Abstract
A century on, Campbell's largely forgotten 1905 monograph on the localization of cerebral function has a distinctly contemporary feel. Although his map of cortical fields has been eclipsed by Brodmann's later contribution, Campbell's project went beyond cytoarchitectonic cartography, attempting to integrate clinical, anatomical and physiological evidence to provide a guide to function. A key component of Campbell's integrative, functional anatomical approach was hodology—the pattern of white matter connections between cortical areas—foreshadowing a recently developed functional anatomical technique: diffusion tensor tractography. Here, we revisit Campbell's model of the human visual system using tractography to illustrate prominent white matter connections within the occipital lobe and from occipital to frontal, parietal and temporal regions. Campbell used his integrative approach to support the view that vision consisted of a ‘visuo-sensory’ and a ‘visuo-psychic’ stage, combining hodological, cytoarchitectonic, physiological and clinicopathological evidence to locate the former within the calcarine cortex and the latter within the cortical field surrounding it. Speaking directly to contemporary debates surrounding the neurobiology of conscious vision and providing a framework with which to shape future developments in tractography, Campbell's integrative functional anatomical approach is as relevant today as it was 100 years ago.
Keywords: tractography, diffusion tensor imaging, occipital lobe, conscious vision
1. Introduction
I have also lying in front of me, a short paper by Prof Vogt, giving the aims of his further researches and containing some excellent microphotographic illustrations of certain types of cell-lamination, for the preparation of which, Brodmann, a worker in the same laboratory, is given credit, and it appears that Brodmann is taking over, or at any rate sharing, the cytological work in this undertaking.
(Campbell 1905, p. 9)
Campbell's prophetic words echo from one century to the next. Brodmann took over far more than Vogt's cytological work, all but wiping Campbell's own contribution to functional anatomy from history. While in contemporary anatomical circles, Brodmann's classificatory system is but one of many, in the cognitive neurosciences, his map has become the lingua franca of cortical localization—numbers used universally as shorthand for a precise cortical locus and, for some cortical fields at least, a precise functional role. Ask of Campbell and, for the most part, one is met with silence or, at least, the assumption that Brodmann both preceded and surpassed Campbell. However, such an assumption would be wrong (figure 1).
Figure 1.
Alfred Walter Campbell (1868–1937; left) and Korbinian Brodmann (1868–1918; right), exact contemporaries and rivals in history. Adapted from Haymaker & Schiller (1970).
Campbell was primarily a clinician. An Australian of Scottish descent, he came to Europe for his medical training, studying for his degree in Edinburgh with further experience in Vienna and Prague. After obtaining his doctorate with a prize-winning thesis on the pathology of alcoholic insanity, he took up a position as resident medical officer and director of the Pathology Laboratory at the Rainhill Asylum, near Liverpool (figure 2). This was a position of some prestige. At the turn of the century, asylum‐linked pathology laboratories led research into mental disease, some even publishing their own scientific journals. In 1891, the Commissioners in Lunacy, a government body responsible for asylums and reporting annually to parliament, wrote of Rainhill:
Figure 2.
The Rainhill Asylum, 1851–1992.
we must not omit to notice the new pathological-room which has been formed; and we would venture to recommend the appointment, at no distant date, of a fifth assistant medical officer, to act, more especially, as a pathologist. Such an appointment is very desirable in all large Asylums, and the additional labour cast on the medical officers by the recent Act will render additional assistance absolutely necessary, when the number of patients has also further increased.
As the chosen appointee, Campbell was to be kept busy. In the years leading up to his appointment, almost one in five of the approximately 1500 patients at Rainhill died, with 213 post-mortem examinations being carried out in 1890. Over the 13 years Campbell remained at Rainhill, the Commissioners' reports testify repeatedly to the quality of his work and to the meticulous records he kept, ‘Pathological research of the finer kind is vigorously pursued, and material stored, which should ultimately become of great scientific value’ (Report of the Commissioners in Lunacy 1896); ‘Of works in progress we may refer to the provision of what will be very excellent pathological laboratories and museum… Dr Campbell is still here as pathologist, and continues to do valuable work of research, the means of prosecuting which will be greatly enhanced by the improvement of this department…’ (Report of the Commissioners in Lunacy 1903).
Campbell had set out to understand the neural pathology of mental illness but realized his endeavours were doomed to fail without prior knowledge of the normal brain:
Particularly do I hope that those who, like myself, have pledged their energies to the apparently hopeless task of elucidating problems connected with mental disease will derive benefit from this research, for it was only after several valuable years had been spent in the cause of scientific research in a laboratory attached to an asylum for the insane that I recognized it was necessary for some worker to begin at the beginning, and attempt to piece together our disjointed knowledge of the structure of the cortex…
(Campbell 1905, p. xix)
Campbell's ‘beginning’ was to record two types of variation across the cortex, that of white matter fibre orientation (myeloarchitecture) and that of cell lamination (cytoarchitecture). By combining the two types of evidence, he was able to produce a map of distinctive cortical fields in man and other primate species and, within man, of changes in the fields related to pathology. The main part of this work was communicated to the Royal Society in November 1903, but refused publication in the Philosophical Transactions on the grounds of length (Dawson 1938; Parker 1938). Instead, a proposal was made to publish the work as a monograph, subsidized by the Royal Society, appearing in 1905 as Histological Studies on the Localisation of Cerebral Function. However, Campbell had left Rainhill and returned to Australia before the monograph was off the press (Fulton 1938).
Campbell's monograph was a monumental achievement and its eclipse by Brodmann's work seems unjust on several grounds. Brodmann worked in Berlin's prestigious neurobiological laboratory on cytoarchitectonics alone, the laboratory's myeloarchitectonic studies being carried out by the Oskar Vogt and Cécile husband and wife team (Garey 1999). In contrast, Campbell worked in comparative isolation, hidden away in the provincial asylum, his main scientific contact being with Sherrington at the University of Liverpool. Furthermore, despite the disadvantage of location and ambitious reach of his project, Campbell had clear priority over Brodmann in the human cortical mapping endeavour. Brodmann presented his earliest architectonic work in April 1903 at a conference in Jena, his main paper on human cortex published in 1908, and in his book in 1909 (Garey 1999) some 6 years after completion of Campbell's monograph. Yet it is not only the priority of publication or more ambitious scope of his work that makes Campbell's disappearance from history unjust. Campbell's and Brodmann's works differ in one key respect: the emphasis given to function. As is implicit in his title, the relationship between brain anatomy and brain function was central to Campbell's monograph, each of the chapters being devoted to a different brain region in terms of its histological anatomy and, together with clinicopathological, comparative and physiological evidence, the functional insights that could be derived from such anatomical observations. Indeed, Campbell's cortical map is labelled not by numbers but by function. Thus, we find descriptions of visuo-sensory, audito-psychic, olfactory, pre central and post central fields among the 17 he defines. In contrast, while surpassing Campbell in anatomical parcellation with more than 40 cytoarchitectonic fields, Brodmann only includes a few pages on clinical pathology and function, the main part of the text being concerned with comparative anatomy. Indeed, Brodmann admits that although his ambitions extended beyond anatomy, his book ended up as a primarily anatomical text: ‘Although my studies of localisation are based on purely anatomical considerations and were initially conceived to resolve only anatomical problems, from the outset my ultimate goal was the advancement of a theory of function and its pathological deviations’ (Brodmann 1909, p. 243). One hundred years on, Campbell's integrative approach, combining anatomical, pathological and physiological insights, resonates far more with contemporary cognitive neuroscience than Brodmann's comparative anatomy. Indeed, Campbell's use of hodology—the white matter connections of each brain region—as a guide to cortical function foreshadows an approach to the study of functional anatomy that has only recently become possible in the living brain—that of diffusion tensor tractography.
2. Diffusion tensor tractography
In 1985, a modification of conventional magnetic resonance imaging sequences permitted quantification of the diffusion characteristics of water molecules in vivo (Le Bihan & Breton 1985). Given that within cerebral white matter, water molecules diffuse more freely along myelinated tracts than across them (a property termed anisotropy of diffusion; Moseley et al. 1990), it is possible to obtain in vivo estimates of white matter fibre orientation (Basser et al. 1994). This has led to the development of diffusion tensor tractography (Conturo et al. 1999; Jones et al. 1999; Mori et al. 1999; Basser et al. 2000; Poupon et al. 2000), in which white matter tracts are reconstructed in three dimensions by sequentially piecing together discrete and shortly spaced estimates of fibre orientation to form continuous trajectories. Although these tracts are ‘virtual’, the connections being defined mathematically and not necessarily implying a true axonal pathway, the technique has been used with some success in the living human brain to study thalamic (Behrens et al. 2003), occipitotemporal (Catani et al. 2003) and other white matter tracts (Mori et al. 1999; Catani et al. 2002; Lehericy et al. 2004) and perisylvian language networks (Catani et al. 2005). While PET and fMRI give insights into the location of functionally defined cortical fields, tractography goes beyond this to reveal how such fields are connected. Although blind to whether such virtually dissected tracts are afferent, efferent or mixed, the technique provides a powerful tool with which to study hodology in the living brain. Just as Campbell used the published post-mortem dissection-defined connectivity of brain regions to derive functional insights 100 years ago, tractography-defined connections can be used today as a functional guide. Below we use tractography to revisit the white matter connections of a cortical region in which Campbell held particular interest, the occipital lobe, re-examining the functional roles he advocated and looking towards future ‘Campbellesque’ integrative hodological approaches to functional anatomy.
3. Visuo-sensory and visuo-psychic cortex
While Campbell endeavoured to map the entire cerebral cortex, three areas interested him in particular: the pre central motor area, the post central sensory area and the visual cortex. For each of these areas, he presented data from specific pathological models (amyotrophic lateral sclerosis, tabes dorsalis and longstanding blindness, respectively) to illustrate points of histological interest and to compare with the normal brain. However, while his work in defining the histological limits of motor, pre motor and somato sensory cortex have long passed into textbooks as unattributed fact, his apportioning of function to two distinct visual cortical areas remains as contested today as it was when first put forward. Campbell's work on vision is closely linked to that of J. S. Bolton, later authors often giving credit equally to both using the attribution ‘Bolton–Campbell’ to describe their anatomical findings. Bolton had worked on the histology of the human visual cortex under Campbell at Rainhill in 1896, continuing his studies in Birmingham and the Claybury Asylum in London. Bolton's visual work was published in the Philosophical Transactions of the Royal Society in 1900, 3 years before the early draft of Campbell's monograph was first communicated to the Royal Society. Time spent with Campbell clearly influenced Bolton and his indebtedness to Campbell was mentioned twice in the acknowledgements section of his paper. In his monograph 5 years later, Campbell refers to Bolton's paper as the definitive histological study of vision to date, naming sulcal features at the occipital pole as the superior and inferior polar fissure of Bolton. However, as will be seen below, while honouring Bolton with one hand, Campbell took pains to point out Bolton's failures with the other.
The neurobiology of vision and visual representation was an issue central to nineteenth century neuroscience, the published literature on clinicopathological correlations and physiology already so large at the time of writing that neither Bolton nor Campbell attempted to review the studies in any depth. Campbell wrote that ‘…there is such a wealth of literature on this subject that a complete retrospect is out of the question’. (Campbell 1905, p. 132); Bolton, less kindly, wrote that, ‘The immense quantity of literature which has been published on the clinico-pathological aspect of this subject renders it impossible to give even a brief résumé of all the facts adduced, but fortunately a considerable amount is relatively useless…’ (Bolton 1900, p. 177). Both Campbell and Bolton inherited the view that vision was a bipartite process—a visuo-sensory stage and a visuo-psychic stage. Campbell wrote,
The term visuo-psychic is of course not of new coinage; it has been employed by many writers in contradistinction to the term visuo-sensory. The employment of such distinguishing terms is rendered necessary because the pathological, the experimental, and the anatomical and embryological researches which have been undertaken to determine the precise regions of the cortex in which visual sensations are centred and elaborated, afford grounds for believing that cortical visual representation is of twofold character; that it consists of a primary station in each hemisphere for the direct reception of impressions derived from homonymous halves of both retinal fields—a centre known by the name visuo-sensory and definitely proved to exist—and a secondary centre which probably serves for the further treatment of the rough impressions, a centre which is probably of psychic nature and hence has been called visuo-psychic. Although this centre, unlike the visuo-sensory, is doubtfully existent and doubtfully located, yet the distinctive histological structure of the field of cortex, which I am about to describe, strongly suggests that it possesses a special physiological function.
(Campbell 1905, p. 125)
Campbell's reference to a doubt concerning the visuo-psychic field was a veiled criticism of Bolton's review in which visuo-psychic cortex had been given the double indignity of being ‘…doubtfully existent and doubtfully located in the angular gyri’ (Bolton 1900, p. 174). A more explicit criticism was made of Bolton in his failure to describe the limits of the visuo-psychic field. ‘Also it is disappointing to find that Dr Bolton says so little about the visuo-psychic area, for, although he clearly recognises and indeed accurately figures the type of cell lamination in the parts bordering on the visuo-sensory area, he makes no attempt to define the limits of the field bearing this type…’ (Campbell 1905, p. 143). The location and extent of Campbell's visuo-sensory and visuo-psychic fields in the human brain are shown in figure 3. The visuo-sensory field corresponds to Brodmann's area 17, the visuo-psychic field encompassing Brodmann's areas 18 and 19. Campbell concluded his defence of a visuo-psychic centre by stating: ‘…its close relation to the visuo-sensory or calcarine area, and other points to which I shall have occasion to refer, suggest in equal measure that it is the cortical centre for psychic visual representation’ (Campbell 1905, p. 125). The ‘other points’ referred to were hodological: the white matter connections of the two centres he had identified histologically and the afferent or efferent direction of these connections.
Figure 3.
Campbell's visuo-sensory and visuo-psychic fields in the human brain. The darker hatching shows the visuo-sensory field and its relation to the calcarine fissure. The lighter dots show the visuo-psychic field that surrounds visuo-sensory cortex. The field is limited by the parieto-occipital sulcus superiorly (PO) and the ramus occipitalis transversus (R Occ T), laterally. Campbell honoured his former colleague J. S. Bolton in P and X by naming these sulcal features the superior and inferior polar fissures of Bolton. Col, collateral sulcus; LO, lateral occipital fissure of Eberstaller; Stem, stem of calcarine fissure; CM, calloso-marginal fissure; Sy, Sylvan [sic] fissure. Adapted from Campbell (1905; fig. 11).
4. The white matter connections of the occipital lobe
Following previous descriptions, Campbell recognized three main classes of occipital white matter connections: the optic radiation, autochthonous fibres (short association or U-shaped fibres connecting adjacent occipital gyri) and long association fibres connecting occipital with other brain regions. As he acknowledged, details of the cortical origin and destination of these fibre tracts was largely unknown at the time, a situation that has changed little over the intervening century.
(a) The optic radiation
On gross anatomical dissection, a large swath of fibres connects the thalamus with the occipital lobe, the radiation of Gratiolet. Citing Flechsig's work on embryological myelination, Campbell argued that the radiation, while appearing homogeneous, consisted of two distinct pathways. Flechsig had shown that within the radiation of Gratiolet, a subset of fibres, myelinated at birth, connected the LGN to the calcarine cortex, a pathway referred to as the ‘narrowly defined’ optic radiation. The remaining fibres of the radiation of Gratiolet were myelinated postnatally and connected the extra-calcarine cortex with the pulvinar (referred to as the optic radiation in its broader sense). Flechsig had hypothesized that the former fibres were cortico-petal (passing from LGN to calcarine cortex), while the latter were cortico-fugal, (passing from extra calcarine regions to the pulvinar). Campbell added weight to this hypothesis with his own myeloarchitectonic observations of fibre orientation. He showed that fibres in the narrowly defined optic radiation pursued a curious oblique course in calcarine cortex. Comparing this myeloarchitectonic pattern to that found in the post central gyrus and transverse temporal regions (the cortical stations for tactile and auditory sensory modalities, respectively), Campbell inferred that this oblique myelination pattern implied a cortico-petal function. In contrast, the broader sense radiation fibres of Gratiolet connected to the visuo-psychic field pursued a straight course within the visuo-psychic cortex, a feature shared with the projection fibres of motor cortex and, hence, suggestive of a cortico-fugal direction.
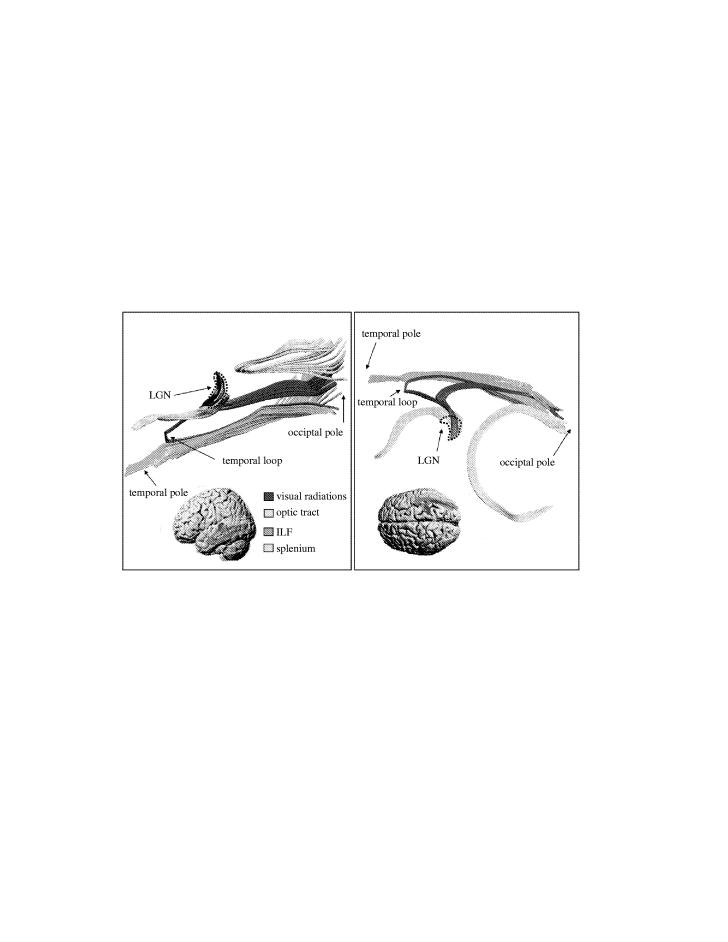
Figure 4 shows the optic radiation fibres in its narrow sense as identified by tractography together with its relations to splenial fibres of the corpus callosum and those of the long association fibres of the ILF. As fibres from the optic nerve leave the chiasm (not shown in the figure) and enter the optic tract (shown in blue), they appear like an italic s-shape and terminate in the anteroventral portion of the LGN. With the image resolution employed in this study, the voxel-averaged anisotropy of grey matter is low and hence the LGN is not visualized directly. However, by following the distribution of terminating fibres, it is possible to define approximately the shape of the area corresponding to the LGN. This bean-shaped space has been defined by the dotted line. The fibres of the optic tract enter the LGN anteroventrally while the fibres of the optic radiation leave the LGN from its posterior dorsolateral surface. The optic radiation fibres divide into two bundles: a smaller temporal bundle and a larger dorsal bundle. The temporal bundle originates from more ventral portions of the LGN. Its fibres pass below those of the dorsal bundle and project forwards and laterally towards the temporal pole. After a short run, the bundle describes a sharp arc around the temporal horn of the lateral ventricle and continues backward towards the occipital pole where it terminates in the lower calcarine lip. The dorsal bundle originates from more dorsal portions of the LGN. Its fibres leave the LGN and assemble into a thick, compact lamina, after a short lateral course, bending posteriorly towards the occipital pole and terminating in the upper calcarine lip (see Catani et al. 2003 for further detail).
Figure 4.
The narrow sense optic radiation. Two views of the right hemispheric radiation fibres (red) are displayed, the left panel as viewed from the medial surface of the hemisphere, the right panel as viewed from above. Also displayed are optic tract (blue) and splenial fibres (yellow) and long associative fibres of the inferior longitudinal fasciculus (ILF; green). Insets show the relation of fibre tracts to the brain surface. The virtual dissections were performed on an average brain derived from the diffusion tensor images of 11 normal subjects. Adapted from Catani et al. (2003).
(b) Autochthonous connections
Campbell referred to three distinct classes of occipital U-shaped association fibre bundles, variously named by previous authors. Although he was confident in the existence of fibres passing between the calcarine and visuo-psychic fields (e.g. from the upper lip of the calcarine to the cuneus), he was less certain of fibres that had been described as passing between upper and lower banks of the calcarine, of which he wrote: ‘…I cannot help thinking that the appearance is due mainly to the presence of the terminal portions of the calcarine division of the optic radiations arching up to the cortex’ (Campbell 1905, p. 141). Similarly, of vertical fibre bundles connecting dorsal with ventral portions of the occipital lobe, he wrote, ‘…as a mater of fact the definition of this band is difficult, and we cannot attach much importance to it’ (Campbell 1905, p. 142).
Autochthonous fibres, although easy to dissect by tractography, pose the same classificatory challenge today as they did for Campbell. Figure 5 shows a series of U-shaped bundles from gyrus to gyrus running superficially to the long associative bundle of the ILF. Such serial links can be traced from the occipital lobe to the anterior temporal lobe, forming an indirect occipitotemporal projection system running parallel to the direct occipitotemporal connections of the ILF (Catani et al. 2003). An equivalent sequence of U-shaped connections can be traced from occipital to parietal lobes and beyond (figure 6), although it is unclear whether a direct long associative bundle equivalent to the ILF connects occipital to parietal lobes. In the monkey brain, the occipital cortex is linked to temporal and parietal cortex through multiple, parallel, cortico-cortical pathways (Mishkin et al. 1983; Van Essen & Maunsell 1983; Zeki & Shipp 1988; Felleman & Van Essen 1991). The collection of tractography-defined pathways to the temporal lobe seem to constitute the human equivalent of ventrally projecting pathways in the monkey, while those to the parietal lobe and beyond seem to constitute the human equivalent of monkey dorsally projecting pathways. Whether the indirect, serial U fibre portions of these tractography-defined pathways can be traced to calcarine cortex is, at present, unclear. It is also uncertain whether a separate U-shaped bundle encompasses the calcarine fissure since, as noted by Campbell, the tractography-defined terminal portions of the temporal and dorsal bundles of the optic radiation arch around calcarine cortex (see blue arrow in the bottom panel of figure 8). A further issue yet to be resolved is the existence of the vertical intra lobar autochthonous connections questioned by Campbell. If found, their significance will go beyond the minimal importance he attached to them (see above) as their implied function would be the direct inter-connection of dorsally and ventrally projecting parallel pathways.
Figure 5.
U-shaped autochthonous fibres. The U-shaped projection system (red) of the right hemisphere as viewed from above. Only that portion of the system projecting to the temporal lobe is shown, together with the inferior longitudinal fasciculus. The virtual dissections were performed on the brain of a single individual. Adapted from Catani et al. (2003).
Figure 6.

The dorsally and ventrally projecting U-shaped fibre systems. Chains of fibres passing to the temporal lobe (ventral pathways, red) and parietal lobe and beyond (dorsal pathways, green) in the left hemisphere are shown, viewed from the lateral surface of the hemisphere. The fibres are co-registered with a parasagittal slice through the diffusion tensor MR volume from which the tracts were derived. The virtual dissections were performed on the brain of a single individual.
Figure 8.

The inferior fronto-occipital tract. The inferior fronto-occipital fibres of the right hemisphere (yellow) are shown as viewed from above (top panel) and from the medial surface of the hemisphere (middle panel). Also displayed are fibres of the optic radiation (red) and inferior longitudinal fasciculus (green). The bottom panel shows the fibres co-registered with a coronal slice through the diffusion tensor MR volume as viewed from the occipital pole. Fibres passing through the co-registered slice are not visible. The position of the slice along the tracts is given by the dotted black line. The blue arrow indicates the approximate position of the calcarine fissure. The virtual dissections were performed on the brain of a single individual.
(c) Long association fibres
Based on previous anatomical descriptions, Campbell recognized three main fibre tracts connecting the occipital lobe to other brain regions within the same hemisphere: the superior longitudinal or arcuate fasciculus, the ILF and the occipitofrontal fasciculus (referred to as the inferior fronto-occipital fasciculus by later authors). Campbell argued that the superior longitudinal/arcuate fasciculus, although predominantly consisting of fibres connecting temporal auditory cortex to Broca's convolution, also carried fibres connecting the occipital and frontal lobes. However, as the fasciculus constantly gave off and received fibres, he acknowledged that it was difficult to be certain of its connections. The ILF was thought to connect occipital and temporal lobes, although Campbell mentions that, in anterior portions, its fibres became confused with those emerging from the geniculate bodies and thalamus, anticipating later debates as to its existence as a bundle distinct from the temporal loop of the optic radiation (Putnam 1926; Polyak 1957; Tusa & Ungerleider 1985). The last of Campbell's long associative bundles—the inferior fronto-occipital fasciculus—was, at the time, the most controversial, being thought to consist of mislabelled callosal fibres by some authors and mislabelled arcuate fasciculus fibres by others. Campbell followed Dejerine and argued that the bundle was distinct from both callosum and arcuate fasciculus and connected the frontal lobe with the lateral surface and inferior border of the occipital lobe.
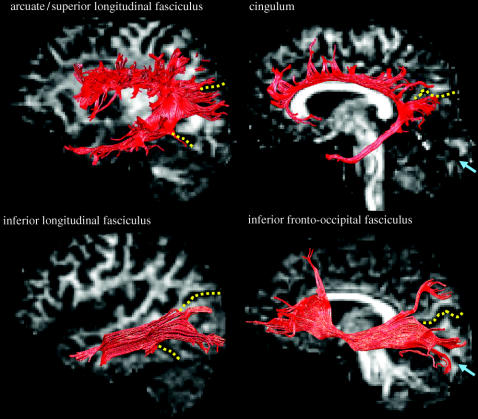
Campbell's long associative fibre bundles as defined by tractography are shown in figure 7, together with the cingulum which, although not mentioned by Campbell, carries fibres from anteromedial portions of the occipital lobe to limbic cortex. The superior longitudinal fasciculus consists of multiple fibre pathways lying in close anatomical relation for part of their trajectory. As held by Campbell, a subset of these fibres connects occipital with other brain regions, although the relation of these fibres to the other pathways in the bundle and their projection territory has yet to be established. A fibre bundle distinct from that of the temporal loop of the optic radiation (see figure 4 for their anatomical relations) connects posterior occipital with anterior temporal regions, corresponding to classical descriptions of the ILF (Catani et al. 2003). The occipital branches of the ILF arise in extra-striate cortical regions on the dorsolateral surface of the occipital lobe, ventromedially from the posterior lingual gyrus and fusiform gyri and dorsomedially from the cuneus. The branches run forward, parallel to the fibres of the splenium and optic radiation and, at the level of the posterior horn of the lateral ventricle, gather in a single bundle. In the anterior temporal lobe, the branches pass to the superior, middle and inferior temporal gyri on the lateral surface of the temporal lobe and medially to the parahippocampal gyrus, hippocampus and amygdala. An observation emphasized by Campbell, and since confirmed by tractography, is that long associative fibres such as those of the ILF arise from the ‘visuo-psychic’ cortex but not calcarine, ‘visuo-sensory’ cortex. As shown in figure 8, the inferior fronto-occipital fasciculus runs lateral to the narrowly defined optic radiation and medially to the ILF. As held by Campbell, it has connections to the inferior and lateral margins of the occipital lobe and to a circumscribed infero- and dorsolateral region of the frontal lobe, passing through the temporal lobe and lying in close relation to the uncinate fasciculus at the fronto-temporal junction (Catani et al. 2002).
Figure 7.
Long associative fibre bundles. Four long associative tracts in the left hemisphere are shown. The tracts are superimposed on sagittal and parasagittal slices through the opposite hemisphere, at a homologous location to the displayed tract. The yellow dotted lines indicate the approximate boundary of Campbell's visuo-psychic cortex; the blue arrows indicate the calcarine fissure. The virtual dissections were performed on the brain of a single individual. Adapted from Catani et al. (2002).
5. Campbell's hodological guide to visual function
Campbell saw the pattern of connectivity between brain regions as an important clue to function. Having inferred the cortico-petal or cortico-fugal nature of specific fibre bundles through a consideration of their myeloarchitectonic signature, he derived a simple model of occipital connectivity. He reasoned that fibres from the LGN passed to the calcarine cortex (the narrowly defined optic radiation) and from there, through autochthonous fibres, to the cortex surrounding it. From this surround, fibres passed back to the pulvinar, (the non geniculo-calcarine portion of the radiation of Gratiolet), to the temporal lobe (the ILF) and the frontal lobe (the arcuate and inferior fronto-occipital fasciculus). For Campbell, the functional implication of the connectivity pattern was clear:
…my own observations have definitely proved that immediately investing the visuo-sensory area there exists a moderately extensive field of cortex, possessing a specialized type of arrangement of nerve cells and nerve fibres, entirely different both from that in the visuo-sensory area and that in the more outlying parts: and granted that the calcarine area is solely devoted to the reception of primary visual stimuli, the mere existence of a second area placed in such immediate contiguity suggests the likelihood that it is concerned with the sorting out and further elaboration of these stimuli. More than this, the arrangement of fibres in this investing area suggests that they carry corticofugal instead of corticopetal impressions, that they are fibres, in other words, which combine to form the strands joining the visual with other centres and helping to make the visual function so complex.
(Campbell 1905, p. 146)
If visual impressions were received in calcarine cortex, the area to which it sent its outputs was likely to be engaged in ‘visuo-psychic’ elaboration and intellectual interpretation, with further, final complexity added by projections to other brain regions.
(a) Visual psychic cortex in the twenty first century
How has Campbell's parcellation of the occipital lobe and his hodologically based model of visual function fared in the twentieth century? Physiological and anatomical studies of monkey visual cortex over its latter half revealed a far greater degree of parcellation than suspected by either Campbell or Brodmann, Brodmann's area 18 alone encompassing areas V2, V3, V3A and V4 (Zeki 1978). This subdivision of cytoarchitectonically defined cortex in the monkey has been confirmed in man with the advent of non invasive functional imaging. Campbell's visuo-psychic cortex (or in Brodmann's terms, areas 18 and 19) contains, at the very least, V2, V3, V3A (Tootell et al. 1998), V4 (the human colour centre; Zeki et al. 1991; Mckeefry & Zeki 1997), and a region of the lateral occipital surface (Malach et al. 1995; Grill-Spector et al. 1998a, b), whose exact function has yet to be determined but which plays a role in object and object part processing. The list is unlikely to be complete. However, advances in our understanding of visual cortical architecture in monkey and man have not progressed hand in hand with advances in our understanding of visual white matter anatomy. This is perhaps surprising as the cortico-cortical connecting fibres which constitute white matter, lie at the heart of our current neurobiological models of the visual system. Such models (e.g. those of Van Essen & Maunsell 1983; Zeki & Shipp 1988; Felleman & Van Essen 1991) use evidence derived from tract tracing studies in the monkey to rank each visual area with respect to another. Thus, an area A is ranked higher than B if the cortico-cortical connections from B to A terminate predominantly in cortical layer 4. Conversely, A is ranked lower than B if the connections from B to A terminate predominantly outside cortical layer 4. Although much emphasis is placed on the cortical layering of cortico-cortical fibre origin and termination points in such models, little attention has been paid to the trajectory of the fibres within white matter. In the human brain, such hodological data are almost entirely absent, our knowledge of cortico-cortical connections and their white matter pathways being much as it was when Campbell wrote his monograph.
What of Campbell's attribution of visual psychic function to the mantle of cortex surrounding his visuo-sensory field? In assessing how this attribution has fared we must first consider what he meant by the ‘visual psyche’. In one sense, the meaning is clear—it refers to the visual mind, the psychic elaboration of visual impressions, a function that today, we might refer to as conscious vision. Indeed, Campbell used the same clinical conditions to illustrate deficits of the visual psyche as are used today as examples of deficits of visual consciousness: visual object agnosia (a deficit of conscious object perception), hemiachromatopsia (a deficit of conscious colour perception) and alexia (in the context of the preserved ability to write, a deficit of the conscious perception of orthography; Zeki & Bartels 1999; ffytche et al. 2004). However, one must exercise caution in translating Campbell's terminology from one century to the next. Campbell never referred to visual psychic cortex as conscious, although does make brief reference to the neural substrate of consciousness with respect to tactile sensation elsewhere in the monograph: ‘…reasoning on lines suggested by Hughlings Jackson's master mind, it may be contended that common sensation is first represented in cortex of the “post-central” area proper, and that after being re-represented in the “intermediate post-central” area it has a still further representation in the physical basis of consciousness, and this may be situated in the “parietal” area’ (Campbell 1905, p. 206).
Whether Campbell intended to imply a conscious correlate within his visuo-psychic field thus remains unclear and, for much of the last century, the issue held little interest. Brodmann, for one, was disdainful of psychic centres in general, let alone in the visual system:
…just as untenable as the idea of a ‘concept cell’ or an ‘association layer’ is the assumption of specific ‘higher-order psychic centres’. Indeed recently theories have abounded which, like phrenology, attempt to localise complex mental activity such as memory, will, fantasy, intelligence, or spatial qualities such as appreciation of shape and position to circumscribed cortical zones. Older authors such as Goltz, Rieger, Wundt and recently, particularly outspokenly, Semon, have already quite rightly expressed their opposition to such a ‘naive view’ and pleaded simple psychological facts against it.
(Brodmann 1909, p. 254)
Despite his own cytoarchitectonic evidence for localized specialization, Brodmann held higher functions to be the result of widespread brain activity:
In reality there is only one psychic centre: the brain as a whole with all its organs activated for every complex psychic event, either all together or most at the same time, and so widespread over the different parts of the cortical surface that one can never justify any separate specially differentiated ‘psychic’ centres within this whole.
(Brodmann 1909 p. 255)
Views such as Brodmann's led to the belief that ‘consciousness’ was not respectable neuroscience, studies of ‘unconscious’ visual abilities such as subliminal visual perception (originating in the 1960s; e.g. Shevrin & Fritzler 1968) and blindsight (originating in the 1970s; Sanders et al. 1974), being as close to ‘consciousness’ as conventional neuroscience dared to tread. However, the advent of functional imaging reopened interest in consciousness, particularly in relation to vision. For the most part, this renaissance has held a unanimity of voice: that Campbell and nineteenth century neuroscience, if it could be said to have had a theory of conscious vision, was wrong; that visual consciousness was located outside the occipital lobe, well beyond visuo-psychic cortex. Yet the strength of the unanimity of voice has been somewhat tempered by a lack of agreement among proponents of ‘extra-occipital’ theories as to where the location of visual consciousness might be. Different authors have placed it in often mutually exclusive locations ranging from dorsolateral prefrontal cortex (Sahraie et al. 1997), the ventral visual pathways (Goodale & Milner 1992; Sheinberg & Logothetis 1997; Milner 1998) and a distributed network of areas including frontal and parietal lobes (Lumer et al. 1998; Dehaene et al. 2001; Dehaene et al. 2003). Echoing Brodmann, others consider consciousness a widely distributed process involving, for example, dynamic interactions within the thalamocortical system (Tononi & Edelman 1998).
As the extra-occipital debate continues, one view stands apart as the conceptual heir to Campbell's visual psychic cortex, a view in which each visual area makes explicit contribution to conscious vision without the necessary requirement of further processing in higher, or lower, brain regions: the theory of micro consciousness (Zeki & Bartels 1999; Zeki 2001). That each specialized visual area contributes directly to conscious vision seems, at first, an unlikely possibility. Surely conscious perception, the apotheosis of visual faculties, requires complex processing and elaboration beyond that available within individual early visual areas? Yet unlikely as the micro conscious view might seem, a weight of evidence suggests it to be correct in a diverse spectrum of normal and pathological perceptual contexts. When we ‘see’ a visual stimulus (‘seeing’ implying a conscious visual perceptual experience), the level of activity within cortex specialized for the attribute perceived increases. This simple relationship was first described for the illusory Kanizsa triangle stimulus (ffytche & Zeki 1996) but has been best characterized for motion perception in studies of patient GY (Zeki & ffytche 1998), a patient with a lesion of left ‘visuo-sensory’ cortex able to consciously perceive certain motion stimuli in his otherwise blind right hemifield. More recently the same association has been described in studies of object and face perception using binocular fusion (Moutoussis & Zeki 2002) and in coloured-hearing synaesthetes, otherwise normal subjects with conscious perceptual experiences of specific colours when hearing specific words (Nunn et al. 2002). However, perhaps the most compelling demonstration of the relationship between activity within individual cortical areas and conscious visual perception is derived from studies of patients with visual hallucinations (ffytche et al. 1998). Here, spontaneous increases in cortical activity lead directly to conscious visual perceptual experiences, with the content of a given percept being determined by the location of the underlying pathological activity. Of difficulty for extra-occipital theories is the fact that whether in hallucinating patients, GY during conscious motion perception, or the perception of illusory figures, the activity correlating with each of these conscious perceptual experiences lies entirely within the occipital lobe. In studies where extra-occipital correlates of perception have been identified, the activity seems to occur too late to contribute directly to perception, the suggestion being that such activity plays a secondary role (Pins & ffytche 2003). If Campbell did indeed hold the view that conscious visual experiences were linked to visuo-psychic cortex, he would have found much of interest in the theory of micro consciousness.
(b) Hodology and the future of functional anatomy
While still in its infancy, tractography offers much future promise. Although issues such as the anatomical validity of the tracts defined and technical problems such as how to deal with fibre crossings need yet to be resolved, it remains the only method of in vivo fibre tracing in man. Indeed, tractography offers some advantages over blunt post-mortem dissection in that, whereas the latter requires stripping superficial layers of fibres to visualize deeper ones, destroying their anatomical relation, virtual dissection (Catani et al. 2002) leaves the brain entirely intact, the dissections and visualizations performed limited only by the patience of the digital anatomist. Although the technique is unlikely to have sufficient resolution to identify individual fibres and cannot ascertain the direction of specific fibre tracts, our current ignorance of white matter anatomy is such that insights gained from tractography dissections are likely to be of use for some time to come. However, as recognized by Campbell, the hodological anatomy itself is simply a means to an end. The strength of tractography is in providing insights into the function of specific areas through the pattern of their connections, insights to be placed alongside those derived from functional imaging and clinical data. The future of tractography lies here, in its integration with other imaging techniques. The results of fMRI, PET or EEG studies can be interpreted with an added layer of complexity if combined with tractography evidence, as can behavioural performance data from normal subjects or pathological cases.
Such integrative approaches are already paying dividends in studies of the visual psyche. Consider, for example, the pattern of brain activity during the very simplest of perceptual tasks, the perception of a grating. In fMRI studies, such stimuli activate several regions in the occipital lobe and, of particular interest here, the frontal operculum/insula (see figure 9). The activation occurs both when subjects see the stimulus and when they do not, although it is greater when the stimulus is perceived (Pins & ffytche 2003). The function of this frontal operculum activation is unclear, falling well outside the dorsolateral frontal region (Sahraie et al. 1997) or fronto-parietal networks (Lumer et al. 1998; Dehaene et al. 2001; Dehaene et al. 2003) implicated by some ‘extra-occipital’ theories as playing a role in conscious perception. However, from the temporal perspective of the evoked potential method, activity in this region is somehow linked to visual function as it exhibits a prominent positive wave, peaking some 10 ms after the prominent late negative wave in the occipital lobe (194 ms occipital negativity, 205 ms frontal positivity; see figure 9). Without the conceptual framework within which to place such EEG and fMRI evidence, these inferior frontal activations have been set aside. However, diffusion tensor tractography suggests we cannot ignore such evidence; indeed, it brings it to the fore. The massive direct projection between occipital and frontal cortex encompasses the inferior frontal region found active in fMRI and EEG studies. Under Campbell's reasoning, the connections, taken together with the functional EEG and fMRI evidence, imply an important role for this frontal region in ‘making the visual function so complex’. Exactly what this role might be and how it relates to the conscious visual psyche are questions for future study.
Figure 9.
Integrative functional hodology. Top left, the bilateral inferior frontal activity evoked by conscious percepts of a grating stimulus as revealed by fMRI (data from Pins & ffytche 2003). Top right, the inferior fronto-occipital tract of the right hemisphere (yellow), co registered on an axial slice through the diffusion tensor MR volume and viewed from above. The white arrows mark approximately corresponding locations in the fMR and diffusion tensor MR images. The bottom panel shows the electroencephalographic evoked response to the same, consciously perceived, grating stimulus (data from Pins & ffytche 2003). Electrodes are arranged as if viewed from above, Oz recording from the occiput, FP1 and FP2 from the frontal pole. The solid vertical line on each trace indicates the onset of the stimulus. Vertical and horizontal electro‐oculogram (VEOG and HEOG, respectively) traces show an absence of vertical (VEOG) and horizontal (HEOG) eye movements at the time of the evoked response, ruling out eye movement artefact as an explanation for the frontal activity. The evoked activity at one frontal and one occipital electrode has been magnified to the left of the panel, the dotted line indicating the latency of peak response over occipital cortex.
6. Conclusion
If Brodmann has largely eclipsed Campbell, it was not always so. Although undoubtedly coloured by the political zeitgeist, Lorente de Nó wrote in 1938:
The only really good cytoarchitectonic pictures are those of Campbell, who let me put in capital letters—HAS BEEN THE ONLY CYTOARCHITECTONIST WHO HAS DESCRIBED FACTS AND ONLY FACTS. The German cytoarchitectonist has mixed facts with theory in such a manner that nobody can tell where facts end and theories begin. I must state that there are perhaps no more than a dozen photographs out of hundreds in which the layers of the cortex have been properly and consistently labelled. On the other hand Campbell's ink drawings beside being good, are easily reproduced.
(Lorente de Nó 1938; see Fulton 1938 p. 566)
In one sense, the ongoing development of integrative approaches to in vivo functional anatomy, combining hodological, functional imaging and clinical evidence, continues Campbell's project. It is to be hoped that the emergence of such integrative, functional anatomical techniques will lead to a wider recognition of Campbell and reinstate for him a justly deserved position of prominence in the history of neuroscience.
Acknowledgments
The tractography studies described above and D. H. ff. were supported by the Wellcome Trust. We thank Professor W. Burke of the University of Sydney for providing biographical material on Campbell and Dr Derek Jones and Professor Rob Howard for access to the tractography software and data.
Glossary
- EEG
electroencephalography
- fMRI
functional magnetic resonance imaging
- ILF
inferior longitudinal fasciculus
- LGN
lateral geniculate nucleus
- PET
positron emission tomography
Footnotes
One contribution of 12 to a Theme Issue ‘Cerebral cartography 1905–2005’.
References
- Basser P.J, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Behrens T.E, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- Bolton J.S. The exact histological localisation of the visual area of the human cerebral cortex. Phil. Trans. R. Soc. B. 1900;193:165–222. [Google Scholar]
- Brodmann K. J.A. Barth; Leipzig: 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. (Translated and edited Laurence J. Garey 1999 London: Imperial College Press) [Google Scholar]
- Campbell A.W. Cambridge University Press; 1905. Histological studies on the localisation of cerebral function. [Google Scholar]
- Catani M, Howard R.J, Pajevic S, Jones D.K. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones D.K, Donato R, ffytche D.H. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones D.K, ffytche D.H. Perisylvian language networks of the human brain. Ann. Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. doi:10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- Conturo T.E, Lori N.F, Cull T.S, Akbudak E, Snyder A.Z, Shimony J.S, McKinstry R.C, Burton H, Raichle M.E. Tracking neuronal fiber pathways in the living human brain. Proc. Natl Acad. Sci. USA. 1999;96:10 422–10 427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W.S. Obituary A W Campbell. Med. J. Aust. 1938;1:183–184. [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan D.L, Mangin J.F, Poline J.B, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Sergent C, Changeux J.P. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc. Natl Acad. Sci. USA. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felleman D.J, Van Essen D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- ffytche D.H, Zeki S. Brain activity related to the perception of illusory contours. Neuroimage. 1996;3:104–108. doi: 10.1006/nimg.1996.0012. [DOI] [PubMed] [Google Scholar]
- ffytche D.H, Howard R.J, Brammer M.J, David A, Woodruff P, Williams S. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat. Neurosci. 1998;1:738–742. doi: 10.1038/3738. [DOI] [PubMed] [Google Scholar]
- ffytche D.H, Lappin J.M, Philpot M. Visual command hallucinations in a patient with pure alexia. J. Neurol. Neurosurg. Psychiatry. 2004;75:80–86. [PMC free article] [PubMed] [Google Scholar]
- Fulton J.F. Obituary A W Campbell. Arch. Neurol. Psychiat. Chicago. 1938;40:566–568. [Google Scholar]
- Garey L.J. Translator's introduction. In: Brodmann K, editor. 1909 Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. J. A. Barth; Leipzig: 1999. (Translated and edited Laurence J. Garey. London: Imperial College Press) [Google Scholar]
- Goodale M.A, Milner A.D. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Itzchak Y, Malach R. Cue-invariant activation in object-related areas of the human occipital lobe. Neuron. 1998;21:191–202. doi: 10.1016/s0896-6273(00)80526-7. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Edelman S, Itzchak Y, Malach R. A sequence of object-processing stages revealed by fMRI in the human occipital lobe. Hum. Brain Mapp. 1998;6:316–328. doi: 10.1002/(SICI)1097-0193(1998)6:4<316::AID-HBM9>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haymaker W, Schiller F. 2nd edn. Charles C Thomas; Springfield, IL: 1970. The founders of neurology. [Google Scholar]
- Jones D.K, Simmons A, Williams S.C, Horsfield M.A. Non-invasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn. Reson. Med. 1999;42:37–41. doi: 10.1002/(sici)1522-2594(199907)42:1<37::aid-mrm7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Breton E. Imagerie de diffusion in-vivo par résonance magnétique nucléaire. C R Acad. Sci. (Paris) 1985;301:1109–1112. [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele P.F, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim D.S. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann. Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Lorente de Nó R. Physiology of the nervous system, pp. 291–327. Oxford University Press; New York: 1938. Architectonics and structure of the cerebral cortex. [Google Scholar]
- Lumer E.D, Friston K.J, Rees G. Neural correlates of perceptual rivalry in the human brain. Science. 1998;280:1930–1934. doi: 10.1126/science.280.5371.1930. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas J.B, Benson R.R, Kwong K.K, Jiang H, Kennedy W.A, Ledden P.J, Brady T.J. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc. Natl Acad. Sci. USA. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckeefry D.J, Zeki S. The position and topography of the human colour centre as revealed by functional magnetic resonance imaging. Brain. 1997;120:2229–2242. doi: 10.1093/brain/120.12.2229. [DOI] [PubMed] [Google Scholar]
- Milner D. Streams and consciousness: visual awareness and the brain. TICS. 1998;2:25–30. doi: 10.1016/s1364-6613(97)01116-9. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider L.G, Macko K.A. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 1983;6:414–417. [Google Scholar]
- Mori S, Crain B.J, Chacko V.P, van Zijl P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Moseley M.E, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari H.S, Wendland M.F, Tsuruda J, Norman D. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology. 1990;176:439–445. doi: 10.1148/radiology.176.2.2367658. [DOI] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. The relationship between cortical activation and perception investigated with invisible stimuli. Proc. Natl Acad. Sci. USA. 2002;99:9527–9532. doi: 10.1073/pnas.142305699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn J.A, et al. Functional magnetic resonance imaging of synesthesia: activation of V4/V8 by spoken words. Nat. Neurosci. 2002;5:371–375. doi: 10.1038/nn818. [DOI] [PubMed] [Google Scholar]
- Parker L.R. Obituary A.W. Campbell. Med. J. Aust. 1938;1:181–183. [Google Scholar]
- Pins D, ffytche D.H. The neural correlates of conscious vision. Cereb. Cortex. 2003;13:461–474. doi: 10.1093/cercor/13.5.461. [DOI] [PubMed] [Google Scholar]
- Polyak S. University of Chicago Press; 1957. The vertebrate visual system. [Google Scholar]
- Poupon C, Clark C.A, Frouin V, Regis J, Bloch I, Le Bihan D, Mangin J. Regularization of diffusion-based direction maps for the tracking of brain white matter fascicles. Neuroimage. 2000;12:184–195. doi: 10.1006/nimg.2000.0607. [DOI] [PubMed] [Google Scholar]
- Putnam T.J. Studies on the central visual connections. Arch. Neurol. Psychiatry. 1926;16:566–596. [Google Scholar]
- Report of the Commissioners in Lunacy to the Lord Chancellor 1891 45, 183–185.
- Report of the Commissioners in Lunacy to the Lord Chancellor 1896 50, 277–280.
- Report of the Commissioners in Lunacy to the Lord Chancellor 1903 57, 303–305.
- Sahraie A, Weiskrantz L, Barbur J.L, Simmons A, Williams S.C, Brammer M.J. Pattern of neuronal activity associated with conscious and unconscious processing of visual signals. Proc. Natl Acad. Sci. USA. 1997;94:9406–9411. doi: 10.1073/pnas.94.17.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M.D, Warrington E.K, Marshall J, Weiskrantz L. “Blindsight”: vision in a field defect. Lancet. 1974;i:707–708. doi: 10.1016/s0140-6736(74)92907-9. [DOI] [PubMed] [Google Scholar]
- Sheinberg D.L, Logothetis N.K. The role of temporal cortical areas in perceptual organization. Proc. Natl Acad. Sci. USA. 1997;94:3408–3413. doi: 10.1073/pnas.94.7.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevrin H, Fritzler D.E. Visual evoked response correlates of unconscious mental processes. Science. 1968;161:295–298. doi: 10.1126/science.161.3838.295. [DOI] [PubMed] [Google Scholar]
- Tononi G, Edelman G.M. Advances in neurology. Consciousness: at the frontiers of neuroscience. Lippincott-Raven; Philadelphia: 1998. Consciousness and the integration of information in the brain. pp. 245–280. [PubMed] [Google Scholar]
- Tootell R.B.H, Hadjikhani N.K, Mendola J.D, Marrett S, Dale A.M. From retinotopy to recognition: fMRI in human visual cortex. TICS. 1998;2:174–183. doi: 10.1016/s1364-6613(98)01171-1. [DOI] [PubMed] [Google Scholar]
- Tusa R.J, Ungerleider L.G. The inferior longitudinal fasciculus: a re-examination in humans and monkeys. Ann. Neurol. 1985;18:583–591. doi: 10.1002/ana.410180512. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C, Maunsell J.H.R. Hierarchical organization and functional streams in the visual cortex. Trends Neurosci. 1983;6:370–375. [Google Scholar]
- Zeki S. Uniformity and diversity of structure and function in Rhesus monkey prestriate visual cortex. J. Physiol. 1978;277:273–290. doi: 10.1113/jphysiol.1978.sp012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S. Localization and globalization in conscious vision. Annu. Rev. Neurosci. 2001;24:57–86. doi: 10.1146/annurev.neuro.24.1.57. [DOI] [PubMed] [Google Scholar]
- Zeki S, Bartels A. Toward a theory of visual consciousness. Conscious. Cogn. 1999;8:225–259. doi: 10.1006/ccog.1999.0390. [DOI] [PubMed] [Google Scholar]
- Zeki S, ffytche D.H. The Riddoch syndrome: insights into the neurobiology of conscious vision. Brain. 1998;121:25–45. doi: 10.1093/brain/121.1.25. [DOI] [PubMed] [Google Scholar]
- Zeki S, Shipp S. The functional logic of cortical connections. Nature. 1988;335:311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson J.D.G, Lueck C.J, Friston K.J, Kennard C, Frackowiak R.S.J. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]