Abstract
Haematopoietic stem cell transplantation (HSCT) offers promise for the treatment of haematological and immune disorders, solid tumours, and as a tolerance inducing regimen for organ transplantation. Allogeneic HSCTs engraftment requires immunosuppression and the anti-tumour effects are dependent upon the immune effector cells that are contained within or generated from the donor graft. However, significant toxicities currently limit its efficacy. These problems include: (i) graft-versus-host disease (GVHD) in which donor T cells attack the recipient resulting in multi-organ attack and morbidity, (ii) a profound period of immune deficiency following HSCT, and (iii) donor graft rejection. Currently available methods to prevent or treat GVHD with systemic immunosuppression can lead to impaired immune recovery, increased opportunistic infections, and higher relapse rates. This review will provide an overview of GVHD pathophysiology and discuss the roles of various cells, pathways, and factors in the GVHD generation process and in the preservation of graft-versus-tumour effects. Variables that need to be taken into consideration in attempting to extrapolate preclinical results to the clinical paradigm will be highlighted.
Keywords: graft-versus-host disease, bone marrow transplantation, graft versus leukaemia, cytokines, allogeneic
Bone marrow transplantation (BMT), or perhaps more accurately, haematopoietic stem cell transplantation (HSCT), offers great promise for the treatment of a variety of diseases ranging from cancer, autoimmunity, aplastic anaemia and other diseases of haematopoietic origin. BMT originated as a means to repopulate the haematopoietic stem cell compartment after myeloablative exposure to radiation as a means of ‘rescue’. It has evolved into a means to induce anti-tumour responses when used in cancer. HSCT can be either autologous (the transferred haematopoietic stem cells (HSCs) are obtained from the patient) or allogeneic (HSCs are from a donor that is, optimally, totally matched at the major histocompatibility complex (MHC) loci and is related to the patient). Classically, HSCT involves some means of cytoreductive conditioning the recipient either by irradiation and/or chemotherapy. Initially HSCs were provided exclusively by bone marrow cells (BMC). Subsequently, other sources that contain HSCs have shown efficacy for haematopoietic reconstitution including mobilized peripheral blood, and more recently, cord blood. The advent of non-myeloablative or reduced intensity conditioning has allowed HSCT3 to be performed in patients with advanced age due to lesser toxicities from the use of lower cytoreductive conditioning. Allogeneic HSCTs engraftment requires immunosuppression and the anti-tumour effects are dependent upon the immune effector cells that are contained within or generated from the donor graft. The latter is supported by earlier observations that the relapse rates for allogeneic BMT were markedly lower than autologous BMT due to the occurrence of graft-versus-tumour (GVT) effects after the transplant. However, allogeneic HSCT is hampered by significant toxicities, which currently limit its efficacy. These problems include: (i) graft-versus-host disease (GVHD) in which donor T cells attack the recipient resulting in multi-organ attack and morbidity, (ii) a profound period of immune deficiency following BMT leaving the patient susceptible to opportunistic infections, and (iii) donor graft rejection. Unfortunately, common means to prevent or treat GVHD with systemic immunosuppression (i.e. by corticosteroids and use of cyclosporin A) can lead to impaired immune recovery, increased opportunistic infections, and higher relapse rates. This review will discuss current strategies in the prevention and treatment of GVHD. The review will begin with an overview on the biology of GVHD, then discuss the roles of various cells, pathways, and factors in the GVHD generation process along with providing an overview on the use of different animal models used to study GVHD and variables that need to be taken into consideration in attempting to extrapolate preclinical results to the clinical paradigm. The review will conclude with discussions on developing means to prevent and treat GVHD without hampering GVT.
1. GVHD
(a) GVHD pathophysiology
GVHD has a complex aetiology but ultimately is the result of donor T cell attack of an immunocompromised and genetically disparate recipient and is a significant cause of morbidity following allogeneic BMT. Unfortunately, these same donor T cells also mediate GVT effects following HSCT as evidenced by studies demonstrating that prior removal of T cells in the donor graft prevents GVHD occurrence but also can increase tumour relapse rates especially for patients with certain types of haematological malignancies.
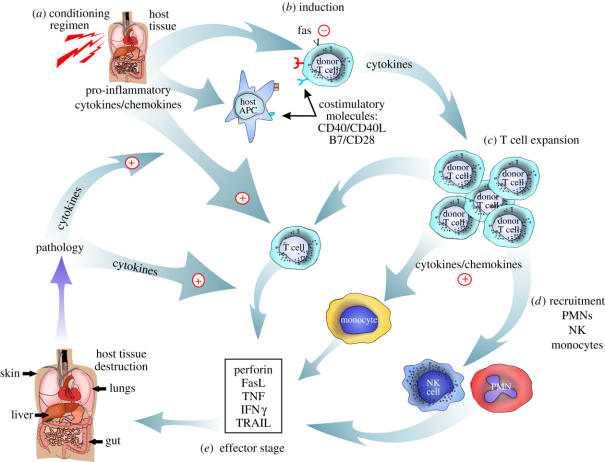
GVHD has been postulated to occur in several stages. The first stage in GVHD induction revolves around the cytoreductive conditioning or immunosuppression used to allow for donor cell engraftment (figure 1). Particularly for conventional (fully myeloablative) BMT, the extensive cytoreductive conditioning results in the release of proinflammatory cytokines which affects host antigen presenting cells (APCs; by increasing their maturation and upregulation of costimulatory molecules/cytokines) and helps fuel the alloreactive donor T cell response. It has been shown in murine models that host APCs can play a particularly important role in acute GVHD induction (Shlomchik et al. 1999). This is followed by the ‘induction phase’ which involves classical T cell recognition involving cell surface events that result in T cell receptor (TCR) ligation (providing a first signal to the T cell) and engagement of one or more costimulatory molecules (providing a second signal to the T cell); both TCR ligation and costimulation must be delivered to achieve full T cell activation and subsequent expansion. Costimulation of donor T cells is typically provided by ligands expressed on the surface of host APCs. As the alloreactive T cells expand (expansion phase) they home to various GVHD target organs (skin, gut, liver, lung), a process controlled by adhesion molecules, integrins and chemokines. The production of chemokines in inflamed and injured tissue also results in the recruitment of other cell-types (neutrophils, natural killer (NK) cells, monocytes) to the GVHD target organ sites further contributing to GVHD pathology. The ‘effector phase’ is noted for the destruction of host tissue by immune effector molecules (e.g. fas ligand, perforin, granzymes, interferon-gamma (IFNγ), tumour necrosis factor (TNF)) and results in the continued production of proinflammatory cytokines which then continues to fuel the entire GVHD process (figure 1). This ‘cytokine storm’ during the recruitment and effector phases is particularly difficult to control. Thus, many attempts to arrest GVHD have focused on targeting early during the induction phase before the massive T cell expansion, homing/migration and cytolytic process has been fully developed.
Figure 1.
Overview of GVHD pathophysiology.
Clinical GVHD occurs in ‘acute’ and ‘chronic’ forms. The designation was initially applied based on differences in time of onset clinically with acute GVHD occurring shortly after BMT and chronic GVHD being more delayed (after 100 days). It now is clear that these are two separate pathophysiological entities with different aetiologies, target organs, therapeutic responses and sequelae. However, there are incidences where they can overlap in time after transplantation and GVHD target organ involvement. Acute GVHD targets skin, gut, liver, and lung whereas chronic GVHD appears with primarily skin and mucosal manifestations, immune perturbations, and has many autoimmune characteristics. Predominant means of controlling GVHD, both acute and chronic, is through the prophylactic use of immunosuppression (i.e. corticosteroids and other agents) as GVHD is difficult to treat once ongoing.
(b) Animals models in the study of GVHD
Murine and canine models are the two predominant animal models used to study GVHD pathophysiology. Within the animal model studies for GVHD there are key variables to take into consideration prior to drawing conclusions regarding the results and attempting to correlate them to the clinical GVHD scenario (table 1). These factors include (but are not limited to): (i) the presence or absence of, and type, of cytoreductive conditioning used on the recipient, which can range from none to lethal. Lethal cytoreductive conditioning must then be followed by some form of HSC rescue. The conditioning regimen intensity is perhaps one of the most important variables in the different GVHD models. (ii) The strain combinations used is a key variable which can affect the kinetics and extent of GVHD lethality as well as the particular target organs involved. For example, in some strain combinations, CD4+ T cells are the only, major, or are not involved at all in GVHD pathophysiology, whereas in others, CD8+ T cells may be the dominant effectors. The MHC and/or minor histocompatibility (miH) antigens recognized could determine the frequency and quality of the immune response of donor T cells that respond to the host. Variations include donor–recipient pairs with semi-allogeneic (parent-into-F1), full mismatch MHC, multiple miH antigens, isolated MHC antigens, or combinations thereof. (iii) The type and number of the donor immune cells transferred. Many models use donor splenocytes to induce GVHD as the extent of contaminating T cells present in BMC preparations are usually inadequate to induce acute GVHD except in certain strain combinations. T cells from lymph node (LN), bone marrow (BM), or peripheral blood can also be used to supplement BMC preparations. The homing and chemokine receptors expressed on T cells in these various locations are distinct and therefore the adoptive transfer of T cells from these sites may differentially affect GVHD. The donor T cells transferred can be purified based on CD3, CD4 or CD8 antigen expression. Contingent on these variables, different organ pathologies (sometimes selective attack occurs in which only the liver or gut is affected versus other models where haematopoietic attack or autoantibody production results) and kinetics of progression (ranging from days to months) results. The number of donor T cells infused and constituency of T cell subpopulations (TCR γ/δ cells; natural killer T (NK/T) cells; naïve versus memory cells; Th1 versus Th2 cells; regulatory T cells) may also influence the kinetics and pathophysiology of GVHD. (iv) The presence of endogenous microenvironmental pathogens. Another variable is the potential presence of environmental pathogens including those that might cause subclinical infections in the mouse colony where the studies are performed and conversely the use of non-absorbable or systemically administered prophylactic antibiotics to decrease intestinal pathogens. This may affect radiosensitivity, particularly in the gut. All of these issues are important, particularly when assessing potential roles of proinflammatory cytokines and chemokines in GVHD pathobiology. Therefore, care must be taken when correlating different murine studies with clinical GVHD paradigms.
Table 1.
Variables in the study of experimental murine BMT.
| parameters | examples |
|---|---|
| intensity of conditioning regimen | no conditioning |
| sublethal conditioning without stem cell rescue | |
| myeloablative conditioning with stem cell rescue | |
| strain disparity | full major and minor MHC disparity |
| full or partial major MHC disparity | |
| minor MHC disparity | |
| parent into F1 | |
| donor graft composition | unfractionated splenocytes and/or lymph node cells |
| purified T cells | |
| purified CD4+ or CD8+ T cells | |
| endogenous microbiota | Helicobacter |
| orphan parvoviruses |
(c) Donor alloreactive T cell homing to GVHD target organs
There has been much interest in altering GVHD progression by affecting T cell trafficking and preventing T cells from arriving at the GVHD target organs. T cell homing involves a complex and coordinated response mediated by chemokines and the appropriate adhesion molecules (Wysocki et al. 2005). Chemokines are a large family of small proteins which function primarily as chemoattractants (Rollins 1997; Moser et al. 2004). The family is subdivided based on the number and position of NH2-terminal cysteine (C) residues. The majority of chemokines fall into the CC (CCL1-28) and CXC (CXCL1-16) subfamilies, while the C family contains only two (XCL1 and XCL2), and CX3 C only one member (CX3CL1; Murphy et al. 2000; Murphy 2002). It has been hypothesized that by selectively targeting certain chemokines involved in T cell trafficking to particular GVHD organs (i.e. skin, gut, liver and lung), GVHD pathology could be blunted without significant impairment of GVT effects, particularly with leukaemia/lymphoma. Since intestinal LNs and specifically Peyer's patches (PP) have been proposed to be critical for the induction of acute GVHD, blocking cellular migration into these organs represents a potential target for GVHD control (Murai et al. 2003). Studies have indicated that alloreactive T cells first home to secondary lymphoid organs such as PP after BMT but the role of these organs in the induction of GVHD was unclear (reviewed in Wysocki et al. 2005). Studies performed by Murai et al. which analysed recipients that received either no conditioning or non-myeloablative conditioning without HSC rescue found that the absence of PP resulted in significant blunting of acute GVHD (Murai et al. 2003). This murine model involved the transfer of parental lymphocytes into F1 hybrid recipients and produced only donor-anti-host T cell reactions under non-inflammatory or low inflammatory conditions. However, recently we have found that intensively conditioned tumour necrosis factor (TNF) alpha knockout (KO) recipients, which totally lack PP, developed acute GVHD affecting both gut and liver pathology that was unimpaired in comparison to wild-type mice (Welniak et al. submitted). These seemingly contradictory data suggest that the induction of proinflammatory cytokines/chemokines after extensive cytoreductive conditioning can have a dramatic impact on the necessity of secondary organs and chemokines in GVHD induction/progression. This was further illustrated by GVHD studies assessing the role of CCR5, a chemokine receptor that was found in the Murai et al. report to be essential for recruitment of donor T cells to PP. Using a similar GVHD model in which no cytoreductive conditioning was applied to examine the role of CCR5 in GVHD, it was reported that use of blocking antibodies to CCR5 resulted in significant abrogation of liver GVHD (Murai et al. 1999). In striking contrast, using models in which lethal cytoreductive conditioning was applied, donor T cells from CCR5KO mice resulted in GVHD that was markedly increased as compared to wild-type donor mice (Welniak et al. 2004). In another study also reporting increased GVHD using cells from CCR5 KO mice, it was found that extensive cytoreductive conditioning could affect the homing of immune suppressor CD4+25+ regulatory T cells (Tregs) which could be correlated with the increased GVHD when CCR5 KO donor T cells were used (Wysocki et al. 2004). Thus, caution must be exercised in interpreting the various GVHD models, and factors such as cytoreductive conditioning, strain combinations used, and donor cells transferred all appear to dramatically affect GVHD induction and progression resulting in apparently contradictory results being reported.
Other chemokines have been examined for roles in GVHD pathophysiology. Fully allogeneic donor CXCR3 KO T cells infused into lethally irradiated recipients resulted in fewer donor T cells in the gut and liver, albeit more in the spleen, resulting in less overall GVHD lethality (Duffner et al. 2003). Chemokines also regulate the homing and migration into the lung which is a target of immune mediated injury during GVHD responses. Donor T cells from RANTES (CCL5) KO mice given to lethally irradiated semi-allogeneic recipients resulted in less severe GVHD-induced lung injury as compared to wild-type donor T cells (Hildebrandt et al. 2004). In another GVHD-induced lung injury model system, the transfer of donor T cells from MIP-1α (CCL3) KO mice infused into mice conditioned with both chemotherapy (cyclophosphamide) and lethal irradiation resulted in accelerated lung pathology (Panoskaltsis-Mortari et al. 2003). In a GVHD model involving CD4-only mediated responses where the recipient mice succumb to bone marrow aplasia, T cells from CCR2 KO donors resulted in increased GVHD suggesting that this pathway may suppress GVHD, much like CCR5 (Rao et al. 2003).
Adhesion molecules which play a role in T cell trafficking have been studied as a potential target in GVHD prevention (i.e. ICAM-1/LFA-1; Blazar et al. 1995a). Blocking L-selectin has been demonstrated to contribute to the inhibition of acute GVHD (Li et al. 2001, 2004), which is perhaps not surprising considering the critical role this molecule plays in T cell trafficking to lymphoid organs. In this study, fully MHC-mismatched splenocytes were incubated with monoclonal antibodies (mAbs) to L-selectin and alpha4-integrin and were then transferred into mice with severe combined immune deficiency that received no conditioning. This resulted in delayed GVHD induction by reducing homing to LNs (Li et al. 2001). This is supported by data using irradiated F1 hybrid recipients of parental donor grafts in which the in vivo administration of anti-alpha4-integrin mAbs resulted in protection from gastrointestinal GVHD pathology (Tanaka et al. 1995). Recently, intriguing data looking at lymphocyte PP adhesion molecule (LPAM or alpha4 beta7 integrin) has suggested that organ-specific effects may be achieved. LPAM is responsible for T cell homing in gut-associated lymphoid tissues through binding the mucosal addressin cellular adhesion molecule (MAdCAM). Using donor T cells which were selected based on LPAM expression, the transfer of LPAM− cells resulted in significantly less gut GVHD compared to recipients receiving LPAM+ T cells (Petrovic et al. 2004). Importantly, GVT responses were maintained with the use of the LPAM− T cells. Thus, the potential of blocking T cell homing to particular GVHD target sites or adoptive transfer of T cells selected based on chemokine receptor or adhesion molecule expression offers great promise in the selective attack of this disease.
A pharmacological agent under investigation is FTY720, a sphingosine-1-phosphate receptor agonist which has been shown to be capable of trapping T cells in secondary lymphoid organs (Chiba et al. 1998). It has been recently shown that FTY720 administration can inhibit GVHD without compromising GVT in a murine BMT model. While GVHD organ pathology was blunted, anti-leukaemia effects were maintained (Kim et al. 2003). However, using a canine model, administration of FTY720, while offering some protection from GVHD, ultimately did not protect survival of recipients (Lee et al. 2003). Thus, while promising, more work on the dosing, timing, and pharmacokineticss of FTY720 in conditioned allogeneic recipients must be performed to optimize its ability to inhibit GVHD and there may be significant species differences of the particular role of chemokines and cytokines in GVHD. Taken together, these studies would suggest that altering the homing of the donor T cells via targeting chemokines/adhesion molecules may provide a means to selectively affect GVHD reactions, in particular organs, thus allowing for preservation of GVT depending on the location of the cancer.
2. Prevention and treatment of GVHD
(a) Targeting of intracellular signalling pathways as a means of preventing GVHD
Recently, through our increased understanding of the intracellular signalling pathways that regulate T cell responses and survival, targeting of such signalling pathways has been applied to inhibit T cell alloresponses, T cell survival and hence, target GVHD. Interestingly, several of the reagents were first evaluated for their ability to directly mediate anti-tumour effects, suggesting that not only preservation of GVT but also a direction induction of anti-tumour effects may be observed when this approach is applied with BMT. For example, histone deacetylase inhibitors (HDACs) have shown promise as anti-tumour agents. HDAC inhibitors increase the acetylation of histone lysine tails in chromatin and affect gene expression, both positively and negatively. One HDAC inhibitor, suberoylanide hydroxamic acid (SAHA), has been demonstrated to: (i) inhibit tumour cell growth (Butler et al. 2000; Almenara et al. 2002; Cohen et al. 2002b) and (ii) inhibit the production of proinflammatory cytokines (interleukin 1 (IL-1), TNF-α and interleukin 12 (IL-12)) in vivo (Leoni et al. 2002). Based upon these biological functions, Ferrara and colleagues evaluated the effects of SAHA on GVHD/GVT in a murine model. The administration of SAHA resulted in significant protection from GVHD in two different murine BMT models involving lethal conditioning. While a reduction of proinflammatory cytokines was observed, no direct inhibition of T cell responses was detected and GVT was spared (Reddy et al. 2004).
Another means to affect GVHD is by targeting the transcription factor nuclear factor kappa B (NFκB). As NFκB has long been known to play a critical role in T cell biology, particularly with respect to cytokine responses, it has been an attractive target but been hampered in finding reagents that have favourable pharmacokinetics resulting in systemic NFκB inhibition without toxicity. Recently, a new class of proteasome inhibitors have been developed which can be administered systemically without excessive toxicity. The proteasome plays a critical role in the degradation of inhibitory kappa B (IκB) which binds and inhibits NFκB activity. Thus proteasome inhibition results in suppression of NFkB although other cellular pathways are also affected. One inhibitor, bortezomib (Velcade; formerly PS-341), has been shown to have direct anti-tumour effects and has been recently approved in the treatment of multiple myeloma (Kane et al. 2003; Bross et al. 2004). We and others have found that bortezomib could also sensitize tumour cells to immune mediated killing (i.e. tumour necrosis factor related apoptosis-inducing ligand (TRAIL); Sayers et al. 2003). We have recently demonstrated that bortezomib given immediately after allogeneic BMT in mice could prevent GVHD (Sun et al. 2004). Bortezomib could inhibit alloreactive T cell proliferation and directly induce alloreactive T cell apoptosis in vitro and in vivo (Sun et al. 2004), which was associated with protection from GVHD and decreased proinflammatory cytokine production. Importantly, GVT effects were still observed in advanced tumour-bearing recipients. Direct cytotoxic T lymphocyte (CTL)-mediated killing was also enhanced with leukaemia cells exposed to bortezomib indicating that the anti-tumour effects could be both direct (i.e. induction of apoptosis) and indirect (increasing tumour cell susceptibility to immune effector cell mediated death pathways). Clinical studies assessing efficacy of bortezomib with allogeneic BMT in multiple myeloma patients are currently underway.
With both of these intracellular signalling pathway targeted approaches, several issues must be taken into consideration. First, both agents have demonstrated direct anti-tumour effects, potentially adding to the anti-tumour effects of cytoreductive conditioning given prior to BMT. In addition, via effects on immune effector cells, these agents may aid in immune-mediated GVT responses. These anti-tumour effects could therefore be obtained at doses much less than if the agent was administered in the absence of BMT. GVHD was reduced but not eliminated; however, such reduction did not preclude GVT responses. While the mechanism(s) responsible for GVHD inhibition have not been fully elucidated, both agents affect multiple cell-types, including immune and parenchymal cells, and this may affect the overall effect on GVHD (figure 1). Finally, as with many studies on GVHD protection, the timing of administration may be critical. In the case of bortezomib, the timing of administration is particularly critical as it appears extremely effective in preventing GVHD early, during the induction phase. Markedly opposing effects were observed when it was administered later during active GVHD in which a lethal GVHD-dependent gut toxicity resulted (Sun et al. 2005). Thus, an agent that can increase death receptor expression on the tumour cell can also increase it on target tissue and subsequently, different effects may be observed contingent on the timing of administration. A recent clinical trial in which multiple myeloma patients who have relapsed after allogeneic BMT were given bortezomib demonstrated no adverse effects but some anti-tumour responses were observed (Giralt et al. 2004). What makes these agents attractive is that prolonged administration may not be required and augmented anti-tumour effects may still be observed.
Another means of suppressing T cell responses is through targeting intracellular signalling pathways. Janus kinase 3 (JAK3) has been demonstrated to play a pivotal role in cytokine signalling of T cells. An inhibitor of JAK3 (by the WHI-P131/JANEX-1 compound) was administered to mice after allogeneic BMT and significant increases in survival were observed (Uckun et al. 2002). Importantly, GVT effects were maintained even though the compound itself had no intrinsic anti-tumour properties. We have also observed that targeting JAK3 on T cells ex vivo using specific inhibitors can result in significant inhibition of allo-responses and subsequent GVHD induction (O'Shaughnessy et al. submitted).
(b) Targeting cytokine responses as a means of preventing GVHD
Given the pivotal role of cytokines in GVHD pathobiology, it is perhaps no surprise that approaches focused upon regulating cytokine production and function have also been studied as a strategy to prevent GVHD (table 2). Blocking cytokines that may participate in GVHD has been a subject of intense investigation. The historical dogma on the role of cytokines in GVHD pathophysiology is that Th1-type cytokines (IFNγ, IL-12) fuel GVHD generation. Interestingly, in several recent studies, it has been shown that Th1 cytokines can be protective if present in high amounts in the very early stage after allogeneic BMT. For example, recipients of donor T cells from IFNγ KO mice were found to exhibit increased GVHD (Murphy et al. 1998). Interleukin 18 (IL-18) has also been shown to inhibit GVHD (Reddy et al. 2001, 2003) but recent studies suggest that this may be dependent on CD4 versus CD8-mediated responses (Min et al. 2004). One mechanism responsible for Th1 cytokine mediated GVHD protection has been the induction of fas-mediated activation-induced death of the donor alloreactive T cells (Sykes et al. 1990, 1995). At the other end of the spectrum, skewing donor T cells to Th2-type cytokine profile (interleukin-4 (IL4), interleukin-10 (IL-10)) responses has also been shown to be protective (Jung et al. 2003). However, the amount and timing of cytokines is likely to be critical to regulating GVHD; high doses of IL-10 can accelerate GVHD (Blazar et al. 1995b). Timing also is critical in influencing the outcome of Th1 cytokine effects on GVHD and long-term survival. For example, the timing of exogenous IL-12 administration in relationship to irradiation can either prevent GVHD or conversely result in rapid peri-BMT lethality (Sykes et al. 1999). Together, these data would suggest that cytokines may have effects, sometimes opposing, contingent on the phase of GVHD where they are applied and that caution must be exercised when attempting systemic administration or global blockade clinically.
Table 2.
Summary of cytokines and GVHD.
| cytokine/growth factor | effects on GVHD | mechanism |
|---|---|---|
| IL-2, IL-12, IFN-γ | inhibits GVHD early | inhibits by activation-induced cell death of alloreactive T cells |
| promotes GVHD late | promotes by augmenting Th1 responses | |
| IL4, IL10 | predominantly inhibits GVHD | promotes Th2 responses and inhibits Th1 |
| IL7 | variable: no effect on or worsening of GVHD | augments T cell responses/thymopoiesis |
| KGF | protects from GVHD | promotes tissue repair |
| TNF | promotes GVHD | increases tissue destruction and inflammatory responses |
Blocking TNF has shown to have efficacy in preclinical models (Wall & Sheehan 1994; Speiser et al. 1997; Brown et al. 2002; Korngold et al. 2003), although the effects may be contingent on the conditioning, strain combination, and donor cell transferred. It has been demonstrated that TNF can play a role in GVHD progression, particularly with the gut (Brown et al. 2002). Clinical trials evaluating efficacy of TNF blockade in acute GVHD progression have been pursued. Given the complex and dual role of TNF as an effector molecule in many protective immune responses to pathogens, resistance to opportunistic infections and effects on relapse are being scrutinized in these studies.
There has been much attention to not only controlling or modulating the T cell response but also tissue damage resulting from GVHD attack. One of the most promising cytokines, keratinocyte growth factor (KGF; a.k.a. fibroblast growth factor-7), has been demonstrated to affect GVHD by multiple pathways. The KGF receptor, fibroblast growth factor receptor II–IIIb is expressed on epithelial cells, which are targets of GVHD. KGF stimulates epithelial cell proliferation increasing the thickness of the epithelial cell layers especially of the gastrointestinal tract, which may permit epithelial cell rich target organs to better withstand GVHD mediated destruction. In rodents, KGF given prior to chemoradiotherapy conditioning clearly can blunt the tissue injury from GVHD attack (particularly in the gut and the lung) but can modulate T cell responses as well (Krijanovski et al. 1999; Panoskaltsis-Mortari et al. 2000). Graft versus leukaemia (GVL) responses were not impaired after KGF administration (Krijanovski et al. 1999). KGF administration has been shown to also promote thymic recovery due to the effect of KGF on thymic epithelial cells which are damaged by chemoradiotherapy conditioning regimens and are targets of GVHD (Min et al. 2002; Rossi et al. 2002). KGF was recently approved as a means of decreasing oral mucositis after HSCT (Spielberger et al. 2004), an effect likely to be the result of the initial induction of epithelial cell proliferation in the oral mucosa. Clinical studies assessing the effects of KGF in allogeneic BMT recipients are underway. Hepatocyte growth factor administration has also been recently shown to protect in liver GVHD, presumably by similar mechanisms as KGF (Kuroiwa et al. 2001; Imado et al. 2004).
Other cytokine infusions specifically are being investigated as means to diminish the period of immune suppression that occurs post-BMT. The potential impact on GVHD remains a paramount concern though. Similar to KGF, interleukin 7 (IL-7) has been shown to promote T cell recovery after BMT. IL-7 has been demonstrated to play a critical role in both thymopoiesis as well as peripheral T cell homeostasis (Alpdogan et al. 2001). Conversely, KGF administration has been shown to induce IL-7 mRNA expression in the thymus (Min et al. 2002). However, the role of IL-7 in GVHD has been conflicting with some reports demonstrating no effects and other showing increased GVHD in murine models (Alpdogan et al. 2001; Sinha et al. 2002; Gendelman et al. 2004).
(c) Targeting costimulatory pathway responses as a means of preventing GVHD
Allogeneic HCT provides a unique situation in which large numbers of donor immune cells are purposefully infused and exposed to allogeneic host MHC and miH antigens typically in the context of a conditioning-regimen induced tissue injury and inflammation which occurs throughout the body and requires several weeks to subside. Such inflammation results in the cell surface upregulation as well as the systemic release of alloantigens that are available for uptake by cells capable of supporting a T cell immune response such as DCs and macrophages. The net result of conditioning regimen injury is a rich environment in which donor T cells need not search far for alloantigen, cytokines or costimulation. Although pan-T cell depletion, if sufficiently rigorous, can prevent GVHD, the lack of mature T cells infused and the slow time to recovery of thymic-derived mature T cells renders the recipient susceptible to infections, tumour recurrence, and host anti-donor BM graft rejection responses. This section will focus on only those approaches that have been shown to affect acute GVHD mortality in lethally irradiated mice to best simulate the human myeloablative allogeneic BMT setting.
Given the important role of host DCs to acute GVHD induction (Teshima et al. 2002a; Zhang et al. 2002; Matte et al. 2004), one approach to GVHD prevention could target DCs for depletion. Since permanent donor and host DC depletion is not feasible yet, even in rodents, a preferred approach would be to block T cell–APC interaction or functions as can be accomplished via costimulatory pathway blockade. In solid organ graft rejection, costimulatory pathway blockade can be highly effective in preventing cardiac and to a lesser extent skin allografts rejection. In allogeneic BMT, positive results may be more difficult to achieve due to the extensive and widely distributed tissue injury caused by conditioning regimen injury. Perhaps the most substantial effects on reducing GVHD lethality have been seen by blocking the CD28/B7 pathway (table 3), which is critically involved in the initiation of T cell responses. Studies using donor CD28−/− mice or fusion proteins that bind B7 ligands, have shown that GVHD lethality is markedly inhibited but not uniformly eliminated in heavily conditioned mice (Blazar et al. 1994, 1996; Hakim et al. 1995; Saito et al. 1998; Yu et al. 1998). These reagents also block the binding of the B7 ligands to cytotoxic lymphocyte antigen-4 (CTLA-4; CD152), a molecule that is homologous to CD28, is expressed predominantly intracellularly, and is upregulated on activated T cells. CTLA-4 binds B7 ligands with higher affinity than CD28. In contrast to CD28/B7 interactions, CTLA-4 ligation confers a negative signal to the T cell to cause cell cycle arrest, terminating proliferation. Interruption of CTLA-4/B7 binding accelerates GVHD lethality (Blazar et al. 1999). In addition to the dual block of CD28/B7 and CTLA-4/B7 interactions, anti-B7 mAbs also may deplete the CD4+25+ Tregs. Since depletion of donor or host Tregs increases acute GVHD lethality (Salomon et al. 2000; Taylor et al. 2001a; Cohen et al. 2002a), the inhibitory effects of anti-B7 mAbs on GVHD initiation are partially offset by the blockade of CTLA-4/B7 pathway and depletion of Tregs. Because CD28/B7 interactions facilitates Treg generation in the thymus, CD28−/− mice have a paucity of Treg cells (Salomon et al. 2000). In a different approach, Yu and Anasetti have used anti-CD28 mAbs to inhibit GVHD generation and have made the surprising observation that alloreactive T cells were specifically depleted via an IFNγ dependent mechanism, opening up new possibilities for selectively targeting the CD28 pathway (Yu et al. 1999, 2000). Moreover, these investigators recently have demonstrated that the combination of CD28 blockade with the pharmacological agent, rapamycin (sirolimus), which binds to the molecular target of rapamycin (mTOR), produced superior responses in inhibiting GVHD (Albert et al. 2005) as compared to either agent alone.
Table 3.
CD28 costimulatory receptor family.
| Receptor: CD28 family | other names | function | expression | ligand: by family | other names | expression |
|---|---|---|---|---|---|---|
| CD28 | T44 | costimulation | T and NK cells | B7.1 | CD80 | activated APC |
| B7.2 | CD86 | APC (upregulated) activated T cells | ||||
| CTLA-4 | CD152 | inhibition | activated T cells | B7.2 | CD86 | APC (upregulated) activated T cells |
| ICOS | H4, CRP-1, AILIM | costimulation | activated T cells | B7RP-1 | ICOSL, GL50, B7-H2, B7h | APC |
| ? | B7-H3 | B, T and NK cells, macrophages, DCs |
In contrast to the CD28 pathway, CD40 ligand (CD154) is expressed on alloactivated CD4+ and not CD8+ T cells. The CD154–CD40 pathway (table 4) directly regulates CD4+ T cell and typically only indirectly affects CD8+ T cell alloresponses. Although anti-CD154 mAbs or the use of CD154−/− CD4+ T cells reduces GVHD lethality, typically about one-half of mice will survive under heavy irradiation conditions and no biological effects are notable in models in which only CD8+ T cells are infused (Blazar et al. 1997). However, in situations in which CD4+ T cells are coinfused to drive CD8+ T cell expansion, signals delivered via this pathway to CD4+ T cells are needed to optimally support CD8+ T cell expansion and GVHD generation (Buhlmann et al. 1999). Recent evidence exists that the effects of CD154/CD40 pathway blockade, dependent upon the conditions, are mediated in part or in whole via Treg cell suppression of alloresponses. In vitro depletion of Tregs from the responding CD4+ T cell population in an allogeneic mixed lymphocyte reaction (MLR) culture precludes the induction of alloantigen hyporesponsiveness by CD154/CD40 pathway (and CD28/CTLA-4–B7 pathway) blockade (Taylor et al. 2001a). Similarly, the in vivo induction of hyporesponsiveness to alloantigen by CD154–CD40 pathway blockade combined with donor specific transfusion also can be dependent upon the presence of Tregs (Jarvinen et al. 2003). The clinical application of anti-CD154 mAb in allogeneic BMT will likely await results of anti-CD154 mAb studies in patients with autoimmunity and solid organ transplants.
Table 4.
Members of the TNF receptor superfamily associated with GVHD.
| TNFR family | other names | function | expression | ligand: TNF family | other names | expression |
|---|---|---|---|---|---|---|
| CD27 | T14 | costimulation | T cells, B subset, NK | CD70 | activated B cells | |
| CD30 | Ki-1 | costimulation, apoptosis | activated T, NK and B cells | CD153 | CD30L | neutrophils, activated B and T cells |
| CD40 | activation | APC, T subset, endothelium, cardiac myocytes, fibroblasts | CD40L | CD154, gp39 TRAP | activated T cells | |
| 4-1BB | CD137 | costimulation | activated T cells | 4-1BBL | activated B, DC, peritoneal cells | |
| OX40 | CD134 | activation, differentiation, apoptosis | activated T cells | OX40L | activated B cells, cardiac myocytes | |
| Fas | CD95, Apo-1 | activation, apoptosis | leukocytes | FasL | CD95L, CD178 | activated T cells |
CD134 is expressed on activated CD4+ and CD8+ T cells in rodents and humans (Mallett et al. 1990; Calderhead et al. 1993; Baum et al. 1994a,b; Latza et al. 1994; Birkeland et al. 1995). Nonetheless, much of the literature indicates that CD134–CD134 ligand (CD134L) interactions may play a more critical role in CD4+ T cell responses than in CD8 T cell responses. CD134 receptor engagement promotes CD4+ T cells to proliferate, produce Th1 and Th2 cytokines and anti-apoptotic proteins, to clonally expand, survive, and develop into memory cells (Flynn et al. 1998; Gramaglia et al. 1998, 2000; Ohshima et al. 1998; Weinberg 1998, 2002; Chen et al. 1999; Kopf et al. 1999; Pippig et al. 1999; Akiba et al. 2000; Murata et al. 2000; Rogers & Croft 2000; Weinberg et al. 2000; Bansal-Pakala et al. 2001; Evans et al. 2001; Rogers et al. 2001; Weatherill et al. 2001; De Smedt et al. 2002). CD134L is expressed on APCs that have been activated by known stimuli such as CD40 or proinflammatory mediators (Stuber et al. 1995; Ohshima et al. 1997; Brocker et al. 1999). Anti-CD134L mAb was able to ameliorate GVHD-mediated lethality (Tsukada et al. 2000; Blazar et al. 2003). Some studies have indicated that CD134 is critical for inducing Th2 responses. However, CD134/CD134L blockade was effective in reducing GVHD mediated by Th1- or Th2-deficient splenocytes as well as when using donor CD28−/−, splenocytes indicating non-redundancy between the CD134 and CD28 pathways. Coblocking CD154/CD40 and CD134/CD134L pathways was superior to either pathway blockade alone or combined CD154/CD40 and CD28/CD152–B7 blockade in a full MHC disparate strain combination (Patricia A. Taylor and BRB, unpublished data).
Similar to CD28 and CD134, 4-1BB (CD137) is expressed on activated CD8+ and CD4+ T cells, in addition to NK cells (Kwon & Weissman 1989; Pollok et al. 1993; Melero et al. 1998). Early data suggested that the CD137–CD137L pathway plays a more prominent role in CD8+ T cell responses than in CD4+ T cell responses (Shuford et al. 1997; Takahashi et al. 1999; Tan et al. 1999, 2000). CD137 signals induce T cells to produce interleukin 2 (IL-2), proliferate, and differentiate, and protect T cells from activation-induced cell death (ACID; Goodwin et al. 1993; Pollok et al. 1993; Alderson et al. 1994; Hurtado et al. 1995, 1997; Saoulli et al. 1998; DeBenedette et al. 1999; Takahashi et al. 1999). CD137 ligand (CD137L) is expressed on APCs (Pollok et al. 1994; DeBenedette et al. 1997). Antagonistic anti-CD137L mAbs significantly reduced GVHD-induced mortality in a lethally irradiated model of acute GVHD (Nozawa et al. 2001). Expansion of donor CD8+ T cells, but not CD4+ T cells, was reduced in anti-CD137L mAb-treated mice. Anti-CD137L mAb ameliorated lethality that was associated with impaired donor CD8+ T cell expansion and IFNγ production without significant effects on CD4+ T cell expansion. Although signalling via CD137 had been reported to be a relatively weak agonist for CD4+ cells (Shuford et al. 1997), we found that the CD137–CD137L pathway was important in regulating GVHD by CD4+ or CD8+ T cells (Blazar et al. 2001a). Taken together these studies indicate that the CD137/CD137L pathway regulates acute GVHD caused by CD8+ T cells and CD4+ Th1 cell generation, whereas the effects of this pathway on CD4+ T cell mediated GVHD lethality may be dependent upon the particular donor-recipient strains tested and conditions used for acute GVHD generation.
Inducible costimulator (ICOS) is expressed on CD4+ and CD8+ T cells within 24–48 h after activation (Hutloff et al. 1999; Coyle et al. 2000; Kopf et al. 2000; Sperling & Bluestone 2001). The ligand for ICOS (ICOS-L is constitutively expressed by B cells, monocytes and some T cells; Swallow et al. 1999; Yoshinaga et al. 1999; Aicher et al. 2000; Ling et al. 2000; Wang et al. 2000; Gonzalo et al. 2001b; Ling et al. 2001; Sperling & Bluestone 2001). Much but not all of the literature indicate that ICOS/ICOS-L ligation has a preferential effect on inducing Th2 rather than Th1 cytokines (Hutloff et al. 1999; Yoshinaga et al. 1999, 2000; Coyle et al. 2000; McAdam et al. 2000; Coyle & Gutierrez-Ramos 2001; Dong et al. 2001; Gonzalo et al. 2001a; McAdam et al. 2001; Sperling & Bluestone 2001; Tafuri et al. 2001; Tamura et al. 2001; Tesciuba et al. 2001; Khayyamian et al. 2002). The inducible expression of ICOS shortly after T cell activation suggests that ICOS may be particularly important in providing costimulatory signals to activated T cells or memory cells (Hutloff et al. 1999; Yoshinaga et al. 1999; Coyle & Gutierrez-Ramos 2001). ICOS signalling does not appear to be required for naïve cell responses with the exception of superantigen (Gonzalo et al. 2001a). ICOS-immunoglobulin (ICOS-Ig) infusion has more pronounced effects when given during induction of the secondary rather than primary immune responses in contrast to CTLA-immunoglobulin (CTLA4-Ig), a fusion protein that binds to B7 ligands thereby reducing or eliminating B7 ligand binding to their endogenous receptors (Coyle et al. 2000; Gonzalo et al. 2001b). ICOS/ICOS-L has been shown to reduce CD4+ and CD8+ T cell mediated GVHD under lethal irradiation conditions (Taylor et al. 2004a). Delaying ICOS blockade until day 5 post-BMT, a time in which T cells have upregulated ICOS expression and have dramatically expanded and gained effector function, improved outcome but did not prevent eventual GVHD-induced lethality. In contrast, anti-CD154 mAb, which is highly protective when initiated at time of BMT, had no effect when mAb infusion was given at that time. Survival was significantly improved by combining anti-ICOS and anti-CD154 mAbs. Thus, ICOS/ICOS-L blockade may be an especially attractive target for downregulating T cell responses once they have been initiated and for use in combined costimulatory pathway blockade.
CD30 is expressed on B cells and mitogen-stimulated T cells (Durkop et al. 1992; Shanebeck et al. 1995; Bowen et al. 1996). CD30 expression is induced on CD8+ and to a lesser extent on CD4+ T cells by alloantigen exposure (Bowen et al. 1996; Martinez et al. 1998; Chan et al. 2002). CD30 signalling regulates CTL generation and also renders T cells sensitive to cell death (Amakawa et al. 1996; Duckett & Thompson 1997; Telford et al. 1997; Chiarle et al. 1999; Kurts et al. 1999). CD30 ligand (CD30L, CD153) is expressed on macrophages, activated B cells and T cells, primarily CD4+ T cells of both Th1 and Th2 phenotype, and can provide signals for B cell growth and differentiation (Shanebeck et al. 1995; Bowen et al. 1996; Wiley et al. 1996; Shimozato et al. 1999). The CD30/CD153 pathway is a potent regulator of CD4+ but not CD8+ T cell-mediated GVHD. Although blocking CD30/CD153 interactions in vivo did not affect alloreactive CD4+ T cell proliferation or apoptosis, a substantial reduction in donor CD4+ T cell migration into the gastrointestinal tract, a GVHD target organ, was readily observed with lesser effects in other GVHD organs. Thus, blockade of the CD30/CD153 pathway represents a viable approach for preventing CD4+ but not CD8+ T cell-mediated GVHD.
Similar to CD152, programmed death-1 (PD-1) is a negative costimulatory pathway with two known ligands: PD-L1 and PD-L2 (table 5; Ishida et al. 1992; Shinohara et al. 1994; Agata et al. 1996; Nishimura et al. 1999; Freeman et al. 2000; Kingsbury et al. 2001; Latchman et al. 2001; Tseng et al. 2001). The expression of PD-1 is induced on mature peripheral T cells, B cells and myeloid cells upon activation (Ishida et al. 1992; Agata et al. 1996; Vibhakar et al. 1997) PD-1 and CD152 have structural similarities and PD-1 has an immunoreceptor tyrosine based inhibitory action suggesting an inhibitory function (Ishida et al. 1992; Shinohara et al. 1994; Vivier & Daeron 1997; Coyle & Gutierrez-Ramos 2001; Sharpe & Freeman 2002). PD-1 ligand, PD-L1, also termed B7-H1, is constitutively expressed on DCs, some activated CD3+ cells, and IFNγ stimulated monocytes and keratinocytes (Freeman et al. 2000) while PD-L2, also termed B7-DC, is constitutively expressed on resting monocytes (Latchman et al. 2001; Tseng et al. 2001). Some tissues (lung, liver, pancreas) express PD-L2 but not PD-L1, whereas others (heart, skeletal muscle) express both. Although investigators have reported that T cell proliferation is enhanced by the PD-1 pathway in vitro under some conditions, others have demonstrated that the dominant function of the PD-1/PD-L pathway is to inhibit T cell responses (Dong et al. 1999; Freeman et al. 2000; Latchman et al. 2001; Tseng et al. 2001). In lethally irradiated recipients of full MHC-disparate donor T cells, PD-1 infiltrating cells were found in increased frequency in GVHD target organs as compared to BMT controls (Blazar et al. 2002). Blockade of the PD-1/PD1-L pathway significantly augmented GVHD mortality. The combined administration of anti-CD152 and anti-PD-1 mAbs was significantly more potent than either alone in accelerating GVHD lethality, indicating that these pathways are not fully redundant. Reagents that may provide a signal to PD-1+ T cells may be of benefit in inhibiting GVHD.
Table 5.
Inhibitory receptors of the B7 family.
| inhibitory molecules | other names | function | expression | ligand: B7 family | other names | expression |
|---|---|---|---|---|---|---|
| PD-1 | inhibition | activated T, B cells and macrophages | PD-L1 | B7-H1 | leukocytes | |
| PD-L2 | B7-DC | monocytes, macrophages, DC | ||||
| CTLA-4 | CD152 | inhibition | activated T cells | B7.1 | CD80 | activated APC |
| B7.2 | CD86 | APC (upregulated) activated T cells |
It is important to point out several factors that must be considered before applying these principles to humans. An important consideration for in vivo costimulatory pathway blockade is that such an approach is likely to be added to conventional immune suppressive pharmacological agents. In order for costimulatory pathway blockade to be effective, donor T cells must receive sufficient TCR signalling. Calcineurin inhibitors such as cyclosporin A or tacrolimus (FK506) blunt effective TCR signalling which may interfere with tolerance induction by blocking T cell apoptosis whilst blocking T cell proliferation. In contrast, sirolimus (rapamycin) as a target interferes with cytokine responsiveness and not TCR signals. Studies in rodents have shown that sirolimus does not interfere with tolerance induction in vivo and in fact massively increases the proportion of proliferating cells destined for apoptosis thereby limiting the size of alloreactive T cells potentially below a threshold level required to cause GVHD (Li et al. 1999). One note of caution in extrapolating these studies from the solid organ grafting to the GVHD arena is that the level of TCR signalling and tissue destruction may not preclude the use of calcineurin inhibitors should T cell proliferation induced by these proinflammatory events proceed, albeit at a reduced level, if such inhibitors merely serve to dampen TCR signals to levels that still exceed a threshold response. Testing in the context of GVHD in rodents and in humans will be required before conclusions can be reached as to whether calcineurin inhibitors are contraindicated in such situations, especially considering that some but not all non-myeloablated BMT rodent models have shown that calcineurin inhibitors can be additive with costimulatory pathway blockade in promoting alloengraftment.
While anti-B7 mAbs have been tested in vivo in solid organ patients and in vivo CTLA4-Ig and anti-CD154 mAb in patients with autoimmune disorders, these reagents have not been tested in vivo in the context of allogeneic BMT and the status of clinical testing of reagents for the other pathways in patients undergoing BMT remains uncertain. The BMT setting poses additional challenges for costimulatory pathway blockade. For example, strategies that block GVHD but have detrimental effects on alloengraftment, GVT effects, or immune recovery must be carefully considered before use in allogeneic BMT patients. Factors found in rodents that may adversely affect the efficacy of mAbs or fusion proteins to block T cell costimulation early post-BMT in fully myeloablated recipients include: (i) the recent observations that profound lymphopenia, which induces homeostatic T cell proliferation to fill up the lymphoid compartment, may be a significant barrier to tolerance induction via costimulatory pathway blockade (Kreisel et al. 2002); (ii) heterologous immunity, a response to an infectious pathogen that can preclude tolerance induction (Williams et al. 2001). Since all fully myeloablated BMT patients have a period of profound lymphopenia and viral infection can occur early post-BMT, although typically significant viral infections do not occur in the first several weeks post-BMT, should the rodent data be translatable to the clinical arena, the potency of costimulatory pathway blockade may be more limited in humans than rodents. Nonetheless, blockade of T cell costimulation may prove to be an important new approach to specifically target those T cells that are alloreactive, especially when used early post-BMT when tumour and viral antigens may be present at far lower amounts than alloantigens.
(d) GVHD prevention by ex vivo approaches
The prior discussions have focused on in vivo mAbs or fusion proteins administration. An ex vivo tolerization approach involves the culturing of donor T cells with host alloantigen in the presence of mAbs or proteins that are capable of blocking T cell costimulation. An ex vivo tolerization procedure has several theoretical advantages over in vivo tolerization attempts. These include: higher likelihood of tolerance induction by ensuring costimulatory pathway blockade is delivered to the site of T cell–APC interactions; achieving tolerance induction before in vivo infusion into an inflammatory milieu which may diminish the likelihood of tolerance induction; capacity to monitor the depth of tolerance induction in vitro; avoidance of potential in vivo toxicities of mAbs or proteins.
We have described an ex vivo approach in which the blockade of the CD154/CD40 or CD28/CD152–B7 pathways added to a 7 to 10-day MLR culture of CD4+ T cells and irradiated MHC class II-disparate stimulators induces tolerance leading to complete GVHD prevention (Blazar et al. 1998; Taylor et al. 2000, 2001b, 2002a). Tolerant CD4+ T cells were hyporesponsive to initial alloantigen challenge and to restimulation in vitro. Tolerance was long-lived and not readily reversible in vivo (Blazar et al. 1998). Tolerance induction resulted in the generation of potent immunoregulatory CD4+25+ T cells that could inhibit both naïve and primed alloresponses as assessed by in vitro and in vivo assays (Taylor et al. 2002a). With respect to the latter, the separate coinfusion of tolerized cells with an otherwise uniformly lethal dose of naïve CD4+ cells prevented GVHD mortality in 75% of mice. The generation of this regulatory capacity during tolerization may be of added benefit by the provision of a fail-safe mechanism to control alloreactive T cells that may escape tolerization. In a clinical trial, donor BM cocultured with irradiated recipient in the presence of CTLA4Ig (Guinan et al. 1999) resulted in a reduction in the frequency of T cells capable of recognizing host alloantigens. The incidence and severity of acute GVHD appeared to be reduced from what might be expected after taking into account the degree of HLA disparity and the number of mature T cells. In a different ex vivo approach, we have shown that CD4+ T cells can be rendered tolerant via exposure to immunoregulatory cytokines (e.g. IL-10 and transforming growth factor-beta, TGFβ; Zeller et al. 1999; Boussiotis et al. 2001; Chen et al. 2003). Tolerization leads to GVHD prevention in most recipients and to the induction of suppressor cells that inhibited naïve T cell mediated GVHD (Zeller et al. 1999; Chen et al. 2003). A clinical trial in humans is underway in a haploidentical BMT setting using exogenous IL-10 to tolerize donor T cells to host alloantigens in vitro before infusing these cells later post-BMT (Bacchetta et al. 2000).
Amongst other approaches that have been explored that show promise include the depletion of alloreactive T cells by affinity columns, magnetic beads, or toxins that bind to T cells expressing activation antigens (CD69; CD25; IFNγ; Montagna et al. 1999; Koh et al. 2002; Solomon et al. 2002; Amrolia et al. 2003). In a different approach, Chen et al. describe the application of a photoactive rhodamine derivative [4,5-dibromorhodamine 123 (TH9402)], that is selectively retained in the mitochondria of activated T cells added to MLR cultures (Chen et al. 2002). Upon exposure to visible light this depletes the TH9402-enriched activated host-reactive cells in the MLR culture. Treatment with photodynamic cell purging process inhibited anti-host CTL and IFNγ responses and importantly prevented GVHD lethality.
Recent data show that memory T cells in rodents have a markedly reduced capacity to cause GVHD lethality (Anderson et al. 2003). These studies have demonstrated that effector memory CD4 T cells (CD62L−, CD44+) induced less GVHD when compared with unfractionated or naïve CD4+ T cells (Anderson et al. 2003). Importantly, engraftment occurred and generation of antigen-specific responses resulted. The observations that the CD62L− T cell infusion can result in markedly reduced GVHD lethality capacity in rodents has been confirmed by others (Chen et al. 2004), and extended into the human preclinical setting with data showing that human CD62L− T cells are less potent in producing MLR responses in vitro when compared to CD62L+ T cells (Foster et al. 2004). These data are intriguing in that assessment of TCR usage showed limited diversity in the memory population. These data also would suggest that isolation and transfer of these cells could prevent GVHD and allow for GVT to be maintained should these data in human in vitro cultures be as reproducible as initial studies have been in rodents.
(e) Cellular therapies to prevent GVHD (table 6)
Table 6.
Cellular therapy in GVHD.
| cells | mechanism of suppression |
|---|---|
| Tregs | suppression of alloreactive T cells |
| NK cells | elimination of host DCs, production of TGF-β and IFN-γ |
| NK/T cells | IL4 production |
| myeloid suppressor cells | inhibition of alloreactive T cell responsiveness |
| mesenchymal stem cells | suppression of alloreactive T cells |
| promotion of tissue repair? |
(i) T regulatory cells (Tregs)
A variety of murine cells that display regulatory function in vitro or in vivo have been described and a review of all types is outside the scope of this manuscript. Instead, we will focus upon CD4+25+ Treg cells shown to have potent suppressor activity (Sakaguchi et al. 1995, 2001; Suri-Payer et al. 1998; Thornton & Shevach 1998; Baecher-Allan et al. 2001; Dieckmann et al. 2001; Jonuleit et al. 2001; Levings et al. 2001; Shevach 2001, 2002; Shevach et al. 2001; Jonuleit et al. 2002). In rodents, this population comprises 8–12% of lymph node CD4+ T cells in most strains, whereas in humans, CD4+CD25bright cells are present in far lower frequencies (1–3% of peripheral blood). There has been agreement that donor or host Treg cell depletion accelerates GVHD and Treg cell add-back inhibits acute GVHD lethality (Cohen et al. 2002a; Hoffmann et al. 2002a,b; Taylor et al. 2002b, 2004b; Jones et al. 2003; Trenado et al. 2003). Whereas fresh Treg cell add-back has had biological effects in reducing GVHD, some studies have shown more striking anti-GVHD effects than others. In direct comparative studies, ex vivo expanded and hence activated Tregs are more effective on a cell-to-cell basis than fresh cells whether Tregs are expanded using polyclonal stimulators (anti-CD3 mAb) or recipient APCs to cross-link the TCR (Taylor et al. 2002b; Jones et al. 2003). These data are consistent with the findings that activated Tregs suppress more potently than resting Tregs (Thornton & Shevach 2000). The additional advantage of ex vivo expansion prior to infusion is to increase the number of Treg in human blood that would be available for infusion. While Tregs can be generated against specific antigens including alloantigens, once activated Tregs can suppress alloresponses in an antigen-non-specific fashion (Godfrey et al. 2005). Nonetheless, it is possible that recipient-specific Tregs may be advantageous as compared to polyclonal Tregs, at least on a per cell basis, if TCR ligation in vivo is required to sustain Treg persistence or increase suppressor cell function. In that event, anti-recipient specific Tregs may be reactivated by host cells in vivo although Treg cell persistence may not necessarily be desirable in patients if such persistence results in global immune suppression during the time of infectious challenge or early tumour recurrence eliminating GVT. Studies in rodents will be needed to address these issues first. In addition, acquiring non-malignant, non-infected host APCs will be needed and, in some instances, the potency of in vitro stimulation of Tregs against host alloantigens (e.g. in miH antigen alone) may be insufficient to permit full expansion or suppressor cell function (table 6).
Strategies have been developed to expand human peripheral blood or cord blood Tregs using a bead-based approach (Godfrey et al. 2004, 2005; Hoffmann et al. 2004). In the majority of these protocols, such purified Tregs will be polyclonally expanded and infused into recipients to prevent GVHD. It will be important to ensure that Treg cells are suppressive after expansion and to utilize available expansion approaches or develop new approaches that lead to the retention of appropriate homing receptors such as CD62L (L-selectin) that in rodents have been shown to improve GVHD prevention via trafficking of Treg cells to putative sites of GVHD initiation (Taylor et al. 2004b). Such trials should commence this year. Lastly, it is worth noting that in contrast to some other forms of GVHD prevention, Treg cell infusions have increased alloengraftment by suppressing host anti-donor interactions and, in most instances, have not eliminated the GVL effect of allogeneic BMT against haematopoietic tumours (Edinger et al. 2003; Jones et al. 2003; Trenado et al. 2003; Joffre et al. 2004; Taylor et al. 2004a,b; Hanash & Levy 2005). GVHD-induced immune suppression has been diminished by Treg cell infusion (Trenado et al. 2003), an approach that might also reduce the need for post-BMT immune suppressive drugs or T cell depletion.
(ii) NK and NK/T cells
Multiple other immunomodulating cell-types are being assessed for their ability to impede GVHD yet still allow for GVT effects. NK and NK/T cells have both been demonstrated to exert such effects. Donor-type NK cells have been demonstrated in preclinical models to inhibit GVHD and promote GVT (Asai et al. 1998). The mechanism underlying this protection is not definitively known. Studies have demonstrated that transforming growth factor-beta (TGF-β), an immunosuppressive cytokine, may be at least partially responsible for the suppression of alloreactivity in this model. Recent studies have also suggested that donor NK cells can attack host DCs which have been shown to play an important role in GVHD (Shlomchik et al. 1999; Zhang et al. 2002; Matte et al. 2004; Anderson et al. 2005). It is possible that all of these pathways may play a role. Interestingly, studies have suggested that the timing of NK cell transfer is also important, paralleling the studies with proteasome inhibition in that NK cell administration needs to be early after BMT. When donor-type NK cells were administered later during ongoing GVHD, increased GVHD mortality was observed (Asai et al. 1998). This is similar to reported results in earlier studies administering IL-2 and IL-12 which showed protection only when given early after BMT (Sykes et al. 1990, 1995).
The characterization of NK cell subsets bearing receptors for MHC and other determinants has revolutionized our understanding of their biology and also how to potentially exploit their clinical potential. NK cells bear inhibitory and activating receptors directed to MHC and other determinants (killing immunoglobin-like receptors (KIR) in humans, Ly49 in mouse, and NKG2D in both), which significantly affects their responses. Clinical BMT studies using KIR ligand mismatch combinations demonstrated heightened anti-tumour effects without increased GVHD in acute myelocytic leukaemia (AML; Ruggeri et al. 2002). Some clinical BMT studies failed to confirm these results but significant differences in the conditioning and BMT protocols may have affected NK cell recovery and ultimate outcome (Davies et al. 2002). Recently, a clinical study examining adoptive transfer of donor-type NK cells after allogeneic BMT yielded encouraging results in which increased anti-tumour effects without increased GVHD (Koehl et al. 2004). These studies suggest that NK cells, either as adoptive immunotherapy or using means to augment their recovery after BMT, may be of use to prevent GVHD and promote GVT responses. Further characterization and exploitation of NK cell subsets may allow for augmentation of these protective effects.
NK/T cells, a subset of lymphocytes which exhibit markers present on both NK and T cells, are functionally heterogeneous. The majority of murine NK/T cells have restricted TCR expression which recognizes glycolipid antigens in the context of the MHC-like molecule, CD1d expressed by APCs (Zhou et al. 2004). They have previously been demonstrated to inhibit GVHD through the production of IL-4 (Hashimoto et al. 2005). A recent study used a means of activating them with the injection of a synthetic ligand, alpha-galactoceramide (alpha-Gal Cer) after allogeneic BMT. Significant skewing to Th2-type cytokine production (IL4) occurred and inhibition of GVHD resulted, suggesting that adoptive transfer of NK/T cells or simply their activation may suppress GVHD (Hashimoto et al. 2005). Interestingly, use of this same ligand in resting mice has been reported to promote Th1-type cytokine responses, suggesting that perhaps the conditioning in BMT models alters the biologic properties of the NK/T cells in response to alpha-Gal Cer. As many of the anti-tumour effects of NK/T cells appear to be due to their ability to promote Th1-type responses, it will be of interest to delineate and reconcile the immunosuppressive ability of the cells in GVHD (via production of Th2 cytokines) combined with their anti-tumour effects.
(iii) Regulation of immune function by manipulating APCs
APCs represent a heterogeneous population of cells with regard to various subsets (lymphoid DCs, myeloid DCs, plasmacytoid DC precursors) capable of mediating distinct effects on immune responses. Using SHIP−/− mice which have an increased population of myeloid suppressor cells (MySC), it was demonstrated that impaired alloreactive responses occurred and protection from GVHD resulted in the recipients (Wang et al. 2002; Ghansah et al. 2004). A similar murine MySC expressing Mac1, Ly6-G, Ly6-C was also demonstrated to protect from GVHD (Billiau et al. 2003). Finally, the pre-BMT depletion of host APCs in the skin and in particular Langerhan's cells has been shown, as the skin is a highly accessible site for manipulation. Langerhan cell depletion prevented GVHD in the skin of mice (Merad et al. 2004), suggesting that it may be possible to perform procedures that target these cells prior and during the transplant and reduce GVHD pathology in the skin.
Cytokines such as Flt3 ligand have also been given to recipients and shown to inhibit GVHD through the induction of CD8 alpha-positive DCs, again suggesting that exploiting the immunodulatory properties of APC subsets may be of use in GVHD (Teshima et al. 2002b), although the post-BMT administration of Flt3 ligand can paradoxically increase acute GVHD in other models (Blazar et al. 2001b). Recent data suggests, at least for chronic GVHD, that both donor and host APCs may play a role, suggesting that both may need to be targeted and that clinically both may of import in acute GVHD as well (Anderson et al. 2005), thus donor APC depletion may be advantageous in GVHD prevention. In all of these studies, effects on GVHD must be balanced with potential adverse effects of GVT.
(iv) Mesenchymal stem cells (MSCs)
Another promising cell therapy that may prevent or treat GVHD are derived from non-haematopoietic stem cells such as mesenchymal stem cells or stromal cells (MSCs) which are an adherent, CD45− BM cell population that can differentiate into a wide-spectrum of cells.
An intriguing feature of MSCs is their potency in inhibiting T cell responses. MSCs can express intermediate levels of MHC class I which is inducible to high levels with IFNγ (Le Blanc et al. 2003) but do not express MHC class II (Di Nicola et al. 2002; Tse et al. 2003) and express low levels of B7 ligands and CD40 (Tse et al. 2003). All but Potian et al. (2003) have shown that irradiated MSCs do not elicit vigorous T cell proliferative responses when used as stimulators (Le Blanc et al. 2003; Tse et al. 2003; Meisel et al. 2004). Human MSCs (hMSCs) are poor allostimulators as has been observed even after differentiation in osteogenic, chondrogenic or adipogenic media (Le Blanc et al. 2003). Autologous and allogeneic human BM stromal cells suppressed both CD4+ and CD8+ T cell proliferation in MLR and mitogen assays. A multi-center non-randomized trial of donor MSC infusion given at the time of BMT has been initiated to inhibit GVHD (Urbano-Ispizua et al. 2002). Acute and chronic GVHD risk appeared significantly reduced compared to historical pair-matched controls. A single case report in the literature provides intriguing evidence that MSCs can be used to treat steroid-resistant GVHD. Given the immune suppressive capacity of MSCs, it may be possible to use third-party MSCs as a universal donor source to prevent or treat GVHD.
3. Future directions
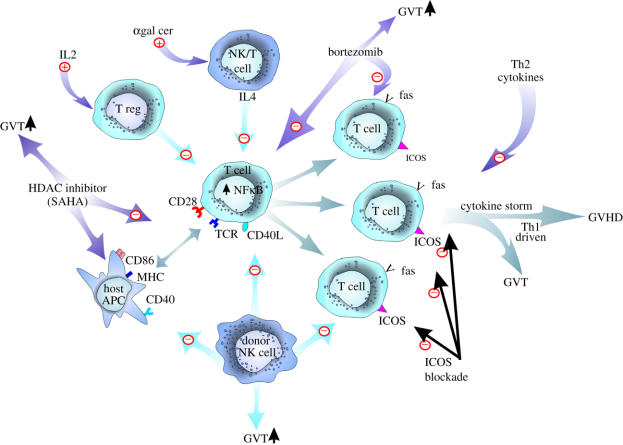
There has been considerable progress in our understanding of GVHD and development of means to overcome this BMT complication without sacrificing GVT. Major areas under intense investigation currently have focused on exploiting Treg transfer, molecular targeting of T cell and GVHD responses, understanding the homing of T cells in part through the use of innovative imaging technologies, use of NK cells, and attempting to understand potential organ-specific responses and effects of interventions (figure 2). As more is understood on basic GVHD processes, T cell biology, and disassociating GVHD with GVT, the likelihood for increased efficacy of BMT looms larger.
Figure 2.
Current means to prevent GVHD.
Acknowledgments
Supported in part by NIH grants R01 AI34495, 2R37 HL56067, R01 HL63452, R01 CA72669, RO1 AG022661 and R01 CA102282.
Footnotes
One contribution of 16 to a Theme Issue ‘Immunoregulation: harnessing T cell biology for therapeutic benefit’.
References
- Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, Honjo T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- Aicher A, Hayden-Ledbetter M, Brady W.A, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter J.A, Clark E.A. Characterization of human inducible costimulator ligand expression and function. J. Immunol. 2000;164:4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- Akiba H, et al. Critical contribution of OX40 ligand to T helper cell type 2 differentiation in experimental leishmaniasis. J. Exp. Med. 2000;191:375–380. doi: 10.1084/jem.191.2.375. doi:10.1084/jem.191.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M.H, Yu X.Z, Martin P.J, Anasetti C. Prevention of lethal acute GVHD with an agonistic CD28 antibody and rapamycin. Blood. 2005;105:1355–1361. doi: 10.1182/blood-2004-08-3305. doi:10.1182/blood-2004-08-3305 [DOI] [PubMed] [Google Scholar]
- Alderson M.R, et al. Molecular and biological characterization of human 4-1BB and its ligand. Eur. J. Immunol. 1994;24:2219–2227. doi: 10.1002/eji.1830240943. [DOI] [PubMed] [Google Scholar]
- Almenara J, Rosato R, Grant S. Synergistic induction of mitochondrial damage and apoptosis in human leukemia cells by flavopiridol and the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) Leukemia. 2002;16:1331–1343. doi: 10.1038/sj.leu.2402535. doi:10.1038/sj.leu.2402535 [DOI] [PubMed] [Google Scholar]
- Alpdogan O, Schmaltz C, Muriglan S.J, Kappel B.J, Perales M.A, Rotolo J.A, Halm J.A, Rich B.E, van den Brink M.R. Administration of interleukin-7 after allogeneic bone marrow transplantation improves immune reconstitution without aggravating graft-versus-host disease. Blood. 2001;98:2256–2265. doi: 10.1182/blood.v98.7.2256. doi:10.1182/blood.V98.7.2256 [DOI] [PubMed] [Google Scholar]
- Amakawa R, et al. Impaired negative selection of T cells in Hodgkin's disease antigen CD30-deficient mice. Cell. 1996;84:551–562. doi: 10.1016/s0092-8674(00)81031-4. doi:10.1016/S0092-8674(00)81031-4 [DOI] [PubMed] [Google Scholar]
- Amrolia P.J, et al. Selective depletion of donor alloreactive T cells without loss of antiviral or antileukemic responses. Blood. 2003;102:2292–2299. doi: 10.1182/blood-2002-11-3516. doi:10.1182/blood-2002-11-3516 [DOI] [PubMed] [Google Scholar]
- Anderson B.E, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik M.J, Shlomchik W.D. Memory CD4+ T cells do not induce graft-versus-host disease. J. Clin. Invest. 2003;112:101–108. doi: 10.1172/JCI17601. doi:10.1172/JCI200317601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B.E, McNiff J.M, Jain D, Blazar B.R, Shlomchik W.D, Shlomchik M.J. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. doi:10.1182/blood-2004-08-3032 [DOI] [PubMed] [Google Scholar]
- Asai O, Longo D.L, Tian Z.G, Hornung R.L, Taub D.D, Ruscetti F.W, Murphy W.J. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J. Clin. Invest. 1998;101:1835–1842. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Zappone E, Zino E, Fleischhauer K, Blazar B, Narula S, Bordignon C, Roncarolo M.G. Use of IL-10 anergized T cells in haploidentical bone marrow transplantation. Blood. 2000;96:580a. [Google Scholar]
- Baecher-Allan C, Brown J.A, Freeman G.J, Hafler D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- Bansal-Pakala P, Jember A.G, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat. Med. 2001;7:907–912. doi: 10.1038/90942. doi:10.1038/90942 [DOI] [PubMed] [Google Scholar]
- Baum P.R, et al. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. EMBO J. 1994a;13:3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum P.R, et al. Identification of OX40 ligand and preliminary characterization of its activities on OX40 receptor. Circ. Shock. 1994b;44:30–34. [PubMed] [Google Scholar]
- Billiau A.D, Fevery S, Rutgeerts O, Landuyt W, Waer M. Transient expansion of Mac1+Ly6-G+Ly6-C+ early myeloid cells with suppressor activity in spleens of murine radiation marrow chimeras: possible implications for the graft-versus-host and graft-versus-leukemia reactivity of donor lymphocyte infusions. Blood. 2003;102:740–748. doi: 10.1182/blood-2002-06-1833. doi:10.1182/blood-2002-06-1833 [DOI] [PubMed] [Google Scholar]
- Birkeland M.L, Copeland N.G, Gilbert D.J, Jenkins N.A, Barclay A.N. Gene structure and chromosomal localization of the mouse homologue of rat OX40 protein. Eur. J. Immunol. 1995;25:926–930. doi: 10.1002/eji.1830250410. [DOI] [PubMed] [Google Scholar]
- Blazar B.R, Taylor P.A, Linsley P.S, Vallera D.A. In vivo blockade of CD28/CTLA4:B7/BB1 interaction with CTLA4-Ig reduces lethal murine graft-versus-host disease across the major histocompatibility complex barrier in mice. Blood. 1994;83:3815–3825. [PubMed] [Google Scholar]
- Blazar B.R, Taylor P.A, Panoskaltsis-Mortari A, Gray G.S, Vallera D.A. Coblockade of the LFA1:ICAM and CD28/CTLA4:B7 pathways is a highly effective means of preventing acute lethal graft-versus-host disease induced by fully major histocompatibility complex-disparate donor grafts. Blood. 1995a;85:2607–2618. [PubMed] [Google Scholar]
- Blazar B.R, Taylor P.A, Smith S, Vallera D.A. Interleukin-10 administration decreases survival in murine recipients of major histocompatibility complex disparate donor bone marrow grafts. Blood. 1995b;85:842–851. [PubMed] [Google Scholar]
- Blazar B.R, Sharpe A.H, Taylor P.A, Panoskaltsis-Mortari A, Gray G.S, Korngold R, Vallera D.A. Infusion of anti-B7.1 (CD80) and anti-B7.2 (CD86) monoclonal antibodies inhibits murine graft-versus-host disease lethality in part via direct effects on CD4+ and CD8+ T cells. J. Immunol. 1996;157:3250–3259. [PubMed] [Google Scholar]
- Blazar B.R, Taylor P.A, Panoskaltsis-Mortari A, Buhlman J, Xu J, Flavell R.A, Korngold R, Noelle R, Vallera D.A. Blockade of CD40 ligand-CD40 interaction impairs CD4+ T cell-mediated alloreactivity by inhibiting mature donor T cell expansion and function after bone marrow transplantation. J. Immunol. 1997;158:29–39. [PubMed] [Google Scholar]
- Blazar B.R, Taylor P.A, Noelle R.J, Vallera D.A. CD4(+) T cells tolerized ex vivo to host alloantigen by anti-CD40 ligand (CD40L:CD154) antibody lose their graft-versus-host disease lethality capacity but retain nominal antigen responses. J. Clin. Invest. 1998;102:473–482. doi: 10.1172/JCI3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazar B.R, Taylor P.A, Panoskaltsis-Mortari A, Sharpe A.H, Vallera D.A. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J. Immunol. 1999;162:6368–6377. [PubMed] [Google Scholar]
- Blazar B.R, Kwon B.S, Panoskaltsis-Mortari A, Kwak K.B, Peschon J.J, Taylor P.A. Ligation of 4-1BB (CDw137) regulates graft-versus-host disease, graft-versus-leukemia, and graft rejection in allogeneic bone marrow transplant recipients. J. Immunol. 2001a;166:3174–3183. doi: 10.4049/jimmunol.166.5.3174. [DOI] [PubMed] [Google Scholar]
- Blazar B.R, McKenna H.J, Panoskaltsis-Mortari A, Taylor P.A. Flt3 ligand (FL) treatment of murine donors does not modify graft-versus-host disease (GVHD) but FL treatment of recipients post-bone marrow transplantation accelerates GVHD lethality. Biol. Blood Marrow Transplant. 2001b;7:197–207. doi: 10.1053/bbmt.2001.v7.pm11349806. doi:10.1053/bbmt.2001.v7.pm11349806 [DOI] [PubMed] [Google Scholar]
- Blazar B.R, Carreno B, Panoskaltsis-Mortari A, Carter L, Iwai Y, Yagita H, Nishimura H, Taylor P.A. PD-1 engagement provides an inhibitory signal which downregulates T cell alloresponses in vivo. Blood. 2002;100:72a. doi: 10.1182/blood.v100.1.72. doi:10.1182/blood.V100.1.72 [DOI] [PubMed] [Google Scholar]
- Blazar B.R, Sharpe A.H, Chen A.I, Panoskaltsis-Mortari A, Lees C, Akiba H, Yagita H, Killeen N, Taylor P.A. Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients. Blood. 2003;101:3741–3748. doi: 10.1182/blood-2002-10-3048. doi:10.1182/blood-2002-10-3048 [DOI] [PubMed] [Google Scholar]
- Boussiotis V.A, Chen Z.M, Zeller J.C, Murphy W.J, Berezovskaya A, Narula S, Roncarolo M.G, Blazar B.R. Altered T-cell receptor+CD28-mediated signaling and blocked cell cycle progression in interleukin 10 and transforming growth factor-beta-treated alloreactive T cells that do not induce graft-versus-host disease. Blood. 2001;97:565–571. doi: 10.1182/blood.v97.2.565. doi:10.1182/blood.V97.2.565 [DOI] [PubMed] [Google Scholar]
- Bowen M.A, Lee R.K, Miragliotta G, Nam S.Y, Podack E.R. Structure and expression of murine CD30 and its role in cytokine production. J. Immunol. 1996;156:442–449. [PubMed] [Google Scholar]
- Brocker T, Gulbranson-Judge A, Flynn S, Riedinger M, Raykundalia C, Lane P. CD4 T cell traffic control: in vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur. J. Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. doi:10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8 [DOI] [PubMed] [Google Scholar]
- Bross P.F, et al. Approval summary for bortezomib for injection in the treatment of multiple myeloma. Clin. Cancer Res. 2004;10:3954–3964. doi: 10.1158/1078-0432.CCR-03-0781. [DOI] [PubMed] [Google Scholar]
- Brown G.R, Lee E, Thiele D.L. TNF–TNFR2 interactions are critical for the development of intestinal graft-versus-host disease in MHC class II-disparate (C57BL/6J→C57BL/6J×bm12)F1 mice. J. Immunol. 2002;168:3065–3071. doi: 10.4049/jimmunol.168.6.3065. [DOI] [PubMed] [Google Scholar]
- Buhlmann J.E, Gonzalez M, Ginther B, Panoskaltsis-Mortari A, Blazar B.R, Greiner D.L, Rossini A.A, Flavell R, Noelle R.J. Cutting edge: sustained expansion of CD8+ T cells requires CD154 expression by Th cells in acute graft versus host disease. J. Immunol. 1999;162:4373–4376. [PubMed] [Google Scholar]
- Butler L.M, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- Calderhead D.M, Buhlmann J.E, van den Eertwegh A.J, Claassen E, Noelle R.J, Fell H.P. Cloning of mouse Ox40: a T cell activation marker that may mediate T–B cell interactions. J. Immunol. 1993;151:5261–5271. [PubMed] [Google Scholar]
- Chan K.W, Hopke C.D, Krams S.M, Martinez O.M. CD30 expression identifies the predominant proliferating T lymphocyte population in human alloimmune responses. J. Immunol. 2002;169:1784–1791. doi: 10.4049/jimmunol.169.4.1784. [DOI] [PubMed] [Google Scholar]
- Chen A.I, et al. Ox40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689–698. doi: 10.1016/s1074-7613(00)80143-0. doi:10.1016/S1074-7613(00)80143-0 [DOI] [PubMed] [Google Scholar]
- Chen B.J, Cui X, Liu C, Chao N.J. Prevention of graft-versus-host disease while preserving graft-versus-leukemia effect after selective depletion of host-reactive T cells by photodynamic cell purging process. Blood. 2002;99:3083–3088. doi: 10.1182/blood.v99.9.3083. doi:10.1182/blood.V99.9.3083 [DOI] [PubMed] [Google Scholar]
- Chen Z.M, O'Shaughnessy M.J, Gramaglia I, Panoskaltsis-Mortari A, Murphy W.J, Narula S, Roncarolo M.G, Blazar B.R. IL-10 and TGF-beta induce alloreactive CD4+CD25− T cells to acquire regulatory cell function. Blood. 2003;101:5076–5083. doi: 10.1182/blood-2002-09-2798. doi:10.1182/blood-2002-09-2798 [DOI] [PubMed] [Google Scholar]
- Chen B.J, Cui X, Sempowski G.D, Liu C, Chao N.J. Transfer of allogeneic CD62L-memory T cells without graft-versus-host disease. Blood. 2004;103:1534–1541. doi: 10.1182/blood-2003-08-2987. doi:10.1182/blood-2003-08-2987 [DOI] [PubMed] [Google Scholar]
- Chiarle R, Podda A, Prolla G, Podack E.R, Thorbecke G.J, Inghirami G. CD30 overexpression enhances negative selection in the thymus and mediates programmed cell death via a Bcl-2-sensitive pathway. J. Immunol. 1999;163:194–205. [PubMed] [Google Scholar]
- Chiba K, Yanagawa Y, Masubuchi Y, Kataoka H, Kawaguchi T, Ohtsuki M, Hoshino Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol. 1998;160:5037–5044. [PubMed] [Google Scholar]
- Cohen J.L, Trenado A, Vasey D, Klatzmann D, Salomon B.L. CD4(+)CD25(+) immunoregulatory T cells: new therapeutics for graft-versus-host disease. J. Exp. Med. 2002a;196:401–406. doi: 10.1084/jem.20020090. doi:10.1084/jem.20020090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L.A, Marks P.A, Rifkind R.A, Amin S, Desai D, Pittman B, Richon V.M. Suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, suppresses the growth of carcinogen-induced mammary tumors. Anticancer Res. 2002b;22:1497–1504. [PubMed] [Google Scholar]
- Coyle A.J, Gutierrez-Ramos J.C. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat. Immunol. 2001;2:203–209. doi: 10.1038/85251. doi:10.1038/85251 [DOI] [PubMed] [Google Scholar]
- Coyle A.J, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. doi:10.1016/S1074-7613(00)00011-X [DOI] [PubMed] [Google Scholar]
- Davies S.M, Ruggieri L, DeFor T, Wagner J.E, Weisdorf D.J, Miller J.S, Velardi A, Blazar B.R. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100:3825–3827. doi: 10.1182/blood-2002-04-1197. doi:10.1182/blood-2002-04-1197 [DOI] [PubMed] [Google Scholar]
- DeBenedette M.A, Shahinian A, Mak T.W, Watts T.H. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J. Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- DeBenedette M.A, Wen T, Bachmann M.F, Ohashi P.S, Barber B.H, Stocking K.L, Peschon J.J, Watts T.H. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J. Immunol. 1999;163:4833–4841. [PubMed] [Google Scholar]
- De Smedt T, Smith J, Baum P, Fanslow W, Butz E, Maliszewski C. Ox40 costimulation enhances the development of T cell responses induced by dendritic cells in vivo. J. Immunol. 2002;168:661–670. doi: 10.4049/jimmunol.168.2.661. [DOI] [PubMed] [Google Scholar]
- Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J. Exp. Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. doi:10.1084/jem.193.11.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni P.D, Matteucci P, Grisanti S, Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. doi:10.1182/blood.V99.10.3838 [DOI] [PubMed] [Google Scholar]
- Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. doi:10.1038/70932 [DOI] [PubMed] [Google Scholar]
- Dong C, Juedes A.E, Temann U.A, Shresta S, Allison J.P, Ruddle N.H, Flavell R.A. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. doi:10.1038/35051100 [DOI] [PubMed] [Google Scholar]
- Duckett C.S, Thompson C.B. CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev. 1997;11:2810–2821. doi: 10.1101/gad.11.21.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffner U, et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp. Hematol. 2003;31:897–902. doi: 10.1016/s0301-472x(03)00198-x. doi:10.1016/S0301-472X(03)00198-X [DOI] [PubMed] [Google Scholar]
- Durkop H, Latza U, Hummel M, Eitelbach F, Seed B, Stein H. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992;68:421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- Edinger M, Hoffman P, Ermann J, Drago K, Fathman C.G, Strober S.S, Negrin R.S. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 2003;9:1144–1150. doi: 10.1038/nm915. doi:10.1038/nm915 [DOI] [PubMed] [Google Scholar]
- Evans D.E, Prell R.A, Thalhofer C.J, Hurwitz A.A, Weinberg A.D. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J. Immunol. 2001;167:6804–6811. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- Flynn S, Toellner K.M, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. doi:10.1084/jem.188.2.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A.E, Marangolo M, Sartor M.M, Alexander S.I, Hu M, Bradstock K.F, Gottlieb D.J. Human CD62L− memory T cells are less responsive to alloantigen stimulation than CD62L+ naive T cells: potential for adoptive immunotherapy and allodepletion. Blood. 2004;104:2403–2409. doi: 10.1182/blood-2003-12-4431. doi:10.1182/blood-2003-12-4431 [DOI] [PubMed] [Google Scholar]
- Freeman G.J, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. doi:10.1084/jem.192.7.1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman M, Hecht T, Logan B, Vodanovic-Jankovic S, Komorowski R, Drobyski W.R. Host conditioning is a primary determinant in modulating the effect of IL-7 on murine graft-versus-host disease. J. Immunol. 2004;172:3328–3336. doi: 10.4049/jimmunol.172.5.3328. [DOI] [PubMed] [Google Scholar]
- Ghansah T, et al. Expansion of myeloid suppressor cells in SHIP-deficient mice represses allogeneic T cell responses. J. Immunol. 2004;173:7324–7330. doi: 10.4049/jimmunol.173.12.7324. [DOI] [PubMed] [Google Scholar]
- Giralt S, Aleman A, Lei X, Davis M, Mickler K, Weber D, Wang M, Champlin R. Results of bortezomib (BTZ) therapy for myeloma (MM) patients relapsing after an allogeneic transplant. Preliminary results show efficacy without induction of GVHD. Blood. 2004;104:459a. [Google Scholar]
- Godfrey W.R, Krampf M.R, Taylor P.A, Blazar B.R. Ex vivo depletion of alloreactive cells based on CFSE dye dilution, activation antigen selection, and dendritic cell stimulation. Blood. 2004;103:1158–1165. doi: 10.1182/blood-2003-04-1098. doi:10.1182/blood-2003-04-1098 [DOI] [PubMed] [Google Scholar]
- Godfrey W.R, Spoden D.J, Ge Y.G, Baker S.R, Liu B, Levine B.L, June C.H, Blazar B.R, Porter S.B. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750–758. doi: 10.1182/blood-2004-06-2467. doi:10.1182/blood-2004-06-2467 [DOI] [PubMed] [Google Scholar]
- Gonzalo J.A, Delaney T, Corcoran J, Goodearl A, Gutierrez-Ramos J.C, Coyle A.J. Cutting edge: the related molecules CD28 and inducible costimulator deliver both unique and complementary signals required for optimal T cell activation. J. Immunol. 2001a;166:1–5. doi: 10.4049/jimmunol.166.1.1. [DOI] [PubMed] [Google Scholar]
- Gonzalo J.A, et al. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat. Immunol. 2001b;2:597–604. doi: 10.1038/89739. doi:10.1038/89739 [DOI] [PubMed] [Google Scholar]
- Goodwin R.G, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur. J. Immunol. 1993;23:2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- Gramaglia I, Weinberg A.D, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- Gramaglia I, Jember A, Pippig S.D, Weinberg A.D, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J. Immunol. 2000;165:3043–3050. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- Guinan E.C, Boussiotis V.A, Neuberg D, Brennan L.L, Hirano N, Nadler L.M, Gribben J.G. Transplantation of anergic histoincompatible bone marrow allografts. N. Engl. J. Med. 1999;340:1704–1714. doi: 10.1056/NEJM199906033402202. doi:10.1056/NEJM199906033402202 [DOI] [PubMed] [Google Scholar]
- Hakim F.T, Cepeda R, Gray G.S, June C.H, Abe R. Acute graft-versus-host reaction can be aborted by blockade of costimulatory molecules. J. Immunol. 1995;155:1757–1766. [PubMed] [Google Scholar]
- Hanash A.M, Levy R.B. Donor CD4+CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood. 2005;105:1828–1836. doi: 10.1182/blood-2004-08-3213. doi:10.1182/blood-2004-08-3213 [DOI] [PubMed] [Google Scholar]
- Hashimoto D, Asakura S, Miyake S, Yamamura T, Van Kaer L, Liu C, Tanimoto M, Teshima T. Stimulation of host NKT cells by synthetic glycolipid regulates acute graft-versus-host disease by inducing Th2 polarization of donor T cells. J. Immunol. 2005;174:551–556. doi: 10.4049/jimmunol.174.1.551. [DOI] [PubMed] [Google Scholar]
- Hildebrandt G.C, Olkiewicz K.M, Choi S, Corrion L.A, Clouthier S.G, Liu C, Serody J.S, Cooke K.R. Donor T cell-production of RANTES significantly contributes to the development of Idiopathic Pneumonia Syndrome after allogeneic stem cell transplantation. Blood. 2004;105:2249–2257. doi: 10.1182/blood-2004-08-3320. doi:10.1182/blood-2004-08-3320 [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Ermann J, Edinger M, Fathman C.G, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 2002a;196:389–399. doi: 10.1084/jem.20020399. doi:10.1084/jem.20020399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P, Ermann J, Edinger M, Fathman C.G, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow trnasplantation. J. Exp. Med. 2002b;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Kunz-Schughart L.A, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. doi:10.1182/blood-2004-01-0086 [DOI] [PubMed] [Google Scholar]
- Hurtado J.C, Kim S.H, Pollok K.E, Lee Z.H, Kwon B.S. Potential role of 4-1BB in T cell activation. Comparison with the costimulatory molecule CD28. J. Immunol. 1995;155:3360–3367. [PubMed] [Google Scholar]
- Hurtado J.C, Kim Y.J, Kwon B.S. Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J. Immunol. 1997;158:2600–2609. [PubMed] [Google Scholar]
- Hutloff A, Dittrich A.M, Beier K.C, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek R.A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. doi:10.1038/16717 [DOI] [PubMed] [Google Scholar]
- Imado T, Iwasaki T, Kataoka Y, Kuroiwa T, Hara H, Fujimoto J, Sano H. Hepatocyte growth factor preserves graft-versus-leukemia effect and T-cell reconstitution after marrow transplantation. Blood. 2004;104:1542–1549. doi: 10.1182/blood-2003-12-4309. doi:10.1182/blood-2003-12-4309 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen L.Z, Blazar B.R, Adeyi O.A, Strom T.B, Noelle R.J. CD154 on the surface of CD4+CD25+ regulatory T cells contributes to skin transplant tolerance. Transplantation. 2003;76:1375–1379. doi: 10.1097/01.TP.0000093462.16309.73. doi:10.1097/01.TP.0000093462.16309.73 [DOI] [PubMed] [Google Scholar]
- Joffre O, Gorsse N, Romagnoli P, van Meerwijk J.P. Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood. 2004;103:4216–4221. doi: 10.1182/blood-2004-01-0005. doi:10.1182/blood-2004-01-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.C, Murphy G.F, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol. Blood Marrow Transplant. 2003;9:243–256. doi: 10.1053/bbmt.2003.50027. doi:10.1053/bbmt.2003.50027 [DOI] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk A.H. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. doi:10.1084/jem.193.11.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk A.H. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J. Exp. Med. 2002;196:255–260. doi: 10.1084/jem.20020394. doi:10.1084/jem.20020394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung U, Foley J.E, Erdmann A.A, Eckhaus M.A, Fowler D.H. CD3/CD28-costimulated T1 and T2 subsets: differential in vivo allosensitization generates distinct GVT and GVHD effects. Blood. 2003;102:3439–3446. doi: 10.1182/blood-2002-12-3936. doi:10.1182/blood-2002-12-3936 [DOI] [PubMed] [Google Scholar]
- Kane R.C, Bross P.F, Farrell A.T, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. doi:10.1634/theoncologist.8-6-508 [DOI] [PubMed] [Google Scholar]
- Khayyamian S, Hutloff A, Buchner K, Grafe M, Henn V, Kroczek R.A, Mages H.W. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc. Natl Acad. Sci. USA. 2002;99:6198–6203. doi: 10.1073/pnas.092576699. doi:10.1073/pnas.092576699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.M, Sachs T, Asavaroengchai W, Bronson R, Sykes M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. J. Clin. Invest. 2003;111:659–669. doi: 10.1172/JCI16950. doi:10.1172/JCI200316950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury G.A, et al. Cloning, expression, and function of BLAME, a novel member of the CD2 family. J. Immunol. 2001;166:5675–5680. doi: 10.4049/jimmunol.166.9.5675. [DOI] [PubMed] [Google Scholar]
- Koehl U, et al. IL-2 activated NK cell immunotherapy of three children after haploidentical stem cell transplantation. Blood Cells Mol. Dis. 2004;33:261–266. doi: 10.1016/j.bcmd.2004.08.013. doi:10.1016/j.bcmd.2004.08.013 [DOI] [PubMed] [Google Scholar]
- Koh M.B, Prentice H.G, Corbo M, Morgan M, Cotter F.E, Lowdell M.W. Alloantigen-specific T-cell depletion in a major histocompatibility complex fully mismatched murine model provides effective graft-versus-host disease prophylaxis in the presence of lymphoid engraftment. Br. J. Haematol. 2002;118:108–116. doi: 10.1046/j.1365-2141.2002.03682.x. doi:10.1046/j.1365-2141.2002.03682.x [DOI] [PubMed] [Google Scholar]
- Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann M.F. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11:699–708. doi: 10.1016/s1074-7613(00)80144-2. doi:10.1016/S1074-7613(00)80144-2 [DOI] [PubMed] [Google Scholar]
- Kopf M, Coyle A.J, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos J.C, Bachmann M.F. Inducible costimulator protein (ICOS) controls T helper cell subset polarization after virus and parasite infection. J. Exp. Med. 2000;192:53–61. doi: 10.1084/jem.192.1.53. doi:10.1084/jem.192.1.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngold R, Marini J.C, de Baca M.E, Murphy G.F, Giles-Komar J. Role of tumor necrosis factor-alpha in graft-versus-host disease and graft-versus-leukemia responses. Biol. Blood Marrow Transplant. 2003;9:292–303. doi: 10.1016/s1083-8791(03)00087-9. doi:10.1016/S1083-8791(03)00087-9 [DOI] [PubMed] [Google Scholar]
- Kreisel D, et al. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat. Med. 2002;8:233–239. doi: 10.1038/nm0302-233. doi:10.1038/nm0302-233 [DOI] [PubMed] [Google Scholar]
- Krijanovski O.I, Hill G.R, Cooke K.R, Teshima T, Crawford J.M, Brinson Y.S, Ferrara J.L. Keratinocyte growth factor separates graft-versus-leukemia effects from graft-versus-host disease. Blood. 1999;94:825–831. [PubMed] [Google Scholar]
- Kuroiwa T, et al. Hepatocyte growth factor ameliorates acute graft-versus-host disease and promotes hematopoietic function. J. Clin. Invest. 2001;107:1365–1373. doi: 10.1172/JCI11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Carbone F.R, Krummel M.F, Koch K.M, Miller J.F, Heath W.R. Signalling through CD30 protects against autoimmune diabetes mediated by CD8 T cells. Nature. 1999;398:341–344. doi: 10.1038/18692. doi:10.1038/18692 [DOI] [PubMed] [Google Scholar]
- Kwon B.S, Weissman S.M. cDNA sequences of two inducible T-cell genes. Proc. Natl Acad. Sci. USA. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman Y, et al. PD-L2 is a second ligand for PD-I and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. doi:10.1038/85330 [DOI] [PubMed] [Google Scholar]
- Latza U, Durkop H, Schnittger S, Ringeling J, Eitelbach F, Hummel M, Fonatsch C, Stein H. The human OX40 homolog: cDNA structure, expression and chromosomal assignment of the ACT35 antigen. Eur. J. Immunol. 1994;24:677–683. doi: 10.1002/eji.1830240329. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. doi:10.1016/S0301-472X(03)00110-3 [DOI] [PubMed] [Google Scholar]
- Lee R.S, Kuhr C.S, Sale G.E, Zellmer E, Hogan W.J, Storb R, Little M.T. FTY720 does not abrogate acute graft-versus-host disease in the dog leukocyte antigen-nonidentical unrelated canine model. Transplantation. 2003;76:1155–1158. doi: 10.1097/01.TP.0000083891.14089.B8. doi:10.1097/01.TP.0000083891.14089.B8 [DOI] [PubMed] [Google Scholar]
- Leoni F, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc. Natl Acad. Sci. USA. 2002;99:2995–3000. doi: 10.1073/pnas.052702999. doi:10.1073/pnas.052702999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings M.K, Sangregorio R, Roncarolo M.G. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. doi:10.1084/jem.193.11.1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li X.C, Zheng X.X, Wells A.D, Turka L.A, Strom T.B. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat. Med. 1999;5:1298–1302. doi: 10.1038/15256. doi:10.1038/15256 [DOI] [PubMed] [Google Scholar]
- Li B, New J.Y, Yap E.H, Lu J, Chan S.H, Hu H. Blocking L-selectin and alpha4-integrin changes donor cell homing pattern and ameliorates murine acute graft versus host disease. Eur. J. Immunol. 2001;31:617–624. doi:10.1002/1521-4141(200102)31:2<617::AID-IMMU617>3.3.CO;2-4 [PubMed] [Google Scholar]
- Li B, New J.Y, Tay Y.K, Goh E, Yap E.H, Chan S.H, Hu H.Z. Delaying acute graft-versus-host disease in mouse bone marrow transplantation by treating donor cells with antibodies directed at l-selectin and alpha4-integrin prior to infusion. Scand J. Immunol. 2004;59:464–468. doi: 10.1111/j.0300-9475.2004.01414.x. doi:10.1111/j.0300-9475.2004.01414.x [DOI] [PubMed] [Google Scholar]
- Ling V, et al. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J. Immunol. 2000;164:1653–1657. doi: 10.4049/jimmunol.164.4.1653. [DOI] [PubMed] [Google Scholar]
- Ling V, Wu P.W, Miyashiro J.S, Marusic S, Finnerty H.F, Collins M. Differential expression of inducible costimulator-ligand splice variants: lymphoid regulation of mouse GL50-B and human GL50 molecules. J. Immunol. 2001;166:7300–7308. doi: 10.4049/jimmunol.166.12.7300. [DOI] [PubMed] [Google Scholar]
- Mallett S, Fossum S, Barclay A.N. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes—a molecule related to nerve growth factor receptor. EMBO J. 1990;9:1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez O.M, Villanueva J, Abtahi S, Beatty P.R, Esquivel C.O, Krams S.M. CD30 expression identifies a functional alloreactive human T-lymphocyte subset. Transplantation. 1998;65:1240–1247. doi: 10.1097/00007890-199805150-00016. doi:10.1097/00007890-199805150-00016 [DOI] [PubMed] [Google Scholar]
- Matte C.C, Liu J, Cormier J, Anderson B.E, Athanasiadis I, Jain D, McNiff J, Shlomchik W.D. Donor APCs are required for maximal GVHD but not for GVL. Nat. Med. 2004;10:987–992. doi: 10.1038/nm1089. doi:10.1038/nm1089 [DOI] [PubMed] [Google Scholar]
- McAdam A.J, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4(+) T cells. J. Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- McAdam A.J, Greenwald R.J, Levin M.A, Chernova T, Malenkovich N, Ling V, Freeman G.J, Sharpe A.H. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. doi:10.1038/35051107 [DOI] [PubMed] [Google Scholar]
- Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. doi:10.1182/blood-2003-11-3909 [DOI] [PubMed] [Google Scholar]
- Melero I, Johnston J.V, Shufford W.W, Mittler R.S, Chen L. NK1.1 cells express 4-1BB (CDw137) costimulatory molecule and are required for tumor immunity elicited by anti-4-1BB monoclonal antibodies. Cell Immunol. 1998;190:167–172. doi: 10.1006/cimm.1998.1396. doi:10.1006/cimm.1998.1396 [DOI] [PubMed] [Google Scholar]
- Merad M, et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat. Med. 2004;10:510–517. doi: 10.1038/nm1038. doi:10.1038/nm1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min D, Taylor P.A, Panoskaltsis-Mortari A, Chung B, Danilenko D.M, Farrell C, Lacey D.L, Blazar B.R, Weinberg K.I. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. doi:10.1182/blood.V99.12.4592 [DOI] [PubMed] [Google Scholar]
- Min C.K, Maeda Y, Lowler K, Liu C, Clouthier S, Lofthus D, Weisiger E, Ferrara J.L, Reddy P. Paradoxical effects of interleukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation. Blood. 2004;104:3393–3399. doi: 10.1182/blood-2004-02-0763. doi:10.1182/blood-2004-02-0763 [DOI] [PubMed] [Google Scholar]
- Montagna D, Yvon E, Calcaterra V, Comoli P, Locatelli F, Maccario R, Fisher A, Cavazzana-Calvo M. Depletion of alloreactive T cells by a specific anti-interleukin-2 receptor p55 chain immunotoxin does not impair in vitro antileukemia and antiviral activity. Blood. 1999;93:3550–3557. [PubMed] [Google Scholar]
- Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. doi:10.1016/j.it.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Murai M, Yoneyama H, Harada A, Yi Z, Vestergaard C, Guo B, Suzuki K, Asakura H, Matsushima K. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J. Clin. Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai M, et al. Peyer's patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat. Immunol. 2003;4:154–160. doi: 10.1038/ni879. doi:10.1038/ni879 [DOI] [PubMed] [Google Scholar]
- Murata K, Ishii N, Takano H, Miura S, Ndhlovu L.C, Nose M, Noda T, Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J. Exp. Med. 2000;191:365–374. doi: 10.1084/jem.191.2.365. doi:10.1084/jem.191.2.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P.M. International union of pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev. 2002;54:227–229. doi: 10.1124/pr.54.2.227. doi:10.1124/pr.54.2.227 [DOI] [PubMed] [Google Scholar]
- Murphy W.J, et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J. Clin. Invest. 1998;102:1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P.M, Baggiolini M, Charo I.F, Hebert C.A, Horuk R, Matsushima K, Miller L.H, Oppenheim J.J, Power C.A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. doi:10.1016/S1074-7613(00)80089-8 [DOI] [PubMed] [Google Scholar]
- Nozawa K, Ohata J, Sakurai J, Hashimoto H, Miyajima H, Yagita H, Okumura K, Azuma M. Preferential blockade of CD8(+) T cell responses by administration of anti-CD137 ligand monoclonal antibody results in differential effect on development of murine acute and chronic graft-versus-host diseases. J. Immunol. 2001;167:4981–4986. doi: 10.4049/jimmunol.167.9.4981. [DOI] [PubMed] [Google Scholar]
- Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- Ohshima Y, Yang L.P, Uchiyama T, Tanaka Y, Baum P, Sergerie M, Hermann P, Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- O'Shaughnessy M.J, Chen Z.-m, Panoskaltsis-Mortari A, Taub D, Palmer E, Berg L.A, Murphy W.I, Blazar B.R. Ex vivo blockade of the Janus kinase 3 pathway in alloreactive T Cells abolishes their capacity to induce murine graft versus host disease. Blood. Submitted [Google Scholar]
- Panoskaltsis-Mortari A, Taylor P.A, Rubin J.S, Uren A, Welniak L.A, Murphy W.J, Farrell C.L, Lacey D.L, Blazar B.R. Keratinocyte growth factor facilitates alloengraftment and ameliorates graft-versus-host disease in mice by a mechanism independent of repair of conditioning-induced tissue injury. Blood. 2000;96:4350–4356. [PubMed] [Google Scholar]
- Panoskaltsis-Mortari A, Hermanson J.R, Taras E, Wangensteen O.D, Serody J.S, Blazar B.R. Acceleration of idiopathic pneumonia syndrome (IPS) in the absence of donor MIP-1 alpha (CCL3) after allogeneic BMT in mice. Blood. 2003;101:3714–3721. doi: 10.1182/blood-2002-08-2465. doi:10.1182/blood-2002-08-2465 [DOI] [PubMed] [Google Scholar]
- Petrovic A, et al. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood. 2004;103:1542–1547. doi: 10.1182/blood-2003-03-0957. doi:10.1182/blood-2003-03-0957 [DOI] [PubMed] [Google Scholar]
- Pippig S.D, et al. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40) J. Immunol. 1999;163:6520–6529. [PubMed] [Google Scholar]
- Pollok K.E, Kim Y.J, Zhou Z, Hurtado J, Kim K.K, Pickard R.T, Kwon B.S. Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- Pollok K.E, Kim Y.J, Hurtado J, Zhou Z, Kim K.K, Kwon B.S. 4-1BB T-cell antigen binds to mature B cells and macrophages, and costimulates anti-mu-primed splenic B cells. Eur. J. Immunol. 1994;24:367–374. doi: 10.1002/eji.1830240215. [DOI] [PubMed] [Google Scholar]
- Potian J.A, Aviv H, Ponzio N.M, Harrison J.S, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J. Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- Rao A.R, et al. CC chemokine receptor 2 expression in donor cells serves an essential role in graft-versus-host-disease. J. Immunol. 2003;171:4875–4885. doi: 10.4049/jimmunol.171.9.4875. [DOI] [PubMed] [Google Scholar]
- Reddy P, Teshima T, Kukuruga M, Ordemann R, Liu C, Lowler K, Ferrara J.L. Interleukin-18 regulates acute graft-versus-host disease by enhancing Fas-mediated donor T cell apoptosis. J. Exp. Med. 2001;194:1433–1440. doi: 10.1084/jem.194.10.1433. doi:10.1084/jem.194.10.1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Teshima T, Hildebrandt G, Williams D.L, Liu C, Cooke K.R, Ferrara J.L. Pretreatment of donors with interleukin-18 attenuates acute graft-versus-host disease via STAT6 and preserves graft-versus-leukemia effects. Blood. 2003;101:2877–2885. doi: 10.1182/blood-2002-08-2566. doi:10.1182/blood-2002-08-2566 [DOI] [PubMed] [Google Scholar]
- Reddy P, Maeda Y, Hotary K, Liu C, Reznikov L.L, Dinarello C.A, Ferrara J.L. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc. Natl Acad. Sci. USA. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. doi:10.1073/pnas.0400380101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers P.R, Croft M. CD28, Ox-40, LFA-1, and CD4 modulation of Th1/Th2 differentiation is directly dependent on the dose of antigen. J. Immunol. 2000;164:2955–2963. doi: 10.4049/jimmunol.164.6.2955. [DOI] [PubMed] [Google Scholar]
- Rogers P.R, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. doi:10.1016/S1074-7613(01)00191-1 [DOI] [PubMed] [Google Scholar]
- Rollins B.J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- Rossi S, Blazar B.R, Farrell C.L, Danilenko D.M, Lacey D.L, Weinberg K.I, Krenger W, Hollander G.A. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. doi:10.1182/blood.V100.2.682 [DOI] [PubMed] [Google Scholar]
- Ruggeri L, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. doi:10.1126/science.1068440 [DOI] [PubMed] [Google Scholar]
- Saito K, Sakurai J, Ohata J, Kohsaka T, Hashimoto H, Okumura K, Abe R, Azuma M. Involvement of CD40 ligand-CD40 and CTLA4-B7 pathways in murine acute graft-versus-host disease induced by allogeneic T cells lacking CD28. J. Immunol. 1998;160:4225–4231. [PubMed] [Google Scholar]
- Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Sakaguchi S, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol. Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. doi:10.1034/j.1600-065X.2001.1820102.x [DOI] [PubMed] [Google Scholar]
- Salomon B, Lenschow D.J, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone J.A. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. doi:10.1016/S1074-7613(00)80195-8 [DOI] [PubMed] [Google Scholar]
- Saoulli K, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J. Exp. Med. 1998;187:1849–1862. doi: 10.1084/jem.187.11.1849. doi:10.1084/jem.187.11.1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers T.J, et al. The proteasome inhibitor PS-341 sensitizes neoplastic cells to TRAIL-mediated apoptosis by reducing levels of c-FLIP. Blood. 2003;102:303–310. doi: 10.1182/blood-2002-09-2975. doi:10.1182/blood-2002-09-2975 [DOI] [PubMed] [Google Scholar]
- Shanebeck K.D, Maliszewski C.R, Kennedy M.K, Picha K.S, Smith C.A, Goodwin R.G, Grabstein K.H. Regulation of murine B cell growth and differentiation by CD30 ligand. Eur. J. Immunol. 1995;25:2147–2153. doi: 10.1002/eji.1830250805. [DOI] [PubMed] [Google Scholar]
- Sharpe A.H, Freeman G.J. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002;2:116–126. doi: 10.1038/nri727. doi:10.1038/nri727 [DOI] [PubMed] [Google Scholar]
- Shevach E.M. Certified professionals: CD4(+)CD25(+) suppressor T cells. J. Exp. Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.f41. doi:10.1084/jem.193.11.F41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E.M. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- Shevach E.M, McHugh R.S, Piccirillo C.A, Thornton A.M. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. doi:10.1034/j.1600-065X.2001.1820104.x [DOI] [PubMed] [Google Scholar]
- Shimozato O, Takeda K, Yagita H, Okumura K. Expression of CD30 ligand (CD153) on murine activated T cells. Biochem. Biophys. Res. Commun. 1999;256:519–526. doi: 10.1006/bbrc.1999.0336. doi:10.1006/bbrc.1999.0336 [DOI] [PubMed] [Google Scholar]
- Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Structure and chromosomal localization of the human PD-1 gene (PDCD1) Genomics. 1994;23:704–706. doi: 10.1006/geno.1994.1562. doi:10.1006/geno.1994.1562 [DOI] [PubMed] [Google Scholar]
- Shlomchik W.D, Couzens M.S, Tang C.B, McNiff J, Robert M.E, Liu J, Shlomchik M.J, Emerson S.G. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. doi:10.1126/science.285.5426.412 [DOI] [PubMed] [Google Scholar]
- Shuford W.W, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. doi:10.1084/jem.186.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M.L, Fry T.J, Fowler D.H, Miller G, Mackall C.L. Interleukin 7 worsens graft-versus-host disease. Blood. 2002;100:2642–2649. doi: 10.1182/blood-2002-04-1082. doi:10.1182/blood-2002-04-1082 [DOI] [PubMed] [Google Scholar]
- Solomon S.R, et al. Optimized clinical-scale culture conditions for ex vivo selective depletion of host-reactive donor lymphocytes: a strategy for GvHD prophylaxis in allogeneic PBSC transplantation. Cytotherapy. 2002;4:395–406. doi: 10.1080/146532402320775982. doi:10.1080/146532402320775982 [DOI] [PubMed] [Google Scholar]
- Speiser D, Bachmann M, Frick T, McKall-Faienza K, Griffiths E, Pfeffer K, Mak T, Ohashi P. TNF receptor p55 controls early acute graft-versus-host disease. J. Immunol. 1997;158:5185–5190. [PubMed] [Google Scholar]
- Sperling A.I, Bluestone J.A. ICOS costimulation: it's not just for TH2 cells anymore. Nat. Immunol. 2001;2:573–574. doi: 10.1038/89709. doi:10.1038/89709 [DOI] [PubMed] [Google Scholar]
- Spielberger R, et al. Palifermin for oral mucostitis after intensive therapy for hematologic cancers. N. Engl. J. Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. doi:10.1056/NEJMoa040125 [DOI] [PubMed] [Google Scholar]
- Stuber E, Neurath M, Calderhead D, Fell H.P, Strober W. Cross-linking of OX40 ligand, a member of the TNF/NGF cytokine family, induces proliferation and differentiation in murine splenic B cells. Immunity. 1995;2:507–521. doi: 10.1016/1074-7613(95)90031-4. doi:10.1016/1074-7613(95)90031-4 [DOI] [PubMed] [Google Scholar]
- Sun K, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc. Natl Acad. Sci. USA. 2004;101:8120–8125. doi: 10.1073/pnas.0401563101. doi:10.1073/pnas.0401563101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wilkins D.E, Anver M.R, Sayers T.J, Panoskaltsis-Mortari A, Blazar B.R, Welniak L.A, Murphy W.J. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GHVD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005 doi: 10.1182/blood-2004-11-4526. Published online 16 June 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri-Payer E, Amar A.Z, Thornton A.M, Shevach E.M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- Swallow M.M, Wallin J.J, Sha W.C. B7h, a novel costimulatory homolog of B7.1 and B7.2, is induced by TNFalpha. Immunity. 1999;11:423–432. doi: 10.1016/s1074-7613(00)80117-x. doi:10.1016/S1074-7613(00)80117-X [DOI] [PubMed] [Google Scholar]
- Sykes M, Romick M.L, Sachs D.H. Interleukin 2 prevents graft-versus-host disease while preserving the graft-versus-leukemia effect of allogeneic T cells. Proc. Natl Acad. Sci. USA. 1990;87:5633–5637. doi: 10.1073/pnas.87.15.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes M, Szot G.L, Nguyen P.L, Pearson D.A. Interleukin-12 inhibits murine graft-versus-host disease. Blood. 1995;86:2429–2438. [PubMed] [Google Scholar]
- Sykes M, Pearson D.A, Taylor P.A, Szot G.L, Goldman S.J, Blazar B.R. Dose and timing of interleukin (IL)-12 and timing and type of total-body irradiation: effects on graft-vs.-host disease inhibition and toxicity of exogenous IL-12 in murine bone marrow transplant recipients. Biol. Blood Marrow Transplant. 1999;5:277–284. doi: 10.1016/s1083-8791(99)70002-9. [DOI] [PubMed] [Google Scholar]
- Tafuri A, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. doi:10.1038/35051113 [DOI] [PubMed] [Google Scholar]
- Takahashi C, Mittler R.S, Vella A.T. Cutting edge: 4-1BB is a bona fide CD8 T cell survival signal. J. Immunol. 1999;162:5037–5040. [PubMed] [Google Scholar]
- Tamura H, Dong H, Zhu G, Sica G.L, Flies D.B, Tamada K, Chen L. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97:1809–1816. doi: 10.1182/blood.v97.6.1809. doi:10.1182/blood.V97.6.1809 [DOI] [PubMed] [Google Scholar]
- Tan J.T, Whitmire J.K, Ahmed R, Pearson T.C, Larsen C.P. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J. Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- Tan J.T, Ha J, Cho H.R, Tucker-Burden C, Hendrix R.C, Mittler R.S, Pearson T.C, Larsen C.P. Analysis of expression and function of the costimulatory molecule 4-1BB in alloimmune responses. Transplantation. 2000;70:175–183. [PubMed] [Google Scholar]
- Tanaka T, Ohtsuka Y, Yagita H, Shiratori Y, Omata M, Okumura K. Involvement of alpha 1 and alpha 4 integrins in gut mucosal injury of graft-versus-host disease. Int. Immunol. 1995;7:1183–1189. doi: 10.1093/intimm/7.8.1183. [DOI] [PubMed] [Google Scholar]
- Taylor P.A, Panoskaltsis-Mortari A, Noelle R.J, Blazar B.R. Analysis of the requirements for the induction of CD4+ T cell alloantigen hyporesponsiveness by ex vivo anti-CD40 ligand antibody. J. Immunol. 2000;164:612–622. doi: 10.4049/jimmunol.164.2.612. [DOI] [PubMed] [Google Scholar]
- Taylor P.A, Lees C.J, Allison J.P, Fournier S, Sharpe A.H, Blazar B.R. B7 expression on T cells downregulates GVHD generation independent of CD4+CD25+ immune regulatory T cells. Blood. 2001a;98:776a. [Google Scholar]
- Taylor P.A, Noelle R.J, Blazar B.R. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J. Exp. Med. 2001b;193:1311–1318. doi: 10.1084/jem.193.11.1311. doi:10.1084/jem.193.11.1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P.A, Friedman T.M, Korngold R, Noelle R.J, Blazar B.R. Tolerance induction of alloreactive T cells via ex vivo blockade of the CD40:CD40L costimulatory pathway results in the generation of a potent immune regulatory cell. Blood. 2002a;99:4601–4609. doi: 10.1182/blood.v99.12.4601. doi:10.1182/blood.V99.12.4601 [DOI] [PubMed] [Google Scholar]
- Taylor P.A, Lees C.J, Blazar B.R. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002b;99:3493–3499. doi: 10.1182/blood.v99.10.3493. doi:10.1182/blood.V99.10.3493 [DOI] [PubMed] [Google Scholar]
- Taylor P.A, Panoskaltsis-Mortari A, Freeman G.J, Sharpe A.H, Noelle R.J, Rudensky A.Y, Mak T.W, Serody J.S, Blazar B.R. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells downregulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2004;105:3372–3380. doi: 10.1182/blood-2004-10-3869. doi:10.1182/blood-2004-10-3869 [DOI] [PubMed] [Google Scholar]
- Taylor P.A, Panoskaltsis-Mortari A, Swedin J.M, Lucas P.J, Gress R.E, Levine B.L, June C.H, Serody J.S, Blazar B.R. L-Selectin(hi) but not the L-selectin(lo) CD4+25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004b;104:3804–3812. doi: 10.1182/blood-2004-05-1850. doi:10.1182/blood-2004-05-1850 [DOI] [PubMed] [Google Scholar]
- Telford W.G, Nam S.Y, Podack E.R, Miller R.A. CD30-regulated apoptosis in murine CD8 T cells after cessation of TCR signals. Cell Immunol. 1997;182:125–136. doi: 10.1006/cimm.1997.1228. doi:10.1006/cimm.1997.1228 [DOI] [PubMed] [Google Scholar]
- Tesciuba A.G, et al. Inducible costimulator regulates Th2-mediated inflammation, but not Th2 differentiation, in a model of allergic airway disease. J. Immunol. 2001;167:1996–2003. doi: 10.4049/jimmunol.167.4.1996. [DOI] [PubMed] [Google Scholar]
- Teshima T, Ordemann R, Reddy P, Gagin S, Liu C, Cooke K.R, Ferrara J.L. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat. Med. 2002a;8:575–581. doi: 10.1038/nm0602-575. doi:10.1038/nm0602-575 [DOI] [PubMed] [Google Scholar]
- Teshima T, Reddy P, Lowler K.P, KuKuruga M.A, Liu C, Cooke K.R, Ferrara J.L. Flt3 ligand therapy for recipients of allogeneic bone marrow transplants expands host CD8 alpha(+) dendritic cells and reduces experimental acute graft-versus-host disease. Blood. 2002b;99:1825–1832. doi: 10.1182/blood.v99.5.1825. doi:10.1182/blood.V99.5.1825 [DOI] [PubMed] [Google Scholar]
- Thornton A.M, Shevach E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. doi:10.1084/jem.188.2.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton A.M, Shevach E.M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- Trenado A, Charlotte F, Fisson S, Yagello M, Klatzmann D, Salomon B.L, Cohen J.L. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J. Clin. Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. doi:10.1172/JCI200317702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse W.T, Pendleton J.D, Beyer W.M, Egalka M.C, Guinan E.C. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. doi:10.1097/01.TP.0000045055.63901.A9 [DOI] [PubMed] [Google Scholar]
- Tseng S.Y, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. doi:10.1084/jem.193.7.839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada N, Akiba H, Kobata T, Aizawa Y, Yagita H, Okumura K. Blockade of CD134 (OX40)-CD134L interaction ameliorates lethal acute graft-versus-host disease in a murine model of allogeneic bone marrow transplantation. Blood. 2000;95:2434–2439. [PubMed] [Google Scholar]
- Uckun F.M, Roers B.A, Waurzyniak B, Liu X.P, Cetkovic-Cvrlje M. Janus kinase 3 inhibitor WHI-P131/JANEX-1 prevents graft-versus-host disease but spares the graft-versus-leukemia function of the bone marrow allografts in a murine bone marrow transplantation model. Blood. 2002;99:4192–4199. doi: 10.1182/blood.v99.11.4192. doi:10.1182/blood.V99.11.4192 [DOI] [PubMed] [Google Scholar]
- Urbano-Ispizua A, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: definitions and current practice in Europe. Bone Marrow Transplant. 2002;29:639–646. doi: 10.1038/sj.bmt.1703535. doi:10.1038/sj.bmt.1703535 [DOI] [PubMed] [Google Scholar]
- Vibhakar R, Juan G, Traganos F, Darzynkiewicz Z, Finger L.R. Activation-induced expression of human programmed death-1 gene in T-lymphocytes. Exp. Cell Res. 1997;232:25–28. doi: 10.1006/excr.1997.3493. doi:10.1006/excr.1997.3493 [DOI] [PubMed] [Google Scholar]
- Vivier E, Daeron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol. Today. 1997;18:286–291. doi: 10.1016/s0167-5699(97)80025-4. doi:10.1016/S0167-5699(97)80025-4 [DOI] [PubMed] [Google Scholar]
- Wall D, Sheehan K. The role of tumor necrosis factor and interferon gamma in graft-versus-host disease and related immunodeficiency. Transplantation. 1994;57:273–279. doi: 10.1097/00007890-199401001-00021. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhu G, Chapoval A.I, Dong H, Tamada K, Ni J, Chen L. Costimulation of T cells by B7-H2, a B7-like molecule that binds ICOS. Blood. 2000;96:2808–2813. [PubMed] [Google Scholar]
- Wang J.W, Howson J.M, Ghansah T, Desponts C, Ninos J.M, May S.L, Nguyen K.H, Toyama-Sorimachi N, Kerr W.G. Influence of SHIP on the NK repertoire and allogeneic bone marrow transplantation. Science. 2002;295:2094–2097. doi: 10.1126/science.1068438. doi:10.1126/science.1068438 [DOI] [PubMed] [Google Scholar]
- Weatherill A.R, Maxwell J.R, Takahashi C, Weinberg A.D, Vella A.T. OX40 ligation enhances cell cycle turnover of Ag-activated CD4 T cells in vivo. Cell Immunol. 2001;209:63–75. doi: 10.1006/cimm.2001.1783. doi:10.1006/cimm.2001.1783 [DOI] [PubMed] [Google Scholar]
- Weinberg A.D. Antibodies to OX-40 (CD134) can identify and eliminate autoreactive T cells: implications for human autoimmune disease. Mol. Med. Today. 1998;4:76–83. doi: 10.1016/S1357-4310(97)01181-7. doi:10.1016/S1357-4310(97)01181-7 [DOI] [PubMed] [Google Scholar]
- Weinberg A.D. OX40: targeted immunotherapy—implications for tempering autoimmunity and enhancing vaccines. Trends Immunol. 2002;23:102–109. doi: 10.1016/s1471-4906(01)02127-5. doi:10.1016/S1471-4906(01)02127-5 [DOI] [PubMed] [Google Scholar]
- Weinberg A.D, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J. Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- Welniak L.A, Wang Z, Sun K, Kuziel W, Anver M.R, Blazar B.R, Murphy W.J. An absence of CCR5 on donor cells results in acceleration of acute graft-vs-host disease. Exp. Hematol. 2004;32:318–324. doi: 10.1016/j.exphem.2003.12.003. doi:10.1016/j.exphem.2003.12.003 [DOI] [PubMed] [Google Scholar]
- Welniak, et al. Peyer's patches are not required for acute graft-versus-host disease after murine allogeneic bone marrow transplantation. Blood. Submitted doi: 10.1182/blood-2004-11-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley S.R, Goodwin R.G, Smith C.A. Reverse signaling via CD30 ligand. J. Immunol. 1996;157:3635–3639. [PubMed] [Google Scholar]
- Williams M.A, et al. Characterization of virus-mediated inhibition of mixed chimerism and allospecific tolerance. J. Immunol. 2001;167:4987–4995. doi: 10.4049/jimmunol.167.9.4987. [DOI] [PubMed] [Google Scholar]
- Wysocki C.A, Burkett S.B, Panoskaltsis-Mortari A, Kirby S.L, Luster A.D, McKinnon K, Blazar B.R, Serody J.S. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J. Immunol. 2004;173:845–854. doi: 10.4049/jimmunol.173.2.845. [DOI] [PubMed] [Google Scholar]
- Wysocki C.A, Panoskaltsis-Mortari A, Blazar B.R, Serody J.S. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. doi:10.1182/blood-2004-12-4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S.K, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. doi:10.1038/45582 [DOI] [PubMed] [Google Scholar]
- Yoshinaga S.K, et al. Characterization of a new human B7-related protein: B7RP-1 is the ligand to the co-stimulatory protein ICOS. Int. Immunol. 2000;12:1439–1447. doi: 10.1093/intimm/12.10.1439. doi:10.1093/intimm/12.10.1439 [DOI] [PubMed] [Google Scholar]
- Yu X.Z, Martin P.J, Anasetti C. Role of CD28 in acute graft-versus-host disease. Blood. 1998;92:2963–2970. [PubMed] [Google Scholar]
- Yu X.Z, Bidwell S, Martin P.J, Anasetti C. Visualization, fate, and pathogenicity of antigen-specific CD8+ T cells in the graft-versus-host reaction. J. Immunol. 1999;163:4780–4787. [PubMed] [Google Scholar]
- Yu X.Z, Bidwell S.J, Martin P.J, Anasetti C. CD28-specific antibody prevents graft-versus-host disease in mice. J. Immunol. 2000;164:4564–4568. doi: 10.4049/jimmunol.164.9.4564. [DOI] [PubMed] [Google Scholar]
- Zeller J.C, Panoskaltsis-Mortari A, Murphy W.J, Ruscetti F.W, Narula S, Roncarolo M.G, Blazar B.R. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J. Immunol. 1999;163:3684–3691. [PubMed] [Google Scholar]
- Zhang Y, Shlomchik W.D, Joe G, Louboutin J.P, Zhu J, Rivera A, Giannola D, Emerson S.G. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J. Immunol. 2002;169:7111–7118. doi: 10.4049/jimmunol.169.12.7111. [DOI] [PubMed] [Google Scholar]
- Zhou D, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. doi:10.1126/science.1103440 [DOI] [PubMed] [Google Scholar]