Abstract
The different regulation of sister chromatid cohesion at centromeres and along chromosome arms is obvious during meiosis, because centromeric cohesion, but not arm cohesion, persists throughout anaphase of the first division. A protein required to protect centromeric cohesin Rec8 from separase cleavage has been identified and named shugoshin (or Sgo1) after shugoshin (‘guardian spirit’ in Japanese). It has become apparent that shugoshin shows marginal homology with Drosophila Mei-S332 and several uncharacterized proteins in other eukaryotic organisms. Because Mei-S332 is a protein previously shown to be required for centromeric cohesion in meiosis, it is now established that shugoshin represents a conserved protein family defined as a centromeric protector of Rec8 cohesin complexes in meiosis. The regional difference of sister chromatid cohesion is also observed during mitosis in vertebrates; the cohesion is much more robust at the centromere at metaphase, where it antagonizes the pulling force of spindle microtubules that attach the kinetochores from opposite poles. The human shugoshin homologue (hSgo1) is required to protect the centromeric localization of the mitotic cohesin, Scc1, until metaphase. Bub1 plays a crucial role in the localization of shugoshin to centromeres in both fission yeast and humans.
Keywords: centromeric protection, shugoshin, cohesin
1. Introduction
Sister chromatid cohesion is carried out by a multi-subunit complex; cohesin comprises two structural maintenance of chromosome (SMC) family proteins, Smc1 and Smc3, and two accessory subunits, Scc1 (also called ‘Mcd1’ in Saccharomyces cerevisiae and ‘Rad21’ in Saccharomyces pombe) and Scc3 (Nasmyth 2001; Uhlmann 2003). Recent studies suggest that Smc1–Smc3 heterodimers, using their long stretches of coiled coil, topologically embrace DNA strands (Haering & Nasmyth 2003). Scc1 interacts with the two ends of the cohesin ring, thereby presumably closing it. Cohesion is maintained until metaphase when sister kinetochores attach to microtubules emanating from the opposite spindle poles. The cohesion at centromeres is especially important at this stage because the establishment of bi-polar spindle attachment depends on the tension generated by the pulling force of spindle microtubules and the counteracting force of centromeric cohesion of sister chromatids. At the onset of anaphase, the anaphase promoting complex (APC) dependent degradation of securin (Pds1 in S. cerevisiae and Cut2 in S. pombe) allows the activation of a specific endopeptidase, separase (Esp1 in S. cerevisiae and Cut1 in S. pombe), which in turn cleaves Scc1/Rad21 (Nasmyth 2001; Uhlmann 2003). Thereby, the cohesin complex is disrupted to release sister chromatid cohesion, resulting in the separation of sister chromatids (figure 1). Although these principles of sister chromatid cohesion and its regulation are presumably applicable to all chromosomes of all eukaryotic organisms, there are some modifications depending on the regions of the chromosome and on whether it is mitosis or meiosis.
Figure 1.
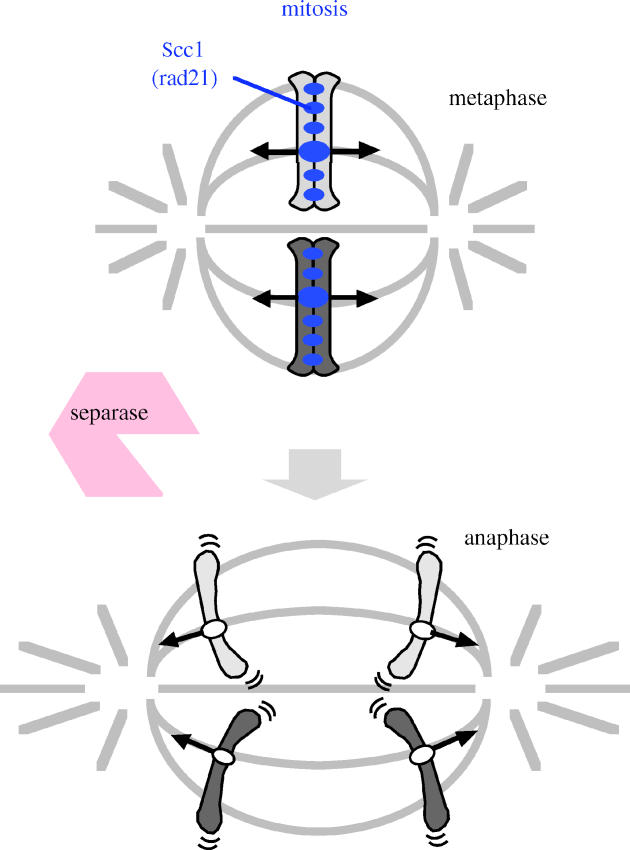
Schematic drawing of mitotic chromosome segregation and behaviour of cohesin Scc1/Rad21. At the onset of anaphase, mitotic cohesin Scc1/Rad21 is cleaved along the whole chromosome length by separase.
2. The mechanism by which cohesin is localized along chromosomes
How is the localization of cohesin along chromosomes regulated? Cohesin associates with chromosomes before DNA replication by a process that depends largely on the Scc2/Scc4 cohesin loading complexes (Ciosk et al. 2000; Tomonaga et al. 2000). Initial inspections of cohesin localization along chromosomes in budding yeast suggested that cohesin is enriched around centromeres and at several sites along the arm regions, spaced roughly every 5–10 kbp (Blat & Kleckner 1999; Tanaka et al. 1999). A precise inspection of cohesin localization performed recently in budding yeast suggests that cohesin is initially loaded onto Scc2/Scc4 binding sites, and may quickly slide to more permanent sites usually located at transcriptionally convergent regions (Glynn et al. 2004; Lengronne et al. 2004). Crucially, the location of cohesin is directed by the active transcription of the flanking genes, but not by the DNA sequences. These and other experiments have suggested that the transcription apparatus pushes cohesin; consequently, cohesin passes through tandem genes until it reaches a site of convergence. It is suggested that this property of cohesin may be important in preventing the obstruction of transcription, although this has not been shown experimentally. Because the transcription convergence sites are distributed randomly along the chromosome length, sister chromatid cohesion is scattered evenly over entire chromosomes. Thus, sister chromatid cohesion along the chromosome arm may be more flexible than previously thought. This feature of cohesin localization seems well conserved between budding yeast and fission yeast (Lengronne et al. 2004) and, therefore, could be applicable to other eukaryotes. The dynamic behaviour of cohesin along the chromosome appears to support the ring model of cohesin, which predicts considerable mobility when cohesin associates with chromatin (Haering & Nasmyth 2003).
Cytological observations in vertebrates have indicated that cohesion is strong in the vicinity of centromeres and, as expected, cohesin is indeed enriched in this region (Hauf et al. 2001). The extensive location of cohesin at centromeres cannot be explained simply by the conventional cohesin loading model. The data obtained in budding yeast suggest that the formation of functional kinetochores actively enriches cohesin to the centromeres, although the identity of the factor that recruits cohesin is still not clear (Megee & Koshland 1999; Tanaka et al. 1999; Weber et al. 2004). In fission yeast, peri-centromeric heterochromatin plays a crucial role in recruiting cohesin to the centromeres, and the inactivation of heterochromatin formation by genetic mutations abolishes the centromere-directed location of cohesin (Bernard, et al. 2001b; Nonaka et al. 2002). Indeed, sister chromatid cohesion in heterochromatin-deficient cells is regionally impaired at centromeres, with an accompanying disturbance of faithful chromosome segregation at mitosis (Bernard 2001b). The recruitment of cohesin by heterochromatin is not specific to centromeres, because cohesin is also enriched at the silent mating type loci on the chromosome arms, where the formed heterochromatin plays a crucial role in inactivating transcription. The cohesin subunit Psc3 (the Scc3 homologue in S. pombe) interacts in a two-hybrid assay with Swi6, an S. pombe homologue of the heterochromatin protein HP1, suggesting a direct role for heterochromatin in recruiting cohesin complexes (Nonaka et al. 2002). These findings may explain why heterochromatin is formed around centromeres in almost all cell species, and stress the importance of centromeric cohesion in eukaryotic chromosome segregation. Indeed, a recent report asserted that heterochromatin formation at centromeres plays a crucial role in concentrating cohesin and robust cohesion at centromeres in animal cells (Fukagawa et al. 2004).
3. The conserved protein shugoshin protects centromeric cohesin in meiosis I
Meiosis consists of two rounds of chromosome segregation following a single round of DNA replication, leading to the formation of four haploid gametes from a diploid germ cell. During the first meiotic division (meiosis I), homologous chromosomes (homologues) pair to recombine, forming chiasmata in which one sister chromatid from one homologue is covalently attached to a sister chromatid from the other homologue. Hence, in order for homologues to segregate at meiosis I, sister chromatid cohesion must be released along the chromosome arms to resolve chiasmata. However, sister chromatid cohesion is retained at the centromeres until meiosis II, when sister chromatids segregate as they do in mitosis, using the residual centromeric cohesion (figure 2). Thus, meiotic divisions require sister chromatid cohesion to be released in two steps. However, the molecular basis for the protection of centromeric cohesion only during meiosis I and only at the centromeres has long remained a mystery. The heterochromatin-dependent cohesin enrichment at centromeres contributes to this centromere-specific protection of cohesin at meiosis I, because fission yeast heterochromatin mutants lose the peri-centromere-associated cohesin as well as persisting centromeric cohesion in meiosis (Kitajima et al. 2003). However, this does not explain meiosis I-specific protection because heterochromatin is present throughout meiosis.
Figure 2.
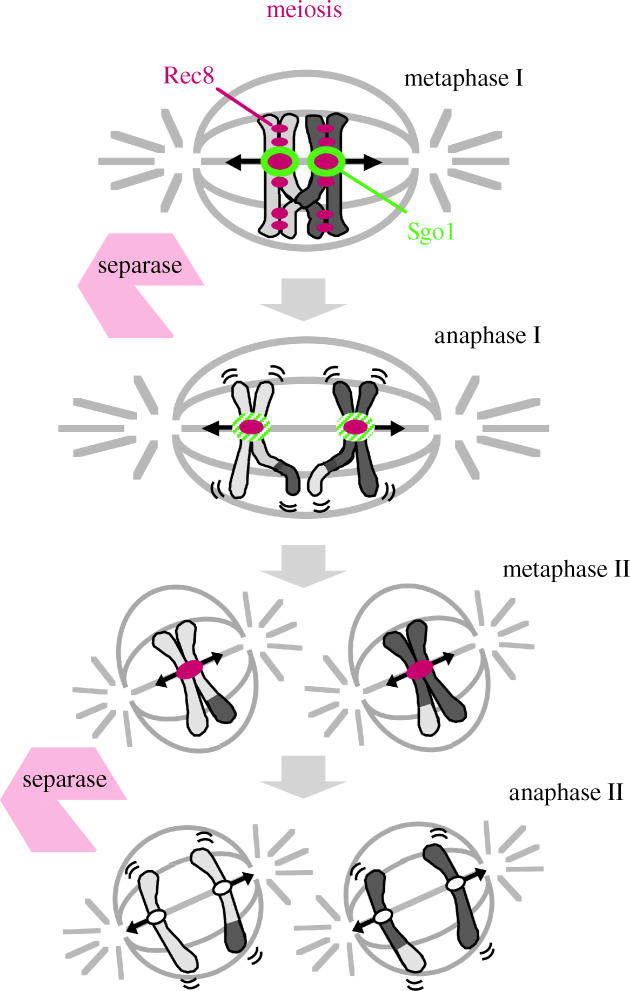
Schematic drawing of meiotic chromosome segregation and behaviour of cohesin Rec8. At the onset of anaphase I, meiotic cohesin Rec8 is cleaved only along the chromosome arms, while centromeric Rec8 is protected by Sgo1.
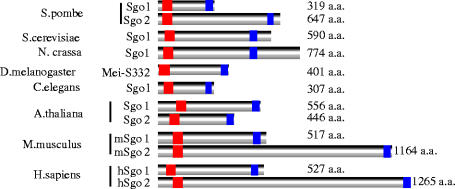
The replacement of the mitotic cohesin, Rad21/Scc1, with the meiotic version, Rec8, is a prerequisite for protecting centromeric sister cohesion through anaphase of meiosis I (Watanabe & Nurse 1999; Toth et al. 2000; Yokobayashi et al. 2003). When Rec8 is expressed ectopically during mitosis, however, Rec8 localization at centromeres disappears at anaphase, with sister chromatids segregating to the opposite sides. These observations suggest a meiosis I-specific centromeric protector of Rec8. Drosophila MEI-S332, a protein that resides at centromeres and is required for the persistence of centromeric cohesion during meiosis I, has features of a candidate protector (Lee & Orr-Weaver 2001). However, the failure to find counterparts in other organisms prevents it being a favourite for the role. To identify a Rec8 protector in fission yeast, we searched a cDNA library prepared from the mRNA of meiotic cells to obtain a gene that yields toxicity during mitotic growth only when co-expressed with Rec8. This screening identified a novel gene encoding a meiosis-specific protein named Sgo1, or ‘shugoshin’ (Japanese for ‘guardian spirit’; Kitajima et al. 2004). Independent ‘knock-out’ screenings in fission yeast and budding yeast also revealed the sgo1/SGO1 gene (Marston et al. 2004; Rabitsch et al. 2004). Fission yeast Sgo1 localizes at the pericentromeric heterochromatin region, the site at which Rec8 was predicted to play a role in centromeric protection during meiosis I (Kitajima et al. 2003). Sgo1 disappeared from centromeres during anaphase I and does not reappear during meiosis II. In the absence of Sgo1, sister chromatids co-segregate to the same pole at meiosis I, suggesting that mono-polar attachment is intact. However, they start to separate precociously during anaphase I. Consequently, because the residual centromeric cohesion is required for bi-polar attachment at metaphase II, the sister chromatids segregate randomly at meiosis II. Thus we conclude that Sgo1 plays a crucial role in protecting centromeric Rec8 from degradation during meiosis I (figure 2). Budding yeast Sgo1 also plays an essential role in protecting centromeric Rec8 at meiosis I (Kitajima et al. 2003; Katis et al. 2004; Marston et al. 2004). Remarkably, it turns out that shugoshin shares a hitherto unperceived similarity to MEI-S332, and constitutes a conserved protein family in eukaryotes (Kitajima et al. 2004; Rabitsch et al. 2004; figure 3).
Figure 3.
Shugoshin constitutes a conserved protein family in eukaryotic organisms.
Curiously, fission yeast possess a paralogue of Sgo1, called Sgo2. Although Sgo1 is expressed only around meiosis I, Sgo2 is ubiquitously expressed throughout mitosis and meiosis. The deletion of Sgo2 is not lethal, but results in a modest defect in the fidelity of chromosome segregation in mitosis, as well as homologue segregation in meiosis I (Kitajima et al. 2004; Rabitsch et al. 2004). Sgo2, like Sgo1, localizes at the pericentromeric region at metaphase; however, it is totally dispensable for the centromeric protection of Rec8 during meiosis I. Similarly, Sgo2 is not required for protecting Rad21/Scc1 at centromeres, because the amount of Rad21/Scc1 at centromeres does not change by Sgo2 depletion. A conserved checkpoint kinase, Bub1, also plays a crucial role in centromeric cohesin in meiosis in fission yeast (Bernard, 2001a). Bub1 apparently regulates shugoshin localization to kinetochores, as the centromeric localization of either Sgo1 or Sgo2 is apparently abolished in bub1 mutants (Kitajima et al. 2004). The precise mechanism for the regulation of shugoshin localization by Bub1 remains unsolved.
4. Sister chromatids are resolved prior to anaphase in vertebrate mitosis
During mitosis in vertebrate cells, chromosomes are compacted along the longitudinal axis, mainly depending on a protein complex, condensin (Hirano 2002). This step probably coordinates with the resolution of sister chromatids, otherwise they would become heavily tangled at anaphase. Evidence in vertebrates indicates that during prophase, most, but not all, cohesin complexes dissociate from the chromosomes while condensin enters the chromosomes (Losada & Hirano 2001). Cytological observations indicate that sister chromatid cohesion is partly resolved by metaphase along the chromosome arm regions but not at the centromeres, and prolonged arrest at this stage brought about by the addition of a microtubule destabilizing drug, nocodazole, leads to the complete separation of chromosome arms, forming X-shaped chromosomes. The expression of a non-cleavable Scc1 in HeLa cells demonstrated that the cleavage of Scc1 is not required for its dissociation at prophase, but is crucial for the separation of sister chromatids at the onset of anaphase (Hauf et al. 2001). The dissociation of cohesin at prophase, which is largely dependent on the activities of two mitotic kinases, Aurora B and polo-like kinase (Plx1), happens at the same time with the association of condensin to chromosomes, which is involved in the process of compaction of metaphase chromosomes (Losada et al. 2002; Sumara et al. 2002; figure 4). However, the simultaneous inactivation of Aurora B and Plx1 inhibits the release of cohesin at prophase, but not the compaction of chromosomes. Remarkably, on compacted chromosomes fully associated with cohesin, sister chromatid resolution at metaphase turns out to be compromised (Losada et al. 2002). Thus, in order for sister chromatid resolution at mitosis to occur, much of the cohesin must be released long before the onset of anaphase, the time when the residual cohesin is finally disrupted by separase cleavage. The arm-specific dissociation of cohesin in pro- and prometaphase suggests that the protection of centromeric cohesin could be applicable to mitosis in animal cells. However, the identities of the molecules that protect cohesin in the vicinity of centromeres in vertebrate mitotic cells have remained elusive.
Figure 4.
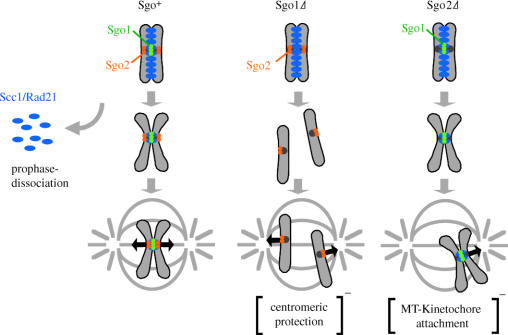
Human Sgo1 protect centromeric Scc1/Rad21 from prophase dissociation in M-phase. In contrast, human Sgo2 is dispensable for centromeric protection but required for the metaphase congression of chromosomes.
5. Two putative human shugoshin homologues are expressed during mitosis
We identified two putative human Sgo proteins, Q9BVA8 (Sgo1) and Q8IZK1 or Tripin (Sgo2), in the database, although their overall sequence homology to known Sgo proteins in other species is marginal (Kitajima et al. 2004; figure 3). To examine whether these proteins are indeed Sgo homologues in humans, we first examine the localization of these proteins. For this, we raised rabbit polyclonal antibodies against recombinant proteins produced in bacteria. Western blotting in HeLa cell extracts indicated that both Sgo1 and Sgo2 are expressed at least in proliferating HeLa cells (our unpublished data).
To examine whether these proteins are indeed human Sgo homologues, we examined the intracellular localization of human Sgo1 and Sgo2 by immunostaining HeLa cells. We found that both Sgo1 and Sgo2 show very similar localizations during the mitotic cell cycle. Neither was detected in interphase cells; however, strong punctate signals appeared throughout prophase until metaphase. These signals dramatically decreased at the onset of anaphase, and almost completely disappeared by telophase. Counterstaining for CENP-A, a variant of histone H3 known to localize at kinetochores, revealed that the Sgo1 and Sgo2 signals localized closely with CENP-A in prometaphase. In prometaphase and metaphase, when the sister kinetochores are pulled outwards by spindle microtubules that stretch the centromeric regions, the locations of Sgo1 were virtually identical to those of Aurora B (an inner centromere protein), whereas Sgo2 localizes just outside the region of Aurora B staining (our unpublished data). Thus, two human Sgo-related proteins localize mainly to the inner centromere throughout prometaphase until metaphase. As S. pombe Sgo1 and Sgo2 localize at pericentromeric heterochromatin regions, which correspond structurally to the mammalian inner centromere, these results thus suggest that human Sgo1 and Sgo2 are indeed Sgo homologues.
6. Sgo1 is required for centromeric protection in vertebrate mitosis
An attractive possibility is that human Sgo proteins are required to protect centromeric cohesion during prophase until metaphase, when most cohesin dissociates from chromosomes. To examine this idea, we knocked down the Sgo protein by RNAi in mitotic HeLa cells and spread the chromosomes to observe the chromosome structure directly. In normal cells, cohesion is tightly preserved in the centromeric region at mitosis, showing a specific structure called ‘primary constriction’. Strikingly, in Sgo1-repressed cells, chromosomes are often resolved along the whole chromosome length with the loss of the primary constriction, although most sister chromatids remained in close proximity. Thus, Sgo1 is apparently responsible for preserving centromeric cohesion, consistent with the recent report by Salic et al. (2004) (figure 4). Moreover, the population of cells with unaligned chromosomes increased in the Sgo1-depleted cells, the phenotype of which was completely suppressed by the repression of BubR1 or Mad2 (our unpublished data). These observations suggest a scenario in which the failure of persisting cohesion at the centromeres caused by Sgo1-repression provokes a spindle checkpoint, resulting in the accumulation of the prometaphase population. Importantly, we found that cohesin Scc1 is displaced from centromeres during prometaphase in the Sgo1-repressed cells whereas Scc1 persists at centromeres in normal cells. These results suggest that the main role of Sgo1 in mitosis is to protect centromeric cohesin from prophase dissociation. It has been reported that Scc1-defective cells show chromosome misalignment probably the loss of tension at the kinetochores has destabilized kinetochore attachment (Sonoda et al. 2001; Hoque & Ishikawa 2002). It is therefore reasonable to think that a loss of sister chromatid cohesion is the primary cause of the aberrant chromosome congression observed in Sgo1-repressed cells.
In parallel experiments, we repressed Sgo2 by RNAi and found that Sgo2-repressed cells also showed a delay in prometaphase, but to a lesser extent compared with Sgo1-repressed cells. In the prometaphase cells, several chromosomes were frequently associated with either spindle pole, whereas the majority of chromosomes aligned at the metaphase plate, a typical phenotype observed when kinetochore microtubule attachment is perturbed (Putkey et al. 2002). In contrast to Sgo1-repressed cells, however, cohesion as well as Scc1 localization at the centromeres was largely intact in the Sgo2-repressed cells (our unpublished data). These results suggest that Sgo2 plays a crucial role in establishing proper kinetochore microtubule attachment, which is different from protecting cohesin or cohesion at centromeres (figure 4).
7. Concluding remarks
The centromere is a crucial part of the chromosome where the kinetochore is assembled, and an essential apparatus for chromosome segregation during mitosis and meiosis. The centromere or kinetochore can be structurally strengthened by the assistance of heterochromatin-dependent chromatin compaction. Heterochromatin plays another role at centromeres in strengthening cohesion by recruiting cohesin to this region. Centromeric cohesion is also strengthened or protected by shugoshin, a conserved kinetochore protein. The role of shugoshin is essential for preserving cohesin at centromeres throughout meiosis I, at least in fission and budding yeast, and Drosophila (and presumably in most eukaryotes). Studies in vertebrate cells have revealed that shugoshin also protects mitotic cohesin at centromeres from dissociating in pro- and prometaphase. It is possible to speculate that shugoshin plays a primary role in strengthening centromeric cohesion together with cohesin even before eukaryotic cells obtained a meiotic system, and that during the course of the establishment of meiosis, shugoshin might have co-developed to protect centromeric cohesion more thoroughly. Remarkably, both fission yeast and vertebrate Sgo2 have no protective role for cohesin, different from Sgo1, which may represent another conserved role of shugoshin. From the data in HeLa cells, we propose that Sgo2 plays a role in regulating the attachment of the kinetochore to microtubules, thereby ensuring proper congression of chromosomes (figure 4). Further studies are required to address this point more precisely.
Note Added in Proof
Our data on human Sgo1 is now published (Kitajima et al. 2005).
Acknowledgments
We thank S. Hauf and M. Ohsugi for their help with some experiments with human cells, S. Kawashima for contribution to S. pombe experiments, and all members of our laboratory for discussion. This work was supported in part by grants from the Ministry of Education, Science and Culture of Japan.
GLOSSARY
- APC
anaphase promoting complex
- Sgo
shugoshin
- SMC
structural maintenance of chromosome
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Chromosome segregation’.
Discussion
T. Hyman (Max Planck Institute,Dresden,Germany). It looks like the localization of Sgo1 is a little different from that reported from Mitchison's lab.
Y. Watanabe. Our data is identical to that just reported by Jan-Michael Peters. We do not know the real reason but this may originate from the fact that Mitchison's lab used a tagged Sgo1 protein instead of the endogenous one for determining localization.
M. Yanagida (Kyoto University,Kyoto,Japan). Is your interpretation that Bub1 restricts localization of Sgo1 at kinetochores? If so, Sgo1 has broad binding specificity to the whole chromosome? Or any other interpretation?
Y. Watanabe. Yes, we think that Bub1 has a role to restrict the Sgo1 localization to centromeres. It is possible that a single modification or interaction will change the specificity of affinity of Bub1 from whole chromosome region to centromeres.
T. Hirano (Cold Spring Harbor Laboratory,New York, USA). Does the distribution of Aurora B alter by Bub1 depletion?
Y. Watanabe. We are currently studying that possibility.
References
- Bernard P, Maure J.F, Javerzat J.P. Fission yeast Bub1 is essential in setting up the meiotic pattern of chromosome segregation. Nat. Cell Biol. 2001;3:522–526. doi: 10.1038/35074598. [DOI] [PubMed] [Google Scholar]
- Bernard P, Maure J.F, Partridge J.F, Genier S, Javerzat J.P, Allshire R.C. Requirement of heterochromatin for cohesion at centromeres. Science. 2001;294:2539–2542. doi: 10.1126/science.1064027. [DOI] [PubMed] [Google Scholar]
- Blat Y, Kleckner N. Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell. 1999;98:249–259. doi: 10.1016/s0092-8674(00)81019-3. [DOI] [PubMed] [Google Scholar]
- Ciosk R, Shirayama M, Shevchenko A, Tanaka T, Toth A, Nasmyth K. Cohesin's binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol. Cell. 2000;5:243–254. doi: 10.1016/s1097-2765(00)80420-7. [DOI] [PubMed] [Google Scholar]
- Fukagawa T, Nogami M, Yoshikawa M, Ikeno M, Okazaki T, Takami Y, Nakayama T, Oshimura M. Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat. Cell Biol. 2004;6:784–791. doi: 10.1038/ncb1155. [DOI] [PubMed] [Google Scholar]
- Glynn E.F, Megee P.C, Yu H.-G, Mistrot C, Unal E, Koshland D.E, DeRisi J.L, Gerton J.L. Genome-wide mapping of the cohesin complex in the yeast Saccharomyces cerevisiae. PLOS Biol. 2004;2:1325–1339. doi: 10.1371/journal.pbio.0020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haering C.H, Nasmyth K. Building and breaking bridges between sister chromatids. Bioessays. 2003;25:1178–1191. doi: 10.1002/bies.10361. [DOI] [PubMed] [Google Scholar]
- Hauf S, Waizenegger I.C, Peters J.M. Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science. 2001;293:1320–1323. doi: 10.1126/science.1061376. [DOI] [PubMed] [Google Scholar]
- Hirano T. The ABCs of SMC proteins: two-armed ATPases for chromosome condensation, cohesion, and repair. Genes Dev. 2002;16:399–414. doi: 10.1101/gad.955102. [DOI] [PubMed] [Google Scholar]
- Hoque M.T, Ishikawa F. Cohesin defects lead to premature sister chromatid separation, kinetochore dysfunction, and spindle-assembly checkpoint activation. J. Biol. Chem. 2002;277:42 306–42 314. doi: 10.1074/jbc.M206836200. [DOI] [PubMed] [Google Scholar]
- Katis V.L, Galova M, Rabitsch K.P, Gregan J, Nasmyth K. Maintenance of cohesin at centromeres after meiosis I in budding yeast requires a kinetochore-associated protein related to MEI-S332. Curr. Biol. 2004;14:560–572. doi: 10.1016/j.cub.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kitajima T.S, Hauf S, Ohsugi M, Yamamoto T, Watanabe Y. Human Bub1 defines the persistent cohesion site along the mitotic chromosome by affecting shugoshin localization. Curr. Biol. 2005;15:353–359. doi: 10.1016/j.cub.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Kitajima T.S, Yokobayashi S, Yamamoto M, Watanabe Y. Distinct cohesin complexes organize meiotic chromosome domains. Science. 2003;300:1152–1155. doi: 10.1126/science.1083634. [DOI] [PubMed] [Google Scholar]
- Kitajima T.S, Kawashima S.A, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature. 2004;427:510–517. doi: 10.1038/nature02312. [DOI] [PubMed] [Google Scholar]
- Lee J.Y, Orr-Weaver T.L. The molecular basis of sister-chromatid cohesion. Annu. Rev. Cell. Dev. Biol. 2001;17:753–777. doi: 10.1146/annurev.cellbio.17.1.753. [DOI] [PubMed] [Google Scholar]
- Lengronne A, Katou Y, Mori S, Yokobayashi S, Kelly G.P, Itoh T, Watanabe Y, Shirahige K, Uhlmann F. Cohesin relocation from sites of chromosomal loading to places of convergent transcription. Nature. 2004;430:573–578. doi: 10.1038/nature02742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losada A, Hirano T. Shaping the metaphase chromosome: coordination of cohesion and condensation. Bioessays. 2001;23:924–935. doi: 10.1002/bies.1133. [DOI] [PubMed] [Google Scholar]
- Losada A, Hirano M, Hirano T. Cohesin release is required for sister chromatid resolution, but not for condensin-mediated compaction, at the onset of mitosis. Genes Dev. 2002;16:3004–3016. doi: 10.1101/gad.249202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston A.L, Tham W.H, Shah H, Amon A. A genome-wide screen identifies genes required for centromeric cohesion. Science. 2004;303:1367–1370. doi: 10.1126/science.1094220. [DOI] [PubMed] [Google Scholar]
- Megee P.C, Koshland D. A functional assay for centromere-associated sister chromatid cohesion. Science. 1999;285:254–257. doi: 10.1126/science.285.5425.254. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 2001;35:673–745. doi: 10.1146/annurev.genet.35.102401.091334. [DOI] [PubMed] [Google Scholar]
- Nonaka N, Kitajima T, Yokobayashi S, Xiao G, Yamamoto M, Grewal S.I, Watanabe Y. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 2002;4:89–93. doi: 10.1038/ncb739. [DOI] [PubMed] [Google Scholar]
- Putkey F.R, Cramer T, Morphew M.K, Silk A.D, Johnson R.S, McIntosh J.R, Cleveland D.W. Unstable kinetochore-microtubule capture and chromosomal instability following deletion of CENP-E. Dev. Cell. 2002;3:351–365. doi: 10.1016/s1534-5807(02)00255-1. [DOI] [PubMed] [Google Scholar]
- Rabitsch K.P, Gregan J, Schleiffer A, Javerzat J.P, Eisenhaber F, Nasmyth K. Two fission yeast homologs of Drosophila Mei-S332 are required for chromosome segregation during meiosis I and II. Curr. Biol. 2004;14:287–301. doi: 10.1016/j.cub.2004.01.051. [DOI] [PubMed] [Google Scholar]
- Salic A, Waters J.C, Mitchison T.J. Vertebrate shugoshin links sister centromere cohesion and kinetochore microtubule stability in mitosis. Cell. 2004;118:567–578. doi: 10.1016/j.cell.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Sonoda E, et al. Scc1/Rad21/Mcd1 is required for sister chromatid cohesion and kinetochore function in vertebrate cells. Dev. Cell. 2001;1:759–770. doi: 10.1016/s1534-5807(01)00088-0. [DOI] [PubMed] [Google Scholar]
- Sumara I, Vorlaufer E, Stukenberg P.T, Kelm O, Redemann N, Nigg E.A, Peters J.M. The dissociation of cohesin from chromosomes in prophase is regulated by polo-like kinase. Mol. Cell. 2002;9:515–525. doi: 10.1016/s1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Cosma M.P, Wirth K, Nasmyth K. Identification of cohesin association sites at centromeres and along chromosome arms. Cell. 1999;98:847–858. doi: 10.1016/s0092-8674(00)81518-4. [DOI] [PubMed] [Google Scholar]
- Tomonaga T, et al. Characterization of fission yeast cohesin: essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev. 2000;14:2757–2770. doi: 10.1101/gad.832000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth A, Rabitsch K.P, Galova M, Schleiffer A, Buonomo S.B, Nasmyth K. Functional genomics identifies monopolin: a kinetochore protein required for segregation of homologs during meiosis I. Cell. 2000;103:1155–1168. doi: 10.1016/s0092-8674(00)00217-8. [DOI] [PubMed] [Google Scholar]
- Uhlmann F. Chromosome cohesion and separation: from men and molecules. Curr. Biol. 2003;13:R104–R114. doi: 10.1016/s0960-9822(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nurse P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature. 1999;400:461–464. doi: 10.1038/22774. [DOI] [PubMed] [Google Scholar]
- Weber S.A, Gerton J.L, Polancic J.E, DeRisi J.L, Koshland D, Megee P.C. The kinetochore is an enhancer of pericentric cohesin binding. PLOS Biol. 2004;2:1340–1353. doi: 10.1371/journal.pbio.0020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokobayashi S, Yamamoto M, Watanabe Y. Cohesins determine the attachment manner of kinetochores to spindle microtubules at meiosis I in fission yeast. Mol. Cell Biol. 2003;23:3965–3973. doi: 10.1128/MCB.23.11.3965-3973.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]