Abstract
The visual system is constantly challenged to organize the retinal pattern of stimulation into coherent percepts. This task is achieved by the cortical visual system, which is composed by topographically organized analytic areas and by synthetic areas of the temporal lobe that have more holistic processing. Additional visual areas of the parietal lobe are related to motion perception and visuomotor control. V1 and V2 represent the entire visual field. MT represents only the binocular field, and V4 only the central 30°–40°. The parietal areas represent more of the periphery. For any eccentricity, the receptive field grows at each step of processing, more at anterior areas in the temporal lobe. Minimal point image size increases towards the temporal lobe, but remains fairly constant toward the parietal lobe. Patterns of projection show asymmetries. Central V2 and V4 project mainly to the temporal lobe, while peripherals V2 (more than 30°) and V4 (more than 10°) also project to the parietal lobe. Visual information that arrives at V1 projects to V2, MT and PO, which then project to other areas. Local lateral propagation and recursive loops corroborate to perceptual completion and filling in. Priority connections to temporal, parietal and parieto-temporal cortices help construct crude early representations of objects, trajectories and movements.
Keywords: topography, connections, immunohistochemistry, single-units, columns, monkey
1. Introduction
One aspect of the organization of the visual cortex in primates is the presence of multiple representations of the visual field. Although the existence of these visuotopic maps has been known for more than 60 years (Talbot & Marshall 1940; Talbot 1942), there are still many open issues regarding their meaning and organization. The processing of visual information takes place in the occipital, temporal and parietal cortices, which contain a large number of visual areas (Zeki 1969, 1971, 1973, 1974, 1978; Gattass & Gross 1981; Gattass et al. 1981, 1986, 1987, 1990; Rosa et al. 1988; Fiorani et al. 1989; Felleman & Van Essen 1991; Rosa et al. 1993; Neuenschwander et al. 1994; Colby et al. 1988; Piñón et al. 1998). Descriptions of the number, boundaries and visuotopic organization of cortical visual areas vary not only among primates, but also between studies in the same species by different groups.
In primates, the main target of the projections of the lateral geniculate nucleus is the striate cortex, also named as primary visual cortex, area 17 of Brodmann, area OC of von Bonin & Bailey (1947) or visual area 1 (V1). The primary visual cortex is the first of a series of visual areas located in the posterior and medial portions of the occipital cortex. Daniel & Whitteridge (1961) studied its topographic organization and described in the macaque monkey a systematic representation of the retinal surface, with large emphasis on the representation of the fovea, with the representation of the vertical meridian located at the V1/V2 border, and that of the horizontal meridian running across the area, from the opercular surface to the depth of the calcarine fissure. Area V2 is the major cortical projection target of V1 (Kuypers et al. 1965; Cragg & Ainsworth 1969; Zeki 1969, 1971, 1976; Jones & Powell 1970; Zeki & Sandeman 1976; Rockland & Pandya 1979, 1981; Weller & Kaas 1983; Van Essen et al. 1986; Shiwa 1987; Lund et al. 1991). It was studied in the Saimiri by Cowey (1964) who showed that the central representation of this area shares the representation of the vertical meridian with V1. The entire retinotopic organization of V2 was described by Allman & Kaas (1974) in the owl monkey Aotus trivirgatus. These authors showed an organization for V2, which they referred to as a second-order transformation of the visual field that has the representation of the vertical meridian at its posterior border and the horizontal meridian at its anterior border. Allman and Kaas extended their studies to more anterior regions relative to V2 and described a number of additional visual areas (Allman & Kaas 1971, 1974; Allman et al. 1972). In the macaque, pioneer anatomical and electrophysiological studies on the central representation of the extrastriate areas completed by Zeki established a basic framework for the cortical organization of visual areas V2, V3 and V4 as concentric rings around area V1 (Zeki 1969, 1971, 1976). In his studies, Zeki used the regularity of the progressions of receptive field centres in oblique penetrations to the cortical layers as criteria to evaluate the topographic organization or re-representations of the retinal projections onto these areas. The observation of re-representations along oblique penetrations in the prelunate gyrus led Zeki to describe the V4 complex as having multiple representations of the visual field. Similarly, using the same method and the observation of a large overlap of V1 projections to the superior temporal sulcus (sts), Zeki described the ‘movement area’ of the superior temporal sulcus as a non-topographically organized visual area. This area was later named area MT by other authors (Gattass & Gross 1981; Van Essen et al. 1981; Desimone & Ungerleider 1986) to acknowledge its homology in location and topographic organization with the visual area located in the middle temporal lobe of New World monkeys named MT by Allman & Kaas (1971). Gattass and his colleagues extended Zeki's observations to more anterior and medial areas in the extrastriate cortex, describing the locations, topographic organizations and extent of the visual field representation of areas V2, V3, V4 and MT (Gattass & Gross 1981; Gattass et al. 1981, 1988a,b).
In the past 30 years, Macaca and Cebus have been used as experimental models, inasmuch as they are diurnal monkeys similar in brain size, sulcal pattern as well as in certain aspects of visual behaviour. In the Macaca, the visuotopic organization of visual areas V2, V3, V4 and MT (Daniel & Whitteridge 1961; Gattass & Gross 1981; Gattass et al. 1981, 1988a,b), as well as the projections of V2 and the connections of V4 have been described (Gattass et al. 1986, 1997; Ungerleider et al. 2005). In the Cebus, we have defined the location and the topographic organization of areas V1, V2, V4, MT (V5), PO and POd (Gattass et al. 1987; Rosa et al. 1988; Fiorani et al. 1989; Neuenschwander et al. 1994; Piñón et al. 1998). In both animals, these areas contain partial or complete representations of the contralateral visual hemi-field in each corresponding hemisphere.
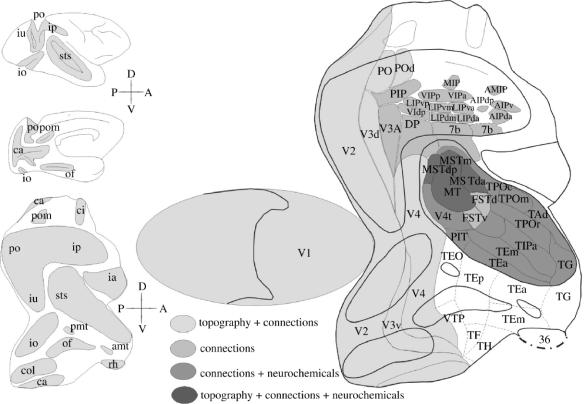
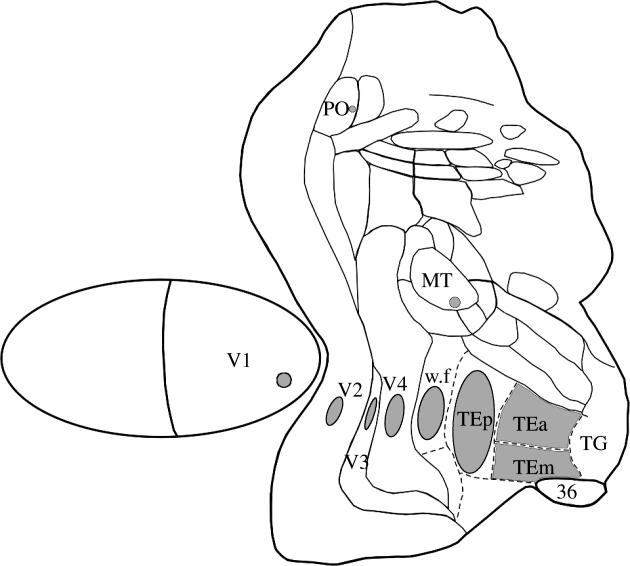
Our studies of V1 and V2 in the Cebus revealed that these areas contain the representation of the entire visual field, whereas area MT (V5) contains only the representation of the binocular portion of the visual field (Gattass et al. 1987, 1990; Rosa et al. 1988; Fiorani et al. 1989). The study of V4 in both Cebus and Macaca revealed that this area has an even more restricted representation of the visual field, extending to only 30° to 40° eccentricity (Gattass 1988; Piñón et al. 1998). We found that the representations of V1 and V2 were anisotropic, predicting the arrangement of cortical modules (columns) orthogonal to the long axis of the anisotropies (Gattass et al. 1987; Rosa et al. 1988). We have also studied the feedback cortical connections to visual area V1 (Sousa et al. 1991). More recently, the areas of the temporal and parietal lobes were divided based on the connections of areas V2 and V4 of the macaque (Gattass et al. 1997; Ungerleider et al. 2005). We also subdivided the intraparietal and superior temporal sulcus of the Cebus into multiple areas based on several criteria such as: afferent and efferent connections, cytoarchitecture, myeloarchitecture, histochemistry for cytochrome c oxidase (CytOx) and for NADPH-diaphorase, and immunohistochemistry for neurofilaments and for calcium-binding proteins (Mariani & Gattass 1996; Nascimento-Silva 2004). The study of single-unit responses and visual topography, connections and architecture of the posterior superior temporal sulcus in Cebus revealed that the area defined by Newsome & Wurtz (1981) and Maunsell & Van Essen (1983) as MST was composed of at least three areas (Nascimento-Silva 2004). Figure 1 shows the location of the cortical visual areas described in Cebus and Macaca. These areas were grouped based on the set of criteria that defined the areas. We also analysed the connection pattern of functionally distinct modules of V2, such as those revealed by CytOx histochemistry, by injecting retrograde fluorescent tracers in V4, MT and PO areas of different streams of visual information processing. The results suggested that different sets of modules of V2 participate in different networks of visual information processing (Nascimento-Silva et al. 2003).
Figure 1.
Two-dimensional reconstruction of the monkey cortex, showing the location of the extrastriate visual areas found in Macaca and in Cebus. The flattened map of V1 was separated from the extrastriate cortex to allow physical flattening of the 3D model of the posterior portion of the hemisphere. Heavy lines indicate the boundaries of the sulci; thin lines indicate the boundaries of the visual, visuomotor and polysensory areas. The dashed lines indicate the boundaries of the temporal areas defined in the macaque, based on cortical connections. The dot–dash lines indicate the boundaries between the neocortex and allocortex. Different grey densities label areas defined by different combination of methods. The grey area on the lateral and medial views of the hemisphere (upper right) and indicated on the 2D reconstruction (lower right) indicate cortex within sulci. Different patterns illustrate the criteria used to define the borders of the areas. White areas in the temporal lobe were defined only in the Macaca. d, dorsal; v, ventral; p, posterior; a, anterior. For names of areas and sulci, see glossary.
In this paper, we review the organization of cortical visual areas in Macaca and Cebus monkeys, focusing on their location, visual topography, connections, columnar organization, cortical plasticity and cortical dynamics. First, we discuss the concept of a cortical visual area and the limitations of each method used to study the areas. Then, we review the organization of the visual areas described by electrophysiological mapping, by connectional methods and by immunohistochemical, myeloarchitectural and cytoarchitectonical methods. In this process, we discuss the types of visual map present in each area. Then, studying the maps in more detail, we focus our attention to local representations in cortical modules, some of them revealed by cytochrome oxidase histochemistry. We also address the contribution of local, feed-forward and feedback connections. Finally, we present a view of the visual system as a topographically organized distributed network.
2. Conceptual and methodological considerations
(a) Definition of cortical visual areas
The definition of a cortical visual area takes into account combined evidences obtained by different experimental paradigms. Anatomical, electrophysiological and behavioural methods can contribute to definition of a cortical visual area. In our view, the method that offers the most complete and precise description of the visuotopic organization of cortical areas is that of systematic electrophysiological mapping of neural responses to visual stimulation. This method was first applied to primate extrastriate areas more than 40 years ago (Cowey 1964; Hubel & Wiesel 1970; Allman & Kaas 1971; Dubner & Zeki 1971; Gross et al. 1972). Electrophysiological mapping has proven to be an adequate method to reveal many retinotopically organized cortical visual areas in the primate brain (Van Essen & Zeki 1978; Gattass & Gross 1981; Gattass et al. 1981, 1987, 1988a, 1990; Burchalter & Van Essen 1983; Maunsell & Van Essen 1983; Felleman & Van Essen 1984; Ungerleider & Desimone 1986b; Rosa et al. 1988, 1993; Fiorani et al. 1989; Boussaoud et al. 1990; Neuenschwander et al. 1994; Piñón et al. 1998).
With new improvements, it is now possible to use multiple electrodes to sample a much larger area in a single animal, therefore reducing the error involved in pooling topographical data obtained in different experimental sessions and in different individuals into a single ‘representative’ map for a given area. In principle, it is possible to combine high-resolution topographic mapping of visual responses with single-unit analysis of response properties, thereby yielding a much more reliable definition of boundaries between areas. In practice, however, one is still confronted with the opposing alternatives of mapping a few simple parameters, such as receptive field size and position, across a large extent of cortex, or conducting quantitative analysis of cell response properties at fewer sites. Long-term chronic preparations, while allowing a detailed study of large populations of single-units, suffer from limited precision regarding the location of the recording sites, especially within the sulci. As high-resolution neural imaging techniques in vivo become more accessible to research laboratories, the precise three-dimensional (3D) reconstruction of recording sites during experimental sessions may become possible. This development will allow the combination of topographic and functional analyses of large populations of neurons in single animals.
(b) Data interpolation, back-transformed maps and topographical sign analyses
In the last two decades, techniques have also been developed for automatic interpretation of electrophysiological data, providing ‘bias-free’ maps of cortical visual topography within a pre-defined area. One such technique involves the use of computer routines to interpolate visuotopic coordinates between points sampled by electrode penetrations within a single area, based on digitized grids of recording sites and receptive field coordinates (Maunsell & Van Essen 1987; Fiorani et al. 1989). This technique allows quantification of certain visuotopic parameters that do not become evident through the analysis of individual receptive fields. However, it does not address the possibility of ambiguous boundaries between areas, and its optimal use requires that the boundaries of the area under study be determined prior to the application of the interpolation algorithm (Neuenschwander et al. 1994). Otherwise, contamination of the data matrix with samples taken from adjacent areas may result in an unreal impression of complex topography, involving duplications or discontinuities. In contrast, the more recently described method of Sereno et al. (1994) does not require predetermination of the boundaries of the areas. This technique, which is based on the interpolation of coordinates across the entire dataset, uses local visual field sign as a criterion for automatic subdivision of the cortex into areas. Visual field sign is a local measure of chirality: whether the visuotopic map, as seen from the cortical surface, forms a mirror- or non-mirror image of the animal's visual field. The assumption behind this technique is that the entire cortex belonging to a single area represents the visual field according to the same field sign; therefore, transitions in field sign should correspond to borders between areas. The main merit of this approach is that it generates an impartial hypothesis regarding both the area boundary and the visual topography, based on the same dataset. However, the ‘human factor’ has not been totally removed, as the exact final configuration of the maps is dependent on parameters used by the interpolation algorithm that are subjectively chosen by the experimenter. Moreover, fine‐scale transitions of visual field sign have been reported within a single area, such as V2 (Roe & Ts'o 1995), as a consequence of segregation of function in stripe-like compartments. Another potential problem, which is common to all of the current ‘bias-free’ methods, is that the algorithms assume local continuity of the maps and cannot be used in areas where discontinuities may be a real feature of the visuotopic map.
(c) Area definition by anatomical methods
The visuotopic organization of one area can also be inferred by tracing the connections to and from another area, for which the visual topography has already been determined (e.g. Cragg & Ainsworth 1969; Zeki 1969; Spatz et al. 1970; Ungerleider & Mishkin 1979; Maunsell & Van Essen 1983; Weller & Kaas 1983; Weller et al. 1984; Newsome et al. 1986; Perkel et al. 1986; Van Essen et al. 1986; Colby et al. 1988; Cusick & Kaas 1988; Rosa et al. 1988, 1993; Krubitzer & Kaas 1990; Sousa et al. 1991; Steele et al. 1991). This method relies on the observation that cortico-cortical connections preferentially link cells that represent the same region of the visual field (Cowey 1964). The definition of the boundaries of the cortical visual areas based on anatomical connections has limitations related to the paucity of data in each cortical area and in each animal. The connectional data, however, have an important role on the definition of the basic framework of the organization of the cortical areas. Connectional data are adequate to show upper and lower field representations in several extrastriate visual areas.
One important assumption that has been used to define borders of a cortical area is the demonstration of coherent patterns of connections with other parts of the brain: different portions of a same area should receive or project to a similar set of areas. The inputs and outputs of an area would remain constant throughout its extension, a rule that we will discuss later in this review. However, for the purposes of a first definition of visuotopic organization, the picture provided by studying connections alone is, at best, coarse. This imprecision stems, in part, from the fact that cortico-cortical connections may also integrate representations of portions of the visual field that are not exactly matching (for a review, see Salin & Bullier 1995). In addition, because adjacent areas often share inputs (or outputs), the possibilities of erroneous interpretations are many.
Finally, metabolic mapping methods such as 2-deoxyglucose, in vivo imaging of intrinsic signals, and functional nuclear magnetic resonance imaging can also be used to study visuotopic representations. To date, these methods have been an important tool for analysis of the visuotopy of the areas already described in non-human primates (Tootell et al. 1982, 1988; DeYoe et al. 1994; Frostig 1994; Grinvald et al. 1994) using traditional electrophysiology, but allowing simultaneous determination of visuotopy, functional response properties and connections in extrastriate cortex, without the need of prohibitively long experiments (Tootell & Hamilton 1989; Malach et al. 1994; Malonek et al. 1994; Sereno et al. 1995).
(d) The use of 2D reconstructions of the visual cortex
In the absence of unequivocal anatomical limits to the cortical visual areas, many authors adopted the use of bi-dimensional reconstructions of the cortical surface to assign recording sites or projections zones to a given site. The assumption of this method is that the organization of the cortical visual areas is fixed and that the location of the areas in relation to the V1/V2 border is stable. We have been using two-dimensional (2D) reconstructions on our electrophysiological mapping and connectional studies, in addition to the use of other anatomical and/or physiological criteria, to define the borders of each visual area (Gattass et al. 1987).
(e) Definition of borders of visuotopically organized areas
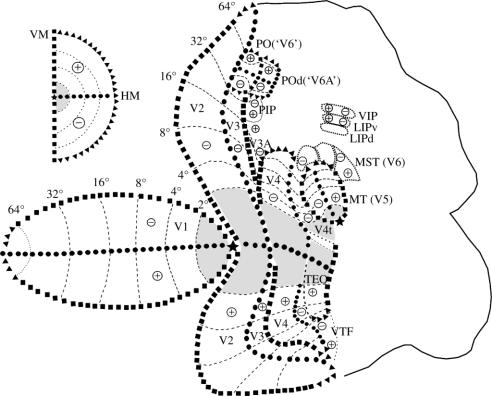
The myeloarchitectonic and the electrophysiological transitions were used to define the borders of the cortical visual areas. Myeloarchitectonic borders were defined at the transitions between two distinct myeloarchitectonic patterns, whereas electrophysiological borders were defined in regions where reversals of receptive field progressions occurred. Figure 2 shows visuotopically organized cortical areas with their boundaries in a flattened representation of occipital, parietal and temporal cortices. Represented in the figure are the visual topography of the first (V1) and second (V2) visual areas, of the ventral and dorsal portions of the third visual area (V3), of the ‘colour area’ of the prelunate sulcus (V4), of the area of the occipitotemporal transition (TEO), of the temporal ventral posterior region (TVP), of the areas of the posterior bank of the superior temporal sulcus, the ‘movement area’ (MT or V5) and its adjacent dorsal zone (V4t or DZ), and of area MST, originally studied by Dubner & Zeki (1971) and later defined by Newsome & Wurtz (1981) and Maunsell & Van Essen (1983). At the anterior bank of the parietal occipital sulcus there are two topographically organized areas now named area PO (or ‘V6’ of Galletti et al. 1996, 1999) and the adjacent parieto-occipital dorsal area POd (or ‘V6A’). In the parietal region are represented the areas VIP, at the fundus of the intraparietal sulcus, and LIP, on the lateral bank.
Figure 2.
Visuotopic organization of the cortical visual areas shown on a 2D reconstruction of a monkey cortex. The vertical meridian is represented by black squares, the horizontal meridian by black circles, the eccentricity lines by dashed lines, the visual field periphery by black triangles. A star illustrates the representation of the fovea. + and − show upper and lower fields, respectively. Insert illustrates the contralateral visual hemi-field. For names of areas, see Glossary.
3. Irregularities in the visual maps
In both V1 and V2 of Cebus, we have observed that at a given eccentricity the cortical magnification factor (CMF) is higher when measured between points located along an isopolar rather than along an isoeccentric line. This anisotropy in visual representation is less pronounced in V1 than in V2, where the isopolar CMF is usually 50% higher than the isoeccentric CMF. This anisotropy in the cortical magnification factor is reflected by the oval shape of the minimum point image size (MPIS), calculated as the product of cortical magnification factor by multiunit receptive field size. More recently, we have been studying the regularity of visual maps in other areas of the Cebus apella. In ventral V3 (V3v), for example, we observed an anisotropy that is even more pronounced than that of V2: isopolar CMF in V3v is at least twice as large as the isoeccentric CMF (Rosa et al. 2000). There is also evidence for a similar amount of anisotropy in the portion of ventral V3 located at the anterior bank of the lunate sulcus and in the ventral portion of V4 (Piñón et al. 1998).
While areas corresponding to the first stages of the ventral pathway are all characterized by anisotropies, a similar analysis carried out in MT did not yield any evidence for anisotropy in its visual map (figure 3). In the areas of the dorsomedial pathway, PO and POd, we found a different type of irregularity in the visual map: it was always possible to define the representations of isopolar lines, but not those of the isoeccentric lines. At present, we have no direct evidence regarding the functional significance of the observed ‘order in the isopolar’ versus ‘disorder in the isoeccentric domain’ in these areas. One may speculate that centrifugal and centripetal organizations of directionality, such as those observed in area PG, which is connected to PO, demand interactions between neurons that analyse regions of space sharing a similar polar angle but with different eccentricities. It may be that the intermixing of eccentricities of receptive fields in adjacent columns in PO and POd allow these interactions to occur at local circuits. This arrangement would be considered a ‘non-visuotopic’ map, but nonetheless a topographically organized isopolar functional map. It would not be surprising to find a similar type of organization at other levels of the central nervous system as, for example, at the oculomotor system. The use of large electrode arrays progressing systematically through the cortical layers may reveal other types of organization in areas of the anaectant gyrus and neighbouring regions of the intraparietal and parieto-occipital sulci.
Figure 3.
Anisotropies in cortical visual maps illustrated by the asymmetries of the minimal point image size in different visual areas, shown on a 2D reconstruction of a monkey cortex.
4. Modular organization
The anisotropies in V1, V2 and V3v in the Cebus have motivated studies on the intrinsic organization of these areas. Tangential sections of the striate cortex of Simiiform primates stained for CytOx showed a periodic pattern of heavily stained, oval-shaped ‘blobs’ in a matrix of less reactive ‘interblob’ regions (Horton & Hubel 1981; Wong-Rilley & Carrol 1984). To evaluate the regularity of the modular organization of V1 in primates, we studied the distribution of CytOx blobs in flattened preparation of V1 (Rosa et al. 1993; Farias et al. 1997). Blobs are present throughout V1 in all already-studied primate species. Another aspect of the modular organization of V1 is the existence of ocular dominance (OD) columns. In the macaque, the observed anisotropy of V1 has been interpreted as a result of the orientation of the OD stripes. The CMF would be greater along the axis perpendicular to the stripes owing to the need of representing twice the same region of the visual field, once for each eye. In Cebus (Rosa et al. 1988a; Gattass et al. 1988), the study of the distribution of OD stripes revealed that their orientation would account for the anisotropy in the representation of the visual field in V1. These results are consistent with the notion that the visual map of V1 may be made up of two partially overlapping visual maps, one for each eye (Gattass et al. 1987).
In V2, modules as revealed by CytOx histochemistry are also stripe-like, running from the anterior border of V2 to its border with striate cortex. Three types of stripes are currently recognized in V2, namely the thin and thick CytOx-rich stripes as well as the CytOx-poor interstripes, which differ in neuronal response properties and connections (Tootell et al. 1982; Livingstone & Hubel 1983; DeYoe & Van Essen 1985; Shipp & Zeki 1985, 1989; Rosa et al. 1988; Levitt et al. 1994; Roe & Ts'o 1995; Gattass et al. 1997; Olavarria & Van Essen 1997). Both the thin stripes and the CytOx-poor interstripes, through their connections with V4, seem to be related to the ventral stream, whereas the thick stripes, by way of their connections with MT, seem to be related to the dorsolateral stream of visual processing. In the Cebus, to evaluate whether there is a segregation of the streams of visual information processing in CytOx modules of V2, we injected fluorescent retrograde tracers into V4, MT and PO and studied the distribution of the labelled cells in these modules. The distribution of labelled cells provided evidence for three streams of visual processing with origins in different CytOx modules in area V2, with the subdivision of the dorsal stream of visual information processing into a dorsomedial and a dorsolateral component (Nascimento-Silva et al. 2003). We have, therefore, reasoned that the same portion of the visual field should be represented multiple times in V2 in stripes of different kinds (Rosa et al. 1988). Taking into consideration the periodical arrangement and orientation of the stripes and assuming little or no overlap between neighbouring modules, one would expect a ratio of isopolar-to-isoeccentric anisotropy in V2 of 3–4 : 1, in comparison with the observed anisotropy ratio of 3 : 2. We have, therefore, suggested that heterogeneous sub-modules should form each stripe (Rosa et al. 1988). In a later study using sections stained for CytOx in V2 of Cebus monkeys (Gattass et al. 1990), we demonstrated that the thin bands are composed of a series of equally spaced puffs. Studies in enucleated animals suggest that the thick bands may be similarly organized.
Regarding area MT, CytOx-stained tangential sections did not reveal a modular organization. In the owl monkey, area MT shows a dark oval region with fuzzy, randomly distributed light sub-regions. In MT of the Cebus, we found no evidence for modularity using the same histochemical method. This finding is coherent with our current interpretation of the data inasmuch as no anisotropies were observed in this area for the Cebus.
5. Adult blobs plasticity
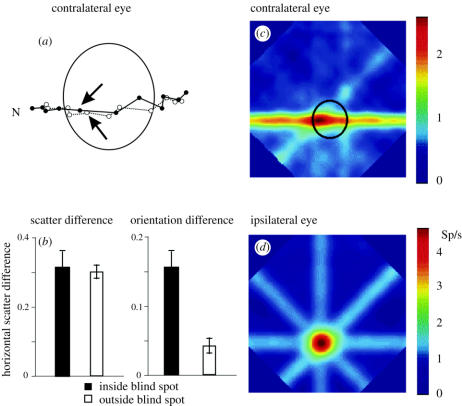
The plastic changes in V1 blobs of adult primates depend on the severity of visual deprivation and on the amount of time of deprivation. Restricted lesions, produced by small laser shots, and massive retinal lesions, produced by laser lesions at the border of the blind spot, induce different degree reorganization on CytOx-rich layer III blobs, with different time courses. These data suggest two independent plastic mechanisms for blob plasticity: one for the decrease of blob size on deprived columns and another for the increase of blob size on non-deprived columns.
Blob cross-sectional area and spatial density were analysed in CytOx-reacted tangential sections of flat mount preparations of V1 in Cebus with focal and massive retinal laser lesions. In cases with focal retinal lesions, the cross-sectional area and spatial density of deprived blobs decrease after 3 days of lesion. There is no variation in cross-sectional area, spatial density or the total area occupied by non-deprived blobs when compared with the control ones. In cases with massive lesions, blob cross-sectional area, blob spatial density and the total area occupied by deprived blobs decrease after 1 day of lesion. The cross-sectional area and the total area occupied by non-deprived blobs increases after 5 days of lesion (Farias 2002). Figure 4a shows a decrease in mean blob size of deprived blobs, with time, in an animal that received focal and restricted laser lesions at different times, whereas figure 4b shows an additional increase of blob size of undeprived blobs, after massive retinal lesions at different times. In both cases there is a significant decrease in blob size of deprived columns (Farias 2002).
Figure 4.
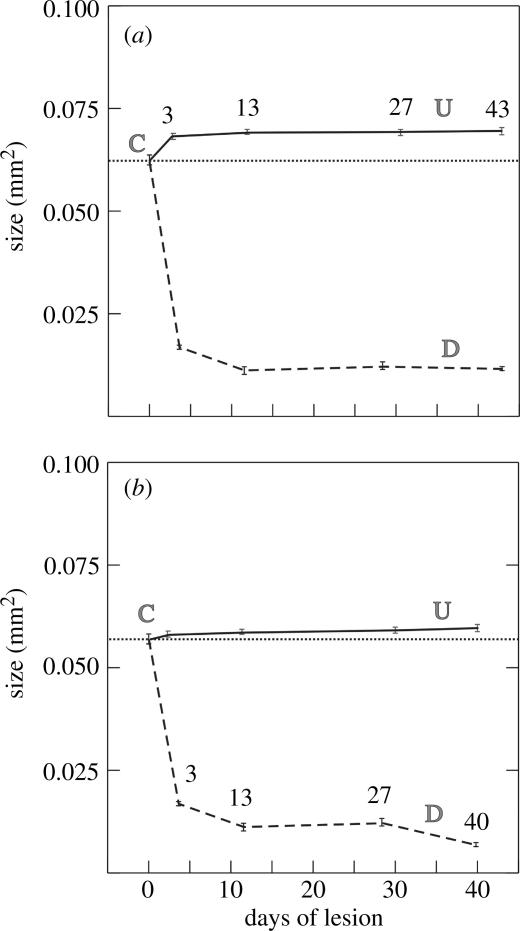
Changes in blob cross-sectional area in V1 after massive (a) and focal (b) retinal laser lesions at different times, from 3 to 43 days. Undeprived blob sizes (continuous lines) increase after massive retinal lesions. Deprived blob sizes (dashed lines) decrease drastically in both cases. Bars, standard error of the mean; C, control (non-lesioned area).
Normal Cebus monkeys showed a complementary labelling in V1 of calbindin (Cb) and parvalbumin (Pv). Layers II, IVb, the superior third of layer IVcα and the inferior third of layer IVcβ are intensely labelled by Cb. Layer V shows stronger labelling than layer VI. In layer III, the interblobs are more densely labelled than the blobs. By contrast, Pv stained preferentially the blobs and layers IVa, IVc and VI. Figure 5 shows the distribution of Cb and Pv in V1, 28 days after a series of laser lesions around the optic disc of one eye. The distributions of Cb and PV were differentially altered. The deprived ocular dominance columns showed a reduction of Cb neuropil (layers I–V) and labelled cell bodies (layers II, III, IVb and IVc). By contrast, the Pv decreased in deprived ocular dominance columns only at the neuropil in layers III, IVa and IVc (Botelho 2004).
Figure 5.
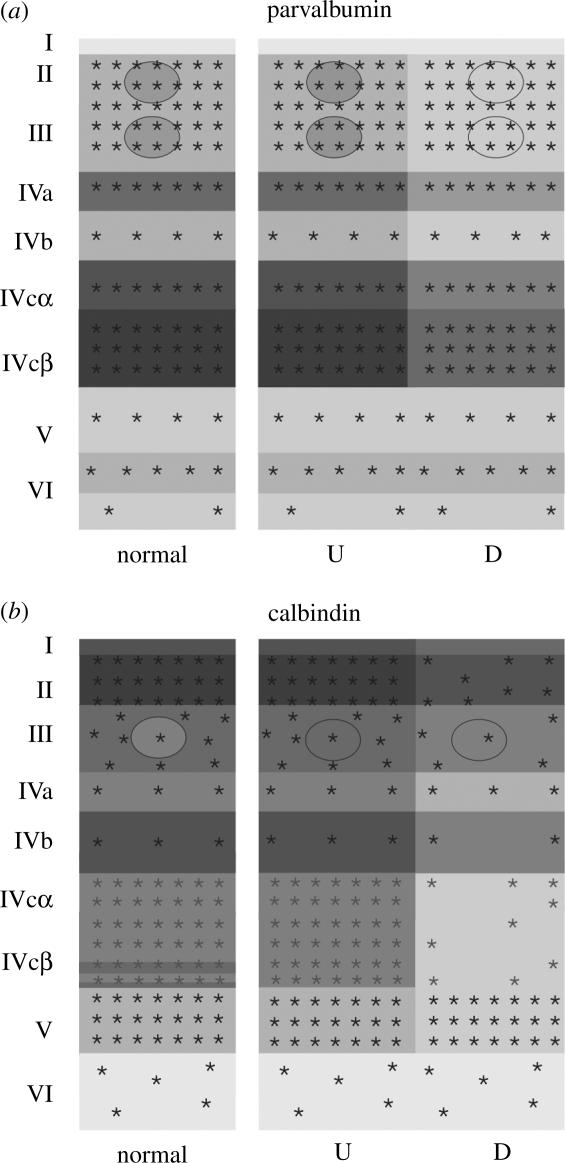
Schematic of the laminar distribution of cell bodies (asterisks) and neuropils (greys) in coronal sections of V1, stained for parvalbumin (a) and calbindin (b), after massive retinal laser lesions. Schematics of the stains are illustrated for normal and for deprived (D) and undeprived (U) columns.
In humans with retinal lesions blob size changes drastically in the region corresponding to lesion, although its spatial density remains constant throughout the binocular field representation in V1. The regions showing conspicuous OD columns showed different degrees of variability of blob plasticity. In the region representing the retinal lesion, blobs are larger and darker above the undeprived OD stripes than in the alternative deprived stripes. Although deprived blobs are on average smaller than those from normal regions, this difference was not significant. Our results suggest that plasticity in V1 after retinal lesions caused by chronic diseases in adult humans is different from that described after acute retinal lesions in non-human primates. In humans, the size increase of undeprived blobs prevails over the decrease of deprived blob sizes. These results suggest the existence of additional mechanisms to regulate blob size other than simple competitive interactions between CytOx-rich blobs (Marcondes 1997).
6. Directional columns in MT
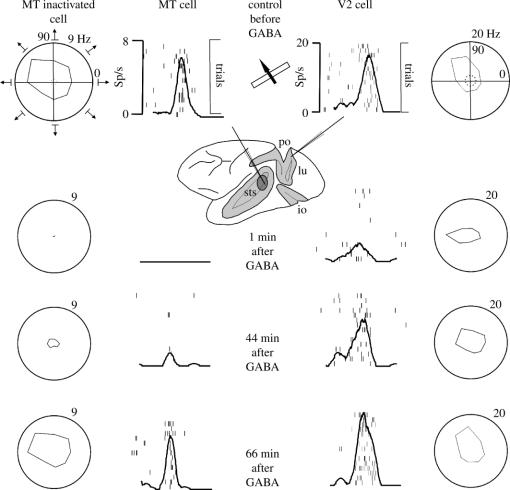
To study the directional columns in MT, we recorded neuronal activity from multielectrode arrays as they were stepped through the area, at a plane parallel to the cortical layers. At each recording site, we determined the preferred direction of motion. Responses recorded at successive locations from the same electrode in the array revealed gradual changes in preferred direction, along with occasional directional reversals. Systematic comparisons of responses from adjacent electrodes, at successive locations, allowed the reconstruction of the 2D pattern of preferred directions across the cortex (Diogo et al. 2002). The data were obtained using the 11 electrode array and 12 sampling nodes, at 200 μm intervals. Between these discrete nodes we interpolated responses to visual motion and then used the interpolated responses to construct 2D maps of preferred direction. We then constructed a set of 12 2D maps of normalized neuronal activity—one for each of the 12 tested directions of motion. Next, each of the 12 conditional activity matrices was multiplied by a unit vector, the direction of which corresponded to the stimulus motion used to obtain the measured neuronal responses for that matrix. These multiplications yielded a new set of 12 matrices, in which each element was a vector of direction corresponding to the stimulus motion and of length proportional to firing rate (measured or interpolated) at that map location. Finally, a matrix representing preferred direction at each map location was obtained by summing the 12 vectorial matrices. The direction and length of the resulting vector sum at each map location reflect, respectively, the neuronal preferred direction and strength of selectivity at that location. This vector-sum matrix was used to produce a 2D map of directionality, in which the preferred direction at each map location was represented by a colour code. The electrophysiological imaging demonstrates a systematic organization for directionality in area MT of the New World Cebus monkey, which is similar to that known to exist in the Old World macaque monkey (Albright 1984). The tangential organization of directional selectivity is characterized by slow continuous changes in directional preference, as well as by the presence of lines (fractures) and points (singularities) that fragment continuous regions into patches (figure 6). These electrophysiological methods also allowed a direct investigation of neuronal selectivity that gives rise to map features. In particular, our results suggest that inhibitory mechanisms may be involved in the generation of fractures and singularities (Diogo et al. 2003).
Figure 6.
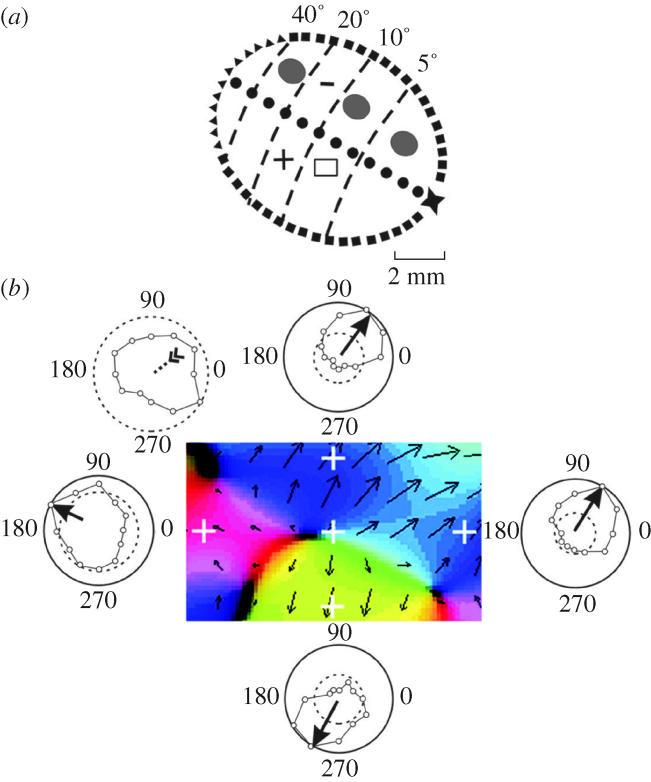
Direction map and single-unit in MT: illustration of neuronal responses that gave rise to a pinwheel map formation. (a) Visual topography of MT showing the location of map region highlighted in panel b. Three minimal point image sizes in MT are illustrated at different eccentricities. (b) Magnified view of a rectangular region of cortex showing a portion of the direction map with a pair of directional singularities (at centre and lower right) with corresponding pinwheel formations, which are linked by a fracture. Each pinwheel is composed of a half-rotation (180°) and a fracture. White crosshairs indicate the locations of five recording sites, which include a site near the pinwheel centre and four sites around the perimeter. The central site exhibited a weak form of directional tuning that was entirely shaped by inhibition. The remaining sites were excitatory and uni-directional. Dashed circle, spontaneous firing rate; external circle, maximum firing rate at the recording site.
7. Cortical visual areas
(a) Areas defined by electrophysiological mapping and connections
(i) Area V1
In the macaque, Daniel & Whitteridge (1961) described a systematic representation of the retinal surface, with large magnification of the fovea representation in the primary visual area, or V1. Their model for V1 established a relation between the representation of the retinal surface or of the ‘cortical map’ with a functional area in the cortex. The studies of Hubel & Wiesel (1967, 1968, 1972) on single-unit properties of V1 established the basis for a hierarchical model and the notion that single-unit properties would become gradually more complex at successive stages of visual processing. They also described functional modules in V1 capable of decoding ocular dominance and orientation of the visual stimuli. We studied the topographic representation of V1 in the Cebus and we found a small anisotropy along the isopolar domain at the peripheral representation of V1. This anisotropy could be related to the orientation of the OD columns of peripheral V1 (Gattass et al. 1987).
We have also addressed the contextual and surrounding influences contributing to the dynamics of receptive-field properties in V1. We found that at the representation of the blind spot, as well as throughout the extension of V1, inputs from large and relatively distant portions of the retina may sometimes influence the response of single cells. This dynamic property of the cell response was characterized as ‘perceptual completion of oriented lines’ and showed that the map of V1 was visuotopic (Fiorani et al. 1992). More recent findings from our group have demonstrated that visuotopic representation of V1 predicts well the receptive field location, but sometimes fails to predict stimulus orientation or direction (Azzi 2004).
(ii) Area V2
In macaques, the major cortical projection target of area V1 is area V2 (Kuypers et al. 1965; Cragg & Ainsworth 1969; Zeki 1969, 1971, 1976; Jones & Powell 1970; Zeki & Sandeman 1976; Rockland & Pandya 1979, 1981; Weller & Kaas 1983; Van Essen et al. 1986; Shiwa 1987; Lund et al. 1991). V2 is located within area 18 of Brodmann and corresponds to area OB of von Bonin & Bailey (1947). The visual map of V2 (Gattass et al. 1981; Rosa et al. 1988) corresponds to a second-order transformation of the visual field with the representation of the vertical meridian at the posterior border of the area. The horizontal meridian splits at about 0.5° and forms the anterior border of the area. The lower field is represented dorsally whereas the upper field is represented ventrally (Allman & Kaas 1971; Gattass et al. 1981; Rosa et al. 1988). Although a number of studies have described projections from V2 back to V1 and feed-forward projections from V2 to several visuotopically organized extrastriate areas, including V3, V4, MT and PO, these reports were based almost entirely on injections of retrograde tracers outside of V2 (Rockland & Pandya 1981; Felleman & Van Essen 1983, 1984; Maunsell & Van Essen 1983; Festemaker et al. 1984; DeYoe & Van Essen 1985; Kennedy & Bullier 1985; Shipp & Zeki 1985, 1989; Burkhalter et al. 1986; Ungerleider & Desimone 1986b; Colby et al. 1988; Zeki & Shipp 1989; Boussaoud et al. 1991; Nakamura et al. 1993). The only exception is a report by Zeki (1971) describing anterograde degeneration in V3, V4 and MT after small lesions in V2, and, in that study, the only part of V2 examined was the representation of the lower half of the central visual field. We studied the projection of V2 in the macaque (Gattass et al. 1997) and found that all V2 sites project topographically back to V1 and forward to V3, V4, and MT. There is also a topographically organized projection from V2 to V4t, but this projection is limited to the lower visual field representation. Thus, V2 appears to project to virtually all visual areas within the occipital lobe. In addition to projections to occipital visual areas, V2 sites representing eccentricities of about 30° and greater project to three visual areas in parietal cortex, namely the medial superior temporal (MST), parieto-occipital (PO) and ventral intraparietal (VIP) areas. This peripheral field representation of V2 also projects to area VTF, a visual area located in area TF on the posterior parahippocampal gyrus.
(iii) Area V3
Currently there are still two different views regarding the organization of V3 in macaques. Based on electrophysiological mapping studies, Gattass and his colleagues (Gattass et al. 1988) have argued that the entire region bordering V2 anteriorly is a single visual area, which contains a representation of the visual field up to 30° to 40° eccentricities in both the upper (V3v) and lower (V3d) visual quadrants. By contrast, Van Essen and his colleagues have argued that V3d and V3v are different visual areas based on differences in projections from V1, myeloarchitecture, and neural response properties (Burkhalter et al. 1986; Newsome et al. 1986; Van Essen et al. 1986). These investigators have termed the upper and lower visual field representations anterior to V2 as areas V3 and VP, respectively. V1 may project asymmetrically to V3, but V2 does not. Whereas the upper field representation of V2 projects to V3v, the lower field representation of V2 projects to V3d. Further, central field representations of V2 project laterally within both V3v and V3d, while more peripheral field representations project more medially. Our results also show that the visual field representation within V3 may extend beyond 40° eccentricity but does not extend to 70°. Thus, there is a reduction in the extent of the visual field represented as one moves from V2 to V3, consistent with the findings from electrophysiology (Gattass et al. 1981; Gattass 1988). Finally, the results in the macaque show that V3d shares the representation of the vertical meridian with areas V4 and PO, which is again consistent with findings from electrophysiology. There was a large inter-animal variability in the visual topography of V3d, with reflections in the organization of V3A (Gattass 1988).
A representation of the upper visual quadrant adjacent to the second visual area (V2) in ventral prestriate cortex was suggested by Cragg & Ainsworth (1969) and Zeki (1969) after anatomical tracing experiments in Old World (macaque) monkeys. This region was considered to be part of a ‘third visual area’ (V3), which wrapped around V2 both dorsally and ventrally. In later years, the organization of the macaque ‘third tier areas’ (Allman & Kaas 1975) became the subject of intense scrutiny, which revealed significant anatomical and physiological differences between the proposed dorsal (lower quadrant representation) and ventral (upper quadrant representation) subdivisions of the original ‘V3’ (Burkhalter et al. 1986; Newsome et al. 1986; Van Essen et al. 1986; Felleman & Van Essen 1987; Krubitzer & Kaas 1993; Beck & Kaas 1999). This has resulted in the hypothesis that, in Old World monkeys, the ventral and dorsal parts of the original ‘V3’ are regarded as separate areas, each containing an incomplete representation of the visual field (e.g. Felleman & Van Essen 1991). This hypothesis does not find support on the connections of dorsal and ventral V2, which projects to V3d and V3v, respectively (Gattass et al. 1987). The width of the middle portion of V3d varies from animal to animal. Some of the discrepancy based on anatomical data of the projection of V1 may be related to such variability.
(iv) Area V4
Zeki first studied the prelunate gyrus in the macaque in 1969 and described it as an area that receives projections from V2 and V3 and represents the central portion of the visual field. Based on these criteria he divided the prelunate gyrus into areas V4, located in the anterior bank of the lunate sulcus, and V4A, located in the prelunate gyrus. Van Essen & Zeki (1978) studied the prelunate gyrus and named this region as ‘V4 complex’ because a single point of the visual space was represented multiple times in this area. The region they studied only contained the representation of the first 10° of the inferior quadrant of the visual field and probably included a large part of area TEO. Zeki (1978) extended his recordings to the superior temporal sulcus in an area lateral to area MT and found a topographic organized area that was later named area V4t (Maguire & Baizer 1984). Maguire & Baizer (1984), studying the visual topography of the prelunate in the awake-behaving macaque monkeys, divided this gyrus into four areas: anterior lateral (AL), posterior medial (PM), dorso-posterior (DP) and V4 transitional or V4t. According to their data, area PM corresponds to area V4 and area AL might correspond to area V4A defined by Zeki (1969). Areas AL and PM have a crude representation of the 30° of the inferior quadrant. Area AL includes the prelunate gyrus and the posterior part of superior temporal sulcus whereas area PM includes the prelunate gyrus and the anterior portion of the lunate sulcus. Dorsal to these two areas is area DP containing large receptive fields. Between MT and AL is V4t, with a distinct and pale myeloarchitecture. Although V4 was originally described as being located on the prelunate gyrus (Zeki 1973; Van Essen & Zeki 1978), it is now clear from more extensive mapping studies that this area extends ventrally into the occipitotemporal cortex. Gattass et al. (1985, 1988a,b) extended Zeki's electrophysiological recordings to the ventral aspect of the cortex, and defined area V4 as a dorsoventral strip of cortex, containing the representation of 30° to 40° of the visual field. This area has the lower visual field represented dorsally in the hemisphere and the upper visual field represented ventrally (Gattass 1988; Piñón et al. 1998). Consistent with these electrophysiological findings, we found projections to both upper and lower visual field representations of V4 from V2 sites representing eccentricities of up to but not greater than 46° eccentricity. In addition, the vertical meridian representation of V2 projects to the V3/V4 border, where the vertical meridian is again represented (Gattass et al. 1987; Piñón et al. 1998). Dorsally in the hemisphere, the representation of the horizontal meridian of V2 projects anteriorly to the V4/V4t border, which also contains a horizontal meridian representation (Desimone & Ungerleider 1986). Ventrally, however, the horizontal meridian of V2 does not appear to project to the V4/TEO border, but rather projects to a region within V4 itself. This anatomical finding agrees with the observation of Gattass et al. (1987) that the horizontal meridian representation of ventral V4 is usually located caudal to the area's anterior border, and it is consistent with the lower field representation of V4 bordering the lower field of VTF (Gattass et al. 1985, 1987; Piñón et al. 1998). We studied the cortical connections of area V4 in the macaque (Ungerleider et al. 2005) and we found that all parts of V4 are connected with V2, V3, V3A, V4t, MT, FST, TEO, TE and the frontal eye field (FEF), confirming prior reports (Zeki 1971; Rockland & Pandya 1981; Maunsell & Van Essen 1983; Festemaker et al. 1984; Kennedy & Bullier 1985; Burkhalter et al. 1986; Ungerleider & Desimone 1986a,b; Shipp & Zeki 1989; Boussaoud et al. 1991; Nakamura et al. 1993). Based on the distribution of labelled cells and of terminals, the projections to V2 and V3 are defined as feedback, those to V3A, V4t and the FEF as intermediate, and those to MT, FST, TEO and TE as feed-forward. Injections in the representation of the fovea in V4 revealed connections from and to V1, confirming previous anatomical findings (Zeki 1971; Rockland & Pandya 1981; Maunsell & Van Essen 1983; Festemaker et al. 1984; Kennedy & Bullier 1985; Burkhalter et al. 1986; Ungerleider & Desimone 1986b; Shipp & Zeki 1989; Boussaoud et al. 1991; Sousa et al. 1991; Nakamura et al. 1993). Injections of anterograde tracers in peripheral V4 did not reveal connections to V1, although injections of retrograde tracers revealed projections in one out of five cases (Ungerleider et al. 2005).
(v) Area V4t
Area V4t has been defined as the region lying between V4 and MT on the lateral bank of the superior temporal sulcus. Injections in V2 and V4 also revealed topographically organized projections from and to V4t (Zeki 1980; Maguire & Baizer 1984; Desimone & Ungerleider 1986; Gattass 1988). Physiological evidence indicates that this area contains a representation of the lower visual field only (Schein et al. 1982; Desimone & Ungerleider 1986; Ungerleider & Desimone 1986a,b; Gattass 1988). We observed projections to V4t from the lower field representations of V2 up to 46° eccentricity, with some evidence for a crude central to peripheral field organization as one progresses from lateral to medial within the area. Frequently, the projection to V4t from lower field V2 was continuous with the projections to V4 and MT (Piñón et al. 1998). This area was named DZ in the Cebus monkey (Fiorani et al. 1989).
(vi) Area MT
Dubner & Zeki (1971) first described the existence of a visual area with directionally selective neurons located in the superior temporal sulcus in macaques. This area has been named MT (Gattass & Gross 1981; Van Essen et al. 1981; Desimone & Ungerleider 1986) to acknowledge its homology with a visual area located in the middle temporal lobe of a New World monkey, the owl monkey (Allman & Kaas 1971). Area MT occupies the lower bank and the floor of the posterior superior temporal sulcus. It contains the representation of the contralateral binocular visual hemi-field, with the vertical meridian represented at the border of the area while the representation of the horizontal meridian runs across the lower bank and floor of the area. It corresponds to area OAa of Seltzer & Pandya (1989). Central field injections (those centred at eccentricities less than 30°) in V2 produced labelled terminals in the heavily myelinated part of MT, whereas peripheral field injections (those centred at eccentricities 30° and greater) produced labelled terminals medial to the heavily myelinated part of MT, i.e. within MTp (Desimone & Ungerleider 1986). A crude central to peripheral field trend and an upper versus lower field segregation was observed in the projections, which is in keeping with the visuotopic map reported for this area (Ungerleider & Mishkin 1979; Gattass & Gross 1981; Van Essen et al. 1981; Desimone & Ungerleider 1986).
(vii) Area TEO
In the macaque, representations of both the lower and upper quadrants were found in the area that occupies the lateral and ventrolateral aspects of the occipitotemporal transition, between area V4 and the non-topographically organized IT complex. This area was named ITC (Weller & Kaas 1985) TEO (Gattass 1988; Boussaoud et al. 1991) because it roughly corresponds to the cytoarchitectonic area defined TEO by Von Bonin & Bailey (1947). It also corresponds to area TEO defined by myeloarchitecture and pattern of connections by Webster et al. (1991). Complete representations of both visual quadrants were observed, although the magnification factor of the upper-quadrant representation was found to be greater than that of the lower-quadrant representation (Boussaoud et al. 1991). The receptive fields in this area are often large and encompass portions of both the vertical and the horizontal meridians. No systematic map-discontinuities were observed within its coarse visual topography. The visual map in this area may be regarded as having a gradient of eccentricities that grows from lateral to medial on the ventrolateral surface, with the upper quadrant represented caudally and the lower quadrant rostrally. Based on anatomical connections other investigators have suggested that the caudal part of TEO should be considered as an additional visual area, VOT (ventral occipitotemporal area; Van Essen et al. 1990; Felleman & Van Essen 1991), located between areas V4 and TEO, which only contains the representation of the upper quadrant. Systematic studies, using additional criteria and high-density sampling, would be needed to support this proposal.
Lesions of area TEO in monkeys produce a severe impairment in visual pattern discrimination. Area TEO receives feed-forward input from prestriate areas V2, V3, V3A, V4, V4t and it sends feedback projections to all these areas, both being topographically organized. Further inputs derive from STS areas MT, FST and areas PP, PITd, TEm, TEa and TIPa (IPa of Seltzer & Pandya 1989). These connections are also reciprocal. Connections with DP, MST and STP could not consistently be demonstrated. Furthermore, TEO receives feedback projections from temporal areas TE, TH, TG, 36 and 35, and intermediate ones from parietal area LIP, parahippocampal area TF, as well as from FEF in the frontal lobe. With the exception of areas TG, 36 and 35, these areas receive in turn a projection from TEO (Webster et al. 1991).
(viii) Area PO
Area PO has been defined as a myeloarchitectonically distinct area, containing a complex visuotopic map with a de-emphasis on the central field representation (Covey et al. 1982; Gattass et al. 1986; Neuenschwander 1989; Neuenschwander et al. 1994). Peripheral field injections in V2 at eccentricities of 30° and greater showed projections to area PO. While upper field cases showed projections medially in PO, lower field cases showed projections laterally, which is consistent with the visuotopic organization described for PO (Covey et al. 1982; Gattass et al. 1986; Colby et al. 1988; Neuenschwander 1989; Neuenschwander et al. 1994). The absence of projections to PO after injections in more central portions of the visual field representation of V2 may be related to the difference in cortical magnification in these two visual areas (Gattass et al. 1986); however, it is unlikely to be related to some technical factor, as Colby et al. (1988) also found projections to PO from peripheral but not central field representation of V2 using retrograde tracers. In Cebus, Neuenschwander et al. (1994) have described two distinct areas in the anterior bank of parieto-occipital sulcus, based on visual topography, receptive field size and myeloarchitecture, and named them PO and POd. Area POd has larger receptive fields and a distinct myeloarchitecture, when compared with area PO. The organization of PO, with emphasis in the isopolar domain, is quite different from that of dorsal V3, which preserves the isoeccentric domain (Neuenschwander et al. 1994).
Adding diversity to the nomenclature, more recently Galletti et al. (1996, 1999) proposed the name of ‘V6’ to visual area PO of the macaque. More recently, POd was also renamed as ‘V6A’ by Galletti and collaborators (2001). There are reasonable arguments to find these names inadequate: the label for visual area PO (PO) can be confused with the label of the parieto-occipital cleft (po), but the name V6 should be reserved for area MST, originally studied with MT (V5), by Dubner & Zeki (1971) in the macaque.
(ix) Area VTF
In Cebus monkeys, the occipitotemporal transition has been subdivided into two areas, on the basis of anatomical connections: a medial area (temporal ventral posterior area, TVP) that projects to both V1 and MT, and a lateral area (TEO) that does not (Sousa et al. 1991; Rosa et al. 1993). The connections between TVP, V1 and MT follow a central–peripheral gradient of eccentricities, from lateral to medial, respectively. In the macaque, the area located medial to TEO, adjacent to the rostral tip of the calcarine fissure, is less understood. In a brief report, Gattass and collaborators (1985) found receptive fields representing the upper and lower quadrant in the ventromedial aspect of the caudal temporal lobe, in a region that they named VF (by analogy with cytoarchitectonic area TF), which encompassed the recording sites in the occipital temporal sulcus. In a study of area TEO, Boussaoud et al. (1991) found evidence for a visual area on the parahippocampal gyrus anterior to V2 and V3v and medial to V4 and TEO. They termed this area VTF because it was the portion of architectonic area TF (von Bonin & Bailey 1947) that was visually responsive. Although occupying the position of the temporal ventral posterior area (area TVP) reported in Cebus (Gattass et al. 1985; Sousa et al. 1991), VTF appears to be located farther medially in the hemisphere. We found projections to VTF from the far peripheral upper field representation of V2 (Sousa et al. 1991). Although it might be argued that this region would be part of ventral V4, VTF and V4 have a markedly different appearance both in cyto- and in myeloarchitecture.
(b) Areas defined by single-unit recordings and anatomical connections
(i) Area MST
Area MST is located in the upper bank of the posterior superior temporal sulcus and contains cells sensitive to direction of motion either in the frontal plane, in depth, or in both (Dubner & Zeki 1971; Van Essen et al. 1981; Maunsell & Van Essen 1983; Saito et al. 1986; Tanaka et al. 1986). It also contains cells that appear to play a role in visuomotor control (Komatsu & Wurtz 1988). Using myeloarchitecture it is not possible to precisely define area MST, an area medial to MT in the superior temporal sulcus. However, the medial portion of the area is characterized by a densely myelinated zone (DMZ; Desimone & Ungerleider 1986). We saw projections within DMZ after peripheral field injections (more than 45°) in V2. The projection within DMZ was separate from the one located medial to MT, i.e. within MTp. Thus, the far peripheral field representation of V2 projects directly to MST and this projection appears to be additional to the one to the far peripheral representation of MTp. MST and MT are reciprocally connected to V4, thus providing the necessary information to extract form information from motion. Anatomical and electrophysiological studies in Cebus suggest that MST is composed of three areas, with crude visual topography (see figures 1 and 2).
(ii) Area VIP
In the present review, and in keeping with the studies by Blatt et al. (1990), Colby & Duhamel (1991) and Colby et al. (1993), we have defined VIP as the area at the fundus of the intraparietal sulcus ventral to the heavily myelinated zone (LIPv) on the lateral bank. Cells in this area present directional property similar to those found in area MT. We saw projections to VIP after peripheral V2 injections (more than 30°) or after peripheral V4 injections (more than 10°). In some cases the projection extended laterally to include a small portion of LIPv after V2 injections. By contrast, they always extended to LIPv and LIPd after peripheral V4 injections (Ungerleider et al. 2005). These results are consistent with those of Cavada & Goldman-Rakic (1989), who observed retrogradely labelled cells in peripheral field V2 following injections that included the ventral portion of the lateral bank of the intraparietal sulcus.
(iii) Area LIPv and LIPd
Areas LIPv and LIPd are located in the lateral bank of the intraparietal sulcus, dorsal to area VIP (Colby et al. 1993). LIPv corresponds to the heavily myelinated region in the lateral bank. At the level of LIPv at more dorsal location in the lateral bank there is a less myelinated region named LIPd. Cells in these areas present re-mapping properties of the location of their visual receptive field depending on oculomotor signals. Injections in peripheral V2 and V4 (Gattass et al. 1997; Ungerleider et al. 2005) show projections to the lower bank of the intraparietal sulcus, which includes two visual areas that have been termed the ventral intraparietal area, or VIP (Maunsell & Van Essen 1983; Ungerleider & Desimone 1986a), and the lateral intraparietal area, or LIP (Andersen et al. 1985, 1990). These two areas largely fall within the cytoarchitectonically defined area POae of Seltzer & Pandya (1980), although some injections were close enough to the cortical surface that areas 7a and 7b may have been minimally involved as well (see Andersen et al. 1985; Cavada & Goldman-Rakic 1989).
(c) Other areas defined by architectural and connectional studies
Based on the anatomical connections of visual area V2 and V4 we define groups of areas in the superior temporal sulcus, and in the parietal and temporal lobes. More recently, using the histochemistry for CytOx, immunohistochemistry for neurofilaments, neurotransmitter, calcium binding proteins and components of the extracellular matrix, we proposed subdivisions for the superior temporal sulcus and for the intraparietal sulcus.
(i) Areas of the superior temporal sulcus
Areas MT, MST and FST all contain many cells sensitive to the direction of motion of the stimulus either in the frontal plane, in depth, or in both (Dubner & Zeki 1971; Bruce et al. 1981; Van Essen et al. 1981; Maunsell & Van Essen 1983; Albright 1984; Desimone & Ungerleider 1986; Saito et al. 1986; Tanaka et al. 1986). Cells in some of these areas are also known to be sensitive to binocular disparity (Zeki 1974; Newsome & Wurtz 1981; Maunsell & Van Essen 1983), and cells in different portions of MST play a role in visuomotor processing (Komatsu & Wurtz 1988; Newsome et al. 1988; Fiorani et al. 1989; Baizer et al. 1991; Boussaoud et al. 1992; Kaas & Morel 1993; Cusick et al. 1995; Stepniewska & Kaas 1996; Felleman et al. 1997; Maiolli et al. 1998). Areas MT and MST are reciprocally connected to more peripheral portions of V2 and V4, thus providing the necessary information to extract form information from motion (Gattass et al. 1997; Ungerleider et al. 2005). In addition, area FST has reciprocal connections with V4 (Ungerleider et al. 2005).
In more anterior portions of the sulcus, labelled cells were located predominantly in the upper bank but they also spread across the floor of the sulcus into the lower bank. The upper bank contains the superior temporal polysensory area (STP of Bruce et al. 1981; or TPO of Seltzer & Pandya 1989) where we did not find projection from either V2 or V4. More recently, using anatomical methods and immunohistochemical markers we found evidence for the existence of several areas (figure 1) in the superior temporal sulcus (STS) and in the intraparietal sulcus (IP; Nascimento-Silva 2004). Table 1 summarizes the anatomical criteria used to delimit these areas. With the exception of one area (IPa, here named TIPa), we labelled the STS-areas using the nomenclature of Seltzer & Pandya (1978). The location and the borders of these areas coincide with the cytoarchitectonic borders determined by using lectin histochemistry (Nascimento-Silva 2004). The distribution of connections shown in table 2 suggests a segregation of the areas of the posterior STS, more related to early (V1, V2 and V4) stages of visual processing, from those of the anterior portion of STS, that are more related to connections of late stages of form perception processing (V4 and TEO). The connection to areas V2, LIP and TEO confirm the subdivisions obtained with the immunohistochemistry. Table 1 suggests the criteria used to subdivide the intraparietal areas. The laminar distribution of the connections to areas V4, TEO, PO and MT were used to segregate the areas. These areas were labelled using topographical landmarks, and they coincide with the nomenclature used by several authors (Seltzer & Pandya 1980; Rockland & Pandya 1981; Felleman & Van Essen 1983; Andersen et al. 1990).
Table 1.
Density of labelled cells in the intraparietal zones after injections in areas V4, TEO, PO and MT. (p, posterior; m, medial; a, anterior; +, isolated cells; ++, sparse; +++, dense; ++++, very dense projections.)
| V4 | TEO | PO | MT | ||||
|---|---|---|---|---|---|---|---|
| superficial | deep | superficial | deep | superficial | deep | ||
| V3A (DM) | ++ | +++ | + | + | |||
| 7A | ++ | +++ | + | ||||
| LIPv-p | +++ | +++ | + | ||||
| LIPv-m | |||||||
| LIPv-a | ++ | +++ | +++ | +++ | ++ | ++ | + |
| LIPd-p | +++ | ++ | +++ | +++ | |||
| LIPd-m | +++ | ++ | ++ | ||||
| LIPd-a | +++ | +++ | ++++ | +++ | + | ++ | + |
| AIPv | ++ | + | +++ | ++ | |||
| AIPd-p | ++ | + | ++ | ++ | |||
| AIPd-a | + | ||||||
| VIP-p | + | + | ++ | ||||
| VIP-a | ++ | ||||||
| 5b-p | + | ||||||
Table 2.
Immunohistochemistry density reaction and density of labelled cells in the STS zones, after injections in areas V2, LIP and TEO
| histochemistry | connections | ||||||
|---|---|---|---|---|---|---|---|
| SMI-32 | CAT-301 | PV | CO | V2 | LIP | TEO | |
| Ipa | ++++ | ++++ | ++++ | ++++ | ++ | ||
| TPOc | ++++ | ++++ | ++++ | ++ | ++++ | ++++ | ++ |
| TAa | ++++ | ++++ | ++ | ++++ | ++++ | ||
| TPOr | ++ | ++ | ++ | ++ | ++ | ++ | |
| V4t | ++++ | ++++ | ++ | ++++ | ++++ | ||
| MSTdp | + | ++++ | ++ | +++ | ++++ | ++++ | + |
| MSTda | ++ | ++ | ++ | ++++ | ++++ | +++ | |
| FSTd | + | + | +++ | ++ | ++ | ||
| FSTv | ++ | ++ | + | ++ | ++ | + | |
| MT | ++++ | ++ | ++ | ++++ | ++ | ||
| Pga | ++++ | ++++ | ++++ | ++++ | ++ | ||
| TG | ++++ | ++++ | |||||
| TEa | ++++ | ++++ | ++++ | ||||
| MSTm | ++++ | + | |||||
| PIT | ++++ | ++++ | ++++ | ++ | |||
| TEm | ++ | ++ | ++ | ||||
| TPOm | ++ | ++++ | |||||
(ii) Areas of the temporal lobe
Central and peripheral V2 and V4 are reciprocally connected to visual area TEO (Gattass et al. 1997; Ungerleider et al. 2005), also named caudal inferotemporal cortex (ITc) while mostly central V4 has additional connections with different portions of cytoarchitectonic area TE (TEp, TEa, TEm), including the portions of TE located within the superior temporal sulcus. Other temporal-lobe areas such as area TG, at the temporal pole, areas TF and TH on the parahippocampal gyrus and the presubiculum are also connected with central V4 (Ungerleider et al. 2005). Connections of the regions of representations of the lower field of V2 and V4 were found within areas TEO, TEp and TEm, while connections from areas V1, V2 and V4 also avoid area TG in the upper bank of STS in the anterior portion of the temporal pole (Desimone & Gross 1979). In the terminology of Seltzer & Pandya (1978), the connections in the anterior portion of the sulcus roughly spanned architectonic areas TAa, TPO, PGa, TIPa (IPa) and TEa, although the labelled region was not sharply demarcated and did not seem to follow any known areal boundary. Projections were also found ventromedially in the temporal lobe both on the parahippocampal gyrus, in area TF, and in the presubiculum. Early horseradish peroxidase (HRP) studies of the projections to area TE showed a more restricted region of input, with labelled cells located in V4 and TEO but not in V2 or V3 (Desimone et al. 1980; Shiwa 1987). The difference between those studies and the more recent ones is probably accounted for by the fact that our dye injections encroached on area TEO (Morel & Bullier 1990), whereas the HRP injections in the earlier studies were limited to TE. Consistent with this explanation, several reports have described direct projections from V2 and V3 to TEO (Festemaker et al. 1984; Shiwa 1987; Morel & Bullier 1990). A further difference may be the greater sensitivity of fluorescent dyes used in the more recent studies (Webster et al. 1991; Ungerleider et al. 2005) compared with HRP, especially as employed by Desimone et al. (1980) without tetramethyl benzidine as chromogene.
The multimodal region in the upper bank (area TPO) is divisible into four rostral-to-caudal architectonic sectors, exhibiting increasing degrees of laminar differentiation and cellularity as one proceeds caudally (Nascimento-Silva 2004). In addition, each rostrocaudal sector of area TPO has reciprocal connections with the laterally adjacent area TAa, at the upper rim of the sulcus, and with the medially adjacent areas PGa and TIPa (IPa), near the depth (Seltzer & Pandya 1989).
The properties of neurons in areas projecting to inferior temporal cortex are consistent with the role of the temporal lobe in object recognition. In addition to area TEO, the primary source of inputs to TE from prestriate cortex is area V4. Cells in V4 are sensitive to many visual features relevant to object perception, including colour, spatial frequency, orientation, length and width (e.g. Zeki 1973; Desimone & Schein 1987a,b; Schein & Desimone 1990; Youakim & Baizer 1990). Moreover, lesions of area V4 produce impairments in both form and colour vision (Wild et al. 1985; Heywood & Cowey 1987; Desimone et al. 1990). Area TE is non-topographically organized, has cells selective to stimulus shape and their receptive field always includes the fovea (Gross et al. 1972). Area TE is subdivided into three areas; one located anteriorly (TEa), another posteriorly (TEp) and another medially (TEm). Area TEa also has reciprocal connections with adjacent segments of area TEm laterally, at the lower rim of the sulcus, and area TIPa (IPa), medially, in the depth. In both the upper and the lower bank, caudal-to-rostral forward connections begin in supragranular layers of cortex and terminate in and around layer IV (Seltzer & Pandya 1989).
(iii) Areas of the parietal lobe
Projections from several visual areas to the intraparietal sulcus have been previously described. A projection from the prelunate portion of V4 to area LIP is well established (Seltzer & Pandya 1980; Rockland & Pandya 1981; Felleman & Van Essen 1983; Andersen et al. 1990), as are the projections from V4 to VIP (Maunsell & Van Essen 1983; Ungerleider & Desimone 1986a) and from V4 to both VIP and LIP (Andersen et al. 1990; also see Colby et al. 1988). More recently, Boussaoud et al. (1990) reported projections from areas MST and FST in the superior temporal sulcus to areas VIP and LIP, and several studies have described a projection from V3 to the intraparietal sulcus (Burchalter & Van Essen 1983; Felleman & Van Essen 1984; Andersen et al. 1990; Morel & Bullier 1990). In cases of injections into peripheral V4, projections were found in dorsal parieto-occipital cortex, including areas PO, V3A, PIP and DP. Projections from the peripheral representation of V2 to the intraparietal sulcus have also been noted in preliminary reports (Ungerleider et al. 1983; Van Essen 1985) and were more clearly demonstrated by Gattass et al. (1997). More recently, Ungerleider et al. (2005) showed that the peripheral representation of V4 has reciprocal connections with the middle portion of areas LIPv and LIPd, as shown in figure 1. The results also demonstrate that the peripheral field representations of V4 project to a number of other visual areas located in occipitoparietal cortex, including DP, VIP, LIPd and LIPv, PIP, MST and PO, as well as to a portion of area TF located on the posterior parahippocampal gyrus. V4 projections to DP, VIP and PIP are of the intermediate type, whereas those to LIPd and LIPv, MST, and TF are of feed-forward type.
8. Upper and lower field representations revealed by anatomical connections
Projections of V2 and V4 allowed to reveal upper and lower field representations in VIP, LIPv, PIP and V3A and confirm the location of upper and lower field representations in V2 (Gattass et al. 1981; Rosa et al. 1988b), V3 (Gattass 1988a,b), PO (Neuenschwander et al. 1994), MT (Gattass & Gross 1981; Ungerleider & Desimone 1986a,b) and TEO (Boussaoud et al. 1990). V4 connectional data were adequate to show superimposed upper and lower field representations in areas VF, TH, TEp and TEa. Although no one has yet reported electrophysiological mapping of VIP or LIP, the connectional results suggest an upper versus lower field segregation within these areas (figure 2). Anatomical and eletrophysiological mapping also suggest crude topographical organization in areas MSTm, MSTda and MSTdp.
9. Preferred flow of information: feed-forward and feedback projections
Using the laminar distribution of labelled cells and terminals as criteria, many authors (Kuypers et al. 1965; Spatz & Tigges 1972; Tigges et al. 1973, 1974; Wong-Rilley 1978; Rockland & Pandya 1979; Wall et al. 1982; Maunsell & Van Essen 1983; Weller et al. 1984; Kennedy & Bullier 1985; Weller & Kaas 1985; Ungerleider & Desimone 1986a,b; Boussaoud et al. 1990; Felleman & Van Essen 1991) distinguished feed-forward projections, originating from cells in superficial layers and terminating in layer IV of a higher-order target areas, from feedback projections, which originate from cells in infragranular layers and terminate mostly outside layer IV of lower-order target areas. Equally strong labelling of cells in layers III and V/VI and rather homogeneous terminal labelling in all cortical laminae indicate an intermediate type or undetermined relative position of two areas in the cortical hierarchy. Using these criteria, we found feedback projections of area TEO to posterior prestriate areas V2, V3, V3A and V4 and caudal STS areas V4t and MT. Mainly feed-forward projections were identified to rostral STS areas TEm, TEa and TIPa and to temporal lobe areas TE and TH. The input from posterior prestriate areas and from V4t and MT was, in turn, classified as feed-forward. The input from temporal areas TE, TH, TG, 36 and 35 had solely feedback characteristics. The connections of TEO with FST and PITd in caudal STS, with the parietal areas, and with the parahippocampal area TF appeared to be of intermediate or undetermined type. By contrast, the laminar distribution of labelled neurons revealed by PITd injection indicated that PITd receives feed-forward input from area V4 and from LIP, and feedback input from MT, FST and TE. The connections with TEO, TEm and TEa were of intermediate nature. A rich pattern of projections interconnects areas of different streams of information processing, at different hierarchical levels, to constitute specific cortical circuit loops. In spite of the multiplicity of connections among visual areas, we favour the notion that direct feed-forward connections relay important information to target areas for the construction of a first crude representation of the object, object trajectory or egocentric motion.
10. Central and peripheral topographically distributed networks
There is accumulating evidence for differences in the cortical projections of central and peripheral visual field representations in extrastriate cortex. Zeki (1969) first noted that the fovea representation of V1, but not the remainder of the area, projects directly to V4, a finding later replicated by Nakamura et al. (1993). In addition, Zeki (1980) reported that peripheral V1 but not central V1 projects to V3A (see also Ungerleider & Mishkin 1982). Moreover, Ungerleider & Desimone (1986a) found that V3A receives a projection from peripheral but not central MT. In addition, Colby et al. (1988) demonstrated direct input to PO from peripheral but not central field representations of V1 and V2. Gattass et al. (1997) also found that peripheral but not central field V2 projects directly to area PO. Injections placed at eccentricities of 30° or greater produced label in PO, but those placed at lesser eccentricities did not. In addition, Gattass et al. (1997) found that peripheral but not central field V2 projects to areas MST, VIP and VTF. These projections also arose from the portions of V2 representing eccentricities of 30° or greater. More recently, Ungerleider et al. (2005) found that central field of V4 projects mainly to temporal areas, whereas peripheral field V4 projects mainly to parietal areas. Contrasting from the V2 connections, the parietal projections arise from the portions of V4 representing eccentricities of 10° or greater.
The difference between peripheral and central field inputs may be related to the visual processing requirements of an area. Which areas of the lateral occipitotemporal cortex receive preferential inputs from central field representations is consistent with the role of these areas in object vision (Ungerleider & Mishkin 1982). By contrast, those within occipitoparietal cortex receive preferential inputs from peripheral field representations, which is consistent with the role of these areas in spatial vision (Gattass et al. 1990, 1999). The projections from peripheral field V4 to parietal areas PO, VIP, LIP, DP and area 7 support this notion.
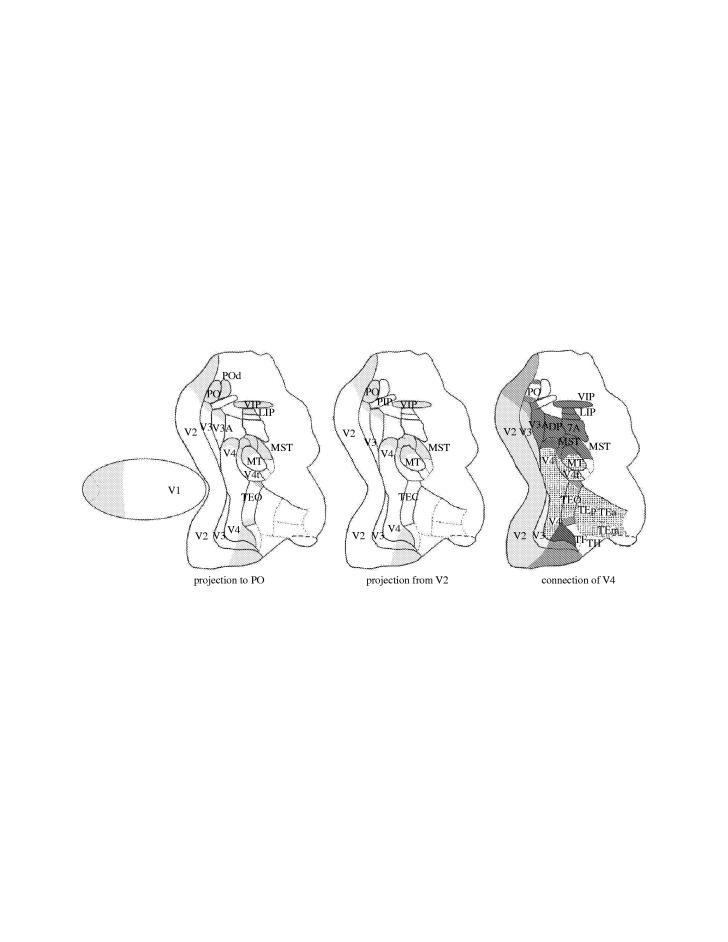
Figure 7 illustrates the connections of area PO, and the segregated projections of central and peripheral representations of V2 and V4. The direct projection from the peripheral representations of V1, V2, V3, V4, MT and MST to the parietal lobe would be equivalent to the direct projection from central V1, V2, V3 and V4 to the temporal lobe. The bypass of the information from the early stages of processing (V1 and V2, for example) would contribute to advance information about the stimulus, and might aid in constructing, within area TE, the initial representation of the overall shape and colour of an object, with the fine-grained information arriving later to fill in the important details. Similarly, the projections from peripheral V2 and V4 to parietal areas could provide a direct route for information about the periphery to quickly reach parietal cortex and thereby rapidly activate circuits for spatial vision and spatial attention.
Figure 7.
Projections to visual area PO (left), projection from peripheral V2 (middle) and connections from central and peripheral V4 (right) represented onto a 2D reconstruction of the monkey visual cortex. The connections to area PO arise from the peripheral representation of areas V1, V2, V3, V3A, V4, V4t, TEO, MT, MST, VIP, LIP and POd; the projection from peripheral V2 encompass areas V1 (not shown), V2, V3, V3A, V4, V4t, MT, MST and VIP; the connection of the central V4 feedback to areas V2 and V3 and project forward to MT, V4t, and to the temporal lobe areas (TEO, TEp, TEm, TEa), while the connection from peripheral V4 projects also to the parietal areas (PO, PIP, V3A, DP, MST, VIP, LIP and 7a).
The study of the connections of the visual areas reveals several interconnections between multiple maps for a particular modality, with each map being of a more or less specialized nature. This arrangement may reflect advantages of modular design proposed by Marr (1976) for the question of computer pattern recognition. Essentially, large computational problems are most easily dealt with through the division of the problem into smaller sub-problems, which are as nearly independent of each other as possible. This allows small changes in one part of the problem not to disrupt other parts of the problem, making the problem as a whole easier to solve, whether by human design or natural selection and mutation. For the visual system, implementing such an approach would be greatly facilitated by an arrangement that could easily reintegrate the visual information once divided among areas. An interconnecting network, which preserves information about position of the stimulus in the visual field and allows different computations of attributes of the stimulus, is an important component of this system. We thus propose two or more classes of central–peripheral segregated, topographically distributed networks, with intrinsic, feed-forward and feedback interactions, as the neuronal basis underlying either visual perception or oculomotor processing. Figure 8 illustrates three of such networks that work in parallel, one with emphasis on central vision, another with emphasis on object trajectory and another with emphasis on peripheral vision.
Figure 8.
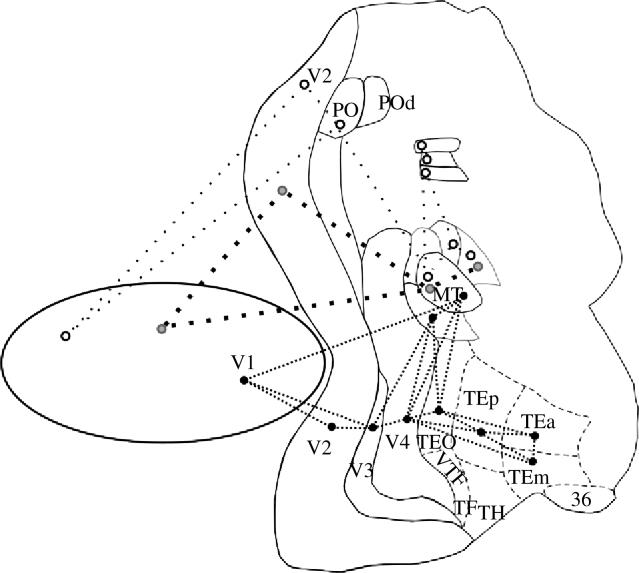
Topographically organized networks in different streams of visual information processing. Three topographically organized networks link different cortical visual areas, at different eccentricities. These networks process the visual information in parallel, one with emphasis on central or object vision (black circle), another with emphasis on object trajectory or object motion (grey circle) and another with emphasis on peripheral vision or forward egocentric motion (white circle).
To evaluate the contribution of the feedback projection at different nodes of the network we chemically inactivate visual areas MT and V4 (Jansen 2002) and the lateral pulvinar (Soares et al. 2004) while recording neurons in V2. The inactivation of visual area MT, with different amounts of GABA, while studying topographically corresponding areas of V2 neurons produces a general decrease in the excitability of the neurons, which includes a decrease of the spontaneous and driven activities. The inactivation of MT is also followed by changes in orientation and direction selectivity of V2 neurons. The changes in direction selectivity are towards gain or loss of selectivity. Thus, feedback connections arising from MT may change receptive field properties of neurons in V2. These feedback projections are capable of not only modulating the spontaneous and driven activity of V2 neurons, but also of modifying V2 receptive field properties, such as its direction selectivity (Jansen et al. 2004). Figure 9 illustrates the change in direction selectivity of a neuron of V2 after GABA inactivation in MT.
Figure 9.
Changes of direction selectivity in V2 after GABA inactivation in MT. Activity of two cells at topographically corresponding locations in MT and V2, before and after GABA inactivation of MT. The directional selectivity (polargram) and the post-stimulus histogram at the preferred direction are shown for both cells 1, 44 and 66 min after GABA injections in MT. The response of the V2 neuron decreases and its direction selectivity changes while the topographically corresponding location in MT is inactivated. Solid circle, maximum spike rate of the cell; dashed circle, spontaneous spike rate.
11. The visuotopic map of V1
In the process of mapping visual area V1 in Cebus, we noticed unequivocal responses at the representation of the blind spot of the contralateral eye. We were using long moving bars on the surface of a transparent globe placed in front of the animal with the representation of the fovea and that of the blind spot drawn onto the surface of the hemisphere. This finding motivated us to do a quantitative study of single-units of V1 in Cebus that constituted the first description of a mechanism of completion of shape in V1 (Fiorani et al. 1992). Also, the automated quantitative mapping of the blind spot region with long moving bars showed a precise organization (see figure 10) that suggests that the visual representation in V1 is visuotopic, rather than retinotopic, i.e. V1 has a continuous representation of the visual field instead of the discontinuous retinal one (Fiorani et al. 2001; Azzi 2004). We propose that distinct forms of perceptual filling-in engage different portions of a wide distributed processing network. The automatic mapping of receptive field in V1 revealed that the orientation selectivity of the ‘blind’ eye is statistically different from that of the other eye, showing the influence of long range iso-orientational connections (Azzi 2004). Figure 10 shows the regularity of the progressions of receptive field centres for both eyes and the difference in orientation selectivity of the cell at the representation of the blind spot, for inputs of the ‘blind’ eye. At the same time, boundary completion associated with illusory contour stimuli probably involve V2 circuits as revealed by the studies of von der Heydt & Peterhans (1989). These two forms of boundary completion should be contrasted with feature completion, such as brightness and texture filling-in. The De Weerd et al. (1995) study strongly suggests that V2 and V3 (but not V1) mediate texture filling-in. Brightness filling-in seems, at least in part, to involve the striate cortex (Rossi & Paradiso 1996). However, the view we advance here is not that of ‘one-effect-one-area’, but instead that a distributed network of topographically organized areas is involved. So, for example, V2 and V3 circuits are important for texture filling-in. Finally, although illusory contours trigger responses in V2, recent evidence suggests that other areas may also be involved (such as V4).
Figure 10.
Precision of receptive field location and discrepancy of orientation selectivity. (a) Location of receptive field centres mapped through the contralateral (filled circles and solid line) and ipsilateral (open circles and dotted line) eyes corresponding to 11 recording sites in V1 across the representation of the contralateral optic disc, N, nasal. (b) Difference in horizontal receptive field position (scatter) and difference in direction/orientation selectivity of the receptive fields for the contralateral and ipsilateral eye, for 123 recording sites. (c,d ) Representations of the receptive field response in the space domain, for the centres marked by arrows in (a), based on the single-unit responses to eight different directions The ellipse in the contralateral eye represents the optic disc position. Spike rate for each eye is colour-coded (right: colour scale).
The visual system is constantly confronted with the problem of organizing the retinal pattern of stimulation into coherent percepts. Somehow, from the luminance pattern stimulating the eyes, the visual system must produce organized, meaningful representations of the world around us. At every instant, multiple organizations of the elementary pattern of stimulation are possible, and the visual system must generate useful representations that can guide behaviour. Therefore, we can view the behaviour of the visual system as, at every instant, recursive loops in topographically organized networks would try different converging algorithms to select the ‘best hypothesis’ consistent with the incoming stimulus. Thus, we propose a framework for understanding visual perception based on topographically organized, functionally synchronized distributed cortical networks (Gattass et al. 1999) that project to more anterior non-topographic shape-sensitive visual areas in the temporal lobe, while other networks project to the parietal lobe to organize oculomotor or egocentred behaviours. Finally, we suggest that multiple representations of the stimulus engage competitive mechanisms that select the ‘most probable hypothesis’ capable of constructing a single neural representation of perceived objects.
In summary, the cortical visual system is composed of a number of specialized visual areas where different visual attributes are segregated and processed in parallel in different areas. Analytic posterior cortical visual areas are topographically organized whereas more anterior temporal areas are not, and they have a more holistic processing. The visual information relayed at the lateral geniculate nucleus projects mainly to the striate cortex, also known as the first visual area or V1. V1 projects primarily to areas V2, MT and PO, and those in turn project to a number of other areas to constitute three main streams of visual information processing. For a given eccentricity, there is an increase in receptive field size at each step of processing. In addition, in most topographically organized areas, there is a gradual increase of the receptive field size with increasing eccentricity. These increases are greater at more anterior areas in the temporal lobe or at more dorsal areas in the parietal lobe. The scatter of receptive field location increases with increasing receptive field size. Thus, the visual topographies are progressively coarser toward the temporal and parietal lobes. The visual areas contain coexisting functional modules (columns) arranged together independently, however preserving a certain degree of overall visual topography. Other types of organization related to perception of egocentric motion or oculomotor behaviour may prevail in dorsal parietal areas. The minimal point image size, i.e. the extent of cortex devoted to the processing of a point in the visual field, increases towards the temporal lobe and remains constant or shows a slight decrease toward the parietal lobe. The concept of topographical distributed networks, connecting areas that contain organized visual maps and different functional modules for visual processing, contrasts to other types of organizations that are related to non-visually organized areas.
The extent of the visual field represented in each area is different. Areas V1 and V2 contain the representation of the entire visual field, area MT, the binocular visual field, while area V4 represents only the central 30° to 40°. Areas of the parietal lobe have larger emphasis in the representation of the periphery. There are asymmetries in the pattern of connections of central and peripheral representations, in different areas. Central V2 and central V4 project mainly to the temporal lobe, whereas peripheral V2 (more than 30°) and peripheral V4 (more than 10°) project mainly to the parietal lobe. Priority connections targeting the temporal, parieto-temporal and parietal lobes may be important to construct crude early representations of objects, objects' trajectories and egocentric movements in the visual space.
Acknowledgments
This work would not be possible without the aid of Maria Thereza A. Monteiro and Liliane H. Pontes in the preparation of the manuscript. This work was supported by grants from FUJB, PRONEX, FINEP, CEPG and CNPq.
Glossary
- CMF
cortical magnification factor
- CytOx
cytochrome oxidase
- DMZ
densely myelinated zone
- FEF
frontal eye field
- HRP
horseradish peroxidase
- OD
ocular dominance
- Cortical visual areas
- FST
visual area FST
- LIPd
dorsal portion of lateral intraparietal area
- LIPv
ventral portion of lateral intraparietal area
- MIP
medial intraparietal area
- MST
medial superior temporal area (V6)
- MT
visual area MT (V5)
- MTp
peripheral portion of MT
- PIP
posterior intraparietal area
- PO
parieto-occipital area
- POd
dorsal parieto-occipital area
- TEa
anterior portion of area TE
- TEm
medial portion of area TE
- TEO
posterior inferior temporal cortex
- TEp
posterior portion of area TE
- TH
cytoarchitectonic area TH
- V1
primary visual cortex
- V2
visual area 2
- V3A
a V3 visual complex part A
- V3d
dorsal portion of visual area 3
- V3v
ventral portion of visual area 3
- V4
visual area 4
- V4t
V4 transition zone
- VIP
ventral intraparietal area
- VTF
visual portion of parahippocampal TF
- Cortical sulci
- amt
anterior middle temporal sulcus
- ar
arcuate sulcus
- ca
calcarine fissure
- ce
central sulcus
- ci
cingulate sulcus
- co
collateral sulcus
- ec
external calcarine sulcus
- io
inferior occipital sulcus
- ip
intraparietal sulcus
- la
lateral sulcus
- lu
lunate sulcus
- orb
orbital sulcus
- ot
occipitotemporal sulcus
- p
principal sulcus
- pmt
posterior middle temporal sulcus
- pom
medial parieto-occipital sulcus
- rh
rhinal sulcus
- sp
subparietal sulcus
- st
superior temporal sulcus
Footnotes
One contribution of 12 to a Theme Issue ‘Cerebral cartography 1905–2005’.
References
- Albright T.D. Direction and orientation selectivity of neurons in visual area MT of the macaque. J. Neurophysiol. 1984;52:1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- Allman J.M, Kaas J.H. A representation of the visual field in the caudal third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus) Brain Res. 1971;31:85–105. doi: 10.1016/0006-8993(71)90635-4. [DOI] [PubMed] [Google Scholar]
- Allman J.M, Kaas J.H. A crescent-shaped cortical visual area surrounding the middle temporal area (MT) in the owl monkey (Aotus trivirgattus) Brain Res. 1974;81:199–213. doi: 10.1016/0006-8993(74)90936-6. [DOI] [PubMed] [Google Scholar]
- Allman J.M, Kaas J.H. The dorsomedial cortical visual area: a third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus) Brain Res. 1975;100:473–487. doi: 10.1016/0006-8993(75)90153-5. [DOI] [PubMed] [Google Scholar]
- Allman J.M, Kaas J.H, Lane R.H, Miezin F.M. A representation of the visual field in the inferior nucleus of the pulvinar in the owl monkey. Brain Res. 1972;40:291–302. doi: 10.1016/0006-8993(72)90135-7. [DOI] [PubMed] [Google Scholar]
- Andersen R.A, Asanuma C, Cowan W.M. Callosal and prefrontal association projecting cells populations in area 7a of the macaque monkey: a study using retrogradely transported fluorescent dyes. J. Comp. Neurol. 1985;232:443–445. doi: 10.1002/cne.902320403. [DOI] [PubMed] [Google Scholar]
- Andersen R.A, Asanuma C, Essick G, Siegel R.M. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J. Comp. Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Azzi, J. 2004 The cortical map of V1 is visuotopically (not retinotopically) organized: visuotopic organization at the blind spot of V1 in primates (Cebus apella). PhD thesis, IBCCF/UFRJ, Federal University of Rio de Janeiro Publication, 121p.
- Baizer J.S, Ungerleider L.G, Desimone R. Organization of visual inputs to the inferior temporal and posterior parietal cortex in macaques. J. Neurosci. 1991;11:168–190. doi: 10.1523/JNEUROSCI.11-01-00168.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P.D, Kaas J.H. Cortical connections of the dorsomedial visual area in old world macaque monkeys. J. Comp. Neurol. 1999;406:487–502. [PubMed] [Google Scholar]
- Blatt G.J, Andersen R.A, Stoner G.R. Visual receptive field organization and cortico-cortical connections of lateral intraparietal area (Area LIP) in the macaque. J. Comp. Neurol. 1990;299:421–445. doi: 10.1002/cne.902990404. [DOI] [PubMed] [Google Scholar]
- Botelho, E. P. 2004 Distribution of Calbindin-28kD, GAP-43, GFAP and Parvalbumin in V1 in adult Cebus apella monkeys with retinal lesions. Master thesis, IBCCF/UFRJ, Federal University Publication, 124p.
- Boussaoud D.R, Ungerleider L.G, Desimone R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J. Comp. Neurol. 1990;296:462–495. doi: 10.1002/cne.902960311. [DOI] [PubMed] [Google Scholar]
- Boussaoud D.R, Desimone R, Ungerleider L.G. Visual topography of area TEO in the macaque. J. Comp. Neurol. 1991;306:554–575. doi: 10.1002/cne.903060403. [DOI] [PubMed] [Google Scholar]
- Boussaoud D.R, Desimone R, Ungerleider L.G. Subcortical connections of visual areas MST and FST in macaques. Vis. Neurosci. 1992;9:291–302. doi: 10.1017/s0952523800010701. [DOI] [PubMed] [Google Scholar]
- Bruce C, Desimone R, Gross C.G. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J. Neurophysiol. 1981;46:369–384. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- Burchalter A, Van Essen D.C. The connections of the ventral posterior area (VP) in the macaque monkey. Soc. Neurosci. Abstr. 1983;9:153. [Google Scholar]
- Burkhalter A, Felleman D.J, Newsome W, Van Essen D.C. Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. Vis. Res. 1986;26:63–80. doi: 10.1016/0042-6989(86)90071-4. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic P.S. Posterior parietalcortex in rhesus monkey. I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J. Comp. Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Colby C.L, Duhamel J.R. Heterogeneity of extrastriate visual areas and multiple parietal areas in the macaque monkey. Neuropsychologia. 1991;29:517–538. doi: 10.1016/0028-3932(91)90008-v. [DOI] [PubMed] [Google Scholar]
- Colby C.L, Gattass R, Olson C.R, Gross C.G. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J. Comp. Neurol. 1988;269:392–413. doi: 10.1002/cne.902690307. [DOI] [PubMed] [Google Scholar]
- Colby C.L, Duhamel J.R, Goldberg M.E. Ventral intraparietal area of the macaque: anatomical location and visual response properties. J. Neurophysiol. 1993;69:902–913. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- Covey E.R, Gattass R, Gross C.G. A new visual area in the parieto-occipital sulcus of the macaque. Soc. Neurosci. Abstr. 1982;8:861. [Google Scholar]
- Cowey A. Projection of the retina on to striate and prestriate cortex in the squirrel monkey, Saimiri sciureus. J. Neurophysiol. 1964;27:366–393. doi: 10.1152/jn.1964.27.3.366. [DOI] [PubMed] [Google Scholar]
- Cragg B.G, Ainsworth A. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vis. Res. 1969;9:733–747. doi: 10.1016/0042-6989(69)90011-x. [DOI] [PubMed] [Google Scholar]
- Cusick C.G, Kaas J.H. Cortical connections of area 18 and dorsolateral visual cortex in squirrel monkey. Vis. Neurosci. 1988;1:211–237. doi: 10.1017/s0952523800001486. [DOI] [PubMed] [Google Scholar]
- Cusick C.G, Seltzer B, Cola M, Griggs E. Chemoarchitectonics and corticocortical terminations within the superior temporal sulcus of the Rhesus monkeys: evidence for subdivisions of superior temporal polysensory cortex. J. Cogn. Neurol. 1995;360:513–535. doi: 10.1002/cne.903600312. [DOI] [PubMed] [Google Scholar]
- Daniel P.M, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. J. Physiol. 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Gross C.G. Visual areas in the temporal cortex of the macaque. Brain Res. 1979;178:363–380. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- Desimone R, Schein S.J. Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. J. Neurophysiol. 1987a;57:835–868. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- Desimone R, Ungerleider L.G. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J. Comp. Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Fleming J, Gross C.G. Prestriate afferents to inferior temporal cortex: an HRP study. Brain Res. 1980;184:41–55. doi: 10.1016/0006-8993(80)90586-7. [DOI] [PubMed] [Google Scholar]
- Desimone R, Wessinger M, Thomas L, Schneider W. Attentional control of visual perception: cortical and subcortical mechanisms. Cold Spring Harb. Symp. Quant. Biol. 1990;55:963–971. doi: 10.1101/sqb.1990.055.01.090. [DOI] [PubMed] [Google Scholar]
- De Weerd P, Gattass R, Desimone R, Ungerleider L.G. Responses of cells in monkey visual cortex during perceptual filling-in of an artificial scotoma. Nature. 1995;377:713–734. doi: 10.1038/377731a0. [DOI] [PubMed] [Google Scholar]
- DeYoe E.A, Van Essen D.C. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985;317:58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- DeYoe E.A, Bandettini P, Neitz J, Miller D, Winans P. Functional magnetic resonance imaging (FMRI) of the human brain. J. Neurosci. Methods. 1994;54:171–187. doi: 10.1016/0165-0270(94)90191-0. [DOI] [PubMed] [Google Scholar]
- Diogo A.C, Soares J.G, Albright T.D, Gattass R. Two-dimensional map of direction selectivity in cortical visual area MT of Cebus monkey. An. Acad. Bras. Cienc. 2002;74:463–476. doi: 10.1590/s0001-37652002000300009. [DOI] [PubMed] [Google Scholar]
- Diogo A.C, Soares J.G, Koulakov A, Albright T.D, Gattass R. Electrophysiological imaging of functional architecture in the cortical middle temporal visual area of Cebus apella monkey. J. Neurosci. 2003;23:3881–3898. doi: 10.1523/JNEUROSCI.23-09-03881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubner R, Zeki S.M. Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res. 1971;35:528–532. doi: 10.1016/0006-8993(71)90494-x. [DOI] [PubMed] [Google Scholar]
- Farias, M. F. 2002 Blob plasticity after focal and massive laser retinal lesions in V1 in Cebus. MS thesis, IBCCF/Federal University of Rio de Janeiro, 83p.
- Farias M.F, Gattass R, Piñón M.C, Ungerleider L.G. Tangential distribution of cytochrome oxidase-rich blobs in the primary visual cortex of macaque monkeys. J. Comp. Neurol. 1997;386:217–228. doi: 10.1002/(sici)1096-9861(19970922)386:2<217::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Felleman D.J, Van Essen D.C. The connections of area V4 of macaque monkey extrastriate cortex. Soc. Neurosci. Abstr. 1983;9:153. [Google Scholar]
- Felleman D.J, Van Essen D.C. Cortical connections of area V3 in macaque extrastriate cortex. Soc. Neurosci. Abstr. 1984;10:933. [Google Scholar]
- Felleman D.J, Van Essen D.C. Receptive field properties of neurons in area V3 of macaque monkey extrastriate cortex. J. Neurophysiol. 1987;57:889–920. doi: 10.1152/jn.1987.57.4.889. [DOI] [PubMed] [Google Scholar]
- Felleman D.J, Van Essen D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Felleman D.J, Burkhalter A, Van Essen D.C. Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. J. Comp. Neurol. 1997;379:21–47. doi: 10.1002/(sici)1096-9861(19970303)379:1<21::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Festemaker S.B, Olson C.R, Gross C.G. Afferent connections of macaque visual areas V4 and TEO. Invest. Ophthalmol. Vis. Sci. 1984;25(Suppl.):213. [Google Scholar]
- Fiorani Jr, M., Gattass R, Rosa M.G.P, Souza A.P.B. Visual area MT in the Cebus monkey: location, visuotopic organization and individual variability. J. Comp. Neurol. 1989;287:98–118. doi: 10.1002/cne.902870108. [DOI] [PubMed] [Google Scholar]
- Fiorani Jr, M., Rosa M.G.P, Gattass R, Rocha-Miranda C.E. Dynamic surrounds of receptive fields in primate striate cortex: a physiological basis for perceptual completion? Proc. Natl Acad. Sci. USA. 1992;89:8547–8551. doi: 10.1073/pnas.89.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorani Jr, M., Azzi J.C, Gattass R. Primate V1 has a visuotopic rather than a retinotopic organization: the topographic organization of the blind spot (BS) in V1. Soc. Neurosci. Abstr. 2001;27(prog.):619.46. [Google Scholar]
- Fiorani Jr, M., Oliveira L, Volchan E, Pessoa L, Gattass R, Rocha-Miranda C.E, Pessoa L. Completion through a permanent scotoma: fast interpolation across the blind spot and the processing of occlusion. In: L. Pessoa, P. De Weerd, editors. Filling-in: from perceptual completion to cortical reorganization. Oxford University Press; New York: 2003. pp. 177–186. [Google Scholar]
- Frostig R.D. What does in vivo optical imaging tell us about the primary visual cortex in primates? In: Peters A, Rockland K.S, editors. Cerebral cortex vol. 10: primary visual cortex in primates. Plenum Press; New York: 1994. pp. 331–358. [Google Scholar]
- Galletti C, Fattori P, Batagilini P.P, Shipp S, Zeki S. Functional demarcation of a border between areas V6 and V6A in the superior parietal gyrus of the macaque monkey. Eur. J. Neurosci. 1996;1:30–52. doi: 10.1111/j.1460-9568.1996.tb01165.x. [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P, Gamberini M, Kutz D.F. The cortical visual area V6: brain location and visual topoghaphy. Eur. J. Neurosci. 1999;11:3922–3936. doi: 10.1046/j.1460-9568.1999.00817.x. [DOI] [PubMed] [Google Scholar]
- Galletti C, Gamberini M, Kutz D.F, Fattori P, Luppino G, Matelli M. The cortical connections of area V6: an occipito-parietal network processing visual information. Eur. J. Neurosci. 2001;8:1572–1581. doi: 10.1046/j.0953-816x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- Gattass R, Gross C.G. Visual topography of striate projection zone (MT) in posterior superior temporal sulcus of the macaque. J. Neurophysiol. 1981;46:621–638. doi: 10.1152/jn.1981.46.3.621. [DOI] [PubMed] [Google Scholar]
- Gattass R, Gross C.G, Sandel J.H. Visual topography of V2 in the macaque. J. Comp. Neurol. 1981;201:519–539. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Gattass R, Souza A.P.B, Covey E. Cortical visual areas of the macaque: possible substrates for pattern recognition mechanisms. In: Chagas C, Gattass R, Gross C.G, editors. Study week on pattern recognition mechanisms. Pontificiae Academiae Scientiarum Scripta Varia. Vol. 54. 1985. pp. 1–20. [Google Scholar]
- Gattass R, Sousa A.P.B, Covey E. Cortical visual areas of the macaque: possible substrates for pattern recognition mechanisms. Exp. Brain Res. 1986;11(Suppl.):1–20. [Google Scholar]
- Gattass R, Souza A.P.B, Rosa M.G.P. Visual topography of V1 in the Cebus monkey. J. Comp. Neurol. 1987;259:529–548. doi: 10.1002/cne.902590404. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa A.P.B, Gross C.G. Visuotopic organization and extent of V3 and V4 of the macaque. J. Neurosci. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattass R, Sousa A.P.B, Rosa M.G.P, Piñón M.C.G.P. Ventral V3 in the Cebus monkey: visual topography and projections to V1. Soc. Neurosci. Abstr. 1988;14:202. [Google Scholar]
- Gattass R, Rosa M.G.P, Sousa A.P.B, Piñón M.C.G.P, Fiorani Jr, M., Neuenschwander S. Cortical streams of visual information processing in primates. Braz. J. Med. Biol. Res. 1990;23:375–393. [PubMed] [Google Scholar]
- Gattass R, Sousa A.P.B, Ungerleider L.G, Mishkin M. Cortical projections of area V2 in the macaque. Cereb. Cortex. 1997;7:110–129. doi: 10.1093/cercor/7.2.110. [DOI] [PubMed] [Google Scholar]
- Gattass R, Pessoa L.A, de Weerd P, Fiorani M. Filling-in in topographically organized distributed networks. An. Acad. Bras. Cienc. 1999;71:997–1015. [PubMed] [Google Scholar]
- Grinvald A, Lieke E.E, Frostig R.D, Hildesheim E. Cortical point-spread function and long-range lateral interactions revealed by real-time optical imaging of macaque monkey primary visual cortex. J. Neurosci. 1994;14:2545–2568. doi: 10.1523/JNEUROSCI.14-05-02545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C.G, Rocha-Miranda C.E, Bender D.B. Visual properties of neurons in inferotemporal cortex of the macaque. J. Neurophysiol. 1972;35:96–111. doi: 10.1152/jn.1972.35.1.96. [DOI] [PubMed] [Google Scholar]
- Heywood C.A, Cowey A. On the role of cortical area V4 in the discrimination of hue and pattern in macaque monkeys. J. Neurosci. 1987;7:2601–2617. doi: 10.1523/JNEUROSCI.07-09-02601.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton J.C, Hubel D.H. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981;292:762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Hubel D.H, Wiesel T.N. Cortical and callosal connections concerned with the vertical meridian of visual fields in the cat. J. Neurophysiol. 1967;6:1561–1573. doi: 10.1152/jn.1967.30.6.1561. [DOI] [PubMed] [Google Scholar]
- Hubel D.H, Wiesel T. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. Lond. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H, Wiesel T.N. Cells sensitive to binocular depth in area 18 of the macaque monkey cortex. Nature. 1970;225:41–42. doi: 10.1038/225041a0. [DOI] [PubMed] [Google Scholar]
- Hubel D.H, Wiesel T.N. Laminar and columnar distribution of geniculo-cortical fibers in the macaque monkey. J. Comp. Neurol. 1972;4:421–450. doi: 10.1002/cne.901460402. [DOI] [PubMed] [Google Scholar]
- Jansen, A. K. 2002 GABA inactivation of visual areas MT and V4 modify direction selectivity of V2 neurons in Cebus monkeys. PhD thesis, IBCCF/UFRJ, Federal University Publication, 81p.
- Jansen A.K, Lima B, Gattass R. GABA inactivation of visual area MT modifies direction selectivity of V2 neurons in Cebus monkeys. Clin. Exp. Pharmacol. Physiol. 2004;31:1580–1590. [Google Scholar]
- Jones E.G, Powell T.P.S. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;19:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kaas J.H, Morel A. Connections of visual areas of the upper temporal lobe of owl monkeys: the MT crescent and dorsal and ventral subdivisions of FST. J. Neurosci. 1993;13:534–546. doi: 10.1523/JNEUROSCI.13-02-00534.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy H, Bullier J. A double-labeling investigation of the afferent connectivity to cortical areas V1 and V2 of the macaque monkey. J. Neurosci. 1985;5:2815–2830. doi: 10.1523/JNEUROSCI.05-10-02815.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Wurtz R.H. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J. Neurophysiol. 1988;60:580–603. doi: 10.1152/jn.1988.60.2.580. [DOI] [PubMed] [Google Scholar]
- Krubitzer L.A, Kaas J.H. Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Vis. Neurosci. 1990;5:165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- Krubitzer L.A, Kaas J.H. The dorsomedial visual area of owl monkeys: connections, myeloarchitecture, and homologies in other primates. J. Comp. Neurol. 1993;334:497–528. doi: 10.1002/cne.903340402. [DOI] [PubMed] [Google Scholar]
- Kuypers H.G, Szwarcbart M.K, Mishkin M, Rosvold H.E. Occipitotemporal corticocortical connections in the rhesus monkey. Exp. Neurol. 1965;11:245–262. doi: 10.1016/0014-4886(65)90016-6. [DOI] [PubMed] [Google Scholar]
- Levitt J.B, Kiper D.C, Movshon J.A. Receptive fields and functional architecture of macaque V2. J. Neurophysiol. 1994;71:2517–2542. doi: 10.1152/jn.1994.71.6.2517. [DOI] [PubMed] [Google Scholar]
- Livingstone M.S, Hubel D.H. Specifity of cortico-cortical connections in monkey visual system. Nature. 1983;304:531–534. doi: 10.1038/304531a0. [DOI] [PubMed] [Google Scholar]
- Lund J.S, Hendrickson A.E, Ogren M.P, Tobin E.A. Anatomical organization of primate visual cortex area Vll. J. Comp. Neurol. 1991;202:19–45. doi: 10.1002/cne.902020104. [DOI] [PubMed] [Google Scholar]
- Maguire W.M, Baizer J.S. Visuotopic organization of the prelunate gyrus in rhesus monkey. J. Neurosci. 1984;4:1690–1704. doi: 10.1523/JNEUROSCI.04-07-01690.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiolli M.G, Squarito S, Samolsky-Dekel B.G, Sanseverino E.R. Cortico-cortical connections between frontal periarcuate regions and visual areas of the superior temporal sulcus and the adjoining inferior parietal lobe in the macaque monkey. Brain Res. 1998;798:118–125. doi: 10.1016/s0006-8993(98)00025-0. [DOI] [PubMed] [Google Scholar]
- Malach R, Tootell R.B.H, Malonek D. Relationship between orientation domains, cytochrome oxidase stripes, and intrinsic horizontal connections in squirrel monkey area V2. Cereb. Cortex. 1994;4:151–165. doi: 10.1093/cercor/4.2.151. [DOI] [PubMed] [Google Scholar]
- Malonek D, Tootell R.B.H, Grinvald A. Optical imaging reveals the functional architecture of neurons processing shape and motion in owl monkey area MT. Proc. R. Soc. B. 1994;258:109–119. doi: 10.1098/rspb.1994.0150. [DOI] [PubMed] [Google Scholar]
- Marcondes, M. 1997 Tangential distribution and plasticity of cytochrome oxidase blobs of V1 in normal adult humans and in humans with chronic retinal lesions. PhD thesis, IBCCF/UFRJ, Federal University of Rio de Janeiro Publication, 102p.
- Mariani O.S, Gattass R. Proceedings of the Federation of Biological and Medical Society (FESBE) 1996. Areas of the intraparietal sulcus segregated by their cortical connections to areas V4, MT, TEO and PO in Cebus. p. 99. [Google Scholar]
- Marr D. Early processing of visual information. Phil. Trans. R. Soc. B. 1976;275:483–519. doi: 10.1098/rstb.1976.0090. [DOI] [PubMed] [Google Scholar]
- Maunsell J.H.R, Van Essen D.C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci. 1983;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsell J.H.R, Van Essen D.C. Topography organization of the middle temporal visual area in the macaque monkey: representational biases and the relationship to callosal connections and myeloarchitectonic boundaries. J. Comp. Neurol. 1987;266:235–255. doi: 10.1002/cne.902660407. [DOI] [PubMed] [Google Scholar]
- Morel A, Bullier J. Anatomical segregation of two cortical visual pathways in the macaque monkey. Vis. Neurosci. 1990;4:555–578. doi: 10.1017/s0952523800005769. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Gattass R, Desimone R, Ungerleider L.G. The modular organization of projections from areas V1 and V2 to areas V4 and TEO in macaque. J. Comp. Neurol. 1993;13:3681–3691. doi: 10.1523/JNEUROSCI.13-09-03681.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento-Silva, S. 2004 Cortical visual areas of the superior temporal sulcus. PhD thesis, IBCCF/UFRJ, Federal University of Rio de Janeiro Publication, 72p.
- Nascimento-Silva S, Gattass R, Fiorani M, Sousa A.P.B. Three streams of visual information processing in V2 of Cebus monkey. J. Comp. Neurol. 2003;466:104–118. doi: 10.1002/cne.10878. [DOI] [PubMed] [Google Scholar]
- Neuenschwander, S. M. 1989 The PO complex in Cebus—anatomical and electrophysiological studies. MS thesis, IBCCF/UFRJ, Federal University of Rio de Janeiro Publication, 67p.
- Neuenschwander S, Gattass R, Sousa A.P.B, Piñón M.C.G.P. Identification and visuotopic organization of areas PO and POd in Cebus monkey. J. Comp. Neurol. 1994;340:65–86. doi: 10.1002/cne.903400106. [DOI] [PubMed] [Google Scholar]
- Newsome W.T, Wurtz R.H. Response properties of single neurons in the middle temporal visual area (MT) of alert macaque monkeys. Soc. Neurosci. Abstr. 1981;7:832. [Google Scholar]
- Newsome W.T, Maunsell J.H.R, Van Essen D.C. The ventral posterior visual area of the macaque: visual topography and areal boundaries. J. Comp. Neurol. 1986;252:139–153. doi: 10.1002/cne.902520202. [DOI] [PubMed] [Google Scholar]
- Newsome W.T, Wurtz R.H, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J. Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Olavarria J.F, Van Essen D.C. The global pattern of cytochrome oxidase stripes in visual area V2 of the macaque monkey. Cereb. Cortex. 1997;5:395–404. doi: 10.1093/cercor/7.5.395. [DOI] [PubMed] [Google Scholar]
- Perkel D.J, Bullier J, Kennedy H. Topography of the afferent connectivity of area 17 in the macaque monkey: a double-labelling study. J. Comp. Neurol. 1986;253:374–402. doi: 10.1002/cne.902530307. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Thompson E, Noe A. Finding out about filling-in: a guide to perceptual completion for visual science and the philosophy of perception. Behav. Brain Sci. 1998;21:723–748. doi: 10.1017/s0140525x98001757. [DOI] [PubMed] [Google Scholar]
- Piñón M.C.G.P, Gattass R, Sousa A.P.B. Area V4 in Cebus monkey: extent and visuotopic organization. Cereb. Cortex. 1998;8:685–701. doi: 10.1093/cercor/8.8.685. [DOI] [PubMed] [Google Scholar]
- Rockland K.S, Pandya D.N. Laminar origins and terminations of cortical connections of the occipital lobe in the rhesus monkey. Brain Res. 1979;179:3–20. doi: 10.1016/0006-8993(79)90485-2. [DOI] [PubMed] [Google Scholar]
- Rockland K.S, Pandya D.N. Cortical connections of occipital lobe in the rhesus monkey: interconnections between areas 17, 18, 19 and the superior temporal sulcus. Brain Res. 1981;212:249–270. doi: 10.1016/0006-8993(81)90461-3. [DOI] [PubMed] [Google Scholar]
- Roe A.W, Ts'o D.Y. Visual topography in primate V2: multiple representation across functional stripes. J. Neurosci. 1995;15:3689–3715. doi: 10.1523/JNEUROSCI.15-05-03689.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M.G.P, Gattass R, Fiorani M. Complete pattern of ocular dominance stripes in V1 of a New World monkey, Cebus apella. Exp. Brain Res. 1988;72:645–648. doi: 10.1007/BF00250609. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Sousa A.P.B, Gattass R. Representation of the visual field in the second visual area in the Cebus monkey. J. Comp. Neurol. 1988;275:326–345. doi: 10.1002/cne.902750303. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Soares J.G.M, Fiorani M, Gattass R. Cortical afferents of visual area MT in the Cebus monkey: possible homologies between New and Old World monkeys. Vis. Neurosci. 1993;10:827–855. doi: 10.1017/s0952523800006064. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Piñón M.C, Gattass R, Sousa A.P.B. ‘Third tier’ ventral extrastriate cortex in the New World monkey, Cebus apella. Exp. Brain Res. 2000;132:287–305. doi: 10.1007/s002210000344. [DOI] [PubMed] [Google Scholar]
- Rossi A, Paradiso M.A. Temporal limits of brightness induction and mechanisms of brightness perception. Vis. Res. 1996;36:1391–1398. doi: 10.1016/0042-6989(95)00206-5. [DOI] [PubMed] [Google Scholar]
- Saito H, Yukie M, Tanaka K, Hikosaka K, Fukada Y, Iwai E. Integration of direction signals of image motion in the superior temporal sulcus of the macaque monkey. J. Neurosci. 1986;6:145–157. doi: 10.1523/JNEUROSCI.06-01-00145.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin P.A, Bullier J. Corticocortical connections in the visual system: structure and function. Physiol. Rev. 1995;75:107–154. doi: 10.1152/physrev.1995.75.1.107. [DOI] [PubMed] [Google Scholar]
- Schein S, Desimone R. Spectral properties of V4 neurons in the macaque. J. Neurosci. 1990;10:3369–3389. doi: 10.1523/JNEUROSCI.10-10-03369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S.J, Marroco R.T, de Monasterio F.M. Is there a high concentration of color selective cells in area V4 of monkey visual cortex? J. Neurophysiol. 1982;2:193–213. doi: 10.1152/jn.1982.47.2.193. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya D.N. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya D.N. Converging visual and somatic sensory cortical input to the intraparietal sulcus of the rhesus monkey. Brain Res. 1980;192:339–351. doi: 10.1016/0006-8993(80)90888-4. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya D.N. Intrinsic connections and architectonics of the superior temporal sulcus in the rhesus monkey. J. Comp. Neurol. 1989;290:451–471. doi: 10.1002/cne.902900402. [DOI] [PubMed] [Google Scholar]
- Sereno M.I, McDonald C.T, Allman J.M. Analysis of retinotopic maps in extrastriate cortex. Cereb. Cortex. 1994;4:601–620. doi: 10.1093/cercor/4.6.601. [DOI] [PubMed] [Google Scholar]
- Sereno M.I, Dale A.M, Reppas J.B, Kwong K.K, Belliveau J.W, Bradu T.J, Rosen B.R, Tootell R.B.H. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;28:889–892. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S.M. Segregation of pathways leading from area V2 to area V4 and V5 of macaque monkey visual cortex. Nature. 1985;315:322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S.M. The organization of connections between areas V5 and V2 in macaque monkey visual cortex. Eur. J. Neurosci. 1989;1:333–353. doi: 10.1111/j.1460-9568.1989.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Shiwa T. Corticocortical projections to the monkey temporal lobe with particular reference to the visual processing pathways. Arch. Ital. Biol. 1987;125:139–154. [PubMed] [Google Scholar]
- Soares J.G.M, Diogo A.C.M, Fiorani M, Jr, Sousa A.P.B, Gattass R. Effects of inactivation of the lateral pulvinar on response properties of second visual area cells in Cebus monkey. Clin. Exp. Pharmacol. Physiol. 2004;31:580–590. doi: 10.1111/j.1440-1681.2004.04051.x. [DOI] [PubMed] [Google Scholar]
- Sousa A.P.B, Piñón M.C.G.P, Gattass R, Rosa M.G.P. Topographical organization of cortical input to striate cortex in the Cebus monkey: a fluorescent tracer study. J. Comp. Neurol. 1991;308:665–682. doi: 10.1002/cne.903080411. [DOI] [PubMed] [Google Scholar]
- Spatz W.B, Tigges J. Experimental–anatomical studies on the ‘middle temporal visual area (MT)’ in primates. I. Efferent cortico-cortical connections in the marmoset Callihrix Jacchus. J. Comp. Neurol. 1972;146:451–464. doi: 10.1002/cne.901460403. [DOI] [PubMed] [Google Scholar]
- Spatz W.B, Tigges J, Tigges M. Subcortical projections, cortical associations, and some intrinsic interlaminar connections of the striate cortex in the squirrel monkey (Saimiri) J. Comp. Neurol. 1970;140:155–174. doi: 10.1002/cne.901400203. [DOI] [PubMed] [Google Scholar]
- Steele G.E, Weller R.E, Cusick C.G. Cortical connections of the caudal subdivision of the dorsolateral area (V4) in monkeys. J. Comp. Neurol. 1991;306:495–520. doi: 10.1002/cne.903060312. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Kaas J. Topographic patterns of V2 cortical connections in macaque monkeys. J. Comp. Neurol. 1996;371:129–152. doi: 10.1002/(SICI)1096-9861(19960715)371:1<129::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Talbot S.A. A lateral localization in the cat's visual cortex. Fed. Proc. 1942;1:84. [Google Scholar]
- Talbot S.A, Marshall W.H. Multiple responses in the optic tract and optic cortex of cat. Am. J. Physiol. 1940;129:478. [Google Scholar]
- Tanaka K, Hikosaka K, Saito H, Yukie M, Fukada Y, Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J. Neurosci. 1986;6:134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges J, Spatz W.B, Tigges M. Reciprocal point-to-point connections between parastriate and striate cortex in the squirrel monkey (Saimiri) J. Comp. Neurol. 1973;148:481–489. doi: 10.1002/cne.901480406. [DOI] [PubMed] [Google Scholar]
- Tigges J, Spatz W.B, Tigges M. Efferent cortico-cortical fiber connections of area 18 in the squirrel monkey (Saimiri) J. Comp. Neurol. 1974;158:219–235. doi: 10.1002/cne.901580208. [DOI] [PubMed] [Google Scholar]
- Tootell R.B, Hamilton S.L. Functional anatomy of the second visual area (V2) in the macaque. J. Neurosci. 1989;9:2620–2644. doi: 10.1523/JNEUROSCI.09-08-02620.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R.B, Silverman M.S, Switkes E, DeValois R.L. Deoxyglucose analysis of retinotopic organization in primate striate cortex. Science. 1982;218:902–904. doi: 10.1126/science.7134981. [DOI] [PubMed] [Google Scholar]
- Tootell R.B, Haminton S.L, Silverman M.S, Switkes E. Functional anatomy of macaque striate cortex. I. Ocular dominance, binocular interactions, and baseline conditions. J. Neurosci. 1988;8:1500–1530. doi: 10.1523/JNEUROSCI.08-05-01500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider L.G, Desimone R. Cortical connections of visual area MT in the macaque. J. Comp. Neurol. 1986a;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G, Desimone R. Projections to the superior temporal sulcus from the central and peripheral field representations of V1 and V2. J. Comp. Neurol. 1986b;248:147–163. doi: 10.1002/cne.902480202. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G, Mishkin M. The striate projection zone in the superior temporal sulcus of Macaca mulatta localization and topographic organization. J. Comp. Neurol. 1979;188:347–366. doi: 10.1002/cne.901880302. [DOI] [PubMed] [Google Scholar]
- Ungerleider L.G, Mishkin M. Two cortical visual systems. In: Ingle D.J, Goodale M.A, Mansfield R.J, editors. Analysis of visual behavior. MIT Press; Cambridge: 1982. pp. 549–586. [Google Scholar]
- Ungerleider L.G, Gattass R, Sousa A.P.B, Mishkin M. Projections of area V2 in the macaque. Soc. Neurosci. Abstr. 1983;9:152. [Google Scholar]
- Ungerleider, L. G., Galkin, T. G., Desimone, R. & Gattass, R. In press. Cortical connections of V4 in the macaque. Cereb. Cortex 15 [DOI] [PubMed]
- Van Essen D.C. Functional organization of primate visual cortex. In: Peters A, Jones E.G, editors. Cerebral cortex. vol. 3. Plenun Publishing Corporation; New York: 1985. pp. 259–329. [Google Scholar]
- Van Essen D.C, Zeki S.M. The topographic organization of rhesus monkey prestriate cortex. J. Physiol. Lond. 1978;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C, Maunsell J.H.R, Bixby J.C. The middle temporal visual area in the macaque: myeloarchitecture, connections, functional properties and topographic organization. J. Comp. Neurol. 1981;199:293–326. doi: 10.1002/cne.901990302. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C, Newsome W.T, Maunsell J.H.R, Bixby J.L. The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: asymmetries, areal boundaries, and patchy connections. J. Comp. Neurol. 1986;244:451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C, Felleman D.J, DeYoe E.A, Olavarria J, Knierim J. Modular and hierarchical organization of extrastriate visual cortex in the macaque monkey. Cold Spring Harb. Symp. Quant. Biol. 1990;55:679–696. doi: 10.1101/sqb.1990.055.01.064. [DOI] [PubMed] [Google Scholar]
- von Bonin G, Bailey P. The University of Illinois Press; Urbana, IL: 1947. The neocortex of the Macaca mulatta. [Google Scholar]
- von der Heydt R, Peterhans E. Mechanisms of contour perception in monkey visual cortex. I. Lines of pattern discontinuity. J. Neurosci. 1989;9:1731–1748. doi: 10.1523/JNEUROSCI.09-05-01731.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J.T, Symonds L.L, Kaas J.H. Cortical and subcortical projections of the middle temporal area (MT) and adjacent cortex in Galagos. J. Comp. Neurol. 1982;211:193–214. doi: 10.1002/cne.902110208. [DOI] [PubMed] [Google Scholar]
- Webster M.J, Ungerleider L.G, Bechevalier J. Connections of inferior temporal areas TE and TEO with medial temporal-lobe structures in infant and adult monkeys. J. Neurosci. 1991;11:1095–1116. doi: 10.1523/JNEUROSCI.11-04-01095.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller R.E, Kaas J.H. Retinotopic patterns of connections of area 17 with areas V-II and MT in macaque monkeys. J. Comp. Neurol. 1983;220:253–279. doi: 10.1002/cne.902200302. [DOI] [PubMed] [Google Scholar]
- Weller R.E, Kaas J.H. Cortical projections of the dorsolateral visual area in owl monkeys: the prestriate relay to inferior temporal cortex. J. Comp. Neurol. 1985;211:193–214. doi: 10.1002/cne.902340104. [DOI] [PubMed] [Google Scholar]
- Weller R.E, Wall J.T, Kaas J.H. Cortical connections of the middle temporal visual area (MT) and the superior temporal cortex in owl monkeys. J. Comp. Neurol. 1984;228:81–104. doi: 10.1002/cne.902280109. [DOI] [PubMed] [Google Scholar]
- Wild H.M, Butter S.R, Carden D, Kulikowski J.J. Primate cortical area V4 important for colour constancy but not for wavelength discrimination. Nature. 1985;313:133–139. [Google Scholar]
- Wong-Rilley M.T.T. Reciprocal connections between striate and prestriate cortex in squirrel monkey as demonstrated by combined peroxidase histochemistry and autoradiography. Brain Res. 1978;147:159–164. doi: 10.1016/0006-8993(78)90781-3. [DOI] [PubMed] [Google Scholar]
- Wong-Rilley M.T.T, Carrol E.W. Effect of impulse blockage on cytochrome activity in monkey visual system. Nature. 1984;307:262–264. doi: 10.1038/307262a0. [DOI] [PubMed] [Google Scholar]
- Youakim M, Baizer J.S. Distribution of response properties across topographic subdivisions of macaque prelunate gyrus. Soc. Neurosci. Abstr. 1990;16:1220. [Google Scholar]
- Zeki S.M. Representation of central visual fields in prestriate cortex of monkey. Brain Res. 1969;14:271–291. doi: 10.1016/0006-8993(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Convergent input from striate cortex (area 17) to the cortex of the superior temporal sulcus in the rhesus monkey. Brain Res. 1971;28:338–340. doi: 10.1016/0006-8993(71)90665-2. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Colour coding in rhesus monkey prestriate cortex. Brain Res. 1973;53:271–291. doi: 10.1016/0006-8993(73)90227-8. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J. Physiol. 1974;236:549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S.M. The projections to the superior temporal sulcus from areas 17 and 18 in rhesus monkey. Proc. R. Soc. B. 1976;193:119–207. doi: 10.1098/rspb.1976.0040. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Uniformity and diversity of structure and function in rhesus monkey prestriate visual cortex. J. Physiol. 1978;277:273–290. doi: 10.1113/jphysiol.1978.sp012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S.M. A direct projection from area V1 to area V3A of rhesus monkey visual cortex. Proc. R. Soc. B. 1980;107:499–506. doi: 10.1098/rspb.1980.0038. [DOI] [PubMed] [Google Scholar]
- Zeki S.M, Sandeman D.R. Combined anatomical and electrophysiological studies on the boundary between the second and the third visual areas of the rhesus monkey cortex. Proc. R. Soc. B. 1976;194:555–562. doi: 10.1098/rspb.1976.0094. [DOI] [PubMed] [Google Scholar]
- Zeki S.M, Shipp S. Modular connections between areas V2 and V4 of macaque monkey visual cortex. Eur. J. Neurosci. 1989;1:494–506. doi: 10.1111/j.1460-9568.1989.tb00356.x. [DOI] [PubMed] [Google Scholar]