Abstract
Patterns in marine fish biodiversity can be assessed by quantifying temporal variation in rate of population change, abundance, life history and demography concomitant with long-term reductions in abundance. Based on data for 177 populations (62 species) from four north-temperate oceanic regions (Northeast Atlantic and Pacific, Northwest Atlantic, North mid-Atlantic), 81% of the populations in decline prior to 1992 experienced reductions in their rate of loss thereafter; species whose rate of population decline accelerated after 1992 were predominantly top predators such as Atlantic cod (Gadus morhua), sole (Solea solea) and pelagic sharks. Combining population data across regions and species, marine fish have declined 35% since 1978 and are currently less than 70% of recorded maxima; demersal species are generally at historic lows, pelagic species are generally stable or increasing in abundance. Declines by demersal species have been associated with substantive increases in pelagic species, a pattern consistent with the hypothesis that increases in the latter may be attributable to reduced predation mortality. There is a need to determine the consequences to population growth effected by the reductions in age (21%) and size (13%) at maturity and in mean age (5%) and size (18%) of spawners, concomitant with population decline. We conclude that reductions in the rate of population decline, in the absence of targets for population increase, will be insufficient to effect a recovery of marine fish biodiversity, and that great care must be exercised when interpreting multi-species patterns in abundance. Of fundamental importance is the need to explain the geographical, species-specific and habitat biases that pervade patterns of marine fish recovery and biodiversity.
Keywords: marine conservation biology, biodiversity index, multi-species index, collapse, recovery, fisheries
1. Collapse and recovery of marine fish
Threats to marine fish biodiversity are particularly acute throughout the temperate and tropical waters overlying the continental shelves (waters typically shallower than 200 m), a spatial bias generated by the observation that roughly 5% of the ocean's waters are responsible for 90% of global catches (Pauly et al. 2002). Among fisheries globally, 75% have been judged to be fully fished, over-fished or depleted (FAO 2002). Population collapses concomitant with increased fishing pressure have led many to conclude that fishing constitutes the primary threat to marine fish biodiversity (e.g. Jackson et al. 2001; Pauly et al. 2002; Myers & Worm 2003), although others have argued that such a conclusion is unduly pessimistic (Hilborn 2004; Mace 2004). In addition to the effects of exploitation, the biodiversity of many coral reef and coastal marine species is also influenced by habitat loss (Friedlander & Parrish 1998; Jones et al. 2002; Gardner et al. 2003). Based on their empirical study of 58 marine fish population extirpations, Dulvy et al. (2003) concluded that exploitation was the sole or primary cause of decline for 40 of the cases examined; the remainder were attributed to habitat loss, although the effects of habitat loss and exploitation on abundance are not always readily separable. Irrespective of the causes, failure to arrest population declines can result, and has resulted, in the extirpation of some marine fish from parts of their former geographical ranges (Dulvy et al. 2003, 2004; Myers & Ottensmeyer 2005).
Losses of marine fish biodiversity can be reflected by various intraspecific metrics. Prominent among these are reductions in population abundance, changes to life history and, in the most extreme cases, population loss (Dulvy et al. 2003, 2004; Hutchings & Reynolds 2004). With some possible exceptions (Jackson et al. 2001), rate of decline among the most severely affected fish has accelerated throughout the latter half of the twentieth century when reductions of more than 80%, relative to recorded (as opposed to true) historical levels, were not uncommon (Hutchings 2000; Myers & Worm 2003), particularly among large predators, e.g. Atlantic cod, Gadus morhua (Hutchings & Reynolds 2004; Myers & Worm 2005) and coastal and oceanic sharks (Baum et al. 2003; Baum & Myers 2004).
Reductions in abundance are an inevitable consequence of harvesting and can constitute a primary objective of fisheries management. Depending on the species and on the single-species model of productivity that is used, the predicted maximum sustainable yield (MSY) of a population may not be achieved until that population has been reduced by at least 50% its size in an unfished state (Hilborn & Walters 1992). This ‘fishing down’ element of population decline has been used as a basis for arguing that substantive declines by marine fish should not be a cause for concern from a conservation or extinction perspective (Musick 1999; Mace 2004). However, notwithstanding often-substantial measurement error, coupled with the caveats associated with using single-species models to estimate MSY (e.g. the effects of interspecific interactions on productivity are often excluded), the strength of this argument depends on the degree to which the documented declines accurately represent population reductions from unfished states. This is rarely the case for most of the world's fisheries, particularly among those prosecuted on the continental shelves of the North Atlantic and North Pacific. Using macroecological theory, Jennings & Blanchard (2004) predicted that the current biomass of large fish (4–66 kg) in the intensively fished North Sea is more than 97% lower than that expected in the absence of fisheries exploitation. Myers & Worm (2005) have estimated that the abundance of many large, predatory fish in temperate marine waters is less than 10% of pre-exploitation levels. Among 21 populations of Atlantic cod, for example, they estimate that all have declined more than 70% relative to their estimated abundance in an unfished state, with 18 of the populations declining by more than 90% (Myers & Worm 2005). Consistent with these studies, Christensen et al. (2003) used ecosystem models to estimate that high-trophic level fish in the North Atlantic have declined by a factor of nine over the past century. An additional caveat associated with single-species models used to estimate MSY is that they treat each kilogram of the spawning component of a population as being equally capable of contributing offspring to future generations, an assumption that is proving to be increasingly difficult to defend (Berkeley et al. 2004,a,b).
Given the extraordinary declines that have been experienced by directly harvested and incidentally caught fish (Hutchings 2000; Myers & Worm 2003, in press; Hutchings & Reynolds 2004), one can raise the question as to whether these reductions are widespread among species, whether the rates of decline have slowed or ceased and whether rapid recovery is as uncommon as the literature might suggest. By addressing these questions, one can assess the argument that, despite rates of decline that are among the greatest recorded for extant vertebrates, much of the recent literature on marine fish collapses has been unduly ‘alarmist’ in nature, inappropriately interpreting the effects that population reduction can have on rates of increase and ignoring examples of recovery that have followed severe reductions in fishing pressure (Mace 2004). Counter to this viewpoint, there is implicit recognition that the biological diversity of all species, including marine fish, is under threat (Royal Society 2003). The most tangible reflection of this concern is the commitment made by the Conference of the Parties to the Convention on Biological Diversity (CBD) to ‘achieve by 2010 a significant reduction of the current rate of biodiversity loss’ (Royal Society 2003).
Within this context, our objectives were to identify potential metrics of biodiversity for marine fish, quantify their patterns over time and assess their utility as biodiversity measures against which progress towards the 2010 CBD target might be evaluated.
2. Metrics of marine fish biodiversity
Qualitative and quantitative changes to various measures of variability within and among species can be used to measure biodiversity (Royal Society 2003). Within species, one can examine rates of change in population size (which relates directly to the 2010 rate-based CBD objective), life-history changes concomitant with population decline, population losses and reductions in genetic variability. At the species level, one can construct multi-species indices of abundance, comparing these by habitat, by geographical region or by some other biologically defensible variable.
(a) Population abundance
Estimates of population size for commercially exploited marine fish are available primarily from two sources. The first are model-based estimates derived from a form of Sequential or Virtual Population Analysis (SPAs or VPAs). These analyses are based on data from commercial catches, calibrated with fisheries-independent survey data and combined with estimates of natural mortality. The second means of estimating population size is to use the catch rates of fish obtained from fisheries-independent, fixed- or mobile-gear surveys conducted under the auspices of the fisheries management agencies responsible for the management of the species in question. The main weaknesses associated with SPA estimates of abundance are that they rely upon accurate reporting of commercial catch data, they do not account for the illegal practices of discarding and catch misreporting, they depend upon reliable estimates of mortality owing to natural causes and they can produce (sometimes serious) overestimates of abundance in the most recent year(s) of the time-series (the so-called ‘retrospective problem’). The primary strength associated with research survey estimates of the size of the breeding population is that the data are obtained from random samples of fish taken throughout the geographical area of each stock. Thus, they are unbiased and do not depend upon the validity of assumptions concerning natural mortality and the accuracy of commercial fishery data. One drawback to some survey estimates of catch rate, however, is that they can be unduly variable in some areas in some years.
From the perspective of assessing rates of change in marine fish biodiversity, a major limitation associated with these two means of estimating abundance is that they are ultimately dependent on fisheries-independent surveys that are very costly to conduct. As a consequence, these surveys tend to be limited primarily to waters adjacent to the richest countries; there are few fisheries-independent catch-rate data for coral reef and other tropical fish. Geographically, the most extensive data are available from the North Atlantic and North Pacific, and the waters off South Africa, Australia and New Zealand.
Compounding this geographical bias is a rather severe taxonomic bias. Population data are available for only a small percentage of the species found in the waters adjacent to a particular country. For example, off eastern Canada, time-series abundance data have been compiled for only about 3–4% of all marine fish species (26 of approximately 700–750 species, the latter estimate having been provided by Claude Renault, Canadian Museum of Nature, Ottawa, Canada). In part, this can be attributed to the fact that comparatively few species are of commercial importance. In addition, although fisheries-independent surveys do provide random samples of the fish available to be caught in a particular region, the survey gear is usually designed to be most effective for the commercially important species and will better sample fish in some habitats than others. For example, eastern Canadian ground-fish surveys are designed to effectively sample Atlantic cod, haddock (Melanogrammus aeglefinus) and other similarly behaving ground-fish. They do not effectively sample pelagic species such as Atlantic herring (Clupea harengus). It is also important to note that fisheries have been conducted on commercially exploited species for a considerably longer period of time than that for which abundance data are available. Thus, when comparing current population sizes with ‘historic’ estimates, one's estimates are almost certainly going to be underestimates.
Notwithstanding these limitations, these survey-based data do provide an extraordinarily important source of demographic information on some marine fish. It is these data on which most of our analyses are based. In addition to SPA- and survey-based estimates of abundance, we also include relative abundance data (standardized catch-per-unit-effort) for sharks that have been estimated from data recorded in fishery log-books (Baum et al. 2003).
(b) Life-history and demographic data
Phenotypic and genetic changes to life-history traits can affect the rate of loss of biodiversity by influencing a population's susceptibility to collapse and probability of recovery. This is because of the direct links that exist between these fitness-related characters, such as age and size at maturity, and population growth rate. The former, manifested ultimately by age-specific schedules of survival and reproductive investment, are the primary determinants of individual fitness. The latter, represented by a population's intrinsic or maximum rate of growth, is a primary determinant of rates of harvesting, probability of persistence and rapidity of recovery (Myers et al. 1999; Hutchings 2002; Roff 2002).
Changes to age and size at maturity, and to the mean age and size of spawners, will affect population growth. Age at maturity reflects an evolutionary compromise between the costs and benefits to fitness of reproducing comparatively early or late in life. Benefits associated with early maturity include increased probability of surviving to reproduce and an increased rate of gene input into the population, resulting in reduced generation time. However, early maturity can also result in reduced fecundity and/or post-reproductive survival because of the smaller body size typically associated with earlier maturity within a population. In contrast, the primary cost of delaying the age of initial spawning is an increased risk of death prior to reproduction. The primary fitness advantage to delaying maturity in fish is the larger initial body size attained by individuals when they first reproduce. Body size has a positive influence on many life-history traits in fish. Most notable among these associations are the observations that larger females produce more eggs of a larger size than smaller females and they frequently have lower age-specific rates of mortality (Wootton 1998; Hutchings 2002; Roff 2002). Although increased fecundity can enhance fitness at the individual level, there is evidence that it does not do so at the species level (Jennings et al. 1998; Hutchings 2001; Denney et al. 2002); indeed marine fish are vulnerable to collapse and extinction in spite of their high fecundity (Myers et al. 1999).
Prolonged fishing pressure can lead to significant changes in life-history traits. A key question is whether these changes are phenotypic or genetic. Reduced population density, for example, should lead to reduced competition for food and may lead to increased growth rate, which in turn usually results in earlier maturity in indeterminately growing organisms (Roff 2002). Alternatively, given that most fisheries target the largest and oldest individuals, fish genetically predisposed to mature at larger sizes and older ages are more likely to be caught before they can reproduce. Such selective harvesting should favour early- and small-maturing genotypes, resulting in genetic responses to exploitation that can alter the average value of a character (Handford et al. 1977; Stokes & Law 2000) or life-history reaction norms (Hutchings 1993; Reznick 1993; Grift et al. 2003; Olsen et al. 2004).
Although data on marine fish are exceedingly limited, Olsen et al. (2004) recently provided compelling evidence that reductions in the age and size at which Newfoundland's northern cod matured are best explained as genetic responses to exploitation. Evidence that fishing has effected genetic changes to exploited marine fish populations has also been documented for North Sea plaice (Pleuronectes platessa; Grift et al. 2003) and for Atlantic cod in the Northeast Arctic (Heino et al. 2002) and Gulf of Maine/Georges Bank (Barot et al. 2004). The magnitude of these life-history changes, and others (Murawksi et al. 2001), can negatively affect recovery (Hutchings 1999). As with many indeterminately growing organisms, including Atlantic cod (Beverton et al. 1994), earlier maturation at a smaller size can be associated with increased survival costs of reproduction, resulting in higher natural mortality and shorter lifespan. Based on the output of a stochastic, age-structured life-history model, Hutchings (2005) found that a reduction in age at maturity from 6 to 4 years can reduce annual population growth in Northwest Atlantic cod by 25–30% and that earlier maturity more than doubles the probability of negative population growth every generation.
Despite clear and fundamental links between individual life history, population growth rate and population recovery, temporal changes in life-history traits concomitant with population decline have yet to be examined for a broad range of marine fish. Indeed, among many species for which abundance estimates are available, life-history data are not. When data are available, the life-history traits most likely to be represented are age and size at maturity; data on fecundity, egg size and metrics of reproductive effort are comparatively few. From a demographic perspective, although the information required to estimate age and length at 50% maturity are frequently unavailable, there are often sufficient data to allow one to quantify changes in the mean age and size of the spawning population over time.
(c) Population loss
Declines experienced by many marine fish have resulted in the extirpation of species from significant parts of their geographical ranges (Dulvy et al. 2003; Myers & Ottensmeyer 2005). However, the degree to which the inevitable population losses associated with these range depletions threaten marine fish biodiversity cannot be reliably assessed with available information. Foremost among the problems in doing so is the difficulty in determining the spatial scale of population differentiation and gene flow in marine fish.
A widely held perception that fish are limited only by the geographical boundaries demarcating their extent of occurrence on a given continental shelf meant that almost no work was directed to the study of small-scale population differentiation. Although population genetics data have provided compelling evidence of comparatively small-scale restrictions in gene flow, accurate identification of geographical boundaries separating putative populations is proving to be exceedingly difficult, if not impossible, even for the best-studied of species (Atlantic cod; Ruzzante et al. 1998; Hutchinson et al. 2003; Nielsen et al. 2003). The primary consequence of this lack of work is that we are unable to determine the extent to which population loss has been a factor in the reduction of marine fish biodiversity, notwithstanding limited evidence that such losses may have been substantial for some heavily exploited species, e.g. Atlantic cod (Ames 2004).
(d) Loss of genetic variability
The viability of collapsed populations is influenced by stochastic factors of demography and genetics that contribute to extinction risk (Beissinger & McCullough 2002). Rate of population decline can serve as a reliable proxy for the degree to which population persistence is threatened by demographic and, more importantly, environmental stochasticity. From a genetic perspective, the major consequence of a reduction in population size is an increase in the rate of loss of genetic variability per generation, a rate of loss that, in the absence of immigration, can be expected to increase as population size declines. However, for many species it is not clear how rate of population decline is related to reductions in genetic variation (notably for loci under selection). This is particularly true for marine fish. Among the studies that have been conducted, the results have been equivocal. Significant reductions in genetic diversity concomitant with population decline have been documented for a population of North Sea cod (Hutchinson et al. 2003) and for New Zealand snapper (Pagrus auratus; Hauser et al. 2002), although such a decline was not evident in a study of Northwest Atlantic cod (Ruzzante et al. 2001).
3. Methods
(a) Overview
We proceed by examining metrics of within-species diversity which pertain to changes in population abundance, life history and demography; there are insufficient data to allow an examination of either rate of population loss or temporal reduction in genetic variability in marine fish. Our analyses focus on temporal changes in (i) the slope of linear regressions of log-transformed abundance plotted against time, (ii) population size, (iii) multi-species abundance indices, (iv) age and size at maturity, and (v) the mean age and size of spawning individuals.
When assessing changes in the rate of biodiversity loss in 2010, at least two time periods will have to be selected for comparison. With this in mind, we compared our metrics of regression slope and relative abundance before and after the arbitrarily selected year of 1992, the year in which the Rio Biodiversity Convention was agreed upon and a year sufficiently close to the present that it permits an examination of recent changes in the rate of change in biodiversity. We are unaware of any bias that the selection of this year would have on our analyses.
(b) The data
Our analyses are based on data obtained from both the primary and the secondary literature; it is the latter in which fisheries stock assessments and their associated data are published. There is a significant geographical bias in our analysis in that we focus on marine fish in the North Atlantic Ocean and the Northeast Pacific Ocean. The reasons for this bias are twofold. First, our analysis is not intended to represent a comprehensive worldwide examination of marine fish biodiversity (although it is fairly comprehensive for the North Atlantic Ocean). Rather, its primary purpose is to evaluate metrics that might be used to measure rates of change in marine fish biodiversity. Second, by concentrating our analyses on species in these regions, we are able to focus on the most extensive abundance and life-history data available for marine fish. Comprehensive time-series data on the abundance of coral reef fish do not exist.
Our literature review yielded data for 177 populations of 62 species in four geographical areas: Northeast Atlantic (including waters east of Greenland, the Baltic Sea westward and the Bay of Biscay northward), the Northwest Atlantic (including waters from Baffin Island and west Greenland southward, and from eastern Georges Bank (the Canadian portion) northward), the North mid-Atlantic (including waters primarily from the American portion of Georges Bank south to the US state of Delaware and secondarily southward to the Gulf of Mexico) and the Northeast Pacific (including waters primarily off British Columbia and secondarily off the northwestern US; tables 1 and 2). The national and international fishery bodies responsible for undertaking stock assessments in these four areas are as follows: Northeast Atlantic assessments are conducted by the International Council for the Exploration of the Sea; Northwest Atlantic assessments by the Canadian Department of Fisheries and Oceans (DFO) and by the Northwest Atlantic Fishery Organization; North mid-Atlantic assessments are conducted under the auspices of the US National Marine Fisheries Service; and Northeast Pacific assessments are also conducted by Canada's DFO. We include the most recent assessment available for each population from the data sources reviewed, excepting those cases for which estimates were considered by the assessors to be either unreliable or unduly problematic. For each population, we also reviewed the availability of data that could be used to estimate temporal changes in two life-history parameters (time-series data on age and length at which 50% of the individuals in a population are mature) and two metrics of demography (mean age and weight of spawners; tables 1 and 2).
Table 1.
Overview of demersal marine fish data (ordered taxonomically) used in analysis and their population trends over time. (Regions: NEA, Northeast Atlantic; NWA, Northwest Atlantic; NMA, North mid-Atlantic; NEP, Northeast Pacific. Abundance metrics: SSB (model-based estimates of spawning stock biomass), BTOT (model-based estimates of total population biomass), NSSB (model-based estimates of the abundance of spawning individuals), CPUETOT (catch-per-unit-effort of all individuals in a fisheries-independent survey), CPUESSB (catch-per-unit-effort of the breeding population in a fisheries-independent survey). Change in abundance: D, decreased; I, increased; S, stable; ?, status unknown. Life-history data review: Amat (age at maturity), Lmat (length at maturity), growth (length-at-age or weight-at-age from fishery or population assessment data), age-structure (numbers-at-age from research survey or population assessment data): (1, time-series of data available; 0, otherwise).)
| names—family common (latin binomial) | region | population | abundance metric | years | pre-1992 slopea | post-1992 slopea | change in abundanceb | Amat | Lmat | growth | age-structure | citationc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rajidae | ||||||||||||
| barndoor skate (Dipturus laevis) | NWA | E Scotian Shelf | CPUETOT | 1958–2001 | −0.020 | 0.000 | D | 0 | 0 | 0 | 0 | 1 |
| W Scotian Shelf | CPUETOT | 1962–2003 | −0.010 | 0.011 | D | 0 | 0 | 0 | 0 | 1 | ||
| NMA | Georges Bank | BTOT | 1986–2001 | −0.160 | 0.289 | I | 0 | 0 | 0 | 0 | 1 | |
| thorny skate (Amblyraja radiata) | NWA | Grand Banks | SSB | 1980–2002 | −0.043 | 0.054 | D | 0 | 0 | 0 | 0 | 2 |
| West Greenland | CPUETOT | 1982–2002 | −0.112 | −0.051 | D | 0 | 0 | 0 | 0 | 3 | ||
| Macrouridae | ||||||||||||
| roughhead grenadier (Macrourus berlgax) | NWA | Labrador, Newfoundland, Grand Banks | BTOT | 1978–1995 | −0.100 | 0.015d | ? | 0 | 0 | 0 | 0 | 4 |
| roundnose grenadier (Coryphaenoides rupsestris) | NEA | Subareas IV, VI, VII | BTOT | 1996–2003 | — | −0.154 | D | 0 | 0 | 1 | 0 | 5 |
| Phycidae | ||||||||||||
| white hake (Urophycis tenuis) | NWA | Grand Banks | BTOT | 1972–2001 | 0.020 | 0.258d | ? | 0 | 0 | 0 | 0 | 6 |
| S Gulf of St Lawrence (4T) | BTOT | 1971–2000 | 0.023 | 0.014 | D | 0 | 0 | 1 | 1 | 7 | ||
| Scotian Shelf (4VWX/5) | NSSB | 1970–2001 | 0.019 | −0.071 | D | 0 | 0 | 0 | 1 | 8 | ||
| NMA | Georges Bank, Gulf of Maine | CPUETOT | 1963–2001 | 0.016 | −0.078 | S | 0 | 0 | 0 | 0 | 9 | |
| Merlucciidae | ||||||||||||
| hake (Merluccius merluccius) | NEA | northern stock | SSB | 1978–2003 | −0.047 | 0.008 | D | 0 | 0 | 1 | 1 | 5 |
| southern stock | SSB | 1982–2003 | −0.099 | −0.031 | D | 0 | 0 | 1 | 1 | 5 | ||
| Pacific hake (M. productus) | NEP | Canada & US | SSB | 1988–1998 | −0.104 | −0.109 | D | 0 | 0 | 1 | 1 | 10 |
| silver hake (M. bilinearis) | NWA | Scotian Shelf | CPUETOT | 1970–2003 | 0.007 | −0.049 | D | 1 | 1 | 1 | 1 | 11 |
| Gadidae | ||||||||||||
| Atlantic cod (Gadus morhua) | NEA | Baltic (22–24) | SSB | 1970–2004 | −0.049 | −0.003 | D | 1 | 0 | 1 | 1 | 12 |
| Baltic (25–32) | SSB | 1966–2003 | 0.010 | −0.044 | D | 1 | 0 | 1 | 1 | 5, 12 | ||
| Celtic Sea (Div. VIIe–k) | SSB | 1971–2003 | 0.035 | −0.034 | I | 0 | 0 | 1 | 1 | 5 | ||
| Faroe Bank | CPUETOT | 1983–2003 | −0.204 | 0.285 | I | 0 | 0 | 0 | 0 | 12 | ||
| Faroe Plateau | SSB | 1961–2004 | −0.013 | 0.022 | S | 1 | 0 | 1 | 1 | 12 | ||
| Iceland | SSB | 1955–2004 | −0.048 | 0.024 | D | 1 | 0 | 1 | 1 | 12 | ||
| Irish Sea | SSB | 1968–2003 | −0.019 | −0.070 | D | 1 | 0 | 1 | 1 | 5 | ||
| Kattegat | SSB | 1971–2004 | −0.061 | −0.200 | D | 1 | 0 | 1 | 1 | 12 | ||
| NE Arctic | SSB | 1946–2004 | −0.020 | −0.028 | D | 1 | 1 | 1 | 1 | 12, 13 | ||
| North Sea | SSB | 1963–2003 | −0.037 | −0.057 | D | 0 | 0 | 1 | 1 | 5 | ||
| Norway coast | SSB | 1984–2001 | 0.000 | −0.145 | D | 1 | 0 | 0 | 1 | 12 | ||
| West of Scotland | SSB | 1978–2003 | −0.061 | −0.186 | D | 0 | 0 | 1 | 1 | 5 | ||
| NWA | Cabot Strait (4Vn) | SSB | 1981–2000 | −0.135 | −0.027 | D | 0 | 0 | 1 | 1 | 14 | |
| E Scotian Shelf (4VsW) | SSB | 1970–2002 | 0.023 | −0.140 | D | 1 | 1 | 1 | 1 | 15 | ||
| E Georges Bank | SSB | 1978–2003 | −0.021 | −0.013 | D | 0 | 0 | 1 | 1 | 16 | ||
| Flemish Cap (3M) | SSB | 1988–2002 | 0.068 | −0.300 | D | 1 | 0 | 1 | 1 | 17, 18 | ||
| Greenland offshore | NSSB | 1982–2003 | −0.134 | 0.268 | D | 0 | 0 | 1 | 1 | 12 | ||
| N Gulf of St Lawrence (3Pn4RS) | SSB | 1974–2003 | −0.064 | 0.030 | D | 1 | 0 | 1 | 1 | 19 | ||
| Northern cod (2J3KL) | SSB | 1962–2002 | −0.081 | −0.099 | D | 1 | 1 | 1 | 1 | 14, 20 | ||
| S Grand Banks (3NO) | SSB | 1959–2003 | −0.061 | −0.101 | D | 1 | 0 | 1 | 1 | 21 | ||
| S Gulf of St Lawrence (4T) | SSB | 1950–2003 | 0.004 | −0.027 | D | 0 | 0 | 1 | 1 | 22 | ||
| St Pierre Bank (3Ps) | SSB | 1959–2001 | −0.018 | 0.007 | D | 1 | 0 | 1 | 1 | 23 | ||
| W Scotian Shelf (4X) | SSB | 1970–2002 | −0.014 | −0.055 | D | 0 | 0 | 1 | 1 | 24 | ||
| NMA | Georges Bank | SSB | 1978–2001 | −0.041 | −0.010 | D | 1 | 1 | 1 | 1 | 9, 25, 26 | |
| Gulf of Maine | SSB | 1982–2001 | −0.003 | 0.032 | D | 1 | 1 | 1 | 1 | 9, 25, 26 | ||
| blue ling (Molva dypterygia) | NEA | Iceland | CPUETOT | 1985–2003 | 0.002 | −0.059 | D | 0 | 0 | 0 | 0 | 12 |
| cusk/torsk (Brosme brosme) | NEA | Iceland | CPUESSB | 1986–2003 | −0.062 | −0.043 | D | 0 | 0 | 0 | 0 | 12 |
| NWA | Scotia–Fundy | CPUETOT | 1970–2001 | −0.035 | −0.001 | D | 0 | 0 | 0 | 0 | 27, 28 | |
| haddock (Melanogrammus aeglefinus) | NEA | Faroe | SSB | 1961–2003 | 0.001 | 0.132 | I | 1 | 0 | 1 | 1 | 5, 12 |
| Iceland | SSB | 1979–2004 | −0.037 | 0.047 | D | 1 | 0 | 1 | 1 | 12 | ||
| NE Arctic | SSB | 1950–2004 | −0.021 | −0.008 | D | 1 | 0 | 1 | 1 | 5, 12 | ||
| North Sea & Skagarrek | SSB | 1963–2003 | −0.050 | 0.064 | D | 0 | 0 | 1 | 1 | 5 | ||
| West of Scotland | SSB | 1978–2003 | −0.049 | −0.001 | S | 0 | 0 | 1 | 1 | 5 | ||
| NWA | S Gulf & E Scotian Shelf (4TVW) | SSB | 1970–2000 | 0.026 | 0.108 | D | 1 | 1 | 1 | 1 | 29, 30 | |
| W Scotian Shelf & Gulf of Maine | SSB | 1970–2003 | −0.022 | 0.073 | D | 0 | 0 | 1 | 1 | 31 | ||
| E Georges Bank (5Zc) | SSB | 1969–2003 | −0.001 | 0.167 | D | 0 | 0 | 1 | 1 | 32 | ||
| NMA | Georges Bank | SSB | 1963–2001 | −0.067 | 0.210 | D | 0 | 0 | 1 | 1 | 9 | |
| Gulf of Maine | CPUETOT | 1963–2001 | −0.135 | 0.520 | D | 0 | 0 | 0 | 0 | 9 | ||
| ling (Molva molva) | NEA | Iceland | CPUETOT | 1985–2003 | −0.067 | −0.023 | D | 0 | 0 | 1 | 1 | 12 |
| Pacific cod (Gadus macrocephalus) | NEP | W coast Vancouver Island | BTOT | 1988–2002 | −0.375 | −0.083 | D | 0 | 0 | 0 | 0 | 33 |
| Hecate Strait | BTOT | 1956–2001 | 0.007 | −0.123 | D | 0 | 0 | 0 | 0 | 34 | ||
| pollock/saithe (Pollachias virens) | NEA | Faroe | SSB | 1961–2004 | −0.016 | 0.057 | I | 1 | 0 | 1 | 1 | 12 |
| Iceland | SSB | 1962–2004 | −0.007 | −0.026 | I | 1 | 0 | 1 | 1 | 12 | ||
| NE Arctic | SSB | 1960–2004 | −0.056 | 0.117 | I | 0 | 0 | 1 | 1 | 12 | ||
| North Sea (IV), VI, IIIa | SSB | 1967–2002 | −0.052 | 0.097 | D | 0 | 0 | 1 | 1 | 5 | ||
| NWA | E Georges Bank & Scotian shelf | SSB | 1982–1999 | −0.042 | 0.041 | D | 0 | 0 | 1 | 1 | 35 | |
| NMA | Georges Bank, Gulf of Maine | SSB | 1963–2001 | −0.038 | 0.119 | I | 0 | 0 | 0 | 0 | 9 | |
| whiting (Merlangius merlangus) | NEA | Celtic Sea | SSB | 1982–2003 | 0.058 | −0.016 | I | 0 | 0 | 1 | 1 | 5 |
| Irish Sea | SSB | 1980–2002 | −0.08 | −0.262 | D | 0 | 0 | 1 | 1 | 5 | ||
| North Sea (IV) & VIId | SSB | 1980–2002 | −0.046 | −0.031 | D | 0 | 0 | 1 | 1 | 5 | ||
| West of Scotland | SSB | 1978–2003 | −0.070 | −0.096 | D | 0 | 0 | 1 | 1 | 5 | ||
| Lophiidae | ||||||||||||
| anglerfish/monkfish (Lophius americanus) | NWA | E Georges Bank & Scotian shelf | CPUETOT | 1970–2000 | −0.061 | 0.055 | D | 0 | 0 | 0 | 0 | 36 |
| W Grand Banks | BTOT | 1977–2000 | 0.064 | 0.117d | ? | 0 | 0 | 0 | 0 | 37, 38 | ||
| anglerfish (L. budegassa) | NEA | Divisions VIIb–k, VIIIa,b | SSB | 1986–2003 | −0.035 | −0.002 | D | 0 | 0 | 1 | 1 | 5 |
| anglerfish (L. piscatorius) | NEA | Divisions VIIb–k, VIIIa,b | SSB | 1986–2003 | −0.080 | 0.004 | D | 0 | 0 | 1 | 1 | 5 |
| Scorpaenidae | ||||||||||||
| Acadian redfish (Sebastes fasciatus) | NMA | Georges Bank, Gulf of Maine | CPUETOT | 1963–2001 | −0.066 | 0.177 | S | 0 | 0 | 0 | 0 | 9 |
| bocaccio (S. paucispinis) | NEP | BC | CPUETOT | 1980–2001 | −0.081 | −0.146 | D | 0 | 0 | 0 | 0 | 39 |
| redfish (S. marinus) | NEA | East Greenland | CPUETOT | 1985–2003 | −0.292 | 0.252 | D | 0 | 0 | 0 | 0 | 12 |
| Iceland | CPUESSB | 1985–2003 | −0.082 | 0.052 | D | 0 | 0 | 0 | 0 | 12 | ||
| Subareas I, II | CPUESSB | 1992–2003 | – | −0.131 | D | 0 | 0 | 0 | 1 | 12 | ||
| redfish (S. mentella, S. fasciatus) | NWA | Gulf of St Lawrence | CPUETOT | 1992–2000 | – | −0.169 | D | 0 | 0 | 0 | 0 | 40 |
| Flemish Cap | CPUESSB | 1979–2002 | −0.120 | −0.032 | D | 1 | 1 | 1 | 1 | 41 | ||
| SW Grand Banks | CPUETOT | 1992–2000 | – | 0.067 | S | 0 | 0 | 0 | 0 | 42, 43 | ||
| redfish (S. mentella, S. marinus) | NWA | W Greenland inshore | CPUESSB | 1982–2002 | −0.327 | −0.053 | D | 0 | 0 | 0 | 0 | 44 |
| redfish (S. mentella, S. fasciatus, S. marinus) | NWA | Labrador & E Newfoundland | CPUETOT | 1978–2000 | −0.208 | 0.055 | D | 0 | 0 | 0 | 0 | 45 |
| Anoplopomatidae | ||||||||||||
| sablefish (Anoplopoma fimbria) | NEP | British Columbia | CPUETOT | 1992–2003 | — | −0.144 | D | 0 | 0 | 0 | 0 | 46 |
| Zoarcidae | ||||||||||||
| ocean pout (Zoarces americanus) Anarhichadidae | NMA | Cape Cod-Delaware | CPUETOT | 1968–2002 | 0.006 | −0.008 | D | 0 | 0 | 0 | 0 | 9 |
| northern wolffish (Anarhichus denticulatus) | NWA | Labrador, Newfoundland, Grand Banks | CPUETOT | 1981–2001 | −0.209 | 0.001 | D | 0 | 0 | 0 | 0 | 47 |
| spotted wolffish (A. minor) | NWA | Labrador, Newfoundland, Grand Banks | CPUETOT | 1981–2001 | −0.207 | 0.146 | D | 0 | 0 | 0 | 0 | 47 |
| W Greenland | CPUESSB | 1982–2002 | −0.224 | 0.150 | D | 0 | 0 | 0 | 0 | 3 | ||
| striped wolffish (A. lupus) | NWA | Labrador, Newfoundland, Grand Banks | CPUETOT | 1977–2001 | −0.190 | 0.152 | D | 0 | 0 | 0 | 0 | 47 |
| W Greenland | CPUESSB | 1982–2002 | −0.211 | 0.022 | D | 0 | 0 | 0 | 0 | 3 | ||
| Ammodytidae | ||||||||||||
| sandeel (Ammodytes spp.) | NEA | North Sea | SSB | 1983–2003 | −0.097 | −0.044 | D | 0 | 0 | 1 | 1 | 5 |
| Scophthalmidae | ||||||||||||
| megrim (Lepidorhombus boscii) | NEA | Spain & Portugal (VIIIc, IXa) | SSB | 1986–2003 | −0.003 | 0.013 | D | 0 | 0 | 1 | 1 | 5 |
| megrim (L. whiffagonis) | NEA | Divisions VII, VIIIa,b,d | SSB | 1984–2003 | −0.055 | 0.023 | D | 0 | 0 | 1 | 1 | 5 |
| Spain & Portugal (VIIIc, IXa) | SSB | 1986–2003 | −0.046 | 0.012 | D | 0 | 0 | 1 | 1 | 5 | ||
| windowpane (Scophthalmus aquosus) | NMA | Georges Bank, Gulf of Maine | CPUETOT | 1963–2001 | 0.033 | 0.094 | I | 0 | 0 | 0 | 0 | 9 |
| S New England & mid-Atlantic Bight | CPUETOT | 1963–2001 | −0.043 | 0.082 | D | 0 | 0 | 0 | 0 | 9 | ||
| Paralichthyidae (Paralichthys dentatus) | NMA | U.S. | SSB | 1982–2002 | −0.126 | 0.157 | I | 0 | 0 | 1 | 1 | 60 |
| Pleuronectidae | ||||||||||||
| American plaice (Hippoglossoides platessoides) | NWA | W Greenland | SSB | 1983–2002 | −0.295 | −0.073 | D | 0 | 0 | 0 | 0 | 3 |
| Labrador & Newfoundland (2J3K) | SSB | 1979–2003 | −0.085 | −0.048 | D | 1 | 1 | 0 | 1 | 48 | ||
| Grand Banks (3LNO) | CPUESSB | 1984–2002 | −0.189 | −0.017 | D | 1 | 1 | 1 | 1 | 49 | ||
| Flemish Cap (3M) | CPUETOT | 1988–2002 | −0.156 | 0.000 | D | 0 | 0 | 1 | 1 | 50 | ||
| St Pierre Bank (3Ps) | CPUESSB | 1983–2002 | −0.175 | 0.065 | D | 1 | 1 | 1 | 1 | 51 | ||
| S Gulf of St Lawrence | CPUESSB | 1967–1999 | −0.06 | 0.043 | D | 0 | 0 | 1 | 1 | 52 | ||
| Scotian Shelf (4VW) | BTOT | 1970–2000 | −0.025 | 0.000 | D | 0 | 1 | 1 | 0 | 53 | ||
| NMA | Georges Bank, Gulf of Maine | SSB | 1980–2000 | −0.138 | 0.025 | D | 1 | 0 | 1 | 1 | 9 | |
| flounder (Platichthyes flesus) | NEA | Baltic (24&25) | SSB | 1978–2003 | −0.024 | −0.016 | S | 1 | 0 | 1 | 1 | 12 |
| Greenland halibut (Reinhardtius hipploglossoides) | NEA | Iceland | CPUESSB | 1996–2003 | — | 0.079 | S | 0 | 0 | 0 | 0 | 5 |
| NE Arctic | SSB | 1964–2004 | −0.058 | 0.054 | D | 1 | 0 | 1 | 1 | 12 | ||
| NWA | Gulf of St Lawrence | CPUETOT | 1992–2002 | — | 0.046 | I | 1 | 0 | 0 | 0 | 54 | |
| Labrador, Newfoundland, Grand Banks (2J3KL) | CPUETOT | 1978–2002 | −0.003 | 0.021 | D | 0 | 0 | 0 | 1 | 55, 56 | ||
| W Greenland | SSB | 1987–2002 | 0.104 | 0.003 | I | 0 | 0 | 1 | 1 | 57,58 | ||
| plaice (Pleuronectes platessa) | NEA | Celtic Sea | SSB | 1977–2003 | 0.070 | −0.053 | I | 0 | 0 | 1 | 1 | 5 |
| Eastern Channel | SSB | 1980–2003 | 0.058 | −0.007 | S | 0 | 0 | 1 | 1 | 5 | ||
| Irish Sea | SSB | 1964–2003 | −0.018 | −0.010 | D | 0 | 0 | 1 | 1 | 5 | ||
| Kattegat & Skaggerak | SSB | 1978–2003 | −0.021 | 0.033 | S | 0 | 0 | 1 | 1 | 5 | ||
| North Sea | SSB | 1957–2003 | −0.002 | −0.039 | D | 1 | 1 | 1 | 1 | 5, 59 | ||
| Western Channel | SSB | 1976–2003 | 0.063 | −0.019 | I | 0 | 0 | 1 | 1 | 5 | ||
| winter flounder (Pseudopleuronectes americanus) | NWA | S Gulf of St. Lawrence | CPUETOT | 1972–2001 | 0.003 | −0.001 | I | 0 | 0 | 1 | 0 | 61 |
| NMA | Georges Bank | BTOT | 1964–2001 | −0.039 | 0.174 | S | 0 | 0 | 0 | 0 | 9 | |
| S New England & mid-Atlantic Bight | SSB | 1981–2001 | −0.129 | 0.090 | D | 0 | 0 | 0 | 1 | 9 | ||
| witch flounder (Glyptocephalus cynoglossus) | NWA | Labrador & Newfoundland | BTOT | 1984–2002 | −0.241 | −0.004 | D | 0 | 0 | 0 | 0 | 62 |
| S Grand Banks | CPUETOT | 1984–2002 | −0.119 | 0.026 | D | 0 | 0 | 0 | 0 | 63 | ||
| St. Pierre Bank | BTOT | 1983–2001 | 0.035 | 0.029 | D | 0 | 0 | 1 | 1 | 64 | ||
| Gulf of St. Lawrence | CPUETOT | 1987–2000 | −0.183 | 0.097 | D | 0 | 0 | 0 | 1 | 65 | ||
| NMA | Gulf of Maine & Georges Bank | SSB | 1982–2001 | −0.125 | 0.000 | D | 0 | 0 | 1 | 1 | 9 | |
| yellowtail flounder (Limanda ferruginea) | NWA | E Scotian Shelf (4VW) | BTOT | 1986–2000 | −0.273 | 0.090 | D | 0 | 0 | 0 | 0 | 53 |
| Grand Banks (3LNO) | CPUETOT | 1984–2002 | −0.120 | 0.179 | S | 1 | 1 | 0 | 1 | 66, 67 | ||
| S Gulf of St. Lawrence | CPUETOT | 1971–2001 | 0.038 | −0.001 | I | 0 | 0 | 0 | 0 | 68 | ||
| NMA | Georges Bank | SSB | 1973–2003 | −0.104 | 0.200 | I | 0 | 0 | 1 | 1 | 69 | |
| Gulf of Maine & Cape Cod | SSB | 1985–2001 | 0.225 | 0.008 | I | 0 | 0 | 1 | 1 | 70 | ||
| S New England & mid-Atlantic Bight | SSB | 1973–2001 | −0.035 | 0.101 | D | 0 | 0 | 1 | 1 | 9, 71 | ||
| Soleidae | ||||||||||||
| sole (Solea solea) | NEA | Bay of Biscay | SSB | 1984–2003 | −0.002 | −0.053 | D | 0 | 0 | 1 | 1 | 5 |
| Celtic Sea | SSB | 1971–2003 | −0.037 | 0.026 | D | 0 | 0 | 1 | 1 | 5 | ||
| Eastern Channel | SSB | 1982–2003 | 0.013 | −0.017 | I | 0 | 0 | 1 | 1 | 5 | ||
| Irish Sea | SSB | 1970–2003 | −0.009 | 0.002 | D | 0 | 0 | 1 | 1 | 5 | ||
| North Sea | SSB | 1957–2003 | −0.016 | −0.076 | D | 0 | 0 | 1 | 1 | 5 | ||
| Skaggerak & Kattegak | SSB | 1984–2004 | 0.180 | −0.126 | I | 0 | 0 | 1 | 1 | 12 | ||
| Western Channel | SSB | 1969–2003 | 0.010 | −0.027 | D | 0 | 0 | 1 | 1 | 5 |
Trends in abundance are represented by the slopes of linear regressions of ln(abundance) plotted against time, in years.
Change in abundance is a comparison of the mean of the earliest 5 years and the mean of the most recent 5 years in each time-series.
(1) Simon et al. 2002, (2) Kulka & Miri 2003a,b, (3) Siegstad et al. 2003a, (4) Murua 2003, (5) ICES 2003, (6) Kulka & Simpson 2002, (7) Hurlbut & Poirier 2001, (8) Bundy et al. 2001, (9) NFSC 2002, (10) Dorn et al. 1999, (11) Showell et al. 2003, (12) ICES 2004, (13) Heino et al. 2002, (14) Mohn et al. 2001, (15) Fanning et al. 2003, (16) Hunt et al. 2003, (17) Vázquez & Cerviño 2002, (18) Cerviño & Vázquez 2003, (19) Fréchet et al. 2003, (20) Lilly et al. 2003, (21) Healey et al. 2003, (22) Chouinard et al. 2003, (23) Brattey et al. 2003, (24) Clark & Hinze 2003, (25) O'Brien 1999, (26) Barot et al. 2004, (27) COSEWIC 2003, (28) Harris et al. 2002, (29) Frank et al. 2001, (30) Mohn & Simon 2002, (31) Hurley et al. 2003, (32) Van Eeckhaute et al. 2003, (33) Starr et al. 2002, (34) Sinclair et al. 2001, (35) Neilson et al. 1999, (36) Beanlands et al. 2000, (37) Kulka & Miri 2001, (38) Kulka & Miri 2003b, (39) Stanley et al. 2004, (40) Morin et al. 2001a, (41) Ávila de Melo et al. 2003, (42) Power 2003a, (43) Power 2003b, (44) Siegstad et al. 2003b, (45) Power 2001, (46) Kronlund et al. 2003, (47) Simpson & Kulka 2002, (48) Dwyer et al. 2003, (49) Morgan et al. 2003, (50) Alpoim 2003, (51) Morgan et al. 2002, (52) Morin et al. 2001b, (53) Fowler & Stobo 2000, (54) Morin & Bernier 2003, (55) Dwyer & Bowering 2003, (56) Darby et al. 2003, (57) Jørgensen 2003, (58) Darby 2003, (59) Grift et al. 2003, (60) Terceiro 2003, (61) Morin et al. 2002, (62) Parsons & Bowering 2003a, (63) Parsons & Bowering 2003b, (64) Bowering & Power 2002, (65) Swain & Poirier 2001, (66) Brodie et al. 2003, (67) Walsh & Morgan 1999, (68) Poirier & Morin 2002, (69) Stone & Legault 2003, (70) Cadrin & King 2003, (71) Cadrin 2003.
Research survey gear changed in this area in 1995, such that for stocks where comparisons of catchability between the gear were not done, time-series prior to and after the change are not comparable. As such, post-1992 slopes for these populations are the slope from 1995 onwards, and total change in abundance is undetermined.
Table 2.
Overview of pelagic marine fish data (ordered taxonomically) used in analysis and their population trends over time. (Column headings same as table 1.)
| names—family common (latin binomial) | region | population | abundance metric | years | pre-1992 slope | post-1992 slope | change in abundance | Amat | Lmat | growth | age-structure | citationa |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carcharhinidae | ||||||||||||
| blue shark (Prionace glauca) | NMA/NWA | Northwest Atlantic | CPUETOTb | 1986–2000 | −0.086 | −0.031 | D | 0 | 0 | 0 | 0 | 1, 2 |
| hammerhead shark (Sphyrna spp.) | NMA/NWA | Northwest Atlantic | CPUETOTb | 1986–2000 | −0.091 | −0.044 | D | 0 | 0 | 0 | 0 | 1 |
| oceanic whitetip shark (Carcharhinus longimanus) | NMA/NWA | Northwest Atlantic | CPUETOTb | 1992–2000 | — | −0.070 | D | 0 | 0 | 0 | 0 | 1 |
| tiger shark (Galeocerdo cuvieri) | NMA/NWA | Northwest Atlantic | CPUETOTb | 1986–2000 | −0.078 | −0.006 | D | 0 | 0 | 0 | 0 | 1 |
| Lamnidae | ||||||||||||
| great white shark (Carcharodon carcharias) | NMA/NWA | Northwest Atlantic | CPUETOTb | 1986–2000 | 0.013 | −0.148 | D | 0 | 0 | 0 | 0 | 1 |
| mako shark (Isurus oxyrinchus) | NMA/NWA | Northwest Atlantic | CPUETOTb | 1986–2000 | −0.027 | −0.037 | D | 0 | 0 | 0 | 0 | 1 |
| porbeagle shark (Lamna nasus) | NWA | Canadian Atlantic | NSSB | 1961–2001 | −0.029 | −0.099 | D | 0 | 0 | 0 | 1 | 3 |
| Alopiidae | ||||||||||||
| thresher shark (Alopias super-ciliousus, A. vulpinus) | NMA/NWA | Northwest Atlantic | CPUETOTb | 1986–2000 | −0.097 | −0.031 | D | 0 | 0 | 0 | 0 | 1 |
| Engraulidae | ||||||||||||
| anchovy (Engraulis encrasicolus) | NEA | Bay of Biscay | SSB | 1987–2002 | 0.080 | 0.021 | I | N/A | 0 | 1 | 1 | 4 |
| Clupeidae | ||||||||||||
| Atlantic menhaden (Brevoortia tyrannus) | NMA | E US | SSB | 1955–2002 | −0.009 | −0.016 | D | 0 | 0 | 1 | 1 | 5 |
| herring (Clupea harengus) | NEA | Baltic (Subdiv. 25–29, 32) | SSB | 1974–2004 | −0.037 | −0.052 | D | 0 | 0 | 1 | 1 | 6 |
| Bothnian Bay (Subdiv. 31) | SSB | 1980–2003 | −0.015 | −0.011 | D | 1 | 0 | 1 | 1 | 6 | ||
| Bothnian Sea (Subdiv. 30) | SSB | 1973–2004 | 0.041 | −0.013 | I | 0 | 0 | 1 | 1 | 6 | ||
| Celtic Sea (Div VIIj) | SSB | 1958–2003 | −0.017 | −0.050 | D | 0 | 0 | 1 | 1 | 6 | ||
| Gulf of Riga | SSB | 1977–2004 | 0.042 | 0 | I | 0 | 0 | 1 | 1 | 6 | ||
| Iceland (summer spawners) | SSB | 1981–2004 | 0.064 | 0.011 | I | 1 | 0 | 1 | 1 | 6 | ||
| North Sea (IV, VIId, IIIa) | SSB | 1960–2004 | −0.019 | 0.122 | D | 1 | 0 | 1 | 1 | 6 | ||
| Norway (spring spawners) | SSB | 1950–2004 | −0.066 | 0.034 | D | 1 | 1 | 1 | 1 | 6 | ||
| West of Scotland | SSB | 1957–2004 | −0.035 | 0.049 | D | 1 | 0 | 1 | 1 | 6 | ||
| Subdiv. 22–24, IIIa | SSB | 1991–2004 | — | −0.042 | D | 0 | 0 | 1 | 1 | 6 | ||
| NWA | E/SE Newfoundland (White & Notre Dame Bay) | CPUETOT | 1988–2002 | 0.384 | −0.303 | D | 1 | 1 | 1 | 1 | 7 | |
| E/SE Newfoundland (Bona-vista & Trinity Bay) | CPUETOT | 1988–2002 | 0.261 | −0.182 | D | 1 | 1 | 1 | 1 | 7 | ||
| E/SE Newfoundland (St Mary's & Placentia Bay) | CPUETOT | 1982–2002 | 0.075 | 0.157 | I | 1 | 1 | 1 | 1 | 7 | ||
| E/SE Newfoundland (Fortune Bay) | CPUETOT | 1982–2002 | 0.266 | 0.067 | I | 1 | 1 | 1 | 1 | 7 | ||
| W Newfoundland (4R spring spawners) | — | — | — | — | ? | 1 | 0 | 1 | 1 | 8 | ||
| W Newfoundland (4R fall spawners) | — | — | — | — | ? | 1 | 0 | 1 | 1 | 8 | ||
| S Gulf of St. Lawrence | NSSB | 1978–2003 | 0.156 | −0.097 | I | 0 | 0 | 1 | 1 | 9 | ||
| Scotian Shelf (4VWX) | CPUETOT | 1970–2002 | 0.122 | 0.189 | I | 0 | 0 | 1 | 1 | 10 | ||
| NMA | Georges Bank, Gulf of Maine | SSB | 1960–2000 | −0.067 | 0.113 | I | 0 | 0 | 1 | 1 | 11 | |
| Pacific herring (Clupea pallasi) | NEP | Queen Charlotte Is., BC | SSB | 1951–2003 | 0.033 | −0.063 | D | 0 | 0 | 1 | 1 | 12, 13 |
| Prince Rupert District, BC | SSB | 1951–2003 | 0.004 | −0.013 | I | 0 | 0 | 1 | 1 | 12, 13 | ||
| Central Coast, BC | SSB | 1951–2003 | 0.019 | −0.064 | I | 0 | 0 | 1 | 1 | 12, 13 | ||
| Georgia Strait, BC | SSB | 1951–2003 | 0.008 | 0 | I | 0 | 0 | 1 | 1 | 12, 13 | ||
| W coast Vancouver Is., BC | SSB | 1951–2003 | 0.024 | −0.057 | I | 0 | 0 | 1 | 1 | 12, 13 | ||
| sardine (Sardina pilchardus) | NEA | Spain & Portugal | SSB | 1978–2003 | −0.001 | −0.032 | D | 1 | 0 | 1 | 1 | 4 |
| sprat (Sprattus sprattus) | NEA | Subdivisions 22–32 | SSB | 1974–2004 | 0.009 | −0.005 | I | 0 | 0 | 1 | 1 | 6 |
| Osmeridae | ||||||||||||
| capelin (Mallotus villosus) | NEA | Barents Sea | SSB | 1973–2003 | −0.076 | 0.166 | I | 0 | 0 | 0 | 1 | 6 |
| Iceland–E Greenland–Jan Mayen | SSB | 1978–2004 | 0.019 | −0.004 | I | 0 | 0 | 1 | 1 | 6 | ||
| Gadidae | ||||||||||||
| blue whiting (Micromesistius poutassou) | NEA | Northeast Atlantic | SSB | 1981–2004 | −0.024 | 0.061 | I | 0 | 0 | 1 | 1 | 6 |
| Norway pout (Trisopterus esmarki) | NEA | North Sea, Skaggerak & Kattegak | SSB | 1974–2003 | −0.032 | −0.003 | S | 0 | 0 | 1 | 1 | 4 |
| Carangidae | ||||||||||||
| horse mackerel (Trachurus trachurus) | NEA | Western (Div. II–VIII) | SSB | 1982–2002 | 0.127 | −0.07 | S | 0 | 0 | 1 | 1 | 4 |
| Scombridae | ||||||||||||
| mackerel (Scomber scombrus) | NEA | S, W & North Sea Components | SSB | 1977–2003 | −0.006 | 0.019 | I | 1 | 0 | 1 | 1 | 4 |
(1) Baum et al. 2003, (2) Campana et al. 2002, (3) Campana et al. 2003, (4) ICES 2003, (5) ASMFC 2004, (6) ICES 2004, (7) Wheeler et al. 2003, (8) Grégoire et al. 2003, (9) LeBlanc et al. 2003, (10) Power et al. 2003, (11) Overholtz et al. 2004, (12) Schweigert 2002, (13) Fu et al. 2004.
Data used in these analyses were from fisheries-dependent (logbook) data.
Most of the analyses presented below distinguish between demersal, or bottom-dwelling, and pelagic, or mid-water/near-surface dwelling, species. On occasion, we also distinguish teleosts from elasmobranchs. The former refers to bony fish (thus excluding hagfish, lampreys, sharks, skates, rays, lungfish and coelacanths); elasmobranchs include the cartilaginous sharks, skates and rays.
(c) Metrics for measuring biodiversity
Data obtained from our examination of almost 180 populations were used to quantify changes in various metrics of marine fish biodiversity. For each metric, we restricted our analysis to those populations for which annual, or nearly annual, estimates were available.
Population abundance data were analysed in several ways. Given that a primary goal of this exercise is to measure a rate, we first estimated the slope associated with linear regressions between log-transformed abundance data and time. We compared these slopes, which measure the rate of change in population size over time, before and after 1992. We also quantified two ratios of abundance. The first compared the average size of each population over the 5 year period ending in 1992 (1988–1992) with the average size of that population during the earliest 5 years for which data were available for that population. The second ratio compared the average population size over the most recent 5 years with that calculated for the earliest 5 years. We term these ratios the pre- and post-1992 relative abundances for each population. To explore the degree to which temporal slopes and relative abundances might vary among fish, we grouped taxa by region and by habitat (pelagic or demersal). There were sufficient data to estimate slopes, as well as changes in relative abundance, before 1992 for 166 populations and after 1992 for 175 populations.
To examine broad temporal patterns in the abundance of marine fish, we constructed aggregated time-series of abundance from individual populations. The time period for which more than 20 years of data were available for the greatest number of populations was that from 1978 to 2001, inclusive. Among the 177 populations, abundance data were available for 87 populations over this 24‐year period. For each population, we standardized the data such that the abundance in 1978 was set to a value of one. Initially, we pooled all data to provide an overall index. We then partitioned the populations by habitat and compared the geometric mean abundance of pelagic and demersal fish combined over the same time period for each of the four geographical regions under study.
One limitation to this exercise is the caveat that changes in abundance are being evaluated over a comparatively short time frame relative to that over which each population has been subjected to directed or incidental exploitation. To address this limitation to some degree, we reconstructed our combined pelagic and demersal fish datasets by dividing each population's annual abundance estimate from 1978 through 2001 by the highest abundance estimate available for that population prior to 2002, before calculating geometric means.
For life-history and demographic traits, we included only those populations that had experienced an overall decline for the time period during which abundance data were available (minimum of 15 years). We used data on both sexes combined, or on females only if data were provided by sex. Proportional changes in life-history and demographic traits were estimated by comparing the average value of the trait for the five most recent years in each dataset with the average value of the trait for the earliest years in the dataset. To assess temporal changes in life-history traits, we used estimates of the age and length at which 50% of individuals had attained maturity, most frequently from probit or logit analyses. Among the 177 datasets, data on age and length at maturity were available for 20 and 21 populations, respectively. As proxies for temporal changes in demography, we quantified changes in the mean age and size of breeding individuals in each population, using annual estimates of age-specific abundance and weight. We defined the breeding population as including all individuals in the population that were at least as old as the age at 50% maturity in each year of the time-series. Our aim was to examine potential truncations in age and size structure, thus by using a constant minimum age for each population, we avoided confounding these metrics with changes in age at maturity. Of the 177 populations, we were able to quantify changes to the average age and weight of breeding individuals for 70 and 49 populations, respectively.
4. Results
(a) Rate of change in abundance
The rate at which abundance changed before and after 1992 differed among populations, regions and habitat. In total, 70% (n=117) of marine populations were in decline prior to 1992 (i.e. time-series for which the slope was less than zero). Among these 117 populations, the rate of decline eased for 80% after 1992, including 82% (NTOT=93) of the demersal teleost populations, 69% (NTOT=13) of the pelagic teleost populations and 82% (NTOT=11) of the elasmobranch populations.
The populations of greatest concern are those that were declining prior to 1992 and whose rates of decline accelerated thereafter. These 23 populations included Atlantic cod (N=9 populations), sole (Solea solea; N=2), Atlantic herring (Clupea harengus; N=2), pelagic sharks (N=2), whiting (Merlangius merlangus; N=2) and one population for each of the following species: plaice (Pleuronectes platessa), bocaccio (Sebastes paucispinis), Pacific hake (Merluccius productus), pollock‐saithe (Pollachias virens), sardine (Sardina pilchardus) and Atlantic menhaden (Brevoortia tyrannus). Based on the trophic level designations for these species provided by Froese & Pauly (2004), the median trophic level of these 23 populations is 4.4 (compared with a maximum of 4.6; Pauly et al. 1998), meaning that they are among the top predators in the marine environment.
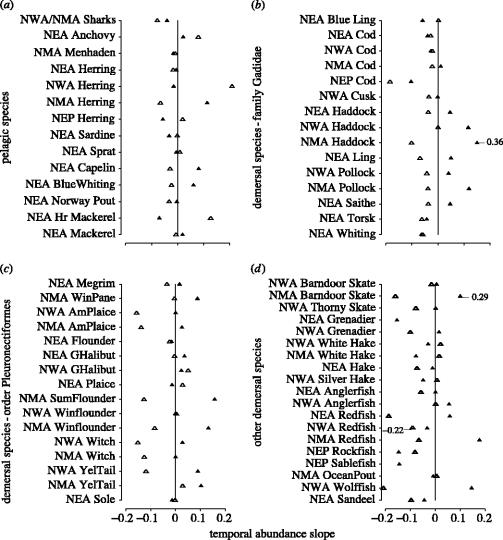
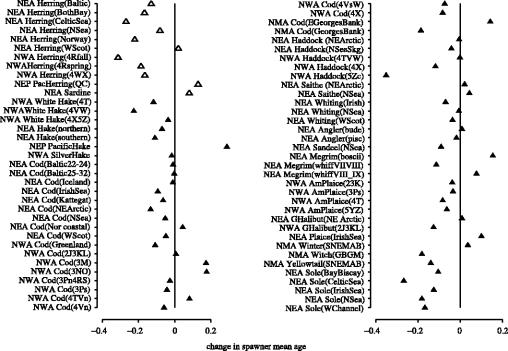
Population differences in the rate of change in abundance before and after 1992 were evident at the species and regional levels. There was considerable variability in the rate of change in abundance for pelagic species; populations exhibited strongly positive changes in some areas, but strongly negative ones in others (figure 1a). Within the Gadidae, the family that includes some of the most over-exploited species of demersal fish, the pre-1992 slopes are generally negative (figure 1b); with a few exceptions (Northeast Atlantic cod, blue ling (Molva dypterygia), whiting (Merlangius merlangus) and Northwest Atlantic cod), the post-1992 slopes were greater than the pre-1992 slopes. Within the flatfish (Pleuronectiformes: soles, plaice, flounders, Greenland halibut), although most species were in decline in most regions prior to 1992, there has been a strong tendency for them to increase since 1992 (figure 1c). Among the remaining demersal species in our dataset, most were declining before 1992 and most have been declining, or have remained stable, since (figure 1d).
Figure 1.
Rate of change in abundance for populations of pelagic and demersal marine fish from four regions (NEA, Northeast Atlantic; NWA, Northwest Atlantic; NMA, North mid-Atlantic; NEP, Northeast Pacific). Data for each population represent slopes of linear regressions between log (abundance) and years for all available data before (open triangle) and after (filled triangle) 1992. The data in each panel are grouped as follows: (a) pelagic fish; (b) demersal fish of the family Gadidae; (c) demersal flatfish of the order Pleuronectiformes; (d) other demersal fish. Values plotted represent the median slope for the populations contained within each species-regional grouping; specific populations and sample sizes can be obtained by consulting tables 1 and 2.
(b) Relative population size
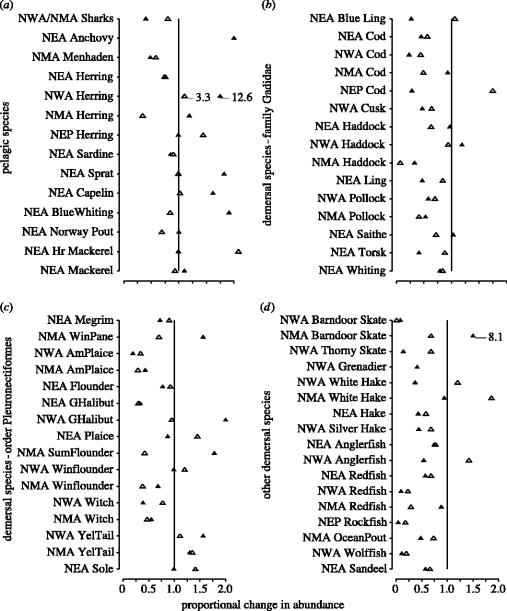
Comparisons of current and 1992 population sizes with those experienced during the earliest years for which data are available suggest that, with a few exceptions, current population sizes of pelagic marine fish exceed those experienced during each population's earliest recorded time period (figure 2a). The opposite is true for demersal species. Although many populations have been increasing since 1992 (figure 1), populations remain small relative to historic sizes (figure 2b–d).
Figure 2.
Changes in the relative abundance of populations of pelagic and demersal marine fish from four regions (NEA, Northeast Atlantic; NWA, Northwest Atlantic; NMA, North mid-Atlantic; NEP, Northeast Pacific). Data for each population represent ratios of the average abundance from 1988 through 1992 (open triangle) and ratios of current abundance (averaged over the most recent 5 years for each population; filled triangle), both relative to the earliest estimates for each population (averaged over the earliest 5 years). The data in each panel are grouped as follows: (a) pelagic fish; (b) demersal fish of the family Gadidae; (c) demersal flatfish of the order Pleuronectiformes; (d) other demersal fish. Each relative abundance estimate represents the median for the populations contained within each species-regional grouping; these sample sizes can be obtained by consulting tables 1 and 2.
These comparisons mask important differences among regions and among species. With the exception of sharks and menhaden in the Northwest Atlantic and herring in the Northeast Atlantic, the current abundance of pelagic fish is similar to or greater than those evident during the earliest years of each population's respective time-series (figure 2a). In contrast, most gadids are at or near historically low levels (figure 2b), notable exceptions being North mid-Atlantic cod, Northwest Atlantic haddock (Melanogrammus aeglefinus) and Northeast Atlantic haddock and saithe. Proportional change in abundance was most highly variable among flatfish (figure 2c), for which some are at or near historic lows (e.g. Northwest Atlantic American plaice (Hippoglossoides platessoides) and witch flounder (Glyptocephalus cynoglossus)) while others are at or near historic highs (e.g. Northwest Atlantic Greenland halibut (Reinhardtius hipploglossoides), North mid-Atlantic summer flounder (Paralichthys dentatus)). Among the non-gadid demersal fish, most are at or near historic lows (figure 2d), the most notable including Northwest Atlantic skates (Dipturus laevis, Amblyraja radiata), redfishes (Sebastes spp.) and wolffishes (Anarhichus spp.) and Northeast Pacific rockfish (Sebastes paucispinis).
Among the top predator populations (N=23) referred to above whose declining population trends prior to 1992 were exacerbated thereafter, median current population size is 64% less than that documented during the earliest years for which data are available (tables 1 and 2). Among the nine populations of Atlantic cod, the median reduction in abundance is 83%.
(c) Trends in multi-species indices of abundance
Combining all available data, the multi-species index of abundance standardized to a value of one at the beginning of the time-series indicated an overall decline of approximately 35% between 1978 and 2001 (figure 3). Compared with the recorded maxima in their respective time-series, which typically extend no further back than the 1950s (usually the 1960s) for most species, the abundance of all marine fish combined in 2001 was less than 70% of recorded highs (figure 3).
Figure 3.
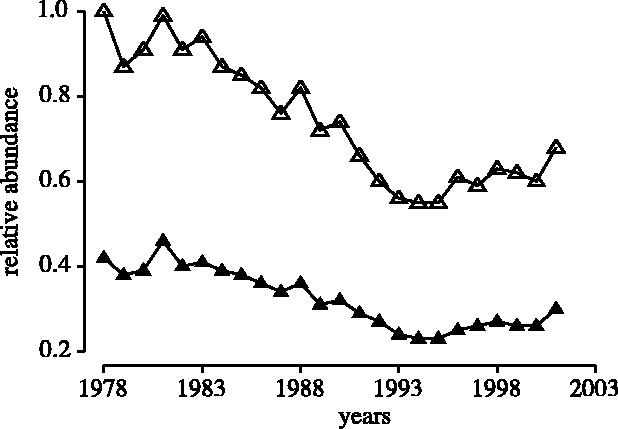
Temporal changes in the abundance of all marine fish (N=87 populations) from four regions in north-temperate oceans from 1978 through 2001. The abundance estimates for each population have been either standardized to a value of one for 1978 (open triangle) or divided by the highest estimate ever recorded for that population prior to 2002 (filled triangle).
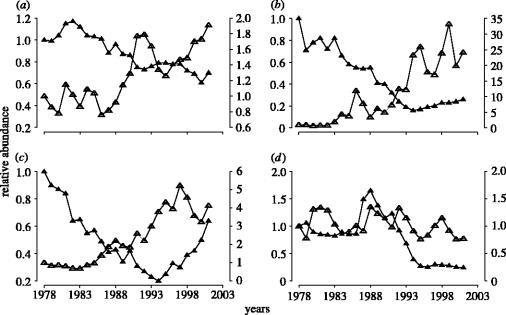
Although the number of populations for which data were available differed considerably among regions, the patterns were remarkably consistent. Between 1978 and 2001, pelagic fish exhibited a general tendency to increase, whereas demersal fish exhibited a general tendency to decline (figure 4). Pelagic fish in the Northeast Pacific (N=5 populations of Pacific herring, Clupea pallasi) were an exception to this pattern, remaining relatively constant (figure 4d). Pelagic species in the Northeast Atlantic (figure 4a) have almost doubled their 1978 levels, whereas those in the Northwest Atlantic (figure 4b) and North mid-Atlantic (figure 4c) have increased even more so. Demersal fish in each of the four regions declined considerably through the 1980s and 1990s, exhibiting declines ranging from 30% in the Northeast Atlantic to 75% in each of the other three regions relative to their 1978 levels of abundance (figure 4); signs of recovery are evident only among North mid-Atlantic demersal fish (figure 4c).
Figure 4.
Temporal changes in the abundance of demersal and pelagic marine fish from four regions in north-temperate oceans from 1978 through 2001, standardized to a value of one for 1978. The relative abundance estimates for demersal species (filled triangle) are scaled according to the left vertical axis in each panel. The relative abundance estimates for pelagic species (open triangle) are scaled according to the right vertical axis in each panel. Number of populations represented in each time-series is as follows: (a) Northeast Atlantic (demersal: N=27; pelagic: N=14); (b) Northwest Atlantic (demersal: N=23; pelagic: N=2); (c) North mid-Atlantic (demersal: N=13; pelagic: N=2); (d) Northeast Pacific (demersal: N=1; pelagic: N=5).
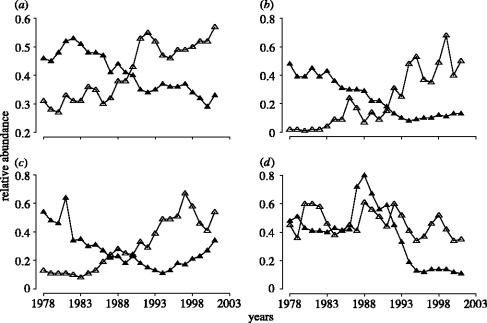
When compared against the highest population estimates recorded for each population, it becomes evident that the data presented in figure 4 present a somewhat misleading representation of the changes in pelagic and demersal fish abundance since 1978. Specifically, the increases in pelagic fish abundance are considerably more modest and the declines by demersal fish considerably more extensive. Although pelagic fish in the North mid-Atlantic and Northwest Atlantic have increased significantly since 1978 (figure 4b,c), their abundance in recent years is approximately 50% of recorded historical highs (figure 5b,c). The same appears to be true for Northeast Atlantic pelagic species (figure 5a). Since 1978, Northeast Pacific pelagic fish have fluctuated between 40 and 60% of their recorded high levels of abundance (figure 5d).
Figure 5.
Temporal changes in the abundance of pelagic (open triangle) and demersal (filled triangle) marine fish from four regions in north-temperate oceans from 1978 through 2001. The abundance estimates for each population have been divided by the highest estimate ever recorded for that population prior to 2002. Number of populations represented in each time-series is as follows: (a) Northeast Atlantic (demersal: N=27; pelagic: N=14); (b) Northwest Atlantic (demersal: N=23; pelagic: N=2); (c) North mid-Atlantic (demersal: N=13; pelagic: N=2); (d) Northeast Pacific (demersal: N=1; pelagic: N=5).
Among demersal marine fish, relative to their recorded historic levels, Northeast Atlantic species have declined almost 70% (figure 5a). The reduction of demersal fish in the other regions has been even greater, populations having experienced declines of more than 80% through the 1980s and 1990s. Although North mid-Atlantic demersal species appear to be recovering, their abundance in 2001, as a group, remained at less than 60% of their highest recorded levels (figure 5c).
(d) Changes to life-history traits and demography concomitant with population decline
The number of marine fish populations for which temporal life-history and demographic data are available is considerably less than that for which abundance estimates are available. One caveat associated with this analysis is that the time frame over which life-history and demographic data is available is usually considerably shorter than that for which abundance estimates are available. A second caveat is that these time periods are biased in that they encompass the most recent years in each population time-series, meaning that data are unavailable for those periods of time when abundance was usually considerably higher. For example, based on published data which extend only to 1983, the estimated proportional reduction in mean age of spawners among Newfoundland's northern cod is less than 1%, despite a severe truncation in age structure that occurred through the 1960s and 1970s (Hutchings & Myers 1994).
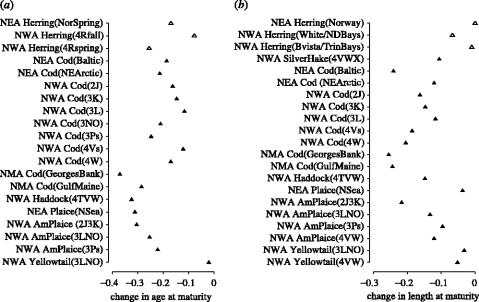
Concomitant with overall reductions in abundance, age and length at maturity have declined consistently within each species for which data are available (figure 6). Notwithstanding the fact that the time periods differ among species, the reduction in age at maturity has been considerable for many populations, averaging 21% for all species (22% for demersal (N=17) populations, 17% for pelagic (N=3) populations). The decline in length at maturity was somewhat less, averaging 13% for all populations, but was considerably greater among demersal populations (15%, N=18) than among pelagic populations (3%, N=3).
Figure 6.
Proportional changes in (a) mean age and (b) length at maturity for pelagic (open triangle) and demersal (filled triangle) marine fish from four geographical regions in the north-temperate Atlantic and Pacific Oceans. The period of time represented by each datum differs among populations. Population data are described more fully in tables 1 and 2.
The observation that population declines are associated with earlier and smaller size at maturity was reflected in changes to the mean age and weight of spawning individuals. Concomitant with population decline, the overall change in mean age of spawning individuals was a decline of 5% (figure 7); pelagic fish experienced considerably greater reductions in spawning age (12%, N=11 populations) than demersal fish (4%, N=59; figure 7). Among the 70 populations examined, the change in mean age was stable among 11% of them (i.e. change was greater or less than 1% for 0 pelagic and 8 demersal populations) and positive among 21% of the others (i.e. increase was >1% for 3 pelagic and 12 demersal populations). Restricting the analysis to the 68% of populations for which mean age of spawners declined, the reduction among pelagic populations was greater (19%; N=8) than that experienced by demersal populations (10%; N=39). It is important to note that, because we held the minimum reproductive age constant within each population, our calculations of mean spawner age will result in an underestimate of the actual changes in mean spawner age within each population.
Figure 7.
Proportional changes in mean age among spawning individuals for pelagic (open triangle) and demersal (filled triangle) marine fish from four geographical regions in the north-temperate Atlantic and Pacific Oceans. The period of time represented by each datum differs among populations. Population data are described more fully in tables 1 and 2.
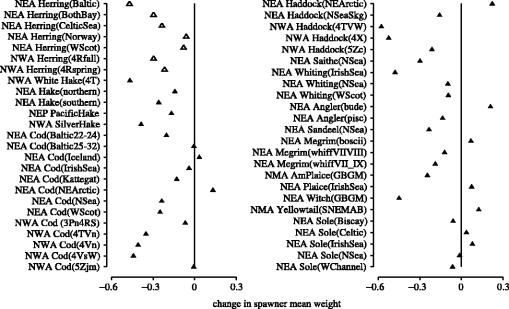
The reduction in mean weight of spawners was similar to the reductions documented for age and length at maturity, declining by an average 18% (N=49 populations); pelagic populations experienced a greater reduction in average weight (24%, N=7 populations) than did demersal populations (17%, N=42 populations; figure 8). Among the 49 populations examined, mean spawner weight remained stable among 4% of the populations (2 demersal populations) and increased among 18% of the populations (9 demersal populations). Restricting the analysis to the 78% of populations for which mean spawner weight exhibited a decline, the reduction among demersal populations (26%, N=31) was slightly greater than that among pelagic populations (24%, N=7).
Figure 8.
Proportional changes in mean weight among spawning individuals for pelagic (open triangle) and demersal (filled triangle) marine fish from four geographical regions in the north-temperate Atlantic and Pacific Oceans. The period of time represented by each datum differs among populations. Population data are described more fully in tables 1 and 2.
5. Discussion
(a) Rate of loss of marine fish biodiversity
Our analysis of temporal patterns in the abundance, life history and demography of pelagic and demersal species in north-temperate waters provides some insight into the complexity of assessing rates of change in the biodiversity of marine fish. At first glance, one might find solace in our observation that among populations in decline prior to 1992, rates of decline since then have eased for 81% of the populations examined. Such a result would presumably be deemed to be consistent with the international commitment to reduce the rate of biodiversity loss by 2010. One might also interpret the increase in abundance of pelagic fish as a sign of optimism. Such an optimist might argue further that, within a multi-species context, we have ‘turned the corner’ in our efforts to reduce the rate of loss of marine fish biodiversity. Positive as these changes are, we caution that such optimism may be premature at best, badly misplaced at worst.
Despite reductions in rate of decline, many demersal species, including most top predators, remain at historically low levels following historically unprecedented declines. Current levels of abundance for demersal fish, as a multi-species group, are 70–90% less than maxima documented in the past 30–40 years (figure 5). Taken singly, several populations have declined more than 90% relative to recorded highs, e.g. northern and Grand Banks cod, Northwest Atlantic spotted wolffish (Anarhichas minor) and northern wolffish (A. denticulatus), Northeast Pacific bocaccio (Sebastes paucispinus) and Northeast Newfoundland American plaice (Hippoglossoides platessoides). Given that the period of time for which data are available is much less than the period of time during which marine fish in the North Atlantic and North Pacific have been harvested, these population declines, as discussed earlier, have almost certainly been underestimated, a conclusion strengthened by recent indications that many predatory marine fish in north-temperate marine waters are 90–95% lower than they would be in the absence of fisheries exploitation (Christensen et al. 2003; Jennings & Blanchard 2004; Myers & Worm 2005).
Appropriate as the 2010 objective of reducing rates of biodiversity loss may be for many species, a more substantive goal for marine fish would have been the establishment of meaningful recovery targets for severely depleted species. One primary consequence of having experienced reductions of 80 and 90% or more is that the rate of population decline must, by necessity, decrease if the population is to persist. Thus, any reduction in the rate of loss of temperate marine fish biodiversity can probably be attributed to long-overdue management actions to reduce fishing effort on targeted species (although the benefits from an ecosystem perspective may be offset if effort is simply shifted to other species). While such effort controls may be insufficient to effect substantive recovery (Hutchings 2001), they should be sufficient to reduce the rate of population decline in the absence of Allee effects.
The question of whether the declines observed among marine fish in the past half-century constitute ‘collapses’, from which the probability of substantive recovery may be low, is fundamental to fisheries management, fisheries science and marine conservation biology. It has been argued that population reductions of 50–70% should not be considered problematic, given that such reductions can be consistent with fisheries policies aimed at achieving MSYs (Musick 1999; Mace 2004). We would argue that such a perspective, taken as an intellectual point of departure from which to interpret the consequences of population declines, is neither precautionary nor wise.
Among other things, such a perspective incorporates the implicit assumption that the observed declines of commercially exploited marine fish can be attributed primarily to the fulfillment of a management objective. This strikes us as an unlikely explanation for the declines documented here for North Atlantic and North Pacific marine fish. To our knowledge, few if any of these declines can be attributed to management plans designed to reduce abundance to stated reduction targets in accordance with decision rules for controlling and eventually stopping the rate of decline. Furthermore, as discussed elsewhere, few if any of these declines describe the reduction in abundance of a population from an unfished state.
Regarding new fisheries, for which one would expect an initial ‘fishing-down’ period of decline, it is incumbent upon the responsible management agency that its management plan for that fishery include: (i) decision rules for controlling the rate of decline in population size; (ii) a biomass reduction target; and (iii) a mechanism for monitoring populations size relative to the target. These recommendations are similar to those made recently by the Groundfish Subcommittee of PSARC (Pacific Scientific Advice Review Committee, Canada) following its assessment of a new fishery for longspine thornyhead (Sebastolobus altivelis) off British Columbia (Romaine 2004). Between 1996 and 2004, the population had decreased 35–55%, a rate of decline that is of considerable concern for a species that matures at 12 years (DFO 1999) and has a generation time of more than 80 years (based on estimates of natural mortality (M=0.013–0.016) provided by Pearson & Gunderson 2003).
(b) Multi-species indices of abundance: caveats
Our examination of temporal changes in the combined abundance of demersal and pelagic species for each of four geographical regions (figures 3–5) is similar to indices that have been constructed for other species (Balmford et al. 2003). The primary difference is that our indices monitor temporal changes in the abundance of populations rather than the abundance of species. The UK Wild Bird Index, for example, comprises three sets of counts of British birds, standardized to a value of one for 1970 and then averaged across all species in one of the following categories: all species (N=105 species), woodland (N=33) and farmland (N=19; DEFRA 2002). The UK government uses this index as one of its indicators for sustainable development (Royal Society 2003). The Living Planet Index (Loh 2002) is similarly constructed and represents a metric for assessing changes in the abundance of a variety of vertebrates inhabiting the forest, marine and freshwater environments. Multi-species indices such as these can be useful in providing relatively simple depictions of the state of species diversity to government agencies and decision-makers. But, as the present study suggests, they can seriously mislead as well.
The value of multi-species indices as reflections of the state of biodiversity loss diminishes as the interdependence among species included in the index increases. As our analysis of temporal trends in the abundance of pelagic and demersal fish indicates, increases by one group of species (pelagic species) may be a direct consequence of reductions in others (demersal species). Although we cannot state with certainty that the rise in pelagic fish abundance can be attributed to a reduction in predation pressure by higher-level demersal predators, the conclusion is not unreasonable, particularly given the evidence that lower-trophic species have benefited similarly from the presumed predation release afforded by the reduction in demersal predators, e.g. increased abundance of shrimp (Worm & Myers 2003) and small pelagic fish (Choi et al. 2004) following the collapse of Atlantic cod, increased abundance of flatfish following the collapse of demersal species (Myers & Worm 2003). On Georges Bank, changes in the abundance of elasmobranchs and sand lance following declines by ground-fish and herring/mackerel, respectively, have also been attributed to reduced competition and predation (Fogarty & Murawski 1998). Thus, a multi-species index for marine fish that combines pelagic and demersal species, both high- and low-trophic level predators, has great potential to provide inaccurate reflections of the current state of marine fish biodiversity (see also Pauly & Watson 2005). This caveat underscores our assertion that marine fish multi-species indices should not be confounded by the consequences of interspecific interactions (such as predation and competition) with rates of change in biodiversity.
Although we have focused on the effects that interspecific interactions can have on one's interpretation of trends in multi-species indices of abundance, our general point is that every such index (e.g. UK Wild Bird Index, Living Planet Index) has its inherent biases. It is incumbent upon those constructing and interpreting multi-species indices to identify these biases and to disentangle their influence from the patterns of biodiversity that these indices produce.
(c) Recommendations
For any group of species, the argument that one requires more data on more species over broader geographical areas is ubiquitous. While we acknowledge that this laudable objective should be pursued for marine fish and that all data be collated and managed within a single electronic database, we recognize that the time required for significant progress to be made towards fulfilling such an objective vastly exceeds the time over which political and management decisions must be made regarding the changing state of marine fish biodiversity.
Based on our analyses to date, we offer the following recommendations regarding the monitoring of marine fish biodiversity. First, the analyses presented here should be extended to include the remaining species assessed by the US National Marine Fisheries Service and the national fishery management agencies of South Africa, Australia and New Zealand. Second, while we believe there can be utility in constructing multi-species indices of abundance, care needs to be taken to ensure that confounding factors do not unduly influence the resulting patterns. Thus, for marine fish, it would be appropriate if multi-species indices were constructed separately for different geographical areas of the world's oceans, for different habitat types (e.g. demersal, pelagic, coral reef, estuarine, mangrove) and for different trophic levels (Pauly & Watson 2005). Distinguishing marine fish on the basis of trophic level is particularly important, given the considerable influence that changes to the abundance of predators, prey and competitors can have on the abundance of other species in the same ecosystem. In this regard, the species-specific trophic level designations provided in FishBase (Froese & Pauly 2004) will prove useful.
We also recommend that effort be allocated to the development of a biodiversity index, or indices, that incorporate data on abundance, life history and demography of the top predatory marine fish. The persistence of these species, preservation of their population components, maintenance of existing life-history, demographic and genetic variability, and their recovery to ecologically meaningful levels of abundance, are fundamentally important to the conservation of marine fish biodiversity. The latter is not achievable without the former. In addition to their importance to the functioning of marine ecosystems, top predators such as cod, tuna, sharks and redfish/rockfish (Sebastes spp.) share characteristics that render them particularly vulnerable to over-exploitation. It is generally these species that have experienced the greatest declines and that tend to have life-history traits (late age and large size at maturity, high longevity, large maximum size, slow growth) that make them less likely to recover than species at lower trophic levels (Denney et al. 2002; Hutchings & Reynolds 2004; Dulvy et al. 2004). The degree to which recovery is more dependent on effectively controlling exploitation (e.g. north and south temperate pelagic and demersal species), or on preservation and rehabilitation of essential habitat (estuarine and coral-reef fish), will clearly differ geographically.
(d) Concluding remarks
Notwithstanding the historically unprecedented depletions experienced by marine fish, our analysis suggests that marine fish biodiversity is recoverable, insofar as rate of change in population size is a reliable metric of biodiversity. Pelagic teleosts are among those most likely to recover in response to reduced fishing pressure (Hilborn 1997; Hutchings 2000; Dulvy et al. 2003), subject to the caveat that this apparent resilience may be attributed, to an unknown degree, to a release from predation and competition pressures. Our analyses also indicate that substantive increases in biomass can be realized among collapsed demersal species. Data from the mid-Atlantic (particularly the Gulf of Maine and Georges Bank) suggest that gadids and flatfish have responded positively to stringent catch and effort controls (figures 4 and 5). However, these positive signs of recovery are not evident at broader geographical scales in the North Atlantic.
It has been asserted that the scientific literature dealing with marine fish collapses has been unduly pessimistic and that insufficient attention has been directed to the significant increases that have been experienced by some collapsed populations. Mace (2004), for example, draws attention to recoveries experienced by 28 marine fish populations. Among her examples, 10 are pelagic species, whose apparent resilience has been well documented (Hilborn 1997; Hutchings 2000; Dulvy et al. 2003). Of the remaining 18 populations, 14 inhabit waters of the North mid-Atlantic area, a region in which substantive increases in demersal fish biomass are indeed evident (figures 4 and 5). Notwithstanding the fact that some populations are capable of rapid recovery (as previous work clearly indicates; Hutchings 2000, 2001), it is unwise to use data taken primarily from a single family known for its apparent resilience (Clupeidae, which includes herrings and sardines) and from a single geographical area to draw general conclusions about the fate of collapsed marine fish.
The question of whether some populations are capable of some level of recovery following collapse is not particularly germane in the broad sense. More fundamental is the question of how one can account for spatial and species-specific variability in recovery rates. For example, in addition to significant reductions in fishing mortality, recovery by demersal fish on Georges Bank and the Gulf of Maine has almost certainly been enhanced by the early maturity and comparatively rapid growth experienced by species in this area, relative to their counterparts elsewhere. The degree to which recovery is enhanced or retarded by changes to life-history or demographic traits, some of which have been substantive among declining fish populations, requires further examination. In part, this will depend on the degree to which the reductions in age and length at maturity documented here (figures 6–8) represent phenotypically plastic or genetic responses to exploitation. It will also depend on the species and its life history. Although they are an inevitable consequence of fishing, truncation in age and size structure of reproductive individuals, reflected here by changes to mean age and size of spawners (figures 7 and 8), can also negatively affect population growth rate, an assertion supported by model simulations (Hutchings 1999, 2005; Murawksi et al. 2001) and by experimental work (Trippel 1998; Berkeley et al. 2004a,b).
Our ability to predict the fate of collapsed marine fish, and the future state of marine fish biodiversity, is poor. Although some species have responded favourably to reductions in fishing mortality, many severely depleted populations have not. To enhance this predictive capacity, we suggest there is utility in undertaking geographically and taxonomically comprehensive examinations of the life-historical, demographic and genetic factors responsible for generating species-specific, spatial and temporal variability in recovery rates in marine fish.
Acknowledgments
We thank Georgina Mace for the invitation to undertake this research. Ray Hilborn and an anonymous reviewer provided extremely helpful comments on an earlier version of the manuscript. We gratefully acknowledge funding provided by the Natural Sciences and Engineering Research Council of Canada.
Footnotes
One contribution of 19 to a Discussion Meeting issue ‘Beyond extinction rates: monitoring wild nature for the 2010 target’.
References
- Alpoim R. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 03/44. 2003. A stock status update of American plaice (Hipplglossoides platessoides) in NAFO Division 3M. [Google Scholar]
- Ames E.P. Atlantic cod stock structure in the Gulf of Maine. Fisheries. 2004;29:10–28. [Google Scholar]
- ASMFC 2004 Atlantic menhaden stock assessment report for peer review. Stock assessment report no. 04-01 (Supplement) of the Atlantic States Marine Fisheries Commission.
- Ávila de Melo A, Alpoim R, Saborido-Rey F. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 03/45. 2003. An assessment of beaked redfish (S. mentella and S. fasciatus) in NAFO Division 3M. [Google Scholar]
- Balmford A, Green R.E, Jenkins M. Measuring the changing state of nature. Trends Ecol. Evol. 2003;18:326–330. [Google Scholar]
- Barot S, Heino M, O'Brien L, Dieckmann U. Estimating reaction norms for age and size at maturation when age at first reproduction is unknown. Evol. Ecol. Res. 2004;6:659–678. [Google Scholar]
- Baum J.K, Myers R.A. Shifting baselines and the decline of pelagic sharks in the Gulf of Mexico. Ecol. Lett. 2004;7:135–145. [Google Scholar]
- Baum J.K, Myers R.A, Kehler D.G, Worm B, Harley S.J, Doherty P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science. 2003;299:389–392. doi: 10.1126/science.1079777. [DOI] [PubMed] [Google Scholar]
- Beanlands D, Branton B, Mohn B. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2000/143. 2000. The status of monkfish in 4VWX5Zc. [Google Scholar]
- Beissinger S.R, McCullough D.R, editors. Population viability analysis. University of Chicago Press; Chicago: 2002. [Google Scholar]
- Berkeley S.A, Chapman C, Sogard S. Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology. 2004;85:1258–1264. [Google Scholar]
- Berkeley S.A, Hixon M.A, Larson R.J, Love M.S. Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries. 2004;29:23–32. [Google Scholar]
- Beverton R.J.H, Hylen A, Ostvedt O.J. Growth, maturation, and longevity of maturation cohorts of Northeast Arctic cod. ICES J. Mar. Sci. Symp. 1994;198:482–501. [Google Scholar]
- Bowering W.R, Power D.J. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/099. 2002. Observations on the witch flounder population in NAFO Subdivision 3Ps from the commercial fishery and research vessel survey data. [Google Scholar]
- Brattey J, Cadigan N.G, Healey B.P, Lilly G.R, Murphy E.F, Stansbury D.E, Mahé J.-C. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/092. 2003. An assessment of the cod (Gadus morhua) stock in NAFO Subdiv. 3Ps in October 2003. [Google Scholar]
- Brodie W.B, Walsh S.J, Power D, Dwyer K.S, Veitch M.F. Northwest Atlantic Fisheries Organization of Scientific Council Research Document. 03/52. 2003. Divisions 3LNO yellowtail flounder—interim monitoring update. [Google Scholar]
- Bundy A, Fowler M, MacEachern W, Fanning P. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/104. 2001. Assessment of the status of 4VWX/5 white hake. 48pp. [Google Scholar]
- Cadrin S. Stock assessment of yellowtail flounder in the southern New England—mid-Atlantic area. Northeast Fisheries Science Center Reference Document. 2003;03-02 112pp. [Google Scholar]
- Cadrin S.X, King J. Northeast Fisheries Science Center Reference Document. 03-03. 2003. Stock assessment of yellowtail flounder in the Cape Cod—Gulf of Maine area. [Google Scholar]
- Campana S, Gonzalez P, Joyce W, Marks L. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/101. 2002. Catch, bycatch and landings of blue shark (Prionace glauca) in the Canadian Atlantic. [Google Scholar]
- Campana S, Joyce W, Marks L. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/007. 2003. Status of the porbeagle shark (Lamna nasus) population in the Northwest Atlantic in the context of species at risk. [Google Scholar]
- Cerviño S, Vázquez A. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 03/38. 2003. Re-opening criteria for Flemish Cap cod: a survey-based method. [Google Scholar]
- Choi J.S, Frank K.T, Leggett W.C, Drinkwater K. Transition to an alternate state in a continental shelf ecosystem. Can. J. Fish. Aquat. Sci. 2004;61:505–510. [Google Scholar]
- Chouinard G.A, Swain D.P, Currie L, Poirier G, Rondeau A, Benoit H, Hurlbut T, Daigle D. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/015. 2003. Assessment of cod in the southern Gulf of St. Lawrence. [Google Scholar]
- Christensen V, Guénette S, Heymans J.J, Walters C.J, Watson R, Zeller D, Pauly D. Hundred-year decline of North Atlantic predatory fish. Fish Fish. 2003;4:1–24. [Google Scholar]
- Clark D.S, Hinze J.M. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/115. 2003. Assessment of cod in Division 4X in 2003. [Google Scholar]
- COSEWIC (Committee on the Status of Endangered Wildlife in Canada) COSEWIC; Ottawa: 2003. COSEWIC Assessment and update status report on the Atlantic cod Gadus morhua, Newfoundland and Labrador population, Laurentian North population, Maritimes population, Arctic population, in Canada. [Google Scholar]
- Darby C. An extended survivors analysis (XSA) of the Greenland halibut in NAFO Divisions 0+1. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 2003;03/54 [Google Scholar]
- Darby C, Bowering W.R, Mahé J.-C. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 03/64. 2003. An assessment of stock status of the Greenland halibut resource in NAFO Subarea 2 and Divisions 3KLMNO based on extended survivors analysis with short and medium-term projections of future stock development. [Google Scholar]
- DEFRA (Department for Environment, Food and Rural Affairs) Achieving a better quality of life. Review of progress towards sustainable development. DEFRA; UK: 2002. Wild Bird Index. Government annual Report 2002. [Google Scholar]
- Denney N.H, Jennings S, Reynolds J.D. Life-history correlates of maximum population growth rates in marine fish. Proc. R. Soc. B. 2002;269:2229–2237. doi: 10.1098/rspb.2002.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DFO (Department of Fisheries and Oceans) 1999 Thornheads (shortspine and longspine) British Columbia coast. Department of Fisheries & Oceans Science Stock Status Report A6-12.
- Dorn M.W, Saunders M.W, Wilson C.D, Guttormsen M.A, Cooke K, Kieser R, Wilkins M.E. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 99/90. 1999. Status of the coastal Pacific hake/whiting stock in U.S. and Canada in 1998. [Google Scholar]
- Dulvy N.K, Sadovy Y, Reynolds J.D. Extinction vulnerability in marine fish populations. Fish Fish. 2003;4:25–64. [Google Scholar]
- Dulvy, N. K., Ellis, J. R., Goodwin, N. B., Grant, A., Reynolds, J. D. & Jennings, S. 2004 Methods of assessing extinction risk in marine fish. Fish Fish.5, 255–276.
- Dwyer K.S, Bowering W.R. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 03/51. 2003. Greenland halibut (Reinhardtius hippoglossoides) in NAFO Subarea 2 and Divisions 3KLMNO: stock trends based on annual Canadian research vessel survey results during 1978–2002. [Google Scholar]
- Dwyer K.S, Brodie W.B, Morgan M.J. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/095. 2003. An assessment of the American plaice stock in NAFO Subarea 2 and Division 3K. [Google Scholar]
- Fanning L.P, Mohn R.K, MacEachern W.J. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/027. 2003. Assessment of 4VsW cod to 2002. [Google Scholar]
- FAO. FAO; Rome: 2002. The state of world fisheries and aquaculture 2002. [Google Scholar]
- Fogarty M.J, Murawski S.A. Large-scale disturbance and the structure of marine systems: fisheries impacts on Georges Bank. Ecol. Appl. 1998;8:S6–S22. [Google Scholar]
- Fowler G.M, Stobo W.T. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2000/144. 2000. Status of 4VW American plaice and yellowtail flounder. [Google Scholar]
- Frank K.T, Mohn R.K, Simon J.E. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/100. 2001. Assessment of the status of Div. 4TVW haddock: 2000. [Google Scholar]
- Fréchet A, et al. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/065. 2003. The status of cod in the northern Gulf of St. Lawrence (3Pn, 4RS) in 2002. [Google Scholar]
- Friedlander A.M, Parrish J.D. Habitat characteristics affecting fish assemblages on a Hawaiian coral reef. J. Exp. Mar. Biol. Ecol. 1998;224:1–30. [Google Scholar]
- Froese R, Pauly D, editors. FishBase. World Wide Web electronic publication; 2004. www.fishbase.org, version (06/2004) [Google Scholar]
- Fu C, Schweigert J, Wood C.C. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2004/011. 2004. An evaluation of alternative age-structured models for risk assessment of Pacific herring stocks in British Columbia. [Google Scholar]
- Gardner T.A, Côté I.M, Gill J.A, Grant A, Watkinson A.R. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- Grégoire F, Lefebvre L, Guérin J, Hudon J, Lavers J. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/090. 2003. Atlantic herring (Clupea harengus harengus L.) on the west coast of Newfoundland (NAFO Division 4R) in 2002. [Google Scholar]
- Grift R.E, Rijnsdorp A.D, Barot S, Heino M, Dieckmann U. Fisheries-induced trends in reaction norms for maturation in North Sea plaice. Mar. Ecol. Prog. Ser. 2003;257:247–257. [Google Scholar]
- Handford P, Bell G, Reimchen T. A gillnet fishery considered as an experiment in artificial selection. J. Fish. Res. Board Can. 1977;34:954–961. [Google Scholar]
- Harris L.E, Comeau P.A, Clark D.S. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/104. 2002. Evaluation of cusk (Brosme brosme) in Canadian waters. [Google Scholar]
- Hauser L, Adcock G.J, Smith P.J, Bernal Ramirez J.H, Carvalho G.R. Loss of microsatellite diversity and low effective population size in an overexploited population of New Zealand snapper (Pagrus auratus) Proc. Natl Acad. Sci. USA. 2002;99:11 742–11 747. doi: 10.1073/pnas.172242899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey B.P, Murphy E.F, Stansbury D.E, Brattey J. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 03/59. 2003. As assessment of the cod stock in NAFO Divisions 3NO. [Google Scholar]
- Heino M.K, Dieckmann U, Godø O.R. International Council for the Exploration of the Sea; Copenhagen: 2002. Reaction norm analysis of fisheries-induced adaptive change and the case of the Northeast cod. ICES CM 2002/Y:14. [Google Scholar]
- Hilborn R. The frequency and severity of fish stock declines and increases. In: Hancock D.A, Smith D.C, Grant A, Beumer J.P, editors. Developing and sustaining world fisheries resources. CSIRO Publishing; Victoria: 1997. pp. 36–38. [Google Scholar]
- Hilborn R. Ecosystem-based fisheries management: the carrot or the stick? Mar. Ecol. Prog. Ser. 2004;274:275–278. [Google Scholar]
- Hilborn R, Walters C.J. Chapman & Hall; London: 1992. Quantitative fisheries stock assessment. [Google Scholar]
- Hunt J.J, Hatt B, O'Brien L. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/096. 2003. Population status of eastern Georges Bank cod (Unit areas 5Zj,m) for 1978–2004. [Google Scholar]
- Hurlbut T, Poirier G. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/024. 2001. The status of white hake (Urophycis tenuis, Mitchill) in the southern Gulf of St. Lawrence (NAFO Division 4T) in 2000. [Google Scholar]
- Hurley P.C.F, Black G.A.P, Simon J.E, Mohn R.K, Comeau P.A. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/104. 2003. Assessment of the status of Division 4X/5Y haddock in 2003. [Google Scholar]
- Hutchings J.A. Reaction norms for reproductive traits in brook trout and their influence on life-history evolution effected by size-selective harvesting. In: Stokes T.K, McGlade J.M, Law R, editors. The exploitation of evolving resources. Springer-Verlag; Berlin: 1993. pp. 107–125. [Google Scholar]
- Hutchings J.A. Influence of growth and survival costs of reproduction on Atlantic cod, Gadus morhua, population growth rate. Can. J. Fish. Aquat. Sci. 1999;56:1612–1623. [Google Scholar]
- Hutchings J.A. Collapse and recovery of marine fish. Nature. 2000;406:882–885. doi: 10.1038/35022565. [DOI] [PubMed] [Google Scholar]
- Hutchings J.A. Influence of population decline, fishing, and spawner variability on the recovery of marine fish. J. Fish Biol. 2001;(Suppl. A):306–322. [Google Scholar]
- Hutchings J.A. Life histories of fish. In: Hart P.J.B, Reynolds J.D, editors. Handbook of fish and fisheries. vol. 1. Blackwell Scientific; Oxford: 2002. pp. 149–174. [Google Scholar]
- Hutchings, J. A. 2005 Consequences of life-history change to population recovery in Atlantic cod, Gadus morhua Can. J. Fish. Aquat. Sci.
- Hutchings J.A, Myers R.A. What can be learned from the collapse of a renewable resource? Atlantic cod, Gadus morhua, of Newfoundland and Labrador. Can. J. Fish. Aquat. Sci. 1994;51:2126–2146. [Google Scholar]
- Hutchings J.A, Reynolds J.D. Marine fish population collapses: consequences for recovery and extinction risk. Bioscience. 2004;54:297–309. [Google Scholar]
- Hutchinson W.F, van Oosterhout C, Rogers S.I, Carvalho G.R. Temporal analysis of archived samples indicates marked genetic changes in declining North Sea cod (Gadus morhua) Proc. R. Soc. B. 2003;270:2125–2132. doi: 10.1098/rspb.2003.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICES. Autumn 2003. International Council for the Exploration of the Sea; Copenhagen, DK: 2003. Advisory committee on fishery management. [Google Scholar]
- ICES. Spring 2004. International Council for the Exploration of the Sea; Copenhagen, DK: 2004. Advisory committee on fishery management. [Google Scholar]
- Jackson J.B.C, et al. Historical overfishing and the collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- Jennings S, Blanchard J.L. Fish abundance with no fishing: predictions based on macroecological theory. J. Anim. Ecol. 2004;73:632–642. [Google Scholar]
- Jennings S, Reynolds J.D, Mills S.C. Life history correlates of responses to fisheries exploitation. Proc. R. Soc. B. 1998;265:333–339. [Google Scholar]
- Jones K.M.M, Fitzgerald D.G, Sale P.F. Comparative ecology of marine fish communities. In: Hart P.J.B, Reynolds J.D, editors. Handbook of fish biology and fisheries. Blackwell; Oxford: 2002. pp. 341–358. [Google Scholar]
- Jørgensen O.A. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 03/53. 2003. Assessment of the Greenland halibut stock component in NAFO Subarea 0 + Division 1A Offshore + Divisions 1B–1F. [Google Scholar]
- Kronlund A.R, Haist V, Wyeth M, Hilborn R. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/071. 2003. Sablefish (Anoplopoma fimbria) in British Columbia, Canada: stock assessment for 2002 and advice to managers for 2003. [Google Scholar]
- Kulka D.W, Miri C.M. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/004. 2001. The status of monkfish (Lophius americanus) in NAFO Divisions 2J, 3K, 3L, 3N, 3O and Subdivision 3Ps. [Google Scholar]
- Kulka D.W, Miri C.M. The status of thorny skate (Amblyraja radiata Donovan, 1808) in NAFO Divisions 3L, 3N, 3O and Subdivision 3Ps. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 2003a;03/57 [Google Scholar]
- Kulka D.W, Miri C.M. The status of monkfish (Lophius americanus Valenciennes 1837; Lophiidae) on the Grand Banks, NAFO Divisions 3L, 3N, 3O, and Subdivision 3Ps. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003b;2003/100 [Google Scholar]
- Kulka D.W, Simpson M.R. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/055. 2002. The status of white hake (Urophycis tenuis), in NAFO Division 3L, 3N, 3O and Subdivison 3Ps. [Google Scholar]
- LeBlanc C.H, Poirier G.A, Chouinard G, MacDougall C. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/040. 2003. Assessment of the NAFO 4T southern Gulf of St. Lawrence herring stocks in 2002. [Google Scholar]
- Lilly G.R, Shelton P.A, Brattey J, Cadigan N.G, Healey B.P, Murphy E.F, Stansbury D.E, Chen N. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/023. 2003. An assessment of the cod stock in NAFO Divisions 2J+3KL in February 2003. [Google Scholar]
- Loh J, et al. WWF International; Switzerland: 2002. Living Planet Report 2002. [Google Scholar]
- Mace P. In defence of fisheries scientists, single-species models and other scapegoats: confronting the real problems. Mar. Ecol. Prog. Ser. 2004;274:285–291. [Google Scholar]
- Mohn R.K, Simon J.E. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/102. 2002. Biological information relevant to the management of 4TVW haddock. [Google Scholar]
- Mohn R.K, Beanlands D, Black G.A.P, Lambert T. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/105. 2001. Assessment of the status of 4Vn cod (May to October) [Google Scholar]
- Morgan M.J, Brodie W.B, Power D, Walsh S.J. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/093. 2002. An assessment of American plaice in NAFO Subdivision 3PS. [Google Scholar]
- Morgan M.J, Brodie W.B, Parsons D.M, Healey B.P. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 03/56. 2003. An assessment of American plaice in NAFO Divisions 3LNO. [Google Scholar]
- Morin B, Bernier B. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/088. 2003. Assessment of Greenland halibut (Reinhardtius hippoglossoides) in the Gulf of St. Lawrence (4RST) in 2002. [Google Scholar]
- Morin B, Bernier B, Bourdages H, Bernier D, Camirand R. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/001. 2001. The status of the redfish stock in Unit 1 (Gulf of St. Lawrence) in 2000. [Google Scholar]
- Morin R, Forest I, Poirier G. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/023. 2001. Status of NAFO Division 4T American plaice, February 2001. [Google Scholar]
- Morin R, Forest I, Benôit H. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/033. 2002. Status of NAFO Division 4T winter flounder, February 2002. [Google Scholar]
- Murawksi S.A, Rago P.J, Trippel E.A. Impacts of demographic variation in spawning characteristics on reference points for fishery management. ICES J. Mar. Sci. 2001;58:1001–1014. [Google Scholar]
- Murua H. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 03/43. 2003. Assessment of roughhead grenadier, Macrourus berglax, in NAFO Subareas 2 and 3. [Google Scholar]
- Musick J.A. Criteria to define extinction risk in marine fish. Fisheries. 1999;24:6–14. [Google Scholar]
- Myers, R. A. & Ottensmeyer, A. C. 2005 Extinction risk in marine species. In Marine conservation biology: the science of maintaining the sea's biodiversity (ed. E. A. Norse & L. B. Crowder), pp. 126–175. Washington, DC: Island Press.
- Myers R.A, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- Myers R.A, Worm B. Extinction, survival, or recovery of large predatory fish. Phil. Trans. R. Soc. B. 2005;360 doi: 10.1098/rstb.2004.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R.A, Bowen K.G, Barrowman N.J. Maximum reproductive rate of fish at low population sizes. Can. J. Fish. Aquat. Sci. 1999;56:2404–2419. [Google Scholar]
- Neilson J.D, Perley P, Nelson C, Johnston T. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 1999/160. 1999. The 1999 assessment of pollock (Pollachius virens) in NAFO Divisions 4VWX and Subdivision 5Zc. [Google Scholar]
- NFSC. Northeast Fisheries Science Center Reference Document. 02-16. 2002. Assessment of 20 Northeast groundfish stocks through 2001: a report of the groundfish assessment review meeting (GARM) Northeast Fisheries Science Center, Woods Hole, Massachusetts, October 8–11, 2002. [Google Scholar]
- Nielsen E.E, Hansen M.M, Ruzzante D.E, Meldrup D, Gronkjaer P. Evidence of a hybrid-zone in Atlantic cod (Gadus morhua) in the Baltic and the Danish Belt Sea revealed by individual admixture analysis. Mol. Ecol. 2003;12:1497–1508. doi: 10.1046/j.1365-294x.2003.01819.x. [DOI] [PubMed] [Google Scholar]
- O'Brien L. Factors influencing the rate of sexual maturity and the effect on spawning stock for Georges Bank and Gulf of Maine cod Gadus morhua stocks. J. Northwest Atlantic Fish. Sci. 1999;25:179–203. [Google Scholar]
- Olsen E.M, Heino M, Lilly G.R, Morgan J, Brattey J, Ernande B, Dieckmann U. Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature. 2004;428:932–935. doi: 10.1038/nature02430. [DOI] [PubMed] [Google Scholar]
- Overholtz W.J, Jacobson L.D, Melvin G.D, Cieri M, Power M, Libby D, Clark K. Northeast Fisheries Science Center Reference Document. 04-06. 2004. Stock assessment of the Gulf of Maine–Georges Bank Atlantic herring complex, 2003. [Google Scholar]
- Parsons D.M, Bowering W.R. Witch flounder population trends in NAFO Divisions 2J, 3K and 3L. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 2003a;03/47 [Google Scholar]
- Parsons D.M, Bowering W.R. Witch flounder in Divisions 3NO: a stock status update. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 2003b;03/46 [Google Scholar]
- Pauly, D. & Watson R. 2005 Background and interpretation of the CBD's Marine Trophic Index as a measure of biodiversity. Phil. Trans. R. Soc. B360 [DOI] [PMC free article] [PubMed]
- Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F., Jr. Fishing down marine food webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- Pauly D, Christensen V, Guennette S, Pitcher T.J, Sumaila U.R, Walters C. Towards sustainability in world fisheries. Nature. 2002;418:689–695. doi: 10.1038/nature01017. [DOI] [PubMed] [Google Scholar]
- Pearson K.E, Gunderson D.R. Reproductive biology and ecology of shortspine thornyhead rockfish, Sebastolobus alascanus, and longspine thornyhead, S. altivelis, from the northeastern Pacific Ocean. Environ. Biol. Fish. 2003;67:117–136. [Google Scholar]
- Poirier G, Morin R. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/034. 2002. The status of yellowtail flounder in NAFO Division 4T in 2001. [Google Scholar]
- Power D. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/102. 2001. The status of redfish in SA2+Div. 3K. [Google Scholar]
- Power D. An assessment of the status of the redfish in NAFO Division 3O. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 2003a;03/63 [Google Scholar]
- Power D. An assessment of the status of the redfish in the NAFO Divisions 3LN. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 2003b;03/55 [Google Scholar]
- Power M.J, Stephenson R.L, Annis L.M, Fife F.J, Clark K.J, Melvin G.D. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/035. 2003. Evaluation of 4VWX herring. [Google Scholar]
- Reznick D.N. Norms of reaction in fish. In: Stokes T.K, McGlade J.M, Law R, editors. The exploitation of evolving resources. Springer-Verlag; Berlin: 1993. pp. 72–90. [Google Scholar]
- Roff D.A. Sinauer Associates; Sunderland, MA: 2002. Life history evolution. [Google Scholar]
- Romaine S. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Proceedings Series. 2004/015. 2004. Proceedings of the PSARC groundfish subcommittee meeting May 18–19, 2004. [Google Scholar]
- Royal Society. Policy document. 11/03. Royal Society; London: 2003. Measuring biodiversity for conservation. [Google Scholar]
- Ruzzante D.E, Taggart C.E, Cook D. A nuclear DNA basis for shelf- and bank-scale population structure in northwest Atlantic cod (Gadus morhua): Labrador to Georges Bank. Mol. Ecol. 1998;7:1663–1680. [Google Scholar]
- Ruzzante D.E, Taggart C.T, Doyle R.W, Cook D. Stability in the historical pattern of genetic structure of Newfoundland cod (Gadus morhua) despite the catastrophic decline in population size from 1964 to 1994. Conserv. Genet. 2001;2:257–269. [Google Scholar]
- Schweigert J. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/110. 2002. Stock assessment for British Columbia herring in 2002 and forecasts of the potential catch in 2003. [Google Scholar]
- Showell M.A, Beanlands D, Mohn R.K, Fowler G.M. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/117. 2003. Assessment of the Scotian Shelf silver hake population to 2003. [Google Scholar]
- Siegstad H, Rätz H.-J, Stransky C. Assessment of other finfish in NAFO Subarea 1. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 2003a;03/34 [Google Scholar]
- Siegstad H, Rätz H.-J, Stransky C. Assessment of demersal redfish in NAFO Subarea 1. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 2003b;03/35 [Google Scholar]
- Simon J.E, Frank K.T, Kulka D.W. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/070. 2002. Distribution and abundance of barndoor skate Dipturus laevis in the Canadian Atlantic based upon research vessel surveys and industry/science surveys. [Google Scholar]
- Simpson M.R, Kulka D.W. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/078. 2002. Status of three wolffish species (Anarhichus lupus, A. minor and A. denticulatus) in Newfoundland waters (NAFO Divisions 2GHJ3KLNOP) [Google Scholar]
- Sinclair A, Martell S, Boutillier J. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/159. 2001. Assessment of Pacific cod off the west coast of Vancouver Island and in Hecate Strait, November 2001. [Google Scholar]
- Stanley R.D, Starr P, Olsen N. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2004/027. 2004. Bocaccio update. [Google Scholar]
- Starr P.J, Sinclair A.S, Boutillier J. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2002/113. 2002. West coast Vancouver Island Pacific cod assessment: 2002. [Google Scholar]
- Stokes K, Law R. Fishing as an evolutionary force. Mar. Ecol. Prog. Ser. 2000;208:307–309. [Google Scholar]
- Stone H.H, Legault C.M. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/055. 2003. Stock assessment of Georges Bank (5Zhjmn) yellowtail flounder for 2003. [Google Scholar]
- Swain D.P, Poirier G.A. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2001/021. 2001. Status of witch flounder in NAFO divisions 4RST, February 2001. [Google Scholar]
- Terceiro M. Northeast Fisheries Science Center Reference Document. 03-09. 2003. Stock assessment of summer flounder for 2003. [Google Scholar]
- Trippel E.A. Egg size, viability and seasonal offspring production of young Atlantic cod. Trans. Am. Fish. Soc. 1998;127:339–359. [Google Scholar]
- Van Eeckhaute L, Gavaris S, Brodziak J. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/076. 2003. Assessment of haddock on eastern Georges Bank. [Google Scholar]
- Vázquez A, Cerviño S. Northwest Atlantic Fisheries Organization Scientific Council Research Document. 02/58. 2002. An assessment of the cod stock in NAFO Division 3M. [Google Scholar]
- Walsh S.J, Morgan M.J. Variation in maturation of yellowtail flounder (Pleuronectes ferruginea) on the Grand Bank. J. Northwest Atlantic Fish. Sci. 1999;25:47–59. [Google Scholar]
- Wheeler J.P, Squires B, Williams P. Department of Fisheries and Oceans Canadian Science Advisory Secretariat Research Document. 2003/084. 2003. Newfoundland east and southeast coast herring—an assessment of stocks to the spring 2002. [Google Scholar]
- Wootton R.J. Chapman & Hall; London: 1998. Ecology of teleost fish. [Google Scholar]
- Worm B, Myers R.A. Meta-analysis of cod–shrimp interactions reveals top–down control in oceanic food webs. Ecology. 2003;84:162–173. [Google Scholar]