Abstract
Human activities have severely affected the condition of freshwater ecosystems worldwide. Physical alteration, habitat loss, water withdrawal, pollution, overexploitation and the introduction of non-native species all contribute to the decline in freshwater species. Today, freshwater species are, in general, at higher risk of extinction than those in forests, grasslands and coastal ecosystems. For North America alone, the projected extinction rate for freshwater fauna is five times greater than that for terrestrial fauna—a rate comparable to the species loss in tropical rainforest. Because many of these extinctions go unseen, the level of assessment and knowledge of the status and trends of freshwater species are still very poor, with species going extinct before they are even taxonomically classified.
Increasing human population growth and achieving the sustainable development targets set forth in 2002 will place even higher demands on the already stressed freshwater ecosystems, unless an integrated approach to managing water for people and ecosystems is implemented by a broad constituency. To inform and implement policies that support an integrated approach to water management, as well as to measure progress in halting the rapid decline in freshwater species, basin-level indicators describing the condition and threats to freshwater ecosystems and species are required. This paper discusses the extent and quality of data available on the number and size of populations of freshwater species, as well as the change in the extent and condition of natural freshwater habitats. The paper presents indicators that can be applied at multiple scales, highlighting the usefulness of using remote sensing and geographical information systems technologies to fill some of the existing information gaps. Finally, the paper includes an analysis of major data gaps and information needs with respect to freshwater species to measure progress towards the 2010 biodiversity targets.
Keywords: freshwater species, freshwater ecosystems, ecosystem condition indicators, freshwater monitoring
1. Introduction
Freshwater ecosystems are rich in species diversity and endemism. Current estimates place 44 000 of the world's 1 868 000 scientifically described species as coming from freshwater ecosystems (Reaka-Kudla 1997), but this figure is believed to be a vast underestimate. A great number of freshwater species have yet to be identified. In fact, about 200 new species of freshwater fishes are described each year (Lundberg et al. 2000). Endemism in freshwater ecosystems is also unusually high with, for example, 500–1000 fish endemic to Lake Malawi, of which only an estimated 315 have been described (Darwall et al., in press).
Freshwater resources are essential to sustaining human existence and people have settled preferentially near to water bodies for millennia. Human alteration of rivers, lakes and wetlands has followed economic development hand in hand throughout human history. As a consequence, freshwater ecosystems and species have suffered from multiple and on-going stresses from use by humans. General analyses and reviews over the past two decades have identified a suite of pressures that cause adverse change in freshwater ecosystems (e.g. Ellison 2004; Revenga & Kura 2003; Revenga et al. 2000; Groombridge & Jenkins 1998; McAllister et al. 1997; Abramovitz 1996). These reviews have shown that physical alteration, habitat loss and degradation, water withdrawal, overexploitation, pollution, and the introduction of non-native species are the leading causes of freshwater species decline and ecosystem degradation. These varied stresses occur all over the world, although their particular effects vary from one river basin to another, and certain freshwater ecosystem types are disproportionately affected by certain threats. Rarely, however, do threats occur singly; with most imperilled species subjected to multiple interacting stresses (Malmqvist & Rundle 2002; Harrison & Stiassny 1999; Miller et al. 1989).
One of the major challenges that human society faces today is achieving those Millennium Development Goals that relate to the use of water resources. These goals include halving the proportion of people without access to safe drinking water and sanitation by 2015, while sustaining functioning freshwater ecosystems and reducing the rate of biodiversity loss. Meeting the targets for a stable global climate through renewable energy development places an additional pressure on freshwater ecosystems and species, because hydropower is a leading potential energy source. Given these pressures, assessing the condition and rates of change of freshwater species and habitats is of critical importance for preserving the integrity of these ecosystems and the goods and services we derive from them.
Multiple indicators for measuring freshwater condition have been tailored to different systems around the world, and in fact the science and practice of freshwater indicator development is relatively advanced compared with work in the terrestrial and marine realms. However, developing and applying standardized indicators for systems across the world, all with different levels of data quality and quantity, is a different matter. Even for a single country, this task is daunting. A recent effort in the United States to develop freshwater indicators found only 3 of 15 that could be fully assessed using available data; of these three, one (changing stream flows) was based on historic gauge data that will be largely unavailable in the future, one (water withdrawals) was based on reporting by political jurisdictions rather than catchments and the last (waterborne human disease outbreaks) is only marginally related to freshwater ecological integrity (The Heinz Center 2002). The United States is data-rich, and we can expect far more extensive data gaps for much of the rest of the world. Data gaps should not prevent us from developing practical indicators that could be assessed through a concerted global investment in basic data development. At the same time, it is important to identify indicators that can be used in the current data situation, assuming little or no new investment.
This paper discusses the extent and quality of data available on the status and trends of freshwater species, as well as the extent and condition of natural freshwater habitats. The paper presents indicators that can be applied at multiple scales, highlighting the usefulness of using remote sensing and geographical information systems technologies to fill some of the existing information gaps. We conclude by providing an analysis of major data gaps and information needs, and highlighting the challenges ahead to meet the water needs of people while sustaining functioning freshwater ecosystems.
2. Monitoring status and trends in freshwater species
Data on the condition and trends of freshwater species are for the most part poor at the global level, although some countries have better inventories and indicators of change of freshwater species (e.g. Australia, Canada, New Zealand, South Africa, United States). This does not mean, however, that there are no data available. There are considerable data on freshwater species and populations, but these are not necessarily accessible. For example, many countries have large inventories of freshwater species in their museum and university collections, but these data are rarely centrally located or electronically archived, and many times the information is found in languages that are not accessible to the larger scientific community—which tends to rely on English, and to some extent French and Spanish literature. Additionally, large numbers of specimens have never been catalogued. Although historic museum records cannot alone be used to monitor the status of populations and species into the future, they can help to establish baseline species distributions against which to evaluate current and future conditions. Such collections are currently being used to validate putative freshwater fish extinctions. In a preliminary global analysis, Harrison & Stiassny (1999) have found quite a few differences between the IUCN Red List's extinct species and those that they have been able to confirm through a rigorous and systematic analysis of museum records and expert input. In addition to determining that a number of species listed as extinct in the 1996 Red List (the most recent version at the time of the analysis) could not be verified definitively as such, the authors also identified 39 species that probably should have been Red Listed as extinct, but were not.
In 2003, the World Resources Institute reviewed the status and trends of inland water biodiversity for the Convention on Biological Diversity (Revenga & Kura 2003), with the purpose of assessing our level of knowledge of the condition of freshwater habitats and species. Two key conclusions of this work noted that:
freshwater fishes and water birds are by far the best studied groups of freshwater species, although with considerable regional differences;
aquatic plants, insects, freshwater molluscs and crustaceans are poorly known or assessed in most parts of the world, with very fragmentary information available; and
in every group of organisms considered, including aquatic plants, invertebrate and vertebrate animal species, examples of extinct, critically endangered, endangered and vulnerable taxa exist, making it clear that freshwaters are among the most threatened of all environments.
Indeed, when looking at the level of assessment of threatened species globally, the coverage of freshwater species is still very poor. Table 1 presents the level of assessment as measured by the 2003 IUCN Red List of threatened species (IUCN 2003). Because of its harmonized category and criteria classification (i.e. all contributing experts follow the same methodology and guidelines), the IUCN Red List is the best source of information, at the global level, on the conservation status of plants and animals. This system is designed to determine the relative risk of extinction, with the main purpose of cataloguing and highlighting those taxa that are facing a higher risk of global extinction (i.e. those listed as critically endangered, endangered and vulnerable). Fortunately, just last year (2004) the IUCN/SSC and Conservation International (CI) completed a global amphibian assessment, which analysed the current level of threat to more than 5500 aquatic and terrestrial amphibian species. This dataset considerably improves our overall knowledge of the condition of freshwater species, although its scope and representativeness are limited by lack of information; 23% of amphibian species could not be assigned to a threat category and were listed as data deficient (Butchart et al. this issue).
Table 1.
Estimated numbers of extant inland water-dependent species and the number of these that are at risk of extinction according to the IUCN Red List. (Only amphibians, waterbirds and aquatic mammals have been fully assessed. Only a small proportion of other taxa has been assessed. DD, data deficient, refers to the number of species assessed, but for which there were insufficient data to assign a threat category. Threatened species information unless otherwise noted is from IUCN (2004).)
| taxon | estimated number of inland water-dependent species or subspecies | number of species assessed as ‘Threatened’ |
|---|---|---|
| aquatic plants | ||
| angiosperms | 2500a | 14 (DD: 1) |
| ferns | 250a | 2 (DD: 0) |
| bryophytes | ?? | 10 (DD: 0) |
| fungi | 1000–10 000 | 0 (DD: 0) |
| aquatic insects | ||
| Odonata | >9000a | 110 (DD: 10) |
| Coleoptera | 35 000a | 17 (DD: 0) |
| Diptera | >20 000b | 0 (DD: 0) |
| Ephemeroptera | 2250b | 1 (DD: 0) |
| Plecoptera | 2140b | 2 (DD: 1) |
| Tricoptera | >50 000a | 0 (DD: 0) |
| Megaloptera | 300b | 0 (DD: 0) |
| Heteroptera | 3200b | 0 (DD: 0) |
| freshwater molluscs | ||
| Gastropoda | 4000a | 310 (DD: 100) |
| Bivalvia | 2000a | 92 (DD: 11) |
| freshwater crustaceans | ||
| Amphipoda | 1700a | 69 (DD: 0) |
| Copepoda | ?? | 0 |
| Isopoda | 850a | 38 (DD: 2) |
| Decapoda | >1000a | 188 (DD: 7) |
| freshwater fishesc | >11 000 | 656 (DD: 254) |
| amphibians | 5743d | 1856 (DD: 1294)d |
| reptiles | ||
| freshwater turtles | 200a | 96 (DD: 10) |
| crocodilians | 23a | 10 (DD: 1) |
| snakes | 43b | 3 (DD: 0) |
| waterbirds | ||
| 33 families | 868e | 132 (DD: 8) |
| mammals | ||
| monotremes | 1a | 0 (DD: 0) |
| marsupials | 1a | 0 (DD: 0) |
| Chiroptera (fishing bats) | 2a | 0 (DD: 0) |
| Insectivora | ca. 23a | 9 (DD: 0) |
| Largomorpha | 3a | 0 (DD: 0) |
| Rodentia | ca. 58a | 11 (DD: 0) |
| otters & minks | 13a | 5 (DD: 3) |
| viverrids | >5a | 1 (DD: 1) |
| felines | 2a | 2 (DD: 0) |
| freshwater seals | 4a | 1 (DD:0) |
| manatees | 3a | 3 (DD: 0) |
| Artiodactyla (hoofed mammals) | 14a | 6 (DD: 0) |
| Cetacea (Freshwater dolphins and porpoises) | 6a | 4 (DD: 7) |
Source: Adapted from Darwall & Revenga (in preparation).
Includes freshwater elasmobranch.
Wetlands International.
It is important to note, however, that only a small proportion of the species in most freshwater taxa has been assessed, and that there is a considerable geographical bias towards North America in the Red List assessments, probably driven by data availability, high level of knowledge, and research capacity in this region and among the IUCN/SSC expert network. In addition it is important to highlight that even the geographical distributions of only a small percentage of the listed taxa have been adequately mapped (Reaka-Kudla 1997). Nonetheless, it should be possible to use those assessed species for which there are good distribution data as indicators of condition, albeit for an unrepresentative sample of species. Change in status over time can serve to indicate not only condition of the individual species, but also of the ecosystems in which they live. It is important to note, though, that changes in species status may reflect past disturbances, because time lags may occur between the occurrence of a stress and its effect on habitats and species populations (Harding et al. 1998).
According to the WRI's Pilot Analysis of Global Ecosystems (PAGE), freshwater systems and their dependent species are faring worse than forest, grassland and coastal ecosystems (WRI et al. 2000; Revenga et al. 2000). At a regional scale, the projected mean future extinction rate for North American freshwater fauna has been estimated to be about five times greater than that for terrestrial fauna and three times that for coastal marine mammals—a rate comparable to the range of estimates predicted for tropical rainforest communities (Ricciardi & Rasmussen 1999).
While data on aquatic plants and invertebrates are not readily available to portray population trends, available data give insight into the condition of freshwater ecosystems and species. In terms of aquatic plants, Revenga & Kura (2003) noted that while many macrophytic species are probably not threatened at global or continental scales, many bryophytes with restricted distributions are rare and threatened. In the United States, one of the few countries to assess more comprehensively the conservation status of freshwater molluscs and crustaceans, one-half of the known crayfish species and two-thirds of freshwater molluscs are at risk of extinction (Master et al. 1998), with severe declines in their populations in recent years. Furthermore, of the freshwater molluscs, at least 1 in 10 is likely to have already gone extinct (Master et al. 1998).
Data on freshwater reptiles, namely freshwater turtles and crocodilians (i.e. crocodiles, caimans and gharials) also show similar trends. According to the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group (1991) and the Asian Turtle Trade Working Group, of the 90 species of Asian freshwater turtles and tortoises, 74% are considered threatened. Over half of Asian freshwater turtle and tortoise species are endangered, including 18 critically endangered species, and one that is already extinct: the Yunnan box turtle Cuora yunnanensis (van Dijk et al. 2000). The number of critically endangered freshwater turtles has more than doubled since the late 1990s (van Dijk et al. 2000). Much of the threat has come from overexploitation and the illegal trade in Asia.
The status of crocodilians presents a similar pattern, particularly in Asia. Of the 17 freshwater-restricted crocodilian species, 4 are listed as Critically Endangered (3 of which are in Asia), 2 as Endangered and 2 as Vulnerable (IUCN 2003). The most critically endangered is the Chinese alligator (Alligator sinensis). The major threats to crocodilians worldwide are: habitat loss and degradation caused by pollution, drainage and conversion of wetlands, deforestation and overexploitation.
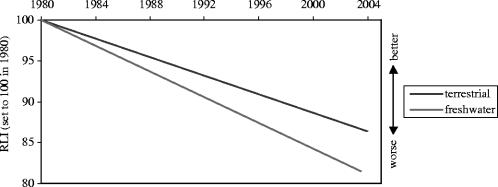
Preliminary results of the IUCN/CI global amphibian assessment also support this declining trend. Assessment findings suggest that the rate of decline in the conservation status of freshwater amphibians is far worse than that of terrestrial species (see figure 1) with more than one-third of the 1003 amphibian species assessed in Asia alone, many of which are freshwater dependent, threatened with extinction. This represents a very high level of threat, especially when compared with the global averages for birds and mammals, which are 11% and 25%, respectively (W. Darwall, personal communication 2003; figure 1).
Figure 1.
Preliminary Red List Index for amphibians in different ecosystems (Red List Consortium 2004).
More recent examples of indices measuring the status and trends in freshwater species include assessments of water birds carried out by BirdLife International for the Red List, and the WWF's Living Planet Index (LPI), which shows a more negative trend than marine or terrestrial biomes, especially since about 1990 (Loh et al. this issue).
Water birds, and particularly migratory water birds, are relatively well studied, with time-series data being available for many species in North America and northwest Europe for about 30 years. Global information on waterbird population status and trends is compiled and regularly updated through the International Waterbird Census, and published as Waterbird Population Estimates (Wetlands International 2002). Detailed information is also available for waterbird species in North America, compiled by the US Geological Survey, and for the Western Palaearctic and southwest Asia by Wetlands International (e.g. Delany et al. 1999). For African–Eurasian waterbird populations, comprehensive analyses have been compiled for Anatidae (ducks, geese and swans; e.g. Scott & Rose 1996) and waders (Charadrii; International Wader Study Group 2003). The trends in waterbird populations are better known in Europe, North America and the neotropics than in regions like Africa, Asia and Oceania. Oceania seems to be the region of highest concern for the conservation of waterbirds with the largest percentage of extinct populations, mostly small island endemic populations.
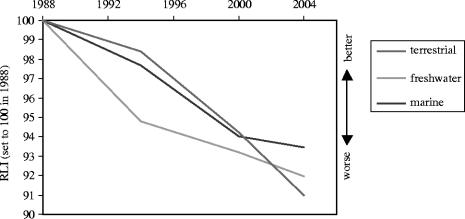
The Red List Index for birds developed by the Red List Consortium (2004) provides the best estimate of net changes over time in the overall threat status of the world's birds. With the exception of marine bird species, freshwater-dependent bird species show the sharpest and most continuous decline over time (see figure 2). The recent sharp decline in marine birds is predominantly caused by an increase in long-line fishing, which has a high bird bycatch rate, particularly for albatrosses and petrels (Red List Consortium 2004; Butchart et al. 2005; figure 2).
Figure 2.
The Red List Index for birds in different ecosystems (Red List Consortium 2004).
The Red List Index focuses only on changes in the threat status of species. This is a relatively insensitive measure because it does not include population trends of non-threatened species. However, it provides a good measure of progress in reducing extinction risk, assessed across all known bird species. Notwithstanding, other measures of the status and trends of water birds for various regions show a similar pattern (e.g. US Breeding Bird Survey, trends in European waterbird populations from Wetlands International), with common species increasing, while populations of more restricted and specialized groups are declining.
Finally, WWF's LPI provides a measure of the trend in 2500 vertebrate species populations around the world for which data are available (see Loh et al. 2005). The index shows that freshwater populations declined to a greater extent between 1970 and 2000 than those in marine and terrestrial systems (Loh et al. 2005). Information from the Nearctic and Palearctic realms predominate in the freshwater LPI, but the method of calculation compensates for this to some extent by giving equal weight to data from all realms (see Loh et al. 2005). At present too few freshwater species contribute to the LPI to allow potential biases due to unevenness in geographical and taxonomic coverage to be corrected in a more refined way.
As these indices and examples show, freshwater species are in serious decline all over the world. However, available data and information are predominantly from northern regions. Some progress is being made to collect and compile information, but progress is slow and the resources needed are high, particularly in developing countries where capacity is limited. There is a need to obtain many more data and much more even coverage for the LPI. This requires a large increase in information from all of the southern and tropical regions (Loh et al. 2005). The IUCN/SSC is making considerable progress in expanding their assessments of threat status and addressing some data gaps in freshwater, but much remains to be done. In addition to completing the global amphibian assessment, global assessments are planned for plants, molluscs and reptiles, as well as a reassessment of 2500 species of mammals. For the purposes of assessing the condition of freshwater systems, a consideration of Red Listed species should include those terrestrial species, such as many mammals that depend on riparian or floodplain habitats, which are intimately tied to the ecological integrity of aquatic systems. Regional assessments have also been completed or planned for Odonata (22 regional assessments completed), East Africa (1700 freshwater taxa assessed), Madagascar (all endemic freshwater fishes assessed) and the Mediterranean (all endemic freshwater fishes assessed by the end of 2004); those for Europe, Mekong, La Plata basin, and Pan-Africa are all planned for 2005–06 (W. Darwall, personal communication 2004).
However, monitoring and measuring change at the species level is very resource intensive. It is unlikely that the scientific community will be able to measure and monitor a large representative sample of freshwater species by 2010, even to establish a baseline. In principle, the extent and condition of habitats are relatively easier to monitor and are a good indicator for species health, plus they integrate ecosystem function and other variables into their assessments. With current technology (i.e. geographic information systems (GISs) and remote sensing) we can map habitats anywhere on the globe, albeit with different degrees of accuracy, and use proxies to assess their condition, inferring species status. The following section looks at examples of indicators to assess freshwater ecosystem condition and change over time and shows some examples of basin-scale monitoring and assessment.
3. Monitoring the extent of freshwater habitats
Global and regional assessments of freshwater habitat extent and condition have typically focused on three separate major ecosystem types: wetlands, lakes and rivers. Broad-scale assessments of wetlands have normally considered extent and number; assessments of lakes have looked at these same measures as well as condition; and assessments of rivers have focused largely on condition, because extent can be a less meaningful measure for non-floodplain river systems. Extent is an important measure, because changes in extent can signal habitat loss owing to the draining of wetlands, water abstractions and diversions, loss of connections between rivers and floodplains, or even flooding of riverine habitats as a result of impoundments. Even when extent is unchanged, though, condition can be severely compromised, such as through pollution or the establishment of invasive species.
The extent, distribution and change of wetlands and lakes is unevenly and even poorly known, partly owing to differences in definitions, as well as difficulties in delineating and mapping habitats with variable boundaries owing to fluctuations in water levels (Finlayson et al. 2001). Data from national inventories, for instance, are often incomplete and difficult to compare with each other because some concentrate on specific habitat types, such as wetlands of importance to migratory birds, whereas others include artificial wetlands, such as rice paddies. The mapping of wetlands, whose boundaries may change seasonally, poses particular difficulties, particularly for wetland complexes and temporary and ephemeral wetlands. Even with the use of remote sensing, the wide range in the sizes and types of wetlands and the problem of combining hydrological and vegetation characteristics to define wetlands make it difficult to produce a global, economical and high-resolution dataset with existing sensors, except for large water bodies in arid and semi-arid regions of the world. Larger wetlands, lakes, major rivers and inland seas have been mapped, but smaller habitats, seasonal and intermittently flooded wetlands and many flooded forests that are critical from a biodiversity standpoint are not well mapped or even delineated in many parts of the world. This introduces a high level of uncertainty into many broad-scale assessments and necessitates caution when attempting to make comparisons between information collected at different spatial scales.
Attempts to estimate the global extent and distribution of freshwater habitats, including lakes and rivers, have used a variety of broad-scale approaches and definitions, and as a result estimates vary considerably. Finlayson & Spiers (1999) published a Global review of wetland resources and priorities for wetland inventory (GRoWI) in which they derived estimates of the global extent of wetlands from compilation of national inventories, and regional and global sources, and compared these with previous estimates. The overall global estimate derived was approximately 1.28 billion ha, which is still considered an underestimate, even though it is considerably higher than previous estimates derived mostly from remotely sensed information. Previous estimates placed the global extent of natural freshwater wetlands at between 530 million ha (i.e. excluding irrigated rice fields; Matthews & Fung 1987) and 970 million ha (including 130 million ha of rice paddies; Finlayson & Davidson 1999). However, a substantial proportion of the inland wetland resource is known to be peatland. Although size estimates vary, peatlands are considered to cover at least 242 million ha (Spiers 1999), and the Global Peatland Initiative has recently estimated coverage of 324 million ha (Wetlands International 2004), some 25–46% of the total area of inland wetlands from different sources.
The GRoWI report concluded that national and regional data for Oceania, Asia, Africa, Eastern Europe and the neotropics allow just a cursory assessment of wetland extent and location, and that further wetland inventory, and studies on the rate and extent of wetland loss are urgently needed. Only for North America and for Western Europe have more robust estimates of wetland extent and loss been published, but even these regions have important information gaps. Of 206 countries for which the state of wetland inventory was assessed, only 7% had adequate or good national inventory coverage. Of the remainder, 69% had only partial coverage, and 24% had little or no national wetland inventory (Finlayson & Davidson 1999).
With respect to wetlands loss the information on the conditions and trends of freshwater habitats for North America is, on the whole, better than that for many other parts of the world. Net loss of wetlands in the USA between 1986 and 1997 was 260 700 ha, an annual loss rate of 23 700 ha (about 0.06% per year), which was an 80% lower rate than previously reported (Dahl 2000). It is estimated that 43 million ha persist of the 89 million ha of wetlands present at the time of European colonization of the USA (Dahl & Johnson 1991). Wetland loss has been minimal in the far north of Canada and Alaska. In recent decades, mitigation of the impact of development on wetlands mostly attributed to Federal protection and restoration programmes has led to a net gain of about 72 870 ha of upland wetlands (Dahl 2000).
Outside Western Europe, North America, Australia, and dryland areas in the Middle East, Central Asia and Northern Africa (Lake Chad), which have recently been mapped and change over time assessed using remote sensing (UNEP/DEWA 2001), there is very little systematic information available on the extent of wetland loss. The loss of wetlands worldwide has been broadly estimated at 50% of those that existed in 1900 (Myers 1997)—a figure that includes inland wetlands and possibly mangroves, but not large estuaries and marine wetlands. However, this figure is not based on comprehensive reliable data and its accuracy is unknown (Finlayson et al. 2001). It is certain though that much loss occurred in the northern temperate zone during the first half of the twentieth century. Unfortunately, many tropical and sub-tropical wetlands, particularly swamp forests, have been lost or degraded since the 1950s, but there is rather little quantitative information on this.
In terms of mapped information, several global exercises have been undertaken for wetlands but the level of detail varies from region to region. The best global GIS database of wetlands currently available is UNEP-WCMC's Global Wetland Distribution. UNEP-WCMC and IUCN estimated the location and extent of the wetlands through expert opinion by delineating wetland boundaries from Operational Navigation Charts (WRI 1995). Unfortunately, wetland characterization and the level of detail vary from region to region, with Africa being the most comprehensively mapped, while most of North America is covered much less accurately. The most recent global map, with a 1 min resolution, covers wetlands, lakes and reservoirs, and was produced by combining various digital maps and data sources of wetlands, lakes and reservoirs, including data from the International Commission on Large Dams (Lehner & Doll 2004), but still suffers from the problems of definition and scale outlined by Finlayson et al. (2001).
Compared with wetlands, lakes have been mapped reasonably well, although issues of scale also occur. However, change in lake extent or condition over time is not regularly monitored except for a few large lakes, such as the North American Great Lakes. The International Lake Environment Committee maintains a database of over 500 lakes worldwide, with some physiographic, biological and socio-economic information (Kurata 1994; ILEC 2002), and while data collected highlight major problem areas that are widespread among lakes and reservoirs, such as lowering of the water level, siltation, acidification, chemical contamination, eutrophication, salinization and the introduction of exotic species (Kira 1997; Jorgensen et al. 2001), the information is questionnaire-based, largely descriptive, often incomplete, and not regularly updated to be used in long-term monitoring.
For river systems, measures of condition require both maps of rivers and delineations of catchments, as well as information on discharge. Global inventories of major river systems, including data on drainage area, length and average runoff are available (e.g. Baumgartner & Reichel 1975; Gleick 1993; Shiklomanov 1997; WRI et al. 2000), but they also suffer from differences in definitions of the extent of a river system and the time period or location for the measurement of discharge (Revenga & Kura 2003). Calculating drainage area, for example, requires the delineation of each catchment, data on river networks, and topographic maps. The Eros Data Centre of the United States Geological Survey developed a GIS database in 1999 called HYDRO 1K, which delineates basin boundaries at a scale of 1 : 1 000 000. HYDRO 1K is currently the most detailed and comprehensive global database with consistent coverage of topographically derived datasets with hydrological modelling applications, including flow direction and drainage basins. However, there are limitations: most of the basins are not named, and the data often require editing to ensure that rivers do not cross basin boundaries (Revenga & Kura 2003).
A new global database, based on 90 m resolution Shuttle Radar Topography Mission data, is under development by WWF and partners and is expected to supersede HYDRO 1K. This database, with an estimated completion date of 2005, should allow managers working anywhere at nearly any scale to perform basic analyses, like deriving the upstream catchment of any point in a hydrological network. However, because the database will be derived from models, it will require field verification. When coupled with flow data, the global drainage map has additional and wide-reaching applications, like the potential to generate stream hydrographs, which in turn should assist groups around the world in establishing new monitoring efforts for their systems of interest.
Modelled discharge data for the world's rivers can be used with the global drainage map, but measured discharge data are far preferable. The World Meteorological Organization's (WMO) Global Runoff Data Centre compiles and maintains a database of observed river discharge data from gauging stations worldwide. Although this is the best global database currently available, the number of operating stations has significantly declined since the 1980s—meaning the discharge data for many rivers have not been updated in the last two decades. The Flow Regimes from International Experimental and Network Data project also compiles hydrological data from several regions of the world, but has a less extensive coverage than the WMO database. For both projects the coverage and reliability of hydrological information obtained through measurements varies from country to country. Better and more reliable information on actual stream and river discharge, and the amount of water withdrawn and consumed at the river basin level, would increase our ability to manage and monitor freshwater ecosystems more efficiently and set conservation measures for ecosystems and species. However, much effort and financial commitment would have to be made to restore hydrological stations.
Given the limitations of our knowledge on the extent and distribution of many wetlands, estimating the global rate of change over time or the extent of loss of these habitats is practically impossible. Similarly, the monitoring of the condition of rivers and lakes requires extensive data collection and capacity, which is out of the reach of many countries and government agencies.
4. Monitoring the condition of freshwater habitats
The condition of freshwater habitats has traditionally been monitored using two different approaches. The first approach, most widely applied, has been to monitor the physical condition of the habitat. More recently, increasing emphasis has been placed on biological monitoring to assess habitat quality. While the second approach has been most widely applied as an indicator of ecosystem health, it often involves direct monitoring of significant components of biodiversity, and is generally at least implicitly believed to reflect biodiversity as a whole.
Physical habitat monitoring commonly encompasses several different components including hydrological components, water chemistry, sediment condition and sometimes bank condition for rivers and streams. In most cases, the original data collection was not initiated because of biodiversity concerns, or even environmental concerns, but for water management reasons or climate change modelling. Data have often been recorded as a basis for making development decisions such as the location and size of reservoirs, or allocations for water abstraction.
Where data are available for a river system it is often difficult to extrapolate from changes in the river discharge to changes in aquatic biodiversity. Aquatic organisms respond to a variety of characteristics in a river's discharge regime, with different species responding in different ways (e.g. see King & Louw 1998). Thus, while we can say that a reduction in discharge will generally lead to a reduced biodiversity, a dam that maintains mean monthly discharges but alters daily or hourly flow patterns may still have a dramatic impact on the aquatic life, sometimes for up to hundreds of kilometres downstream.
Water chemistry and physical parameters such as temperature, have been the most widely used tools to evaluate physical habitat quality in freshwaters. Most national governments have some sort of chemical water quality monitoring programme in place, although geographical coverage is limited and quality assurance procedures may be less than satisfactory in many developing countries. Different countries, and even different regions within a country, such as states or provinces, may analyse water for different parameters, depending on their perceived problems and their analytical and financial capacity. Global chemical water quality data is collected through the UN GEMS (Global Environmental Monitoring System) programme which collects data from 106 countries (www.gemswater.org/global_network/index-e.html). The actual data contributed differ between countries for the reasons previously indicated. Some analyses of the data have been produced (Fraser et al. 1995), but data are not available online.
The value of chemical water quality data as an indicator of freshwater biodiversity is limited. Aquatic organisms do not respond to water chemistry in isolation, and chemical data are highly specific and inevitably limited. This is why biomonitoring programmes have been established (e.g. see Campbell 2002; Rosenberg & Resh 1996). Nevertheless, trends of deterioration in water chemistry parameters are likely to be accompanied by declines in aquatic biodiversity, although improving water quality will not necessarily lead to increased biodiversity.
Sediment condition or river bed condition, and stream bank condition are not widely monitored. There have been several procedures developed to conduct such assessments or to include them as part of more extensive evaluations. For example, Platts et al. (1987) developed methods to evaluate riparian habitats for the US Department of Agriculture, and Ladson et al. (1999) incorporated assessments of bed and bank conditions into their Index of Stream Condition (ISC). The ISC is now being used to monitor streams in the Australian state of Victoria.
In the Orange-Vaal River basin in South Africa three different indices are used to assess physical habitat condition in the river: the Index of Habitat Integrity (IHI) which assesses the effect of disturbances in instream and riparian zone habitat; the Geomorphology Index, which assesses the channel condition and channel stability; and the Riparian Vegetation Index which measures the degree of modification of the riparian zone from its natural state. However, as far as we are aware, physical monitoring procedures have not as yet been implemented on national or regional scales anywhere in the world.
Monitoring components of the biota as a way to track the condition of freshwater systems has a long history. Possibly the first monitoring system proposed was the Saprobien system developed by Kolkwitz & Marsson (1908, 1909) which used assemblages of plants and animals to assess levels of sewage pollution in streams in Germany. Their system has since been extensively elaborated (e.g. see Sládecek 1973) and is now being adapted to conform to the European Union Water Framework Directive (Rolauffs et al. 2004).
A variety of different components of the biota have been employed for biological monitoring, including fishes, algae and invertebrates. The choice for any given monitoring exercise depends on a number of factors including the type of habitat present, the significance of the component to the region and the skills available for collection and processing of samples. In each case, the components selected are chosen in the belief that they represent the biota as a whole, that is, they represent the biodiversity present.
Several approaches have been taken to collecting and evaluating the data from biological monitoring exercises (e.g. see Rosenberg & Resh 1996). For invertebrates, Campbell (2002) identified four main groups of data analysis methods for species assemblage data: multivariate methods, indices and metrics, predictive models and the analysis of selected species or species groups. Multivariate assessment methods generally compare samples from different sites and/or sampling times and use statistical similarity measures to determine which are more alike. This is often useful as a means of detecting impact, but tells little about biodiversity. Similarly, analysis of selected species, often using biochemical or morphological markers, may or may not inform about biodiversity as a whole.
There are a wide variety of indices and metrics used in biological monitoring. Some use measures of species diversity or species richness, and can be considered direct measures of biodiversity. Indices such as SIGNAL (Chessman 1995) and SASS (Dallas & Day 1993) use numbers of higher level taxa (such as families) while others such as the BMWP Index (National Water Council 1981), the Index of Biotic Integrity (Karr 1999) and the various Saprobien indices (Sládecek 1973) use the presence or relative abundance of particular taxa. Most of these can be expected to correlate reasonably well with biodiversity. Predictive models such as RIVPACS (Wright 2000) and AUSRIVAS (Davies 2000) use empirically selected physico-chemical parameters in a model to predict the invertebrate assemblage at a site. Actual biotic composition is then compared with the prediction to assess the level of impairment at the site. A conceptually similar approach is being adopted in Europe where the biota at reference sites is being used to evaluate impairment at test sites (Hering et al. 2004). Both the reference site and predictive model approach should indicate biodiversity loss as long as the model successfully predicts unimpaired faunal composition at the site, or suitable reference sites can be located.
Comparing and contrasting monitoring procedures in the Orange-Vaal River system in South Africa with those in the lower Mekong River in Southeast Asia is a useful way of identifying the possibilities for, and obstacles to, using bio-assessment data to evaluate changes in freshwater biodiversity on a global scale. South Africa is a developed country with limited freshwater resources and relatively low freshwater biodiversity. The four lower Mekong countries (Cambodia, Laos, Thailand and Vietnam) are developing countries with high freshwater biodiversity.
South Africa has been developing tools for freshwater bio-assessment for more than 30 years. Chutter (1972) developed the first South African index for bio-assessment using stream invertebrates. This has now been developed into the SASS system, which is used within the Orange-Vaal system and nationally (Dallas & Day 1993). Support tools for the SASS monitoring system now include a series of taxonomic keys which allow invertebrates to be identified, the first of which was published in 2000 (Day et al. 2000). Most rivers in southern Africa are relatively small in terms of discharge, so sampling methods and strategies developed for small rivers and streams elsewhere can be readily adapted for local conditions.
In addition to these bio-assessment tools, South Africa has developed a number of tools for assessing the physical state of streams and the impacts of altered flow regimes. The physical indices were previously mentioned, but in addition the Building Block Method for assessing environmental flows (King et al. 2000) and the more recent DRIFT method (King et al. 2003) were both developed by South African freshwater ecologists and are currently being applied in the Orange-Vaal system, as well as elsewhere in South Africa and neighbouring countries. The sophistication and range of the tools used to monitor South African streams and their biodiversity reflects the high level of capacity available in South Africa, as well as the level of attention and funding support that National and Provincial governments have devoted to the conservation and management of South Africa's scarce freshwater resources.
For many waters in less developed countries, biological monitoring methods have not yet been developed. Most invertebrate methods, for example, have been applied to small ‘wadeable’ streams rather than big rivers, and few bio-assessment methods have been tested adequately in tropical freshwaters. As a consequence, for most of the world's largest rivers, such as the Amazon, Zaire, Nile and Mekong, bio-assessment methods are still lacking.
The lower Mekong River, where biological assessment methods are currently being developed, provides an instructive example. Because capacity in the four countries is limited, the Mekong River Commission has assembled a single bio-assessment team including experts from all four lower Mekong countries, to work with experienced international mentors. Benthic invertebrates, attached diatoms and zooplankton are now being monitored at about 20 sites in the lower Mekong each year and sites will be assessed over a 3–5 year rotation. Because of the absence of regional taxonomic keys to facilitate identification of the biota, the Mekong River Commission is supporting local experts to develop keys, the first of which should be published in 2005. So far, data are being analysed using multivariate statistical methods that would not allow a ready evaluation of biodiversity impacts, but the need for some sort of robust metric which would allow such an evaluation has been identified. However, with large rivers such as the Mekong it is difficult to find reference sites that can be used for comparison with sites on the mainstream which may be impacted. This is especially true for sites in the delta, which is now densely populated throughout.
Fishes are not being included at present in the Mekong bio-assessment work for several reasons. The Mekong freshwater fish fauna is extremely diverse (with an estimated 1200 species present (MRC 2003)) and very important to the livelihoods of the people. However, in a system such as the Mekong it is very difficult to obtain a representative sample of the fish fauna. There is no single sampling method that can be used at the full range of locations, and some preferred sampling methods, such as electrofishing, would be interpreted by local communities as poaching. To obtain a sufficiently large fishes sample by methods such as trapping or gill-netting requires overnight sampling, which is too time consuming to allow sufficient sites to be sampled within the constraints of the programme.
The lower Mekong is a pulsed floodplain river system, so management of floodplain habitats is the largest physical habitat monitoring concern. The physical condition indices applied elsewhere are essentially designed to evaluate the condition of river banks and narrow riparian vegetation strips. The floodplain of the Mekong is up to 100 km wide, so different techniques, probably using remote sensing technology, need to be developed to monitor changes in physical condition.
It is evident that development and implementation of bio-assessment protocols that can be used as indicators of changes in freshwater biodiversity in tropical developing countries is not a trivial exercise. Yet the work in the Mekong basin demonstrates that such systems can be implemented in a relatively short time, using local expertise even in a region where capacity is low, where there is sufficient will and sufficient support.
Two additional issues which need to be resolved if bio-assessment data are to be used to monitor freshwater biodiversity per se at large scales are the problems of sampling and the variety of methods in use. The problems of sampling include the difficulty of extrapolating over large areas from data collected at single points, and the problem of data gaps. The problems of extrapolation are the same problems facing agencies attempting to assess river health over a river basin, or province or country with data collected only from a limited number of sites. They can be resolved to some extent through increased sampling effort.
Data gaps are a particular issue at the global scale since in some countries, especially developing countries, there are as yet no bio-assessment data. As noted previously, in many of those countries the capacity to conduct such assessments is minimal and their waters are sufficiently different to most of those in developed countries that existing assessment methods will need to be adapted before being used. In addition, tools such as fishes and invertebrate identification keys will need to be developed. Such countries will need international support to train and assist water resource and pollution control agencies to develop the necessary capacity.
The variety of bio-assessment methods in use in different countries also makes it difficult to determine large-scale patterns or trends in freshwater biodiversity. In the United States the IBI is used quite widely, but many State agencies still use other metrics (e.g. Davies & Tsomides 1997). In Europe, a variety of methods have been used in the past; however, the European Union Water Framework Directive (European Union 2000) now requires all countries to use consistent methods of assessment. All assessments are to be water body specific, and compare the biota present to that expected under near-natural reference conditions. Thus, all assessments are based on estimates of biodiversity lost at a particular site. Australia has a national system which is not dissimilar, comparing faunal composition at the family level at a test site with the fauna predicted using a model (Davies 2000), while in South Africa an index is used based on the number of families present.
While such differences make it difficult to compare absolute biodiversity, it should be possible to use such data to determine the strength and direction of trends. It would be possible, if the data were to be compiled, to determine the proportion of sites or river basins at which indices or metrics are declining versus those in which metrics were stable or increasing over a significant part of the globe. Such a compilation could be conducted through the existing GEMS network, since many of the same agencies collecting chemical water quality data are also collecting bio-assessment data.
5. Monitoring drivers of freshwater condition at the global scale
Considerable obstacles need to be overcome to achieve global monitoring of the extent and condition of freshwater ecosystems, but the major drivers affecting their condition are quite clear and, for the most part, easier to assess and monitor. This is especially true in those areas lacking sufficient data on species imperilment or the resources for extensive fieldwork. For example, using data on the extent of agriculture in a watershed, or the size and location of dams, we can draw some conclusions about the relative degree of alteration or stress affecting a system. These geospatial indicators are often called proxies or surrogates, because they are indicators of current threat and give only indirect information about actual ecological integrity. Physical habitat modification, land use change, water withdrawals, pollution, and the presence of invasive species can all be used as proxies to assess ecosystem condition and infer species status. We develop these indicators at larger spatial scales using geospatial datasets and GIS. GIS allows us to analyse the spatial relationships between anthropogenic activities and freshwater systems. Innumerable analyses can be run using geospatial data; the challenge is to select the most appropriate tools based on our best ecological knowledge and available data, and then to undertake a judicious interpretation of the results.
Geospatial proxies are most often used when field-measured data for assessing integrity are unavailable, or to augment those data. Conservation planners and decision-makers typically put these proxies into service to evaluate current status and sometimes to forecast future threats. A monitoring plan to assess condition over time could also make use of these proxies, by analysing temporal changes in the data. While this approach still fails to measure actual change in species or habitats, it provides a method applicable to broad, inaccessible areas and to situations where funds are unavailable for detailed field studies.
The examples we present here focus on threat assessments over large areas with limited data available, as is the case in much of the developing world. Analyses that address multiple-scales of biodiversity within a landscape context are used to protect not only rare and endangered species, but also broader-scale organizational levels among species and their ecological and evolutionary contexts. Here we describe some examples of threat analyses at the global scale and offer caveats that should accompany their use.
Global-scale assessments of threats are necessarily unrefined because input data are rarely of uniformly high quality across the world, if they are available at all. Additionally, many global analyses focus on large river basins or use models derived from climate datasets that are coarse-scaled. Despite their limitations, global assessments can illustrate spatial and sometimes temporal patterns; maps showing these patterns can be powerful tools for raising awareness and catalysing action. They can also help international conservation groups and funding agencies set priorities for action.
All monitoring programmes, no matter what their scale, must designate units of analysis. For global analyses, these units are often predetermined by the given resolution of available datasets. Many global assessments use grid cells as their unit of analysis, with typical cell sizes in the range of 0.5 degrees to 30 seconds (about 50–1 km). Some global data, such as human population densities, are provided in different resolutions and data formats, or are the product of post-processing. For example, the Gridded Population of the World dataset (CIESIN et al. 2000) provides census data as derived from sub-national administrative units. For freshwater threat assessments, the most meaningful unit of analysis is often the drainage basin. The particular data formats, resolutions and data manipulations of each of these datasets provide advantages and disadvantages for answering different types of questions.
Examples of existing global analyses of freshwater biodiversity and their threats are rare. One of the most comprehensive assessments of condition of the world's freshwater systems is the freshwater analysis in the World Resources Institute's PAGE: Freshwater Systems (Revenga et al. 2000). Although the measures of condition are focused on the maintenance of ecosystem services rather than on biodiversity, many of the measures are broadly applicable as proxies for aquatic ecological integrity. Two indicator examples that follow this approach are presented here.
(a) Indicator of river fragmentation and flow regulation
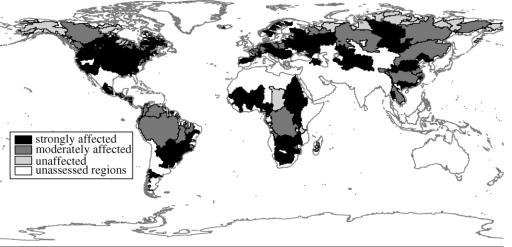
River fragmentation, which is the interruption of a river's natural flow by dams, inter-basin transfers, or water withdrawal, is an indicator of the degree to which rivers have been modified by humans. The impoundment of main channels, the presence of dams on major tributaries, the storage volume of reservoirs, and the overall reduction of discharge are all considered to be substantial threats to the integrity of river systems (Revenga et al. 2000). The results of this indicator analysis, presented in figure 3, demonstrated that of the 227 large river systems assessed, 60% are highly or moderately fragmented, affecting 90% of the water volume in these rivers (Revenga et al. 2000). All river systems with parts of their basins in arid areas or that have internal drainage systems are highly fragmented.
Figure 3.
River fragmentation and flow regulation. Source: Revenga et al. 2000.
The only remaining large free-flowing rivers in the world are found in the tundra regions of North America and Russia, and in smaller coastal basins in Africa and Latin America. It should be noted, however, that considerable parts of some of the large rivers in the tropics, such as the Amazon, the Orinoco and the Congo, would be classified as unaffected rivers if an analysis at the sub-basin level were done. The Yangtze River in China, which currently is classified as moderately affected, will become strongly affected once the Three Gorges Dam is completed.
This indicator is derived using information on dam location, river basin transfers, river channel alteration and changes in flow owing to reservoirs and irrigation water consumption. Some of these data can be updated and the analysis redone to measure change over time. For example, information on new dams planned or under construction can be used to assess future change, or assess increased fragmentation on a given basin. However, it should be noted that these data are not readily available for many countries. On the other hand, such an analysis at the subcatchment level, where more data are available, may be useful in tracking change over time, especially as it refers to altered flows (figure 3).
(b) Indicator of water stress
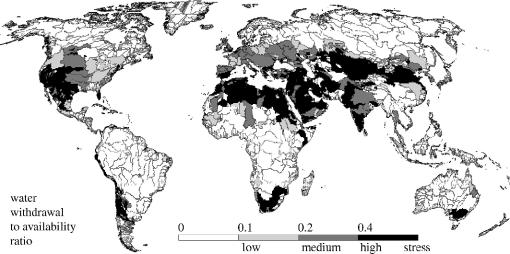
Water stress is another indicator of relevance to aquatic habitat threats, though it is traditionally applied to evaluate pressures on human societies. Water stress is variously defined, but one measure is the annual water withdrawals to water availability ratio (wta). In principle, the higher the wta ratio is, the more intensively water is used in a river basin, and hence the more stress is placed on the affected freshwater system. Commonly used thresholds can be applied to identify areas of ‘low’ (0.1<wta≦0.2), ‘medium’ (0.2<wta≦0.4) and ‘high’ (wta>0.4) water stress. However, the significance of these numbers for freshwater ecological integrity requires testing. To capture inter-annual variability or seasonal fluctuations in water stress, additional measures for critical low flows, like the Q90 (discharge that is exceeded 90% of time), or duration and severity of large-scale droughts, can be assessed. It is important to note that this measure of water stress does not take into account the water requirement of the ecosystem; this ‘environmental reserve’ is the water that should remain in the system to ensure its ecological integrity. When environmental water requirements are taken into account, more river basins show a higher degree of stress (Smakhtin et al. 2004).
Water stress maps have been generated for the entire world, based on calculations for individual large river basins. One such global example is derived from the WaterGAP2 model (Döll et al. 2003; Alcamo et al. 2004), which provides discharge and human water use calculations for the present time and for future scenarios, on a global 0.5 degree grid (figure 4). These global maps require modelled discharge estimates for the Earth's entire land surface (e.g. Fekete et al. 2000; Döll et al. 2003). For individual basin monitoring efforts, however, measured discharge data could be substituted, where available, to give a more accurate assessment of water stress. Like other proxies, water stress measurements have potential as relative indicators of change of time, but their widespread use would require a marked reversal of the current decline in the number of gauging stations around the world.
Figure 4.
Water withdrawal to water availability ratio (wta) by river basin. Sources: Döll et al. 2003 and Alcamo et al. 2003.
6. River basin scale analysis
It is at the scale of the large river basin that monitoring becomes most relevant, as this is the scale at which interventions begin to have the potential to make a difference for freshwater ecosystems. Of course, not all freshwaters are within large river basins, and systems within the upper reaches of large basins will not always be affected by downstream activities. Nevertheless, nearly all of the large river basin monitoring-approaches discussed here apply within smaller drainages.
Surprisingly, the quality and resolution are not necessarily better at the scale of river basins than at continental and global scales. For example, water use may be estimated for a country or large river basin through summing annual industrial, household and agricultural water withdrawals across the entire area, but characterizing the spatio-temporal distribution of water withdrawals within a given river basin would require locational and other data that rarely exist. Additionally, local water transfers or water trading may cause significant small-scale fluctuations. Evaluating whole-basin changes by indicators such as water stress, then, is possible, but within-basin evaluations will elude managers in most parts of the world.
On the other hand, at the river basin scale, high-resolution remote sensing imagery analyses may be feasible, both from a financial and workload perspective, owing to the smaller region of analysis. Remote sensing of freshwater systems is becoming increasingly sophisticated, with applications such as assessments of water quality (Glasgow et al. 2004; Bilge et al. 2003; BirdLife International 2004; Sawaya et al. 2003), aquatic and floodplain vegetation mapping (Costa 2004; Vis et al. 2003; Williams et al. 2003), invasive species mapping (Verma et al. 2003), wetland flooding assessments (Hess et al. 2003; Kasischke et al. 2003), land cover mapping (Ballester et al. 2003), wetland restoration success (Shuman & Ambrose 2003), instream habitats (Whited et al. 2002) and lake change area (Yu & Jiang 2003).
Some problems with low precision that permeate global and continental analyses are easier to resolve for individual river basins, where specific data manipulations and manual corrections also become feasible. Within a relatively small study area, gauging stations and dam locations may be reliably linked to river stretches; digital elevation models can be improved to reproduce the actual river system; rivers, lakes and wetlands can be aligned and topologically connected; and species occurrences can be mapped to particular streams, river reaches, or other freshwater systems.
7. Conclusions
The indicators and information presented in the previous sections show that human activities have severely affected the condition of freshwater ecosystems and species all over the world. Habitat loss and degradation, invasive species, overexploitation, and pollution, are all stressing the capacity of these systems to support biodiversity, with many species facing rapid population declines or extinction. Critical freshwater habitats, such as wetlands and rivers, are subject to increased pressures and demands on their use, causing the disappearance of some of the remaining refuges for many species as well as areas for food production and water availability for local communities, particularly the poor.
Although physical alteration to inland waterways have increased the amount of water available for human use, more than 40% of the world's human population still experience water stress, with this percentage predicted to increase to almost 50% by 2025. In addition, surface and groundwater is being degraded in almost all regions of the world by intensive agriculture and rapid urbanization, aggravating the water scarcity problem. Achieving the Millennium Development Goals for drinking water, food and sanitation will further challenge our ability to manage water to meet human and ecosystem needs.
Current management of water resources has not been sustainable, and in large part has been inefficient. In order to manage water resources to meet the needs of people while sustaining functioning ecosystems we need to implement a more balanced and integrated approach to water management. For this to occur, several key factors need to be overcome. These factors include current institutional and management structures that favour single-sector approaches and generate cross-sectoral conflicts; transboundary conflicts in managing entire river basins; the undervaluing of water, which makes its use inefficient and wasteful; and the lack of understanding of the key role that ecosystems play as legitimate users of water.
Many of the options available to improve water resources management to benefit both people and nature fall within the economic and political realm. However, monitoring and assessment of freshwater ecosystems and species is crucial to informing and guiding policy or economic action. Governments, international agencies, NGOs, river basin authorities and civil society need data and information on the condition of freshwater resources, their ecosystem functions, their dependent species and the livelihoods of people dependent upon them, in order to formulate and implement policy options that are sustainable. For example, better information on actual stream and river discharge, and the amount of water withdrawn and consumed in each basin, would increase our ability to manage freshwater systems more efficiently and evaluate trade-offs. To fill in the information gaps, much effort and financial commitment would have to be made, but the rewards for doing so are significant.
In general, all areas related to freshwater ecosystems require more data and information, from water availability and quality to the status and population trends of freshwater species. Because adopting such an information gathering effort at the global level would be a daunting task, we propose selected areas where an incremental amount of effort would produce fruitful results.
(a) Species information
In general, information on freshwater species and trends in species' populations in most freshwaters is poor. Even economically important groups such as fishes tend to be poorly covered at both local and global scales. In addition, the existing species inventories are organized by taxonomic group and not by ecosystem type, which makes it hard to assess the condition of freshwater ecosystems. Freshwater species have traditionally been less studied, and because of their distribution within water bodies, they are harder to map than terrestrial species. Without population trends of species, it is hard, for example, to assess the effects of pressures or the risk of extinction of species. Because monitoring and assessing all freshwater species is a daunting task, countries and institutions are encouraged to monitor key indicator species for freshwater systems, as well as monitoring the presence or introduction of exotic species and their impacts on native fauna and flora. This paper has presented several initiatives that may help identify, catalogue and map species around the world, but to monitor ecosystem condition it is also necessary to assess change over time, ideally by looking at population trends. In order to do so, however, baseline information on the distribution and abundance of species is needed. There is great potential to improve the availability of such information by drawing from the existing museum collections and databases around the world. Mobilizing the data and integrating it into a standard format could help fill many of the existing gaps and establish baselines against which change can be monitored. However, monitoring programmes can be implemented without first having a full picture of every species present in an ecosystem. There is an urgent need to implement repeatable monitoring of species selected to be representative of wider biodiversity or of other valued attributes of the system.
Finally, because of the large impact that introduced species can have on inland water ecosystems, information on the location of introduced species as well as the presence or absence of invasive species is urgently needed. Regional or national monitoring programmes often have more detailed information. For example, the Group on Aquatic Alien Species (GAAS) in Russia has compiled documentation on six aquatic invasive invertebrates found in enclosed seas of Europe and the Great Lakes region of North America, including a mapped range of original and current occurrence (GAAS 2002). Detailed information and distribution maps of a number of non-indigenous aquatic species including vertebrates, invertebrates and plants, are also available for the USA (USGS 2001).
(b) Information on habitat extent and condition
There is a very limited capacity to measure the extent of freshwater habitats and the rate at which their area is changing. Currently we lack widely applicable, repeatable methods for recognizing and mapping wetland habitats. Remote sensing technology, for example, has not been very successful in mapping wetlands so far. Part of the problem is the coarse resolution of most satellite imagery, although this is improving every year. The other more problematic area is the difficulty in mapping seasonal wetlands and forested wetlands. Radar, which can sense flooding underneath vegetation and can penetrate cloud cover, is probably the best option for developing a global wetland extent monitoring programme. However, work in this area is minimal with most remote sensing groups focusing on terrestrial habitats.
Our ability to reverse current trends in the condition of freshwater ecosystems is in large part dependent on our understanding of threats and our capacity to manage them in an integrated fashion. In the absence of actual measures of ecosystem condition, we can use proxy measures that evaluate the relative degree of threat facing different systems. As presented in the paper, geospatial datasets can serve as proxies for evaluating levels of threat at global, regional, and river basin scales, though these datasets have limitations. Future efforts should focus on enhancing the quality of existing data, and on providing additional reliable datasets of important hydrologic features. Examples include geographically referenced databases of dams and their operational schemes, of lakes and their biogeochemical conditions, of altered versus pristine river courses, and of quantity and quality of surface and groundwater pollution.
Research is also needed to understand how disturbances associated with different land uses affect freshwater habitats, with a focus on the scales over which these disturbances operate and the thresholds above which biotically meaningful changes occur. Current work on indexes of ecological integrity will continue to support these research aims, however, the current limited investment into these research questions is inadequate to generate meaningful progress in the near term, during which time more species are expected to go extinct.
(c) Water resource and socio-economic data
Although water is essential for human survival, information of this resource is lacking in many parts of the world. Most data on water availability and use are generally only available at the national level, which makes management of river basins, especially those that cross national borders difficult. Data and information on basic variables, such as river flow, water withdrawals, aquifer recharge rates, etc. are not often available at the basin level and most of the data available are based on models developed from climate and precipitation data. Consequently, these datasets are coarse and only applicable at larger scales.
If information on water availability and use is lacking, the amount of information on water quality is even more depressing. Better information on water quality can provide nations with immediate benefits because of the direct connection between water quality and human health. But gathering such information generally requires expensive monitoring networks that are beyond the reach of many developing countries. Even though surface water monitoring programmes are well developed in most countries of the Organization for Economic Cooperation and Development (OECD), water quality monitoring in most parts of the world is rudimentary or nonexistent. Even those developed countries that have water quality monitoring programmes in place focus on chemical parameters that leave out important biological information. One of the biggest challenges in future water monitoring programmes is the integration of chemical and biological measures of water quality.
In addition to ecosystem-specific datasets, greatly improved socio-economic information that can be aggregated at the basin level is essential for a more integrated approach to water management. Some socio-economic variables needed include: population density and distribution in relation to water resources; income distribution; the degree of dependence on inland waters and the biodiversity they support; irrigated area per basin; and food production from freshwaters.
In summary, if we are to achieve long-term sustainability and reduce the rate of biodiversity loss in freshwater systems, we will need to shift the way that water resources are managed from single-objective and narrow focus to a resource that is used in an integrated fashion and that has long-term sustainability as a guiding principle. For this integrated approach to be implemented, resources have to be focused on rebuilding and maintaining monitoring stations for water quality and quantity in much of the world's rivers and water bodies.
What we hope to see in the coming years is a paradigm shift from the traditional water management approach that believes that ‘water that reaches the sea is water wasted’ to an ecosystem-based belief that water that remains in the river is an integral part of water management. This vision implies that: (i) there is cross-sectoral collaboration in managing water resources that includes stakeholder participation in large basin-wide development plans, specifically the agricultural sector; (ii) river basin institutions have the authority and mandate to allocate water resources to meet the basic needs of people and ecosystems; (iii) ecosystems and freshwater species are considered in water allocations and basin plans and the threat level to freshwater species is reduced; and (iv) many more uses of water are valued correctly and there are economic incentives to improve efficiency and limit pollution. Well-designed monitoring programmes are an integral part of any plan to turn this vision into reality, but, except in a few restricted areas, we are far from having them in place by 2010.
Glossary
- DEWA
Division of Early Warning and Assessment
- GAAS
Group on Aquatic Alien Species
- GRoWI
Global Review of Wetland Resources and Priorities for Wetland Inventory
- GSI
Geographic Information System
- IHI
Index of Habitat Integrity
- ISC
Index of Stream Condition
- LPI
Living Planet Index
- OECD
Organization for Economic Cooperation and Development
- PAGE
Pilot Analysis of Global Ecosystems
- SSC
Conservation International
- UNEP
United Nations Environment Program
- UN GEMS
Global Environment Monitoring System
- WMO
World Meteorological Organization
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Beyond extinction rates: monitoring wild nature for the 2010 target’.
References
- Abramovitz J.N. Worldwatch paper 128. Worldwatch Institute; Washington, DC: 1996. Imperiled waters, impoverished future: the decline of freshwater ecosystems. [Google Scholar]
- Alcamo, J., Döll, P., Henrichs, T., Kaspar, F., Lehner, B., Rösch, T. & Siebert, S. In press. Global estimates of water withdrawals and availability under current and future ‘business-as-usual’ conditions, Hydrol. Sci. J.48, 339–349.
- Ballester M.V. R, Victoria D.de C, Krusche A.V, Coburn R, Victoria R.L, Richey J.E, Logsdon M.G, Mayorga E, Matricardi E. A remote sensing/GIS-based physical template to understand the biogeochemistry of the Ji-Parana river basin (Western Amazonia) Remote Sens. Environ. 2003;87:429–445. [Google Scholar]
- Baumgartner A, Reichel E. Elsevier Science Publishers; Amsterdam, The Netherlands: 1975. The world water balance: mean annual global, continental, and maritime precipitation, evaporation, and runoff. [Google Scholar]
- Bilge F, Yazici B, Dogeroglu T, Ayday C. Statistical evaluation of remotely sensed data for water quality monitoring. Int. J. Remote Sens. 2003;24:5317–5326. [Google Scholar]
- BirdLife International. BirdLife International; Cambridge, UK: 2004. State of the world's birds 2004: indicators for our changing world. [Google Scholar]
- Butchart S.H.M, Stattersfield A.J, Bennun L.A, Akçakaya H.R, Baillie J.E.M, Stuart S.N, Hilton-Taylor C, Mace G.M. Using Red List Indices to measure progress towards the 2010 target and beyond. Phil. Trans. R. Soc. B. 2005;360 doi: 10.1098/rstb.2004.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I.C. Biological monitoring and assessment using invertebrates. In: Burden F.R, McKelvie I, Föstner U, Guenther A, editors. Environmental monitoring handbook. McGraw-Hill; New York: 2002. pp. 5.0–5.1. [Google Scholar]
- Chessman B.C. Rapid assessment of rivers using macroinvertebrates: a procedure based on habitat specific sampling, family level identification and a biotic index. Aust. J. Ecol. 1995;20:122–129. [Google Scholar]
- Chutter F.M. An empirical biotic index of the quality of water in South African streams and rivers. Water Res. 1972;6:19–30. [Google Scholar]
- CIESIN (Center For International Earth Science Information Network), International Food Policy Research Institute and World Resources Institute . CIESIN/Columbia University; Palisades, NY: 2000. Gridded Population of the World (GPW), Version 2. [Google Scholar]
- Costa M.P.F. Use of SAR satellites for mapping zonation of vegetation communities in the Amazon floodplain. Int. J. Remote Sens. 2004;25:1817–1835. [Google Scholar]
- Dahl T.E. US Department of the Interior, Fish and Wildlife Service; Washington: 2000. Status and trends of wetlands in the conterminous United States 1986 to 1997. [Google Scholar]
- Dahl T.E, Johnson C.E. US Department of the Interior, Fish and Wildlife Service; Washington, DC: 1991. Status and trends of wetlands in the conterminous United States, Mid-1970s to Mid-1980s. [Google Scholar]
- Dallas H.F, Day J.A. Water Research Commission; South Africa: 1993. The effect of water quality variables on riverine ecosystems: a review. Technical Report Series No. TT 61/93. [Google Scholar]
- Darwall, W. & Revenga, C. In press. Wetlands. In The World's Protected Areas (ed. M. Spalding, M. Jenkins & S. Chape). California University Press.
- Darwall, W., Smith. K., Lowe, T. & Vié, J.-C. In press. The Status and Distribution of Freshwater Biodiversity in Eastern Africa Gland, Switzerland and Cambridge, UK: IUCN.
- Davies P.E. Development of a national river bioassessment system (AUSRIVAS) in Australia. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of fresh waters: RIVPACS and other techniques. Freshwater Biological Association; Cumbria, UK: 2000. [Google Scholar]
- Davies S.P, Tsomides L. Maine Department of Environmental Protection; Augusta, ME: 1997. Methods for biological sampling and analysis of Maine's inland waters. DEPLW-107-A97. [Google Scholar]
- Day J.A, Stewart B.A, de Moor F.J, Louw A.E. Guides to the freshwater invertebrates of Southern Africa. Crustacea I. Water Research Commission, Pretoria. In: Dahl T.E, editor. Stats and trends of wetlands in the conterminous United States 1986–1997. US Department of the Interior, Fish and Wildlife Service; Washington, DC: 2000. [Google Scholar]
- Delany S.N, Reyes C, Hubert E, Pihl S, Rees E, Haanstra L, van Strien A. Wetlands International Publication 54. Wetlands International; Wageningen, The Netherlands: 1999. Results from the International Waterbird Census in the western Palearctic and Southwest Asia, 1995 and 1996. [Google Scholar]
- Döll P, Kaspar F, Lehner B. A global hydrological model for deriving water availability indicators: model tuning and validation. J. Hydrol. 2003;270:105–134. [Google Scholar]
- Ellison A.M. Wetlands of Central America. Wetlands Ecol. Mngmt. 2004;12:3–55. [Google Scholar]
- European Union 2000. The EU Water Framework Directive – Integrated River Basin Management in Europe. Official Journal of the European Community (OJL 327).
- Fekete, B.M., Vörösmarty, C.J. & Grabs, W. 2000 UNH/GRDC Composite Runoff Fields, Version 1.0. Durham, NH: Complex Systems Research Center, University of New Hampshire; Koblenz, Germany: Global Runoff Data Center (GRDC). See http://www.grdc.sr.unh.edu/
- Finlayson C.M, Davidson N.C. Global review of wetland resources and priorities for wetland inventory: summary report. In: Finlayson C.M, Spiers A.G, editors. Global review of wetland resources and priorities for wetland inventory. 2nd edn. Environmental Research Institute of the Supervising Scientist; Supervising Scientist Report 144. Canberra, Australia: 1999. pp. 1–14. [Google Scholar]
- Finlayson C.M, Spiers A.G, editors. Global review of wetland resources and priorities for wetland inventory. Supervising Scientist Report 144. 2nd edn. Environmental Research Institute of the Supervising Scientist; Canberra, Australia: 1999. [Google Scholar]
- Finlayson C.M, Davidson N.C, Stevenson N.J. Report from Workshop 4, 2nd International Conference on Wetlands and Development, 8–14 November, 1998, Dakar, Senegal. Supervising Scientist Report 161, Darwin, Australia: Supervising Scientist. Wetlands International; Wageningen, The Netherlands: 2001. Wetland inventory, assessment and monitoring: practical techniques and identification of major issues. [Google Scholar]
- Fraser A.S, Meybeck M, Ongley E.D. UNEP Environment Library No. 14. UNEP; Nairobi: 1995. Global environment monitoring system (GEMS) water quality of world river basins. [Google Scholar]
- Glasgow H.B, Burkholder J.M, Reed R.E, Lewitus A.J, Kleinman J.E. Real-time remote monitoring of water quality: a review of current applications, and advancements in sensor, telemetry, and computing technologies. J. Exp. Mar. Biol. Ecol. 2004;300:409–448. [Google Scholar]
- Gleick P.H. Part II: freshwater data. In: Gleick P.H, editor. Water in crisis: a guide to the world's fresh water resources. Oxford University Press; New York: 1993. [Google Scholar]
- Groombridge B, Jenkins M. WCMC-World Conservation Press; Cambridge, UK: 1998. Freshwater biodiversity: a preliminary global assessment. [Google Scholar]
- Groombridge B, Jenkins M.D. WCMC-World Conservation Press; Cambridge, UK: 2000. Global biodiversity: Earth's living resources in the 21st century. [Google Scholar]
- Harding J.S, Benfield E.F, Bolstad P.V, Helfman G.S, Jones E.B.D., III Stream biodiversity: the ghost of land use past. Proc. Natl Acad. Sci. USA. 1998;95:14 843–14 847. doi: 10.1073/pnas.95.25.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison I.J, Stiassny M.J. The quiet crisis: a preliminary listing of the freshwater fishes of the world that are extinct or ‘Missing in Action’. In: MacPhee R.D.E, editor. Extinctions in near time: causes, contexts, and consequences. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 271–332. [Google Scholar]
- Hering D, Moog O, Sandin L, Verdonschot P.F.M. Overview and application of the AQEM assessment system. Hydrobiologia. 2004;516:1–20. [Google Scholar]
- Hess L.L, Melack J.M, Novo E.M.L, Barbosa C.C.F, Gastil M. Dual-season mapping of wetland inundation and vegetation for the central Amazon basin. Remote Sens. Environ. 2003;87:404–428. [Google Scholar]
- ILEC (International Lake Environment Committee). 2002 See http://www.ilec.or.jp/database/database.html
- International Wader Study Group. Waders are declining worldwide. Conclusions from the 2003 International Wader study group Conference, Cadiz, Spain. Wader Stud. Group Bull. 2003;101:8–12. [Google Scholar]
- IUCN. The IUCN Species Survival Commission; Gland, Switzerland: 2003. 2003 IUCN Red List of threatened species.http://www.redlist.org [Google Scholar]
- IUCN. IUCN; Gland, Switzerland: 2004. 2004 IUCN Red List of threatened species.http://www.redlist.org [Google Scholar]
- IUCN Species Survival Commission, Conservation International Center for Applied Biodiversity Science and NatureServe 2004 IUCN Global Amphibian Assessment. See http://www.globalamphibians.org
- IUCN/SSC Tortoise and Freshwater Turtle Specialist Group. IUCN/SSC tortoise and freshwater turtle specialist group. IUCN; Gland, Switzerland: 1991. Tortoises and freshwater turtles: an action plan for their conservation. [Google Scholar]
- Jorgensen, S.E., de Bernardi, R. Ballatore, T. & Muhandiki, V. 2001 Lake Watch 25: changes in the world's lakes. Draft report. Kusatsu, Japan: International Lake Environment Committee.
- Karr J.R. Defining and measuring river health. Freshwat. Biol. 1999;41:221–234. [Google Scholar]
- Kasischke E.S, Smith K.B, Bourgeau-Chavez L.L, Romanowicz Edwin A, Brunzell S, Richardson C.J. Effects of seasonal hydrologic patterns in south Florida wetlands on radar backscatter measured from ERS-2 SAR imagery. Remote Sens. Environ. 2003;88:423–441. [Google Scholar]
- King J.M, Louw D. Instream flow assessments for regulated rivers in South Africa using the building block methodology. Aquat. Ecosyst. Health Mngmt. 1998;1:109–124. [Google Scholar]
- King, J. M., Tharme, R.E. & de Villiers, M. 2000 Environmental flow assessments for rivers: manual for the building block methodology. Technology Transfer Report TT131/100. Pretoria, South Africa: Water Research Commission.
- King J.M, Brown C.A, Sabet H. A scenario-based holistic approach for environmental flow assessments. Rivers Res. Appl. 2003;19:619–639. [Google Scholar]
- Kira T. Survey of the state of world lakes. In: Jørgensen S.E, Matsui S, editors. The world lakes in crisis: guidelines of lake management. Vol. 8. International Lake Environment Committee and United Nations Environment Programme; Kusatsu, Japan: 1997. pp. 147–155. [Google Scholar]
- Kolkwitz R, Marsson M. Ökologie der pflanzlichen Saprobien. Ber. Deutsch Botan. Ges. 1908;26a:505–519. [Google Scholar]
- Kolkwitz R, Marsson M. Ökologie der tierischen Saprobien. Int. Rev. Hydrobiol. 1909;2:126–152. [Google Scholar]
- Kurata A. International Lake Environment Committee and United Nations Environment Programme (UNEP); Kusatsu, Japan and Nairobi, Kenya: 1994. Data book of world lake environments: a survey of the state of world lakes. (Five volumes.) [Google Scholar]
- Ladson A.R, White L.J, Doolan J.A, Finlayson B.L, Hart B.T, Lake P.S, Tilleard J.W. Development and testing of an index of stream condition for waterway management in Australia. Freshw. Biol. 1999;41:453. [Google Scholar]
- Lehner B, Döll P. Development and validation of a global database of lakes, reservoirs and wetlands. J. Hydrol. 2004;296:1–22. [Google Scholar]
- Loh et al. In preparation. The living planet Report 2004.
- Lundberg J.G, Kottelat M, Smith G.R, Stiassny M.L.J, Gill A.C. So many fishes, so little time: an overview of recent ichthyological discovery in continental waters. Ann. Mo. Bot. Gard. 2000;87:26–62. [Google Scholar]
- Malmqvist B, Rundle S. Threats to the running water ecosystems of the world. Environ. Conserv. 2002;29:134–153. [Google Scholar]
- Master L.L, Flack S.R, Stein B.A, editors. Rivers of life: critical watersheds for protecting freshwater biodiversity. The Nature Conservancy; Arlington, VA, USA: 1998. [Google Scholar]
- Matthews E, Fung I. Methane emissions from natural wetlands: global distribution, area, and environmental characteristics of sources. Global Biogeochem. Cycles. 1987;1:61–86. [Google Scholar]
- McAllister D.E, Hamilton A.L, Harvey B. Global freshwater biodiversity: striving for the integrity of freshwater ecosystems. Sea Wind Bull. Ocean Voice Int. 1997;11:1–140. [Google Scholar]
- Miller R.R, Williams J.D, Williams J.E. Extinctions of North American fishes during the past century. Fisheries. 1989;14:22–38. [Google Scholar]
- MRC 2003 State of the Basin report. Phnom Penh, Mekong: River Commission.
- Myers N. The rich diversity of biodiversity issues. In: Reaka-Kudla M.L, Wilson D.E, Wilson E.O, editors. Biodiversity II: understanding and protecting our biological resources. Joseph Henry Press; Washington, DC: 1997. pp. 125–138. [Google Scholar]
- National Water Council. NWC; London: 1981. River quality: the 1980 survey and future outlook. [Google Scholar]
- Platts W.S, et al. 1987. Methods for evaluating riparian habitats with applications to management. US Department of Agriculture, Forest Service, General Technical Report INT-221. Ogden, UT: USDA. [Google Scholar]
- Reaka-Kudla M.L. The global biodiversity of coral reefs: a comparison with rain forests. In: Reaka-Kudla M.L, Wilson D.E, Wilson E.O, editors. Biodiversity II: understanding and protecting our biological resources. Joseph Henry Press; Washington, DC: 1997. pp. 83–108. [Google Scholar]
- Red List Consortium. 2004 Red List Indices Gland, Switzerland and Cambridge, UK: IUCN and SSC, BirdLife International, CI and CABS and NatureServe.
- Revenga C, Kura Y. Technical Series no. 11. Secretariat of the Convention on Biological Diversity; Montreal, Canada: 2003. Status and trends of biodiversity of inland water ecosystems. [Google Scholar]
- Revenga C, Brunner J, Henninger N, Kassem K, Payne R. World Resources Institute; Washington, DC: 2000. Pilot analysis of global ecosystems: freshwater systems. [Google Scholar]
- Ricciardi A, Rasmussen J.B. Extinction rates of North American freshwater fauna. Conserv. Biol. 1999;13:1220–1222. [Google Scholar]
- RHP 2003 State-of-rivers report: free state province region river systems Pretoria, South Africa: Department of Water Affairs and Forestry.
- Rolauffs P, Stubauer I, Zahrádkhová S, Brabec K, Moog O. Integration of the saprobic system into the European Water Framework Directive. Hydrobiologia. 2004;516:285–298. [Google Scholar]
- Rosenberg D.M, Resh V.H. Use of aquatic insects in biomonitoring. In: Merritt R.W, Cummins K.W, editors. An introduction to the aquatic insects of North America. 3rd edn. Kendall/Hunt Publishing Company; Dubuque, IA, USA: 1996. pp. 87–97. [Google Scholar]
- Sawaya K.E, Olmanson L.G, Heinert N.J, Brezonik P.L, Bauer M.E. Extending satellite remote sensing to local scales: land and water resource monitoring using high-resolution imagery. Remote Sens. Environ. 2003;88:144–156. [Google Scholar]
- Scott D.A, Rose P.M. Wetlands International Publication No. 41. Wetlands International; Wageningen, The Netherlands: 1996. Atlas of Anatidae populations in Africa and western Eurasia. [Google Scholar]
- Shiklomanov I.A. World Meteorological Organization and Stockholm Environment Institute; Stockholm, Sweden: 1997. Comprehensive assessment of the freshwater resources of the world: assessment of water resource and water availability in the world. [Google Scholar]
- Shuman C.S, Ambrose R.F. A comparison of remote sensing and ground-based methods for monitoring wetland restoration success. Restor. Ecol. 2003;11:325–333. [Google Scholar]
- Sládecek V. System of water quality from the biological point of view. Arch. Hydrobiol. Beih. Ergebnisse Limnol. 1973;7:1–218. [Google Scholar]
- Smakhtin V, Revenga C, Döll P. Comprehensive assessment of water management in Agriculture. Research Report No. 2. International Water Management Institute; Colombo, Sri Lanka: 2004. Taking into account environmental water requirements in global-scale water resources assessments. [Google Scholar]
- Spiers A.G. Global review of wetland resources and priorities for wetland inventory. In: Finlayson C.M, Spiers A.G, editors. Global review of wetland resources and priorities for wetland inventory, Supervising Scientist Report 144. Environmental Research Institute of the Supervising Scientist; Canberra, Australia: 1999. pp. 63–104. [Google Scholar]
- The Heinz Center. Environmental Research Institute of the Supervising Scientist; Canberra, Australia: 2002. Global review of wetland resources and priorities for wetland inventory, Supervising Scientist Report 144. [Google Scholar]
- UNEP/DEWA 2001. The Mesopotamian marshlands: demise of an ecosystem. Early warning and assessment. (46 pages.) Technical Report No. TR.01-3. Nairobi, Kenya: UNEP.
- van Dijk, P. P., Stuart, B. L. & Rhodin, A. G. J. 2000 Asian turtle trade: proceedings of a workshop on conservation and trade of freshwater turtles and tortoises in Asia. In Chelonian research monographs No. 2. Lunenburg, MA: Chelonian Research Foundation in association with WCS, TRAFFIC, WWF, Kadoorie Farm and Botanic Gardens and the US Fish and Wildlife Service.
- Verma R, Singh S.P, Raj K.G. Assessment of changes in water-hyacinth coverage of water bodies in northern part of Bangalore city using temporal remote sensing data. Curr. Sci. 2003;84:795–804. [Google Scholar]
- Vis C, Hudon C, Carignan R. An evaluation of approaches used to determine the distribution and biomass of emergent and submerged aquatic macrophytes over large spatial scales. Aquat. Bot. 2003;77:187–201. [Google Scholar]
- Wetlands International. Wetlands International Global Series No. 12. 3rd edn. Wetlands International; Wageningen, The Netherlands: 2002. Waterbird population estimates. [Google Scholar]
- Wetlands International. Wetlands International; Wageningen, The Netherlands: 2004. Global peatlands initiative.http://www.wetlands.org/projects/GPI/peatland.htm. [Google Scholar]
- Whited D, Stanford J.A, Kimball J.S. Application of airborne multispectral digital imagery to quantify riverine habitats at different base flows. River Res. Appl. 2002;18:583–594. [Google Scholar]
- Williams D.J, Rybicki N.B, Lombana A.V, O'Brien T.M, Gomez R.B. Preliminary investigation of submerged aquatic vegetation mapping using hyperspectral remote sensing. Environ. Monit. Assess. 2003;81:383–392. doi: 10.1023/a:1021318217654. [DOI] [PubMed] [Google Scholar]
- WRI. WRI; Washington, DC: 1995. Africa data sampler user's guide; a georeferenced data base for all African countries. [Google Scholar]
- WRI in collaboration with United Nations Development Programme, United Nations Environment Programme and the World Bank . World Resources Institute; Washington, DC: 2000. World Resources 2000–2001: people and ecosystems: the fraying web of life. [Google Scholar]
- Wright J.F. An introduction to RIVPACS. In: Wright J.F, Sutcliffe D.W, Furse M.T, editors. Assessing the biological quality of fresh waters: RIVPACS and other techniques. Freshwater Biological Association; Cumbria, UK: 2000. pp. 1–24. [Google Scholar]
- Yu X, Jiang N. Analyzing lake area change in Ebinur by integration of RS and GIS techniques. Hupo Kexue. 2003;15:81–84. [Google Scholar]