Abstract
The global pledge to deliver ‘a significant reduction in the current rate of biodiversity loss by 2010’ is echoed in a number of regional and national level targets. There is broad consensus, however, that in the absence of conservation action, biodiversity will continue to be lost at a rate unprecedented in the recent era. Remarkably, we lack a basic system to measure progress towards these targets and, in particular, we lack standard measures of biodiversity and procedures to construct and assess summary statistics. Here, we develop a simple classification of biodiversity indicators to assist their development and clarify purpose. We use European birds, as example taxa, to show how robust indicators can be constructed and how they can be interpreted. We have developed statistical methods to calculate supranational, multi-species indices using population data from national annual breeding bird surveys in Europe. Skilled volunteers using standardized field methods undertake data collection where methods and survey designs differ slightly across countries. Survey plots tend to be widely distributed at a national level, covering many bird species and habitats with reasonable representation. National species' indices are calculated using log-linear regression, which allows for plot turnover. Supranational species' indices are constructed by combining the national species' indices weighted by national population sizes of each species. Supranational, multi-species indicators are calculated by averaging the resulting indices. We show that common farmland birds in Europe have declined steeply over the last two decades, whereas woodland birds have not. Evidence elsewhere shows that the main driver of farmland bird declines is increased agricultural intensification. We argue that the farmland bird indicator is a useful surrogate for trends in other elements of biodiversity in this habitat.
Keywords: indicators, biodiversity targets, European birds, population trends, summary statistics, policy relevance
1. Introduction
Government representatives at the 2002 World Summit of Sustainable Development pledged ‘a significant reduction in the current rate of biodiversity loss by 2010’ and similar commitments have been made at regional and national levels. There is broad consensus, however, that in the absence of conservation action, biodiversity will continue to be lost at a rate unprecedented in the recent era, and yet we lack basic systems to measure progress towards these objectives (Balmford et al. 2003; Jenkins et al. 2003; Royal Society 2003; Green et al. 2005). Furthermore, we lack agreement on those elements of biodiversity of greatest relevance in relation to the targets and hence on the specific set of measures required. Numerous studies have documented biodiversity loss in ecosystems across the globe; the size of these losses, measured as habitat area lost or degraded, or population decline, is considerable (e.g. May et al. 1995; Pimm et al. 1995; Jenkins et al. 2003).
Of course, biodiversity is a multifaceted term, defined as the sum total of all biotic variation from the level of genes to ecosystems (Purvis & Hector 2000). As such, it can be measured in various ways and no single metric is likely to adequately describe biodiversity as a whole. The gauntlet thrown down to ecologists by the global and regional targets is to develop summary statistics that accurately and robustly describe trends in components of biodiversity in such a way as to communicate this information to a policy audience. The information available on biodiversity, however, is often patchy and biased in its coverage of species, habitats and regions, and synthesis is rare (Balmford et al. 2003; Jenkins et al. 2003; Royal Society 2003). Taxonomic bias strongly colours our view of biodiversity and all the indicators we describe suffer in this respect. The challenge in the medium term is to combine in a representative way population trends and other information for multiple taxa from a range of sites, habitats and biomes.
High-level summaries tend to focus on threatened taxa (e.g. IUCN 2002), or population trends gleaned from the literature (e.g. Loh 2002). The former is undoubtedly a useful approach in describing a key element of biodiversity loss, particularly in well-studied taxa, but because it overlooks other more common species, it is not necessarily a good measure of the general state of nature and how it is changing. By definition, many species are considered threatened because their population is declining. Any indicator based on trends of these species will properly capture species loss in this group, but may not capture other changes in species composition. Trends in threatened species might be different from other species for a variety of reasons; for example, they live in particular places, differ systematically in their ecology (Kunin & Gaston 1997), or are subject to special beneficial conservation measures (Aebischer et al. 2000b). Information on threat status often accrues slowly, typically over a number of years, and so status can only be updated at intervals. The rate of population change must also be relatively large to trigger the IUCN criteria. Average population declines of 3.5% per annum over 10 years qualify species for listing as Vulnerable and 14.9% per annum for Critical, yet a species falling by 3.4% per annum will still have halved in number over 20 years, but would go unnoted in this system. Change in threat status can also be associated with artefacts such as increased knowledge, increased sampling efforts or changes in taxonomy, or a combination of these factors, rather than genuine population change (Possingham et al. 2002). Some have suggested that while extinction rate provides an important measure of human impacts over the long term, it is an inherently poor measure of contemporary biodiversity loss (McKinney & Lockwood 1999; Possingham et al. 2002; Royal Society 2003; Jenkins 2003; Balmford et al. 2003).
The other main method of generating summary statistics is to use population trends and here too there are a number of problems. Compared with threat status, population trends can be updated more frequently and thus have a higher temporal resolution, but they too can suffer from bias owing to non-random selection of species and localities. This is especially the case when trends are extracted from the literature (e.g. Houlahan et al. 2000, 2001; Alford et al. 2001; Loh 2002), because the underlying data might come from published studies with inherent bias towards, for example, well-studied localities or strongly positive or negative trends, or towards threatened species. Missing values also complicate analysis of such time-series data.
An alternative approach is to extract population trends from existing wide-scale monitoring schemes in order to be able to control and reduce any selection bias. A good example of this approach at a national scale is the wildlife indicator in the UK, which is based on population trends of common breeding birds and is taken to represent the state of the countryside. This indicator has been adopted by Government as 1 of 15 headline indicators of the sustainability of lifestyles in the UK (Anon. 2002). The indicator shows that on average common birds have increased by 10%, while common woodland and common farmland birds have fallen by 15 and 42%, respectively, from 1970 to 2002 (figure 1; Gregory 2004b). Healthy wildlife populations are seen as a useful barometer of sustainable land use policies and of the general quality of life (Anon. 2002). The Government has adopted a Public Service Agreement to ‘care for our living heritage and preserve natural diversity by reversing the long-term decline in the number of farmland birds by 2020, as measured annually against underlying trends’ (Anon. 2002; Gregory et al. 2004b). With this target is a detailed delivery plan that defines how the target is measured and how it will be achieved. The adoption of the indicator has provided a significant impetus and focus for research on farmland and woodland birds. At the same time, the indicator has played a central role in wholesale change in land use policy in the UK, particularly in a shift to agricultural production that is coupled with the needs of maintaining and restoring biodiversity (Vickery et al. 2004). The introduction of an Environmental Stewardship Scheme in England in 2005 will see large numbers of farmers rewarded financially for implementing a range of management prescriptions designed to enhance biodiversity interest, including priority birds. Similar agri-environment schemes are being deployed in the other countries of the UK. The UK wild bird index is a good example of an indicator that has turned science into policy.
Figure 1.
The UK wild bird indicator is based on the population trends of wild breeding birds. This indicator, adopted by the UK Government, is 1 of 15 headline indicators of the sustainability of lifestyles in the UK.
The decline of once common taxa associated with lowland farmland has become one of the most pressing issues in British nature conservation (Krebs et al. 1999; Aebischer et al. 2000a; Vickery et al. 2004). There is compelling evidence to show that the recent declines among farmland birds in north and west Europe have been driven by changes in agricultural methods and specialization (Tucker & Heath 1994; Krebs et al. 1999; Aebischer et al. 2000a; Chamberlain et al. 2000; Pitkänen & Tiainen 2001; Donald et al. 2001; Hole et al. 2002; Vickery et al. 2004). The nature of evidence linking farmland bird trends with increased agricultural modernization and intensification is of two kinds. Autoecological studies have shown how and why individual species have responded negatively, or occasionally positively, to agricultural change. Broader-scale analyses and modelling have tested the hypothesis of agricultural change driving the decline of farmland birds and examined the probable mechanisms. The level of knowledge of the interaction between farmland management and biodiversity is exceptional (Aebischer et al. 2000a; Vickery et al. 2004). The most important changes affecting birds have been hedgerow loss, land drainage, increased mechanization, increased fertilizer and pesticide use, reduction of spring cultivation, simplification of crop rotations, changes in crop use and loss of farm diversity (Krebs et al. 1999; Aebischer et al. 2000a; Donald et al. 2001; Robinson & Sutherland 2002; Vickery et al. 2004). Agricultural practices during the nesting season are known to have adverse effects on the breeding performance of corn bunting Miliaria calandra (Brickle et al. 2000), grey partridge Perdix perdix (Potts 1986), stone curlew Burhinus oedicnemus (Aebischer et al. 2000b), lapwing Vanellus vanellus (Shrubb 1990), and corncrake Crex crex (Green & Stowe 1993). Survival, as opposed to productivity, is implicated as a key factor in the population declines of seed-eating birds, such as cirl bunting Emberiza cirlus (Evans & Smith 1994), reed bunting Emberiza schoeniclus (Peach et al. 1999), house sparrow Passer domesticus and goldfinch Carduelis carduelis (Siriwardena et al. 1999; Hole et al. 2002).
The decline of lowland farmland birds in the UK was striking both in the sheer scale of changes (many birds have more than halved in numbers over the last 30 years: Gregory et al. 2004b), but also in the similarity of pattern across species (Fuller et al. 1995; Siriwardena et al. 1998; Fewster et al. 2000). One consequence of severe population declines is that many widespread and still relatively abundant birds have become priorities for conservation action in the UK (Gregory et al. 2002). The official Red List of birds of highest conservation concern in the UK contains 16 out of 40 species with current populations in excess of 10 000 pairs (Gregory et al. 2002). These tend to be farmland birds, but also include woodland birds whose populations are now much depleted. Of course, the choice of conservation priorities is at some point based on value judgements and is part of a wider socio-political debate about the sort of environment people wish to live in and the relative value of biodiversity. In the UK, at least, there is public pressure on decision-makers to improve the quality of the countryside around them. This has been translated, for example, into the UK Biodiversity Action Plan (DETR 2001), which responds specifically to the severe decline of once common species. Furthermore, even if severely declining common species were to be dismissed as conservation priorities (which would seem to be a mistake), we would argue that trends in their populations are relevant in measuring the sustainable use of resources, which is a central pillar of the Convention on Biological Diversity (CBD). Plainly, the choice of conservation priorities will differ in different situations to reflect the threats, opportunities, legal frameworks and resources available.
Against this backcloth, in this paper we develop an indicator to describe the composite population trends of European birds, building on previous work (Hustings 1988, 1992; Gibbons 2000; van Strien et al. 2001, 2004; Gregory et al. 2003). Our aim was to measure the mean population change within a set of species, measuring biodiversity as the number of individuals in a species population and determining the rate of change and how this rate itself was changing. In this way, the indicator describes changes in species composition within a chosen habitat. We focused on changes in the abundance of widespread and populous species through time, taking birds as our example.
The paper is structured as follows. First, we define what we mean by an indicator and consider the ideal properties of an indicator of biodiversity. Next, we introduce a framework to help define the purpose of different kinds of indicators. Based on these principles, we have developed an indicator based on the breeding populations of common European birds. We go on to discuss how this indicator can be interpreted, to what extent it is fit for the purpose and finally discuss the development of indicators for biodiversity more broadly.
2. Developing indicators for biodiversity
(a) Defining the ideal indicator
At the outset, it is helpful to define what we mean by an ideal indicator in this context. This is a group of species whose population trends, when taken together, reflect the average behaviour of the constituent species, but also cast light on trends in attributes of other taxa and act as a surrogate for ecosystem health (see Caro & O'Doherty 1999). Indicators are meant to quantify and communicate complex phenomena in a simple manner (Bibby 1999). These surrogate measures are frequently used as a proxy for ecosystem function and health, because of the complexity, cost and difficulty of measuring these processes directly (Hilty & Merenlender 2000). The purpose of indicators is to help decision-makers formulate policy and then to continue to review it in response to changes in the indicator. To some degree, indicators might be seen as a bridge between science and policy. In the classification of Caro & O'Doherty (1999) such an indicator is termed a health or population indicator, as opposed to a biodiversity indicator, which is used typically to identify areas of high species richness across taxa. These trend indicators tend to measure aspects of ‘state’ in the pressure-state-response (PSR) and driver-pressure-state-impact-response (DPSIR) models (OECD 1993; EEA 1997).
For indicators to be effective at a general level they must meet a number of competing scientific and practical criteria (Landres et al. 1988; Bibby 1999; Caro & O'Doherty 1999; ten Brink 2003; Gregory et al. 2003; SBSTTA 2003). These include qualities such as scientific credibility, sensitivity to environmental change, links to drivers, clarity of message, affordability, ease of update and so forth (table 1).
Table 1.
Key attributes of effective indicators of biodiversity.
| attribute | details |
|---|---|
| representative | includes all species in a chosen taxon, or a representative group. |
| immediate | capable of regular update, ideally, at least on an annual basis. |
| simplifying information | transparent, easy to interpret and visually attractive. Complex information must be presented simply to have impact and communicate. |
| easily understood | non-experts, from policy makers to members of the public, must be able to grasp the issues to have any ownership of them. |
| quantitative | accurate measurement with assessment of error. Shows trends over time, measures a rate of change and changes in the rate. |
| responsive to change | sensitive to environmental change over relatively short time-scales. |
| timeliness | allows rapid identification of trends—an early warning of issues. |
| susceptible to analysis | data can be disaggregated to help understand the underlying patterns and shed light on the potential causes of trends. |
| realistic to collect | quantitative data are available or can be collected readily. Does not require excessive or unrealistic financial resources. |
| indicative | representing more general components or attributes of biodiversity than just the constituent species trends, ideally reflecting ecosystem health. |
| user driven | developed in response to the need of stakeholders. |
| policy relevant | indicators aim to provide signals to policy customers to help them develop and then review policy measures. |
| stability | buffered from irregular, large natural fluctuations. |
| tractable | susceptible to human influence and change. |
To develop indicators of species and populations further, we need statistical procedures to construct and judge the resulting indicators, comparable, in some ways, to those for the more familiar economic statistics. From a statistical viewpoint, the question is how to get timely and unbiased information in a cost-effective manner. This is not only a matter of applying an appropriate sampling design and statistical method, but also a matter of defining the purpose of the indicator at the start.
(b) Purpose, fitness and practicality
Having described the qualities of an ideal indicator, next we must flesh out the specific purpose, or purposes, of an indicator, whether the indicator is fit to deliver that purpose, and consider the practicality of production (table 2).
Table 2.
Assessing the soundness of indicators.
| 1. purpose | what does the indicator aim to indicate? |
| is the aim described clearly? | |
| is the aim to indicate changes in specific taxa only? Or is the aim to indicate change in biodiversity more generally, thus beyond its constituent parts? | |
| is the aim to show how taxa or biodiversity responds to a particular environmental driver? | |
| what spatial scale is the indicator designed for? Is the aim to indicate changes at a national scale, a regional scale or something else? Is the aim to indicate changes per ecosystem and which ecosystems are distinguished? | |
| is the indicator intended to respond rapidly to environmental change? If not immediately, then on what time-scale? | |
| who are the key stakeholders, policy- and decision-makers? | |
| is the driver susceptible to human influence through policy or other measures? | |
| can the indicator be disaggregated to shed light on the underlying ecological processes? | |
| 2. fit for purpose | do sampling design and statistical method correspond to the purpose? |
| are the methods of species and sample site selection sound? Do they ensure representation of species groups, habitats or geographical areas? If not, can this be adjusted to reduce bias? | |
| is the statistical analysis sound? Have missing values been taken into account? Are confidence limits around the indicator available? Failing this, can the sensitivity be measured in other ways? | |
| if the purpose is to show changes in biodiversity more generally, how can this be substantiated? | |
| if the purpose is to link changes with drivers, what is the evidence for this link? Is a positive change in the indicator associated with an improving or deteriorating situation for species/habitats in the environment? Could other environmental factors explain the behaviour of the indicator? | |
| 3. practicality | can the indicator be constructed and updated easily? |
| does the indicator use existing data and expertise, or require new data collection and expertise? | |
| is the indicator available immediately? If not, on what time-scale can it be produced? | |
| can the indicator be updated frequently, e.g. annually, or less frequently? | |
| what level of resource is required to produce the indicator? Is it cost-effective? Is further investment required and justified? |
In considering purpose, it is helpful to articulate the specific aims and limitations of an indicator—for example, whether trends in the indicator are thought to mirror trends in other biodiversity components or not and whether these trends are linked to known or suspected drivers. It is also useful to define the spatial and temporal scales over which the indicator is expected to react to environmental change and to identify, at least in principle, whether such drivers are susceptible to human influence through policy. The speed of response to environmental change will have important ramifications for the utility of the indicator; inertia in a system will inevitably delay any potential response. Under fitness, practitioners need to ask themselves a series of questions about sampling design and statistical treatment of data, as well as about inferences, in order to check that their ambitions for an indicator can be realized. A detailed ecological knowledge of the species and systems in question is extremely helpful at this stage in judging fitness for use and reasonable inference. It is important to stress that indicators must be capable of disaggregation (to species' groups, species and sample sites) in order to better understand the underlying ecological processes and to explore the connections between an indicator and potential drivers (both natural and man-induced). Indicators are not a substitute for detailed knowledge, which is essential in assessing the causes of change and in formulating strategies or plans in response to such changes (Bibby 1999).
Finally, in addition to theoretical considerations the feasibility of indicator production requires attention. Preferably, indicators need to be developed and updated relatively easily without considerable new investment in analysis or data collection. It makes sense to use the best available information, unless such data do not exist, or are seriously flawed. A feedback loop may be necessary to balance purpose, fitness for purpose and practicality. For example, weaknesses in sampling design might lead you to revise down your expectations of the generality of an indicator and the reasonable inferences that could be drawn from it. Practical issues might lead to the conclusion that the current datasets are simply inadequate for the stated purposes, or at least require strong health warnings on their inference. Equally, practicality might limit the speed with which indicators can be created and updated to such a degree that this limits their relevance to policy makers.
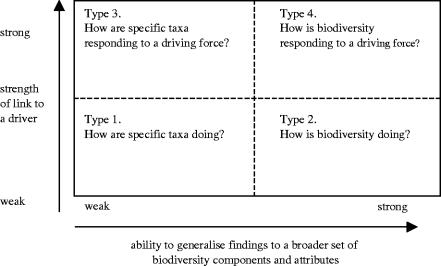
(c) A framework for indicators
In an attempt to clarify the use of indicators linked to biodiversity trends, we have developed a simple framework (figure 2). We distinguish four broad types of indicators, based on two axes: ability to generalize findings to a broader set of biodiversity components and attributes, and strength of relationship with a potential driver in the environment (figure 2). Type 1 indicators are designed to measure how specific taxa are faring; Type 2 consider how biodiversity is doing more generally; Type 3 show how specific taxa are responding to drivers; and Type 4 how biodiversity is responding to a driver or drivers in general. In reality, many indicators are Type 1 or Type 3 indicators because they are likely to have a quite limited or specific scope. This is not a criticism, but it does limit their broader applicability and emphasizes the need for clarity of purpose at the beginning and for realism in judging indicators.
Figure 2.
A classification of indicators for biodiversity based on our ability to generalize their findings to a broader set of biodiversity components and attributes, and potential links to natural or man-induced drivers.
By default, poor indicators will be created when measurements suffer from selection bias of species or sites measured and when inferior statistical methods have been applied. Further problems arise when inferences are made about an indicator that go beyond what may be reasonably drawn from the information available. Our aim, of course, is to avoid poor indicators and to ensure indicators are interpreted appropriately. Thus, consideration should be given to sample design and statistical analysis, in order to produce unbiased estimates and to use statistical methods that take into account missing values, estimation of precision and removal of bias (see Olsen et al. 1999). Estimation of precision helps in assessing whether the indicator is sufficiently sensitive to detect change.
The procedures required to select sites as well as species (design) and to aggregate species information and make causality plausible (analysis), differ between the four indicator types. Type 1 indicators are chosen where the purpose is to assess sets of species of specific interest, such as endemic species, or amphibians, or the species listed in a piece of legislation. The choice of species is defined. The aim is neither to link changes directly to causes, nor to generalize the changes beyond the set of species. Type 2 indicators are deigned to tell us about the general state and changes of biodiversity (e.g. the Living Planet Index—Loh 2002). The purpose is not to link these indicators with specific environmental factors. However, the criterion to select species from all groups in a representative way is problematic and there is often considerable variety of trends between species and species groups (e.g. Thomas et al. 2004). Thus, species selection is a critical issue for Type 2 indicators and there is a strong risk of selection bias. This is not a matter of finding the taxa that best indicate ecosystem health (as discussed by Hilty & Merenlender 2000), but finding the set of species from different species groups that together may produce an unbiased estimate of biodiversity change. As described above, however, the information available is frequently biased in its coverage of species, habitats and biomes.
Type 3 indicators are used to show how particular environmental pressures, such as air pollution, climatic change or agricultural intensification, might drive changes in a group of species. An example is the decline of birds owing to fragmentation (Foppen 2001). There is no aim to generalize the changes beyond the species included. Species are selected that are known or expected to be sensitive to these factors based on published evidence. Sites are selected in order to detect the link with the key driver. The indicators in category 4 resemble those in category 3, but aim to have a wider reach and direct linkage to potential drivers, again based on the best evidence. Species and site selection criteria should take into account the ability to generalize findings to all taxa under the same pressure and to make a link with the proposed driver. In this way, Type 3, and especially Type 2 and Type 4 indicators are more ambitious in their aims than Type 1 and require extra attention to design and analysis.
3. Indicators for European birds
(a) Aims
The bird indicators which we now examine in detail come from the Pan-European Common Bird Monitoring scheme (PECBM), which has been developed through a consortium of individuals and organizations from many countries, cooperating through the European Bird Census Council (EBCC) to measure mean population change in breeding bird populations. The overall project goal was to explore the use of bird population trends as indicators of biodiversity in Europe and to develop indices capable of measuring the 2010 targets. The specific aim was an assessment of the mean change in breeding bird populations of European farmland and woodland (including woods, parks and gardens). The two habitats were chosen because agricultural land and grassland make up roughly 50%, and boreal and temperate forest 30% of the land surface of Europe (Tucker & Evans 1997), so these represent the predominant land types in Europe. Note that the vast majority, if not all, of these habitats are heavily man-modified. Our aim was to create an index that could be updated annually and thus provide feedback to policy makers on a reasonable time-scale. The work we describe on common birds forms one part of a three-pronged approach to delivering indicators for sustainability in Europe based on birds, also incorporating monitoring of important sites and threatened species.
(b) Methods: sample design and data
The number of European countries with annual breeding bird surveys based on nationwide samples has increased from 3–7 in 1980–89 to 10–18 in 1990–2000 (figure 3). In the first phase of the project in 2003, 18 countries supplied trend information (European Union (EU) countries: Austria, Belgium, Denmark, France, Germany, Ireland, Italy, Netherlands, Spain, Sweden and UK; EU Accession countries (i.e. the group of eastern European countries that joined the EU in May 2004): Estonia, Latvia, Poland, Czech Republic and Hungary; others: Norway and Switzerland). The data were collected using a variety of field methods (spot/territory mapping method, line or point transects, each with between 1 and 12 visits to each site per year; see Bibby et al. 2000; Gregory et al. 2004a). These sample surveys record all bird species encountered, but by their very nature, they are unlikely to cover very rare species and so the trends represent the commoner and more widespread birds in the environment.
Figure 3.

Number of countries (open symbols) and number of schemes (filled symbols) engaged in national common bird monitoring programmes in Europe.
(c) Methods: species selection
Expert ornithologists selected 24 native bird species characteristic of woodland, parks and gardens and 24 typical of agricultural habitats in Europe (table 3). The birds selected had large European ranges and were abundant enough to be monitored accurately in the majority of countries by common bird monitoring schemes, were well monitored by standard field methods and were considered to some degree dependent on the habitat for nesting or feeding. The majority of these species are resident in Europe, but several are long-distance migrants wintering in Africa (table 3). Note that for a small number of farmland birds, population indices could not be computed for some countries and some groupings because of the sparseness of data. The result is that the European and EU trends exclude the quail, Coturnix coturnix, which is highly volatile in numbers and has an erratic migrant breeding population, and the indicators for the Accession countries also exclude little owl, Athene noctua, and hobby, Falco subbuteo, which are comparatively rare in these countries. The small number of species included in the specialist groups makes strict interpretation of their trends difficult, but nonetheless, they help to shed light on potential drivers of trends.
Table 3.
Species selected for analysis: (a) agricultural birds and (b) woodland, park and garden birds. (Species were classified as specialists of these habitats according to the EBCC European breeding bird Atlas (Hagemeijer and Blair 1997), Birds in Europe (Tucker & Heath 1994) and national coordinators' assessments of the proportion of each species' national population breeding in a given habitat type. ‘Non-specialists’ are either generalists or specialists of other habitat types. Migration strategy was coded simply as long-distance migrant, or short-distance migrant/resident in Europe following Cramp (1977–1994). The first group was defined as a species migrating from Europe to Africa. The second group was defined as migrant birds that chiefly winter within Europe, but also included resident, sedentary and eruptive species.)
| species name | common name | specialist | migration strategy |
|---|---|---|---|
| (a) agricultural birds | |||
| Alauda arvensis | skylark | specialist | short-distance/resident |
| Athene noctua | little owl | non-specialist | short-distance/resident |
| Carduelis cannabina | linnet | specialist | short-distance/resident |
| Carduelis carduelis | goldfinch | specialist | short-distance/resident |
| Carduelis chloris | greenfinch | non-specialist | short-distance/resident |
| Columba palumbus | woodpigeon | non-specialist | short-distance/resident |
| Corvus corone | carrion/hooded crow | non-specialist | short-distance/resident |
| Corvus monedula | jackdaw | non-specialist | short-distance/resident |
| Coturnix coturnix | quail | specialist | long-distance |
| Emberiza citrinella | yellowhammer | specialist | short-distance/resident |
| Emberiza schoeniclus | reed bunting | non-specialist | short-distance/resident |
| Falco subbuteo | hobby | non-specialist | long-distance |
| Falco tinnunculus | kestrel | non-specialist | short-distance/resident |
| Hirundo rustica | swallow | non-specialist | long-distance |
| Lanius collurio | red-backed shrike | specialist | long-distance |
| Miliaria calandra | corn bunting | specialist | short-distance/resident |
| Motacilla flava | yellow wagtail | non-specialist | short-distance/resident |
| Passer montanus | tree sparrow | specialist | short-distance/resident |
| Pica pica | magpie | non-specialist | short-distance/resident |
| Saxicola rubetra | stonechat | specialist | long-distance |
| Streptopelia turtur | turtle dove | specialist | long-distance |
| Sturnus vulgaris | starling | specialist | short-distance/resident |
| Sylvia communis | whitethroat | specialist | long-distance |
| Vanellus vanellus | lapwing | specialist | short-distance/resident |
| (b) woodland, park and garden birds | |||
| Accipiter nisus | sparrowhawk | non-specialist | short-distance/resident |
| Aegithalos caudatus | long-tailed tit | specialist | short-distance/resident |
| Anthus trivialis | tree pipit | specialist | long-distance |
| Buteo buteo | buzzard | non-specialist | short-distance/resident |
| Dendrocopos major | great-spotted woodpecker | non-specialist | short-distance/resident |
| Erithacus rubecula | robin | non-specialist | short-distance/resident |
| Fringilla coelebs | chaffinch | non-specialist | short-distance/resident |
| Garrulus glandarius | jay | specialist | short-distance/resident |
| Jynx torquilla | wryneck | non-specialist | long-distance |
| Muscicapa striata | spotted flycatcher | specialist | long-distance |
| Parus ater | coal tit | specialist | short-distance/resident |
| Parus caeruleus | blue tit | non-specialist | short-distance/resident |
| Parus major | great tit | non-specialist | short-distance/resident |
| Phoenicurus phoenicurus | redstart | specialist | long-distance |
| Phylloscopus collybita | chiffchaff | specialist | long-distance |
| Phylloscopus trochilus | willow warbler | non-specialist | long-distance |
| Prunella modularis | dunnock | specialist | short-distance/resident |
| Regulus regulus | goldcrest | specialist | short-distance/resident |
| Sylvia atricapilla | blackcap | specialist | short-distance/resident |
| Sylvia borin | garden warbler | non-specialist | long-distance |
| Troglodytes troglodytes | wren | specialist | short-distance/resident |
| Turdus merula | blackbird | non-specialist | short-distance/resident |
| Turdus philomelos | song thrush | specialist | short-distance/resident |
| Turdus viscivorus | mistle thrush | specialist | short-distance/resident |
(d) Methods: site selection
For practical reasons, we selected all sites from national count schemes, rather than sites in woodland or farmland only. We assume that the great majority of the data for the species selected came from farmland and woodland, respectively, because the bulk of their populations breed within these preferred and extensive habitats. The alternative of calculating habitat-specific trends for these species, while attractive theoretically, was simply impractical because this would be beyond the capability of some national schemes at present. The calculation of habitat-specific trends necessitates the extraction of bird counts by habitat, and in some cases, basic habitat information was not collected, or was unavailable from other sources. While countries routinely calculate species-by-species indices, they rarely produce habitat-specific trends. We judged that this extra step would have been time-consuming and potentially off-putting for contributors. Work in the UK has shown that bird indicators based on all sample plots and those based on specific habitats (i.e. farmland plots for farmland birds) are almost identical in pattern and trend for both farmland and woodland birds (Newson et al. 2004).
(e) Methods: population estimates
Information on species-specific national population sizes was obtained for a particular year from the European Bird Database (Tucker & Heath 1994; BirdLife International/European Bird Census Council 2000). It is difficult to judge the accuracy of the population estimates and this is likely to vary from country to country; however, the general level of knowledge of European birds suggests that these are probably among the best estimates of their kind, a suggestion supported by theoretical studies (Gregory 2000).
(f) Methods: data analysis
A European index was produced for each species by combining national results for that species. Difficulties, such as gaps in data, both at site and country level, were taken into account using a standard indexing programme. The individual European species indices were combined (averaged) to create multi-species supranational indicators. Details of the method are outlined below.
(i) National level
The indices for each species were produced for each country, using TRIM (TRends and Indices for Monitoring data—Pannekoek & van Strien 2001). TRIM is a programme to analyse time-series of counts with missing observations using Poisson regression (log-linear models; McCullagh & Nelder 1989). The basic model with effects for each site and year is
with α i the effect for site i and γ j the effect for year j on the log of expected counts μ ij.
Missing counts of particular sites were estimated (‘imputed’) from changes in all other sites, or sites with the same characteristics by using covariates. In addition, serial correlation was taken into account. The programme produced imputed yearly indices and scheme totals for each species. These yearly scheme totals, together with their standard errors and covariances were collated by the PECBM scheme.
(ii) Supranational level
Since our aim was to generate European trends, the difference in national population size of each species in each country needed to be taken into account. This weighting allowed for the fact that different countries hold different proportions of a species' European population (van Strien et al. 2001). This means a change in a larger national population has greater impact on the overall trend than a change in a smaller population. The alternative, of weighting national population trends equally, makes little sense in this context because changes in small, insignificant populations could dominate and obscure the genuine European trend. Therefore, the yearly scheme totals were first converted into yearly national population sizes. A weighting factor was calculated as the national population size for a particular year divided by the estimated yearly scheme total for that year. This weighting factor was applied to all years of the scheme in order to obtain yearly national population sizes for each year. If the weight is treated as a known constant, estimates of the variances of these weighted year totals can be obtained by multiplying the variances of the estimated unweighted year totals by the square of the weight.
The next step was to combine the yearly totals from each country. Combining total numbers across countries is straightforward in cases where we restricted the analysis to the period for which data were available for all countries; we simply summed the estimated totals for each country. Since the estimates of the year totals are independent between countries, the variance of each combined total is the sum of the variances of the corresponding country totals. However, missing year totals for many countries, owing to differences in the length of the time-series, made the combination of year totals more complicated. The missing year totals were estimated by TRIM in a way equivalent to imputing missing counts for particular sites within countries (van Strien et al. 2001). Missing year totals of particular country sites were thus estimated from other countries of the same European region, assuming that all countries within the same region have had similar changes in population numbers. Four regions were identified for this purpose alone: Central and East (Estonia, Latvia, Poland, Hungary, Czech Republic and former East Germany); North (Norway, Sweden and Denmark); South (France, Spain and Italy); and West (Ireland, UK, Belgium, Netherlands, former West Germany, Switzerland and Austria).
The computed indices and confidence intervals are in fact extremely similar to those that would have been calculated had we received the raw data (van Strien et al. 2001). After estimating the year totals for the European regions, these regions were then combined to generate European indices for each species. Countries were also combined to assess separate EU indices and indices for the group of EU Accession countries.
(iii) Multi-species level
We averaged indices rather than abundances in order to give each species an equal weight in the resulting indicators. When positive and negative changes of indices are in balance, then we would expect their mean to remain stable. If more species decline than increase, the mean should go down and vice versa. Thus, the index mean is considered a measure of biodiversity change. We used geometric means rather than arithmetic means because we consider an index change from 100 to 200 equivalent, but opposite, to a decrease from 100 to 50.
We combined indices for species to produce multi-species indicators for European regions and Europe. Standard errors for geometric means were computed from the indices and standard errors of individual species (Appendix).
(g) Results
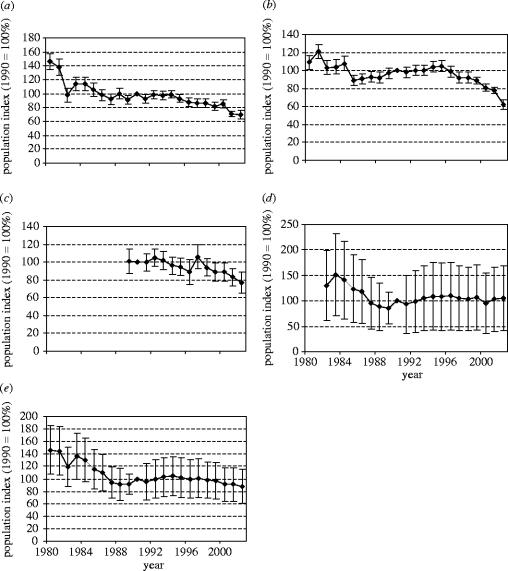
The procedures we describe allowed us to construct population indices with standard errors for individual species at national, then regional, and finally Pan-European levels. This is illustrated for the skylark, Alauda arvensis, at regional and Pan-European levels (figure 4). Having constructed European indices for species, these were then grouped into composite trends for the two habitats of interest, farmland and woodland, parks and gardens. We are able to summarize these data for the four regions we used in constructing the indices (figure 5) and for Europe as a whole (figure 6).
Figure 4.
An example of Pan-European indices (±1.96 s.e.) for the skylark, Alauda arvensis, for (a) Western Europe, (b) Northern Europe, (c) Southern Europe, (d) Central and Eastern Europe and (e) all Europe. See text for definitions of these areas. The index for the base year (1990) is set at 100.
Figure 5.
Pan-European common bird indicators for (a) farmland and (b) woodland, park and garden birds for the four regions used to construct indictors.
Figure 6.
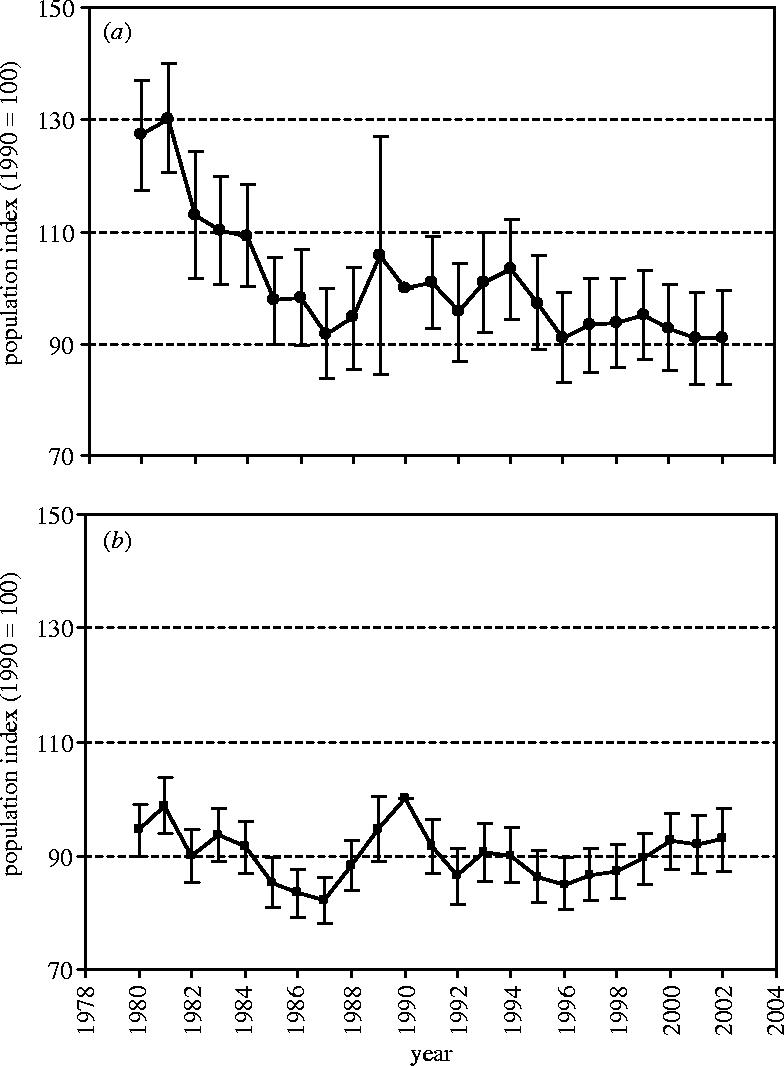
Supranational, multi-species indicators of European bird populations 1980–2002. (a) European farmland birds (countries=18, species=23, ±1.96 s.e.) and (b) European woodland, park and garden birds (countries=18, species=24, ±1.96 s.e.). The index for the base year (1990) is set to 100.
Confidence limits on the trends are wider for farmland birds compared with woodland birds because the latter data were more sparse (typically the counts for woodland birds were larger and contained fewer zeros). Large standard errors in 1989 came about because the German monitoring scheme entered at this stage and data from former Eastern Germany were very sparse in the first year of the scheme.
On average, populations of common birds of woods, parks and gardens in Europe have remained relatively stable over the last 20 years, whereas common farmland birds have declined sharply, especially in the 1980s (table 4; figure 6). This difference is maintained if we focus on birds that were judged specialists of woodland and farmland in Europe, although farmland specialists have declined more precipitously than farmland birds generally (table 4). The decline in farmland birds in Europe is associated with increased agricultural intensification: the European farmland bird index correlates negatively with an index of total cereal production for the constituent countries (analysis across years: r 23=−0.57, p=0.005; data from FAOSTAT http://apps.fao.org/; see Donald et al. 2001).
Table 4.
Long- and short-term population trends in the Pan-European wild bird indicators for farmland and woodland, park and garden birds. (Trends in parenthesis represent specialist species. The trend is the difference in the index from the starting value, either 1980 or 1990, to the final value in 2002, expressed as a percentage change.)
| habitat grouping (n=number of species) | country grouping (n=number of countries) | 22-year trend (1980–2002; %) | 12-year trend (1990–2002; %) |
|---|---|---|---|
| farmland indicator: n=23 (specialists n=12) | Europe: n=18 | −29 (−42) | −9 (−9) |
| European Union: n=11 | −32 (−47) | −13 (−21) | |
| Accession: n=5 | −23 (−9) | +3 (+12) | |
| woodland, parks & garden indicator: n=24 (specialists n=13) | Europe: n=18 | −2 (−7) | −7 (−8) |
| European Union: n=11 | +2 (−10) | −8 (−12) | |
| Accession: n=5 | +34 (+79) | −2 (+14) |
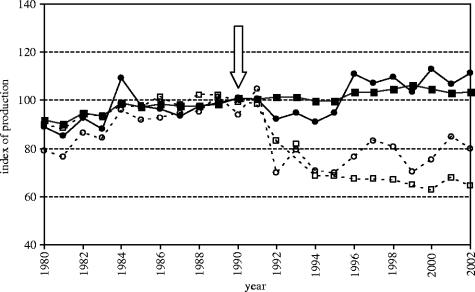
There is a contrast between farmland bird trends in EU and EU Accession countries (table 4; figure 7). While the long-term trends were universally downwards for farmland birds, the more recent short-term trends were positive for Accession countries; and they were more positive for the specialists on farmland (table 4; figure 7). Farmland populations of Accession countries began to show signs of recovery around 1990, coincident with the break-up of the former Eastern Bloc and an associated reduction in agricultural intensity (figure 8). There has been no similar recovery of farmland birds in the EU, where intensification has continued (figure 8). Agricultural production, measured as total overall or total cereal production, correlated negatively with the farmland index for the EU countries (r 23=−0.82, p<0.0001 and r 23=−0.54, p=0.008, respectively). Neither statistic was correlated significantly with the farmland index for the Accession countries (r 23=−0.05, p=0.83 and r 23=−0.32, p=0.13, respectively). If we repeat the analysis pre- and post-1990, however, significant negative correlations emerge (pre-1990: r 11=−0.87, p=0.0001 and r 11=−0.85, p=0.001, respectively; post-1990: r 12=−0.82, p=0.001 and r 12=−0.55, p=0.07, respectively).
Figure 7.
Supranational, multi-species indicators of EU farmland bird populations 1980–2002. (a) Common farmland birds. EU (solid line, countries=11, species=23) and Accession countries (dashed line, countries=5, species=21), (b) specialist farmland birds. EU (solid line, countries=11, species=12) and Accession countries (dashed line, countries=5, species=12). The arrow indicates the break-up of the Eastern Bloc in 1990.
Figure 8.
Indices of agricultural production in EU (solid lines, n=11) and Accession countries (dashed lines, n=5) within this study. Squares represent total agricultural production and circles total cereal production fixed to an average value of 100 in 1981–89 (data come from FAOSTAT, Food and Agricultural Organisation of the United Nations http://apps.fao.org/). The arrow again indicates the break-up of the Eastern Bloc in 1990.
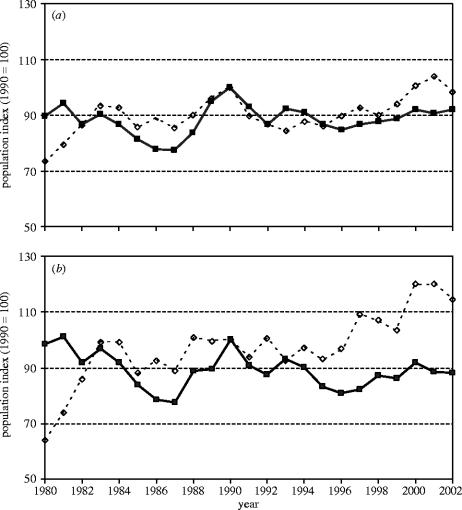
Population trends among birds of wooded habitats showed little variation across country groupings (figure 5). There was again a difference between EU countries with relatively flat, or even slightly downwards long-term trends among specialist birds (table 4; figure 9), and strong positive trends in EU Accession countries. We can only speculate as to why the trends should be more positive among Accession countries; some of the recent trends may be linked to reduced agricultural production too, which has allowed invasion of scrub and trees on what was formerly agricultural land. While such changes will continue to favour woodland birds, the beneficial effects on farmland birds are likely to be short-lived and we would predict a downturn in their populations as succession proceeds and habitat suitability declines.
Figure 9.
Supranational, multi-species indicators of European bird populations of woodland, parks and gardens 1980–2002. (a) Common woodland birds. EU (solid line, countries=11, species=24) and Accession countries (dashed line, countries=5, species=21), (b) specialist woodland birds. EU (solid line, countries=11, species=13) and Accession countries (dashed line, countries=5, species=13).
4. Discussion
(a) Indicators
The concept of indicator species has been a matter of debate in ecology (Landres et al. 1988; Simberloff 1998; Caro & O'Doherty 1999; Hilty & Merenlender 2000). The basic question of whether an individual species, or group of species, can indicate anything about species and environmental health more broadly remains contentious; in some cases this appears to be true (e.g. Gregory et al. 2003; Gregory et al. 2004b), in others, not. Confusion is added because the term ‘indicator’ can have a range of different and specific meanings, such as health or population indicators, bio-indicators, surrogate, keystone, umbrella and flagship species (Furness & Greenwood 1993; Caro & O'Doherty 1999). In a review of indicator taxa, Hilty & Merenlender (2000) concluded that ambiguous and sometimes conflicting selection criteria often brought the utility of the indicators into question. They were critical of the selection of invertebrate and vertebrate taxa. In the latter case, they suggested that a lack of knowledge of tolerance levels of species and of correlations with ecosystem changes were key issues. In addition, they criticized the choice of low density, high mobility generalist species, where species choice was sometimes driven by external agendas. Clearly, species selection lies at the heart of improving the utility of indicators and they suggested a framework to aid this process. In a similar vein, Caro & O'Doherty (1999) suggested some of the desirable ecological characteristics of different kinds of indicator.
A further technical problem facing composite trend indicators is the presence of missing counts in time-series. Debate over the extent and timing of global amphibian declines offers an insight into potential problems (Houlahan et al. 2000, 2001; Alford et al. 2001). Two sets of researchers agreed on the spatial and temporal variability of sampling effort and of trends among amphibians, but came to different conclusions about the timing of declines, even though they were analysing the same data. The difference revolved around the estimation of missing values; Houlahan et al. (2000, 2001) chose not to estimate, whereas Alford et al. (2001) incorporated estimation. Whatever the merits of this case, it is clear that trend analysis must pay careful attention to missing counts when using this kind of unbalanced information, because failure to do so can lead to bias.
Despite misgivings, however, the political imperative to generate summary information in a cost-effective and practical manner has repeatedly brought ecologists and land managers back to consider and develop indicators (Bibby 1999; Caro & O'Doherty 1999; Hilty & Merenlender 2000; Loh 2002; Gregory et al. 2003). What has been missing to date is a recipe book to help practitioners create robust indicators for biodiversity. Progress has been made in defining the purpose of different indicators in conservation biology and in species selection (Caro & O'Doherty 1999; Hilty & Merenlender 2000), but more work is needed in the construction of a new generation of multi-species indicators, as exemplified by the UK and European wild bird indices, and the Living Planet Index (Loh 2002; Gregory et al. 2003; Gregory et al. 2004b).
As Bibby (1999) pointed out, ‘never previously has there been such an opportunity for biologists to inform and advise environmental decision-makers’; he went on to suggest that ‘ornithologists have a special contribution to make because of the extent and quality of the information (on birds) available to us’. In the next section, we consider the central proposition that birds can act as indicators of other taxa and of environmental health.
(b) Birds as indicators
Birds are often, but not always, regarded as good general indicators of the broad state of wildlife and of the countryside, for both scientific and practical reasons (Furness & Greenwood 1993). Some of the advantages of using birds are as follows. Birds are relatively easy to detect, identify and census. Their taxonomy is well resolved and the general level of our understanding of their population biology and behaviour is extremely high. Birds are wide-ranging in habitat distribution, moderately abundant, are of moderate body size and have moderate life spans. These characteristics result in population responses to environmental change at moderate spatial and temporal scales. Birds tend to be at, or near, the top of the food chain and are thus responsive to signals that accumulate through the chain (the most obvious examples being persistent pollutants). There are often good historical and contemporary data on bird population changes and these data are realistic and relatively inexpensive to collect. In some situations, at least, birds can reflect changes in other biodiversity and are responsive to environmental change. In addition, and importantly, birds can have considerable resonance and symbolic value with many audiences, from the public to decision-makers, in a way that other taxa do not.
That said, some of the same, or different, characteristics of birds make them less suitable as indicators. For example, migratory habits and their wide-ranging nature make it difficult to link their populations with specific drivers on the ground. Moderate body size means birds are slower to respond numerically to change compared with smaller-bodied taxa and they do so at a larger spatial scale. A low degree of specialism in some species makes any link to specific environmental conditions and drivers much more difficult to discern. There are also situations in which positive population trends in birds can be associated with environmental degradation (e.g. the euthrophication of wetlands and the response of some waterbirds—van Impe 1985; over-fishing of large predatory fishes and the response of some seabirds—Furness 1982; the increase of non-native populations—Hughes et al. 1999).
It is instructive to assess birds against the criteria for health and population indicators suggested by Caro & O'Doherty (1999). Strict comparison is difficult because the attributes are described in broad terms, but birds seem to score well on four of the five classes of attribute considered. They compare worst in relation to life-history traits where Caro and O'Doherty (1999) advocate small body size, short generation times and high metabolic rates, so that environmental change can be detected rapidly. As described above, this means bird populations will respond to change on a moderate time-scale, but this in itself does not appear to preclude their use as indicators.
In short, there are numerous difficulties in using birds (in fact, any individual taxon) as indicators of wider components or attributes of biodiversity, but we would argue that robust indicators could be constructed when sufficient attention is paid to their design. In the next section, we look in some depth at the provisional European indicators for breeding birds.
(c) Strengths and weaknesses of the European wild bird indicator
(i) Projects goals
Our aim was to develop an annually updated assessment of the mean change in farmland and woodland breeding bird populations in Europe. We have been successful in creating summary measurements for a large group of species and countries, although there is room for improvement (see below). Encouragingly, the precision of the European indices per species and of the multi-species indicators is sufficient to be able to detect substantial and biologically significant trends. In the following discussion, we examine the suitability of the indicator by looking at species selection, data and analysis. We then consider the degree to which the farmland and woodland bird indicators might be representative of trends in other elements of biodiversity and assess potential links to environmental drivers.
(ii) Species selection
Having identified species selection as one of the critical steps in indicator production, we need to revisit and review our selection criteria. Species selection was based on expert judgement guided by additional information, which in theory could have led to bias if, for example, species whose populations were known, or suspected, to be declining had been selected preferentially. We do not believe that this occurred, but we will move to a formal species selection procedure in the future. We are currently considering a more objective classification of species to habitats published by Tucker & Evans (1997). The species chosen varied considerably in the nature of their association with the given habitats—some were considered habitat specialists, others not (table 3). It would be highly desirable to have a quantitative measure of the degree to which birds specialize in particular habitats and to know more about the nature of the association. Habitat specialists are frequently promoted as indicator taxa and yet defining such a trait in a quantitative manner remains a challenge and there is a risk of circularity and subjectivity in this process. We have also included a small number of migratory species within our list and it may be worth considering whether their presence strongly influences the trends and whether they should be excluded in the future. We intend keeping species selection under careful review.
(iii) Data and analysis
Although national count schemes for common birds differed in field methods and in other respects, these differences should not have influenced the supranational results because the standardized indices were combined (van Strien et al. 2001). We cannot, however, exclude the possibility that bias within national schemes, owing to non-representative selection of sites, might introduce bias into the European trends. It is true to say that only the most recent count schemes have adopted a formal stratified random sample (e.g. Gregory & Baillie 1998) and that earlier schemes were mostly based on volunteer-selected sites (Vorisek & Marchant 2003). We would argue, however, that this does not result in strong directional bias, although we will work to reduce site selection bias in the future.
With respect to analysis, we treated missing counts using a statistical procedure that has been developed for this specific purpose. Missing counts for particular sites were estimated from other sites within the same country, or even better, from other sites with similar characteristics within the same country. We have thus addressed one of the key difficulties in analysing this kind of time-series data. In addition, missing yearly indices for particular countries were estimated from other countries within the same region. The assumption is that changes in other sites and countries were representative for the sites and countries that were not measured. We believe this assumption is appropriate, although the assumption of true representativeness in 1980–1989, when only seven country schemes were in operation (figure 3), may be questioned. This latter assumption, however, is confirmed by the independent information on population trend estimates in Europe (BirdLife International/European Bird Census Council 2000). Specifically, species trends among the group of seven countries do appear to closely mirror those in the wider group of countries under consideration.
(iv) Linking the farmland bird index with other biodiversity trends and drivers
Our analysis revealed a consistent pattern of decline in common farmland bird populations across Europe, reinforcing the results of earlier work (table 4; Tucker & Heath 1994; Donald et al. 2001). There is extensive evidence elsewhere showing a link between farmland bird declines and agricultural intensification in Europe (Tucker & Heath 1994; Krebs et al. 1999; Aebischer et al. 2000a; Chamberlain et al. 2000; Donald et al. 2001; Pitkänen & Tiainen 2001; Hole et al. 2002; Vickery et al. 2004). The conclusion that changes in farming practice have driven bird declines is supported by the reversal in the trends of farmland birds when intensification of farming was reversed with the post-1990 collapse of the state farming system in the former Eastern Bloc (table 4; figures 7 and 8). Of course, such correlations do not prove causation, but the reversal of the farmland bird index in the former Eastern Bloc is at least suggestive of a cause and consistent with previous research. There are alternative explanations for the decline of farmland birds, such as climate change or increased predation, but there is little evidence to substantiate these hypotheses. In the case of farmland birds, land use changes can be traced back to national agricultural policy, which in turn is shaped strongly by the Common Agricultural Policy within the EU.
A critical question for the wild bird index is the degree to which trends in bird populations mirror those in other taxa. A large body of work has examined the degree to which different taxa co-occur in space. This is often, but not always the case, depending on the ecological similarity of the taxa compared and the scale of analysis (Balmford 2002). Very few studies, however, have examined the degree of correspondence in the trends of different taxa through time or space. Studies in Europe have shown that many vertebrate, insect and plant species of farmland have declined in parallel, whereas only a few species have increased, and these changes are thought to be driven by agricultural intensification and specialization (Wilson 1992; Donald 1998; Southerton & Self 2000; Pitkänen & Tiainen 2001; Benton et al. 2002; Robinson & Sutherland 2002). The nature of the information available, which is often incomplete in coverage of taxa, is set out in table 5. A consistent pattern emerges of concomitant change in different taxa and declines are widespread. There is also a perceived shift in species composition from specialist to generalist or pest species (Southerton & Self 2000; Robinson & Sutherland 2002). This pattern is echoed in our findings where specialist farmland birds have declined more strongly than the group as a whole (table 4). Thomas et al. (2004) showed that national range declines of birds and butterflies in the UK were correlated, but that declines among the butterflies were larger, suggesting that birds may be a conservative estimator of declines in other taxa, although the authors concede that there is little additional evidence to substantiate this view. van Strien et al. (2004) have also shown parallel declines of butterflies and birds in grassland in The Netherlands.
Table 5.
The nature of evidence showing concomitant declines of farmland biodiversity in the UK and in continental Europe.
| authors | type of study | taxa | nature of evidence |
|---|---|---|---|
| Donald (1998) | review | invertebrates & plants | significant change in populations with a preponderance of declines. Plant diversity, abundance and seed bank have declined. Trends in invertebrate populations have been stable, or have declined |
| Southerton & Self (2000) | review | plants & arthropods | increase and decline of species associated with farmland. Many arable plants have become rare, but some attained pest status. Many arthropods have declined |
| Benton et al. (2002) | correlation | arthopods & birds | Temporal links between the declines of farmland bird and invertebrate populations and changes in agricultural practices |
| Robinson & Sutherland (2002) | review | plants, invertebrates, vertebrates (reptiles, birds & mammals) | widespread decline in the populations of many groups of organisms associated with farmland. Marked loss of specialized taxa in favour of generalist species |
| Pitkänen & Tiainen (2001) | review of comprehensive monitoring | plants, birds, butterflies, bees & dung beetles | widespread declines in many taxa, loss of diversity and some extinction |
| Thomas et al. (2004) | comparison of changes in national geographical ranges | plants, birds & butterflies | 28% of plants, 54% of birds and 71% of butterflies had declined in range size |
| van Strien et al. (2004) | comparison of species trends in grassland | birds & butterflies | parallel declines of butterflies and birds |
Surveys of birds and butterflies on prairie grasslands in the United States have shown that the abundance of specialist butterflies was positively correlated with that of songbirds, although correlations with grassland and generalist butterflies were not as good (Swengel & Swengel 1999). The authors concluded that, within a habitat and region, conservation programmes benefiting grassland birds can favour prairie specialist butterflies and that certain birds and butterflies can be effective indicators of each other. They also cite a number of studies showing that grassland conservation has benefited many species simultaneously, including birds and insects.
The best information available to us, which is admittedly lacking in its coverage of taxa and is non-experimental in nature, supports the view that bird population trends on lowland farmland are correlated positively with trends in other taxa in the UK and probably in continental Europe. We recognize, however, that the nature of evidence is weak (based largely on correlation) and recommend further work to explore the temporal and spatial correspondence of across-taxa trends in different systems. Our tentative conclusion, however, is that the farmland bird indicator is probably a useful surrogate for broad changes in biodiversity in the wider environment of Europe, but that these trends need to be interpreted with some caution. Returning to the indicator framework described above (figure 2), the farmland indicator is arguably a Type 4 indicator, having relevance to trends in other farmland biodiversity and a clear link to an anthropogenic driver. If, instead, one dismissed the evidence available because it is based on correlation, this would downgrade the indicator to a Type 2 or even Type 1 indicator.
(v) Linking the woodland bird index with other biodiversity trends and drivers
The woodland bird trend has shown relative stability over the last 20 years and little difference in the trends of specialist and non-specialist species (table 4; figures 6 and 9). The indicator appears to faithfully describe the trends in this group of common birds and many national monitoring schemes have reported similar findings (BirdLife International/European Bird Census Council 2000; Donald et al. personal communication); note that the situation in the UK is unusual in revealing moderate declines among some common woodland birds (Anon. 2002; Gregory et al. 2003).
We were not able to find a link with a driver in this indicator, nor were we able to find evidence to show that the woodland indicator, as constituted, was a useful surrogate for other taxa in woodland. Few studies have compared trends among different taxa in woodland. Interestingly, van Strien et al. (2004) have shown that woodland trends among birds, butterflies and fungi differed in The Netherlands. It is important to note that the group of birds live in a range of wooded habitats from forest, woodland, copse and hedgerows to parks and gardens; and some species are relatively catholic in habitat choice (table 3). This indicator was not designed as an indicator of forest health because the species were not selected with that purpose in mind. Evidence elsewhere shows that some specialists of forest, particularly birds associated with old growth European forests, have declined severely and are threatened by modern commercial forestry practices (Virkkala 1991; Tucker & Heath 1994; Kouki & Vaananen 2000).
Returning to the indicator framework (figure 2), the evidence available to us suggests the woodland bird indicator is a Type 1 indicator, with specific, but limited scope. The woodland indicator might be upgraded to a Type 2 or Type 4 indicator by altering species selection, by combining the trends with those from other taxa, or by connecting it more clearly with a driver. It would be sensible to try to combine this indicator, for example, with the information collected in the EU Forest Focus Program and other similar programmes.
(vi) Using and developing the wild bird indicators
The indicators we have developed for European birds provide a potentially powerful tool to enable scientific information to be communicated to policy makers. The divergence in the fortunes of farmland bird populations within EU and Accession countries illustrates the rapid impact political decisions can have on bird populations and hence the relevance to policy of current biodiversity measurements that are capable of update. The European wild bird indicators are already being used widely to inform debate on biodiversity targets and sustainability in Europe. They have been used in: the EU's 2003 Environment Policy Review and environment related indicators pamphlet; in Eurostat's Yearbook 2004; in the European Environment Agency's (EEA) Signals 2004 report and High Nature Value Farmland 2004 report; in EEA's core biodiversity indicator set; and in the IRENA indicator set (Indicator Reporting on the integration of ENvironmental concerns into Agricultural policy). Indeed, the farmland bird index has been adopted as a Structural Indicator of the European Union to represent trends in biodiversity. The wild bird index is by far the most advanced summary statistic of its kind available in Europe. The speed with which the indicator has been used reflects a policy need and a desire to incorporate biodiversity trends in environmental monitoring, prompted to a degree by the EU target to halt biodiversity loss by 2010.
There are important caveats attached to the wild bird indicator. For example, it covers only two broad habitat types in Europe and the species chosen were classified to habitats in a simplistic fashion. There are also temporal gaps in the data, particularly early in the time-series, potential bias within national schemes and significant spatial gaps in the south and east of Europe. This indicator covers only one component of bird diversity (i.e. trends in the common and widespread species) and only one small element of biodiversity. We would argue, however, that we have been able to produce an indicator for an important element of biodiversity that is capable of meaningful measurement against the 2010 target in Europe. As such, this is a small but significant step towards truly representative indicators for biodiversity. In the future, we plan to improve the indicator by increasing the number of countries contributing data, increasing the number of species for which we collect data, formalizing species selection and species grouping, streamlining data entry and analysis, and exploring the potential links between the bird trends and drivers.
(d) Indicators of biodiversity trends
The pledge made by politicians at the 2002 World Summit of Sustainable Development was to achieve ‘a significant reduction in the current rate of biodiversity loss by 2010’. The task for scientists therefore is to develop effective indicators of biodiversity trends so that progress towards the target can be measured (Balmford et al. 2003, 2005; Jenkins et al. 2003; Royal Society 2003; Green et al. 2005). Indicators are central to the CBD framework to evaluate progress towards the 2010 biodiversity target. Specifically, ‘trends in abundance and distribution of selected species’ was identified as an indicator for immediate testing. Indicators are also central in European deliberations to assess their stated biodiversity goals and to measure sustainability of land use. There is an urgent need for scientists to engage fully in the debate to develop indicators and get smarter at using them to describe the state of biodiversity.
Composite population trend indicators, as we have described in this paper, provide a tangible basis for measuring progress towards the biodiversity targets at global and regional scales, and complement information on habitats and other aspects of populations (Balmford et al. 2003, 2005; Royal Society 2003). Such indicators might provide a template for other continents, taxa and biomes, and for other aspects of global biodiversity. The potential strength of this approach is its statistical robustness, its relative simplicity, its efficient use of existing data, its sensitivity to environmental change, its ability to communicate and its ease of update, which is often practical on an annual basis.
We are fortunate in having such extensive, high quality and long-running data for birds in Europe, but many of the principles of trend analysis and indicator construction apply to other types of population data for animals and plants. We have been able to mobilize and access large amounts of data collected in many countries by cooperating within a network of national organizations. Rather than asking for raw datasets, which may be difficult to obtain and politically contentious, we asked national contacts to provide processed national results, computed using standard software, and we are able to combine the national results as if we had the raw data at our disposal. By using existing data, the indicators are cost-effective and the cost-effectiveness is even higher if the taxa in question are viewed as surrogates for other taxa. The model of data collation, analysis and cooperation we describe might be usefully mimicked in other places and with other kinds of population or habitat data. Clearly, our methods are data hungry and shortage of data on biodiversity is the biggest limiting factor in many areas, and especially in the tropics, where most diversity resides. There are, naturally, limits to the degree to which our methods could be applied elsewhere, and questions about the degree to which bird trends represent trends in other taxa.
The process of ecological change we are attempting to capture in our indicators has been described as ‘biotic homogenization’ (McKinney & Lockwood 1999). Widespread human activity results in the decline of many species and the increase in a few that thrive in disturbed environments. In this way, a few ‘winners’ replace the many ‘losers’ in wholesale change in the environment. Change is driven by two main human influences across the globe, environmental modification and the transportation of exotic species (McKinney & Lockwood 1999). The result is a more homogenized environment with lower diversity at national, regional and global scales. The challenge for ecologists is to mobilize, or create, new data and then to use that information to describe adequately the state of biodiversity and how it is changing. The paradox, identified by Pimm et al. (2001), is not that we are limited by knowledge of biodiversity, but by our failure to synthesize and distribute what we know.
Acknowledgments
We thank all those who have supported the Pan-European Common Bird Monitoring scheme, which is funded by the Royal Society for the Protection of Birds in association with the European Bird Census Council, BirdLife International, Czech Society for Ornithology and Statistics Netherlands. Special thanks to Ian Burfield, Norbert Teufelbauer, Christian Vansteenwegen, Anne Weiserbs, Michael Dvorak, Jean-Paul Jacob, Anny Anselin, Karel Št'astný, Vladimír Bejček, Michael Grell, Erik Mandrup Jacobsen, Henning Heldbjerg, Andres Kuresoo, Frederic Jiguet, Martin Flade, Johannes Schwarz, Tibor Szép, Olivia Crowe, Lorenzo Fornasari, Ainars Aunins, Magne Husby, Przemek Chylarecki, Juan Carlos del Moral, Ramón Martí, Åke Lindström, Hans Schmid, Nicola Crockford, Norbert Schäffer and Ward Hagemeijer. Thanks to the many individuals and organizations that have played a part in developing bird monitoring through the European Bird Census Council and BirdLife International. The European Environment Agency (through the European Topic Centre/Nature Protection & Biodiversity) has supported the development of this indicator. Thanks also to SOVON, British Trust for Ornithology and the Czech Society for Ornithology. We thank the Royal Society for the opportunity to present this work at the Scientific Discussion Meeting ‘Beyond extinction rates: monitoring wild nature for the 2010 target’ and for valuable discussion. We thank Andrew Balmford and an anonymous referee for constructive comments on the manuscript.
Appendix A:
Standard error of geometric mean of indices
By J. Pannekoek (Statistics Netherlands)
This appendix describes the variance (or standard error) estimate for a geometric mean. If the number of indices is T and the index for each species is denoted by I t, then the geometric mean can be expressed as
| (A1) |
The geometric mean is thus a nonlinear function of the component indices. The variance of a linear function of random variables can easily be expressed in terms of the (co)variances of the component variables but this is not so for the variance of a nonlinear function. The usual approach to obtain a variance estimate for a nonlinear function is to use a linear approximation for the nonlinear function, calculate the variance of this linear approximation and then use this variance as an approximation for the variance of the original nonlinear function. If the component variables can be assumed to be independent, as is the case for the species indices I t, this technique (Taylor linearization or delta method, see e.g. Agresti 1990, chapter 12) yields a variance approximation of the form
| (A2) |
where is the derivative of the geometric mean with respect to the index I t. Since this derivative is given by , the variance approximation for can be expressed as:
| (A 3) |
Now, an estimate of the variance (and standard error) of the geometric mean of the species indices can be obtained from (A 3) by plugging in estimates of the variances of the species indices I t.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Beyond extinction rates: monitoring wild nature for the 2010 target’.
References
- Aebischer N.J, Evans A.D, Grice P.V, Vickery J.A, editors. Ecology and conservation of lowland farmland birds. British Ornithologists Union; Tring, UK: 2000. [Google Scholar]
- Aebischer N.J, Green R.E, Evans A.D. From science to recovery: four case studies of how research has been translated into conservation action in the UK. In: Aebischer N.J, Evans A.D, Grice P.V, Vickery J.A, editors. Ecology and conservation of lowland farmland birds. British Ornithologists Union; Tring, UK: 2000. pp. 43–54. [Google Scholar]
- Agresti A. John Wiley; New York: 1990. Categorical data analysis. [Google Scholar]
- Alford A.P, Dixon R.M, Pechmann J.H.K. Global amphibian declines. Nature. 2001;412:499–500. doi: 10.1038/35087658. [DOI] [PubMed] [Google Scholar]
- Anon. Department for the Environment, Food & Rural Affairs; London: 2002. Achieving a better quality of life: review of progress towards sustainable development. http://www.defra.gov.uk/environment/statistics/wildlife. [Google Scholar]
- Balmford A. Selecting sites for conservation. In: Norris K, Pain D.J, editors. Conserving bird biodiversity: general principles and their application. Cambridge University Press; 2002. pp. 74–104. [Google Scholar]
- Balmford A, Green R.E, Jenkins M. Measuring the changing state of nature. Trends Ecol. Evol. 2003;18:326–330. [Google Scholar]
- Balmford A, et al. The convention on biological diversity's 2010 target. Science. 2005;307:212–213. doi: 10.1126/science.1106281. [DOI] [PubMed] [Google Scholar]
- Benton T.G, Bryant D.M, Cole L, Crick H.Q.P. Linking agricultural practice to insect and bird populations: a historical study over three decades. J. Appl. Ecol. 2002;39:673–687. [Google Scholar]
- Bibby C.J. Making the most of birds as environmental indicators. Ostrich. 1999;70:81–88. [Google Scholar]
- Bibby C.J, Burgess N.D, Hill D.A, Mustoe S. 2nd edn. Academic Press; London: 2000. Birds census techniques. [Google Scholar]
- BirdLife International/European Bird Census Council 2000 European bird populations: estimates and trends BirdLife Conservation Series No. 10. Cambridge: BirdLife International.
- Brickle N.W, Harper D.G.C, Aebischer N.J, Cockayne S.H. Effects of agricultural intensification on the breeding success of corn buntings Miliaria calandra . J. Appl. Ecol. 2000;37:742–755. [Google Scholar]
- Caro T.M, O'Doherty G. On the use of surrogate species in conservation biology. Conserv. Biol. 1999;13:805–814. [Google Scholar]
- Chamberlain D.E, Fuller R.J, Bunce R.G.H, Duckworth J.C, Shrubb M. Changes in the abundance of farmland birds in relation to the timing of agricultural intensification in England and Wales. J. Appl. Ecol. 2000;37:771–788. [Google Scholar]
- Cramp S, editor. Handbook of the birds of Europe, the Middle East and North Africa: the birds of the Western Palearctic. vols 1–11. Oxford University Press; 1977–1994. [Google Scholar]
- DETR 2001 Sustaining the variety of life: 5 years of the UK Biodiversity Action Plan Report of the UK Biodiversity Group to the UK Government, the Scottish Executive, the National Assembly for Wales and the Northern Ireland Executive. London: Department of the Environment Transport and the Regions www.ukbap.org.uk/library/BIODIV1.PDF
- Donald P.F. Changes in the abundance of invertebrates and plants on British farmland. Br. Wildl. 1998;9:279–289. [Google Scholar]
- Donald P.F, Green R.E, Heath M.F. Agricultural intensification and the collapse of Europe' farmland bird populations. Proc. R. Soc. B. 2001;268:25–29. doi: 10.1098/rspb.2000.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EEA 1997 Annual Report 1996 Copenhagen: European Environment Agency.
- Evans A.D, Smith K.W. Habitat selection of Cirl Buntings Emberiza cirlus wintering in Britain. Bird Study. 1994;41:81–87. [Google Scholar]
- Fewster R.M, Buckland S.T, Siriwardena G.M, Baillie S.R, Wilson J.D. Analysis of population trends for farmland birds using generalized additive models. Ecology. 2000;81:1970–1984. [Google Scholar]
- Foppen R.P.B. Alterra scientific contributions 4. Alterra; Wageningen: 2001. Bridging gaps in fragmented marshland. Applying landscape ecology for bird conservation. [Google Scholar]
- Fuller R.J, Gregory R.D, Gibbons D.W, Marchant J.H, Wilson J.D, Baillie S.R, Carter N. Population declines and range contractions among lowland farmland birds in Britain. Conserv. Biol. 1995;9:1425–1441. [Google Scholar]
- Furness R.W. Seabird–fisheries relationships in the northeast Atlantic and North Sea. In: Nettleship D.N, Sanger G.A, Springer P.F, editors. Marine birds: their feeding ecology and commercial fisheries relationships. Proceedings of the Pacific Seabird Group Symposium. Canadian Wildlife Service Special Publication; Seattle, WA: 1982. pp. 162–167. [Google Scholar]
- Furness R.W, Greenwood J.J.D, editors. Birds as monitors of environmental change. Chapman & Hall; London: 1993. [Google Scholar]
- Gibbons D.W. Development of Pan-European breeding bird monitoring. Ring. 2000;22:25–33. [Google Scholar]
- Green R.E, Stowe T.J. The decline of the corncrake in Britain and Ireland in relation to habitat change. J. Appl. Ecol. 1993;30:689–695. [Google Scholar]
- Green R.E, Balmford A.P, Crane P.R, Mace G.M, Reynolds J.D, Turner R.K. A framework for improved monitoring of biodiversity: responses to the World Summit on Sustainable Development. Conserv. Biol. 2005;19:56–65. [Google Scholar]
- Gregory R.D. Abundance patterns of European breeding birds. Ecography. 2000;23:201–208. [Google Scholar]
- Gregory R.D, Baillie S.R. Large-scale habitat use of some declining British birds. J. Appl. Ecol. 1998;35:785–799. [Google Scholar]
- Gregory R.D, Wilkinson N.I, Noble D.G, Brown A.F, Robinson J.A, Hughes J, Procter D.A, Gibbons D.W, Galbraith C.A. The population status of birds in the United Kingdom, Channel Islands and Isle of Man: an analysis of conservation concern 2002–2007. Br. Birds. 2002;95:410–448. [Google Scholar]
- Gregory R.D, Noble D, Field R, Marchant J.H, Raven M, Gibbons D.W. Using birds as indicators of biodiversity. Ornis Hungarica. 2003;12–13:11–24. [Google Scholar]
- Gregory R.D, Gibbons D.W, Donald P.F. Bird census and survey techniques. In: Sutherland W.J, Newton I, Green R.E, editors. Bird ecology and conservation: a handbook of techniques. Cambridge University Press; 2004. pp. 17–55. [Google Scholar]
- Gregory R.D, Noble D.A, Custance J. The state of play of farmland birds: population trends and conservation status of farmland birds in the United Kingdom. Ibis. 2004b;146(Suppl. 2):1–13. [Google Scholar]
- Hagemeijer E.J.M, Blair M.J, editors. The EBCC Atlas of European breeding birds: their distribution and abundance. Poyser; London: 1997. [Google Scholar]
- Hilty J, Merenlender A. Faunal indicator taxa selection for monitoring ecosystem health. Biol. Conserv. 2000;92:185–197. [Google Scholar]
- Hole D.G, Whittingham M.J, Bradbury R.B, Anderson G.Q.A, Lee P.L.M, Wilson J.D, Krebs J.R. Widespread local house-sparrow extinctions—agricultural intensification is blamed for the plummeting populations of these birds. Nature. 2002;418:931–932. doi: 10.1038/418931a. [DOI] [PubMed] [Google Scholar]
- Houlahan J.E, Findlay C.S, Schmidt B.R, Meyer A.H, Kuzmin S.L. Quantitative evidence for global amphibian declines. Nature. 2000;404:752–755. doi: 10.1038/35008052. [DOI] [PubMed] [Google Scholar]
- Houlahan J.E, Findlay C.S, Meyer A.H, Kuzmin S.L, Schmidt B.R. Global amphibian declines: reply. Nature. 2001;412:500. doi: 10.1038/35008052. [DOI] [PubMed] [Google Scholar]
- Hughes B, Criado J, Delany S, Gallo-Orsi U, Green A.J, Grussu M, Perennou C, Torres J.A. Council of Europe Publication T-PVS/Birds (99) 9. Council of Europe Publishing; Strasbourg: 1999. The status of the North American Ruddy Duck Oxyura jamaicensis in the Western Palearctic: towards an action plan for eradication. [Google Scholar]
- Hustings F. SOVON; Beek-Ubbergen: 1988. European monitoring studies on breeding birds. [Google Scholar]
- Hustings F. European monitoring studies on breeding birds: an update. Bird Census News. 1992;2:1–56. [Google Scholar]
- IUCN 2002 2002 IUCN Red List of threatened species Gland: IUCN www.redlist.org
- Jenkins M. Prospects for biodiversity. Science. 2003;302:1175–1177. doi: 10.1126/science.1088666. [DOI] [PubMed] [Google Scholar]
- Jenkins M, Green R.E, Madden J. The challenge of measuring global change in wild nature: are things getting better or worse? Conserv. Biol. 2003;17:20–23. [Google Scholar]
- Kouki J, Vaananen A. Impoverishment of resident old-growth forest bird assemblages along an isolation gradient of protected areas in eastern Finland. Ornis Fennica. 2000;77:145–154. [Google Scholar]
- Krebs J.R, Wilson J.D, Bradbury R.B, Siriwardena G.M. The second silent spring? Nature. 1999;400:611–612. [Google Scholar]
- Kunin W.E, Gaston K.J, editors. The biology of rarity. Chapman & Hall; London: 1997. [Google Scholar]
- Landres P.H, Verner J, Thomas J.W. Ecological uses of vertebrate indicator species—a critique. Conserv. Biol. 1988;2:316–328. [Google Scholar]
- Loh J, editor. Living Planet Report 2002. WWF International; Gland, Switzerland: 2002. [Google Scholar]
- May R.M, Lawton J.H, Stork N.E. Assessing extinction rates. In: Lawton J.H, May R.M, editors. Extinction rates. Oxford University Press; 1995. pp. 1–24. [Google Scholar]
- McCullagh P, Nelder J.A. 2nd edn. Chapman & Hall; London: 1989. Generalized linear models. [Google Scholar]
- McKinney M.L, Lockwood J.L. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 1999;11:450–453. doi: 10.1016/s0169-5347(99)01679-1. [DOI] [PubMed] [Google Scholar]
- Newson S.E, Noble D.G, Eaton M. BTO Research Report No. 368. British Trust for Ornithology; Thetford: 2004. Preliminary BBS-based habitat-specific indicators for wild bird populations: 1994–2002. [Google Scholar]
- OECD 1993 Shaping the 21st century: the contribution of development co-operation Paris: OECD.
- Olsen A.R, Sedransk J, Edwards D, Gotway C.A, Liggett W, Rathbun S, Reckhow K.H, Young L.J. Statistical issues for monitoring ecological and natural resources in the United States. Environ. Monit. Assess. 1999;54:1–45. [Google Scholar]
- Pannekoek J, van Strien A.J. Research paper no. 0102. Statistics Netherlands; Voorburg, The Netherlands: 2001. TRIM 3 manual. TRends and Indices for Monitoring data. Available freely at http://www.ebcc.info. [Google Scholar]
- Peach W.J, Siriwardena G.M, Gregory R.D. Long-term changes in over-winter survival rates explain the decline of reed buntings Emberiza schoeniclus in Britain. J. Appl. Ecol. 1999;36:798–811. [Google Scholar]
- Pimm S.L, Russell G.J, Gittleman J.L, Brooks T.M. The future of biodiversity. Science. 1995;269:347–350. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- Pimm S.L, et al. Can we defy nature's end? Science. 2001;293:2207–2208. doi: 10.1126/science.1061626. [DOI] [PubMed] [Google Scholar]
- Pitkänen M, Tiainen J. BirdLife Conservation Series (No. 3) BirdLife; Finland: 2001. Biodiversity of agricultural landscapes in Finland. [Google Scholar]
- Possingham H.P, Andelman S.J, Burgman M.A, Medellin R.A, Master L.L, Keith D.A. Limits to the use of threatened species lists. Trends Ecol. Evol. 2002;17:503–507. [Google Scholar]
- Potts G.R. Collins; London: 1986. The partridge: pesticides, predation and conservation. [Google Scholar]
- Purvis A, Hector H. Getting the measure of biodiversity. Nature. 2000;405:212–219. doi: 10.1038/35012221. [DOI] [PubMed] [Google Scholar]
- Robinson R.A, Sutherland W.J. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 2002;39:157–176. [Google Scholar]
- Royal Society 2003 Measuring biodiversity for conservation Policy document 11/03. London: The Royal Society.
- Shrubb M. Effects of agricultural change on nesting lapwings Vanellus vanellus on farmland. Bird Study. 1990;37:115–128. [Google Scholar]
- Simberloff D. Flagships, umbrellas, and keystones: is single-species management passé in the landscape era? Biol. Conserv. 1998;83:247–257. [Google Scholar]
- Siriwardena G.M, Baillie S.R, Buckland S.T, Fewster R.M, Marchant J.H, Wilson J.D. Trends in the abundance of farmland birds: a quantitative comparison of smoothed Common Birds Census indices. J. Appl. Ecol. 1998;35:24–43. [Google Scholar]
- Siriwardena G.M, Baillie S.R, Wilson J.D. Temporal variation in the annual survival rates of six granivorous birds with contrasting population trends. Ibis. 1999;141:621–636. [Google Scholar]
- Sotherton N.W, Self M.J. Changes in plant and arthropod biodiversity on lowland farmland: an overview. In: Aebischer N.J, Evans A.D, Grice P.V, Vickery J.A, editors. Ecology and conservation of lowland farmland birds. British Ornithologists Union; Tring, UK: 2000. pp. 26–35. [Google Scholar]
- Subsidiary Body on Scientific, Technical and Technological Advise (SBSTTA) 2003 Proposed biodiversity indicators relevant to the 2010 target Montreal: Bureau of Convention of Biological Diversity.
- Swengel S.R, Swengel A.B. Correlations in abundance of grassland songbirds and prairie butterflies. Biol. Conserv. 1999;90:1–11. [Google Scholar]
- ten Brink, B. 2003 The state of agro-biodiversity in The Netherlands: integrating habitat and species indicators. Proceedings of OECD expert meeting in 2001 Agriculture and biodiversity. Developing indicators for policy analysis, pp. 264–275. Paris: OECD.
- Thomas J.A, Telfer M.G, Roy D.B, Preston C.D, Greenwood J.J.D, Asher J, Fox R, Clarke R.T, Lawton J.H. Comparative losses of British butterflies, birds and plants and the global extinction crisis. Science. 2004;303:1879–1881. doi: 10.1126/science.1095046. [DOI] [PubMed] [Google Scholar]
- Tucker G.M, Evans M.I. BirdLife International; Cambridge: 1997. Habitats for birds in Europe: a conservation strategy for the wider environment. [Google Scholar]
- Tucker G.M, Heath M.H. BirdLife International; Cambridge: 1994. Birds in Europe: their conservation status. [Google Scholar]
- van Impe J. Estuarine pollution as a probable cause of increase of estuarine birds. Mar. Pollut. Bull. 1985;16:271–276. [Google Scholar]
- van Strien A.J, Pannekoek J, Gibbons D.W. Indexing European bird population trends using results of national monitoring schemes: a trial of a new method. Bird Study. 2001;48:200–213. [Google Scholar]
- van Strien A.J, Knol O, van Duuren L, ten Cate B, Leewis R. 2004. Natuurcompendium 2004. Natuur in cijfers. Milieu- en Natuurplanbureau en CBS. www.natuurcompendium.nl. [Google Scholar]
- Vickery J.A, Evans A.D, Grice P, Brand-Hardy R, Aebischer N.A. Ecology and conservation of lowland farmland birds II: the road to recovery. Ibis. 2004;146(Suppl. 2):1–258. [Google Scholar]
- Virkkala R. Population trends of forest birds in a Finnish Lapland landscape of large habitat blocks—consequences of stochastic environmental variation or regional habitat alteration. Biol. Conserv. 1991;56:223–240. [Google Scholar]
- Vorisek P, Marchant J.H. Review of large-scale generic population monitoring schemes in Europe. Bird Census News. 2003;16:14–38. [Google Scholar]
- Wilson P. Britain's arable weeds. Br. Wildl. 1992;3:149–161. [Google Scholar]