Abstract
The study of tolerance in the clinic can be divided into three areas: (i) focused evaluation of existing tolerant transplant recipients as to their mechanism of tolerance; (ii) prospective tolerance trials, such as combined bone marrow and kidney transplantation as well as T cell depletion followed by subsequent weaning of immunosuppression; and (iii) immunologic assays to assess the likelihood of rejection or tolerance. Frankly, a very small number of patients have been transplanted with the intention of removing all immunosuppressive therapy, but several clinical trials with this aim are currently in progress, largely sponsored by the Immune Tolerance Network, a joint venture between the National Institutes of Health and the Juvenile Diabetes Research Foundation. Similarly, a reliable assay to assess tolerance has not yet been developed but a variety of approaches towards assessing rejection, and in some cases tolerance, are being developed. It would be accurate to state that many of the experimental and preclinical approaches to the induction of tolerance have resulted in better immunosuppression for human transplantation, but reliable tolerance strategies in humans have not yet been achieved. Combined bone marrow and kidney transplantation may be considered as one exception to this, but such a strategy is not generally applicable to the vast majority of solid organ transplant recipients. This review will summarize efforts to date, particularly focusing on kidney transplantation.
Keywords: tolerance, depletion, co-stimulation blockade, chimerism, monitoring
1. Introduction
While immunological tolerance has been achieved via multiple strategies in rodent models, fewer strategies have succeeded in outbred large animal models of solid organ transplantation and even fewer strategies have succeeded in human organ transplantation. The clinical successes that have been achieved in human transplantation and that can be described as tolerance will be reviewed and some related strategies that seek to minimize immunosuppressive drugs will be included. While scientific definitions of immunologic tolerance continue to be debated, a clinical definition of tolerance would be long-term allograft function in the absence of continuous immunosuppressive therapy yet with intact immunity to potential infection. Lessons learned from tolerant transplant patients will be reviewed followed by a review of the strategies that have been developed with the intention of inducing tolerance. Finally, in vitro tests that would allow us to recognize the presence of immunologic tolerance will be discussed.
2. Lessons learned from tolerant patients
The difficulty investigators had in inducing transplantation tolerance in humans had led many to question whether immunologic tolerance can actually exist in large animals or humans. Pertinent to this discussion is the original discovery of tolerance by Ray Owen who observed in dizygotic cattle twins the persistence of sibling-derived blood cells in a stable manner through adult life. This observation led Owen to hypothesize that in utero exposure and persistent expression of alloantigen permitted the development of allogeneic chimerism and tolerance that continued throughout the life of the animal without rejection and without adverse effects (Owen 1945). This description later led Billingham et al. (1953) to recapitulate this situation in a neonatal mouse model of allogeneic skin grafting. Despite the early success of this rodent model of transplantation tolerance, duplication of this result in humans has proven exceedingly challenging. The first success of clinical renal transplantation in humans depended instead on the identification of identical twin siblings for whom renal transplantation was possible from one healthy donor to the recipient without necessitating immunosuppression, since a state of genetic identity permitted a successful long-term outcome (Merrill et al. 1956). While this uncommon biological situation of identical twin siblings permitted the genesis of the field of renal transplantation, pharmacologic immunosuppression subsequently developed to permit the more common situation of allogeneic renal transplantation.
3. Tolerance in allogeneic transplantation
HLA-identical renal transplants have the best long-term outcomes owing to the lesser degree of disparity between donor and recipient immunologically, and even since the 1960s have had excellent outcomes with relatively minimal immunosuppression such as azathioprine and steroids. Nevertheless, it is well known that even HLA-identical sibling renal transplants can experience rejection leading to graft loss. An outstanding exception to this principal of rejection was demonstrated by a patient transplanted at the University of Wisconsin in 1968 under azathioprine and steroid immunosuppression. The patient received an HLA-identical transplant from her sister who was 2 years younger than her. Of her own volition, she discontinued azathioprine and, subsequently, prednisone about 5 years after the transplant (Uehling et al. 1976). Unlike the vast majority of patients who discontinue immunosuppression on their own, she has maintained excellent graft function for over 32 years off all immunosuppressive drugs. In an effort to understand this remarkable outcome, Burlingham did extensive studies of the patient and her family to establish that she is microchimeric with her sister, at least at the level of her T cells and peripheral blood dendritic cells, that she is mismatched at the minor histocompatibility antigen HA-1 and that her CD8-positive T lymphocytes suppress her immune responsiveness to the HA-1 disparity (Cai et al. 2004a). Furthermore, family genetic testing has determined that she would have been exposed to HA-1 disparity during her own neonatal life, at the time of her pregnancy at age 18 and subsequently at the time of her kidney transplant at age 35 and thereafter. Elegant studies using HLA-A2 tetramers, which have the HA-1 antigen in their peptide-binding groove, demonstrate that the patient has a population of low-avidity CD8 cells that regulate or suppress her own T cell response to HA-1. Furthermore, this regulation is mediated at least in part by TGF-β and by CTLA4 (figures 1 and 2) (Cai et al. 2004a). This patient beautifully demonstrates that allogeneic tolerance is possible in human renal transplantation, suggesting that immune regulation is an important mediator of such tolerance, but is somewhat a limited success in that tolerance is to a minor histocompatibility antigen rather than to a major histocompatibility antigen. While a minor histocompatibility antigen may be equally immunogenic as a major histocompatibility antigen, the MHC is well-described genetically and immunologically and far more familiar than the minor antigens, which are, for the most part, unidentified and poorly understood.
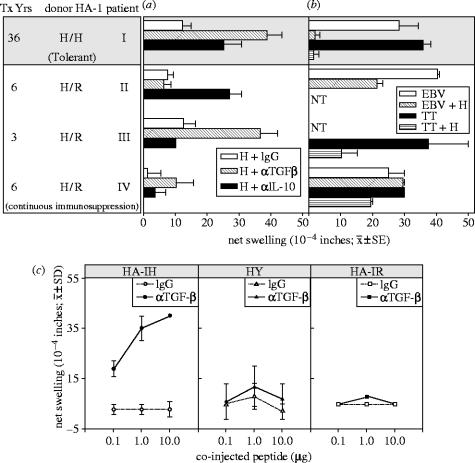
Figure 1.
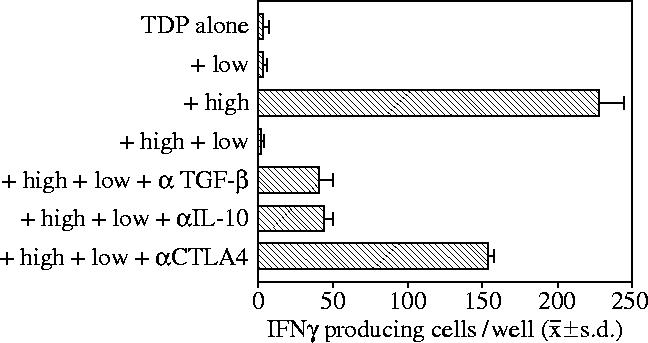
Suppression of IFNγ response of HA-1A2-high by HA-1A2-low tetramer-binding CD8+ T cells. Total dissolved phosphorus (TDP), flow-sorted HA-1 tetramer-low (low, 3×104 cells per well) and tetramer-high (high, 103 cells per well) CD8+ T cells were tested separately or in combination for response to HA-1H (n=3 wells). Control responses of sorted T cells alone to H peptide (no TDP added) were less than five spots per well (Cai et al. 2004a).
Figure 2.
Minor H antigen HA-1-mismatched renal transplant recipients had regulated DTH responses to HA-1H peptide. (a) PBMCs were tested for DTH responses to H peptide with control IgG, anti-TGFβ and anti-IL-10 antibodies. Donor HA-1 typing, years after transplant and patient status are indicated (grey background, tolerant; white background, continuous immunosuppression). Bars represent mean ±s.e. of 2–3 determinations. (b) PBMCs were injected with a recall antigen (TT or EBV) alone, or with recall antigen plus H peptide. NT, not tested. (c) Patient I PBMCs were tested for DTH responses in the presence or absence of anti-TGFβ1 neutralizing antibody, with varying doses of HA-1H (left), HY (centre) and HA-1R peptide (right; Cai et al. 2004a).
The observation that better outcomes are associated with greater degrees of MHC identity between donor and recipient supports the notion that T cell regulation to achieve tolerance is more likely to occur with greater donor–recipient matching. This indeed has also been supported by studies of Burlingham showing that trans vivo delayed-type hypersensitivity (DTH) measurement of regulation occurs with a higher frequency in HLA-identical sibling transplants (100%) compared to lesser degrees of mismatch (table 1) (Rodriguez et al. 2003). We do not yet understand when and why regulation, when it exists, can be lost leading to graft rejection. For this reason, immunologic tolerance to organ transplants can be viewed as a metastable situation which can be lost for a variety of reasons (Knechtle & Burlingham 2004). One reason for loss of tolerance might be heterologous immunity as described by Larsen and co-workers, meaning that crossreactivity of viral or other infectious antigens with alloantigen may stimulate the alloimmune response (Adams et al. 2003). Presumably, there is a dynamic equilibrium between memory effector T cells capable of damaging a graft and regulatory T cells which function to dampen or suppress such an effector response.
Table 1.
Summary of DTH results by transplant type.
| Tx type | N | regulator | non-regulator | sensitized |
|---|---|---|---|---|
| HLA-identicala | 6 | 6 (100%) | 0 (0%) | 0 (0%) |
| 1 haplo LRDa | 27 | 18 (67%) | 9 (33%) | 0 (0%) |
| cadavera | 29 | 9 (31%) | 15 (52%) | 5 (17%) |
| 2 DR match | 11 | 6 (55%) | 4 (36%) | 1 (9%) |
| 1 DR match | 14 | 2 (14%) | 10 (72%) | 2 (14%) |
| 0 DR match | 4 | 1 (25%) | 1 (25%) | 2 (50%) |
| total | 62 | 33 (53%) | 24 (39%) | 2 (8%) |
p=0.002; statistical comparison of the three groups using the Mantel–Haenzel chi-square test (Rodriguez et al. 2003).
4. Chimerism and tolerance
Patients who have experienced a successful bone marrow transplant and have multi-lineage chimerism with their bone marrow donor are known to accept a subsequent renal transplant in the absence of additional immunosuppression (Sayegh et al. 1991). While such patients are relatively rare, many have been described and this biological situation is a recapitulation of the rodent experiments by Billingham et al. (1953). However, the safety and ease of their subsequent renal transplant from an immunologic perspective is possible owing to the earlier success of their far riskier bone marrow transplant and associated immunosuppression. Because of the risk of graft-versus-host disease associated with allogeneic bone marrow transplantation, this strategy has generally not been attractive to adopt for patients who do not require a therapeutic bone marrow transplant.
Nevertheless, the strategy of bone marrow transplantation with simultaneous renal transplantation has been studied and developed in renal transplantation by Sachs et al. since the first description in the mouse model of this approach (Ildstad et al. 1986). Recently, six patients at Massachusetts General Hospital have been simultaneously transplanted with bone marrow and kidney for the indication of multiple myeloma with associated renal failure (Cosimi & Sachs 2004). These HLA-identical combined transplants have succeeded both technically and from the perspective of establishing tolerance although one graft failed owing to recurrence of myeloma. The experimental work in both rodents and inbred mini-pigs by this group demonstrated the necessity of stable multi-lineage chimerism in order to maintain tolerance. However, in non-human primates, transient chimerism led to prolonged allograft survival. Remarkably, despite the loss of chimerism in the six human patients done to date, tolerance has been maintained though outcomes are still short-term (less than 7 years). Whether tolerance in these patients is as robust as in animal models with stable chimerism or whether the human situation will be better described as metastable remains to be seen.
The same group has extended the method of combined kidney and bone marrow transplantation to haploidentical donor–recipient pairs who do not need therapeutic bone marrow transplantation. Three such renal transplants have been performed with two successes and one antibody-mediated rejection leading to graft loss. One of the successful haploidentical transplants experienced a C4d-positive humoral rejection with successful reversal by aggressive anti-rejection therapy. This patient demonstrates that tolerance may develop after an antibody-mediated rejection episode, although alloantibody did not persist in the patient.
Strober reported the successful application of pre-transplant total lymphoid irradiation (TLI) in three kidney transplant recipients who were subsequently weaned off all immunosuppression (Strober et al. 1989). One such patient, completely MHC disparate with the donor, had stable allograft function for at least 12 years (Strober et al. 2000). However, recent attempts to combine haematopoietic cell and kidney transplants conditioned with post-transplantation TLI and antithymocyte globulin (ATG) have met with limited success. Briefly, three out of four such patients developed transient microchimerism, lasting less than three months, and two were withdrawn from immunosuppression at 1 year based on donor-specific unresponsiveness by mixed lymphocyte reaction (MLR). Both experienced acute rejection five months later that was reversed with steroids and both had reinstitution of immunosuppression (Strober et al. 2004).
5. Tolerance-inducing cells of donor origin
Although the principle of bone marrow transplantation leading to subsequent tolerance to solid organ transplants demonstrates the value of multi-lineage stable chimerism of bone marrow-derived cells, the question remains as to which of these cellular elements is actually responsible for mediating tolerance and how it does so. It may be true, for instance, that only a particular subset of donor-derived blood cells is necessary or desirable to promote and maintain tolerance. Other groups have studied such strategies with variable degrees of success.
Fändrich and Geissler have developed a novel strategy to promote tolerance by culturing donor peripheral blood cells in the presence of monocyte colony stimulating factor and subsequently gamma-interferon (IFNγ; Fändrich et al. 2004; Exner et al. in preparation). The cells that develop over about 5 days can be subsequently administered to recipients of donor solid organs who are then weaned off immunosuppression successfully. Models illustrating this principle have been developed in rodents and pigs. The limitations of this observation to date are: (i) morphologic but not full phenotypic description of the cells and (ii) lack of consistency of results in large animals making an application to humans somewhat premature, although preliminary results have been obtained. How such cells work, the precise cell type and whether such cells can be potent enough to truly induce tolerance in humans are the questions that require further study. Nevertheless, such studies are intriguing in that they are potentially applicable to the clinic and would reduce or eliminate reliance on pharmacologic immunosuppression.
A related mechanistic explanation of tolerance has been developed by Thompson et al. who propose that an important cell regulating tolerance is the dendritic cell. Dendritic cells may have suppressor function passively by preventing antigen presentation or they may actively suppress effector cells by a variety of mechanisms (Thompson & Thomas 2002; Thompson et al. 2004). Related work in rodent models has been published by Cobbold and Waldmann in which dendritic cells treated with tolerance-enhancing agents, IL-10 or vitamin D3, are able to promote tolerance (Nolan et al. 2004).
6. Co-stimulation blockade
Initial experiments in a mouse cardiac transplant model by Larsen suggested that blockade of the B7-CD28 pathway by CTLA4-Ig in combination with blockade of the CD40–CD154 pathway by monoclonal antibody permitted long-term graft survival and indeed tolerance (Larsen et al. 1996). Extension of this approach by Kirk et al. in a rhesus monkey renal allograft model confirmed not only the usefulness of the combined blockade, but suggested that CD154 blockade alone provided a potent and effective means of preventing rejection (Kirk et al. 1997). However, long-term follow-up studies suggested that this was not a tolerance mechanism but rather a strategy that could effectively prevent acute rejection using ongoing therapy (Kirk et al. 1999). In other words, continued monthly therapy with CD154 antibody was necessary in order to prevent acute rejection from occurring in most instances. This approach was also extended into the baboon islet transplant model by Kenyon (Kenyon et al. 1999). Unfortunately, when CD154 blockade was attempted in the clinic, two unexpected results were: (i) a significant amount of acute rejection of renal allografts despite theoretically adequate CD154 blockade and (ii) thrombotic complications not anticipated from preclinical experience (Knechtle 2000). The reason for lesser efficacy in humans with CD154 and the thrombotic complications has still not been adequately explained. Using another anti-CD154 antibody based on the hypothesis that the unexpected properties might be related to the particular formulation of the antibody, Kanmaz et al. found similar outcomes in a non-human primate renal allograft model; namely, the antibody to CD154 effectively prevented rejection during therapy, but did not induce tolerance (Kanmaz et al. 2004). Thorough pathological analysis of these monkeys at the time of sacrifice suggested that two of seven monkeys might have had thrombotic complications. Therefore, it is possible, although not confirmed, that thrombosis may be an intrinsic complication associated with targeting CD154. An even greater issue may be the lesser degree of efficacy in humans compared to monkeys in preventing rejection.
Larsen and Pearson have combined donor bone marrow administration with co-stimulation blockade, targeting the CD28-B7 pathway as a strategy in both rodents and non-human primates to induce tolerance. While tolerogenic strategies have successfully been developed in the rodent (Adams et al. 2001), it has not been possible to translate these into true tolerance strategies in the non-human primate (Pearson et al. 2002). Analogous strategies have also been reported by Cosimi's group using combined bone marrow and CD154 blockade with encouraging long-term survival (Kawai et al. 2004). Co-stimulation blockade with LEA29Y, a high affinity modification of CTLA4-Ig, is being evaluated in humans as a means of replacing cyclosporine as maintenance immunosuppression (Vincenti et al. 2004). While not tolerogenic, this approach may perhaps be less toxic to renal tissues than long-term calcineurin-inhibitor (CNI) use.
7. T Cell depletion
Non-human primate studies in a renal allograft model using an anti-CD3 immunotoxin demonstrated the usefulness of profound T cell depletion to substantially promote tolerance in this outbred large animal model (Knechtle et al. 1997). This work has been previously summarized in this journal (Knechtle 2001). While anti-CD3 immunotoxin continues to be developed in the form of a fusion protein targeting CDε (Thompson et al. 2001), an analogous approach to T cell depletion has been applied in human transplantation using a highly lytic antibody directed at CD52. This monoclonal antibody called Campath-1H or alemtuzumab (Berlex) was first used by Calne et al. in human renal allografts (Calne et al. 1998). The approach to ‘near tolerance’ termed prope tolerance relied on low-dose cyclosporine monotherapy following Campath induction. Excellent results were achieved in 31 patients with a low incidence of infection and malignancy. Five year results have been reported by Watson et al. (2004) again with excellent outcomes, although this series has not been extended since the initial pilot study.
Building on the initial experience of the Cambridge group, a pilot trial was performed at the University of Wisconsin essentially duplicating the Cambridge series, but substituted sirolimus monotherapy for cyclosporine monotherapy for the maintenance phase following Campath-1H depletion (Knechtle et al. 2003). This series included 29 patients, was characterized by a relatively high incidence of early acute rejection seen in 10 patients and included humoral rejection in six of the 10 patients. It was particularly noticeable that rejection occurred frequently in patients younger than 45 years of age and rarely in those older than 45. All but one of the early acute rejection episodes were reversed and remarkably now at 3 year follow-up, there is 93% graft survival. Twelve of the original 29 patients remain on sirolimus monotherapy and three additional patients on monotherapy with steroid, Prograf or mycophenolate mofetil (one each). An addition of 11 patients are on two-drug therapy with only three patients requiring conventional three-drug therapy. Studies of antibody production in this cohort show that 10 of 24 patients developed alloantibody at some point post-transplant, although antibody disappeared from the circulation in many of these (Cai et al. 2004b). Compared to control patients treated with Simulect, calcineurin-inhibitors, mycophenolate and prednisone, patients initially treated with Campath-1H have prolonged CD3, CD4 and CD8 cell depletion as shown in figure 3. carboxyfluorescein diacetate succinimidyl ester (CFSE) MLR studies at 6 and 24 months also demonstrate that Campath-1H-treated patients have donor-specific hyporesponsiveness of CD4 and CD8 cells in most cases, whereas conventional immunosuppression controls seldom have donor-specific hyporesponsiveness. By measuring IFNγ in the supernatants of MLR daily for 5 days, it is also clear that Campath-treated patients usually have a blunted IFNγ response to donor with intact IFNγ production following third-party stimulation. Finally, the PBLs of these Campath-treated patients have approximately one-tenth the number of lymphocytes as control patients, but equivalent levels of Foxp3 expression by quantitative PCR (Bloom et al. submitted). Together these results suggest that T cell depletion with Campath-1H is able to frequently promote donor-specific hyporesponsiveness.
Figure 3.
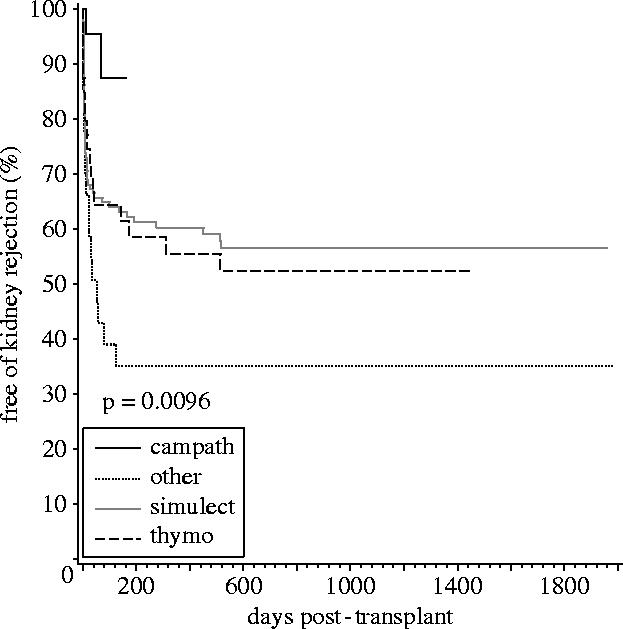
Incidence of rejection in patients with delayed graft function is compared between the four cohorts. Campath-1H-treated patients had significantly less rejection compared to the other three groups (Knechtle et al. 2004).
Campath-1H treatment has been broadly applied to renal transplantation at the University of Wisconsin with over 500 patients now treated, but maintenance therapy had consisted of a calcineurin-inhibitor, mycophenolate mofetil or Myfortic (Novartis) and low-dose prednisone (10 mg starting day 2 after surgery) (Knechtle et al. 2004). The greatest impact on outcomes to date has been a dramatic reduction in the incidence of acute rejection in patients experiencing delayed graft function. With Simulect or Thymoglobulin induction, the incidence of acute rejection was approximately 45%; under Campath, the incidence has dropped to 12% acute rejection. The overall incidence of acute rejection which includes both primary and non-primary renal transplants has also been reduced to about 10%.
An ongoing study at the University of Wisconsin to evaluate calcineurin-inhibitor withdrawal following Campath induction has enrolled 23 patients to date. Results are preliminary, but at this point, 10 patients have been weaned off of calcineurin-inhibitors with some off for over 1 year. There has been one biopsy-proven rejection in the withdrawal group and one presumed rejection in a patient on anticoagulation who could not be safely biopsied. Both rejections were easily reversed and the patients resumed calcineurin-inhibitor therapy.
Encouraging results using Campath-1H to deplete lymphocytes at the time of renal transplantation have also been reported by the Northwestern University group which has also adopted routine Campath-1H therapy for kidney and pancreas transplants. Kidney transplants are performed using a combination of Prograf and mycophenolate mofetil, while pancreas–kidney transplants depend on tacrolimus and sirolimus (Kaufman et al. 2003; Leventhal et al. 2003). The manner in which Campath is currently used clinically in transplantation is best described as a drug minimization strategy rather than a tolerance strategy. Encouraging results in liver (Marcos et al. 2004; Tzakis et al. 2004) and small intestinal (Kato et al. 2005) transplantation have been reported with Campath-1H at the University of Miami and the University of Pittsburgh. Campath-1H is also being used for lung transplantation at the University of Pittsburgh. In general, profound T cell depletion permits minimization of maintenance immunotherapy with infection and malignancy rates not significantly different from those observed using conventional immunosuppressive regimens. The rejection incidence, however, is minimized with Campath-1H. Mechanistic studies continue to be done in order to evaluate long-term T- and B-cell immunity following immune cell depletion by Campath-1H.
Finally, Campath-1H is currently under evaluation in two immune tolerance network (ITN)-sponsored studies to evaluate whether in kidney transplants three doses of Campath (30 mg each) will permit discontinuation of Prograf at two months and discontinuation of sirolimus at 1 year. This pilot study will seek to evaluate whether patients can indeed be rendered tolerant by Campath-1H immune cell depletion with gradual weaning of maintenance immunosuppression. A similar trial has been initiated for liver transplant patients who receive two doses of Campath (30 mg each) with subsequent slow weaning of Prograf (the only maintenance immunosuppressive agent in this study).
8. Need for monitoring
All of the efforts to identify tolerance strategies in solid organ transplantation are handicapped by our inability to recognize tolerance in our patients. Even recognition of acute and chronic rejection is dependent on time-honoured methodologies at this time, namely, measuring serum creatinine in the case of kidney transplants and evaluation of histologic changes on biopsy. There is an urgent need for better non-invasive monitoring tools that will allow early detection of acute and chronic rejection and potential interventions. The other obvious missing piece is a phenotypic or genotypic description of tolerance from an immunologic perspective. The trans vivo DTH assay, as defined by Burlingham, has the ability to identify donor-specific unresponsiveness with linked recognition (VanBuskirk et al. 2000). Generally, transplant patients studied with the trans vivo DTH who are clinically tolerant, that is with stable graft function off all drugs, have a suppressed DTH response to donor but intact third-party responsiveness. However, this situation has been described as metastable in that unresponsiveness may be lost for unclear reasons (Torrealba et al. 2004). Nevertheless, the trans vivo DTH assay may be of some use in monitoring patients considered to be tolerant. Other potential assays include the enzyme-linked immunosorbent spot (ELISPOT) assay to evaluate IFNγ production (Hricik et al. 2003), a cytokine kinetics assay described by Kwun et al. (submitted) and non-invasive urine assays evaluating chemokines and other proteins as described by Hu et al. (2004; figure 4). Also under development are PCR-based methodologies to measure RNA encoding mediators of inflammation. RNA of urine sediment suggests that granzyme B, perforin and CD3 are elevated in patients with acute rejection (Li et al. 2001). Other approaches to urine proteomics may yield new markers that indicate the immune status of a transplanted organ (Clarke et al. 2003; Schaub et al. 2004). Markers of tolerance are not known at this time and beg the question of whether tolerance will have a phenotype of its own or rather will be indicated by the absence of rejection markers. Whether tolerance will become recognized as a phenotypically defined state of the immune system, or as simply the absence of rejection, monitoring the immune system will certainly take on increasing importance. Whether patients are entirely drug-free or on minimal maintenance immunosuppression, assays that recognize and predict acute and chronic rejection before morphologic injury to the transplanted organ will help prolong graft and, hence, patient survival. This area will likely be the next step forward in pursuit of the goal of immunologic tolerance in organ transplantation.
Figure 4.
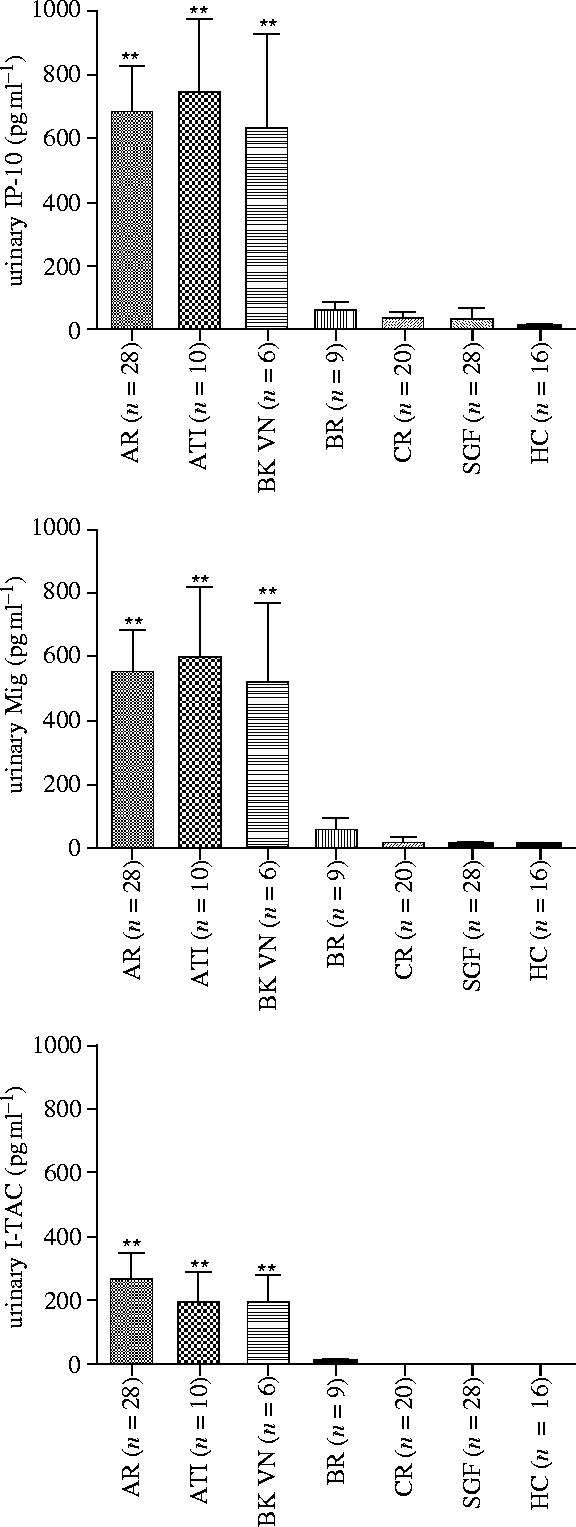
Urinary levels of CXCR3-binding chemokines IP-10, Mig and I-TAC in recipients of renal allografts. Urinary samples were obtained and the chemokine levels were determined by the triplex immunoassay. The levels of all three chemokines in the recipients with acute rejection (AR), acute tubular injury (ATI) or BK virus nephritis (BK VN) were significantly (**p<0.01) higher than the levels in the recipients with borderline rejection (BR), chronic rejection (CR), stable graft function (SGF) and also significantly (p<0.01) higher than levels in healthy controls (HC) (Hu et al. 2004).
Footnotes
One contribution of 16 to a Theme Issue ‘Immunoregulation: harnessing T cell biology for therapeutic benefit’.
References
- Adams A.B, et al. Costimulation blockade, busulfan, and bone marrow promote titratable macrochimerism, induce transplantation tolerance, and correct genetic hemoglobinopathies with minimal myelosuppression. J. Immunol. 2001;167:1103–1111. doi: 10.4049/jimmunol.167.2.1103. [DOI] [PubMed] [Google Scholar]
- Adams A.B, Pearson T.C, Larsen C.P. Heterologous immunity: an overlooked barrier to tolerance. Immunol. Rev. 2003;196:147–160. doi: 10.1046/j.1600-065x.2003.00082.x. doi:10.1046/j.1600-065X.2003.00082.x [DOI] [PubMed] [Google Scholar]
- Billingham R.E, Brent L, Medawar P.B. Activity acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bloom, D. D., Hu, H., Fechner, J. H. & Knechtle, S. J. Submitted. T lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation [DOI] [PubMed]
- Cai J, Lee J, Jankowska-Gan E, Derks R, Pool J, Mutis T, Goulmy E, Burlingham W.J. Minor H antigen HA-1-specific regulator and effector CD8+T cells, and HA-1 microchimerism, in allograft tolerance. J. Exp. Med. 2004a;199:1017–1023. doi: 10.1084/jem.20031012. doi:10.1084/jem.20031012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Terasaki P.I, Bloom D.D, Torrealba J.R, Friedl A, Sollinger H.W, Knechtle S.J. Correlation between human leukocyte antigen antibody production and serum creatinine in patients receiving sirolimus monotherapy after Campath-1H induction. Transplantation. 2004b;78:919–924. doi: 10.1097/01.tp.0000134398.86243.81. doi:10.1097/01.TP.0000134398.86243.81 [DOI] [PubMed] [Google Scholar]
- Calne R, Friend P, Moffatt S, Bradley A, Hale G, Firth J, Bradley J, Smith K, Waldmann H. Prope tolerance, perioperative campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. doi:10.1016/S0140-6736(05)77739-4 [DOI] [PubMed] [Google Scholar]
- Clarke W, Silverman B.C, Zhang Z, Chan D.W, Klein A.S, Molmenti E.P. Characterization of renal allograft rejection by urinary proteomic analysis. Ann. Surg. 2003;237:660–665. doi: 10.1097/01.SLA.0000064293.57770.42. doi:10.1097/00000658-200305000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosimi A.B, Sachs D.H. Mixed chimerism and transplantation tolerance. Transplantation. 2004;77:943–946. doi: 10.1097/01.tp.0000117779.23431.3f. doi:10.1097/01.TP.0000117779.23431.3F [DOI] [PubMed] [Google Scholar]
- Exner, B. G. et al Submitted. PD-L1-expressing monocyte-derived cells delete and regulate lymphocytes that mediate inflammatory bowel disease in mice. J. Exp. Med
- Fändrich F, Schulze M, Zehle G, Lange H, Ungefroren H. Stem cell-mediated tolerance inducing strategies in organ transplantation. Kidney Int. 2004;65:1548–1550. doi: 10.1111/j.1523-1755.2004.05408.x. doi:10.1111/j.1523-1755.2004.05408.x [DOI] [PubMed] [Google Scholar]
- Hricik D.E, Rodriguez V, Riley J, Bryan K, Tary-Lehmann M, Greenspan N, Dejelo C, Schulak J.A, Heeger P.S. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am. J. Transplant. 2003;3:878–884. doi: 10.1034/j.1600-6143.2003.00132.x. doi:10.1034/j.1600-6143.2003.00132.x [DOI] [PubMed] [Google Scholar]
- Hu H, Aizenstein B.D, Puchalski A, Burmania J.A, Hamawy M.M, Knechtle S.J. Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am. J. Transplant. 2004;4:432–437. doi: 10.1111/j.1600-6143.2004.00354.x. doi:10.1111/j.1600-6143.2004.00354.x [DOI] [PubMed] [Google Scholar]
- Ildstad S.T, Wren S.M, Bluestone J.A, Barbieri S.A, Stephany D, Sachs D.H. Effect of selective T cell depletion of host and/or donor bone marrow on lymphopoietic repopulation, tolerance, and graft-vs-host disease in mixed allogeneic chimeras (B10+B10⋅D2–-B10) J. Immunol. 1986;136:28–33. [PubMed] [Google Scholar]
- Kanmaz T, et al. Monotherapy with the novel human anti-CD154 monoclonal antibody ABI793 in rhesus monkey renal transplantation model. Transplantation. 2004;77:914–920. doi: 10.1097/01.tp.0000116392.72152.75. doi:10.1097/01.TP.0000116392.72152.75 [DOI] [PubMed] [Google Scholar]
- Kato T, et al. Intestinal transplantation in children: a summary of clinical outcomes and prognostic factors in 108 patients from a single center. J. Gastrointest. Surg. 2005;9:75–89. doi: 10.1016/j.gassur.2004.10.012. doi:10.1016/j.gassur.2004.10.012 [DOI] [PubMed] [Google Scholar]
- Kaufman D.B, Leventhal J.R, Gallon L.G, Parker M.A, Koffron A.J, Fryer J.P, Abecassis M.M, Stuart F.P. Pancreas transplantation in the prednisone-free era. Am. J. Transplant. 2003;3:322. (abstract.) [Google Scholar]
- Kawai T, et al. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am. J. Transplant. 2004;4:1391–1398. doi: 10.1111/j.1600-6143.2004.00523.x. doi:10.1111/j.1600-6143.2004.00523.x [DOI] [PubMed] [Google Scholar]
- Kenyon N.S, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc. Natl Acad. Sci. USA. 1999;96:8132–8137. doi: 10.1073/pnas.96.14.8132. doi:10.1073/pnas.96.14.8132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk A.D, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc. Natl Acad. Sci. USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. doi:10.1073/pnas.94.16.8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk A.D, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat. Med. 1999;5:686–693. doi: 10.1038/9536. doi:10.1038/9536 [DOI] [PubMed] [Google Scholar]
- Knechtle S.J. Knowledge about transplantation tolerance gained in primates. Curr. Opin. Immunol. 2000;12:552–556. doi: 10.1016/s0952-7915(00)00137-0. doi:10.1016/S0952-7915(00)00137-0 [DOI] [PubMed] [Google Scholar]
- Knechtle S.J. Treatment with immunotoxin. Phil. Trans. R. Soc. B. 2001;356:681–689. doi: 10.1098/rstb.2001.0839. doi:10.1098/rstb.2001.0839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knechtle S.J, Burlingham W.J. Metastable tolerance in nonhuman primates and humans. Transplantation. 2004;77:936–939. doi: 10.1097/01.tp.0000117777.70887.3c. doi:10.1097/01.TP.0000117777.70887.3C [DOI] [PubMed] [Google Scholar]
- Knechtle S.J, et al. FN18-CRM9 immunotoxin promotes tolerance in primate renal allografts. Transplantation. 1997;63:1–6. doi: 10.1097/00007890-199701150-00002. doi:10.1097/00007890-199701150-00002 [DOI] [PubMed] [Google Scholar]
- Knechtle S.J, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am. J. Transplant. 2003;3:722–730. doi: 10.1034/j.1600-6143.2003.00120.x. doi:10.1034/j.1600-6143.2003.00120.x [DOI] [PubMed] [Google Scholar]
- Knechtle S.J, Fernandez L.A, Pirsch J.D, Becker B.N, Chin L.T, Becker Y.T, Odorico J.S, D'Alessandro A.M, Sollinger H.W. Campath-1H in renal transplantation: the University of Wisconsin experience. Surgery. 2004;136:754–760. doi: 10.1016/j.surg.2004.06.015. doi:10.1016/j.surg.2004.06.015 [DOI] [PubMed] [Google Scholar]
- Kwun, J., Knechtle, S. J. & Hu, H. Submitted. Determination of the functional status of alloreactive T cells by interferon-gamma kinetics. Transplantation [DOI] [PubMed]
- Larsen C.P, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. doi:10.1038/381434a0 [DOI] [PubMed] [Google Scholar]
- Leventhal J.R, Gallon L, Kaufman D.B, Stuart J, Koffron A.J, Fryer J.P, Abecassis M, Stuart F.P. Alemtuzumab (Campath-1H) facilitates prednisone-free immunosuppression (IP) in kidney transplant recipients. Am. J. Transplant. 2003;3:310. (abstract.) [Google Scholar]
- Li B, et al. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N. Engl. J. Med. 2001;344:947–954. doi: 10.1056/NEJM200103293441301. doi:10.1056/NEJM200103293441301 [DOI] [PubMed] [Google Scholar]
- Marcos A, et al. Use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation: with particular reference to hepatitis C virus. Transplantation. 2004;78:966–971. doi: 10.1097/01.tp.0000142674.78268.01. doi:10.1097/01.TP.0000142674.78268.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill J.P, Murray J.E, Harrison J.H. Successful homotransplantation of the human kidney between identical twins. JAMA. 1956;160:277–282. doi: 10.1001/jama.1956.02960390027008. [DOI] [PubMed] [Google Scholar]
- Nolan K.F, et al. IL-10-conditioned dendritic cells, decommissioned for recruitment of adaptive immunity, elicit innate inflammatory gene products in response to danger signals. J. Immunol. 2004;172:2201–2209. doi: 10.4049/jimmunol.172.4.2201. [DOI] [PubMed] [Google Scholar]
- Owen R.D. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- Pearson T.C, et al. Anti-CD40 therapy extends renal allograft survival in rhesus macaques. Transplantation. 2002;74:933–940. doi: 10.1097/00007890-200210150-00006. doi:10.1097/00007890-200210150-00006 [DOI] [PubMed] [Google Scholar]
- Rodriguez D.S, Jankowska-Gan E, Haynes L.D, Leverson G, Munoz A, Heisey D, Sollinger H.W, Burlingham W.J. Immune regulation and graft survival in kidney transplant recipients are both enhanced by human leukocyte antigen matching. Am. J. Transplant. 2003;4:537–543. doi: 10.1111/j.1600-6143.2004.00385.x. doi:10.1111/j.1600-6143.2004.00385.x [DOI] [PubMed] [Google Scholar]
- Sayegh M.H, Fine N.A, Smith J.L, Rennke H.G, Milford E.L, Tilney N.L. Immunologic tolerance to renal allografts after bone marrow transplants from the same donors. Ann. Intern. Med. 1991;114:954–955. doi: 10.7326/0003-4819-114-11-954. [DOI] [PubMed] [Google Scholar]
- Schaub S, et al. Proteomic-based detection of urine proteins associated with acute renal allograft rejection. J. Am. Soc. Nephrol. 2004;15:219–227. doi: 10.1097/01.asn.0000101031.52826.be. doi:10.1097/01.ASN.0000101031.52826.BE [DOI] [PubMed] [Google Scholar]
- Strober S, et al. Acquired immune tolerance to cadaveric renal allografts. A study of three patients treated with total lymphoid irradiation. N. Engl. J. Med. 1989;321:28–33. doi: 10.1056/NEJM198907063210106. [DOI] [PubMed] [Google Scholar]
- Strober S, Benike C, Krishnaswamy S, Engleman E.G, Grumet F.C. Clinical transplantation tolerance twelve years after prospective withdrawal of immunosuppressive drugs: studies of chimerism and anti-donor reactivity. Transplantation. 2000;69:1549–1554. doi: 10.1097/00007890-200004270-00005. doi:10.1097/00007890-200004270-00005 [DOI] [PubMed] [Google Scholar]
- Strober S, Lowsky R.J, Shizuru J.A, Scandling J.D, Millan M.T. Approaches to transplantation tolerance in humans. Transplantation. 2004;77:932–936. doi: 10.1097/01.tp.0000117782.93598.6e. doi:10.1097/01.TP.0000117782.93598.6E [DOI] [PubMed] [Google Scholar]
- Thompson J, Stavrou S, Weetall M, Hexham J.M, Digan M.E, Wang Z, Woo J.H, Yu Y, Mathias A, Liu Y.Y, Ma S, Gordienko I, Lake P, Neville D.M., Jr. Improved binding of a bivalent single-chain immunotoxin results in increased efficacy for in vivo T-cell depletion. Protein Eng. 2001;14:1035–1041. doi: 10.1093/protein/14.12.1035. doi:10.1093/protein/14.12.1035 [DOI] [PubMed] [Google Scholar]
- Thompson A.G, O'Sullivan B.J, Beamish H, Thomas R. T cells signaled by NF-kappa B-dendritic cells are sensitized not anergic to subsequent activation. J. Immunol. 2004;173:1671–1680. doi: 10.4049/jimmunol.173.3.1671. [DOI] [PubMed] [Google Scholar]
- Thompson A.G, Thomas R. Induction of immune tolerance by dendritic cells: implications for preventative and therapeutic immunotherapy of autoimmune disease. Immunol. Cell Biol. 2002;80:509–519. doi: 10.1046/j.1440-1711.2002.01114.x. doi:10.1046/j.1440-1711.2002.01114.x [DOI] [PubMed] [Google Scholar]
- Torrealba J, et al. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGFβ1+CD4+T-regulatory cell infiltrates. J. Immunol. 2004;172:5753–5764. doi: 10.4049/jimmunol.172.9.5753. [DOI] [PubMed] [Google Scholar]
- Tzakis A.G, et al. Preliminary experience with alemtuzumab (Campath-1H) and low-dose tacrolimus immunosuppression in adult liver transplantation. Transplantation. 2004;77:1209–1214. doi: 10.1097/01.tp.0000116562.15920.43. doi:10.1097/01.TP.0000116562.15920.43 [DOI] [PubMed] [Google Scholar]
- Uehling D.T, Hussey J.L, Weinstein A.B, Wank R, Bach F.H. Cessation of immunosuppression after renal transplantation. Surgery. 1976;79:278–282. [PubMed] [Google Scholar]
- VanBuskirk A.M, Burlingham W.J, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, Pelletier R.P, Orosz C.G. Human allograft acceptance is associated with immune regulation. J. Clin. Invest. 2000;106:145–155. doi: 10.1172/JCI9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti F, Muehlbacher F, Nashan B, Larsen C, Atillasoy E, Natarajan K, Chatenoud L, LEA29Y Study Group Co-stimulation blockade with LEA29Y in a calcineurin inhibitor free maintenance regimen in renal transplant: 6-month efficacy and safety. Am. J. Transplant. 2004;4:442. (abstract.) [Google Scholar]
- Watson C.J.E, et al. Campath 1H (Alemtuzumab) in renal transplantation: 5-year comparative follow up. Transplantation. 2004;78:55–56. doi:10.1097/00007890-200407271-00156 (abstract.) [Google Scholar]