Abstract
Context: Fatigue appears to influence musculoskeletal injury rates during athletic activities, but whether males and females respond differently to fatigue is unknown.
Objective: To determine the influence of fatigue on vertical leg stiffness (K VERT) and muscle activation and joint movement strategies and whether healthy males and females respond similarly to fatigue.
Design: Repeated-measures design with all data collected during a single laboratory session.
Setting: Laboratory.
Patients or Other Participants: Physically active males (n = 11) and females (n = 10).
Intervention(s): Subjects performed hopping protocols at 2 frequencies before and after fatigue, which was induced by repeated squatting at submaximal loads.
Main Outcome Measure(s): We measured K VERT with a forceplate and peak muscle activity of the quadriceps, hamstrings, gastrocnemius, soleus, and anterior tibialis muscles with surface electromyography. Sagittal-plane kinematics at the knee and ankle were recorded with an electrogoniometer.
Results: After fatigue, K VERT was unchanged for all subjects. However, both males and females demonstrated reduced peak hamstrings ( P = .002) and anterior tibialis ( P = .001) activation, coupled with increased gastrocnemius ( P = .005) and soleus ( P = .001) peak activity, as well as increased quadriceps-hamstrings ( P = .005) and gastrocnemius/soleus-anterior tibialis coactivation ratios ( P = .03) after fatigue. Overall, females demonstrated greater quadriceps-hamstrings coactivation ratios than males, regardless of the fatigue condition ( P = .026). Only females showed increased knee flexion at initial contact after fatigue during hopping ( P = .03).
Conclusions: Although K VERT was unaffected, the peak muscle activation and joint movement strategies used to modulate K VERT were affected after fatigue. Once fatigued, both males and females used an ankle-dominant strategy, with greater reliance on the ankle musculature and less on the knee musculature. Also, once fatigued, all subjects used an antagonist inhibition strategy by minimizing antagonist coactivation. Overall, females used a more quadriceps-dominant strategy than males, showing greater quadriceps activity and a larger quadriceps-hamstrings coactivation ratio. Changes in muscle activation and coactivation ratios because of fatigue and sex are suggested to alter knee joint stability and increase anterior cruciate ligament injury risk.
Keywords: electromyography, anterior cruciate ligament, coactivation, injury prevention
Fatigue has been hypothesized to alter the biomechanical and neuromuscular factors associated with the risk of sustaining musculoskeletal injury. 1 Biomechanical and neuromuscular factors include muscle strength, rate of force production, muscle activation strategies, movement strategies, and stiffness properties of the muscle and joint complex. 2 These variables are believed to be of particular importance because they involve active muscle contributions. After a fatiguing exercise bout, biomechanical and neuromuscular factors such as muscle activation patterns, 3–7 coactivation, 8–10 kinematics and kinetics, 4, 5, 11–14 and stiffness properties 5, 15, 16 are altered. In addition, epidemiologic research suggests a relationship between fatigue and musculoskeletal injury rates. 17, 18 Investigators have demonstrated a greater incidence of injuries occurring during the later stages of athletic competitions and practices. 17, 18 The psychosocial perception of fatigue has also been implicated as a factor influencing injury, because the perception of fatigue measured during preseason and midseason strongly predicted injury rates over the course of a season. 19 Based on these research findings, fatigue does appear to influence musculoskeletal injury rates during athletic activities.
Preexisting sex differences in various biomechanical and neuromuscular factors 20–25 may be amplified by fatigue. For example, females have been shown to have less muscle strength than males 24 and may respond differently to a fatiguing bout of exercise because of this inherent strength difference. However, the effects of fatigue in males and females have not been compared. If females respond differently than males to fatigue, this may be an additional risk factor for anterior cruciate ligament (ACL) injury. 1 Most of the previous authors investigating the influence of fatigue on biomechanical and neuromuscular factors have studied open kinetic chain exercise fatigue protocols that were performed under maximal effort conditions for a short duration of time. 1, 9–12 14, 16 Unfortunately, these types of fatigue protocols do not mimic the loading conditions sustained by the lower extremity. It may be important to assess the effects of fatigue using movement patterns similar to those experienced by the lower extremity (closed kinetic chain) and performed under submaximal loading conditions for sustained periods of time. In addition, no previous investigators have assessed the influence of sex and fatigue induced via closed kinetic chain exercise on vertical leg stiffness (K VERT) and control strategies for modulating K VERT. Control strategies for modulating K VERT may be operationally defined as the multijoint coordination (joint kinematics) and muscular recruitment plan (muscle activation) an individual executes to modulate joint torsional stiffness and lower extremity stiffness, hence satisfying the objectives of the functional task. 26–28 Therefore, our primary purpose was to investigate the effect of lower extremity muscle fatigue induced through closed kinetic chain exercise on K VERT and control strategies for modulating K VERT (muscle activation peak amplitude, coactivation, and sagittal-plane knee and ankle joint kinematics) during a functional hopping task. Our secondary purpose was to determine whether the response to lower extremity fatigue differed between healthy males and females.
METHODS
Subjects
Eleven physically active males (age = 27.81 ± 4.35 years, height = 176.54 ± 7.54 cm, mass = 80.11 ± 9.21 kg) and 10 females (age = 24.10 ± 3.75 years, height = 168.50 ± 5.91 cm, mass = 66.92 ± 12.39 kg) with no known knee abnormalities or recent musculoskeletal injuries participated in this study. We performed an a priori statistical power analysis based on previously published data comparing K VERT between males and females. 23 Granata et al 23 noted that a 20% difference in K VERT between males and females was significant. Thus, we hypothesized that a 20% change in K VERT from prefatigue to postfatigue measures would represent a clinically significant change in K VERT. Based on these data, a sample size of 20 subjects would provide a power of .98 to detect a 20% change in K VERT from prefatigue to postfatigue. A subset of the data collected from these subjects during these testing procedures has been previously reported in a separate study. 25 Before participating in the study, all subjects read and signed an informed consent form approved by the university's institutional review board, which also approved the study. All testing was performed in a research laboratory during a single test session.
The preferred landing leg served as the test limb for all muscle activity and kinematic data. We determined the preferred landing leg by having subjects perform a single-leg landing from a 30-cm-high box. The preferred landing leg was defined as the self-selected lower extremity limb on which the subject chose to land in 2 of 3 trials. Pilot testing revealed that this method of determining the preferred landing leg had excellent intersession reliability (intraclass correlation coefficient [2,k] = .92).
Testing Procedures
Hopping Protocol
Upon arrival at the laboratory, subjects completed a verbal questionnaire to ensure that they had no known knee abnormalities or recent musculoskeletal injuries (within the past 3 months). Before testing, subjects received an explanation of all testing procedures and were allowed practice trials to become acquainted with the protocol. We assessed K VERT, peak muscle activation, and joint movement strategies by having subjects perform 2-legged hopping in place with their hands on their hips while barefoot. Because subjects performed 2-legged hopping for all trials, K VERT was equivalent to the combined stiffness for both legs. Hopping conditions consisted of 2 hopping frequencies. Subjects hopped at their preferred, self-selected rates (FREQ PREF) and a controlled hopping rate of 3.0 Hz (FREQ 3.0). The FREQ 3.0 was controlled by having subjects hop in time with a digital metronome. Subjects were instructed that each hop must be a continuous motion, and they were allowed as much practice as needed until they felt comfortable. We estimate that it took approximately 30 seconds for subjects to become comfortable with hopping in time with the digital metronome at the controlled hopping frequency.
During preliminary testing, we determined that use of the digital metronome during controlled FREQ 3.0 hopping influenced individuals' FREQ PREF. When performing FREQ 3.0 hopping before FREQ PREF, the individual's FREQ PREF increased compared with the reverse sequence. Thus, we chose to have subjects perform the FREQ PREF hopping first, followed by FREQ 3.0 hopping, to avoid any alteration to the FREQ PREF. Approximately 45 continuous hops were performed for each hopping condition during testing.
Fatigue Protocol
The fatigue protocol followed the prefatigue assessment. A 5-minute rest period was allowed after the prefatigue assessment before the fatigue protocol was initiated. To ensure that postfatigue assessments truly represented fatigue, subjects performed the postfatigue hopping protocol immediately (within 30 seconds) after the fatiguing protocol. In addition, no rest was allowed between the hopping conditions performed after fatigue.
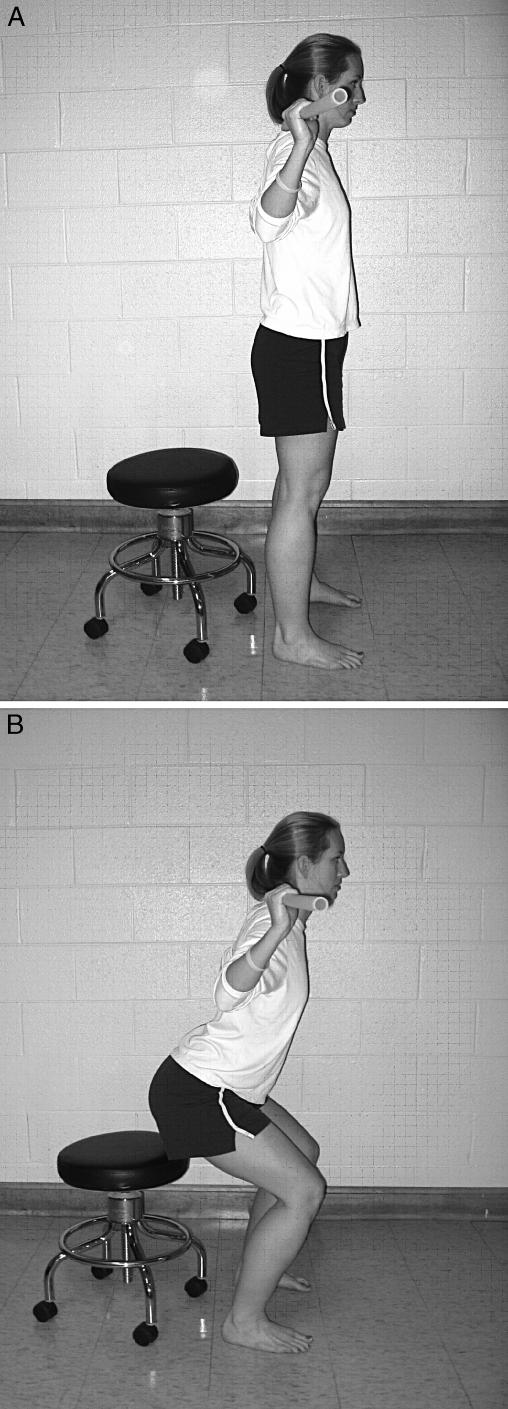
We induced lower extremity muscle fatigue using a functional, closed kinetic chain exercise protocol. Subjects performed repeated squatting motions while carrying one third of their body weight. A barbell loaded with the appropriate weight was positioned along the posterior aspect of the subject's shoulders, similar to that used while performing a loaded squat maneuver. Each squatting motion was performed through a knee flexion range of 0° to 60°. Knee range of motion during the fatigue protocol was controlled by instructing subjects to come to an extended knee position (0°) when moving upward (Figure 1A), then lightly touch an end range-of-motion block set at 60° of knee flexion when moving downward (Figure 1B). An electrogoniometer was used to monitor knee motion during the fatigue protocol. Movement frequency was controlled by having subjects perform the repeated squatting movements at a frequency of 50 squats/min to a digital metronome. Subjects were instructed to maintain a constant movement rate for both the concentric and the eccentric movement phases. The fatigue protocol was modified from previous research by Yates et al, 29 who investigated elbow flexor muscle fatigue. We altered the fatigue protocol to accommodate the squat task by changing the movement frequency, knee range of motion, and load based on pilot testing.
Figure 1. Subject setup during the lower extremity fatigue protocol. A, Starting position. B, Subjects performed closed kinetic chain exercise (repeated squatting motions) through a controlled knee range of motion while carrying one third of their body weight.
Each subject performed the squatting maneuver until the point of fatigue. Fatigue was defined as the time when the subject fell 4 squat cycles behind the set pace or failed to complete 2 successive squat cycles. Before beginning, we emphasized the need to come into full knee extension and lightly touch the range-of-motion block when moving into knee flexion, even at the expense of falling behind the set cadence.
Data Processing and Analyses
All data were acquired using DATAPAC III Lab Application Systems software (version 2000; Run Technologies, Laguna Hills, CA) and stored in a personal computer for later analysis with a customized program developed in MATLAB (version 6.1; The MathWorks, Natick, MA).
Data Selection
We analyzed the first 10 acceptable hopping trials of the total 45 from each hopping condition. Hopping trials were determined to be acceptable based on 2 criteria. First, for FREQ 3.0 hopping conditions, only those trials in which the subject's hopping frequency was within 5% of 3.0 Hz were accepted for analysis; during FREQ PREF hopping conditions, trials within 5% of the subject's average self-selected hopping rate were analyzed. Hopping frequency was determined through the vertical ground reaction force data. Second, the correlation between vertical displacement and vertical ground reaction force during the ground-contact phases of hopping must have been greater than r = .80 to be accepted for analysis. 23, 30 This criterion was selected to ensure that the lower extremity behaved like a simple spring-mass system during the hopping conditions.
Vertical Leg Stiffness
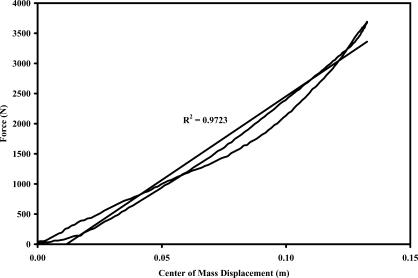
The K VERT was assessed as subjects performed the hopping protocol on a forceplate (model 6700; Kistler Corp, Winterthur, Switzerland; natural frequency = 400 Hz, linearity = ±0.2% full scale, dimensions = 40 × 60 cm) sampling at 1000 Hz. Specifically, we used vertical ground reaction force data for K VERT calculations. The K VERT was calculated from the regression slope of the profile when vertical ground reaction force was plotted versus center-of-mass displacement (kN/m) (Figure 2,). 31, We have previously described these measures. 23, 25 Because K VERT is strongly related to body size, 32 we normalized K VERT to each subject's body weight (N) (K VERT-NORM).
Figure 2. Vertical leg stiffness assessment. The linear relationship is an indicator that the lower extremity behaved like a simple spring-mass system. The slope of these force-displacement curves represents the vertical leg stiffness (K VERT) during hopping .
Peak Muscle Activity and Coactivation
An 8-channel telemetry electromyography (EMG) system (model 900; Noraxon Telemyo Inc, Scottsdale, AZ) sampling at 1000 Hz was used to record peak muscle activity and coactivation. Unit specifications included a differential amplifier gain of 1000 fixed, a frequency bandwidth of 16 Hz to 500 Hz, a common mode rejection ratio of 114 dB, and input resistance from 20 MOhm to 1 GOhm. Bipolar Ag-AgCl surface electrodes (Medicotest, Rolling Meadows, IL) measuring 10 mm in diameter with a center-to-center distance of approximately 2.0 cm were arranged in parallel over the muscle bellies of the rectus femoris (RF), vastus medialis (VM), semimembranosus (SM), biceps femoris (BF), medial gastrocnemius (MG), lateral gastrocnemius (LG), soleus (SO), and anterior tibialis (AT). 33 These muscles were selected to be representative of muscle activation of the quadriceps (Q), hamstrings (H), triceps surae (TS), and AT muscles, respectively. Before applying surface electrodes, we shaved the subject's skin and cleaned it with isopropyl alcohol. Manual muscle testing was performed to confirm electrode placement and check for cross-talk. Surface electrodes were secured with an elastic bandage to prevent cable movement artifact during hopping. The subject wore a battery-operated FM transmitter/amplifier (Noraxon Telemyo Inc), which collected muscle activity data from the surface electrodes. The signal was sent via telemetry from the transmitter to the computer, where the raw EMG data were stored for later analysis. All EMG data were low-pass filtered at 250 Hz, high-pass filtered at 30 Hz, rectified, and smoothed using a Hanning integrator set to 20 points.
After proper surface electrode placement was achieved, subjects were placed in a sitting position on a commercial isokinetic dynamometer (model 835-000; Biodex Medical Systems, Shirley, NY) according to the manufacturer's guidelines. The dynamometer was set to the isometric mode, and subjects performed maximal voluntary isometric contractions (MVICs) for each muscle tested. The isokinetic dynamometer provided an immovable lever arm against which the subject exerted maximal force during MVIC testing. The MVIC levels for the 8 muscles tested were established for each subject by collecting 3 maximal 5-second trials. The first and last second of the MVIC trials were removed from the data to assure only steady-state results during MVIC trials. The peak amplitude across the 3 trials was averaged for each muscle. The peak muscle amplitude during MVIC trials was used to normalize all EMG data collected during hopping. Thus, EMG data are expressed as a percentage of MVIC (% MVIC).
Peak muscle activity and coactivation ratios were assessed during the preparatory response (PR) and loading response (LR) phases while the subject hopped. We defined the PR phase as the 50 milliseconds preceding the initial ground contact, as determined from the vertical ground reaction force. We chose the PR phase because it is believed to represent an individual's preprogrammed muscle recruitment strategy. 28, 34, 35 Previous researchers 25, 28, 34, 35 have selected similar time periods before ground impact when investigating preparatory muscle activation. The LR phase was defined as the 50-millisecond interval immediately after ground contact. This time interval was selected in an attempt to assess the peak muscle activation response immediately after lower extremity perturbation from ground contact and to be consistent with previous investigations of muscle recruitment after landing. 3, 6, 36, 37 In addition, a knee injury during jumping activities typically occurs immediately after initial foot strike. 38, 39 Although a specific time interval after foot strike has not been determined, the temporal window for injury after foot strike appears to be quite small. We believe that assessing muscle peak activation during the 50-millisecond time window after foot strike (LR phase) may lend insight into the muscle recruitment strategy used to stiffen and stabilize the lower extremity during times when injury is estimated to occur.
The average peak muscle activation was measured by averaging the peak muscle activation amplitude from each of the first 10 acceptable hopping trials for each muscle tested. Coactivation ratios for the Q and H (Q:H) as well as the TS (MG, LG, and SO muscles) and AT (TS:AT) muscle groups were also assessed. The Q:H coactivation was computed as the sum of peak Q (RF and VM) (agonist) activity divided by the sum of peak H (SM and BF) (antagonist) activity for each trial. Similarly, TS:AT coactivation was computed as the sum of peak TS (agonist) activity divided by the peak AT (antagonist) activity for each trial. We computed the corresponding coactivation ratio separately for each of the 10 acceptable trials, and then the average was taken for the Q:H and TS:AT coactivation ratios. Coactivation ratios of 1.0 indicate equal peak activation of the agonist and antagonist muscles. Coactivation ratios greater than 1.0 indicate increased agonist peak activation (Q or TS) compared with the antagonist (H and AT) muscles. Coactivation ratios less than 1.0 indicate the opposite: greater antagonist peak activity than agonist peak activity.
Knee and Ankle Kinematics
We assessed sagittal-plane knee and ankle motion using an electrogoniometer (models SG110 and SG150; Penny and Giles Biometrics Limited, Gwent, UK). To assess knee motion, we placed the electrogoniometer over the lateral aspect of the dominant leg, using the joint line as the axis of rotation. To assess ankle motion, we placed the electrogoniometer over the dorsum of the foot, in line with the third metatarsal and along the anterior shaft of the tibia. The electrogoniometer was secured to the subject's skin using double-sided medical tape and an elastic bandage to prevent cable tensioning and movement artifact during hopping.
Knee and ankle kinematic variables included joint flexion-angle measurements at the time of initial ground contact (IC KNEE and IC ANKLE) as well as joint flexion excursions (EX KNEE and EX ANKLE). Joint excursion was defined as the angular displacement occurring from the initial ground contact until the maximal joint flexion angle during the ground contact portion of hopping. 24, 27 Researchers 27, 30, 40 have demonstrated that knee and ankle joint excursion influence leg stiffness and ground reaction forces during hopping and jumping maneuvers.
Statistical Analyses
We performed separate, mixed-model, repeated-measures analyses of variance for each of the dependent variables to determine whether sex and fatigue had an effect on the variables tested. All analyses involved only 1 between-subjects variable (sex), whereas the number of within-subjects variables differed, depending on the factors tested. The K VERT, IC KNEE, IC ANKLE, EX KNEE, and EX ANKLE analyses involved 2 within-subject variables: fatigue (2 levels: prefatigue, postfatigue) and hopping frequency (2 levels: FREQ PREF, FREQ 3.0). Peak muscle activation amplitude of the SO and AT and the Q:H and TS:AT coactivation ratios involved 3 within-subjects variables: fatigue, hopping frequency, and phase (2 levels: PR, LR). Peak muscle activation amplitude of the Q (MQ, LQ), H (SM, BF), and gastrocnemius (MG, LG) involved 4 within-subjects variables: fatigue, hopping frequency, phase, and muscle side (2 levels: medial, lateral). A significance level of α < .05 was set a priori for all analyses. We used the Tukey Honestly Significant Difference method to perform post hoc analyses on all significant main effects and interactions.
RESULTS
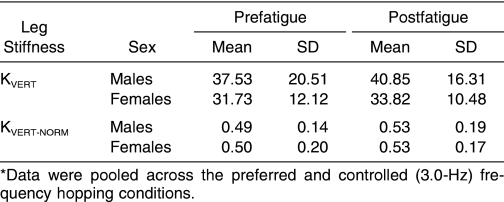
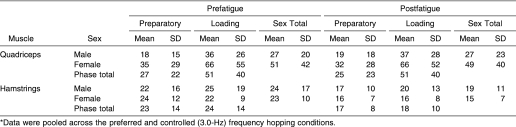
The data for K VERT, thigh muscle peak activation, lower leg muscle peak activation, and coactivation ratios, respectively ( Tables 1 through 4), have been pooled across hopping frequency and muscle side, as no significant interactions involved these variables (P > .05). Lower extremity muscle fatigue induced through closed kinetic chain exercise did not significantly alter K VERT (F 1,19 = 2.497, P = .131), K VERT-NORM (F 1,19 = 2.284, P = .147), peak Q muscle activity (F 1,19 = 0.025, P = .877), EX KNEE (F 1,19 = 1.034, P = .322), EX ANKLE (F 1,19 = 0.149, P = .705), or IC ANKLE (F 1,19 = 0.396, P = .538). These variables were unchanged in both males and females after the fatigue protocol.
Table 1. Vertical Leg Stiffness (K VERT) and Normalized Vertical Leg Stiffness (K VERT-NORM) Values During Prefatigue and Postfatigue Hopping Conditions (kN/m)* .
Peak Muscle Activation
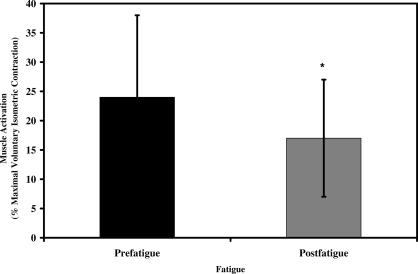
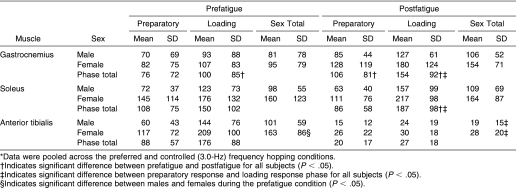
Although K VERT and K VERT-NORM were not affected, peak muscle activation and joint movement strategy were altered after fatigue. We observed significant reductions in peak H and AT muscle activation. A significant fatigue main effect was noted for the H muscles (F 1,19 = 12.073, P = .002), with postfatigue H muscle activity reduced by 26% compared with prefatigue measures for all subjects (Figure 3). Hamstring activation was not significantly affected by sex, as no significant main effect or interactions involving sex were seen for H activation (see Table 2). However, AT activity after fatigue was influenced by sex, with a significant fatigue-by-sex interaction (F 1,19 = 4.777, P = .042). Before fatigue, females displayed 37% more AT activation than males. Anterior tibialis activity significantly decreased for males and females by 83% and 81%, respectively, from prefatigue to postfatigue measures. Sex differences in AT muscle activity were no longer present after fatigue (see Table 3).
Figure 3. Influence of fatigue on hamstring muscle activation (data pooled across sex, muscle response phase, and hopping frequency). *Indicates a significant decrease in hamstring muscle activation from prefatigue to postfatigue ( P < .05) .
Table 2. Thigh Muscle Activity Values (% Maximal Voluntary Isometric Contraction) for the Preparatory and Loading Response Phases During Prefatigue and Postfatigue Hopping Conditions*.
Table 3. Ankle Muscle Activity Values (% Maximal Voluntary Isometric Contraction) for the Preparatory and Loading Response Phases During Prefatigue and Postfatigue Hopping Conditions*.
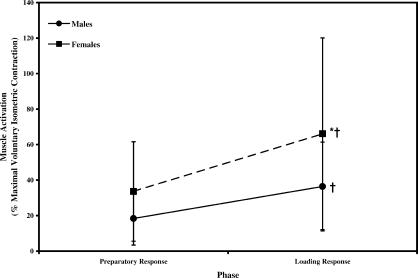
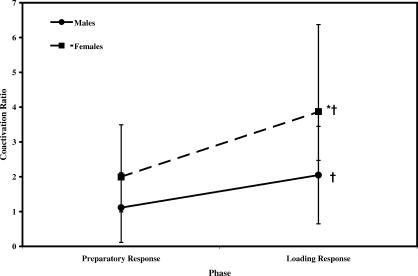
Postfatigue reductions in peak H and AT activity were accompanied by significant increases in peak MG, LG, and SO muscle activity. We observed a fatigue-by-phase interaction for gastrocnemius (F 1,19 = 10.368, P = .005) and SO activity (F 1,19 = 16.976, P = .001). Gastrocnemius activity increased during both the PR and LR phases by 38% and 36%, respectively, from prefatigue to postfatigue (see Table 3). The fatigue-by-phase interaction for SO activity demonstrated that prefatigue SO activity during the LR phase was 28% greater than during the PR phase. In the LR phase, SO activity increased 20% from prefatigue to postfatigue; however, PR-phase SO activity did not change after fatigue. Thus, SO activity in the LR phase was 53% greater than in the PR phase postfatigue (see Table 3). Changes in MG, LG, and SO muscle activity after fatigue were similar between males and females, with no significant main effect or interaction involving sex ( P > .05). The Q peak activity was not affected by the fatigue protocol, as no significant main effects or interactions involving fatigue were seen ( P > .05). Thus, Q peak activation was maintained after the fatigue protocol. A significant sex-by-phase interaction was noted for Q peak activity (F 1,19 = 4.660, P = .044) (Figure 4). Post hoc analyses revealed that Q activity was 45% greater in females than in males during the PR and LR phases. Both males and females increased Q activity by 50% from the PR to the LR phase.
Figure 4. Influence of sex and muscle response phase on quadriceps activation (data pooled across fatigue, hopping frequency, and muscle side). *Indicates a significant difference in quadriceps activity between males and females ( P < .05). †Indicates a significant increase in quadriceps activity from PR to LR ( P < .05). PR indicates preparatory response phase; LR, loading response phase .
Coactivation
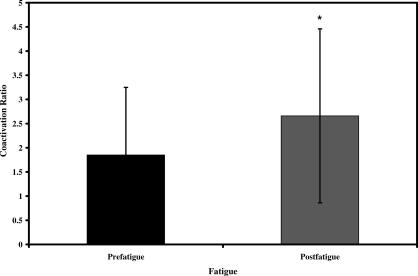
The Q:H and TS:AT coactivation ratios were altered after lower extremity fatigue induced through closed kinetic chain exercise. Analyses of Q:H coactivation ratios revealed a significant main effect for fatigue (F 1,19 = 10.093, P = .005) as the Q:H coactivation ratio doubled from prefatigue to postfatigue for all subjects (Figure 5). Also, a significant sex-by-phase interaction (F 1,19 = 5.812, P = .026) demonstrated sex differences in Q:H coactivation ratios. Tukey post hoc analyses revealed that Q:H coactivation ratios increased in both males and females from the PR to the LR phase. However, females demonstrated greater Q:H coactivation ratios than males during the PR and LR phases (Figure 6). Sex differences in Q:H coactivation ratios were not affected by the fatigue protocol, as no interaction involving sex and fatigue was seen ( P > .05) (see Table 4).
Figure 5. Influence of fatigue on quadriceps:hamstrings coactivation (data pooled across sex, muscle response phase, and hopping frequency). *Indicates a significant increase in quadriceps:hamstrings coactivation ratio from prefatigue to postfatigue ( P < .05) .
Figure 6. Influence of sex and muscle response phase on quadriceps:hamstrings coactivation ratios (data pooled across fatigue and hopping frequency). *Indicates a significant difference in the quadriceps:hamstrings coactivation ratio between males and females. †Indicates a significant increase in the quadriceps:hamstrings coactivation ratio from PR to LR ( P < .05). PR indicates preparatory response phase; LR, loading response phase .
Table 4. Coactivation Ratios (Agonist:Antagonist) for the Preparatory and Loading Response Phases During Prefatigue and Postfatigue Hopping Conditions*.
The TS:AT coactivation ratio was greatly influenced by the fatigue protocol, as shown by a significant fatigue-by-phase interaction (F 1,19 = 5.037, P = .037). The TS:AT coactivation ratios were 17.8 times greater during the PR phase and 19.5 times greater during the LR phase from prefatigue to postfatigue (see Table 4). No significant difference was noted in TS:AT between the PR and LR phases before fatigue. However, after fatigue, the TS:AT coactivation ratio during the LR phase was significantly greater than during the PR phase. No significant main effect or interaction involved sex for TS:AT ( P > .05).
Knee Flexion at Initial Contact
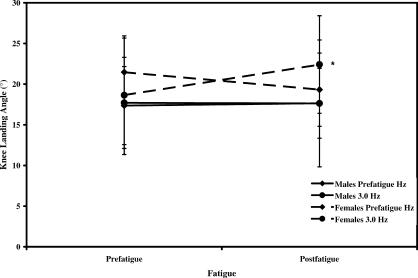
We found a significant fatigue-by-frequency-by-sex interaction for IC KNEE data (F 1,19 = 5.324, P = .032). After fatigue and hopping at FREQ 3.0, females landed with significantly greater knee flexion (4.8° more) than they did prefatigue (Figure 7).
Figure 7. Influence of fatigue, sex, and hopping frequency on knee flexion angle at initial contact. *Indicates females greater than males at postfatigue 3.0-Hz hopping frequency ( P < .05) .
DISCUSSION
We hypothesized that lower extremity muscle fatigue induced through submaximal, closed kinetic chain exercise would alter K VERT, peak muscle activation, and joint movement strategies during a functional hopping task. The hopping task was selected based on epidemiologic injury data suggesting that one of the primary noncontact mechanisms for lower extremity injury, most notably to the ACL, is landing from a jump. 41 Although distinct differences exist between 2-legged hopping and landing from a jump, 2-legged hopping provides a highly controlled method to assess the neuromuscular response to fatigue. Given the lack of studies investigating sex differences in response to fatigue, we felt it important to study a highly controlled and well-documented functional task such as 2-legged hopping. Our primary finding was that even though K VERT was not affected, peak muscle activation and joint movement strategy were altered during the functional hopping task after the fatigue protocol. We observed reduced peak activation in the hamstrings and anterior tibialis muscles, which was coupled with increased peak activity in the gastrocnemius and soleus muscles. Thus, fatigue altered coactivation ratios between the agonist and antagonist muscle groups, and individuals adopted different muscle activation strategies to maintain K VERT after fatigue. We further hypothesized that fatigue-induced alterations would differ between males and females. Although significant differences between the sexes for several variables were seen, no findings indicated that males and females responded in a different manner to the fatigue task.
Vertical Leg Stiffness Not Affected by Fatigue
Contrary to previous research demonstrating a slight but significant increase (9%) in K VERT, 35 in our study K VERT was maintained after the closed kinetic chain fatigue protocol. We observed a trend toward greater K VERT postfatigue (8% increase from prefatigue to postfatigue); however, this increase was not significant ( P = .131). The lack of statistical significance may be because of an inadequate number of subjects, with resultant low statistical power. The observed power for the effects of fatigue on K VERT was relatively low (.323), but the question is whether the low power was the result of a limited number of subjects or a small effect size. Given the significant changes observed in peak muscle activation and knee kinematics after fatigue, we believe that a small effect size and not a limited number of subjects was the primary factor influencing the low observed power. The effect size for K VERT between the prefatigue and postfatigue hopping conditions was .247, which by convention indicates a very small magnitude of difference between prefatigue and postfatigue K VERT measures. 42, 43 Based on the small effect size, we would have needed to add substantially more subjects to achieve a statistically significant difference that might have no clinical significance. Thus, K VERT does not appear to have been influenced by the fatigue task, a finding that was not caused by a limited number of subjects.
Contrary to our original hypothesis of K VERT being altered after fatigue, we believe that after fatigue, individuals attempt to maintain K VERT in order to sustain functional performance. However, in order to maintain K VERT after fatigue, individuals used different control strategies (eg, altered muscle activation patterns and coactivation ratios) than when they were not fatigued.
Control Strategy Alterations After Fatigue
An individual's control strategy for K VERT can be defined as the muscular recruitment (muscle activation) and multijoint coordination (movement) plan an individual executes to satisfy the physical demands of the functional task being performed. During functional loading conditions, K VERT properties are affected by muscles, tendons, ligaments, and skeletal alignment working as an integrated unit to modulate torsional joint stiffness during ground contact periods. 26 Torsional joint stiffness properties are controlled by several neuromuscular and biomechanical factors, including muscle activation and force, 44 reflexes, 45 antagonist muscle coactivation, 46, 47 and lower extremity kinematics during ground contact. 30, 44, 48 Essentially, in a multijointed system, the potential control strategies available to modulate K VERT are limitless. Our results demonstrate that muscle activation strategies were primarily altered as a result of fatigue, as sagittal-plane knee and ankle kinematics were largely unaffected by the fatigue protocol. Three fatigue-induced muscle activation strategy alterations were identified for all subjects: (1) ankle-dominant strategy, (2) antagonist inhibition strategy, and (3) quadriceps-dominant strategy.
The only change in kinematics after fatigue was a significant increase in IC KNEE postfatigue compared with prefatigue. Interestingly, increases in IC KNEE after fatigue were observed only in females during the FREQ 3.0 hopping condition. No such alterations were demonstrated during the FREQ PREF hopping condition or in the male subjects ( P > .05). It is unclear why IC KNEE was increased in females during the FREQ 3.0 hopping condition and not the FREQ PREF hopping condition after the fatigue protocol. A limitation of this study is that we only measured sagittal-plane motion at the knee and ankle. Movement pattern alterations may have occurred in the transverse or frontal planes of motion at these joints as well as in all planes of hip joint motion.
Ankle-Dominant Strategy After Fatigue
Altered peak muscle activation during the preparatory or ground contact phases of motion (or both) is believed to represent compensatory strategies to maintain K VERT during physical activities. 4–6, 15 Individuals using an ankle-dominant strategy place greater reliance on the ankle musculature (increased peak gastrocnemius and soleus activation) and less on the knee musculature (decreased peak hamstrings activation) to maintain K VERT after the fatigue protocol in this study. The shift toward an ankle-dominant strategy was largely influenced by the increased peak gastrocnemius activity observed in females. Gastrocnemius activity was increased by 38% in females and by only 24% in males from prefatigue to postfatigue. However, the fatigue-by-sex interaction was not significant (P = .20). The lack of a significant fatigue-by-sex interaction for gastrocnemius activity is likely influenced by low statistical power because of sample size and the variability of peak gastrocnemius muscle activation.
Previous researchers 4–6, 15 using closed kinetic chain exercise to induce fatigue have demonstrated similar shifts toward greater reliance on the less fatigued or nonfatigued musculature. Fatigue induced through continuous hopping causes reduced gastrocnemius and soleus activation after fatigue. 4–6, 15 Vertical leg stiffness during 2-legged hopping is largely controlled by the ankle musculature. 30 Findings of reduced gastrocnemius and soleus activation after the fatigue task of continuous hopping 4–6, 15 may represent a compensatory shift toward greater reliance on muscles that were not fatigued by the fatigue protocol. In our study, fatigue was induced through repeated squatting. The squat exercise primarily facilitates the knee and hip extensor musculature, with little stress placed on the gastrocnemius and soleus. 49 Although the patterns of muscle recruitment after fatigue differ in our study and previous studies, the observation of altered muscle recruitment toward greater reliance on less fatigued musculature (increased gastrocnemius and soleus peak activation) is consistent. A limitation of our study is that gluteal muscle activation was not measured. The fatigue protocol we used may have altered gluteal muscle activation in order to compensate for fatigue and modulate K VERT. Future researchers should consider the effects of fatigue on the hip musculature.
Antagonist Inhibition Strategy After Fatigue
After the closed kinetic chain fatigue protocol, antagonist muscle (hamstrings and anterior tibialis) peak activation was reduced and agonist muscle peak activation was maintained (quadriceps) or increased (gastrocnemius and soleus) in all subjects during the 2-legged hopping task. Previous investigators 50 have observed similar findings during a cycling task, with significantly reduced muscle activation of the antagonist knee flexors (hamstring and gastrocnemius) and unchanged activation of the agonist knee extensors (quadriceps). We believe that the presence of an antagonist inhibition strategy may be further demonstrated by coactivation ratio alterations after fatigue. A 2-fold increase in quadriceps:hamstrings coactivation ratios (prefatigue = 1.8, postfatigue = 2.6) for all subjects followed the fatigue task. Increased quadriceps:hamstrings coactivation ratios occurred because of a 25% decrease in the peak activation of the antagonistic hamstring muscles from prefatigue values, which was coupled with virtually no change in the peak activation of the agonist quadriceps muscles after the fatigue task.
Further demonstrating the antagonist muscle inhibition strategy, anterior tibialis muscle peak activation was also considerably reduced after fatigue (82% relative decrease), whereas agonist muscle peak activity of the gastrocnemius and soleus was increased. These changes led to a 20-fold increase in the triceps surae:anterior tibialis coactivation ratios during the PR and LR phases from prefatigue to postfatigue for all subjects. Interestingly, antagonist muscle activation of the anterior tibialis was reduced more in females than in males. This finding suggests that an antagonist inhibition strategy of ankle musculature after fatigue may be more prominent in females than males. Females tended to display a more prominent antagonist inhibition strategy for the knee musculature than males, with a trend toward greater increases in the quadriceps:hamstrings coactivation ratio after fatigue (see Table 4). However, these differences were not significant, as no significant sex-by-fatigue interaction was shown (P = .13). Both sexes displayed an antagonist inhibition strategy at the knee and ankle (more evident in females at the ankle) after the fatiguing task.
The antagonist inhibition strategy may represent a compensatory mechanism to offset fatigue by maximizing mechanical efficiency at the joint. Knee and ankle joint moments during hopping are products of both agonist and antagonist muscle moments. Shifting to an antagonist inhibition strategy would maximize efficiency in transferring agonist muscle force to produce the joint moments necessary to perform the hopping motion. Therefore, altered coactivation ratios after fatigue may represent a compensatory mechanism to enhance mechanical efficiency by maximizing agonist and minimizing antagonist muscle contributions to the overall joint moments. Recent findings 50 indicate that similar changes occur during cycling. When subjects were fatigued during cycling, no changes occurred in either prime mover muscle activation levels (gluteus maximus and quadriceps) or the torque about the crank. However, antagonist muscle activation (gastrocnemius and hamstrings) was significantly reduced. 50 The authors hypothesized that this was a compensatory mechanism to adapt for contractile loss in the prime mover muscles in order to maintain the overall moment needed for maintenance of functional performance. 50
Quadriceps-Dominant Strategy
After the fatigue protocol, all subjects displayed a quadriceps-dominant strategy by placing greater reliance on the quadriceps muscles, which was demonstrated by the significant increase in quadriceps:hamstrings coactivation ratios from prefatigue to postfatigue. The quadriceps:hamstrings coactivation ratios were 2.6 postfatigue, indicating that peak quadriceps activity was more than 2.5 times that of the hamstrings. The shift to a quadriceps-dominant strategy after fatigue was likely an attempt to compensate for quadriceps muscle fatigue and maximize the internal knee extension moment generated by the quadriceps muscles.
Our findings also demonstrated that females used a more prominent quadriceps-dominant strategy than males. Females displayed greater peak quadriceps activity and quadriceps: hamstrings coactivation ratios than males during the PR and LR phases (see Figures 4 and 5). In preparation for ground contact (PR phase), females used a quadriceps-dominant strategy by activating their quadriceps 45% more than males, which resulted in females' peak quadriceps activity being nearly twice that of their hamstrings (quadriceps:hamstrings = 1.9). In contrast, males recruited nearly equivalent quadriceps and hamstrings peak activity during the PR phase (quadriceps: hamstrings = 1.1). We believe that the sex differences in quadriceps peak activity and quadriceps:hamstrings coactivation ratios during the PR phase were voluntary; muscle activation before ground contact (ie, PR phase, preactivation, or preparatory activation) is believed to represent voluntary muscle recruitment using higher-order centers. 28, 51
The quadriceps-dominant strategy was displayed by males and females immediately after ground contact (LR phase), as quadriceps peak activity increased by 50% and quadriceps: hamstrings coactivation ratios increased to values above 2.0 from the PR to LR phases for all individuals (see Figures 4 and 5). Although both sexes demonstrated increased quadriceps activity and quadriceps:hamstrings coactivation ratios from the PR to the LR phase, females continued to demonstrate significantly greater quadriceps:hamstrings coactivation ratios than their male counterparts. During the LR phase, females had 45% more peak quadriceps activity than males and displayed quadriceps:hamstrings coactivation ratios that were again 2-fold greater than those of males. Quadriceps peak activation amplitude was approximately 4 times greater than that of the hamstrings in females during the LR phase (quadriceps: hamstrings = 3.9). In contrast, the quadriceps:hamstrings coactivation ratio in males was only 2.0 during the LR phase, half that observed in females and essentially equivalent to female quadriceps:hamstrings values during the non–weight-bearing PR phase. Our finding of a quadriceps-dominant strategy in females is in agreement with previous research. 20, 25, 52, 53 Contrary to our original hypothesis, no sex difference was evident in quadriceps:hamstrings coactivation ratios after the fatiguing task. Thus, females exhibited greater quadriceps:hamstrings coactivation ratios than males, and no sex difference was noted in how the Q:H coactivation ratios were affected by the fatigue task.
Clinical Relevance
We demonstrated that after submaximal closed kinetic chain exercise, an individual's control strategy for modulating K VERT is significantly altered. Altered control strategies after fatigue may influence lower extremity injury risk, specifically injury to the ACL. Increasing gastrocnemius peak activity as a compensatory mechanism (ankle-dominant strategy) to facilitate ankle joint torsional stiffness and maintain K VERT may increase ACL strain. Also, the gastrocnemius muscle is an antagonist of the ACL and is able to increase ACL strain. 54 Increased reliance on the gastrocnemius muscle after fatigue may cause greater ACL strain and place the ligament closer to its ultimate failure point. The shift to an ankle-dominant strategy may also negatively affect knee joint stability. Greater reliance on the ankle musculature and less reliance on the knee musculature (decreased peak hamstrings activity) was an effective strategy to maintain K VERT after fatigue. However, our theory is that K VERT was maintained after fatigue by increasing ankle joint torsional stiffness (increased peak gastrocnemius and soleus activity) and minimizing knee joint torsional stiffness (decreased peak hamstrings activity). Reduced knee joint torsional stiffness after fatigue may allow for decreased knee joint stability, as stiffness is a key factor influencing stability.
The fatigue-induced quadriceps-dominant strategy, demonstrated by an increased quadriceps:hamstrings coactivation ratio after fatigue, may be effective in modulating K VERT; however, it may also negatively affect knee joint stability and increase ACL strain. Large quadriceps forces increase proximal anterior tibial shear force, thus facilitating ACL strain. 54–56 The quadriceps' ability to facilitate ACL strain is maximized when hamstrings activity is minimal and unable to counteract the quadriceps. Minimizing ACL strain and injury risk requires strong antagonist hamstrings coactivation to counteract forceful quadriceps contraction and maintain a balanced muscular recruitment. 54, 57, 58 However, all individuals demonstrated an antagonist inhibition strategy after fatigue by reducing hamstring peak activity with no change in quadriceps peak activity, which facilitated an increased quadriceps:hamstrings coactivation ratio. Thus, after fatigue, individuals used less hamstrings coactivation to modulate knee joint torsional stiffness and instead relied more on the quadriceps muscles. Greater reliance on the quadriceps muscles was more pronounced in females, who displayed greater quadriceps activity and quadriceps:hamstrings coactivation ratios than males. Although all individuals relied more on the quadriceps muscles after fatigue, which might have facilitated increased ACL strain, females may be at particular risk for increasing ACL strain because of their greater reliance on quadriceps muscles to modulate knee joint torsional stiffness. Females employing a greater quadriceps-dominant strategy may be at higher risk for ACL injury once fatigued. However, it should be noted that we do not know at this time whether a quadriceps-dominant strategy places an individual at significantly greater risk for ACL injury.
CONCLUSIONS
We evaluated the influence of fatigue induced through prolonged, submaximal closed kinetic chain exercise on K VERT, as well as muscle activation and joint movement strategies in males and females. Once fatigued, individuals appear to shift to an ankle strategy, relying more on the ankle musculature and less on the knee musculature (in particular, the hamstrings) to modulate total K VERT. An ankle-dominant control strategy alteration is theorized to reduce knee joint stability and facilitate gastrocnemius-induced ACL strain. It was also apparent that after fatigue, subjects used an antagonist inhibition strategy. Both males and females demonstrated this strategy at the ankle and knee joints by reducing antagonist peak muscle activation (eg, anterior tibialis, hamstrings) and relying more on the agonist musculature (eg, gastrocnemius, soleus, quadriceps), which may also negatively affect knee joint stability. In addition, females placed greater reliance on the quadriceps musculature, both before and after fatigue. This finding supports the argument that females use quadriceps-dominant strategies during physical activity. Vigorous quadriceps contraction unopposed by antagonist hamstring recruitment may elicit increased ACL strain. In theory, the combined influence of these alterations in females may place them at greater risk for ACL injury when fatigued, as they would maximize quadriceps force and potentially reduce knee joint stability. Whether or not ankle-dominant, antagonist-inhibition, or quadriceps-dominant control strategy alterations after fatigue actually place the ACL at greater risk for injury is still unknown and requires further study.
Acknowledgments
We thank Sara E. Wilson, PhD, Assistant Professor in the Department of Mechanical Engineering at the University of Kansas, Lawrence, for her assistance with the reduction and processing of the data.
REFERENCES
- Rozzi SL, Lephart SM, Fu FH. Effects of muscular fatigue on knee joint laxity and neuromuscular characteristics of male and female athletes. J Athl Train. 1999;34:106–114. [PMC free article] [PubMed] [Google Scholar]
- Griffin LY, Agel J, Albohm MJ. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141–150. doi: 10.5435/00124635-200005000-00001. et al. [DOI] [PubMed] [Google Scholar]
- Avela J, Komi PV. Reduced stretch reflex sensitivity and muscle stiffness after long-lasting stretch-shortening cycle exercise in humans. Eur J Appl Physiol Occup Physiol. 1998;78:403–410. doi: 10.1007/s004210050438. [DOI] [PubMed] [Google Scholar]
- Bonnard M, Sirin AV, Oddsson L, Thorstensson A. Different strategies to compensate for the effects of fatigue revealed by neuromuscular adaptation processes in humans. Neurosci Lett. 1994;166:101–105. doi: 10.1016/0304-3940(94)90850-8. [DOI] [PubMed] [Google Scholar]
- Horita T, Komi PV, Nicol C, Kyrolainen H. Stretch shortening cycle fatigue: interactions among joint stiffness, reflex, and muscle mechanical performance in the drop jump. Eur J Appl Physiol Occup Physiol. 1996;73:393–403. doi: 10.1007/BF00334415. [DOI] [PubMed] [Google Scholar]
- Nicol C, Komi PV, Horita T, Kyrolainen H, Takala TE. Reduced stretch-reflex sensitivity after exhausting stretch-shortening cycle exercise. Eur J Appl Physiol Occup Physiol. 1996;72:401–409. doi: 10.1007/BF00242268. [DOI] [PubMed] [Google Scholar]
- Rodacki AL, Fowler NE, Bennett SJ. Multi-segment coordination: fatigue effects. Med Sci Sports Exerc. 2001;33:1157–1167. doi: 10.1097/00005768-200107000-00013. [DOI] [PubMed] [Google Scholar]
- Potvin JR, O'Brien PR. Trunk muscle co-contraction increases during fatiguing, isometric, lateral bend exertions: possible implications for spine stability. Spine. 1998;23:774–781. doi: 10.1097/00007632-199804010-00006. [DOI] [PubMed] [Google Scholar]
- Psek JA, Cafarelli E. Behavior of coactive muscles during fatigue. J Appl Physiol. 1993;74:170–175. doi: 10.1152/jappl.1993.74.1.170. [DOI] [PubMed] [Google Scholar]
- Weir JP, Keefe DA, Eaton JF, Augustine RT, Tobin DM. Effect of fatigue on hamstring coactivation during isokinetic knee extensions. Eur J Appl Physiol Occup Physiol. 1998;78:555–559. doi: 10.1007/s004210050460. [DOI] [PubMed] [Google Scholar]
- Marras WS, Granata KP. Changes in trunk dynamics and spine loading during repeated trunk extensions. Spine. 1997;22:2564–2570. doi: 10.1097/00007632-199711010-00019. [DOI] [PubMed] [Google Scholar]
- Nyland JA, Caborn NM, Shapiro R, Johnson DL. Crossover cutting during hamstring fatigue produces transverse plane knee control deficits. J Athl Train. 1999;34:137–143. [PMC free article] [PubMed] [Google Scholar]
- Pinniger GJ, Steele JR, Groeller H. Does fatigue induced by repeated dynamic efforts affect hamstring muscle function? Med Sci Sports Exerc. 2000;32:647–653. doi: 10.1097/00005768-200003000-00015. [DOI] [PubMed] [Google Scholar]
- Sparto PJ, Parnianpour M, Reinsel TE, Simon S. The effect of fatigue on multijoint kinematics and load sharing during a repetitive lifting test. Spine. 1997;22:2647–2654. doi: 10.1097/00007632-199711150-00013. [DOI] [PubMed] [Google Scholar]
- Avela J, Komi PV. Interaction between muscle stiffness and stretch reflex sensitivity after long-term stretch-shortening cycle exercise. Muscle Nerve. 1998;21:1224–1227. doi: 10.1002/(sici)1097-4598(199809)21:9<1224::aid-mus19>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Wilson S, Granata K. Active knee stiffness decreases after fatigue. Paper presented at: 24th Annual Meeting of the American Society of Biomechanics; July 19–22, 2000; Chicago, IL.
- Kersey RD, Rowan L. Injury account during the 1980 NCAA wrestling championships. Am J Sports Med. 1983;11:147–151. doi: 10.1177/036354658301100307. [DOI] [PubMed] [Google Scholar]
- Pettrone FA, Ricciardelli E. Gymnastic injuries: the Virginia experience 1982–1983. Am J Sports Med. 1987;15:59–62. doi: 10.1177/036354658701500108. [DOI] [PubMed] [Google Scholar]
- Smith AM, Stuart MJ, Wiese-Bjornstal DM, Gunnon C. Predictors of injury in ice hockey players: a multivariate, multidisciplinary approach. Am J Sports Med. 1997;25:500–507. doi: 10.1177/036354659702500413. [DOI] [PubMed] [Google Scholar]
- Malinzak RA, Colby SM, Kirkendall DT, Yu B, Garrett WE. A comparison of knee joint motion patterns between men and women in selected athletic tasks. Clin Biomech (Bristol, Avon) 2001;16:438–445. doi: 10.1016/s0268-0033(01)00019-5. [DOI] [PubMed] [Google Scholar]
- Chappell JO, Yu B, Kirkendall DT, Garrett WE. A comparison of knee kinetics between male and female recreational athletes in stop-jump tasks. Am J Sports Med. 2002;30:261–267. doi: 10.1177/03635465020300021901. [DOI] [PubMed] [Google Scholar]
- Granata KP, Wilson SE, Padua DA. Sex differences in active musculoskeletal stiffness, part I: quantification in controlled measurements of knee joint dynamics. J Electromyogr Kinesiol. 2002;12:119–126. doi: 10.1016/s1050-6411(02)00002-0. [DOI] [PubMed] [Google Scholar]
- Granata KP, Padua DA, Wilson SE. Sex differences in active musculoskeletal stiffness, part II: quantification of leg stiffness during functional hopping tasks. J Electromyogr Kinesiol. 2002;12:127–135. doi: 10.1016/s1050-6411(02)00003-2. [DOI] [PubMed] [Google Scholar]
- Lephart SM, Ferris CM, Riemann BL, Myers JB, Fu FH. Sex differences in strength and lower extremity kinematics during landing. Clin Orthop Relat Res. 2002;401:162–169. doi: 10.1097/00003086-200208000-00019. [DOI] [PubMed] [Google Scholar]
- Padua DA, Carcia CR, Arnold BL, Granata KP. Sex differences in leg stiffness and stiffness recruitment strategy during two-legged hopping. J Mot Behav. 2005;37:111–125. doi: 10.3200/JMBR.37.2.111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley CT, Houdijk HHP, van Strien C, Louie M. Mechanism of leg stiffness adjustment for hopping on surfaces of different stiffnesses. J Appl Physiol. 1998;85:1044–1055. doi: 10.1152/jappl.1998.85.3.1044. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, DeVita P. Altered movement strategy increases lower extremity stiffness during stepping down in the aged. J Gerontol A Biol Sci Med Sci. 1999;54:B63–B70. doi: 10.1093/gerona/54.2.b63. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, DeVita P. Muscle pre- and coactivity during downward stepping are associated with leg stiffness in aging. J Electromyogr Kinesiol. 2000;10:117–126. doi: 10.1016/s1050-6411(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Yates JW, Kearney JT, Noland MP, Felts WM. Recovery of dynamic muscular endurance. Eur J Appl Physiol Occup Physiol. 1987;56:662–667. doi: 10.1007/BF00424807. [DOI] [PubMed] [Google Scholar]
- Farley CT, Morgenroth DC. Leg stiffness primarily depends on ankle stiffness during human hopping. J Biomech. 1999;32:267–273. doi: 10.1016/s0021-9290(98)00170-5. [DOI] [PubMed] [Google Scholar]
- Farley CT, Gonzalez O. Leg stiffness and stride frequency in human running. J Biomech. 1996;29:181–186. doi: 10.1016/0021-9290(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Farley CT, Glasheen J, McMahon TA. Running springs: speed and animal size. J Exp Biol. 1993;185:71–86. doi: 10.1242/jeb.185.1.71. [DOI] [PubMed] [Google Scholar]
- Cram J, Kasman G. Introduction to Surface Electromyography. Gaithersburg, MD: Aspen; 1998.
- Horita T, Komi P, Nicol C, Kyrolainen H. Effect of exhausting stretch-shortening cycle exercise on the time course of mechanical behaviour in the drop jump: possible role of muscle damage. Eur J Appl Physiol Occup Physiol. 1999;79:160–167. doi: 10.1007/s004210050490. [DOI] [PubMed] [Google Scholar]
- Moritani T, Oddson L, Thorstensson A. Electromyographic evidence of selective fatigue during the eccentric phase of stretch/shortening cycles in man. Eur J Appl Physiol Occup Physiol. 1990;60:425–429. doi: 10.1007/BF00705031. [DOI] [PubMed] [Google Scholar]
- Avela J, Kyrolainen H, Komi PV, Rama D. Reduced reflex sensitivity persists several days after long-lasting stretch-shortening cycle exercise. J Appl Physiol. 1999;86:1292–1300. doi: 10.1152/jappl.1999.86.4.1292. [DOI] [PubMed] [Google Scholar]
- Maton B, Le Pellec A. Adaptation of the short latency component of the stretch reflex plays only a minor role in compensating for muscle fatigue induced by spontaneous hopping in humans. Eur J Appl Physiol. 2001;84:26–35. doi: 10.1007/s004210000324. [DOI] [PubMed] [Google Scholar]
- Boden BP, Griffin LY, Garrett WE., Jr. Etiology and prevention of noncontact ACL injury. Physician Sportsmed. 2000;28(4):53–60. doi: 10.3810/psm.2000.04.841. [DOI] [PubMed] [Google Scholar]
- Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr. Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23:573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- Arampatzis A, Bruggemann GP, Metzler V. The effect of speed on leg stiffness and joint kinetics in human running. J Biomech. 1999;32:1349–1353. doi: 10.1016/s0021-9290(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34:86–92. [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Assoc; 1988.
- Portney L, Watkins M. Foundations of Clinical Research: Applications to Practice. 2nd ed. Upper Saddle River, NJ: Prentice Hall; 2000.
- Zhang LQ, Nuber G, Butler J, Bowen M, Rymer WZ. In vivo human knee joint dynamic properties as functions of muscle contraction and joint position. J Biomech. 1998;31:71–76. doi: 10.1016/s0021-9290(97)00106-1. [DOI] [PubMed] [Google Scholar]
- Kearney RE, Stein RB, Parameswaran L. Identification of intrinsic and reflex contributions to human ankle stiffness dynamics. IEEE Trans Biomed Eng. 1997;44:493–504. doi: 10.1109/10.581944. [DOI] [PubMed] [Google Scholar]
- Cannon SC, Zahalak GI. The mechanical behavior of active human skeletal muscle in small oscillations. J Biomech. 1982;15:111–121. doi: 10.1016/0021-9290(82)90043-4. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Licata F, Soechting JF. The mechanical behavior of the human forearm in response to transient perturbations. Biol Cybern. 1982;44:35–46. doi: 10.1007/BF00353954. [DOI] [PubMed] [Google Scholar]
- McMahon TA, Valiant G, Frederick EC. Groucho running. J Appl Physiol. 1987;62:2326–2337. doi: 10.1152/jappl.1987.62.6.2326. [DOI] [PubMed] [Google Scholar]
- Escamilla RF. Knee biomechanics of the dynamic squat exercise. Med Sci Sports Exerc. 2001;33:127–141. doi: 10.1097/00005768-200101000-00020. [DOI] [PubMed] [Google Scholar]
- Hautier CA, Arsac LM, Deghdegh K, Souquet D, Belli A, Lacour JR. Influence of fatigue on EMG/force ratio and cocontraction in cycling. Med Sci Sports Exerc. 2000;32:839–843. doi: 10.1097/00005768-200004000-00017. [DOI] [PubMed] [Google Scholar]
- Dietz V, Noth J, Schmidtbleicher D. Interaction between pre-activity and stretch reflex in human triceps brachii during landing from forward falls. J Physiol. 1981;311:113–125. doi: 10.1113/jphysiol.1981.sp013576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett TE, Lindenfeld TN, Riccobene JV, Noyes FR. The effect of neuromuscular training on the incidence of knee injury in female athletes: a prospective study. Am J Sports Med. 1999;27:699–706. doi: 10.1177/03635465990270060301. [DOI] [PubMed] [Google Scholar]
- Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sport Med. 1996;18:427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- Fleming BC, Renstrom PA, Ohlen G. The gastrocnemius is an antagonist of the anterior cruciate ligament. J Orthop Res. 2001;19:1178–1184. doi: 10.1016/S0736-0266(01)00057-2. et al. [DOI] [PubMed] [Google Scholar]
- Beynnon BD, Johnson RJ, Fleming BC, Stankewich CJ, Renstrom PA, Nichols CE. The strain behavior of the anterior cruciate ligament during squatting and active flexion-extension: a comparison of an open and a closed kinetic chain exercise. Am J Sports Med. 1997;25:823–829. doi: 10.1177/036354659702500616. [DOI] [PubMed] [Google Scholar]
- Hirokawa S, Solomonow M, Lu Y, Lou Z, D'Ambrosia R. Anterior-posterior and rotational displacement of the tibia elicited by quadriceps contraction. Am J Sports Med. 1992;20:299–306. doi: 10.1177/036354659202000311. [DOI] [PubMed] [Google Scholar]
- Beynnon BD, Fleming BC, Johnson RJ, Nichols CE, Renstrom PA, Pope MH. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23:24–34. doi: 10.1177/036354659502300105. [DOI] [PubMed] [Google Scholar]
- Li G, Rudy TW, Sakane M, Kanamori A, Ma CB, Woo SL. The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech. 1999;32:395–400. doi: 10.1016/s0021-9290(98)00181-x. [DOI] [PubMed] [Google Scholar]