Abstract
Merotelic kinetochore attachment is a major source of aneuploidy in mammalian tissue cells in culture. Mammalian kinetochores typically have binding sites for about 20–25 kinetochore microtubules. In prometaphase, kinetochores become merotelic if they attach to microtubules from opposite poles rather than to just one pole as normally occurs. Merotelic attachments support chromosome bi-orientation and alignment near the metaphase plate and they are not detected by the mitotic spindle checkpoint. At anaphase onset, sister chromatids separate, but a chromatid with a merotelic kinetochore may not be segregated correctly, and may lag near the spindle equator because of pulling forces toward opposite poles, or move in the direction of the wrong pole. Correction mechanisms are important for preventing segregation errors. There are probably more than 100 times as many PtK1 tissue cells with merotelic kinetochores in early mitosis, and about 16 times as many entering anaphase as the 1% of cells with lagging chromosomes seen in late anaphase. The role of spindle mechanics and potential functions of the Ndc80/Nuf2 protein complex at the kinetochore/microtubule interface is discussed for two correction mechanisms: one that functions before anaphase to reduce the number of kinetochore microtubules to the wrong pole, and one that functions after anaphase onset to move merotelic kinetochores based on the ratio of kinetochore microtubules to the correct versus incorrect pole.
Keywords: mitosis, microtubule, kinetochore, aneuploidy, Ndc80, chromosome
1. Introduction
Aneuploidy is the condition of a cell that has lost its normal diploid chromosome number because it has lost or gained one or more chromosomes during cell division. The role of aneuploidy in human meiotic cells is well known for inducing severe pathological genetic syndromes (e.g. Down syndrome; for review see Nicolaidis & Petersen 1998). In addition, an increasing number of studies have shown that abnormalities like aneuploidy and whole-chromosome loss of heterozygosity are commonly present in tumour cells, suggesting that chromosome segregation defects play a critical role in tumour development and progression (Lengauer et al. 1997; Cahill et al. 1998; Stoler et al. 1999; Saunders et al. 2000; Sen 2000; Duesberg et al. 2000).
Merotelic kinetochores are a major source of aneuploidy in mammalian tissue cells because their attachment by microtubules to opposite poles produces errors in chromosome segregation not prevented by the mitotic spindle checkpoint (Cimini et al. 2001). The 15–30 attachment sites at vertebrate kinetochores for the plus ends of spindle microtubules (Rieder 1982) provide considerable opportunity for generating merotelic kinetochores. One well-established error produced by merotelic kinetochores is lagging chromosomes near the spindle equator at anaphase (figure 1). Before anaphase, merotelic kinetochores support chromosome alignment into a metaphase plate and their presence is not detected by the spindle checkpoint (figure 1a; Cimini et al. 2001, 2002). As a result, cells with merotelic kinetochores are not delayed in metaphase by the spindle checkpoint and enter anaphase with timing similar to controls (Cimini et al. 2002). In anaphase, after sister chromosome separation, a sister with a merotelic kinetochore does not exhibit normal anaphase movement because it is pulled in opposite directions by attachments to microtubules from opposite poles (figures 1b,c and 2d; Cimini et al. 2001). Sister kinetochores are normally segregated to opposite poles in anaphase because each kinetochore is attached only to microtubules from the same pole and sisters are attached to microtubules from opposite poles. The frequency of anaphase cells with lagging chromosomes in normal culture media is significant: about 1% for PtK1 and 0.5% for low-passage human lung fibroblasts cells (not immortalized; Cimini et al. 1999, 2002). There is also another anaphase error in chromosome segregation that occurs after all the chromosomes have become aligned near the spindle equator and sisters separate in anaphase. This is the movement of separated sister chromosomes toward the same pole; movement that has been detected by the Degrassi lab using FISH staining techniques (Cimini et al. 1999). This error is termed ‘sister chromosome non-disjunction’ and it occurs at frequencies higher than anaphase lagging chromosomes. It is not established how this chromosome non-disjunction is produced, but we propose in this paper a mechanism based on merotelic kinetochore attachment. Both lagging chromosomes and chromosome non-disjunction are of particular interest because they do not result in extensive variation of the karyotype per cell division. A gain or loss of one chromosome may be more compatible with cell viability than large changes in chromosome number or polyploidy. The aneuploid cells produced by these malsegregation events are expected to have a higher probability of propagation and inducing birth defects during development, or cancer cells in tissues (Rasnick & Duesberg 1999).
Figure 1.
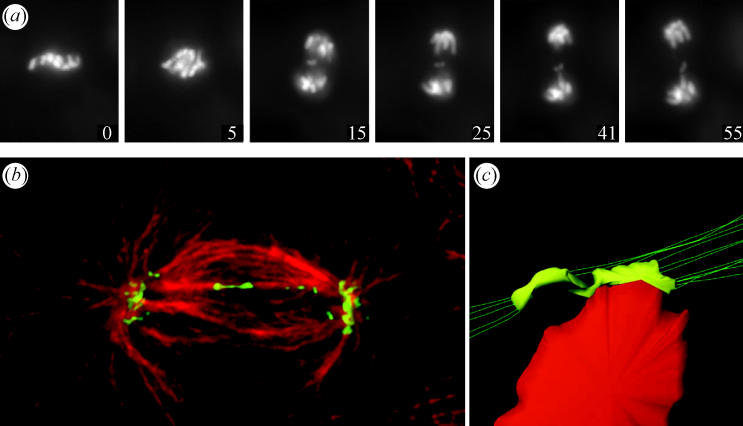
Merotelic kinetochores can produce lagging chromosomes near the spindle equator in anaphase. (a) Live-cell imaging of a PtK1 cell expressing GFP-H2B. A lagging chromosome remains near the spindle equator as the rest of the chromosomes segregate to their poles. Time on each frame given in minutes. From Cimini et al. (2002). (b) Spinning disk confocal fluorescence microscopy of PtK1 cell showing how microtubule attachments to the opposite poles stretch a merotelic kinetochore laterally from its normal width of about 0.4 to 2 μm or more. The kinetochores (green) were labelled by immunofluorescence with CREST antibodies to inner kinetochore proteins while microtubules (red) were labelled with tubulin antibodies. From Cimini et al. (2001). (c) 3D reconstruction from electron micrographs of a merotelic kinetochore of an anaphase lagging chromosome done by Alexey Khodjakov. From Cimini et al. 2001.
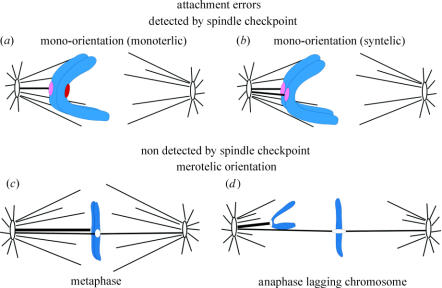
Figure 2.
Attachment errors of kinetochores to spindle microtubules. The intensity of red at the kinetochores is an indication of their spindle checkpoint activity. See text for details.
Nicklas (1997) describes his pioneering work and contributions from others on kinetochore attachment errors and their correction, with a major focus on results from studies in meiotic insect spermatocytes. In this paper, we initially review the properties of merotelic attachment relative to other errors in kinetochore attachment in mitotic cells. We then examine merotelic kinetochore formation in mammalian tissue cells, including evidence for a high frequency of merotelic attachments in the early stages of mitosis and factors that promote this attachment error. There appear to be two major correction mechanisms. One functions before anaphase onset to reduce kinetochore microtubule attachments to the wrong pole (Cimini et al. 2003). There is evidence from budding yeast that this mechanism depends on Aurora B kinase (Ipl1 in budding yeast) and the Ndc80/Nuf2 complex at kinetochore microtubule attachment sites (Maiato et al. 2004). There are now some supporting data for mammalian tissue cells because both Aurora B and the Ndc80/Nuf2 complex control the stability of microtubule attachment (Maiato et al. 2004). However, a big surprise in mammalian tissue cells is that this mechanism fails to correct a substantial number of merotelic kinetochores before anaphase onset, even when metaphase is experimentally prolonged by 2 h (Cimini et al. 2003). We propose that this is not because of a slow rate of detachment, but because of structural issues that prevent recruitment of microtubules from the correct pole into unoccupied sites of a merotelic kinetochore. We have found that the number of anaphase cells with lagging chromosomes is 16-fold lower than the number of chromosomes with merotelic kinetochores entering anaphase (Cimini et al. 2003, 2004). We suggest from high-resolution imaging of fixed and live preparations that the segregation of merotelic kinetochores in anaphase is determined by spindle mechanics and depends on the ratio of the number of kinetochore microtubules to the correct versus incorrect poles. When this ratio is greater than one, merotelic kinetochores move their chromosomes in the direction of the correct pole, preventing the lagging chromosome error in segregation. This same mechanism may also produce segregation of separated sisters to the same pole (chromosome non-disjunction) when this ratio is less than one. Finally, we discuss further the potential roles of the Ndc80/Nuf2 complex and other potential attachment molecules at the kinetochore/microtubule interface.
2. Merotelic attachments differ from other attachment errors in that they are not sensed by the spindle checkpoint
Kinetochore attachments and the spindle checkpoint have been extensively reviewed (Rieder & Salmon 1998; Musacchio & Hardwick 2002; Cleveland et al. 2003; Maiato et al. 2004). Mono-oriented sister chromosome pairs have kinetochore microtubules to only one pole (figure 2a). Attachment by only one kinetochore, monotelic attachment, is typical of early prometaphase, but occasionally, both sisters become attached to the same pole, producing syntelic attachment (figure 2b). Unattached kinetochores have high concentrations of spindle checkpoint proteins, and a single unattached kinetochore is capable of sustaining the spindle checkpoint and preventing anaphase. The concentration of spindle checkpoint proteins at monotelic and syntelic kinetochores is partially reduced. These kinetochores often have neither a full complement of attached kinetochore microtubules, nor the high tensions typical of bi-oriented chromosomes near the spindle equator.
Bi-oriented chromosomes (figure 2c) have one sister attached to microtubules from one pole, while the other sister has attachments to microtubules from the opposite pole. Bi-orientation stretches the centromere between sister kinetochores to about twice their rest lengths on average. Tension promotes stability of microtubule attachment and helps inactivate kinetochore spindle checkpoint activity either directly or indirectly through increasing kinetochore microtubule number. Spindle checkpoint protein concentration is reduced and activity is inactivated at kinetochores of bi-oriented chromosomes that are under tension with most of their attachment sites filled with kinetochore microtubules. We still do not know if spindle checkpoint activity is the sum of checkpoint activities surrounding each individual microtubule attachment site or whether activity at the kinetochore is controlled by integration of regulatory signals from individual attachment sites.
Bi-oriented chromosomes whose sister kinetochores are attached by microtubules to just one pole have amphitelically attached kinetochores. This is the correct attachment to ensure accurate chromosome segregation.
Bi-oriented chromosomes can also have one or both kinetochores merotelically oriented (figure 2d). One is most frequent, but both have been seen to occur (Cimini et al. 2004). The concentration of spindle checkpoint proteins Mad2 and the 3F3/2 epitope are reduced to the same low levels at merotelic kinetochores in anaphase as amphitelically attached kinetochores on late metaphase aligned chromosomes (Cimini et al. 2001). The depletion of Mad2 signals filled attachment sites, while loss of 3F3/2 antibody staining signals the loss of phosphorylation at kinetochores induced by tension. Neither disappears from unattached kinetochores in prometaphase cells induced into anaphase by inactivation of the spindle checkpoint (Canman et al. 2002). Cells with merotelic kinetochores align their chromosomes and enter anaphase with similar timing to amphitelically oriented kinetochores (Cimini et al. 2002). This suggests that merotelic kinetochores have bound a similar number of kinetochore microtubules as normal, although a fraction of these are to the incorrect pole, the pole attached to the sister kinetochore.
3. Chromosome and spindle factors that promote merotelic kinetochore formation
There are several possible mechanisms and perturbations that can contribute to inducing formation of merotelic kinetochores. We will first summarize these, beginning with those that may be important for normal, unperturbed, tissue cells. Two mechanisms are considered. One is geometrical defects in microtubule attachment by the polar microtubule ‘search and capture’ mechanism based on microtubule plus-end dynamic instability (Rieder & Salmon 1998; Maiato et al. 2004). The other is kinetochore/centromere initiation of kinetochore fibre growth (Khodjakov et al. 2003).
Consider first, cells that have normally separated their spindle poles (centrosomes) before spindle formation after nuclear envelope breakdown and the entry into prometaphase. In the absence of kinetochore microtubules, the kinetochore outer domain motors, the kinesin CENP-E and cytoplasmic dynein, and spindle checkpoint proteins like Mad1, Mad2 and BubR1 assemble into expanded crescents around unattached kinetochores, expansion that is exaggerated when all microtubules are depolymerized with nocodazole (Hoffman et al. 2001). The motors in particular may serve an ‘antenna function’ for recruiting microtubule plus ends into the attachment sites within the outer plate of the vertebrate kinetochore (Rieder & Salmon 1998). If, following nuclear envelope breakdown, the orientation of the axis between sister kinetochores is perpendicular to the spindle interpolar axis, this would prevent the chromosome arms from blocking access to the expanded kinetochore so that capture of plus end from both poles could occur (figure 3a; Cimini et al. 2003). Another potential source of merotelic kinetochores in normal cells is mono-oriented chromosomes that occasionally become syntelic (figure 2b). One of the syntelic kinetochores could become attached to microtubules to the opposite pole. This would produce bi-orientation and induce congression of the sister chromosome pair to near the equator (figure 2c).
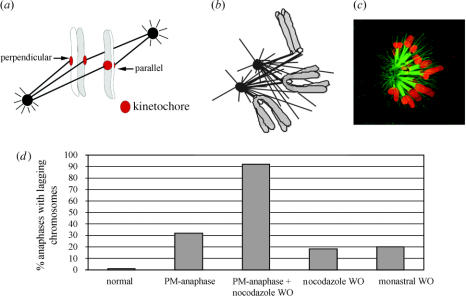
Figure 3.
Some factors affecting merotelic kinetochore formation during prometaphase (a–c) and affecting the percent cells with lagging chromosomes in anaphase (d). See text for details. (a) Two different sister chromatid orientations relative to the spindle pole-to-pole axis; one favours merotelic attachment, one does not. From Cimini et al. (2003). (b) Centrosomes often come close together in nocodazole-treated mitotic cells and centrosome separation is slow compared with microtubule polymerization after nocodazole washout producing opportunities for merotelic attachments when the centres separate. (c) Spinning disk confocal fluorescence image of a prometaphase PtK1 cell treated with monastrol to prevent centrosome separation before fixation. Note: kinetochore attachments to both poles and syntelic attachments occur in monastrol blocked cells; both errors can lead to merotelic attachments after drug washout and subsequent centre separation as described in the text. Tubulin immunofluorescence is green while chromosome fluorescence is red. From Canman et al. (2003). (d) The frequency of anaphase cells with lagging chromosomes is substantially increased by experimental treatments that induce premature anaphase or prevent centrosome separation before bipolar spindle assembly or both.
The ability of the kinetochore/centromere region to initiate kinetochore microtubule assembly has been recently reported for tissue cells, but evidence for this mechanism was initially found by Inoué in 1963 from UV microbeam studies of plant kinetochore fibre assembly (Inoué 1963). As discovered by Khodjakov et al. (2003), unattached kinetochores on mono-oriented chromosomes in Ptk1 cells will infrequently grow a kinetochore fibre away from the kinetochore toward the periphery of the spindle. When the minus end of this fibre interacts with polar microtubules from the proximal or distal poles, it is transported by dynein mediated forces toward the pole attached to the polar microtubule. If it goes to the proximal pole, this provides a mechanism for syntelic attachment. If the minus ends of the fibre splays, and these splayed ends move to opposite poles, then this would provide a mechanism for merotelic kinetochore formation. At the poles, the minus ends become anchored along with the minus ends of the other spindle fibres. We do not know if this mechanism contributes to the formation of merotelic kinetochores, but this possibility should be investigated. Although infrequent in mammalian tissue cells (Khodjakov et al. 2003), there is still much to be learned about this interesting aspect of kinetochore function.
Abnormal spindle geometry has a big influence on the frequency of merotelic kinetochore formation. If the spindle poles fail to separate before spindle assembly at nuclear envelope breakdown, or are prevented from separation before spindle assembly, then individual kinetochores have the opportunity to capture microtubules from, or extend microtubules to, both pole because of close proximity of the two spindle poles (figure 3b,c). A delay in spindle pole separation relative to spindle formation may naturally occur in some dividing mammalian tissue culture cells, but it can be produced experimentally by transient treatment with nocodazole or cold to reversibly depolymerize the spindle (Janicke & LaFountain 1982; Cimini et al. 2003), or transient use of the drug monastrol (an inhibitor of the kinesin Eg5) to delay centrosome separation and initially cause the formation of monopolar spindles (figure 3c; Canman et al. 2003). When nocodazole is washed out, the two centrosomes are usually close to the chromosomes and abundant microtubule assembly occurs from each centrosome (figure 3b) and from NuMA positive centres clustered around the chromosomes (not shown in figure 3b) within 5 min of washout (Cimini et al. 2003). Subsequently, both the centrosomes and centres are sorted into two separated spindle poles over a period of 15 min in treated PtK1 cells, but most Mad2 is lost from kinetochores within several minutes after washout of the drug, an indicator of rapid kinetochore microtubule formation (Cimini et al. 2003). During this rapid burst of kinetochore microtubule formation, both merotelic and syntelic connections are possible given the close proximity of multiple polar centres (figure 3b). For cells treated with monastral, many of the mono-oriented chromosomes also become syntelically attached over time (Lampson et al. 2004; figure 3c). When the drug is washed out, the centres separate into poles on opposite sides of the chromosomes. If a single kinetochore is attached by microtubules to centres that migrate into the opposite poles, then merotelic attachments will occur. When nocodazole- or monastrol-treated cells progress into anaphase, the frequency of lagging chromosomes is about 18–20%, about 18- to 20-fold higher than the 1% for normal cells entering anaphase (figure 3d; Cimini et al. 2003). By single cell imaging, we found for PtK1 cells in culture that spindle pole separation occurs for 29 out of 30 cells before nuclear envelope breakdown (Cimini et al. 2003). Thus, merotelic kinetochore formation initiated by unseparated poles in early spindle assembly is rare in these cells, but it could occur at high frequencies in other tissue cell types or by defects in proteins that regulate the timing of centrosome separation at entry into mitosis, such as Aurora A. Finally, merotelic kinetochore orientation and lagging chromosomes in anaphase occur frequently in cells with multipolar spindles (Sluder et al. 1997).
Perturbations of chromosome structure can also induce merotelic kinetochores in cells with bipolar spindles. In particular, chromosomes with single kinetochores are prone to establishing merotelic attachments. Single kinetochore chromosomes have been produced by premature sister chromatid separation (Yu & Dawe 2000), blocking replication before mitosis (Brinkley et al. 1988; Wise & Brinkley 1997) or by severing away a sister chromatid by laser microsurgery (Khodjakov et al. 1997). In all studies, single kinetochore chromosomes became merotelically attached and became aligned near the spindle equator at metaphase in the absence of pulling forces from a sister kinetochore. This result is mechanistically important for understanding kinetochore function in chromosome alignment because it shows that the multiple microtubule attachment sites on a single merotelically attached kinetochore can function as independent force generators for chromosome alignment near the spindle equator.
In yeast, proteins have been identified that control the structure of the centromeres and constrain adjacent kinetochore microtubule attachment sites to face the same direction, and mutants in these proteins promote lagging chromosomes (Pidoux et al.. 2000; Pidoux & Allshire 2003; Rabitsch et al. 2003; Allshire 2005). Many aspects of how such proteins constrain the attachment sites of vertebrate kinetochore to face the same direction are a major unsolved mystery.
4. Merotelic kinetochore formation is frequent in early prometaphase for normal cells
For normal tissue cells in culture, lagging chromosomes are seen in about 1% of anaphase cells (figure 3d). Does this represent the frequency of occurrence of merotelic kinetochores during all of mitosis, or does merotelic attachment occur much more frequently, and do correction mechanisms exist? We addressed this issue by microinjecting early prometaphase PtK1 cells with dominant negative inhibitors of the spindle checkpoint protein Mad2 (Cimini et al. 2003). These injections induce premature anaphase within about 15 min, before many chromosomes have had time to align at the spindle equator. We found that about 32% of these injected cells had one or more lagging chromosomes in anaphase. This frequency was increased to 92% for cells recovering from a nocodazole block (figure 3d).
These results suggest two conclusions. First, merotelic kinetochore formation is frequent during early mitosis in unperturbed cells that have separated their centrosomes before spindle assembly. As described below, only about 6% of merotelic kinetochores in metaphase produce lagging chromosomes in anaphase. If the same percentage holds for the premature anaphases, then every normal cell has one or more chromosomes with a merotelic kinetochore early in mitosis, and this number is greatly enhanced in the cells that fail to establish spindle bipolarity before spindle assembly (e.g. like cells transiently treated with nocodazole or monastrol). The second conclusion concerns the high incidence of lagging chromosomes reported for anaphase cells with mutant spindle checkpoint proteins (reviewed in Cleveland et al. 2003). Based on our studies (Cimini et al. 2003), this high incidence occurs simply because cells with a defective spindle checkpoint spend an unusually short time in prometaphase.
5. Merotelic kinetochore correction occurs before anaphase onset, but it is incomplete
Does the 1% of anaphase cells with lagging chromosomes represent the number of cells with merotelic kinetochores at the end of metaphase? If so, this would indicate that correction mechanisms function before anaphase to eliminate most merotelic kinetochores. Initially, we addressed this question by fixing PtK1 cells to preserve metaphase kinetochore microtubules, spindle interpolar length and kinetochore structure (Cimini et al. 2003). We labelled the microtubules and kinetochores by immunofluorescence. We then obtained high-resolution spinning disk confocal images acquired at 200 nm steps through the spindle and chromosomes. These image stacks were deconvolved to give us 3D views of kinetochore microtubule connections. At metaphase, sister kinetochores have stretched their centromeres by about 2–2.5 μm in PtK1 cells and they achieve average positions on either side of the spindle equator (figure 4). Surprisingly, this assay revealed a high frequency of merotelic kinetochores; about 16% of untreated metaphase cells had at least one identifiable merotelic kinetochore, a frequency 16-fold higher than expected from the 1% frequency of lagging chromosomes in anaphase. Clearly, correction mechanisms function before anaphase to eliminate merotelic kinetochores, since 16% is far fewer than the number estimated for early prometaphase as summarized in figure 5. We then asked if, given more time, these pre-anaphase correction mechanisms could eliminate all merotelic kinetochores and lagging chromosomes. We prolonged metaphase by 2 h using MG-132 to reversibly inhibit the 26S proteosome and delay anaphase onset. We found only a small decrease in the frequency of detectable merotelics at metaphase but a 4-fold decrease in the frequency of anaphase cells with lagging chromosomes (figure 5). We also showed that the ratio of fluorescence of microtubule bundles to opposite poles increased during the delayed anaphase.
Figure 4.
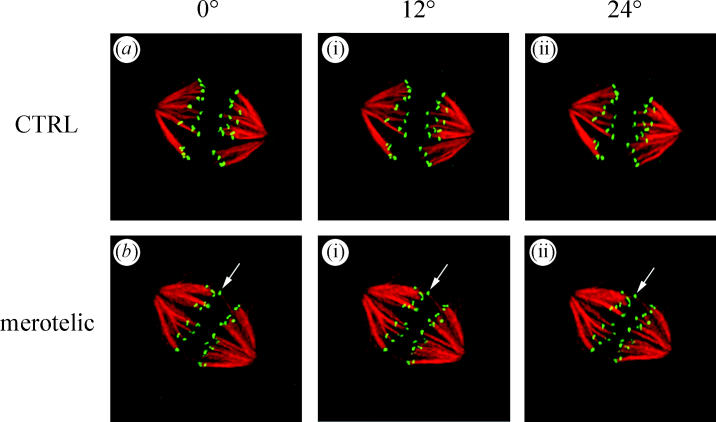
Projection views at three different angles through 3D reconstructions of two metaphase PtK1 spindles (a and b) that were fixed and immunofluorescently labelled with CREST antibodies to the kinetochore (green) and tubulin antibodies to stain kinetochore fibre microtubules (red). Cells had progressed normally through mitosis to metaphase before fixation. Note the cell in b has a merotelic kinetochore shifted toward the equator in between the other pairs of sister kinetochores (arrows). From Cimini et al. (2003).
Figure 5.
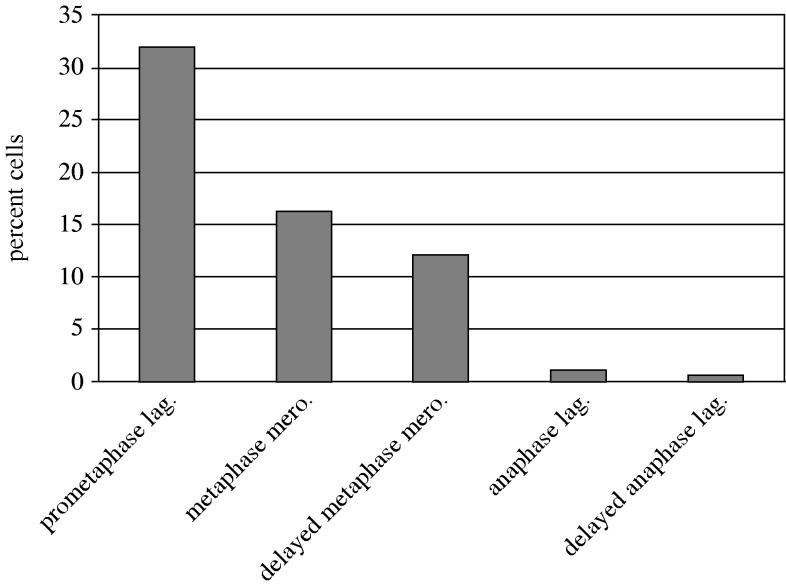
Merotelic kinetochore formation occurs frequently in early prometaphase and merotelic kinetochores are only partially corrected by late metaphase or by prolonging metaphase by 2 h. Only 1 out of 16 merotelic kinetochores entering normal anaphase produce lagging chromosomes, and this reduction is enhanced by prolonging metaphase. See text for details.
The above results (Cimini et al. 2003) suggest that there are two mechanisms that prevent lagging chromosomes produced by merotelic kinetochores: one that functions before anaphase to reduce the number of merotelic kinetochores or the number of microtubules to the incorrect pole, and one that functions after anaphase onset to prevent most merotelic kinetochores from producing lagging chromosomes.
6. Correction of merotelic attachment before anaphase onset
How does the first mechanism function to reduce the number of microtubules to the incorrect pole and eliminate merotelic kinetochores? In budding yeast, correction of mis-attachments depends on the activity of the Aurora B kinase (Ipl1 in yeast) and kinetochore tension (Biggins et al. 1999; Biggins & Murray 2001; Tanaka et al. 2002). Normally, stable kinetochore microtubule attachment is achieved when chromosomes bi-orient and sister kinetochores become amphitelically attached. Tension is generated by kinetochores pulling toward opposite poles restrained by the cohesions holding the sisters together. This stretches the intervening chromatin, inactivates Ipl1 activity, and stabilizes microtubule attachment. When syntelic attachments are made, these kinetochores are not under this high tension, Ipl1 is active and attachment is unstable. Ipl1 mutants have been shown to maintain stable syntelic attachments at the lower tensions (Biggins et al. 1999; Biggins & Murray 2001; Tanaka et al. 2002). There is evidence that inhibiting Aurora B in mammalian tissue cells, Drosophila and Caenorhabditis elegans promotes stability of microtubule attachment and errors in segregation like anaphase lagging chromosomes (reviewed in Maiato et al. 2004). However, inhibiting Aurora B also abrogates the spindle checkpoint and disrupts kinetochore assembly, effects that complicate interpretation of Aurora B function in correcting merotelic attachments. To address this issue, we have used low doses of an Aurora B inhibitor on PtK1 cells that do not compromise the spindle checkpoint and normal chromosome movements, and we see enhanced frequencies of anaphase lagging chromosomes (Cimini et al. unpublished observations). These results support a role for Aurora B in destabilizing incorrect microtubule attachments in mammalian tissue cells.
However, merotelic kinetochores, unlike syntelic kinetochores in yeast, are under high tension from pulling forces from opposite poles and the sister kinetochore. Based on experiments in yeast, this high tension is expected to inactivate Aurora B activity. One possibility is that Aurora B regulation is different for the larger vertebrate kinetochores. In yeast, Ipl1 localizes to the kinetochore; in vertebrate cells, it is concentrated within the inner centromere region, not the kinetochore (Maiato et al. 2004). Figure 6 shows a possible model for how Aurora B might function to destabilize incorrect attachments at merotelic kinetochores. The idea is that the region of the kinetochore containing correctly attached microtubules is pulled away from the Aurora B at the inner centromere, so that its phosphatase, phosphatase 1 (PP1), concentrated at the kinetochore (Maiato et al. 2004) keeps the targets for the Aurora B at the kinetochore microtubule attachment site dephophorylated, promoting stable attachment. In contrast, the region of the kinetochore attached to microtubules from the wrong pole is pulled back toward the centromere and near the location of the Aurora B kinase, enhancing the probability of phophorylation and destabilization at these attachment sites.
Figure 6.
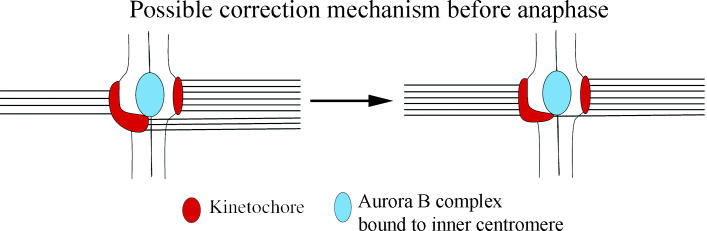
Microtubules attached to the wrong pole may bring their kinetochore attachment sites closer to the Aurora B kinase, which is located within the inner centromere region between sister kinetochores before anaphase. From Cimini et al. (2003).
It surprised us that the additional 2 h delay in metaphase did not change very much the number of detectable merotelic kinetochores before anaphase, although it did produce a four-fold decrease in detectable anaphase lagging chromosomes (figure 3). A further puzzle stems from the turnover rate measured for kinetochore microtubules of amphitelic kinetochores on bi-oriented chromosomes. Using microtubule fluorescent marking techniques, the half-life has been measured for metaphase PtK1 cells at about 5 min, or 300 s (Zhai et al. 1995). Thus in mammalian tissue cells, microtubule attachment at normal kinetochores under maximum tension at metaphase is not completely stable. If we assume a similar turnover half-life for microtubules attached to merotelic kinetochores at metaphase, then if detachment of a microtubule to the incorrect pole is followed by attachment of a microtubule to the correct pole, we expect to see complete correction of merotelic kinetochores during a period about five times the half-life of attachment, or 25 min, which is the time for nearly complete turnover of all microtubules. Yet, in 2 h, this correction did not occur. Either attachments at merotelic kinetochores are much more stable than expected, or reattachment depends on previous history. The latter seems possible, since an attachment site surrounded by microtubules to the wrong pole is likely to face that pole and regain a new attachment from that direction or initiate new microtubule growth in that direction.
The microtubule depolymerase, MCAK, concentrates at the inner centromere of bi-oriented chromosomes (Maiato et al. 2004). Inhibition of MCAK or inhibition of its co-factor ICIS elevates the frequency of lagging chromosomes in anaphase (Ohi et al. 2003; Kline-Smith et al. 2004). Both MCAK and ICIS have been proposed to be targets for the Aurora B-based mechanism that eliminates merotelic attachments before anaphase. We will discuss potential kinetochore targets for the Aurora B kinase, like the Ndc80 protein, and other possible molecular regulators of attachment stability in the last section of this paper.
We conclude from our studies that the spindle checkpoint and Aurora B mechanisms that operate to allow correction of mis-attachments like syntelic or merotelic attachment, fail to ensure complete correction of merotelic kinetochores before anaphase in vertebrate tissue cells. In fact, 16% of unperturbed late metaphase PtK1 cells enter anaphase with at least one merotelic kinetochore.
7. Anaphase spindle mechanics prevent merotelic kinetochores from producing lagging chromosomes
When cells enter anaphase, the Aurora B complex leaves the centromere (Maiato et al. 2004) and the rate of turnover of kinetochore microtubules decreases almost 10-fold (Zhai et al. 1995). Nevertheless, only 1/16th of the merotelic kinetochores at late metaphase in unperturbed cells produce a detectable lagging chromosome near the spindle equator in anaphase (Cimini et al. 2003). The above studies suggested that the correction mechanisms that operate before anaphase increase the number of microtubules to the correct pole and reduce the number to the incorrect pole. We suspected that the correction mechanisms that operate in anaphase depend on the ratio of the number of microtubules to the correct versus incorrect pole, R. In anaphase, merotelic kinetochores with ratios near one would produce lagging chromosomes because of nearly equal numbers of microtubules to the correct versus incorrect pole, while ratios sufficiently greater than one would result in movement away from the equator in the direction of the correct pole. In this way, the correction mechanisms operating before anaphase can prevent most merotelic kinetochores from producing lagging chromosomes near the equator in anaphase by increasing this ratio, R, without completely eliminating merotelic attachment.
To test this hypothesis, we developed live cell imaging techniques for PtK1 cells to track the movements of individual merotelic kinetochores from metaphase through anaphase relative to their spindle poles and relative to the ratio of microtubules connecting merotelic kinetochores to opposite poles (Cimini et al. 2004). We microinjected cells with low concentrations of Alexa-488-antibodies to the outer-plate protein CENP-F to fluorescently mark kinetochores and poles green and X-rhodamine labelled tubulin to fluorescently label kinetochore fibre microtubules red. A high-resolution spinning disk confocal microscope system (Maddox et al. 2003b) was used to obtain time-lapse recordings (figure 7a). As in the fixed preparations described above, the merotelic kinetochores we could identify at metaphase were usually positioned in between the separated sisters of bi-oriented chromosomes with normally attached kinetochores. The faces of these merotelic kinetochores were tilted and stretched laterally toward opposite poles. In anaphase, after sister separation, only a few merotelic kinetochores had fluorescent fibres of similar intensity to opposite poles, and these became lagging chromosomes (figure 7b). Most had fluorescent ratios of two or greater and these moved significant distances away from the equator in the direction of the correct pole (figure 7c,d). We never saw kinetochore fibre detachment from merotelic kinetochores or breakage of stretched kinetochores. In anaphase, merotelic kinetochores were often laterally stretched from their normal width of about 0.4 μm (McEwen et al. 1998) to about 2 μm by their microtubule attachments to opposite poles. In a few cases, where the fibre fluorescence ratio was about two, the kinetochore became pulled out toward the incorrect pole into thin strands interconnected by small beads as seen by the CENP-F antibody fluorescence (figure 7d). Usually, however, the merotelic kinetochore moved away from the equator in the direction of the more intense kinetochore fibre connected to the correct pole with about 2 μm stretch (figure 7c). This movement was always coupled to changes in the length of the kinetochore fibres to opposite poles, where the fibre to the incorrect pole became much longer than that to the correct pole as the ratio of their fluorescent intensities increased. Figure 8 compares for the above study the average changes in length of kinetochore fibres in anaphase for normally attached kinetochores with the changes in length of those attached to merotelic kinetochores. Normally attached kinetochores in anaphase shorten their kinetochore microtubules mainly by plus-end depolymerization at the kinetochore (‘Pac-Man’ mechanism; Mitchison & Salmon 2001; Maiato et al. 2004), and these shortened by 2 μm or more. For merotelic kinetochores, their fibres typically became longer as interpolar spindle elongation (anaphase B) occurred, but growth depended on the fluorescence intensity of the fibre. Kinetochore fibres to the incorrect pole with fewer microtubules typically grew much longer than at metaphase, while those to the correct pole with more microtubules grew much less (figure 8), and in a few cases shortened by 0.5 μm (Cimini et al. 2004). The presence of merotelic attachments to microtubules from opposite poles slowed the average velocity (0.72–0.42 μm min−1), but did not reduce significantly the extent of interpolar elongation (figure 8). The slower velocities are probably caused by the load generated by the lateral stretch typical of merotelic kinetochores in anaphase.
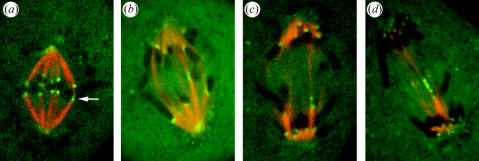
Figure 7.
Live imaging of merotelic kinetochores (green) and spindle microtubules (red) in PtK1 cells using a spinning disk confocal fluorescence microscope as described in the text. Frames are from time-lapse movies of different cells. (a) Metaphase aligned sister chromatid pair near the equator with one merotelic kinetochore (arrow). (b) Merotelic kinetochore in anaphase remained near the spindle equator with the brightness of its kinetochore fibres to opposite poles nearly equal and fibre polymerization nearly equal (R∼1). (c) Merotelic kinetochore in anaphase moved away from the equator in the direction of its brighter kinetochore fibre to the correct pole as the dimmer fibre to the opposite pole polymerized much longer (R>1). (d) A merotelic kinetochore in anaphase that became unusually stretched laterally as it moved away from the equator in the direction of the brighter kinetochore fibre (R>1).
Figure 8.
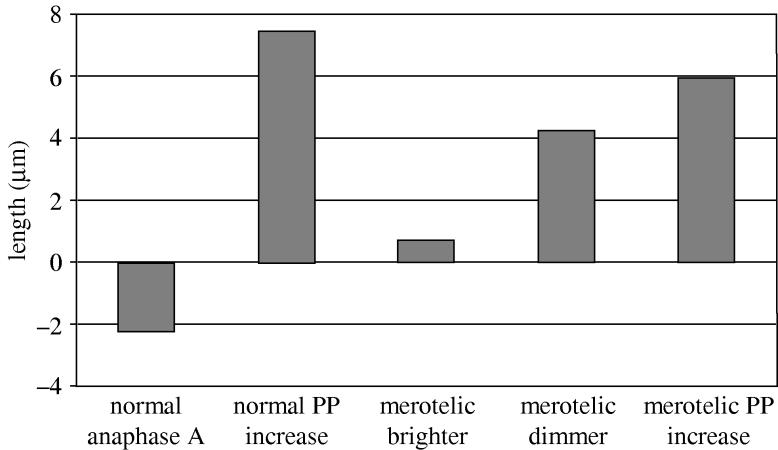
Comparison of the changes in length of kinetochore fibres and interpolar distance during anaphase in PtK1 cells for cells with only normally oriented kinetochores and for cells with a merotelic kinetochore in anaphase as described by Cimini et al. (2004).
We conclude from the above study that lagging chromosomes are prevented in anaphase for merotelic kinetochores whose numbers of kinetochore microtubules to the correct pole are bigger than those to the incorrect pole. Correction occurs not by detachment, but by differences in the polymerization dynamics of microtubules to the correct versus incorrect pole.
Note that in the anaphase example in figure 7d, the merotelic kinetochore became stretched into puncta separated by thin strands of CENP-F-stained material, as previously observed by CREST staining (Cimini et al. 2001). This pattern of lateral stretch indicates that the supporting coiled centromeric DNA–protein subunits of the inner kinetochore have been locally pulled apart or partially uncoiled consistent with the model of inner kinetochore DNA–protein interactions proposed by Zinkowski and coworkers (1991).
8. Model for merotelic kinetochore movements in anaphase
Why do merotelic kinetochore fibres with fewer microtubules grow longer, while fibres with more microtubules grow less or shorten? A model we have proposed (Cimini et al. 2004) is shown in figure 9. At anaphase onset in PtK1 cells, interpolar spindle elongation begins and moves poles apart (anaphase B). Microtubules attached to merotelic kinetochores are moved away from the equator in the direction of their poles by a velocity equal to the sum of half the velocity of interpolar elongation (Vpp/2) plus the velocity of poleward flux of kinetochore microtubules (Vflux) that is coupled to depolymerization at the poles (figure 9a). Interpolar elongation and poleward flux pull and stretch the kinetochore in opposite directions. We assume that, in anaphase, minus ends of merotelic kinetochore microtubules behave like those of normally oriented chromosomes. They remain anchored at the spindle poles and exhibit slower rates of poleward flux compared with metaphase (0.2 versus 0.55 μm min−1 for PtK1 cells; Zhai et al. 1995). Poleward flux velocity may also be slowed by high tension at their kinetochore microtubule attachment sites, but reduction may be small (Maddox et al. 2003a) and poleward flux velocity is assumed constant in the analysis below. We also assume that mechanisms that move chromosomes to the equator in prometaphase, such as polar ejection forces (Rieder & Salmon 1998), that are in part produced by plus-directed kinesin motors bound to the chromosomes (Funabiki & Murray 2000; Levesque & Compton 2001), are inactivated as normally appears to occur in anaphase (Funabiki & Murray 2000; Canman et al. 2003). Finally, we assume each kinetochore MT attachment site can function independently of its neighbours, as discussed previously for the alignment of merotelic kinetochores at the spindle equator (Khodjakov et al. 1997).
Figure 9.
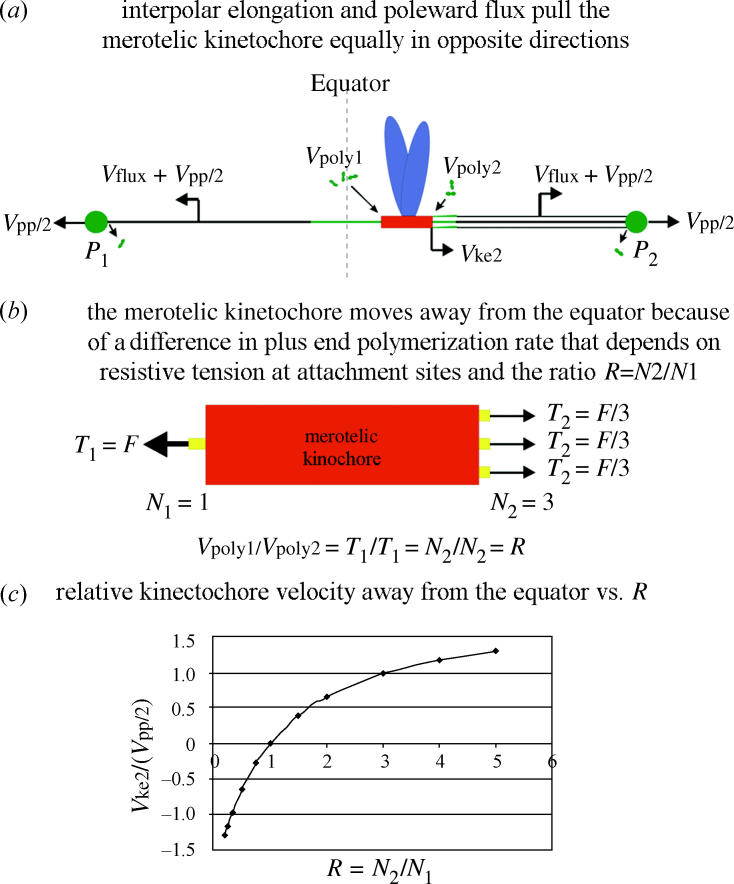
Model for how anaphase spindle and kinetochore mechanics move chromosomes with merotelic kinetochores in anaphase. See text for details.
In our model, the velocities of movement away from the equator of the kinetochore ends facing the incorrect pole, P1, and the correct pole, pole P2 (figure 9a), depend on the rate of microtubule polymerization at kinetochore plus-end attachment sites. The velocities are given by
| (8.1) |
where i=1 or 2 for P1 or P2, respectively, and is the rate of polymerization at attachment sites facing pole Pi.
We propose that the kinetochore moves away from the equator because the average rate of plus-end polymerization, Vpoly, at a kinetochore microtubule attachment site depends on tension generated at the attachment site/microtubule interface. Attachment sites with higher tension will polymerize faster than sites with lower tension. The magnitude of tension will depend directly on the number of kinetochore microtubules to the same pole since kinetochore fibre forces (F) toward opposite poles are approximately equal (figure 9b). This is because the viscous drag on chromosomes at the slow velocities of merotelic kinetochore movement are not significant (0.1 pN; Nicklas 1983, 1988; Alexander & Reider 1991) compared with the forces needed to stall a chromosome in metaphase or anaphase, about 12 pN/kMT attachment site (Nicklas 1983, 1988). Since the forces to opposite poles are equal, then the ratio of tensions at attachment sites facing opposite directions will depend on the ratio (R) of the number (N2) of microtubules attached to the correct pole (P2) versus the number (N1) attached to the incorrect pole (P1) as
| (8.2) |
The tensions at kinetochore microtubule attachment sites facing opposite poles are only equal when the microtubule number is equal. When the numbers are unequal, then the side with the fewer microtubules will have the higher tension relative to the side with the greater number of microtubules (figure 9b). If polymerization rate to a first approximation is proportional to tension, then the ratios of polymerization rates for attachment sites facing opposite poles are related to R by
| (8.3) |
From the above analysis, the velocity of kinetochore movement away from the equator toward pole P2, Vke2, is related to R, Vpp/2, Vflux, and the rate of kinetochore lateral stretch during anaphase, Vks, by the following:
| (8.4) |
From equation (8.4), Vpoly1=RVpoly2, so that from equation (8.6),
| (8.5) |
and
| (8.6) |
Substituting equation (8.6) into equation (8.1) gives for Vke2:
| (8.7) |
Figure 9c shows a plot of Vke2 relative to Vpp/2 as a function of R from 0.25 to 5.0 using the average value of Vpp=0.42 μm min−1 measured in Cimini et al. (2004) for spindles with merotelic kinetochores, Vflux=0.2 μm min−1 as measured by Zhai et al. (1995) for normally attached kinetochores in anaphase. Changes in the stretch of the kinetochore are neglected in the curve in figure 9c, but increased stretch would add to the predicted values of Vke2 relative to Vpp/2. Note that for large values of R, Vke2 becomes faster than Vpp/2 when attachment site tension for the larger fibre becomes low enough so that the rate of polymerization is less than Vflux and the kinetochore fibre shortens.
Our assumption in the above model that polymerization rate depends on tension is based on the findings of Maddox et al. (2003a) for force generation at kinetochores of bi-oriented chromosomes at the spindle equator of Xenopus egg extract spindles. They proposed that the attachment site acts like a ‘slip-clutch’ mechanism where at high tensions, the kinetochore persists in polymerization and resists the poleward flux of kinetochore microtubules as these microtubules are pulled out of the attachment site by spindle forces. Resistive tension and polymerization rate both appeared to depend on the rate at which the microtubule lattice was pulled by spindle forces through the attachment site. This resistance may depend on dynamic linkages between the attachment site and the microtubule lattice produced by motor or non-motor proteins (Howard 2001). Tension could also depend on thermal ratchet mechanisms that act to maintain polymerizing ends within the attachment site similar to the sleeve model proposed by Hill in 1985 (Hill 1985; Inoué & Salmon 1995; Joglekar & Hunt 2002; Mogilner & Oster 2003).
How plus-end polymerization is promoted by attachment site tension is not understood. One possibility is that higher tension promotes a higher net rate of polymerization by a ‘finger cuff’ mechanism, where tension causes the fibrous structure of the attachment site to contract more tightly around the microtubule lattice, blocking the protofilament inside-out curvature associated with loss of lateral bonding between dimers and tubulin dimer dissociation (Maddox et al. 2003a). Attachment site constriction at high tension could also in some way prevent the action of depolymerases thought to function in dimer dissociation within the kinetochore and/or promote the action of the plus-end stabilizing proteins like EB1 that concentrate at polymerizing ends as discussed below.
9. Merotelic kinetochores may also produce chromosome non-disjunction
An important prediction of the model in figure 9c is that for ratios R=N2/N1 that are less than 1, the merotelic kinetochore will move toward the incorrect pole in anaphase. Such movement will produce non-disjunction of separated sister chromosomes (figure 10). As mentioned earlier, this error in chromosome segregation actually occurs slightly more frequently in mammalian tissue cells than the occurrence of anaphase lagging chromosomes near the equator (Cimini et al. 1999). Note that lagging chromosomes only occur for ratios near one, and the vast majority of merotelic kinetochores we identified in metaphase and followed into anaphase migrated toward the correct pole because of ratios greater than 1 (Cimini et al. 2004). We have not yet detected merotelic kinetochores at metaphase that migrate to the incorrect pole in anaphase. We think these merotelic kinetochores are difficult to detect at metaphase because they achieve positions away from the equator and toward the incorrect pole that are similar to the positions of sister kinetochores that are correctly oriented to that pole. If such merotelic kinetochores exist, how do they form and achieve more kinetochore microtubules to the incorrect pole versus correct pole? One possibility is that these merotelic kinetochores are derived from the bi-orientation of sister pairs that initially form syntelic attachments to one pole (figure 10). When one of these kinetochores makes attachments to the opposite (correct) pole, this bi-orientation induces chromosome movement closer to the equator and inactivates the spindle checkpoint. Because of the initial syntelic orientation, the numbers of microtubules to the correct pole could be significantly less than the number to the incorrect pole. If this ratio persists into anaphase after separation of sister chromatids, then the chromosome with the merotelic kinetochore will follow its sister to the same pole.
Figure 10.
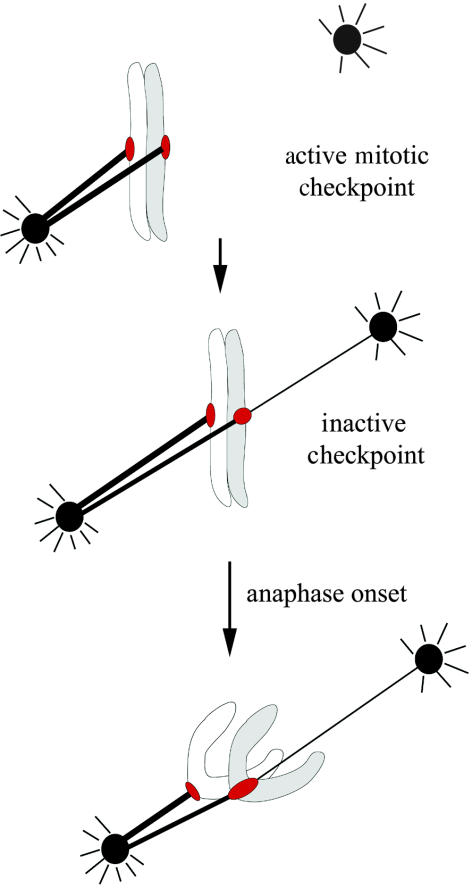
Hypothesis for how syntelic attachment of both sister kinetochores to one pole in prometaphase before merotelic kinetochore formation and bi-orientation can lead to both sisters moving to the same pole in anaphase by the mechanism described in figure 9. Chromosome non-disjunction occurs because the merotelic kinetochore in anaphase has kinetochore microtubules to the same pole as its sister kinetochore with a number that is larger than the number to the opposite, correct pole (R<1).
McEwen et al. (1997) showed for PtK1 cells that mono-oriented chromosomes with 10 or more microtubules attached to one sister kinetochore will congress to the spindle equator when only two to three microtubules from the opposite pole become attached to the previously unattached sister kinetochore. This indicates that only a few microtubules to the opposite pole would be sufficient to induce an initially syntelically oriented sister pair to become aligned near the equator. This equatorial alignment of sister pairs with unequal numbers of microtubules to opposite poles is probably dependent on centring forces that are inactivated upon anaphase onset. A major contributor to the centring force are polar ejection forces on the chromosome arms produced in part by plus-end directed chromokinesins like Kid (Funabiki & Murray 2000; Levesque & Compton 2001), whose activity appears inactivated in anaphase. However, chromosomes still achieve positions near the spindle equator when Kid is substantially inhibited (Levesque & Compton 2001), so other unknown molecular mechanisms need to be discovered.
10. The Ndc80 complex and other molecules that may function in the stability of plus-end attachment and resistive force generation at the kinetochore microtubule attachment site
The mitosis field is still at the early stages of understanding how individual kinetochore microtubule attachment sites are constructed and regulated within the kinetochore. One possible model is shown in figure 11. Our focus for the past several years has been on the function of Nuf2 and Hec1, two protein components of the highly conserved Ndc80 complex. The Ndc80 complex includes Ndc80 (highly enhanced in cancer cells (HEC1) in humans), Nuf2, Spc24 and Spc25. These proteins were initially identified in yeast and more recently their homologues were found in vertebrates and C. elegans (Howe et al. 2001; Nabetani et al. 2001; Wigge & Kilmartin 2001; DeLuca et al. 2002; Martin-Lluesma et al. 2002; Bharadwaj et al. 2003; Desai et al. 2003; Hori et al. 2003; McCleland et al. 2003, 2004). Inner kinetochore proteins like CENPA, C, H and I, and Mis 12 and the KNL proteins are important for localization of the Ndc80 complex to the kinetochore (Desai et al. 2003; Hori et al. 2003). DeLuca et al. (2005) have recently shown by electron microscopy that Hec1 specifically localizes to the kinetochore outer plate, and not the corona. Also, RNAi for Nuf2 results in loss of kinetochore microtubules as well as outer plate structure. Unlike the motors CENP-E and cytoplasmic dynein, and many spindle checkpoint proteins, Nuf2 and Hec1 are part of a stable core of the kinetochore that remains constant in concentration during mitosis and does not change in concentration with microtubule depolymerization or kinetochore microtubule formation to the extent exhibited by the motors and the checkpoint proteins, which are prominent components of the kinetochore corona (Hoffman et al. 2001; DeLuca et al. 2005).
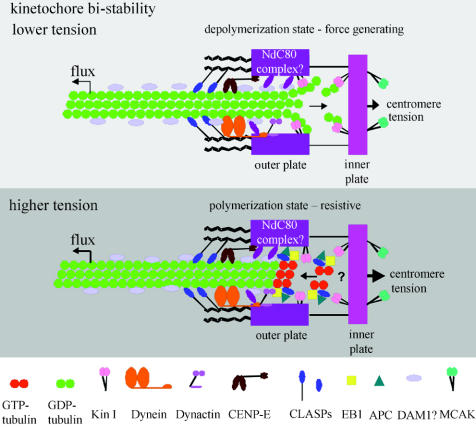
Figure 11.
A model for molecular organization at a vertebrate kinetochore microtubule attachment site. Kinetochores exhibit bi-stability, switching to depolymerization at low attachment site tensions and switching to polymerization at high attachment site tensions (Inoué & Salmon 1995; Rieder & Salmon 1998; Mitchison & Salmon 2001; Maiato et al. 2004). These two persistent states are probably dependent on the dynamic instability of microtubule plus ends. At depolymerizing ends, GDP-dimers within the microtubule lattice lose their lateral connections and their protofilaments curve inside-out promoting dimer dissociation. Energy for producing this curvature is provided by the hydrolysis of GTP that occurred previously during polymerization. The depolymerization state of the kinetochore is force generating and pulls the attachment site in a minus direction along the microtubule lattice coupled to depolymerization within the attachment site. An attachment site appears to switch to the polymerization state when attached plus ends of microtubules switch to polymerization. Polymerizing ends are capped and stabilized by unhydrolysed GTP-tubulin dimer, the polymerizing subunit. Polymerizing ends rarely push kinetochores during mitosis (Rieder & Salmon 1998), but attachment sites with polymerizing ends generate resistive tension to pulling forces produced by poleward microtubule flux, spindle interpolar elongation and centromere stretch. The Ndc80 complex of proteins is required to organize kinetochore outer plate attachment site structure and, based on budding yeast studies, probably provides dynamic linkage (projection in drawing) between the outer plate and microtubule associated proteins on the microtubule lattice similar to the DAM1 complex in yeast. Kin I kinesins promote depolymerization at depolymerizing ends and the Kin I kinesin MCAK at the inner centromere may assist in eliminating merotelic kinetochore attachments. Cytoplasmic dynein, dynactin and the kinesin CENP-E are components of the kinetochore corona. Dynein minus-motor activity may enhance depolymerase activity by pulling the microtubule lattice into the attachment site. CENP-E and dynein have major functions in the spindle checkpoint (Cleveland et al. 2003). Polymerizing ends concentrate EB1 at their tips, which stabilizes growth and antagonizes the depolymerases. Adenomatous polyposis coli (APC) protein binds EB1 and may help link polymerizing ends at kinetochores. Another protein that binds polymerizing ends is CLASP1, and CLASP1 is required to prevent persistent depolymerization (Maiato et al. 2004). Not shown in the drawing are potential roles of Cdc42 and mDia3 in linking APC, EB1 and microtubules to kinetochores (Green & Kaplan 2003; Yasuda et al. 2004) and RanBP1 and RanGap for stable kinetochore microtubule attachment (reviewed in Maiato et al. 2004). Drawing is modified from Maiato et al. (2004).
Like in yeast, Nuf2, Hec1 and the other Ndc80 components are needed to form stable kinetochore microtubule attachments in metazoan cells that can sustain the high tensions typical of bi-oriented chromosomes. HeLa cells reduced from 5 to 10% of normal levels of Hec1 or Nuf2 become blocked in prometaphase with unaligned chromosomes, unoriented and unstretched centromeres, and spindles much longer than occurs during normal metaphase after pulling forces are generated by kinetochore microtubule formation. Kinetochores in Ndc80 complex depleted cells also have no stable kinetochore microtubules as normally occurs following rapid cooling (Brinkley & Cartwright 1975; DeLuca et al. 2002; McCleland et al. 2004). If cells with depleted levels of Nuf2 and Hec1are induced into anaphase by abrogating the spindle checkpoint, some chromosomes do move toward the poles, indicating weak interactions between kinetochores and spindle microtubules (DeLuca et al. 2003). These weak interactions may be produced by motors like CENP-E that are not depleted by loss of the Ndc80 complex components, or by transient weak attachments to kinetochores that have low concentrations of Ndc80 components and cytoplasmic dynein (DeLuca et al. 2002, 2005; Martin-Lluesma et al. 2002; McCleland et al. 2004).
The identities of the proteins that dynamically link the Ndc80 complex to the microtubule lattice within the kinetochore attachment site are not yet known in metazoans. In budding yeast, linkers include the microtubule associated protein complex, DAM1 (DASH1) and the EB1 homologue, Bim1. Ipl1 phosphorylation of Ndc80 (or mutants that mimic phosphorylation) and components of the DAM1 complex detach microtubules from the kinetochore while dephosphorylation by PP1 or mutants that mimic dephosphorylation result in tight attachment (Cheeseman et al. 2002; Shang et al. 2003). This suggests a similar mechanism operates at the vertebrate kinetochore, but no data are yet available. Microtubule attachment to merotelic kinetochores in anaphase appears very stable from our live cell imaging. This stability is correlated with the absence of Aurora B at centromeres in anaphase and the low rate of microtubule turnover at anaphase for normally oriented kinetochores (Zhai et al. 1995). EB1 may also contribute to linking polymerizing plus ends to kinetochores under tension. Live cell imaging of GFP-EB1 shows that it binds transiently to polymerizing plus ends of microtubules (Tirnauer et al. 2002). EB1 concentrates at normally oriented kinetochores of chromosomes in PtK1 cells when they are polymerizing and oscillating away from the pole, but not at kinetochores that are moving poleward either before or after anaphase onset (Tirnauer et al. 2002).
Other proteins that may contribute to generating tension at polymerizing kinetochores are the molecular motors CENP-E and dynein. However, substantial inhibition of these proteins does not prevent bi-orientation, accumulation of kinetochore microtubules or development of centromere tension in mammalian tissue cells (Howell et al. 2001; Cleveland et al. 2003). The microtubule binding protein Sgo has recently been shown to have a function in regulating stability of attachment as does RanGap1 and RanBP2 (Maiato et al. 2004). How these proteins function between the Ndc80 complex and the microtubule lattice will hopefully be revealed in the near future. There is also a lot of interest in the roles of microtubule depolymerases like the Kin I kinesins HsMCAK, HsKif2, DmKLP10A, and DmKLp67F and yeast Kip3 and its homologues in metazoans in linking plus ends dynamically to the kinetochore (Maiato et al. 2004). These proteins use ATP hydrolysis to drive tubulin dimer dissociation from the ends of microtubules. Although most attention is currently being focused on how these proteins contribute to depolymerization at either the kinetochore (figure 11) or the pole, it is equally interesting to know whether they also contribute to dynamically linking the kinetochore to the microtubule lattice during polymerization. CLASP1, and another plus-end tracking protein, like EB1, may have a significant roles in preventing depolymerase activity at kinetochores and promoting polymerization, as well as linking polymerizing ends to the kinetochore (Maiato et al. 2004).
There is also very recent evidence that the formin protein, mDia3, and the RhoGTPase, Cdc42, have critical roles for achieving microtubule attachment to kinetochores of sufficient strength to resist the high centromere tensions generated by normal sister chromosome bi-orientation. Cdc42 is needed for mDia3 to bind to inner kinetochore proteins, and mDia3 may link the kinetochore to the microtubule lattice through APC and EB1 (Kaplan et al. 2001; Green & Kaplan 2003; Yasuda et al. 2004). This suggests that these proteins, like the Ndc80 complex and its microtubule linkers (DAM1?), may play key roles in maintaining the attachment of merotelic kinetochores to polymerizing plus ends of microtubules under the high tensions generated in anaphase when merotelic kinetochores are highly stretched toward opposite poles. Another important question that remains unresolved is whether EB1 and other microtubule linker proteins are regulated by the Aurora B complex before anaphase, like the Ndc80 and DAM1 complexes in budding yeast (Cheeseman et al. 2002; Shang et al. 2003), to promote release of mis-attached microtubules.
11. How often do mitotic cells make errors in chromosome segregation in situ?
Our analysis of errors in chromosome segregation based on spindle mechanics predicts a frequency of the order of 1% for unperturbed mammalian tissue cells progressing through anaphase in culture. This rate per chromosome is of the order of 1/12% (0.000 85) for PtK1 cells with 12 chromosomes and 1/46% (0.0002) for human tissue cells with 46 chromosomes. Unlike mammalian cells with multiple kinetochore microtubule attachment sites, budding yeast has only one per kinetochore. The chromosome loss rate in these cells is on the order of 1/100 000 (0.000 01; Hartwell & Smith 1985). This is about 100-fold lower than measured for PtK1 tissue culture cells and 20-fold lower than for primary human lung cells in culture. Micronuclei are one possible product of a lagging chromosomes near the equator in anaphase. In human lymphocytes, the frequency of cells with micronuclei containing whole chromosomes is reported to be about 0.6–0.8% at 52 h of culture following initial purification (Falck et al. 1997). We do not know of any data regarding the accuracy of chromosome segregation in human or other mammalian stem cells. Since tissue culture conditions could be a factor in promoting errors in chromosome segregation, it will be of great interest to know the error rate for chromosome segregation for stem cells in situ or, as a first step, in primary cultures.
Acknowledgments
We thank The Royal Society and the organizers of the meeting on chromosome segregation, Professor Kim Nasmyth and Professor Yanagida, and our continued support from NIH GM24364.
References
- Alexander S.P, Rieder C.L. Chromosome motion during attachment to the vertebrate spindle: initial saltatory-like behaviour of chromosomes and quantitative analysis of force production by nascent kinetochore fibers. J. Cell Biol. 1991;113:805–815. doi: 10.1083/jcb.113.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj, R., Qi, W. & Yu., H. T. 2004 Identification of two novel components of the human NDC80 kinetochore complex. J. Biol. Chem.279, 13076–13085. [DOI] [PubMed]
- Biggins S, Murray A.W. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15:3118–3129. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S, Severin F.F, Bhalla N, Sassoon I, Hyman A.A, Murray A.W. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 1999;13:532–544. doi: 10.1101/gad.13.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley, B. R. & Cartwright, Jr., J. 1975 Cold-labile and cold-stable microtubules in the mitotic spindle of mammalian cells. Ann NY Acad. Sci.253, 428–439. [DOI] [PubMed]
- Brinkley B.R, Zinkowski R.P, Mollon W.L, Davis F.M, Pisegna M.A, Pershouse M, Rao P.N. Movement and segregation of kinetochores experimentally detached from mammalian chromosomes. Nature. 1988;336:251–254. doi: 10.1038/336251a0. [DOI] [PubMed] [Google Scholar]
- Cahill D.P, Lengauer C, Yu J, Riggins G.J, Willson J.K.V, Markowitz S.D, Kinzler K.W, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- Canman J.C, Sharma C.N, Straight A, Shannon K.B, Fang G, Salmon E.D. Anaphase onset does not require the microtubule-dependent depletion of kinetochore and centromere-binding proteins. J. Cell Sci. 2002;115:3787–3795. doi: 10.1242/jcs.00057. [DOI] [PubMed] [Google Scholar]
- Canman J.C, Cameron L.A, Maddox P.S, Straight A, Tirnauer J.S, Mitchison T.J, Fang G, Kapoor T.M, Salmon E.D. Determining the position of the cell division plane. Nature. 2003;424:1074–1078. doi: 10.1038/nature01860. [DOI] [PubMed] [Google Scholar]
- Cheeseman I.M, Anderson S, Jwa M, Green E.M, Kang J.S, Yates J.R, Chan C.S.M, Drubin D.G, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- Cimini D, Tanzarella C, Degrassi F. Differences in malsegregation rates obtained by scoring ana-telophases or binucleate cells. Mutagenesis. 1999;14:563–568. doi: 10.1093/mutage/14.6.563. [DOI] [PubMed] [Google Scholar]
- Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon E.D. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J. Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, Fioravanti D, Salmon E.D, Degrassi F. Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J. Cell Sci. 2002;115:507–515. doi: 10.1242/jcs.115.3.507. [DOI] [PubMed] [Google Scholar]
- Cimini D, Moree B, Canman J.C, Salmon E.D. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 2003;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- Cimini, D., Cameron, L., Salmon, E. D. In press Anaphase spindle mechanics prevent mis-segregation of merotelically-oriented chromosomes. Curr Biol 14, 2149–2155. [DOI] [PubMed]
- Cleveland D.W, Mao Y, Sullivan K.F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- DeLuca, J. G., Moree, B., Hickey, J. M., Kilmartin, J. V. & Salmon. E. D. 2002 hNuf2 inhibition blocks stable kinetochore-microtubule attachment and induces mitotic cell death in HeLa cells. J. Cell Biol.159, 549–555. [DOI] [PMC free article] [PubMed]
- DeLuca, J. G., Howell, B. J., Canman, J. C., Hickey, J. M., Fang, G. & Salmon. E. D. 2003 Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr. Biol.13, 2103–2109. [DOI] [PubMed]
- DeLuca J.G, Dong Y, Hergert P, Strauss J, Hickey J, Salmon E.D, McEwen B.F. Hec-1 and Nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell. 2005;16:519–531. doi: 10.1091/mbc.E04-09-0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, A., Rybina, S., Muller-Reichert, T., Shevchenko, A., Hyman, A. & Oegema. K. 2003 KNL-1 directs assembly of the microtubule-binding interface of the kinetochore in C. elegans Genes Dev.17, 2421–2435. [DOI] [PMC free article] [PubMed]
- Duesberg P, Li R, Rasnik D, Rausch C, Willer A, Kraemer A, Yerganian G, Helmann R. Aneuploidy precedes and segregates with chemical carcinogenesis. Cancer Genet. Cytogenet. 2000;119:83–93. doi: 10.1016/s0165-4608(99)00236-8. [DOI] [PubMed] [Google Scholar]
- Falck G, Catalan J, Norppa H. Influence of culture time on the frequency and contents of human lymphocyte micronuclei with and without cytochalasin B. Mutat. Res. 1997;392:71–79. doi: 10.1016/s0165-1218(97)00046-3. [DOI] [PubMed] [Google Scholar]
- Funabiki H, Murray A.W. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell. 2000;102:411–424. doi: 10.1016/s0092-8674(00)00047-7. [DOI] [PubMed] [Google Scholar]
- Green R.A, Kaplan K.B. Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. J. Cell Biol. 2003;163:949–961. doi: 10.1083/jcb.200307070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L.H, Smith D. Altered fidelity of mitotic chromosome transmission in cell cycle mutants of S. cerevisiae. Genetics. 1985;110:381–395. doi: 10.1093/genetics/110.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T.L. Theoretical problems related to the attachment of microtubules to kinetochores. Proc. Natl Acad. Sci. USA. 1985;82:4404–4408. doi: 10.1073/pnas.82.13.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D.B, Pearson C.G, Yen T.J, Howell B.J, Salmon E.D. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at ptk1 kinetochores. Mol. Biol. Cell. 2001;12:1995–2009. doi: 10.1091/mbc.12.7.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori, T., Haraguchi, T., Hiraoka, Y., Kimura, H. & Fukagawa, T. 2003 Dynamic behavior of Nuf2-Hec1 complex that localizes to the centrosome and centromere and is essential for mitotic progression in vertebrate cells. J. Cell Sci.116, 3347–3362. [DOI] [PubMed]
- Howard J. Sinauer Associates; Sunderland, MA: 2001. Mechanics of motor proteins and the cytoskeleton. p. 367. [Google Scholar]
- Howe, M., McDonald, K. L., Albertson, D. G. & Meyer B. J. 2001 HIM-10 is required for kinetochore structure and function on Caenorhabditis elegans holocentric chromosomes. J. Cell Biol.153, 1227–1238. [DOI] [PMC free article] [PubMed]
- Howell B.J, McEwen B.F, Canman J.C, Hoffman D.B, Farrar E.M, Rieder C.L, Salmon E.D. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoué S. Organization and function of the mitotic spindle. In: Allen R.D, Kamiya N, editors. Primitive motile systems in cell biology. Academic Press; New York: 1963. pp. 549–598. [Google Scholar]
- Inoué S, Salmon E.D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol. Biol. Cell. 1995;6:1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke, M. A. & LaFountain, Jr., J. R. 1982 Chromosome segregation in crane-fly spermatocytes: cold treatment and cold recovery induce anaphase lag. Chromosoma.85, 619–631. [DOI] [PubMed]
- Joglekar A.P, Hunt A.J. A simple, mechanistic model for directional instability during mitotic chromosome movements. Biophys. J. 2002;83:42–58. doi: 10.1016/S0006-3495(02)75148-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan K.B, Burds A.A, Swedlow J.R, Bekir S.S, Sorger P.K, Nathke I.S. A role for the Adenomatous polyposis coli protein in chromosome segregation. Nature Cell Biol. 2001;3:429–432. doi: 10.1038/35070123. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Cole R.W, McEwen B.F, Buttle K.F, Rieder C.L. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J. Cell Biol. 1997;136:229–240. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Gopenagle L, Gordon M.B, Compton D.A, Kapoor T.M. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J. Cell Biol. 2003;160:671–683. doi: 10.1083/jcb.200208143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith S.L, Khodjakov A, Hergert P, Walczak C.E. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell. 2004;15:1146–1159. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson M.A, Renduchitala K, Khodjakov A, Kapoor T.M. Correcting improper chromosome-spindle attachments during cell division. Nature Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- Lengauer C, Kinzler K.W, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–627. doi: 10.1038/386623a0. [DOI] [PubMed] [Google Scholar]
- Levesque A.A, Compton D.A. The chromokinesin Kid is necessary for chromosome arm orientation and oscillation, but not congression, on mitotic spindles. J. Cell Biol. 2001;154:1135–1146. doi: 10.1083/jcb.200106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox P, Straight A, Coughlin P, Mitchison T.J, Salmon E.D. Direct observation of microtubule dynamics at kinetochores in Xenopus extract spindles: implications for spindle mechanics. J. Cell Biol. 2003a;162:377–382. doi: 10.1083/jcb.200301088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox P.S, Moree B, Canman J.C, Salmon E.D. Spinning disk confocal microscope system for rapid high-resolution, multimode, fluorescence speckle microscopy and green fluorescent protein imaging in living cells. Methods Enzymol. 2003b;360:597–617. doi: 10.1016/s0076-6879(03)60130-8. [DOI] [PubMed] [Google Scholar]
- Maiato H, DeLuca J.G, Salmon E.D, Earnshaw W.J. The dynamic kinetochore-microtubule interface. J. Cell Sci. 2004;117:5461–5477. doi: 10.1242/jcs.01536. [DOI] [PubMed] [Google Scholar]
- Martin-Lluesma, S., Stucke, V. M. & Nigg, E. A. 2002 Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science297, 2267–2270. [DOI] [PubMed]
- McCleland, M. L., Gardner, R. D., Kallio, M. J., Daum, J. R., Gorbsky, G. J., Burke, D. J. & Stukenberg, P. T. 2003 The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev.17, 101–114. [DOI] [PMC free article] [PubMed]
- McCleland, M. L., Kallio, M. J., Barrett-Wilt, G. A., Kestner, C. A., Shabanowitz, J., Hunt, D. F., Gorbsky, G. J. & Stukenberg. P. T. 2004 The vertebrate Ndc80 complex contains Spc24 and Spc25 homologs, which are required to establish and maintain kinetochore-microtubule attachment. Curr. Biol.14, 131–137. [DOI] [PubMed]
- McEwen B.F, Heagle A.B, Cassels G.O, Buttle K.F, Rieder C.L. Kinetochore fiber maturation in PtK1 cells and its implications for the mechanisms of chromosome congression and anaphase onset. J. Cell Biol. 1997;137:1567–1580. doi: 10.1083/jcb.137.7.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.F, Ding Y, Heagle A.B. Relavance of the kinetochore size and microtubule binding capacity of stable chromosme attachment during mitosis in PtK1 cells. Chromatogr. Res. 1998;6:123–132. doi: 10.1023/a:1009239013215. [DOI] [PubMed] [Google Scholar]
- Mitchison T.J, Salmon E.D. Mitosis: a history of division. Nature Cell Biol. 2001;3:E17–E21. doi: 10.1038/35050656. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Oster G. Cell biology. Shrinking gels pull cells. Science. 2003;302:1340–1341. doi: 10.1126/science.1092041. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Hardwick K.G. The spindle checkpoint: structural insights into dynamic signalling. Nature Rev. Mol. Cell Biol. 2002;3:731–741. doi: 10.1038/nrm929. [DOI] [PubMed] [Google Scholar]
- Nabetani, A., Koujin, T., Tsutsumi, C., Haraguchi, T. & Hiraoka Y. 2001 A conserved protein, Nuf2, is implicated in connecting the centromere to the spindle during chromosome segregation: a link between the kinetochore function and the spindle checkpoint. Chromosoma 110, 322–334. [DOI] [PubMed]
- Nicklas R.B. Measurements of the force produced by the mitotic spindle in anaphase. J. Cell Biol. 1983;97:542–548. doi: 10.1083/jcb.97.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R.B. The forces that move chromosomes in mitosis. Annu. Rev. Biophys. Chem. 1988;17:431–449. doi: 10.1146/annurev.bb.17.060188.002243. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Nicolaidis P, Petersen M.B. Origin and mechanisms of non-disjunction in human autosomal trisomies. Hum. Reprod. 1998;13:313–319. doi: 10.1093/humrep/13.2.313. [DOI] [PubMed] [Google Scholar]
- Ohi R, Coughlin M.L, Lane W.S, Mitchison T.J. An inner centromere protein that stimulates the microtubule depolymerizing activity of a KinI kinesin. Dev. Cell. 2003;5:309–321. doi: 10.1016/s1534-5807(03)00229-6. [DOI] [PubMed] [Google Scholar]
- Pidoux A, Allshire R. Chromosome segregation: clamping down on deviant orientations. Curr. Biol. 2003;13:R385–R387. doi: 10.1016/s0960-9822(03)00316-6. [DOI] [PubMed] [Google Scholar]
- Pidoux A.L, Allshire R.C. The role of heterochromatin in centromere function. Phil. Trans. R. Soc. B. 2005;360:569–579. doi: 10.1098/rstb.2004.1611. 10.1098/rstb.2004.1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux A.L, Uzawa S, Perry P.E, Cande W.Z, Allshire R.C. Live analysis of lagging chromosomes during anaphase and their effect on spindle elongation rate in fission yeast. J. Cell Sci. 2000;113:4177–4191. doi: 10.1242/jcs.113.23.4177. [DOI] [PubMed] [Google Scholar]
- Rabitsch K.P, Petronczki M, Javerzat J.P, Genier S, Chwalla B, Schleiffer A, Tanaka T.U, Nasmyth K. Kinetochore recruitment of two nucleolar proteins is required for homolog segregation in meiosis I. Dev. Cell. 2003;4:535–548. doi: 10.1016/s1534-5807(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Rasnick, D. & Duesberg, P. H. 1999 How aneuploidy affects metabolic control and causes cancer. Biochem. J.340, 621–630. [PMC free article] [PubMed]
- Rieder C.L. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int. Rev. Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Rieder C.L, Salmon E.D. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 1998;8:310–318. doi: 10.1016/s0962-8924(98)01299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders W.S, Shuster M, Huang X, Gharaibeh B, Enyenihi A, Petersen I, Gollin S.M. Chromosomal instability and cytoskeletal defects in oral cancer cells. Proc. Natl Acad. Sci. USA. 2000;97:303–308. doi: 10.1073/pnas.97.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S. Aneuploidy and cancer. Curr. Opin. Oncol. 2000;12:82–88. doi: 10.1097/00001622-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shang C, Hazbun T.R, Cheeseman I.M, Aranda J, Fields S, Drubin D.G, Barnes G. Kinetochore protein interactions and their regulation by the Aurora kinase Ipl1p. Mol. Biol. Cell. 2003;14:3342–3355. doi: 10.1091/mbc.E02-11-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Thompson E.A, Miller F.J, Hayes J, Rieder C.L. The checkpoint control foranaphase onset does not monitor excess numbers of spindle poles or bipolar spindle symmetry. J. Cell Sci. 1997;110:421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- Stoler D.L, Chen N, Basik M, Kahlenberg M.S, Rodrigues-Bigas M.A, Petrelli N.J, Anderson G.R. The onset and extent of genomic instability in sporadic colorectal tumor progression. Proc. Natl Acad. Sci. USA. 1999;96:15 121–15 126. doi: 10.1073/pnas.96.26.15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T.U, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark M.J, Nasmyth K. Evidence that the Ipl1–Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Tirnauer J.S, Canman J.C, Salmon E.D, Mitchison T.J. EB1 targets to kinetochores with attached, polymerizing microtubules. Mol. Biol. Cell. 2002;13:4308–4316. doi: 10.1091/mbc.E02-04-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge, P. A. & Kilmartin, J. V. 2001 The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J. Cell Biol.152, 349–360. [DOI] [PMC free article] [PubMed]
- Wise D.A, Brinkley B.R. Mitosis in cells with unreplicated genomes (MUGs): spindle assembly and behavior of centromere fragments. Cell Motil. Cytoskeleton. 1997;36:291–302. doi: 10.1002/(SICI)1097-0169(1997)36:3<291::AID-CM9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, Ishizaki T, Narumiya S. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature. 2004;428:767–771. doi: 10.1038/nature02452. [DOI] [PubMed] [Google Scholar]
- Yu H.G, Dawe R.K. Functional redundancy in the maize meiotic kinetochore. J. Cell Biol. 2000;151:131–142. doi: 10.1083/jcb.151.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y, Kronebusch P.J, Borisy G.G. Kinetochore microtubule dynamics and the metaphase–anaphase transition. J. Cell Biol. 1995;131:721–734. doi: 10.1083/jcb.131.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkowski R.P, Meyne J, Brinkley B.R. The centromere–kinetochore complex: a repeat subunit model. J. Cell Biol. 1991;113:1091–1110. doi: 10.1083/jcb.113.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]