Abstract
Context: Isokinetic and isotonic resistance training exercises are commonly used to increase strength during musculoskeletal rehabilitation programs. Our study was designed to examine the efficacy of isokinetic and isotonic muscle actions using surface electromyographic (EMG) amplitude-to-work ratios (EMG/WK) and to extend previous findings to include a range of isokinetic velocities and isotonic loads.
Objective: To examine work (WK), surface EMG amplitude, and EMG/WK during concentric-only maximal isokinetic muscle actions at 60, 120, 180, 240, and 300°/s and isotonic muscle actions at 10%, 20%, 30%, 40%, and 50% of the maximal voluntary isometric contraction (MVIC) torque during leg extension exercises.
Design: A randomized, counterbalanced, cross-sectional, repeated-measures design.
Setting: A university-based human muscle physiology research laboratory.
Patients or Other Participants: Ten women (mean age = 22.0 ± 2.6 years) and 10 men (mean age = 20.8 ± 1.7 years) who were apparently healthy and recreationally active.
Intervention(s): Using the dominant leg, each participant performed 5 maximal voluntary concentric isokinetic leg extension exercises at randomly ordered angular velocities of 60, 120, 180, 240, and 300°/s and 5 concentric isotonic leg extension exercises at randomly ordered loads of 10%, 20%, 30%, 40%, and 50% of the isometric MVIC.
Main Outcome Measure(s): Work was recorded by a Biodex System 3 dynamometer, and surface EMG was recorded from the superficial quadriceps femoris muscles (vastus lateralis, rectus femoris, and vastus medialis) during the testing and was normalized to the MVIC. The EMG/WK ratios were calculated as the quotient of EMG amplitude (μVrms) and WK (J) during the concentric phase of each exercise.
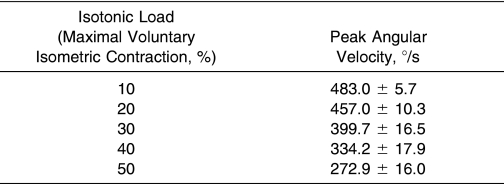
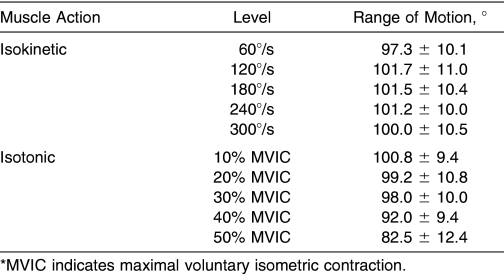
Results: Isotonic EMG/WK remained unchanged ( P > .05) from 10% to 50% MVIC, but isokinetic EMG/WK increased ( P < .05) from 60 to 300°/s. Isotonic EMG/WK was greater ( P < .05) than isokinetic EMG/WK for 50% MVIC versus 60°/s, 40% MVIC versus 120°/s, and 30% MVIC versus 180°/s; however, no differences were noted ( P > .05) between 20% MVIC versus 240°/s or 10% MVIC versus 300°/s. An 18% decrease in active range of motion was seen for the isotonic muscle actions, from 10% to 50% MVIC, and a 3% increase in range of motion for the isokinetic muscle actions from 60 to 300°/s was also observed. Furthermore, the peak angular velocities for the isotonic muscle actions ranged from 272.9 to 483.0°/s for 50% and 10% MVIC, respectively.
Conclusions: When considering EMG/WK, peak angular velocity, and range of motion together, our data indicate that maximal isokinetic muscle actions at 240°/s or controlled-velocity isotonic muscle actions at 10%, 20%, or 30% MVIC may maximize the amount of muscle activation per unit of WK done during the early stages of musculoskeletal rehabilitation. These results may be useful to allied health professionals who incorporate open-chain resistance training exercises during the early phases of rehabilitation and researchers who use isotonic or isokinetic modes of resistance exercise to examine muscle function.
Keywords: range of motion, angular velocity, muscle activation, leg extension, rehabilitation
I sokinetic and isotonic exercises are 2 modes of resistance training that can be used to improve muscle strength and performance. Isotonic muscle actions are performed with a constant resistance throughout the active range of motion (ROM). Isotonic exercises are cost effective and relatively simple to execute and have been used for many years to increase muscle strength in the rehabilitative setting. 1, 2 However, concentric isotonic exercises can only provide loads that can be overcome by the weakest point in the ROM, and accurately controlling the velocity of movement can be difficult. In contrast, isokinetic exercise provides an accommodating resistance at a constant velocity, which theoretically allows for maximal force production at all points throughout the active ROM and provides an angle-torque curve for each separate muscle action. 3 Isokinetic resistance exercise has also been used for strength testing and training in clinical settings 4, 5; however, a costly dynamometer and trained technicians are required. In addition, the ROM that actually occurs at a constant velocity can be limited at the faster angular velocities because of the acceleration and deceleration phases of movement. 6
Previous authors have compared isokinetic and isotonic exercises during acute bouts of testing 7–13 and in response to training programs. 14–17 For example, Hinson and Rosentswieg 7 and Rosentswieg and Hinson, 10 using surface electromyography (EMG) to compare isometric, isotonic, and isokinetic muscle actions, concluded that isokinetic exercises should be favored over isotonic exercises because the EMG amplitude was greatest for isokinetic muscle actions in one study, 10 whereas in the other investigation, 7 EMG amplitude and ROM were greater for the isokinetic than for the isotonic contractions. In another study, 8 heart rate and arterial pressure were investigated, but no differences were observed between isokinetic and isotonic exercises. In response to a 6-week training period, 2 groups suggested that isokinetic resistance training might be better than isotonic training for improving strength and performance 16 and time to peak torque. 17 In contrast, earlier investigators 14 reported that isokinetic and isotonic training were equally effective modes of resistance exercise and concluded that an isokinetic dynamometer “…appears to be an excellent tool for kinesiologic research, but for simple strengthening of the quadriceps it is well matched by a set of weights.” In a study incorporating a fatiguing protocol, participants were able to maintain greater levels of force production during isotonic than isokinetic leg extensions as the muscles fatigued. 9 In a well-controlled study, participants completed 6 weeks of resistance training on a dynamometer with either isokinetic or isotonic resistance, and the difference scores for strength and power from pretraining to posttraining were significantly better for the isotonic group. 15 Therefore, some conflicting evidence exists about the use of isokinetic versus isotonic exercises for testing 7–13 and training, 14–17 particularly with regard to clinical applications.
Schmitz and Westwood 13 proposed a novel technique termed EMG amplitude-to-work (EMG/WK) ratios to examine the efficacy of isokinetic and/or isotonic leg extension exercises during the early stages of a rehabilitation program. Surface EMG is defined as the algebraic sum of muscle action potentials that pass within the recording areas of the surface electrodes located directly over a force-producing muscle or muscle group. 18–21 Thus, EMG amplitude provides a global quantification of muscle activation, which is affected by the number of motor units recruited and the firing rates of the activated motor units. 22–26 Mechanical work (WK) is defined as the product of force × displacement, and during dynamic muscle actions, WK can be calculated as the integrated area under the angle-torque relationship. 14 Therefore, EMG/WK ratios, which are expressed as the quotient of EMG amplitude divided by WK, may quantify the amount of muscle activation per unit of work accomplished and may provide a global indication of the efficiency of dynamic muscle actions.
Muscle activation may be more important than muscle force production during the early stages of musculoskeletal rehabilitation when emphasizing the training-induced adaptations of the nervous system. 13, 27 Therefore, higher EMG/WK ratios would reflect more muscle activation per unit area of work done by a muscle, which would theoretically emphasize neural adaptations, such as increased motor unit recruitment and firing rate, without subjecting the muscle to unnecessarily heavy loads during rehabilitation. In a previous study, EMG/WK ratios during isotonic muscle actions at 50% of maximal voluntary isometric contraction (MVIC) were greater than EMG/ WK ratios recorded during maximal isokinetic muscle actions at 180°/s. 13 The authors concluded, therefore, that isotonic rather than isokinetic exercises should be incorporated during the early stages of rehabilitation to emphasize improvements in muscle activation. 13
Little is known, however, about EMG/WK ratios during a range of isotonic loads or a range of isokinetic velocities. Lighter isotonic loads or different isokinetic velocities may improve EMG/WK ratios, which in turn may help to refine the use of isokinetic and/or isotonic exercises in clinical rehabilitative settings. Our purpose, therefore, was to extend the findings of Schmitz and Westwood 13 and examine WK, surface EMG amplitude, and EMG/WK ratios during maximal voluntary concentric isokinetic muscle actions at 60, 120, 180, 240, and 300°/s and isotonic muscle actions at 10%, 20%, 30%, 40%, and 50% MVIC during leg extension exercises.
METHODS
Design
We used a randomized, counterbalanced, cross-sectional, repeated-measures experimental design to examine the WK, EMG amplitude, and EMG/WK ratio values for concentric isokinetic and isotonic leg extension exercises. The independent variables were mode (isokinetic versus isotonic), level (1, 2, 3, 4, or 5), and muscle (vastus lateralis [VL], rectus femoris [RF], or vastus medialis [VM]). The independent variable, level, consisted of the following partitions: level 1 corresponded to an isotonic intensity of 50% MVIC and isokinetic velocity of 60°/s; level 2 corresponded to 40% MVIC and 120°/s; level 3 corresponded to 30% MVIC and 180°/s; level 4 corresponded to 20% MVIC and 240°/s; and level 5 corresponded to 10% MVIC and 300°/s. The dependent variables were WK, normalized EMG amplitude, and EMG/WK ratio. Each participant performed both the isokinetic and isotonic testing protocols in random order during the same laboratory visit.
Participants
Ten men (mean age = 20.8 ± 1.7 years, height = 182.9 ± 6.5 cm, mass = 87.6 ± 16.1 kg) and 10 women (age = 22.0 ± 2.6 years, height = 160.8 ± 6.7 cm, mass = 58.9 ± 6.4 kg) volunteered to participate in this study. The subjects were apparently healthy, recreationally active (engaging in 1–5 hours of regular physical activity per week), and indicated no current or recent knee-, hip-, or ankle-related injuries. This study was approved by the university review board for human subjects, and all participants completed a health history questionnaire and signed informed consent forms before testing began.
Instrumentation
A calibrated Biodex System 3 isokinetic dynamometer (Biodex Medical Systems, Inc, Shirley, NY) was used to record the angle-torque curves during the concentric isokinetic and isotonic muscle actions. The subjects were in a seated position with restraining straps over the pelvis and torso, in accordance with the Biodex instructions. 28 The input axis of the dynamometer was aligned with the axis of the knee. The surface EMG signals were recorded with a Biopac data acquisition system (model MP150WSW; Biopac Systems, Inc, Santa Barbara, CA). The EMG signals (recorded in μV) were differentially amplified with a band width of 1 to 5000 Hz, input impedance of 2 MΩ (differential), common mode rejection ratio of 110 dB, maximum input voltage of ±10 V, sampling rate of 1000 Hz, and gain of 1000 (model EMG100C; Biopac Systems). Pregelled, disposable EMG electrodes (Moore Medical, LLC, New Britain, CT) containing 1-cm-diameter Ag-AgCl discs were used for this study.
Concentric Isokinetic and Isotonic Testing
Each participant began with a 5-minute warm-up on a stationary cycle ergometer (model 818E; Monark, Varberg, Sweden) at a resistance of 50 W and a cadence of 60 to 70 revolutions per minute. After the warm-up, the subject was seated on the dynamometer. The testing began with each participant completing two 6-second MVICs of the dominant leg extensors (based on kicking preference) at a knee joint angle of 90° below full extension, in accordance with the procedure of a previous study. 13 Each participant performed 3 or 4 submaximal practice trials before the MVICs, and the MVIC that resulted in the highest peak torque was selected as the representative score.
After the MVIC measurements, the concentric isokinetic and isotonic testing protocols were performed in random order by each participant with 5 minutes of rest between protocols. The isokinetic testing protocol consisted of maximal, concentric isokinetic muscle actions of the leg extensors at randomly ordered angular velocities of 60, 120, 180, 240, and 300°/s. Three or four submaximal warm-up trials preceded 3 maximal muscle actions at each velocity, and a 2-minute rest period was allowed between tests at each velocity. The isotonic testing protocol consisted of concentric isotonic muscle actions of the leg extensors at randomly ordered loads of 10%, 20%, 30%, 40%, and 50% of the previously determined MVIC. Three or four warm-up trials preceded 3 muscle actions for each isotonic load, and a 2-minute rest was allowed between tests at each load. To ensure consistency with a previous protocol 13 for the isotonic muscle actions, we instructed the participant to complete the full ROM in approximately 1 second. Of the 3 repetitions completed for each isokinetic velocity and isotonic load, the repetition that yielded the highest WK (J) value (calculated by integrating the area under the angle-torque curve) was selected for analysis. The WK for the concentric isokinetic and isotonic muscle actions was derived from the Biodex System 3 software (Biodex Medical Systems).
Surface Electromyography
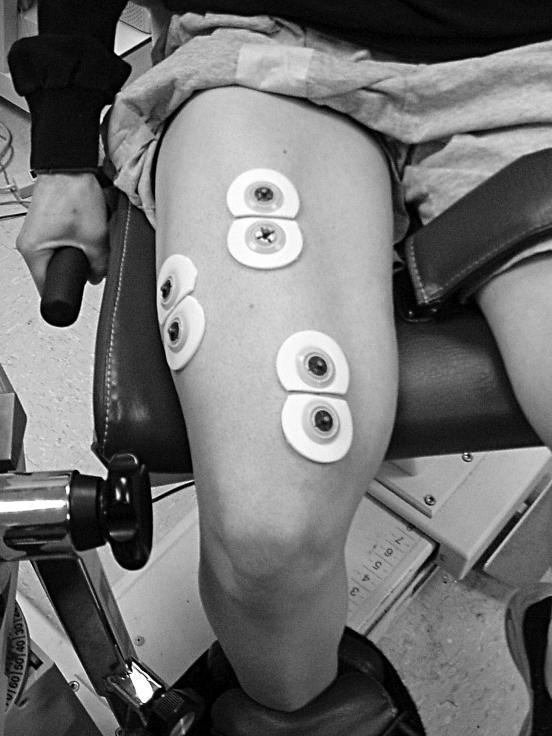
Bipolar surface electrode arrangements were placed along the longitudinal axes of the VL, RF, and VM muscles (Figure 1). For the VL, electrodes were placed at 66% of the distance from the anterior superior iliac spine to the lateral border of the patella, with interelectrode distances of 3.53 ± 0.64 cm. For the RF, electrodes were placed at 50% of the distance from the anterior superior iliac spine to the superior aspect of the patella, with a mean interelectrode distance of 4.54 ± 0.35 cm. For the VM, electrodes were placed at 80% of the distance from the anterior superior iliac spine to the medial border of the patella, with a mean interelectrode distance of 4.65 ± 0.33 cm. The reference electrodes were placed over the iliac crest. Electrodes were placed in accordance with the recommendations of Hermens et al 29 to avoid overlap with the innervation zones and to reduce the risk of cross-talk between muscles. Interelectrode impedance for each muscle was kept below 2000 Ω by swabbing with isopropyl alcohol and carefully abrading the skin, which involved 8 to 12 light strokes over the electrode site with emery paper (3M Red Dot Trace Prep; 3M Canada Inc, London, Ontario, Canada).
Figure 1. Surface electromyographic electrode placements.
Signal Processing
The analog EMG (μV) signals were sampled at a frequency of 1 KHz, stored on a personal computer, and expressed as root mean square (rms) amplitude values (AcqKnowledge version 3; Biopac Systems, Inc). The EMG signals were band-pass filtered (second-order Butterworth filter) at 10 to 500 Hz. For all subsequent analyses, we used the filtered rms EMG (μVrms) amplitude values. To be consistent with the WK values, we analyzed the EMG amplitude values corresponding to the full ROM. This allowed for comparisons between the isokinetic velocities and isotonic loads that were based on a standardized ROM. For statistical comparisons, the EMG amplitude values recorded during the concentric isokinetic and isotonic muscle actions were normalized as a percentage of the EMG amplitude (μVrms) value corresponding to the representative isometric MVIC (% MVIC). For the EMG/WK ratios (% rms/J), the rms EMG amplitude values (μVrms) were normalized to WK (J) separately for each muscle action by dividing the EMG amplitude for the VL, RF, and/or VM by the respective isokinetic or isotonic WK values.
Statistical Analysis
We calculated a 2-way repeated-measures analysis of variance (mode [isotonic versus isokinetic] × level [1, 2, 3, 4, or 5]) to analyze the WK data and 2 separate 3-way repeated-measures analyses of variance (mode [isotonic versus isokinetic] × level [1, 2, 3, 4, or 5] × muscle [VL, RF, or VM]) to analyze the normalized EMG amplitude and EMG/WK data. When appropriate, follow-up analyses included additional 2-way and 1-way repeated-measures analyses of variance with Bonferroni-corrected alpha levels for all pairwise comparisons. An alpha level of P ≤ .05 was considered statistically significant. We used SPSS (version 11.5; SPSS Inc, Chicago, IL) for all statistical analyses.
RESULTS
Work
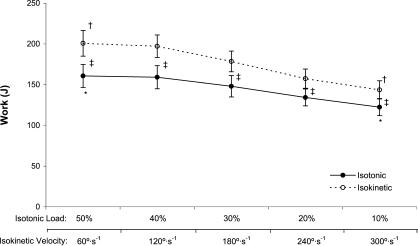
A significant 2-way interaction was noted (mode × level, P = .004) (Figure 2). Isotonic WK decreased from 50% to 30% MVIC ( P = .042), 50% to 20% MVIC ( P = .001), 50% to 10% MVIC ( P < .001), 40% to 30% MVIC ( P = .001), 40% to 20% MVIC ( P = .004), 40% to 10% MVIC ( P < .001), and 30% to 10% MVIC ( P < .001). Isokinetic WK decreased from 60 to 180°/s ( P = .012), 60 to 240°/s ( P = .001), 60 to 300°/s ( P < .001), 120 to 180°/s ( P < .001), 120 to 240°/s ( P < .001), 120 to 300°/s ( P < .001), and 180 to 300°/s ( P < .001) (see Figure 2). In addition, isokinetic WK was greater than isotonic WK at 60°/s versus 50% MVIC ( P < .001), 120°/s versus 40% MVIC ( P < .001), 180°/s versus 30% MVIC ( P < .001), 240°/s versus 20% MVIC ( P = .004), and 300°/s versus 10% MVIC ( P = .001).
Figure 2. Work (J) versus isotonic loads (% maximal voluntary isometric contraction) (solid line). *Indicates work decreased from 50% to 10% maximal voluntary isometric contraction. Work (J) versus isokinetic velocity (°/s) (dashed line). †Indicates work decreased from 60°/s to 300°/s. ‡Indicates isokinetic work was greater than isotonic work for all comparisons ( P < .05). Values are mean ± SEM .
Normalized Electromyographic Amplitude
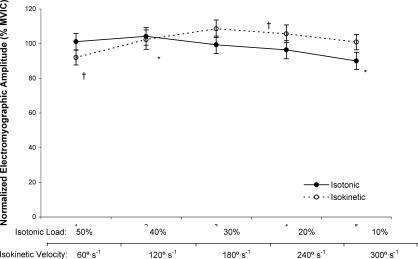
For the isotonic muscle actions, the marginal means for normalized EMG amplitude (collapsed across muscle) decreased from 40% to 10% MVIC ( P = .032), but no other significant differences were seen among any other isotonic loads ( P = .063–.998) (Figure 3). For the isokinetic muscle actions, the marginal means for normalized EMG amplitude (collapsed across muscle) increased from 60°/s to 180°/s ( P = .004) and 240°/s ( P = .032) (see Figure 3). However, the analysis indicated no significant 3-way interaction (mode × level × muscle, P = .344); or 2-way interactions for mode × muscle ( P = .527) or level × muscle ( P = .066) but a significant 2-way interaction for mode × level ( P = .001). No differences ( P = .078–.712) were demonstrated between normalized EMG amplitude values recorded at 50% MVIC and 60°/s, 40% MVIC and 120°/s, 30% MVIC and 180°/s, 20% MVIC and 240°/s, or 10% MVIC and 300°/s.
Figure 3. The marginal means for normalized electromyographic amplitude (collapsed across muscle, % maximal voluntary isometric contraction [MVIC]) versus isotonic load (% MVIC) (solid line). *Indicates decrease from 40% to 10% MVIC ( P = .032). The marginal means for normalized electromyographic amplitude (collapsed across muscle, % MVIC) versus isokinetic velocity (°/s) (dashed line). †Indicates increase from 60°/s to 180°/s ( P = .004) and 240°/s ( P = .032). No differences were noted between isotonic and isokinetic electromyographic amplitude values for any level ( P = .078–.712). Values are mean ± SEM .
Electromyographic Amplitude-to-Work Ratios
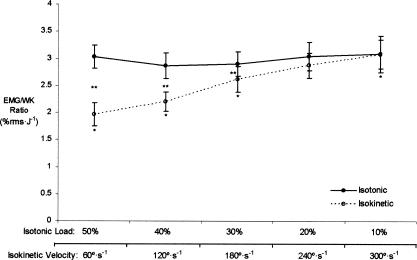
The marginal means for isotonic EMG/WK (collapsed across muscle) did not change from 50% to 10% MVIC ( P = .662) (Figure 4). The marginal means for isokinetic EMG/WK (collapsed across muscle), however, increased from 60 to 180°/s ( P < .001), 120 to 180°/s ( P = .003), and 180 to 300°/s ( P = .010) (see Figure 4). Paired-samples t tests indicated that isotonic EMG/WK was greater than isokinetic EMG/WK at 50% MVIC versus 60°/s ( P = .009), 40% MVIC versus 120°/s ( P < .001), and 30% MVIC versus 180°/s ( P = .004). No differences, however, were found between the isotonic EMG/WK and isokinetic EMG/WK at 20% MVIC versus 240°/s ( P = .103) or 10% MVIC versus 300°/s ( P = .989). No significant 3-way interaction (action × level × muscle, P = .303) or 2-way interactions for action × muscle ( P = .420) or level × muscle ( P = .261) were shown, but a significant 2-way interaction was seen for action × level ( P = .002).
Figure 4. The marginal means for electromyographic amplitude-to-work ratio (EMG/WK) (collapsed across muscle, % root mean square [rms]/J) versus isotonic load (% maximal voluntary isometric contraction [MVIC]) (solid line); no changes from 50% to 10% MVIC ( P = .662). The marginal means for EMG/WK ratio (collapsed across muscle, % rms/J) versus isokinetic velocity (°/s) (dashed line). *Indicates increases from 60°/s to 180°/s ( P < .001), 120°/s to 180°/s ( P = .003), and 180°/s to 300°/s ( P = .010). †EMG/ WK was greater for isotonic muscle actions at 50% MVIC versus 60°/s ( P = .009), 40% MVIC versus 120°/s ( P < .001), and 30% MVIC versus 180°/s ( P = .009). However, no other differences were noted in EMG/WK between isotonic and isokinetic at the other 2 levels ( P = .103–.989). Values are mean ± SEM .
DISCUSSION
In 2001, Schmitz and Westwood 13 introduced a novel EMG normalization procedure, termed EMG/WK ratio, to examine the amount of muscle activation (surface EMG amplitude) per unit of WK during isokinetic muscle actions at 180°/s and isotonic muscle actions at 50% MVIC. The authors reported that the isotonic EMG/WK at 50% MVIC was greater than the isokinetic EMG/WK at 180°/s and concluded that isotonic resistance training should be included in the early stages of open chain musculoskeletal rehabilitation involving the knee joint, especially when emphasizing muscle activation is more important than muscle force production. 13 Our results indicated that isotonic EMG/WK at 50% MVIC (mean ± SEM = 3.0% ± 0.3% rms/J) was 15% greater than the EMG/WK at 180°/s (2.6% ± 0.2% rms/J); however, this difference was not significant ( P = .205). In addition, these findings indicated that the isotonic EMG/WK ratios from 50% to 10% MVIC were greater than the isokinetic EMG/WK ratios at 60, 120, and 180°/s (see Figure 4), but we found no differences among the isotonic EMG/WK ratios from 50% to 10% MVIC and the isokinetic EMG/WK ratios at 240 and 300°/s. It is possible, therefore, that the isokinetic muscle actions at 240 and 300°/s may be as effective as the isotonic muscle actions at 50% to 10% MVIC for maximizing the amount of muscle activation per unit of work (EMG/WK ratio). When considering only the EMG/WK ratio, our findings extend those of Schmitz and Westwood 13 and support the use of isokinetic muscle actions at 240°/s and/or 300°/s or isotonic muscle actions at 50% to 10% MVIC in “…the early stages of rehabilitation when central drive to the muscle (motor unit recruitment) may be more important than absolute muscle force production.” 13
One concern that may arise with the use of isokinetic muscle actions at 240 and 300°/s during rehabilitation is the relatively high angular velocities. The mean peak angular velocities for the isotonic muscle actions are presented in Table 1. Interestingly, the isotonic muscle actions exhibited peak angular velocities ranging from 272.9 to 483.0°/s (50% to 10% MVIC, respectively). Therefore, the muscle actions with the lowest peak angular velocity and equivalent EMG/WK ratios were the isokinetic muscle actions at 240°/s. Thus, when considering both EMG/WK ratios and peak angular velocities, maximal concentric isokinetic muscle actions at 240°/s provided the same EMG/WK ratios as the isotonic muscle actions (10%–50% MVIC) as well as the lowest peak angular velocity. For this reason, perhaps isokinetic exercises at 240°/s could be considered for rehabilitative exercises that maximize the amount of muscle activation per unit of work while minimizing peak angular velocity. However, although 240°/s and higher angular velocities are relatively safe for healthy adults, these velocities may not be safe for injured participants undergoing rehabilitation. Therefore, another solution is to avoid these relatively high peak angular velocities during isotonic exercises by carefully controlling the speed of movement to prevent fast muscle actions that may put premature stress on a recently injured muscle. It is possible that the high peak angular velocities during the isotonic muscle actions were due to the force necessary to overcome the “preload” in order to accelerate the limb through the ROM. 15 Participants were instructed to complete the full ROM in approximately 1 second for the isotonic muscle actions, which was difficult using an isokinetic dynamometer to provide an isotonic resistance, because the movement was not particularly smooth. Future authors should examine the EMG/WK ratios during dynamic constant external resistance exercises, which can be completed on a plate-loaded resistance training device (rather than dynamometer-simulated isotonic muscle actions). This type of resistance training equipment may allow the allied health professional more manual control over the movement velocities.
Table 1. Mean Peak Angular Velocities During the Isotonic Muscle Actions (Mean ± SD).
The value WK is defined as the product of force × displacement. 30 In the present study, therefore, we calculated WK as the integrated area under the angle-torque relationships during the isotonic and isokinetic muscle actions. Theoretically, if the ROM remains unchanged, isotonic WK increases as torque increases from 10% to 50% MVIC. Overall, our findings support this hypothesis and indicate increases in isotonic WK from 10% to 40% MVIC but no change from 40% to 50% MVIC (see Figure 2). The plateau in isotonic WK from 40% to 50% MVIC may have been due, in part, to the 18% decrease in mean ROM from 10% to 50% MVIC (Table 2). This decrease in ROM was consistent with previous findings 7 and was likely due to the added isotonic resistance that limited each participant's ability to extend throughout the entire ROM, despite strong verbal encouragement. In contrast, if the ROM remains unchanged, isokinetic WK would be expected to decrease as the angular velocity is increased as a result of the well-characterized force-velocity relationship. 31 Our results were consistent with this hypothesis and indicated decreases in isokinetic WK from 60 to 300°/s (see Figure 2) in lieu of a slight increase in ROM from 60°/s to 300°/s (see Table 2). Thus, the 18% decrease in mean ROM from 10% to 50% MVIC during the isotonic muscle actions compared with the 3% increase in mean ROM from 60 to 300°/s during the isokinetic muscle actions (see Table 2) indicated that isotonic loads near 10% to 30% MVIC and/or any of the isokinetic velocities (60, 120, 180, 240, or 300°/s) should be used to allow extension throughout the preselected ROM. Although it is sometimes necessary to limit ROM during rehabilitative exercises, 32, 33 the ROM limits should be selected by a qualified allied health professional rather than limited by excessive resistance placed on the muscle.
Table 2. Range of Motion During the Isokinetic and Isotonic Muscle Actions (Mean ± SD).
Several previous groups have investigated the relationship between muscle force production and EMG amplitude during isometric, 34, 35 isokinetic, 36 and isotonic 37, 38 muscle actions, but to our knowledge, no previous authors have examined EMG amplitude response patterns during different isotonic loads simulated by an isokinetic dynamometer. Based on the isometric, isokinetic, and isotonic studies, 34–38 we expected EMG amplitude to increase with increasing isotonic loads from 10% to 50%. Indeed, normalized EMG amplitude increased from 10% to 40% MVIC for each muscle, but no other differences were seen among isotonic loads (see Figure 3). It is possible that this discrepancy to some extent may be related to the relative contributions of the vastus intermedius muscle, which was not examined with EMG. It is likely, however, that the level of muscle activation required to overcome the isotonic preload at all levels resulted in relatively high normalized EMG amplitudes. This hypothesis is supported by the data in Figure 3, because all the normalized EMG amplitude values recorded during the isotonic muscle actions were near 100% of the EMG amplitude values recorded during the isometric MVIC. These findings further support the need to examine EMG amplitude values recorded during plate-loaded resistance training device exercises to avoid the isotonic preload that is present with dynamometers.
In theory, during maximal muscle actions, all available motor units are recruited and firing at their optimal frequencies. 39 Therefore, EMG amplitude should have remained constant during the maximal concentric isokinetic muscle actions across the angular velocities. However, normalized EMG amplitude increased from 60 to 180°/s and then plateaued (see Figure 3) for each muscle (VL, RF, and VM). Previous authors 40–44 hypothesized that velocity-related increases in EMG amplitude during maximal isokinetic muscle actions may be attributable to submaximal muscle activation as a result of central nervous system inhibition under slow-velocity, high-tension conditions. Conversely, Barnes 45 suggested that decreasing patterns of EMG amplitude may result from velocity-related changes in qualitative recruitment, peripheral feedback, and/or antagonistic muscle cross-talk. Overall, the minor fluctuation we found reflected the conflicting results of previous groups who reported increases, 40–44 decreases, 45 and no change 46 in the patterns of EMG amplitude across angular velocity during maximal, concentric isokinetic muscle actions. It is possible that this conflicting evidence is due to differences among the muscle or muscle group involved and/or the range of velocities tested.
CONCLUSIONS
Our results extend the findings of Schmitz and Westwood 13 and indicate that isotonic loads ranging from 10% to 50% MVIC and isokinetic velocities of 240 and 300°/s resulted in similar EMG/WK ratios for the superficial muscles of the QF during leg extension exercises. Further examination of the isotonic and isokinetic muscle actions indicated that peak angular velocities for the isotonic muscle actions (see Table 1) ranged from 272.9 to 483.0°/s (50%–10% MVIC, respectively), whereas the lowest peak angular velocity with an equivalent EMG/WK ratio occurred during the isokinetic muscle actions at 240°/s. In addition, the mean ROM (see Table 2) decreased from 100.8° to 82.5° (10%–50% MVIC, respectively) during the isotonic muscle actions but slightly increased from 60 to 300°/s during the isokinetic muscle actions. Therefore, when considering EMG/WK, peak angular velocity, and ROM together, our data indicate that isokinetic muscle actions at 240°/s provided the highest EMG/WK ratio that minimized the peak angular velocity and allowed for extension throughout the preselected ROM. These findings also indicate that if the velocity of movement is carefully controlled during isotonic muscle actions at 10%, 20%, or 30% MVIC (see Table 1), ROM is not substantially compromised (see Table 2), and the EMG/WK ratios are no different than those at 40% or 50% MVIC or 240 or 300°/s. Overall, we propose that maximal isokinetic muscle actions at 240°/s or controlled-velocity isotonic muscle actions at 10%, 20%, or 30% MVIC may maximize the amount of muscle activation per unit of work done and may be appropriate during the early stages of musculoskeletal rehabilitation. These results may be useful to allied health professionals who incorporate open chain resistance training exercises during the early phases of rehabilitation and to researchers who use isotonic and/or isokinetic modes of resistance exercise to examine muscle function. It is possible, however, that muscle activation patterns could be different for those who are injured and undergoing rehabilitation, as a result of joint swelling and pain. Therefore, additional studies are necessary to examine EMG/WK ratios in injured muscles or joints. In addition, future authors should examine the EMG/ WK ratios during dynamic constant external resistance muscle actions on a plate-loaded device to compare with the isokinetic and isotonic muscle actions and to determine optimal loads for rehabilitation exercises.
Acknowledgments
We thank Laurie L. Massey, Suzanne M. Dangelmaier, Julie Y. Culbertson, and Kristi A. Fitz for aiding in the data collection process during this study.
REFERENCES
- DeLorme TL. Restoration of muscle power by heavy-resistance exercises. J Bone Joint Surg. 1945;27:645–667. [Google Scholar]
- DeLorme TL, Watkins AL. Techniques of progressive resistance exercise. Arch Phys Med Rehabil. 1948;29:263–273. [PubMed] [Google Scholar]
- Brown LE. Isokinetics in Human Performance. Champaign, IL: Human Kinetics; 2000.
- Timm KE. Postsurgical knee rehabilitation: a five year study of four methods and 5,381 patients. Am J Sports Med. 1988;16:463–468. doi: 10.1177/036354658801600506. [DOI] [PubMed] [Google Scholar]
- Morrissey MC, Harman EA, Johnson MJ. Resistance training modes: specificity and effectiveness. Med Sci Sports Exerc. 1995;27:648–660. [PubMed] [Google Scholar]
- Brown LE, Whitehurst M, Gilbert R, Buchalter DN. The effect of velocity and gender on load range during knee extension and flexion exercise on an isokinetic device. J Orthop Sports Phys Ther. 1995;21:107–112. doi: 10.2519/jospt.1995.21.2.107. [DOI] [PubMed] [Google Scholar]
- Hinson M, Rosentswieg J. Comparative electromyographic values of isometric, isotonic, and isokinetic contraction. Res Q. 1973;44:71–78. [PubMed] [Google Scholar]
- Iellamo F, Legramante JM, Raimondi G. Effects of isokinetic, isotonic and isometric submaximal exercise on heart rate and blood pressure. Eur J Appl Physiol Occup Physiol. 1997;75:89–96. doi: 10.1007/s004210050131. et al. [DOI] [PubMed] [Google Scholar]
- Knight KL, Ingersoll CD, Bartholomew J. Isotonic contractions might be more effective than isokinetic contractions in developing muscle strength. J Sport Rehabil. 2001;10:124–131. [Google Scholar]
- Rosentswieg J, Hinson MM. Comparison of isometric, isotonic and isokinetic exercises by electromyography. Arch Phys Med Rehabil. 1972;53:249–252. [PubMed] [Google Scholar]
- Knapik JJ, Wright JE, Mawdsley RH, Braun JM. Isokinetic, isometric and isotonic strength relationships. Arch Phys Med Rehabil. 1983;64:77–80. [PubMed] [Google Scholar]
- Knapik JJ, Wright JE, Mawdsley RH, Braun J. Isometric, isotonic, and isokinetic torque variations in four muscle groups through a range of joint motion. Phys Ther. 1983;63:938–947. doi: 10.1093/ptj/63.6.938. [DOI] [PubMed] [Google Scholar]
- Schmitz RJ, Westwood KC. Knee extensor electromyographic activity-to-work ratio is greater with isotonic than isokinetic contractions. J Athl Train. 2001;36:384–387. [PMC free article] [PubMed] [Google Scholar]
- DeLateur B, Lehmann JF, Warren CG. Comparison of effectiveness of isokinetic and isotonic exercise in quadriceps strengthening. Arch Phys Med Rehabil. 1972;53:60–64. et al. [PubMed] [Google Scholar]
- Kovaleski JE, Heitman RH, Trundle TL, Gilley WF. Isotonic preload versus isokinetic knee extension resistance training. Med Sci Sports Exerc. 1995;27:895–899. [PubMed] [Google Scholar]
- Smith MJ, Melton P. Isokinetic versus isotonic variable resistance training. Am J Sports Med. 1981;9:275–279. doi: 10.1177/036354658100900420. [DOI] [PubMed] [Google Scholar]
- Wojtys EM, Huston LJ, Taylor PD, Bastian SD. Neuromuscular adaptations in isokinetic, isotonic, and agility training programs. Am J Sports Med. 1996;24:187–192. doi: 10.1177/036354659602400212. [DOI] [PubMed] [Google Scholar]
- deVries HA, Housh TJ. Physiology of Exercise: For Physical Education, Athletics and Exercise Science. 5th ed. Dubuque, IA: WCB Brown & Benchmark; 1994.
- Day SJ, Hulliger M. Experimental simulation of cat electromyogram: evidence for algebraic summation of motor-unit action-potential trains. J Neurophysiol. 2001;86:2144–2158. doi: 10.1152/jn.2001.86.5.2144. [DOI] [PubMed] [Google Scholar]
- Enoka RM. Neuromechanics of Human Movement. 3rd ed. Champaign, IL: Human Kinetics; 2002.
- Keenan KG, Farina D, Maluf KS, Merletti R, Enoka RM. Influence of amplitude cancellation on the simulated surface electromyogram. J Appl Physiol. 2005;98:120–131. doi: 10.1152/japplphysiol.00894.2004. [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Mills KR. The relationship between mean power frequency of the EMG spectrum and muscle fibre conduction velocity. Electroencephalogr Clin Neurophysiol. 1985;60:130–134. doi: 10.1016/0013-4694(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Komi PV, Tesch P. EMG frequency spectrum, muscle structure, and fatigue during dynamic contractions in man. Eur J Appl Physiol Occup Physiol. 1979;42:41–50. doi: 10.1007/BF00421103. [DOI] [PubMed] [Google Scholar]
- Moritani T, Muro M, Kijima A, Gaffney FA, Parsons D. Electromechanical changes during electrically induced and maximal voluntary contractions: surface and intramuscular EMG responses during sustained maximal voluntary contraction. Exp Neurol. 1985;88:484–499. doi: 10.1016/0014-4886(85)90065-2. [DOI] [PubMed] [Google Scholar]
- Solomonow M, Baten C, Smit J. Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J Appl Physiol. 1990;68:1177–1185. doi: 10.1152/jappl.1990.68.3.1177. et al. [DOI] [PubMed] [Google Scholar]
- Westbury JR, Shaughnessy TG. Associations between spectral representation of the surface electromyogram and fiber type distribution and size in human masseter muscle. Electromyogr Clin Neurophysiol. 1987;27:427–435. [PubMed] [Google Scholar]
- Kasman GS, Cram JR, Wolf SL. Clinical Applications in Surface Electromyography: Chronic Musculoskeletal Pain. Gaithersburg, FL: Aspen Publishers Inc; 1998.
- Biodex Medical Systems Inc. Biodex Pro Manual. Shirley, NY: Biodex Medical Systems Inc; 1998.
- Hermens HJ, Freriks B, Merletti R. SENIAM 8: European Recommendations for Surface Electromyography: Results of the SENIAM Project. et al. Enschede, The Netherlands: Roessingh Research and Development b.v.; 1999.
- Cutnell JD, Johnson KW. Physics. Vol 1. 4th ed. New York, NY: John Wiley & Sons Inc; 1998.
- Perrine JJ, Edgerton VR. Muscle force-velocity and power-velocity relationships under isokinetic loading. Med Sci Sports. 1978;10:159–166. [PubMed] [Google Scholar]
- Beynnon BD, Fleming BC, Johnson RJ, Nichols CE, Renstrom PA, Pope MH. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23:24–34. doi: 10.1177/036354659502300105. [DOI] [PubMed] [Google Scholar]
- Houglum PA. Therapeutic Exercise for Athletic Injuries. Champaign, IL: Human Kinetics; 2001.
- Ebersole KT, Housh TJ, Johnson GO, Evetovich TK, Smith DB, Perry SR. MMG and EMG responses of the superficial quadriceps femoris muscles. J Electromyogr Kinesiol. 1999;9:219–227. doi: 10.1016/s1050-6411(98)00036-4. [DOI] [PubMed] [Google Scholar]
- Lawrence JH, De Luca CJ. Myoelectric signal versus force relationship in different human muscles. J Appl Physiol. 1983;54:1653–1659. doi: 10.1152/jappl.1983.54.6.1653. [DOI] [PubMed] [Google Scholar]
- Beck TW, Housh TJ, Johnson GO. Mechanomyographic and electromyographic time and frequency domain responses during submaximal to maximal isokinetic muscle actions of the biceps brachii. Eur J Appl Physiol. 2004;92:352–359. doi: 10.1007/s00421-004-1110-9. et al. [DOI] [PubMed] [Google Scholar]
- Dalton PA, Stokes MJ. Acoustic myography reflects force changes during dynamic concentric and eccentric contractions of the human biceps brachii muscle. Eur J Appl Physiol Occup Physiol. 1991;63:412–416. doi: 10.1007/BF00868071. [DOI] [PubMed] [Google Scholar]
- Petitjean M, Maton B, Cnockaert JC. Evaluation of human dynamic contraction by phonomyography. J Appl Physiol. 1992;73:2567–2573. doi: 10.1152/jappl.1992.73.6.2567. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, De Luca CJ. Muscles Alive: Their Functions Revealed by Electromyography. 5th ed. Baltimore, MD: Williams & Wilkins; 1985.
- Cramer JT, Housh TJ, Johnson GO, Ebersole KT, Perry SR, Bull AJ. Mechanomyographic amplitude and mean power output during maximal, concentric, isokinetic muscle actions. Muscle Nerve. 2000;23:1826–1831. doi: 10.1002/1097-4598(200012)23:12<1826::aid-mus5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cramer JT, Housh TJ, Weir JP. Power output, mechanomyographic, and electromyographic responses to maximal, concentric, isokinetic muscle actions in men and women. J Strength Cond Res. 2002;16:399–408. et al. [PubMed] [Google Scholar]
- Cramer JT, Housh TJ, Weir JP. Gender, muscle, and velocity comparisons of mechanomyographic and electromyographic responses during isokinetic muscle actions. Scand J Med Sci Sports. 2004;14:116–127. doi: 10.1111/j.1600-0838.2003.00317.x. et al. [DOI] [PubMed] [Google Scholar]
- Seger JY, Thorstensson A. Muscle strength and myoelectric activity in prepubertal and adult males and females. Eur J Appl Physiol Occup Physiol. 1994;69:81–87. doi: 10.1007/BF00867932. [DOI] [PubMed] [Google Scholar]
- Westing SH, Cresswell AG, Thorstensson A. Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur J Appl Physiol Occup Physiol. 1991;62:104–108. doi: 10.1007/BF00626764. [DOI] [PubMed] [Google Scholar]
- Barnes WS. The relationship of motor-unit activation to isokinetic muscular contraction at different contractile velocities. Phys Ther. 1980;60:1152–1158. doi: 10.1093/ptj/60.9.1152. [DOI] [PubMed] [Google Scholar]
- Rothstein JM, Delitto A, Sinacore DR, Rose SJ. Electromyographic, peak torque, and power relationships during isokinetic movement. Phys Ther. 1983;63:926–933. doi: 10.1093/ptj/63.6.926. [DOI] [PubMed] [Google Scholar]