Abstract
Mad2 is an essential component of the spindle assembly checkpoint (SAC), a molecular device designed to coordinate anaphase onset with the completion of chromosome attachment to the spindle. Capture of chromosome by microtubules occur on protein scaffolds known as kinetochores. The SAC proteins are recruited to kinetochores in prometaphase where they generate a signal that halts anaphase until all sister chromatid pairs are bipolarly oriented. Mad2 is a subunit of the mitotic checkpoint complex, which is regarded as the effector of the spindle checkpoint. Its function is the sequestration of Cdc20, a protein required for progression into anaphase. The function of Mad2 in the checkpoint correlates with a dramatic conformational rearrangement of the Mad2 protein. Mad2 adopts a closed conformation (C-Mad2) when bound to Cdc20, and an open conformation (O-Mad2) when unbound to this ligand. Checkpoint activation promotes the conversion of O-Mad2 to Cdc20-bound C-Mad2. We show that this conversion requires a C-Mad2 template and we identify this in Mad1-bound Mad2. In our proposition, Mad1-bound C-Mad2 recruits O-Mad2 to kinetochores, stimulating Cdc20 capture, implying that O-Mad2 and C-Mad2 form dimers. We discuss Mad2 oligomerization and link our discoveries to previous observations related to Mad2 oligomerization.
Keywords: spindle checkpoint, Mad1, Mad2, Cdc20, metaphase, kinetochore
1. The spindle assembly checkpoint
The separation of sister chromatids into two equal complements during mitosis is a complex endeavour (Nasmyth 2002). Loss of sister chromatid cohesion at the metaphase–anaphase transition requires attachment of all sister chromatid pairs to the mitotic spindle. This dependency is maintained by the SAC and protects cells from the undesired loss or gain of chromosomes (aneuploidy). Aneuploidy has been implicated as a possible trigger of cellular transformation based on the observation that cancer cells are often highly aneuploid (Bharadwaj & Yu 2004). Furthermore, aneuploidy resulting from defective meioses has been identified as a cause of several genetic disorders (Petronczki et al. 2003).
Kinetochores are protein scaffolds assembled on centromeric chromatin that mediate the end-on attachment of spindle microtubules (Cleveland et al. 2003). The attachment process consists of the creation of a stable link between the kinetochores and the microtubules of the mitotic spindle into what is called bipolar orientation. Chromosomes are bipolarly oriented if the sisters are attached to opposite poles of the spindle and are, therefore, subject to a force that operates to separate them. At least in vertebrates, this force can be visualized as an increase in the distance between sister kinetochores in metaphase (i.e. after attachment) relative to that in prometaphase (i.e. before attachment; Cleveland et al. 2003). The dependency of anaphase on attachment requires functional kinetochores (Musacchio & Hardwick 2002). A single unattached kinetochore acts as a source of a diffusible signal that inhibits anaphase within the whole spindle to which that unattached kinetochore belongs (Rieder et al. 1995). The creation of the diffusible signal is operated by the SAC, a molecular device whose components localize at kinetochores and at the centromere during prometaphase and, in some cases, also in the subsequent phases of mitosis (Musacchio & Hardwick 2002; Bharadwaj & Yu 2004). The MAD proteins Mad1 and Mad2, and the BUB proteins, Bub1, BubR1 (Bub1-related) and Bub3, compose the molecular core of the spindle checkpoint (Hoyt et al. 1991; Li & Murray 1991). Other proteins, including the kinases Mps1 and Aurora B/Ipl1, and limited to higher eukaryotes, the ZW10 and Rod proteins, are also required for the spindle checkpoint (Musacchio & Hardwick 2002; Bharadwaj & Yu 2004). The role of Aurora B/Ipl1 is particularly important, as the activity of this kinase is believed to be required to sever faulty microtubule–kinetochore connections that prevent the generation of bipolar attachment (Tanaka et al. 2002).
To understand how the SAC prevents anaphase, it must be mentioned that the metaphase–anaphase transition requires the activity of the APC/C (Peters 2002). This multi-subunit ubiquitin (Ub) ligase mediates the ubiquitination and subsequent degradation of cyclin B and securin. The latter is an inhibitor of separase, the protease that causes the loss of sister chromatid cohesion by cleaving one of the subunits of cohesin. The degradation of securin and the concomitant degradation of cyclin B activate separase, causing anaphase (Peters 2002). The target of the SAC is Cdc20, an activator of the APC/C that directs the activity of this Ub-ligase towards securin and cyclin B (Fang et al. 1998; Hwang et al. 1998; Kallio et al. 1998; Kim et al. 1998; Wassmann & Benezra 1998). Two proteins in the SAC, Mad2 and BubR1, bind Cdc20 directly and have been reported to enter a single complex in budding yeast and HeLa cells, now usually referred to as mitotic checkpoint complex (MCC; Sudakin et al. 2001; Tang et al. 2001; Chen 2002; Fang 2002; Millband & Hardwick 2002; Shannon et al. 2002; Meraldi et al. 2004). This complex also contains Bub3, which appears to be constitutively bound to BubR1 (reviewed in Musacchio & Hardwick 2002).
The MCC is believed to be the critical effector of the SAC, and it is important to understand how this complex is formed and the role played by kinetochores in promoting its formation. There is considerable evidence indicating that unattached kinetochores enhance the formation of the MCC. For instance, interventions that completely disrupt kinetochore assembly, thus preventing the localization of the spindle checkpoint proteins at the kinetochore, ablate the checkpoint response (see for instance Rieder et al. 1995; McCleland et al. 2003; Meraldi et al. 2004). All SAC proteins, but also Cdc20 and the subunits of the APC/C, can be identified at kinetochores in prometaphase (reviewed in Musacchio & Hardwick 2002). Using FRAP, it has been shown that several SAC proteins, including Mad2, Bub3, BubR1 and Cdc20, are continuously recruited and released from kinetochores during checkpoint activation (Howell et al. 2001, 2004; Kallio et al. 2002; Shah et al. 2004). This suggests that the kinetochore surface acts as a platform for the recruitment of these proteins and for the formation of a complex between them. Intriguingly, other SAC proteins, including Mad1 and Bub1, are stably bound at kinetochores during checkpoint activation (Howell et al. 2004; Shah et al. 2004). This suggests that they work as components of a catalytic platform at the kinetochore, whose function is the recruitment of Mad2, BubR1, and possibly Cdc20 and the assembly of the MCC.
2. The two conformations of Mad2 and the ‘Mad2 exchange’ model
Our laboratory is interested in understanding the molecular bases of the kinetochore cycle of recruitment and release of SAC proteins during checkpoint activation. Critical goals of our investigations are determining the precise mechanisms through which Mad2 and the other checkpoint proteins are recruited to the kinetochores, the mechanisms that allow the interaction of Mad2 with other components of the SAC at the kinetochore and the mechanisms of propagation and amplification of the spindle checkpoint signal away from the kinetochore. In particular, we have studied the role of Mad1 in activating Mad2 for binding Cdc20 (Sironi et al. 2001, 2002; De Antoni et al. 2005). Although this interaction does not requires Mad1 when purified proteins are mixed in vitro, evidence from the budding yeast Saccharomyces cerevisiae and from Xenopus laevis egg extracts indicates that Mad1 is definitely required for Mad2 to bind Cdc20 in these systems, and that both Mad1-free Mad2 and Mad1-bound Mad2 are required to maintain the spindle checkpoint (Hwang et al. 1998; Fraschini et al. 2001; Sironi et al. 2001; Chung & Chen 2002; Luo et al. 2002; Martin-Lluesma et al. 2002). On the other hand, BubR1, or more precisely, its budding yeast orthologue Mad3, is not required for the interaction of Mad2 with Cdc20 (Hwang et al. 1998), implying that its function may be required downstream from Mad1 and Mad2.
In what way does Mad1 promote the formation of Mad2/Cdc20 complexes? This question turned out to be particularly complex for reasons that will become clear in the remainder of this paper. A first relevant discovery towards the elucidation of this problem was that Mad1 and Mad2 bind to each other directly in the absence of other proteins and that Mad1 is definitely required for the localization of Mad2 to kinetochores, indicating that Mad1 is the kinetochore receptor of Mad2 (Chen et al. 1998, 1999; Sironi et al. 2001; Chung & Chen 2002; Luo et al. 2002; Sironi et al. 2002). Given the relevance of kinetochores for the generation of the spindle checkpoint effector signal, these observations suggested that the positive role of Mad1 in promoting the formation of the Mad2/Cdc20 complex may be based on the recruitment of Mad2 to the kinetochore, which, in turn, would promote the encounter of Mad2 with Cdc20 (reviewed in Musacchio & Hardwick 2002; Bharadwaj & Yu 2004).
We asked what relationship existed between the complexes of Mad2 with Mad1 and Cdc20. In particular, we wished to know whether Mad2, Mad1 and Cdc20 entered a unique ternary complex or whether these interactions of Mad2 were competitive. A critical finding, independently derived in our laboratory and in Hongtao Yu's laboratory, is that Mad2 recognizes similar short linear motifs in Cdc20 and Mad1, which bind into the same pocket of Mad2 (Luo et al. 2002; Sironi et al. 2002). This suggests that Mad1 and Cdc20 may compete for Mad2 binding. A second decisive observation is that the binding of Mad2 to Mad1 or Cdc20 is accompanied by a dramatic conformational change in the C-terminal region of Mad2, the so-called ‘safety-belt’ (Luo et al. 2002; Sironi et al. 2002). The cytosolic form of Mad2, free of Mad1 or Cdc20, adopts what we call open conformation (O-Mad2). In this conformation, the safety belt forms a β-hairpin that completes one edge of the large exposed β-sheet of Mad2. On the other hand, upon binding to Mad1 or Cdc20 the safety belt of Mad2 relocates to the opposite edge of the β-sheet, creating in this manner a topological link with its Mad1 or Cdc20 ligands (Luo et al. 2002, 2004; Sironi et al. 2002; De Antoni et al. 2005). We have defined this as the closed conformation of Mad2 (C-Mad2). An alternative nomenclature has been recently proposed (Luo et al. 2005) in which O-Mad2 and C-Mad2 are named N1 and N2, respectively (where N indicates the native conformation of Mad2, and 1 and 2 indicates that Mad2 adopts two distinct native conformations). We prefer to use the names O-Mad2 and C-Mad2, however, because they refer to a recognizable physical state of the safety belt.
The structural properties of Mad2 have profound implications for understanding the catalytic role played by kinetochores in the formation of the MCC complex (of which Mad2/Cdc20 is a subcomplex). The ‘Mad2 exchange model’, shown in figure 1a, represents an attempt to reconcile these observations. In this model, the conformational change in the safety belt accompanying the binding of Mad2 to Cdc20 is predicted to be rate limiting for the formation of the MCC complex, owing to the large activation barrier that separates the two Mad2 conformers (Luo et al. 2002, 2004; Sironi et al. 2002; De Antoni et al. 2005). Mad1-dependent recruitment of Mad2 to kinetochores may facilitate the conversion of O-Mad2 to Cdc20-bound C-Mad2 by creating a form of C-Mad2 bound to Mad1, which would then become available for Cdc20 binding (Luo et al. 2004).
Figure 1.
Schematic view of the Mad2 exchange model and the Mad2 template model. (a) The Mad2 exchange model predicts that in the presence of Cdc20 the Mad1/C-Mad2 core complex, a 2 : 2 tetramer (Luo et al. 2002; Sironi et al. 2002) dissociates and releases Mad2 for Cdc20 binding. A Cdc20/C-Mad2 complex is formed and a C-Mad2 vacancy is left on Mad1. This vacancy will be filled by cytosolic O-Mad2. An implication of this model is that Mad1 and Cdc20 compete for Mad2 binding, because both contain a Mad2-binding motif. (b) The Mad1/Mad2 tetramer is stable in the Mad2 template model (De Antoni et al. 2005). C-Mad2 on this tetramer acts as a receptor for cytosolic O-Mad2. Thus, a C-Mad2/O-Mad2 oligomer is formed at kinetochores, and O-Mad2 within this oligomer is a source of Mad2 for Cdc20, which leaves an O-Mad2 vacancy (but not a C-Mad2 vacancy as in the exchange model).
3. The MAD2 exchange and the ‘MAD2 template’ models
Our recent studies on the molecular mechanism put forward by the Mad2 exchange model unveiled several weaknesses that question its biological relevance (De Antoni et al. 2005). Our main concern with this model stemmed from the observation introduced above that Mad1 and Cdc20 share a Mad2-binding motif that binds Mad2 into the same binding pocket. Thus, a mechanistic implication of the Mad2 exchange model is that Mad1 and Cdc20 compete for Mad2 binding because before Cdc20 can bind to Mad2, it has to be displaced from Mad1 (figure 1a). While this is not a problem per se, it appears to contrast with existing genetic evidence indicating that Mad1 is required for forming the Mad2/Cdc20 complex. If Cdc20 competed with Mad1 for Mad2 binding, then loss of Mad1 would increase the interaction between Mad2 and Cdc20, rather that antagonizing it.
A second weakness of the Mad2 exchange model is that it assumes that Mad2 dissociates from its complex with Mad1. Although reasonable, the possibility that the tetrameric Mad1/Mad2 complex breaks to release Mad2 has not received experimental support, and conditions that may provoke a fast dissociation of this very stable complex are unknown (Sironi et al. 2002; De Antoni et al. 2005). Finally, the kinetic implications of the safety belt for Mad1 and Cdc20 binding by Mad2 also suggest that the Mad2 exchange model is unlikely to be correct. An in-depth analysis of the kinetic implications of the safety belt is beyond the scope of this article, but the reader is referred to previous reports in which such implications were carefully discussed (Musacchio & Hardwick 2002; Sironi et al. 2002).
Recently, we have gathered substantial evidence in favour of a new model, which we have named the Mad2 template model (De Antoni et al. 2005), which satisfies the available genetic, biochemical, and structural evidence relating Mad1, Mad2 and Cdc20 more smoothly with respect to the Mad2 exchange model. The backbone of the Mad2 template model is that the Mad1/Mad2 complex is stable and does not dissociate during checkpoint activation (figure 1b). In other words, Mad2 bound to Mad1 is not a source of Mad2 for Cdc20 binding, as predicted by the Mad2 exchange model. Rather, Mad1 is required to lock Mad2 into a stable C-Mad2 conformation that resides at mitotic kinetochores during checkpoint activation without any significant dissociation of the Mad1/Mad2 complex. The function of C-Mad2 bound to Mad1 is the recruitment of O-Mad2 from its cytosolic pool. The latter, not the former, is then transferred onto Cdc20 (figure 1b). An interesting corollary of the Mad2 template model is that it represents the C-Mad2/Cdc20 complex as a cytosolic copy of the C-Mad2/Mad1 ‘template’ existing at the kinetochore, suggesting the possibility that once released from the kinetochore, the Mad2/Cdc20 complex acts as an amplifier for the spindle checkpoint signal into the cytosol (De Antoni et al. 2005).
4. Mad2 oligomers. Old and new interpretations
The Mad2 template model proposes that the recruitment of O-Mad2 to kinetochores is based on the interaction of O-Mad2 with Mad1-bound C-Mad2 (figure 1b). We reached this conclusion after showing that O-Mad2 mutants that are unable to bind to Mad1 directly, but are capable of binding to C-Mad2, are normally recruited to kinetochores in mitosis (De Antoni et al. 2005). Conversely, Mad2 mutants that are capable of binding to Mad1 but fail to sustain the interaction with C-Mad2 are not recruited to the kinetochore (De Antoni et al. 2005). This behaviour correlates very well with the ability of the same mutants to interact in vitro with a recombinant form of the Mad1/Mad2 complex, in which Mad2 holds the C-Mad2 conformation (De Antoni et al. 2005). Furthermore, we showed that the interaction of O-Mad2 with C-Mad2 is essential to maintain the SAC in HeLa cells. Mutant alleles of Mad2 that are capable of binding Mad1 and Cdc20, but are impaired in the O-Mad2/C-Mad2 interaction, are also unable to sustain the spindle checkpoint after RNAi-mediated knock down of the endogenous Mad2 protein (De Antoni et al. 2005).
In the course of our studies, we had an opportunity to re-evaluate initial observations by Kirschner and collaborators regarding the oligomerization of Mad2 (Fang et al. 1998). These authors showed that Mad2 purified from bacteria forms oligomers and suggested that oligomerization is essential for Mad2 function (Fang et al. 1998). Although the oligomerization of Mad2 has been confirmed in subsequent reports, its functional and structural significance has remained elusive (Luo et al. 2000, 2002, 2004; Sironi et al. 2001, 2002; Tunquist et al. 2003; De Antoni et al. 2005). From a structural perspective, the difficulty in assessing the structure of the Mad2 oligomers has been ascribed to the fact that pure preparations of bacterially expressed Mad2 tend to be polydisperse and are not amenable to direct structural analysis (Luo et al. 2000, 2002; Sironi et al. 2001, 2002). Because O-Mad2 and C-Mad2 bind to each other to promote the recruitment of O-Mad2 to kinetochore-bound C-Mad2, we considered the possibility that the oligomerization of bacterially expressed Mad2 may also be explained by an interaction between O-Mad2 and C-Mad2.
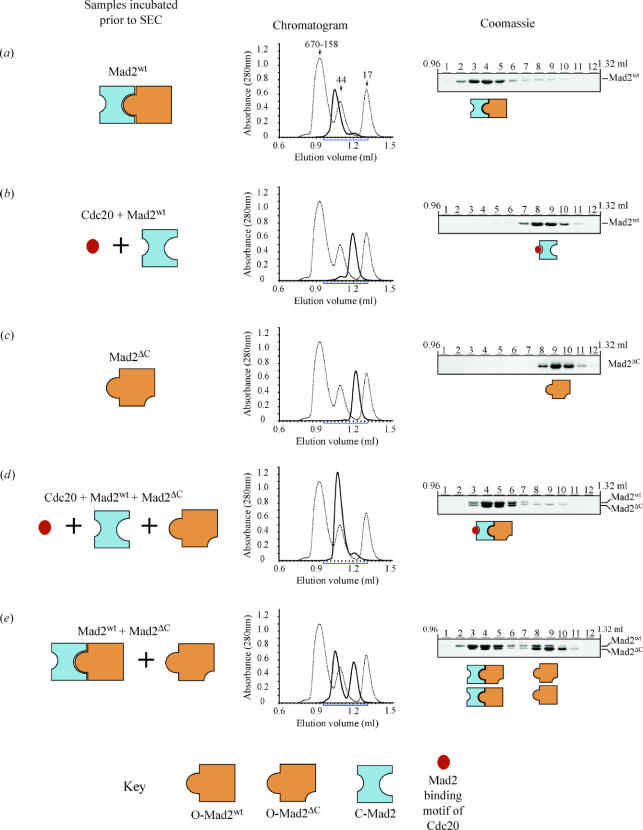
To test this hypothesis, we made use of purified recombinant versions of Mad2 and a defined set of Mad2 mutants (see below). These proteins and their complexes were analysed on a Superdex-75 SEC column, and the content of defined fractions was analysed by SDS-PAGE (figure 2). Using this approach, we first showed that bacterially expressed Mad2wt was oligomeric by SEC, as reported previously, with an average molecular weight at least double that expected for a 24 kDa Mad2 monomer (figure 2a). Next, we inquired about the oligomerization state of a pure preparation of C-Mad2. To obtain a pure preparation of C-Mad2 from Mad2wt, we mixed Mad2wt with an excess of a synthetic peptide corresponding to the Mad2-binding region of Cdc20 to generate a pure C-Mad2/Cdc20 complex (figure 2b). In separate ITC experiments, we have shown that the Cdc20 peptide used in this experiment binds Mad2 with high-affinity (Sironi et al. 2001, 2002; De Antoni et al. 2005). After 1 h, we separated the products of the incubation by SEC. The elution volume of Mad2wt after the incubation with the Cdc20 peptide was as expected for a 1 : 1 Mad2–peptide complex (figure 2b). This experiment shows that the oligomerization of Mad2wt can be reverted by the addition of a peptide that binds Mad2 and turns it into C-Mad2. Thus, a pure population of C-Mad2 is monomeric, in agreement with previous reports (Luo et al. 2000, 2002, 2004; Sironi et al. 2001, 2002; De Antoni et al. 2005).
Figure 2.
Opposite. The oligomerization state of Mad2 can be explained as an interaction of the two conformers of Mad2, O-Mad2 and C-Mad2. (a) The elution profile of bacterially expressed Mad2wt was typical of a Mad2 oligomer, in agreement with previous reports (Fang et al. 1998; Luo et al. 2000, 2002; Sironi et al. 2001). We interpret this result as the formation of a complex between O-Mad2 and empty C-Mad2 forming in bacterial preparations of Mad2. (b) When challenged with a Cdc20 peptide, Mad2 is turned into a monomeric Cdc20-bound C-Mad2 species, which agrees with previous reports (Fang et al. 1998; Luo et al. 2000, 2002; Sironi et al. 2001). This experiment shows that the accumulation of C-Mad2 reverts the oligomerization of Mad2. To generate Mad2/Cdc20, Mad2wt was incubated for 1 h with a 10 fold excess of a synthetic peptide corresponding to residues between 111 and 138 of Cdc20 (Cdc20111–138). This segment is a stronger Mad2 ligand than full-length Cdc20, probably because the Mad2-binding region is partially buried in full-length Cdc20 (Tang et al. 2001; Zhang & Lees 2001). The resulting Mad2/Cdc20 complex runs as a Mad2 monomer because the contribution of the Cdc20 peptide to the overall shape and molecular weight of Mad2 is near to negligible. (c) Mad2ΔC is locked in the O-Mad2 conformation and is also monomeric, which agrees with previous observations (Fang et al. 1998; Luo et al. 2000, 2002; Sironi et al. 2001). (d) When O-Mad2ΔC and Cdc20-bound C-Mad2wt (generated as described in panel b) are mixed in roughly equimolar amounts, a stoichiometric complex form that runs very similarly to the Mad2wt oligomers. (e) The existence of C-Mad2 in Mad2wt is supported by partial binding of Mad2ΔC to Mad2wt also in the absence of Cdc20 peptide. The shift in elution volume of Mad2ΔC caused by its incorporation into a complex with C-Mad2wt can be appreciated by a comparison with panel c. Some monomeric Mad2wt, presumably in the O-Mad2 conformation, is competed by Mad2ΔC off the wild-type oligomer and it appears as a monomeric species. The Mad2 proteins used in panels a–e were purified and analysed by SEC on a Superdex-75 PC 3.2/30 column essentially as described (Sironi et al. 2001, 2002; De Antoni et al. 2005). For every chromatogram, pure proteins were loaded onto the column and the contents of 12 consecutive 30 μl fractions eluting between 0.96 and 1.32 ml were analysed by SDS-PAGE and stained with Coomassie.
Next, we wished to assess the oligomerization state of a pure preparation of O-Mad2. For this, we took advantage of the Mad2ΔC deletion mutant, which lacks 10 residues from the C-terminus of Mad2. Mad2ΔC is locked in the O-Mad2 conformation. The structural bases for this property of Mad2ΔC have been thoroughly discussed before (see Luo et al. 2002; Sironi et al. 2002), and will not be repeated here. It suffices to remind the reader that being locked in the open conformation, the Mad2ΔC protein is utterly unable to bind Mad1 or Cdc20 (Luo et al. 2000, 2002, 2004; Sironi et al. 2001, 2002; De Antoni et al. 2005). When analysed by SEC, a pure preparation of Mad2ΔC was homogeneously monomeric, as shown in figure 2c. This result is consistent with the possibility that O-Mad2, like C-Mad2, is unable to self-associate and that the dimerization of Mad2 requires opposite conformers. However, an alternative hypothesis is that the Mad2 oligomers are formed from O-Mad2 and that the 10-residue C-terminal deletion in O-Mad2ΔC disrupts the dimerization interface. To test this, we asked if O-Mad2ΔC was capable of forming dimers if challenged with C-Mad2. For this, we mixed O-Mad2 and C-Mad2 (in the form of Mad2ΔC and Mad2wt/Cdc20, respectively) in roughly equimolar amounts and analysed the products of this incubation by SEC. As shown in figure 2d, O-Mad2ΔC and Cdc20-bound C-Mad2wt entered a stoichiometric dimeric complex, indicating that the ability of Mad2ΔC to dimerize is largely untouched by the ΔC deletion (at least at the level of resolution allowed by this analysis). We conclude that Mad2 oligomerization can be reproduced as an interaction of O-Mad2 with C-Mad2, while pure forms of O-Mad2 or C-Mad2 are unable to form dimers.
The oligomers shown in figure 2d contain a form of C-Mad2 obtained by incubation of Mad2wt with a synthetic peptide containing the Mad2 binding site of Cdc20. Bacterially expressed Mad2wt, however, forms oligomers in the absence of Cdc20 or Mad1 peptides. If these oligomers of apo-Mad2 are based on the interaction of O-Mad2 with C-Mad2, then the implication is that C-Mad2 can also appear spontaneously in the absence of Mad2 ligands as an empty C-Mad2 form (where ‘empty’ indicates that the Mad2-binding site is not occupied by Mad1 or Cdc20). This is not unreasonable given that the C-terminal tail of Mad2 is designed to undergo a conformational change from the O-Mad2 to the C-Mad2 position and vice versa. Testing this possibility directly, however, is rather difficult, as we do not have a direct sensor to assess the state of the Mad2 C-terminal tail in solution. We reasoned that if C-Mad2wt existed in pure preparations of Mad2, we ought to be able to use O-Mad2ΔC as a probe for detecting C-Mad2, to which Mad2ΔC binds tightly (figure 2d). Binding of Mad2ΔC to C-Mad2wt may be revealed as a shift in the elution profile of any Mad2ΔC that were incorporated into a high molecular weight complex with C-Mad2wt. To test this, we mixed equimolar amounts of Mad2ΔC and Mad2wt, and analysed the elution of Mad2ΔC from the SEC column (figure 2e). Confirming our hypothesis, about one-quarter of total Mad2ΔC shifted from the monomeric state of O-Mad2ΔC into a high molecular weight complex with Mad2wt. Concomitantly, part of Mad2wt was released in a monomeric form, which we suspect to be O-Mad2wt previously bound to C-Mad2 and displaced after addition of O-Mad2ΔC (figure 2e).
This result strongly suggests that C-Mad2 exists in preparations of Mad2wt, even in the absence of Mad2 ligands. Using a different chromatographic approach, Yu and collaborators reached what is essentially the same conclusion (Luo et al. 2004). Because pure preparations of Mad2wt are entirely oligomeric, it seems sensible to conclude that they contain an equal concentration of O-Mad2 and C-Mad2 bound into dimers. Previous attempts to define the stoichiometry of the Mad2wt oligomers suffered from the polydispersity of these preparations, probably caused by the intrinsic instability of empty C-Mad2 occurring in the absence of Mad1 or Cdc20. In this respect, it is worth mentioning that the Mad2-binding motifs of Mad1 and Cdc20 bind Mad2 with high affinity, significantly stabilizing C-Mad2 relative to its empty variant. From this, we infer that also the interaction of C-Mad2 with O-Mad2 will be significantly stabilized in the presence of a Mad2 ligand (Mad1 or Cdc20), creating a stable form of C-Mad2. Because the complex of Mad2ΔC with C-Mad2/Cdc20 is perfectly monodisperse, we suspect that the lack of a ligand stabilizing C-Mad2 causes the polydispersity of the pure preparations of oligomeric Mad2wt, although we cannot provide a direct mechanistic explanation for this observation.
In summary, we propose that the oligomerization of Mad2wt in solution and its oligomerization on Mad1 are two aspects of the same interaction between opposite conformers of Mad2. In our view, while the latter is relevant to the biology of Mad2, the former is only an interesting epiphenomenon caused by the dynamic nature of the Mad2 C-terminal tail. In fact, cytosolic apo-Mad2 has not been found to form oligomers in HeLa cells or other systems (reviewed in Musacchio & Hardwick 2002). We decided to re-evaluate these results (figure 3). Mad2ΔC and Mad2wt were expressed and purified from bacteria and loaded onto a Superose-12 PC 3.2/30 column equilibrated as described in the legend to figure 3. SDS-PAGE and Coomassie staining were used to analyse the content of equivalent fractions (1–12) in each run. The elution profiles of Mad2ΔC and Mad2wt were assumed to represent the elution of monomeric and dimeric forms of Mad2, respectively (figure 3a,b).
Figure 3.

Oligomeric state of different forms of Mad2 after purification and in cell extracts. The elution of bacterially expressed and purified monomeric and oligomeric Mad2 proteins was used to discern the oligomerization state of Mad2 in HeLa cell extracts. Protein samples were loaded on a Superose-12 PC 3.2/30 column equilibrated in 50 mM Hepes pH 7.5, 150 mM NaCl, 1 mM DTT, 5 mM EDTA. Elution was carried out at 40 μl min−1. For each run, identical 35 μl fractions were collected and then subjected to SDS-PAGE, followed by Coomassie staining or western blotting. Arrows indicated the position of the peaks of three molecular weight markers. (a) SDS-PAGE analysis of monomeric O-Mad2ΔC. (b) SDS-PAGE analysis of monomeric O-Mad2wt. (c) HeLa cells were harvested by scraper detaching and lysed in 50 mM Hepes pH 7.5, 150 mM NaCl, 1 mM DTT, 5 mM EDTA, 50 mM NaF, 20 mM Na4-pyrophosphate, 0.5 μM okadaic acid, 100 ng ml−1 leupeptine, 100 ng ml−1 aprotinin, 1 mM PMSF, protease inhibitors (Roche), 0.1% NP40 for 20 min on ice. Cell lysates were clarified by centrifugation for 15 min at 13 000 r.p.m. at 4 °C in an Eppendorf microcentrifuge. Cell lysates (70 μg) were subjected to SEC analysis.
To assess the state of oligomerization of Mad2 in HeLa cells, a HeLa cell lysate was loaded onto the same column and the presence of Mad2 in the eluate was analysed by western blotting with an anti-Mad2 antibody (figure 3c). Although we detected some Mad2 in high-molecular weight fractions, the majority of Mad2 eluted precisely as expected for a monomeric protein (figure 3c). That the conformation of this monomeric cytosolic form of Mad2 is O-Mad2 is based on a recent report of Luo and collaborators and from our own work showing that this is the conformation of Mad2 which is recruited to the kinetochore from the cytosol (Luo et al. 2004; De Antoni et al. 2005). Because bacterially expressed Mad2wt has a tendency to oligomerize, it is possible that cytosolic Mad2 in HeLa cells is kept in an open conformation by cellular chaperones. However, this is a possibility that will require further investigation. An alternative possibility, which will also need to be explored, is that p31comet (previously known as CMT2), a protein that binds exclusively to C-Mad2, binds to and ‘buffers’ any empty C-Mad2 forming spontaneously in living cells (Habu et al. 2002; Hagan et al. Submitted; Xia et al. 2004). Finally, we cannot formally exclude the unlikely possibility that the appearance of what we have defined above as empty C-Mad2 results from the binding of small molecules or peptides picked up from the bacterial cytoplasm into the Mad2 ligand-binding site.
5. R133 is required for the interaction of O-Mad2 with C-Mad2
We have previously described a Mad2 point mutant, Mad2R133A, as a monomeric species of Mad2 that retains the C-terminal dependent tight binding to Mad1 and Cdc20 (Sironi et al. 2001, 2002). Thus, Mad2R133A seems to have retained a functional safety belt and is, therefore, expected to populate an equilibrium between C and O conformers like Mad2wt. However, as reported previously (Sironi et al. 2001, 2002; Tunquist et al. 2003; De Antoni et al. 2005; Luo et al. 2004), pure preparations of Mad2R133A are monomeric by SEC, and they remain monomeric when turned into C-Mad2 with a Cdc20 peptide (figure 4a,b). The binding of the Cdc20 peptide cannot be gauged by loss of oligomerization as in the case of Mad2wt, however, because Mad2R133A is already monomeric. For this, we had to use isothermal titration calorimetry, which shows that the Cdc20 peptide binds Mad2R133A as well as Mad2wt (De Antoni et al. 2005). Because Mad2ΔC is locked in the open conformation (see above), we could build an O-Mad2 version of Mad2R133A in the form of the Mad2ΔC-R133A double mutant. As expected, Mad2ΔC-R133A was also monomeric based on the monomeric nature of the R133A and ΔC mutants (figure 4c).
Figure 4.
Opposite. Behaviour of the Mad2R133A mutant. (a) Mad2R133A is monomeric because the R133A mutation (yellow triangle) impairs the interaction between O- and C-Mad2. (b) Mad2R133A/Cdc20 runs as a 1:1 Mad2/peptide complex. (c) Mad2ΔC-R133A is monomeric and locked as O-Mad2, like Mad2ΔC, owing to the ΔC deletion. (d) O-Mad2ΔC-R133A does not bind C-Mad2R133A/Cdc20 and the individual profiles of the monomers largely overlap, although a small shift is observed, possibly revealing small residual binding. (e) When the R133A mutation is present only on the C-Mad2 moiety, an interaction with O-Mad2 is observed. Note that the elution of this complex is shifted to the lower molecular weights relative to Mad2wt (see figure 2a,d). (f) As in f, the R133A mutation is present on only one face of the interaction, which, in this case, is the O-Mad2 moiety. As in panel e, an interaction with C-Mad2 is observed. The Mad2 proteins used in panels a–f were purified and analysed by SEC on a Superdex-75 PC 3.2/30 column essentially as described (Sironi et al. 2001, 2002; De Antoni et al. 2005). For every chromatogram, pure proteins were loaded onto the column and the contents of 12 consecutive 30 μl fractions eluting between 0.96 and 1.32 ml were analysed by SDS-PAGE and stained with Coomassie.
Most probably, the monomeric nature of Mad2R133A owes to the fact that the mutation, which hits a fully conserved exposed residue of Mad2 (Aravind & Koonin 1998), is located on a patch required for dimerization of O-Mad2 with C-Mad2. To test this, we mixed Cdc20-bound C-Mad2R133A with O-Mad2ΔC-R133A and analysed the product of their incubation by SEC (figure 4d). Both proteins eluted in a peak situated between the 44 and the 17 kDa markers, indicating a lack of interaction if the R133A mutation is present on both O-Mad2 and C-Mad2. This result agrees with the hypothesis that the R133A mutation impairs the binding of O-Mad2 to C-Mad2.
Our studies, as depicted in figure 2, introduce the concept that O-Mad2 and C-Mad2 bind each other, but pure forms of each conformer are unable to form oligomers. Because O-Mad2 and C-Mad2 are chemically identical (i.e. they are not modified and have equal masses; Fang et al. 1998; Luo et al. 2004), it follows that the binding surface that allows the interaction of O-Mad2 and C-Mad2 must be at least partially asymmetric. The reason for this is that a perfectly symmetric interface would be expected to cause the oligomerization of the pure O-Mad2 or C-Mad2 forms as well. Thus, at least in principle, it is possible that the effects of the R133A mutation on the disruption of Mad2 dimerization are limited to one of the two conformers on Mad2, but not to both.
To test this, we analysed the ability of open and closed R133A mutants to bind wild-type dimerization surfaces of the opposite conformation. In particular, to test the effect of the R133A mutation on the ability of C-Mad2 to bind O-Mad2, we incubated stoichiometric amounts of Cdc20-bound C-Mad2R133A (the mutant) and O-Mad2ΔC (viewed as a wild-type surface with respect to oligomerization based on our results in figure 2). We then analysed the products by SEC (figure 4e). Conversely, to determine the effects of the R133A mutation on the ability of O-Mad2 to bind C-Mad2, we used SEC to analyse the binding of O-Mad2R133A-ΔC (the mutant) to Cdc20-bound C-Mad2wt (wild-type C-Mad2, figure 4f). The SEC elution profiles from these incubations were then compared with those of the incubation of Cdc20-bound C-Mad2R133A and O-Mad2ΔC-R133A (see above), which represented a lack of any interaction.
The elution profile of the incubation of Cdc20-bound C-Mad2R133A with O-Mad2ΔC (figure 4e) revealed significant residual binding because the proteins co-eluted close to the 44 kDa marker, which indicated oligomerization. The elution volume, however, was distinct from that of the incubation of Cdc20-bound C-Mad2wt and O-Mad2ΔC (figure 2d). The elution volume shifted towards the lower molecular weights as if the interaction between C-Mad2R133A and O-Mad2ΔC were weaker. The elution profile of the products of the incubation O-Mad2R133A-ΔC and Cdc20-bound C-Mad2wt revealed what we also interpreted as an intermediate level of binding (figure 4f). Possibly, the partial shift in elution volume relative to the incubation of Cdc20-bound C-Mad2wt and O-Mad2ΔC can be interpreted as a faster dissociation of the C-Mad2/O-Mad2 complex caused by one R133A mutation at the interface (which is indeed the case; data not shown). Why does the decreased affinity of Mad2R133A oligomers not result in the appearance of discrete oligomer and monomer peaks, rather than in the continuous shift in elution volumes actually observed? We suspect that this effect owes to the continuous dissociation and rebinding of O- and C-Mad2 in the column, caused by a faster off rate, which results in an elution profile representing a weighted average of the molecular weight of the dimer and the monomer.
Overall, these results suggest that R133 is part of both surfaces involved in the C-Mad2/O-Mad2 interaction. Complete disruption of binding occurs when both surfaces are affected, while intermediate binding is observed when the mutation is present on only one binding partner, regardless of whether this is O-Mad2 or C-Mad2. The result of this characterization is interesting because it suggests that the interaction surface between O-Mad2 and C-Mad2 may be partially symmetric. This suggests that both conformers require the R133 residue for high-affinity binding. A structural analysis of the binding interface is currently underway in our laboratory and will help shedding light on this important issue.
6. Conclusions
This characterization of Mad2R133A triggered our search for new mutations with more penetrant effects on Mad2 dimerization (De Antoni et al. 2005). Our search identified the double mutant Mad2R133E/Q134A as being almost completely impaired in the binding of a wild-type version of the opposite Mad2 conformer, both in the O-Mad2 and in the C-Mad2 configuration (De Antoni et al. 2005). Notably, both residues are exposed at the surface of Mad2 and are fully conserved in Mad2 orthologues across species (Aravind & Koonin 1998; Luo et al. 2000; Sironi et al. 2002). Using Mad2R133E/Q134A, we were able to show that monomeric forms of Mad2 are unable to maintain the spindle checkpoint.
We have suggested previously that the oligomerization of Mad2 may not be required to sustain the checkpoint (Sironi et al. 2001). This conclusion was based on the observation that the over-expression of Mad2R133A caused an ectopic activation of the spindle checkpoint similar to that obtained upon overexpression in HeLa cells of wild-type Mad2 (Sironi et al. 2001). Because Mad2R133A is monomeric, this result suggested that the oligomerization of Mad2 is not required to sustain the checkpoint. In retrospect, it is easier to see that the interpretation of the results of Mad2R133A overexpression (which we have recently confirmed; De Antoni et al. 2005) is very complex, and a number of factors can contribute to explain the observed phenotype. To mention just one, we show here that the Mad2R133A mutant at least partially retains the ability to interact with Mad2wt. Furthermore, there remain doubts as to the requirement for Mad1 in the metaphase arrest caused by Mad2 overexpression, and it is possible that high amounts of Mad2 bind Cdc20 in a Mad1-independent fashion. Although we have initially reported efficient metaphase arrest only upon co-expression of Mad1 and Mad2 (Sironi et al. 2001), we have now also identified conditions in which Mad2 overexpression is sufficient for a robust mitotic arrest (De Antoni et al. 2005), suggesting that the requirement for Mad1 for an efficient mitotic arrest is bypassed if Mad2 is overexpressed. Finally, it is also possible that Mad2R133A fails to be inhibited by a checkpoint inhibitor like p31comet (see above).
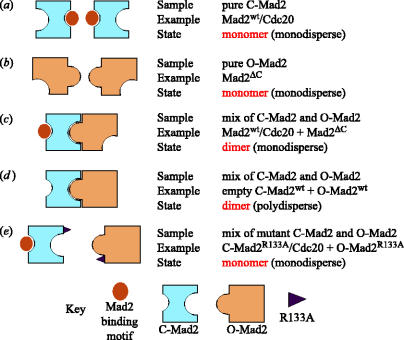
Thus, our initial caution about the relevance of Mad2 oligomerization for the spindle checkpoint can now be lifted. Our recent investigations show that the dimerization of Mad2 can be interpreted on the basis of an interaction between the O-Mad2 and C-Mad2 conformers of Mad2, and that this interaction is essential for the spindle checkpoint (De Antoni et al. 2005). From a biochemical perspective, we suggest that our hypothesis is sufficient to explain most (and possibly all) observations related to the oligomerization properties of Mad2 previously described, as summarized in figure 5.
Figure 5.
Summary of Mad2 oligomerization mechanism. Although neither conformer interacts with itself, the idea that the oligomerization of Mad2 is caused by an interaction of O-Mad2 with C-Mad2 explains all observations regarding the oligomerization state of Mad2. In particular, pure preparations of C-Mad2 (panel a) are monomeric. We show this in figure 2b, but this effect of Cdc20 and Mad1 binding on Mad2 has also been described before (Luo et al. 2000, 2002, 2004; Sironi et al. 2001, 2002; De Antoni et al. 2005). Similarly, pure O-Mad2ΔC is monomeric (panel b), a result that has also already been discussed (Fang et al. 1998; Luo et al. 2000, 2002, 2004; Sironi et al. 2001, 2002; De Antoni et al. 2005). In panels c and d, we summarize our finding that the oligomerization of Mad2 can be explained as an interaction of O-Mad2 with C-Mad2. The difference between panels c and d is the presence of a Mad2 ligand, which will cure the polydispersity of oligomeric preparations of empty Mad2 (Sironi et al. 2001; De Antoni et al. 2005; Luo et al. 2004). Recently, we have shown that the interaction of O-Mad2 with C-Mad2, which we have ‘simulated’ here with purified components, is essential for Mad2 recruitment to the kinetochore and maintenance of the spindle checkpoint (De Antoni et al. 2005). Finally, we postulate that the reason why the R133A mutant is monomeric is that this residue lies at the interface between the O-Mad2 and C-Mad2 monomers (panel e).
Acknowledgments
Work on the SAC in the Musacchio laboratory is supported by the Italian Association for Cancer Research, the Human Frontier Science Program, and the FP6 of the European Union. A.D.A. is an EMBO postdoctoral fellow. A.M. is an EMBO Young Investigator and a fellow of the Italian Foundation for Cancer Research. We thank all members of the Musacchio laboratory for helpful discussions.
Glossary
- APC/C
anaphase promoting complex/cyclosome
- BUB
budding uninhibited by benomyl
- FRAP
fluorescence recovery after photobleaching
- ITC
isothermal titration calorimetry
- MAD
mitotic-arrest deficient
- MCC
mitotic checkpoint complex
- SAC
spindle assembly checkpoint
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Chromosome segregation’.
Discussion
M. Herbert (Newcastle Fertility Centre, Centre for Life, University of Newcastle, Newcastle upon Tyne NE1 4EP, UK). Is it correct to say that inactivation of the spindle checkpoint requires release of Cdc20 by the closed form of Mad2? If yes, do we have any ideas on the mechanism by which this might occur?
A. Musacchio. The answer to this question is necessarily incomplete because we lack vital information on the role that other proteins like BubR1 and Bub3 play within the MCC to affect its stability. At any rate, the inactivation of the SAC does indeed require the release of Cdc20 from the closed form of Mad2. We suspect that the timing out of the checkpoint signal does not need to be a regulated event, and that most of the control may be played on the rates at which Mad2/Cdc20 is formed at the kinetochore. If the source of Mad2/Cdc20 is arrested, as it is likely to occur once the attachment process is complete, the existing Mad2/Cdc20 might dissociate at its own rate until a threshold of free Cdc20 is reached that is sufficient to trigger anaphase. CMT2 has been recently identified as a protein that might play an essential role to arrest the production of Mad2/Cdc20. However, we are in the realm of pure speculation at this point.
J.-K. Heriche (The Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge CB10 1RQ, UK). Is there any evidence that other proteins other than Mad1 and Mad2 are required for the Mad2 conformational change?
A. Musacchio. At this time, we can only say that in vitro the conformational change may occur in the absence of other proteins. Whether additional levels of regulation exist in living cells is currently unknown.
T. Mitchison (Department of Systems Biology, Harvard Medical School, Boston, MA 02115, USA). What is the nature of the catalysis of the open–closed transformation of Mad2?
A. Musaccio. This is a very important question for which we have no answer at the moment. Indeed, we cannot say that the interaction of O-Mad2 with C-Mad2 is catalytic with respect to the transformation of O-Mad2 into C-Mad2, as we have no evidence for this. All we can say is that, genetically, Mad1 is absolutely required to sustain the checkpoint and the interaction of Mad2 with Cdc20, and that its function seems to be linked to the localization of a stable form of C-Mad2 at the kinetochore. Whether the Mad1/C-Mad2 complex has catalytic properties on the transformation of O-Mad2 into C-Mad2 bound to Cdc20 is unclear at this stage. The problem we are facing in answering this question in vitro with purified material is that we do not observe a dependency on Mad1/C-Mad2 for the formation of Mad2/Cdc20 as it exists for living cells. For this reason, measuring catalysis by the Mad1/C-Mad2 complex on the transformation of O-Mad2 into Cdc20-bound C-Mad2 in vitro is particularly difficult, although probably not impossible. At this time, we cannot exclude that the ‘catalytic’ nature of Mad1 is limited to its receptor activity on O-Mad2, which together with other kinetochore-based function may be instrumental in ensuring a kinetochore dependency of the checkpoint signal. More studies will be required to answer this very interesting question.
References
- Aravind L, Koonin E.V. The HORMA domain: a common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem. Sci. 1998;23:284–286. doi: 10.1016/s0968-0004(98)01257-2. [DOI] [PubMed] [Google Scholar]
- Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23:2016–2027. doi: 10.1038/sj.onc.1207374. [DOI] [PubMed] [Google Scholar]
- Chen R.-H. BubR1 is essential for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 2002;158:487–96. doi: 10.1083/jcb.200204048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.H, Shevchenko A, Mann M, Murray A.W. Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J. Cell Biol. 1998;143:283–295. doi: 10.1083/jcb.143.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R.H, Brady D.M, Smith D, Murray A.W, Hardwick K.G. The spindle checkpoint of budding yeast depends on a tight complex between the Mad1 and Mad2 proteins. Mol. Biol. Cell. 1999;10:2607–2618. doi: 10.1091/mbc.10.8.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Chen R.-H. Spindle checkpoint requires Mad1-bound and Mad1-free Mad2. Mol. Biol. Cell. 2002;13:1501–1511. doi: 10.1091/mbc.02-01-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D.W, Mao Y, Sullivan K.F. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112:407–421. doi: 10.1016/s0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- De Antoni A. et al 2005 The Mad1/Mad2 complex as a template for Mad2 activation in the spindle assembly checkpoint. Curr. Biol.15, 214–225. [DOI] [PubMed]
- Fang G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell. 2002;13:755–766. doi: 10.1091/mbc.01-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner M.W. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 1998;12:1871–1883. doi: 10.1101/gad.12.12.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini R, Beretta A, Sironi L, Musacchio A, Lucchini G, Piatti S. Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J. 2001;20:6648–6659. doi: 10.1093/emboj/20.23.6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habu T, Kim S.H, Weinstein J, Matsumoto T. Identification of a MAD2-binding protein, CMT2, and its role in mitosis. EMBO J. 2002;21:6419–6428. doi: 10.1093/emboj/cdf659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan, R., Dobles M., Meraldi P., Mapelli M., Musacchio A. & Sorger P.K. Submitted. Distinctive Mad2-binding properties confer on CMT2 a roles as an inhibitor of the spindle checkpoint.
- Howell B.J, McEwen B.F, Canman J.C, Hoffman D.B, Farrar E.M, Reider C.L, Salmon E.D. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 2001;155:1159–1172. doi: 10.1083/jcb.200105093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.J, Moree B, Farrar E.M, Stewart S, Fang G, Salmon E.D. Spindle checkpoint protein dynamics at kinetochores in living cells. Curr. Biol. 2004;14:953–964. doi: 10.1016/j.cub.2004.05.053. [DOI] [PubMed] [Google Scholar]
- Hoyt M.A, Totis L, Roberts B.T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Hwang L.H, Lau L.F, Smith D.L, Mistrot C.A, Hardwick K.G, Hwang E.S, Amon A, Murray A.W. Budding yeast Cdc20: a target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- Kallio M, Weinstein J, Daum J.R, Burke D.J, Gorbsky G.J. Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J. Cell Biol. 1998;141:1393–1406. doi: 10.1083/jcb.141.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallio M.J, Beardmore V.A, Weinstein J, Gorbsky G.J. Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells. J. Cell Biol. 2002;158:841–847. doi: 10.1083/jcb.200201135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H, Lin D.P, Matsumoto S, Kitazono A, Matsumoto T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- Li R, Murray A. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Luo X, Fang G, Coldiron M, Lin Y, Yu H, Kirschner M.W, Wagner G. Structure of the mad2 spindle assembly checkpoint protein and its interaction with cdc20. Nat. Struct. Biol. 2000;7:224–229. doi: 10.1038/73338. [DOI] [PubMed] [Google Scholar]
- Luo X, Tang Z, Rizo J, Yu H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell. 2002;9:59–71. doi: 10.1016/s1097-2765(01)00435-x. [DOI] [PubMed] [Google Scholar]
- Luo X, Tang Z, Xia G, Wassmann K, Matsumoto T, Rizo J, Yu H. The Mad2 spindle checkpoint protein has two distinct natively folded states. Nat. Struct. Mol. Biol. 2004;11:338–345. doi: 10.1038/nsmb748. [DOI] [PubMed] [Google Scholar]
- Martin-Lluesma S, Stucke V.M, Nigg E.A. Role of hec1 in spindle checkpoint signaling and kinetochore recruitment of mad1/mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- McCleland M.L, Gardner R.D, Kallio M.J, Daum J.R, Gorbsky G.J, Burke D.J, Stukenberg P.T. The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 2003;17:101–114. doi: 10.1101/gad.1040903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Draviam V.M, Sorger P.K. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Millband D.N, Hardwick K.G. Fission yeast Mad3p is required for Mad2p to inhibit the anaphase-promoting complex and localizes to kinetochores in a Bub1p-, Bub3p-, and Mph1p-dependent manner. Mol. Cell. Biol. 2002;22:2728–2742. doi: 10.1128/MCB.22.8.2728-2742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Hardwick K.G. The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell Biol. 2002;3:731–741. doi: 10.1038/nrm929. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Segregating sister genomes: the molecular biology of chromosome separation. Science. 2002;297:559–565. doi: 10.1126/science.1074757. [DOI] [PubMed] [Google Scholar]
- Peters J.M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell. 2002;9:931–943. doi: 10.1016/s1097-2765(02)00540-3. [DOI] [PubMed] [Google Scholar]
- Petronczki M, Siomos M.F, Nasmyth K. Un menage a quatre: the molecular biology of chromosome segregation in meiosis. Cell. 2003;112:423–440. doi: 10.1016/s0092-8674(03)00083-7. [DOI] [PubMed] [Google Scholar]
- Rieder C.L, Cole R.W, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J.V, Botvinick E, Bonday Z, Furnari F, Berns M, Cleveland D.W. Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr. Biol. 2004;14:942–952. doi: 10.1016/j.cub.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Shannon K.B, Canman J.C, Salmon E.D. Mad2 and BubR1 function in a single checkpoint pathway that responds to a loss of tension. Mol. Biol. Cell. 2002;13:3706–3719. doi: 10.1091/mbc.E02-03-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, Melixetian M, Faretta M, Prosperini E, Helin K, Musacchio A. Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J. 2001;20:6371–6382. doi: 10.1093/emboj/20.22.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi L, Mapelli M, Knapp S, Antoni A.D, Jeang K.-T, Musacchio A. Crystal structure of the tetrameric Mad1-Mad2 core complex: implications of a ‘safety belt’ binding mechanism for the spindle checkpoint. EMBO J. 2002;21:2496–2506. doi: 10.1093/emboj/21.10.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudakin V, Chan G.K, Yen T.J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T.U, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark M.J, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Tang Z, Bharadwaj R, Li B, Yu H. Mad2-independent inhibition of APC-Cdc20 by the mitotic checkpoint protein Bub1R. Dev. Cell. 2001;1:227–237. doi: 10.1016/s1534-5807(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Tunquist B.J, Eyers P.A, Chen L.G, Lewellyn A.L, Maller J.L. Spindle checkpoint proteins Mad1 and Mad2 are required for cytostatic factor-mediated metaphase arrest. J. Cell Biol. 2003;163:1231–1242. doi: 10.1083/jcb.200306153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassmann K, Benezra R. Mad2 transiently associates with an APC/p55Cdc complex during mitosis. Proc. Natl Acad. Sci. USA. 1998;95:11 193–11 198. doi: 10.1073/pnas.95.19.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia G, Luo X, Habu T, Rizo J, Matsumoto T, Yu H. Conformation-specific binding of p31(comet) antagonizes the function of Mad2 in the spindle checkpoint. EMBO J. 2004;23:3133–3143. doi: 10.1038/sj.emboj.7600322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lees E. Identification of an overlapping binding domain on Cdc20 for Mad2 and anaphase-promoting complex: model for spindle checkpoint regulation. Mol. Cell. Biol. 2001;21:5190–5199. doi: 10.1128/MCB.21.15.5190-5199.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]