Abstract
A comparison of the architecture of the human prefrontal cortex with that of the macaque monkey showed a very similar architectonic organization in these two primate species. There is no doubt that the prefrontal cortical areas of the human brain have undergone considerable development, but it is equally clear that the basic architectonic organization is the same in the two species. Thus, a comparative approach to the study of the functional organization of the primate prefrontal cortex is more likely to reveal the essential aspects of the various complex control processes that are the domain of frontal function. The lateral frontal cortex appears to be functionally organized along both a rostral–caudal axis and a dorsal–ventral axis. The most caudal frontal region, the motor region on the precentral gyrus, is involved in fine motor control and direct sensorimotor mappings, whereas the caudal lateral prefrontal region is involved in higher order control processes that regulate the selection among multiple competing responses and stimuli based on conditional operations. Further rostrally, the mid-lateral prefrontal region plays an even more abstract role in cognitive control. The mid-lateral prefrontal region is itself organized along a dorsal–ventral axis of organization, with the mid-dorsolateral prefrontal cortex being involved in the monitoring of information in working memory and the mid-ventrolateral prefrontal region being involved in active judgments on information held in posterior cortical association regions that are necessary for active retrieval and encoding of information.
Keywords: prefrontal cortex, frontal cortex, cytoarchitecture, monkey
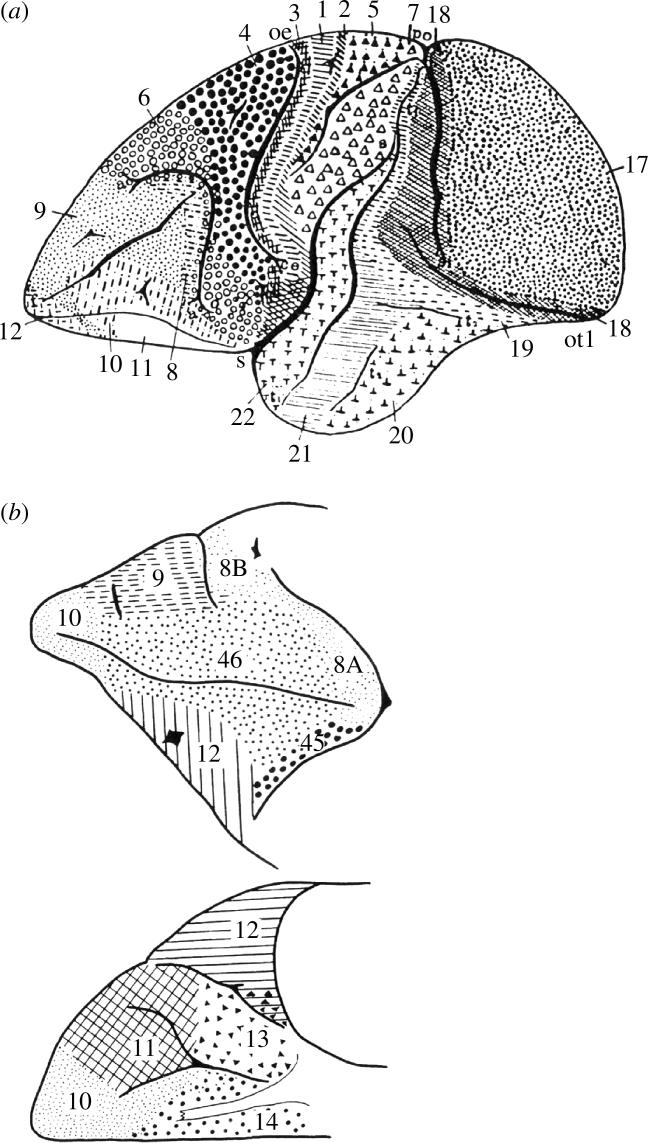
The cerebral cortex can be subdivided into several areas based on differences in the arrangement of their cellular elements into layers, such as differences in cell packing density across layers, in cell size or type in one or more layers, in the relative thickness of the layers, or even in overall cortical thickness. Some regional differences in the structure of the cerebral cortex were noted as early as the end of the eighteenth century, such as the white stripe in the visual cortex of primates (Vicq d'Azyr 1786). In the nineteenth century, Baillarger (1840) described the white stripes in the cerebral cortex that are now known as the outer and inner stripes of Baillarger. These limited early findings were not based on histological observations and it was only later with the introduction of microscopic examination of fixed, sectioned and cell-stained tissue that a serious study of the cellular architecture of the cerebral cortex could begin. Meynert (1867, 1885), a pioneer in this type of investigation, realized that the cerebral cortex is not a homogeneous sheet of grey matter, but rather that it consists of several different areas; with this realization, he proceeded to demonstrate cellular differences between the rhinencephalic region and the neocortex. Several such cytoarchitectonic studies followed in the latter part of the nineteenth century (e.g. Betz 1874; Lewis & Clarke 1878; Lewis 1881). In 1905, Campbell's classic treatise on Histological Studies on the Localisation of Cerebral Function presented the first complete cytoarchitectonic map of the human cerebral cortex based on the investigation of eight cerebral hemispheres (Campbell 1905). Interestingly, in the same year, Brodmann (1905) published his architectonic map of the monkey (cercopithecus) cerebral cortex (figure 1a), followed in 1908 by his architectonic map of the human cerebral cortex (figure 2a). Brodmann, who between 1901 and 1910 worked in the neurobiological laboratory in Berlin (directed by O. Vogt), carried out cytoarchitectonic analysis on the cerebral cortex in several mammals. This work, which complemented the myeloarchitectonic research that was carried out in the same laboratory by Vogt & Vogt (1919), was to have a profound influence on modern architectonic studies. Other major maps of the human cerebral cortex were published by Elliot Smith (1907) and by Economo & Koskinas (1925). In the 1950s, two more atlases of the human cerebral cortex appeared, one by Bailey & Bonin (1951) and the other by Sarkissov et al. (1955), the latter being largely a modified version of the Brodmann map based on extensive investigations on several brains at the Moscow Brain Research Institute (figure 2b). Of note is the cytoarchitectonic map of the frontal cortex published by Sanides in 1962.
Figure 1.
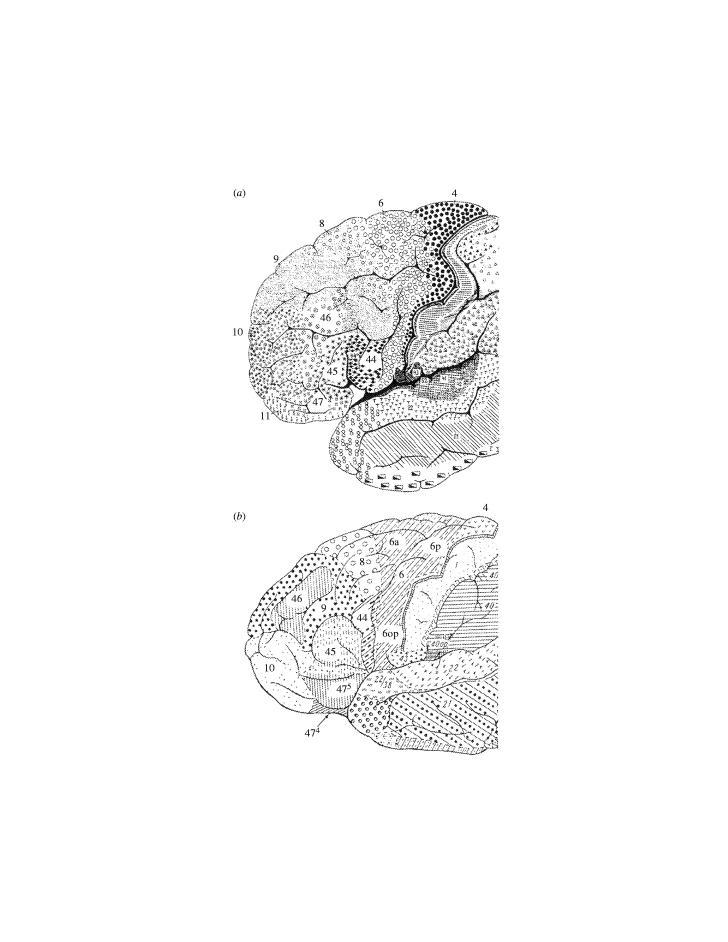
(a) Cytoarchitectonic map of the lateral surface of the cerebral cortex of the monkey by Brodmann (1905). Note that the orbital frontal cortex is also partially shown as an extension of the ventral part of the lateral surface. (b) Cytoarchitectonic map of the lateral and orbital prefrontal cortex of the macaque monkey by Walker (1940).
Figure 2.
(a) Cytoarchitectonic map of the lateral surface of the human cerebral cortex by Brodmann (1909). Note that the orbital frontal cortex is also shown as an extension of the ventral part of the lateral surface. (b) Cytoarchitectonic map of the lateral surface of the human cerebral cortex by Sarkissov et al. (1955).
With the emergence of modern functional neuroimaging in the 1980s, the map of Brodmann (1908, 1909) became the basis for the description of the location of foci of activation in the human cerebral cortex. The primary reason for the widespread adoption of the Brodmann map is its use in the Talairach & Tournoux (1988) proportional stereotaxic atlas of the human brain, namely, the standard stereotaxic atlas for functional neuroimaging studies. It is important to point out that the location of cortical areas in the Talairach and Tournoux atlas was based on a simple projection of the Brodmann map onto the brain sections of the atlas and not on architectonic analysis of those particular sections. Thus, the Brodmann numbers in the Talairach and Tournoux atlas are, at best, approximate estimations of the location of cortical areas. Several modern architectonic investigations have attempted to correct this problem by studying the architecture of the cerebral cortex in several brains and by describing the variability in the location of cortical areas in the proportional Talairach stereotaxic space (e.g. Amunts et al. 1999; Morosan et al. 2001).
Functional neuroimaging studies in human subjects permit the visualization of changes in neuronal activity in specific regions of the human brain in relation to particular aspects of cognitive processing. These changes in neuronal activity are indexed, indirectly, by changes in local blood flow that are presumably the result of the particular cognitive requirements of the tasks performed. The findings of every functional neuroimaging study reduce to a statement that there has been a change in the measured blood flow ‘signal’ in certain areas of the brain under the conditions studied. Thus, the value of such a study is ultimately dependent on the extent to which one can (i) specify the area(s) of the brain where activity changed and (ii) understand what those changes in the blood flow ‘signal’ represent in terms of actual neuronal computations. The actual neuronal computations in a particular cortical area can be investigated in experimental studies in behaving monkeys performing appropriate cognitive tasks while the activity of single neurons in the area of interest is being recorded. Furthermore, the significance of the computations occurring in a given area for the complex neuronal network within which it is embedded can be explored, in monkeys, by observing the consequences on cognitive/behavioural function of removal or disconnection of the particular area or manipulations of its neurotransmitter activity.
There is, however, one major problem impeding effective crosstalk between functional neuroimaging investigations of the human brain and experimental work in the monkey. The architectonic maps of the human cerebral cortex and those of the cortex of the macaque monkey (the most common experimental primate) are, in several important respects, not consistent with each other. For instance, the same architectonic designation may refer to areas that are obviously not homologous in the two species, and even when the same designation is used for what appears to be the same area in the two species, the similarity may be deceptive because the criteria applied in delineating the area in the two species may have not been the same. This situation is an unavoidable consequence of the fact that the maps of the human cerebral cortex were constructed in the first half of the twentieth century (Brodmann 1909; Economo & Koskinas 1925; Sarkissov et al. 1955) and did not change much, whereas the maps of the monkey cortex continued to evolve as physiological and anatomical studies burgeoned during the second half of the twentieth century.
Architectonic studies of the cortex of monkeys appeared at approximately the same time as those of the human cortex (Brodmann 1905; Vogt & Vogt 1919). It is unfortunate that the numerical designations employed by Brodmann in his maps of the human and the monkey brain were not always consistent, even for obviously comparable areas. For instance, Brodmann (1905) designated an area 9 in his map of the monkey cortex, but not an area 46 (see figure 1a). Furthermore, he stated in his 1909 monograph that area 9 in the monkey corresponds to the granular frontal area 9 and frontopolar area 10 in the human brain. In his monkey map, however, which was constructed before his human map, Brodmann (1905) used the designation area 10 for parts of the orbital and ventrolateral frontal region and designated the frontopolar region as area 12 (compare figures 1a and 2a). This kind of discrepancy in nomenclature, as well as the considerable uncertainty that Brodmann expressed in his subdivisions of the frontal cortex in the monkey, has been a source of considerable confusion in the anatomical literature. It was, in fact, for this reason that Walker (1940) investigated the cytoarchitecture of the frontal cortex of the macaque monkey, and attempted, to some extent, to use a numerical scheme similar to that used by Brodmann in the human brain (figure 1b). For instance, Walker designated the frontopolar cortex of the monkey as area 10 (as in the Brodmann map of the human cortex) and designated areas 46 and 45 that were missing from the Brodmann monkey map. Walker's map of the prefrontal cortex subsequently became the basis of all subsequent investigations of the cytoarchitecture of the frontal cortex of the monkey (e.g. Barbas & Pandya 1989; Preuss & Goldman-Rakic 1991).
Although Walker (1940) harmonized the designations of some of the areas of the monkey prefrontal cortex with those used by Brodmann for the human frontal cortex, he did not carry out an explicit comparison between the human and the monkey frontal cortex, and consequently the correspondence of some of the areas he identified in the monkey with those of the human brain raise several questions. For instance, in the mid-lateral prefrontal cortex of the monkey, Walker identified a large granular region as area 46, abutting posteriorly onto area 8 (see figure 1b). Yet, in all maps of the human frontal cortex, area 46 is never shown to have a common border with area 8, being separated from it by a cortical region that Brodmann (1909) included as part of area 9 (figure 2a,b). It is important to note that both Walker (1940) and subsequent investigators of the monkey prefrontal cortex (e.g. Barbas & Pandya 1989; Preuss & Goldman-Rakic 1991) noted that the region he labelled as area 46 in the monkey is not homogeneous and that it can be further subdivided. Walker (1940) basically used the designation ‘area 46’ for the large granular region at the mid-lateral part of the frontal cortex, but it was not known whether all or only part of this heterogeneous region corresponds to area 46 in the Brodmann map of the human brain. Furthermore, Walker (1940) used the term ‘area 12’ for the most ventrolateral part of the prefrontal cortex extending onto the lateral orbital surface, a label that was not used by Brodmann for this region in the human map. Walker (1940) also introduced the term ‘area 45’ for a part of the monkey frontal cortex, speculating that it might correspond to Brodmann's area 45 of the human cortex, but he was not confident on this issue since he had not examined the human cortex.
The above discrepancies in the architectonic parcellation of the human and the macaque monkey prefrontal cortex are a serious problem for modern neuroscience. They impede a meaningful crosstalk between functional neuroimaging work with human subjects and experimental anatomical, physiological and behavioural work on non-human primates that is necessary for a proper understanding of the significance of the blood flow signal changes observed within particular cortical areas in the human brain. It is because of these glaring discrepancies that we undertook a re-examination of the cytoarchitecture of the human prefrontal cortex and that of the macaque monkey (Petrides & Pandya 1994, 1999, 2002). The aim of this work was to define prefrontal architectonic areas in the two species by the same cytoarchitectonic and topographical criteria so that crosstalk between experimental research on monkeys and functional neuroimaging work on human subjects can proceed in a meaningful fashion. This cytoarchitectonic research yielded a parcellation of the prefrontal cortex that is comparable in the two species, thus resolving major problems that had arisen from discrepancies between the parcellations in the classic maps of the human and the monkey prefrontal cortex (figure 3). There is no doubt that there has been considerable development of the prefrontal cortical areas in the human brain, but it is also clear that the basic architectonic plan is similar in these two primate brains. In the present article, the cytoarchitectonic organization of the dorsolateral prefrontal cortex will be described first, followed by that of the ventrolateral prefrontal cortex.
Figure 3.
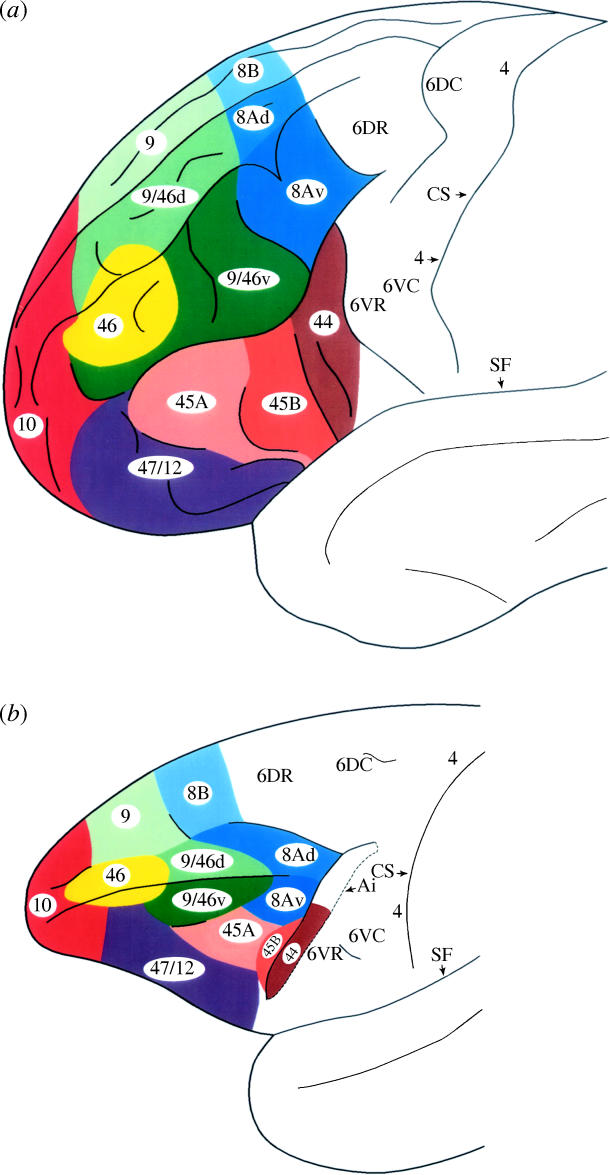
Cytoarchitectonic map of the lateral surface of the prefrontal cortex of (a) the human brain and (b) the macaque monkey brain by Petrides & Pandya (1994). Abbreviations: Ai, the inferior arcuate sulcus; CS, central sulcus; SF, Sylvian fissure.
1. Dorsolateral prefrontal cortex
In the classic cytoarchitectonic maps of the human cerebral cortex (Brodmann 1908, 1909; Sarkissov et al. 1955), two areas are shown on the mid-lateral prefrontal cortex: area 9 and area 46 (figure 2a,b). Area 46 is shown on the middle frontal gyrus, whereas area 9 is shown both on the superior frontal gyrus and on the middle frontal gyrus. In all the maps of the human frontal cortex, area 46 is separated from area 8 by the portion of area 9 that lies on the middle frontal gyrus (figure 2a,b), whereas in the map of the macaque monkey brain by Walker (1940), area 46 is shown to have a common border with area 8 because area 9 is restricted to the superior part of the dorsolateral prefrontal cortex (figure 1b). Subsequent studies of the monkey frontal lobe have followed Walker in defining the limits of areas 46 and 9, although both Walker (1940) and subsequent investigators (e.g. Barbas & Pandya 1989; Preuss & Goldman-Rakic 1991) acknowledged the fact that the region labelled as area 46 in the monkey is not homogeneous and that it can be further subdivided. In comparing the cytoarchitecture of the human and the macaque monkey prefrontal cortex (Petrides & Pandya 1994, 1999), we observed that the cortex lying on the superior frontal gyrus in the human brain, above area 46, and labelled as area 9 by Brodmann, has architectonic features that are similar to those of area 9 in the monkey as defined by Walker (1940): namely a poorly developed layer IV and the existence of large pyramidal cells in the deeper part of layer III. We have therefore designated this region of the human frontal lobe in both the human and the macaque monkey as area 9 (figure 3a,b).
In the human brain, however, the designation area 9 was also used by Brodmann (1908, 1909) and by Sarkissov et al. (1955) to refer not only to a part of the cortex on the superior frontal gyrus, but also to a large part of the cortex occupying the middle frontal gyrus caudal to area 46 and thus separating area 46 from area 8 (figure 2a,b). We observed that the part of the middle frontal gyrus included in area 9 in the classic maps has a well-developed layer IV and, in this respect, it is closer in architecture to area 46 (which also has a well-developed layer IV) than the part of area 9 that lies on the superior frontal gyrus and which exhibits a poorly developed layer IV. The portion of area 9 on the middle frontal gyrus, although sharing with area 46 a well-developed layer IV, can be discriminated from the latter area by the presence of large, deeply stained pyramidal neurons in the lower part of layer III. In contrast, area 46 has a layer III that contains small to medium size pyramidal neurons, giving it a rather uniform appearance (Petrides & Pandya 1994, 1999). In comparing the architecture of the monkey and human prefrontal cortex, we noted that only a limited part of the large region that Walker labelled as area 46 has features similar to those of area 46 of the human brain, namely a well-developed layer IV and a layer III that has a rather uniform appearance owing to the lack of many large pyramidal neurons in its deeper part. This part of Walker's area 46 lies mostly in the rostral extent of the sulcus principalis. We have therefore restricted the designation area 46 to this cortical region of the monkey to acknowledge the fact that only this portion is architectonically comparable to area 46 of the human brain. The cortex on the lips of the caudal portion of the sulcus principalis and the immediately adjacent cortex (which Walker included as part of area 46) exhibits, in addition to a well-developed layer IV, prominent large neurons in the deeper part of layer III, namely architectonic features that, in the human brain, characterize the part of the middle frontal gyrus that Brodmann (1908, 1909) and Sarkissov et al. (1955) labelled as area 9 (caudal to area 46). On the basis of these observations, we have designated this part of the mid-dorsolateral prefrontal cortex, in both the human and the monkey brain, as area 9/46 (figure 3a,b) to emphasize two important facts: (i) this portion of the middle frontal gyrus in the human brain, although labelled as area 9 by Brodmann (1908, 1909) and Sarkissov et al. (1955), is in fact closer in architecture to area 46 which extends anterior to it on the same gyrus, than to the cortex labelled as area 9 on the superior frontal gyrus. (ii) The designation 9/46 denotes the fact that this area had been included as part of area 9 in the classic maps of the human cortex, but as part of area 46 in the widely followed map of the monkey frontal cortex by Walker (1940). There is agreement in all architectonic maps of the human and the monkey frontal cortex that the posterior part of the dorsolateral frontal cortex comprises area 8 and the rostral part of dorsal area 6 (figures 1–3).
Areas 9, 46 and 9/46 receive input from the multimodal superior temporal sulcal cortex, the rostral superior temporal gyrus, the anterior and posterior cingulate cortex and the retrosplenial cortex. Thus, these areas maintain preferential connections with multimodal temporal areas, on the one hand, and paralimbic cortical areas, such as the cingulate, the retrosplenial and the rostral temporal cortex, on the other hand. The major difference in the connections of these areas is the lack of input from lateral and medial parietal cortex in the case of area 9 (Petrides & Pandya 1984, 1999; Cavada & Goldman-Rakic 1989; Andersen et al. 1990). It is interesting to note that posterior dorsolateral areas 8Ad and 8Av which adjoin, caudally, mid-dorsolateral area 9/46, lack connections with the paralimbic retrosplenial cortex, which is a hallmark of the mid-dorsolateral areas 9/46, 46 and 9 (Morris et al. 1999a, b; Petrides & Pandya 1999). Thus, there is a unique and bidirectional relation between the mid-dorsolateral prefrontal cortex and the paralimbic retrosplenial cortex. The caudally adjacent area 8 primarily has connections with visuo-spatial parietal and posterior visual temporal areas (Barbas & Mesulam 1981; Andersen et al. 1990; Petrides & Pandya 1999).
2. Ventrolateral prefrontal cortex
The major part of the ventrolateral prefrontal cortex of the human brain lies on the inferior frontal gyrus. In front of the ventral part of the precentral gyrus (agranular area 6) lies a distinct cortical area labelled as area 44 by Brodmann (1908, 1909; figure 2a). Area 44 is a dysgranular area in which layer IV is present but not well-developed and is further characterized by large pyramidal neurons in the lower part of layer III and in layer V (Petrides & Pandya 1994; Amunts et al. 1999). Area 44 is succeeded rostrally by area 45 which differs from area 44 by the presence of a well-developed layer IV and strikingly large pyramidal neurons in the deeper part of layer III. Area 45 occupies the pars triangularis of the inferior frontal gyrus (Brodmann 1908, 1909; Sarkissov et al. 1955; Petrides & Pandya 1994; Amunts et al. 1999). Rostroventral to area 45, there is a cortical region that occupies the most ventral part of the lateral frontal cortex, extending onto the orbital surface. This distinct cortical region was labelled as area 47 by Brodmann (1909) (figure 2a). Sarkissov et al. (1955), who studied in detail this heterogeneous region, have identified five distinct subdivisions of 47 (figure 2b).
Major discrepancies exist between the classical cytoarchitectonic maps of the human ventrolateral prefrontal cortex and those of the monkey. Whereas the presence of the agranular areas 4 and 6 in the ventral part of the precentral gyrus of the monkey has not been the subject of debate, the identification of areas 44, 45 and 47 in the monkey brain has been problematic. Brodmann (1905) did not identify these areas in the monkey frontal cortex (figure 1a). Walker (1940) identified a part of the monkey ventrolateral prefrontal cortex as area 45 (figure 1b), but he only tentatively suggested that it might correspond to area 45 of the human brain because he had not compared monkey with human cytoarchitecture (Walker 1940, see p. 67). The issue was further complicated by the adoption in the 1990s by some oculomotor neurophysiologists of the term ‘area 45’ to refer to the ventral part of the frontal eye field, from which small amplitude saccades can be evoked with electrical microstimulation, while referring to the part of the frontal eye field where large amplitude saccades can be evoked as caudal area 8A (Schall et al. 1995). This usage was driven by the fact that in the Walker (1940) map, area 45 is shown to extend in the anterior bank of the arcuate sulcus as far as area 8 where the classic frontal eye field region is located. However, using the term ‘area 45’ to refer to a part of the frontal eye field is unfortunate since oculomotor responses from microstimulation have never been observed in the lower part of the inferior ramus of the arcuate sulcus where the bulk of Walker's ‘area 45’ extends (Bruce et al. 1985; Stanton et al. 1989; Schall et al. 1995), and, furthermore, area 45 in the human prefrontal cortex has never been linked to eye movement control, but rather with verbal and non-verbal retrieval from long-term memory (e.g. Petrides 1996).
The above considerations raised the following questions. Is there a dysgranular area in the ventrolateral prefrontal cortex immediately in front of ventral area 6 of the macaque monkey that has the characteristics of area 44 of the human brain? Is all or part of the strip of cortex that Walker (1940) labelled as ‘area 45’ in the monkey ventrolateral prefrontal cortex comparable in architectonic characteristics to area 45 in the human brain? Finally, is there an area 47 in the rostroventral part of the ventrolateral prefrontal cortex? In our architectonic studies, we searched the prefrontal cortex of the macaque monkey for areas that have the cytoarchitectonic characteristics of areas 44, 45 and 47 of the human ventrolateral prefrontal cortex (Petrides & Pandya 1994, 2002).
The ventral part of the precentral gyrus of the monkey brain, as in the human brain, is occupied by agranular areas 4 and 6 (figures 1 and 3b). There are two subdivisions of lower area 6: a ventrocaudal area 6 (area 6VC) and a ventrorostral area 6 (area 6VR), which have been referred to as areas F4 and F5, respectively, by Matelli et al. (1985). The ventrorostral area 6 (area 6VR or F5) exhibits a better lamination than the ventrocaudal area 6 (area 6VC or F4; Matelli et al. 1985). According to our cytoarchitectonic studies, anterior to ventral area 6 and buried mostly within the posterior bank and the fundus of the arcuate sulcus, there is a dysgranular area that exhibits a rudimentary layer IV and conspicuous deeply stained large pyramidal neurons in the deeper part of layer III and layer V (Petrides & Pandya 1994, 2002). Since these are the cytoarchitectonic characteristics of area 44 in the human brain and the area that exhibits them in the monkey occupies a comparable location (i.e. immediately anterior to the ventral agranular area 6), we consider it to be comparable to human area 44 (figure 3b).
In the human brain, in front of area 44, lies area 45 that is characterized by the presence of clusters of large deeply stained pyramidal neurons in the deeper part of layer III combined with a well-developed layer IV and medium size neurons in layer V (Economo & Koskinas 1925; Sarkissov et al. 1955; Petrides & Pandya 1994; Amunts et al. 1999). The lower part of the anterior bank of the inferior ramus of the arcuate sulcus that we defined as area 45, using the criteria of area 45 in the human brain, does not extend dorsally to the region where short amplitude saccades are generated and extends for a considerable distance anteriorly within the ventrolateral frontal cortex (figure 3b). In the microstimulation-defined frontal eye field, which lies within the anterior bank of the arcuate sulcus in the region that curves just caudal to the sulcus principalis, the cortex exhibits large and dense pyramidal neurons in layer V (Stanton et al. 1989). These large layer V neurons diminish sharply as one proceeds into the lower part of the anterior bank of the inferior limb of the arcuate sulcus, i.e. as one moves away from the region where eye movements can be evoked (Stanton et al. 1989). In the lower part of the inferior ramus of the arcuate sulcus that we consider to be comparable to area 45 of the human brain, one rarely encounters the very large pyramidal neurons in layer V that are typical in the dorsal part where the frontal eye field is located. We have included the upper part of the inferior limb of the arcuate cortex that exhibits large neurons in layer V as part of caudal area 8, as other investigators had previously done (e.g. Brodmann 1905; Barbas & Pandya 1989). Thus, area 45 in the monkey ventrolateral prefrontal cortex, when defined by criteria comparable to those of human area 45, is not coincidental with Walker's area 45 and does not include any part of the frontal-eye field. Injection of retrograde fluorescent tracers into the part of the monkey prefrontal cortex that is comparable to area 45 of the human cortex revealed cortical inputs from the superior temporal gyrus (i.e. the auditory system) and the multimodal areas of the superior temporal sulcus and not from areas that are known to be connected with the frontal eye field (Petrides & Pandya 2002).
Another major issue when attempting to compare the human and the monkey ventrolateral prefrontal cortex concerns the relationship between area 47 of the human brain and area 12 of the monkey brain. The designation ‘area 47’ was used by Brodmann for a very large zone, extending from the ventralmost part of the lateral prefrontal cortex to the posterior part of the orbital frontal cortex as far as the medial orbital sulcus (figure 2a; Brodmann 1908, 1909). This is architectonically a heterogeneous region that Sarkissov et al. (1955) subdivided into five parts (figure 2b). The designation ‘area 47’ has not been used in any of the maps of the monkey brain, but Walker (1940) identified a large area on the ventrolateral part of the macaque frontal lobe extending onto the orbital surface which he called area 12 (figure 1b). Medial to area 12, on the orbital frontal surface, Walker (1940) identified two other areas: area 13, caudally, and area 11, rostrally.
Here, it is important to note that in Walker's map, area 12 occupies the ventralmost part of the ventrolateral convexity (figure 1b). In our comparative architectonic analysis, it was evident that the region occupying the ventralmost part of the ventrolateral prefrontal cortex and extending onto the orbital surface that Walker (1940) labelled as area 12 has characteristics comparable to those of the part of the human area 47 that lies anterior and below area 45 and which also extends as far as the lateral orbital sulcus. We have labelled this region, in both the human and the monkey brain, as area 47/12 (figure 3b) to acknowledge the similarity in topography and cytoarchitecture of this part of the frontal cortex in these two primate brains (Petrides & Pandya 1994, 2002). The part of Brodmann's area 47 that extends medial to the lateral orbital sulcus in the human brain is a dysgranular cortex that has characteristics similar to those of the caudal orbital frontal cortex that Walker labelled as area 13 in the monkey (see Petrides & Pandya 1994). Thus, the inferior convexity of the macaque monkey cortex comprises two architectonic areas, areas 45 and 47/12, that, in the human brain, occupy the pars triangularis and pars orbitalis of the inferior frontal gyrus. Area 47/12 is strongly linked with the rostral inferotemporal visual association cortex and ventral limbic areas (i.e. perirhinal cortex and rostral parahippocampal gyrus; Barbas 1988; Carmichael & Price 1995; Petrides & Pandya 2002), whereas area 45 is strongly connected with the auditory superior temporal region and the multimodal superior temporal sulcal cortex (Petrides & Pandya 2002).
3. Functional organization of the prefrontal cortex
There is considerable evidence by now that the prefrontal cortex plays a major role in high-order control processes that exercise a top-down regulation of cognition and behaviour (e.g. Luria 1969; Stuss & Benson 1986; Fuster 1989; Petrides 1996; Robbins 1996; Shallice & Burgess 1996; Duncan & Owen 2000; Postle & D'Esposito 2000). The structural differences between the various prefrontal cortical areas, which define their cytoarchitecture, and their distinct connections with other cortical and subcortical brain structures, suggest that these areas are involved in distinct aspects of the high-level control of cognitive processing and behaviour that is the domain of prefrontal function. In order to uncover the fundamental principles of functional organization of higher order control processing in the primate frontal cortex, we have studied the cognitive effects of lesions of the frontal cortex in both human patients and macaque monkeys in a comparative manner. Since the fundamental organizational scheme is likely to be the same across all primate brains, a comparative approach is more likely to reveal the essential aspects of frontal cortex organization. In addition, since lesions in patients are rarely restricted precisely to particular anatomically defined architectonic areas of the frontal cortex, research on monkeys in which lesions can be made with great precision can establish dissociations in the functional contributions of various sectors of the prefrontal cortex that can be only imperfectly studied in the human brain. This work has revealed both a rostral–caudal axis in the organization of cognitive control in the lateral prefrontal cortex and a dorsal–ventral axis in the mid-lateral part of the prefrontal cortex. The rostral–caudal distinction was established in the 1980s from monkey lesion studies that examined differences in the effects of mid-dorsolateral prefrontal lesions and caudal dorsolateral frontal lesions in conditional learning and working memory (see Petrides 1987, 2005), and the dorsal–ventral distinction in executive control was proposed in the early 1990s (Petrides 1994, 1996).
(a) The rostral–caudal axis of frontal cortex organization
There is strong evidence from studies with both human patients and monkeys that there is a rostral–caudal axis of functional organization within the lateral frontal cortex (figure 4). As is well known, the most caudal region of the frontal lobe, namely the motor region that occupies the precentral gyrus (area 4 and caudal area 6) is involved in fine motor control and direct sensorimotor transformations for reaching, grasping and manipulation of objects (He et al. 1993; Rizzolatti & Luppino 2001). In front of the motor precentral region, a further distinction can be made between the caudal prefrontal region (rostral area 6 and area 8) and the mid-dorsolateral prefrontal cortex (areas 46 and 9/46). Whereas lesions restricted to the caudal dorsolateral prefrontal region (area 8 and rostral area 6) yield a massive impairment on tasks that require the selection between alternative competing responses based on conditional operations, lesions of the mid-dorsolateral prefrontal cortex (area 46 and 9/46) yield a severe deficit on tasks designed to measure the monitoring of information in working memory (Petrides 1987, 1994, 2005).
Figure 4.
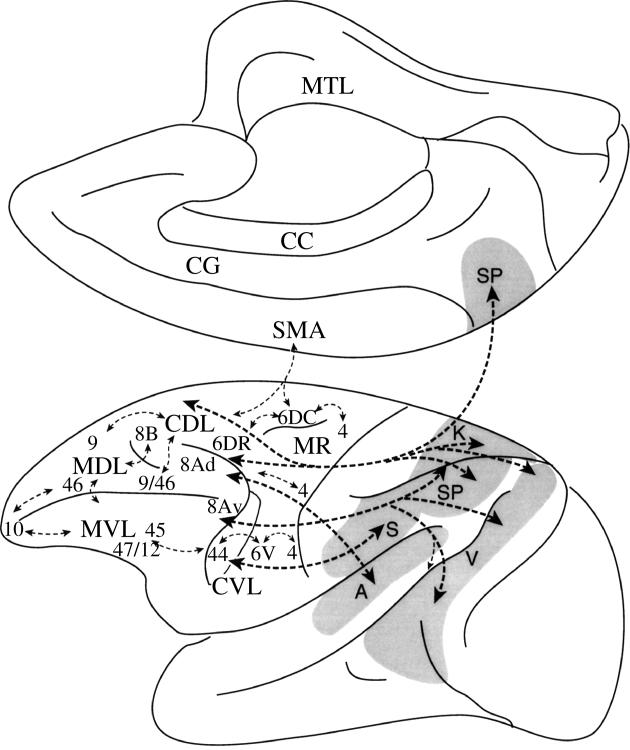
Schematic diagram of the monkey brain illustrating the rostral–caudal axis of lateral frontal cortex organization. Some of the interactions of the caudal lateral frontal region with post-rolandic cortical regions are shown by the thick dashed lines and interactions within the lateral frontal cortex are shown by the thin dashed lines. Abbreviations: A, auditory processing in the superior temporal gyrus; CC, corpus callosum; CDL, caudolateral frontal region; CG, cingulate gyrus; CVL, caudal ventrolateral frontal region; K, kinaesthetic processing in the superior parietal lobule; MDL, mid-dorsolateral prefrontal region; MR, motor region; MTL, medial temporal lobe region; MVL, mid-ventrolateral prefrontal region; S, body-centred (i.e. somato-centric) amodal processing in rostral inferior parietal lobule; SMA, supplementary motor area; SP, spatial processing in lateral and medial posterior parietal cortex; V, visual processing in prestriate cortex and the occipito-temporal cortical region. The numbers refer to architectonic areas in the lateral frontal cortex. Note that the posterior bank of the inferior branch of the arcuate sulcus is displayed opened in order to illustrate area 44 that lies within the inferior bank of the arcuate sulcus.
The two lesions in the monkey that were used to study fundamental differences in function along the rostral–caudal axis of the lateral frontal cortex are shown in figure 5. The mid-dorsolateral prefrontal lesions included cortex in the sulcus principalis and above it and, therefore, involved areas 46, 9/46 and 9. The caudal dorsolateral frontal lesions involved the cortex within the dorsal part of the arcuate sulcus and the immediately surrounding region, namely the rostral dorsal area 6 and area 8A. We refer to these caudal dorsolateral frontal lesions as the periarcuate (PA) lesions since they involved cortex within and surrounding the arcuate sulcus. One example of the impaired performance of monkeys with caudal dorsolateral frontal lesions in conditional tasks is provided in figure 6. In this visual–visual conditional associative task, the monkeys were faced with two white perspex boxes. Inside each one of these boxes there was a light bulb that could be remotely turned on and off by the experimenter (Petrides 1985). On each trial, one of the boxes, chosen according to a random sequence, was lit and the other remained unlit. One of two objects was then presented. The monkeys were rewarded if they opened the lit box when object A was shown and if they opened the unlit box when object B was shown. Thus, the monkeys had to select between two visual non-spatial stimuli (i.e. the lit or the unlit box, the position of which varied from trial to trial) based on an acquired conditional rule. The animals with the mid-dorsolateral prefrontal lesions and the normal control animals reached criterion (90% correct performance over 3 consecutive days of testing, i.e. 90 trials) within a mean of 300 and 330 trials, respectively. In sharp contrast, the animals with the caudal dorsolateral frontal lesions (i.e. PA lesions) failed to reach criterion within the limits of testing (1020 trials) and the mean level of correct performance achieved during the last 3 days of testing (i.e. the last 90 trials) was only 58% correct.
Figure 5.
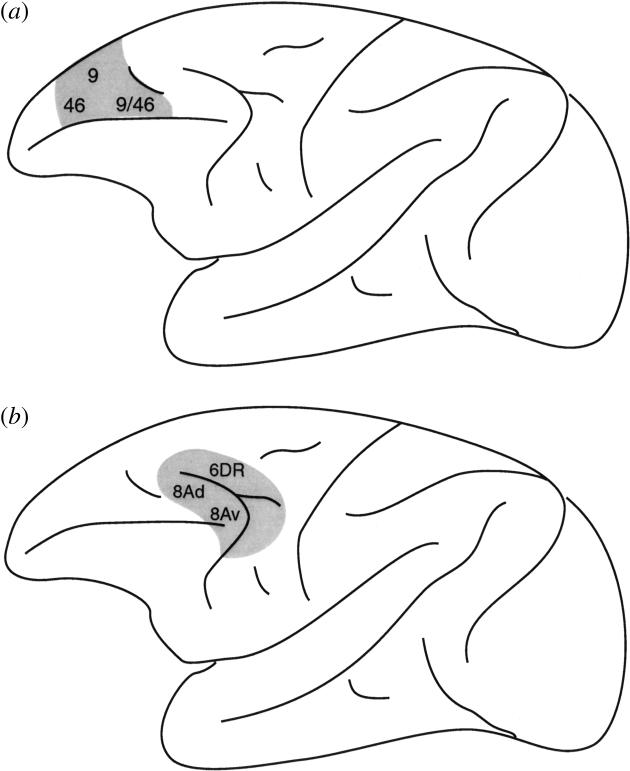
Schematic illustration of (a) the mid-dorsolateral (MDL) prefrontal lesion and (b) the caudal dorsolateral prefrontal lesion, which involved the cortex within and around the dorsal arcuate sulcus, i.e. the periarcuate (PA) region. These lesions in the monkey were used to study fundamental differences in function along the rostral–caudal axis of lateral frontal cortex. The numbers refer to the architectonic areas involved in these lesions.
Figure 6.
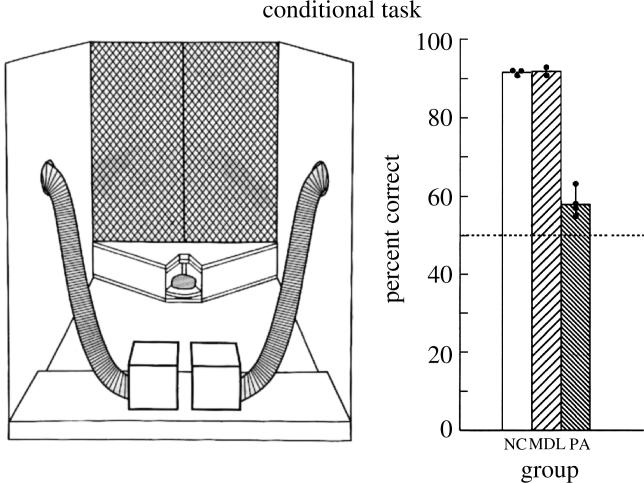
(Left): schematic diagram of the experimental arrangement in the visual–visual object conditional task administered to monkeys. On each trial, one of the two white perspex boxes is lit and the other remains unlit by remotely turning on a light bulb that is inside them. One of two conditional stimuli is then presented in front of the opaque screen hiding the experimenter and the animal responds by pushing back one of the two boxes. The reward is delivered via the tubes that are attached to the boxes. (Right): performance of animals with mid-dorsolateral prefrontal lesions (MDL), animals with periarcuate lesions (PA) and normal control animals (NC). Solid circles indicate the scores of individual animals in each group. The animals with MDL and NC lesions reached criterion (90% correct performance across three consecutive days of testing, i.e. 90 trials) within a mean of 300 and 330 trials, respectively. None of the animals with PA lesions was able to reach criterion within the limits of testing (i.e. 1020 trials) and the mean level of correct performance achieved during the last 3 days of testing (i.e. last 90 trials) was 58% correct. Data from Petrides (1985).
The critical role of the caudal dorsolateral prefrontal cortex in the selection between different aspects of the visual, auditory and somatomotor environment based on conditional operations can be thought of as the conditional allocation of attention to competing stimuli in the environment. Thus, learned conditional rules provide a means by which attention can be flexibly switched between different stimuli or responses in a given situation under different conditions. The caudal lateral frontal region comprises various parts that exhibit differences in their connections with posterior association cortex (figure 4). It has been argued (Petrides 1987, 2005) that all these sectors of the caudal lateral frontal region are involved in conditional selection, but that the conditional operations are applied to different types of information depending on the distinct connections of the various sectors of the caudal lateral frontal cortex with posterior association cortex. For instance, it has been shown that lesions of area 8 yield severe impairments on the visual–visual conditional task described above, but not on visuo-motor conditional tasks (Petrides 1987). As can be seen in figure 4, area 8 is linked with the prestriate cortical region and the caudal inferior parietal lobule, both of which are involved with oculomotor and visuo-spatial processing (Mountcastle et al. 1975; Andersen & Gnadt 1989). Thus, area 8 can be said to control the selection between alternative visual stimuli in the environment based on conditional rules. By contrast, there is strong evidence that lesions of rostral area 6 impair selectively visuo-motor conditional tasks (Petrides 1982, 1987; Halsband & Passingham 1982). This result is perfectly consistent with the connections of rostral area 6 (figure 4). This area is strongly connected, locally, with motor areas, such as caudal area 6 and the supplementary motor area, as well as with the superior parietal lobule and the caudal part of the inferior parietal lobule. Neurons of the cortex of the superior parietal lobule code the location of body parts (e.g. the arm) in a body-centred coordinate system (Duffy & Burchfield 1971; Sakata et al. 1973; Mountcastle et al. 1975; Lacquaniti et al. 1995). Thus, rostral area 6, by virtue of its connections with the motor system and the superior parietal lobule, can play a major role in the selection between alternative competing motor acts based on conditional operations.
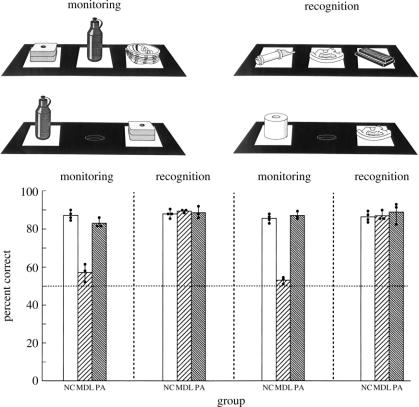
In sharp contrast to the critical role of the caudal prefrontal region in conditional operations, lesions limited to the mid-dorsolateral prefrontal cortex (i.e. area 46 and 9/46) impair performance on working memory tasks that require monitoring of selections from a set of stimuli or the occurrence of stimuli from an expected set (Petrides 1991, 2000a). Monkeys with dorsolateral frontal lesions can remember recently presented stimuli as demonstrated by normal performance on recognition memory tests and on several short-term memory tasks (Bachevalier & Mishkin 1986; Petrides 1991, 2000a). It has been shown that the fundamental problem on working memory tasks of monkeys with mid-dorsolateral prefrontal lesions stems from the monitoring requirements of these tasks (Petrides 1991, 2000a). An example is provided in figure 7.
Figure 7.
(Upper panel): schematic diagram of the experimental arrangement in the self-ordered monitoring working memory condition and the recognition memory condition administered to the monkeys. The upper displays illustrate the presentation trials and the lower displays the test trials in both the monitoring and recognition conditions. (Lower panel): postoperative performance of animals with mid-dorsolateral frontal lesions (MDL), animals with periarcuate lesions (PA) and normal control animals (NC). The mean per cent correct performance over the four postoperative testing blocks (20 days of testing per block) is shown. Solid circles indicate the scores of individual animals in each group. In the monitoring condition, the animals with MDL lesions were severely impaired, whereas the animals with PA lesions performed as well as the NC animals. Both groups with lesions performed as well as the normal control animals in the recognition memory condition. Data from Petrides (1991).
In this experiment, there were two conditions: a monitoring and a recognition memory condition (Petrides 1991). In both conditions, there was first a presentation trial during which the monkey was faced with three objects on white plaques that covered three foodwells, all of which were baited with a food pellet. The monkey selected one of these objects (any one he wished) by displacing the plaque under the object to uncover the foodwell and receive the reward. The presentation trials were identical in both conditions. After a delay of 10 s, there was a test trial during which the monkey faced a choice between two objects. In the monitoring condition, the monkey was faced with the object that he had previously selected and one of the objects that he had not selected. The monkey was required to select the object not previously selected, i.e. reward was available only under the object not selected on the presentation trial. Since both stimuli were equally familiar, the monkey could only perform well on the test trials if he could monitor (track) his earlier choice on the presentation trial. By contrast, in the test trials of the recognition condition, the monkey was faced with the object he previously chose together with a novel object and the reward was under the novel object. Thus, the monkey could perform well on these test trials even if he was not able to monitor his earlier choices provided that he could discriminate the familiar from the novel stimulus. As can be seen in figure 7, the monkeys with mid-dorsolateral prefrontal lesions were severely impaired when they had to decide which one of two equally familiar objects they had previously selected (monitoring condition). Note that the monkeys with PA lesions performed as well as the control animals in this condition. Furthermore, all monkeys performed well if the decision could be made on the basis of recognizing the familiar from the unfamiliar object. Thus, recognition memory that is so severely impaired by lesions of the medial temporal lobe in both human subjects (Milner 1972) and monkeys (Mishkin 1982; Squire & Zola-Morgan 1991) is normal in monkeys with mid-dorsolateral and caudal prefrontal lesions.
(b) Two levels of executive control within the mid-lateral prefrontal cortex: a dorsal–ventral axis of organization
In addition to the caudal–rostral axis of organization that was outlined above, there is a dorsal–ventral axis of organization within the lateral prefrontal cortex. It has been proposed that the mid-dorsolateral and the mid-ventrolateral prefrontal cortex underlie two distinct levels of executive control of cognition (Petrides 1994, 1996; figure 8). As pointed out above, the analysis of the effects of mid-dorsolateral prefrontal lesions on memory established that information can still be maintained in memory, but the capacity to consider (i.e. monitor) multiple pieces of information in working memory is severely reduced (e.g. Petrides 1991, 2000a). I have proposed that the mid-dorsolateral prefrontal cortex (areas 46 and 9/46) is a specialized region where stimuli or events that are first interpreted and maintained in posterior association cortical areas can be re-coded in an abstract form for the purpose of the monitoring of expected acts or events (Petrides 1994, 1996). Once the task at hand is completed, these temporary abstract representations of events or stimuli are deleted. I have argued that this region of the prefrontal cortex evolved, not in order to maintain information for short-periods of time (a process that can easily be sustained by posterior cortical association areas in the absence of the prefrontal cortex), but rather as a system for the conscious active control of planned behaviour and cognition. Such a system must have the capacity to hold abstract coded representations of events that are expected to occur, so as to mark their occurrence or non-occurrence (i.e. monitor their relative status in relation to each other and the intended plan). Furthermore, such a system would be involved in the manipulation of these cognitive representations (i.e. planning) since such manipulation would require constant monitoring of the relative status of intended acts or events. Thus, the involvement of the mid-dorsolateral prefrontal cortex in stimulus manipulation is secondary to its primary role in monitoring. These specific functional contributions of the mid-dorsolateral prefrontal cortex, a region that is very well developed in the primate brain, make possible some aspects of high-level planning and organization of behaviour (Petrides 1994, 1996).
Figure 8.
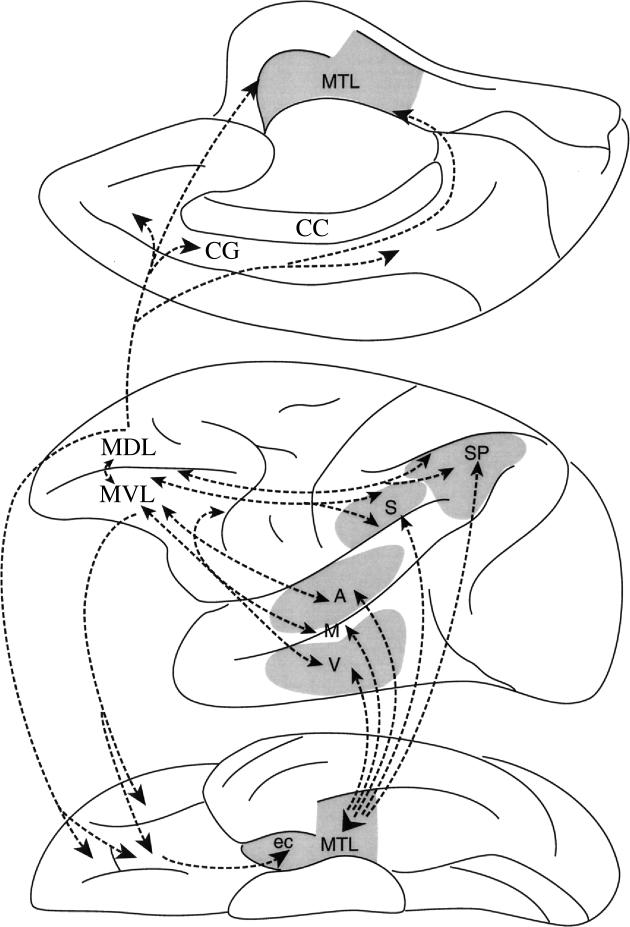
Schematic diagram of the monkey brain illustrating the dorsal–ventral axis of lateral frontal cortex organization. Some of the interactions postulated to underlie the mid-dorsolateral (MDL) and the mid-ventrolateral (MVL) prefrontal region functional organization. Abbreviations: A, auditory processing in superior temporal gyrus; CC, corpus callosum; CG, cingulate gyrus; ec, entorhinal cortex; M, multimodal processing in superior temporal sulcus; MTL, medial temporal lobe region; S, body-centred (i.e. somato-centric) amodal processing in rostral inferior parietal lobule; SP, spatial processing in posterior parietal cortex; V, visual object processing in rostral inferotemporal cortex.
Although not much has been clearly established of the functional contribution of the frontopolar area 10, its cellular structure and connections indicate strong similarities with mid-dorsolateral prefrontal area 46. Area 10 has a sparser cellular appearance than all adjacent areas (including area 46), but it has a well-developed granular layer IV and small to medium size pyramidal cells in layer III that are also characteristic of area 46 (Petrides & Pandya 1994, 1999). There are also some striking similarities in connectional patterns. Anatomical studies have established that the mid-dorsolateral prefrontal region (areas 46, 9/46, 9) has unique access to the hippocampal/parahippocampal region via the retrosplenial cortex (Morris et al. 1999a, b; Petrides & Pandya 1999). Axons originating in the mid-dorsolateral prefrontal cortex are directed medially and, of course, caudally, as part of the cingulum bundle to reach the caudal cingulate region and the adjacent retrosplenial cortex (figure 8), which in turn provides input to the hippocampal region. This medially directed fibre system linking the mid-dorsolateral prefrontal areas with the retrosplenial region is a unique mode of interaction with the hippocampal region, and is probably the anatomical basis of the control that the mid-dorsolateral prefrontal cortex exercises on working memory. This unique access to memory processing in the hippocampal region via the cingulum bundle and the retrosplenial region that is so characteristic of mid-dorsolateral prefrontal cortex is shared by frontopolar area 10, but not by ventrolateral (areas 45, 47/12) or caudal (areas 8A, 6) prefrontal cortex (Petrides & Pandya 2004). Furthermore, both areas 46 and 10 have strong connections with the multimodal cortical region in the upper bank of the superior temporal sulcus (Petrides & Pandya 2004). With regard to local connections within the frontal cortex, area 10 is connected both with adjacent mid-dorsolateral prefrontal cortex (areas 9 and 46) and adjacent mid-ventrolateral prefrontal cortex (areas 47/12 and 45; figure 4). Thus, frontopolar area 10 is in an ideal position to exercise control over adjacent mid-dorsolateral and mid-ventrolateral prefrontal cortex, while sharing with area 46 influences on memory and other cognative processing via unique connections with the retrosplenial cortex and the polysensory temporal region. Furthermore, both areas 10 and 46 are in strong interaction with polysensory processing in the superior temporal sulcus regions. On the basis of these anatomical facts, it seems to me that frontopolar area 10 is in an ideal position to monitor the monitoring process in the mid-dorsolateral prefrontal cortex, namely to engage in what might be called ‘hyper-monitoring’. Such control processing would constitute yet another more abstract level of cognitive control along the rostral–caudal axis of lateral frontal cortex that would be critical in multi-tasking and high-level planning. Thus, area 10 may be thought of as being the highest level in the rostral–caudal hierarchy of lateral frontal control processes (figure 4).
In sharp contrast to the mid-dorsolateral prefrontal cortex, according to the two-level hypothesis of mid-lateral prefrontal control (Petrides 1994, 1996), the mid-ventrolateral prefrontal cortex, in interaction with posterior cortical association areas, subserves the expression of various first-order executive processes, such as active selection, comparison and judgment of stimuli held in short-term and long-term memory (see Petrides 1994, 1996 for details). This type of interaction is necessary for the active (explicit) encoding and the active retrieval of information, i.e. processing initiated under conscious effort by the subject and guided by the subject's plans and intentions, but not for automatic stimulus-driven or context-driven encoding and retrieval of information. There is now considerable evidence from lesion studies in the monkey and functional neuroimaging data obtained in normal human subjects in support of the above proposal (for reviews see Owen 1997; Petrides 2000b).
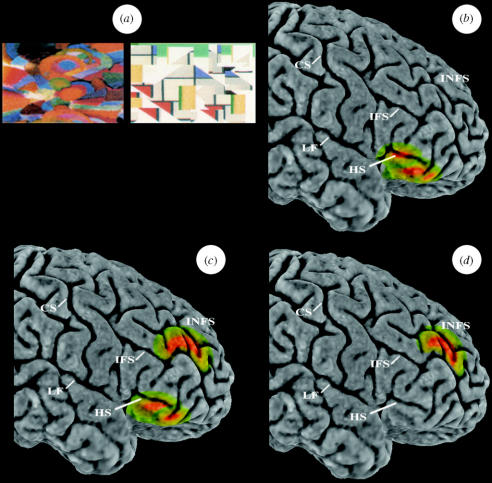
An example of a functional neuroimaging study with normal human subjects that provided support for the above dorsal–ventral distinction is presented here (figure 9). In this study with positron emission tomography, normal human subjects were scanned under different memory conditions. In all conditions, the subjects viewed pairs of abstract visual designs on the screen and had to respond by touching the screen (figure 9a). In the familiarity/novelty decision condition, during scanning, the subjects viewed pairs of abstract designs, one of which had been seen just before scanning, and had to select the novel one by touching it. Activation in this condition was compared with a control condition in which novel and familiar stimuli were presented, but the subjects were now instructed simply to view the images and touch in the space between them to view the next pair. These conditions were designed to test the hypothesis that the mid-ventrolateral prefrontal cortex would be engaged when an active explicit decision was made regarding the relative familiarity of stimuli as opposed to passively viewing novel and familiar stimuli. As can be seen in figure 9b, there was increased activity in the right mid-ventrolateral prefrontal region (area 47/12) in the familiarity/novelty explicit decision condition relative to the control condition. Note the absence of activity in the mid-dorsolateral prefrontal region as a result of this comparison. In the monitoring condition, the subjects again saw pairs of abstract designs and were required to select one of them and touch it. The subjects were told that some of the pairs of stimuli would recur and that in such cases they would have to select the stimulus that they had not previously selected. Thus, during scanning, the subjects were required to decide that some pairs of stimuli were novel and proceed to select one of them, and that others pairs of stimuli were familiar (i.e. the recurring stimuli) and then proceed to select the stimulus that they had not previously selected. Thus, unlike the familiarity/novelty decision condition which consisted of a series of independent trials requiring judgments of the familiarity of stimuli, in the monitoring condition the subjects had, in addition, to keep track of (i.e. monitor) their earlier choices because correct decisions depended on them. When the monitoring condition was compared with the control condition, there was increased activity in both the mid-ventrolateral and mid-dorsolateral prefrontal cortex (figure 9c). Furthermore, when the monitoring condition was compared with the familiarity/novelty explicit decision condition, there was only increased activity in the mid-dorsolateral prefrontal cortex (figure 9d). Thus, the increased requirements for monitoring resulted in a selective increase of activity in the mid-dorsolateral prefrontal cortex.
Figure 9.
(a) An example of a pair of abstract designs used in the functional neuroimaging experiment requiring different types of mnemonic decisions on such visual stimuli. (b) Increased activity in the right mid-ventrolateral prefrontal cortex (area 47/12) during the making of active judgments on the familiarity of stimuli (comparison: familiarity/novelty decision condition minus control condition). (c) Note the additional increase of activity in the right mid-dorsolateral prefrontal cortex (areas 46 and 9/46) in the monitoring condition minus control condition comparison. The observed increase of activity in the mid-ventrolateral prefrontal cortex (area 47/12) was expected because an active decision was included in the monitoring condition. (d) Note that only the mid-dorsolateral prefrontal cortex (areas 46 and 9/46) showed increased activity when the comparison was the monitoring condition minus the familiarity/novelty decision condition. This comparison isolated the mid-dorsolateral prefrontal involvement in monitoring because the active memory decision, which leads to increased activity in the mid-ventrolateral prefrontal cortex, was common to both the familiarity/novelty decision condition and the monitoring condition, while the monitoring requirement was present only in the monitoring condition. Abbreviations: CS, central sulcus; HS, horizontal sulcus; IFS, inferior frontal sulcus; INFS, intermediate frontal sulcus; LF, lateral fissure. Data from Petrides et al. (2002).
Note that the monitoring trials in this functional neuroimaging experiment are identical in requirements to the monitoring trials in the monkey experiment described above (figure 7; monitoring): in both cases the subject (monkey or human) is faced with two equally familiar stimuli and has to decide which one has been selected before. In the monkey, lesions of the mid-dorsolateral prefrontal cortex selectively impair this decision; in the human imaging experiment, there was a selective increase of activity in the architectonically comparable mid-dorsolateral prefrontal cortex during such decisions. Furthermore, note the lack of increased activity in the human mid-dorsolateral prefrontal cortex when familiarity/novelty decisions are made (figure 9b), which is perfectly consistent with the fact that monkeys with mid-dorsolateral prefrontal lesions perform normally on recognition tasks that require such judgments (figure 7: recognition). Thus, the functional neuroimaging results in the normal human brain were entirely consistent with and were predicted by the results of lesion studies in the monkey.
The fundamental contribution to the control of cognitive processing made by specific prefrontal cortical regions (e.g. monitoring by the mid-dorsolateral prefrontal cortex) and which can be isolated in its essential aspects in monkey research will, of course, be involved in all types of cognitive processing (e.g. perceptual, spatial, mnemonic) and, in the more complex human brain, will be adapted for use in linguistic and numerical processing. For instance, the involvement of the mid-ventrolateral prefrontal cortex in active memory retrieval that can be studied in the monkey at the non-verbal level can also be clearly observed in the right mid-ventrolateral prefrontal cortex of the human brain in imaging studies (e.g. Petrides et al. 2002; Kostopoulos & Petrides 2003). In the left hemisphere of the human brain, the mid-ventrolateral prefrontal cortex is also used for verbal episodic and semantic retrieval (Poldrack et al. 1999; Petrides et al. 1995). Thus, the neurobiological correlates of active retrieval can be pursued in an animal model.
In conclusion, the lateral frontal cortex is functionally organized both along a rostral–caudal axis and a dorsal–ventral axis. The most caudal frontal region, the motor region on the precentral gyrus, is involved in fine motor control and direct sensorimotor mappings, whereas the caudal lateral frontal region is involved in higher order control processes that regulate the selection among multiple competing responses and stimuli based on conditional operations. Further, rostrally, the mid-lateral prefrontal region plays an even more abstract role in cognitive control. The mid-lateral prefrontal region is itself organized along a dorsal–ventral axis of organization, with the mid-dorsolateral prefrontal cortex being involved in the monitoring of information in working memory and the mid-ventrolateral prefrontal region being involved in active judgments on information held in posterior cortical association regions that are necessary for active retrieval and encoding of information (see Petrides 1994, 1996).
It must be emphasized that the various levels of executive control posited above are likely to be involved during the performance of several cognitive tasks, often simultaneously. The successful demonstration of the specific contribution of the different prefrontal areas will, therefore, depend on selective lesion studies (e.g. in non-human primates) in which impaired performance on certain tasks designed to tax the proposed executive process (e.g. monitoring) is contrasted with normal performance on appropriate control tasks that do not tax the particular executive process. Most functional neuroimaging studies are not specifically designed to isolate particular abstract executive processes that are hypothesized to depend on particular prefrontal areas. They are simply aimed at comparisons of brain activity evoked during the performance of various cognitive tasks with activity on baseline tasks. Not surprisingly, in such studies, greater activity in several prefrontal areas is often observed because the cognitive tasks used are inevitably tapping, to varying (and often unknown) degrees, the specific abstract executive processes that depend on the various prefrontal areas. As would be expected from the fact that the prefrontal areas are involved in abstract cognitive control processes, meta-analyses reveal greater activity in several prefrontal areas in diverse cognitive tasks relative to their comparison tasks (e.g. Duncan & Owen 2000). Only functional neuroimaging studies that are designed to load particular scanning conditions with aspects of high-level control processing thought to involve one or the other prefrontal region, while carefully maintaining other control processes to the same level, can reveal the specific contributions of particular prefrontal areas.
Acknowledgments
This work was supported by grants from NSERC, CIHR and the James S. McDonnell Foundation.
Footnotes
One contribution of 12 to a Theme Issue ‘Cerebral cartography 1905–2005’.
References
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings H.B.M, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J. Comp. Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Andersen R, Gnadt J.W. Role of posterior parietal cortex in saccadic eye movements. In: Wurtz R, Goldberg M, editors. The neurobiology of saccadic eye movements. Elsevier; Amsterdam: 1989. pp. 315–335. [Google Scholar]
- Andersen R.A, Asanuma C, Essick G, Siegel R.M. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J. Comp. Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav. Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Bailey P, Bonin G. University of Illinois Press; Urbana: 1951. The isocortex of man. [Google Scholar]
- Baillarger J. Recherches sur la structure de la couche corticale des circonvolutions du cerveau. Mém. Acad. R. Méd. 1840;8:149–183. [Google Scholar]
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J. Comp. Neurol. 1988;276:313–342. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam M.-M. Organization afferent input to subdivisions of area 8 in the rhesus monkey. J. Comp. Neurol. 1981;200:407–431. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya D.N. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J. Comp. Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Betz W. Anatomischer Nachweis zweier Gehirncentra. Centralblatt für die medicinischen Wissenschaften. 1874:578–580. (see also pp. 595–599) [Google Scholar]
- Brodmann K. Beitraege zur histologischen Lokalisation der Grosshirnrinde. III. Mitteilung: Die Rindenfelder der niederen Affen. J. Psychol. Neurol. (Lzp) 1905;4:177–226. [Google Scholar]
- Brodmann K. Beitraege zur histologischen Lokalisation der Grosshirnrinde. VI. Mitteilung: Die Cortexgliederung des Menschen. J. Psychol. Neurol. (Lzp) 1908;10:231–246T. [Google Scholar]
- Brodmann K. Barth; Leipzig: 1909. Vergleichende localisationslehre der grosshirnrinde in ihren prinzipien dargestellt auf grund des zellenbaues. [Google Scholar]
- Bruce C.J, Goldberg M.E, Fushnell M.C, Stanton G.B. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Campbell A.W. Cambridge University Press; Cambridge: 1905. Histological studies on the localisation of cerebral function. [Google Scholar]
- Carmichael S.T, Price J.L. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic P.S. Posterior parietal cortex in rhesus monkey. II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J. Comp. Neurol. 1989;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen A.M. Common regions of the human frontal recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Duffy F, Burchfield J. Somatosensory system: organizational hierarchy from single units in monkey area 5. Science. 1971;172:273–275. doi: 10.1126/science.172.3980.273. [DOI] [PubMed] [Google Scholar]
- Economo C, Koskinas G.N. Springer; Wien: 1925. Die cytoarchitektonik der hirnrinde des erwachsenen menschen. [Google Scholar]
- Fuster J.M. Raven Press; New York: 1989. The prefrontal cortex. Anatomy, physiology, and neuropsychology of the frontal lobe. [Google Scholar]
- Halsband U, Passingham R. The role of premotor and parietal cortex in the direction of action. Brain Res. 1982;240:368–372. doi: 10.1016/0006-8993(82)90239-6. [DOI] [PubMed] [Google Scholar]
- He S.Q, Dum R.P, Strick P.L. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J. Neurosci. 1993;13:952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostopoulos P, Petrides M. The mid-ventrolateral prefrontal cortex: insights into its role in memory retrieval. Eur. J. Neurosci. 2003;17:1489–1497. doi: 10.1046/j.1460-9568.2003.02574.x. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Guigon E, Bianchi L, Ferraina S, Caminiti R. Representing spatial information for limb movement: role of area 5 in the monkey. Cereb. Cortex. 1995;5:391–409. doi: 10.1093/cercor/5.5.391. [DOI] [PubMed] [Google Scholar]
- Lewis B.W. Researches on the comparative structure of the cortex cerebri. Phil. Trans. R. Soc. Lond. 1881;171:35–64. [Google Scholar]
- Lewis B.W, Clarke H. The cortical lamination of the motor area of the brain. Proc. R. Soc. Lond. 1878;27:38–49. [Google Scholar]
- Luria A.R. Frontal-lobe syndromes. In: Vinken P.J, Bruyn G.W, editors. Handbook of clinical neurology. vol. 2. North Holland; Amsterdam: 1969. pp. 725–757. [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. Patterns of cytochrome oxidase activity in the frontal agranular cortex of the macaque monkey. Behav. Brain Res. 1985;18:125–136. doi: 10.1016/0166-4328(85)90068-3. [DOI] [PubMed] [Google Scholar]
- Meynert T. Der Bau der Grosshirnrinde und seine ortlichen Verschiedenheiten, nebst einem pathologisch–anatomischen Corollarium. Vierteljahresschrift für Psychiatrie. 1867:77–93. (see also pp. 198–217) [Google Scholar]
- Meynert T. G.P. Putnam's Sons; New York: 1885. A clinical treatise on diseases of the fore-brain based upon a study of its structure, functions, and nutrition. Part I. The anatomy, physiology, and chemistry of the brain. [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin. Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Mishkin M. A memory system in the monkey. Phil. Trans. R. Soc. B. 1982;298:85–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. NeuroImage. 2001;13:684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- Morris R, Pandya D.N, Petrides M. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J. Comp. Neurol. 1999a;407:183–192. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya D.N. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur. J. Neurosci. 1999b;11:2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Mountcastle V.B, Lynch J.C, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J. Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Owen A.M. The functional organization of working memory processes within the human lateral frontal cortex: The contribution of functional neuroimaging. Eur. J. Neurosci. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Motor conditional associative-learning after selective prefrontal lesions in the monkey. Behav. Brain Res. 1982;5:407–413. doi: 10.1016/0166-4328(82)90044-4. [DOI] [PubMed] [Google Scholar]
- Petrides M. Deficits in non-spatial conditional associative learning after periarcuate lesions in the monkey. Behav. Brain Res. 1985;16:95–101. doi: 10.1016/0166-4328(85)90085-3. [DOI] [PubMed] [Google Scholar]
- Petrides M. Conditional learning and the primate frontal cortex. In: Perecman E, editor. The frontal lobes revisited. IRBN Press; New York: 1987. pp. 91–108. [Google Scholar]
- Petrides M. Monitoring of selections of visual stimuli and the primate frontal cortex. Proc. R. Soc. B. 1991;246:293–298. doi: 10.1098/rspb.1991.0157. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and working memory: evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J, editors. Handbook of neuropsychology. vol. 9. Elsevier; Amsterdam: 1994. pp. 59–82. [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Phil. Trans. R. Soc. B. 1996;351:1455–1462. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Petrides M. Dissociable roles of mid-dorsolateral and anterior inferotemporal cortex in visual working memory. J. Neurosci. 2000a;20:7496–7503. doi: 10.1523/JNEUROSCI.20-19-07496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Mapping prefrontal cortical systems for the control of cognition. In: Toga A.W, Mazziotta J.C, editors. Brain mapping: the systems. Academic Press; San Diego: 2000b. pp. 159–176. [Google Scholar]
- Petrides M. The rostral–caudal axis of cognitive control within the lateral frontal cortex. In: Dehaene S, Duhamel G.R, Hauser M, Rizzolatti G, editors. From monkey brain to human brain. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Petrides M, Pandya D.N. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J. Comp. Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D.N. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of neuropsychology. vol. 9. Elsevier; Amsterdam: 1994. pp. 17–58. [Google Scholar]
- Petrides M, Pandya D.N. Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D.N. Comparative cytoarchitectonic analysis in the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D.N. The frontal cortex. In: Paxinos G, Mai J.K, editors. The human nervous system. 2nd edn. Elsevier Academic Press; San Diego: 2004. pp. 950–972. ch 25. [Google Scholar]
- Petrides M, Alivisatos B, Frey S. Differential activation of the human orbital, mid-ventrolateral, and mid-dorsolateral prefrontal cortex during the processing of visual stimuli. Proc. Natl Acad. Sci. USA. 2002;99:5649–5654. doi: 10.1073/pnas.072092299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans A.C. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proc. Natl Acad. Sci. USA. 1995;92:5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A, Wagner A.D, Prull M.W, Desmond J.E, Glover G.H, Gabrieli J.D.E. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Postle B.R, D'Esposito M. Evaluating models of the topographical organization of working memory function in frontal cortex with event-related fMRI study. Psychobiology. 2000;28:132–145. [Google Scholar]
- Preuss T.M, Goldman-Rakic P.S. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate galago and the anthropoid primate macaca. J. Comp. Neurol. 1991;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Robbins T.W. Dissociating executive functions of the prefrontal cortex. Phil. Trans. R. Soc. B. 1996;351:1463–1470. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Sakata H, Takaoka Y, Kawarasaki A, Shibutani H. Somatosensory properties of neurons in the superior parietal cortex (area 5) of the rhesus monkey. Brain Res. 1973;64:85–102. doi: 10.1016/0006-8993(73)90172-8. [DOI] [PubMed] [Google Scholar]
- Sanides F. Springer; Berlin: 1962. Die architektonik des menschlichen stirnhirns. [Google Scholar]
- Sarkissov S.A, Filimonoff I.N, Kononowa E.P, Preobraschenskaja I.S, Kukuew L.A. Medgiz; Moscow: 1955. Atlas of the cytoarchitectonics of the human cerebral cortex. [Google Scholar]
- Schall J.D, Morel A, King D.J, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J. Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Burgess D.F. The domain of supervisory processes and temporal organisation of behaviour. Phil. Trans. R. Soc. B. 1996;351:1405–1411. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Smith G.E. A new topographical survey of the human cerebral cortex, being an account of the distribution of the anatomically distinct cortical areas and their relationship to the cerebral sulci. J. Anat. Physiol. 1907;41:237–254. [PMC free article] [PubMed] [Google Scholar]
- Stanton G.B, Deng S.-Y, Goldberg M.E, McMullen N.T. Cytoarchitectural characteristic of the frontal eye fields in macaque monkeys. J. Comp. Neurol. 1989;282:415–427. doi: 10.1002/cne.902820308. [DOI] [PubMed] [Google Scholar]
- Stuss D.T, Benson D.F. Raven Press; New York: 1986. The frontal lobes. [Google Scholar]
- Squire L.R, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Thieme; Stuttgart: 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- Vicq d'Azyr F. Paris; F.A. Didot: 1786. Traité d'anatomie et de physiologie. [Google Scholar]
- Vogt C, Vogt O. Allgemeinere Ergebnisse unserer Hirnforschung. J. Psychol. Neurol. 1919;25:279–462. [Google Scholar]
- Walker A.E. A cytoarchitectural study of the prefrontal area of the macaque monkey. J. Comp. Neurol. 1940;73:59–86. [Google Scholar]