Abstract
Since the demonstration, in 1998, of the phenomenon now widely known as ‘fishing down marine food webs’, and the publication of a critical rejoinder by Food and Agricultural Organization (FAO) staff, a number of studies have been conducted in different parts of the world, based on more detailed data than the global FAO fisheries statistics originally used, which established the validity and ubiquity of this phenomenon. In this contribution, we briefly review how, rather than being an artefact of biased data, this phenomenon was in fact largely masked by such data, and is in fact more widespread than was initially anticipated. This is made visible here by comparing two global maps of trophic level (TL) changes from the early 1950s to the present. The first presents the 50-year difference of the grand mean TL values originally used to demonstrate the fishing down effect, while the second is based on means above a cut-off TL (here set at 3.25), thus eliminating the highly variable and abundant small pelagic fishes caught throughout the world. Based on this, we suggest that using mean TL as ‘Marine Trophic Index’ (MTI), as endorsed by the Convention on Biological Diversity , always be done with an explicitly stated cut-off TL (i.e. cutMTI), chosen (as is the case with our proposed value of 3.25) to emphasize changes in the relative abundance of the more threatened, high-TL fishes. We also point out the need to improve the taxonomic resolution, completeness and accuracy of the national and international fisheries catch data series upon which the cutMTI is to be based.
Keywords: food webs, trophic level, overfishing, ecosystem impact, predatory fishes
1. Introduction
In February 2004, the Conference of the Parties to the Convention on Biological Diversity (CBD) identified a number of indicators to monitor progress toward reaching the target to ‘achieve by 2010 a significant reduction in the current rate of biodiversity loss’ (CBD 2004). This target, which is part of the Convention's Strategic Plan, demonstrates a desire by the CBD member countries (188 at the present time) to translate broad international policy commitments into action, leading to measurable results. The Marine Trophic Index (MTI) is one of the eight indicators that the Conference of the Parties of the CBD identified for ‘immediate testing’ of their ability to measure progress towards the 2010 target.
The term ‘MTI’ is in fact the CBD's name for the mean trophic level (TL) of fisheries landings, originally used by Pauly et al. (1998a) to demonstrate that fisheries, since 1950, are increasingly relying on the smaller, short-lived fishes and on the invertebrates from the lower parts of both marine and freshwater food webs. Thus, this contribution briefly reviews the origin of the TL concept, with emphasis on aquatic ecosystem. Also, we briefly review the work which, since the publication of Pauly et al. (1998a), established the widespread nature of TL changes in marine fisheries catches, and their usefulness in summarizing fisheries impact on marine ecosystems. In fact, it is these features that led to the mean TL of fisheries catches being suggested for ‘immediate testing’ as a biodiversity indicator by the Parties to the CBD. Also, we show how a slight modification of the manner in which mean TL are computed can make the resulting estimates less sensitive to environmental fluctuations than in the form that was originally proposed, and thus a better reflection of fisheries and other human impacts on marine biodiversity.
2. Trophic level: history and definitions
Predation is one of the key processes shaping the structure of marine ecosystems (Darwin 1876; Volterra 1926; Jones 1982; Walters & Juanes 1993; Pimm 2002). This has been widely understood for a long time, and we illustrate this here with a quote from William Shakespeare, where in response to a query about ‘how the fishes live in the sea’, the master fisher responds that that they do what we do on land, where ‘the great ones eat up the little ones’ (Pericles, Act I, Scene 1), which articulates a major feature of aquatic ecosystems. Owing to the viscosity of water, body shape—at least in pelagic fishes—is tightly constrained, and thus body size impacts on mouth size, and thence on prey size, the result being predictable predator/prey size ratios, with predators generally being three to four times longer than their prey (Ursin 1973).
The modern definition of TL, expressing the tendency of larger (and less abundant) fishes feeding on smaller (more abundant) fishes, which themselves feed on zooplankton, and all these animals resting upon primary producers, originated with the classics of Elton (1927) and Lindeman (1942). Therein, TL is a categorical entity, relying on the existence of other categorical entities such as primary producers, first-order consumers, second-order consumers, etc. Here, the role of biologists attempting to describe a food web consists of assigning organisms to TL-as-entities. Hence there came about the tendency in the literature inspired by this approach to simply list, and sometimes count, the species of fishes or the number of individuals in each of these categories (see, e.g. Bell & Harmelin-Vivien 1983), then perhaps construct ‘Eltonian’ or ‘Lindeman’ pyramids, instead of manipulating the TL themselves. This approach, which also involves archaeologists who describe fishes' remains (see, e.g. Wendrich & Van Neer 1994), was also used by the International Biological Programme (IBP), which described the world's biomes and ecosystems by quantifying biomasses and flows by categorical TL, and estimated crude transfer efficiencies between them (Golley 1993).
This approach, which treated TL as qualitative ‘concepts’, was rightly questioned by Rigler (1975), who argued that such concepts have no legitimate role in natural sciences such as ecology, which should rely on measurable objects or processes. Rigler's harsh criticism may have been of the elements leading to the demise of IBP. Yet, in the very same year, Odum & Heald (1975) published a solution to the problem Rigler had articulated, i.e. a method for computing the (fractional) TL of a consumer from the mean TL of its prey, plus one, starting from primary producers with a definitional TL of 1. This resulted, given the catholic nature of the diet of most marine consumers, in their having non-integer, species- and stage-specific TL, a theme to which we return below.
With this redefinition, TL became a definable property of organisms, similar to body temperature in humans, which can be measured by various methods, although it varies over time as a function of our state of health, this being the very reason why it can serve as an indicator of that state. The equation corresponding to this redefinition is
| (2.1) |
where TLj is the fractional TL of the prey j, and DCij represents the fraction of j in the diet of i (Christensen & Pauly 1992).
Thus defined, the TL of most fishes and other aquatic consumers can take any value between 2.0 and 5.0, the latter being rare even in large fishes (Cortés 1999), occurring only in specialized predators of marine mammals, such as killer whales or polar bears (Pauly et al. 1998a). Moreover, an index of omnivory (OI) can be computed from
where TLj and DCij are defined as in equation (2.1), and TLav as the weighted mean TL of the prey. Thus defined, OI also expresses the uncertainty associated with a TL estimate, and hence its square root can be used as a preliminary estimate of the standard error of TL (Christensen & Pauly 1992).
Note that the above definition of TL does not necessarily imply that its estimation must be based on diet composition data. An alternative method is to use the ratio of stable isotopes of nitrogen in their tissues, following calibration of isotope composition differences in consumers with different, known TL (DeNiro & Epstein 1981; Wada et al. 1991). Kline & Pauly (1998) validated this alternative approach through a comparison of TL estimates based on stable isotope and diet composition data for species and functional groups of the same ecosystems, which yielded a close match. We shall return in the following to issues of TL estimation after noting, however, that they are not crucial to the analyses of mean TL trends or of the fishing-in-balance (FiB) and MTI indices presented below.
3. Evolution and integrity of marine food webs
Here, to facilitate appreciation of how trends in the mean TL of fisheries catches reflect human impacts on marine ecosystems, we briefly recapitulate how, in the course of evolutionary time, marine food webs have acquired successive TLs and why, therefore, high TLs reflect high levels of evolved biodiversity.
The first living organisms to evolve on Earth, billions of years ago, were probably chemotrophs and, in the absence of grazers, they will have rapidly filled all suitable space (Lane 2002). This created opportunities for organisms that, rather than functioning as primary producers, could feed on the primary producers themselves; these were the first consumers. The emergence of photosynthesis and of metazoan consumers then led to food webs roughly similar to those we have now, with second- and third-order predators at the top, all invertebrates, and many belonging to now-extinct groups (Shrock & Twenhofel 1953; Lane 2002).
The evolution of vertebrates led to fishes, then reptilians replacing invertebrates on top of marine food webs, with only a few species of large squid able to compete (Froese et al. 2004). About 50 million years ago, the newly evolved marine mammals (Zimmer 1998) positioned themselves on top of the marine food web (Pauly et al. 1998c), a process facilitated by a number of attributes shared with the then extinct large marine reptiles (ichthyosaurs, mesosaurs, plesiosaurs). Among those was the breathing of atmospheric air, which not only enabled marine mammals to reach large size much more rapidly than fishes and invertebrates owing to the limiting effect of gills (Pauly 1997), but also to carry along their own supply into the oxygen-poor, deep-scattering layer of the ocean, thus overwhelming the main defence of the abundant lantern-fish (Family: Myctophidae) and associated fauna therein (Gjøsaeter & Kawaguchi 1980; Papastavrou et al. 1988).
Overall, though we are not better equipped than the founder of evolutionary biology to tell whether such addition of complexity can be labelled as ‘progress’ (Darwin 1876; pp. 98–99), it is clear that, over evolutionary time, more energetic and larger animals have tended to move onto the top of marine food webs. Moreover, it also evident that human impacts on marine food webs tend to reverse this evolutionary sequence (Pauly 1979; Parssons 1996; Jackson et al. 2001), in the worse, and increasingly common case, all the way back to the ill-named and bacteria-rich ‘dead zones’ that mimic major features of our primordial world (Rabalais et al. 2002).
4. Fishing down marine food webs
The original demonstration of the effect now widely known as ‘fishing down marine food webs’ by Pauly et al. (1998a) relied upon the global database of fishes landing assembled and maintained by the Food and Agricultural Organization (FAO) of the United Nations. This database includes, based on voluntary submissions, the annual fisheries catches (since 1950) of member countries, by species or groups of species (genera or families or larger groupings such as ‘miscellaneous fishes’). Importantly, these statistics are aggregated by the countries where the catches were landed, and not by the countries where they were taken (Watson et al. 2004). However, FAO also assigns the marine components of these catches to 18 large statistical areas (e.g. Northeast Atlantic; West Central Pacific), thus allowing at least some spatial disaggregation. Using the FAO data and TL estimates for over 200 species (or groups thereof; see below), mean TLs were computed, for each year k from
| (4.1) |
where Yi refers to the landings of species (group) i, as included in fisheries statistics. (Note that, ideally, mean TL should be based on catches, i.e. all animals killed by fishing (i.e. landings and discards; Alverson et al. 1994), rather than only on the landings included in FAO statistics, a problem to which we return below.)
The time-series of mean TL thus obtained showed, for most FAO areas, a smoothly declining trend, as did the time-series computed by combining the statistics from all FAO areas to represent global fisheries. Based on the assumption that the relative abundance of taxa in the landing data used in this analysis correlated with the relative abundance of the same taxa in the ecosystem, these declining trends were interpreted by Pauly et al. (1998a) as representing a decline in the abundance of high-TL fishes relative to low-TL fishes. From this, and given that high-TL fishes tend to grow slowly toward large sizes and are thus very sensitive to fishing effort (Jennings et al. 1998), it can be straightforwardly inferred that declining TL trends indicate declining abundances among the larger fishes on top of marine food webs, and thus impact on their biodiversity (both in terms of within-species abundance and, in the longer term, in term of number of species).
The demonstration of declining mean TL trends by Pauly et al. (1998a) had a large impact in the mass media, its message being relatively simple and easy to convey (e.g. Stevens 1998). Moreover, it also inspired a strong response and a large number of replications, our next topic.
5. Fishing down marine food webs: critique and replication
A number of FAO staff (Caddy et al. 1998), in an important critique, suggested that Pauly et al. (1998a) had overlooked that:
the composition of landings does not necessarily reflect relative abundance in the underlying ecosystems, and hence the taxonomic composition of the landings cannot be assumed to represent relative abundances in the ecosystem;
TLs change with size or age, and hence it is inappropriate to perform analyses of multispecies time-series using one TL estimate per species;
over-aggregated statistics may bias results, because the mean TL of ‘miscellaneous fishes’ and other such broad categories cannot be estimated reliably;
fishing down may be the deliberate policy of a given country to catch more of the abundant fishes at the bottom of marine food webs, and thus TL declines would not reflect more than a policy decision;
fishing down does not account for ‘bottom-up’ effects, for example, increases in low-TL fishes owing to increased eutrophication and thus primary production.
A brief response, addressing each of these five points, was published in Pauly et al. (1998b), but they will still be used to structure this contribution as they cover the entire range of features required for mean TL to perform adequately as an ecosystem indicator.
(a) The composition of landings does not necessarily reflect relative abundance in underlying ecosystem
While it is undoubtedly true that fishers tend to be selective (aiming at the most profitable species) when they develop a new fishery, it is also the case that, currently, with gears such as trawls to catch demersal fishes and driftnets and longlines to catch pelagic fishes, essentially all fishes and associated fauna in the areas where fisheries operate are impacted by them. Note that not all the animals killed need to appear in the landings, e.g. because they belong to that part of the by-catch (non-targeted species) that is discarded (Alverson et al. 1994). However, catches as defined above (i.e. landings and discards), will generally, if often incompletely, reflect the relative abundance of species in a given ecosystem. Exceptions do occur, one example being the above-mentioned lantern-fish, which, although extremely abundant throughout the world's oceans, are, with about 1 g of fish per m3 of water, generally too dilute to be exploited by fisheries (Gjøsaeter & Kawaguchi 1980). Cases of this sort, where a potential resource remains unexploited, can be quickly identified, however. Indeed, they may be viewed as the exception that challenges the rule, as in the case of Namibia, where a low-TL species (the pelagic goby Sufflogobius bibarbatus) has become very abundant but remains underexploited, thus precluding catches from reflecting changes in ecosystem abundance (Willemse & Pauly 2004).
One well-studied case, that of the Gulf of Thailand, documents that essentially the same mean TL trends are obtained, whether one uses landings data or data from trawl surveys, which directly sample ecosystems (Christensen 1998; Pauly & Chuenpagdee 2003). Moreover, empirical work by Pinnegar et al. (2002), who studied the Celtic Sea, documents that the skippers of fishing vessels do attempt to maintain in their catch a high proportion of high-value (and usually high-TL) fishes, irrespective of their relative abundance in the ecosystem. In such a case, the fishing down effect that is detected by monitoring catches will tend to underestimate corresponding changes in the ecosystem.
Other examples of ‘groundtruthing’ are the work of Valtysson & Pauly (2003) and other work reviewed in Pauly & Palomares (in press). Jointly, they confirm that the observation of downward TL trends is not an artefact of the difference between relative abundance in catches and in the ecosystem.
(b) Trophic levels change with size or age
The TLs of fishes do change with size, and consequently with age, and for most fishes, this change is an increase of TL from the larval stage, where TL≈3.0, owing to the larvae consuming mainly herbivorous zooplankton, to TL values well above 3 in zooplanktivores such as herring or sardine or above 4 in piscivores such as hake or cod (Pauly et al. 2001). The only exceptions to this pattern are detritivores such as grey mullet (Family: Mugilidae) and herbivores, common on coral reefs (see www.fishbase.org) and to which we return in §6.
One of the strongest, direct effects of fishing is to reduce the mean size and age of the species that are caught, and hence, in most cases, their mean TL. Pauly et al. (2001) developed two analytic models to express this, one size-based, the other age-based. Their application to data from the fisheries of Eastern Canada indicated that considering life-history changes in TL adds, if not strongly, to the fishing down effect. Thus, ontogenic changes in TL are not the reason that declines in mean TL are observed in typical fisheries catch time-series. In fact, explicit consideration of ontogenic TL changes accentuates such declines. The magnitude of this effect is low, however, relative to the impact of change in species composition, and this is the reason why we put emphasis on the quality of underlying catch data (see below).
(c) Over-aggregated catch statistics may bias results
When the detailed fisheries statistics for Western Europe (FAO area 27, covering the northeastern Atlantic), which document the catches in this region at the species level, are aggregated into genera, families and orders, the rate of mean TL declines. This is not surprising: the fishing down effect is about changes in species composition and the effect should thus disappear when the taxonomic information in a dataset is lost (Pauly & Palomares 2001).
The bulk of the fisheries statistics from low-latitude countries are reported by FAO at a very high degree of aggregation, i.e. as ‘miscellaneous fishes’ or ‘miscellaneous crustaceans’, etc., and hence contain little information about changes in species composition (Pauly & Palomares 2001). Thus, detecting the fishing down effect using data from a mix of low- and high-latitude areas, in spite of the masking effect of taxonomic over-aggregation, implies that the fishing down effect was even stronger than originally assumed (Pauly & Palomares 2005).
Over-aggregation of catch statistics is not only a taxonomic issue, but also a spatial problem (or a combination of both spatial and taxonomic problems). Thus, a small island state may report catch statistics pertaining to its inshore (reef) catch, implying a decline in mean TL, as groupers and other large, sensitive reef predators are fished out. The fisheries in this island state may then shift to offshore operations and target large pelagics such as tuna and billfish in offshore areas (see Zeller et al. (2003) for several case studies from the Caribbean). This would cause an increase in mean TL for the overall landing for that island, although both the reef and the large pelagic fishes in the waters around the country in question would display (if they were investigated separately) declining trends in mean TL. Pauly & Palomeres (2005) document several instances of spatial aggregation of this sort masking the fishing down effect.
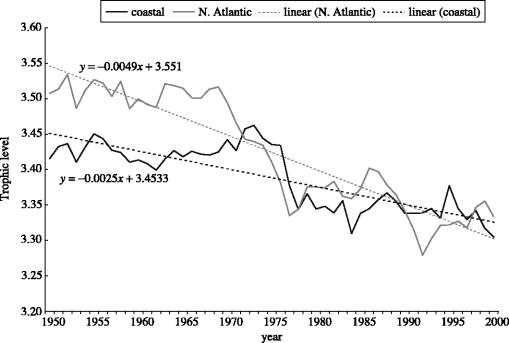
This spatial aggregation effect is largely overcome when catches are analysed that have been plotted on a sufficiently fine spatial scale, as in the case of the 180 000 half-degree latitude/longitude cells used by Watson et al. (2004) to map the global FAO fisheries statistics. Thus, figure 1, based on mean TL computed by pooling data from these 180 000 cells, documents a decline of mean TL stronger than originally documented by Pauly et al. (1998a).
Figure 1.
Trends in mean trophic levels of fisheries landings, 1950–2000, based on aggregation of data from over 180 000 half-degree latitude/longitude cells (based on spatial disaggregation method of Watson et al. (2004)). Data for the North Atlantic is shown in grey and for Coastal waters is shown in black. Observed and fitted data are represented by solid and dashed lines respectively. Note: strong decline, particularly in the North Atlantic.
As for the previous two points, what were perceived by Caddy et al. (1998) as a potential source of bias artificially generating a fishing down effect where none occurred, were, in fact, processes that masked the strength and ubiquity of this phenomenon.
(d) Fishing down as a deliberate policy to catch more fishes
Marine ecosystems operate as pyramids wherein the primary production generated at TL one is moved up toward the higher TL, with a huge fraction of that production being wasted in the process for the maintenance, reproduction and other activities of the animals in the systems (Winberg 1971; Pauly & Christensen 1995). Thus, it can be argued that, notwithstanding our preference for catching and consuming large predators, deliberately fishing down should enable more of an ecosystem's biological production to be captured by humans. However, to avoid waste here as well, any decline in the mean TL of the fisheries catches should, in this case, be matched by an ecologically appropriate increase in these catches, the appropriateness of that increase being determined by the transfer efficiency (TE) between TL. (Note that this refers to catches in biomass units. If individual fish numbers were to be counted, these considerations would imply an even stronger imbalance, i.e. that huge numbers of small fishes must be caught to compensate, in ecological term, for the large fishes caught at high TLs. However, this aspect of fishing down is not pursued here.)
Thus, an FiB index can be defined, which
will remain constant (remains=0) if TL changes are matched by ‘ecologically correct’ changes in catch;
will increase (>0) either if bottom-up effect occurs, for example, increase in primary production in the Mediterranean (which triggered Caddy et al.'s concerns), or if a geographical expansion of the fishery occurs, and the ‘ecosystem’ that is exploited by the fishery has been in fact expanded;
will decrease (<0) if discarding occurs that is not considered in the ‘catches’, or if the fisheries withdraw so much biomass from the ecosystem that its functioning is impaired.
The FiB index is defined as
| (3) |
where Y is the catch in year k, TL the mean trophic level in the catch, TE the mean TE (specific to an ecosystem, often set at 0.1; Pauly & Christensen 1995), i refers to species (groups) in the catch and 0 refers to any year used as a baseline to normalize the index.
Application of this index to the North Atlantic (Pauly et al. 2000) indicates that the observed decline in mean TL, though initially matched by an increase in catches, eventually led to decreasing FiB indices, i.e. the decline in catches did not compensate for the decline in mean TL. This effect also occurs for the world catch as a whole, which follows a similar trajectory of FiB increase, then decrease. Here, we conclude that, while fishing down the marine food web may be a deliberate policy, it is still reflective of an unsustainable trend, which in the long term threatens the integrity of marine ecosystems.
(e) Bottom-up effects are not accounted for
With this, Caddy et al. (1998) had in mind processes such as the eutrophication of the Mediterranean, which has indeed led to increases in the biomass and production of small pelagic fishes such as anchovies and sardines (Caddy 1993). Analysed naïvely, such increase of small pelagic fishes would lead, via a decrease of computed mean TL, to an inference of high-TL fishes becoming scarcer, even though the latter may not have declined in absolute terms. Pinnegar et al. (2003) document an effect of this sort for the Mediterranean, where decline in mean TL reported by Pauly et al. (1998a) may have resulted from the unwarranted inclusion of farmed (and planktivorous, and thus low-TL) bivalves in catch statistics.
Pauly et al. (1998a) noted a related problem owing to fluctuations in the abundance of Peruvian anchoveta (Engraulis ringens), whose enormous catches strongly influenced the mean TL of global catches. This, indeed, was one of the main reasons why they disaggregated their results by FAO areas. On the other hand, they did not want to exclude such abundant, widely variable species from their analysis, and thus expose themselves to the risk of being accused of having massaged their data by removing ‘inconvenient’ species.
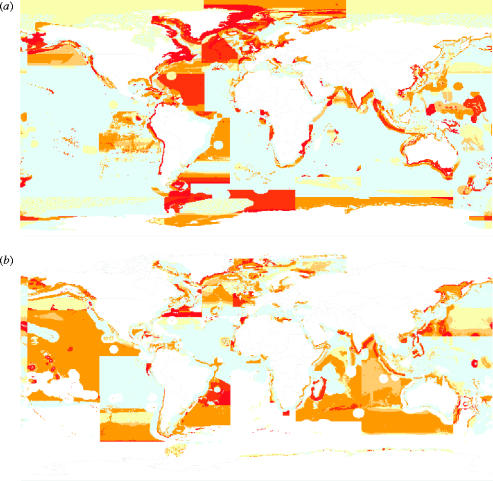
However, now that the fishing down effect has been established, in spite of the biasing effect of small pelagic fishes (or of farmed bivalves erroneously included in fisheries catch statistics), the time has come to propose that mean TL, if used to document fisheries impact on marine ecosystems, should generally be computed after excluding low-TL species from the analysis. Thus, we propose that the CBD's MTI, which is based on mean TL, should be in fact based on time-series that exclude low-TL organisms. This would lead to an indicator that may be labelled ‘cutMTI’, with the superscript referring to the lowest (cut-off) TL value used in the computation. We illustrate this with a global map of long-term change in 3.25MTI (figure 2), the value of 3.25 being here proposed as standard cut-off TL to eliminate (besides herbivores and detritivores) the planktivores whose high biomass tends to vary widely in response to environmental factors (e.g. in the case of the Peruvian anchoveta, Engraulis ringens; Muck 1989), and thus mask TL changes induced by fishing.
Figure 2.
Maps illustrating changes in MTI (i.e. mean trophic level of fisheries catches) from the early 1950s to the present. (a) Changes in grand mean TLs, corresponding to 2.0MTI (see text), based on FAO statistics disaggregated using method of Watson et al. (2004); (b) same data, but with trophic levels below 3.25 not included, and hence with maps representing changes in the values of 3.25MTI. Note: increase of the areas with negative changes, indicating that 3.25MTI is more sensitive than 2.0MTI. (In both panels, the straight borders between colours, and the areas of positive change, largely represent artefacts of the underlying FAO statistics; the white areas in panel (b) represent cells with catches of less than 1000 t.)
Figure 2 shows changes occurring over an area 1.6 times larger than suggested by using overall mean TL, corresponding to 2.0MTI. This demonstrates that cutMTI, when the cut-off TL is judiciously chosen, is a more powerful indicator than the grand mean TL levels used so far by the authors and their colleagues. Figure 2 also shows, by the same token, that bottom-up effects, like the other potential sources of bias listed by Caddy et al. (1998), only masked rather than generated the fishing down phenomenon.
6. Discussion
Recently, there has been considerable interest in the development and application of ecosystem-based indicators, notably through IOC/SCOR Working Group 119, on ‘Ecosystem Indicators for Fisheries Management’, whose work culminated, in April 2004, in an international conference held at the UNESCO Headquarters and devoted to this topic. This work, although it led to important advances, also illustrated that much confusion surrounds the notion of ecosystem indicators. Some believe that ecosystem indicators are whatever one can measure that impacts ecosystems, for example sea-surface temperatures. However, to be of any use, indicators must summarize in a single number a variety of complex processes that are otherwise hard to apprehend. Moreover, besides description, indicators must also allow for communication and, ideally, for intervention as well (Degnbol, in press). This is the case for the mean TL and the related MTI index, which describe a major aspect of the complex interactions between fisheries and marine ecosystems and communicate a measure of species replacement induced by fisheries. Specific MTI values, moreover, may eventually be used as targets for management interventions, though the present state of our knowledge on this does not allow for the identification of critical threshold TL values.
However, the present usefulness of the indicator is not based on a certain number (e.g. a 3.25MTI value of 3.72 being important); rather, it is the presence of a downward trend that matters. Sustainability, however defined, must imply some notion of permanence in at least some of the entities or processes being evaluated. Thus, if there is, in a given ecosystem, a clear trend of the relative abundance of high-TL vis-à-vis low-TL fishes, as indicated by declining MTI values, then this indicates the absence of sustainability and the need for intervention. A multispecies fishery can safely be assumed to be unsustainable if the mean TL of the species it exploits keeps going down.
Moreover, there are a number of countries in which TL declines are accompanied by stagnating or even declining catches, inducing the sharp declines in the FiB index corresponding to what Pauly et al. (1998a) called ‘backward bending curves’ (of TL versus catches). Such trends, which also describe what is happening to global fisheries, imply a collapse of the underlying ecosystems, and detecting the TL at which such collapse can be expected in different ecosystem types should become a major research topic.
Selecting cutMTI as an indicator will have a number of implications for the biodiversity, conservation and fisheries research communities. One such implication is that the quality of the underlying fisheries catch data must be improved. There are currently two major sources of global catch data: one is the FAO fisheries statistics (see www.fao.org). The other is the website of the Sea Around Us Project, which presents FAO catch data complemented with regional and national catch statistics, all re-expressed on a spatial basis (see Watson et al. (2004) and www.seaaroundus.org).
Ideally, FAO member countries, most of which also happen to be parties to the CBD, will have to increase the quality of the data they submit to FAO: these countries cannot monitor marine biodiversity if they do not monitor, in some taxonomic details, the fisheries catches extracted from their waters. In the meantime, the Sea Around Us Project will continue to work at improving the catch statistics of various countries with inaccurate and/or over-aggregated data, as already documented for China (Watson & Pauly 2001) or for the Eastern Caribbean in the contribution edited by Zeller et al. (2003).
The major source of TL values for the fish species caught by marine fisheries is FishBase (Froese & Pauly 2000; see www.fishbase.org), which contains TL estimates for thousands of species, based mainly on diet composition data. Various authors have suggested using, instead, estimates based on stable-isotope data. Although differences occur between these two sources of estimates, they have been shown to be closely correlated in at least in one case where both sets of data were available (Kline & Pauly 1998). This will be important, for example, for developing countries where the cost of performing stable-isotope analyses will often be prohibitive, but where, in many cases, a long tradition exists of studying the feeding habits of fishes through stomach contents analyses.
For some countries, notably those where coral reef fisheries are important, the cut-off TL proposed here of 3.25 is probably too high, as it eliminates the very herbivores whose occurrence in fisheries catches (and thus decline in the ecosystem) induces massive ecological changes, all detrimental to coral reef biodiversity (Pandolfi et al. 2003). Research will also be needed on the implications (e.g. in terms of forgone benefits and risks to biodiversity) of stagnating, low TL values, and on the TL recovery rates that can be expected under successful, ecosystem-based management.
Finally, a formal approach will have to be developed for combining the uncertainty associated with fisheries catches with the more easily estimated imprecision of TL estimates (see equation (4.1)), such that formal confidence intervals around cutMTI can be calculated. This is not presented here given our perception that the first task at hand is to reduce the uncertainty associated with national and international catch statistics datasets, notably by adding to these datasets as much of the IUU (illegal, unreported and unregulated) catches as possible.
Acknowledgments
We thank Mr Adrian Kitchingman for preparing the panels of figure 2; Dr Marjo Vierros of the Secretariat of the CBD for helpful comments, and Dr N. Balmford, Dr P. Crane, Dr R. Green and Dr G. Mace for their invitation to present our results at The Royal Society meeting devoted to moving ‘Beyond extinction rates: monitoring wild nature for the 2010 target’, held on 19–20 July 2004 in London. We also thank the Pew Charitable Trusts for initiating and supporting the Sea Around Us Project. D.P. also acknowledges support from Canada's National Science and Engineering Research Council.
Footnotes
One contribution of 19 to a Discussion Meeting Issue ‘Beyond extinction rates: monitoring wild nature for the 2010 target’.
References
- Alverson D.L, Freeberg M.H, Murawski S.A, Pope J.G. FAO Fisheries Technical Paper 339. Food and Agriculture Organization; Rome: 1994. A global assessment of fisheries bycatch and discards. [Google Scholar]
- Bell J.D, Harmelin-Vivien M.L. Fish fauna of Posidonia oceanica seagrass meadows. 2. Feeding habits. Tethys. 1983;11:1–14. [Google Scholar]
- Caddy J. Toward a comparative evaluation of human impacts on fisheries ecosystems of enclosed and semi-enclosed seas. Rev. Fish. Sci. 1993;1:57–95. [Google Scholar]
- Caddy J, Csirke J, Garcia S.M, Grainger R.J.L. How pervasive is ‘Fishing down marine food webs’? Science. 1998;282:1383. (full text (p. 1383a) on www.sciencemag.org/cgi/content/full/282/5393/1383a?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&author1=PAULY&searchid=1108594059175_11362&stored_search=&FIRSTINDEX=0&fdate=1/1/1998&tdate=12/31/1998) [Google Scholar]
- CBD 2004 Annex I, decision VII/30. The 2020 biodiversity target: a framework for implementation, p. 351. Decisions from the Seventh Meeting of the Conference of the Parties of the Convention on Biological Diversity, Kuala Lumpur, 9–10 and 27 February 2004. Montreal: Secretariat of the CBD.
- Christensen V. Fishery-induced changes in a marine ecosystem: insights from the models of the Gulf of Thailand. J. Fish Biol. 1998;53(Suppl. A):128–142. [Google Scholar]
- Christensen V, Pauly D. The ECOPATH II—a software for balancing steady-state ecosystem models and calculating network characteristics. Ecol. Modell. 1992;61:169–185. [Google Scholar]
- Cortés E. Standardized diet compositions and trophic levels of sharks. ICES J. Mar. Sci. 1999;56:707–717. [Google Scholar]
- Darwin C. 6th edn. John Murray; London: 1876. On the origin of species by means of natural selection, or on the preservation of favoured races in the struggle for life. [Google Scholar]
- Degnbol, P. In press. Indicators as a means to communicate knowledge. ICES J. Mar. Sci.
- DeNiro M.J, Epstein S. Influence of diet on the distribution of nitrogen isotope in animals. Geochim. Cosmochim. Acta. 1981;45:341–353. [Google Scholar]
- Elton C. Sidgwick & Jackson; London: 1927. Animal ecology. [Google Scholar]
- Froese R, Pauly D, editors. FishBase 2000: concepts, design and data sources. International Centre for Living Aquatic Resources Management; Los Baños: 2000. (distributed with four CD-ROMs; see www.fishbase.org for updates) [Google Scholar]
- Froese R, Garthe S, Piatowski U, Pauly D. Trophic signatures of marine organisms in the Mediterranean as compared with other ecosystem. Belg. J. Zool. 2004;134(Suppl. 1):25–32. [Google Scholar]
- Gjøsaeter J, Kawaguchi K. Fisheries Technical Paper 193. Food and Agriculture Organization; Rome: 1980. A review of the world resources of mesopelagic fish. [Google Scholar]
- Golley F.B. Yale University Press; New Haven: 1993. A history of the ecosystem concept in ecology. [Google Scholar]
- Jackson J.B.C, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- Jennings S, Reynolds J.D, Mills S.C. Life history correlates of responses to fisheries exploitation. Proc. R. Soc. B. 1998;265:333–339. [Google Scholar]
- Jones R. Ecosystems, food chains and fish yields. In: Pauly D, Murphy G, editors. Theory and management of tropical fisheries. ICLARM Conference Proceedings 9. International Centre for Living Aquatic Resources Management; Manila: 1982. pp. 195–239. [Google Scholar]
- Kline T.C, Jr, Pauly D. Cross-validation of trophic level estimates from a mass-balance model of Prince William Sound using 15N/14N data. In: Quinn T.J II, Funk F, Heifetz J, Ianelli J.N, Powers J.E, Schweigert J.F, Sullivan P.J, Zhang C.-I, editors. Proceedings of the International Symposium on fishery stock assessment models. Alaska Sea Grant College Program Report No. AK-SG-98-01. University of Alaska; Fairbanks: 1998. pp. 693–702. [Google Scholar]
- Lane N. Oxford University Press; Oxford: 2002. Oxygen: the molecule that made the world. [Google Scholar]
- Lindeman R.L. The trophic dynamic concept in ecology. Ecology. 1942;23(4):399–418. [Google Scholar]
- Muck P. Major trends in the Peruvian upwelling ecosystem and their implications for management. In: Pauly D, Muck P, Mendo J, Tsukayama I, editors. The Peruvian upwelling ecosystem: dynamics and interactions. ICLARM Conference Proceedings 18. International Centre for Living Aquatic Resources Management; Manilla: 1989. pp. 386–403. [Google Scholar]
- Odum W.E, Heald E.J. The detritus-based food web of an estuarine mangrove community. In: Cronin L.E, editor. Estuarine research. vol. 1. Academic Press; New York: 1975. pp. 265–286. [Google Scholar]
- Pandolfi J.M, et al. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- Papastavrou V, Smith S.C, Whitehead H. Diving behaviour of the sperm whale, Physeter macrocephalus, off the Galápagos Islands. Can. J. Zool. 1988;67:839–846. [Google Scholar]
- Parssons, T. R. 1996 The impact of industrial fisheries on the trophic structure of marine ecosystems. In Food webs: integration of patterns and dynamics (ed. G. A. Polis & K. D. Winnemiller), ch. 33. New York: Chapman & Hall.
- Pauly D. Biological overfishing of tropical stocks. ICLARM Newslett. 1979;2:3–4. [Google Scholar]
- Pauly D. Geometrical constraints on body size. Trends Ecol. Evol. 1997;12:442–443. doi: 10.1016/s0169-5347(97)85745-x. [DOI] [PubMed] [Google Scholar]
- Pauly D, Christensen V. Primary production required to sustain global fisheries. Nature. 1995;374:255–257. [Google Scholar]
- Pauly D, Chuenpagdee R. Development of fisheries in the Gulf of Thailand large marine ecosystem: analysis of an unplanned experiment. In: Hempel G, Sherman K, editors. Large marine ecosystems of the world 12: change and sustainability. Elsevier Science; Amsterdam: 2003. pp. 337–354. [Google Scholar]
- Pauly D, Palomares M.L. Fishing down marine food webs: an update. In: Bendell-Young L, Gallaugher P, editors. Waters in peril. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. pp. 47–56. [Google Scholar]
- Pauly, D. & Palomares, M.L. 2005 Fishing down marine food web: it is far more pervasive than we thought. Bull. Mar. Sci.
- Pauly D, Christensen V, Dalsgaard J, Froese R, Torres F.C., Jr Fishing down marine food webs. Science. 1998;279:860–863. doi: 10.1126/science.279.5352.860. [DOI] [PubMed] [Google Scholar]
- Pauly D, Froese R, Christensen V. How pervasive is ‘Fishing down marine food webs’: response to Caddy et al. Science. 1998;282:1383. doi: 10.1126/science.279.5352.860. (full text (p. 1383a) on www.sciencemag.org/cgi/content/full/282/5393/1383a?maxtoshow=&HITS=10&hits=10&RESULTFORMAT=&author1=PAULY&searchid=1108594059175_11362&stored_search=&FIRSTINDEX=0&fdate=1/1/1998&tdate=12/31/1998) [DOI] [PubMed] [Google Scholar]
- Pauly, Trites A, Capuli E, Christensen V. Diet composition and trophic levels of marine mammals. ICES J. Mar. Sci. 1998;55:467–481. [Google Scholar]
- Pauly D, Christensen V, Walters C. Ecopath, ecosim and ecospace as tools for evaluating ecosystem impact of fisheries. ICES J. Mar. Sci. 2000;57:697–706. [Google Scholar]
- Pauly D, Palomares M.L, Froese R, Sa-a P, Vakily M, Preikshot D, Wallace S. Fishing down Canadian aquatic food webs. Can. J. Fish. Aquat. Sci. 2001;58:51–62. [Google Scholar]
- Pimm S.L. University of Chicago Press; Chicago: 2002. Food webs. [Google Scholar]
- Pinnegar J.K, Jennings S, O'Brien C.M, Polunin N.V.C. The effects of exploitation and environmental variability on the trophic structure of the Celtic Sea fish community. J. Appl. Ecol. 2002;39:377–390. [Google Scholar]
- Pinnegar J.K, Badalamenti F, Polunin N.V.C. Long-term changes in the trophic level of western Mediterranean fishery and aquaculture landings. Can. J. Fish. Aquat. Sci. 2003;60:222–235. [Google Scholar]
- Rabalais N.N, Turner R.E, Wiseman W.J., Jr Hypoxia in the Gulf of Mexico, a.k.a. ‘the dead zone’. Annu. Rev. Ecol. Syst. 2002;33:235–263. [Google Scholar]
- Rigler F.H. In: The concept of energy flow and nutrients flows between trophic levels. van Dobben W.H, Lowe McConnell R.H, editors. Dr W. Junk B.V. Publishers; The Hague: 1975. pp. 15–26. [Google Scholar]
- Shrock R.R, Twenhofel W.H. McGraw-Hill; New York: 1953. Principles of invertebrate paleontology. [Google Scholar]
- Stevens, W. K. 1998 Man moves down marine food chain, creating havoc. The New York Times, 10 February 1998, B: p 12.
- Ursin E. On the prey preference of cod and dab. Meddelelser fra Danmarks Fiskeri-og Havundersogelser N.S. 1973;7:85–98. [Google Scholar]
- Valtysson H.Þ, Pauly D. Fishing down the food web: an Icelandic case study. In: Guðmundsson E, Valtysson H.Þ, editors. Competitiveness within the global fisheries. Proceedings of a Conference held in Akureyri, Iceland, on April 6–7th 2000. University of Akureyri; Akureyri, Iceland: 2003. pp. 12–24. [Google Scholar]
- Volterra V. Variations and fluctuations of individuals of animals living together. In: Chapman R.N, editor. Animal ecology, with special reference to insects. McGraw Hill; New York: 1926. pp. 409–448. [Google Scholar]
- Wada E, Mizutani H, Minagawa M. The use of stable isotopes for food web analysis. Crit. Rev. Food Sci. Nutr. 1991;30:361–371. doi: 10.1080/10408399109527547. [DOI] [PubMed] [Google Scholar]
- Walters C.J, Juanes F. Recruitment limitation as a consequence of natural selection for use of restricted feeding habitat and predation risk-taking by juvenile fishes. Can. J. Fish. Aquat. Sci. 1993;50:2058–2070. [Google Scholar]
- Watson R, Pauly D. Systematic distortions in world fisheries catch trends. Nature. 2001;414:534–536. doi: 10.1038/35107050. [DOI] [PubMed] [Google Scholar]
- Watson R, Kitchingman A, Gelchu A, Pauly D. Mapping global fisheries: sharpening our focus. Fish Fish. 2004;5:168–177. [Google Scholar]
- Wendrich, W. Z. & Van Neer, W. 1994 Preliminary notes on fishing gears and fish at the late Roman fort at ‘Abu Sha'ar’ (Egyptian Red Sea coast). In Fish Exploitation in the Past: Proceedings of the Seventh Meeting of the ICAZ Fish Remains Working Group (ed. W. Van Neer), 274. Tervuren, Belgium: Annales du Musee Royale pour L'Afrique Central.
- Willemse N.E, Pauly D. Reconstruction and interpretation of marine fisheries catches from Namibian waters, 1950 to 2000. In: Sumaila U.R, Boyer D, Skog M, Steinshamm S.I, editors. Namibia's fisheries: ecological, economic and social aspects. Eburon; The Netherlands: 2004. pp. 99–112. [Google Scholar]
- Winberg G.G. Academic Press; London: 1971. Methods for the estimation of production in aquatic animals. [Google Scholar]
- Zeller, D., Booth, S., Mohammed, E. & Pauly, D. 2003 From Mexico to Brazil: Central Atlantic fisheries catch trends and ecosystem models. Fisheries Centre Research Reports 11, Fisheries Centre, University of British Columbia, Vancouver.
- Zimmer C. Simon & Schuster; New York: 1998. At the water's edge: fish with fingers, whales with legs and how life came ashore and went back to sea. [Google Scholar]