Abstract
After 100 years of progress in understanding the organization of cerebral cortex, three issues have persisted over the last 35 years, which are revisited in this paper. First, is V3 an established or questionable area of visual cortex? Second, does taste cortex include part of area 3b (S1 proper) and other somatosensory areas? Third, is primary auditory cortex, A1, of primates the homologue of A1 in cats? The existence of such questions about even the early stages of cortical processing reflects the difficulties in mapping cerebral cortex, and reminds us that the era of basic discovery is far from over.
Keywords: gustatory cortex, V3, auditory cortex, somatosensory cortex, visual cortex
1. Introduction
This special issue paper marks the 100-year anniversary of two major publications on the organization of cerebral cortex in primates (Brodmann 1905; Campbell 1905). These investigators struggled within the limits of the histological techniques, experimental findings and clinical reports of the time, and yet started to formulate our modern concepts of how cortex is subdivided into areas of specialized functions and how these areas form parts of processing hierarchies within systems. Brodmann, in particular, recognized that anthropoid primates have a large number of cortical areas, and that different mammals both share a number of areas and differ in a number of areas. Brodmann (1909) had the more lasting impact, probably because he considered all regions of cortex and compared many species.
While Brodmann's maps of cerebral cortex usefully guided subsequent investigators, they could also give the misleading impression that the organization of cortex is well understood. If all the areas of cortex are delimited, named and numbered, what is there left to do? Of course, experienced investigators realize that a number of alternative proposals for how the cortex is divided exist, and in regions of disagreement, they cannot all be right. Gradually, and now at an accelerating pace, new and powerful methods have been added to the early histological approaches, and greatly improved understanding of how cortical organization emerged. However, we should not be too complacent or adopt the attitude that the age of discovery is over. Data on cortical organization are often ambiguous and there remain alternative interpretations of cortical organization. Very few cortical areas are so well defined that they are universally accepted as valid, with known boundaries and properties. This uncertainty was apparent in the influential review of cerebral cortex organization in macaque monkeys by Felleman & Van Essen (1991). These investigators concluded that there was evidence for 32 visual areas (a current estimate is over 40; Van Essen 2003), but only five were well defined (areas V1, V2, V3d, VP and MT). Other proposed visual areas of uncertain significance could be subsumed in alternative schemes of organization or at least had uncertain extents. Clearly, there is much left for us to do.
This review focuses on three fundamental issues about cortex organization. The first issue concerns ongoing efforts to establish proposed visual areas as valid subdivisions of the cortex. Recent evidence suggests that two of the ‘well-established’ visual areas of macaque monkeys and other anthropoid primates, V3d and VP, are not separate visual areas. Instead, these proposed visual areas appear to be halves of the same visual area, V3, as originally postulated some 35 years ago (Cragg 1969; Zeki 1969).
The second issue concerns how taste is represented in cortex. Over 35 years ago, Benjamin and co-workers proposed that two cortical regions are involved in taste: a portion of primary somatosensory cortex, S1, and a nearby gustatory area in cortex of the ventral tip of the lateral sulcus (Benjamin & Burton 1968; Benjamin et al. 1968). The apparent involvement of S1 indicates that at some levels, the processing of touch and taste on the tongue is closely intertwined. However, current concepts of how the somatosensory cortex is organized in primates include more areas than proposals at the time of the studies of Benjamin and co-workers. The proposed primary area, S1, of that time included four representations of the body in areas 3a, 3b, 1 and 2. In addition, cortex in the lateral sulcus, in the region of the proposed gustatory area, has several representations of the body in addition to the second somatosensory area, S2 (see Kaas 2004a,b,c for reviews). The greater complexity of the currently proposed cortical system for processing somatosensory inputs leads to the suggestion that the cortical system for processing taste is comparably complex.
The third issue concerns the difficulty of defining the same cortical area across taxonomic groups. Because homologous cortical areas are often less differentiated histologically in some species than others, identifying areas as the same across species was a serious challenge for Brodmann (1909) and his contemporaries, and many misidentifications were made. This challenge is one that remains, especially for higher-order areas. Given the power of functional magnetic resonance imaging (fMRI) to distinguish functionally distinct regions of cortex in humans, the issue of how similar or dissimilar cortex organization is in humans and monkeys comes to the forefront, especially since proposals of macaque monkey cortical organization commonly serve as models for human cortex organization (Preuss 2000). Here, we return to the early discoveries of Woolsey & Walzl (1942, 1944, 1982) of tonotopic (cochleotopic) organization of primary auditory cortex, A1, of cats and monkeys, and ask if the same area has been defined as A1 in both mammals.
2. Is V3V to be or not to be?
One of the proposed visual areas for primates, the third visual area, V3, remains contentious (figure 1). Early concepts of a primary visual area (area 17 in Brodmann 1905 and the visuosensory field in Campbell 1905), bordered by one (the visuopsychic field in Campbell 1905) or two higher levels of visual processing (areas 18 and 19 of Brodmann 1905), the parastriate and peristriate areas in Smith (1908), and areas OB and OA of Von Economo (1929) were based on studies of cortical architecture, supported by the evidence of more obvious visual impairments (‘psychic blindness’; Munk 1890) for more posterior lesions. In retrospect, these early proposals were not very convincing because the extents and types of depicted extrastriate areas varied from investigator to investigator. However, the concept of a primary area (17 or V1) surrounded or nearly surrounded by two ring-like higher areas (area 18 or V2 and area 19 or V3) persisted in the schematics of subsequent investigators (e.g. McCulloch 1944; Konorski 1967). The modern concept of V3 emerged from the studies of Hubel & Wiesel (1965) in cats, in which V1, V2 and V3 (or V-I, V-II and V-III) were conceptualized as three representations of the contralateral visual field corresponding to three architectonic fields termed areas 17, 18 and 19. V2 formed a retinotopically congruent border with V1 along the representation of the zero vertical meridian, and was ‘split’ so that the horizontal meridian formed the outer boundary of the belt-like area (Bilge et al. 1967). V3 adjoined V2 along the representation of the horizontal meridian, where V3 mirrored V2 in retinotopy. Thus, the outer border of V3 was formed by the vertical meridian. The primary area, V1, projected to retinotopically matched locations in V2 and V3, and V2 projected to retinotopic locations in V3 and V1 (Wilson 1968).
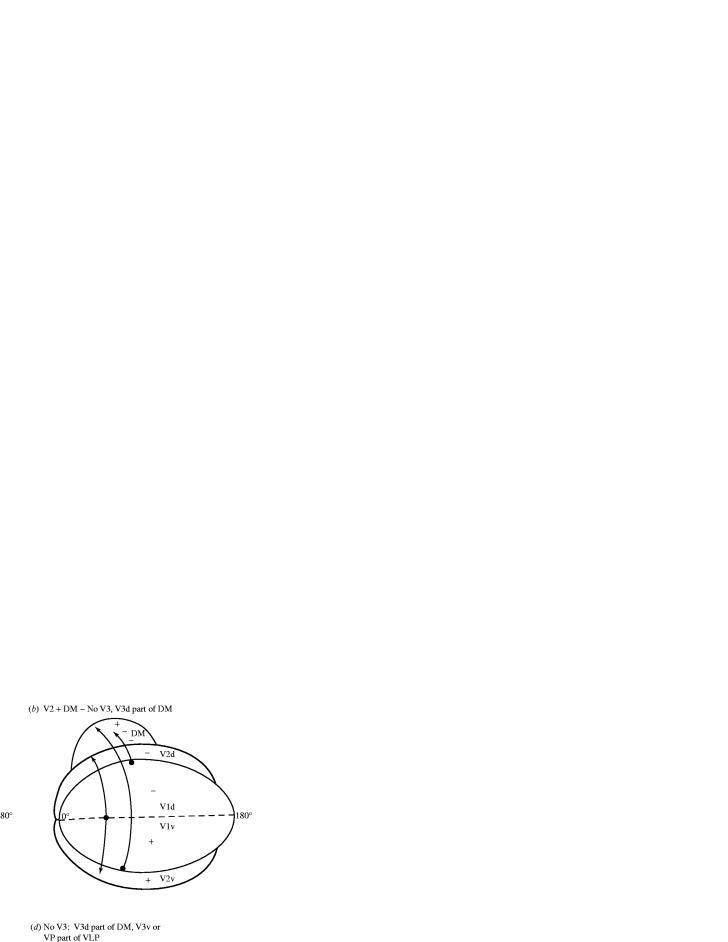
Figure 1.
Different proposals for how visual cortex in the region of V3 is organized. (a) Traditional proposals stemming from the descriptions of Cragg (1969) and Zeki (1969) include a V3 with a dorsal half, V3d, representing the lower visual quadrant, and a ventral half, V3v, representing the upper visual quadrant. The second visual area, V2, is split along the zero horizontal meridian (HM) to border primary visual cortex, V1, along the representation of the zero vertical meridian (VM). Area V3 borders much of V2, roughly as a mirror image of V2. V1 projects to retinotopically matched sets in both V2 and V3, as well as other areas including the dorsomedial visual area, DM, possibly equivalent to V3A. Sites along the outer border of V1 correspond to the VM and project to the outer border of V3, while sites along the midline of V1 correspond to the HM and project to the V2/V3 border. In the original portrayals of V3 of Cragg (1969) and Zeki (1969), V2 and V3 were continuous and of equal size. It now appears likely that in at least some primates, V3 has separated dorsal (V3d) and ventral (V3v) halves, and V3 is smaller than V2 (e.g. Kaas & Lyon 2001). (b) An alternative proposal is that a number of visual areas border V2, and there is no V3 (Allman & Kaas 1974). In this view, much of V3d is subsumed in DM, and projections of V1 that were considered to be to V3d are instead to DM (Lin et al., 1982). Because the evidence for V1 projections to V3v was uncertain, the region of V3v was considered part of another visual area (a combination of ventroposterior, VP, and ventroanterior, VA, visual areas (see Newsome & Allman 1980). (c) Perhaps the dominant theory today is that V3d is a visual area by itself even though it represents only the lower visual quadrant. The V3v region is also considered a separate visual area, the ventroposterior area, VP, which represents only the upper visual quadrant. This theory (as well as those in (b) and (d)) was supported by the erroneous conclusion that V3v does not have connections with V1 (Weller & Kaas 1983; Van Essen et al. 1986). (d) Another possibility is that much of V3d is part of DM (as in (b) above), while V3v is part of a larger area, the ventrolateral posterior area (VLP). The dorsolateral complex (DL or V4) of monkeys has been divided into rostral (DLr) and caudal (DLc) areas (see Stepniewska & Kaas 1996) and DLc has been combined with V3v/VP to form VLP by Rosa & Tweedale (2000). They have also combined the territory of DLr with the region of VA or V4v to form the ventrolateral anterior area, VLA.
Many studies later showed that this model of visual cortex organization for cats also applied to monkeys for V1 and V2 (for complete early maps of V1 and V2 in monkeys, see Allman & Kaas 1971, 1974). Furthermore, the studies of Zeki (1969) and Cragg (1969) in macaque monkeys demonstrated projections from dorsolateral V1 to two locations in adjoining visual cortex, providing strong evidence for V3. Both investigators proposed similar schemes of cortical organization for monkeys (figure 1a). As in cats, V2 bordered V1 along the representation of the vertical meridian and V3 bordered V2 along the representation of the horizontal meridian. The outer border of V3 corresponded to the vertical meridian. The locations of projections in V2 and V3 from different parts of V1 supported this contention. Cragg (1969) referred to the three areas as VI, VII and VIII, whereas Zeki (1969) originally referred to the areas as 17, 18 and 19, while recognizing that his areas 18 and 19 did not correspond to Brodmann's (1909) areas 18 and 19. Subsequently, perhaps the most powerful evidence for V3 in primates came from Gattass et al.'s (1988) microelectrode mapping study. These investigators described a complete representation of the contralateral visual hemifield in a strip of cortex bordering V2, which they termed V3. This V3 conformed closely, but not precisely, to the V3 of the early proposals of Cragg (1969) and Zeki (1969).
Given this early evidence for V3 in monkeys and its compatibility with the earlier evidence for V3 in cats, it may seem surprising that the majority of current depictions of cortical organization in primates do not include a V3. Several other emergent theories for the organization of visual cortex in the V3 region have persisted. These alternative theories involve distinctions between dorsal and ventral halves of V3.
One alternative proposal stemming from early microelectrode mapping studies in owl monkeys (Allman & Kaas 1971) is that dorsal V3 is part of a larger area that includes adjoining cortex representing the upper visual quadrant to form a complete, continuous representation of the contralateral visual hemifield, the dorsomedial visual area, DM (figure 1b). It is now apparent from various proposals, for example, DM (Allman & Kaas 1971), V3a (Zeki 1978), M (Allman & Kaas 1976), PO (Covey et al., 1982), V6 and V6A (Galletti et al., 1996), that there are several representations of the upper visual quadrant near V3d. One of these representations of the upper visual quadrant could be combined with the representation of the lower visual quadrant in V3d to form a complete representation of the contralateral hemifield. Because of these nearby representations of the upper visual quadrant, the microelectrode and fMRI data on the visuotopy of the V3d region are at least somewhat ambiguous, and they allow the interpretation that V3d is part of a larger field that includes adjoining cortex. Variations of this theory are seen in several recent reports. Rosa & Tweedale (2000) have added most of the V3d territory to DM while combining V3v (VP) with the caudal division of DL (V4) to form a ventrolateral posterior area (figure 1d). Further evidence in support of the theory that V3d is part of DM or some other area rests on similarities in connections between and in the architecture of the V3d region and adjoining cortex. Both regions have connections with V1 (e.g. Lyon & Kaas 2002a,b) and both regions appear to be more myelinated than the surrounding cortex (see Van Essen et al. 1986; Beck & Kaas 1999). Thus, the theory that DM subsumes V3d is at least somewhat consistent with much of the microelectrode mapping and anatomical evidence. However, mapping evidence and anatomical evidence for DM also seem compatible with the proposal that DM is a visual area that includes representations of both the upper and lower visual quadrants, has inputs from V1, is moderately myelinated, and is adjacent to and separate from V3d (Kaas & Lyon 2001; Lyon & Kaas 2001).
Another interpretation of the V3 region in primates, which dominates the field today, patently violates a basic definition of a sensory area. While cortical sensory representations characteristically devote much of their map to parts of receptor surfaces of high receptor density, they also represent the complete receptor surface, or at least nearly so. In visual cortex, this means that representations include both the upper and lower visual quadrants, as V1, V2 and MT clearly do. However, the dominant current proposal is that ventral V3 (V3v) is not part of V3 at all, but instead is a visual area by itself, the ventral posterior visual area, VP, representing only the upper visual quadrant (figure 1c; Newsome et al. 1986). A similar dorsal V3 (V3d) is also a separate visual area that represents only the lower visual quadrant (Burkhalter & Van Essen 1986; Burkhalter et al. 1986; Felleman & Van Essen 1987). The proposal that V3d and VP are both visual areas, rather than parts of a visual area, implies that these visual areas do not mediate distinct sets of visual functions or that visual functions for the upper and lower quadrants are distinct. Thus, for this reason alone, V3d and VP were judged to be ‘improbable’ visual areas (Kaas 1993; see also Zeki 2004). One wonders what visual functions are possible only in the upper or lower visual quadrants, and to the extent that they exist, whether they depend on incomplete visual areas rather than specialized parts of complete visual areas.
The primary evidence for the V3d–VP theory is the notion that V3d is interconnected with V1 whereas VP is not (e.g. Weller & Kaas 1983; Van Essen et al. 1986; Felleman et al. 1997; Nakamura et al. 2004). However, this evidence is undermined by a failure to adequately reveal the connections of V3v (VP). Early studies of V1 connections generally failed to adequately examine the connections of ‘ventral’ V1, the half representing the upper visual quadrant, as this cortex is less accessible on the ventral surface, medial wall or calcarine fissure of the occipital lobe. Injections or lesions in dorsolateral V1, representing the lower visual quadrant, of course, would not reveal connections with V3v. Later studies, in which injections of tracers in VP (V3v) failed to label neurons in V1, provided uncertain evidence that the injections included the territory of V3v (Felleman et al. 1997; Nakamura et al. 2004). In an attempt to resolve this issue of V1 connections, Lyon & Kaas (2001; 2002a,b,c) injected tracers into both ventral and dorsal portions of V1 in macaque monkeys, four species of New World monkeys (marmoset, squirrel, titi and owl monkeys) and prosimian galagos, and demonstrated a pattern of connections between ventral V1 and ventral V3 (VP) that mirrors the pattern between dorsal V1 and dorsal V3 (V3d). The injections in V1 labelled cells in at least 12 visual areas. Nearly half of these labelled cells were in V2. Approximately one-fifth of the total were in V3 with dense populations of labelled cells in both dorsal and ventral V3, depending on the locations of the injection sites in V1. Similar results were reported by Sousa et al. (1991) for New World cebus monkeys (also see Rosa et al. 2000). Thus, there seems to be compelling evidence that the V3v or VP region is densely connected with V1, not only in macaques but in all examined primates, including members of the three major primate clades (strepsirhine prosimians, platyrhine anthropoids and catarhine anthropoids). Strangely, Nakamura et al. (2004) accepted the evidence for V1 connecting with V3v in New World monkeys (‘in New World monkeys VP receives V1 projections’), but not for macaque monkeys. Admittedly, the Lyon & Kaas (2001, 2002a,b,c) series of studies was largely based on counts of cells labelled in V3 after the injections of retrograde and bi-directional tracers in V1. Thus, it can be argued that only projections from V3v to V1 have been convincingly demonstrated (see Nakamura et al. 2004). However, the existence of dense, one-way connections from one visual area to another would be a major violation of the principle of reciprocity in connections. Although weak feedback connections to V1 have been reported from regions of cortex with no known inputs from V1 (see Felleman & Van Essen 1991; Salin & Bullier 1995; Rockland 2004), this may simply reflect the detectability of labelled cells compared with axons, as well as the overall greater magnitude of feedback connections.
The other less compelling features that have been used to distinguish VP from V3d include proposed differences in architecture (Burkhalter et al. 1986; Van Essen et al. 1986) and single neuron response properties (e.g. Burkhalter & Van Essen 1986; Felleman & Van Essen 1987). In reality, there is little evidence from architecture and neuron response properties that V3v and V3d do not belong together. In myelin-stained brain sections, V3d has been variously described as a broad myelinated zone of 4–5 mm in width (Girard et al. 1991; Gegenfurtner et al. 1997) to a strip that was highly variable across individuals and as narrow as 1–2 mm (Van Essen et al. 1986). Gattass et al. (1988) concluded that V3 was difficult to distinguish in myelin stains from V2 caudally and V3a (DM) rostrally. Burkhalter et al. (1986) did not find a difference in myelination between V2 and VP (V3v) ‘to be consistent enough to permit reliable identification’. In several previous studies, V3d appears to have been included in the moderately to densely myelinated area defined as DM (e.g. Beck & Kaas 1999). In recent studies of V1–V3 connections, cytochrome oxidase (CO)-stained sections cut parallel to the cortical surface in New World monkeys (Lyon & Kaas 2001, 2002a) and macaques (Lyon & Kaas 2002b) revealed a pattern of dark and light CO bands across V3d and V3v. These bands were broader and less obvious than bands in V2, but of a similar orientation. In the macaques studied, the banding was most obvious in V3v. Others have described a variable pattern of CO banding and patches in the V3v and V3d regions in sections from flattened cortex that did not usefully delimit and unite the regions (Tootell et al. 1985; Olavarria & Van Essen 1997; Sincich et al. 2003). However, Sincich et al. (2003) described a macaque monkey where V3d was distinctly delimited by a series of alternations of light and dark broad stripes. In the absence of a consistent and compelling histological marker for either V3d or V3v, architectonic approaches add little to the arguments for or against V3v being VP.
As V3d and V3v/VP have not been consistently and reliably delimited, the results of studies that characterized these regions in terms of single neuron properties or the transport of tracers to other areas should be treated with considerable caution. However, these approaches could lead to more certain results in future experiments. The locations of these fields could be closely approximated by measurements from the V2 border in flattened cortex, at least in those cases where a V2 border is obvious (see Olavarria & Van Essen 1997). Alternatively, rows of small injections along the V1/V2 border could be used to mark the rostral border of V3 with labelled patches of connections (e.g. Lyon & Kaas 2002a,b; Sincich et al. 2003). At present, we do not know if the few studies of single neurons in the V3d and V3v/VP regions (Burkhalter & Van Essen 1986; Felleman & Van Essen 1987; Gegenfurtner et al. 1997) show differences because these are different fields. Moreover, we do not know whether these differences resulted from recordings outside these fields. Similar concerns apply to the few studies that compare connections based on injections judged to be in the two regions (Felleman et al. 1997).
In summary, V3d and V3v/VP mirror each other in size, retinotopy of upper or lower visual quadrants and location along the V2 border. They have similar patterns of connections with V1. The rest is uncertain. It seems most reasonable to conclude that they are halves of the same field. This conclusion resolves the incongruity of separate fields along the border of V2, each representing opposite halves of the visual hemifield.
While future research will hopefully soon provide data that will offer further insight into how cortex in the V3 region is organized in primates, there are many similar issues that also need to be resolved (see Kaas 2004a). For example, as presently proposed, V4 seems to over-represent the upper visual quadrant and boundaries are not well established. Further studies of connection patterns might help define boundaries. In addition, there are several proposals for subdividing the V4 region into areas, and these differences need to be resolved. Are the proposed areas DM and V3a largely or completely the same visual area? Does the FST contain two areas with different patterns of connections? Is the incomplete representation of proposed area V4t part of a complete representation? Does the MST contain two or more areas? Indeed, numerous questions await answers.
3. Defining the cortical network for taste
The visual, auditory and somatosensory systems of primates are each characterized by a number of areas that interconnect into processing networks (e.g. Felleman & Van Essen 1991). In contrast, the traditional view of the gustatory system is that only a few areas of cortex are involved: a somatosensory-taste region of primary somatosensory cortex (S1), a large primary gustatory area (G) and a hedonic taste region in orbital–frontal cortex. Supporting evidence for this scheme comes from a number of early studies. Most importantly, Benjamin et al. (1968) and Benjamin & Burton (1968) used electrical stimulation of nerves of the tongue to activate two regions of cortex in squirrel monkeys, one judged to be the tongue representation of primary somatosensory cortex in its most laterorostral extension into frontal cortex on the lateral surface of the brain, and the other nearby in the opercular–insular cortex close to the end of the lateral fissure. Both regions were thought to receive inputs from the taste nucleus of the thalamus, VPMpc, as a large cortical lesion involving both areas produced retrograde degeneration in VPMpc (Benjamin & Burton 1968). Pritchard et al. (1986) provided further evidence for these connections in macaque monkeys when injections of tracers into VPMpc labelled both regions of cortex, but ‘area G’ was much more densely labelled than S1. However, the significance of the sparser label in ‘S1’ was subsequently questioned as a possible artifact of the spread of the injected tracer from VPMpc into the adjacent VPM, which represents mechanoreceptors of the face and oral cavity (Pritchard & Norgren 2004). Thus, a role for S1 in taste was uncertain. Later, a third region of cortex involved in taste was revealed by recordings from a location in orbito-frontal cortex (OFC) in macaque monkeys where neurons respond to taste substances, as well as visual and olfactory stimuli (e.g. Rolls et al. 1989, 1990; Rolls & Baylis 1994; Rolls 2000). Neurons in OFC are said to reflect the hedonic aspects of taste (Pritchard & Norgren 2004). Rolls (2000) considers that the caudolateral part of OFC is secondary taste cortex and that neurons in adjoining regions of OFC constitute a tertiary taste area.
Clearly, there are reasons for questioning this scheme as too simple (see Pritchard & Norgren 2004). For example, we now have a much better understanding of the organization of the S1 region of cortex in primates (figure 2). At the time that the landmark studies of Benjamin et al. (1968) implicated S1 of squirrel monkeys in taste, several different fields were confounded in the concept of S1. Areas 3a, 3b, 1 and 2 were all thought to be parts of the same field S1 (see Kaas et al. 1979 for review). At present, each of these areas is widely recognized as a separate processing station, with area 3b being the primary tactile area and the homologue of S1 in other mammals (Kaas 1983), while the areas 1 and 2 are successively higher levels of processing (see Kaas 2004c). Area 3a combines muscle spindle receptor information from the thalamus with inputs from area 3b, 1 and 2, and is more directly involved in sensorimotor control. The focus of activity in the lateral frontal cortex evoked by electrically stimulating the nerves of the tongue by Benjamin et al. (1968) involved both part of area 3b and adjoining regions of the cortex. Thus, three or more areas of cortex in this focus of activity could be parts of the cortical taste network. This assumption is supported by the results of recent studies of the connections of the part of area 3b that represents the tongue.
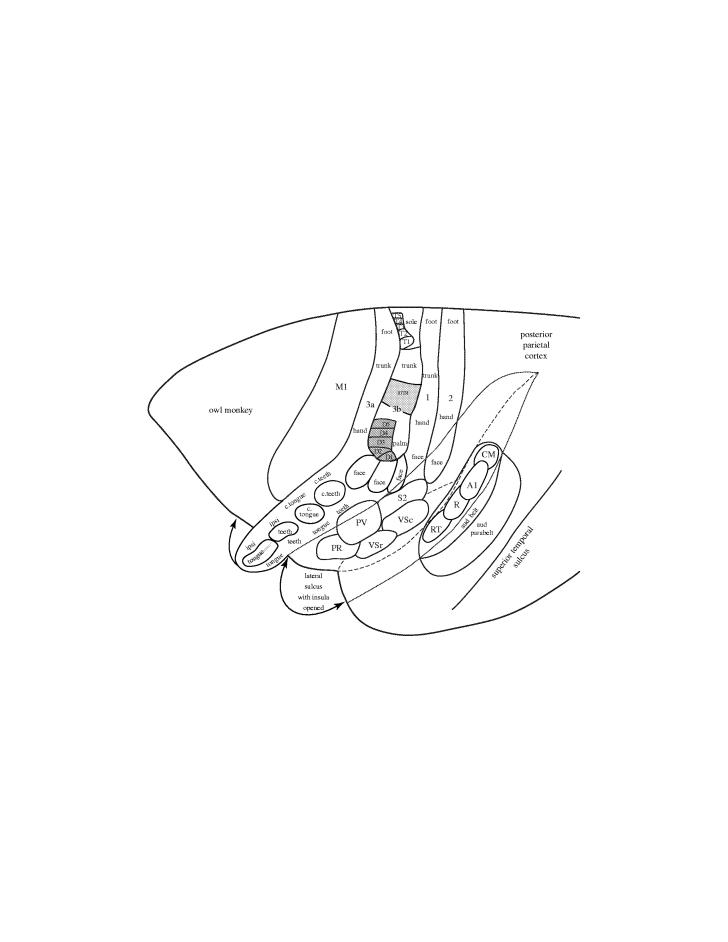
Figure 2.
A lateral view of the rostral two-thirds of the left cerebral hemisphere of an owl monkey. The full extent of primary somatosensory cortex (area 3b) in New World owl and squirrel monkeys, as recently defined by Jain et al. (2001), includes a large RL representation of the oral cavity with representations of the periodontal receptors of the contralateral teeth (c. teeth), the contralateral tongue (c. tongue), ipsilateral teeth (ipsi teeth) and ipsilateral tongue (ipsi tongue) in a dorsocaudal to ventrorostral sequence. In the more medial area 3b, three ovals of cortex represent the lower face and lower lip, the upper lip and the upper face. These ovals are followed by representations of the hand with digits (1–5), arm, trunk and sole and toes of the foot. Area 3b is bordered by rostral and caudal parallel representations of the contralateral body in areas 3a and 1. These areas appear to extend rostrolaterally to form representations of the oral cavity that adjoin that of area 3b. Additional somatosensory representations include the second somatosensory area, S2, the parietal ventral area, PV, the rostral ventral somatosensory area, VSr, the caudal ventral somatosensory area, VSc, and the parietal rostral area, PR (see Coq et al. 2004). The lateral sulcus has been opened to show these areas and the insular cortex. Area 2 contains another representation of the contralateral body. Many or most of these somatosensory areas represent the tongue, and some of them receive direct inputs from the taste nucleus of the thalamus. Thus, tongue portions of all or most of these areas, in addition to other fields in the orbital–frontal cortex, may be involved in a complex cortical network that mediates aspects of taste. Primary motor cortex, M1 and auditory areas are shown for reference. Primary auditory cortex, A1, the primary-like rostral area, R, and rostral temporal area, RT. The auditory (aud) belt, of secondary auditory areas includes the caudomedial area, CM. The auditory (aud) parabelt constitutes a third level of cortical processing.
One of the useful features of area 3b of monkeys is that aspects of the representation of the contralateral body surface are visible in brain sections processed for myelin or the metabolic enzyme, CO. Thus, territories for the representations of each of the five digits are apparent in the hand portion of area 3b of New World (Jain et al. 1998) and Old World monkeys (Qi & Kaas 2004) as myelin-dense bands or ovals separated by narrow myelin-poor septa (see Kaas & Catania (2002) for other mammals). Lateral to the myelin-dense bands for digits, monkeys have a series of myelin-dense ovals separated by myelin-poor septa that identify parts of area 3b representing upper and lower face, contralateral teeth, contralateral tongue, ipsilateral teeth and the ipsilateral tongue (Jain et al. 2001; Qi et al. 2004). These ovals extend area 3b (somatosensory koniocortex) much further ventrorostrally than was portrayed by early investigators (e.g. Sanides 1968; Merzenich et al. 1978). In owl and squirrel monkeys, the three ovals just lateral to the representation of the hand represented upper face, lip and lower face and lip (Jain et al. 2001). The next more ventral oval represents the contralateral teeth (periodontal receptors), followed by an oval for contralateral tongue, and then by ovals for ipsilateral teeth and tongue (figure 2). Neurons in the oval for the contralateral tongue were highly responsive to touch on the tongue, but neurons just medial and lateral to this oval also responded to touch and taps on the tongue, albeit less vigorously than area 3b neurons. Although these neurons were not evaluated for responsiveness to taste stimuli, injections of tracers into the contralateral tongue oval densely labelled large numbers of neurons in the thalamic taste nucleus, VPMpc (Iyengar et al. 2002).
The contralateral tongue oval is located in the middle of a larger region where stimulation of either ipsilateral or contralateral nerves of the tongue evoked responses in the experiments of Benjamin et al. (1968). It is uncertain why this region responded to only touch and taps on the contralateral half of the tongue in the experiments of Jain et al. (2001), but the responsiveness of neurons to the tongue, along with inputs from VPMpc, clearly implicates the area 3b tongue representation in the processing of taste. Because VPMpc has both neurons that respond to taste and neurons that respond to touch (Pritchard et al. 1989), it should not be surprising that touch and taste processing in area 3b are intimately related. In addition, the previously questionable evidence for VPMpc projections to the 3b region of Benjamin & Burton (1968) and Pritchard et al. (1986) now seems to reflect real projections. The area 3b tongue oval projects to adjacent dorsorostral and ventrocaudal regions of the cortex (Iyengar et al. 2002) and these regions of cortex were also responsive to tongue stimulation. In terms of position, these two regions correspond to parts of areas 3a and 1, and thus higher-order processing in these regions might be analogous to area 3b and area 1 processing for other parts of somatosensory cortex.
The second representation of the tongue in area 3b in the most rostral myelin-dense oval (figure 2) was of the ipsilateral tongue (Jain et al. 2001). Although this oval is located close to the orbital–frontal region for hedonic aspects of taste, it appears to be caudal and separate from it. This oval could also be involved in taste, but there is no evidence of this possibility. Most notably, no projection from VPMpc to this region of the cortex has been described. Because of its less accessible position, we have not attempted to place injections in the oval for ipsilateral tongue.
Most of the cortex of the upper bank of the lateral sulcus and the adjoining insula is responsive to somatosensory stimuli, and several somatosensory areas have been described within this cortex (see Kaas 2004b,c). Most notably, the classical area, S2, borders the face representation in area 3b, and a more recently defined parietal ventral area, PV, occupies cortex along the rostral border of S2 (Krubitzer & Kaas 1990; Krubitzer et al. 1995; Qi et al. 2002; Coq et al. 2004). These two areas have face representations along or near the face portion of area 3b and limb representations deeper in the lateral fissure. S2 and PV mirror each other in crude somatotopic organization, while receiving inputs from areas 3a, 3b, 1 and 2. Presumably, both S2 and PV have tongue representations with inputs from the tongue ovals in 3b. Thus, parts of S2 and PV are probably involved in taste. S2 and PV are connected with other regions of lateral parietal cortex, including a proposed parietal rostral area, PR, of uncertain significance, and rostral (VSr) and caudal (VSc) ventral somatosensory areas, which represent the contralateral body (Cusick et al. 1989; Coq et al. 2004) and possibly contain additional representations of the tongue.
In squirrel monkeys, the rostral part of the lateral parietal cortex, including the territories of PR and much of PV and VSr, appears to be within the primary gustatory area, G, as described by Benajmin et al. (1968) and Sanides (1968). In macaques, the opercular–insular region of dense VPMpc projections of Pritchard et al. (1986) appears to be more focused. However, the gustatory area G of macaques has been described as a rather large area where only a few neurons (between 2 and 10%) respond to taste, and many more respond to mouth movements and other stimuli including touch on the tongue (e.g. Smith-Swintosky et al. 1991; Plata-Solomán et al. 1993; Scott et al. 1999). Neurons that are responsive to touch on the tongue appear to be infrequent, but they are scattered over much of parietal cortex of the lateral sulcus (Ogawa et al. 1989; Ito & Ogawa 1994). This result could simply reflect recordings from a number of scattered representations of the tongue that are parts of larger representations of the body. Thus, many of the recording sites would be in cortex that is not devoted to the tongue. While some of the cortex of the lateral sulcus has a well-developed layer 4 of granule cells (Sanides 1968) and expresses moderate levels of CO in layer 4 (Ito & Ogawa 1991), none of this cortex has the marked architectonic characteristics of primary sensory cortex. Moreover, the large size of area G appears to include much or all of several proposed areas. Such considerations bring the concept of a pure primary taste area, G, into question.
Thus, a simple interpretation of the opercular–insular region seems questionable. Instead, it seems likely that many (perhaps all) somatosensory representations of the lateral parietal cortex include representations of the tongue, and these parts of larger fields are involved in taste. As areas S2 and PV both receive inputs from areas 3b, 3a and 1, one or both of these two areas would probably receive connections from VPMpc tongue ovals in area 3b and the tongue representations presumed to be part of areas 3a and 1. These fields would connect with others, and some would relay to the orbital–frontal hedonic taste region, forming a distribution of interconnected cortical locations involved in taste. However, it seems likely that few or none of these locations would be involved exclusively in taste.
4. The problem of identifying homologous areas across taxa: would the real a1 please stand up?
In a once popular television show, contestants were invited to distinguish a real person from two impostors by answering a series of questions. After choices were made, the real person was revealed when asked to stand up. In neuroscience, we not only face the problem of identifying regions of cortex as functionally distinct areas, but also the difficulty of identifying areas across taxa as homologous (present in a common ancestor). This is generally accomplished by asking a series of questions about the similarities between the areas in question. While the homology of areas can be obvious for such distinctive areas as primary visual cortex (V1 or area 17), identifying areas as the same is much more difficult than one would imagine from the summary maps of cortical areas of various species by early investigators such as Brodmann (1909). As a notable example, there has been much confusion over the identity of primary somatosensory cortex in mammals, not only by Brodmann (1909) who denoted areas 3, 1 and 2 in variously inconsistent ways across species, but also more recently when investigators combined four fields (3a, 3b, 1 and 2) into S1 in anthropoid primates, rather than recognizing the area 3b representation as a homologue of S1 of other mammals (see Kaas 1983 for review). Correctly identifying homologues is at the heart of making reliable inferences about an area in one species using data from an area in another species. Thus, we infer functions and features of visual area MT in humans from the results of studies of MT in monkeys. The question here is if the area is termed A1 (primary auditory cortex) in monkeys (and humans) is the area identified as A1 in cats? At first, the question seems absurd, as investigators have already widely accepted the same name for the areas in primates and carnivores without serious question. However, there are problems inherent in this tendency.
The current model of the early stages of cortical processing in anthropoid primates (see Kaas & Hackett 2000 for review) includes a core of three primary or primary-like areas, aligned caudal to rostral along the lower bank of the lateral sulcus: primary auditory cortex, A1, the rostral area, R, and the rostral–temporal area, RT (figure 2). All three areas of the core have the architectonic features of primary sensory cortex (koniocellular cortex), as well as direct sensory inputs from the principal (ventral) division of the medial geniculate complex of the auditory thalamus. While all studied mammals have an auditory field that has been termed A1 (see Luethke et al. 1988), the term A1 originated in early electrophysiological studies on cats in an effort to distinguish two patterns of cochleotopic (tonotopic) organization in auditory as AI and AII (Woolsey & Walzl 1942, 1944). At present, this A1 of cats is recognized as within the cortex with koniocellular (sensory) characteristics and inputs from the ventral (principal) nucleus of the medial geniculate complex, MGv (e.g. Ehret 1997). The field is tonotopically organized with contours of isofrequency coursing from dorsal to ventral (Merzenich et al. 1975). Frequencies are represented from high to low in a rostrocaudal sequence across A1 (figure 3).
Figure 3.
The tonotopic organization of three primary-like auditory fields in (a) cats (and other carnivores) and monkeys (b). Cats have an anterior auditory field, AAF, a primary field, A1, and a posterior field, P. These fields represent high to low frequencies in isofrequency bands that progress from rostral to caudal in A1, but in the opposite (mirror-image) patterns in the two adjoining fields. Monkeys have a rostral–temporal field, RT, a rostral field, R, and a primary field, A1. The rostrocaudal tonotopic sequence of representation in R matches that of A1 of cats, except for a tilt, while RT and A1 mirror R in tonotopic organization. The question mark (?) for RT denotes the sparseness of data on the tonotopy of the area; R, rostral in the brain; C, caudal.
However, A1 is not the only field in cats with primary-like characteristics. A1 is bordered rostrally by the anterior auditory field, which represents frequencies from low to high in a rostrocaudal sequence that mirrors that of A1 (Knight 1977) and A1 is bordered caudally by a posterior field, P, with a tonotopic organization in a mirror reversal of that in A1 (Reale & Imig 1980). MGv projects to all three fields (Morel & Imig 1987; Huang & Winer 2000).
Early evidence for a systematic representation of the cochlea in a region of cortex termed A1 in monkeys came from the electrophysiological studies of Woolsey & Walzl (1944; see also Woolsey & Walzl 1982). Later, Merzenich & Brugge (1973) redefined A1 as a smaller field and added a rostro-lateral field (RL) with primary-like features including a tonotopic organization that mirrored that of A1. RL subsequently became known as R for rostral (Imig et al. 1977). Morel & Kaas (1992) recognized another tonotopically organized RT at the rostral border of R. These three fields have the histological features of primary sensory cortex (e.g. Hackett et al. 2001) and inputs from MGv (e.g. Morel & Kaas 1992). The sequences of primary-like areas in cats and monkeys roughly parallel each other in number and orientation relative to primary visual and somatosensory areas. The obvious difference is that A1 is in the middle of the three tonotopic areas in cats and at the caudal end in monkeys (figure 3). More importantly, the tonotopic organization of A1 in cats is opposite that of monkeys. Is there a way of accounting for these basic differences or are we talking about two different auditory areas?
One way of resolving these differences in the tonotopic gradients is to conclude that A1 of monkeys has rotated nearly 180° with growth of the temporal lobe of primates (Jones 1985). However, this interpretation seems questionable when the orientation of the auditory core of A1, R and RT is considered relative to somatosensory fields in flattened cortex, especially in those primates with less expanded temporal lobes. In such a view (see Tootell et al. 1985), the RT end of the core appears to be rotated ventrally away from S1 as a result of an expansion of the somatosensory cortex in the lateral fissure, but the rotation is less than 90°. Alternatively, the tonotopic gradient of A1 could have reversed at some point in evolution, but this seems unlikely given the stability of topographic organizations in visual and somatosensory areas (however, see Calford et al. 1985). A third possibility is that A1 of cats and A1 in monkeys are not the same field. Instead, area R could be the homologue of A1 in cats. For this to be the case, the two fields need not be identical because cats and primates have evolved separately for some 100 million years (e.g. Novacek 1992). Although the most caudal field ‘A1’ in monkeys is more primary-like overall than the most caudal field ‘P’ in cats, they could be homologous fields that more closely resembled each other in ancestry, closer to the time of divergence. If early mammals had two or more primary-like fields, then different fields in different lines of descent could have evolved more pronounced, primary-like features. A fourth possibility is that A1 and R in monkeys are mirror reversal duplications of a single area A1 of an ancestor, and that A1 and R of monkeys are serial homologues of A1 in cats. Although this may seem unlikely, mammals differ in number of sensory areas (see Kaas 1989) and one proposed mechanism of evolving more areas is through the duplication and the subsequent differentiation of areas (Allman & Kaas 1974). Recently, the feasibility of this mechanism was experimentally demonstrated when the development of primary somatosensory cortex was manipulated with an in utero gene transfer technique to alter the production of a growth factor. By creating two concentration gradients of the growth factor in cortex instead of one, two mirror-image representations of S1 formed instead of one (Fukuchi-Shimugori & Grove 2001).
While the homologue of A1 of cats in primates remains uncertain, the problem is part of a larger issue of identifying homologues of cortical areas across species (Kaas 2002). This problem seems especially pronounced for auditory areas. For those unfamiliar with current proposals of how the auditory cortex is organized, it might seem surprising that an auditory area with a high to low frequency representation from rostral to caudal in cortex has been identified as A1 in some rodents, while an area with the opposite tonotopic organization has been termed A1 in other rodents. Thus, the area proposed to be A1 in guinea pigs (Redies et al. 1989; Wallace et al. 2000), chinchillas (Harrison et al. 1996) and squirrels (Luethke et al. 1988) represents high to low tones in a caudorostral direction, while the area proposed to be A1 in mice (Stiebler et al. 1997), rats (Sally & Kelly 1988; Doron et al. 2002) and gerbils (Thomas et al. 1993) represents high to low tones in the opposite rostrocaudal direction. As it seems extremely unlikely that A1 would have opposite patterns of tonotopy in different rodents, a more parsimonious conclusion is that it is not easy to identify A1 and different areas have been called A1. The problem of identifying homologues is undoubtedly even greater for many of the higher-order sensory representations, and this problem is confounded by the likelihood that many higher-order areas evolved independently in various lines of descent (Kaas 1995, 2004b).
5. Conclusions
The main point of the present review is that 100 years after the landmark publications of Campbell (1905) and Brodmann (1905) on cortical organization, there is still important work to do. For each sensory system, only a few cortical areas have been unambiguously established and are widely accepted by current investigators. For the visual cortex of anthropoid primates, nearly all investigators recognize V1, V2 and MT as valid areas with established boundaries, but we need to increase that number to the likely 30 to 40 visual areas that characterize these primates. In this review, we suggested that V3 might soon be widely recognized as a valid visual area. A number of other areas seem close to being well established. Considerable progress in defining areas in auditory and somatosensory systems has occurred as well, but many uncertainties remain.
The cortical network for taste is not well understood, but progress could be rapid. The close relationship of taste processing and touch in the somatosensory thalamus, and the recognition that anterior and lateral somatosensory cortex of primates contains a number of areas suggests that tongue representations in some or most of these areas are involved in taste.
The homologies of proposed areas of anthropoid primates and those in other clades are not well established. As a carryover from the time when areas 3a, 1 and 2 were considered to be parts of S1 in anthropoid primates, investigators persist in placing these fields in a single area, S1, of other mammals such as rats, ferrets and cats. Homologues of primate visual areas other than V1 and V2 have not been established in cats and other non-primate mammals. In auditory cortex, the homologies of even the area identified as A1 in anthropoids, rodents and carnivores are uncertain. Thus, there are many important questions about identifying areas across taxa yet to be resolved.
Progress in addressing these issues may have been hindered by an uncritical acceptance of both earlier and more recent depictions of cortical organization. These depictions represent theories or models that are variously supported by evidence that can be quite ambiguous. If depictions of cortical organization were more clearly presented as theories or models that are open to modification and replacement, then investigators would be encouraged to more fully challenge these theories.
Glossary
- FST
fundal area of the superior temporal sulcus
- MT
middle temporal area
- MST
the medial superior temporal area
- VPMpc
parvicellular ventroposteromedial nucleus
Footnotes
One contribution of 12 to a Theme Issue ‘Cerebral cartography 1905–2005’.
References
- Allman J.M, Kaas J.H. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus) Brain Res. 1971;35:89–106. doi: 10.1016/0006-8993(71)90596-8. [DOI] [PubMed] [Google Scholar]
- Allman J.M, Kaas J.H. The organization of the second visual area (VII) in the owl monkey: a second order transformation of the visual hemifield. Brain Res. 1974;76:247–265. doi: 10.1016/0006-8993(74)90458-2. [DOI] [PubMed] [Google Scholar]
- Allman J.M, Kaas J.H. Representation of the visual field on the medial wall of occipital–parietal cortex in the owl monkey. Science. 1976;191:572–575. doi: 10.1126/science.814619. [DOI] [PubMed] [Google Scholar]
- Beck P.D, Kaas J.H. Cortical connections of the dorsomedial visual area in old world macaque monkeys. J. Comp. Neurol. 1999;406:487–502. [PubMed] [Google Scholar]
- Benjamin R.M, Burton H. Projection of taste nerve afferents to anterior opercular–insular cortex in squirrel monkey (Saimiri sciureus) Brain Res. 1968;7:221–231. doi: 10.1016/0006-8993(68)90100-5. [DOI] [PubMed] [Google Scholar]
- Benjamin R.M, Emmers R, Blomquist A.J. Projection of tongue nerve afferents to somatic sensory area I in squirrel monkey (Saimiri sciureus) Brain Res. 1968;7:208–220. doi: 10.1016/0006-8993(68)90099-1. [DOI] [PubMed] [Google Scholar]
- Bilge M, Bingle A, Senevirathe K.N, Whitteridge D.W. A map of the visual cortex in the cat. J. Physiol. Lond. 1967;191:116. [PubMed] [Google Scholar]
- Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. Dritte Mitteilung: Die Rindenfelder der niederen Affen. J. Psych. Neurol. 1905;4:177–226. [Google Scholar]
- Brodmann K. Leipzig. Barth; Leipzig: 1909. Vergleichende Lokalizationlehre der Grosshirnrinde. [Google Scholar]
- Burkhalter A, Van Essen D.C. Processing of color, form and disparity information in visual areas VP and V2 of ventral extrastriate cortex in the macaque monkey. J. Neurosci. 1986;6:2327–2351. doi: 10.1523/JNEUROSCI.06-08-02327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter A, Felleman D.J, Newsome W.T, Van Essen D.C. Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. Vision Res. 1986;26:63–80. doi: 10.1016/0042-6989(86)90071-4. [DOI] [PubMed] [Google Scholar]
- Calford M.B, Graydon M.L, Huerta M.F, Kaas J.H, Pettigrew J.D. Altered somatotopy in the brain of a flying mammal. Nature. 1985;313:477–479. doi: 10.1038/313477a0. [DOI] [PubMed] [Google Scholar]
- Campbell A.W. Cambridge University; Cambridge: 1905. Histological studies in the localisation of cerebral function. [Google Scholar]
- Coq J.O, Qi H.-X, Collins C.E, Kaas J.H. Anatomical and functional organization of somatosensory areas of the lateral fissure of the New World titi monkey. J. Comp. Neurol. 2004;476:363–386. doi: 10.1002/cne.20237. [DOI] [PubMed] [Google Scholar]
- Covey E, Gattass R, Gross C.G. A new visual area in the parietooccipital sulcus of the macaque. Soc. Neurosci. Abstr. 1982;8:861. [Google Scholar]
- Cragg B.C. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 1969;9:733–747. doi: 10.1016/0042-6989(69)90011-x. [DOI] [PubMed] [Google Scholar]
- Cusick C.G, Wall J.T, Felleman D.J, Kaas J.H. Somatotopic organization of the lateral sulcus of owl monkeys: area 3b, S-II, and a ventral somatosensory area. J. Comp. Neurol. 1989;282:169–190. doi: 10.1002/cne.902820203. [DOI] [PubMed] [Google Scholar]
- Doron N.N, Ledoux J.E, Simple M.N. Redefining the tonotopic core of rat auditory cortex: physiological evidence for a posterior field. J. Comp. Neurol. 2002;453:345–360. doi: 10.1002/cne.10412. [DOI] [PubMed] [Google Scholar]
- Ehret G. The auditory cortex. J. Comp. Physiol. 1997;181:547–557. doi: 10.1007/s003590050139. [DOI] [PubMed] [Google Scholar]
- Felleman D.J, Van Essen D.C. Receptive field properties of neurons in area V3 of macaque monkey extrastriate cortex. J. Neurophysiol. 1987;57:889–920. doi: 10.1152/jn.1987.57.4.889. [DOI] [PubMed] [Google Scholar]
- Felleman D.J, Van Essen D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Felleman D.J, Burkhalter A, Van Essen D.C. Cortical connections of area V3 and VP of macaque monkey extrastriate visual cortex. J. Comp. Neurol. 1997;379:21–47. doi: 10.1002/(sici)1096-9861(19970303)379:1<21::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Grove E.A. Neocortex patterning by the secreted signaling molecule FGFS. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P, Battaglini P.P, Shipp S, Zeki S.M. Functional demarcation of a border between areas V6 and V6A in the superior parietal gyrus of the macaque monkey. Eur. J. Neurosci. 1996;8:30–52. doi: 10.1111/j.1460-9568.1996.tb01165.x. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa A.P.B, Gross C.G. Visuotopic organization and extent of V3 and V4 of the macaque. J. Neurosci. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegenfurtner K.R, Kiper D.C, Levitt J.B. Functional properties of neurons in macaque area V3. J. Neurophysiol. 1997;77:1906–1923. doi: 10.1152/jn.1997.77.4.1906. [DOI] [PubMed] [Google Scholar]
- Girard P, Salin P.-A, Bullier J. Visual activity in areas V3a and V3 during reversible inactivation of area V1 in the macaque monkey. J. Neurophysiol. 1991;66:1492–1503. doi: 10.1152/jn.1991.66.5.1493. [DOI] [PubMed] [Google Scholar]
- Hackett T.A, Preuss T.M, Kaas J.H. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J. Comp. Neurol. 2001;441:197–222. doi: 10.1002/cne.1407. [DOI] [PubMed] [Google Scholar]
- Harrison R.V, Kakigi A, Hirakawa H, Harel N, Mount R.J. Tonotopic mapping in auditory cortex of the chinchilla. Hear. Res. 1996;100:157–163. doi: 10.1016/0378-5955(96)00120-7. [DOI] [PubMed] [Google Scholar]
- Huang C.L, Winer J.A. Auditory thalamocortical projections in the cat: laminar and areal patterns of input. J. Comp. Neurol. 2000;427:302–331. doi: 10.1002/1096-9861(20001113)427:2<302::aid-cne10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hubel D.H, Wiesel T.N. Receptive fields and functional architecture in two straite visual areas (18 and 19) of the cat. J. Neurophysiol. 1965;30:1561–1573. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Imig T.J, Ruggero M.A, Kitzes L.M, Javel E, Brugge J.F. Organization of auditory cortex in the owl monkey (Aotus trivirgatus) J. Comp. Neurol. 1977;171:111–128. doi: 10.1002/cne.901710108. [DOI] [PubMed] [Google Scholar]
- Ito S.I, Ogawa H. Cytochrome oxidase staining facilitates unequivocal visualization of the primary gustatory area in the fronto-operculo-insular cortex of macaque monkeys. Neurosci. Lett. 1991;130:61–64. doi: 10.1016/0304-3940(91)90227-k. [DOI] [PubMed] [Google Scholar]
- Ito S.I, Ogawa H. Neural activities in the fronto-opercular cortex of macaque monkeys during tasting and mastication. Jpn. J. Physiol. 1994;44:141–156. doi: 10.2170/jjphysiol.44.141. [DOI] [PubMed] [Google Scholar]
- Iyengar S, Jain N, Qi H.-X, Kaas J.H. Cortical and thalamocortical connections of the oral cavity representations in area 3b of New World monkeys. Soc. Neurosci. Abstr. 2002;28:6509. [Google Scholar]
- Jain N, Catania K.C, Kaas J.H. A histologically visible representation of the fingers and palm in primate area 3b and its immutability following long-term deafferentations. Cereb. Cortex. 1998;8:227–236. doi: 10.1093/cercor/8.3.227. [DOI] [PubMed] [Google Scholar]
- Jain N, Qi H-X, Catania K.C, Kaas J.H. Anatomical correlates of the face and oral cavity representations in somatosensory area 3b of monkeys. J. Comp. Neurol. 2001;429:455–468. doi: 10.1002/1096-9861(20010115)429:3<455::aid-cne7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Jones E.G. Plenum Press; New York: 1985. The thalamus. [Google Scholar]
- Kaas J.H. What, if anything, is SI? The organization of the “first somatosensory area” of cortex. Physiol. Rev. 1983;63:206–231. doi: 10.1152/physrev.1983.63.1.206. [DOI] [PubMed] [Google Scholar]
- Kaas J.H. The evolution of complex sensory systems in mammals. J. Exp. Biol. 1989;146:165–176. doi: 10.1242/jeb.146.1.165. [DOI] [PubMed] [Google Scholar]
- Kaas J.H. Evolution of multiple areas and modules within neocortex. Perspect. Dev. Neurobiol. 1993;1:101–107. [PubMed] [Google Scholar]
- Kaas J.H. The evolution of isocortex. Brain Behav. Evol. 1995;46:187–196. doi: 10.1159/000113273. [DOI] [PubMed] [Google Scholar]
- Kaas J.H. Convergences in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behav. Evol. 2002;59:262–272. doi: 10.1159/000063563. [DOI] [PubMed] [Google Scholar]
- Kaas J.H. Early visual areas: V1, V2, V3, DM, DL, and MT. In: Kaas J.H, Collins C.E, editors. The primate visual system. CRC Press; Boca Raton: 2004a. [Google Scholar]
- Kaas J.H. The evolution of the large, complex sensorimotor systems of anthropoid primates. Int. J. Comp. Psychol. 2004b;17:34–52. [Google Scholar]
- Kaas J.H. Somatosensory system. In: Paxinos G, Mai J.K, editors. The human nervous system. 2nd edn. Elsevier Academic Press; New York: 2004c. pp. 1059–1092. [Google Scholar]
- Kaas J.H, Catania K.C. How do features of sensory representations develop? BioEssays. 2002;24:334–343. doi: 10.1002/bies.10076. [DOI] [PubMed] [Google Scholar]
- Kaas J.H, Hackett T.A. Subdivisions of auditory cortex and processing streams in primates. Proc. Natl Acad. Sci. USA. 2000;97:11 793–11 799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J.H, Lyon D.C. Visual cortex organization in primates: theories of V3 and adjoining visual areas. Prog. Brain Res. 2001;134:285–295. doi: 10.1016/s0079-6123(01)34019-0. [DOI] [PubMed] [Google Scholar]
- Kaas J.H, Nelson R.J, Sur M, Lin C.S, Merzenich M.M. Multiple representations of the body within the primary somatosensory cortex of primates. Science. 1979;204:521–523. doi: 10.1126/science.107591. [DOI] [PubMed] [Google Scholar]
- Knight P.L. Representation of the cochlea within the anterior auditory field (AAF) of the cat. Brain Res. 1977;130:447–467. doi: 10.1016/0006-8993(77)90108-1. [DOI] [PubMed] [Google Scholar]
- Konorski M.D. University of Chicago Press; Chicago: 1967. Integrative activity of the brain. [Google Scholar]
- Krubitzer L.A, Kaas J.H. The organization and connections of somatosensory cortex in marmosets. J. Neurosci. 1990;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L.A, Manger P, Pettigrew J, Calford M. The organization of somatosensory cortex in monotremes: in search of the prototypical plan. J. Comp. Neurol. 1995;351:261–306. doi: 10.1002/cne.903510206. [DOI] [PubMed] [Google Scholar]
- Lin C.S, Weller R.E, Kaas J.H. Cortical connections of striate cortex in the owl monkey. J. Comp. Neurol. 1982;211:165–176. doi: 10.1002/cne.902110206. [DOI] [PubMed] [Google Scholar]
- Luethke L.E, Krubitzer L.A, Kaas J.H. Cortical connections of electrophysiologically and architectonically defined subdivisions of auditory cortex in squirrels. J. Comp. Neurol. 1988;268:181–203. doi: 10.1002/cne.902680205. [DOI] [PubMed] [Google Scholar]
- Lyon D.C, Kaas J.H. Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. J. Neurosci. 2001;21:249–261. doi: 10.1523/JNEUROSCI.21-01-00249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon D.C, Kaas J.H. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in the prosimian primate, Galago garnetti. Brain Behav. Evol. 2002a;59:114–129. doi: 10.1159/000064159. [DOI] [PubMed] [Google Scholar]
- Lyon D.C, Kaas J.H. Evidence for a modified V3 with dorsal and ventral halves in macaque monkeys. Neuron. 2002b;33:453–461. doi: 10.1016/s0896-6273(02)00580-9. [DOI] [PubMed] [Google Scholar]
- Lyon D.C, Kaas J.H. Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of New World monkeys. J. Comp. Neurol. 2002c;449:281–297. doi: 10.1002/cne.10297. [DOI] [PubMed] [Google Scholar]
- McCulloch W.S. Functional organization of cerebral cortex. Physiol. Rev. 1944;24:390–407. [Google Scholar]
- Merzenich M.M, Brugge J.F. Representation of the cochlear partition on the superior temporal plane of the macaque monkey. Brain Res. 1973;50:275–296. doi: 10.1016/0006-8993(73)90731-2. [DOI] [PubMed] [Google Scholar]
- Merzenich M.M, Knight P.L, Roth G.L. Representation of cochlea within primary auditory cortex in the cat. J. Neurophysiol. 1975;38:231–249. doi: 10.1152/jn.1975.38.2.231. [DOI] [PubMed] [Google Scholar]
- Merzenich M.M, Kaas J.H, Sur M, Lin C.S. Double representation of the body surface within cytoarchitectonic areas 3b and 1 in “SI” in the owl monkey (Aotus trivirgatus) J. Comp. Neurol. 1978;181:41–73. doi: 10.1002/cne.901810104. [DOI] [PubMed] [Google Scholar]
- Morel A, Imig T.J. Thalamic projections to fields A, AI, P, and VP in the cat auditory cortex. J. Comp. Neurol. 1987;265:119–144. doi: 10.1002/cne.902650109. [DOI] [PubMed] [Google Scholar]
- Morel A, Kaas J.H. Subdivisions and connections of auditory cortex in owl monkeys. J. Comp. Neurol. 1992;318:27–63. doi: 10.1002/cne.903180104. [DOI] [PubMed] [Google Scholar]
- Munk H. Of the visual area of the cerebral cortex, and its relation to eye movements. Brain. 1890;13:45. [Google Scholar]
- Nakamura H, Le W.R, Wakita M, Mikami A, Itoh K. Projections from the cytochrome oxidase modules of visual area V2 to the ventral posterior area in the macaque. Exp. Brain Res. 2004;155:102–110. doi: 10.1007/s00221-003-1698-8. [DOI] [PubMed] [Google Scholar]
- Newsome W.T, Allman J.M. Interhemispheric connections of visual cortex in the owl monkey Aotus trivirgatus, and the bushbaby, Galago senegalensis. J. Comp. Neurol. 1980;194:209–233. doi: 10.1002/cne.901940111. [DOI] [PubMed] [Google Scholar]
- Newsome W.T, Maunsell J.H.R, Van Essen D.C. Ventral visual area of the macaque: visual topography and area boundaries. J. Comp. Neurol. 1986;252:139–153. doi: 10.1002/cne.902520202. [DOI] [PubMed] [Google Scholar]
- Novacek M.J. Mammalian phylogeny: shaking the tree. Nature. 1992;356:121–125. doi: 10.1038/356121a0. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Ito S.I, Nomura T. Oral cavity representation at the frontal operculum of macaque monkeys. Neurosci. Res. 1989;6:283–298. doi: 10.1016/0168-0102(89)90021-7. [DOI] [PubMed] [Google Scholar]
- Olavarria J.F, Van Essen D.C. The global pattern of cytochrome oxidase stripes in visual area V2 of the macaque monkey. Cereb. Cortex. 1997;7:395–404. doi: 10.1093/cercor/7.5.395. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C.R, Scott T.R, Smith-Swintosky V.L. Gustatory neural coding in the monkey cortex: the quality of sweetness. J. Neurophysiol. 1993;69:482–493. doi: 10.1152/jn.1993.69.2.482. [DOI] [PubMed] [Google Scholar]
- Preuss T.M. Taking the measure of diversity: comparative alternatives to the model-animal paradigm in cortical neuroscience. Brain Behav. Evol. 2000;55:287–299. doi: 10.1159/000006664. [DOI] [PubMed] [Google Scholar]
- Pritchard T.C, Norgren R. Gustatory system. In: Paxinos G, Mai J.K, editors. The human nervous system. Elsevier Press; New York: 2004. pp. 1171–1198. [Google Scholar]
- Pritchard T.C, Hamilton R.B, Morse J, Norgren R. Projections from thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J. Comp. Neurol. 1986;244:213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Pritchard T.C, Hamilton R.B, Norgren R. Neural coding of gustatory information in the thalmus of Macaca mulatta. J. Neurophysiol. 1989;61:1–14. doi: 10.1152/jn.1989.61.1.1. [DOI] [PubMed] [Google Scholar]
- Qi H.-X, Kaas J.H. Myelin stains reveal an anatomical framework for the representation of the digits in somatosensory area 3b of macaque monkeys. J. Comp. Neurol. 2004;477:172–187. doi: 10.1002/cne.20247. [DOI] [PubMed] [Google Scholar]
- Qi H.-X, Lyon D.C, Kaas J.H. Cortical and thalamic connections of the parietal ventral somatosensory area in marmoset monkeys (Callithrix jacchus) J. Comp. Neurol. 2002;443:168–182. doi: 10.1002/cne.10113. [DOI] [PubMed] [Google Scholar]
- Qi H.-X, Phillips W.S, Kaas J.H. Connections of neurons in the lumbar ventral horn of the spinal cord are altered after long-standing limb loss in a macaque monkey. Somatosens. Mot. Res. 2004;21:1–11. doi: 10.1080/08990220400012588. [DOI] [PubMed] [Google Scholar]
- Reale R.A, Imig T.J. Tonotopic organization in auditory cortex of the cat. J. Comp. Neurol. 1980;192:265–291. doi: 10.1002/cne.901920207. [DOI] [PubMed] [Google Scholar]
- Redies H, Sieben U, Creutzfeldt O.D. Functional subdivisions in the auditory cortex of the guinea pig. J. Comp. Neurol. 1989;282:473–488. doi: 10.1002/cne.902820402. [DOI] [PubMed] [Google Scholar]
- Rockland K.S. Feedback connections: splitting the arrow. In: Kaas J.H, Collins C.E, editors. The primate visual system. CRC Press; Boca Raton: 2004. [Google Scholar]
- Rolls E.T. The orbitofrontal cortex and reward. Cereb. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Baylis L.L. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J. Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T, Sienkiewicz Z.J, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur. J. Neurosci. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Rolls E.T, Yaxley S, Sienkiewicz Z.J. Gustatory responses of single neurons in the orbitofrontal cortex of the macaque monkey. J. Neurophysiol. 1990;64:1055–1066. doi: 10.1152/jn.1990.64.4.1055. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Tweedale R. Visual areas in lateral and ventral extrastriate cortices of the marmoset monkey. J. Comp. Neurol. 2000;422:621–651. doi: 10.1002/1096-9861(20000710)422:4<621::aid-cne10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Gattass R, Sousa A.P.B. “Third tier” ventral extrastriate cortex in the New World monkey, Cebus apella. Exp. Brain Res. 2000;132:287–305. doi: 10.1007/s002210000344. [DOI] [PubMed] [Google Scholar]
- Salin P.-A, Bullier J. Corticocortical connections in the visual system: structure and function. Physiol. Rev. 1995;75:107–154. doi: 10.1152/physrev.1995.75.1.107. [DOI] [PubMed] [Google Scholar]
- Sally S.L, Kelly J.B. Organization of auditory cortex in the albino rat: sound frequency. J. Neurophysiol. 1988;59:1627–1638. doi: 10.1152/jn.1988.59.5.1627. [DOI] [PubMed] [Google Scholar]
- Sanides F. The architecture of the cortical taste nerve areas in squirrel monkey (Saimiri sciureus) and their relationships to insular, sensorimotor and prefrontal regions. Brain Res. 1968;8:97–124. doi: 10.1016/0006-8993(68)90174-1. [DOI] [PubMed] [Google Scholar]
- Scott T.R, Giza B.K, Yan J. Gustatory neural coding in the cortex of the alert cynomolgus macaque: the quality of bitterness. J. Neurophysiol. 1999;81:60–71. doi: 10.1152/jn.1999.81.1.60. [DOI] [PubMed] [Google Scholar]
- Sincich L.C, Adams D.L, Horton J.C. Complete flatmounting of the macaque cerebral cortex. Vis. Neurosci. 2003;20:663–686. doi: 10.1017/s0952523803206088. [DOI] [PubMed] [Google Scholar]
- Smith G.E. On the form of the brain in extinct lemurs of Madagascar, with some remarks on the affinities of the Indrisinae. Trans. Zool. Soc. Lond. 1908;18:163. [Google Scholar]
- Smith-Swintosky V.L, Plata-Salamán C.R. Gustatory neural coding in the monkey cortex: stimulus quality. J. Neurophysiol. 1991;66:1156–1165. doi: 10.1152/jn.1991.66.4.1156. [DOI] [PubMed] [Google Scholar]
- Sousa A.P.B, Piñon M.C.G, Gattass R, Rosa M.G.P. Topographic organization of cortical input to striate cortex in the Cebus monkey: a fluorescent tracer study. J. Comp. Neurol. 1991;308:665–682. doi: 10.1002/cne.903080411. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Kaas J.H. Topographic patterns of V2 cortical connections in macaque monkeys. J. Comp. Neurol. 1996;371:129–152. doi: 10.1002/(SICI)1096-9861(19960715)371:1<129::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. J. Comp. Neurol. 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- Thomas H, Tillein J, Heil P, Scheich H. Functional organization of auditory cortex in the mongolian gerbil (Meriones unguiculatus). I. Electrophysiological mapping of frequency representation and distinction of fields. Eur. J. Neurosci. 1993;5:882–897. doi: 10.1111/j.1460-9568.1993.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Tootell R.B.H, Hamilton S.L, Silverman M.S. Topography of cytochrome oxidase activity in owl monkey cortex. J. Neurosci. 1985;5:2786–2800. doi: 10.1523/JNEUROSCI.05-10-02786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C. Organization of visual areas in macaque and human cerebral cortex. In: Chalupa L.M, Werner J.S, editors. The visual neurosciences. MIT Press; Cambridge, MA: 2003. [Google Scholar]
- Van Essen D.C, Newsome W.T, Maunsell J.H.R, Bixby J.L. The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: asymmetries, areal boundaries, and patchy connections. J. Comp. Neurol. 1986;244:451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- Von Economo C. Oxford University Press; London: 1929. The cytoarchitectonics of the human cortex. [Google Scholar]
- Wallace M.N, Rutkowski R.G, Palmer A.R. Identification and localisation of auditory areas in guinea pig cortex. Exp. Brain Res. 2000;132:445–456. doi: 10.1007/s002210000362. [DOI] [PubMed] [Google Scholar]
- Weller R.E, Kaas J.H. Retinotopic patterns of connections of area 17 with visual areas V-II and MT in macaque monkeys. J. Comp. Neurol. 1983;220:253–279. doi: 10.1002/cne.902200302. [DOI] [PubMed] [Google Scholar]
- Wilson M.E. Cortico-cortical connections of the cat visual areas. J. Anat. 1968;102:375–386. [PMC free article] [PubMed] [Google Scholar]
- Woolsey C.N, Walzl E.M. Topical projection of nerve fibers from local regions of the cochlea to the cerebral cortex of the cat. Bull. Johns Hopkins Hosp. 1942;71:315–344. [Google Scholar]
- Woolsey C.N, Walzl E.M. Topical projection of the cochlea to the cerebral cortex of the monkey. Am. J. Med. Sci. 1944;207:685–686. [Google Scholar]
- Woolsey C.N, Walzl E.M. Cortical auditory area of Macaca mulatta and its relation to the second somatic sensory area (SM II) In: Woolsey C.N, editor. In Cortical sensory organization. Multiple auditory areas. Humana; New Jersey: 1982. [Google Scholar]
- Zeki S.M. Representation of central visual fields in prestriate cortex of monkey. Brain Res. 1969;14:271–291. doi: 10.1016/0006-8993(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. The third visual complex of rhesus monkey prestriate cortex. J. Physiol. 1978;277:245–272. doi: 10.1113/jphysiol.1978.sp012271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S.M. Improbable areas in color vision. In: Werner J.S, Chalupa L.M, editors. The visual neurosicences. MIT Press; Cambridge: 2004. pp. 1029–1039. [Google Scholar]