Abstract
CD4+ regulatory T (TR) cells represent a unique lineage of thymically generated lymphocytes capable of powerfully suppressing immune responses. A large body of experimental data has now confirmed the key role played by these cells in the maintenance of self-tolerance. Increasingly, the importance of these cells is also being recognized in a host of other clinically relevant areas such as transplantation, tumour immunity, allergy and microbial immunity. Additionally, it is also possible to generate TR cells by using a variety of ex vivo experimental approaches. We will focus here on harnessing the suppressive abilities of both these families of regulatory cells and how this should give us access to a potent cell-based immunotherapy appropriate for clinical application.
Keywords: adaptive regulatory cell, Foxp3, immunotherapy, natural regulatory cell, self-tolerance
1. Introduction
The random nature of T cell receptor (TCR) generation and its selection on self-major histocompatability complex (MHC) inevitably leads to the appearance of deleterious autoreactive clones. The vast majority of such cells are purged in the thymus during the processes of negative selection. This process is, however, imperfect, as there is abundant experimental evidence that harmful autoreactive T cells can be activated outside of the thymus by the administration of adjuvant and self-proteins (Zamvil et al. 1985; Salamero et al. 1987; Myers et al. 1997). This seems also to be the case in humans, wherein it is possible to clone-out autoreactive T cells from the periphery of both normal individuals and those with manifest autoimmunity (Burns et al. 1983; Allegretta et al. 1990; Ota et al. 1990). Recent studies using MHC Class II tetramers have indeed unequivocally demonstrated that peripheral T cells recognizing self-antigen exist in normal humans (Danke et al. 2004). The fact that perfectly healthy individuals can harbour auto-aggressive cells connotes the existence of tolerance mechanisms operational in the periphery. Textbooks classically divide such mechanisms into immunological ignorance, immunological privilege, anergy/deletion and dominant tolerance, though in reality the distinctions are probably not nearly so clear-cut. In recent years, the role played by dominant tolerance mediated through the action of regulatory T (TR) cells has come to pre-eminence. We herein focus on this latter mechanism of peripheral tolerance and investigate the clinical potential held by the successful therapeutic manipulation of TR cells.
2. Naturally occurring and adaptive regulatory cells
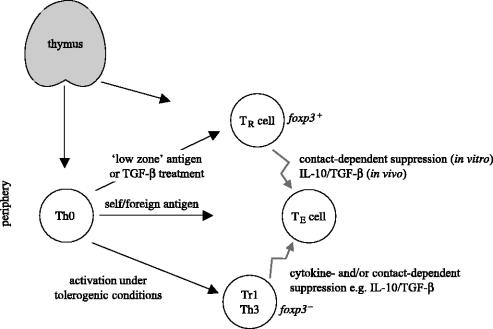
Broadly speaking, CD4+ T cells possessing the ability to suppress immune responses can be divided into two types: naturally occurring and adaptive regulatory cells (figure 1; Bluestone & Abbas 2003). Naturally occurring regulatory cells (defined hereafter as ‘TR cells’) are generated in the thymus under poorly understood conditions. Adaptive regulatory cells (also referred to variously as ‘Th3’ or ‘Tr1’) are generated outside of the thymus under specialized activation conditions. We will consider each of these populations in turn.
Figure 1.
Relationship of thymically and extrathymically generated regulatory cells. T cells emerge from the thymus as either regulatory cells (TR) or conventional naive T cells (Th0). The pathological responses of autoreactive effector cells (TE) can be suppressed by the action of both thymically (TR) and peripherally (Tr1/Th3) generated regulatory cells. The developmental relationship between the two classes of regulatory cells still needs to be fully clarified but some evidence suggests that Th0 are able to differentiate into TR cells in the periphery under special conditions.
(a) Naturally occurring regulatory T cells
Experimental evidence for TR cells has been suggested by animal models of autoimmune disease for many years (Sakaguchi 2000a). One of the earliest clues to the existence of TR cells (though it was not appreciated as such at the time) was found when neonatal thymectomy of certain strains of mice was shown to elicit autoimmune oophoritis (Nishizuka & Sakakura 1969). Thymectomy of adult rats and mice followed by fractionated X-irradiation could also produce autoimmune disease (Penhale et al. 1973; Sakaguchi et al. 1994). Crucially, it was shown that the induction of such autoimmunity could be prevented by the transfer of normal CD4+ splenocytes or CD4+CD8− thymocytes (Sakaguchi et al. 1994). Collectively, these data were strongly suggestive of the existence of a thymically produced suppressive T cell population, which was responsible for the establishment and maintenance of peripheral self-tolerance. These regulatory cells appeared to migrate out from the thymus at a relatively late stage compared to conventional/autoreactive T cells (more than 3 days after birth in mice). This lag-phase enabled the experimental induction of autoimmunity by the careful timing of neonatal thymectomy or an immunosuppressive regimen which preferentially targeted the suppressive T cell population.
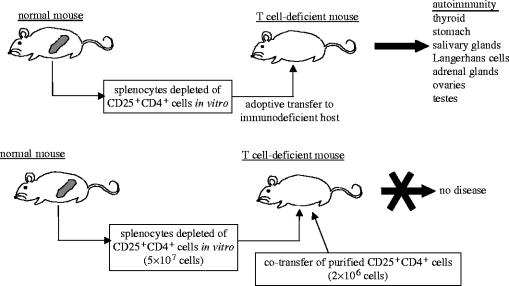
Attempts were subsequently made to characterize these suppressor or TR cells as they came to be known, by identifying the surface phenotype that harboured regulatory activity. Initially, CD5 was proposed as a marker for TR cells by demonstrating that otherwise normal lymphocytes depleted of CD5highCD4+ cells elicited autoimmunity when transferred into athymic nude mice (Sakaguchi et al. 1985). Unfractionated CD4+ cells (which contain CD5high expressers) prevented the induction of autoimmunity when co-transferred along with the CD5low cells, implying that TR cells were contained specifically within the CD5high compartment. Similarly, the CD45RB molecule appears to divide T cells into two distinct functional subsets: CD45RBhigh and CD45RBlow cells (Powrie et al. 1993). The CD45RBhigh population triggers inflammatory bowel disease (IBD) when transferred to lymphopenic mice, by eliciting an immunopathological reaction against normal gut-flora, whereas the CD45RBlow counterpart prevents such disease induction. To date, the most functionally useful surface marker for TR cells has proven to be their constitutive expression of the IL-2 receptor α-chain (IL-2R), CD25 (Sakaguchi et al. 1995). Approximately 5–10% of CD4+ peripheral T cells constitutively express CD25 in normal naive mice and healthy humans (Sakaguchi et al. 1995; Baecher-Allan et al. 2001). In mice, these CD25+ T cells are found in both the CD5high and CD45RBlow T cell fractions. Indeed, transfer of CD25-depleted CD4+ T cells to athymic mice elicits a variety of autoimmune diseases such as thyroiditis, gastritis and insulitis, whereas co-transfer with CD25+CD4+ cells inhibits such disease development (see figure 2).
Figure 2.
Demonstrating TR cell-mediated maintenance of self-tolerance. Induction of autoimmune diseases in T cell-deficient mice by transferring CD4+ splenocyte suspensions depleted of CD25+ cells to T cell-deficient mice. Co-transfer of purified CD25+CD4+ T cells prevents the induction of autoimmunity. Based on Sakaguchi et al. (1995).
Interestingly, CD25 does not appear to be merely a marker for TR cells, but rather reflects an absolute dependence on IL-2 for their peripheral maintenance and function. This is dramatically demonstrated by the loss of TR cells and consequent autoimmunity which occurs if IL-2 signalling is perturbed, e.g. in the case of knock-out mice (Almeida et al. 2002; Malek et al. 2002) or antibody blockade (Setoguchi et al. 2005). In vitro TR cells are anergic to conventional TCR stimuli but this is not observed physiologically in vivo where they show a seemingly high rate of turnover, which again probably reflects a dependency on IL-2 (Takahashi et al. 1998; Almeida et al. 2002; Setoguchi et al. in press).
More recently a number of other cell surface molecules have been shown to be associated with TR cells, among them: CTLA4 (CD152), αEβ7-integrin (CD103), glucocorticoid induced TNF family receptor (GITR) and neuropilin-1 (a receptor more usually involved in axon guidance) (Read et al. 2000; Takahashi et al. 2000; Lehmann et al. 2002; McHugh et al. 2002; Shimizu et al. 2002; Bruder et al. 2004). It should be noted, however, that no single uniquely expressed cell surface molecule has thus far been identified for TR cells and in many cases relatively specific molecules such as GITR or CD25 are upregulated to high levels on activated non-regulatory T cells as well. This problem is made especially acute in humans who naturally show large numbers of activated CD25+ T cells and, therefore, a definitive identification is usually only possible by sorting the highest CD25+ expressers (Baecher-Allan et al. 2004). Experimental evidence now also appears to demonstrate the existence of a small TR population lacking CD25 but showing high levels of GITR expression (Lehmann et al. 2002; Nishimura et al. 2004; M. Ono, personal communication). Where these CD25−CD4+ TR cells fit into overall TR cell taxonomy remains unclear, but it further highlights the difficulties involved in unambiguously pinpointing natural TR cells for clinical application.
The molecular understanding of TR cell biology took a leap forward following the delineation of the functional and developmental role played by the transcriptional repressor Foxp3. The importance of Foxp3 was first appreciated in studies using the Scurfy mouse. This mouse exhibits a fatal X-linked lymphoproliferative disease characterized by a very severe multi-organ immunopathology, allergy and IBD (Lyon et al. 1990). The Scurfy phenotype is very similar to the human immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome both in presentation and severity, and mutations in the Foxp3 (FOXP3 in humans) gene were found to underlie both conditions (Bennett et al. 2001; Brunkow et al. 2001; Wildin et al. 2002). The overt similarities seen between mutations in Foxp3 and experimental depletion of CD25+CD4+ cells led to its identification as a key transcription factor involved in the development and function of TR cells (Fontenot et al. 2003; Hori et al. 2003; Khattri et al. 2003). These mouse studies demonstrated Foxp3 mRNA and protein to be expressed specifically in CD25+CD4+ T cells and, in contrast to the cell surface markers used to date, was never observed in conventional T cells following activation or differentiation to Th1 or Th2. Critically, and probably most significantly from a potential therapeutic perspective, retroviral transduction of Foxp3 to both mouse (Hori et al. 2003) and human (Yagi et al. 2004) non-regulatory CD4+ cells bestowed them with a fully functional TR cell phenotype; i.e. they became anergic, expressed TR cell surface markers and could mediate suppression both in vitro and in vivo. Collectively, the data from mice show Foxp3 to be not only a seemingly unambiguous marker for naturally occurring TR cells, whose genetically programmed expression is sufficient to drive their development and function, but also demonstrates them to be a genuinely distinct lineage rather than simply another activation state of conventional T cells. Essentially, a similar pattern of FOXP3 expression is seen in human cells (Yagi et al. 2004) as in the mouse, although one report has suggested an important difference: both FOXP3 mRNA and protein appear to be induced in conventional CD4+ T cells following activation (Walker et al. 2003). However, it should be noted that a second study was unable to observe this effect in human cells and reported a largely stable expression of FOXP3 (Yagi et al. 2004). By way of a possible resolution, one can speculate that the appearance of FOXP3+ in the human cell cultures represents a selective expansion of a small contaminating FOXP3+ population. We will return to this confusion later on.
The mechanism of TR cell suppression remains rather confusing. Abundant in vitro evidence has demonstrated that TR cells require cell contact to mediate suppression, with no suggestion of any role played by soluble factors (Takahashi et al. 1998; Thornton & Shevach 1998; Sakaguchi 2004). Candidate molecules employed by TR cells to mediate suppression have been suggested, e.g. CTLA4 and cell surface TGF-β (Nakamura et al. 2001; Fallarino et al. 2003; Munn et al. 2004; Paust et al. 2004) and the CD4 homologue LAG-3 (Huang et al. 2004); however, none of these findings appear to be definitive. For instance, in the case of CTLA4, TR cells from CTLA4−/− mice appear to be just as effective as their wild-type counterparts at mediating suppression in vitro (Takahashi et al. 2000; Tang et al. 2004a). In vivo, however, there appears to be an indispensable role for soluble factors such as IL-10 and TGF-β in TR cell mediated control of immunopathology or allograft rejection (Powrie et al. 1996; Asseman et al. 1999; Hara et al. 2001; Singh et al. 2001; Maloy et al. 2003). The importance of both these cytokines to TR cell suppressive functions in vivo can be clearly demonstrated by using cells from IL-10−/− mice or blocking TGF-β and/or IL-10R with monoclonal antibodies (mAbs); in each case suppressive functions are abrogated.
(b) Adaptive regulatory cells
Abundant evidence has also demonstrated the extra-thymic generation of CD4+ T cells with suppressive properties. Several quite different experimental approaches can lead to the generation of such regulatory cells (see table 1). In the original experimental demonstrations of extra-thymically generated regulatory cells, the cells were termed Tr1 or Th3 cells (Chen et al. 1994; Roncarolo et al. 2001; Weiner 2001). Th3 cells were first cloned-out from the mesenteric lymph nodes of mice orally tolerized with myelin basic protein. The majority of such cells produce TGF-β, low/absent IL-10 and varying levels of Th2 cytokines and are able to suppress the induction of experimental autoimmune encephalitis (EAE). Th3-like cells have also been shown to be important in the control of a variety of other experimental autoimmune disease models (Weiner 2001). Some clinical data has also observed a perturbation of TGF-β/Th3 function in some cases of human autoimmunity and allergy (Andersson et al. 2002; Perez-Machado et al. 2003).
Table 1.
Experimental approaches to the extrathymic generation of (adaptive) regulatory T cells.
| approach | references |
|---|---|
| (1) oral tolerance induction with MBP | Chen et al. (1994) |
| (2) ex vivo stimulation in the presence of IL-10 | Groux et al. (1997) |
| (3) stimulation with immature, cytokine modified or specialized DC subsets | Jonuleit et al. (2000), Levings et al. (2004), Sato et al. (2003) and Wakkach et al. (2003) |
| (4) ex vivo treatment with the pharmacological immunosuppressants vitamin D3 and dexamethasone | Barrat et al. (2002) and Vieira et al. (2004) |
| (5) ex vivo triggering of specific molecules: CD2, CD46 and Notch-1 | Hoyne et al. (2001), Kemper et al. (2003), Vigouroux et al. (2003) and Wakkach et al. (2001) |
| (6) infection with particular bacteria or parasites | McGuirk et al. (2002) and Zaccone et al. (2003) |
| (7) ex vivo treatment with TGF-β | Chen et al. (2003) |
| (8) low-zone tolerance induction by systemic administration of minute quantities of antigenic peptide | Apostolou & Von Boehmer (2004) |
A large number of experimental approaches have been shown to induce regulatory cells outside of the thymus. They may take the form of ex vivo treatment (e.g. the addition of specific cytokines or pharmacological compounds in vitro) or in vivo induction (e.g. oral tolerance). The relationship among these various regulatory cell types is still largely unclear as is their relationship to thymically generated CD25+CD4+ TR cells, though the approaches (7) and (8) listed below have resulted in the production of Foxp3+ cells. The ‘tailor-made’ generation of such regulatory cells provides hope for a potent cell-based therapy.
Tr1 cells were first generated under conditions involving mouse T cell activation in the presence of the immunomodulating cytokine IL-10 (Groux et al. 1997). Such cells secrete a pattern of cytokines quite distinct from that of the more usual Th1–Th2 profile and are characterized by high levels of IL-10 and generally low levels of TGF-β and IL-5. Moreover, Tr1 cells are anergic, functionally suppressive in vitro and are able to prevent the development of experimentally induced Th1 autoimmune diseases such as colitis (Groux et al. 1997). A variety of other ex vivo experimental treatments have also led to the generation of Tr1-like cells, suppressive in vitro or in vivo, chief among these being the stimulation of normally non-suppressive conventional CD4+ T cells by immature or cytokine modified dendritic cells (DC) (Jonuleit et al. 2000; Sato et al. 2003; Verhasselt et al. 2004). A common principle which may underlie such tolerizing DC is their unique pattern of stimulatory ligands, e.g. high MHC class II coupled to low co-stimulation (CD80 and CD86). A recent paper, with potentially important therapeutic implications, apparently identified a DC subset responsible for the generation of regulatory cells in vivo (Wakkach et al. 2003). Such DCs possessed a plasmacytoid morphology and expressed the CD45RB molecule. Other molecular signals which seem to play a role in Tr1 generation (not necessarily in the presence of DC) appear to be those acting through CD2, Notch-1 and interestingly the complement receptor CD46 (Hoyne et al. 2001; Wakkach et al. 2001; Kemper et al. 2003; Vigouroux et al. 2003; Yvon et al. 2003). Finally, it is also possible to generate regulatory cells ex vivo by treating human and mouse T cells with the pharmacological immunosuppressants vitamin D3 and dexamethasone (Barrat et al. 2002; Vieira et al. 2004). However, these particular cells also appear to be somewhat distinct from the previously characterized Tr1/Th3 cells in that they appear to secrete solely IL-10 as well as retain a robust proliferative capacity; such characteristics would certainly be desirable for their use in any therapeutic capacity.
(c) What is the relationship between naturally occurring TR cells and adaptive regulatory cells such as Tr1 or Th3 cells?
A degree of uncertainty persists as to the precise developmental relationship between thymically generated TR cells and peripherally generated adaptive regulatory cells. Although both CD4+ regulatory cell types are certainly suppressive in one form or another, it is likely that TR cells and adaptive regulatory cells actually represent distinct cell types, with any similarities being a result of convergence rather than a closely shared lineage. A number of observations lead to this conclusion. Firstly, TR cells and adaptive regulatory cells appear to differ fundamentally in their in vitro suppressive mechanisms, with the former requiring cell contact and the latter utilizing secreted IL-10 and TGF-β either alone or in combination. Secondly, it is still unclear which of the peripherally generated regulatory cells express Foxp3, which is now thought to be critical for the generation of natural TR cells. To be fair, a comprehensive analysis of Foxp3 in all the various types of adaptive regulatory cells has not yet been made, although a couple of studies have suggested that Tr1 cells generated on immature DC (Levings et al. 2004) or by treatment with vitamin D3–dexamethasone (Vieira et al. 2004) are both Foxp3 negative. Such results would imply that they represent a differentiation state quite distinct from TR cells. One could conclude then that extra-thymically generated regulatory cells represent a heterogeneous assemblage whose primary physiological function would most likely be the homeostatic control of immune responses to exogenous antigens. Such adaptive regulatory cells would be generated concurrent/subsequent to a microbial infection with the aim of mitigating immunopathology. The corollary of this is that adaptive regulatory cells are an alternative lineage of conventional CD4+ helper T cells, akin to Th1 or Th2. In contrast, thymically generated CD25+CD4+ TR cells, by virtue of their Foxp3 expression, can be considered a de facto terminally differentiated lineage primarily, but not exclusively, committed to the maintenance of self-tolerance. Unfortunately this rather neat compartmentalization is challenged by recent data suggesting that TR cells, as defined by Foxp3 expression and suppressive function, can also be generated extra-thymically under particular conditions (Chen et al. 2003; Apostolou & Von Boehmer 2004). The addition of exogenous TGF-β to ex vivo cultures of conventional non-TR cells results in the generation of suppressive Foxp3+ TR-like cells (Chen et al. 2003; our own unpublished observations). Similarly, a ‘low-zone tolerance’ approach involving carefully controlled dosing of minute quantities of systemic antigen generates Foxp3+ regulatory cells in otherwise TR cell-deficient and thymectomized mice (Apostolou & Von Boehmer 2004). Therefore, if one accepts Foxp3/FOXP3 to be an unambiguous marker for TR cells then the ineluctable conclusion would be that the extrathymic generation of ‘true’ TR cells can, in actual fact, occur under very particular conditions. The finding in the human system that FOXP3+ cells can be generated in vitro following standard TCR-mediated stimulation of conventional CD4+ T cells (Walker et al. 2003) is similarly problematic to the formulation of a clear distinction between thymically and extrathymically generated regulatory cells. From a therapeutic perspective such epistemological questions may not even be important; what matters is the ability to controllably generate regulatory cells that are able to suppress harmful immunopathology.
3. Experimental evidence for regulatory cells as a potential immunotherapy
(a) Autoimmunity and allergy
As described in the previous sections there is an abundance of evidence that TR cells are engaged in the maintenance of self-tolerance and both TR and adaptive TR cells can be used to control immunopathology in various animal models of experimental autoimmunity (Roncarolo et al. 2001; Sakaguchi 2004). Importantly, a focus on TR cell therapy is validated by a small but growing body of clinical data showing that their dysfunction may be relevant to human autoimmune disease and allergy (Akdis et al. 2004; Baecher-Allan & Hafler 2004; Taylor et al. 2004). For example, in the case of multiple sclerosis patients, there seems to be normal numbers of peripheral CD25+ TR cells but their in vitro suppressive capacity is impaired relative to normal controls (Baecher-Allan & Hafler 2004; Viglietta et al. 2004). A similar impairment of TR cell function has also been reported in patients with rheumatoid arthritis (Ehrenstein et al. 2004).
Allergic diseases such as asthma, rhinitis and atopic dermatitis are thought to result chiefly from the differentiation and activation of Th2 cells in response to allergens. In vitro studies have shown that CD25+CD4+ cells are also able to suppress Th2 responses; therefore, moderating the reaction to allergens is potentially within the TR cell remit of control (Stassen et al. 2004). Suppression of allergic responses appears to occur primarily through the action of IL-10, most likely secreted by TR cells, whether natural or adaptive. IL-10 can suppress both the innate (mast cells, basophils and eosinophils) and adaptive (Th2 and B cell secretion of IgE) arms of the allergic response (Taylor et al. 2004). Several clinical studies have indeed found an association between diminished levels of patients' T cell IL-10 and both asthma and atopic dermatitis (Borish et al. 1996; Koning et al. 1997). More direct evidence for the role of regulatory cells in allergy is shown by a relative preponderance of allergen-specific Tr1-like cells in healthy non-atopic individuals but Th2 cells in allergic individuals (Akdis et al. 2004). Similarly, the in vitro ability of CD25+CD4+ cells to inhibit allergen-specific responses appears diminished in atopic individuals (Ling et al. 2004). Most interestingly, this relative impairment of suppression was even more pronounced in CD25+CD4+ cells isolated from hayfever sufferers during the grass pollen season than out of it. Whether the majority of human autoimmune and allergy cases implicate a component of TR cell dysfunction remains to be seen.
Although no clinical therapies involving TR cells have yet been attempted, some recent works using non-obese diabetic (NOD) mice (a model of autoimmune diabetes) have suggested that such an approach may well be successful (Tang et al. 2004b; Tarbell et al. 2004). The authors of these studies were able to demonstrate not only a large in vitro expansion of TR cells but, most excitingly, the ability to bring ongoing type 1 diabetes (T1D) under control by the transfer of TR cells combined with islet transplantation. However, in the case of one of the studies, in vivo suppression of diabetogenic cells was only possible by using pancreatic islet antigen-specific TR cells, presumably because only a tiny fraction of cells from a normal polyclonal TR population would actually home to the pancreas and be able to exert any meaningful suppression. A similar ‘specificity’ limitation was found for the suppression of T1D using Foxp3 transduced cells (Jaeckel et al. 2004). This has significant implications for any clinical immunotherapy, since it demands the identification of islet reactive human T or TR cell clones.
(b) Transplantation
The Holy Grail of organ transplantation would be to establish tolerance to the foreign graft as effectively as that to self-tissues, but without the need for generalized immunosuppression (Wood & Sakaguchi 2003). One way in which this ambitious goal may be realized is through the exploitation of TR cells. BALB/c nude mice transplanted with B6 skin and subsequently reconstituted with normal BALB/c T cells show robust graft rejection, which is accelerated by the removal of CD25+ cells from the reconstituting T cell population (Sakaguchi et al. 1995). This finding suggests that the small population (5–10%) of TR cells resident in the BALB/c T cell transfer is able to somewhat retard the normal allogeneic response and, therefore, increasing the numbers (or function) of transferred TR cells may engender a permanent state of allograft survival. This does indeed appear to be the case, since the transfer of highly purified CD25+CD4+ TR cells to nude recipients, either prior or simultaneous to reconstitution with naive T cells, significantly prolongs graft survival (Sakaguchi et al. 2001). Furthermore, permanent allograft survival may be established by the transfer of large doses of TR cells. The scarcity of TR cells means that obtaining large numbers (as would be required for clinical applications) is highly problematic. However, it is possible to expand, in vitro, purified CD25+ TR cells on donor-specific splenocytes (plus IL-2 to break TR cell in vitro anergy) prior to in vivo transfer (Sakaguchi et al. 2001; Trenado et al. 2003; Nishimura et al. 2004). As well as generating large numbers of TR cells for potential in vivo use, such expansion has the additional advantage of selectively expanding the donor-responsive cells and, hence, improving the efficiency of suppression. Potentially TR cells may also be expanded in vitro using mature DC yet still retain their suppressive functions, which may represent an appealing technique for therapy (Yamazaki et al. 2003; Tarbell et al. 2004).
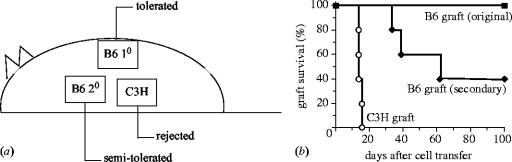
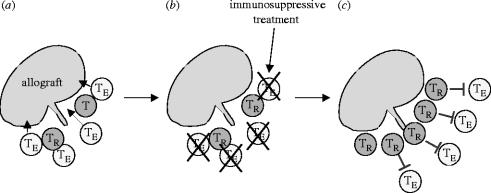
TR cells are also able to expand on an allograft in vivo, in much the same way as normal alloreactive TE (effector) cells; however, in the case of TR cells this expansion is non-destructive and beneficially suppressive (Nishimura et al. 2004). Experimental skin transplantation systems have clearly demonstrated that in vivo pre-expansion of regulatory cells on an allograft prior to exposure to alloreactive CD25− cells significantly improves graft survival, when compared to a simultaneous transfer of both CD25+ TR and CD25− cells (Nishimura et al. 2004). TR cells themselves home to, and can actually be found within, the graft proper (Graca et al. 2002; our own unpublished observations) and once this population is established the graft is very stably tolerated, whereas a fresh graft of identical haplotype shows a lower survival (figure 3; Nishimura et al. 2004). Rather than being a systemic phenomenon, TR cell-maintained tolerance instead appears to take the form of stable ‘islands’ of suppression. From the standpoint of immunotherapy, such properties are ideal, since it would engender a state of localized tolerance without the dangers of generalized immunosuppression. This process is summarized in figure 4.
Figure 3.
Population of a graft with allospecific TR cells results in highly stable tolerance and demonstrates dominant suppression by TR cells. BALB/c nude mice recipients received MHC Class II mismatched B6 allografts in conjunction with adoptively transferred CD25− and CD25+ cells (B6 10). Approximately 70% of such grafts are retained more than 100 days. Mice with original surviving grafts received fresh grafts of identical haplotype (B6 20) and third party grafts (C3H) and survival examined (a). Animals maintained a high localized tolerance to the original graft but only partially so to the secondary graft of identical haplotype, which was rejected albeit at a slower tempo than the third party graft (b) (kindly reproduced with permission from Oxford University Press).
Figure 4.
Blockade of effector T cells allowing the in vivo expansion of alloreactive/self-reactive TR cells: a putative immunotherapy for transplantation or autoimmunity. Following transplantation both harmful effector T cells (TE) and suppressive natural regulatory cells (TR) are recruited to the graft. In the normal physiological state, there are too few TR cells or their function is too weak to beneficially suppress the large precursor frequency of graft destructive TE cells (a). Administration of an immunotherapeutic agent (e.g. non-depleting anti-CD4) specifically blocks TE cells but allows the function and, more importantly, alloreactive expansion of TR cells (b). The large expanded population of TR cells then renders the graft operationally tolerant (c). Essentially similar principles can be applied in the case of autoimmunity.
A large number of studies have also been able to induce prolonged graft survival by the administration of mAbs to various T cell- and antigen presenting cell (APC)-associated molecules such as CD4, CD8, CD40L, CD80 and CD86 (Hara et al. 2001; Taylor et al. 2001; Graca et al. 2002; Nathan et al. 2002; Taylor et al. 2002; Cobbold et al. 2004). Many such studies employing mAbs implicate the role of regulatory cells in maintaining graft tolerance, and interestingly there is a suggestion that co-receptor blockade by non-depleting anti-CD4 mAbs in particular may act by inducing TR cells de novo (Cobbold et al. 2004; Karim et al. 2004; Waldmann & Cobbold 2004). Non-depleting anti-CD4 may yet lend itself as the TE cell-specific form of immunosuppression depicted in figure 4; however, the optimization of anti-CD4 for the clinic has still not been achieved.
Bone marrow transplantation for the treatment of haematological malignancies presents particular clinical problems. Such treatments must carefully balance the immunosuppression of potentially lethal graft versus host disease (GVHD) yet preserve a robust and beneficial lethal graft versus tumour (GVT) response. TR cells have also been shown to be potentially effective in such a setting. Initially, studies demonstrated that the transfer of donor-type CD25+CD4+ TR cells with bone marrow precursor cells could markedly protect from lethal GVHD and this effect was dependent in part on IL-10 production (Hoffmann et al. 2002). In subsequent related studies, it was shown that TR cells were able to not only protect from GVHD following bone marrow transplantation, but also retain strong enough immunity for an effective GVT response (Edinger et al. 2003; Trenado et al. 2003). TR cells thus appear to offer the flexibility of function and potency required for bone marrow transplantation, by balancing the conflicting requirements of immunosuppression and immunopermissiveness.
(c) Tumour immunity
It is now clear that many tumour-associated antigens recognized by autologous T cells are normal self-constituents and, thus, presumably within the remit of control by self-reactive TR cells. TR cells may even actively be recruited by tumours as a means of immune evasion (Hontsu et al. 2004). The desirable elimination of tumours by cytotoxic effector T cells may thus be impeded by the action of TR cells and evidence is accumulating that this in fact appears to be the case (Sakaguchi 2000b; Takahashi & Sakaguchi 2003). Experiments suggest that the simple elimination of TR cells from splenocyte preparations by treatment with anti-CD25 mAb results in productive responses to syngeneic tumours when transferred together to lymphopenic recipients (Onizuka et al. 1999; Shimizu et al. 1999; Sutmuller et al. 2001). Similarly beneficial effects are seen with the in vivo administration of antibodies. Data appear to suggest that CD25+CD4+ TR cells interfere with tumour-specific CD8+ responses and their removal, thus enhancing anti-tumour cytolysis (Chen et al. 2004). Another fruitful approach may simply be to interfere with TR cell suppression as opposed to depleting them. Signalling through GITR on TR or activated TE cells with either mAbs or specific ligand (GITRL) has been shown to either perturb TR cell suppression or render TE cells resistant to suppression (Shimizu et al. 2002; Stephens et al. 2004). A therapy embracing both depletion and perturbation of suppression may be the most effective. In parallel to the experimental data, clinical evidence is emerging that suppressive TR cells are present in increased numbers within tumour draining nodes or within the tumour microenvironment itself (Liyanage et al. 2002; Viguier et al. 2004). Perturbing TR cell abrogation within the tumour may thus represent a viable treatment strategy.
(d) Microbial immunity
Although the main function of TR cells is thought to be the maintenance of self-tolerance, it is clear that they can also control excessive immune responses to pathogens or inappropriate responses to commensal/mutualistic microbes. As described above, it is well known that severe combined immunodeficiency (SCID) mice transferred with CD25−CD45RBhiCD4+ cells develop IBD, whereas similarly treated mice lacking intestinal microbes under germ-free conditions show no such immunopathology (Singh et al. 2001). The sensitivity of the gut in general to perturbation of TR cell number or function intimates the critical involvement that these cells have in the control of immune responses to non-self but otherwise harmless symbiotes. The fundamental nature of this microbial interaction is further suggested by the expression on TR cells of the TLR4 pattern recognition receptor (Caramalho et al. 2003). It now seems that inflammatory signals, whether host (e.g. IL-6) or pathogen (e.g. lipopolysaccharide, LPS) derived either directly or indirectly, disable TR cell suppressive function (Pasare & Medzhitov 2003; Yamazaki et al. 2003; Fehervari & Sakaguchi 2004). This suggests a model whereby immune responses are freed-up from TR cell suppression only in the presence of microbial ‘danger signals’. In fact, it is becoming increasingly clear that naive T cell responses can only be initiated by DC matured with ‘danger signals’ such as LPS and that this control is maintained, at least in part, through the action of regulatory cells (Pasare & Medzhitov 2004; Sporri & Reis e Sousa 2005).
TR cells also have a role to play in infectious diseases by blunting overactive and damaging immune responses to pathogens. Evidence for this comes from the rapid and fatal pulmonary inflammation seen following CD25+CD4+ TR cell depletion during chronic infection with Pneumocystis carinii (Hori et al. 2002). In this model, immunopathology was mediated by CD25−CD4+ effector T cells and their damage could be prevented by the presence of CD25+CD4+ TR cells. It may well be that the necessity to attenuate harmful immunopathology was a key selective pressure leading to the evolution of the TR subset. Another study has examined the role of TR cells during chronic infection with the protozoan parasite Leishmania major (Belkaid et al. 2002). This model suggested that the long-term persistence of L. major is maintained through the action of TR cells and the removal of CD25+CD4+ cells resulted in a sterilizing response, i.e. the complete elimination of the parasite. Interestingly, eradication of the parasite in this way prevented immunity to subsequent re-infection and so demonstrated the importance of TR cells for the support of long-term immunological memory. The perseverance of immunological memory has been long-suspected to be mediated by the presence of a small ‘antigen depot’ and the action of TR cells may thus provide a hitherto unappreciated mechanism by which this can be achieved. This observation with L. major has obvious implications for a vaccine development strategy, i.e. using an adjuvant formulation that enables a reasonable level of TR cell function would encourage the generation of memory. These findings may also throw light on the puzzling nature of the so-called Koch's Phenomenon, i.e. a tuberculous individual is often highly resistant to a new infection yet unable to overcome the original infection. This could be seen as akin to the dual transplant tolerance effect depicted in figure 3, with the original infection being protected by an established TR population, while fresh infections are subject to the full force of the immune response (Sakaguchi 2003).
Multicellular parasites such as helminths and nematodes provide a particularly compelling area in which to study regulatory cells. Owing to their size such organisms are unable to pursue a classic pathogen evasive strategy of sequestering themselves in host cells, yet they are still able to reside in immunologically exposed environments of their host often for many years. To achieve such a feat they appear to possess multiple and potent mechanisms of immune subversion (Maizels et al. 2004). There is much evidence that helminths and nematodes trigger a switch from an immunopathological Th1 to a less harmful Th2 response; however, this cannot explain all the data and increasingly it appears that regulatory cell function is being co-opted by such parasites (Yazdanbakhsh et al. 2002). Basic evidence for the involvement of regulatory cells comes from the observations of elevated lymphocyte IL-10 and TGF-β production following nematode infection (Mahanty et al. 1996). A further example is seen in the infection of mice with the helminth Schistosoma mansoni. In this case, TR cell-derived IL-10 appears critical for the prevention of immunopathology following such infection and IL-10-producing CD25+ cells can be recovered from parasite-induced granulomas (Hesse et al. 2004). Several reports have also shown that infection with S. mansoni, along with other microbes, can prevent a host of experimental autoimmune diseases such as T1D, EAE and collagen induced arthritis (CIA) (Cooke et al. 2004). Interestingly, the T1D-protective effect of S. mansoni is also observed with adult parasite and egg-derived antigens (Cooke et al. 1999; Zaccone et al. 2003). Importantly, exposure to such antigens may result in the in vivo generation of regulatory cells, since parasite antigen-treated mice were unable to cause diabetes in NOD-SCID recipients following adoptive transfer. More direct evidence for TR cell involvement has been shown in a mouse model of experimental filariasis, where increased expression of Foxp3 and TR cell surface markers are observed in draining lymph nodes following nematode infection (Maizels et al. 2004). Increasingly, such parasite data are painting a more complex picture of immune evasion, i.e. it is not just a product of simple Th1–Th2 switching but rather the recruitment of TR cell functions. As with many such host–parasite interactions, it is often difficult to disentangle which party benefits from a particular immune response; i.e. is dampening down immunity beneficial to the host through the prevention of immunopathology or is the parasite ‘co-opting’ the TR cells to ensure its survival/transmission? One thing is clear though: the efficient ability of parasites to engage TR cell functions may be exploited for immunotherapeutic benefit. This has, in fact, already been achieved, with one study demonstrating encouraging results in the treatment of Crohn's disease and IBD following ingestion of Whipworm (Trichuris suis) ova (Summers et al. 2003, 2005). In this particular case, the nematode parasite is by-and-large non-pathological, but hopefully more advanced therapies should be able to utilize totally harmless parasite-derived antigens with similar efficacy.
4. A putative therapeutic scheme involving the use of regulatory cells
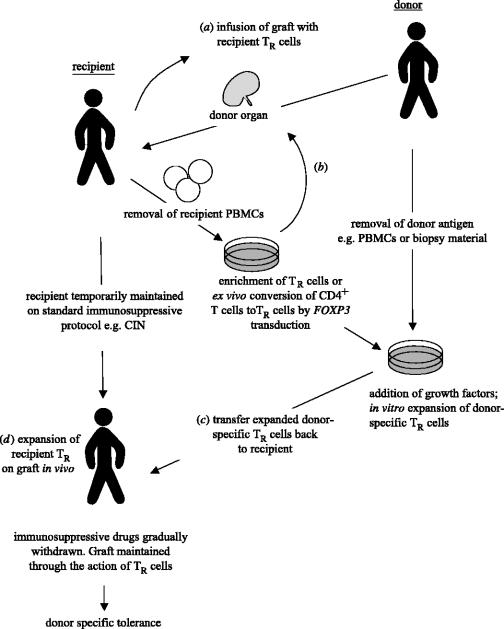
Naturally occurring TR cells exhibit several properties that make them potentially useful for therapeutic applications. Firstly, they have a diverse repertoire reactive not only with self- but also with non-self antigens. Secondly, they exhibit a functionally stable phenotype, which appears terminally differentiated for the control of immune responses. Finally, TR cells can be expanded both in vitro and in vivo yet retain a suppressive phenotype. Such properties could be applied to a number of clinically important areas. Figure 5 summarizes a potential therapeutic scheme involving TR cells as applied to the control of transplant rejection. Once a donor is identified the organ is harvested and a source of donor antigen taken, e.g. biopsy material or peripheral blood mononuclear cells (PBMCs), to be used for later expansion of donor-specific regulatory cells. Such donor-specific antigen can be banked if necessary. In the case of a non-solid organ transplant such as bone marrow, recipient TR cells could be pre-mixed with the graft prior to surgery (a), such an enforced localization may reduce the number of TR cells ultimately required for tolerance induction by encouraging in situ regulatory cell expansion. Following grafting, the recipient is put onto a standard immunosuppressive regimen. Two routes of therapy could then potentially be taken. In the first, the recipient undergoes leukapheresis followed by enrichment of TR cells or conversion of conventional T cells to regulatory cells ex vivo, either by gene transduction of FOXP3 or the generation of Tr1-like cells using a pharmacological approach (b). These newly generated regulatory cells are then sorted and expanded on the previously frozen donor-derived antigenic source. To support expansion, growth factors such as IL-2 may need to be added at this stage. The expanded and more importantly donor-specific, regulatory cells are then re-sorted and transferred back to the patient (c). Once an effective population of regulatory cells has been established in the recipient, pharmacological immunosuppression is gradually withdrawn and the graft is maintained long-term by dominant tolerance alone. If one views an organ targeted by autoimmunity as an allograft, then a broadly similar scheme could also be used to alleviate autoimmunity.
Figure 5.
A putative therapeutic scheme using regulatory cells applied to organ transplantation. Both TR and adaptive regulatory cells could potentially be used to establish allograft tolerance in the absence of standard immunosuppression. Intervention could be made at several stages. (a) In the case of bone marrow grafting, fresh recipient TR cells or (b) ex vivo-expanded TR cells could be suffused with the graft. (c) Donor-specific TR or adaptive regulatory cells could be generated and expanded ex vivo on a source of donor antigen (e.g. PBMCs or biopsy material). (d) Recipient TR cells could expand naturally on the graft in vivo following transplantation. Meanwhile host effector cell responses would be held in check by immunosuppressive treatment allowing expansion of TR cells to effective levels, before drug treatment is phased-out. CIN, Calcineurin inhibitors.
As outlined here, such a therapeutic approach seems rather straightforward, however applying it to a real-life situation would present formidable technical challenges. Basically, these could be summarized as problems of number and specificity, i.e. obtaining enough regulatory cells which are reactive with the allograft or, in the case of autoimmunity, self-antigen. These problems could be ameliorated to some extent by the use of living related donors which is becoming an increasingly attractive transplant modality (Prasad et al. 2002; Takada & Tanaka 2004). The luxury of foreknowing the donor haplotype and time of grafting would give ample opportunity for pre-expanding donor-reactive recipient TR cells. The most desirable scheme would involve the use of an immunosuppressive agent that selectively targeted effector cells, so staving-off rejection, yet allow the expansion of beneficial graft-reactive TR cells (figure 5d and as depicted in figure 4). Again, once TR cell mediated suppression had reached a critical threshold of efficacy, immunosuppression could be withdrawn. Immunosuppressive drugs routinely used in transplantation, such as cyclosporin and basiliximab (a depleting anti-IL2R mAb), are probably wholly inappropriate at engendering such a situation, since they would likely impair TR cell function (Sakaguchi & Sakaguchi 1989; Kovarik et al. 1999). However, numerous animal models have demonstrated the ability of anti-CD4 and to a lesser extent, anti-CD40L mAb to facilitate the in vivo expansion and/or function of TR cells or adaptive regulatory cells (Nathan et al. 2002; Cobbold et al. 2004; Waldmann & Cobbold 2004).
5. Major obstacles to the use of regulatory cells as an immunotherapy
As detailed earlier, a large body of data from murine studies have demonstrated the potent ability of both natural and adaptive regulatory cells to control immune responses under a wide range of clinically important conditions (table 2). Although, of course, this should be seen as very encouraging, it is now almost axiomatic that many efficacious therapies in the mouse are very difficult to translate into humans or at least if there is a beneficial effect then the results are more subdued. So in order to avoid regulatory cells from becoming a failed ‘next big thing’, a number of significant hurdles need to be overcome (see table 3).
Table 2.
Potential clinical applications of CD25+CD4+ TR cells.
| target condition | potential therapeutic approach | |
|---|---|---|
| increase of TR cell number or function | transplantation | ex vivo gene transduction of Foxp3 to conventional T cells |
| autoimmune disease | ex vivo generation of regulatory cells from conventional T cells using cytokines, pharmacological agents or modified DC | |
| allergy | expansion of host TR cells ex vivo using transplantation antigens or known autoantigens plus growth factors such as IL-2 | |
| reduction of TR cell number or perturbation of function in vivo | cancer | numerical reduction or transient elimination of TR cells and/or perturbation of suppressive function in vivo (anti-CD25, anti-CTLA4) |
| infectious disease | rendering effector cells resistant to suppression (signalling through GITR with anti-GITR or GITRL) |
Depending on the target condition, clinical benefit may be accrued by either enhancing or attenuating/depleting TR cell number or function. While none of these approaches has yet been approved for clinical trials, abundant experimental data have demonstrated the potential of a regulatory cell based therapy.
Table 3.
Major hurdles to the development of regulatory T cells as an immunotherapy.
| identification of unambiguous surface markers for the isolation of human TR cells |
| clear delineation of the molecular mechanism of TR cell mediated suppression |
| characterization of the signals both downstream and upstream of Foxp3 |
| identification of factors capable of viably expanding TR cells ex vivo |
| resolving the accuracy to which FOXP3 can be used as a marker in human cells |
A number of theoretical and experimental hurdles need to be overcome before the clinical potential of TR cells can be fully realized.
(a) Ex vivo generation of TR cells
The identification of FOXP3 as a critical gene for TR cell development and function was a major advance, since it opened the possibility of in vitro TR cell generation with the obvious clinical benefits that this implies. However, a sober analysis of the experimental details of the mouse work should temper our excitement somewhat. For instance, a simple linear extrapolation of cell numbers used to control immunopathology in the mouse colitis model or skin transplant rejection would require the generation of hundreds of millions of TR cells for a single dose of systemic therapy in a human patient. Relative to the spleen, human peripheral blood yields only small numbers of transduction targets and, furthermore, even if the requisite number of TR cells could be generated then sort times alone would be prohibitive. Clearly, such an approach is impractical and alternatives need to be found, whether theoretical or technical. High-volume magnetic bead-based sorting could be much more practical and would only require the generation of a cell-surface reporter for isolation of TR cell transductants. It is also likely that such exorbitant numbers of TR cells are not required if the antigen is known, such as in the case of transplantation antigens, where there is a high precursor frequency of responders and less-so if there is a defined autoantigen, e.g. myelin basic protein in multiple sclerosis or glutamic dehydrogenase in T1D. A TR cell therapy designed to control autoimmunity may therefore require the identification of real human autoantigens and the cloning of specific cells. A more targeted, rather than systemic, administration of the TR cells may also enable the use of significantly fewer cell numbers. Another technical hurdle concerns the vectors used to introduce Foxp3. All the Foxp3 gene transduction studies in the mouse have been performed using retroviral vectors, which would be an undesirable approach in humans, since it could risk both the transformation of the target cells as well as resulting in sensitization of the host immune system to viral antigens. Ideally, efficient non-viral transfection would need to be used in the clinical setting.
(b) Potential activation of autoimmunity by perturbation of TR cell function
Blocking TR cell function or their numerical reduction/elimination, e.g. in the case of cancer therapy, raises a different problem, namely the potential induction of autoimmunity. At one level it could be argued that a transient autoimmunity would be an acceptable price to pay to be rid of an otherwise intractable cancer, but clinically this is clearly far from ideal. Thankfully, though there may be no need for a systemic reduction of TR cell numbers to elicit effective cancer reactivity, rather only within the tumour mass itself. Similarly, an incomplete reduction of TR cell number/function may be enough to elicit potent tumour immunity yet preserve self-tolerance. Such risks need to be carefully assessed prior to abrogation of TR cell function in the clinical setting.
(c) In vivo expansion/enhancement of TR cells
A TR cell-based therapy, while being ‘intelligent’ in that it is highly targeted as well as flexible, would also be incredibly laborious and expensive to perform. Therefore, from a large-scale therapeutic perspective, the identification of compounds which could specifically target TR cells in vivo by either intensifying/stymieing their function or increasing/decreasing their numbers would be the most desirable approach. This would also make it amenable to the kind of high throughput technologies at which the pharmaceutical and biotech industry excel. Attaining such a goal would require a considerable extension of our knowledge of fundamental TR cell biology. Specifically it would be critical to understand the signalling events both upstream and downstream of FOXP3. How is the FOXP3 gene switched on? How does it exert its effects, is it through direct action or via an intermediary? Finally, the discrepancy between mouse and human as regards the induction of FOXP3 following conventional TCR triggering needs to be resolved (Walker et al. 2003; Yagi et al. 2004). If this were to be substantiated, it would alter our understanding of how regulation is generated and maintained in humans, as well as have important implications for the usefulness of FOXP3 as a TR cell specific marker and the validity of mice as a model of human TR cells.
(d) Identification and perturbation of TR cell suppressive mechanisms
The inhibition of TR cell-mediated suppression should also be a major therapeutic target. Ideally, this would lead on from the identification of a TR cell-associated molecule specifically involved in suppression, should one actually exist. As outlined in previous sections here, this is still a controversial area. Alternatively, rendering effector cells resistant to suppression could also be a feasible approach. Clearly, identifying target molecules on either effector or TR cells and unambiguously delineating the mechanism of suppression would be absolutely fundamental to the success of any such therapy.
(e) Identification of highly specific human TR cell surface marker(s)
Finally, there is a lacuna regarding a highly specific surface marker to identify TR cells. Although several candidate molecules such as CD25 or GITR have been identified, none of them are able to definitively discriminate between activated T cells and TR cells. This is an especially acute problem in humans who naturally have large numbers of activated T cells and where TR cell isolation can be performed definitively only by flow sorting the very highest CD25+ expressers. The ability to confidently identify and isolate TR cells from patients would be fundamental to their clinical exploitation; therefore, the discovery of appropriate cell surface markers should remain a priority.
6. Conclusions
Abundant evidence now strongly supports the existence of TR cells as key controllers of self-tolerance. It now seems that their suppressive role can be expanded to many areas of immunology, in fact potentially to any scenario, where the suppression and/or tuning of an immune response is required. A strategic manipulation of regulatory cells, whether naturally occurring or adaptive, to dampen or enhance their functions as appropriate promises great clinical benefit. Already manipulation of CD25+CD4+ TR cells in various animal models has provided encouraging results for the enhancement of tumour immunity, maintenance of allograft tolerance or the control of autoimmunity. Increasingly, studies are also demonstrating the significant roles CD25+CD4+ TR cells can play in human pathologies as varied as autoimmunity, HIV infection and allergy. Recent advances in our understanding of CD25+CD4+ TR cell development and important functional markers such as the association with Foxp3/FOXP3, has permitted the accurate isolation and manipulation of these cells in mice and importantly their human counterparts. Understanding the events both upstream and downstream of Foxp3/FOXP3 may enable us to ‘tailor-make’ large numbers of CD25+CD4+ TR cells for the specific suppression of immune responses or antagonizing them, where a boost of immunity is required. It is the strong belief of many researchers in this area that TR cells will, in a not-too-distant futurity, become a potent cell based therapy.
Acknowledgments
Z.F. would like to thank Kanji Nagahama for helpful discussion and Masahiro Ono and Naoshi Sugimoto for mention of their unpublished data. The authors would like to apologize for any omissions owing to constraints of space. The authors are funded by a Core Research for Evolutional Science and Technology (CREST) grant.
Footnotes
One contribution of 16 to a Theme Issue ‘Immunoregulation: harnessing T cell biology for therapeutic benefit’.
References
- Akdis M, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 2004;199:1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegretta M, Nicklas J.A, Sriram S, Albertini R.J. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990;247:718–721. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- Almeida A.R, Legrand N, Papiernik M, Freitas A.A. Homeostasis of peripheral CD4+T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J. Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- Andersson P.O, Olsson A, Wadenvik H. Reduced transforming growth factor-beta1 production by mononuclear cells from patients with active chronic idiopathic thrombocytopenic purpura. Br. J. Haematol. 2002;116:862–867. doi: 10.1046/j.0007-1048.2002.03345.x. [DOI] [PubMed] [Google Scholar]
- Apostolou I, Von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J. Exp. Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach M.W, Coffman R.L, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C, Hafler D.A. Suppressor T cells in human diseases. J. Exp. Med. 2004;200:273–276. doi: 10.1084/jem.20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baecher-Allan C, Brown J.A, Freeman G.J, Hafler D.A. CD4+CD25 high regulatory cells in human peripheral blood. J. Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Viglietta V, Hafler D.A. Human CD4+CD25+ regulatory T cells. Semin. Immunol. 2004;16:89–98. doi: 10.1016/j.smim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Barrat F.J, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Piccirillo C.A, Mendez S, Shevach E.M, Sacks D.L. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Bennett C.L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Bluestone J.A, Abbas A.K. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J. Allergy Clin. Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- Bruder D, Probst-Kepper M, Westendorf A.M, Geffers R, Beissert S, Loser K, von Boehmer H, Buer J, Hansen W. Neuropilin-1: a surface marker of regulatory T cells. Eur. J. Immunol. 2004;34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- Brunkow M.E, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Burns J, Rosenzweig A, Zweiman B, Lisak R.P. Isolation of myelin basic protein-reactive T-cell lines from normal human blood. Cell. Immunol. 1983;81:435–440. doi: 10.1016/0008-8749(83)90250-2. [DOI] [PubMed] [Google Scholar]
- Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J. Exp. Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Kuchroo V.K, Inobe J, Hafler D.A, Weiner H.L. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei K.J, Li L, Marinos N, McGrady G, Wahl S.M. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.L, Pittet M.J, Gorelik L, Flavell R.A, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc. Natl Acad. Sci. USA. 2004;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S.P, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J. Immunol. 2004;172:6003–6010. doi: 10.4049/jimmunol.172.10.6003. [DOI] [PubMed] [Google Scholar]
- Cooke A, Tonks P, Jones F.M, O'Shea H, Hutchings P, Fulford A.J, Dunne D.W. Infection with Schistosoma mansoni prevents insulin dependent diabetes mellitus in non-obese diabetic mice. Parasite Immunol. 1999;21:169–176. doi: 10.1046/j.1365-3024.1999.00213.x. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zaccone P, Raine T, Phillips J.M, Dunne D.W. Infection and autoimmunity: are we winning the war, only to lose the peace? Trends Parasitol. 2004;20:316–321. doi: 10.1016/j.pt.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Danke N.A, Koelle D.M, Yee C, Beheray S, Kwok W.W. Autoreactive T cells in healthy individuals. J. Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- Edinger M, Hoffmann P, Ermann J, Drago K, Fathman C.G, Strober S, Negrin R.S. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- Ehrenstein M.R, Evans J.G, Singh A, Moore S, Warnes G, Isenberg D.A, Mauri C. Compromised function of regulatory t cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- Fehervari Z, Sakaguchi S. Control of Foxp3+ CD25+CD4+ regulatory cell activation and function by dendritic cells. Int. Immunol. 2004;16:1769–1780. doi: 10.1093/intimm/dxh178. [DOI] [PubMed] [Google Scholar]
- Fontenot J.D, Gavin M.A, Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Graca L, Cobbold S.P, Waldmann H. Identification of regulatory T cells in tolerated allografts. J. Exp. Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries J.E, Roncarolo M.G. A CD4+T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Hara M, Kingsley C.I, Niimi M, Read S, Turvey S.E, Bushell A.R, Morris P.J, Powrie F, Wood K.J. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J. Immunol. 2001;166:3789–3796. doi: 10.4049/jimmunol.166.6.3789. [DOI] [PubMed] [Google Scholar]
- Hesse M, Piccirillo C.A, Belkaid Y, Prufer J, Mentink-Kane M, Leusink M, Cheever A.W, Shevach E.M, Wynn T.A. The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J. Immunol. 2004;172:3157–3166. doi: 10.4049/jimmunol.172.5.3157. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Ermann J, Edinger M, Fathman C.G, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hontsu S, Yoneyama H, Ueha S, Terashima Y, Kitabatake M, Nakano A, Ito T, Kimura H, Matsushima K. Visualization of naturally occurring Foxp3+ regulatory T cells in normal and tumor-bearing mice. Int. Immunopharmacol. 2004;4:1785–1793. doi: 10.1016/j.intimp.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Hori S, Carvalho T.L, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 2002;32:1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Hoyne G.F, Dallman M.J, Champion B.R, Lamb J.R. Notch signalling in the regulation of peripheral immunity. Immunol. Rev. 2001;182:215–227. doi: 10.1034/j.1600-065x.2001.1820118.x. [DOI] [PubMed] [Google Scholar]
- Huang C.T, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Jaeckel E, von Boehmer H, Manns M.P. Antigen-specific FoxP3-transduced T-cells can control established type 1 diabetes. Diabetes. 2004;54:306–310. doi: 10.2337/diabetes.54.2.306. [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Schuler G, Knop J, Enk A.H. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J. Exp. Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M, Kingsley C.I, Bushell A.R, Sawitzki B.S, Wood K.J. Alloantigen-induced CD25+CD4+ regulatory T cells can develop in vivo from CD25-CD4+ precursors in a thymus-independent process. J. Immunol. 2004;172:923–928. doi: 10.4049/jimmunol.172.2.923. [DOI] [PubMed] [Google Scholar]
- Kemper C, Chan A.C, Green J.M, Brett K.A, Murphy K.M, Atkinson J.P. Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- Khattri R, Cox T, Yasayko S.A, Ramsdell F. An essential role for Scurfin in CD4+CD25+T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Koning H, Neijens H.J, Baert M.R, Oranje A.P, Savelkoul H.F. T cells subsets and cytokines in allergic and non-allergic children. II. Analysis and IL-5 and IL-10 mRNA expression and protein production. Cytokine. 1997;9:427–436. doi: 10.1006/cyto.1996.0185. [DOI] [PubMed] [Google Scholar]
- Kovarik J.M, Moore R, Wolf P, Abendroth D, Landsberg D, Soulillou J.P, Gerbeau C, Schmidt A.G. Screening for basiliximab exposure–response relationships in renal allotransplantation. Clin. Transplant. 1999;13:32–38. doi: 10.1034/j.1399-0012.1999.t01-2-130105.x. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25-regulatory T cells. Proc. Natl Acad. Sci. USA. 2002;99:13 031–13 036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings M.K, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo M.G. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Treg cells. Blood. 2004;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- Ling E.M, Smith T, Nguyen X.D, Pridgeon C, Dallman M, Arbery J, Carr V.A, Robinson D.S. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- Liyanage U.K, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- Lyon M.F, Peters J, Glenister P.H, Ball S, Wright E. The scurfy mouse mutant has previously unrecognized hematological abnormalities and resembles Wiskott–Aldrich syndrome. Proc. Natl Acad. Sci. USA. 1990;87:2433–2437. doi: 10.1073/pnas.87.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty S, Mollis S.N, Ravichandran M, Abrams J.S, Kumaraswami V, Jayaraman K, Ottesen E.A, Nutman T.B. High levels of spontaneous and parasite antigen-driven interleukin-10 production are associated with antigen-specific hyporesponsiveness in human lymphatic filariasis. J. Infect. Dis. 1996;173:769–773. doi: 10.1093/infdis/173.3.769. [DOI] [PubMed] [Google Scholar]
- Maizels R.M, Balic A, Gomez-Escobar N, Nair M, Taylor M.D, Allen J.E. Helminth parasites—masters of regulation. Immunol. Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Malek T.R, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- Maloy K.J, Salaun L, Cahill R, Dougan G, Saunders N.J, Powrie F. CD4+CD25+T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuirk P, McCann C, Mills K.H. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J. Exp. Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh R.S, Whitters M.J, Piccirillo C.A, Young D.A, Shevach E.M, Collins M, Byrne M.C. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Munn D.H, Sharma M.D, Mellor A.L. Ligation of B7-1/B7-2 by human CD4(+) T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- Myers L.K, Rosloniec E.F, Cremer M.A, Kang A.H. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61:1861–1878. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J. Exp. Med. 2001;194:629–644. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan M.J, Yin D, Eichwald E.J, Bishop D.K. The immunobiology of inductive anti-CD40L therapy in transplantation: allograft acceptance is not dependent upon the deletion of graft-reactive T cells. Am. J. Transplant. 2002;2:323–332. doi: 10.1034/j.1600-6143.2002.20406.x. [DOI] [PubMed] [Google Scholar]
- Nishimura E, Sakihama T, Setoguchi R, Tanaka K, Sakaguchi S. Induction of antigen-specific immunologic tolerance by in vivo and in vitro antigen-specific expansion of naturally arising Foxp3+CD25+CD4+ regulatory T cells. Int. Immunol. 2004;16:1189–1201. doi: 10.1093/intimm/dxh122. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–755. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- Ota K, Matsui M, Milford E.L, Mackin G.A, Weiner H.L, Hafler D.A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 2004;6:1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc. Natl Acad. Sci. USA. 2004;101:10 398–10 403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhale W.J, Farmer A, McKenna R.P, Irvine W.J. Spontaneous thyroiditis in thymectomized and irradiated Wistar rats. Clin. Exp. Immunol. 1973;15:225–236. [PMC free article] [PubMed] [Google Scholar]
- Perez-Machado M.A, Ashwood P, Thomson M.A, Latcham F, Sim R, Walker-Smith J.A, Murch S.H. Reduced transforming growth factor-beta1-producing T cells in the duodenal mucosa of children with food allergy. Eur. J. Immunol. 2003;33:2307–2315. doi: 10.1002/eji.200323308. [DOI] [PubMed] [Google Scholar]
- Powrie F, Leach M.W, Mauze S, Caddle L.B, Coffman R.L. Phenotypically distinct subsets of CD4+T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Powrie F, Carlino J, Leach M.W, Mauze S, Coffman R.L. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+T cells. J. Exp. Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Russ G, Faull R. Live donor renal transplantation in Australia 1964–1999: an evolving practice. Intern. Med. J. 2002;32:569–574. doi: 10.1046/j.1445-5994.2002.00325.x. [DOI] [PubMed] [Google Scholar]
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo M.G, Bacchetta R, Bordignon C, Narula S, Levings M.K. Type 1 T regulatory cells. Immunol. Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Animal models of autoimmunity and their relevance to human diseases. Curr. Opin. Immunol. 2000a;12:684–690. doi: 10.1016/s0952-7915(00)00163-1. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000b;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Regulatory T cells: mediating compromises between host and parasite. Nat. Immunol. 2003;4:10–11. doi: 10.1038/ni0103-10. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N. Organ-specific autoimmune disease induced in mice by elimination of T cell subsets. V. Neonatal administration of cyclosporin A causes autoimmune disease. J. Immunol. 1989;142:471–480. [PubMed] [Google Scholar]
- Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J. Exp. Med. 1985;161:72–87. doi: 10.1084/jem.161.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N, Miyai K, Sakaguchi S. Ionizing radiation and autoimmunity. Induction of autoimmune disease in mice by high dose fractionated total lymphoid irradiation and its prevention by inoculating normal T cells. J. Immunol. 1994;152:2586–2595. [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Sakaguchi S, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- Salamero J, Remy J.J, Michel-Bechet M, Charreire J. Experimental autoimmune thyroiditis induced by a 5-10-kDa tryptic fragment from porcine thyroglobulin. Eur. J. Immunol. 1987;17:843–848. doi: 10.1002/eji.1830170617. [DOI] [PubMed] [Google Scholar]
- Sato K, Yamashita N, Baba M, Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood. 2003;101:3581–3589. doi: 10.1182/blood-2002-09-2712. [DOI] [PubMed] [Google Scholar]
- Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J. Exp. Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- Singh B, Read S, Asseman C, Malmstrom V, Mottet C, Stephens L.A, Stepankova R, Tlaskalova H, Powrie F. Control of intestinal inflammation by regulatory T cells. Immunol. Rev. 2001;182:190–200. doi: 10.1034/j.1600-065x.2001.1820115.x. [DOI] [PubMed] [Google Scholar]
- Sporri R, Reis e Sousa C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+T cell populations lacking helper function. Nat. Immunol. 2005;6:163–170. doi: 10.1038/ni1162. [DOI] [PubMed] [Google Scholar]
- Stassen M, Jonuleit H, Muller C, Klein M, Richter C, Bopp T, Schmitt S, Schmitt E. Differential regulatory capacity of CD25+T regulatory cells and preactivated CD25+T regulatory cells on development, functional activation, and proliferation of Th2 cells. J. Immunol. 2004;173:267–274. doi: 10.4049/jimmunol.173.1.267. [DOI] [PubMed] [Google Scholar]
- Stephens G.L, McHugh R.S, Whitters M.J, Young D.A, Luxenberg D, Carreno B.M, Collins M, Shevach E.M. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+T cells. J. Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- Summers R.W, Elliott D.E, Qadir K, Urban J.F, Jr, Thompson R, Weinstock J.V. Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 2003;98:2034–2041. doi: 10.1111/j.1572-0241.2003.07660.x. [DOI] [PubMed] [Google Scholar]
- Summers R.W, Elliott D.E, Urban J.F, Jr, Thompson R, Weinstock J.V. Trichuris suis therapy in Crohn's disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutmuller R.P, van Duivenvoorde L.M, van Elsas A, Schumacher T.N, Wildenberg M.E, Allison J.P, Toes R.E, Offringa R, Melief C.J. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J. Exp. Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Tanaka K. Living related liver transplantation. Transplant. Proc. 2004;36:271S–273S. doi: 10.1016/j.transproceed.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Sakaguchi S. The role of regulatory T cells in controlling immunologic self-tolerance. Int. Rev. Cytol. 2003;225:1–32. doi: 10.1016/s0074-7696(05)25001-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak T.W, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Boden E.K, Henriksen K.J, Bour-Jordan H, Bi M, Bluestone J.A. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur. J. Immunol. 2004a;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 2004b;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell K.V, Yamazaki S, Olson K, Toy P, Steinman R.M. CD25+ CD4+T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.A, Noelle R.J, Blazar B.R. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.A, Lees C.J, Blazar B.R. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- Taylor A, Verhagen J, Akdis C.A, Akdis M. T regulatory cells in allergy and health: a question of allergen specificity and balance. Int. Arch. Allergy Immunol. 2004;135:73–82. doi: 10.1159/000080523. [DOI] [PubMed] [Google Scholar]
- Thornton A.M, Shevach E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon B.L, Cohen J.L. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J. Clin. Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhasselt V, Vosters O, Beuneu C, Nicaise C, Stordeur P, Goldman M. Induction of FOXP3-expressing regulatory CD4pos T cells by human mature autologous dendritic cells. Eur. J. Immunol. 2004;34:762–772. doi: 10.1002/eji.200324552. [DOI] [PubMed] [Google Scholar]
- Vieira P.L, et al. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol. 2004;172:5986–5993. doi: 10.4049/jimmunol.172.10.5986. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner H.L, Hafler D.A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigouroux S, Yvon E, Wagner H.J, Biagi E, Dotti G, Sili U, Lira C, Rooney C.M, Brenner M.K. Induction of antigen-specific regulatory T cells following overexpression of a Notch ligand by human B lymphocytes. J. Virol. 2003;77:10 872–10 880. doi: 10.1128/JVI.77.20.10872-10880.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viguier M, Lemaitre F, Verola O, Cho M.S, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- Wakkach A, Cottrez F, Groux H. Differentiation of regulatory T cells 1 is induced by CD2 costimulation. J. Immunol. 2001;167:3107–3113. doi: 10.4049/jimmunol.167.6.3107. [DOI] [PubMed] [Google Scholar]
- Wakkach A, Fournier N, Brun V, Breittmayer J.P, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- Waldmann H, Cobbold S. Exploiting tolerance processes in transplantation. Science. 2004;305:209–212. doi: 10.1126/science.1099538. [DOI] [PubMed] [Google Scholar]
- Walker M, Kasprowicz D.J, Gersuk V.H, Benard A, Van Landeghen M, Buckner J.H, Ziegler S.F. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25-T cells. J. Clin. Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H.L. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- Wildin R.S, Smyk-Pearson S, Filipovich A.H. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med. Genet. 2002;39:537–545. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood K.J, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- Yagi H, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int. Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman R.M. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh M, Kremsner P.G, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- Yvon E.S, Vigouroux S, Rousseau R.F, Biagi E, Amrolia P, Dotti G, Wagner H.J, Brenner M.K. Over expression of the Notch ligand, Jagged-1 induces alloantigen-specific human regulatory T cells. Blood. 2003;102:3815–3821. doi: 10.1182/blood-2002-12-3826. [DOI] [PubMed] [Google Scholar]
- Zaccone P, Fehervari Z, Jones F.M, Sidobre S, Kronenberg M, Dunne D.W, Cooke A. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol. 2003;33:1439–1449. doi: 10.1002/eji.200323910. [DOI] [PubMed] [Google Scholar]
- Zamvil S, Nelson P, Trotter J, Mitchell D, Knobler R, Fritz R, Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 1985;317:355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]