Abstract
The claustrum is a thin, irregular, sheet-like neuronal structure hidden beneath the inner surface of the neocortex in the general region of the insula. Its function is enigmatic. Its anatomy is quite remarkable in that it receives input from almost all regions of cortex and projects back to almost all regions of cortex. We here briefly summarize what is known about the claustrum, speculate on its possible relationship to the processes that give rise to integrated conscious percepts, propose mechanisms that enable information to travel widely within the claustrum and discuss experiments to address these questions.
Keywords: neuroanatomy, connectivity, primate, electrophysiology, gap junctions, cortex
1. Introduction
Most people working on the brain have heard of the claustrum—it was known to Ramón y Cajal—but very few have any idea what it does. It is thin and fairly small—in humans, its volume is a quarter of one percentage of that of the cerebral cortex (Kowianski et al. 1999)—and so it is easily overlooked. Crick (1994) described the claustrum briefly, but since then we have left it to one side. So what prompted this article?
A key property of conscious sensations is their integrated nature. You are not aware of isolated percepts, but of a single, unifying experience. When holding a rose, you smell its fragrance and see its red petals while feeling its textured stem with your fingers. The philosopher Searle (2004) refers to the ‘conscious field’ in this context.1
There is an approximate consensus among scholars who speculate on the neuronal basis of consciousness that its correlate must involve some form of cooperative activity, mediated by electrical and chemical synapses between forebrain neurons which are responding to different aspects of the same conscious experience. In vision, to which we have paid special attention, this would mean correlated activity among the different neuronal representations that encode the different visual aspects of the same object or event. We have suggested that this takes the form of coalitions of active neurons in cortex, thalamus and closely associated structures whose spiking activity (in some form) has reached a special threshold, and whose interactions tend to support each other (Crick & Koch 2003; Koch 2004).
These neurons are distributed over large distances (spanning, in humans, many centimetres) that include (for vision) visual cortex at the back of the brain, the frontal eye field and other frontal regions, posterior parietal and inferior temporal cortices, the hippocampus, and the associated thalamic and basal ganglia nuclei. Many of the neurons in these areas code for local aspects of any one scene, such as the orientation of an edge, or the colour and depth of a surface patch. Much of this information is ambiguous and is compatible with many different interpretations of the overall scene. In mathematical terms, the visual input is ill-posed (Poggio et al. 1984), if only because the brain is trying to reconstruct a three-dimensional representation of object and events occurring in the outside world from two-dimensional and noisy retinal inputs. Resolving these ambiguities must involve interactions among large groups of cells, most likely cortical pyramidal neurons, since their axons carry the bulk of long-distance communications within, and to structures outside, the cortex. These synaptically mediated interactions among assemblies of neurons involve both cooperation as well as winner-takes-all style competition (Hebb 1949; Palm 1990). This competition can be biased by bottom-up, saliency-driven or top-down volitionally controlled attention (Desimone & Duncan 1995), as well as by the expected reward (Maunsell 2004).
A key feature of almost all neuronal theories of consciousness is the need for continuous interactions among groups of widely dispersed pyramidal neurons that express themselves in the ongoing stream of conscious percepts, images and thoughts.
This is apparent in Edelman and Tononi's concept of the dynamical core. This is a shifting assembly of spiking neurons throughout the forebrain that is stabilized using massive re-entrant feedback connections (Tononi & Edelman 1998; Edelman & Tononi 2000). Its representational content, integrated, yet at the same time highly differentiated, corresponds to the unitary yet amazingly particular content of phenomenal consciousness.
Dehaene & Changeux (2004; see also Dehaene et al. 2003) postulate a widely dispersed set of reciprocally connected pyramidal neurons with long-distance axons linking most, if not all, the cortical and thalamic regions. This network implements a neuronal workspace, a term borrowed from Baars's (1997, 2002) cognitive theory of consciousness. It distinguishes a large array of unconscious specialized processors running in parallel from a unified, limited-capacity ‘workspace’ that allows the local processors to exchange information.
Although using different terminologies, the basic ideas of these neuroscientists are surprisingly similar to ours: the need to rapidly integrate and bind information in neurons that are situated across distinct cortical and thalamic regions (see also Bachmann 2000; Llinas 2001).
It is in the light of this consensus and the existing, albeit limited, knowledge of the anatomical and functional organization of the claustrum, that the structure attracted us again. It appears to be in an ideal position to integrate the most diverse kinds of information that underlie conscious perception, cognition and action. What follows is an attempt to illustrate this and advocate the need for research examining its role.
2. The neuroanatomy of the claustrum
The claustrum is present in all mammalian species so far examined (Kowianski et al. 1999), from insectivore to man, though its precise shape and some of its connections appear to vary from species to species.2 We shall consider mainly the cat and primate claustrum (for comprehensive reviews, consult Sherk 1986; Tanné-Gariépy et al. 2002; Edelstein & Denaro 2004).
So what and where is the claustrum? The word claustrum means ‘hidden away’, and indeed, the claustrum is a thin, irregular sheet of grey matter, one sheet on each side of the head, concealed between the inner surface of the neocortex. It lies below the general region of the insula, and above the outer surface of the putamen, with a fibre tract on each side of it (the extreme and external capsulae). Viewed face on, it has an irregular outline, for primates not unlike that of the contiguous United States (figure 1). The latero-medial thickness varies from a fraction of a millimetre to several millimetres. The sheet is not flat but curved in a curious way. The claustrum is not located everywhere beneath the cerebral cortex. It is mainly, but not entirely, in the general region of the insular cortex. The exact shape varies from species to species. Figure 2 shows the general shape of the human claustrum and how it is tucked away underneath the cortex. There is a general tendency for any cortical area to connect to the area of the claustrum nearest to it (figure 3).
Figure 1.
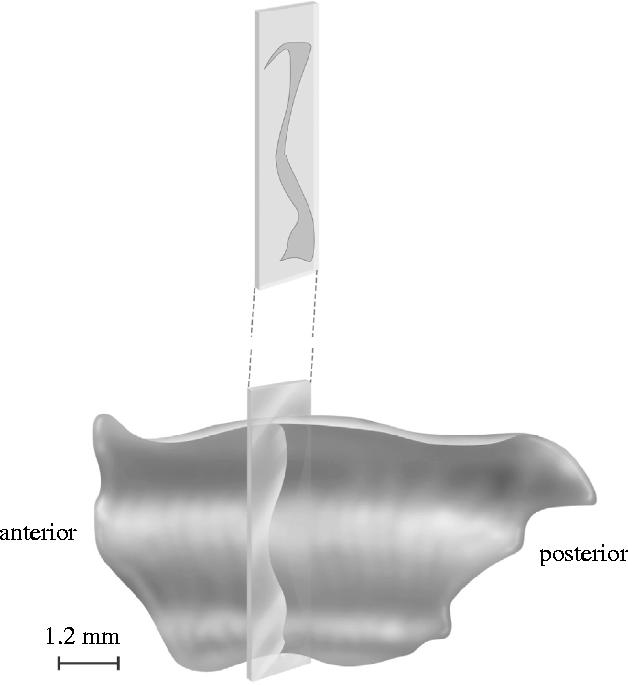
A drawing of the claustrum of a young squirrel monkey. The inset shows a single coronal section, illustrating the sheet-like structure of the claustrum. Modified from Brand (1981).
Figure 2.
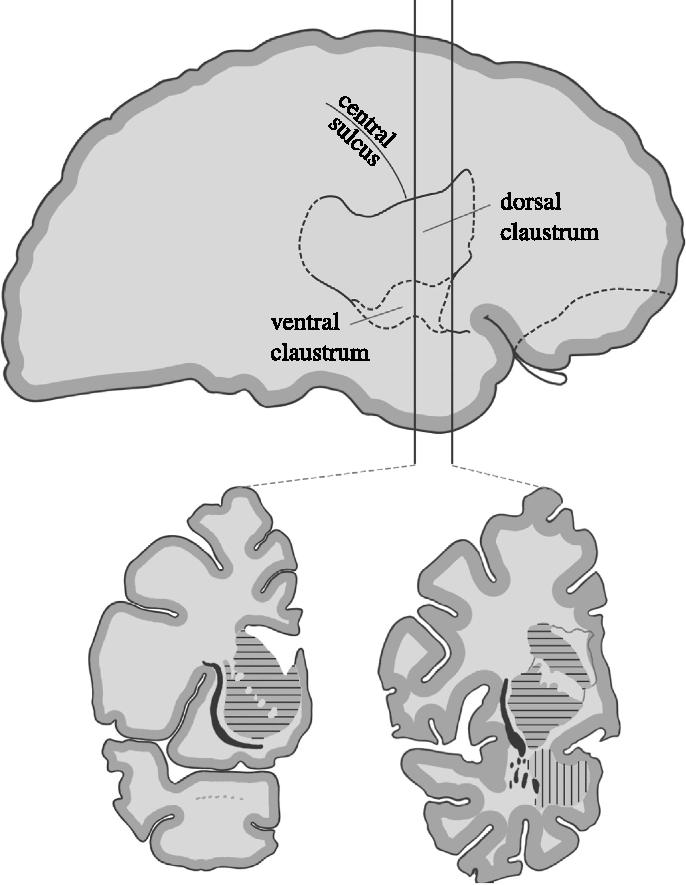
The approximate location of the human claustrum beneath the insular cortex. The vertical lines correspond to the two coronal sections shown at 60 and 70 mm posterior to the frontal pole. The external capsule consists of fibres making up the white matter lying between the putamen and the claustrum and the extreme capsule fibres that constitute the white matter between the claustrum and neocortex. The horizontal stripes correspond to the putamen and caudate nucleus and the vertical lines to the amygdala. Modified from Rae (1954).
Figure 3.
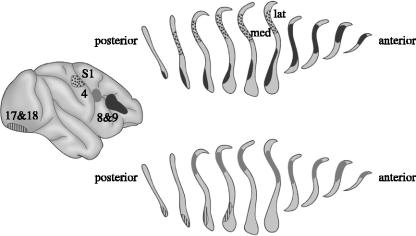
Horseradish peroxidase (HRP) was injected into four distinct neocortical regions in the monkey and the distribution of labelled cells and extracellular granules (shown in coronal sections) in the claustrum was recovered. Note the overlap between claustral zones that communicate with somatosensory cortex (S1) and area four in the precentral gyrus, between claustral regions linked to striate and extrastriate visual cortex (areas 17 and 18) and areas eight and nine of the prefrontal lobe, and between claustral regions that are connected with area four and those connected to areas eight and nine. Modified from Pearson et al. (1982).
3. Cell types in the claustrum
A striking feature of the claustrum is the few neuronal types it has compared with those of the cerebral cortex (figure 4). Moreover, while the latter is clearly laminated, the former is not.
Figure 4.
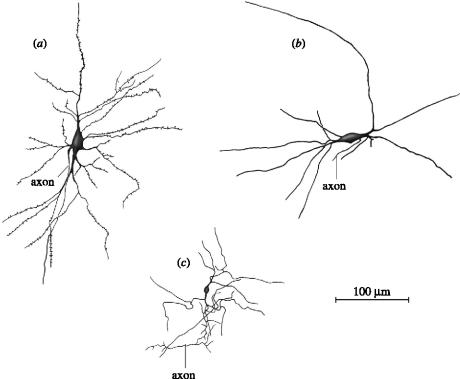
Neuronal cell types in the human claustrum as seen in Golgi preparations. (a) The dominant cell type (type I). They both receive input from cortex and send their axons back there. Their dendrites are covered with spines. (b), (c) At least two types of neurons whose dendrites are devoid of spines have been identified. Their axons do not leave the claustrum. They are most likely inhibitory interneurons. Modified from Braak & Braak (1982).
There is general agreement that the most common cell type, called type I, is a large cell whose dendrites are covered by spines (for the relevant Golgi studies in cats, monkeys and humans see, respectively; LeVay & Sherk 1981a; Brand 1981; Braak & Braak 1982). The axons of these type I cells, after throwing off local collaterals, often leave the claustrum either medially or laterally (figure 4a). They are the principal cells of the claustrum in that they can both receive an input from the cortex and project back. Their shape varies considerably, the soma of some being pyramidal, some fusiform and others with more spherical somas. All authors agree that they cannot find discontinuous sub-groups of type I neurons and have therefore lumped them together under one heading. The dendrites of these cells do not have a preferred orientation (unlike, for example, the apical dendrites of cortical pyramidal cells).
Other claustral neurons lack spines and so have largely smooth dendrites (figure 4b,c). There appear to be two types of aspiny neurons, one with ‘large’ and the other with ‘small’ cell bodies. The latter are fairly compact cells, whereas the dendrite and axons of the large type are more extensive. The axon of these cells does not leave the claustrum, so they are both classed as interneurons.
Immunoreactivity to calcium-binding proteins such as parvalbumin and calbindin have been invaluable in revealing compartments and other large-scale cell populations within brain tissue that may otherwise look homogeneous. This is particularly true for the thalamus (Jones 1998) and the brainstem (Parvizi & Damasio 2003). Reynhout & Baizer (1999) localized different calcium-binding proteins to the claustral cell types and comment upon the remarkable uniformity in the types and numbers of claustral cells, with no functional segregation or structural inhomogeneity. If this is indeed borne out, the clear modality specificity of the claustrum (see below) must be imposed by its inputs.
We conclude that the claustrum appears to possess a relative uniform architecture. What then is the nature of the computations performed by claustral neurons? If waves of information can travel within the claustrum (as we suggest further below), neurons could be especially sensitive to the timing of the inputs, cementing the role of the claustrum in binding disparate events into a single percept, experienced at one point in time.
4. Connections between cortex and the claustrum
What can be said of the connections between the cortex and the claustrum? Its principal cells project to the cortex on the same side, usually to many parts of it. Some claustral neurons also project to the contralateral cortex. Most regions of the cortex send a projection to the claustrum, usually to many parts of it. Thus their mappings are far from being a precise local mapping and tend more to be somewhat global (that is, all to all), though not completely so.
The best studied example is the claustrum of the cat. The mapping from visual cortex to the claustrum forms a single map with contributions from V1 and three neighbouring visual areas (Olson & Graybiel 1980). The map is folded in a curious way (LeVay & Sherk 1981b), with two unexpected features. (i) A point on the cortex maps onto a line of neurons in the claustrum (and thus a line of neurons in the cortex maps on to a sheet in the claustrum; Sherk 1986). (ii) There is considerably less emphasis on the centre (the area centralis) compared with V1. The map of the claustrum is nearer in proportion to that of the outside world.
The cells in cat V1 that project to the claustrum are a minority (4–10%) of the cells in layer 6, and are quite distinct from the cells in that layer that project to the lateral geniculate nucleus (LGN) (Katz 1987; Grieve & Sillito 1995). They probably use glutamate as a transmitter (Baughman & Gilbert 1981). The principal cells of the claustrum which project back to visual cortex usually go heavily to layer 4 and also to layer 6.
It has been claimed (Narkiewicz 1964) that the cat claustrum has three broad zones. An anterior dorsal zone connects to somatosensory and motor cortex, a posterior dorsal zone connects to visual cortex, and a third zone, ventral to the visual one, is connected to auditory areas. There could well be others. All areas of the cortex tested received input from roughly corresponding regions from both claustra, though projections from the contralateral claustrum are always considerably weaker than those from the ipsilateral claustrum (Norita 1977; see also Cortimiglia et al. 1991).
Numerous neuroanatomical studies in the monkey have revealed widespread connections between the claustrum and most allocortical and neocortical regions (figure 3; for references see Tanné-Gariépy et al. 2002). The claustrum is interconnected with the frontal lobe—including motor cortex, prefrontal cortex and cingulate cortex—visual cortical regions in the occipital lobe, temporal and temporopolar cortices, parietooccipital and posterior parietal cortex, the frontoparietal operculum, somatosensory areas, prepiriform olfactory cortex and the entorhinal cortex. The claustrum also projects to the hippocampus (Amaral & Cowan 1980), the amygdala (Amaral & Insausti 1992) and to the caudate nucleus (Arikuni & Kubota 1985).
It is not known whether there is any one cortical region that does not receive an input from the claustrum.
While most of the claustrum-to-cortex projections are reciprocated, there appear to be a few exceptions. In particular, no projection from primary visual cortex to the claustrum has been found in the macaque monkey (as discussed by Sherk 1986), while there is little doubt about the existence of axons from the claustrum to V1 (e.g. Mizuno et al. 1981; Perkel et al. 1986; Lysakowski et al. 1988).
The great discrepancy between the sizes of cortex and the claustrum raises the question of the extent of overlap of these diverse connections in the claustrum. Sherk's (1986) summary illustrates the complexity of this widespread claustrum-centred net. There are many projections—most bi-directional—which often overlap. No region projects everywhere, yet there are a number of more local maps which overlap somewhat. To give an example, orbital frontal cortex projects to the entire claustrum except the visual part. All these complicated interactions show that the cortical areas act in groups. There is strong interaction within each group and lesser interactions between groups. Likewise, there is at least one visual region in the monkey claustrum that sits at the nexus of a network linking it to multiple visual areas (V1, V2, V4, MT/V5, FST, MST, TEO and TE; Webster et al. 1993; Baizer et al. 1997). There may be more than one such visual area in the claustrum.
It has been suggested by Pearson et al. (1982) for monkeys that (i) if cortical area A, which is located in either the occipital, parietal or temporal lobe, projects to cortical area B in the frontal lobe, then the target zones of cortical areas A and B in the claustrum will overlap along a dorsal ventral axis; (ii) if cortical area C in the frontal lobe is connected to cortical area D, also in the frontal lobe, their respective claustral target zones will overlap along a dorsoventral axis and (iii) if area A is not connected to cortical area B, no matter where in the cortex they are located, their target zones in the claustral will not overlap. The picture emerging then from tracer studies is that cortical areas having widespread cortical connections, such as area 46 or the cingulate gyrus, are linked to extensive zones within the claustrum and that these large zones are likely to overlap with other claustrum territories.3
5. Physiological considerations
Insights into the physiological role of the claustrum, just like that of any other brain structure, has been obtained from lesion experiments, single cell recordings and stimulation studies in animals as well as from clinical observations and functional imaging in people.
What happens when the claustrum, or parts of it, have been transiently or permanently removed? The answer to this important question is, unfortunately, not known. It is very difficult to solely inactivate the claustrum by pharmacological substances or surgical ablation. Given its extended and sheet-like topography, ablating or otherwise shutting this structure down in a controlled manner—without interfering with fibres of passage or nearby regions—would require numerous, precisely targeted injections. Molecular biology could help here: if one or more genes were to be specifically and strongly expressed in neurons of the claustrum, it might be possible to silence this population by the judicious use of genetic techniques (Lechner et al. 2002; Slimko et al. 2002; Yamamoto et al. 2003) and observe the behaviour of such aclaustral animals.
As far as we know, there are no humans born without claustra and with all nearby regions intact. Furthermore, we know of only one report in the clinical literature (Morys et al. 1988) of patients with unilateral lesion in the claustrum. Indeed, damage restricted to the claustrum, without collateral damage to the nearby putamen or insula is not very likely, since the claustrum is located in watershed territory in terms of its vascularization; it can be supplied by both the deeper and the more superficial part of the middle cerebral artery (A. R. Damasio, personal communication). Finally, it is likely that any loss of function would require loss of both claustra.
There are remarkably few microelectrode investigations of claustral receptive-field properties and almost none in awake animals. LeVay & Sherk (1981b; see also Olson & Graybiel 1980; Sherk & LeVay 1981) focused on responses of claustral neurons to visual inputs in anaesthesized cats. Stimuli were oriented slits of light swept over the screen. What was remarkable was the shape of the receptive fields, in most cases being very long and thin, so that the claustral cells were highly selective for orientation. Cells are almost all binocular, summate strongly as the stimulus is lengthened and lack selectivity for other stimulus features, such as width, direction of movement, velocity and contrast. Sherk & LeVay (1981) suggest that ‘the claustrum performs some rather simple service that does not require a complex transformation of information it receives.’ Note that the animals were not alert, though.
Tsumoto & Suda (1982) applied electric shocks to the dorsocaudal claustrum of cats, eliciting bimodal excitatory responses in the visual cortex with 12–26 ms latencies. About one quarter of the cells had inhibition with onset latencies longer than excitation.
The dorsal claustrum has bi-directional connections with cortical motor structures (figure 3; Kunzle & Akert 1977; Riche & Lanoir 1978; Barbas & Mesulam 1981; Tanné-Gariépy et al. 2002). What is the relation between an animal's movements and the manner in which neurons in the dorsal claustrum behave? Shima et al. (1996) found that the majority (70%) of movement-related neurons were non-selective, firing to any of three different forelimb movements (push, pull or turn) made by the monkey. A small fraction of cells was more discerning, only responding to one of the three actions (as were the majority of cells in the primary motor cortex).
Cortimiglia et al. (1991) recorded from frontal oculomotor neurons while stimulating the motor part of the claustrum. They report ipsilateral excitatory effects with onset latencies of 5–15 ms and, less frequently, contralateral effects with a latency of 20–50 ms. These short-lived facilitatory effects can be followed by delayed inhibitory influences lasting up to 250 ms. Latencies are of some interest because of the suspicion that the claustrum is important in the timing of cortical activity. A systematic study of the latency, in both directions, for a variety of cortical areas would be straightforward and might be of considerable interest.
Given the amazing rise of functional brain imaging over the past decade, what has been learned about haemodynamic activity in the claustrum of normal human volunteers? Two positron emission tomography (PET) and one functional magnetic resonance imaging studies investigated male arousal during viewing of sexually explicit material or during ejaculation (Redoute et al. 2000; Arnow et al. 2002; Holstege et al. 2003). In all three experiments, strong haemodynamic responses were reported in the putamen, the insula and the claustrum. However, given the low spatial resolution of current imaging techniques compared with the thin (5 mm or less) width of the sheet of claustrum neurons, and the known involvement of the nearby insula in interoceptive awareness of bodily states (Craig 2002; Critchley et al. 2004), it is uncertain as to what extent these signals correspond to a specific neural activity in the claustrum. The same comment also applies to the innovative study of awareness for air hunger (breathlessness induced by breathing CO2) that reported enhanced PET activity in the region of the insula/claustrum (Liotti et al. 2001).
Lastly, a PET study (Hadjikhani & Roland 1998) revealed the involvement of the insula/claustrum region in cross-model matching, in tasks that require the simultaneous evaluation of information from more than one sensory domain (e.g. visual–tactile, audio–visual and so on).
It is clear that our existing knowledge is insufficient for understanding how claustral neurons respond when a human or animal is engaged in any task of moderate complexity. This cries out for additional work.
6. Functional considerations
Given these data, what role or roles does the claustrum play?
It has been suggested (Ettlinger & Wilson 1990; Calvert 2001) that the claustrum is involved in cross-model processing. The main exhibit for this hypothesis is the previously mentioned imaging study (Hadjikhani & Roland 1998).
Within the context of the neurobiological theories of consciousness mentioned in the introduction, the highly networked nature of the claustrum raises the question of whether it acts as a sort of ‘Cartesian theatre’. This is a metaphor, introduced and ridiculed by the philosopher Dennett (1991), for the fictitious centre where the mind and brain meet, where ‘it all comes together’ and consciousness occurs.
We think that a more appropriate analogy for the claustrum is that of a conductor coordinating a group of players in the orchestra, the various cortical regions. Without the conductor, the players can still play but they fall increasingly out of synchrony with each other. The result is a cacophony of sounds.
This metaphor would suggest that different attributes of objects, both within (e.g. colour and motion) and across modalities (e.g. visual form and sound location), are rapidly combined and bound in the claustrum. Without this structure—and that of its twin in the other hemisphere—the subject may still be able to respond to simple, isolated or to highly familiar stimuli, but not to complex or unfamiliar ones. Objects or events in the real world have many simultaneous attributes: colour, shape, distance, velocity, smell, sound, feel and so on. In the absence of both claustra, these attributes may not be experienced in an integrated manner and the subject may fail to altogether perceive these objects or events or only be consciously aware of some isolated attribute.
It is clear that the claustrum lies at the confluence of a large number of simple loops with cortex. This widespread and reciprocal connectivity with many, if not most, cortical regions raises the obvious question: why is all this information brought together, since this involves most of the loops being much longer than if the claustrum lay more uniformly under the cortex? Even more unusually, there appears to be no long-range connections within the claustrum.
In biology, if seeking to understand function, it is usually a good idea to study structure. Thus, if the claustrum is critical to binding information within and across sensory and motor modalities, certain anatomical constraints would have to be met. In particular, the information from say, a visual cortical region would need to be combined with information from somatosensory, auditory or motor cortices. This demands some sort of intermixing of the associated signals within the claustrum. Several, non-exclusive, anatomical and biophysical substrates for such widespread intra-claustral interactions are possible.
The axonal arbours of incoming fibres would have to spread over a considerable distance in the anterior–posterior and ventral–dorsal directions.
A hitherto unrecognized, possibly quite sparse, interneuron type with very long dendrites and extensive axonal terminals in the plane of the claustrum.
Dendro-dendritic chemical synapses among claustral cells, as found in the intraglomular microcircuits of the olfactory bulb (Shepherd & Greer 1998).
Gap junctions between aspiny claustral interneurons. Formed from the connexin family of proteins, they provide a low-resistance, bi-directional, electrical pathway between neurons. Such ‘electrical synapses’ interconnect specific types of inhibitory interneurons in the thalamic reticular nucleus and in the cortex (Gibson et al. 1999; Landisman et al. 2002; LeBeau et al. 2003; Bennett & Zukin 2004). Different from conventional chemical synapses that would inhibit the firing activity of their postsynaptic targets, gap junctions can cause the membrane depolarization in one interneuron to spread to others. It has been hypothesized that gap junctions help synchronize the firing of interneurons, enabling the entire population to fire in lock-step in the 30–70 Hz (gamma) range. Rhythmic firing of claustral interneurons might be critical to help synchronize far-flung populations of cortical neurons.
In summary, we suggest that the claustrum may contain specialized mechanisms that permit information to travel widely within its anterior–posterior and ventral–dorsal extent to synchronize different perceptual, cognitive and motor modalities. This postulated intra-claustrum mixing of information would make it quite different from the thalamus, a subcortical structure that also enjoys widespread and reciprocal relations with most cortical regions, but that does not possess any obvious mechanism to directly link its various constitutive nuclei.
7. Future experiments
A great deal can be learned about the claustrum by means of neuroanatomical connectivity studies and combined behavioural and physiological experiments. Given its wide-reaching connections, it might be more instructive to know which neocortical regions are not innervated by claustral fibres and which ones do not send axons to the claustrum.4 Do projections from the claustrum to the cortex terminate in layer 4? Does this imply that claustral input is driving, rather than modulatory (Crick & Koch 1998)? That is, that the input from the claustrum can, by itself, give rise to firing in its cortical target cells rather than just modulating their discharge. Is it plausible that the claustrum plays a key role in assembling the cortical coalitions of neurons that are sufficient for any one conscious percept?
Understanding the function of the claustrum requires a local circuit diagram, including the detailed neuroanatomical wiring and biophysical knowledge of the extent and quality of information circulating within the claustrum. How elongated are the axon collaterals? Are there dendro-dendritic chemical synapses or gap junctions among claustral neurons? Are there any substantial differences between type 1 neurons having different shapes (e.g. pyramidal, fusiform)?
In order to move from correlation towards causation, experimentalists need to perturb the claustrum and observe any changes in the behaviour of the organism. This requires (i) practical methods to selectively, deliberately, reversibly and delicately silence (sub)populations of claustral neurons in appropriate organisms and (ii) relevant behaviours that depend on the claustrum.
While the shape of the claustrum makes surgical or pharmacological intervention difficult, conditional knock-in or knock-down molecular techniques are more promising. They demand the urgent identification of one or more proteins strongly expressed in the claustrum but not in adjacent regions (the insula and the putamen).
What behavioural paradigms can be usefully studied using such ‘silencing’ protocols? If the claustrum were to be involved in combining information within and across modalities, it is likely that its bilateral loss would only interfere minimally with the host of sensory-motor systems that we have dubbed zombie modes (Koch & Crick 2001). That is, removing both claustra would not affect routine and stereotyped behaviours in response to some simple inputs (e.g. an eye or hand movement in response to a single, isolated visual stimulus that moves one way or another). Thus, more complex tasks may be needed to reveal loss-of-function.
It has been argued (Clark & Squire 1998; Koch 2004) that trace associative conditioning of mice is a task that requires awareness of the contingency between neutral and aversive stimuli. Mice can be both delay- and trace-conditioned and distracting procedures have been devised that selectively interfere with the latter but not the former (Han et al. 2003). This may therefore be a very promising behavioural assay for testing consciousness-related functional loss.
The neuroanatomy of the claustrum is compatible with a global role in integrating information at the fast time-scale. This should be further experimentally investigated, in particular if this structure plays a key role in consciousness. What could be more important? So why wait?
Acknowledgments
The first draft of this manuscript was completed on 19 July 2004 by F.C. He was refining the manuscript on 28 July, the day he died in hospital. I (C.K.) wish to thank the many people who helped Francis and myself in various ways with the manuscripts and the ideas it contains: Ann Butler, Ed Callaway, Antonio Damasio, Emiko Koike, Nikos Logothetis, Kathleen Murray, Tomaso Poggio, Ralph Siegel and Terry Sejnowski.
Endnotes
We do not like the term ‘field’ since it is so easily misunderstood to imply the working of an electro-magnetic field that carries this integrated information in the brain. For biophysical reasons, this is unlikely to be the case (Koch 2004).
Whether the claustrum occurs in monotremes is controversial (Butler et al. 2002).
This gives rise to a possible neuroanatomical hypothesis: some or all long-range direct cortico-cortical projections, front to back, or vice versa, give off collaterals to the claustrum.
In particular, is it true that the macaque V1 does not project to the claustrum? This would be compatible with our idea (Crick & Koch 1995) that neurons in V1, like retinal neurons, are not strictly necessary for visual consciousness.
References
- Amaral D.G, Cowan W.M. Subcortical afferents to the hippocampal formation in the monkey. J. Comp. Neurol. 1980;189:573–591. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- Amaral D.G, Insausti R. Retrograde transport of d-[3H]aspartate injected into the monkey amygdaloid complex. Exp. Brain Res. 1992;88:375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- Arikuni T, Kubota K. Claustral and amygdaloid afferents to the head of the caudate nucleus in macaque monkeys. Neurosci. Res. 1985;2:239–254. doi: 10.1016/0168-0102(85)90003-3. [DOI] [PubMed] [Google Scholar]
- Arnow B.A, Desmond J.E, Banner L.L, Glover G.H, Solomon A, Polan M.L, Lue T.F, Atlas S.W. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125:1014–1023. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- Baars B.J. Oxford University Press; New York: 1997. Theater of consciousness. [Google Scholar]
- Baars B.J. The conscious access hypothesis: origins and recent evidence. Trends Cogn. Sci. 2002;6:47–52. doi: 10.1016/s1364-6613(00)01819-2. [DOI] [PubMed] [Google Scholar]
- Bachmann T. Johns Benjamins; Amsterdam: 2000. Microgenetic approach to the conscious mind. [Google Scholar]
- Baizer J.S, Lock T.M, Youakim M. Projections from the claustrum to the prelunate gyrus in the monkey. Exp. Brain Res. 1997;113:564–568. doi: 10.1007/pl00005607. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam M.M. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J. Comp. Neurol. 1981;200:407–431. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Baughman R.W, Gilbert C.D. Aspartate and glutamate as possible neurotransmitters in the visual cortex. J. Neurosci. 1981;1:427–439. doi: 10.1523/JNEUROSCI.01-04-00427.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M.V.L, Zukin R.S. Electrical coupling and neuronal synchronization in the mammalian brain. Neuron. 2004;41:495–511. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuronal types in the claustrum of man. Anat. Embryol. 1982;163:447–460. doi: 10.1007/BF00305558. [DOI] [PubMed] [Google Scholar]
- Brand S. A serial section Golgi analysis of the primate claustrum. Anat. Embryol. 1981;162:475–488. doi: 10.1007/BF00301872. [DOI] [PubMed] [Google Scholar]
- Butler A.B, Molnar Z, Manger P.R. Apparent absence of claustrum in monotremes: implications for forebrain evolution in amniotes. Brain Behav. Evol. 2002;60:230–240. doi: 10.1159/000066698. [DOI] [PubMed] [Google Scholar]
- Calvert G.A. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb. Cortex. 2001;11:1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- Clark R.E, Squire L.R. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Cortimiglia R, Crescimanno G, Salerno M.T, Amato G. The role of the claustrum in the bilateral control of frontal oculomotor neurons in the cat. Exp. Brain Res. 1991;84:471–477. doi: 10.1007/BF00230958. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Crick F.C. Charles Scribner's Sons; New York: 1994. The astonishing hypothesis. [Google Scholar]
- Crick F.C, Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375:121–123. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- Crick F.C, Koch C. Constraints on cortical and thalamic projections: the no-strong-loops hypothesis. Nature. 1998;391:245–250. doi: 10.1038/34584. [DOI] [PubMed] [Google Scholar]
- Crick F.C, Koch C. A framework for consciousness. Nat. Neurosci. 2003;6:119–126. doi: 10.1038/nn0203-119. [DOI] [PubMed] [Google Scholar]
- Critchley H.D, Wiens S, Rotshtein P, Öhman A, Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux J.-P. Neural mechanisms for access to consciousness. In: Gazzaniga M, editor. The cognitive neurosciences. 3rd edn. MIT Press; Cambridge, MA: 2004. [Google Scholar]
- Dehaene S, Sergent C, Changeux J.P. A neuronal model linking subjective report and objective neurophysiological data during conscious perception. Proc. Natl Acad. Sci. USA. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennett D. Little & Brown; Boston, MA: 1991. Consciousness explained. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Edelman G.M, Tononi G. Basic Books; New York: 2000. A universe of consciousness. [Google Scholar]
- Edelstein L.R, Denaro F.J. The claustrum: a historical review of its anatomy, physiology, cytochemistry and functional significance. Cell. Mol. Biol. 2004;50:675–702. [PubMed] [Google Scholar]
- Ettlinger G, Wilson W.A. Cross-model performance: behavioral processes, phylogenetic considerations and neural mechanisms. Behav. Brain Res. 1990;40:169–192. doi: 10.1016/0166-4328(90)90075-p. [DOI] [PubMed] [Google Scholar]
- Gibson J.R, Beierlein M, Connors B.W. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Grieve K.L, Sillito A.M. Differential properties of cells in the feline primary visual cortex providing the corticofugal feedback to the lateral geniculate nucleus and visual claustrum. J. Neurosci. 1995;15:4868–4874. doi: 10.1523/JNEUROSCI.15-07-04868.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Roland P.E. Cross-modal transfer of information between the tactile and the visual representations in the human brain: a positron emission tomographic study. J. Neurosci. 1998;18:1072–1084. doi: 10.1523/JNEUROSCI.18-03-01072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C.J, O'Tuathaigh C.M, van Trigt L, Quinn J.J, Fanselow M.S, Mongeau R, Koch C, Anderson D.J. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc. Natl Acad. Sci. USA. 2003;100:13 087–13 092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D.O. Wiley; New York: 1949. The organization of behavior: a neuropsychological theory. [Google Scholar]
- Holstege G, Georgiadis J.R, Paans A.M, Meiners L.C, van der Graaf F.H, Reinders A.A. Brain activation during human male ejaculation. J. Neurosci. 2003;8:9185–9193. doi: 10.1523/JNEUROSCI.23-27-09185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E.G. The core and matrix of thalamic organization. Neuroscience. 1998;85:331–345. doi: 10.1016/s0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- Katz L.C. Local circuitry of identified projection neurons in cat visual cortex brain slices. J. Neurosci. 1987;7:1223–1249. doi: 10.1523/JNEUROSCI.07-04-01223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. Roberts & Company; Englewood, CO: 2004. The quest for consciousness: a neurobiological approach. [Google Scholar]
- Koch C, Crick F.C. On the zombie within. Nature. 2001;411:893. doi: 10.1038/35082161. [DOI] [PubMed] [Google Scholar]
- Kowianski P, Dziewiatkowski J, Kowianska J, Morys J. Comparative anatomy of the claustrum in selected species: a morphometric analysis. Brain Behav. Evol. 1999;53:44–54. doi: 10.1159/000006581. [DOI] [PubMed] [Google Scholar]
- Kunzle H, Akert K. Efferent connections of cortical area 8 (frontal eye field) in Macaca fascicularis: a reinvestigation using the autoradiographic technique. J. Comp. Neurol. 1977;173:147–164. doi: 10.1002/cne.901730108. [DOI] [PubMed] [Google Scholar]
- Landisman C.E, Long M.A, Beierlein M, Deans M.R, Paul D.L, Connors B.W. Electrical synapses in the thalamic reticular nucleus. J. Neurosci. 2002;22:1002–1009. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBeau E.N, Traub R.D, Monyer H, Whittington M.A, Buhl E.H. The role of electrical signaling via gap junctions in the generation of fast network oscillations. Brain Res. Bull. 2003;62:3–13. doi: 10.1016/j.brainresbull.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Lechner H.A.E, Lein E.S, Callaway E.M. A genetic method for selective and quickly reversible silencing of mammalian neurons. J. Neurosci. 2002;22:5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, Sherk H. The visual claustrum of the cat. I. Structure and connections. J. Neurosci. 1981a;1:956–980. doi: 10.1523/JNEUROSCI.01-09-00956.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S, Sherk H. The visual claustrum of the cat. II. The visual field map. J. Neurosci. 1981b;1:981–992. doi: 10.1523/JNEUROSCI.01-09-00981.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotti M, et al. Brain responses associated with consciousness of breathlessness (air hunger) Proc. Natl Acad. Sci. USA. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R.R. MIT Press; Cambridge, MA: 2001. I of the vortex: from neurons to self. [Google Scholar]
- Lysakowski A, Standage G.P, Benevento L.A. An investigation of collateral projections of the dorsal lateral geniculate nucleus and other subcortical structures to cortical areas V1 and V4 in the macaque monkey: a double label retrograde tracer study. Exp. Brain Res. 1988;69:651–661. doi: 10.1007/BF00247317. [DOI] [PubMed] [Google Scholar]
- Maunsell J.H. Neuronal representations of cognitive state: reward or attention? Trends Cogn. Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Mizuno N, et al. Extrageniculate projections to the visual cortex in the macaque monkey: an HRP study. Brain Res. 1981;212:454–459. doi: 10.1016/0006-8993(81)90477-7. [DOI] [PubMed] [Google Scholar]
- Morys J, Sloniewski P, Narkiewicz O. Somatosensory evoked potentials following lesions of the claustrum. Acta Physiol. Pol. 1988;39:475–483. [PubMed] [Google Scholar]
- Narkiewicz O. Degenerations in the claustrum after regional neocortical ablations in the cat. J. Comp. Neurol. 1964;123:335–356. doi: 10.1002/cne.901230304. [DOI] [PubMed] [Google Scholar]
- Norita M. Demonstration of bilateral claustro-cortical connections in the cat with the method of retrograde axonal transport of horseradish peroxidase. Arch. Histol. Jpn. 1977;40:1–10. doi: 10.1679/aohc1950.40.1. [DOI] [PubMed] [Google Scholar]
- Olson C.R, Graybiel A.M. Sensory maps in the claustrum of the cat. Nature. 1980;288:479–481. doi: 10.1038/288479a0. [DOI] [PubMed] [Google Scholar]
- Palm G. Cell assemblies as a guideline for brain research. Concept. Neurosci. 1990;1:133–147. [Google Scholar]
- Parvizi J, Damasio A.R. Differential distribution of calbindin D28k and parvalbumin among functionally distinct sets of structures in the macaque brainstem. J. Comp. Neurol. 2003;462:153–167. doi: 10.1002/cne.10711. [DOI] [PubMed] [Google Scholar]
- Pearson R.C, Brodal P, Gatter K.C, Powell T.P. The organization of the connections between the cortex and the claustrum in the monkey. Brain Res. 1982;234:435–441. doi: 10.1016/0006-8993(82)90883-6. [DOI] [PubMed] [Google Scholar]
- Perkel D.J, Bullier J, Kennedy H. Topography of the afferent connectivity of area 17 in the macaque monkey: a double-labeling study. J. Comp. Neurol. 1986;253:374–402. doi: 10.1002/cne.902530307. [DOI] [PubMed] [Google Scholar]
- Rae A.S. The form and structure of the human claustrum. J. Comp. Neurol. 1954;100:15–39. doi: 10.1002/cne.901000103. [DOI] [PubMed] [Google Scholar]
- Redoute J, Stoleru S, Gregoire M.C, Costes N, Cinotti L, Lavenne F, Le Bars D, Forest M.G, Pujol J.F. Brain processing of visual sexual stimuli in human males. Hum. Brain Mapp. 2000;11:162–177. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynhout K, Baizer J.S. Immunoreactivity for calcium-binding proteins in the claustrum of the monkey. Anat. Embryol. 1999;199:75–83. doi: 10.1007/s004290050211. [DOI] [PubMed] [Google Scholar]
- Riche D, Lanoir J. Some claustro-cortical connections in the cat and baboon as studied by retrograde horseradish peroxidase transport. J. Comp. Neurol. 1978;177:435–444. doi: 10.1002/cne.901770306. [DOI] [PubMed] [Google Scholar]
- Searle J. Oxford University Press; New York: 2004. Mind: a brief introduction. [Google Scholar]
- Shepherd G.M, Greer C.A. Olfactory bulb. In: Shepherd G.M, editor. The synaptic organization of the brain. Oxford University Press; New York: 1998. pp. 159–204. [Google Scholar]
- Sherk H. The claustrum and the cerebral cortex. In: Jones E.G, Peters A, editors. Cerebral cortex. vol. 5. Plenum Press; New York: 1986. pp. 467–499. [Google Scholar]
- Sherk H, LeVay S. The visual claustrum of the cat. III. Receptive field properties. J. Neurosci. 1981;1:993–1002. doi: 10.1523/JNEUROSCI.01-09-00993.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Hoshi E, Tanj J. Neuronal activity in the claustrum of the monkey during performance of multiple movements. J. Neurophysiol. 1996;76:2115–2119. doi: 10.1152/jn.1996.76.3.2115. [DOI] [PubMed] [Google Scholar]
- Slimko E.M, McKinney S, Anderson D.J, Davidson N, Lester H.A. Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. J. Neurosci. 2002;22:7373–7379. doi: 10.1523/JNEUROSCI.22-17-07373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanné-Gariépy J, Boussaoud D, Rouiller E.M. Projections of the claustrum to the primary motor, premotor, and prefrontal cortices in the macaque monkey. J. Comp. Neurol. 2002;454:140–157. doi: 10.1002/cne.10425. [DOI] [PubMed] [Google Scholar]
- Tononi G, Edelman G.M. Consciousness and complexity. Science. 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Suda K. Effects of stimulation of the dorsocaudal claustrum on activities of striate cortex neurons in the cat. Brain Res. 1982;240:345–349. doi: 10.1016/0006-8993(82)90233-5. [DOI] [PubMed] [Google Scholar]
- Webster M.J, Bachevalier J, Ungerleider L.G. Subcortical connections of inferior temporal areas TE and TEO in macaque monkeys. J. Comp. Neurol. 1993;335:73–91. doi: 10.1002/cne.903350106. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Wada N, Kitabatake Y, Watanabe D, Anzai M, Yokoyama M, Teranishi Y, Nakanishi S. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J. Neurosci. 2003;23:6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]