Abstract
The neuron doctrine represents nerve cells as polarized structures that contact each other at specialized (synaptic) junctions and form the developmental, functional, structural and trophic units of nervous systems. The doctrine provided a powerful analytical tool in the past, but is now seldom used in educating neuroscientists. Early observations of, and speculations about, sites of neuronal communication, which were made in the early 1860s, almost 30 years before the neuron doctrine was developed, are presented in relation to later accounts, particularly those made in support of, or opposition to, the neuron doctrine. These markedly differing accounts are considered in relation to limitations imposed by preparative and microscopical methods, and are discussed briefly as representing a post-Darwinian, reductionist view, on the one hand, opposed to a holistic view of mankind as a special part of creation, on the other. The widely misunderstood relationship of the neuron doctrine to the cell theory is discussed, as is the degree to which the neuron doctrine is still strictly applicable to an analysis of nervous systems. Current research represents a ‘post-neuronist’ era. The neuron doctrine provided a strong analytical approach in the past, but can no longer be seen as central to contemporary advances in neuroscience.
Keywords: history, neuronists, reticularists, microscopy
1. Introduction
Neuroscience owes a major debt to the neuron doctrine, and to the closely related law of dynamic polarization. The neuron doctrine defines nerve cells as structural, functional, developmental and trophically independent units, and the law of dynamic polarization defines the nerve cell body and dendrites as providing receptor surfaces for incoming messages, whereas the axon serves as the output of the nerve cell. In the following I will, for the sake of simplicity, refer to this combination, of the nerve cell as unit and the nerve cell as polarized, as the neuron doctrine.
Although one can find statements that claim the neuron doctrine as central to neuroscience, drawing comparisons with Darwinian evolution or the quantum theory,1 details about the doctrine are no longer seen as a generally accepted, essential part of neuroscience courses, and are described ever more briefly, or not at all, in contemporary textbooks. A recent informal survey of graduate students approaching the end of their first year of graduate course work showed that 10 out of 10 students did not know what the neuron doctrine is. One of the students wrote that the law of dynamic polarization related to the fact that epithelial cells are polarized, the others knew nothing about it; a small sample from a medical class did not differ significantly from this.
In this essay I will look at some early observations that led to the formulation of the neuron doctrine. I will also consider the way the neuron doctrine can be seen today, so as to explore why ideas that have played a vital role in the development of neuroscience seem no longer to be of much importance in the training that is provided for young neuroscientists.
Today it is not possible to imagine what would have happened if investigations of the nervous system, carried out over the past century and more, had not been based on the neuron doctrine. The view of nerve cells as the ‘building blocks’, and synapses as the one-way communication link between them, provided the structures upon which analysis of the long pathways of the vertebrate brain depended heavily for 50 years. Without these analytical tools we might still be roughly where we are today, but we would have travelled a different route. Almost certainly, the route would have been more tortuous and difficult even though the plateau on which we stand today could, perhaps, have been the same. The neuron doctrine provided a powerful means for taking the nervous system apart and for studying it in manageable bits. Much has been written about the neuron doctrine; first in its formulation, then in its denial defence or clarification, and finally in its celebration. However, today many younger neuroscientists know very little about the doctrine or about the significant battles between ‘neuronists’ who defended the neuron doctrine and ‘reticularists’, who attacked it from several different points of view. These arguments formed an important part of the origin of the neuron doctrine, and recent summaries have done scan justice to the complex history. For example, Albright et al. (2000), writing about investigators they (wrongly) classified as reticularists, stated that a ‘…chaotic view of the brain…emerged from the work of Golgi, Gerlach and Deiters who conceived of the brain as a diffuse nerve net in which every type of interaction appeared possible’. Such historically inaccurate views trivialize the issues that the neuronists had to address, and call for a closer look at the observations that were actually made.
This review will not cover the whole of the history of the neuron doctrine, but will focus on early observations on the structure of the synapse, a key issue in the debate. I shall first consider the neuron doctrine itself, and then look particularly at how knowledge about synaptic junctions grew during the second half of the nineteenth century, from observations made at the limits of resolution of the available microscopes. The details of the difficult interpretations of microscopic sections are often overlooked in earlier accounts of this history. I will try to show how some of the early observations were made, how some of them led to interpretations that were contrary to the neuron doctrine, and indicate the basis on which the neuron doctrine won through and shaped our current views of what nerve cells, particularly their synaptic junctions, ‘really’ look like under the microscope. This is not part of a partisan debate about the neuron doctrine; the days for that are long since past. It is an exploration of the difficulties of making observations under a microscope, and also looks at the relationship between the appearances reported and the theoretical framework into which those reports have often been thought to fit.
It is difficult to write a historical account that truly represents the points of view of those who, between 1850 and 1900, were seeking to discover how nerve cells relate to each other. In an old account or figure it is sometimes possible to understand how a particular feature relates to current knowledge, but often this is not possible. Many old observations remain puzzling, dependent on a particular original misinterpretation or on a preparation that produced artefacts, whose precise nature and origins remain mysterious unless one repeats the author's (often sketchy) recipes, which I have not done. Often, one has to conclude that the observations were wrong, and that a more careful observer would have seen something else. It is not possible for me to cover all of the different viewpoints that were raised for or against the neuron doctrine. To some extent this has been done by Cajal, writing late in his long career (for the English translation see Cajal 1954) and defending the neuron doctrine against many of its opponents. Nissl (1903) wrote a long and detailed summary of many of the same and some other arguments from one opposing point of view, as did Bielschowsky (1904, 1928) from another. More recently, others have summarized the development of the neuron doctrine (e.g. Bullock 1959; Liddell 1960; Andreoli 1961; Van der Loos 1967; Shepherd 1991; Jacobson 1993; Clarke & O'Malley 1996; Albright et al. 2000; Bennett 2002; Cowan & Kandel 2002), focusing on some of the original discoveries and disagreements, and generally (but see Andreoli 1961) aiming to show how the doctrine developed, without providing much of a view of the opposition, except for that coming from Golgi, who presented perhaps the most notable opposition.
From these published historical accounts it is difficult to gain a view of how the observations of synaptic structures were actually made, or why they led to the discordant views that characterized the early days. Nor do they show where the neuron doctrine stands in relation to contemporary neuroscience, which is considered in the second part of this review. To what extent can the neuron doctrine still play the vital role it played 100, or even 50 years ago, and to what extent is it no longer relevant to the way in which we teach students to think about nervous systems? I shall argue that the neuron doctrine has done its job, that contemporary neuroscience is best served by thinking of nerve cells as basically like other cells, capable of going against the dogma of the neuron doctrine: for example, forming cell to cell fusions, not necessarily functioning as a single unit, and in several other ways. It is important to stress that this is not an argument about the neuron doctrine itself, which has proved its analytical power for over 100 years. Throughout this essay I assume that the reader understands the analytical strengths that the neuron doctrine has provided in the past and shares my interest in exploring its origins and its current role.
2. The neuron doctrine itself
von Waldeyer-Hartz (1891) is generally credited with formulating the neuron doctrine, although as soon as one writes that one is obliged to cite Cajal (see Cajal (1954) for the English translation) who wrote: ‘Professor Waldeyer, to whom poorly informed persons attribute the neuron theory, supported it with the prestige of his authority but did not contribute a single personal observation. He limited himself to a short, brilliant exposition (1891) of the objective proofs adduced by His, Kölliker, Retzius, Van Gehuchten and myself, and he invented the fortunate term of neuron’.
That is a fair summary of von Waldeyer-Hartz's long and thoughtful review, which not only owed much to Cajal's observations, and those of His and Van Gehuchten, but which would later be strongly defended by Cajal on the basis of wide ranging observations on vertebrate and invertebrate nervous systems. Near the end of the last section of the review von Waldeyer-Hartz wrote:2
‘Axons…all arise from nerve cells. There is no connection with a fibrous network, no origin from such a network’. This was followed by ample examples.
‘All of these axons end freely, with terminal arbours with no networks or anastomoses’. Again there followed examples, the most important of which were the branching motor nerves described by Kühne (1862) (see §6).
In his summary von Waldeyer-Hartz said: ‘If we take an overview of the main conclusion (Hauptgewinn)…then it lies particularly in allowing a sharper definition of the anatomical and functional elements of the nervous system, which we have to regard as the neural elements (Neurons)’ (stress added).
His had earlier argued (His 1886), on the basis of many different examples, in favour of free nerve endings, and had written further: ‘…every nerve fibre arises as the outgrowth of a single cell. This is its genetic, its nutritive and its functional centre’.2
That is, basing himself on the observations and interpretations of many predecessors (see Shepherd (1991) and Clarke & O'Malley (1996) for details) von Waldeyer-Hartz defined the structural and functional unit of the nervous system. Two further points were added by others; that these elements were: (i) the units in the development of the nervous system; and (ii) the units in its degenerative changes (the trophic units). His (1883, 1886, 1889) had earlier shown that nerve cells arise as independent elements during development and that the neural processes, axons first, and then dendrites, arise from these cells. Forel (1887) had argued, mainly from studies of neural degeneration produced by experimentally produced lesions, that nerve cells and their axons formed a trophic unit.
At a later date, primarily Cajal and Van Gehuchten in complex discussions that extended from 1891 to 1897 (see Van Gehuchten (1891) and Van Gehuchten & Martin (1891), Cajal (1911) or (1995) for details) added the law of dynamic (or functional) polarization. This stated that nerve cells have a single axon, which serves as an effector and that the dendrites and the cell body serve as the receptive surfaces of the neuron. Essentially then, the fully developed neuron doctrine states that the nerve cell is a polarized structure and is the unit of neural structure, the functional unit, the developmental unit and the trophic unit. A key part of the neuron doctrine is the statement that the individual nerve cells communicate at regions of cell-to-cell contact; there is no continuity between cells.
Cajal is recognized as the leading protagonist of the neuron doctrine. He also made many of the critical observations: He described, in many different situations, and in great detail, the regions of contact between neurons that Sherrington (1897) later named ‘synapses’.3 Cajal's careful, wonderfully accurate and detailed illustrations provided the key documentation (see especially Cajal 1911), upon which rest not only the neuron doctrine, but also much of our current understanding of neural interrelationships. It was his richly documented view, obtained from many different parts of the nervous system, of nerve cells as functionally polarized units linked at contact regions by synapses, which provided the key for a systematic and highly successful reductionist analysis of nervous systems.
3. Opposition to the neuron doctrine
It is hardly necessary to stress that the neuron doctrine was not accepted without opposition. The fight between those supporting the neuron doctrine, the ‘neuronists’, and the ‘reticularists’ who opposed it, was fierce and drawn out, fought over several decades. To understand the often heated nature of the argument, it is important to recognize that the debate occurred at a time when the relationship of mankind to other organisms was being challenged by scientific conclusions that were the subject of widespread public debate. The scientific issues included the cell theory (Schwann 1838), which claimed a common structure for plants and animals, and the theory of evolution (Darwin 1859) for biology in general. Also directly relevant, were theories of cerebral localization (1860s and 1870s4) and the neuron doctrine (von Waldeyer-Hartz 1891) for the brain in particular. Each of these concerned either the relationship of humans to other species, or the related subject: the nature of mind. Each provided an entry into a reductionist analysis of areas that were long regarded as the province of theologians, unknowable to science. That is, there was a controversy between a reductionist view of the brain on the one hand and a holistic, often mystical view, on the other. Although these ‘deeper’ concerns are often left entirely unexpressed in the scientific writings, it is impossible to avoid a sense of their presence in the fierce arguments.
The arguments about the neuronal structures were mostly expressed in terms that were appropriately dry, distant and impersonal.5 However, there were heated passages and flashes of hostility, indicating that something else was also at stake. To some extent, competitive interactions and a striving for priority are clearly discernible, but almost certainly, the public issues that dominated the period were more important than these personal issues. There are not many glimpses of the broader issues that lay beyond the arguments about how nerve cells relate to each other. Two examples cited by Shepherd (1991) provide a view of the deeper issues. Forel, often thought of as one of the ‘founders’ of the neuron doctrine, wrote about the influence that his reading of Darwin had on his views on the physiology of the brain. Golgi, in explaining the rich interconnections formed by his proposed neural reticulum linked this to an argument against the localization of function in the brain, using one holistic obscurantist argument to justify another (see 4).
In practical terms, it is important to recognize that observations in opposition to the neuron doctrine came from many directions. There was no ‘reticularist school’, and at times someone regarded as a reticularist by one investigator is described as a neuronist by another. The different lines of opposition will be briefly outlined here, and only some will be considered in more detail later.6
Initially, there was a long-standing view, arising from the fact that it was not easy to trace a long nerve fibre back to its cell of origin, that nerve fibres were distinct from the neurons; structures we call neurons today were often called ‘ganglion cells’, and the term ‘nerve cell’ was then reserved for other cells that were thought to give rise to nerve fibres;7 in some accounts these were what we now call Schwann cells, rather than true nerve cells. There was a ‘catenary’ theory of the origin of nerve fibres as being composed of a chain or a necklace of several different parts. Once it was accepted that neurons give rise to axons and dendrites (see below), there was then controversy about how these processes relate to each other. Where the neuronists saw regions of contact between one neuron and another, others saw continuities, some of axons with each other (e.g. Golgi 1908), some of axons and dendrites with other dendrites (e.g. Gerlach 1871, 1872), some of axons with dendrites or cell bodies (e.g. Held 1897). There was argument about neurofibrils (now recognized as a cytoskeletal element of nerve cells and their processes), which were seen by some as independent elements running through neurons, passing from one neuron to another and forming the proposed conducting element (Apáthy 1897); these neurofibrils were also seen by others as contributing to an extraneural element (of the cerebral ‘grey’) intercalated between the axons and dendrites of the neuronal components (Nissl 1903).
4. The neuron doctrine in relation to the cell theory
The neuron doctrine is often presented as an extension of the cell theory, with the reticularist view seen as a challenge not just to the neuron doctrine but also to the cell theory. Brodal (1969) wrote: ‘The neuron theory is in reality nothing more than the cell theory applied to nervous tissue’. Kuffler & Nicholls (1976), writing about the history of the neuron, stated: ‘The cell theory won general acceptance and most biologists started to think of nerve cells as being similar to other cells in the body’. This is now the generally stated view of how the neuron doctrine relates to the cell theory. Cowan & Kandel (2002) write about the reticularist view of the nervous system as one that ‘challenged both the cell theory in general and the neuron doctrine in particular…’ and Albright et al. (2000) also represent this view.
These statements are not historically correct. The neuron doctrine is clearly based on the cell theory, but the cell theory is far more accommodating than is the neuron doctrine. That is, apart from the properties that distinguish neurons from other cells, the rules for belonging to the class of neurons under the neuron doctrine are far more rigorous than are the rules for belonging to the class of cells under the cell theory. No one, to my knowledge, has ever suggested that a syncytial structure challenges the cell theory. The syncytiotrophoblast seems to be comfortably accommodated within the cell theory, as are multinucleate muscle cells and extracellular tissue components. Maximow & Bloom (1930), having introduced the cell as the structural unit of plants and animals, have no problem in describing epithelia where ‘… no limits between cells can be detected and the epithelial sheet has the character of a syncytium’. That is, the idea that any argument against the neuron doctrine is consequently an argument against the cell theory is patently false. For some reticularists the issue was regarded as a challenge to the cell theory,8 but for many the issues were specific to an understanding of how nerve cells relate to each other. Kölliker in the early editions of his book (Kölliker 1852, 1853, 1863, 1867) presented a strong case for the cell theory and also presented, in 1867, an early view of neural connections that were essentially reticularist in postulating a network of fused neural processes (figure 1). It is necessary to understand that for many histologists, and Koelliker was one of the most eminent at the time, the possible continuity of nerve processes with each other was not in conflict with the cell theory. Given the occurrence of cell fusions in non-neural tissues, fused neural processes cannot be seen as a challenge to the cell theory, only to the neuron doctrine.9
Figure 1.
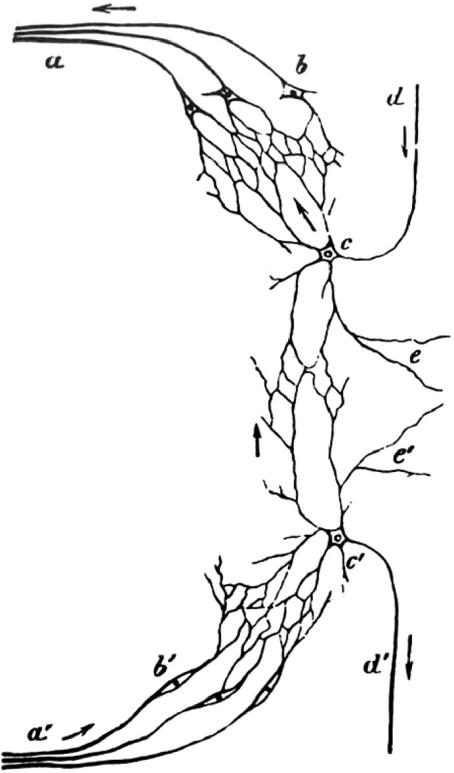
Interconnected neurons suggesting a ‘reticular’ structure of interneuronal connections (see also figure 2) from Kölliker (1867). Although he was clear that he could not see neural continuities, Kölliker showed a scheme that could explain how messages are transmitted from one nerve root to another. a, Axons of the motor root; b, motor cells in the ventral horn; c, ‘motor conducting cell’; d, ‘motor conducting fibre’; e, the process for connecting to the other half of the cord. All cells are connected by networks (Netze) of their branching processes. a′–e′ indicate the corresponding sensory components. In order to preserve the original character of the figures the lettering has not been changed in any of the old figures, even though some of the lettering is not referred to in the present figure legends or in the text.
5. Some of the methods of investigation
To understand the nature of the observations that led neuronists and reticularists to their opposing conclusions, it is necessary to understand the methods of study that were used. To a significant extent, what was seen depended on the methods. The analysis of the detailed relationships between nerves had to be carried out at the limits of resolution of the light microscope. This was difficult and heavily dependent on a variety of different techniques that were introduced during the nineteenth century. One was the development of lenses without chromatic aberration (1820s). To gain the maximum benefit from these lenses it was necessary to look at very thin pieces of tissue. At first, this was achieved by carefully teasing or dissecting the tissues, but by the 1870s methods for producing good thin sections had been developed. It therefore became necessary to develop methods for embedding tissues so that they could be held firmly as they were cut, and to design microtomes for producing a regular and even cut. Soon, it was possible to study sections that were just 2–3 μm in thickness, but, of course, in such very thin sections there is a serious problem about studying neurons, which extend far beyond a few microns. Also, to embed tissues successfully they had to be dehydrated and passed through solutions that were miscible with the embedding substance, a process that can produce significant shrinkage and deformation of fine details.
Cytologists have traditionally, and rightly, insisted on very thin sections of just a few microns for the best optical conditions. However, in the best thin sections, which at 2–3 μm approach the diameter of many relatively fine axons and dendrites, it is not only impossible to trace the long thin, often winding processes of nerve cells for any distance, but also, at the surface of such sections, it is difficult to distinguish regions of apparent fusion, artefactually produced by the action of the knife, from regions of contact. There is a conflict. Neural processes must be followed for long distances if one is to determine how they relate to each other, and this requires thick sections, but to see the details of the relationship one needs thin sections. Further, since the neural processes that need to be studied often form an extremely dense feltwork, thick sections are often not sufficiently transparent for study.
His (1883, 1886) resolved this difficulty by studying very early stages when nerve processes were short and not densely packed. However, it was the introduction of Golgi's method of selectively staining a few nerve cells and their processes (Golgi 1873, cited by Cowan & Kandel 2002; figures 2 and 3) that provided a method for tracing neural processes over long distances. Its success depended on the fact that it left most of the cells entirely unstained and appeared to stain a few (ca. 1% in most preparations) completely,10 showing the terminal portions of axons and dendrites as isolated structures in an otherwise translucent tissue. Since only a few of the nerve cells are stained, one can cut thick sections (often up to 100 μm) and see essentially all parts of a nerve cell, its dendrites and its local axon branches. The reduced silver methods (figures 4–6) generally also stained only a part of the dense neural network, and could also be used on relatively thick sections (up to ca. 10 μm).
Figure 2.
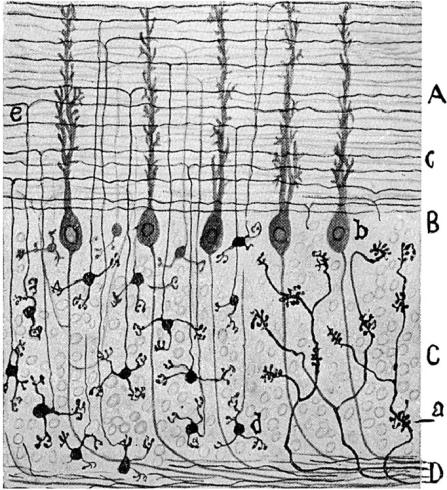
Figure drawn by Cajal (1954). Golgi preparation showing cerebellar granule cells in the lower left part of the figure and the mossy fibres in the lower right. Note that, although this was not shown by Cajal, in the cerebellar cortex illustrated here each mossy fibre terminal relates to several of the granule cell dendrites in a close synaptic relationship that Cajal describes as a connection by ‘gearing’. If a contact region between the mossy axon and the granule cell dendrite were shown for such a preparation, then the processes would appear to be continuous because the relationship is so close.
Figure 3.
Drawing of a Golgi preparation from Cajal (1954). This shows the nature of the Golgi impregnation, revealing a few neurons clearly and also shows Cajal's view of the direction of impulse propagation in the retina from the receptors at the top through the bipolar cells (A and B) to the ganglion cells at the bottom of the figure (see §7). The axons of the ganglion cells run to the right, towards the optic nerve and the brain, and their dendrites run up towards the bipolar cells. Notice that the six amacrine cells in the mid-section of the figure have dendrites that run downwards to relate to the dendrites of the retinal ganglion cells and to the axons of the bipolar cells (A, B), but they have no axon. Further details in the text.
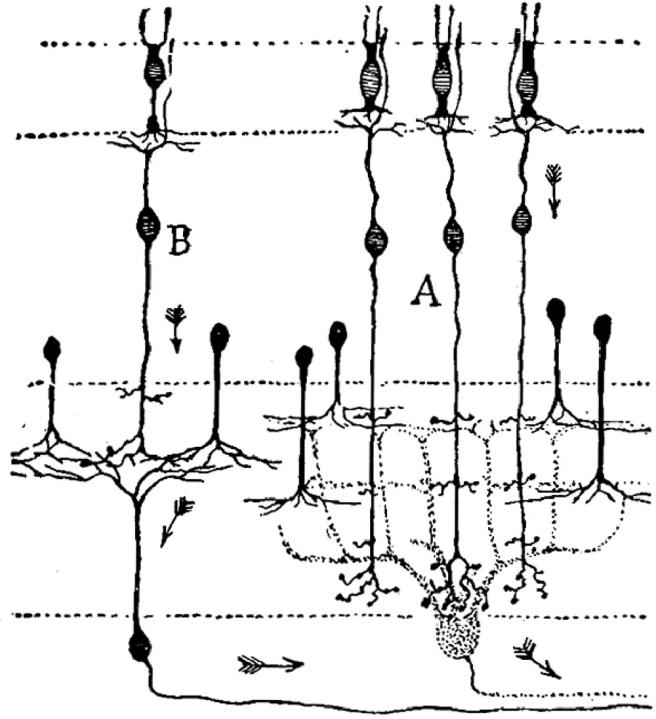
Figure 4.
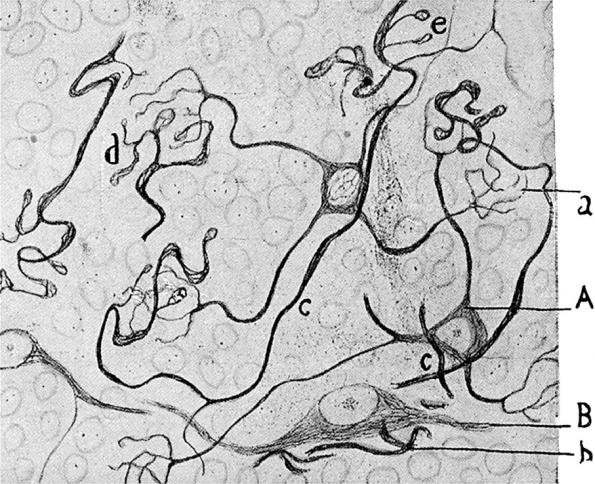
Figure drawn by Cajal (1954). A reduced silver stain showing the neurofibrils that form a part of the cytoskeleton in the mossy fibre axons, labelled (c), (d) and (e) and in the granule cell dendrites (a). The neurofibrils are stained and there is a clear gap between the axons and the dendrites (see §7).
Figure 5.
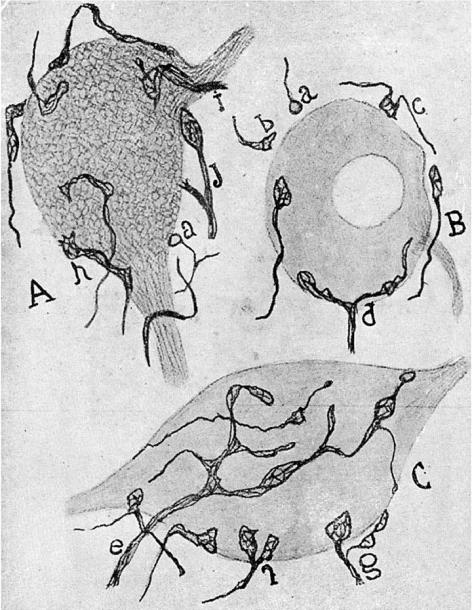
Figure drawn by Cajal (1954). Reduced silver stains showing synaptic terminals (boutons) ending in relation to nerve cells of the cochlear nucleus. This figure is discussed in further detail in §7. The terminals that form boutons on the ventral horn cells are shown and for some of the boutons there is a visible gap between the axon and the ventral horn cell.
Figure 6.
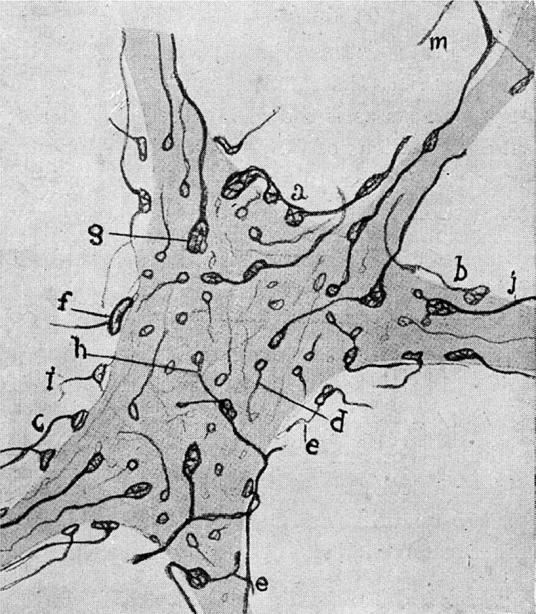
Figure drawn by Cajal (1954). Reduced silver stains showing synaptic terminals (boutons) ending in relation to the ventral horn cells of the spinal cord. This figure is discussed in further detail in §7. The terminals that form boutons on the ventral horn cells are shown and for some of the boutons there is a visible gap between the axon and the ventral horn cell.
Both of these methods, described more fully in §7, were widely used as the controversy developed, but questions were then raised as to whether a highly selective method such as the Golgi method was really revealing all that there was in the tissue. Further, the purist cytologists were not inclined to take seriously details that could not be examined under optimal optical conditions in very thin sections.
Apart from the methods of sectioning and embedding, cutting, and teasing tissues, methods of fixing and staining advanced considerably as the controversy grew. Many of the relevant techniques were derived from the concurrent development of industrial or other processes that were adopted and adapted by the microscopists. For example, fixative solutions such as chromic acid and osmium tetroxide were based on uses in the tanning industry; the dye industry provided stains such as carmine for revealing structures in fixed tissues or methylene blue for staining live (unfixed) nerve cells; the use of silver salts and gold chloride for staining nerve cells and nerve fibres came from photographic processing.
There were thus many different methods of studying neural tissues. The dyes and fixatives were used in a great variety of, often idiosyncratic, combinations and concentrations. Histology has always been rather like cooking, essentially an empirical approach, but with deeper chemical roots. Many methods were introduced for more or less well-defined chemical reasons, but the results, particularly for nervous tissues, were often surprising, and their chemical foundations unknown. One important, indeed overriding issue, was to distinguish the extent to which the appearance seen under the microscope represented the tissue as it is in life and not some artefactual relationships produced by the particular methods used. The structures that were seen had to be interpreted, and one important skill was the ability to distinguish significant relationships that provided clues about what the structures might be like in the living organism, from relationships that were purely due to damage or distortions produced by fixation, sectioning or staining. The degree of faith that any one observer had in any one method was often critical to the interpretation of how the images seen related to the nervous system itself.
Artistic skill was also important. Although photography was available, photographs of microscopic images were not available in the early days of the controversy. Many of the illustrations were extremely detailed, carefully prepared and remarkably beautiful. The difference between a rough sketch of what an investigator thought he had seen, and a detailed, careful drawing that at its best showed much more than a photograph commonly does, was often a critical factor in the debate. For example, Cajal's illustrations (Cajal (1911); not Cajal (1995) which reproduces the beauty of his drawings but poorly) are remarkably true to features that can still be seen by contemporary investigators, and these served, to a significant extent, to convince others. Many of the participants in these debates were first class artists, with skills that are only rarely on display today. In addition, it was also a general practice to bring preparations to meetings and convince others, by study of the actual preparations under a microscope.
6. Early observations of neuronal interrelations
To appreciate how views about neuronal connections developed, it is necessary to look briefly at early accounts of nerve cells and nerve fibres that preceded the neuron doctrine and the reticularists. One early step was the recognition that dorsal roots are sensory and ventral roots are motor. This was due to Bell (1811) and Magendie (1822, cited by Clarke & O'Malley (1996), pp. 299–303), and is often called the law of Bell and Magendie, although there has been considerable debate about exactly what was contributed by Bell (see Clarke & O'Malley 1996). This identification of distinct functions for the roots led to the concept of messages travelling into the cord, and out of the cord with, necessarily, some means of communication between the dorsal and the ventral roots, and also with the rest of the brain. von Waldeyer-Hartz's (1891) review, mentioned above, was significantly based on the relevant spinal connections, as were the views of His (1886, 1889). However, much of the critical evidence about neuronal connections produced by Cajal and others actually came from other parts of the brain, from the retina, or from the cerebellum and the hippocampus, whose functions were far less clearly understood.11
Descriptions of nerve cells in the vertebrate central nervous system, roughly corresponding to what would today be called the perikaryon, go back to Purkinje (1838, cited by Clarke & O'Malley (1996), pp. 52–53), and the connections between nerve cells and nerve fibres were established at approximately the same time by observations of the spinal ganglia, the sympathetic nervous system and invertebrate nervous systems. These observations, made by Wagner, Hannover, Helmholtz and Kölliker and others, were summarized by Kölliker in his 1852 textbook,12 which also included detailed drawings of nerve cells and their processes from the spinal cord, cerebral cortex and thalamus (see Andreoli 1961; Shepherd 1991; Clarke & O'Malley 1996).
The problem of how these nerve cells could communicate with each other was recognized as important at the time, but the methods available allowed no clear resolution. In the early editions of his textbook, Kölliker (1852, 1853, 1863) clearly explained the difficulties of resolving the details, and left the problem unresolved; but then in the fifth edition he sketched a postulated network that would allow the necessary communications between nerve cells (figure 1) and this is probably one of the earliest versions, though a hypothetical one, of a frankly reticular view of the nervous system.
The period 1862–1863 can be regarded as a crucial time when the nature of axonal branching patterns, axonal terminals, axonal fusions and dendritic structures came into sharp focus. This was very shortly after the publication of Darwin's Origin of species, although I have no information about how most of those contributing to the advancing knowledge about neuronal structures reacted to Darwin's book. The point is worth exploring.
We have seen how Kölliker, in the 1863 edition of his textbook, was concerned to define how dorsal and ventral roots might communicate, recognizing that simple observation down a microscope could not resolve the issue. At this time, Kühne (1862) and Krause (1863) first described the terminal branches of peripheral axons innervating muscle at the endplates (Kühne's ‘Endorgane’). It is particularly relevant that Kühne recognized the axon branches as freely ending terminal structures, not in continuity with the muscle. He showed their independence of the muscle by demonstrating that the nerve endings survived even when he was able to induce the muscle to disintegrate. Such free-ending axons would later be seen as a strong argument against a network such as that drawn by Kölliker in 1867. von Waldeyer-Hartz (1891), when he was citing examples to illustrate his statement that all axons end freely with terminal arbours and with no networks or anastomoses, used the motor nerve branches described by Kühne as one of his examples, as did His (1886, 1889) when he argued that axons end freely in the central as in the peripheral nervous system.
During this same period (1862–1863), three young investigators, Max Schultze, Georg Walter and Otto Deiters, were studying nerve cells in the Anatomy Department at Bonn, where Max Schultze was the professor of Anatomy.13 Max Schultze had earlier exploited the use of silver stains for the study of nerve fibres, and had demonstrated neurofibrils in the peripheral nervous system, although it is generally not clear from his published accounts whether he was looking at fine unmyelinated axons which (as we know today) share a Schwann sheath and thus often look like a single fibre, or whether he was looking at actual neurofibrils within a larger axon. The dimensions involved would have made it essentially impossible for a light microscopist to make the necessary distinction between the closely packed thin axons and the cytoskeletal elements within a larger unmyelinated single axon.
In 1863, Schultze published an account of the nasal mucosa, in which he described the olfactory receptor cells within the epithelium of the nasal mucous membrane. Most of his account was concerned with showing that these cells, even though they were within the epithelial sheet (a region where nerve cells were then not expected), were, indeed, nerve cells and gave origin to axons that passed centrally to the brain, specifically to the olfactory bulb, where they related to what are now recognized as olfactory ‘glomeruli’, structures that had previously been briefly described, at Schultze's suggestion, by Walter (1861). Schultze (1863) traced some of these fine axons centrally from the epithelium, and described them apparently fusing with each other on the way to the olfactory bulb. The fact that he saw such fusions suggests that, indeed, some of the structures he interpreted as individual axons were actually bundles of very fine axons grouped together. In discussing these fusions, Schultze, who was then not in a position to make a rigorous distinction between axons and dendrites (see below), treated the fusions as evidence that, in general, axons in the brain were formed by the fusion of several of the fine processes that characterize central ganglion cells.
A striking feature of Schultze's account is the detailed way in which he stresses the importance of the solutions in which he teased out the very fine nerve cells and fibres of the olfactory nerve, using cerebrospinal fluid or fluid from the aqueous chamber of the eye, dichromate solutions, acetic acid, salt solutions, etc., to preserve the tissues in a lifelike condition and yet give them sufficient strength so that he could tease them out for display under the highest power of the microscope. He has a long methods section in this study, a feature that was unusual at the time.
Georg Walter, who was then working as a young medical practitioner near Bonn, concurrently studied the nervous system of several invertebrates, including the leech, which had previously been described by Helmholtz (1842). Walter had earlier published an account of the olfactory bulb (Walter 1861), in which he had shown a number of surprising, and from a modern view entirely improbable, nerve fusions: dendrodendritic and axodendritic fusions, large cells fusing with small cells, large cells with more than one myelinated axon; a neuronist's nightmare. His figures did, however, clearly distinguish some of the axons, identifiable by their myelin sheath, from the dendrites.
In his 1863 study of invertebrate nervous systems Walter (1863) again described and drew several other, equally improbable, examples of nerve fusions. Some were fusions of relatively large nerve fibres close to the cell bodies which, on the basis of current knowledge, are most reasonably regarded as misinterpretations, or as artefactually produced appearances; others were fusions of thinner peripheral motor branches. These, he wrote, come very close to each other and after a short course acquire a common wrapping (Hülle) and become surrounded by a common sheath (not strictly a myelin sheath in these invertebrates, he stresses earlier).
Walter's publication includes a preface dated autumn 1862, and a final, undated passage added after some of the manuscript was already with the printer, so written some time in 1863. This appears to have been written after a discussion, perhaps an examination, at which the author was criticized for not citing others, since here Walter refers to recent publications by Kölliker and Schultze. He discusses Kölliker's (1863) newest (fourth) edition of the highly influential Handbuch der Gewebelehre, as well as the then new study by Max Schultze (1863) of the nasal mucous membrane, both of which appeared after the first part of the manuscript had already gone to the printers. Kölliker, in the fourth edition clearly stated that, although he recognized the functional importance of the issue, he was not in a position to come to any clear conclusion about how the nerve cells of the spinal cord relate to the nerve fibres in the spinal roots. That is, he was entirely negative about the types of fusions that Walter was showing in his invertebrate material. It was not until the fifth edition that Kölliker (1867) introduced the schema shown in figure 1, which can be regarded as an important forerunner of a strictly reticularist view of neural connections. As regards Schultze's observations on nerve fusions, Walter wrote (see 2).
The fifth sheet of the present study was already in print when I received the newest study of Max Schultze's ‘Investigations of the nasal mucosa etc.’ On p. 66 he says in a comment ‘I hold as not nonsense (nicht ungereimt), also to propose the hypothesis, with others, that a certain number of the fine processes which actually arise from separate ganglion cells here and there join as a single bundle which will later form an axon (Achsencylinder) of a myelinated nerve.’
Walter then expresses his joy at having made a discovery that was in accord with the observations of such an outstanding investigator, particularly since both observations, his and Schultze's, were made independently. It is worth stressing that in 1863 Walter was already 34 years old, possibly struggling to establish some sort of research reputation, and that Schultze was only 4 years older but was already a well-recognized figure in the field, who had made important contributions to a broad range of histological problems, and was head of the department. Walter's claim for priority14 indicates that he had significant ambition and a sense of pride in his own work. The rather fawning note about Schultze may merely reflect the usage of the time, but as it is followed immediately by the priority claim, it may reflect a more complex relationship. Walter died in 1865, and I know of no publications of his after the 1863 study.
These accounts of nerve fusions are of interest for several reasons. One important point is that the serious interest in nerve fusions that was recorded by Walter, and then added to by Kölliker (1867; see figure 1), actually referred to three quite distinct reports of ‘fusions’, all published within a few years of each other. All preceded any thoughts about a neuron doctrine, although all came well after the formulation of the cell theory. One of these types of fusion is a theoretical proposal of relationships that could account for neural communications between dorsal root and ventral root, illustrated in figure 1. These were offered by an experienced histologist, Kölliker, who had previously, in the same volume, stated that the images he could see under his microscope could not show the relationships he was proposing, and drawing. Another was by a relatively inexperienced, and today forgotten investigator, Walter, who saw relationships at least some of which (the fusions of large processes near cell bodies) were likely to have been artefactually produced or the results of poor observation. The third was of fusions, again described by an experienced histologist, Schultze, who almost certainly misinterpreted bundles of very fine axons as single nerve fibres, and went on to propose the occurrence of other comparable fusions to produce single axons. Reading Walter's study and some later accounts, it becomes clear that these quite different views about nerve fusions all contributed to early ‘reticular’ interpretations of the nervous system. They were all taken to be about the same issue, even though they had been produced by different means, at different sites, and for different reasons. This is the earliest view of what was often to be the reticularist case: a confusing mixture of reports, some largely theoretical, some plain bad histology (a point that is repeatedly and forcefully made by Cajal), and some misinterpretations of structures that were beyond the resolving power of the light microscope.
A second interesting but less important point is that the tradition for describing nerve fusions was continued in Bonn for many years. Long after most investigators regarded the neuron doctrine as well established, P. Stöhr Jr continued to publish light microscopic images of nerve fusions in autonomic ganglia and peripheral nerve plexuses (Stöhr 1928, 1957). These can today all be regarded as probably based on extremely fine, unmyelinated axons forming thin bundles that were interpreted as single axons. I can remember a visit to University College London in the 1950s of one of the Bonn investigators, who brought with him a bottle of the special fixative that allowed the beautiful staining of very fine peripheral nerve fibres. The strongest impression made on me at the time was by the derogatory comments made by the head of the Anatomy Department (J. Z. Young) after the visitor had left, about scientists who thought they had evidence for axonal fusions that could be regarded as evidence against the neuron doctrine. At the time I failed to recognize the significance of these comments (see §8b(i)).
At the same time that Walter was working on invertebrate nervous systems in Bonn, Deiters was studying the mammalian spinal cord and medulla in the same department. Deiters (figure 7) was 5 years younger than Walter. He was also a medical practitioner in Bonn and had a University appointment at Bonn. In addition, he had a number of earlier publications to his credit (see Andreoli 1961; Deiters & Guillery 1963), including an account of the inner ear and another of muscle cells. Deiters' contribution to neuroscience has been well summarized by Shepherd (1991). Deiters died when he was only 29, towards the end of 1863, the year of Schultze's and Walter's publications, so the three studies must have been closely concurrent. Deiters' research was published posthumously (Deiters 1865) as a book, edited by Max Schultze, after Deiters' older brother, a music critic and historian (my great-grandfather), had copied the original cramped and almost illegible material into a legible form for Schultze.
Figure 7.

Otto Deiters.
Deiters described individual nerve cells and glial cells (figures 8–10) that he had gently dissected free of their surroundings in carefully prepared fixative solutions that in many respects resembled the methods described by Schultze (1863). He also published a series of plates showing sections of brain stem and spinal cord. He is remembered for structures named after him: the supporting cells of the cochlea, described earlier, and the large-celled lateral vestibular nucleus illustrated in the 1865 book.15 However, his major contribution and the one most relevant for understanding the origins of the reticularist controversy is his description of individual nerve cells of the spinal cord and brain stem (see figures 8–10). He distinguished the several dendrites, his ‘protoplasmic processes’ from the single axon of the nerve cells, and he showed that the axon had a characteristic dark border, the myelin sheath. That is, he provided the first clear evidence of what would later be seen as the polarized neuron. In addition, he also showed some fine fibres that he identified as fine branches of axons, which any contemporary neuroanatomist can recognize as incoming axons making contact with the dendrites at triangular swellings (‘b’ in figures 8–10; see Shepherd 1991). On p. 73 and 74 he states: ‘… a number of very fine fibres (Faserchen), which in the manner illustrated sit on the dendrites under a triangular swelling, I take to be axons of the finest nerve fibres, and I find in these a second system of fibrous neural elements, whose central point is the nerve cell’. He then goes on to admit how difficult the study of these fine fibres is, and how there can be doubts but, ‘there are sitting on the dendrites fine fibres having a characteristic form that distinguishes them from the dendrites so that they cannot be the product of dendritic divisions’. He labels them ‘b’ in his figures 1, 6 and 7 (almost as though he knew they would later be identified as synaptic ‘boutons’) and most tellingly goes on to draw special attention to his figure 7 (figure 10), which shows one such fine fibre with a dark border, that is, with a myelin sheath that Deiters recognized as characteristic of axons. This fibre contacts the dendrite at b near the bottom of the figure. That is, he shows a fine myelinated axon, losing its myelin shortly before it terminates on a dendrite in what today would be regarded as an axodendritic synaptic junction.16 This terminal portion of an afferent axon has survived his gentle dissection method because the synaptic contact region has adherent properties. The tendency for axons to stick by their terminals to the postsynaptic site, somatic or dendritic, has been documented by others more recently (Gray 1959; Shapiro & Coleman 1999).
Figure 8.
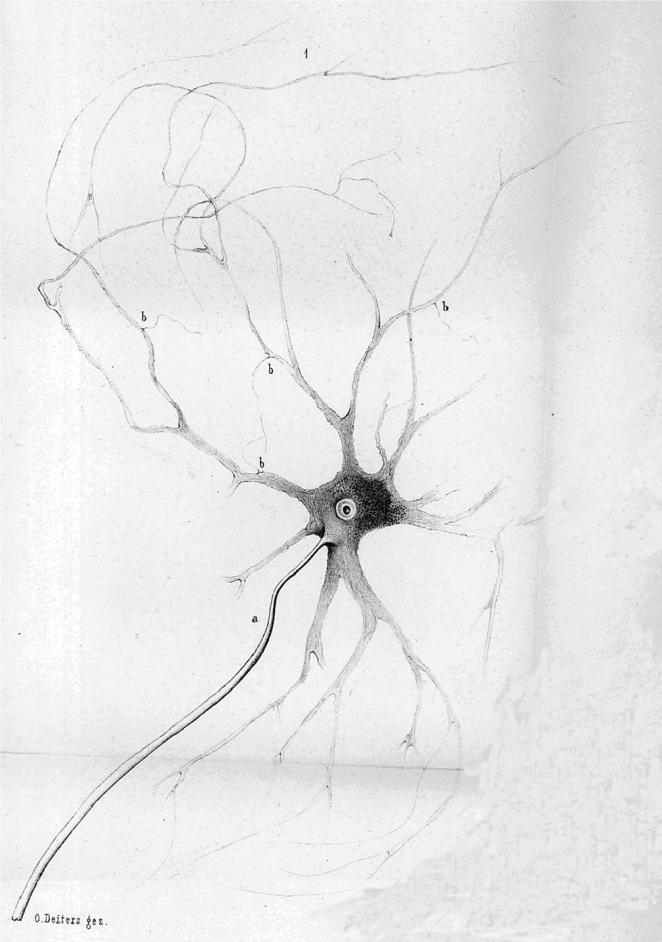
Deiters' (1865) original fig. 1. a, axon; b, see text. Further details in the text.
Figure 9.
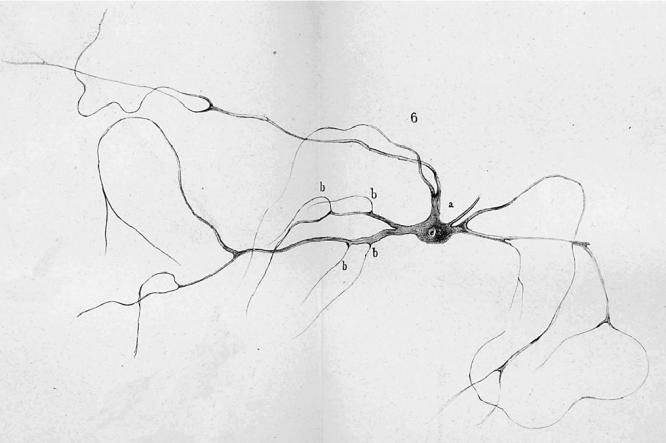
Deiters' (1865) original fig. 6. a, axon; b, see text. Further details in the text.
Figure 10.
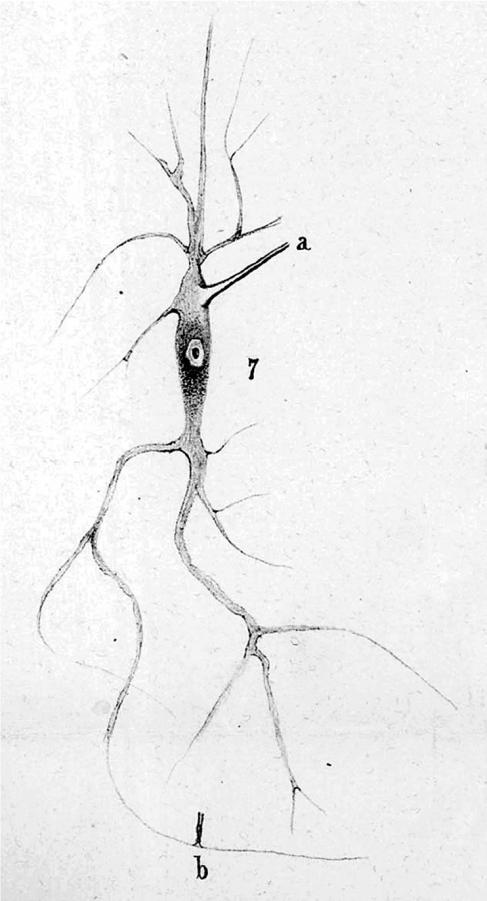
Deiters' (1865) original fig. 7. a, axon; b, see text. Further details in the text.
In Deiters' drawing there is no indication of any synaptic gap, and from what is known today, there is no reason to think that even with the finest optics and most careful observation such a gap could have been visible (see §7 for further discussion of this point). Deiters was not sure what to make of this ‘second’ system of nerve fibres. He saw that some of these axons branched before attaching to the dendrite, but he was unable to trace them far, and essentially left them as a mysterious second system, distinct from the single axon and the several beautifully clear dendrites shown in his drawing. Perhaps, by describing them as centred on the cell, which in contemporary terms is correct if we see the cell as the postsynaptic structure, he missed the interpretation of them as arising from other cells, which came later.
Deiters appears to have drawn essentially what he saw. The impressive care taken over these drawings and their remarkable accuracy is a point that is relevant for an evaluation of his work. There are only a few of his contemporaries who communicated their results in such beautiful and clear drawings. We have to go to Cajal and Kölliker for something comparable, and for each, the visual representation of their results was one important ingredient of their success.
Deiters discussed the possibility of fusions of dendrites but stated firmly that he could find no evidence of fusions.17 He noted that others, who claimed to have seen fusions, were studying sections, where the distortions of the knife may have caused fine processes to appear fused, but he saw none in his dissected specimens. He recognized the importance, from a functional point of view, of connections linking dorsal and ventral roots, and stated: ‘As regards the circumstance that physiology demands connections of this sort, I hold that such an assumption does not give us the right to assume particular formulations of anatomical facts, particularly in an area where the unknown dominates’. A fine creed for an anatomist, and one that would not have turned him into a dogmatic neuronist or reticularist had he lived to witness these later arguments. His statement was similar to those made by Kölliker (1863) at the same time; it is difficult to avoid speculation about what Deiters would have made of the schema of Kölliker (1867) shown in figure 1.
Deiters' account of what can actually be seen was remarkably like Kölliker's earlier statement: careful, measured, conservative. However, it is clear that, far from the ‘chaotic view’ ascribed to him by Albright et al. (2000), he also had a serious commitment to understanding the functional significance of his material. He recognized the importance of defining the relationships of the neural processes, knowing that messages had to traverse the spinal cord not only from the sensory dorsal roots to the motor ventral roots, but also from and to the long descending and ascending pathways of the cord. In another chapter of his book, unrelated to his description of nerve cells but clearly illustrative of his functional turn of mind, he was concerned to determine whether nerve tracts that have different functions have nerve fibres that can be differentiated from each other structurally, particularly on the basis of their size.18 Here one is seeing the influence of Johannes Müller and his law of specific nerve energies. Müller had earlier been Professor at Bonn and later moved to Berlin, where Deiters also studied for a year and a half. In his consideration of different fibre diameters in different fibre pathways Deiters is asking questions about central nerve tracts that would not be answered for the peripheral nervous system until the 1920s by Erlanger & Gasser (1937), and would be studied for the central nervous system mainly in the 1950s, by P. O. Bishop, G. Bishop, H. T. Chang, and others (Bishop et al. 1953; Bishop & Clare 1955; Chang 1956).
Deiters has been included in this account of early descriptions of nerve fusions, not because he was a reticularist, as Albright et al. (2000), rather oddly, claim19 (he was not), but because his work provides an interesting key to the next major contribution to the reticularist view, which is perhaps what Albright et al. had in mind if, indeed, their account was based on an evaluation of the relevant original material. This was by Gerlach (1872), who is generally regarded as an originator of the reticularist view, based on his description of fused neural processes in the mammalian spinal cord.
Gerlach's (1872) account was probably influenced by Kölliker's schematic representation (compare figures 1 and 11) but his approach was significantly based on Deiters' earlier study. He used the same tissues (the spinal cord of the ox), and he referred to Deiters' description of the second system of axons. He used sections stained with carmine and gold chloride, and it appears that these stains allowed him to trace axons more readily than Deiters had been able to without the stains. He wrote: ‘If Deiters had taken a step further he would have discovered the fine nerve fibre plexus…’. With the Deiters figures (figures 8–10) in mind, the Gerlach figure (figure 11) looks much more interesting than Kölliker's figure (figure 1). Instead of showing a fusion of the dendrites of the two cells, as Kölliker had done (figure 1), Gerlach traced an axon (‘b’ in figure 11), that branched forming two daughter axons (‘a’ in figure 1) and then terminated in relation to the dendrites of the two nerve cells illustrated. He considered that these branches corresponded to the second axon system described by Deiters, and he traced these axons into apparent continuity with the dendrites, as would be expected from the Deiters figure.
Figure 11.
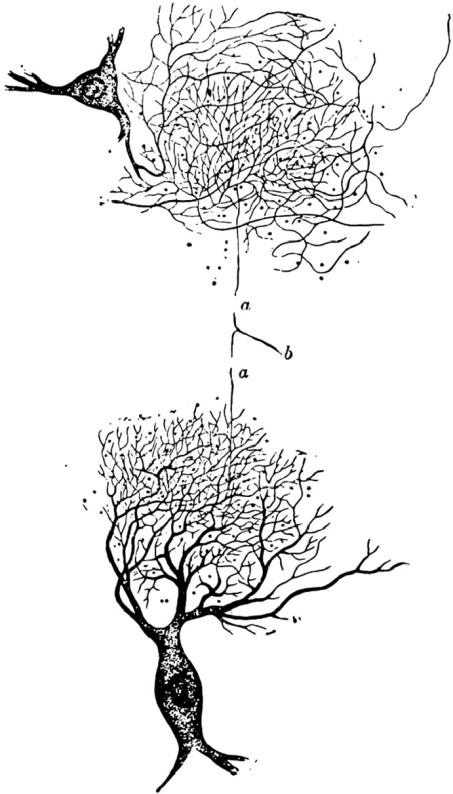
Interconnected neurons suggesting a ‘reticular’ structure of interneuronal connections from Gerlach (1872). Gerlach showed drawings that seem to correspond roughly to the cells labelled c and c′ in the Kölliker (1867) figure (figure 1), but added an axon ‘b’ that he had traced to its bifurcations (a, a) and beyond that to apparent fusions with the dendrites of the two cells, and apparently with many other axons. Further details in text.
The Gerlach account and illustration show why the quality of the illustrations was so important. Gerlach's figure, in terms of details, is little better than Kölliker's schema, but it purports to represent what was actually seen under the microscope. Further, Gerlach's account seemed to create an intermediate network that intervened between the axons and dendrites. It fails to do justice to the difference that Deiters clearly recorded between the dendrites and the ‘second system of fibres’. One can wonder what influence Gerlach's account might have had, had his illustration of the contact region of the axonal branches and the dendrites been as fine as that of Deiters,20 particularly if he had, as did Deiters, made a clear distinction between the axons and the dendrites, and not produced a drawing in which one cannot be sure to what extent fine axons join dendrites or dendrites join each other. As it was, a key illustration of the reticularist view could justifiably be dismissed as inadequate, and was so dismissed by Cajal and by others subsequently. However, from what we know about the observations made by Deiters, and the close relationship to these of the structures described by Gerlach, it seems reasonable to conclude that Gerlach was not reporting artefacts or structures that simply were not present in the tissues, as was true of the earlier account by Walter. It is worth noting that von Waldeyer-Hartz (1891) in his important essay on the neuron doctrine spoke highly of Gerlach's observations. It seems most probable that Gerlach, because he used carmine to stain the axons, actually saw rather more than Deiters had seen, and was able to trace the axons from the Deiters ‘second system of fibres’ back towards their branching parent axons.
7. The synaptic gap and the methods that appear to reveal it
The reader who knows something of the history will want to argue at this point: perhaps Gerlach did trace an axon and its branches to one or more synaptic terminals on one or more dendrites, but since we now know that there is a synaptic gap (see figure 12) (De Robertis 1959), and since that gap was clearly described by Cajal and others, Gerlach should have seen that gap, and should have been able to see that the ‘second system’ of axons described by Deiters was nowhere continuous with the dendrites. This is an important point because it represents a serious misunderstanding of what can be seen under the light microscope.
Figure 12.
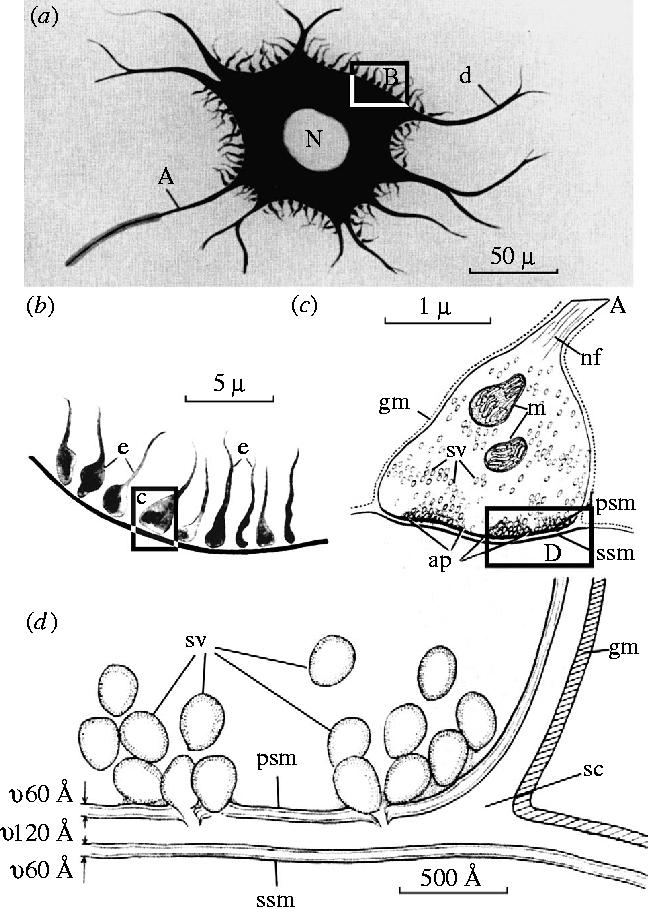
Schematic representation of light (a), (b) and electron microscopic (c), (d) images of synaptic contact, upon a nerve cell. Reproduced from De Robertis (1959), courtesy of Academic Press, San Diego, CA. Further details in the text.
The drawing of the axodendritic connection that Deiters showed (figures 8–10) is accurate. The gap between the presynaptic axon and the neuronal cell body or dendrite seen today in electron micrographs is ca. 20 nm;21 it is not a gap that could be resolved in even the best modem bright-field light microscopic images. It could not have been visible to Deiters or to Gerlach, even though figure 12 clearly suggests that this gap should have been seen. The issue is intriguing and has been briefly discussed before (Gray & Guillery 1966; Guillery 1996, 2000).
The observations made by Deiters and Gerlach were made on preparations that showed the neural processes including essentially all of their cytoplasmic contents, and when such methods are used a synaptic gap cannot be seen. Such methods were used by Held (1897) and Auer-bach (1898a,b), and are discussed later in this section. The gap, that is, the evidence for a discontinuity, can be seen under one of two distinct conditions. Neither was available to Deiters or Gerlach. One is seen in Golgi preparations and the other in reduced silver preparations. I will consider each in turn.
The Golgi method, because it only stains a small proportion of nerve cells, shows the free-ending axons and dendrites in thick sections (see §5), and these processes can then be seen as evidence for a discontinuity, even where the preparation does not allow a precise identification of the (unstained) postsynaptic element (figures 2 and 3). That is, when the incoming axons are stained, and the postsynaptic cell body and dendrites are unstained, one can see the free termination of the axons; when the dendrites are stained and the incoming axons are unstained one can see the freely ending dendrites.
Golgi claimed that the axons are not free-ending but that they fuse with each other to form a reticulum, and that the dendrites serve a nutritive function. Although Golgi played a leading role in the controversy about the neuron doctrine, I will not consider his proposals further, since they tell us nothing about the synapse. I will focus on evidence, particularly Cajal's, about how Golgi preparations are relevant to the structure of synaptic junctions and to Gerlach's account, noting that Gerlach's account was published just 1 year before Golgi described the Golgi method.
In figure 2 the postsynaptic dendrites of the cerebellar granule cells are shown at the lower left, and the presynaptic, mossy fibre axon terminals which contact them are shown at the lower right. If the pre- and postsynaptic processes of a single synapse are both impregnated then, in a Golgi preparation, there will be no visible gap and the two processes will appear to be continuous, except in rare preparations where one process may be paler than the other. Cajal's illustration, for obvious reasons, does not include such a relationship. The selective nature of the Golgi method, essentially limiting the impregnation to single, generally isolated cells, and apparently revealing those cells completely,22 provided a strong argument for the structural discontinuity of nerve cells. The argument presented here for the granule cells and mossy fibres also applies to many other synapses in many other regions of the brain. It is difficult to look at Golgi preparations in detail without coming away with a clear impression that the method reveals the nerve cells as distinct separate entities. Cajai (1954) wrote: ‘… this sharp interruption of the staining reaction is a fact favourable to the neuron theory because it indicates that at the level of the membrane there exists an obstacle which is almost always resistant to the reducing agents’. This is an important conclusion to be derived from the Golgi methods. It argues in favour of a discontinuity but it says nothing about the presence of a gap that is visible light microscopically.
In spite of the striking evidence of the Golgi methods, not all observers agreed with the conclusion about the discontinuity. Some have seen a complex network of fusing axons (Golgi, see above) and others have tended to distrust the Golgi methods because they could not understand the physical or chemical basis of its curiously selective action. Nissl (1903) argued that there was intercellular ‘grey’ between the axons and the dendrites and that the Golgi method failed to reveal this.23 Held (1897) distrusted the Golgi method. He commented: ‘In order to determine whether two things are in contact with each other, it would seem to be necessary to be able to observe them both together’ (p. 282).2 Further, he wanted to be able to study that relationship in thin sections with optimal optics.
The Golgi preparations provided some of the strongest visual evidence for considering neurons to be independent units and for many years provided the main source of information about what nerve cells looked like. There cannot be many who doubt that these methods demonstrate a discontinuity between pre- and postsynaptic processes. However, even today the biochemical basis of the Golgi method is not understood, and no one knows why some cells are impregnated and others are not. Moreover, the Golgi method does not demonstrate a synaptic gap such as that shown in figure 12, which might have convinced Gerlach, had he seen it, that he was not looking at processes that are continuous with each other and form a net. The method demonstrates a cellular discontinuity (of impregnation) across a gap that we now know is beyond the resolving power of the light microscope.
The reduced silver methods24 do appear to show this gap, and in the spinal cord and many other parts of the brain stem, views of synapses depended more on the reduced silver methods than on the Golgi methods (figures 4–6). These stains are successors to Schultze's silver staining, and were developed, at a later stage of the history of the neuron doctrine, by Cajal (1903) and Bielschowsky (1904) on the basis of the current photographic processes. There are a great many varieties of reduced silver methods. Some are suitable for block staining with subsequent sectioning and some are suitable for staining free-floating sections. Generally, they do not stain all of the neural cytoplasm but only reveal some of the cytoskeletal elements inside the cells and their processes. That is, they show the neurofibrils25 of the nerve cells selectively, and at the axon terminals one commonly, but far from invariably, sees bundles of fibrils that form rings or dense club-like structures in the axon terminals (figures 5 and 6). Cajal's representation of this cytoskeletal element in the cerebellar mossy fibres and granule cell dendrites is shown in figure 4. With reference to this figure, Cajal clearly described the gaps that lie between the mossy fibre terminals and the dendrites of the granule cells. He compared these gaps to windows or buttonholes, and stressed that these gaps are never seen in Golgi preparations where the two processes interdigitate closely forming ‘axo-dendritic connections by gearing’. That is, in a Golgi preparation, as indicated above, when a mossy axon and its postsynaptic granule cell dendrite are both stained, the two appear to be continuous. The conclusion that they are not continuous was based on the selectivity of the Golgi preparation and on the discontinuity of their cytoskeletal elements.
The gap between pre- and postsynaptic elements seen in a reduced silver preparation is not the synaptic gap. It is the gap between the cytoskeletal elements. That is, the gap seen in reduced silver preparations is occupied by neural cytoplasm other than the fibrils. Golgi preparations, where they show two processes in synaptic contact, demonstrate the absence of a (light microscopically) visible true synaptic gap.
Electron microscopic studies confirm this. They show that at synapses such as those made by mossy fibres or on motor neurons, the light microscopically identifiable cytoskeletal fibrils are made up of intermediate filaments (neurofilaments) (Gray & Guillery 1966; see figures 13–16). Some axon terminals in the spinal cord contain such filamentous rings or clubs, but others do not. Generally, only those parts of axons that contain the intermediate filaments are stained by the reduced silver methods and, where they are stained, the light microscope shows a clear gap between the presynaptic and the postsynaptic process because the cytoplasm that lies next to the synapse itself, filled with synaptic vesicles in figures 15 and 16, is not stained by the reduced silver methods.
Figure 13.
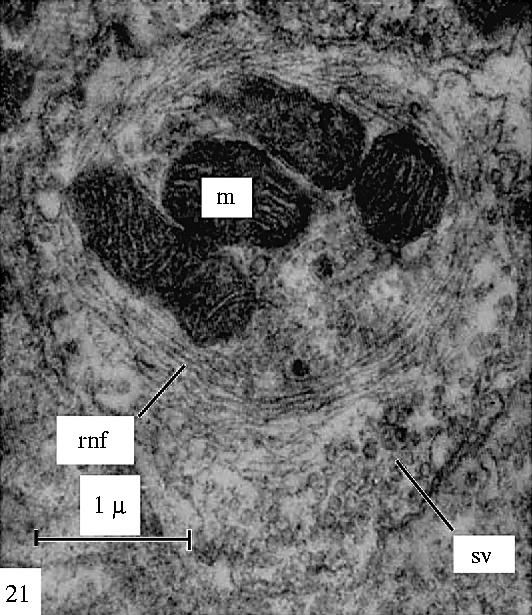
Electron microscopic view of a filamentous ring in axon terminals from the brain stem of a lizard to show the cytoskeletal element, the neurofilaments, which correspond to the neurofibrillar structures seen in reduced silver preparations (figures 4–6) of these synapses. Reproduced from Gray & Guillery (1966), courtesy of Academic Press, San Diego, CA.
Figure 14.
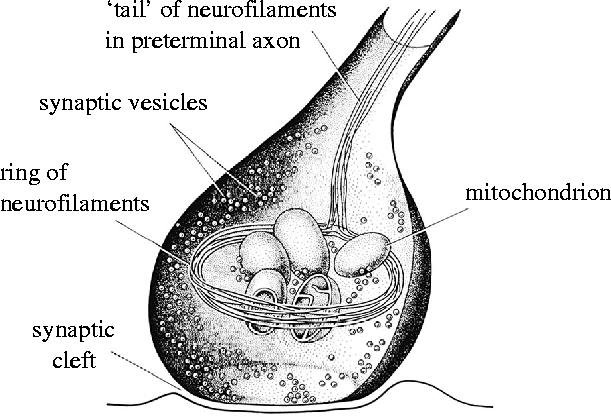
A schematic view of how the filaments, which correspond to the neurofibrillar structures seen in reduced silver preparations (figures 4–6) of these synapses, relate to the other parts of the synaptic terminal. Reproduced from Gray & Guillery (1966), courtesy of Academic Press, San Diego, CA.
Figure 15.
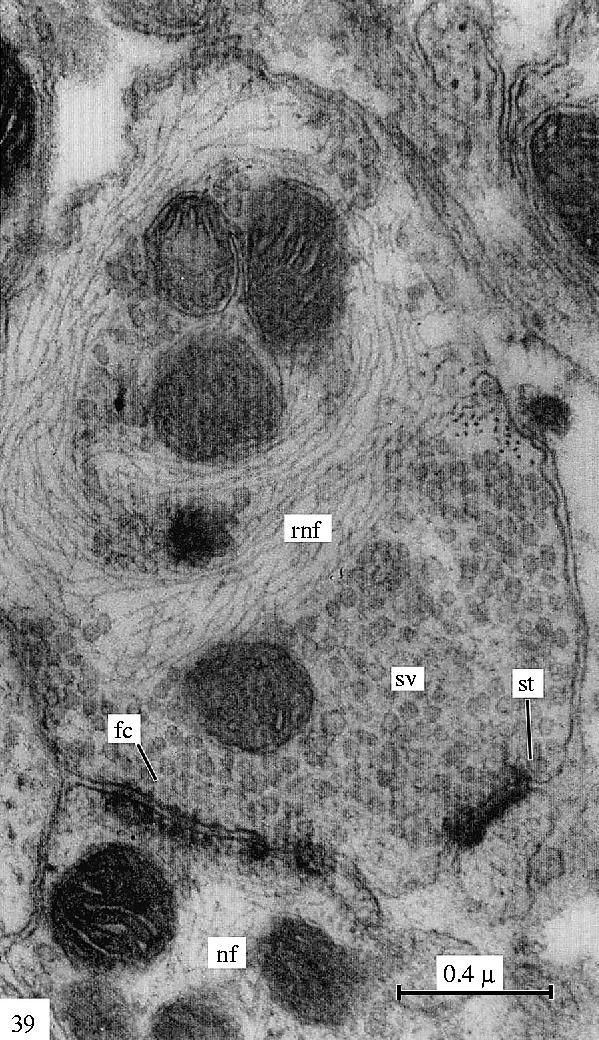
Electron microscopic view of a filamentous ring in a degenerating retinogeniculate axon of a monkey. Reproduced from Gray & Guillery (1966), courtesy of Academic Press, San Diego, CA.
Figure 16.
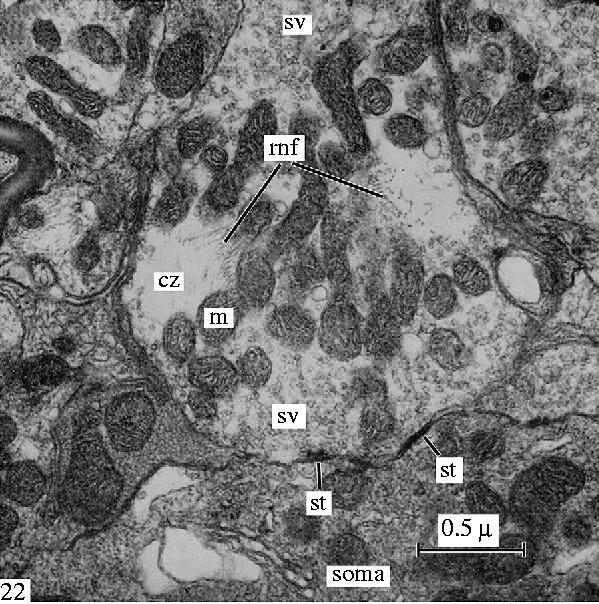
Another electron microscopic view (see also figure 13) of a filamentous ring in axon terminals from the brain stem of a lizard to show the cytoskeletal element, the neurofilaments, which correspond to the neurofibrillar structures seen in reduced silver preparations (figures 4–6) of these synapses. Reproduced from Gray & Guillery (1966), courtesy of Academic Press, San Diego, CA.
Where the filaments are not present in the presynaptic terminal, one does not see the synapse at all in a reduced silver preparation. In the cerebral cortex, axon terminals generally contain no filaments, and it was not until the cortex was studied with the electron microscope that any clear ideas about the nature of synapses in the cortex could be developed (see Gray 1959; Gray & Guillery 1961; Guillery 2000). Whereas in the cerebellum, Cajal (1954) clearly recognized that the reduced silver methods revealed the cytoskeletal elements, in the spinal cord he regarded them as showing the outlines of the axon terminals (that is of the end bulbs of Held and Auerbach which are described later in this section). This is probably the basis of the synaptic gap shown in figure 12; a misinterpretation of the structures revealed by the reduced silver methods.
Figure 12 can now be seen to be misleading in an instructive way. The axon terminals shown in figure 12a are drawn as though they represent the whole of the axonal terminal, including all of the cytoplasmic contents, as they might appear in a Golgi preparation. They are shown densely distributed over the cell body, far more densely than one would expect to see in a reduced silver preparation of this region (see figures 5 and 6; see Haggar & Barr 1950), but for reasons that are not clear are not shown on the dendrites at all except close to the cell body. We now know that in many cells the terminals also extend densely along the dendrites. Figure 12b is clearly based on the electron microscopic image of figure 12c, except for the synaptic gap, which looks as though it must have been based on the appearance of a reduced silver preparation; it cannot, by the laws of optics, have been based on the gap shown in the electron microscopic image, which is only 20 nm as shown in figure 12d.
Figure 12 shows how the neuron doctrine dominated our thinking about synaptic structure. Even the careful and scholarly account published by Shepherd (1991) includes this figure (Sheperd's fig. 39), which clearly misrepresents what can actually be seen under the light microscope, and appears to do so in order to make the appearances fit to the dogma of the neuron doctrine, that there is a discontinuity. The belief in this visible gap between pre- and postsynaptic processes was so firmly established in the mythology of the first half of the twentieth century, that De Castro (1942, 1950), publishing after Cajal's death, from the Cajal Institute in Madrid, and recognizing that such a gap could not be empty, proposed a third element to the synapse, a thin sheet of glial cytoplasm interposed between the two neuronal processes. He thought of this as a trophic barrier, and as a part of the polarized structure of the synapse. He cited an ‘interneuronal fluid’ proposed by Lorente de Nó (another of Cajal's pupils) in the same relationship. It is surprising how close these accounts from the headquarters of the neuronists camp were to Nissl's intercellular ‘grey’, particularly since both described the essential nature of this third component as essentially ‘unknown’. Today the electron microscope shows a thin layer of extracellular material in some (but not all) synaptic junctions of the central nervous system. This appears to serve an adhesive function (Shapiro & Coleman 1999), but it widens the narrow extracellular space by only a small amount and is still well beyond the resolving powers of the light microscope.
The issue of exactly what the light microscope does show at the synapse, in preparations where both pre- and postsynaptic processes are stained at the same time, in accordance with the comment that Held (1897) made about seeing both at the same time (see above), is best addressed by looking at Held's account, and at an account published one year later by Auerbach (1898a,b). Both worked with thin sections (2–5 μm) in which pre- and postsynaptic processes were both revealed essentially completely. Both used methods that appear to have stained the mitochondria in the axon terminals (see Bodian 1942), although other parts of the cytoplasm such as the synaptic vesicles were probably stained as well, especially in Auerbach's preparations.
Held described the terminal structures of axons in the trapezoid nucleus, where the axon branches form a dense basketwork or ‘calyx’ around the cell bodies. He noted that the axon terminals looked different from the passing axons, having a more granular appearance. Today we can interpret this as probably due to the mitochondria in the terminals, which are sparser or absent in the preterminal axons. He saw a borderline (Grenzlinie) between the axon terminal and the postsynaptic cell in a 9 day old dog and in younger kittens, but this line was not present in the adults. He argued that in accordance with the developmental account of His (see above), the nerve cells developed as independent elements, but that during post-natal development there was a fusion across the neural junction, and this borderline disappeared. He further argued that since most Golgi studies used immature animals,26 that was why these preparations failed to show this fusion.
Held's interpretation is interesting, but wrong. We know that even in the adult, the calyces are like other axon terminals, separated from the postsynaptic cell by the membrane of the axon, the membrane of the cell and the usual 20 nm gap. A study by Ryugo & Fekete (1982) of the development of calyces in the cochlear nucleus shows their early postnatal development from rather large solid terminal structures to a more complex, branched form, with finer processes. For large axon terminals, similar to those of the young animals, one can expect to see the two closely adjacent membranes as a thin (refractive) line when the two membranes are roughly perpendicular to the plane of the section (see reference to Bodian's study below). The membranes will have refractive properties that differ from the adjacent pre- and postsynaptic cytoplasm and will thus be visible as a single thin line wherever the membranes are viewed end on. Where the membranes are viewed face on or obliquely, they will not be visible because they are very thin relative to the thickness of even the thin sections used by Held and Auerbach. For smaller terminal structures, the membranes would mostly be curved, and so would not be seen in a perpendicular view. The clear line seen for a large terminal would change to several small somewhat blurred and non-interpretable areas for the smaller terminals. That is, the borderline would not be visible, and the developmental change in appearance described by Held would be expected from the maturational changes described by Ryugo & Fekete (1982).
Held also looked at a number of other brain regions, and he compared the axonal end-feet that he saw with the second system of axons described by Deiters, recognizing these (as did Gerlach) as afferent terminals (of incoming axons) and stressing that Deiters saw these afferents as being in continuity with the dendrites, not as contact zones. Held specifically looked for this second system using a method that was an improvement on the one used by Deiters and he reports seeing the same second system of axons sitting on the dendrites and also on the cell body at triangular swellings.
There is a granular and vacuolated appearance to some of the preparations that Held illustrated, and this confirms the interpretations of Cajal (1954) who thought poorly of the quality of the preservation in Held's tissues. However, Kölliker (1899) while disagreeing with Held's interpretation, wrote about Held's ‘schöne Beobachtungen’ (nice/beautiful observations). It is possible that some of the tissues studied by Held were not suitable for showing whether there was a cytoplasmic discontinuity at the synapse. However, his account of the borderline in young animals and of its disappearance with maturity, suggests that what he described is closer to reality than are figure 12a,b. That is, a line that represents an edge-on view of the two synaptic membranes can, on occasion, be seen, and this is quite distinct from a synaptic gap. The gap would lie within this single visible line.
Auerbach (1898a,b) also described a hair-sharp line marking the border between the nerve ending and the postsynaptic cell, and wrote that ‘there can be no doubt about where the one stops and the other starts’. He stated that he could not confirm Held's report of any continuity. In this sense, Auerbach is a follower of what he describes as the ‘Contactlehre’ and, from the point of view of this discussion of synaptic structure that is where he belongs. However, Auerbach cannot be regarded as a neuronist, because he described the fine axons, which surrounded the postsynaptic cell, passing through a pericellular ‘feltwork’ before they give off the ‘Knoten’ or ‘Endknöpfchen’ (knots or end-buttons) that form a remarkably dense covering for the cell body shown in his drawing (figure 17).
Figure 17.
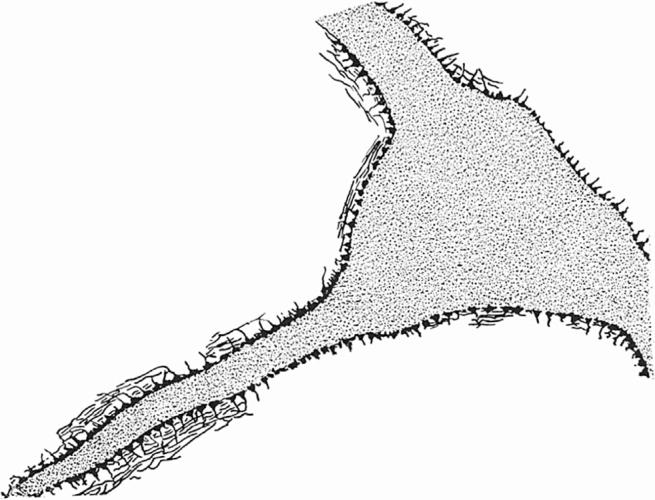
Axon terminals contacting a motor neuron from the seventh cranial nerve of a rabbit. Notice the dense covering of these terminals on the motor neuron and compare with figures 5 and 6, which show the sparser covering revealed by the reduced silver methods. Redrawn from Auerbach (1898a,b). Reproduced from Gray & Guillery (1966), courtesy of Academic Press, San Diego, CA.
In his very thin sections Auerbach could not trace the individual course of these densely arranged very thin axons, and they appeared to him to form a sort of presynaptic reticulum, quite distinct from Golgi's reticulum of axon terminals and also quite different from the reticulum described by Gerlach.
One striking feature of Auerbach's illustration (figure 17) is the very dense distribution of the axonal end-buttons or end-feet on the surface of the nerve cell. This is much denser than anything ever seen with the reduced silver methods (compare figures 5 and 6 with figures 17–19), and may have helped to make Cajal and Kölliker doubt whether they corresponded to the synaptic terminal structures shown by the Golgi and reduced silver methods. I return to this problem below.
Figure 18.
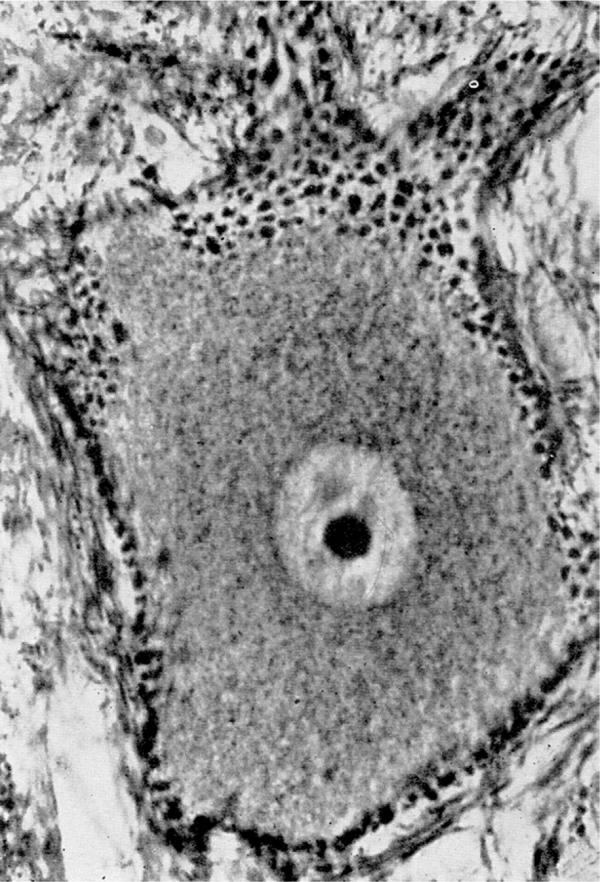
Synaptic contacts on cells of the reticular formation of a cat's medulla oblongata, revealed more recently by Rasmussen (1957). Notice the rich distribution of the small terminal boutons and their close apposition to the surface of the cell body and dendrites. Reproduced from Rasmussen (1957) with permission purchased from C. C. Thomas, Springfield, IL.
Figure 19.
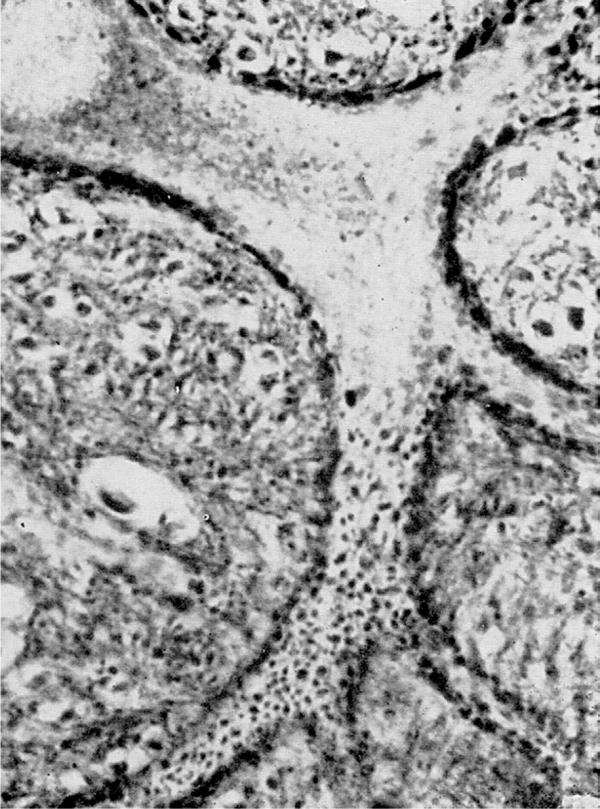
Synaptic contacts on cells of the reticular formation of a cat's medulla oblongata, revealed more recently by Rasmussen (1957). Notice the rich distribution of the small terminal boutons and their close apposition to the surface of the cell body and dendrites. Reproduced from Rasmussen (1957) with permission purchased from C. C. Thomas, Springfield IL.
It is important to note that neither Auerbach nor Held reported a synaptic gap. The supposed gap needs to be distinguished from the discontinuity that Auerbach described and Held saw in his young tissues. Recognizing the discontinuity in light microscopic material depends on two features. One is the thin dark line (the border line) that separates the two processes, and the other is the change in staining properties that can often be recognized on each side of that line. The change in staining properties depends almost entirely on the quality of the fixation and the nature of the stains used. The visibility of the dark line will depend on the size of the structures involved, on the curvature of the membranes, on the optics of the tissue, and therefore also on the clearing solutions used and possibly also on the stain used.
More recent light microscopic studies of synapses in vertebrate or invertebrate nervous systems exploited relatively large synaptic junctions (Bartelmez & Hoerr 1933; Young 1936, 1939; Bodian 1942), and clearly showed the discontinuity across the synapse in terms of staining properties and on the basis of the borderline. Bodian (1942) described this line (he called it the synaptolemma), and recognized it as representing the two membranes one presynaptic and the other postsynaptic, so close to each other that the gap could not be resolved. Bodian pointed out that there had been very little change in the 50 years that preceded his review, as regards what could be seen under a microscope, but that at the time that he was writing there was ‘voluminous’ evidence in favour of a ‘membranous synaptic barrier’. By that time the reticularists could be seen as an endangered species.
When electron microscopists first showed the fine structural relationships at a synapse, they confirmed that both pre- and postsynaptic processes are membrane bound (De Robertis & Bennett 1955; Palay & Palade 1955). This was the first visual display of the two distinct separate, parallel neuronal membranes and of the space that separated them at the synapse. The widely stated conclusion that this confirmed the neuron doctrine (see Peters et al. 1991; Albright et al. 2000; Cowan & Kandel 2002), is not based on knowledge of what the reticularists had claimed, nor does it do justice to the position of the neuron doctrine at the time. It is valid insofar as the membranes at the synaptic barrier could now be seen as two individual membranes separated by a ca. 20 nm gap. However, the electron microscopic image would have been as welcome to Auerbach as to Cajal, and Apáthy would have asked to see an image with some neurofibrils in the junctional region.
The neuron doctrine was not significantly strengthened by the electron microscopic evidence, because at that stage it did not need to be. Nor did anyone bother to refute the various claims of the reticularists. To my knowledge, no one has ever shown electron microscopic evidence against axonal fusions of the sort claimed by Golgi or Auerbach; it is difficult to know what one would have been looking for in an electron micrograph, short of an extensive study of serial sections. It is equally difficult to know what sort of electron microscopic evidence would serve to convince someone that Nissl's intercellular ‘grey’ does not exist. When George Gray and I studied the leech nervous system (Gray & Guillery 1963), it never occurred to us to check whether Apáthy had been correct about reporting neurofibrils that run from one nerve cell into another; we wanted to know why the leech neurofibrils looked so much thicker than those of the vertebrates we had studied. Early electron micrographs never did demonstrate the absence of membrane breaks or possible channels of continuity from one nerve cell to another. This, too, would have required serial reconstructions, although almost any electron micrograph will show a number of membrane breaks which are generally (and almost certainly correctly) interpreted as artefacts. The important point is that by then (1955) no one was asking for that sort of evidence in favour of the neuron doctrine; it was not needed because the reticularist views were no longer taken seriously.
The early electron microscopic studies were not really designed to challenge the neuron doctrine or the reticularist views: they were not needed for that nor should they now be celebrated for that. These electron micrographs demonstrated the clear asymmetry (polarization) at the synapse. They should be celebrated for that, for their high quality, and for showing us synaptic vesicles and specialized synaptic contact regions, which opened entirely new doors for the study of synaptic structures. These features are functionally significant, and their importance far exceeds the contribution that electron micrographs have made in establishing the neuron doctrine. Further, it is worth stressing that light microscopists had essentially no information about the structure or the distribution of synaptic junctions in the mammalian neocortex. Information, commonplace today, about axo-spinous, axo-dendritic and axo-somatic synaptic junctions in cortex had to await electron microscopic studies. Cortical synapses were a great terra incognita until 1959 (see Gray 1959). This was partly because the axon terminals contained no fibrils and were not revealed by the reduced silver methods, partly because the organization of the neuronal elements is less regular than it is in the cerebellum or the retina, so that it was far more difficult to use Golgi preparations and arrive at logical deductions about connectivity patterns.
Two other aspects of our reaction to early electron micrographs merit consideration. One, implicit in what has been said so far, is that no one asked about the supposed visibility of the synaptic gap in light micrographs, illustrated in figure 12 and widely accepted before electron microscope images were available. Why did no one comment that the 20 nm synaptic gap shown by the electron microscope was not consistent with a synaptic gap identifiable with the light microscope? I am inclined to believe that this, too, was because the neuron doctrine was generally accepted, no one was really interested in challenging it any more, and the evidence provided by the electron microscope, though widely celebrated, was no longer really needed for establishing the power of the neuron doctrine. Arguments about the visibility of the synaptic gap, which had been important earlier, were no longer of any interest.
The second point was that the electron microscope showed a dense distribution of synaptic terminals on motor neurons, which was far denser than anything seen in the reduced silver preparations (compare figures 5, 6, 12a and 17–19; Haggar & Barr 1950; De Robertis 1959; Wykoff & Young 1956). That is, the distribution of the synaptic terminals was very like that drawn by Auerbach (1898a,b; figure l7). This discrepancy between the electron micrographs and the reduced silver preparations was not generally recognized or acknowledged, although Wykoff & Young (1956) did express some surprise at the dense covering of end-feet that they saw on ventral horn cells in their electron micrographs, a surprise that is easily understood on the basis of Young's earlier experience with reduced silver stains in the ventral horn of the spinal cord.
Shortly after electron microscopic images became available, Richardson and Rasmussen actually developed methods not unlike those of Auerbach (Armstrong et al. 1956; Rasmussen 1957) and published micrographs that confirmed the general accuracy of some of the drawings prepared by Auerbach and Held (see figures 18 and 19). These showed that the extremely dense covering of synaptic terminals upon axons and dendrites could be reliably revealed for light microscopy, and that the axon terminals were as closely applied to the postsynaptic surface as Deiters, Gerlach, Held and Auerbach had shown. Further, they confirmed that the reduced silver stains revealed only a small fraction of the terminal structures and failed to reveal their true structure.
I have explored some of the early descriptions of synapses in detail to show that even the best, most careful and accurate observers could be in error about important details of appearances seen under the microscope. The problems of defining what is there under the microscope are often subtle. It is not enough to avoid the presentation of critical structures by poorly drawn sketches (Gerlach) or the illustration of structures that could not have been present (Walter). It is necessary to follow each candidate observation to its logical conclusion, to interpret an appearance obtained in one region or with one method in the light of observations made in other regions and by different methods, and to maintain a fine balance between observation and interpretation, recognizing clearly what cannot currently be known. It would be a mistake to conclude that either side in the dispute between neuronists and reticularists was entirely right, but it is important to recognize that the neuronists were far more successful in moving the subject forward. On the whole, once the controversy had been clearly formulated, the neuronists were more concerned with understanding how the system worked, whereas the reticularists seemed more focused on what they could (or could not) see.
One further question can be raised about the early presentation of the neuron doctrine. Why was it so much less accepting of exceptions than was the cell theory? Was it just that its proponents were that much more dogmatic? Or was it that the neuron doctrine was seen as providing a powerful tool for a reductionist analysis of neural function? Any ground given to neuronal continuities, to non-polarized neurons, to trophic actions across neuronal junctions, was seen as a concession to the other side, a concession to what Cowan & Kandel in 2002 still saw as a ‘chaotic’ view of the brain.
8. The neuron doctrine today
Although one can find statements that claim the neuron doctrine as central to neuroscience (see 1), today there has to be serious doubt about this. The doctrine has undoubtedly played a central role in the past; my generation of neuroscientists was raised on the neuron doctrine as though it were a Doctrine of the Neuroscience Church, and is still writing about the doctrine in considerable detail (e.g. Albright et al. 2000; Bennett 2002; Cowan & Kandel 2002) from that point of view. By contrast, a generation of students is growing up either in complete ignorance, or with seriously defective knowledge, of the subject. It is worth asking what, if anything, the neuron doctrine tells us about the nervous system that, perhaps, our students should still be taught. Are the claims that the neuron doctrine is central to neuroscience justified, and if they are, then what are the key issues, and how do they relate to the bitter controversies of the past?
Contemporary students, asked about the unit of function of the nervous system, could well conclude that the nerve cell is not the unit of function that is of primary interest to them. The units of contemporary studies are the packets of transmitter molecules, the channels and receptors by means of which nerve cells communicate with each other. Although many nerve cells are polarized in the classical sense, others are not: parts of the classical neuronal output system, the axons, can serve as receptors, and the classical receptor portions of the dendrites can serve as effectors. It is readily accepted that some nerve cells may be able to ‘multiplex’, doing one thing with one process and something different with another process. That is, a single nerve cell can operate as more than one distinct functional unit. There are many examples of nerve cells linked to each other by specialized ‘gap junctions’ that provide electrical coupling and allow the passage of small molecules from one nerve cell to another. Horizontal cells in the retina, linked by gap junctions have been described as a ‘syncytium’ (Amzica 2002), and neocortical neurons as forming a ‘network’ through extensive dendro–dendritic gap junctions (Fukuda & Kosaka 2003) but, to my knowledge, no one has objected that these represent ‘reticularist’ views of the nervous system, as many would no doubt have done 50 or 100 years ago. There are nerve fibres that are produced by the fusion of processes from several cells. The effects of neural damage can cross synapses, so that ‘transneuronal (or trans-synaptic) degeneration’ can be seen in some parts of the nervous system, either crossing the synapse in the direction of conduction or in the opposite direction.
These facts are all contrary to the neuron doctrine, as originally expressed. However, they are widely recognized, and in the following they will be briefly documented. Yet they have had little influence on the view of the neuron doctrine that is still current among many older neuroscientists. The most recent summaries of the neuron doctrine and the law of dynamic polarization still present them as though they were basic to our concept of what comprises the nervous system (Albright et al. 2000; Bennett 2002; Cowan & Kandel 2002; and other papers in volume 136 of Progress in Brain Research). While recognizing some of the areas where the facts no longer fit the doctrine, these issues are sidestepped, without any serious exploration of the significance of a doctrine that has such varied loopholes.
(a) The polarized neuron
The view of the neuron as a polarized structure, with the dendrites (and cell body) providing a receptor surface and the axon acting as the effector was based by Cajal primarily on observations of cells in the olfactory bulb, retina and cerebellum, where he was able to analyse the circuitry in detail and to present schematic connectional diagrams with arrows indicating the direction of information flow (figure 3). Cajal (1995; and see Shepherd 1991) provides details of the significant contribution made particularly by Van Gehuchten (1891; and see Van Gehuchten & Martin 1891), who pointed out some of the difficulties relating to the view of the neuron as a polarized structure. Once the polarized structure of nerve cells was accepted, it led to the important recognition of synapses as asymmetrical structures specialized for one-way transmission. This proved to be powerful for an analysis of neuronal circuits in vertebrate brains, particularly in its application to the ventral horn cell, where Sherrington and his successors laid the foundations for many key studies of synaptic functions (see Cowan & Kandel 2002).
It has long been recognized that there are situations in the vertebrate brain where the model of the polarized neuron could not be readily applied, and for invertebrate brains, which may perhaps house the majority of neurons in the world, the model of the polarized neuron is not readily applicable for analysis of many neurons. One difficulty arises from neurons, such as the dorsal root ganglion cells of adult mammals, that are unipolar, so that distinguishing axons from dendrites is somewhat arbitrary. This problem, which formed a significant part of the original debate undertaken by Cajal and Van Gehuchten (see above) has been addressed repeatedly (Maximow & Bloom 1930, pp. 232–233; Bodian 1952) especially for dorsal root ganglion cells, and is often raised in textbooks, but has never been satisfactorily resolved other than by providing an arbitrary definition of terms.
For dorsal root ganglion cells there is, at first sight, not a serious problem functionally. The peripheral receptor portions of the axons serve the functions ascribed to dendrites elsewhere, and the other parts of the axon, including both the central and the peripheral portions, but most importantly the central terminals in the spinal cord, serve as the effector processes. This is the issue of terminology so often discussed in the past. However, there is an added complication, because Eccles et al. (1961, 1962) showed that the central processes of dorsal root axons could be depolarized by stimulation of adjacent dorsal root fibres. They interpreted this as ‘presynaptic inhibition’ and postulated that there must be some axon terminals that are presynaptic to the central terminals of dorsal root axons. That is, the dorsal root axon terminals were postulated to be postsynaptic, contrary to the law of dynamic polarization. Gray (1962), stimulated by the observations of Eccles and colleagues, described such axo-axonal or ‘serial’ synaptic junctions in electron micrographs, adding to the problems of treating dorsal root ganglion cells as examples of a ‘dynamically polarized neuron’.
At about the time of the Eccles and Gray observations two other populations of vertebrate nerve cells that had long been exceptions to the law of dynamic polarization came under study (Kidd 1962; Phillips et al. 1963; Rall et al. 1966).27 These were the amacrine cells in the retina (see figure 3, where they are represented by six unipolar cells distributed in the mid-layers of the retina) and the granule cells in the olfactory bulb. They had been recognized since the days of Cajal and Van Gehuchten, as cells that lacked an axon, and thus, on a strict view of dynamic polarization, as cells that lacked an output. They had been a puzzle for everyone. Kidd (1962) showed serial synapses in electron micrographs of the inner plexiform layer, the retinal layer containing the dendrites of amacrine cells, and Phillips et al. (1963) described the inhibitory (presynaptic) actions of granule cells of the olfactory bulb, an observation later followed by a comparable demonstration of serial synapses involving presynaptic dendrites of olfactory granule cells (Rall et al. 1966). That is, the presence of presynaptic synaptic vesicles in dendrites, and of postsynaptic specializations in axons came to be accepted as a feature of vertebrate nervous systems, and the common occurrence of serial synapses in many invertebrate brains (Shepherd 1999) showed a breakdown of a strict view of neurons as polarized, with axons and dendrites having clearly distinct functions.
Serial synaptic connections are now widely recognized, but they cannot be regarded as having changed views of the dynamically polarized neurons to a significant extent. These connections are regarded as relatively minor exceptions in the vertebrate brain to a widely applicable and extremely useful general rule. However, a point that is not widely recognized is that serial synapses of any sort raise a serious question, not only about the functionally polarized neuron, but also go beyond that to challenge the idea of the neuron as a functional unit. This question arises because, if (for example) a part of a dendrite can be both presynaptic and postsynaptic, then it may act on its own, independent of the action of other dendrites belonging to the same cell. That is, there arises the possibility that a cell can ‘multiplex’, performing distinct concurrent functions with several of its dendritic processes. Similarly, if an axon can be postsynaptic, then there is a possibility that one terminal of a single axon will have actions that are distinct from the other terminals of the same axon, and these possibilities clearly undermine the concept of the nerve as a functional unit.
In particular, this possibility of cells multiplexing has been raised for thalamic interneurons, which have an axon and several dendrites (Ralston 1971; Cox & Sherman 2000; Sherman & Guillery 2004). The dendrites of these interneurons are extremely slender, and have axon-like terminals that contain synaptic vesicles. These terminals are presynaptic to dendrites of thalamic relay cells and postsynaptic to incoming afferent axons. Because the dendrites are long and very thin, voltage changes in one dendrite or dendritic terminal will be severely attenuated before they reach other parts of the dendritic tree. That is, it is probable that an incoming afferent that is presynaptic to one of these dendritic terminals can produce a local, voltage-dependent release of transmitter from that one interneuronal dendrite, but that the voltage changes in the dendrite will not spread to other parts of the dendritic tree to any significant extent. Each part of the dendritic tree can then act as an independent functional unit. It seems probable that cells such as this can act as multiple, distinct functional units through their presynaptic dendrites and as a single ‘classical’ dynamically polarized unit through their axon.
Similar arguments can be applied to retinal amacrine cells (Miller & Bloomfield 1983; Taylor &Wässle 1995; Peters & Masland 1996; Euler & Denk 2001; Euler et al. 2002), to horizontal cells and to the granule cells of the olfactory bulb (Shepherd 1999). Relationships such as this may be relatively uncommon in vertebrate brains, perhaps leaving the model of a dynamically polarized neuron as a useful even if not a generalizable model for vertebrates. However, such serial synapses are likely to be relatively common in invertebrates, and when it comes to defining axons that may be postsynaptic, the possibility has not been fully explored for any brain, vertebrate or invertebrate.
The fact that currently a variety of receptors are being localized on presynaptic axonal terminals (e.g. Chen & Regehr 2003), suggests that axons may prove to be receptors far more commonly than is generally recognized. As the distribution and the functions of receptors on the terminal portions of richly branching axons become defined, it will become more and more difficult to think of an axon as the single effector responsible for the output of a neuron acting as a single unit, while all of the inputs are delivered to dendritic and somatic surfaces. That is, it is becoming necessary to recognize that for any one neuron there may be several more or less independent axon terminals serving as outputs; unless all of them are activated in exactly the same way, each may be controlled by, or modulated by, different independent input mechanisms (see also Nusbaum et al. 2001).
(b) The neuron as a developmental and structural unit
An understanding of the developmental history of nerve cells provided some of the crucial evidence for the neuron doctrine. Earlier concepts about how nerve fibres were related to nerve cells varied greatly, and a distinction, often found between ‘nerve cells’ as cells related to the developmental formation of axons, and ganglion cells as cells that connected to the axons (see §3) had a strong life of its own. His (1883) reviewed many of these earlier observations as well as others that saw the nerve fibres as outgrowths from ‘ganglion cells’. His's own observations (1883, 1886, 1889) on the development of axons as the outgrowth of nerve cells, particularly for the dorsal root ganglia and the ventral horn, were critical in establishing the modern view, as were the later experimental studies of Harrison (1908, 1910, 1924), showing in vivo and in vitro that the outgrowth of the nerves was independent of the satellite Schwann cells or of any other cells. These observations eliminated all thoughts that axons were anything other than the outgrowth of a single cell, the neuron. The history has been well covered by others and here only two quite different points regarding the structural and developmental identity and individuality of nerve cells will be considered. These concern fusions of axons and, by contrast, small zones of cytoplasmic continuity that can produce a functional coupling of neurons.
(i) The squid giant axons
Young (1936, 1939) described the giant axons of squid as fused neuronal processes. He described first-order giant axons, which are ‘joined across the middle by a complete protoplasmic bridge’ and considered how the fusion occurs during embryogenesis. He also described the third-order giant axon as formed by the fusion of many smaller axons, stating that ‘Each third-order axon is therefore a syncytium’. It fires impulses as a whole; there is no evidence for separate parts responding at different thresholds. In discussing these fusions Young writes: ‘It is not necessary to delay over the question of whether we should save the letter of the neuron theory by saying that such cells are, by definition, not neurons (see Maximow & Bloom 1930)’28 (citing the histology textbook) ‘It is important to recognize that the occurrence of such fusions does not invalidate the neuron theory in general’ (his italics).
For him, the importance of the one-way synapse for preventing the spread of activity through the whole brain was the major contribution of the neuron doctrine. For a contemporary reader, the idea of treating these giant axons as (by definition) not neurons must seem bizarre, but it accurately reflects the dogmatic rigour with which the neuron doctrine has often been defended in the past.
Young's view of the neuron doctrine, which can be regarded as the ‘generous view’, allowing for exceptions but recognizing the force of its conclusions for understanding basic connectivity patterns, is one that is also to be found in the broad survey of invertebrate nervous systems published by Bullock & Horridge (1965). Perhaps it comes more readily to those concerned with invertebrate nervous systems, or perhaps it represents a more modem view of the neuron doctrine, one that sees it as having served in the analysis of connectivity patterns, but expects little from it beyond that. That is, it is seen less as a doctrine or a law and more as a guide.
(ii) Gap junctions
Specialized functional regions that provide electrical coupling between neurons were first described by Furshpan & Potter (1957) in crayfish abdominal nerve cord, and have subsequently been recorded in many other situations in vertebrate and invertebrate nervous systems (see Bennett 2002). Structurally, these are the gap junctions that provide direct cytoplasmic continuity between two nerve cells allowing the passage of small molecules from one cell to the other. That is, these are regions where nerve cells are in structural and functional continuity with each other, and it is reasonable to regard them as continuities that develop between nerve cells that are originally distinct elements in accordance with the observations of His. Often they occur in regions where a rapid response is required from several linked nerve cells. These, like the fused squid neurons, are contrary to a strict interpretation of the neuron doctrine. Bennett (2002) writing about these junctions describes his earlier view of them, based on an ‘iconoclastic predilection’, as significant evidence against the neuron doctrine. However, he now writes about them from a more generous viewpoint, representing them, as Young wrote about the fused squid axons, as leaving the basic tenet of the neuron doctrine (that neurons are by and large independent elements of the nervous system) largely untouched. There is a serious question here as to whether the passage of time has mellowed Bennett's youthful iconoclastic inclinations, whether it has reduced the importance of the neuron doctrine, or whether it has trimmed some parts of the neuron doctrine that once seemed important to the early protagonists.
(c) The neuron as the trophic unit
This is perhaps the least clear and most poorly documented aspect of the neuron doctrine. Its clearest roots lie in the observations, published by Waller in 1850, that when a nerve is cut, the peripheral processes, which are (in contemporary terms) separated from their neuronal cell bodies, degenerate. This provided evidence (as did Harrison's later 1908 and 1910 observations) that the nerve fibre is continuous with, and depends for its survival on, the cell body. These observations, together with some of the observations made by von Gudden (1870, 1889), were taken by Forel (1887) as a part of the foundation of the neuron doctrine.
Forel had been a student with von Gudden, who, apart perhaps from Waller, can be regarded as the father of experimental neuroanatomy. von Gudden's experiments included some in which he cut the facial nerve and looked at changes centrally, and others where he made lesions at very early postnatal stages, removing eyes or pieces of cerebral cortex, and then after the animals had matured studying the central pathways that had been affected. Forel (1937; cited by Shepherd 1991) wrote: ‘I considered the findings of von Gudden's atrophic method, and above all the fact that total atrophy is always confined to the processes of the same group of ganglion-cells, and does not extend to the remoter elements in merely functional connection with them’. That is, he interpreted the degenerative change described by Waller (called Wallerian degeneration) and also the changes that occur in the nerve cell bodies centrally as demonstrating the unity of the nerve cell, and from this he was inspired to argue towards the independence of nerve cells from each other. However, making the trophic independence of neurons into a part of the neuron doctrine was an extreme extension of the anti-reticularist dogma and can now be seen as an error. Already in 1889, His, writing about the trophic dependence of a nerve fibre upon the cell from which it had formed, added, in an evident critique of Forel: ‘The relationships seen in Gudden's degeneration are less clear, including the atrophy in neural centres after peripheral lesions as well as the retrograde degeneration of intracerebral motor roots. These phenomena appear to me to be not yet sufficiently developed (ripe) for general discussion'.2 Today, neurotrophic substances, which are produced by one cell and act upon another, represent a major occupation for many neuroscientists, and it would be hard to defend the trophic independence of nerve cells.
Forel's view of the experimental results is puzzling from a contemporary point of view. The results of Gudden's experiments with young animals are, as His pointed out, difficult to fit into Forel's interpretation. von Gudden had written (von Gudden 1889, p. 143): ‘… of two central organs, if one is destroyed, the other atrophies only if it receives inputs from the other, not if it sends inputs to the other’.2 Later he also described a loss of fibres in the medial lemniscus after forebrain lesions. Further, he described atrophic changes in the mamillary bodies after cortical removals and in the visual centres after eye removals. These are all changes that cross a synapse: we now recognize that those in the mamillary bodies are ‘retrograde transneuronal’ changes (cells in the mamillary bodies send their axons to the anterior thalamus and the anterior thalamic cells send their axons to cortex); those in the visual centres are anterograde transneuronal (retinal ganglion cells send their axons to the visual centres). Forel interpreted the change in the visual centres as a loss of the incoming axons, but in fact it also represents marked neuronal shrinkage in adults, and even more severe and rapid changes in young animals (Cook et al. 1951; Matthews et al. 1960; Guillery 1973; Guillery et al. 1985). Many other experiments also demonstrate many instances that go against Forel's account. Apart from the ‘total atrophy’ that is invariably seen in the peripheral nerve processes undergoing the classical Wallerian degeneration, changes are highly variable in the central processes, including the nerve cell body of the damaged neuron. For lesions in the peripheral nervous system the central degeneration can be rapid and complete in very young animals but it is often mild or absent in the adult, depending on the group of nerve cells damaged, on the species, on the method of injury, and on the distance from the cell to the lesion (Bielschowsky 1928; Geist 1933; Brodal 1940). Lesions in the central nervous system produce reactions that are also highly variable. Whereas in an adult animal, thalamic cells undergo rapid and severe degenerative changes leading to cell death after their thalamocortical axons are cut by a cortical removal, cells in the hippocampus or cerebellum can survive with relatively little or no change after their axons are cut.
von Gudden's demonstration that removal of the cingulate cortex produces changes, not only in the anterior thalamic nuclei but also in the cells of the mamillary bodies, has been confirmed by others (see Cowan 1970) with more modem techniques. Transneuronal changes can also be seen in the thalamic reticular nucleus when cortical lesions destroy the axons that innervate this nucleus (Rose 1952; Carman et al. 1964; Guillery & Harting 2004), in some of the central auditory nuclei after damage to the eighth nerve (Powell & Erulkar 1962) and in the retina after lesions of the visual cortex (Van Buren 1963; Cowey 1974).
Overall, the evidence on the trophic unity of nerve cells makes sense only for Wallerian degeneration. Here, the changes clearly represent the dependence of the axon on the nerve cell body and relate in an importaant way to the axon as an outgrowth of the nerve cell and as a freely ending entity. However, it is impossible to understand how the highly variable changes that are seen centrally, in the cell body or across a synapse, in synaptically connected neurons, can ever have been considered to contribute to a view of the nerve cell as an independent entity, or can be seriously considered in this light in contemporary reviews.29 The available observations did not fit when the trophic independence of the neuron was first proposed, and are an even worse fit today. The neuron doctrine would have lost none of its power without the proposed trophic independence of neurons. von Gudden's important experiments, which were basic to the elucidation of some central pathways, should not have been considered as playing a role in defining the neuron as an independent element. The experiments provided a conceptual stepping-stone for Forel when he first started thinking about how neurons relate to each other, but they were out of place in the final theory 100 years ago, and are glaringly out of place today. The continued inclusion of the trophic independence of neurons in contemporary accounts of the neuron doctrine represents perhaps the most extreme example of the dogmatism that neuronists have used to defend their position over the years.
9. A brief overview
It is not easy to unravel the motivations of all those involved in the controversies that surrounded the birth of the neuron doctrine. However, all of those considered here were committed to using the microscope to define the structures that make up the living nervous system. Our understanding of the history is obscured by failures to understand what individual observers were actually able to see; it is significantly hindered by our limited knowledge about the philosophical stand upon which any one observer may have based his interpretations. I have tried to show how difficult the business of microscopy is and how important it is to understand optical limitations and interpretative hazards when one is studying preparations that have been significantly altered from their living state. It is easy to see more than is really there, to miss features that are obvious to others, to misinterpret artefacts, and to let favoured presuppositions about what the nervous system should be like to influence unduly what is seen and reported. It is also important to be bold in interpreting what is seen, accurate in reporting and drawing it, and then to check the interpretation over and again in different parts of the brain and with different methods, and in this Cajal excelled above all others.
I have been concerned only with those early observations that were relevant to identifying the synaptic junction between axon and postsynaptic structure (dendrite or cell body). I have said little or nothing about Golgi's proposed axo-axonal fusions, about the supposed nutritive functions of the dendrites, about Nissl's poorly defined intercellular grey, or about Apáthy's supposed transcellular neurofibrils. They are a part of the opposition that the neuronists had to fight, but they made no significant contribution to our knowledge of synapses. The microscopic observations I have described interested me because each forms a part of a whole. We can see how each of the observations, whether made by neuronists or reticularists, fits the picture of the synapse that we have today. In the well worn analogy of the blind men and the elephant, we can now pretty well see most of the elephant (the synapse), and we can understand how each of the investigators I have discussed arrived at a particular description of that elephant. However, the analogy is inappropriate to the extent that we are not dealing with just one elephant. Synapses and nerve cells vary greatly from one part of the brain to another. It is difficult to conceive of general rules or ‘laws’ that can be applied to all nerve cells in all brains. For many years our understanding of synaptic transmission was dominated by studies of ventral horn cells of the spinal cord, whereas today the focus is heavily on cells in the hippocampus. Each cell type, when studied intensively, can serve to demonstrate functional specializations that are possible in nerve cells, but it is unlikely that even the most intensive study of any one cell type will reveal the rich array of specializations that nerve cells can develop.
10. Conclusions
When viewed in terms of currently available evidence, nerve cells can be seen to be very similar to other cells: they can develop from several fused structures, show small areas of cytoplasmic continuity, have several functionally independent units within a single cell, have significant trophic influences upon each other, and even have one part (the cell body) that can survive injury to another (the axon). There is no reason for the neuron doctrine to be more rigorous than the original cell theory. Nerve cells represent one example of the many classes of cell that have arisen during the course of evolution, and appear to be governed by the same general rules. It is possible that an analysis of nervous systems in terms of independent neuronal units could have been undertaken successfully even if these several points had been recognized early on, although it is reasonable to see the dogmatic form of the neuron doctrine, one that allows no exceptions, as having served a useful simplifying function in the past.
What about the present? Do our students need to learn about the neuron doctrine and the law of dynamic polarization? If it is thought that time can be made to introduce students to some of the historical developments of our subject then, clearly, students should have an opportunity to look at some of the early observations of neurons, and at some of the arguments that led investigators to different conclusions about how neurons communicate with each other. But this is history and unfortunately, today, most courses in neuroscience have all too little time for history. The most recent papers in journals with high impact factors are considered to be far more important. In terms of the current knowledge of nervous systems, a dogmatic statement of the neuron doctrine and the law of dynamic polarization is more likely to be confusing than enlightening to students.
Students need to know that nerve cells originate developmentally as independent entities. They need to understand that much (but not all) of the communication between nerve cells occurs at one-way (i.e. polarized) synaptic junctions, where two neurons are in contact with each other, not in continuity. In addition, they need to appreciate that fused neurons, neurons coupled by gap junctions, presynaptic dendrites and postsynaptic axons, nerve cells that multiplex, which all had an air of surprise about them when they were first introduced to neuroscience, do not ‘break any rules’. They can no longer be regarded as against the generally accepted view of what nerve cells should be like. We learnt about all of these after the neuron doctrine had done its main job, and so they seemed not really to challenge the neuron doctrine. This can be seen in Bennett's (2002) account of gap junctions and in Shepherd's (1991) account of presynaptic dendrites. Each was treated as being to one side of the neuron doctrine, somehow not really relevant to the classical debate (which was over) and therefore something that could easily be added to the neuron doctrine without modifying it in any way. Cowan & Kandel (2002) have written about the discovery of gap junctions between neurons as having been ‘quickly seized upon by a number of ‘latent reticularists’ who saw in it a modern-day challenge to the neuron doctrine’. They cite no latent reticularists. Perhaps they existed; perhaps Bennett was one (Bennett 2002). Today, there are likely to be no latent reticularists. Today, those of us who are not dedicated neuronists, are more likely to be post-neuronists.
One other question merits consideration. Can we regard the neuron doctrine as ‘one of the great ideas of modern thought’ (Shepherd 1991), comparable to the quantum theory, the periodic table or the theory of evolution? I suspect that in the long run it will be seen simply as the cell theory applied to neurons, necessarily having to be as generous as the cell theory in allowing for cell fusions, extra-cellular materials, subcellular functional units, etc. The original version of the neuron doctrine was stronger and more rigorous than the cell theory, and was presented as a ‘doctrine’, so as to provide a clear and practical reductionist approach to the study of neuronal connections. That has been its main strength and its important contribution to neuroscience. Today it is appropriate to celebrate the success of the neuron doctrine and to admire the histological skills and the strength of purpose that animated the founders of the doctrine. However, it is now necessary to recognize that a full, dogmatic application of all that the neuron doctrine stood for in its early days would tend to obscure a student's appreciation of many recent advances. The neuron doctrine as something over and above the cell theory is not likely to survive as a fundamental idea, and perhaps that is why it is no longer taught to a significant extent. Brains are a piece of the biological world, and our nerve cells share their organizational rules with the other systems of the body, the immune system and the endocrine system in particular. If we ever do find some powerful generalization that applies to brains in particular then it will probably not be about brains as a piece of biology but about brains as computing entities.
Acknowledgments
The author thanks Sherry Feig, Peter Guillery, Anthony LaMantia, Jenny LaVail, Ray Lund, Jeff Mayne, Donata Oertel, H.J. (Peter) Ralston and Murray Sherman, who all contributed helpful comments on an earlier version of this essay, and to the librarians of the Health Sciences Library at the University of Wisconsin, Madison, particularly Micaela Sullivan Fowler, for help with finding much of the material. Supported by grant no. EY12936 from the NIH.
Endnotes
Shepherd (1991) writes ‘Of broader interest is the potential significance of the neuron doctrine as one of the great ideas of modern thought. One thinks here for comparison of such great achievements of the human intellect as quantum theory and relativity in physics; the periodic table and the chemical bond in chemistry; the cell theory, evolution and the gene in biology’.
My translation.
Sherrington wrote: ‘If…the axon continues to run and finally ends in the central nervous system, its mode of termination as well as that of the collaterals to which it may give rise is in the form of an arborescent tuft, which is applied to the body or dendrites of some other cells. So far as our present knowledge goes we are led to think that the tip of a twig in the arborescence is not continuous with but merely in contact with the substance of the dendrites or cell-body on which it impinges. Such a special connection of one nerve-cell with another might be called a synapsis’ (stress in original).
The history of cerebral localization of function runs closely parallel to the controversy of the neuron doctrine, but started much earlier. Whereas Newton and Descartes both recognized the importance of topographical maps in the visual pathways (Polyak 1957), and therefore assumed that functions were localized in the brain, subsequent workers, summarized by Clarke & O'Malley (1996), either denied localization of function in the brain (Fluorens) or produced the unscientific speculations of the phrenologists. It was not until Broca, Hughlings Jackson, Fritsch and Hitzig, Ferrier and others (1860s–1870s) started to look at the problems of localization on the basis of experimental and clinical evidence, that a serious consideration of the localization of function became an important part of neuroscience. It is interesting to note that these observations were being made just as early efforts at defining how nerve cells relate to each other were also underway.
The fact that the science was written in terms of objective statements about observations, not about the deeper issues, is not surprising. For example, in the fierce arguments concerning evolution between R. Owen and T. H. Huxley about the structure of the human brain in the region of the ‘hippocampus minor’ (see Brown 2002), the scientific papers were written in terms of apparently objective statements about the structure of human and monkey brains. However, there was no doubt that these were arguments about the supposedly special nature of mankind, and that was how they were perceived by the wider public.
Cajal (1954, p. 63), discussing the anatomical relationships described by Held and other reticularists at the synapse, wrote ‘All this complicated system of relationships, difficult to demonstrate…leads to endless confusion, so much the more so, since each reticularist upholds a different interpretation…’.
Today the terms are often used interchangeably, although the use of ‘ganglion cell’ tends to be limited to nerve cells that actually lie in peripheral ganglia or to the cells in the innermost layer of the retina.
For example, Schultze (1861), basing himself on a study of multinucleate muscle cells, argued against the view that cells must be bounded by a membrane.
Swanson & Swanson (1995) have pointed out that Schwann, a parent of the cell theory, considered that nerve fibres were formed by cell fusions.
An important feature of recent single cell injections with small marker molecules that can be visualized to show all of the processes of the single cell, is that the Golgi method must now be regarded as commonly not staining nerve cells in their entirety, as appeared to be the case when no competing method was available. Single cells stained by injections commonly have richer dendritic arbours than are seen for the same class of cells in Golgi preparations.
This is an interesting issue: the spinal connections were a focus of attention for many investigators because the functional implications of the connectivity patterns could be clearly understood. However, the cerebellum and the hippocampus provided tissues where the neural connections are remarkably regular and arranged in a well-defined geometrical pattern, feature not found in the cord. The retina provided a combination of regular geometric layout with a clearly defined functional role. One aspect of Cajal's particularly powerful contribution was his broad approach to the nervous system; he had studied all of these regions and chosen his arguments from all over the brain.
It is worth noting that the first edition of 1852 had been translated by Thomas Henry Huxley (Darwin's ‘bulldog’ and an active protagonist in later arguments about the hippocampus minor; see endnote 5).
Walter and Deiters were not related to each other, but they were both my great-grand uncles. Walter was my paternal grandfather's uncle, and Deiters was my paternal grandmother's uncle. I know nothing about their interaction, although they were both sons of law professors at the University at Bonn and were both working on similar projects on the nervous system in Bonn, so they must have known each other over a period of many years. Both came from musical families, and since Schultze was a violinist who liked to have music in his home (Schwalbe 1874), it is intriguing to think about the social interactions of these three early neuroanatomists, Schultze, Walter and Deiters, who were relatively close to each other in age; to what extent did they interact and to what extent did they influence each other? The only hint on the subject comes from a comment made by a niece of Deiters (Dr Elizabeth Deiters), who commented that Otto Deiters was not regarded as a sociable person in the family (see Deiters & Guillery 1963). Certainly, in terms of his published account Deiters showed a remarkable independence of spirit, apparently not shared by the older Walter.
It is odd that Walter did not claim his earlier account of fusions in the olfactory bulb as giving him priority in the description of such fusions of neuronal processes, particularly since Schultze in his 1863 study cites the earlier Walter (1861) paper.
He also illustrated glial cells (astrocytes) and their processes.
Kölliker (1867) in the fifth edition of his textbook, confirmed the presence of these fine axons, but was unable to see any that had a myelin sheath on their preterminal portions.
Andreoli (1961) describes Deiters as a founder (Begründer) of ‘contact theory’ and Schultze as a founder of a theory that sees axons as comprising several independent fused components. It is clear that there was a major difference between the two, but we know too little about their relationship as the work was progressing to judge how much of this was discussed, or how much Schultze knew about Deiters' findings as the work progressed. We have to recognize Schultze's significant contribution for undertaking the considerable labour of bringing the work of Deiters, a junior colleague, with whom he appears to have disagreed, to publication after Deiters' death, and also to recognize Deiters for his independent spirit. Whereas the published record suggests that Schultze was closely involved with the studies that Walter undertook, I am not clear about the extent to which Schultze involved himself with the work that the younger but far more able Deiters was doing at the same time.
On p. 108 he says ‘since Volkmann's attempt to differentiate peripheral nerves on the basis of diameter into functional classes foundered, there has been no talk of similar such efforts. But none the less, … it seems worth while to look for a principle here…’. He goes on to discuss mistakes made by earlier workers on the distribution of fibre diameters and then records particularly the fibre diameter differences on the motor and the sensory sides of the cord.
It seems likely that this classification of Deiters as a reticularist (see also Swanson & Swanson 1995) was not based on a reading of his work, nor on the fine account given by Shepherd (1991), but rather on the translation of a Cajal paper, cited by Shepherd (1991): ‘The doctrine of intercellular anastomoses due to Gerlach and supported by Deisters (sic)…’.
His (1886) wrote: ‘Gerlach, whose viewpoint I share in some respects, assumes that in the grey matter there is an intermediate element, or nerve net, between the fibres and the cells’.2 He points out, quite rightly, that Gerlach failed to show this.
For some synapses it is slightly thicker, with the wider extracellular space occupied by a thin layer of extracellular material (see Gray 1959).
See endnote 10.
Clarke & O'Malley (1996) describe Nissl's writing style in an earlier article as ‘awkward and long-winded’, a description that also fits the 1903, long paper on the neuron doctrine. He describes the postulated ‘nervöse Grau’ (neural grey) as a ‘specific neuronal, non-cellular component of the grey matter, which is firmly established even though we know nothing of its histological structure’. I will say no more about Nissl's interpretation in what follows.
They are called that to distinguish them from the Golgi methods, which also use silver salts, but in combination with dichromate. They are also often called neurofibrillar methods because they reveal the neurofibrils in the nerve cells, the dendrites and the axons.
‘Neurofibril' refers to the light microscopically identifiable component. ‘Neurofilament’ refers to the intermediate filaments that can be seen with the electron microscope and that at many sites correspond to the neurofibrils. That is, neurofibrils are made up of bundles of neurofilaments and represent the light microscopic image of neurofilaments.
Early versions of the Golgi method produced poor results in tissues that contained significant amounts of myelin. For this reason most of the results published before 1960 were based on new born or very young animals.
The fact that these observations were made 100 years after axon and dendrites were first distinguished may seem trivial, but it underlines the slow and difficult progress that characterized those 100 years.
I have been unable to find this statement in the referenced book, but have to believe that Young's characteristically dismissive comment was stimulated by a published statement.
Cowan & Kandel (2002) have addressed this issue, stating that: ‘Gudden had noted that when a nerve is severed, the resulting neuronal atrophy (or retrograde degeneration as we now call it) is confined to the relevant cell group and does not spread to involve neighbouring populations of neurons, as might be expected if the cells were physically continuous’. Since Cowan had earlier studied transneuronal degeneration in the lateral geniculate nucleus and the mamillary bodies, and had contributed significantly to our understanding of the transneuronal changes in the thalamic reticular nucleus (Cowan 1970) this statement looks as though here the history of the subject was being made to fit into a dogmatic view of the doctrine. A footnote that was added to the Cowan and Kandel account does nothing to strengthen one's faith in the trophic independence of nerve cells. It reads: ‘Later work showed that in some situations degenerative changes extend to other cell populations. Indeed, in some of von Gudden's experiments (which involved lesions of the cerebral cortex in young rabbits), he reported an atrophy of the mamillary body, which we now know to be secondary to the retrograde degeneration in the anterior thalamic nuclei (see Cowan 1970, for review). But at the time Forel wrote (1887), his interpretation of von Gudden's finding was widely considered a significant ancillary line of evidence for the ‘trophic independence’ of neurons’.
References
- Albright T.D, Jessel T.M, Kandel E.R, Posner M.I. Neural science: a century of progress and the mysteries that remain. Neuron. 2000;25:S1–S55. doi: 10.1016/s0896-6273(00)80912-5. [DOI] [PubMed] [Google Scholar]
- Amzica F. Physiology of sleep and wakefulness as it relates to the physiology of epilepsy. J. Clin. Neurophysiol. 2002;19:488–503. doi: 10.1097/00004691-200212000-00002. [DOI] [PubMed] [Google Scholar]
- Andreoli A. Schwabe; Basel: 1961. Zur geschichtlichen Entwicklung der Neuronentheorie. pp. 1–88. [Google Scholar]
- Apáthy S. Das leitende Element des Nervensystems und seine topographischen Beziehungen zu den Zellen. Mitteil. zool. Station. Neapel. 1897;12:495–772. [Google Scholar]
- Armstrong J, Richardson K.C, Young J.Z. Staining neural end feet and mitochondria after postchroming and carbowax embedding. Stain Technol. 1956;31:263–270. doi: 10.3109/10520295609113816. [DOI] [PubMed] [Google Scholar]
- Auerbach L. Nervenendigungen in den centralorganen. Neurol. Centralblatt. 1898a;16:439–441. [Google Scholar]
- Auerbach L. Nervenendigungen in den centralorganen. Neurol. Centralblatt. 1898b;17:445–454. [Google Scholar]
- Bartelmez G.W, Hoerr N.L. The vestibular club endings of Ameiurus: Further evidence on the morphology of the synapse. J. Comp. Neurol. 1933;57:401–428. [Google Scholar]
- Bell C. Cited in The human brain and spinal cord. In: Clarke E, O'Malley C.D, editors. Norman Publishing; San Francisco, CA: 1811. pp. 296–299. [Google Scholar]
- Bennett M.V. Neoreticularism and neuronal polarization. Prog. Brain Res. 2002;136:189–201. doi: 10.1016/s0079-6123(02)36017-5. [DOI] [PubMed] [Google Scholar]
- Bielschowsky M. Die Silberimpregnation der Neurofibrillen. J. Psychol. Neurol. (Lpz) 1904;3:169–189. [Google Scholar]
- Bielschowsky M. Nervengewebe. In: von Möllendorf W, editor. Handbuch der mikroskopischen Anatomie des Menschen. vol. 4. Springer; Berlin: 1928. pp. 1–142. [Google Scholar]
- Bishop G.H, Clare M.H. Organization and distribution of fibers in the optic tract of the cat. J. Comp. Neurol. 1955;103:269–304. doi: 10.1002/cne.901030204. [DOI] [PubMed] [Google Scholar]
- Bishop P.O, Jeremy D, Lance J.W. The optic nerve: properties of a central tract. J. Physiol. 1953;121:415–432. doi: 10.1113/jphysiol.1953.sp004955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodian D. Cytological aspects of synaptic function. Physiol. Rev. 1942;22:146–169. [Google Scholar]
- Bodian D. Introductory survey of neurons. Cold Spring Harb. Symp. Quant. Biol. 1952;17:1–13. doi: 10.1101/sqb.1952.017.01.003. [DOI] [PubMed] [Google Scholar]
- Brodal A. Modification of Gudden method for study of cerebral localization. Arch. Neurol. Psychiat. Chicago. 1940;43:46–58. [Google Scholar]
- Brodal A. 2nd edn. Oxford University Press; Oxford: 1969. Neurological anatomy in relation to clinical medicine. [Google Scholar]
- Brown J. A.A. Knopf; New York: 2002. Charles Darwin: the power of place. [Google Scholar]
- Bullock T.H. Neuron doctrine and electrophysiology. Science. 1959;129:997–1002. doi: 10.1126/science.129.3355.997. [DOI] [PubMed] [Google Scholar]
- Bullock T.H, Horridge G.A. Structure and function of nervous systems in invertebrates. vol. 1. Freeman; San Francisco, CA: 1965. [Google Scholar]
- Cajal S.R.Y. Un sencillo metodo de coloracion selectiv del reticulo protoplasmico y sus effectos en los diversos organos nerviosos. Trab. Lab. Invest. Biol. Univ. Madrid. 1903;2:129–222. [Google Scholar]
- Cajal S.R.Y. Hisrologie du système nerveaux de l'homme et des vértébris. vol. 1. Maloine; Paris: 1911. [Google Scholar]
- Cajal S.R.Y. Consejo Superio De Investigaciones Cientificas; Madrid: 1954. Neuron theory or reticular theory. Translated by M. U. Purkiss and C. A. Fox. [Google Scholar]
- Cajal S.R.Y. Histology of the nervous system of man and vertebrates. vol. 1. Oxford University Press; Oxford: 1995. Translated from the 1911 French edition by N. Swanson and L. W. Swanson. [Google Scholar]
- Carman J, Cowan W.M, Powell T.P.S. Cortical connexions of the thalamic reticular nucleus. J. Anat. 1964;98:587–598. [PMC free article] [PubMed] [Google Scholar]
- Chang H.T. Fiber groups in primary optic pathways of cat. J. Neurophysiol. 1956;19:224–231. doi: 10.1152/jn.1956.19.3.224. [DOI] [PubMed] [Google Scholar]
- Chen C, Regehr W.G. Presynaptic modulation of the retinogeniculate synapse. J. Neurosci. 2003;23:3130–3135. doi: 10.1523/JNEUROSCI.23-08-03130.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke E, O'Malley C.D. Norman Publishing; San Francisco, CA: 1996. The human brain and spinal cord. [Google Scholar]
- Cook W.H, Walker J.H, Barr M.L. A cytological study of transneuronal atrophy in the lateral geniculate nucleus of the cat. J. Comp. Neurol. 1951;94:267–292. doi: 10.1002/cne.900940207. [DOI] [PubMed] [Google Scholar]
- Cowan W.M. Anterograde and retrograde transneuronal degeneration in the central and peripheral nervous system. In: Nauta W.J, Ebbesson S.O.E, editors. Contemporary research methods in neuroanatomy. Springer; New York: 1970. pp. 217–249. [Google Scholar]
- Cowan W.M, Kandel E.R. A brief history of synapses and synaptic transmission. In: Cowan W.M, Südhof T.C, Stevens C.F, Davies K, editors. Synapses. Johns Hopkins University Press; Baltimore, MD: 2002. pp. 1–87. [Google Scholar]
- Cowey A. Atrophy of retinal ganglion cells after removal of striate cortex in rhesus monkeys. Perception. 1974;3:257–260. doi: 10.1068/p030257. [DOI] [PubMed] [Google Scholar]
- Cox C.L, Sherman S.M. Control of dendritic outputs of inhibitory intemeurons in the lateral geniculate nucleus. Neuron. 2000;27:597–610. doi: 10.1016/s0896-6273(00)00069-6. [DOI] [PubMed] [Google Scholar]
- Darwin, C. R. 1859 The origin of species by means of natural selection, or the preservation of favoured races in the struggle for life Facsimile edition with an introduction by Ernst Mayr. Cambridge, MA: Harvard University Press, 1964, cited by Brown (2002).
- De Castro F. Modelación de un arco reflejo en el simpático, uniéndolo con la raiz aferente central del vago. Nuevas ideas sobre la sinapsis. Trab. Inst. Cajal Invest. Biol. Madrid. 1942;34:217–301. [Google Scholar]
- De Castro F. Die normale Histologie des peripheren vegetativen Nervensystems. Das Synapsen-Problem: Anatomisch-experimentelle Untersuchungen. Verhandl. deut. Gesellsch. Pathol. 1950;34:1–52. [Google Scholar]
- De Robertis E. Submicroscopic morphology of the synapse. Int. Rev. Cytol. 1959;8:61–96. doi: 10.1016/s0074-7696(08)62728-x. [DOI] [PubMed] [Google Scholar]
- De Robertis E, Bennett H.S. Some features of the submicroscopic morphology of synapses in frog and earthworm. J. Biophys. Biochem. Cytol. 1955;1:47–58. doi: 10.1083/jcb.1.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiters O.F.K. Vieweg; Braunschweig, Germany: 1865. Untersuchungen über Gehirn und Rückenmark des Menschen und der Säugethiere. pp. 1–318. [Google Scholar]
- Deiters E, Guillery R.W. Otto Deiters, 1834–1863. Exp. Neurol. 1963;9:iii–vi. [Google Scholar]
- Eccles J.C, Eccles R.M, Magni F. Central inhibitory action attributed to presynaptic depolarization produced by muscle afferent volleys. J. Physiol. 1961;159:147–166. doi: 10.1113/jphysiol.1961.sp006798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J.C, Schmidt R.F, Willis W.D. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J. Physiol. 1962;161:282–297. doi: 10.1113/jphysiol.1962.sp006886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger J, Gasser H.S. University of Pennsylvania Press; Philadelphia, PA: 1937. Electrical signs of nervous activity. [Google Scholar]
- Euler T, Denk W. Dendritic processing. Curr. Opin. Neurobiol. 2001;11:415–422. doi: 10.1016/s0959-4388(00)00228-2. [DOI] [PubMed] [Google Scholar]
- Euler T, Detwiler P.B, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Forel A. Einige hirnanatomische betrachtungen und Ergebnisse. Arch. Psychiat. Berlin. 1887;18:162–198. [Google Scholar]
- Forel A.H. Norton; New York: 1937. Out of my life work. [Google Scholar]
- Fukuda T, Kosaka T. Ultrastructural study of gap junctions between dendrites of parvalbumin-containing GABAergic neurons in various neocortical areas of the adult rat. Neuroscience. 2003;120:5–20. doi: 10.1016/s0306-4522(03)00328-2. [DOI] [PubMed] [Google Scholar]
- Furshpan E.J, Potter D.D. Mechanism of nerve impulse transmission at a crayfish synapse. Nature. 1957;180:342–343. doi: 10.1038/180342a0. [DOI] [PubMed] [Google Scholar]
- Geist F.D. Chromatolysis of efferent neurons. Arch. Neurol. Psychiat. Chicago. 1933;29:88–103. [Google Scholar]
- Gerlach J. Von dem Rückenmark. In: Stricker S, editor. Handbuch der Lehre der Gewebe des Menschen und der Thiere. W. Engelmann; Leipzig: 1871. pp. 665–693. [Google Scholar]
- Gerlach J. The spinal cord. In: Stricker S, editor. Manual of histology. Williams and Wood & Co; New York: 1872. pp. 327–366. [Google Scholar]
- Golgi C. La doctrine du neurone, théorie et faits. In: Hasselberg K.B, Pettersson S.O, Mörner K.A.H, Wirsén C.D, Santesson M.C.G, editors. Les prix Nobel 1906. Imprimerie Royale, Norstedt & Söner; Stockholm: 1908. pp. 1–31. [Google Scholar]
- Gray E.G. Axosomatic and axodendritic synapses of the cerebral cortex: an electron microscopic study. J. Anat. 1959;93:420–433. [PMC free article] [PubMed] [Google Scholar]
- Gray E.G. A morphological basis for presynaptic inhibition? Nature. 1962;193:82–83. doi: 10.1038/193082a0. [DOI] [PubMed] [Google Scholar]
- Gray E.G, Guillery R.W. The basis of silver staining of synapses of the mammalian spinal cord: a light and electron microscope study. J. Physiol. 1961;157:581–588. doi: 10.1113/jphysiol.1961.sp006744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E.G, Guillery R.W. An electron microscopical study of the ventral nerve cord of the leech. Z. Zellfforsch. 1963;60:826–849. doi: 10.1007/BF00339095. [DOI] [PubMed] [Google Scholar]
- Gray E.G, Guillery R.W. Synaptic morphology in the normal and degenerating nervous system. Int. Rev. Cytol. 1966;19:111–182. doi: 10.1016/s0074-7696(08)60566-5. [DOI] [PubMed] [Google Scholar]
- Guillery R.W. Quantitative studies of transneuronal atrophy in the dorsal lateral geniculate nucleus of cats and kittens. J. Comp. Neurol. 1973;149:423–438. doi: 10.1002/cne.901490403. [DOI] [PubMed] [Google Scholar]
- Guillery R.W. Histology of the nervous system (book review) Trends Neurosci. 1996;19:156–157. [Google Scholar]
- Guillery R.W. Early electron microscopic observations of synaptic structures in the cerebral cortex: a view of the contributions made by George Gray (1924–1999) Trends Neurosci. 2000;23:594–598. doi: 10.1016/s0166-2236(00)01635-0. [DOI] [PubMed] [Google Scholar]
- Guillery R.W, Harting J.K. The structure and connections of the thalamic reticular nucleus: advancing views over half a century. J. Comp. Neurol. 2004;472:360–371. doi: 10.1002/cne.10738. [DOI] [PubMed] [Google Scholar]
- Guillery R.W, LaMantia A.S, Robson J.A, Huang K. The influence of retinal afferents upon the development of layers in the dorsal lateral geniculate nucleus of mustelids. J. Neurosci. 1985;5:1370–1379. doi: 10.1523/JNEUROSCI.05-05-01370.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggar R.A, Barr M.L. Quantitative data of synaptic end-bulbs in the cat's spinal cord. J. Comp. Neurol. 1950;93:17–32. doi: 10.1002/cne.900930103. [DOI] [PubMed] [Google Scholar]
- Harrison R.G. Embryonic transplantation and development of the nervous system. Anat. Rec. 1908;2:385–410. [Google Scholar]
- Harrison R.G. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J. Exp. Zool. 1910;9:787–848. doi: 10.1002/jez.1401420103. [DOI] [PubMed] [Google Scholar]
- Harrison R.G. Neuroblast versus sheath cell in the development of peripheral nerves. J. Comp. Neurol. 1924;37:123–205. [Google Scholar]
- Held H. Beiträge zur structur der nervenzellen und ihrer Fortsätze. Zweite Abhandlung. Arch. Anat. Physiol. (Anat. Abt) 1897:204–294. [Google Scholar]
- Helmholtz, H. 1842 De fabrica systematis nervosa evertebratorum. Berlin. Inaugural dissertation, cited by Walter (1863) and by Shepherd (1991).
- His W. Ueber das Auftreten der weissen substanz und der Wurzelfasern am Rückenmark menschlicher Embryonen. Arch. Anat. Entwicklungs. 1883:163–170. [Google Scholar]
- His W. Zur Geschichte des menschlichen Rückenmarkes und der Nervenwurzeln. Abhandlungen Math-Phys. Classe Königl. säch Gesellsch. Wiss. Leipzig. 1886;13:377–513. [Google Scholar]
- His W. Die Neuroblasten und deren Enstehung im embryonalen Mark. Abhandlungen Math-Phys. Classe Königl. säch Gesellsch. Wiss. Leipzig. 1889;15:311–372. [Google Scholar]
- Jacobson M. Plenum Press; New York: 1993. Foundations of neuroscience. [Google Scholar]
- Kidd M. Electron microscopy of the inner plexiform layer of the retina in cat and pigeon. J. Anat. 1962;96:179–187. [PMC free article] [PubMed] [Google Scholar]
- Kölliker A. W. Engelmann; Leipzig: 1852. Handbuch der Gewebelehre des Menschen. [Google Scholar]
- Kölliker A. Human histology. vols. 1 and 2. Sydenham Society; London: 1853. [Translated by G. Busk and T. H. Huxley] [Google Scholar]
- Kölliker A. 4th edn. W. Engelmann; Leipzig: 1863. Handbuch der Gewebelehre des Menschen. [Google Scholar]
- Kölliker A. 5th edn. W. Engelmann; Leipzig: 1867. Handbuch der Gewebelehre des Menschen. [Google Scholar]
- Kölliker A. W. Englemann; Leipzig: 1899. Erinnerungen aus meinem Leben. [Google Scholar]
- Krause W. Über die Endigung der Muskelnerven. Z. Rat. Med. 1863;18:136–160. [Google Scholar]
- Kuffler S.W, Nicholls J.G. Sinauer; Sunderland, MA: 1976. From neuron to brain: a cellular approach to the function of the nervous system. [Google Scholar]
- Kühne W. W. Engelmann; Leipzig: 1862. Über die peripherischen Endorgane der motor-ischen Nerven. [Google Scholar]
- Liddell E.G.T. Clarendon; Oxford: 1960. The discovery of the reflexes. [Google Scholar]
- Matthews M.R, Powell T.P.S, Cowan W.M. Transneuronal cell degeneration in the lateral geniculate nucleus of the macaque monkey. J. Anat. 1960;94:145–169. [PMC free article] [PubMed] [Google Scholar]
- Maximow, A. A. & Bloom, W. 1930 A text-book of histology, by Alexander A. Maximow…completed and edited by William Bloom. Philadelphia, PA: W. B. Saunders Company.
- Miller R.F, Bloomfield S.A. Electroanatomy of a unique amacrine cell in the rabbit retina. Proc. Natl Acad. Sci. USA. 1983;80:3069–3073. doi: 10.1073/pnas.80.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissl F. Gustav Fisher; Jena: 1903. Die Neuronenlehre und ihre Anhänger; ein Beitrag zur Lösung des Problems der Beziehungen zwischen Nervenzelle, Faser und Grau. [Google Scholar]
- Nusbaum M.P, Blitz D.M, Swensen A.M, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Palay S.L, Palade G.E. The fine structure of neurons. J. Biophys. Biochem. Cytol. 1955;1:69–88. doi: 10.1083/jcb.1.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay S.L, Webster deF.H. 3rd edn. Oxford University Press; New York: 1991. The fine structure of the nervous system. [Google Scholar]
- Peters B.N, Masland R.H. Responses to light of starburst amacrine cells. J. Neurophysiol. 1996;75:469–480. doi: 10.1152/jn.1996.75.1.469. [DOI] [PubMed] [Google Scholar]
- Phillips C.G, Powell T.P.S, Shepherd G.M. Responses of mitral cells to stimulation of the lateral olfactory tract in the rabbit. J. Physiol. 1963;168:89–100. doi: 10.1113/jphysiol.1963.sp007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak S. Chicago University Press; 1957. The vertebrate visual system. [Google Scholar]
- Powell T.P.S, Erulkar S.D. Transneuronal cell degeneration in the auditory nuclei of the cat. J. Anat. 1962;96:249–268. [PMC free article] [PubMed] [Google Scholar]
- Rall W, Shepherd G.M, Reese T.S, Brightman M.W. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp. Neurol. 1966;14:44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Ralston H.J., III Evidence for presynaptic dendrites and a proposal for their mechanism of action. Nature. 1971;230:585–587. doi: 10.1038/230585a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen G.L. Selective silver impregnation of synaptic endings. In: Windle W.F, editor. New research techniques of neuroanatomy. C. C. Thomas; Springfield, IL: 1957. pp. 27–39. [Google Scholar]
- Rose J.E. The cortical connections of the reticular complex of the thalamus. Res. Publ. Assn Nerv. Ment. Dis. 1952;30:455–479. [PubMed] [Google Scholar]
- Ryugo D.K, Fekete D.M. Morphology of primary axosomatic endings in the anteroventral cochlear nucleus of the cat: a study of the endbulbs of Held. J. Comp. Neurol. 1982;210:239–257. doi: 10.1002/cne.902100304. [DOI] [PubMed] [Google Scholar]
- Schultze M. Über Muskelkörperchen und das, was man eine Zelle nennen habe. Arch. F. Anat. Physiol. Wissentsch. Medicin. 1861:1–27. [Google Scholar]
- Schultze M. Abhandlungen der naturforsch Gesellschaft zu Halle. VII. 1863. Untersuchungen über den Bau der Nasenschleimhaut, namentlich die structur und Endigung-sweise der Geruchsnerven bei dem Menschen und den Wir-belthieren. 1–100 plus plates I–V. [Google Scholar]
- Schwalbe G. Biography of M Schultze. Arch. mikroskop. Anat. (Bonn) 1874;X:I–XXIII. [Google Scholar]
- Schwann T. G. E. Reimer; Berlin: 1838. Mikroskopische Untersuchungen über die Über einstimmung in der Structur und dem Wachstum der Thiere and der Pflanzen. [Google Scholar]
- Shapiro L, Coleman D.R. The diversity of cadherins and the implications for a synaptic adhesive code in the CNS. Neuron. 1999;23:427–430. doi: 10.1016/s0896-6273(00)80796-5. [DOI] [PubMed] [Google Scholar]
- Shepherd G.M. Oxford University Press; New York: 1991. Foundations of the neuron doctrine. [Google Scholar]
- Shepherd G.M. Information processing in dendrites. In: Zigmond M.J, Bloom F.E, Landis S.C, Roberts J.L, Squire L.R, editors. Fundamental neuroscience. Academic Press; San Diego, CA: 1999. pp. 363–388. [Google Scholar]
- Sherman S.M, Guillery R.W. The thalamus. In: Shepherd G, editor. Synaptic organization of the brain. Oxford University Press; 2004. pp. 311–359. [Google Scholar]
- Sherrington C.S. The central nervous system. In: Foster M, editor. A text-book of physiology. 7th edn. 7th edn, pt III. Macmillan; London: 1897. pp. 928–929. [Google Scholar]
- Stöhr P., Jr . Die peripherische Nervenfaser. In: von Möllendorff W, editor. Handbuch der mikroskopiscchen Anatomie des Menschen. vol. 4. Springer; Berlin: 1928. pp. 143–201. [Google Scholar]
- Stöhr P., Jr . Mikroskopische Anatomie des vegetativen Nervensystems. In: Bargmann W, editor. Handbuch der mikroskopischen Anatomie des Menschen. vol. IV. Springer; Berlin: 1957. pp. 1–678. [Google Scholar]
- Swanson N, Swanson L.W. Preface. In: Cajal S.R.Y, editor. Histology of the nervous system of man and vertebrates. vol. 1. Oxford University Press; 1995. pp. xxi–xxxiv. [Google Scholar]
- Taylor W.R, Wässle H. Receptive field properties of starburst cholinergic amacrine cells in the rabbit retina. Eur. J. Neurosci. 1995;7:2308–2321. doi: 10.1111/j.1460-9568.1995.tb00652.x. [DOI] [PubMed] [Google Scholar]
- Van der Loos H. The history of the neuron. In: Hydén H, editor. The neuron. Elsevier; Amsterdam: 1967. pp. 1–47. [Google Scholar]
- Van Buren J.N. Trans-synaptic retrograde degeneration in the visual system of primates. Neurol. Neurosurg. Psychiat. 1963;26:402–409. doi: 10.1136/jnnp.26.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gehuchten A. La structure des centres neveus. La moelle épinière et le cervelet. La Cellule. 1891;7:79–122. [Google Scholar]
- Van Gehuchten A, Martin I. Le bulbe olfactiv ches quelques mammiféres. La Cellule. 1891;7:205–237. [Google Scholar]
- von Gudden B. Experimentaluntersuchungen über das peripherische und centrale Nervensystem. Arch. Psychiat. Nervenkr. 1870:693–723. [Google Scholar]
- von Gudden, B. 1889 Gesammelte un hinterlassene Abhandlungen (ed. H. Grashey). Wiesbaden: J. F. Bergmann.
- von Waldeyer-Hartz, H. W. G. 1891 Über einige neuere Forschungen im Gebiete der Anatomie des Centralnervensysystems. Deutsche Med. Wochenschr. 17, 1213–1218, 1244–1246, 1267–1269, 1287–1289, 1331–1332, 1352–1356.
- Waller A.V. Experiments on the section of the glosso-pharyngeal and hypoglossal nerves of the frog and observations of the alterations produced thereby in the structure of their primitive fibres. Phil. Trans. R. Soc. 1850;140:423–429. [PMC free article] [PubMed] [Google Scholar]
- Walter G. Über den feineren Bau des Bulbus olfactorius. Virch. Arch. Path. Anat. 1861;22:241–259. [Google Scholar]
- Walter G. A. Henry; Bonn: 1863. Mikroskopische Studien über das Central Nervensystem wirbeloser Thiere. [Google Scholar]
- Wykoff R.W, Young J.Z. The motor neuron surface. Proc. R. Soc. B. 1956;144:440–450. doi: 10.1098/rspb.1956.0002. [DOI] [PubMed] [Google Scholar]
- Young J.Z. Structure of nerve fibres and synapses in some invertebrates. Cold Spring Harb. Symp. Quant. Biol. 1936;4:1–6. [Google Scholar]
- Young J.Z. Fused neurons and synaptic contacts in the giant nerve fibres of cephalopods. Phil Trans. R. Soc. B. 1939;229:465–503. [Google Scholar]


