listed a century ago recorded national extinction rates were less than half those of groups in which 100% of species had been known. Given the rigour of early butterfly recording in Britain, their documented declines were not unusual (Thomas & Clarke 2004).
(e) Other indicator groups in terrestrial neotropical ecosystems
While still relying much on butterflies, Brown (1991, 1995a,b) has made successful broad-brush assessments of change over three decades in Brazilian rain forests using inventories of additional ‘colourful, conspicuous, easily observed, highly diversified ecologically, and habitat-restricted’ taxa, including selected groups within the Odonata, Heteroptera, Coleoptera, Diptera and Hymenoptera. Following 25 years of practical experience, Brown (1991, 1996a) was able to score each group against a list of 12 key attributes: taxonomic and ecological diversification; fidelity of species to narrow niches; low vagility; endemism; ease of identification; conspicuousness; ease of sampling; biologically well-studied groups; damped fluctuations; sensitivity to perturbation; contribution to ecosystem function and association with other species. He concluded that Heliconiini and Ithomiinae butterflies along with ants, selected Odonata and certain beetle groups (carabids, scarabs, etc.) were the most useful indicators for locating biodiversity hotspots and for measuring change, being marginally superior to most other butterflies, Hymenoptera, isopods and Collembola. Hemiptera, Homoptera, sphingid moths and bait-attracted nymphalid butterflies all scored low as indicators. Spector & Forsyth (1998) independently make a strong case for using scarab (dung) beetles to assess neotropical biodiversity. My own assessment of Brown's (1991) table elevates ants above the rest, because he took small account of the ca 100 000 (often specialist) myrmecophilous insect species that depend on them (Elmes 1996), or of the dominant keystone role played by ants in shaping characteristic communities of plants and other organisms at their scale (Huxley & Cutler 1991).
6. Freshwater invertebrates
I have noted that freshwater invertebrates may have experienced greater declines than terrestrial species (Master et al. 2000) and that butterflies cannot be used as indicators of them (§5). Fortunately, the demand for a sustainable supply of clean water for human use has resulted in large resources being spent in developed countries to monitor the quality of natural waterbodies, and one of the most sensitive indicators available has proved to be their macro-invertebrate communities (Wright et al. 2000). Although developed as a biodiversity indicator to promote human health, for example to meet the EU Water Framework Directive target of 2006, current schemes that generate indexes based on freshwater invertebrates exist throughout Europe, the USA, Canada, Australia and New Zealand, and could be extended to monitor change in biodiversity per se (Wright et al. 1993), including to assess the goals of the 2010 Johannesburg summit.
The assumption in current schemes is that a strong correlation exists between the quality of water and the richness of the invertebrate community that it supports. Of course, the baseline communities vary with geography and according to local physical and chemical features, and the starting point in every scheme is to classify the variety of pristine communities found throughout the geographic region (often nation) to be monitored. Thus, RIVPACS (River InVertebrate Prediction And Classification System), developed initially for British waters, had two main objectives: to develop a biological classification of the unpolluted running-water sites of the country based on the macro-invertebrate fauna; and to assess whether the macro-invertebrate community at a site could be predicted using physical and chemical features (Wright 2000). The original 268 unstressed reference sites selected across Britain demonstrated a strong relationship between environmental features and faunal characteristics (Wright 1994). This enabled the RIVPACS model to be developed: it predicts, from the physical environment, the macro-invertebrate community expected for any site if it were in an unstressed condition, and compares that prediction with the observed community found on the site, thereby generating an index of its ecological quality status (Clarke et al. 2003). Since its initial calibration, the RIVPACS model has been refined (Moss 2000), extended to new regions, tested and shown to be remarkably robust (Clarke 2000).
The RIVPACS technique has been applied across Britain to assess changes in invertebrate communities, and hence water quality. About 6000 river sites are sampled for 640 ‘species groups’ representing 121 families of macro-invertebrate, on a 3 year rotation of 2000 sites sampled in both spring and autumn each year. Unlike the terrestrial invertebrate schemes, sampling and identification is carried out by professionals employed by Britain's Environment Agency, reflecting the large resources available when a biodiversity indicator is directly useful to monitor a human commodity. The British scheme does not require identification to the species level, but many samples are of immature stages and strict quality control is essential. This is provided through an audit in which a random 10% of all samples are checked annually by expert taxonomists employed by CEH (NERC). The recent (RIVPACS III+) software package uses statistical simulations to integrate the effects of sampling variation and errors in estimating the environmental predictor variables (both quantified in replicated field studies), and biases due to sample sorting and taxonomic identification errors (quantified from the audit), to provide an assessment of the uncertainty in O/E values, the assignment of sites to quality bands, spatial or temporal differences in O/E values and potential changes in quality bands (Clarke 2000).
Variations of the RIVPACS approach are employed across Australia (Davies 2000; Simpson & Norris 2000), Canada (Rosenberg et al. 2000), the USA (Hawkins et al. 2000; Reynoldson et al. 2000), New Zealand and in many European countries (Alba-Tercedor & Pujante 2000; Johnson & Goedkoop 2000; Verdonschot & Nijboer 2000). Already, it is incorporated in the EU's Water Framework Directive on the quality and management of rivers and lakes, which will be mandatory for EU nations after 2006. There is, however, variation in the methods employed in different countries. The two main variants involve the standardized technique used to sample, subsample and identify invertebrates, and whether a predictive model or a multi-metric system is used for analysis (Wright et al. 2000). In Europe, an EU-funded project (STAR—STAndardization of River classification) is engaged in testing the comparability of the biodiversity indices currently being used in 14 nations to assess the ecological quality of fresh water, including the use of diatoms, fish, macrophytes and habitat measures as well as macro-invertebrates (M. T. Furse, personal communication).
The large annual investment in sampling freshwater macro-invertebrates to measure water quality for human use could be exploited (and to some extent has been) to assess change in biodiversity in its own right (Wright et al. 1993; Wright 1994). A drawback of most current schemes is that taxa are identified to ‘species groups’ or families; only in a few counties, for example Germany and Austria, is identification to the species level (J. Davy-Bowker, personal communication). There appears to be considerable potential for conservation agencies that require more sensitive indicators of change to measure 2010 targets, to capitalize on the existing infrastructure by subsampling to identify to species level the annual and past (stored) samples collected by water agencies.
In contrast to the situation for vertebrates, butterflies and plants in some regions, there are no nationally reliable recording schemes for freshwater invertebrates which can be used to estimate long-term (i.e. 20+ years) changes in species' frequency and distribution. However, one approach that could be used in regions like Great Britain, where RIVPACS models have been developed, is to compare the ratio of the current observed frequency of occurrence of any particular taxon (family, genus or species) in the country obtained from national surveys with its expected total frequency of occurrence in the absence of any environmental stress as predicted from the RIVPACS model applied to each of the survey sites (i.e. summing the site-specific expected probabilities of occurrence; Clarke, in preparation).
7. Conclusions
The unexpected observation that insects (represented by butterflies) have experienced greater recent extinction rates than birds or plants (Thomas et al. 2004) increases the pressure on conservationists to monitor this difficult group. Unfortunately, although the described species of insect, freshwater and terrestrial arthropod comprise more than half of all organisms known on Earth, probably less than a third of the existing morpho-species has been found. And despite Brown's (1991, 1996a,b) repeated inventories in Brazilian rain forests, for roughly 99.9% of the world's known species of insect there is no scientific process that currently measures change with the rigour that is required to report against the 2010 target for sustainable development. Those schemes that do exist are largely restricted to prosperous developed nations, where biodiversity is typically low. Five years is too short a time to obtain meaningful results from extending tested schemes to new regions and taxa, so my conclusions focus briefly on providing a structure beyond the next decade.
In all ecosystems, the use of indicator groups is inevitable if generalizations are to be made about trends in insect biodiversity and abundance. In the terrestrial neotropics, research is urgently required to determine the priorities to be placed, when compiling inventories, on the various indicator groups recommended by Brown (1991, 1996a) and Spector & Forsyth (1998) and also to devise simple, tested methods to convert inventories from the mere listing of species to the measurement of population sizes.
In temperate countries, there is an over-dependence on monitoring butterflies, even though, having examined their life-history traits (§5b), relative sensitivity to climate change (§5c) and adjusted extinction rates (§5d), I conclude that they are sufficiently representative of all except saproxylic groups to be employed and are the only invertebrate taxon for which it is currently possible to estimate rates of decline among terrestrial insects in many parts of the world (Thomas & Clarke 2004). To obtain useful measures of range change using butterflies (or other groups), full atlas surveys should be repeated at intervals of 10–20 years, with regular checks by plotting accumulation curves that near-saturation levels of recording have been achieved in all sub-areas and by taking particular care to measure recorder effort if surveys are likely to be incomplete (see supplementary material in Warren et al. 2001; Thomas et al. 2004). Time-series of adult butterfly population changes, using standardized repeated transects, should also be established across the developed world and, wherever feasible, in primary ecosystems. The UK butterfly mapping and monitoring schemes both grew from very humble beginnings, and the current level of support, which was unimaginable 30–40 years ago, was generated largely by the enthusiasm of the volunteers who undertook early recording. In my view, it will be possible to repeat these successes in many other nations, as has already been demonstrated in parts of Europe. To be successful, however, it is essential to have a well-funded institutional group to organize data gathering and to collate and analyse the results, as well as a determined leader to establish each scheme at the outset.
These comments apply to the mapping and monitoring of other insect taxa in the UK and comparable nations. Current mapping schemes for several groups in the UK are more advanced than was the case for butterflies around 1970 and, with concerted promotion (and a forceful leader), could achieve equally useful results. I recommend priority be placed on Odonata (dragonflies and damselflies) as conspicuous representatives of aquatic carnivores, bumblebees (social insects), hoverflies (mainly terrestrial or wetland carnivores) and ants (social keystone species supporting many myrmecophiles). I also recommend that the ability of transect counts to monitor genuine population change in adult Odonata, bumblebee and hoverfly species be tested as rigorously as was the case with butterflies (Pollard et al. 1975; Pollard 1977; Thomas 1983a). If this key assumption is confirmed, similar schemes to the BMS should be established to generate time-series of population changes in those taxa. As with butterfly monitoring beyond the UK, it is not essential that every species be included in these schemes: groups containing confusing or cryptic species can be omitted. No current scheme monitors the fate of saproxylic insects: until adequate methods are developed, I recommend that the entire resource of this important, but localized, ecosystem be monitored.
Finally, declines in freshwater ecosystems may be more severe than in terrestrial or marine ones (Master et al. 2000; Anon. 2003). I strongly recommend that those authorities responsible for the supply and quality of water in all nations apply the same methods of using invertebrate diversity to measure water quality that are currently employed across Europe, North America and Australia. I recommend that conservation organizations capitalize on this infrastructure to use existing schemes to monitor change in invertebrate biodiversity per se.
Acknowledgments
I thank J. A. Asher, H. Arnold, T. Brereton, R. T. Clarke, J. Davy-Bowker, R. L. H. Dennis, R. Fox, M. T. Furse, J. Murphy, D. B. Roy, A. E. Stubbs, M. G. Morris, J. Settele, M. S. Warren and the Butterfly Conservation society for data, advice or comments, and the European Commission for RTD research grant Macman (EVK2-CT-2001-00126).
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Beyond extinction rates: monitoring wild nature for the 2010 target’.
References
- Alba-Tercedor J, Pujante M. Running-water biomonitoring in Spain: opportunities for a predictive approach. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 207–216. [Google Scholar]
- Anon. The Royal Society; London: 2003. Measuring biodiversity for conservation. [Google Scholar]
- Anon. Butterflies for the new millennium. Butterfly. 2004;86:18. [Google Scholar]
- Asher J.A, Warren M.S, Fox R, Harding P, Jeffcoate G, Jeffcoate S. Oxford University Press; Oxford: 2001. The millennium atlas of butterflies in Britain and Ireland. [Google Scholar]
- Attenborough D. The millennium atlas of butterflies in Britain and Ireland. Oxford University Press; Oxford: 2001. Foreword. [Google Scholar]
- Beneš J, Konvička M, editors. Butterflies of the Czech Republic: distribution and conservation, I, II. Vydal SOM; Praha: 2002. [Google Scholar]
- Bibby C.J. Recent past and future extinctions in birds. Phil. Trans. R. Soc. B. 1994;344:35–40. [Google Scholar]
- Bourn N.A.D, Thomas J.A. The challenge of conserving butterflies at range margins in Europe. Biol. Conserv. 2002;104:285–292. [Google Scholar]
- Bratton J.H, editor. British Red Data Books: 3. Invertebrates other the insects. Joint Nature Conservation Committee; Peterborough: 1991. [Google Scholar]
- Brereton T, Greatorex-Davies N, Fox R, Roy D, Stewart K, Warren M.S. Butterfly Conservation; Wareham: 2003. Annual monitoring coverage of conservation priority butterflies. [Google Scholar]
- Brown J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984;124:255–279. [Google Scholar]
- Brown K.S. Conservation of neotropical environments: insects as indicators. In: Collines N.M, Thomas J.A, editors. The conservation of insects and their habitats. Academic Press; London: 1991. pp. 350–404. [Google Scholar]
- Brown K.S. The use of insects in the study, inventory, conservation and monitoring of biological diversity in Neotropical habitats, in relation to traditional land use systems. In: Ae S.A, Hirowatari T, Ishii M, Brower L.P, editors. Decline and conservation of butterflies in Japan III. Lepidopterological Society of Japan; Aichi: 1996a. pp. 128–149. [Google Scholar]
- Brown K.S. Conservation of threatened species of Brazilian butterflies. In: Ae S.A, Hirowatari T, Ishii M, Brower L.P, editors. Decline and conservation of butterflies in Japan III. Lepidopterological Society of Japan; Aichi: 1996b. pp. 45–62. [Google Scholar]
- Brown K.S, Brown G.G. In: Tropical forest deforestation and species extinction. Whitmore T.C, Sayer J.A, editors. Chapman and Hall; London: 1992. pp. 119–142. [Google Scholar]
- Burnett J, Copp C, Harding P. DoE; Ruislip: 1995. Biological recording in the United Kingdom: present practice and future development. [Google Scholar]
- Clarke R.T. Uncertainty in estimates of river quality based on RIVPACS. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 39–54. [Google Scholar]
- Clarke R.T, Wright J.F, Furse M.T. RIVPACS models for predicting the expected macroinvertebrate fauna and assessing the ecological quality of rivers. Ecol. Model. 2003;160:219–233. [Google Scholar]
- Collins N.M, Thomas J.A, editors. The conservation of insects and their habitats. Academic Press; London: 1991. [Google Scholar]
- Conrad, K. F., Woiwod, I. P., Parsons, M., Fox, R. & Warren, M. S. 2004 Long-term population trends in widespread British moths. J. Insect Conserv.8, 119–136.
- Coope G.R. The response of insect faunas to glacial–intergalcial climatic fluctuations. Phil. Trans. R. Soc. B. 1994;344:19–26. [Google Scholar]
- Cowley M.J.R, Thomas C.D, Thomas J.A, Warren M.S. Flight areas of British butterflies: assessing species status and decline. Proc. R. Soc. B. 1999;266:1587–1592. [Google Scholar]
- Davies P.E. Development of a national river bioassessment system (AUSRIVAS) in Australia. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 113–124. [Google Scholar]
- Dempster J.P, McLean I.F.G. Kluwer; Dordrecht: 1998. Insect populations in theory and in practice. [Google Scholar]
- Dempster J.P, Pollard E. Fluctuations in resource availability and insect populations. Oecologia. 1981;50:412–416. doi: 10.1007/BF00344984. [DOI] [PubMed] [Google Scholar]
- Denholm I, Chapman J.W, Denholm C, Woiwod I.P. Institute of arable crops research report 2000–2001. Rothamsted; Harpenden: 2001. Insect population dynamics. [Google Scholar]
- Dennis R.L.H. Manchester University Press; Manchester: 1993. Butterflies & climate change. [Google Scholar]
- Ehrlich P.R. Energy use and biodiversity loss. Phil. Trans. R. Soc. B. 1994;344:99–104. [Google Scholar]
- Elmes G.W. Biological diversity of ants and their role in ecosystem function. In: Lee B.H, Kim T.H, Sun B.Y, editors. Biodiversity research and its perspectives in the east Asia. Proceedings of Inaugural Seminar of KIBIO. Chonbuk National University; Korea: 1996. pp. 33–48. [Google Scholar]
- Elmes G.W, Barr B, Thomas J.A, Clarke R.T. Extreme host specificity by Microdon mutabilis (Diptera: Syrphidae), a social parasite of ants. Proc. R. Soc. B. 1999;266:447–453. [Google Scholar]
- Emmett A.M, Heath J. Harley Books; Colchester: 1990. The butterflies of Great Britain and Ireland. [Google Scholar]
- Erwin T.L. Tropical forests: their richness in Coleoptera and other arthropod species. Coleopts. Bull. 1982;36:74–75. [Google Scholar]
- Erwin T.L. How many species are there? Revisited. Conserv. Biol. 1991;5:1–4. [Google Scholar]
- Finlay B.J. Global dispersal of free-living microbial eukarytote species. Science. 2002;296:1061–1063. doi: 10.1126/science.1070710. [DOI] [PubMed] [Google Scholar]
- Finlay B.J. Protist taxonomy: an ecological perspective. Phil. Trans. R. Soc. B. 2004;359:599–610. doi: 10.1098/rstb.2003.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E.B. Collins; London: 1945. Butterflies. [Google Scholar]
- Gaston K.J. The magnitude of global insect species richness. Conserv. Biol. 1991;5:283–296. [Google Scholar]
- Gaston K.J, Lawton J.H. Patterns in the distribution and abundance of insect populations. Nature. 1988;331:709–711. [Google Scholar]
- Gaston K.J, Blackburn T.M, Loder N. Which species are described first—the case of North-American butterflies. Biodiv. Conserv. 1995;4:119–127. [Google Scholar]
- Geiger W, editor. Tagfalter und ihr Lebensraum. Schweizerisches Bund fur naturschutz; Basel: 1987. [Google Scholar]
- Gibbons D.W, Reid J.B, Chapman R.A. Poyser; London: 1993. The new atlas of breeding birds in Britain and Ireland 1988–1991. [Google Scholar]
- Godfray H.C.J, Hassell M.P. Natural enemies can cause discrete generations in tropical insects. Nature. 1987;327:144–147. [Google Scholar]
- Godfray H.C.J, Hassell M.P. Long time series reveal density dependence. Nature. 1992;359:673–674. [Google Scholar]
- Godfray H.C.J, Műller C.B. Host–parasitoid dynamics. In: Dempster J.P, McLean I.F.G, editors. Insect populations in theory and in practice. Kluwer; Dordrecht: 1998. pp. 135–166. [Google Scholar]
- Groombridge B, editor. Global biodiversity: status of the Earth's living resources. Chapman and Hall; London: 1992. [Google Scholar]
- Hambler C, Speight M.R. Extinction rates in British nonmarine invertebrates since 1900. Conserv. Biol. 1996;10:892–896. [Google Scholar]
- Hambler C, Speight M.R. Extinction rates and butterflies. Science. 2004;305:1563. doi: 10.1126/science.305.5690.1563b. [DOI] [PubMed] [Google Scholar]
- Hanski I. Spatial structure and dynamics of insect populations. In: Dempster J.P, McLean I.F.G, editors. Insect populations in theory and in practice. Kluwer; Dordrecht: 1998. pp. 3–28. [Google Scholar]
- Hanski I. Oxford University Press; Oxford: 1999. Metapopulation ecology. [Google Scholar]
- Harding P.T. National species distribution surveys. In: Goldsmith F.B, editor. Monitoring for conservation and ecology. Chapman and Hall; London: 1991. pp. 133–154. [Google Scholar]
- Harding P.T. Famous laboratories: the Biological Records Centre. Biologist. 1991b;37:162–164. [Google Scholar]
- Harding P.T. Data for nature conservation in statutory and voluntary heritage agencies. In: Berry A.Q, Brown I.W, editors. Managing ancient monuments: an integrated approach. Clwyd County Council; Mold: 1995. pp. 1–13. [Google Scholar]
- Harding P.T, Sheail J. The Biological Records Centre: a pioneer in data gathering and retrieval. In: Harding P, editor. Biological recording of changes in British wildlife. HMSO; London: 1992. pp. 5–18. [Google Scholar]
- Hassell M.P. The regulation of populations by density-dependent processes. In: Dempster J.P, McLean I.F.G, editors. Insect populations in theory and in practice. Kluwer; Dordrecht: 1998. pp. 29–52. [Google Scholar]
- Hassell M.P. Oxford University Press; Oxford: 1998b. Insect parasitoid population dynamics. [Google Scholar]
- Hawkins C.P, Norris R.H, Hogue J.N, Feminella J.W. Development and evaluation of predictive models for measuring the biological integrity of streams. Ecol. Appl. 2000;10:1456–1477. [Google Scholar]
- Heath J, Pollard E, Thomas J.A. Viking; Harmondsworth: 1984. Atlas of butterflies in Britain and Ireland. [Google Scholar]
- Hochberg M.E, Ives A.R, editors. Parasitoid population biology. Princeton University Press; Princeton: 2000. [Google Scholar]
- Huxley C.R, Cutler D.F, editors. Ant–plant interactions. Oxford University Press; Oxford: 1991. [Google Scholar]
- Wells S.M, Pyle R.M, Collins N.M, editors. IUCN. The IUCN invertebrate Red Data Book. IUCN; Gland: 1983. [Google Scholar]
- Johnson R.K, Goedkoop W. The 1995 national survey of Swedish lakes and streams: assessment of ecological status using macroinvertebrates. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 229–240. [Google Scholar]
- Lawton J.H. Population dynamic principles. In: Lawton J.H, May R.M, editors. Extinction rates. Oxford University Press; Oxford: 1995. pp. 147–163. [Google Scholar]
- Lawton J.H, May R.M, editors. Extinction rates. Oxford University Press; Oxford: 1995. [Google Scholar]
- Loder N. ITE; Huntingdon: 1990. Invertebrate geographic ranges and climate. [Google Scholar]
- Mace G.M. Classifying threatened species: means and ends. Phil. Trans. R. Soc. B. 1994;344:91–97. [Google Scholar]
- Mace G.M. Classification of threatened species and its role in conservation planning. In: Lawton J.H, May R.M, editors. Extinction rates. Oxford University Press; Oxford: 1995. pp. 197–213. [Google Scholar]
- Maes D, Van Dyck H. Stichting Leefmilieu; Antwerp: 1999. Dagvlinders in Vlaanderen. [Google Scholar]
- Master L.L, Stein B.A, Kutner L.S, Hammerson G.A. Vanishing assets. In: Stein B.A, Kutner L.S, Adams J.S, editors. Precious heritage: the status of biodiversity in the United States. Oxford University Press; Oxford: 2000. pp. 93–118. [Google Scholar]
- May R.M. Biological populations with non-overlapping generations: stable points, stable cycles and chaos. Science. 1974;186:645–647. doi: 10.1126/science.186.4164.645. [DOI] [PubMed] [Google Scholar]
- May R.M. How many species are there on earth? Science. 1988;241:1441–1449. doi: 10.1126/science.241.4872.1441. [DOI] [PubMed] [Google Scholar]
- May R.M. How many species? Phil. Trans. R. Soc. B. 1990;330:292–304. [Google Scholar]
- May R.M, Lawton J.H, Stork N.E. Assessing extinction rates. In: Lawton J.H, May R.M, editors. Extinction rates. Oxford University Press; Oxford: 1995. pp. 1–24. [Google Scholar]
- McKinney M.L. High rates of extinction and threat in poorly studied taxa. Conserv. Biol. 1999;13:1273–1281. [Google Scholar]
- Moore N.W. Butterfly transects in a linear habitat, 1964–73. Entomol. Gaz. 1975;26:71–78. [Google Scholar]
- Morris M.G, Plant R. Response of grassland invertebrates to management by cutting V. Changes in Hemiptera following cessation of management. J. Appl. Ecol. 1983;20:157–177. [Google Scholar]
- Moss D. Evolution of statistical methods in RIVPACS. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 25–38. [Google Scholar]
- Moss D, Pollard E. Calculation of collated indices of abundance of butterflies based on monitored sites. Ecol. Entomol. 1993;18:77–83. [Google Scholar]
- Murdoch W.W, Briggs C.J, Collier T.R. Biological control of insects: implications for theory in population ecology. In: Dempster J.P, McLean I.F.G, editors. Insect populations in theory and in practice. Kluwer; Dordrecht: 1998. pp. 167–186. [Google Scholar]
- Ødegaard F. How many species of arthropods? Erwin's estimate revised. Biol. J. Linn. Soc. 2000;71:583–597. [Google Scholar]
- Parmesan C, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. [Google Scholar]
- Perring F.H, Walters S.M, editors. Atlas of the British flora. Thomas Nelson & Sons; London: 1962. [Google Scholar]
- Pierce N.E, Braby M.F, Heath A, Lohman D.J, Mathew J, Rand D.B, Travassos M.A. The ecology and evolution of ant association in the Lycaenidae (Lepidoptera) Ann. Rev. Entomol. 2002;47:733–771. doi: 10.1146/annurev.ento.47.091201.145257. [DOI] [PubMed] [Google Scholar]
- Pimm S.L. University of Chicago Press; Chicago: 1991. The balance of nature? [Google Scholar]
- Pimm S.L, Lawton J.H. Number of trophic levels in ecological communities. Nature. 1977;268:329–331. [Google Scholar]
- Pimm S.L, Moulton M.P, Justice L.J. Bird extinctions in the central Pacific. Phil. Trans. R. Soc. B. 1994;344:27–33. [Google Scholar]
- Pimm S.L, Russell G.J, Gittleman J.L, Brooks T.M. Science. 1995;269:347. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- Pollard E. A method for assessing changes in the abundance of butterflies. Biol. Conserv. 1977;12:115–134. [Google Scholar]
- Pollard E. A national scheme for monitoring the abundance of butterflies: the first three years. Br. Entomol. Nat. Hist. Soc. Proc. Trans. 1979;12:77–90. [Google Scholar]
- Pollard E. Temperature, rainfall and butterfly numbers. J. Appl. Ecol. 1988;25:819–828. [Google Scholar]
- Pollard E. Synchrony of population fluctuations: the dominant influence of widespread factors on local butterfly populations. Oikos. 1991;60:7–10. [Google Scholar]
- Pollard E, Eversham B.C. Butterfly monitoring 2—interpreting the changes. In: Pullin A.S, editor. Ecology and conservation of butterflies. Chapman and Hall; London: 1995. pp. 23–36. [Google Scholar]
- Pollard E, Yates T.J. Chapman and Hall; London: 1993. Monitoring butterflies for ecology and conservation. [Google Scholar]
- Pollard E, Elias D.O, Skelton M.J, Thomas J.A. A method of assessing the abundance of butterflies in Monks Wood National Nature reserve in 1973. Entomol. Gaz. 1975;26:79–88. [Google Scholar]
- Pollard E, Moss D, Yates T.J. Population trends of common British butterflies at monitored sites. J. Appl. Ecol. 1995;32:9–16. [Google Scholar]
- Pollard E, Greatorex-Davies J.N, Thomas J.A. Drought reduces breeding success of the butterfly Aglais urticae. Ecol. Entomol. 1997;22:315–318. [Google Scholar]
- Preston C.D, Pearman D.A, Dines T.D, editors. New atlas of the British and Irish flora. Oxford University Press; Oxford: 2002. [Google Scholar]
- Raimondo S, Strazanac J.S, Butler L. Comparison of sampling techniques used in studying Lepidoptera population dynamics. Environ. Entomol. 2003;33:418–425. [Google Scholar]
- Reynoldson D.M, Day E.D, Pascoe T. The development of the BEAST: a predictive approach for assessing sediment quality in the North American Great Lakes. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 165–180. [Google Scholar]
- Rosenberg D.M, Reynoldson T.R, Resh V.H. Establishing reference conditions in the Fraser River catchment, British Columbia, using the BEAST (Benthic Assessment of SedimenT) predictive model. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 181–194. [Google Scholar]
- Roy D.B, Rothery P, Moss D, Pollard E, Thomas J.A. Butterfly numbers and weather: predicting historical trends in abundance and the future effects of climate change. J. Anim. Ecol. 2001;70:201–217. [Google Scholar]
- Schönrogge K, Barr B, Wardlaw J.C, Napper E, Gardner M.G, Breen J, Elmes G.W, Thomas J.A. When rare species become endangered: cryptic speciation in myrmecophilous hoverflies. Biol. J. Linn. Soc. 2002;75:291–300. [Google Scholar]
- Sharrock J.T.R. Poyser; Staffordshire: 1976. The atlas of breeding birds in Britain and Ireland. [Google Scholar]
- Shirt D.B, editor. British Red Data Books 2: insects. Nature Conservancy Council; Peterborough: 1987. [Google Scholar]
- Simpson J.C, Norris R.H. Biological assessment of river quality: development of AUSRIVAS models and outputs. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 125–142. [Google Scholar]
- Sparks T, Crick H, Woiwod I, Beebee T. Climate change and phenology in the United Kingdom. In: Green R.E, Harley M, Spalding M, Zöckler C, editors. Impacts of climate change on wildlife. RSPB; Sandy: 2001. pp. 53–55. [Google Scholar]
- Spector, S. & Forsyth, A. B. 1998 Indicator taxa in the vanishing tropics. In Conservation in a changing world (ed. G. M. Mace, A. Balmford & J. R. Ginsberg) Symposium of the Zoological Society of London, pp. 181–210. Cambridge University Press.
- Stoltze M. Gyldendal; Copenhagen: 1996. Danske dagsommerfugle. [Google Scholar]
- Stork N. How many species are there? Biodiv. Conserv. 1997;2:215–232. [Google Scholar]
- Stubbs A.E, Drake M. BENHS; Reading: 2001. British soldierflies and their allies. [Google Scholar]
- Stubbs A.E, Falk S.J. 2nd edn. BENHS; Reading: 2002. British hoverflies. [Google Scholar]
- Tax M.H. Vlinderstichting; Wageningen: 1989. Atlas van de nederlandse dagvlinders. [Google Scholar]
- Thomas J.A. A quick method for estimating butterfly numbers during surveys. Biol. Conserv. 1983a;27:195–211. [Google Scholar]
- Thomas J.A. The ecology and conservation of Lysandra bellargus (Lepidoptera: Lycaenidae) in Britain. J. Appl. Ecol. 1983b;20:59–83. [Google Scholar]
- Thomas J.A. The conservation of butterflies in temperate countries: past efforts and lessons for the future. In: Vane-Wright R.I, Ackery P, editors. Biology of butterflies. Symposium of the Royal Entomological Society. Vol. 11. Academic Press; New York: 1984. pp. 333–353. [Google Scholar]
- Thomas J.A. Country Life Books (Hamlyn); London: 1986. RSNC guide to butterflies of the British Isles. [Google Scholar]
- Thomas J.A. Rare species conservation: case studies of European butterflies. In: Spellerberg I.F, Goldsmith F.B, Morris M.G, editors. The scientific management of temperate communities for conservation. BES Symposium (1989) Vol. 31. Blackwells; Oxford: 1991. [Google Scholar]
- Thomas J.A. Holocene climate changes and warm man-made refugia may explain why a sixth of British butterflies possess unnatural early-successional habitats. Ecography. 1993;16:278–284. [Google Scholar]
- Thomas J.A. The conservation of declining butterfly populations in Britain and Europe: priorities, problems and successes. Biol. J. Linn. Soc. 1995;56:55–72. [Google Scholar]
- Thomas J.A, Clarke R.T. Extinction rates and butterflies. Science. 2004;305:1563–1564. doi: 10.1126/science.305.5690.1563b. [DOI] [PubMed] [Google Scholar]
- Thomas J.A, Morris M.G. Patterns, mechanisms and rates of extinction among invertebrates in the United Kingdom. Phil. Trans. R. Soc. B. 1994;344:47–54. [Google Scholar]
- Thomas J.A, Morris M.G. Rates and patterns of extinction among British invertebrates. In: Lawton J.H, May R.M, editors. Extinction rates. Oxford University Press; Oxford: 1995. pp. 111–130. [Google Scholar]
- Thomas C.D, Thomas J.A, Warren M.S. Distributions of occupied and vacant butterfly habitats in fragmented landscapes. Oecologia. 1992;92:563–567. doi: 10.1007/BF00317850. [DOI] [PubMed] [Google Scholar]
- Thomas J.A, Moss D, Pollard E. Increased fluctuations of butterfly populations towards the northern edges of species' ranges. Ecography. 1994;17:215–220. [Google Scholar]
- Thomas J.A, Clarke R.T, Elmes G.W, Hochberg M.E. Population dynamics in the genus Maculinea (Lepidoptera: Lycaenidae) In: Dempster J.P, McLean I.F.G, editors. Insect population dynamics: in theory and practice. Chapman & Hall; London: 1998. pp. 261–290. [Google Scholar]
- Thomas J.A, Surry R, Shreeves W, Steele C. DNHAS; Dorchester: 1998. New atlas of Dorset butterflies. [Google Scholar]
- Thomas C.D, Jordano D, Lewis O.T, Hill J.K, Sutcliffe O.S, Thomas J.A. Butterfly distributional patterns, processes and conservation. In: Mace G.M, Balmford A, Ginsberg J.R, editors. Conservation in a changing world. Symposium of the Zoological Society of London. Cambridge University Press; Cambridge: 1999. pp. 107–138. [Google Scholar]
- Thomas J.A, Rose R.J, Clarke R.T, Thomas C.D, Webb N.R. Intraspecific variation in habitat availability among ectothermic animals near their climatic limits and their centres of range. Funct. Ecol. 1999;13:55–64. [Google Scholar]
- Thomas C.D, Bodsworth E.J, Wilson R.J, Simmons A.D, Davies Z.G, Musche M, Conradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. [DOI] [PubMed] [Google Scholar]
- Thomas J.A, Bourn N.A.D, Clarke R.T, Stewart K.E, Simcox D.J, Pearman G.S, Curtis R, Goodger B. The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc. R. Soc. B. 2001;268:1791–1796. doi: 10.1098/rspb.2001.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.A, Telfer M.G, Roy D.B, Preston C.D, Greenwood J.J.D, Asher J, Fox R, Clarke R.T, Lawton J.H. Comparative losses of British butterflies, birds and plants and the global extinction crisis. Science. 2004;303:1879–1881. doi: 10.1126/science.1095046. [DOI] [PubMed] [Google Scholar]
- Thomas, J. A., Schönrogge, K. & Elmes, G. W. 2005 Specialisations and host associations of social parasites of ants. In Evolutionary ecology (ed. J. Rolff, M. Fellowes & G. Holloway). Symposium of the Royal Entomological Society XXI, pp. 475–514.
- Thompson J.N. University of Chicago Press; Chicago: 1994. The coevolutionary process. [Google Scholar]
- van Swaay C, Warren M.S, editors. Prime butterfly areas in Europe. Ministry of Agriculture, Nature Management & Fisheries; The Netherlands: 2003. [Google Scholar]
- van Swaay C.A.M, Plate C.L, van Strien A.J. Monitoring butterflies in the Netherlands: how to get unbiased indices. Proc. Exp. Appl. Entomol. 2002;13:21–27. [Google Scholar]
- Verdonschot P.F.M, Nijboer R.C. Typology of macrofaunal assemblages applied to water and nature management: a Dutch approach. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 241–262. [Google Scholar]
- Warren M.S, et al. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature. 2001;414:65–69. doi: 10.1038/35102054. [DOI] [PubMed] [Google Scholar]
- Wright J.F. Development of RIVPACS in the UK and the value of the underlying data-base. Limnetica. 1994;10:15–31. [Google Scholar]
- Wright J.F. An introduction to RIVPACS. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. pp. 1–24. [Google Scholar]
- Wright J.F, Furse M.T, Armitage P.D, Moss D. New procedures for identifying running-water sites subject to environmental stress and for evaluating sites for conservation, based on the macroinvertebrate fauna. Arch. Hydrobiol. 1993;127:319–326. [Google Scholar]
- Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of freshwaters: RIVPACS and other techniques. Freshwater Biological Association; Ambleside: 2000. [Google Scholar]