Abstract
By agreeing to strive for ‘a significant reduction in the current rate of loss of biological diversity’ by the year 2010, political leaders at the 2002 World Summit on Sustainable Development (held in Johannesburg, South Africa) presented conservation scientists with a great opportunity, but also one of their most significant challenges. This is an extremely exciting and laudable development, but this reporting process could be made yet more powerful if it incorporates, from the outset, independent scientific assessment of the measures, how they are analysed, and practical ways of plugging key gaps. This input is crucial if the measures are to be widely owned, credible and robust to the vigorous external scrutiny to which they will doubtless be exposed. Assessing how rates of biodiversity loss have changed from current levels by 2010 will require that a given attribute has been measured at least three times; however, most habitats, species, populations and ecosystem services have not been assessed even once. Furthermore, the best data on which to base estimates of biodiversity loss are biased towards the charismatic vertebrate species; unfortunately, these supply minimal services to the human economy. We have to find ways to redress this taxonomic imbalance and expand our analyses to consider the vast diversity of invertebrate, fungal and microbial species that play a role in determining human health and economic welfare.
In the first part of this paper I will use examples from local and regional monitoring of biological diversity to examine the desired properties of ‘ideal indicators’. I will then change focus and examine an initial framework that asks how we might monitor changes in the economic goods and services provided by natural ecosystems. I will use this exercise to examine how the set of possible indicators given by the Convention on Biological Diversity might be modified in ways that provide a more critical assay of the economic value of biological diversity. Here I will emphasize that we need not only to monitor these benefits, but also to significantly increase public awareness of human dependence upon the role that non-voting species play in driving the world's financial economy.
Keywords: biodiversity, monitoring, ecosystem service, economic trade-off, global change
1. Introduction
The abundant diversity of life forms that have evolved on Earth is beginning to disappear (Wilson 1988; Pimm et al. 1995); this decline in life on Earth is both a consequence of anthropogenic climate change and more directly a response to anthropogenic modification of natural habitats (Meyer & Turner 1992; Vitousek et al. 1997a, b). Paradoxically, considerable scientific energy and media attention are currently focused on determining whether life of any form has ever existed on Mars. It took around 4 billion years for life to evolve to its current state on Earth; several predictions suggest we may lose between one quarter and one half of this over the course of the next century (Ehrlich 1995; Pimm et al. 1995). Perhaps a more urgent task for scientists interested in alternative forms of life in the universe is to quantify the diversity of life on Earth and estimate rates at which it is declining. The Convention on Biological Diversity (CBD) 2010 Biodiversity Targets set a major challenge to the ecological and conservation community by requesting a detailed understanding of rates of biodiversity change by 2010 (UNEP, 2003). The urgency of making this assessment is underscored by our increasing understanding that the quality of human life is intrinsically dependent upon services provided by other species. As biodiversity declines through habitat modification and other anthropogenic disturbances, how can we quantify the changes in goods and services that directly affect human health and economic welfare? In this essay I will present a broad overview of the scientific criteria that need to be met if we are to meet the CBD 2010 Biodiversity goals. In particular, I will focus on developing methods for monitoring rates of change in biodiversity and upon the economic motivation for setting these goals.
2. Features that need to be measured
The CBD 2010 Biodiversity indicators seek to measure change at a hierarchy of levels. These range from changes in species population size, through changes in the distribution and condition of habitats, to changes in rates of delivery of goods and services to the human economy. Once set up, the measures should be able to quantify how different populations and habitats respond to changes in interventions and threats such as nitrogen deposition, alien invasion and climate change. Hopefully, once the mechanisms to detect change are in place, they can also be used to monitor the efficacy of legal and economic measures that are designed to protect biological diversity.
3. Which sort of data do WE already have?
Ecologists and conservation biologists have collected a huge canon of information on the distribution, abundance, diversity and behaviour of a large number of animal, plant and fungal species (Levin 2000). Most of these data were collected to address specific questions about the behaviour, dynamics, or physiology of different species. We have also collected data on the diversity of different animal and plant communities, and on the interactions between a limited number of the species that inhabit these communities. Similarly, we have data on the rates at which ecosystems process and cycle different key elements, as well as their ability to absorb and break down pollutants and natural toxins. Unfortunately, these data are rarely collected using the same protocols or with the measurement of change on decadal time-scales as an objective. Furthermore, the data are stored in ways that vary from the traditional ‘write in the rain’ field notebook that is stored on a shelf ‘somewhere’ in an office, through to highly sophisticated, but system specific, computer databases. While some of these data could provide powerful and important information about the ways in which different populations, communities and habitats have changed and are changing, there is a pressing need for ecologists, conservation biologists and epidemiologists to adopt a common, globally accessible format for all environmental monitoring (Palmer et al. 2003, 2004). Nevertheless, ecologists and conservation biologists already have at their disposal a powerful and diverse set of tools for monitoring natural communities and the way in which they function. Once the currently extant databases are collated, they will serve as useful baseline measurements for areas at which future surveys might be taken.
4. Ideal properties of data and indicators
The currently available data contrast with the ideal datasets that would provide comprehensive and sensitive monitoring of the environment. Yet, if we asked ecologists and conservation biologists what the ideal dataset would be, I suspect we would get a diverse set of answers. Certainly, an ideal dataset should make significant steps to correct the inherent taxonomic bias in current surveys that focus upon mammals, birds, herptiles and flowering plants. More focus is needed upon the nematodes, bacteria, fungi and arthropods that represent a higher proportion of the diversity which, while less charismatic, actually undertake the key functions in natural ecosystems (Wilson 1987). Unfortunately, this would require significant levels of training both in field collection and in basic taxonomy (Gaston & May 1992; May 1992). A Martian visiting the Earth would be aghast to realize that we have only a rudimentary taxonomic knowledge of the species that drive most of the fundamental processes that make life possible on Earth (Nee 2004). This strongly suggests that if we are truly interested in alternative forms of life, or are interested in how ecosystems function in ways that make life on Earth possible, we should make a considerable investment in studying both the taxonomy of lower life forms and how their complex nonlinear interactions give rise to important emerging properties that drive ecosystem function. The continued well-being of the human economy is critically dependent on the level of redundancy in these systems. Developing a detailed theoretical and empirical understanding of how ecosystems are organized and how we can reassemble damaged ecosystems are some of the ultimate goals of ecology (Palmer et al. 2003, 2004). Understanding the way in which ecosystems function and ‘self-organize’ into efficient systems is one of the biggest challenges in science; at its heart is a nested series of problems that involve nonlinear interactions between multiple groups of particles all with different birth, death and movement rates (May 1974; Levin 1999). When posed as a mathematical problem, understanding ecosystem function (and its restoration) may be more challenging than understanding the structure of atoms, or the origin of the universe; it is certainly not something we should investigate as a series of uncontrolled experiments.
A short-term alternative to hastily attempting to quantify the species-by-species contributions to ecosystem function is to acknowledge that, until we develop the expertise to understand ecosystem function and resilience at the species level, we can only indirectly measure how changes in biodiversity lead to changes in ecosystem function and in the goods and services supplied by these systems. This will lead us to focus our biodiversity monitoring on the more charismatic vertebrate species and upon the plants that form the superficial outer layers of the more complex web of life. This inherently assumes that we will be able to monitor changes in the lower levels of biodiversity by monitoring what happens in the upper trophic levels. In some ways this is similar to studying the oceans by observing surface currents and wave movements. It is also directly analogous to using stock prices and employment statistics to monitor the health and future trajectory of an economy.
If we are going to initially rely upon the higher forms of life to monitor underlying biodiversity change we need to ensure that monitoring at this scale is balanced across habitats, ecosystems, and across the species we can sample within these ecosystems. More acutely, if we are to monitor changes in biodiversity by 2010 we need to sample regularly and have at least three data points for each chosen species, community and habitat at each location. This will require an increased reliance on long-term datasets that have already been collected for other purposes and the collation of this information at local, national and international centres. One way to stimulate collection and curation of such data would be to set up a network of data centres, in which major locations for biodiversity around the world were represented. There are existing centres: for example, the Centre for Population Biology at Imperial College, Silwood Park, London (www.cpb.bio.ic.ac.uk); CABS (Center for Applied Biodiversity Science, Washington, DC) (www.biodiversityscience.org/xp/CABS/home); NCEAS (National Center for Ecological Analysis and Synthesis, University of California, Santa Barbara) (www.nceas.ucsb.edu); and the LTER (Long-term Ecological Research) network (www.lternet.edu). They all give important examples of how collation of data and its analysis by teams of experts can provide major insights into local and global patterns of ecological change, yet they are all located in the species-poor northern temperate zone. We need to fund and develop similar facilities in the tropics.
There is plainly a need to expand the geographical and taxonomic range of the data we collect if we are to provide global, broad-scale monitoring of environmental change. Collating new data into a form in which it is compatible across studies with currently extant data creates a second whole set of additional problems because there is no common database into which all of these data may be assimilated. This creates a major logistic and innovative challenge to the environmental community, yet it is one whose solution would benefit us all. Several groups are working to develop computer databasing languages that would provide a common framework for the storage and retrieval of all information collected by ecologists, conservation biologists and epidemiologists. The rapid developments in computer and information technology over the last 20 years make this possible; there is also significant financial support to facilitate the development of such a database (Palmer et al. 2003). Similar initiatives are already in place to monitor up-to-the-minute movements in the prices of stocks and shares; banks can move financial resources around with equal facility because of common computer languages and databases. The equivalent environmental initiative simply requires more logistic and political support. Much of this support must come from within the environmental community; having a common databasing language will benefit us all; furthermore, having a centralized database of geo-referenced changes in species abundance and diversity will allow ecologists to ask much bigger questions about how populations and communities interact and respond to climate and anthropogenic change. This will require the ecological and conservation community to minimize our inherent resistance to sharing data; instead we have to realize that the magnitude of the world's environmental problems requires us to undertake larger and more detailed projects and experiments. This can only be done if we pool our resources and expertise while seeking new collaborations from manufacturing, health and insurance industries who will benefit from a better understanding of future potential environmental change.
5. Would I get the same answer if I asked another species?
Obviously we will never be able to monitor every species within a region. Even if we could do so, the temporal differences in the life histories of different taxa would require us to monitor biodiversity on a complex hierarchy of time-scales. Similarly, while it may be possible to sample the dynamics of aphids on a nested sample of host plants, elephants and migratory species such as elk or caribou need to be sampled across a huge geographical area. Thus it is essential that local initiatives that contribute to national and global monitoring schemes focus on expanding from detailed monitoring of individual species to include regular estimates of the abundance of species that interact with these focal species as well as important information about the overall species diversity and resilience of the habitat. Here, a careful trade-off needs to be made between the correct temporal resolution of the data and the confidence we can place in its quality.
6. How will you detect change?
The CBD 2010 Biodiversity Targets are curiously worded in that they hope for ‘a significant reduction in the current rate of biodiversity loss.’ This seems to presuppose that we will continue to lose biological diversity, but our principal aim is to slow the rate of loss by 2010. Framing the goals in terms of ‘rates of loss’ significantly increases the scale of the problem; whereas two samples will tell us whether biodiversity at any location is decreasing or remaining stable, at least three samples are needed to estimate change in the rate of biodiversity loss. Even then, statisticians will view the analysis as very outdated when we explain we have estimated the change in rates from three data-points.
Realistically, estimating rates with any form of statistical confidence will require considerably more intense sampling. Let us consider data from a local long-term study of a single species population, the African elephants at Amboseli National Park in Kenya. Cynthia Moss and her colleagues have monitored every elephant in this population for the last 25 years (Moss 2001). The population has steadily increased in size over the main period of study, mainly owing to its protection from poaching (Moss 1988). Although a steady increase is apparent from the data on total population size, if we plot these data as annual rate of increase then we see periods of sustained growth and periods when the rate of growth declines (figure 1b). Furthermore, when we compare the estimates of other East African elephant populations for the same period we see very different patterns. Most of the other elephant populations have been surveyed less regularly than those at Amboseli, so it is considerably harder to obtain estimates of rates of change. Some of the other elephant populations occupy much larger areas, so we can never hope to identify all of the animals as individuals, as is the case at Amboseli. So when can we say with any statistical confidence that rates of population of growth of Amboseli elephants have changed? Furthermore, although we know a lot about elephants and baboons at Amboseli (Samuels & Altmann 1991; Combes & Altmann 2001), we have only anecdotal information about other species following the cessation of regular aerial surveys in the 1980s (Western 1975). Satellite images are now available that provide detailed monthly spatial data on the vegetation cover and net primary production across the park, but only for the later years of the elephant study. Moreover, there is almost no overlap between the satellite vegetation data and studies of community diversity.
Figure 1.
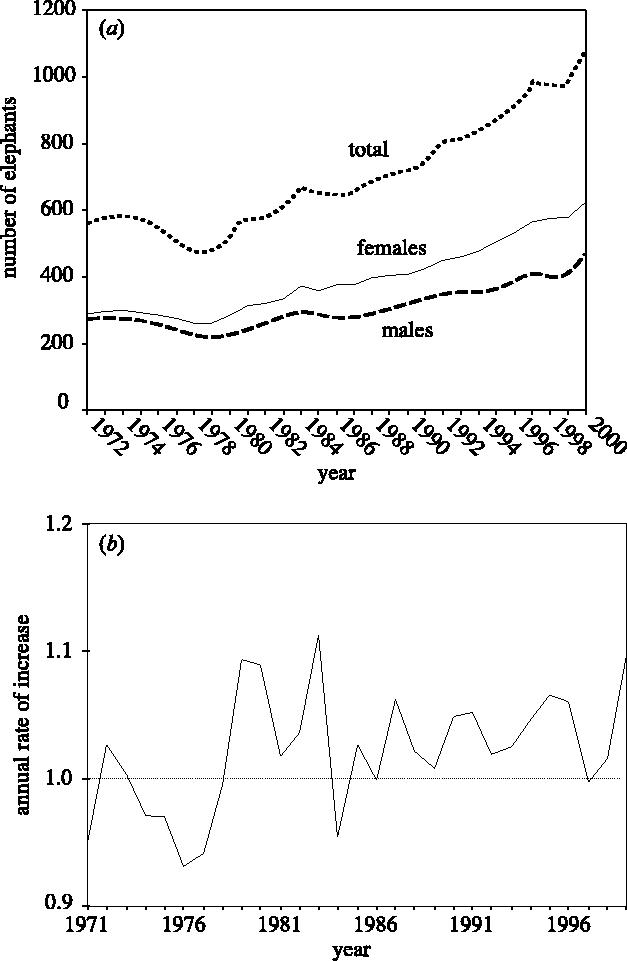
The dynamics of the African elephant population at Amboseli N.P., Kenya (after Moss 2001). (a) Recent temporal trends in the total number of male and female elephants and the total population size, Nt, (b) the annual rate of population increase Nt+1/Nt.
Similar problems arise when we examine long-term data for elk, bison and wolves in Yellowstone National Park in the United States (figure 2). Elk supply is economically important to the local community because there is an elk hunt each autumn where sportsman pay for licences to shoot elk (Coughenour & Singer 1996). Bison hunts were abandoned in the 1960s, although hypothetical worries about disease transmission to cattle have led to occasional ‘removals’ when bison cross the park boundary in winter (Dobson & Meagher 1996). Wolves were reintroduced in the mid-1990s immediately following a natural re-colonization (McNamee 1998). Like those collected at Amboseli, these data provide important insights into the dynamics of the large vertebrate populations. The data on removals for both elk and bison strongly suggest that using a population as a resource leads to reductions in its abundance. However, there are periods of time when both populations increase, even when hunted, and periods when they decrease, even when hunting removals are low or absent. In particular, the elk population illustrates a recent dramatic decline. Is this owing to the reintroduction of wolves, or to a series of bad winters, or a combination of these two effects? Plainly, more data are needed to address these questions, yet these are some of the best-quality wildlife data available; if we are to use this data to monitor the environment, it is essential that we have some way of interpreting the mechanisms that lead to changes in its long-term trajectories. Also included in figure 2b are data from three other elk populations, one at the National Elk refuge within 100 miles of Yellowstone (Boyce 1990), one 500 miles away at Rocky Mountain National Park (Lubow et al. 2002), and one nearly a thousand miles away on the coast of California (Howell et al. 2002). There is very little correlation between the annual rates of change of these populations and it would be very hard to say anything about the short-term future population trajectory of any of them from examining the dynamics of one of the others. Yet many of the underlying demographic characteristics of the populations will be almost identical, and if we could collate this information with data about their sensitivity to available resources, natural enemies and climate variation, we could begin to extrapolate between studies.
Figure 2.
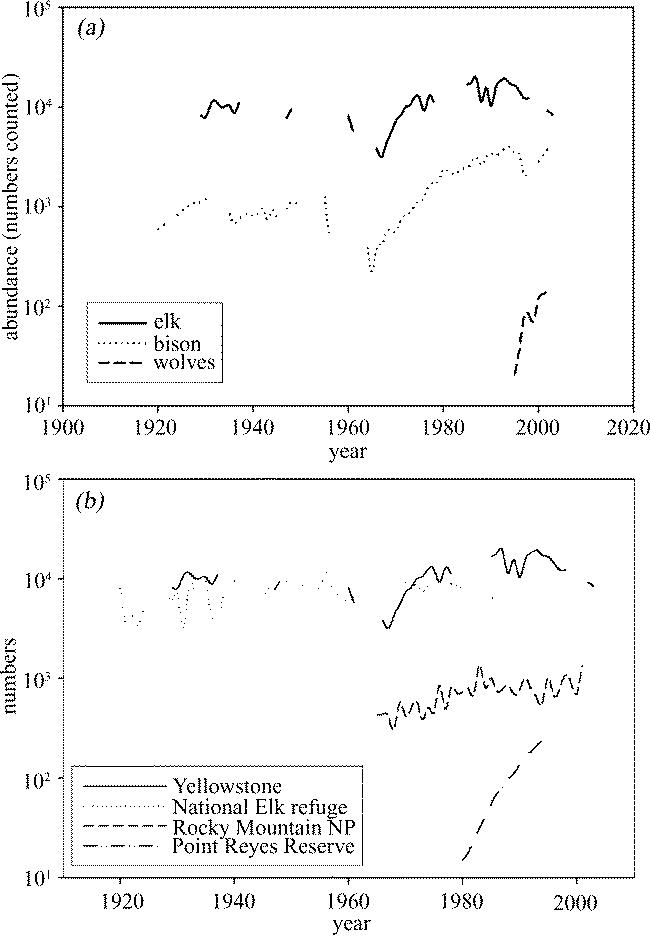
(a) Populations of elk, bison and wolves in Yellowstone National Park, USA; (b) Population of elk in Yellowstone National Park; National Elk Refuge, Wyoming USA; Rocky Mountain National Park, Colorado, USA; Point Reyes Reserve, California, USA.
All of the problems described for Amboseli and Yellowstone will arise when we try to monitor trends in biodiversity at other locations and at even larger spatial scales. While the need to monitor rates immediately requires one to ask ‘how frequently will we monitor?’, the need to monitor ‘biological diversity’ requires that we embed classic single-species studies into ones that involve many species from the surrounding community. Here we have to at least focus on animals and plants (or at least vegetation cover and net primary production (NPP). If we are to monitor the health of the major habitat types, or biomes, on each of the continents and larger islands, then we have to balance studies in national parks with studies of the same species in areas where they receive considerably less protection. We then need to examine data that we have already collected and sub-sample it to examine what level of sampling will allow us to detect trends. It may not be enough to simply monitor a set of populations or habitats at least three times prior to 2010. Instead we may need to collate historical data, establish an excess of possible monitoring sites (an excess because some will be converted to new uses even while we monitor them), and then sample these every 2, 3 or 5 years at both local and regional scales. I am not convinced we can do this in a totally effective way by 2010. However, I am convinced we can both produce important indications of rates of change for a large subset of species and habitats by 2010 and set in place the mechanisms that will allow us to have regular comprehensive biannual estimates of rates of change by 2020. If we fail to do this, our discipline will become redundant and silent in the policy arena.
7. What is a significant decline?
A second major set of problems arise when we attempt to examine whether populations, communities or habitats have significantly altered their rate of change (sensu CBD 2010). Mace and Lande (Mace & Lande 1991; Mace et al. 1992) have developed an important set of criteria that are widely used in population viability analyses for species that may require legal classification of their risk of extinction. The method builds on a large body of extinction and risk theory for populations (Burgman et al. 1993). It explicitly acknowledges that it is unlikely that quantitative data will be available for all of the demographic and habitat variables that provide a detailed estimate of extinction risk for any species. Instead, it suggests that synergistic interactions between habitat loss, fragmentation and population decline will all increase risk of extinction. Thus it should be possible to broadly classify the risk to a species by focusing on the best-quality subsets of information that are available. The methods developed by Mace & Lande (1991) could readily be modified to classify habitats and ecosystems on both national and global scales. This approach will require only limited modification if it is to be used to examine changes in the magnitude and quality of endangered habitats (Balch & Rodriguez 2004). Increasingly, we will rely upon satellite data to monitor the extent of natural ecosystems and habitats, yet this methodology needs to be expanded to include regular surveys of a large diversity of habitats at sites that are not too frequently obscured by clouds. The current focus on forests needs to be expanded to savannahs, agricultural lands and even estuaries and coral reefs. Most importantly, any attempts to interpret changes in habitat extent and condition that are discernible from space need to be verified using terrestrial surveys so that the impact on species, diversity, ecosystem function and services may be quantified.
8. Status and trends of components of biological diversity
An essential factor in developing regional, national and global indices of biological diversity is our need to inform the public about biodiversity change and to illustrate how the quality of human life is intimately coupled with the well-being of other species. Combining data from many time-series, as in the Living Planet Index (Loh et al. 2005), the Wild Bird Index (Gregory et al. 2005) and the index of coral cover (Cote et al. 2005), is potentially a very useful and powerful way of getting information about the state of the world's biodiversity into the minds of the world's public and the media. The Heinz Center's report on the state of ecosystems in the United States provides an excellent national example (H. John Heinz III Center for Science, Economics and The Environment 2002) that is already finding an audience with policy makers in the United States.
Here we should also consider the audience whose attention we wish to grasp when we present results of our analyses. In particular, we should examine how our index might be compared with the consumer price index ‘bag of household goods’, which is supposed to represent the financial outlay required for a family of four to meet their dietary and healthcare needs (www.bls.gov/cpi/home.htm). What is the environmental equivalent to this economic indicator? In many ways it may be necessary to subdivide the index into components that reflect how the environment affects each of the major subclasses of ecosystem service. The Millennium Ecosystem Assessment (Millennium Ecosystem Assessment 2003) has adopted a classification of ecosystem services that provides a useful way of framing a discussion about how we might measure changes in the rates at which they are delivered. The services are divided into supporting services and provisioning, regulating and cultural services (table 1). We would thus need to subdivide our population and habitat monitoring data in ways that allowed it to reflect contributions to these different services.
Table 1.
Ecosystem Services as benefits people obtain from ecosystems (Millenium Ecosystem Assessment 2003).
| provisioning services | |
| products obtained from ecosystems | |
| food | |
| fresh water | |
| fuel-wood | |
| fibre | |
| biochemicals | |
| genetic resources | |
| supporting services | regulating services |
| services necessary for the production of all other ecosystem services | benefits obtained from regulation of ecosystem services |
| soil formation | climate regulation |
| nutrient cycling | disease regulation |
| primary production | water regulation |
| water purification | |
| cultural services | |
| nonmaterial benefits obtained from ecosystems | |
| spiritual and religious | |
| recreation and tourism | |
| aesthetic inspirational | |
| educational | |
| sense of place | |
| cultural heritage |
A bizarrely appropriate role model is provided by the Zagat surveys of restaurants in major US and European cities (Zagat & Zagat 2004); these use large surveys of the public to rank huge numbers of restaurants by their food, décor, service and the average cost of a meal. The guides have a major impact on the choices people make, and thus a huge effect on the economy and consumer choice available in the restaurant industry. From an economic perspective, this is exactly what the major goal of the Living Planet Index should be; something that influences consumers' choice and modifies the services available to them.
Once we have set up a modified methodology for biodiversity indicators, a useful exercise would be to illustrate the sensitivity of the methodology by using known sets of detailed data (e.g. those for Serengeti (Sinclair & Arcese 1995); Krakatau (Thornton 1996); and Cabin John Island in the Potomac (Terborgh 1989)), even fossil data from past extinctions (Knoll 1984; Labandeira & Sepkoski 1993; Benton 1995). The results of testing the methodology in this way should then be published in a high-profile journal—Science, Nature or Public Library of Science; the primary goal here is to convince a broad audience of scientists that these indices provide a powerful and sensitive way of monitoring environmental change. Ultimately, funds should be sought to greatly increase the public's exposure to these indices. If we have interactive billboards in Times Square and other major cities relaying ‘up-to-the-minute’ stock prices and baseball scores, we should aim to have similar scoreboards for the environment. These should be advertised in a way analogous to the way the physicists advertised the Doomsday atomic clock. The Biodiversity Indices should form the centre of each news broadcast on Earth Day (Hayes 2000) and even New Year's day. Ultimately, it has to be a regular feature of weekly and monthly news reports. The Economist and Financial Times report the weekly average financial exchange rates between different currencies; we need an equivalent coverage of weekly exchange rates between the human and non-human economy.
9. Monitoring economic goods and services provided by natural ecosystems
Ecologists and economists have spent much of the past decade wrestling with how to quantify the goods and services provided by natural ecosystems (Daily 1997; Daily et al. 1997, 2000). At one extreme this discussion has focused on attempting to quantify the net annual economic benefit provided to the human economy by natural ecosystems (Costanza et al. 1997). An alternative approach to this question has been a heated discussion among ecologists on the dependence of ecosystem function upon species diversity (Tilman et al. 1997; Chapin et al. 2000; Kinzig et al. 2001; Loreau et al. 2001; Bond & Chase 2002). Here we have to acknowledge that ecosystem functions, such as nitrogen cycling, succession, primary production, pollination, soil retention and water movement, are explicitly undertaking services that feed into the human economy.
Quantifying ecosystem services on a species-by-species basis clearly is an impossible task, particularly as many ecosystem services are undertaken by microscopic species whose taxonomic status is unclear (Nee 2004). Nevertheless, it is probable that different types of services will predominantly be undertaken by species on different trophic levels. For example, regulating services such as climate regulation, water regulation and purification will be predominantly undertaken by interactions between species at the lowest trophic levels. In contrast, cultural services, such as recreation and tourism, and aesthetic and inspirational services will require ecosystems that contain a complete suite of species; this requires that the upper trophic levels remain intact. Furthermore, species at higher trophic levels will play a crucial role in regulating the abundance of species at lower trophic levels; when predators and pathogens are lost from a system we often see huge increases in the abundance of their prey species at lower trophic levels (Terborgh et al. 2001). Thus the trophic diversity of ecosystems will provide important information on their ability to provide different types of services in a way that is directly analogous to the length of marine trophic webs used by fisheries biologists (Pauly et al. 1998). Ultimately, it may be that a trophic diversity index for different habitats will fulfil our need for the equivalent of the consumer price index used in the ‘cost of living’ calculations made by economists. Figure 3 illustrates one possible approach using data from the biological recovery of Krakatau following the volcanic eruption that totally destroyed the island in 1883. Colonization of the barren rock was initiated by plants, which were then joined by a variety of primary and secondary consumers (Thornton 1996). If we follow the approach used by marine biologists and calculate mean trophic position we are only able to monitor fairly coarse changes in species diversity. If instead we calculate the standard deviation of mean trophic position then we obtain a more sensitive index of trophic diversity that captures more of the change in trophic diversity as the community recovers from the ultimate perturbation.
Figure 3.
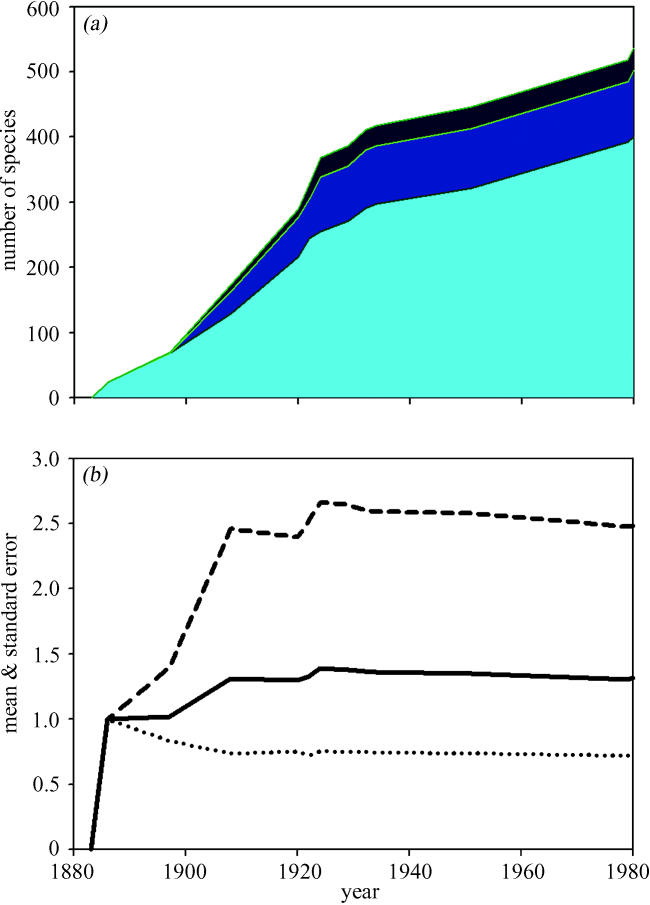
(a) Trophic diversity on Krakatau, the palest blue shading represents the number of plant species, the medium blue are the number of primary consumers, and the darkest shading is the number of secondary consumers or carnivores. Data collated from surveys reported in Thornton (1996); (b) the solid line represents mean trophic level for all the species recorded in each published survey on Krakatau. The upper dashed line illustrates mean trophic level plus two standard deviations, the lower dotted line illustrates mean trophic level minus one standard deviation.
A second major consideration when quantifying rates of change of ecosystem services is to consider how well the modified habitat continues to supply services. When forests or savannahs are converted to agricultural land their aesthetic value changes in ways that some people will find pleasing, and others will find abhorrent. Certainly the ability of the land to produce food will change and the diversity of food produced will certainly decline, but the net value of agricultural produce may well increase. Furthermore, the rate at which food and other services are produced in the modified habitat may be determined by services supplied by the non-converted habitat. For example, the diversity and abundance of pollinators for food crops in farmland may be strongly dependent upon the presence of local areas of non-converted habitat (Kremen et al. 2002; Ricketts et al. 2004).
A handful of studies have examined the relationship between economic goods and services provided by natural systems and the services supplied in adjacent, or equivalent, modified systems (Peters et al. 1989; Bonnie et al. 2000; Kremen et al. 2000; Balmford et al. 2002). Cost–benefit analyses of these studies suggest that in most cases the net economic value of converted habitat declines by an average of around 50% and the cost–benefit ratio of conserving remaining unconverted habitats may be as high as 100:1 (Balmford et al. 2002). Let us briefly use insights from these studies to examine how we might monitor ‘change in the rate’ at which economic services are provided by natural habitats.
10. How will the value of ecosystem services change as habitat is converted?
The most general form of the problem we are interested in consists of estimating the net economic value of a land system with two boundary conditions: at one point it is entirely pristine and supplies only ‘natural resources’; at the opposite extreme it is completely converted to a habitat devoted entirely to anthropogenic activities such as agriculture, housing, or industry; at all points along the spectrum it supplies economic goods and services that provide both local and more diffuse ‘global’ benefits. Thus in its pristine state it may supply a stable off-take of food and fibre resources (e.g. fruit and timber), it will cleanse air and water, and fulfil the spiritual, recreational and aesthetic needs of people who need to spend time in pristine forests, savannahs, or deserts. Some of these functions will also be supplied by the fully modified habitat and at each point along the continuum of levels of conversion that separates them. Depending upon whether the habitat has been converted to agricultural land, housing, or manufacturing, then the economic benefits will accrue to different numbers of people in different places. When considering the net economic benefit of habitat conversion, one additional consideration is to divide the economic activities into those that are independent of the remaining area of natural habitat and those that are dependent upon natural habitat. Similarly we can divide the economic benefits of the ‘pristine’ habitat into provisioning and sustaining services (table 1) and examine how habitat conversion impacts these in different ways.
11. Which species supply key ecosystem goods and services?
Monitoring the diversity and abundance of the species that undertake all the key ecosystem services is an impossible task. Identifying the level of redundancy in the diversity of species undertaking ecosystem processes is a major research agenda in ecology, but we are not yet at the level where we can quantify the impact of the loss of any species, or group of species, on the efficiency with which ecosystems function. Instead we need to adopt a phenomenological approach that is analogous to those used in the study of chemical and physiological reactions and assume that we can define the point at which the efficiency at which a task is performed declines to 50% of its maximum observed value. This would allow us to use Michaelis–Menton style kinetics to examine changes in ecosystem function in a way that is central to our understanding of resource consumer dynamics (Tilman 1976, 1982; Hansen & Hubbell 1980).
Even this simplification creates two initial problems: (i) how should we measure the efficiency with which ecosystem services are supplied? (ii) How do we quantify the biological diversity supplying the services? The first question requires us to measure either the impact of the service on the local economy, which may be reasonably simple for food harvested and entering local markets, or ‘bed-nights’ in local hotels for ecotourism. It is harder to quantify for amount of soil retained, CO2 stored, water purified or O2 produced. Here, however, regular (weekly) satellite monitoring may allow us to estimate NPP, which can be used to indirectly quantify some of these services. When quantifying biodiversity our choices are to sample the diversity in a region, where we are doomed to distort our knowledge in favour of the vertebrates, or alternatively, we can assume that biological diversity follows some species–area function, and that the proportional decline in area as natural habitat is converted will give rise to a proportional decline in species diversity that will follow some form of species–area curve (MacArthur & Wilson 1967). Here we should acknowledge that species on different trophic levels may have different exponents on this power function and thus ecosystem services provided by species on different trophic levels may decline at different rates (Holt et al. 1999; Dobson et al. in preparation).
12. The dynamics of ecosystem services and habitat conversion
A number of important insights may be gained by developing a very general, two-equation model of land use change and ecosystem services that are based on the above series of observations. One equation will describe the change in the rate at which the ecosystems services provided by the landscape change as it is modified to contain different proportions of ‘pristine’ and modified habitats. The second equation describes how different types of ecosystem service decline as habitat is converted, and net biodiversity in the landscape decreases. Both of these expressions could be infinitely more complicated and inelegant and each of their components could take a large variety of possible functional forms. Some of this will be explored elsewhere (Dobson et al. in preparation). Here I have simply focused on the most general linear form of the model to examine some of the basic underlying factors that confound our ability to detect ‘changes in the rate’ at which ecosystem services are supplied.
Let us assume that the quantity we wish to monitor is the net relative value (NRV) of the goods and services produced from an area of land a proportion, p, of which has been converted from its pristine state into some new form of land use. We wish to define its current value as a simple function of its value in the pristine state when all it supplied were indirect services to the human economy. This naively assumes we have some way of quantifying this value; at present, all we really know is that we tend to undervalue it (Daily 1997; Daily et al. 1997; Balmford et al. 2002). The proportion of land that is converted will also produce goods and services and we assume that we can express these as a proportional value, s, of the goods produced in the unmodified pristine habitat. We will also assume that the quality of the goods produced in the modified habitat has some dependence, d, on the services supplied by the unmodified proportion of the habitat (for example crop pollination by wild insects, or water quality and retention). Finally, we will include a function f(ES, p) that describes the relationship between the efficiency with which an ecosystem service is supplied and the proportion of habitat that remains pristine, (1−p); we will again assume that this is at a maximum value (of unity) when the habitat is pristine. Here we will implicitly assume that proportion of pristine habitat acts as a surrogate for the amount of biological diversity present in the system. This allows us to write a simple expression for the NRV of the land in terms of the proportion of habitat that has been converted from its pristine state:
| 12.1 |
We then need to consider some functional form for the relationship between the proportion of habitat converted and the efficiency with which different ecosystem services are supplied. Here we assume that we can define a proportional change in habitat, ES50, when the ecosystem services have declined to 50% of their maximum value. If the services are relatively resilient then it is probable that the value of ES50 will be close to unity; in contrast, if the services decline relatively rapidly with land conversion, then the value of ES50 will be substantially less than unity. We can normalize the relative value of services supplied by using a Michaelis–Menton type formulation (Hansen & Hubbell 1980; Tilman 1982):
| (2) |
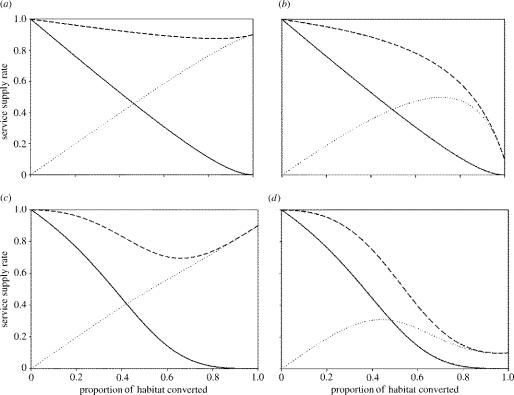
Note that I have included a second shape parameter, τ, which determines the rate at which services decline. Here I again assume that relatively resilient services will have values of this parameter close to unity. In contrast, brittle and less resilient services will have larger values of τ. One possible biological interpretation is to consider τ as the index of the trophic level that primarily drives the ecosystem function supplying the process (figure 4). Thus the cleansing of air and water that is undertaken by plants, soil nematodes and bacteria will tend to have low values of τ and values of ES50 close to unity. In contrast, ecotourism and aesthetic services that require the presence of top carnivores will have values of τ around 3–4 and relatively low values of ES50. A more complicated formulation would be required if we are to consider strong regulatory interactions between species on different trophic levels; these modifications will be explored elsewhere (they do not affect the main conclusions described here).
Figure 4.
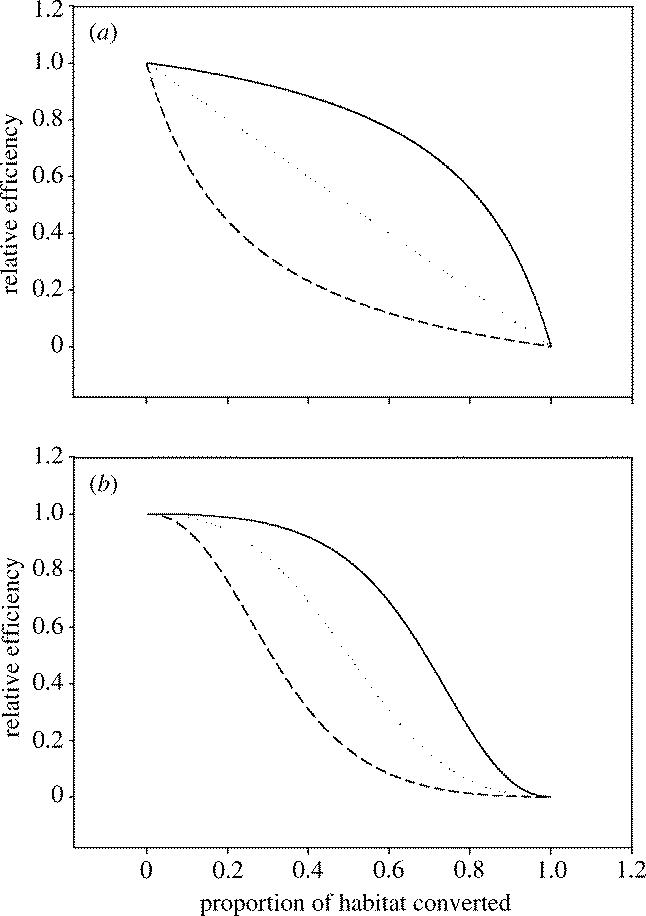
The phenomenological relationship between the supply of ecosystem services and either the proportion of habitat converted or the proportion of the original host community that has been lost. In (a) we assume the τ-term in equation 12.2 equals unity, the curves are then drawn for ES50=0.8 (upper solid line); ES50=1 (middle dotted line); and ES50=5 (lowest dashed line). In (b) we have set τ=2 (corresponding to services from a higher trophic level), the same three values of ES50 are then used as in (a).
The central point I am trying to make here is that if we are to monitor decline in ecosystems' function and the good services they supply, then we need to know more about the shapes of the curves that map change in ecosystem function onto change in area and hence species diversity. In particular, we need to know whether the species–area curve, which translates habitat loss into species loss, has different exponents for species on different trophic levels. This could be done by an expanded survey of the literature (Holt et al. 1999). However, it is essential that such a survey focus on studies of invertebrate, protozoan and fungal diversity; if we continue to focus on species–area relationships for charismatic vertebrates, we will only obtain data on species that supply a subset of ecosystem services. The second problem that arises here is the need to develop a deeper understanding of the relationship between species diversity and the efficiency with which the ecosystem functions (De Leo & Levin 1997; Hector et al. 1999; Huston 1997; Tilman et al. 1997; Schwartz et al. 2000; Kinzig et al. 2001; Bond & Chase 2002). In particular, we need to know how much redundancy there is in the system for different ecosystem functions and services, and whether there are any simple mappings of ecosystem function onto the trophic level (Dobson et al. in preparation). We also need to know which ecosystem functions are dependent upon a very limited number of species; these functions may be brittle and collapse suddenly. I suspect this will be the case for ecotourism. In contrast, ecosystem functions, such as water cleansing and soil retention, may be dependent upon a diversity of species from lower trophic levels. These processes may continue at modified rates in the converted habitats, and their species–area slopes may be so shallow that the net decline in service is a simple linear function of habitat loss, which may even be partially compensated for the same services being undertaken in the modified habitat. Again it would be nice to know more about the shape and redundancy of these species–function relationships, rather than to perform a post hoc analyses of a large-scale, uncontrolled experiment in land use change.
A second set of questions arises when we examine the hypothetical relationship between habitat conversion and economic services (as characterized by equation 12.1). Here, our ability to detect changes in the economic value of natural habitats will be a subtle function of the relative value of services supplied by the pristine and modified habitats, the dependence of the modified services on the presence of unmodified habitat, and the resilience of the relationship between diversity and ecosystem (figure 5). There are again a handful of simple messages we can take from this exercise: if the value of the services provided by the modified habitat is similar to those provided by the pristine habitat, then we will only be able to detect changes in services when those provided by the modified habitat are highly dependent upon the pristine habitat. When the services provided by the modified habitat are almost independent of the pristine habitat then the rate of change of land value may be too shallow to be detected as the habitat degrades or is converted. Where the economic activities on the modified habitat are only weakly dependent upon ecosystem services, the initial decline in land value is reversed as the new land use comes to dominate the landscape.
Figure 5.
Net services provided by a habitat at different levels of conversion. Four different scenarios are presented, in each the services provided by the natural habitat are presented as a (downward sloping) solid line, the new services provided by the modified habitat are depicted by upward sloping dotted lines. The net services are illustrated by the uppermost, broken line. Scenarios (a) and (b) illustrate the case for resilient services (p=0.8) that are undertaken by species at low trophic levels. Scenarios (c) and (d) are for brittle and less resilient services (p=0.2) undertaken by species at higher trophic levels. In (a) and (c) the services in the modified area of habitat are only weakly dependent upon services in the pristine habitat (d=0.1). In (b) and (d) the services in the modified habitat are strongly dependent upon services provided by the remaining pristine habitat.
As I implied in §4, one major worry is that we are attempting to understand these processes using a global series of uncontrolled experiments. If the ecosystem services are initially resilient, but ultimately brittle, we may have little indication that we need to reverse the trend until it is too late. By contrast, if our dependence on ecosystem services is fairly strong and these services break down steadily, we may be able to detect and reverse the trend before it is totally irreversible. A key consideration here is the cost of restoration; this will probably increase exponentially with the proportion of habitat converted (figure 6; Chen 2001). The increase is exponential because in general, it will take longer to recover when less pristine habitat is available as a natural species pool from which to colonize the converted habitat. Moreover, the greater the level of conversion, the more restoration is needed; it may take millennia to replace soils, while locally extinct top predators that may play a major regulatory role (Terborgh 1988), could be reintroduced provided a suitable source is available. Ironically, conservation biology has focused a lot of attention on reintroduction of charismatic vertebrates (Campbell 1980; Lyles & May 1987; Cade 1988; Kleiman 1989). In contrast, restoration ecology, which focuses on ecosystem processes, has only recently assumed significance on the conservation radar screen (Dobson et al. 1997; Higgs 1997; Simberloff et al. 1998; Palmer et al. 2004). Furthermore, any cost to restoration has to be offset against the present economic uses of the land. As the benefits of restoration have to be discounted in any cost–benefit analysis, it will become increasingly hard to make the argument that the simple long-term benefits of restoration exceed the net costs of restoration and the lost benefits of business as usual. When discount rates are high we will quickly cross a threshold where it will be impossible to ever recoup the cost of restoration. All of these considerations suggest we should treat environmental alarms more seriously (Pacala et al. 2003), particularly when sounded at a relatively early stage of habitat conversion; the relative cost of ignoring them may be very high.
Figure 6.
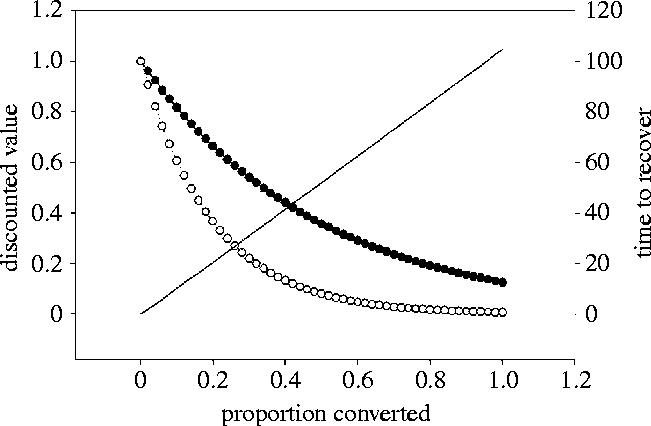
The relationship between proportion of habitat converted, the time to complete restoration and the discounted benefits of undertaking restoration. The solid line illustrates the hypothetical restoration time, which increases as more land has to be recovered (time is on the right-hand y-axis). The two broken lines illustrate the discounted value of undertaking restoration, assuming the system is returned to its completely unconverted state. Solid circles, discount rate of 2%; open circles, discount rate of 5%.
13. Final thoughts
There are many features of the CBD 2010 Biodiversity Target that I have failed to discuss here. In this final discussion I briefly touch upon a couple of areas where we need to develop new initiatives that require either new funding, redistribution of current efforts or some simple creative initiatives. The most glaring omission here is some more general way of monitoring ecosystem services and their impact on the local, regional and global economies. Here I think we need to assemble a working group to examine how we can develop the equivalent of the economist's ‘consumer price index’ (e.g. the average cost of a weekly bag of standardized household goods). We could also examine the possibility of developing the ecosystem services equivalent of the Economist's Big MAC index (Economist 2004), an index that estimates the cost people pay in different countries for a common standardized commodity, and the hours they have to work to pay for this commodity. From an ecosystem service potential, this might be a day's supply of drinkable water. This could be defined and quantified in one of three possible ways: (i) how much you would have to pay to safely drink the water at this place and time (measured as hours of work it required to pay for safe daily water consumption); (ii) relative life expectancy if you lived in this place and drank the water; (iii) proportion of native fishes, invertebrate and plant species that could survive in water of this quality; here again, it may be possible to express this as a mean trophic index, or the standard deviation of mean trophic level.
14. Nitrogen and Phosphorus deposition and monitoring
Intrinsic to the quality of drinking water is the amount of nitrogen and phosphorous entering the water supply. The use of industrial fertilizers is both a testimony to the success of human ingenuity in increasing the global food supply (Vitousek et al. 1997a,b) and, simultaneously, to our inability to recognize that the benefits this produces create problems and economic costs that are not borne by those who profit from the local increased agricultural activity. Thus monitoring of nitrogen levels in streams and rivers needs to be expanded to include phosphorus and also to examine how river flows of nitrogen and phosphorus are modified by the presence or increasing absence of wetlands and marshes. These data on nutrient loading in rivers then need to be integrated with data on numbers and sizes of harmful algal blooms (HABs) and ‘dead zones’ of near-shore oceans, particularly with respect to areas of high nitrogen and phosphorus outflow into the oceans (Turner & Rabalais 1994).
15. Role of space agencies
NASA has just spent many billions of dollars searching for signs of life on Mars. The current absence of water on Mars suggests it will take at least 4 billion years for the diversity of life on Mars to match that on Earth. This will also require a significant change in climate and atmosphere on Mars. Indeed, the absence of life on Mars formed one of the strongest arguments that changing the Earth's climate would not be good for biodiversity (Dobson et al. 1989; Peters & Lovejoy 1992). But Earth's climate and atmosphere are changing and the abundant diversity of life forms that have evolved on Earth are beginning to disappear; this decline in life on Earth is both a consequence of climate change and a response to anthropogenic modification of natural habitats. Perhaps a more urgent task for scientists interested in alternative forms of life is to quantify the diversity of life on Earth and estimate the rates at which it is declining. If it took 4 billion years for life to evolve to its current state on Earth, how much longer can we expect it to sustain its current level of diversity? Ultimately, it is less costly, more urgent and important to quantify and complete our understanding of life on Earth than to engage in a biologically fruitless search for signs of life on Mars. If there were intelligent life on Mars, it is probable that their distant perspective would allow them to assume that quantifying biodiversity was the greatest scientific challenge currently facing the scientists of Earth. That we have alternatively decided to invest considerable resources in focusing upon discerning the possibility that life once existed on their planet may lead Martians to question the presence of intelligent life on Earth.
NASA and the European Space Agency (ESA) can instead play an essential role in providing regular remote estimates of forest cover for all areas of the World's land surface that are visible at least four times in a year. For those areas perennially covered in cloud, these estimates should be made whenever possible and a global estimate provided every 3–4 years. We should also focus on more than just forests: savannahs, grasslands, wetlands, mangroves and estuaries should also be surveyed annually, as should cities, glaciers and agricultural areas. I personally think that this should be NASA's foremost priority if it is to retain any credibility with the global public, US tax payers and scientists not involved in the exploration of space. If NASA cannot achieve better coverage of Earth, then it should be split into two agencies and amalgamated with the ESA. One should continue to be sponsored by taxpayers' money and should focus on the Earth as observed from space, the other should be privatized, perhaps aided by a tax on astrology; this ‘agency’ can focus on whatever it wishes, including attempts to colonize the moon and nearby planets. The open market can then solve the problem of who can use this information and who may chose to colonize these new regions (Hardin 1993).
16. Conclusions
Monitoring the Earth's biological diversity in a way that will allow us to meet the CBD 2010 goals presents a formidable challenge to the global environmental sciences community. As we develop measures and indices of biodiversity it is essential that we develop protocols to look at each measure and examine the repeatability of the measure to ensure we can detect change in ways that are both statistically robust and that provide insight into the underlying mechanisms of change. To achieve this we need to ensure that the data we collect are representative and hierarchically structured at all geographical, taxonomic and trophic scales. We need to increase the quality and diversity of data we have on habitats, and in particular, need to focus on ways to monitor changes in the goods and services supplied by natural ecosystems. Here we need to build a consensus on the indicators ahead of time, where it is essential to separate the indicators from their policy implications. Many of the groups who are most likely to use the indicators will not support their use if they think they will only provide bad news about the environment. There is much to be done if we are to provide an answer to the questions posed by the CBD 2010 Biodiversity Targets. It sets an challenging and exciting agenda that should steer conservation biology and ecology onto the trajectory they need to take if they are to become the most important and relevant sciences of the twenty-first century.
Acknowledgments
I would like to thank Andrew Balmford, Rhys Green, Claire Kremen, Georgina Mace, Tom Lovejoy and Jon Paul Rodriguez for many insightful discussions on this topic. I also benefited from many discussions on related topics with the Ecological Society of America's ‘Visions’ Committee (Palmer et al. 2004). I thank the Royal Society for support to attend the Discussion Meeting.
Footnotes
One contribution of 19 to a Discussion Meeting Issue `Beyond extinction rates: monitoring wild nature for the 2010 target'.
References
- Balch, J. K. & Rodriguez, J. P. 2004 The design of quantitative methods for estimating habitat extinction risk. Oral presentation delivered at the 18th Annual Meeting Society for Conservation Biology, Columbia University, New York. 30 July to 2 August 2004.
- Balmford A, et al. Economic reasons for conserving wild nature. Science. 2002;297:950–953. doi: 10.1126/science.1073947. [DOI] [PubMed] [Google Scholar]
- Benton M.J. Diversification and extinction in the history of life. Science. 1995;268:52–58. doi: 10.1126/science.7701342. [DOI] [PubMed] [Google Scholar]
- Bond E.M, Chase J.M. Biodiversity and ecosystem functioning at local and regional spatial scales. Ecol. Lett. 2002;5:467–470. [Google Scholar]
- Bonnie R, Schwartzman S, Oppenheimer M, Bloomfield J. Counting the cost of deforestation. Science. 2000;288:1763–1764. doi: 10.1126/science.288.5472.1763. [DOI] [PubMed] [Google Scholar]
- Boyce M.S. Cambridge University Press; Cambridge: 1990. The Jackson Elk Herd. Intensive wildlife management in North America. [Google Scholar]
- Burgman M.A, Ferson S, Akcakaya H.R. Chapman & Hall; London: 1993. Risk assessment in conservation biology. [Google Scholar]
- Cade T.J. Using science and technology to reestablish species lost in nature. In: Wilson E.O, editor. Biodiversity. National Academy Press; Washington, DC: 1988. pp. 279–288. [Google Scholar]
- Campbell S. Is reintroduction a realistic goal? In: Soulé M.E, Wilcox B.A, editors. Conservation biology: an evolutionary-ecological perspective. Sinauer Associates, Inc; Sunderland, MA, USA: 1980. pp. 263–270. [Google Scholar]
- Chapin F.S, et al. Consequences of changing biodiversity. Nature. 2000;405:234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- Chen L.Y. Cost savings from properly managing endangered species. Nat. Areas J. 2001;21:197–203. [Google Scholar]
- Combes S.L, Altmann J. Status change during adulthood: life-history by-product or kin selection based on reproductive value? Proc. R. Soc. B. 2001;268:1367–1373. doi: 10.1098/rspb.2001.1631. (doi: 10.1098/rspb.2001.1631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanza R, et al. The value of the world's ecosystem services and natural capital. Nature. 1997;387:253–260. [Google Scholar]
- Côté I.M, Gill J.A, Gardner T.A, Watkinson A.R. Measuring coral reef decline through meta-analyses. Phil. Trans. R. Soc. B. 2005;360 doi: 10.1098/rstb.2004.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughenour M.B, Singer F.J. Elk population processes in Yellowstone National Park under the policy of natural regulation. Ecol. Appl. 1996;6:573–593. [Google Scholar]
- Daily G.C. Island Press; Washington, DC: 1997. Nature's services. Societal dependence on natural ecosystems. [Google Scholar]
- Daily G.C, et al. Ecosystem services: benefits supplied to human societies by natural ecosystems. Issues Ecol. 1997;2:1–16. [Google Scholar]
- Daily G.C, et al. The value of nature and the nature of value. Science. 2000;289:395–396. doi: 10.1126/science.289.5478.395. [DOI] [PubMed] [Google Scholar]
- De Leo, G. A. & Levin, S. A. 1997 The multifaceted aspects of ecosystem integrity. Conserv. Ecol. 1, 3. Available online at: http://www.consecol.org/vol1/iss1/art3
- Dobson, A. & Meagher, M. 1996 The population dynamics of brucellosis in the Yellowstone National Park. Ecology77, 1026–1036.
- Dobson A.P, Jolly A, Rubenstein D. The greenhouse-effect and biological diversity. Trends Ecol. Evol. 1989;4:64–68. [Google Scholar]
- Dobson A.P, Bradshaw A.D, Baker A.J.M. Hopes for the future: restoration ecology and conservation biology. Science. 1997;277:515–521. [Google Scholar]
- Dobson, A. P., et al In press. Habitat loss, trophic collapse and the decline of ecosystem services. Ecology [DOI] [PubMed]
- Economist 2004 The Big Mac Index. Food for thought. London: http://www.economist.com/markets/bigmac
- Ehrlich P.R. The scale of the human enterprise and biodiversity loss. In: Lawton J.H, May R.M, editors. Extinction rates. Oxford University Press; Oxford: 1995. pp. 214–226. [Google Scholar]
- Gaston K.J, May R.M. Taxonomy of taxonomists. Nature. 1992;356:281–282. [Google Scholar]
- Gregory R.D, van Strien A, Vorisek P, Gmelig Meyling A.W, Noble D.G, Foppen R.P.B, Gibbons D.W. Developing indicators for European birds. Phil. Trans. R. Soc. B. 2005;360 doi: 10.1098/rstb.2004.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S.R, Hubbell S.P. Single-nutrient microbial competition: qualitative agreement between experimental and theoretically forecast outcomes. Science. 1980;207:1491–1493. doi: 10.1126/science.6767274. [DOI] [PubMed] [Google Scholar]
- Hardin G. Oxford University Press; Oxford: 1993. Living within limits. Ecology, economics and population taboos. [Google Scholar]
- Hayes D. Island Press; Washington, DC: 2000. The Official Earth Day Guide to Planet Repair. [Google Scholar]
- Hector A, et al. Plant diversity and productivity experiments in European grasslands. Science. 1999;286:1123–1127. doi: 10.1126/science.286.5442.1123. [DOI] [PubMed] [Google Scholar]
- Heinz, H. J. III Center for Science, Economics and the Environment. 2002 The state of the nation's ecosystems. Measuring the lands, waters, and living resources of the United States. Cambridge: Cambridge University Press.
- Higgs E.S. What is good ecological restoration? Conserv. Biol. 1997;11:338–348. [Google Scholar]
- Holt R.D, Lawton J.H, Polis G.A, Martinez N.D. Trophic rank and the species–area relationship. Ecology. 1999;80:1495–1504. [Google Scholar]
- Howell J.A, Brooks G.C, Semenoff-Irving M, Greene C. Population dynamics of Tule elk at Point Reyes National Seashore, California. J. Wildl. Manage. 2002;66:478–490. [Google Scholar]
- Huston M.A. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. [DOI] [PubMed] [Google Scholar]
- Kinzig A.P, Pacala S.W, Tilman D. Monographs in population biology. Princeton University Press; Princeton: 2001. The functional consequences of biodiversity. Empirical progress and theoretical extensions. [Google Scholar]
- Kleiman D.G. Reintroduction of captive mammals for conservation. Bioscience. 1989;39:152–161. [Google Scholar]
- Knoll A.H. Patterns of extinction in the fossil record of vascular plants. In: Nitecki M.H, editor. Extinctions. The University of Chicago Press; Chicago: 1984. pp. 21–68. [Google Scholar]
- Kremen C, Niles J.O, Dalton M.G, Daily G.C, Ehrlich P.R, Fay J.P, Grewal D, Guillery R.P. Economic incentives for rain forest conservation across scales. Science. 2000;288:1828–1832. doi: 10.1126/science.288.5472.1828. [DOI] [PubMed] [Google Scholar]
- Kremen C, Willimas N.M, Thorp R.W. Crop pollination from native bees at risk from agricultural intensification. PNAS. 2002;99:16 812–16 816. doi: 10.1073/pnas.262413599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira C.C, Sepkoski J.J., Jr Insect diversity in the fossil record. Science. 1993;261:310–315. doi: 10.1126/science.11536548. [DOI] [PubMed] [Google Scholar]
- Levin S. Perseus Books; Reading, MA: 1999. Fragile dominion. Complexity and the commons. [Google Scholar]
- Levin S. Academic Press; London: 2000. Encyclopedia of biodiversity: five volumes. [Google Scholar]
- Loh J, Green R.E, Ricketts T, Lamoreux J, Jenkins M, Kapos V, Jorgen R. The Living Planet Index: using species population time series to track trends in biodiversity. Phil. Trans. R. Soc. B. 2005;360 doi: 10.1098/rstb.2004.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau M, et al. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- Lubow B.C, Singer F.J, Johnson T.L, Bowden D.C. Dynamics of interacting elk populations within and adjacent to Rocky Mountain National Park. J. Wildl. Manage. 2002;66:757–775. [Google Scholar]
- Lyles A.M, May R.M. Problems in leaving the ark. Nature. 1987;326:245–246. [Google Scholar]
- MacArthur R.H, Wilson E.O. Princeton University Press; Princeton: 1967. The theory of island biogeography. [Google Scholar]
- Mace G.M, Lande R. Assessing extinction threats: toward a reevaluation of IUCN threatened species categories. Conserv. Biol. 1991;5:148–157. [Google Scholar]
- Mace G, Collar N, Cooke J, Gaston K, Ginsberg J, Leader Williams N, Maunder M, Milner-Gulland E.J. The development of new criteria for listing species on the IUCN Red List. Species. 1992;19:16–22. [Google Scholar]
- May R.M. Princeton University Press; Princeton: 1974. Stability and complexity in model ecosystems. [Google Scholar]
- May R.M. How many species inhabit the Earth? Sci. Am. 1992;267:42–48. [Google Scholar]
- McNamee T. Henry Holt; New York: 1998. The return of the wolf to Yellowstone. [Google Scholar]
- Meyer W.B, Turner B.L., II Human population growth and global land-use/cover change. Annu. Rev. Ecol. Syst. 1992;23:39–61. [Google Scholar]
- Millenium Ecosystem Assessment. Island Press; Washington, DC: 2003. Ecosystems and human well-being: a framework for assessment. [Google Scholar]
- Moss C. William Morrow; New York: 1988. Elephant memories: thirteen years in the life of an elephant family. [Google Scholar]
- Moss C.J. The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya. J. Zool. Lond. 2001;255:145–156. [Google Scholar]
- Nee S. More than meets the eye. Earth's real biodiversity is invisible, whether we like it or not. Nature. 2004;429:804–805. doi: 10.1038/429804a. [DOI] [PubMed] [Google Scholar]
- Pacala S.W, Bulte E, Levin S.A. False alarm over environemental false alarms. Science. 2003;301:1187–1188. doi: 10.1126/science.1086646. [DOI] [PubMed] [Google Scholar]
- Palmer M.A, et al. Ecological science and sustainability for the 21st century. Front. Ecol. 2005;3:4–11. [Google Scholar]
- Palmer M, et al. Ecology: ecology for a crowded planet. Science. 2004;304:1251–1252. doi: 10.1126/science.1095780. [DOI] [PubMed] [Google Scholar]
- Pauly D, Christensen V, Dalsgaard J, Froese R, Torres J.F. Fishing down marine food webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- Peters R.L, Lovejoy T.E. Yale University Press; New Haven: 1992. Global warming and biological diversity. [Google Scholar]
- Peters C.M, Gentry A.H, Mendelsohn R.O. Valuation of an Amazonian rainforest. Nature. 1989;339:655–656. [Google Scholar]
- Pimm S.L, Russell G.J, Gittleman J.L, Brooks T.M. The future of biodiversity. Science. 1995;269:347–350. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- Ricketts T.H, Daily G.C, Ehrlich P.R, Michener C.D. Economic value of tropical forest to coffee production. PNAS. 2004;101:12 579–12 582. doi: 10.1073/pnas.0405147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels A, Altmann J. Baboons of the Amboseli basin—demographic stability and change. Int. J. Primatol. 1991;12:1–19. [Google Scholar]
- Schwartz M.W, Brigham C.A, Hoeksema J.D, Lyons K.G, Mills M.H, van Mantgen P.J. Linking biodiversity to ecosystem function: implications for conservation biology. Oecologia. 2000;122:297–305. doi: 10.1007/s004420050035. [DOI] [PubMed] [Google Scholar]
- Simberloff D, Doak D, Groom M, Trombulak S, Dobson A.P, Gatewood S, Soule M, Gilpin M, DelRio C, Mills L. Regional and continental restoration. In: Soule M, Terborgh J, editors. Continental conservation: scientific foundations of regional reserve networks. Island Press; Washington, DC: 1998. [Google Scholar]
- Sinclair A.R.E, Arcese P. Chicago University Press; Chicago: 1995. Serengeti II. Dynamics, management, and conservation of an ecosystem. [Google Scholar]
- Terborgh J. The big things that run the world—a sequel to E. O. Wilson. Conserv. Biol. 1988;2:402–403. [Google Scholar]
- Terborgh J. Princeton University Press; Princeton: 1989. Where have all the birds gone? [Google Scholar]
- Terborgh J, et al. Ecological meltdown in predator-free forest fragments. Science. 2001;294:1923–1926. doi: 10.1126/science.1064397. [DOI] [PubMed] [Google Scholar]
- Thornton I. Harvard University Press; Cambridge, MA: 1996. Krakatau. The destruction and reassembly of an island ecosystem. [Google Scholar]
- Tilman D. Ecological competition between algae: experimental confirmation of resource-based competition theory. Science. 1976;192:463–465. doi: 10.1126/science.192.4238.463. [DOI] [PubMed] [Google Scholar]
- Tilman D. Princeton University Press; Princeton: 1982. Resource competition and community structure. [PubMed] [Google Scholar]
- Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Sieman E. The influence of functional diversity and composition on ecosystem processes. Science. 1997;277:1300–1302. [Google Scholar]
- Turner R.E, Rabalais N.N. Coastal eutrophication near the Mississipi river delta. Nature. 1994;368:619–621. [Google Scholar]
- UNEP 2003 Proposed biodiversity indicators relevant to the 2010 target UNEP/CBD/SBSTTA/9/INF/26, Montreal.
- Vitousek P.M, Aber J.D, Howarth R.W, Likens G.E, Matson P.A, Schindler D.W, Schlesinger W.H, Tilman D.G. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 1997;7:737–750. [Google Scholar]
- Vitousek P.M, Mooney H.A, Lubchenco J, Melillo J.M. Human domination of earth's ecosystems. Science. 1997;277:494–499. [Google Scholar]
- Western D. Water availability and its influence on the structure and dynamics of a savannah large mammal community. East Afr. Wildl. J. 1975;13:265–286. [Google Scholar]
- Wilson E.O. The little things that run the world (the importance and conservation of invertebrates) Conserv. Biol. 1987;1:344–346. [Google Scholar]
- Wilson E.O. The current state of biological diversity. In: Wilson E.O, editor. Biodiversity. National Academy Press; Washington, DC: 1988. pp. 3–18. [Google Scholar]
- Zagat E.H, Zagat N.S. Zagat Survey; New York: 2004. Zagat Survey. New York City Restaurants. [Google Scholar]