Abstract
For proper chromosome segregation, sister kinetochores must attach to microtubules extending from opposite spindle poles prior to anaphase onset. This state is called sister kinetochore bi-orientation or chromosome bi-orientation. The mechanism ensuring chromosome bi-orientation lies at the heart of chromosome segregation, but is still poorly understood. Recent evidence suggests that mal-oriented kinetochore-to-pole connections are corrected in a tension-dependent mechanism. The cohesin complex and the Ipl1/Aurora B protein kinase seem to be key regulators for this correction. In this article, I discuss how cells ensure sister kinetochore bi-orientation for all chromosomes, mainly focusing on our recent findings in budding yeast.
Keywords: chromosome bi-orientation, amphitelic attachment, syntelic attachment, the cohesin complex, the Ipl1/Aurora B protein kinase
1. Sister kinetochore bi-orientation (chromosome bi-orientation)
The proper segregation of chromosomes to opposite poles of the cell during mitosis is crucial for maintenance of genetic integrity in eukaryotic cells. Revelation of the mechanisms for high-fidelity chromosome segregation should improve our understanding of various human diseases such as cancers and congenital disorders, which are characterized by chromosome instability and aneuploidy (Lengauer et al. 1998; Hassold & Hunt 2001). The segregation of sister chromatids during mitosis depends on the pulling forces exerted by microtubules that attach to kinetochores (McIntosh et al. 2002). For proper chromosome segregation, sister kinetochores must attach to microtubules extending from opposite spindle poles (bi-orientation or amphitelic attachment; figure 1) prior to anaphase onset (Tanaka 2002).
Figure 1.

Modes of kinetochore–microtubule interactions. Monotelic attachment: one of the sister kinetochores attaches to microtubules, whereas the other does not attach to any microtubules. Syntelic attachment: both sister kinetochores attach to microtubules extending from one spindle pole. Amphitelic attachment: each sister kinetochore attaches to microtubules extending from opposite spindle poles. Merotelic attachment: a single kinetochore simultaneously attaches to microtubules extending from both spindle poles.
Sister chromatids attach to each other by a multiprotein complex called cohesin from their production during DNA replication until the onset of anaphase (Nasmyth 2001). Sister chromatid cohesion resists the tendency of microtubules to pull chromatids apart during their bi-orientation on the mitotic spindle, whereas cleavage of the cohesin subunit Scc1 (also called Mcd1 or Rad21) by separase triggers the segregation of sister chromatids (Yanagida 2000; Uhlmann 2001; Nasmyth 2002). A surveillance mechanism, called the spindle checkpoint, delays activation of separase until all pairs of sister kinetochores have bi-oriented on the spindle (Lew & Burke 2003). The spindle checkpoint is crucial to maintain cell viability when the cell cycle is disturbed, for instance, when the spindle is disrupted by drugs causing microtubule depolymerization. However, at least in yeast cells, the spindle checkpoint is not essential for bi-orientation in a normal cell cycle. For instance, mutations of checkpoint proteins such as Mad2 have little effect on cell growth and on the fidelity of chromosome bi-orientation (Warren et al. 2002). Therefore, the spindle checkpoint is not an integral part of the mechanism that promotes bi-orientation.
We have chosen to address mechanisms for chromosome bi-orientation in the budding yeast Saccharomyces cerevisiae since the attachment of kinetochores to only a single microtubule in this organism (Winey et al. 1995) makes our study particularly simple and easy. Recent identification of dozens of kinetochore components and their mutants in this organism (McAinsh et al. 2003) also provides a great advantage for our study. Moreover, the centromeres (DNA underlying the kinetochore protein complex) have been pinpointed to sequences no longer than 120 bp (Hegemann & Fleig 1993), which allows us to regulate the activity of centromeres. For instance, we can conditionally turn off the centromere activity by transcription from an adjacent GAL1-10 promoter (Hill & Bloom 1987). The behaviour of yeast centromeres can be followed by marking them with arrays of bacterial operators bound by repressor proteins fused to green fluorescent protein (GFP; Goshima & Yanagida 2000; He et al. 2000; Tanaka et al. 2000; Pearson et al. 2001). Traction owing to amphitelic attachments overwhelms sister chromatid cohesion at centromeres but not in their flanking sequences, which leads to precocious sister centromere separation before the onset of anaphase.
2. Mal-orientation: avoided or corrected?
It has long been thought that different mechanisms might work for chromosome bi-orientation in mitosis and in meiosis I (discussed in Oestergren 1951). In mitosis (and meiosis II), two sister kinetochores seem to be positioned back-to-back because of sister chromatid cohesion at centromeres. Therefore, if one sister kinetochore attaches to microtubules and faces a given pole, the other kinetochore may usually face the opposite pole in such a way that it is captured only by microtubules extending from the opposite pole (discussed in Oestergren 1951; Nicklas 1971). Thus, mal-orientation (syntelic and merotelic attachments; figure 1) might be avoided by inflexibility of sister kinetochore geometry (geometry mechanism). By contrast, in meiosis I, two homologous kinetochore pairs (sister kinetochores are somehow fused to make each pair) must attach to microtubules extending from opposite spindle poles. Because two homologous centromeres (there is no cohesion between them) are only connected by chiasmata formed on the chromosome arms (Page & Hawley 2003), homologous kinetochores can flexibly change their geometry (although this may not be the case in the organisms where two homologous centromeres show significant pairing; Kemp et al. 2004). This flexibility precludes a key role of the geometry mechanism in facilitating bi-orientation of homologous kinetochores. What, then, is an alternative mechanism to facilitate bi-orientation in meiosis I? In grasshopper spermatocytes, whose kinetochores and spindle poles are repeatedly connected and disconnected by microtubules until they bi-orient, Nicklas and colleagues showed that the kinetochore-to-pole connection of a ‘maternal’ chromosome is stabilized by using a micro-needle to pull on the ‘paternal’ chromosome attached to it (Nicklas & Koch 1969; Nicklas 1997). This experiment suggests that mal-orientation (syntelic attachment in meiosis I) is corrected until tension is applied on kinetochore–spindle pole connections and thereby stabilizes these connections (tension-dependent mechanism).
Whether the geometry mechanism is sufficient to ensure bi-orientation in mitosis has been a source of argument (Nicklas 1971; Ault & Rieder 1992). If this were the case, and if mal-orientation were never corrected after this happened inadvertently, the frequency of mal-orientation should be very low throughout mitosis (otherwise cells cannot maintain their ploidy with high fidelity). On the other hand, if the mal-orientation were corrected during mitosis, mal-orientation might be found more frequently in early mitosis (prometaphase) than in late mitosis (metaphase, anaphase). Although mal-orientation was previously identified by electron microscopy (Ault & Rieder 1992), it has become possible only recently to address the frequency of mal-orientation in early mitosis using advanced light microscopy techniques. It was indeed suggested that mal-orientation (syntelic and merotelic attachments; figure 1) is more frequent in early mitosis than in late mitosis (Cimini et al. 2003; Hauf et al. 2003).
3. Tension-dependent mechanism to ensure bi-orientation in mitosis
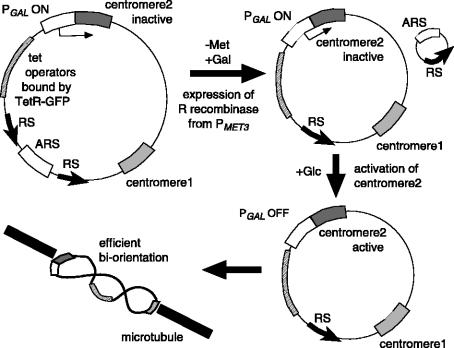
If mal-orientation is indeed corrected during mitosis, what is the mechanism to promote this correction? Does the tension-dependent mechanism, which ensures bi-orientation in meiosis I, also work in mitosis? If the tension-dependent mechanism works to facilitate bi-orientation in mitosis, we can predict that any type of connections between two kinetochores (including connections that do not support back-to-back geometry of the two kinetochores) will suffice for their bi-orientation, so long as these connections generate tension upon bi-orientation of the two kinetochores. To test this, we studied the behaviour of the two kinetochores on an engineered, unreplicated dicentric circular minichromosome in budding yeast (figure 2; Dewar et al. 2004). The two kinetochores are not sisters born by DNA replication, and therefore should not have back-to-back geometry. Nonetheless, the two kinetochores are connected by chromatin, on which tension could be applied if they bi-orient on the spindle. Because such minichromosomes cannot be propagated, we needed to establish an induction system by which we can make them from minichromosomes that are propagated stably (figure 2; Dewar et al. 2004). The original minichromosome carried two centromeres; the first one was constitutively active and the second one was conditionally inactivated owing to its juxtaposition to the GAL1-10 promoter (Hill & Bloom 1987). The chromosome also possessed a single replication origin flanked by recombination sites for the R recombinase (Matsuzaki et al. 1990). To visualize the behaviour of this minichromosome, we integrated a tandem array of tet operators that bind Tet repressorGFP fusion proteins (Michaelis et al. 1997). Moreover, the host cell could express the R recombinase conditionally from the MET3 promoter. To visualize spindle pole bodies (SPBs) in host cells, a SPB component (Spc42) was fused to yellow fluorescent protein. The minichromosome was stably propagated as long as cells were grown without expression of the R recombinase and the second centromere was maintained inactive. To obtain an unreplicated dicentric chromosome, we first removed the replication origin on the minichromosome by expressing the R recombinase. Then, the second centromere was activated by turning off the adjacent GAL1-10 promoter. As a control, we also created an unreplicated monocentric chromosome that was the same as the unreplicated dicentric chromosome except that the former did not have the second centromere regulated by the GAL1-10 promoter. We observed the motion of these two kinds of minichromosomes with time-lapse microscopy in metaphase and anaphase (Dewar et al. 2004). The unreplicated monocentric chromosomes always stayed in the vicinity of one of two SPBs and did not spend any appreciable time in the space between two SPBs. By contrast, all unreplicated dicentric minichromosomes localized halfway between two SPBs, moved vigorously back and forth along the axis connecting SPBs, and most of them had stretched GFP signals. These data suggest that two kinetochores on unreplicated dicentric minichromosomes bi-orient efficiently on the mitotic spindle. It could be argued that proximity of the two kinetochores (4.2 kb, spanning 30 nm; Bressan et al. 2004) on the unreplicated dicentric minichromosomes precluded their syntelic attachment of microtubules (their diameter is 25 nm) and that this ensured bi-orientation. However, we found that this is unlikely because, after the distance between the two centromeres was enlarged to 10 kb (spanning 70 nm) on both sides of the circular minichromosome, the unreplicated dicentric minichromosome still bi-oriented efficiently (Dewar et al. 2004). We therefore propose that any connection between kinetochores, which can provide tension upon bi-orientation, is sufficient to facilitate bi-orientation probably by stabilizing kinetochore-to-pole microtubule connections in mitosis in a similar way to meiosis I. The corollary is that geometry of two kinetochores is dispensable for their efficient bi-orientation on the mitotic spindle (although this does not exclude the possibility that the geometry mechanism functions redundantly to facilitate bi-orientation of sister centromeres in mitosis).
Figure 2.
The process of generating an unreplicated dicentric circular minichromosome (Dewar et al. 2004) is illustrated. ARS, DNA replication origin; RS, recombination site; PMET3, MET3 promoter; PGAL, GAL1-10 promoter; Met, methionine; Gal, galactose; Glc, glucose. The original minichromosome had two centromeres; one (centromere 1) was constitutively active, and the other (centromere 2) was conditionally inactivated owing to its juxtaposition to the GAL1-10 promoter (Hill & Bloom 1987). The chromosome also possessed a single replication origin flanked by recombination sites for the R recombinase (Matsuzaki et al. 1990). The R recombinase was conditionally expressed from the MET3 promoter that is turned on in the absence of methionine. To visualize the behaviour of this minichromosome, we integrated a tandem array of tet operators that bind Tet repressor–GFP fusion proteins (Michaelis et al. 1997). The minichromosome was stably propagated as long as cells were grown without expression of the R recombinase and the centromere 2 was maintained inactive. To obtain an unreplicated dicentric chromosome, we first removed the replication origin on the minichromosome by expressing the R recombinase. Then, we activated the centromere 2 by turning off the adjacent GAL1-10 promoter.
4. Roles of cohesin in chromosome bi-orientation
In addition to proteins necessary for the kinetochore–microtubule attachment, what kinds of factors are required to ensure chromosome bi-orientation on the mitotic spindle? We found that the cohesin complex is crucial to ensure bi-orientation in budding yeast (Tanaka et al. 2000; Dewar et al. 2004). For instance, after the cohesin subunit Scc1 is depleted, 45% of sister centromeres mono-oriented, whereas 55% of them still bi-oriented. Nonetheless, in Scc1-depleted cells, kinetochores were pulled towards spindle poles whether they mono-oriented or bi-oriented, suggesting that cohesin is not required for the kinetochore–microtubule attachment itself. Meanwhile, similar results were also obtained in chicken DT40 cells (Sonoda et al. 2001). Given that cohesin is necessary to ensure chromosome bi-orientation, how does cohesin accomplish this job? For this, there are two possible explanations (not mutually exclusive): cohesin might facilitate bi-orientation of sister kinetochores by ensuring their back-to-back geometry or by generating tension between sister kinetochores when bi-orientation is established. If the second tension-generating mechanism is operative, then it should be possible to restore bi-orientation in Scc1-depleted cells by providing an alternative means of connection between sister chromatids. A possible way to achieve this would be by inactivating topoisomerase II, which is required to decatenate sister chromatids after DNA replication. We therefore compared the efficiency of sister kinetochore bi-orientation in TOP2+ and top2 mutant cells after depleting Scc1 (Dewar et al. 2004). Sister centromeres bi-oriented in 55% of TOP2+ cells and in 77% of top2 mutant cells. Thus bi-orientation was at least partially restored when topoisomerase II was impaired in cohesin-depleted cells. A similar result was obtained when Scc1-depleted DT40 cells were treated with an inhibitor of topoisomerase II (Vagnarelli et al. 2004). These data suggest that the one, if not the only, role of cohesin in facilitating bi-orientation is to provide the physical connection between sister centromeres necessary to generate tension upon their bi-orientation.
5. Roles of the Ipl1–Sli15 (Aurora B-INCENP) kinase complex in chromosome bi-orientation
Recent evidence suggests that the Ipl–Sli15 kinase complex plays crucial roles in chromosome bi-orientation. Ipl1 is the only Aurora kinase in Saccharomyces cerevisiae, whereas Sli15 encodes a yeast ortholog of INCENP protein in animal cells (Andrews et al. 2003; Carmena & Earnshaw 2003). Ipl1 and Sli15 form a complex in yeast as do Aurora B and INCENP in animal cells. Initial studies indicated that ipl1 and sli15 mutant cells showed frequent chromosome missegregation (Chan & Botstein 1993; Kim et al. 1999). Subsequently, we and other groups discovered that sister centromeres were frequently pulled to one pole (∼70%) when a bipolar spindle was established in these mutant cells (Biggins et al. 1999; He et al. 2001; Tanaka et al. 2002). Once this happened, sister centromeres stayed at this spindle pole (i.e. mono-oriented), which eventually resulted in chromosome missegregation in subsequent anaphase. We also found that mono-orientation in these mutant cells was not owing to defects in kinetochore–microtubule attachments or in the microtubule-organizing function of the SPB (Tanaka et al. 2002), raising the possibility that kinetochore-to-pole microtubule connections are largely fine but that they are not properly oriented in the mutants.
Given that the Ipl1–Sli15 complex facilitates chromosome bi-orientation, does the complex accomplish this by ensuring back-to-back geometry of sister kinetochores or by promoting tension-dependent correction of kinetochore-to-pole orientation? To address this, we studied the behaviour of unreplicated dicentric minichromosomes whose bi-orientation should rely on the tension-dependent mechanism but not on the geometry mechanism (see above) in ipl1 and sli15 mutants (Dewar et al. 2004). Remarkably, the two kinetochores of unreplicated dicentric minichromosomes frequently (∼70%) mono-oriented in these mutants, whereas they rarely mono-oriented in control wild-type cells. The Ipl1–Sli15 complex must therefore be able to facilitate bi-orientation independently of sister kinetochore geometry. It would seem that the complex facilitates bi-orientation by promoting tension-dependent correction of kinetochore-to-pole orientation. If this is the case, we can envision two possible ways for the Ipl1–Sli15 complex to promote this tension-dependent correction: first, the complex might be required to destabilize kinetochore-to-pole microtubule connections in the absence of tension (e.g. syntelic attachment) and, therefore, to promote their reorientation; second, the complex might be necessary to stabilize kinetochore-to-pole microtubule connections when tension is applied on them. To test the second possibility, we arrested ipl1 and sli15 mutants (both are temperature-sensitive mutants) at the permissive temperature in metaphase by depleting Cdc20, which is required for sister chromatid separation and for anaphase onset, and shifted them to the restrictive temperature while cells were still in metaphase (Tanaka et al. 2002). After temperature shift, chromosome bi-orientation (separation of sister centromeres) was maintained, suggesting that the Ipl1–Sli15 complex is not required to stabilize bi-orientation once tension is applied.
This result prompted us to study the first possibility, i.e. the complex might be necessary to destabilize kinetochore-to-pole connections and thereby to promote their reorientation in the absence of tension. To address this possibility, we devised the following two assays. First, we depleted Cdc6, which is required for DNA replication initiation, and studied reorientation of unreplicated centromeres (where tension cannot be exerted) between two SPBs (figure 3; Tanaka et al. 2002). To score reorientation, we needed to distinguish the two SPBs from each other. This was done as follows: the old SPB, inherited from the previous cell cycle, remained intact during SPB duplication (SPBs duplicate in a conservative manner; Adams & Kilmartin 2000) and always entered the bud during anaphase (Pereira et al. 2001); on the other hand, the new SPB was formed in the vicinity of the old SPB during S phase and remained in the mother cell during anaphase (SPB duplication and segregation still occurred without Cdc6; Piatti et al. 1995). In yeast, centromeres are tethered to the old SPB by microtubules in G1 phase (Winey & O'Toole 2001; Tanaka et al. 2002). During anaphase of Cdc6-depleted cells (IPL1+, SLI15+), unreplicated centromeres segregated with the old and new SPBs with equal frequency (figure 3; Piatti et al. 1995; Stern & Murray 2001). This suggests that, after the new SPB had been formed, kinetochores had detached from the old SPB and reoriented between the old and new SPBs. By contrast, in Cdc6-depleted ipl1 and sli15 mutant cells, unreplicated centromeres predominantly segregated with the old SPB during anaphase (figure 3; Tanaka et al. 2002). This implies that the frequency of reorientation from the old SPB to the new SPB is considerably reduced when Ipl1 or Sli15 is defective.
Figure 3.

Reorientation of unreplicated centromeres between the new and the old SPBs. The behaviours of unreplicated centromeres (four representative ones are illustrated) in IPL1+ and ipl1 mutant cells (Tanaka et al. 2002) are shown. To inhibit DNA replication (tension is not applied on unreplicated centromeres), we depleted Cdc6 that is required for DNA replication initiation. The old SPB, inherited from the previous cell cycle, remained intact during SPB duplication (Adams & Kilmartin 2000) and always entered the bud during anaphase (Pereira et al. 2001). The new SPB was formed in the vicinity of the old SPB during S phase and remained in the mother cell during anaphase. In yeast, centromeres are tethered to the old SPB by microtubules in G1 phase (Winey & O'Toole 2001; Tanaka et al. 2002). During anaphase of Cdc6-depleted IPL1+ cells, unreplicated centromeres segregated with the old and new SPBs with equal frequency (Piatti et al. 1995; Stern & Murray 2001). This suggests that, after the new SPB had been formed, kinetochores had detached from the old SPB and reoriented between the old and new SPBs. By contrast, in Cdc6-depleted ipl1 mutant cells, unreplicated centromeres predominantly segregated with the old SPB during anaphase (Tanaka et al. 2002). This implies that the frequency of reorientation from the old SPB to the new SPB is considerably reduced when Ipl1 is defective.
In the second assay, we directly visualized reorientation of kinetochore-to-pole microtubule connections with time-lapse microscopy (Dewar et al. 2004). To obtain centromeres on which tension cannot be applied, we generated an unreplicated monocentric minichromosome by removing a replication origin as described above. To increase the chance of detecting reorientation, we made nuclei with four SPBs instead of two SPBs. To make such nuclei, we aborted anaphase by conditional expression of separase-resistant Scc1 (Uhlmann et al. 1999) in bub2-deleted cells that entered the next cell cycle without completion of anaphase (Lew & Burke 2003). Time-lapse imaging showed that an unreplicated monocentric minichromosome changed its associated SPB in 17 out of 46 IPL1+ nuclei but in only 3 out of 36 ipl1 mutant nuclei (p<0.005; Dewar et al. 2004). These data suggest that Ipl1 does indeed have an important role in promoting reorientation of kinetochore-to-pole microtubule connections when they do not come under tension that is normally generated by sister kinetochore bi-orientation. This notion was consistent with the observations that Ipl1 activates spindle checkpoint in the absence of tension and that the reduced Ipl1 activity restores defective kinetochore-microtubule interactions (Biggins & Murray 2001; Pinsky et al. 2003).
In summary, the Ipl1–Sli15 complex facilitates bi-orientation by promoting reorientation of kinetochore–spindle pole connections in a tension-dependent manner (figure 4). Because the syntelic attachment does not generate tension on kinetochore-to-pole connections, the Ipl1–Sli15 complex promotes reorientation of these connections (probably by phosphorylation of kinetochore components; see below). When amphitelic attachment is established, tension is applied on kinetochore-to-pole connections, and thereby the Ipl1–Sli15 complex stops promoting their reorientation, leading to preferential selection of the amphitelic mode of attachment. It was recently shown that Aurora B is required to correct syntelic attachments to amphitelic ones in mammalian cells (Hauf et al. 2003; Lampson et al. 2004). Therefore, Ipl1/Aurora B kinase ensures chromosome bi-orientation in a conserved manner from yeast to mammalian cells.
Figure 4.
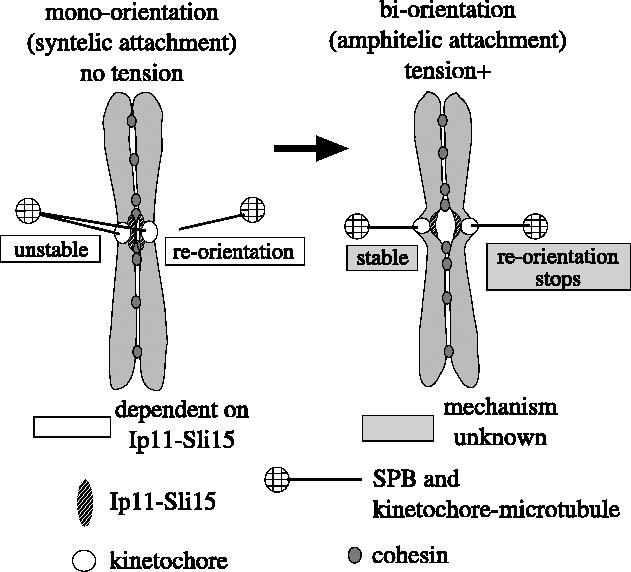
A model on how the Ipl1–Sli15 complex facilitates chromosome bi-orientation. The Ipl1–Sli15 kinase complex facilitates bi-orientation by promoting reorientation of kinetochore–spindle pole connections in a tension-dependent manner (Tanaka et al. 2002; Dewar et al. 2004). Because the syntelic attachment does not generate tension on kinetochore-to-pole connections, the Ipl1–Sli15 complex promotes reorientation of these connections (by phosphorylating kinetochore components; see text). When amphitelic attachment is established, tension is applied on kinetochore-to-pole connections and, thereby, the Ipl1–Sli15 complex stops promoting their reorientation, resulting in preferential selection of the amphitelic attachment. Using a chromatin immunoprecipitation assay and fluorescence microscopy, we and others found that Ipl1 localizes at centromeres similarly in the presence and absence of tension exerted on kinetochores during pre-anaphase (K. Tanaka et al. unpublished; Buvelot et al. 2003). It is still unclear how tension leads to cease of the reorientation.
6. Roles of the Dam1 complex in chromosome bi-orientation
It was reasonably predicted that the Ipl1–Sli15 kinase complex promotes reorientation of kinetochore-to-pole connections by phosphorylating kinetochore components, because the complex localized at kinetochores from G1 phase until anaphase onset (Kang et al. 2001; Tanaka et al. 2002; Buvelot et al. 2003). Indeed, it was subsequently found that Dam1 and Spc34 (two components of the Dam1 kinetochore complex) are phosphorylated by Ipl1 kinase and such phosphorylation is crucial to ensure chromosome bi-orientation (Cheeseman et al. 2002; Shang et al. 2003). Intriguingly, it was also reported that the Dam1 complex itself is required to maintain sister kinetochore bi-orientation after its establishment (Cheeseman et al. 2001; Janke et al. 2002; Li et al. 2002). This is in contrast with the fact that the Ipl1–Sli15 complex is not required for maintenance of bi-orientation once it is established (see above). Therefore, it seems that the Dam1 complex is an important target of the Ipl1 kinase to promote reorientation of the kinetochore-to-pole connections, and moreover, that the Dam1 complex works on its own to stabilize bi-orientation (this may be independent of Ipl1). It is still unclear whether the Dam1 complex stabilizes bi-orientation by simply strengthening the kinetochore–microtubule attachment or by stopping its reorientation when tension is applied on kinetochores.
While orthologs of the Dam1 complex have not yet been identified in vertebrate cells, it was reported that the Aurora B-dependent phosphorylation of MCAK (a kinetochore-localizing Kin I kinesin; now called kinesin-13; Lawrence et al. 2004) and CENP-A (a centromeric histone H3 variant) is required to ensure bi-orientation in mammalian cells (Kunitoku et al. 2003; Andrews et al. 2004; Lan et al. 2004; Ohi et al. 2004). It is therefore probable that the Ipl1/Aurora B kinase facilitates bi-orientation through phosphorylation of multiple targets.
7. Clues to early kinetochore–microtubule interactions
Because reorientation of kinetochore-to-pole connections is blocked in ipl1 mutant cells, ipl1 mutant cells give important clues to early kinetochore–microtubule interactions that have happened before the reorientation is initiated. For instance, mono-orientation of sister kinetochores was more frequently found at the old SPB than at the new SPBs in ipl1 mutant cells that had undergone DNA replication (figure 5; Tanaka et al. 2002). One explanation for the mono-orientation bias in favour of the old SPB is that centromere DNA replication occurs early during S phase and is completed before the new SPB has become fully operative. As a result, the majority of nascent kinetochores may be initially connected to the old SPB by microtubules, even in wild-type cells. If this hypothesis were correct, the rate of mono-orientation at the old SPBs would decrease when we delay centromere DNA replication and give more time for the new SPB to become fully operative in ipl1 mutants. To test this, we scored mono-orientation of early-replicating and late-replicating minichromosomes in ipl1 mutants (figure 5; Tanaka et al. 2002). While early-replicating minichromosomes showed a strong bias towards mono-orientation in favour of the old SPB (similarly to authentic chromosomes), late-replicating minichromosomes mono-oriented to the old and new SPBs with similar frequency in ipl1 mutants (but there was no increase in bi-orientation, suggesting a natural bias of sister kinetochores to attach to the same SPB). These data are consistent with the notion that, in wild-type cells, nascent kinetochores (on authentic chromosomes) frequently attach to the old SPB first, and then reorient, facilitated by the Ipl1 kinase, between the old and new SPBs after the new SPB becomes operative. These results also confirm that there may be nothing intrinsically wrong with the new SPB in ipl1 mutants.
Figure 5.

Orientation of the initial kinetochore-to-pole microtubule connections. The ipl1 mutant cells give important clues to early kinetochore–microtubule interactions that have happened before reorientation of kinetochore-to-pole connections is initiated, because the reorientation is blocked in ipl1 mutant cells. The initial microtubule connections of early- and late-replicating centromeres (Tanaka et al. 2002) are illustrated. Thin and thick lines represent microtubules and chromosomes, respectively. The actual experiments were done using circular minichromosomes. Here, to simplify the model, linear chromosomes are shown where replication is initiated in the vicinity of centromeres. When centromeres replicate in early S phase, mono-orientation of sister kinetochores is predominantly found at the old SPB in ipl1 mutant cells. By contrast, when centromeres replicate in late S phase, mono-orientation is found equally at the old and new SPBs. These results address whether centromere DNA replication abolishes pre-existing attachment to microtubules or not (note that centromeres are tethered to the SPB in G1 phase). Formation of mono-orientation to the new SPB suggests that DNA replication indeed transiently disrupts kinetochore–microtubule interactions (see rectangles).
In budding yeast, centromeres are tethered to SPBs during most of its cell cycle, including G1 phase (Winey & O'Toole 2001; Tanaka et al. 2002). The above experiments also addressed whether centromere DNA replication abolishes any pre-existing attachment to microtubules or whether kinetochore replication happens in a conservative manner like that of SPBs (Tanaka et al. 2002). The above result (that upon delayed centromere replication, the rate of mono-orientation to the new SPB increases whereas the rate of bi-orientation does not) is consistent with the idea that DNA replication does indeed transiently disrupt kinetochore-to-pole microtubule connections (figure 5, rectangles). In fact, kinetochore replication does not seem to occur in a conservative manner, because a recent report suggested that the majority of Cse4 (a centromere histone H3 variant) proteins are turned over at kinetochores during S phase (Pearson et al. 2004). In summary, in spite of the presence of kinetochore-to-pole microtubule connections during most of the cell cycle in budding yeast, two kinds of mechanisms can disrupt these connections: one is by kinetochore disassembly upon centromere DNA replication, independent of Ipl1 function; the other is dependent on Ipl1 and is regulated by tension applied on these connections. The fact that centromeres are detached from microtubules upon their replication (probably accompanied by transient kinetochore disassembly) raised the possibility of analysing the capture of centromeres by microtubules upon kinetochore reassembly. Indeed, we recently succeeded in visualizing kinetochore capture by individual microtubules in budding yeast (Tanaka et al. in press).
8. Perspectives
Our data suggest that cohesin provides the physical connection between sister kinetochores necessary to generate tension when they bi-orient, and that a crucial function of Ipl1 is to eliminate kinetochore-to-pole connections that do not generate such tension (figure 4; Tanaka et al. 2000, 2002; Dewar et al. 2004). These mechanisms seem to be conserved from yeast to vertebrate cells (Sonoda et al. 2001; Hauf et al. 2003; Lampson et al. 2004; Vagnarelli et al. 2004). On the other hand, it is still unclear how tension is actually sensed or how this affects whether or not Ipl1–Sli15 complex promotes reorientation of kinetochore-to-pole connections. It also remains to be clarified how greatly sister kinetochore geometry may contribute, redundantly with the tension-dependent mechanism, towards facilitating bi-orientation. For instance, Ipl1/Aurora B and cohesin might have additional roles in promoting kinetochore geometry (e.g. Ono et al. 2004). Moreover, in contrast to budding yeast, fission yeast and metazoan cells must have a single kinetochore attached to more than one microtubule (Bloom 1993). Chromosome bi-orientation fails if a single kinetochore is captured by microtubules from the opposite spindle poles (merotelic attachment; figure 1). Recent data suggest that not only syntelic attachment but also merotelic attachment is corrected during M phase (Cimini et al. 2003). Aurora B might be involved in correction of merotelic attachment (Kaitna et al. 2002). It will be an intriguing challenge to study whether and, if so, how, Aurora B and cohesin cooperate to correct merotelic attachment that would otherwise cause chromosome missegregation and aneuplody in metazoan cells.
Acknowledgments
I thank M.J.R. Stark and H. Dewar for their comments on the manuscript. The author's collaborators (designated as ‘we’ in the text) include H. Dewar, K. Tanaka, K. Nasmyth, N. Rachidi, C. Janke, G. Pereira, M. Galova, E. Schiebel and M.J.R. Stark. Work in the author's laboratory was supported by The Wellcome Trust, The Cancer Research UK and The EMBO Young Investigator Program.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Chromosome segregation’.
References
- Adams I.R, Kilmartin J.V. Spindle pole body duplication: a model for centrosome duplication? Trends Cell Biol. 2000;10:329–335. doi: 10.1016/s0962-8924(00)01798-0. [DOI] [PubMed] [Google Scholar]
- Andrews P.D, Knatko E, Moore W.J, Swedlow J.R. Mitotic mechanics: the auroras come into view. Curr. Opin. Cell Biol. 2003;15:672–683. doi: 10.1016/j.ceb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Andrews P.D, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow J.R. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- Ault J.G, Rieder C.L. Chromosome mal-orientation and reorientation during mitosis. Cell Motil. Cytoskeleton. 1992;22:155–159. doi: 10.1002/cm.970220302. [DOI] [PubMed] [Google Scholar]
- Biggins S, Severin F.F, Bhalla N, Sassoon I, Hyman A.A, Murray A.W. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 1999;13:532–544. doi: 10.1101/gad.13.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S, Murray A.W. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15:3118–3129. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K. The centromere frontier: kinetochore components, microtubule-based motility, and the CEN-value paradox. Cell. 1993;73:621–624. doi: 10.1016/0092-8674(93)90242-i. [DOI] [PubMed] [Google Scholar]
- Bressan D.A, Vazquez J, Haber J.E. Mating type-dependent constraints on the mobility of the left arm of yeast chromosome III. J. Cell Biol. 2004;164:361–371. doi: 10.1083/jcb.200311063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvelot S, Tatsutani S.Y, Vermaak D, Biggins S. The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. J. Cell Biol. 2003;160:329–339. doi: 10.1083/jcb.200209018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena M, Earnshaw W.C. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- Chan C.S, Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993;135:677–691. doi: 10.1093/genetics/135.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I.M, Enquist-Neuman M, Muller-Reichert T, Drubin D.G, Barnes G. Mitotic spindle integrity and kinetochore function linked by the Duo1p/Dam1p complex. J. Cell Biol. 2001;152:197–212. doi: 10.1083/jcb.152.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I.M, Anderson S, Jwa M, Green E.M, Kang J, Yates J.R, III, Chan C.S, Drubin D.G, Barnes G. Phospho-regulation of kinetochore–microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- Cimini D, Moree B, Canman J.C, Salmon E.D. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J. Cell Sci. 2003;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- Dewar H, Tanaka K, Nasmyth K, Tanaka T.U. Tension between two kinetochores suffices for their bi-orientation on the mitotic spindle. Nature. 2004;428:93–97. doi: 10.1038/nature02328. [DOI] [PubMed] [Google Scholar]
- Goshima G, Yanagida M. Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell. 2000;100:619–633. doi: 10.1016/s0092-8674(00)80699-6. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Hauf S, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Asthana S, Sorger P.K. Transient sister chromatid separation and elastic deformation of chromosomes during mitosis in budding yeast. Cell. 2000;101:763–775. doi: 10.1016/s0092-8674(00)80888-0. [DOI] [PubMed] [Google Scholar]
- He X, Rines D.R, Espelin C.W, Sorger P.K. Molecular analysis of kinetochore–microtubule attachment in budding yeast. Cell. 2001;106:195–206. doi: 10.1016/s0092-8674(01)00438-x. [DOI] [PubMed] [Google Scholar]
- Hegemann J.H, Fleig U.N. The centromere of budding yeast. Bioessays. 1993;15:451–460. doi: 10.1002/bies.950150704. [DOI] [PubMed] [Google Scholar]
- Hill A, Bloom K. Genetic manipulation of centromere function. Mol. Cell Biol. 1987;7:2397–2405. doi: 10.1128/mcb.7.7.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Ortiz J, Tanaka T.U, Lechner J, Schiebel E. Four new subunits of the Dam1–Duo1 complex reveal novel functions in sister kinetochore biorientation. EMBO J. 2002;21:181–193. doi: 10.1093/emboj/21.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitna S, Pasierbek P, Jantsch M, Loidl J, Glotzer M. The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous chromosomes during meiosis. Curr. Biol. 2002;12:798–812. doi: 10.1016/s0960-9822(02)00820-5. [DOI] [PubMed] [Google Scholar]
- Kang J, Cheeseman I.M, Kallstrom G, Velmurugan S, Barnes G, Chan C.S. Functional cooperation of Dam1, Ipl1, and the inner centromere protein (INCENP)-related protein Sli15 during chromosome segregation. J. Cell Biol. 2001;155:763–774. doi: 10.1083/jcb.200105029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B, Boumil R.M, Stewart M.N, Dawson D.S. A role for centromere pairing in meiotic chromosome segregation. Genes Dev. 2004;18:1946–1951. doi: 10.1101/gad.1227304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H, Kang J.S, Chan C.S. Sli15 associates with the ipl1 protein kinase to promote proper chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol. 1999;145:1381–1394. doi: 10.1083/jcb.145.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunitoku N, et al. CENP-A phosphorylation by Aurora-A in prophase is required for enrichment of Aurora-B at inner centromeres and for kinetochore function. Dev. Cell. 2003;5:853–864. doi: 10.1016/s1534-5807(03)00364-2. [DOI] [PubMed] [Google Scholar]
- Lampson M.A, Renduchitala K, Khodjakov A, Kapoor T.M. Correcting improper chromosome–spindle attachments during cell division. Nat. Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- Lan W, Zhang X, Kline-Smith S.L, Rosasco S.E, Barrett-Wilt G.A, Shabanowitz J, Hunt D.F, Walczak C.E, Stukenberg P.T. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- Lawrence C.J, et al. A standardized kinesin nomenclature. J. Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengauer C, Kinzler K.W, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- Lew D.J, Burke D.J. The spindle assembly and spindle position checkpoints. Annu. Rev. Genet. 2003;37:251–282. doi: 10.1146/annurev.genet.37.042203.120656. [DOI] [PubMed] [Google Scholar]
- Li Y, Bachant J, Alcasabas A.A, Wang Y, Qin J, Elledge S.E. The mitotic spindle is required for loading of the DASH complex onto the kinetochore. Gene Dev. 2002;16:183–197. doi: 10.1101/gad.959402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H, Nakajima R, Nishiyama J, Araki H, Oshima Y. Chromosome engineering in Saccharomyces cerevisiae by using a site-specific recombination system of a yeast plasmid. J. Bacteriol. 1990;172:610–618. doi: 10.1128/jb.172.2.610-618.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh A.D, Tytell J.D, Sorger P.K. Structure, function, and regulation of budding yeast kinetochores. Annu. Rev. Cell Dev. Biol. 2003;19:519–539. doi: 10.1146/annurev.cellbio.19.111301.155607. [DOI] [PubMed] [Google Scholar]
- McIntosh J.R, Grishchuk E.L, West R.R. Chromosome–microtubule interactions during mitosis. Annu. Rev. Cell Dev. Biol. 2002;18:193–219. doi: 10.1146/annurev.cellbio.18.032002.132412. [DOI] [PubMed] [Google Scholar]
- Michaelis C, Ciosk R, Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 2001;35:673–745. doi: 10.1146/annurev.genet.35.102401.091334. [DOI] [PubMed] [Google Scholar]
- Nasmyth K. Segregating sister genomes: the molecular biology of chromosome separation. Science. 2002;297:559–565. doi: 10.1126/science.1074757. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. Mitosis. Adv. Cell Biol. 1971;2:225–297. doi: 10.1007/978-1-4615-9588-5_5. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Nicklas R.B, Koch C.A. Chromosome micromanipulation. 3. Spindle fiber tension and the reorientation of mal-oriented chromosomes. J. Cell Biol. 1969;43:40–50. doi: 10.1083/jcb.43.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestergren G. The mechanism of co-orientation in bivalents and multivalents. Hereditas. 1951;37:85–156. [Google Scholar]
- Ohi R, Sapra T, Howard J, Mitchison T.J. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell. 2004;15:2895–2906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Fang Y, Spector D.L, Hirano T. Spatial and temporal regulation of condensins I and II in mitotic chromosome assembly in human cells. Mol. Biol. Cell. 2004;15:3296–3308. doi: 10.1091/mbc.E04-03-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S.L, Hawley R.S. Chromosome choreography: the meiotic ballet. Science. 2003;301:785–789. doi: 10.1126/science.1086605. [DOI] [PubMed] [Google Scholar]
- Pearson C.G, Maddox P.S, Salmon E.D, Bloom K. Budding yeast chromosome structure and dynamics during mitosis. J. Cell Biol. 2001;152:1255–1266. doi: 10.1083/jcb.152.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C.G, Yeh E, Gardner M, Odde D, Salmon E.D, Bloom K. Stable kinetochore–microtubule attachment constrains centromere positioning in metaphase. Curr. Biol. 2004;14:1962–1967. doi: 10.1016/j.cub.2004.09.086. [DOI] [PubMed] [Google Scholar]
- Pereira G, Tanaka T.U, Nasmyth K, Schiebel E. Modes of spindle pole body inheritance and segregation of the Bfa1p-Bub2p checkpoint protein complex. EMBO J. 2001;20:6359–6370. doi: 10.1093/emboj/20.22.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti S, Lengauer C, Nasmyth K. Cdc6 is an unstable protein whose de novo synthesis in G1 is important for the onset of S phase and for preventing a ‘reductional’ anaphase in the budding yeast Saccharomyces cerevisiae. EMBO J. 1995;14:3788–3799. doi: 10.1002/j.1460-2075.1995.tb00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky B.A, Tatsutani S.Y, Collins K.A, Biggins S. An Mtw1 complex promotes kinetochore biorientation that is monitored by the Ipl1/Aurora protein kinase. Dev Cell. 2003;5:735–745. doi: 10.1016/s1534-5807(03)00322-8. [DOI] [PubMed] [Google Scholar]
- Shang C, Hazbun T.R, Cheeseman I.M, Aranda J, Fields S, Drubin D.G, Barnes G. Kinetochore protein interactions and their regulation by the Aurora kinase Ipl1p. Mol Biol. Cell. 2003;14:3342–3355. doi: 10.1091/mbc.E02-11-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E, et al. Scc1/Rad21/Mcd1 is required for sister chromatid cohesion and kinetochore function in vertebrate cells. Dev. Cell. 2001;1:759–770. doi: 10.1016/s1534-5807(01)00088-0. [DOI] [PubMed] [Google Scholar]
- Stern B.M, Murray A.W. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr. Biol. 2001;11:1462–1467. doi: 10.1016/s0960-9822(01)00451-1. [DOI] [PubMed] [Google Scholar]
- Tanaka T.U. Bi-orienting chromosomes on the mitotic spindle. Curr. Opin. Cell Biol. 2002;14:365–371. doi: 10.1016/s0955-0674(02)00328-9. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Fuchs J, Loidl J, Nasmyth K. Cohesin ensures bipolar attachment of microtubules to sister centromeres and resists their precocious separation. Nat. Cell Biol. 2000;2:492–499. doi: 10.1038/35019529. [DOI] [PubMed] [Google Scholar]
- Tanaka T.U, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark M.J.R, Nasmyth K. Evidence that the Ipl1–Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore–spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Mukae N, Dewar H, van Breugel M, James E.K, Prescott A.R, Antony C, Tanaka T.U. In Press. Molecular mechanisms of kinetochore capture by spindle microtubules. Nature.10.1038/nature03483 [DOI] [PubMed] [Google Scholar]
- Uhlmann F. Secured cutting: controlling separase at the metaphase to anaphase transition. EMBO Rep. 2001;2:487–492. doi: 10.1093/embo-reports/kve113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann F, Lottspeich F, Nasmyth K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature. 1999;400:37–42. doi: 10.1038/21831. [DOI] [PubMed] [Google Scholar]
- Vagnarelli P, Morrison C, Dodson H, Sonoda E, Takeda S, Earnshaw W.C. Analysis of Scc1-deficient cells defines a key metaphase role of vertebrate cohesin in linking sister kinetochores. EMBO Rep. 2004;5:167–171. doi: 10.1038/sj.embor.7400077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren C.D, Brady D.M, Johnston R.C, Hanna J.S, Hardwick K.G, Spencer F.A. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol. Biol. Cell. 2002;13:3029–3041. doi: 10.1091/mbc.E02-04-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, O'Toole E.T. The spindle cycle in budding yeast. Nat. Cell Biol. 2001;3:E23–E27. doi: 10.1038/35050663. [DOI] [PubMed] [Google Scholar]
- Winey M, Mamay C.L, O'Toole E.T, Mastronarde D.N, Giddings T.H, Jr, McDonald K.L, McIntosh J.R. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J. Cell Biol. 1995;129:1601–1615. doi: 10.1083/jcb.129.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida M. Cell cycle mechanisms of sister chromatid separation; roles of Cut1/separin and Cut2/securin. Genes Cells. 2000;5:1–8. doi: 10.1046/j.1365-2443.2000.00306.x. [DOI] [PubMed] [Google Scholar]