Abstract
Vascular plants are often considered to be among the better known large groups of organisms, but gaps in the available baseline data are extensive, and recent estimates of total known (described) seed plant species range from 200 000 to 422 000. Of these, global assessments of conservation status using International Union for the Conservation of Nature (IUCN) categories and criteria are available for only approximately 10 000 species. In response to recommendations from the Conference of the Parties to the Convention on Biological Diversity to develop biodiversity indicators based on changes in the status of threatened species, and trends in the abundance and distribution of selected species, we examine how existing data, in combination with limited new data collection, can be used to maximum effect. We argue that future work should produce Red List Indices based on a representative subset of plant species so that the limited resources currently available are directed towards redressing taxonomic and geographical biases apparent in existing datasets. Sampling the data held in the world's major herbaria, in combination with Geographical Information Systems techniques, can produce preliminary conservation assessments and help to direct selective survey work using existing field networks to verify distributions and gather population data. Such data can also be used to backcast threats and potential distributions through time. We outline an approach that could result in: (i) preliminary assessments of the conservation status of tens of thousands of species not previously assessed, (ii) significant enhancements in the coverage and representation of plant species on the IUCN Red List, and (iii) repeat and/or retrospective assessments for a significant proportion of these. This would result in more robust Sampled Red List Indices that can be defended as more representative of plant diversity as a whole; and eventually, comprehensive assessments at species level for one or more major families of angiosperms. The combined results would allow scientifically defensible generalizations about the current status of plant diversity by 2010 as well as tentative comments on trends. Together with other efforts already underway, this approach would establish a firmer basis for ongoing monitoring of the status of plant diversity beyond 2010 and a basis for comparison with the trend data available for vertebrates.
Keywords: global biodiversity, species richness, conservation assessments, extinction risk, IUCN Red List, Living Planet Index
1. Introduction
Parties to the Convention on Biological Diversity (CBD) are committed to achieving ‘a significant reduction in the current rate of loss of biological diversity’ by 2010, and have proposed several potential indicators to assess progress towards this important goal (http://www.biodiv.org/2010-target). These include monitoring the changing abundance and distribution of selected species, as well as measuring changes in the status of rare or threatened species. The aim of this paper is to consider the feasibility of applying these and other approaches to vascular plant species to assess the rate of loss of botanical diversity by 2010. Guided by the CBD's call for global-level indicators that draw on existing data, we review the strengths and limitations of current data on vascular plant diversity, and their suitability for inclusion in those composite indices being considered as vehicles for assessing and communicating progress towards the 2010 target. Based on this review, we outline a preferred approach that would yield useful results in the time available.
2. Background
(a) Targets and indicators
In February 2004, the seventh Conference of the Parties to the CBD (COP VII, see www.biodiv.org/convention/cops.asp) adopted a framework for the evaluation of progress towards the target of achieving ‘a significant reduction in the current rate of loss of biological diversity’ by 2010 (Decision VII/30, www.biodiv.org/decisions/default.aspx?m=COP-07&id=7767&lg=0). The framework comprises seven focal areas in which goals and sub-targets were to be established, and indicators identified. For the first focal area, ‘Status and trends of the components of biological diversity’, three provisional goals were identified (see Annex I, column A, and Annex II at www.biodiv.org/decisions/default.aspx?m=COP-07&id=7767&lg=0) (i) Goal 1. Promote the conservation of the biological diversity of ecosystems, habitats and biomes. (ii) Goal 2. Promote the conservation of species diversity. (iii) Goal 3. Promote the conservation of genetic diversity. For each of these goals one or more targets were proposed. For goal 2, the most relevant to this paper, two targets were proposed: (i) target 2.1: restore, maintain or reduce the decline of populations of species of selected taxonomic groups; and (ii) target 2.2: status of threatened species improved.
For the components of biological diversity (focal area 1), three indicators were identified for immediate testing: (i) trends in the extent of selected biomes, ecosystems and habitats; (ii) trends in the abundance and distribution of selected species; and (iii) coverage of protected areas. In addition, two further possible indicators were highlighted ‘for development by SBSTTA (Subsidiary Body on Scientific, Technical and Technological Advice) or Working Groups’ (see Annex I, column C, at www.biodiv.org/decisions/default.aspx?m=COP-07&id=7767&lg=0): (i) change in status of threatened species (Red List indicator under development) and (ii) trends in genetic diversity of domesticated animal, cultivated plants and fish species of major socio-economic importance. All indicators were intended for assessing and communicating progress towards the 2010 target at the global level. However, it was also emphasized that, as far as is feasible, the indicators should draw on existing datasets and be identified or developed in such a way that they may be used at global, regional, national and local levels (www.biodiv.org/2010-target/indicators.aspx; www.biodiv.org/decisions/default.aspx?m=COP-07&id=7767&lg=0).
A further set of targets for delivery by 2010 are outlined in the Global Strategy for Plant Conservation (GSPC) adopted by the sixth Conference of the Parties to the CBD (COP VI, Decision VI/9, see www.biodiv.org/decisions/default.aspx?m=COP-06&id=7183&lg=0). Of particular relevance in this context are targets 1 and 2, which recognize the urgent need for improved baseline information on plant diversity to facilitate practical conservation action. GSPC target 1 calls for the production of a working list of all known plant species by 2010. GSPC target 2 proposes a preliminary assessment of the conservation status of all known plant species by the same date. These targets are heavily interdependent. GSPC target 2 helps to set a baseline of assessments, but it is dependent on target 1 to ensure that all currently recognized species are assessed, and progress on both will feed into monitoring for the 2010 target on reducing the rate of biodiversity loss.
(b) Why plants?
Plants are responsible for the bulk of primary production in almost all terrestrial ecosystems and are thus fundamental to their functioning. They are also the principal structural elements of some of the most biodiverse ecosystems and, because of the close association between plants and diverse groups of terrestrial organisms, such as insects, the diversity of plants is probably one of the best available predictors of the total diversity of living organisms across all land ecosystems. For instance, Barthlott et al. (1999b) found a very close correlation between overall plant species richness at country level and species numbers of insects for the few areas where overall insect diversity can be at least estimated (insect species numbers based on Gaston 1996). Vascular plants are also diverse in themselves, with more than 200 000 species (but see § 2c). They account for at least 10% of all the species known to science (i.e. described to date), far more than birds, mammals or amphibians that currently tend to dominate conservation assessments.
(c) Baseline knowledge available
Vascular plants are often considered to be relatively well known for a group of their size and it is generally agreed that most plant species have already been described (www.biodiv.org/doc/ref/gti-diversitas.PDF). This contrasts sharply with the situation for other groups of comparable size: just 25 000 of an estimated 400 000 species of nematodes have been described; 40 000 of an estimated 150 000 crustacean species have been described, and there are estimated to be about 200 000 mollusc species of which some 70 000 have been described (Groombridge & Jenkins 2000). Overviews of global patterns of diversity are also more readily available for plants than for most other major groups (e.g. Barthlott et al. 1999a; Mutke & Barthlott in press) and these data have been used, for example, for the original delimitation of biodiversity hotspots (Myers 1988). Plants remain the primary criterion and principal determinant of Conservation International's high-profile hotspots (Mittermeier et al. 1999).
However, behind the perception of a relatively well-known group, the reality is more complex and heterogeneous. Almost a million names have been published for seed plant species, but there is no authoritative list indicating which of these names should be accepted and which should be treated as synonyms. The debate as to how many distinct seed plant species have been described—recent estimates range from 220 000 (Scotland & Wortley 2003) to 422 000 (Govaerts 2001)—hinges on different estimates of overall synonymy. In addition, some 2000 seed plant species new to science are described each year (R. Davies, personal communication) and, in some areas of the world, specialist collectors achieve rates of more than five new species discovered for every hundred herbarium specimens prepared (e.g. Western Cameroon; M. Cheek, unpublished data). The remaining groups of vascular plants are less species-rich than the seed plants and are generally estimated to comprise fewer than 15 000 species in total (Groombridge & Jenkins 2000). Many ‘known’ vascular plant species are known only from a single herbarium specimen and a short description, and have not been documented in the field for 100 years or more. Formal assessments of global conservation status using current International Union for the Conservation of Nature (IUCN) criteria are available for just 9598 vascular plant species.
Against this background, GSPC targets 1 and 2 (outlined in § 2a) are recognized to be challenging objectives and opinions differ as to whether they are achievable. Target 2, in particular, is problematic, since it will involve assessing the conservation status of hundreds of thousands of species for the first time. The best case outcome is that targets 1 and 2 will be in place for vascular plants and bryophytes by 2010, and will deliver a firm baseline for monitoring future trends in rates of loss of plant diversity. The target of achieving a significant reduction in the rate of loss of biodiversity by 2010 is a far more challenging one, and even providing the data to assess progress towards this target will be out of the question for most groups of organisms. For plants, target 2, if achieved, would provide an assessment of current status—a single data point; an assessment of the rate of loss would require at least two data points; and to detect any change in the rate of loss would require at least three data points. A global assessment based on rates of change for all vascular plant species cannot be delivered by 2010. How then can we hope to use plant data to monitor progress towards achieving a significant reduction in the rate of loss of biodiversity by 2010?
If trend data on all vascular plants cannot be delivered in time for inclusion in an assessment of progress towards the 2010 biodiversity target then any plant-based contribution will have to be based on some manageable subset of total vascular plant diversity. The subset should be defensible as representative of plant diversity more broadly, so that the resulting indices are potentially representative of global trends in plant diversity. In theory, a random or stratified sampling approach could deliver a suitable subset. In practice, however, the size and composition of the subset will be governed by a variety of more pragmatic considerations including: (i) availability of existing data; (ii) availability of resources to gather new data; (iii) taxonomic stability; and (iv) nature of the index in which the data are to be included.
The variety of biodiversity indices in current use or under development is illustrated by other papers in this issue, as are the range of criteria against which the merits of a particular index can be assessed (Buckland et al. 2005). In this paper we address the question of whether certain features of the existing or proposed indices could be extended to incorporate data for vascular plants, whether there are existing data that are suitable for this purpose, and whether the resulting index would be representative of vascular plant diversity more broadly. The Living Planet Index (LPI) and the Red List Index (RLI) are considered in detail because they are global indices for which sufficient information on methodology is available to assess the scope and possible consequences of incorporating plant data. Both also feature prominently in CBD background documents on indicators (LPI: https://www.biodiv.org/doc/2010/2010-indicator-species-trends-first-review-draft.PDF; RLI: https://www.biodiv.org/doc/2010/2010-indicator-red-list-index-first-review-draft.PDF; both documents accessible by registering at: http://www.biodiv.org/doc/notifications/2004/ntf-2004-063-2010-ind-en.PDF) and may, therefore, be probable candidates for adoption as official indicators of progress towards the 2010 biodiversity target. It is not our intention to provide detailed new critiques of these indices and their fit to purpose. Instead we seek to summarize their strengths and limitations (which are for the most part documented and discussed in detail elsewhere in this issue) and to discuss them in relation to the available plant data.
3. Choosing a biodiversity index
(a) Living Planet Index
The LPI (Loh et al 2005.) is the result of a collaboration between the World Wide Fund for Nature and the United Nations Environment Programme World Conservation Monitoring Centre (UNEP-WCMC) and is based on published and unpublished time-series data on vertebrate populations from 1970 onwards. The LPI is a measure of global biodiversity only to the extent that trends in populations of vertebrate species are representative of wider trends in all species, genes and ecosystems (Loh et al. this volume). Time-series for vertebrate populations are included in the database provided that they meet criteria relating to data quality. Numbers or densities of animals harvested by hunting or fisheries are specifically excluded from the index, but studies focusing on species known to be of conservation concern are not differentiated from studies undertaken for other reasons. It is therefore possible that inclusion of data from monitoring programmes, undertaken because a species or population was thought to be declining, or to monitor response to management, may introduce bias. Also acknowledged are a series of weaknesses inherent in the LPI that relate to the representativeness of the population data on which it is based.
The data on which the LPI is based are gleaned from studies undertaken for diverse reasons. In aggregate, the underlying data are not the results of a designed programme that samples representative sites within a given species' range, or representative species within a biogeographical realm and taxonomic group. Trends indicated by time-series data available for some species may not be representative of those for the species as a whole, whereas trends for the species included may not be representative of species of that taxonomic group in the biogeographical realm as a whole. In addition, some taxonomic groups and biogeographical realms have more species included in the index than others. Nearctic and Palearctic species are over-represented compared with species from the Australasian, Afrotropical, Indo-Malayan or Neotropical realms. Temperate and boreal forest are over-represented compared with species of tropical and subtropical forest. Temperate grassland and tundra species are over-represented compared with tropical grassland and desert species. In general, data availability is lowest where species diversity is highest. Calculation of the index corrects for over-representation of certain realms but adjustments have not been made for differences in representation of different taxonomic groups.
Despite these limitations, the LPI has considerable strength as an indicator of trends in global biodiversity, because time-series dating back to the 1970s are available for many species. Trends and changes in trends can therefore be discerned and are easy to understand and communicate. Species population trends are easy to apply at any level and to aggregate or disaggregate (Loh et al. 2005). They have also been widely used as proxy indicators of the state of ecosystems.
(i) Applying LPI methodology to existing plant data
Could the methodology used in preparing the LPI be applied to existing vascular plant population time-series, and to what extent would the results be meaningful as a global indicator of the status of plant species diversity? Loh et al. (2005) explain that the restriction of the LPI to vertebrates ‘is for reasons of data availability: time-series data for invertebrate or plant populations exist, but for relatively few, geographically restricted populations.’ We agree that vascular plant species for which population data are available are relatively few as a proportion of all vascular plant species. Nevertheless, hundreds of such datasets do exist, and while many deal only with geographically restricted populations, this is also the case for many vertebrate datasets included in the LPI.
Our experience suggests that the fundamental problem in applying LPI methodology to plant data is not the number of datasets available, but rather their representativeness. For example, the Global Population Dynamics Database (GPDD, NERC Centre for Population Biology, Imperial College 1999, see http://www.sw.ic.ac.uk/cpb/cpb/gpdd.html) is a significant source of vertebrate data for the LPI and is generally acknowledged to be the largest collection of animal and plant population data in the world. The GPDD comprises about 5000 time-series, including 58 datasets for vascular plants derived from 11 different published and unpublished sources. Four of these 11 meet the criteria for inclusion in the LPI and include 46 time-series referring to 32 different vascular plant species. Of these, 39 datasets referring to 25 species were from a single unpublished Argentinian arrey designed to study the effects of flooding in grassland. The remaining seven datasets were derived from studies designed, respectively, to document the invasion of annual grassland by a native shrub species, the effect of the invasion of an exotic aquatic species on native aquatic species (both in the USA) and the reasons for the rarity of an orchid species in the UK. These plant data collectively illustrate in miniature the over-representation of temperate and grassland species already described for the LPI, but they also illustrate a further potential problem: such studies tend to be undertaken where change is expected. The populations studied may be more likely to exhibit significant changes (increases or decreases) than a randomly selected sample.
Long-term ecological studies include much data on vascular plant population time-series that could be incorporated into a summary index such as the LPI. For example, the worldwide network of Forest Dynamics Plots established and coordinated by the Center for Tropical Forest Science of the Smithsonian Tropical Research Institute (http://www.ctfs.si.edu) includes 17 sites in 14 countries and is currently monitoring more than 3 million trees of about 6000 species. The combined results from those studies comprise the world's largest database on tropical tree demography (http://www.ctfs.si.edu/CTFS%20Research%20Grants%208-04.PDF). However, because of the objectives and long-term nature of the enterprise, the plots tend to be located in relatively secure areas, whether officially protected or not. As a result, any observed population reductions owing to habitat degradation or destruction might be expected to be lower than global averages for forest species. In most of the major biomes of the African continent, this problem is addressed by the BIOTA Africa programme (http://www.biota-africa.org) in which pairs of permanent biodiversity observatories are always placed close to each other in contrasting land use systems (e.g. protected area versus agriculture or commercial versus communal land use).
Conservation-focused studies represent another potentially rich source of plant population time-series data. For example, the Center for Plant Conservation (http://www.centerforplantconservation.org) lists over 600 species of conservation concern in the USA and cites references concerning population change for many of these. By its very nature, this list can be expected to over-represent species currently under threat, but since many of the plants listed are the subject of active monitoring and/or management programmes, it is to be expected that trend data for these species might, on average, present a more encouraging picture than perhaps is the case for a more representative sample of plant species.
We conclude that, given sufficient investment of resources, plant population time-series data could be gathered from existing published and unpublished sources in numbers comparable with those currently available for vertebrates included in the LPI. However, the results of such an exercise might not be particularly valuable in assessing progress towards the 2010 target. The species included would be demonstrably unrepresentative of plant diversity in terms of biogeography and biomes, and could also be expected to over-represent threatened species, thus over-estimating the rates of plant diversity loss since the 1970s. Conversely, they may also overstate the degree of improvement in recent years by over-representing species now actively managed for conservation purposes.
(ii) Gathering new plant data for the LPI
The limitations of the LPI are largely a function of reliance on available data that have been gathered for other diverse purposes that are often quite different from that for which they are now being employed. A careful sampling strategy should be designed to yield population time-series for a group of plant species selected as more broadly representative of vascular plant diversity. However, this is likely to be resource-intensive and the need to obtain at least three reliable data points means that, even if started immediately, it would be unlikely to produce sufficient data to detect any changes in the rate of loss of biodiversity between now and 2010.
(b) Red List Indices
RLIs are designed to illustrate the relative rate at which a particular set of species change in projected relative extinction risk, based on population and range size trends as quantified by the IUCN Red List Categories and Criteria (Butchart et al. in press; http://www.redlist.org). In broad terms, the RLI relates to the rate at which species are slipping towards (or away from) extinction.
RLIs can be derived for any representative set of species that has been fully assessed by IUCN Red List criteria at least twice. For the index to be meaningful, genuine changes in the status of species must be distinguished from category changes that are due to other reasons, such as changes in knowledge about the situation on the ground, taxonomic changes or changes in the criteria applied. Birds were the first group for which RLIs were calculated. A preliminary RLI for amphibians has also been prepared, based on a recent global assessment and a retrospective assignment of categories estimated to have applied in 1980. A sampled index is being developed (Butchart et al. 2005), based on species from all major taxonomic groups, realms, ecosystems and Red List Categories, to provide trends in extinction risks representative of all biodiversity, including groups in which all species have not yet been assessed.
Butchart et al. (2005) discuss the strengths and weaknesses of the RLIs in terms of representativeness and resolution. The most significant strength of the RLIs for birds and amphibians is that they are based on comprehensive assessments of all species in that taxonomic group across the world. This contrasts with other global indicators based on population estimates, which are derived from sampled data that are biased towards common, well-studied species in the developed world. Thus, the capacity to incorporate information on species that are rare, localized, or difficult to survey, including those most susceptible to extinction, is a particular strength of the RLIs. However, the requirement for two successive assessments of all the species in the group under study means that RLIs can be generated for only a small fraction of species diversity because of limited resources and the practical problems associated with reassessing large numbers of species at regular intervals. The Sampled RLI under development is intended to address this weakness and is discussed further in § 3b(i).
A further limitation of the RLIs is the coarse level of resolution of the categories on which they are based. Butchart et al. (2005) acknowledge this limitation and argue that the RLIs are very complementary to population-based indices since the former are derived from (potentially) cruder data that can be collected for all species in a taxonomic group, whereas the latter are based on much more detailed information that can only be collected for a small (and potentially biased) subset of species.
(i) Applying Red List Index methodology to plants
Could the RLI methodology be applied to plants based on existing data? Here we assess the scope for applying the RLI methodology to existing data to generate RLIs for plants, or to incorporate plants into a Sampled RLI.
The preparation of an RLI for plants that is comparable with those for birds or amphibians requires two successive assessments of the whole group in question. Only two vascular plant groups of more than 100 species have been fully assessed by IUCN criteria on more than one occasion (IUCN 1994, 2001): conifers were assessed in 1993 and again in 1999 (Farjon et al. 1993; Farjon & Page 1999), while cycads were assessed in the early 1990s and again in 2003 (Donaldson 2003). The first step in calculating a RLI for either of these groups is to distinguish which species changed category between the two assessments owing to genuine change in status and which species changed for other reasons. Unfortunately, this is not straightforward because in both cases the IUCN assessment criteria underwent major changes in the interval between the earlier and later assessments. One of us (AF), first author of both the conifer assessments (Farjon et al. 1993; Farjon & Page 1999), was able to provide documented (unpublished) examples of species that changed category owing to genuine change in status, and of others that changed category for other reasons, such as a change in knowledge of the situation on the ground. However, he did not consider it feasible to attribute reasons in retrospect for all of the changes in category reported over the interval between assessments. This was largely because the earlier IUCN criteria applied in the first assessment were very subjective and there is often a lack of documentary evidence to support distinctions between genuine status changes and circumstantial changes. Similar difficulties may apply to the earlier assessment of cycads, which are in any case probably not sufficiently species-rich to justify a dedicated RLI. Thus for RLI purposes these groups may need to be treated as having been assessed on just one occasion. Nonetheless, they remain the only two classes of plants for which baseline assessments are in place for all species.
A Sampled RLI is proposed as a solution to the problem of ensuring that species-rich groups in which relatively few species have been evaluated can be represented in the overall assessment of progress towards the 2010 biodiversity target. Butchart et al. (2005) outline an approach to this problem. They propose using a random or representative sample of species from a broad set of major taxonomic groups, including plants. They discuss the practical difficulties of designing a sampling approach across taxonomic groups that differ greatly in number of species and in level of our existing knowledge. They advocate an approach based on stratifying species equally across taxonomic groups, Red List Categories, realms and ecosystems. Preliminary testing indicates that a sample size of about 300 species per taxonomic group would provide sufficient resolution to detect important changes in the status of species. Applied to plants, this approach would involve a stratified sample of 300 species from the approximately 10 000 included in the current Red List. Certainly this approach would be practical and affordable (Butchart et al. 2005); it probably represents the best current prospect for inclusion of vascular plant data in an indicator designed to assess progress towards the 2010 biodiversity target. However, given the acknowledged geographical and taxonomic biases in the current Red List, the resulting sample of plant species cannot be justified as representative of vascular plant diversity more broadly.
Butchart et al. (2005) identify a major expansion of taxonomic coverage as a very high priority to develop representative biodiversity indicators from the IUCN Red List. A cursory examination of the range of plant species included in the current Red List is sufficient to establish that they are not representative of vascular plant diversity in terms of major taxonomic groups, nor in terms of ecosystems. The list includes approximately 10 000 species from an estimated total of approximately 280 000 vascular plants (Groombridge & Jenkins 2000). This equates to a sample of approximately 3% of all known plant species. If this sample was broadly representative taxonomically one might expect approximately 3% of the species in some of the major plant families to have been evaluated. A summary of Red List coverage for some major vascular plant families shows that rather few families have been assessed at about the level (c. 3%) one might expect if coverage was even in a taxonomic sense (table 1).
Table 1.
2003 Red List coverage for angiosperm families with >3000 species (according to Mabberley 1997) and for selected woody families.
| family name | total species (Mabberley 1997) | number evaluated | % evaluated | number threatened | % threatened of those evaluated |
|---|---|---|---|---|---|
| angiosperm families with at least 3000 species | |||||
| Compositae | 22 750 | 419 | 1.8 | 305 | 73 |
| Orchidaceae | 18 500 | 23 | 0.1 | 20 | 87 |
| Leguminosae | 18 000 | 689 | 3.8 | 548 | 80 |
| Rubiaceae | 10 200 | 330 | 3.2 | 274 | 83 |
| Poaceae (Gramineae) | 9500 | 14 | 0.1 | 6 | 43 |
| Lamiaceae | 6700 | 6 | 0.1 | 6 | 100 |
| Scrophulariaceae | 5100 | 3 | 0.1 | 2 | 67 |
| Euphorbiaceae | 4950 | 283 | 5.7 | 223 | 79 |
| Melastomataceae | 4950 | 167 | 3.4 | 139 | 83 |
| Myrtaceae | 4620 | 334 | 7.2 | 250 | 75 |
| Cyperaceae | 4350 | 3 | 0.1 | 0 | 0 |
| Umbelliferae | 3540 | 11 | 0.3 | 7 | 64 |
| Acanthaceae | 3450 | 47 | 1.4 | 35 | 74 |
| Ericaceae | 3400 | 27 | 0.8 | 18 | 67 |
| Cruciferae | 3250 | 15 | 0.5 | 13 | 87 |
| Piperaceae | 3000 | 9 | 0.3 | 1 | 11 |
| selected woody families | |||||
| Lauraceae | 2850 | 268 | 9.4 | 196 | 73 |
| Annonaceae | 2150 | 216 | 10.0 | 146 | 68 |
| Myrsinaceae | 1225 | 114 | 9.3 | 72 | 63 |
| Sapotaceae | 975 | 331 | 33.9 | 238 | 72 |
| Fagaceae | 700 | 108 | 15.4 | 64 | 59 |
| Dipterocarpaceae | 680 | 394 | 57.9 | 369 | 94 |
Leguminosae, Rubiaceae and Melastomataceae are represented about in proportion to their total diversity but most major families are grossly under-represented (for example Orchidaceae, Poaceae, Lamiaceae and Scrophulariaceae) while some rather smaller woody families such as Dipterocarpaceae, Fagaceae and Sapotaceae are greatly over-represented (table 1). The over-representation of woody species in the Red List is attributable to the fact that the database is dominated by assessments generated as a result of a single major project reviewing the conservation status of 10 091 trees of the world (Oldfield et al. 1998). Even for those families that do appear to have been evaluated in numbers roughly proportional to their total diversity, closer examination often shows great unevenness in treatment. The family Myrtaceae might appear to be somewhat over-represented in the database, with some 7% of known species having been evaluated. However, the species evaluated are very unevenly distributed across the family. Eucalyptus, one of the best known and most species-rich genera, is scarcely represented.
The under-representation of major monocot families such as grasses and orchids is of particular concern. It is difficult to envisage a stratification scheme that could redress this imbalance. If grasses and herbs are so poorly represented in the database, could the world's grassland habitats be adequately represented by the resulting sample of 300 species? If orchids are scarcely represented in the Red List, how can the resulting Index reflect the status of Neotropical cloud forests where a high proportion of the total plant species diversity is contributed by epiphytes, especially orchids?
A further issue is the extent to which species listed as ‘Least Concern’ are representative of the whole class of non-threatened species. Instances where whole groups of plant species are evaluated for their conservation status using IUCN Red List Categories and Criteria are the exception rather than the rule. More usually, botanists prepare formal assessments, primarily or exclusively, for species that they consider likely to meet the criteria indicative of some level of threat. The very high percentage of species evaluated that are listed as being of conservation concern reflects this tendency to focus effort on the species most likely to yield a ‘positive’ result (table 1). This practice is cost-effective and efficient if the overall objective is to increase understanding of, and draw attention to, the plant species most in need of conservation action. However, it does not meet the needs of a programme now endeavouring to provide a global index of the changing state of biodiversity. The plant species listed as being of Least Concern are likely to include many that were originally assessed because a specialist considered them likely to qualify for one of the threat categories. The original assessment will often have been prompted by some factor(s), such as restricted distribution or loss of habitat, which, though not always sufficient to result in the species being evaluated as ‘Near Threatened’ at the time of the assessment, would generally mean that the species was of greater conservation concern than the average plant species not yet assessed. In other words, many of the plant species currently listed as being of Least Concern are probably already closer to meeting the criteria for the Near Threatened category than is the average randomly selected plant species. As a result, the rate at which species move between Least Concern and Near Threatened in the future is likely to exaggerate the rate at which plant species as a whole are slipping towards extinction. This deficiency, which may well also apply to groups other than plants, needs to be addressed if the Sampled RLI is to be defensible in the face of detailed criticism.
(c) Relative prospects for and merits of the inclusion of plant data within the two indices
Many of the limitations of the LPI and the RLI methods are also likely to be relevant to other kinds of approaches to measuring biodiversity. The overriding theme to emerge from our analysis of the feasibility of applying the two indices to plants is that the available data are far from representative in terms of taxonomic and geographical coverage. A further, and less easily quantifiable difficulty is that the original purpose for which the data were gathered may render them inappropriate for inclusion in trend indices. In these respects the two indices have comparable strengths and weaknesses as indicators of progress towards the 2010 biodiversity target.
However, in terms of current and future availability of data for plants, the Red Data Index offers the following advantages: (i) the underlying data points (individual Red List assessments for particular species at a given time are derived from the application of standard methodologies (IUCN 1994, 2001)); (ii) the available data are already assembled in a single database that is under effective management by an organization committed to its further development and expansion; (iii) effective, extensive and expanding networks already exist for gathering data and carrying out assessments in certain plant groups; (iv) globally, the botanical and plant conservation communities are committed to a significant acceleration in the rate of production of ‘preliminary assessments of the conservation status’ of plant species, in order to address target 2 of the GSPC; and (v) the relatively coarse nature of the Red List Categories means that it may be possible to retrospectively assign Red List Categories for plant species for given dates in the past, based on a rather limited amount of historical data and an understanding of the ecology of the species in question. Such ‘backcasting’ has already been undertaken for amphibians (Butchart et al. 2005) and may be feasible for some plant species (see § 6a).
We conclude that, under current circumstances, a Sampled RLI approach represents the best prospect for incorporating existing plant data into an indicator to assess progress towards the 2010 target, although other, and perhaps less problematic approaches may be possible if current constraints of timing and funding were ameliorated. However, the existing pool of conservation status assessments from which the plant element of the Sampled RLI would be derived is not representative of plant diversity more broadly. Biases in the current sample could be redressed by careful targeting of effort during the drive to assess the conservation status of all known plant species by 2010 as part of the attempt to deliver target 2 of the GSPC.
4. Constructing a representative sample of plant diversity
Unfortunately, it is easier to pinpoint ways in which a particular plant dataset is demonstrably unrepresentative than it is to construct a sample of plant species that is defensible as representative of vascular plant species as a whole. The lack of a complete listing of all known plant species is a serious impediment to the construction of a sample of any significant size. This need is being actively addressed in order to meet target 1 of the GSPC, but the complete product will not be available before 2008 at the earliest (UNEP/CBD/SBSTTA/9/INF/24, www.biodiv.org/doc/meetings/sbstta/sbstta-09/information/sbstta-09-inf-24-en.PDF).
(a) Sampling the International Plant Names Index
One potential approach to constructing a representative sample of plant species in the absence of a complete list might be to develop a sample from the International Plant Names Index (http://www.ipni.org), which attempts to list all published names for vascular plants but does not distinguish between accepted names and synonyms. Such an approach would: (i) obtain a random sample of plant names at species level from IPNI; (ii) determine the current accepted name for each name included in the sample; (iii) add geographical distribution data for each record where available; and (iv) use the resulting enhanced sample as the data source for randomized and/or stratified subsamples to be targeted for ongoing monitoring or periodic conservation status assessment. We have trialled this approach on a small scale (L. J. Pleasants & K. A. Hardwick, unpublished data). The difficulties encountered illustrate the complexity of this seemingly straightforward task.
Initially, we planned to tackle the task of updating the names in the random sample by working through the (randomly ordered) sample list from the top so that at any given point in time the portion completed would in itself represent a random subsample. However, it proved much more efficient to order the list taxonomically. This re-ordering highlighted the fact that ratios of synonyms to accepted names at species level varies dramatically between plant groups. Thus, a random sample of names at species level from IPNI will result in over-representation of families with higher levels of synonymy and under-representation of families with lower numbers of synonyms per accepted species. This problem could be overcome by using a weighted resampling methodology once the names have been updated. However, the feasibility of this is limited because precise ratios of accepted names to synonyms are known only for those very few plant families for which there is a complete and up-to-date listing of accepted names and synonyms.
Kirschner & Kaplan (2002) provide a telling illustration of the pitfalls inherent in any attempt to assess the conservation status of taxa that are not well understood and circumscribed. Having completed taxonomic treatments of Juncaceae and Potamogetonaceae, they prepared conservation status assessments of all the species recognized. They then compared their results with the listings for these families in the 1997 IUCN Red List of Threatened Plants (Walter & Gillett 1998). This is the most comprehensive compilation of plants assessed as threatened at global level, but the assessments are not included in the current IUCN Red List because they are based largely on in-country assessments of presumed endemics and on criteria that predate the new, more objective criteria adopted by the IUCN in 1994. Kirschner & Kaplan showed that a substantial proportion of the names Red Listed in 1997 are synonyms or taxonomically doubtful. More worryingly, even when nomenclatural changes and synonymy are taken into account, the accuracy of many of the assessments is highly questionable. For instance, only half of the Juncaceae names on the 1997 Red List refer to taxa now considered to be of conservation concern, and a similar situation is seen in Potamogetonaceae: four of the nine Red Listed names are of widespread, not threatened, taxa.
Global taxonomic monographs are a crucial source of basic data for the accurate compilation of Red Lists (Kirschner & Kaplan 2002). Gaps in baseline knowledge of many plant groups continue to represent a significant impediment to their conservation and can only be addressed by ongoing investment in basic research on the diversity of plant species, their circumscription, relationships, distribution and ecology. The importance of this work simply cannot be overstated. However, this taxonomic impediment cannot be used as an excuse not to provide expert input into assessing progress towards the 2010 biodiversity target. In the medium term, the situation can be expected to improve as more taxonomists become actively involved in the Red Data listing process (Lowry & Smith 2003; Golding & Timberlake 2003) and when non-specialists are able to consult a global list of accepted names (GSPC target 1; see § 2a). In the short-term, however, we need to address the question of whether we can construct a sample of plant diversity comprising taxa that are sufficiently well known to lend themselves to reliable assessment of their conservation status, but that are nonetheless defensible as representative of global plant diversity.
(b) Analysing global distributions of families and genera
To establish whether some plant families are more representative of global diversity patterns across regions than other families, we used a database recording the presence or absence of all 14 724 vascular plant genera across 52 major regions of the world (Level 2 units of the Taxonomic Databases Working Group international data standard as described in Brummitt 2001). This database was developed by one of us (NAB) based on a comprehensive survey of the herbarium collections at the Royal Botanic Gardens, Kew, supplemented by literature records where available. The majority (>96%) of distribution records are documented by herbarium specimens; of 70 550 distribution records, 2560 (3.63%) are from literature sources only. Many individual distribution records that were represented either by only a few or by doubtfully determined specimens were further corroborated using literature records.
Criteria for selecting families representative of global diversity patterns were: (i) taxa should be well-known taxonomically, to minimize the biases in the analysis outlined above; (ii) taxa should be speciose and widely distributed, so that species from the same family can be studied across the world; and (iii) the diversity of the family for different regions of the world should be highly correlated with the distribution of total diversity for all families. The analysis was restricted to families of angiosperms. Although gymnosperms are relatively well-known taxonomically, they are not sufficiently widely distributed to serve alone as indicator taxa in a standardized global monitoring programme. Similarly, ferns, despite (as a whole) being widely distributed, are not always numerous across a diversity of different habitats. Furthermore, ferns still lack a generally accepted taxonomic baseline.
The analysis focused on large, widely distributed families of angiosperms, and all families that have more than 100 genera (32 families) were used, even though not all of these have a global distribution. For each family, Spearman non-parametric rank correlations were performed on the numbers of genera within each region against the total number of genera in each region for all other families (not just those with more than 100 genera). Correlations were also performed for all pairs and also all triplets of these families, against the distribution of total diversity across the world for all other families. The 10 highest non-parametric correlations of both single families and pairs of families are given in table 2; correlations for triplets of families are not presented since almost every triplet had a correlation of greater than 0.9.
Table 2.
The 10 highest Spearman non-parametric correlation coefficients between numbers of genera for single families and pairs of families across the world against global generic diversity across the world; n=52, p<0.001 in each case.
| family | rs | family pair | rs |
|---|---|---|---|
| Leguminosae | 0.940 | Orchidaceae & Gramineae | 0.973 |
| Gramineae | 0.915 | Scrophulariaceae & Palmae | 0.972 |
| Cucurbitaceae | 0.908 | Gramineae & Melastomataceae | 0.966 |
| Araceae | 0.899 | Leguminoseae & Rosaceae | 0.961 |
| Asclepiadaceae | 0.887 | Gramineae & Palmae | 0.961 |
| Apocynaceae | 0.870 | Leguminoseae & Ericaceae | 0.960 |
| Cyperaceae | 0.870 | Euphorbiaceae & Scrophulariaceae | 0.955 |
| Euphorbiaceae | 0.855 | Gramineae & Euphorbiaceae | 0.955 |
| Malvaceae | 0.851 | Scrophulariaceae & Melastomataceae | 0.954 |
| Rubiaceae | 0.843 | Gramineae & Gesneriaceae | 0.953 |
The individual family, for which generic diversity is best-correlated with overall patterns of angiosperm generic diversity, is clearly the Leguminosae, which has a cosmopolitan distribution and is well represented in tropical, temperate, dry and wet habitats. For pairs of families, the highest correlation was not for the pair of largest families of angiosperms (Compositae and Orchidaceae), but for family pairs with distributions that complement each other. The pair that are best-correlated with total generic diversity of other angiosperm families is Orchidaceae and Gramineae. Both families have a cosmopolitan distribution, but the former are conspicuously diverse in tropical, wet habitats, whereas the latter are more diverse in temperate and drier tropical habitats. Scrophulariaceae and Palmae (table 2) provide a more extreme example of the complementarity shown by Orchidaceae and Gramineae: Scrophulariaceae are a family mostly known from drier temperate habitats, while the Palmae are almost exclusively known from wet tropical habitats.
As a test of these genus-level results, preliminary analyses at the species level were conducted based on a combined dataset of several country checklists. This includes the PLANTS Checklist (see http://plants.usda.gov/index.html) for the USA (at state resolution, including Puerto Rico and Hawaii), the Flora Europaea (country resolution) and the checklists for Panama, Peru, and the Guianas (country resolution). Although this dataset is biased to northern temperate floras, it allows analyses with higher taxonomic and geographical resolution. (Details of the dataset are provided by Mutke & Barthlott (2000)). Families occurring in most of the 97 resulting operational geographical units, and having the highest correlation with overall plant species richness, overlap to a high degree with the families listed in table 2. Gramineae, Leguminosae, Compositae, Malvaceae, Solanaceae and Gentianaceae occur in at least 94 out of the 97 units (including all 7 tropical units) and have Spearman rank correlation coefficients with overall species richness of greater than 0.8. Regarding family pairs, in this species-level dataset, Leguminosae/Gramineae and Orchidaceae/Gramineae show highest r (0.97 and 0.96) with overall species richness.
Legumes, grasses and orchids are among the most species-rich of all angiosperm families, with numbers of species known to science estimated at approximately 18 000, 9500, and 18 500 species, respectively. All three families can be counted among the better known groups of angiosperms, with world checklists at species level complete or nearly complete (table 3) and significant taxonomic expertise available that could underpin assessments of the status of individual species. None of these families is particularly well represented on the current IUCN Red List. Legumes are represented approximately in proportion to their diversity, but grasses and orchids are significantly under-represented. Complete assessments of the conservation status of all species in these three families (some 46 000 in total, approximately equivalent to the diversity of birds, reptiles, amphibians and fishes combined) would represent a very significant contribution towards target 2 of the GSPC and would enhance both the coverage and the representation of the IUCN Red List for Plants, while avoiding many of the taxonomic black holes and grey areas characteristic of less well-known plant groups. On a practical note, the fact that these families happen to include many species of great economic and/or aesthetic value may prove an advantage, both in securing funding for the work and in communicating the results to the general public.
Table 3.
Global checklists for major families.
| estimated no. species | URL for world checklist | |
|---|---|---|
| Leguminosae | 18 000 | http://www.ildis.org |
| Orchids | 18 500 | http://www.kew.org/monocotChecklist/home.do |
| Grasses | 9500 | http://www.rbgkew.org.uk/data/grasses-db.html |
These results are derived from limited datasets and our analyses address patterns in diversity at rather coarse taxonomic and geographical scales. Therefore, these preliminary conclusions need to be tested using other datasets at finer scales. There is also a need to investigate whether these groups are likely to be representative in terms of their vulnerability to anthropogenic change. Nonetheless, the results obtained are encouraging because of the high correlation values reported. The families emerging as most representative of plant diversity more broadly are not surprising, but they ‘make sense’ in ecological terms and we have a fair expectation that they may be borne out in analyses of other, more finely grained, global datasets if and when these become available.
5. Accelerating the rate of production of species-level conservation assessments
Even though legumes, grasses and orchids are relatively well-known taxonomically and may be broadly representative of plant diversity, the task of assessing trends in the status of approximately 50 000 species is a major challenge. The current IUCN Red List (Baillie et al. 2004) includes approximately 12 000 plant species of which ca. 2 000 were added in the past year. Not all of the assessments submitted to date have been processed: resources in the IUCN/SSC Red List Programme Office are a significant limiting factor in the system. A fivefold increase in the rate of production of assessments is required in order to have even these groups fully assessed by 2010. This will require significant additional resources both to enable the generation of assessments by specialists and to allow the results to be processed and incorporated in the database. And, if achieved, this would yield just a snapshot of current status for these groups, a single point for each species rather than the minimum of three data points per species needed to detect a change in rate of loss over time. However, there is potential to obtain preliminary trend data using a combination of existing data, Geographical Information Systems (GIS) and modelling techniques.
The herbaria of the world are extraordinary repositories of data on the distribution of plant species through space and time. For instance, each of the approximately 7 million specimens in the Kew herbarium documents the presence of a particular plant species at a particular point in space and time. There is growing recognition of how such data can be integrated with environmental spatial data in order to document and predict spatial patterns of biological diversity (Graham et al. 2004). Specifically, herbarium data can be used to provide the information on which IUCN Red List assessments can be based.
Plotting distribution maps based on specimen data is a long-established practice among taxonomists, but new approaches using GIS have the potential to offer tremendous added value (Willis et al. 2003). In particular, it is possible to use macros developed by one of us (J. Moat) to automate the measurements of geographical range that are used in IUCN assessments (http://www.redlist.org/info/categories_criteria2001.html). Herbarium data can be used to make evaluations based largely on criterion B—small range, defined in terms of extent of occurrence or area of occupancy, combined with estimates of fragmentation, continuing decline and/or fluctuation, and also on criterion D—population with a very restricted area of occupancy or number of locations (see Butchart et al. 2005; Schatz 2002; Willis et al. 2003). Given a series of plotted distribution points, both extent of occurrence and area of occupancy can be easily calculated and two alternative approaches have been implemented to allow assessment of the number of subpopulations and fragmentation of the population (Willis et al. 2003).
A drawback of this approach, because it focuses primarily on criterion B, is the possibility of underestimating extinction risk by overlooking species that might be listed if data were available to use criteria A, C or D. However, as a method of generating preliminary assessments for subsequent verification by experts and/or fieldwork, the approach is a useful starting point and a promising shortcut. Among the plant species on the current Red List, assessments based on criterion B predominate (e.g. Oldfield et al. 1998). GIS-based methods are now being applied to generate preliminary assessments of the conservation status of species being treated for floras and monographs or described as new to science. Currently, at the Royal Botanic Gardens, Kew and the Missouri Botanical Garden, they are applied to hundreds of species per year and are expected to be extended to thousands of species over the next few years. The techniques could just as easily be applied to tens of thousands of species given sufficient distribution data in appropriately geo-referenced form. The limiting factor is the rate of conversion of relevant specimen label data into electronic records suitable for GIS analysis.
Converting the information held in the world's herbaria into electronic form has long been recognized as an important but challenging task. In many cases, type holdings have been prioritized for databasing (and often for imaging), since they are especially valuable to taxonomists. There are also logistical advantages to selecting types based on the way that herbaria are commonly organized. However, there is an equally valid argument to prioritize databasing of label data from specimens of species most likely to be of conservation concern, so that assessments of conservation status can be based on all of the available information. However, in the absence of a complete listing of species of conservation concern, how can we construct a sample of herbarium material that is as rich as possible in specimens of these species?
Du Puy et al. (2002) monographed the legumes of Madagascar and undertook assessments of the conservation status of all woody endemic species. The specimen database developed during the course of this study includes all relevant material from herbaria, with significant holdings from Madagascar (including Kew, Missouri and Paris), and is believed to include the vast majority of the world's herbarium collections for these taxa. The database includes 8932 specimen records for 435 endemic species (woody and herbaceous). Of these, 228 species can be considered to be of conservation concern (CR, EN, VU and NT) when assessed using the GIS routines described above (see figure 1). Almost half (49%) of all the species surveyed were represented by 10 specimens or fewer, and these accounted for 82% of the species that were ultimately assessed as being of conservation concern. Most strikingly, these specimens comprise just 11% of all the specimens databased. In other words, if all species of Malagasy endemic legumes represented by no more than 10 specimens in the world's herbaria had been assessed, the greatly reduced investment in capturing label data would still have enabled specimen-based assessments for almost half of the endemic species, including the majority (82%) of species that can be established by this method to be of conservation concern. A threshold of 20 specimens per species would have captured information to support conservation assessments for 69% of the endemic species, including 96% of those provisionally rated as being of conservation concern. This result could have been achieved by capturing label data for just 26% of the available specimens. Species represented by more than 40 specimens accounted for more than half of the investment in specimen data capture, but not one of these was rated as of conservation concern. While these data have value in increasing the representation of species of Least Concern, there are clearly efficiencies to be achieved by gathering full datasets for just a few of such well-represented species.
Figure 1.
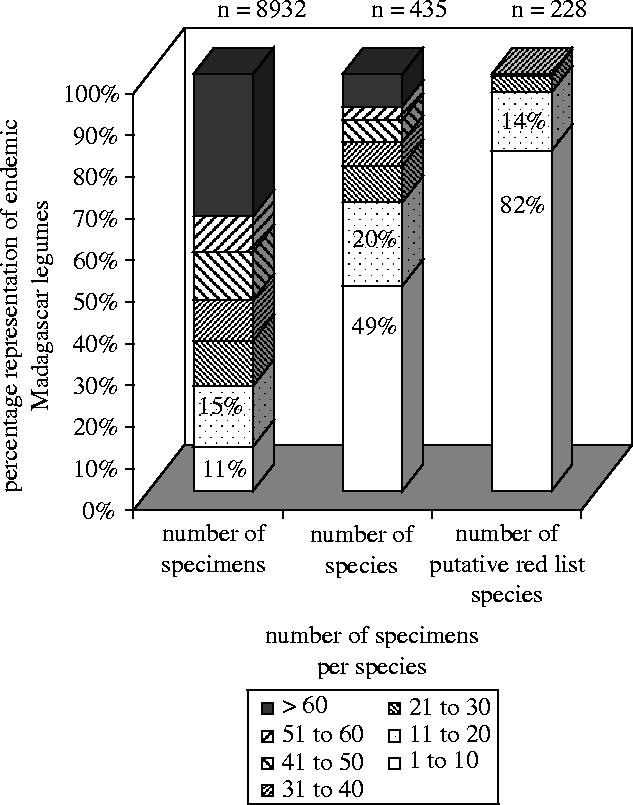
Percentage representation of endemic Madagascar legumes plotted against number of specimens per species, n.
Madagascar is extraordinary in its levels of endemism and, perhaps, unusual in the degree to which its collections are concentrated in a few major herbaria. However, the same general pattern is also seen in other areas of the world (e.g. Western Cameroon; M. Cheek & G. Gosline, unpublished data). The same pattern is also seen in the numbers of specimens available for species already on the Red List. A random sample of 100 vascular plant species from the 2003 Red List included 62 species represented in the Kew herbarium. Of these, 74% (46) were represented by no more than 10 specimens. A collaboration between the Royal Botanic Gardens, Kew, the Missouri Botanical Garden and New York Botanical Garden is now exploring how these patterns can be further understood and exploited to help maximize the efficiency with which herbarium specimen data are captured for conservation purposes and to identify, and where possible quantify, any biases which may be inherent in this approach (A. Paton, E. Nic Lughadha, R. E. Magill, B. Thiers, K. Harman, M. Tulig & C. Ulloa, unpublished data). Rarity in the herbarium does not always correlate with rarity in the field, but specimen numbers and specimen-based distributions are the best readily available indicator of relative rarity that we have at our disposal and, as such, the best basis for large-scale preliminary conservation assessments.
Automated GIS-based approaches do not produce authoritative conservation status assessments ready for submission to IUCN for inclusion in the Red List, but they do produce useful preliminary conservation assessments that allow efficient screening of many hundreds of species. Species that appear most likely to be of conservation concern can then be assessed by specialists using additional information, such as knowledge of the ecological requirements of particular species and/or remote sensing data on the extent and quality of habitats, to provide as complete as possible a picture of the current conservation status of the species. Of course, it is essential to ensure that a proportion of these specimen-based ‘desk’ assessments are ground-truthed. Conservation status assessment exercises of this kind are best concentrated in areas where they are supported by extensive field experience and/or active networks of in-country collaborators. To apply such an approach, at anything beyond a local scale, will require the coordinated effort of many institutions and individuals.
6. From species conservation status assessments to trends
(a) Backcasting (retrodiction)
Specimen data and GIS analyses are now routinely employed to calculate current conservation status and can also be used, in combination with remote sensing data, environmental variables and GIS techniques, to produce retrospective estimates of the probable distribution of selected species in previous decades, and thereby to derive retrospective preliminary conservation assessments for selected species. It is becoming commonplace to predict distributions of species (see Franklin (1995) for an overview of these techniques) and it would be no great leap of logic to apply this historically to retrodict a species' distribution using data on vegetation type, soils, elevation and other environmental variables. Such retrospective classification or ‘backcasting’ has already been applied extensively to amphibians (Butchart et al. 2005) and should also be feasible for thousands of plant species back to the 1970s and even to the 1950s where suitable satellite imagery or aerial photography data are available. Thus, for some plant species, it should be possible to generate a preliminary estimate of the rate at which they are moving towards (or away from) extinction.
(b) The elusive third data point
The approaches sketched out above are already ambitious, but one further element is required to detect changes in the rate of loss of plant diversity by 2010: measurement of at least three points in time is needed for as many species as possible. Retrodiction can be used to generate estimates at several points in time: readily available satellite imagery allows estimation for the 1980s, 1990s and up to the present day. Although the number of species for which this is feasible may be limited, the prospects are not entirely gloomy. One significant source of such observations could come from targeted fieldwork, including that undertaken in connection with other projects: for example, the network of seed collectors currently operating in 17 countries across the world gathering seed for the Millennium Seed Bank Project (http://www.kew.org/msbp). Although the project team use all the resources at their disposal to ensure that seed collectors visit populations of endemic, threatened or economically important species at the time when they are most likely to bear mature seed, repeat visits to the same population are often necessary. Assessment of the status of each population is now a routine element of each such visit and provides a much more reliable basis for evaluating overall conservation status, albeit of a limited number of species. Collectively, these observations, coordinated with the work of other existing networks (e.g. the IUCN Species Survival Commission network), could underpin reassessments of the conservation status of many species before the 2010 deadline.
Field-based methods can generate more comprehensive data for analysis of rates of loss than the area-based retrodiction. In addition, they facilitate calculation of the relationship between the area of distribution and the population size of particular species. Retrodicted area could then be extended to estimate population size at various times in the past. This, in turn, could allow IUCN assessments for the past to be estimated using both area and population size. The data might also be considered suitable for incorporation into a LPI or equivalent. Trends in distribution, population size and IUCN category could all be examined. The actual measurement of any of these parameters at any time may have errors, but these will be similar for each time point. Calculations concerning the changes in parameters between two given points in time, and the rates of change across three or more points in time, should not be significantly affected by these errors.
(c) Monitoring representative species not considered threatened
Data capture and fieldwork directed towards the species most likely to be of conservation concern are desirable, but broadening the representation of species classed as Least Concern is also be important to ensure that this class is not dominated by species that were ‘near misses’ for being listed as of conservation concern. The size and structure of this representative sample would depend on available resources, but a focus on families for which complete checklists are available would enable stratification by realm and biome, and perhaps by life-form and other relevant attributes.
(d) Potential significance and limitations of the approach
The approach outlined here will not give us what we might ideally want from a measure to monitor the state of plant diversity, but it would establish a foundation for the future and yield a large amount of data on the current status of plant species. Trend data could probably be derived for a small proportion of these species. In the short term, plant data are unlikely to show clear and robust trends that are defensible in isolation from other data sources. Nevertheless, the plant data would offer an important opportunity to address the question raised both by Butchart et al. (2005), and by Loh et al. (2005): the extent to which the rich vertebrate data already available are representative of biodiversity more broadly. Furthermore, insofar as plants are a fundamental element of most terrestrial ecosystems and represent a large proportion of the total known species diversity, any attempt to summarize progress towards the 2010 target without taking account of plants would be open to criticism for ignoring a large body of available data of great potential relevance.
7. Conclusions
The most high-profile biodiversity indicators currently in use and under development do not include plants, even though significant volumes of vascular plant data are available in both published and unpublished form that could be captured for inclusion in such indices. However, pervasive sampling biases in these data have the potential to render the resulting trends unrepresentative of plant diversity more broadly. Therefore, careful sampling and targeting of resources are needed to ensure that any plant-based contribution to the 2010 assessment makes best use of existing data and is also defensible as representative of plant diversity. It is possible to partially reconcile these twin aims by focusing on major vascular plant families, for which the taxonomic baseline is already largely in place and whose global distribution at the generic level or below suggests that they may be broadly representative of the remainder of plant families in terms of diversity. Assessment of the conservation status of large numbers of species from these families would greatly enhance the representativeness of the plant component on the IUCN Red List, providing an enhanced pool of data for the proposed new RLIs and at the same time making a major contribution to target 2 of the GSPC, a preliminary assessment of the conservation status of all known plant species. Such assessments can be generated cost-effectively using label data from selected herbarium specimens in combination with GIS techniques. Retrospective assignment of conservation status estimated for decades in the past would also be possible in many instances. Ground-truthing (verifying by ground survey data) will be essential to verify these preliminary evaluations and could also provide subsequent, more complete and robust assessments against all the IUCN Red List Criteria. This could be achieved in part through existing networks of fieldworkers. The value of this approach lies in the trend data for plants generated in time for the 2010 target, and also in the scope to compare status and trends for groups of plants with those already described for vertebrates. It will also establish a firmer and more representative baseline for future monitoring of the fate of plant diversity.
Acknowledgments
The authors acknowledge many contributions to the development of the ideas presented in this paper, ranging from unpublished data to thought-provoking questions. Contributors included Andrew Balmford, Steve Blackmore, Steve Buckland, David Goyder, Rhys Green, Kehan Harman, Mireille de Heer, Sally Hinchcliffe, Laura Jennings, Peter Jorgensen, Chris Leon, Bob Magill, Matthew Mustard, Nigel Taylor, Barbara Thiers, Carmen Ulloa, Kerry Walter, Daniela Zappi. We are particularly grateful to Georgina Mace for her support and encouragement throughout the process, and to Adrian Newton and an anonymous referee for thoughtful reviews of the manuscript.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Beyond extinction rates: monitoring wild nature for the 2010 target.’
References
- Baillie, J. E. M., Hilton-Taylor, C. & Stuart, S. N. (eds) 2004 2004 IUCN Red List of Threatened Species. A Global Species Assessment Gland, Switzerland/Cambridge, UK: IUCN. http://www.iucn.org/themes/ssc/red_list_2004/main_EN.htm.
- Barthlott W, Biedinger N, Braun G, Feig F, Kier G, Mutke J. Terminological and methodological aspects of the mapping and analysis of global biodiversity. Acta Bot. Fenn. 1999a;162:103–110. [Google Scholar]
- Barthlott W, Kier G, Mutke J. Globale Artenvielfalt und ihre ungleiche Verteilung. Cour. Forsch. Inst. Senck. 1999b;215:7–22. [Google Scholar]
- Brummitt R.K. 2nd edn. Hunt Institute for Botanical Documentation, Carnegie Mellon University; Pittsburgh, USA: 2001. World geographical scheme for recording plant distributions. [Google Scholar]
- Buckland S.T, Magurran A.E, Green R.E, Fewster R.M. Monitoring change in biodiversity through composite indices. Phil. Trans. R. Soc. B. 2005;360 doi: 10.1098/rstb.2004.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butchart, S. H. M., Stattersfield, A. J., Bennun, L. A., Shutes, S. M., Akçakaya, H. R., Baillie, J. E. M., Stuart, S. N., Hilton-Taylor, C., Mace, G. M. 2004. Measuring global trends in the status of biodiversity: Red List Indices for birds. PLoS Biol 2, e383. [DOI] [PMC free article] [PubMed]
- Butchart, S. H. M., Stattersfield, A. J., Baillie, J., Bennun, L. A., Stuart, S. N., Akçakaya, H. R., Hilton-Taylor, C. & Mace, G. M. 2005 Using red list indices to measure progress towards the 2010 target and beyond. Phil. Trans. R. Soc. B 360. [DOI] [PMC free article] [PubMed]
- Donaldson J.S, editor. Cycads. Status survey and conservation action plan. IUCN/SSC Cycad Specialist Group. IUCN; Gland, Switzerland and Cambridge, UK: 2003. [Google Scholar]
- Du Puy D.J, Labat J.-N, Rabevohitra R, Villiers J.-F, Bosser J, Moat J. Royal Botanic Gardens; Kew, UK: 2002. The Leguminosae of Madagascar. [Google Scholar]
- Farjon A, Page C.N. IUCN; Gland, Switzerland and Cambridge, UK: 1999. Conifers status survey and conservation action plan. [Google Scholar]
- Farjon A, Page C.N, Schellevis N. A preliminary world list of threatened conifer taxa. Biodiversity and Conservation. 1993;2:304–326. [Google Scholar]
- Franklin J. Predictve vegetation mapping: geographic modeling of biospatial patterns in relation to environmental gradients. Prog. Phys. Geogr. 1995;19:474–499. [Google Scholar]
- Gaston K.J. Species richness: measure and measurement. In: Gaston K.J, editor. Biodiversity. A biology of numbers and difference. Blackwell Science; Oxford: 1996. pp. 77–113. [Google Scholar]
- Golding J.S, Timberlake J. How taxonomists can bridge the gap between taxonomy and conservation science. Conserv. Biol. 2003;17:1177–1178. [Google Scholar]
- Govaerts R. How many species of seed plants are there? Taxon. 2001;50:1085–1090. [Google Scholar]
- Graham C.H, Ferrier S, Huettman F, Moritz C, Townsend Paterson A. New developments in museum-based informatics and applications in biodiversity analysis. TRENDS Ecol. Evol. 2004;19:497–503. doi: 10.1016/j.tree.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Groombridge B, Jenkins M.D. World Conservation Monitoring Centre; UK: 2000. Global biodiversity: earth's living resources in the 21st century. [Google Scholar]
- IUCN. Prepared by the IUCN Species Survival Commission. IUCN; Gland, Switzerland: 1994. Red List Categories. [Google Scholar]
- IUCN. Prepared by the IUCN Species Survival Commission. IUCN; Gland, Switzerland: 2001. IUCN Red List Categories and Criteria Version 3.1. [Google Scholar]
- Kirschner J, Kaplan Z. Taxonomic monographs in relation to global Red Lists. Taxon. 2002;51:155–158. [Google Scholar]
- Loh, J., Green, R. E., Ricketts, T., Lamoreux, J., Jenkins, M., Kapos, V. & Randers, J. 2005 The Living Planet Index: using species population time series to track trends in biodiversity. Phil. Trans. R. Soc. B. 2005;360 doi: 10.1098/rstb.2004.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry P.P, II, Smith P.P. Closing the gulf between botanists and conservationists. Conserv. Biol. 2003;17:1175–1176. [Google Scholar]
- Mabberley D.J. 2nd edn. Cambridge University Press; 1997. The plant book. [Google Scholar]
- Mittermeier R.A, Myers N, Gil P.R, Mittermeier C.G. CEMEX, Conservation International and Agrupacion Sierra Madre; Mexico City: 1999. Hotspots: earth's biologically richest and most endangered terrestrial ecoregions. [Google Scholar]
- Mutke J, Barthlott W. Some aspects of North American phytodiversity and its biogeographic relationships. In: Breckle S.-W, Schweizer B, Arndt U, editors. Results of worldwide ecological studies. Verlag Günter Heimbach; Stuttgart: 2000. pp. 435–447. [Google Scholar]
- Mutke, J. & Barthlott, W. In press. Patterns of vascular plant diversity at continental to global scales. In Plant diversity and complexity patterns: local, regional and global dimensions (ed. I. Friis & H. Balslev). Copenhagen: CA Reitzels Forlag for Royal Danish Academy of Sciences and Letters.
- Myers N. Threatened biotas: ‘hotspots’ in tropical forests. Environmentalist. 1988;8:187–208. doi: 10.1007/BF02240252. [DOI] [PubMed] [Google Scholar]
- NERC Centre for Population Biology, Imperial College. 1999. The Global Population Dynamics Database. [Google Scholar]
- Oldfield S, Lusty C, MacKinven A. World Conservation Press; Cambridge, UK: 1998. The world list of threatened trees. [Google Scholar]
- Schatz G.E. Taxonomy and herbaria in service of plant conservation: lessons from Madagascar's endemic families. Ann. Mo. Bot. Gard. 2002;89:145–152. [Google Scholar]
- Scotland R.W, Wortley A.H. How many species of seed plants are there? Taxon. 2003;52:101–104. [Google Scholar]
- Walter, K. S. & Gillett, H. J. (eds) 1998 1997 IUCN Red List of Threatened Plants. Cambridge, UK/Gland, Switzerland: UNEP – World Conservation Monitoring Centre/IUCN – The World Conservation Union.
- Willis F, Moat J, Paton A. Defining a role for herbarium data in Red List assessments: a case study of Plectranthus from eastern and southern tropical Africa. Biodivers. Conserv. 2003;12:1537–1552. [Google Scholar]


