Abstract
The immune and nervous systems play distinct roles in maintaining physiological homeostasis. Recent data indicates that these systems influence one another and share many proteins and pathways that are essential for their normal function and development. Molecules originally shown to be critical for the development of proper immune responses have recently been found to function in the nervous system. Conversely, neuronal guidance cues can modulate immune functions. Although semaphorins were originally identified as axon guidance factors active during neuronal development, several recent studies have identified indispensable functions for these molecules in the immune system. This review provides an overview of the rapidly emerging functions of semaphorins and their receptors in the immune system.
Keywords: Sema4D, CD72, Sema4A, Tim-2
1. Introduction
The semaphorins are a large family of phylogenetically conserved proteins, some of which were originally identified as repulsive axon guidance cues active during neuronal development. More than 20 members have been identified in a variety of species from viruses to human. Semaphorin family members can be soluble or membrane-bound molecules, and they have been categorized into eight subclasses, based on sequence similarity and distinctive structural features (Semaphorin Nomenclature Committee 1999). Semaphorin subclasses I and II exist in invertebrate species, and Subclasses III–VII are expressed in vertebrates. In addition, some non-neurotrophic DNA viruses encode functional semaphorin molecules (figure 1).
Figure 1.
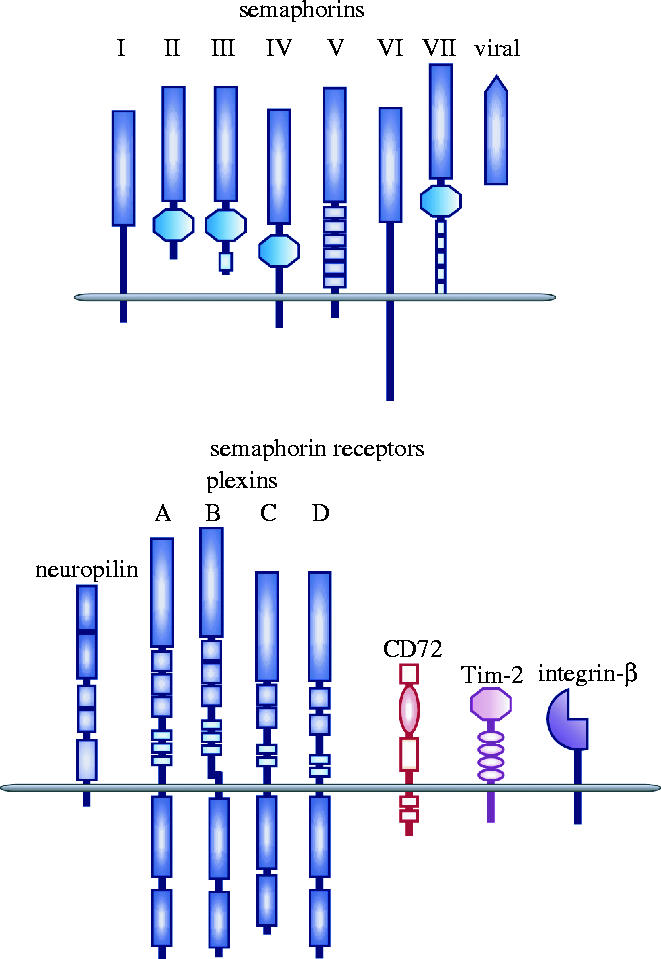
The semaphorin family and their receptors. The semaphorin family contains a large number of phylogenetically conserved proteins consisting of secreted and transmembrane members. Based on structural features, the members have been divided into eight classes, including a unique viral class. The members of the semaphorin family share a common sema domain. The plexin family also possesses a sema domain within their extracellular region. Neuropilins and plexins differ, however, in the structural motifs found in their extracellular domains. Within the immune system, class IV semaphorins, CD100/Sema4D and Sema4A, bind to CD72 and Tim-2, respectively. GPI-anchored Sema7A binds to integrin-β.
Two families of semaphorin receptors have been identified including plexins, a family of large transmembrane molecules conserved from invertebrates to humans, and neuropilins (NP1 and NP2), molecules found only in vertebrates (Tamagnone & Comoglio 2000). Membrane-bound vertebrate semaphorins bind directly to plexins, while class III secreted semaphorins require neuropilins as obligate co-receptors. In addition, two molecules that are unrelated to plexins and neuropilins, CD72 and Tim-2, functionally interact with class IV transmembrane semaphorins in the immune system (Kumanogoh et al. 2000, 2002a,b). Moreover, although GPI-linked Sema7A binds plexin-C1 (Tamagnone et al. 1999), it also binds the integrin β1 chain independent of plexins (Pasterkamp et al. 2003).
Several members of the semaphorin family are involved in axonal steering, zonal segregation of axon populations, axonal fasciculation, neuronal polarity, and neuronal cell migration as chemorepellents during neuronal development (Tessier-Lavigne & Goodman 1996). However, recent evidence indicates that semaphorins play diverse roles unrelated to axon guidance, including organogenesis, vascularization, angiogenesis, neuronal apoptosis and neoplastic transformation (Kitsukawa et al. 1995; Behar et al. 1996; Sekido et al. 1996; Soker et al. 1998). Additionally, it is becoming increasingly clear that several semaphorins play critical roles in the immune system (Kikutani & Kumanogoh 2003). Although the physiologic and pathologic significance of these semaphorins in the immune system is not fully known, we will review the rapidly emerging data on the biological functions of immune semaphorins.
2. A class III semaphorin: Sema3A in immune cell migration
Sema3A, a classical semaphorin member, is involved in repulsive growth cone guidance during neuronal development. Sema3A mediates these effects via its interaction with NP-1. Boumsell and colleagues recently identified a role for Sema3A in regulating monocyte migration (Delaire et al. 2001) by demonstrating that Sema3A inhibits spontaneous monocytic-cell migration in a transwell assay. Interestingly, Sema3A was recognized by some anti-human Sema4D monoclonal antibodies (mAbs), and Sema3A mediated cell migration was abolished by pre-incubation with these mAbs. These data suggest that a domain common to both human Sema3A and Sema4D might be important for cell migration. No NP-1 was found on the immune cells in this assay, raising the possibility of alternative Sema3A ligands in the immune system compared to those found in the nervous system. However, NP-1 is expressed on the surface of human T cells and DCs (Tordjman et al. 2002), and it remains possible that immune cell migration may be mediated by mechanisms similar to those identified in the nervous system. Cell migration, a fundamental developmental process common across a broad range species, is essential to proper immune and nervous system functioning. Analysis of Sema3A function in the immune system may reveal conserved mechanisms governing cell migration in these different systems.
3. Class IV semaphorins
(a) Sema4D/CD100
(i) Structure
Sema4D, a 150 kDa transmembrane protein, is a class IV semaphorin (Delaire et al. 1998; Kumanogoh & Kikutani 2001; Kikutani & Kumanogoh 2003). It contains an amino-terminal signal sequence followed by a sema domain, an Ig-like domain, a lysine-rich stretch, a hydrophobic transmembrane region, and a cytoplasmic tail (Furuyama et al. 1996; Hall et al. 1996). Mutational analysis of human Sema4D revealed that C679 in the sema domain is required for homodimerization (Klostermann et al. 1998; Delaire et al. 2001). Although Sema4D does not have a catalytic domain within the cytoplasmic region, it has consensus sites for both tyrosine and serine phosphorylation. Both human and mouse Sema4D are proteolytically cleaved and released from the membrane as a 120 kDa soluble form (Herold et al. 1995; Delaire et al. 2001; Elhabazi et al. 2001; Wang et al. 2001). The crystal structure of human Sema4D (residues 1–657) has been solved, and, surprisingly, the sema domain bears a high degree of structural homology with integrins by folding into a seven-bladed beta-propeller (Love et al. 2003).
(ii) Expression
Sema4D transcripts are observed at high levels in both lymphoid and non-lymphoid organs including the brain, heart, kidney, spleen, thymus, and lymph nodes. Within the lymphoid organs, Sema4D is highly expressed on resting T cells, but its expression is very low on resting B cells and antigen-presenting cells (APCs), such as dendritic cells (DCs). The expression of Sema4D on these cells is up-regulated upon their activation. The release of soluble Sema4D from the cell surface of immune cells also depends on cell activation.
(iii) Receptors of Sema4D: CD72 and plexin-B1
Two receptor families, the neuropilins and plexins, mediate the functions of many semaphorins such as axonal growth cone collapse (He & Tessier-Lavigne 1997; Kolodkin et al. 1997; Comeau et al. 1998; Winberg et al. 1998; Takahashi et al. 1999; Tamagnone et al. 1999), and Sema4D utilizes two receptors, CD72 and plexin-B1, which are preferentially expressed in lymphoid and non-lymphoid tissues, respectively (figure 2; Kumanogoh & Kikutani 2001).
Figure 2.
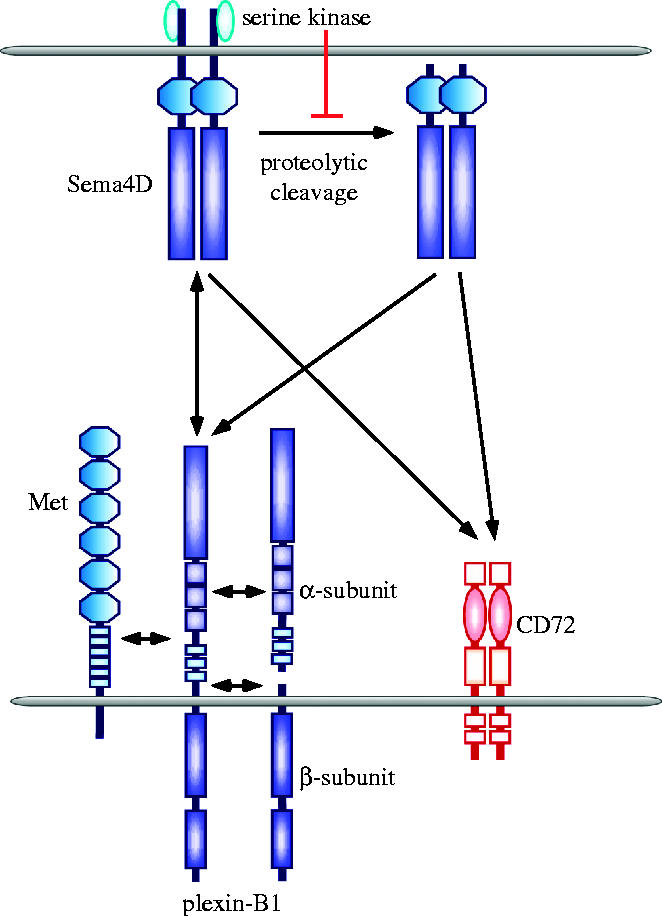
Two types of receptors for Sema4D. Sema4D is a 150 kDa transmembrane class IV. It contains a sema domain, an Ig-like domain, a transmembrane region and a cytoplasmic tail. A 120 kDa soluble form of Sema4D can be released from the cell surface following proteolytic cleavage. The generation of soluble Sema4D may be dependent on metalloprotease activity and is regulated by serine kinases associated with the cytoplasmic tail of Sema4D. Sema4D utilises two receptors; plexin-B1 and CD72. Binding of Sema4D to CD72 enhances immune responses. During the regulation of epithelial cell invasion, Sema4D signals through a plexin-B1/Met receptor complex.
CD72. This is the major Sema4D receptor in lymphoid tissues (Kumanogoh et al. 2000; Kumanogoh & Kikutani 2001; Kikutani & Kumanogoh 2003; Suzuki et al. 2003). CD72 can be detected throughout B cell differentiation from early progenitors to mature B cells (Nakayama et al. 1989; Von Hoegen et al. 1990; Gordon 1994), but its expression is downregulated upon terminal differentiation into plasma cells. In addition to B-cells, CD72 is expressed by professional APCs, such as macrophages and DCs (Tutt Landolfi & Parnes 1997; Kumanogoh et al. 2002b). Several lines of evidence indicate that CD72 is a functional lymphocyte receptor for Sema4D (Kumanogoh et al. 2000). Specifically, recombinant soluble Sema4D proteins bind to CD72-expressing transfectants, and Abs specific for CD72 inhibit the binding of soluble Sema4D to B cells. In addition, soluble mouse Sema4D fails to stimulate CD72-deficient B cells. A direct protein–protein interaction between soluble, recombinant mouse Sema4D and CD72 has also been demonstrated by surface plasmon resonance. CD72, a member of the C-type lectin family, may function as a negative regulator of B cell responses by recruiting SHP-1, a tyrosine phosphatase, to an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic tail (Adachi et al. 1998). Both agonistic anti-mouse CD72 mAb and soluble mouse Sema4D protein can induce dephosphorylation of mouse CD72, resulting in the dissociation of SHP-1 from CD72 (Wu et al. 1998; Kumanogoh et al. 2000). Sema4D may enhance B-cell activation by switching off negative signalling mediated by CD72 (Kumanogoh et al. 2000; Kumanogoh & Kikutani 2001).
Plexin-B1. This is highly expressed in the foetal brain and kidney. The extracellular domain of plexin-B1 exhibits 28% similarity to Met, and it contains a sema domain and a putative subtilisin-like proprotein convertase (PCs) cleavage site (Tamagnone et al. 2000; Artigiani et al. 2003). Plexin-B1 is synthesized as a single protein, but, following proteolytic cleavage by PCs, it is expressed at the cell surface as a heterodimer of α and β subunits (Artigiani et al. 2003). The β transmembrane subunit contains a short extracellular sequence and the cytoplasmic domains, while the α subunit, comprising the majority of the extracellular domain, remains associated with the cell surface via weak interactions with the β subunit. The proteolytic conversion of plexin-B1 significantly enhances the binding and functional responses to Sema4D. Interestingly, plexin-B1 forms a functional receptor complex with Met in epithelial cells (Giordano et al. 2002). Met is structurally similar to the plexins and semaphorins, also containing a Sema domain. Sema4D binding to plexin-B1 stimulates the intrinsic tyrosine kinase activity of Met, leading to the phosphorylation of both the receptors and a Met substrate, Gab1, which promotes epithelial invasion of surrounding tissues. Recently, Swiercz et al. reported that plexin-B1 is associated with the receptor tyrosine kinase ErbB-2, and binding of Sema4D to ErbB-2 stimulates the intrinsic activity of ErbB-2 (Swiercz et al. 2004). Plexin-B1 transcripts have been found in activated T cells, but a role for plexin-B1 in the immune system has yet to be demonstrated.
(iv) Biological functions of Sema4D in the immune system
Sema4D affects the biological activity of a wide variety of cells including neuronal cells, epithelial cells, and cells of the immune system. Within the immune system, Sema4D influences the behaviour of B cells, DCs, and monocytes, and many of these roles have been confirmed by analysis of Sema4D-deficient mice (Shi et al. 2000). Transfection of Sema4D into human B cells enhances their in vitro aggregation and survival (Hall et al. 1996). Stimulation of Sema4D on human B cells also induces the shedding of CD23 (a low affinity receptor for IgE used as an activation marker for B-cells) from the cell surface (Hall et al. 1996). Both the transfection of Sema4D into mouse B cells and treatment of cells with soluble recombinant mouse Sema4D enhances CD40-induced proliferation and immunoglobulin production (Kumanogoh et al. 2000). Thus, signalling events downstream of the Sema4D receptor, not Sema4D, seem to be important for the observed phenomena. In mice deficient for Sema4D, B cell responses to CD40 or LPS stimulation in vitro and humoral immune responses against T cell dependent antigens in vivo are impaired (Shi et al. 2000). Sema4D also plays a role in the activation and maturation of DCs (Kumanogoh et al. 2002b); soluble recombinant Sema4D enhances co-stimulatory molecule expression and IL-12 production during CD40-induced DC maturation. In Sema4D-deficient mice, T cell priming, in which DCs play a central role, is severely impaired (Shi et al. 2000). Sema4D also plays a role in monocyte and macrophage migration; soluble human Sema4D inhibits the spontaneous and MCP-3-induced migration of freshly isolated monocytes and monocytic cell lines (Delaire et al. 2001). Additionally, both recombinant soluble human Sema4D and agonistic anti-human CD72 mAb induce the production of proinflammatory cytokines, such as IL-6 and IL-8, by human monocytes (Ishida et al. 2003). Collectively, these findings suggest essential roles for Sema4D and its receptors in a variety of immune responses.
Accumulating data strongly suggests a role for Sema4D in the development of pathologic immune responses and autoimmunity in both mice and humans (Wang et al. 2001; Giraudon et al. 2004). In mice, high levels of soluble Sema4D are detectable in the sera of animals immunized with T cell dependent antigens, as well as in the sera of autoimmunity-prone MRL/lpr mice. In these systems, the levels of soluble Sema4D correlate well with both antigen-specific and autoantibody titres. Soluble Sema4D is undetectable in the sera of unimmunized normal mice. Interestingly, elevated levels of soluble Sema4D are found in the cerebrospinal fluid of patients suffering from neuroinflammatory demyelination. While not conclusive, this observation suggests a role for soluble Sema4D derived from infiltrating T cells in this disease process.
In contrast, several studies suggest that Sema4D may be active as a receptor in the immune system. In early studies using mAbs against human Sema4D, antibody-crosslinking of human Sema4D promoted T cell proliferation in the presence of suboptimal doses of anti-CD3 or anti-CD2 mAbs (Bougeret et al. 1992). Thus, Sema4D was thought to mediate signals through its cytoplasmic domain. Furthermore, in human T cells, Sema4D is associated with both serine/threonine kinases and protein tyrosine phosphatases (PTP; Elhabazi et al. 1997). In addition, PTPs differentially associate with human Sema4D during the terminal stages of B-cell differentiation (Herold et al. 1996; Dorfman et al. 1998; Billard et al. 2000), suggesting that Sema4D may function as a receptor transmitting signals to lymphocytes. Granziero et al. recently reported that human plexin-B1-expressing transfectants promote the proliferation of normal and leukaemic CD5+ cells, both of which express human Sema4D (Granziero et al. 2003), suggesting that human Sema4D functions as a receptor for human plexin-B1. Although the physiological significance of Sema4D as a receptor remains to be determined, it is plausible that Sema4D possesses bi-directional functions in cognate cell–cell contacts.
Collectively, these findings indicate that Sema4D plays a critical role in the immune system. Although the only abnormalities seen in Sema4D-deficient mice occur in lymphoid tissues, it is possible that compensatory mechanisms lacking in lymphocytes may function outside lymphoid tissues (Shi et al. 2000).
(b) Sema4A
(i) Structure and expression
Sema4A, a class IV semaphorin originally identified as semB (Puschel et al. 1995), is similar in structure to Sema4D. Its expression gradually increases during embryonic development, and Sema4A transcripts are detectable in a broad range of adult tissues, including the brain, spleen, lungs, kidneys, and testes (Kumanogoh et al. 2002a). Sema4A is expressed on bone marrow-derived and splenic DCs and B cells, but not on resting T cells. No differences in Sema4A expression are observed between CD8α+ and CD8α− DCs. Expression of Sema4A becomes detectable upon T cell activation (Kumanogoh et al. 2002a). Recently, we found that Sema4A expression is selectively and significantly upregulated during T helper cell 1 (Th1) differentiation (Kumanogoh et al. 2005).
(ii) Tim-2 as a Sema4A receptor
Unlike Sema4D, the binding of Sema4A to plexins has not been seen. Using recombinant soluble Sema4A, we detected Sema4A-binding partners on the surface of activated T cells, but not B cells or DCs. Binding of Sema4A to the cell surface of a subset of T cell lines, such as EL-4 cells, is also observed. Expression cloning using Sema4A-Fc identified Tim-2, a transmembrane protein and member of the T cell, immunoglobulin and mucin domain proteins (Tim) family, as a receptor for Sema4A (Kumanogoh et al. 2002a). Sema4A-Fc binds COS7 cells transfected with Tim-2 cDNA (Kumanogoh et al. 2002a), and the binding of Sema4A to Tim-2-expressing COS7 cells is abrogated by excess Tim-2-Fc. Surface plasmon resonance estimates the affinity constant (Kd) of Sema4A binding to Tim-2 at 7.0×10−8 M. Sema4A stimulation also induces Tim-2 tyrosine phosphorylation in COS7 cells. However, it remains unclear whether Tim-2 is the sole receptor for Sema4A in the immune system (figure 3).
Figure 3.
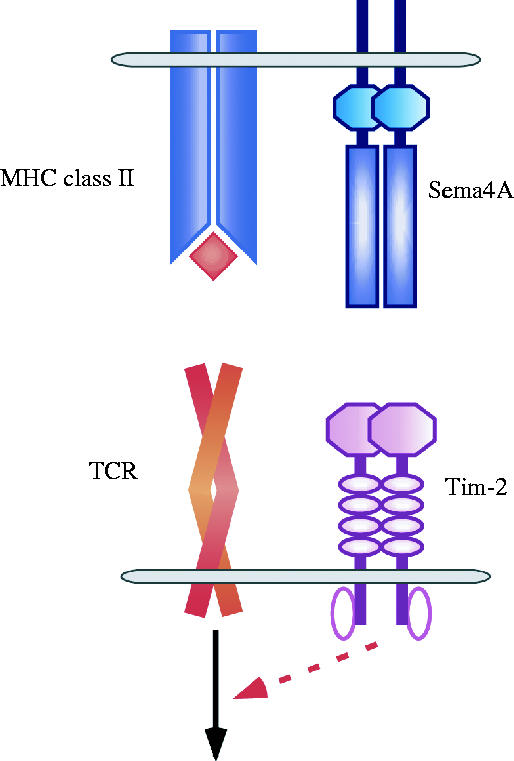
Involvement of Sema4A in T cell activation through Tim-2. Sema4A, preferentially expressed on DCs, is a class IV transmembrane-type semaphorin member. Sema4A contains a sema domain, an Ig-like domain, a transmembrane region, and a cytoplasmic region. Tim-2, a member of the Tim-protein family, possesses an Ig-like domain, a mucin domain, a transmembrane region, and a cytoplasmic region containing consensus tyrosine phosphorylation sites. The mucin domain has multiple putative sites for O-linked glycosylation, while the Ig domain has several sites for putative N-linked glycosylation. Following T cell activation through TCR ligation, Tim-2 expression is induced on T cells to interact with Sema4A, resulting in enhanced T cell activation through unknown signalling molecules associated with tyrosine-phosphorylated Tim-2.
Mclntire et al. identified a locus conferring susceptibility to mouse allergen-induced airway hypersensitivity, which they dubbed T cell and airway phenotype regulator (Tapr; McIntire et al. 2001). Subsequent positional cloning revealed that this locus contains a new family of genes, designated Tims. There are a remarkable number of sequence polymorphisms within both mouse and human Tim-1 (also designated as human hepatitis A virus cellular receptor-1 (HAVcr-1); McIntire et al. 2001), and these different genotypes may contribute to the aetiology of both mouse and human T helper cell (Th) 2-dependent diseases such as asthma. Additionally, Kuchroo and colleagues independently identified Tim-3 in a screen for Th1 cell specific surface proteins (Monney et al. 2002). Administration of anti-Tim-3 mAb or soluble Tim-3 proteins promoted the development of Th1-dependent immune responses including experimental autoimmune encephalomyelitis (EAE). This suggests a role for Tim-3 in the regulation of Th1 responses. Although the natural ligands of Tim-1 and Tim-3 have not been identified, the Tim protein ligands, including Sema4A, are likely to be regulatory molecules influencing the activation and differentiation of T cells.
(iii) Activities
Although recombinant soluble Sema4A does not stimulate B cells and DCs, Sema4A can provide a co-stimulatory signal to T cells (Kumanogoh et al. 2002a). The addition of recombinant soluble Sema4A enhances T cell proliferation and IL-2 production following stimulation with anti-CD3 mAb. Additionally, soluble Sema4A enhanced the mixed lymphocyte reactions (MLR) between allogeneic T cells and DCs, while an anti-Sema4A mAb blocked the MLR, suggesting that Sema4A plays a role in T cell activation by influencing the stimulatory interactions between T cells and DCs.
The role of Sema4A in immune responses in vivo has been clarified using soluble Sema4A and anti-Sema4A mAb (Kumanogoh et al. 2002a). The administration of soluble Sema4A significantly enhances the generation of antigen-specific T cells. In contrast, administration of anti-Sema4A mAb blocks antigen-specific T cell priming. Treatment of mice with anti-Sema4A mAb inhibits the development of EAE induced by myelin oligodendrocyte glycoprotein (MOG)—peptide administration. Delayed administration of soluble Sema4A or anti-Sema4A does not affect the generation of antigen-specific T cells, suggesting that Sema4A acts early in T cell activation (Kumanogoh et al. 2002a; Kikutani & Kumanogoh 2003). Recently, we generated and characterized Sema4A-deficient mice. Mutant mice develop normally despite the broad range of expression of Sema4A throughout development. However, these mice display several functional defects in the immune system. Sema4A-deficient mice exhibit impaired T cell priming, consistent with the data obtained with recombinant Sema4A and anti-Sema4A mAb (Kumanogoh et al. 2005). Furthermore, the mutant mice display defective Th1 responses, suggesting a regulatory role of Sema4A in Th1/Th2 responses. As described above, Sema4A is expressed not only in DCs but also in Th1 cells, which raises a question of which Sema4A is important for different phases of immune response. To clarify this, we performed a reconstitution study using antigen-pulsed DCs and found that DC-derived Sema4A is critical for generation of antigen-specific T cells, while Th1-derived Sema4A is important for promoting Th1 responses (Kumanogoh et al. 2005).
4. Virus-encoded semaphorins and Sema7A
Many virally encoded immune modulators have significant homology with host proteins, and many of these factors likely arose by viral incorporation of host genetic information during the co-evolution of virus and host. In recent years, the study of these viral immune modulators has significantly advanced our understanding of viral pathogenesis. Many of these proteins interfere with the development of a proper immune response, and deletion of some of the genes encoding viral immune modulators greatly attenuates the pathogenicity of these viruses. Several viruses also encode semaphorins, suggesting a function for these molecules as immune modulators in an infected host.
(a) A39R/SEMAVA and AHVsema/SEMAVB
Vaccinia virus, a member of the poxvirus family, contains an open reading frame (ORF), A39R/SEMAVA, related to the semaphorin family (Kolodkin et al. 1993). A39R/SEMAVA, the smallest semaphorin, consists of only a truncated extracellular sema domain, and it is thought to act as a monomer, in contrast to all other semaphorins. The mammalian homologue A39R/SEMAVA has yet to be identified. Skin lesions develop at the site of vaccinia virus infection, and this is followed by secondary viral replication in lymphoid organs. When human monocytes are treated with A39R/SEMAVA, they undergo aggregation, produce proinflammatory cytokines, and upregulate the monocyte cell surface marker CD54 (ICAM-1; Comeau et al. 1998). The precise in vivo function of A39R/SEMAVA is not clear, but it can clearly modulate the behaviour of some immune cells.
Ensser and Fleckenstein identified a semaphorin, AHVsema/SEMAVB, encoded by the alcelaphine herpesvirus (AHV; Ensser & Fleckenstein 1995). Unlike other herpes viruses, AHV is not neurotropic, but causes wildebeest-associated malignant catarrhal fever, a lymphoproliferative syndrome occurring in ungulate species other than the natural host. Both mouse and human homologues of AHVsema/SEMAVB have been identified.
Spriggs and colleagues identified the cellular receptor of both A39R/SEMAVA and AHVsema/SEMAVB as VESPR/CD232/plexin-C1 (Comeau et al. 1998). A blocking antibody targeting VESPR/CD232/plexin-C1 inhibits the A39R/SEMAVA-induced production of inflammatory cytokines targeting. The precise role of these virally encoded semaphorins is not clear, but identification of their mammalian counterparts will facilitate studies into the pathogenicity of these viruses.
(b) Sema7A
Two groups independently identified the class VII semaphorin Sema7A, also known as CDw108 or Sema-K1, when searching for vertebrate homologues of virally encoded semaphorins. Sema7A is a membrane-associated GPI-linked protein homologous to AVHsema/SEMAVB (Lange et al. 1998; Xu et al. 1998). Sema7A is expressed by a variety of lymphoid and myeloid cells as well as several pre- and post-natal neuronal populations. Like SEMAVB, Sema7A promotes chemotaxis and cytokine production in vitro (Holmes et al. 2002). Stimulation of Sema7A on monocytes promotes granulocyte-macrophage colony-stimulating factor (GM-CSF) production and induces these cells to adopt a dendritic cell morphology. In addition, Sema7A defines the John–Milton–Hagen human blood group antigen on erythrocytes, which has been implicated in a clinically benign autoimmune disorder. Tamagnone and colleagues reported that Sema7A specifically binds to plexin-C1 (Tamagnone et al. 1999). However, Pasterkamp et al. reported that Sema7A exerts plexin-C1-independent activity mediated by integrins. Specifically, Sema7A activates integrin-β1 and mitogens-activated protein kinase (MAPK) signalling in a plexin-C1-independent manner (Pasterkamp et al. 2003). Similarly, the immunological functions of Sema7A are thought to be mediated by plexin-C1-independent pathways. Clearly, further studies are needed to identify the physiologic roles of Sema7A as well as its viral homologue.
5. Semaphorin receptors in the immune system: NP-1 and plexin-A1
(a) NP-1
(i) NP-1 in initial T/DC contacts
NP-1 was the first receptor identified for class III semaphorins. Recently, Tordjman et al. determined that DCs and resting T cells express NP-1 (Tordjman et al. 2002). The homophilic interaction of NP-1 on DCs and resting T cells is critically involved in the initiation of primary immune responses. During the initial contact between DCs and resting T cells a variety of molecules facilitate the formation of the immunological synapse including DC-SIGN with ICAM-3, CD58 (LFA-3) with CD2, or LFA-1 with either ICAM-1 or ICAM-2. Following the interaction of resting T cells with DCs, the distribution of NP-1 becomes polarized towards sites of cell/cell contact. NP-1 and CD3 co-localize by double immunofluorescence staining, indicating that T cell derived NP-1 localizes to the immunological synapse during interactions with professional APCs. In addition, soluble NP-1 binds both DCs and resting T cells, and anti-NP-1 blocked the induction of T cell proliferation by allogeneic DCs. Collectively, these findings indicate a role for NP-1 in the initiation of the primary immune responses. These studies, however, do not exclude the possibility that unidentified transmembrane proteins, including semaphorins, are involved in stabilizing the initial interaction between DCs and resting T cells via NP-1.
(ii) NP-1 on regulatory T cells
It is increasingly becoming clear that CD4+CD25+ regulatory T cells (Treg cells) are key modulators of immune responses and are essential for the maintenance of immunological homeostasis. Because these cells hold great therapeutic promise, extensive efforts have been undertaken to identify reliable surface markers that are selectively upregulated on Treg cells. Hansen and colleagues recently identified NP-1 as a specific surface marker for CD4+CD25+ Treg cells (Bruder et al. 2004), and its expression is not affected by cellular activation status. Importantly, CD4+ NP-1high T cells express high levels of Foxp3 and exhibit strong Treg activities. In contrast, the expression of NP-1 is downregulated in CD4+CD25+ T cells after cellular activation. The regulated expression of NP-1 by T cells may have functional significance, and, consequently, semaphorins may play a role in Treg mediated immune modulation through their interactions with NP-1. Alternatively, NP-1 may participate in homophilic interactions to regulate immunological homeostasis. Further comprehensive studies are needed to clarify the involvement of NP-1 in the functions of Treg cells.
6. Plexin-A1
Plexin-A1 associates with NP-1 in the nervous system and functions as a signal-transducer downstream of class III semaphorins. Interestingly, plexin-A1 functions as a receptor for Sema6D, and it could form a receptor complex with VEGFR2 or Off-track in a region-specific manner during cardiac morphogenesis, suggesting its pleiotropic functions in vivo (Toyofuku et al. 2004).
Using DNA microarray analysis, Wong et al. found that plexin-A1 is a target of CIITA, a transcriptional factor that controls MHC class II expression (Wong et al. 2003). Plexin-A1 is expressed in DCs and is involved in DC functions including T cell stimulatory activities. DCs in which plexin-A1 was knocked-down by RNAi have a severe impairment in their ability to activate antigen-specific- or allogeneic-T cells in vitro and in vivo. These DCs exhibit normal antigen-presentation, MHC-expression and peptide loading. Plexin-A1 is involved in T cell–DC interactions but not antigen processing or binding. As plexin-signalling induces neuronal cytoskeleton rearrangement, it is possible that plexin-A1 is involved in the modulation of cytoskeletal rearrangement in DCs during interactions with T cells. Of note, class III semaphorin/NP-1 complexes can be associated with plexin-A1 (Takahashi et al. 1999); plexin-A1 might mediate signals from both class III and VI semaphorins. Further comprehensive studies are required to identify the functions of plexin-A1 in the immune system.
7. Perspectives
The etymology of the term ‘semaphorin’ is ‘semaphore’, a primitive but effective communication tool. Correspondingly, semaphorins exert their roles as communication tools between neuronal cells in developing embryos. As reviewed in this article, several semaphorins play critical roles in the immune system. In addition to the semaphorins described here, we recently identified additional semaphorin molecules involved in various phases of the immune response. Further studies are needed to more clearly address the roles of semaphorin family members in disease pathogensis and infection, but these molecules may be promising targets of immunomodulatory therapies. Additionally, increased understanding of semaphorins in the immune system could establish a new paradigm of immune cell communication through the immune semaphorin network. Finally, the cellular consequences of semaphorin expression in the immune system should inform studies on neural semaphorins to create a comprehensive body of knowledge detailing the wide array of physiologic process known to be influenced by this interesting family of proteins.
Acknowledgments
We would like to thank K. Kubota for excellent secretarial assistance. A.K. and H.K were supported by research grants from the Ministry of Education, Culture, Science, and Technology of Japan.
Footnotes
One contribution of 16 to a Theme Issue ‘Immunoregulation: harnessing T cell biology for therapeutic benefit’.
References
- Adachi T, Flaswinkel H, Yakura H, Reth M, Tsubata T. The B cell surface protein CD72 recruits the tyrosine phosphatase SHP-1 upon tyrosine phosphorylation. J. Immunol. 1998;160:4662–4665. [PubMed] [Google Scholar]
- Artigiani S, et al. Functional regulation of semaphorin receptors by proprotein convertases. J. Biol. Chem. 2003;278:10 094–10 101. doi: 10.1074/jbc.M210156200. [DOI] [PubMed] [Google Scholar]
- Behar O, Golden J.A, Mashimo H, Schoen F.J, Fishman M.C. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- Billard C, Delaire S, Raffoux E, Bensussan A, Boumsell L. Switch in the protein tyrosine phosphatase associated with human CD100 semaphorin at terminal B-cell differentiation stage. Blood. 2000;95:965–972. [PubMed] [Google Scholar]
- Bougeret C, Mansur I.G, Dastot H, Schmid M, Mahouy G, Bensussan A, Boumsell L. Increased surface expression of a newly identified 150-kDa dimer early after human T lymphocyte activation. J. Immunol. 1992;148:318–323. [PubMed] [Google Scholar]
- Bruder D, et al. Neuropilin-1: a surface marker of regulatory T cells. Eur. J. Immunol. 2004;34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- Comeau M.R, et al. A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor, VESPR. Immunity. 1998;8:473–482. doi: 10.1016/s1074-7613(00)80552-x. [DOI] [PubMed] [Google Scholar]
- Delaire S, Elhabazi A, Bensussan A, Boumsell L. CD100 is a leukocyte semaphorin. Cell Mol. Life Sci. 1998;54:1265–1276. doi: 10.1007/s000180050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaire S, Billard C, Tordjman R, Chedotal A, Elhabazi A, Bensussan A, Boumsell L. Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. J. Immunol. 2001;166:4348–4354. doi: 10.4049/jimmunol.166.7.4348. [DOI] [PubMed] [Google Scholar]
- Dorfman D.M, Shahsafaei A, Nadler L.M, Freeman G.J. The leukocyte semaphorin CD100 is expressed in most T-cell, but few B-cell, non-Hodgkin's lymphomas. Am. J. Pathol. 1998;153:255–262. doi: 10.1016/S0002-9440(10)65566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhabazi A, Lang V, Herold C, Freeman G.J, Bensussan A, Boumsell L, Bismuth G. The human semaphorin-like leukocyte cell surface molecule CD100 associates with a serine kinase activity. J. Biol. Chem. 1997;272:23 515–23 520. doi: 10.1074/jbc.272.38.23515. [DOI] [PubMed] [Google Scholar]
- Elhabazi A, Delaire S, Bensussan A, Boumsell L, Bismuth G. Biological activity of soluble CD100. I. The extracellular region of CD100 is released from the surface of T lymphocytes by regulated proteolysis. J. Immunol. 2001;166:4341–4347. doi: 10.4049/jimmunol.166.7.4341. [DOI] [PubMed] [Google Scholar]
- Ensser A, Fleckenstein B. Alcelaphine herpesvirus type I has a semaphorin-like gene. J. Gen. Virol. 1995;76:1063–1067. doi: 10.1099/0022-1317-76-4-1063. [DOI] [PubMed] [Google Scholar]
- Furuyama T, et al. Identification of a novel transmembrane semaphorin expressed on lymphocytes. J. Biol. Chem. 1996;271:33 376–33 381. doi: 10.1074/jbc.271.52.33376. [DOI] [PubMed] [Google Scholar]
- Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio P.M. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat. Cell Biol. 2002;4:720–724. doi: 10.1038/ncb843. [DOI] [PubMed] [Google Scholar]
- Giraudon P, et al. Semaphorin CD100 from activated T lymphocytes induces process extension collapse in oligodendrocytes and death of immature neural cells. J. Immunol. 2004;172:1246–1255. doi: 10.4049/jimmunol.172.2.1246. [DOI] [PubMed] [Google Scholar]
- Gordon J. B-cell signalling via the C-type lectins CD23 and CD72. Immunol. Today. 1994;15:411–417. doi: 10.1016/0167-5699(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Granziero L, Circosta P, Scielzo C, Frisaldi E, Stella S, Geuna M, Giordano S, Ghia P, Caligaris-Cappio F. CD100/Plexin-B1 interactions sustain proliferation and survival of normal and leukemic CD5+ B lymphocytes. Blood. 2003;101:1962–1969. doi: 10.1182/blood-2002-05-1339. [DOI] [PubMed] [Google Scholar]
- Hall K.T, Boumsell L, Schultze J.L, Boussiotis V.A, Dorfman D.M, Cardoso A.A, Bensussan A, Nadler L.M, Freeman G.J. Human CD100, a novel leukocyte semaphorin that promotes B-cell aggregation and differentiation. Proc. Natl Acad. Sci. USA. 1996;93:11 780–11 785. doi: 10.1073/pnas.93.21.11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- Herold C, Bismuth G, Bensussan A, Boumsell L. Activation signals are delivered through two distinct epitopes of CD100, a unique 150 kDa human lymphocyte surface structure previously defined by BB18 mAb. Int. Immunol. 1995;7:1–8. doi: 10.1093/intimm/7.1.1. [DOI] [PubMed] [Google Scholar]
- Herold C, Elhabazi A, Bismuth G, Bensussan A, Boumsell L. CD100 is associated with CD45 at the surface of human T lymphocytes. Role in T cell homotypic adhesion. J. Immunol. 1996;157:5262–5268. [PubMed] [Google Scholar]
- Holmes S, et al. Sema7A is a potent monocyte stimulator. Scand. J. Immunol. 2002;56:270–275. doi: 10.1046/j.1365-3083.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- Ishida I, Kumanogoh A, Suzuki K, Akahani S, Noda K, Kikutani H. Involvement of CD100, a lymphocyte semaphorin, in the activation of the human immune system via CD72: implications for the regulation of immune and inflammatory responses. Int. Immunol. 2003;15:1027–1034. doi: 10.1093/intimm/dxg098. [DOI] [PubMed] [Google Scholar]
- Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat. Rev. Immunol. 2003;3:159–167. doi: 10.1038/nri1003. [DOI] [PubMed] [Google Scholar]
- Kitsukawa T, Shimono A, Kawakami A, Kondoh H, Fujisawa H. Overexpression of a membrane protein, neuropilin, in chimeric mice causes anomalies in the cardiovascular system, nervous system and limbs. Development. 1995;121:4309–4318. doi: 10.1242/dev.121.12.4309. [DOI] [PubMed] [Google Scholar]
- Klostermann A, Lohrum M, Adams R.H, Puschel A.W. The chemorepulsive activity of the axonal guidance signal semaphorin D requires dimerization. J. Biol. Chem. 1998;273:7326–7331. doi: 10.1074/jbc.273.13.7326. [DOI] [PubMed] [Google Scholar]
- Kolodkin A.L, Matthes D.J, Goodman C.S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- Kolodkin A.L, Levengood D.V, Rowe E.G, Tai Y.T, Giger R.J, Ginty D.D. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, Kikutani H. The CD100–CD72 interaction: a novel mechanism of immune regulation. Trends Immunol. 2001;22:670–676. doi: 10.1016/s1471-4906(01)02087-7. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity. 2000;13:621–631. doi: 10.1016/s1074-7613(00)00062-5. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002a;419:629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, et al. Requirement for the lymphocyte semaphorin, CD100, in the induction of antigen-specific T cells and the maturation of dendritic cells. J. Immunol. 2002b;169:1175–1181. doi: 10.4049/jimmunol.169.3.1175. [DOI] [PubMed] [Google Scholar]
- Kumanogoh A, et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity. 2005;22:305–316. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Lange C, Liehr T, Goen M, Gebhart E, Fleckenstein B, Ensser A. New eukaryotic semaphorins with close homology to semaphorins of DNA viruses. Genomics. 1998;51:340–350. doi: 10.1006/geno.1998.5256. [DOI] [PubMed] [Google Scholar]
- Love C.A, Harlos K, Mavaddat N, Davis S.J, Stuart D.I, Jones E.Y, Esnouf R.M. The ligand-binding face of the semaphorins revealed by the high-resolution crystal structure of SEMA4D. Nat. Struct. Biol. 2003;10:843–848. doi: 10.1038/nsb977. [DOI] [PubMed] [Google Scholar]
- McIntire J.J, Umetsu S.E, Akbari O, Potter M, Kuchroo V.K, Barsh G.S, Freeman G.J, Umetsu D.T, DeKruyff R.H. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2001;2:1109–1116. doi: 10.1038/ni739. [DOI] [PubMed] [Google Scholar]
- Monney L, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- Nakayama E, von Hoegen I, Parnes J.R. Sequence of the Lyb-2 B-cell differentiation antigen defines a gene superfamily of receptors with inverted membrane orientation. Proc. Natl Acad. Sci. USA. 1989;86:1352–1356. doi: 10.1073/pnas.86.4.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp R.J, Peschon J.J, Spriggs M.K, Kolodkin A.L. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- Puschel A.W, Adams R.H, Betz H. Murine semaphorin D/collapsin is a member of a diverse gene family and creates domains inhibitory for axonal extension. Neuron. 1995;14:941–948. doi: 10.1016/0896-6273(95)90332-1. [DOI] [PubMed] [Google Scholar]
- Sekido Y, et al. Human semaphorins A (V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc. Natl Acad. Sci. USA. 1996;93:4120–4125. doi: 10.1073/pnas.93.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaphorin Nomenclature Committee. Unified nomenclature for the semaphorins/collapsins. Cell. 1999;97:551–552. doi: 10.1016/s0092-8674(00)80766-7. [DOI] [PubMed] [Google Scholar]
- Shi W, et al. The class IV semaphorin CD100 plays nonredundant roles in the immune system: defective B and T cell activation in CD100-deficient mice. Immunity. 2000;13:633–642. doi: 10.1016/s1074-7613(00)00063-7. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao H.Q, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kumanogoh A, Kikutani H. CD100/Sema4D, a lymphocyte semaphorin involved in the regulation of humoral and cellular immune responses. Cytokine Growth Factor Rev. 2003;14:17–24. doi: 10.1016/s1359-6101(02)00073-4. [DOI] [PubMed] [Google Scholar]
- Swiercz J.M, Kuner R, Offermans S. Plexin-B1/RhoGEF-mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J. Cell Biol. 2004;165:869–880. doi: 10.1083/jcb.200312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang L.H, Murakami Y, Kalb R.G, Fujisawa H, Strittmatter S.M. Plexin–neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio P.M. Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol. 2000;10:377–383. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman C.S. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tordjman R, Lepelletier Y, Lemarchandel V, Cambot M, Gaulard P, Hermine O, Romeo P.H. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune responses. Nat. Immunol. 2002;3:477–482. doi: 10.1038/ni789. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutt Landolfi M, Parnes J.R. CD72 workshop panel report. In: Kishimoto T, Kikutani H, Von dem Borne A. E. G. Kr, et al., editors. Leukocyte typing VI. Garland Publishing; New York: 1997. pp. 162–164. [Google Scholar]
- Von Hoegen I, Nakayama E, Parnes J.R. Identification of a human protein homologous to the mouse Lyb-2 B cell differentiation antigen and sequence of the corresponding cDNA. J. Immunol. 1990;144:4870–4877. [PubMed] [Google Scholar]
- Wang X, Kumanogoh A, Watanabe C, Shi W, Yoshida K, Kikutani H. Functional soluble CD100/Sema4D released from activated lymphocytes: possible role in normal and pathologic immune responses. Blood. 2001;97:3498–3504. doi: 10.1182/blood.v97.11.3498. [DOI] [PubMed] [Google Scholar]
- Winberg M.L, Noordermeer J.N, Tamagnone L, Comoglio P.M, Spriggs M.K, Tessier-Lavigne M, Goodman C.S. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Wong A.W, et al. CIITA-regulated plexin-A1 affects T-cell–dendritic cell interactions. Nat. Immunol. 2003;4:891–898. doi: 10.1038/ni960. [DOI] [PubMed] [Google Scholar]
- Wu Y, et al. The B-cell transmembrane protein CD72 binds to and is an in vivo substrate of the protein tyrosine phosphatase SHP-1. Curr. Biol. 1998;8:1009–1017. doi: 10.1016/s0960-9822(07)00421-6. [DOI] [PubMed] [Google Scholar]
- Xu X, Ng S, Wu Z.L, Nguyen D, Homburger S, Seidel-Dugan C, Ebens A, Luo Y. Human semaphorin K1 is glycosylphosphatidylinositol-linked and defines a new subfamily of viral-related semaphorins. J. Biol. Chem. 1998;273:22 428–22 434. doi: 10.1074/jbc.273.35.22428. [DOI] [PubMed] [Google Scholar]


