Abstract
Context: A neuromuscular relationship exists between the lumbar extensor and quadriceps muscles during fatiguing exercise. However, this relationship may be different for persons with low back pain (LBP).
Objective: To compare quadriceps inhibition after isometric, fatiguing lumbar extension exercise between persons with a history of LBP and control subjects.
Design: A 2 × 3 factorial, repeated-measures, time-series design with independent variables of group (persons with a history of LBP, controls) and time (baseline, postexercise set 1, postexercise set 2).
Setting: University research laboratory.
Patients or Other Participants: Twenty-five subjects with a history of LBP were matched by sex, height, and mass to 25 healthy control subjects.
Intervention(s): Electromyography median frequency indexed lumbar paraspinal muscular fatigue while subjects performed 2 sets of isometric lumbar extension exercise. Subjects exercised until a 15% downward shift in median frequency for the first set and a 25% shift for the second set were demonstrated.
Main Outcome Measure(s): Knee extension force was measured while subjects performed an isometric maximal quadriceps contraction. During this maximal effort, a percutaneous electric stimulus was applied to the quadriceps, causing a transient, supramaximal increase in force output. We used the ratio between the 2 forces to estimate quadriceps inhibition. Quadriceps electromyographic activity was recorded during the maximal contractions to compare median frequencies over time.
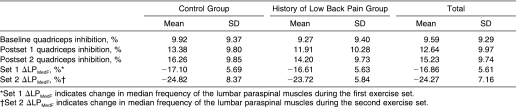
Results: Both groups exhibited significantly increased quadriceps inhibition after the first (12.6% ± 10.0%, P < .001) and second (15.2% ± 9.7%, P < .001) exercise sets compared with baseline (9.6% ± 9.3%). However, quadriceps inhibition was not different between groups.
Conclusions: Persons with a history of LBP do not appear to be any more or less vulnerable to quadriceps inhibition after fatiguing lumbar extension exercise.
Keywords: superimposed burst technique, neuromuscular activity, knee
Muscle inhibition describes the failure to completely activate all motor units in a given motor neuron pool. Muscle inhibition is an important component of motor control during human movement and is vital for proper functioning. 1 Although inhibition of a healthy muscle is a normal finding, activation failure in undamaged muscles can occur after muscle fatigue 2 or joint injury. 3–6 Quadriceps inhibition (QI) may be present in persons with knee injuries such as patellofemoral joint pain 7 or osteoarthritis 5 and is correlated with reduced knee extension strength. 4 In theory, inadequate quadriceps strength resulting in altered gait 8 may lead to degenerative joint injury 4, 9 because altered gait patterns may expose the knee joint surfaces to abnormal forces. 10 However, this relationship is not yet clearly understood. Investigating possible sources of inhibition in patient populations may provide a better understanding of the lower extremity overuse or degenerative joint injuries that may result from QI.
Persons with chronic low back pain (LBP) commonly exhibit weak 11 or unbalanced 12 trunk muscles and tend to experience a quicker rate of fatigue during sustained lumbar extension exercise. 13 This muscular deficiency may impose lower extremity muscular adaptations during fatiguing exercise to maintain stability and preserve normal function. Recently, we 14 observed more QI after fatiguing lumbar extension exercise in 16 persons with healthy knees, indicating that the quadriceps may be adapting in response to lumbar paraspinal fatigue. Understanding the neuromuscular relationship between the quadriceps and lumbar paraspinal muscles will help us learn more about a possible source of QI during fatiguing exercise, which may be different in persons with a history of LBP (HxLBP). 14 However, more research in a larger group of individuals with more severe low back injury will help us learn more about how persons with LBP adapt during fatiguing exercise.
Our purpose was to compare QI in persons with a history of repeated LBP episodes and control subjects after fatiguing lumbar paraspinal exercise.
METHODS
This study consisted of a 2 × 3 factorial, repeated-measures, time-series design with a static group comparison. The independent variables were time (baseline, postexercise set 1, or postexercise set 2) and group (history of LBP or control). The dependent variables were percentage of QI and quadriceps electromyography (EMG) median frequency (MedF).
Subjects
Twenty-five subjects with HxLBP (13 women: age = 21.7 ± 1.9 years, height = 169.0 ± 7.1 cm, mass = 64.6 ± 6.8 kg; 12 men: age = 22.8 ± 3.5 years, height = 180.2 ± 6.6 cm, mass = 80.5 ± 8.3 kg) were matched by sex, height, and mass with 25 control subjects (13 women: age = 20.9 ± 1.5 years, height = 171.4 ± 6.3 cm, mass = 64.2 ± 7.5 kg; 12 men: age = 23.8 ± 3.5 years, height = 181.8 ± 6.7 cm, mass = 79.9 ± 11.7 kg). All subjects voluntarily participated after they read and signed an informed consent form. This study was approved by our university's institutional review board.
All subjects were recreationally active (exercised at least 3 days per week for at least 30 minutes per session) and reported no current knee pain or LBP. Subjects also reported no hip, knee, or ankle injury within the past 6 months and had never had surgery to any lower extremity joint. The HxLBP group consisted of subjects who reported at least 3 episodes of LBP in the past 3 years or 5 episodes in their lifetime. We defined an episode of LBP as being sufficient to cause modification or limitations in daily activities. Subjects in the HxLBP group had no pain during data collection and reported no known history of spinal fracture, disc injury, tumor, or lower extremity radicular symptoms. Subjects in the control group had never experienced LBP.
Instruments
Knee extension force was measured with a dynamometer (System 3 No. 900-550; Biodex Medical Systems, Inc, Shirley, NY). Signals from the dynamometer were exported from a remote access port and digitized at 125 Hz (MP150; Biopac Systems, Inc, Goleta, CA).
The S88 dual-output square-pulse stimulator with the SIU8T transformer stimulus isolation unit (Grass-Telefactor, West Warwick, RI) was used to deliver a 100-millisecond train of 10 square-wave pulses at an intensity of 125 V. Individual pulse duration was 600 μs delivered at a carrier frequency of 100 pulses per second.
Electric activity in the lumbar paraspinal and quadriceps muscles was collected with surface EMG. Signals were amplified with a high-gain, differential-input biopotential amplifier with a gain of 1000 and digitized with a 16-bit data acquisition system (Biopac Systems) at 2000 Hz with a common-mode rejection ratio of 110 dB, an input impedance of 1.0 MΩ, and a noise voltage of 0.2 μV.
Procedures
Before data collection, we screened subjects for LBP history in order to assign them to the appropriate group. After screening, a physical examination was performed by an experienced, licensed, and certified athletic trainer (J.M.H.). Subjects were excluded if they exhibited any of the following: bilateral asymmetry of dermatomes, myotomes, or deep tendon reflexes; intolerable pain (pain score of >3 on a scale of 10) with standing lumbar extension; inability to extend the spine at least 15°; or positive straight-leg test (pain, numbness).
We first prepared the skin at the EMG electrode placement sites. The skin was shaved, lightly debrided with fine sandpaper, and cleaned thoroughly with isopropyl alcohol. Self-adhesive, round (35-mm), pregelled Ag-AgCl surface electrodes were placed at the vastus lateralis (10 cm proximal to the patella base) and lumbar paraspinal muscles (L4-L5 level). A pair of EMG electrodes was placed parallel to the muscle fiber orientation (interelectrode distance of approximately 2 cm) at each site over active muscle, verified by palpation during active contraction. A ground electrode was placed on the anterior mid-tibia.
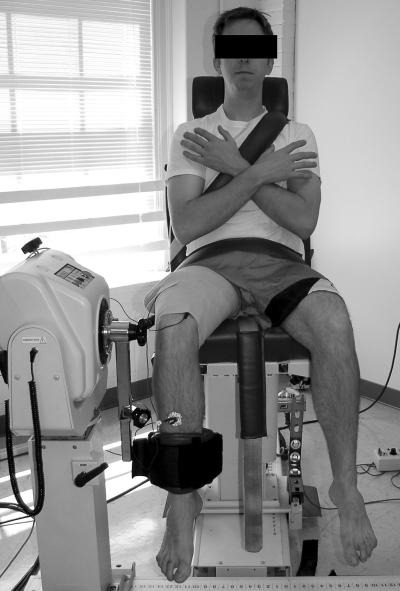
Two 8 × 14-cm rubber stimulating electrodes coated with aqueous conductive gel were secured to the proximal-lateral and distal-medial thigh with a compression wrap. Then subjects were securely positioned for baseline assessment of QI (Figure 1).
Figure 1. Subject setup for measuring knee extension force. (Reprinted with permission from Hart JM, Kerrigan DC, Fritz JM, Saliba EN, Gansneder BM, Ingersoll CD. Contribution of hamstring fatigue to quadriceps inhibition following lumbar extension exercise. J Sport Sci Med. 2006;5:70–79) .
Quadriceps Inhibition
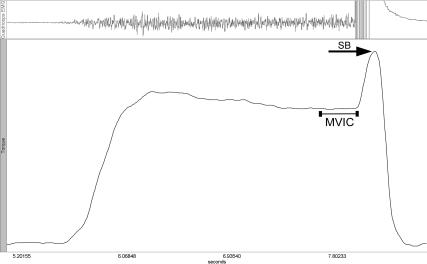
Quadriceps inhibition was measured using the superimposed burst technique. 5, 15, 16 Baseline QI was measured before and immediately after each lumbar paraspinal fatiguing exercise set. During QI measurements, subjects performed a maximal voluntary isometric contraction (MVIC) using their knee extensors while receiving continuous verbal encouragement from the tester. Once the MVIC reached a plateau (representing the subject's maximal effort), an electric stimulus was manually triggered and was delivered directly to the quadriceps through the stimulating electrodes. The superimposed stimulus is theoretically intended to recruit all motor units in the quadriceps motor neuron pool, thus causing a transient increase of force, called a superimposed burst (SB), over the MVIC force (Figure 2). We averaged 3 trials at each time. A ratio between the mean MVIC force value for a 100-millisecond period immediately before the stimulation and the peak superimposed burst force was used to calculate the percentage of QI:
| QI = 1 − (FMVIC/F MVIC+SB) |
This ratio is similar to formulae used previously to describe the extent of QI. 5, 16–18 The QI values are presented as percentages and indicate the percentage of the subject's quadriceps motor neuron pool that cannot be voluntarily activated.
Figure 2. Sample tracing of force and electromyographic activity measured during the superimposed burst (SB) technique, with labels showing maximal voluntary isometric contraction (MVIC) and SB force components of the curve.
We collected the EMG activity from the vastus lateralis during each quadriceps MVIC while measuring QI in order to calculate MedF as an index of muscular fatigue that possibly resulted from repeated maximal contractions.
After the baseline measure of QI, subjects moved to a lumbar extension machine (Figure 3), where they performed a set of lumbar extension exercises and then moved back to the dynamometer chair for a postexercise QI measure. Quadriceps inhibition was measured again after the second lumbar-extension exercise set.
Figure 3. A subject performing fatiguing lumbar extension exercise.
Lumbar Paraspinal Fatiguing Exercise
Subjects were positioned prone in a lumbar extension machine (see Figure 3), where they were instructed to perform repeated 10-second periods of gravity-resisted isometric contractions, followed by a 10-second rest. Subjects were verbally encouraged to keep the position of the upper torso parallel to the floor during all contractions. Activity of the right-side lumbar paraspinal muscles was recorded during each active repetition and analyzed to calculate the MedF. We were able to calculate the MedF from each repetition using a method described previously. 14 Baseline MedF was established during the first repetition. Subjects continued the repeated 10-second isometric contractions until an approximate 15% reduction in MedF from baseline was observed. Similarly, the second exercise set ended once a reduction of approximately 25% in MedF was observed. For example, if the baseline MedF was 100 Hz, then the subject was instructed to stop the exercise set once the MedF from a repetition fell to approximately 85 Hz for the first exercise set and 75 Hz for the second set. We chose the MedF shifts of 15% and 25% to represent mild and moderate levels of fatigue, respectively, in the lumbar paraspinal muscles.
Exercise sets also ended if a subject was unable to continue as a result of intolerable fatigue. If a subject experienced pain in the lumbar or sacroiliac area, the session was ended and the data were discarded.
For the lumbar paraspinal MedF calculations, 1-second clips of raw signal were band-pass filtered (10–500 Hz) and decomposed into the frequency domain through a fast Fourier transform with Hamming window. For the quadriceps MedF calculations, we selected 200 milliseconds of raw EMG signal while subjects performed MVICs that corresponded to the same time epoch used to record MVIC force for QI calculations. Raw EMG signal was band-pass filtered (10–500 Hz) and decomposed into the frequency domain through a fast Fourier transform with Hamming window. The MedF changes are calculated as a percentage shift from baseline, with negative values indicating a leftward (decreasing MedF) shift in the skewness of the frequency spectrum and positive values indicating a rightward (increasing MedF) shift.
Statistical Analysis
A 2 × 3 mixed-model analysis of variance was used to assess the effects of group and time and the interaction of these factors on QI. Simple contrast testing was performed post hoc to locate specific group differences after a significant main effect. Between-group comparisons of QI were made. We also calculated a 1 × 3 analysis of variance with repeated measures on time to compare the quadriceps' MedF during MVICs while measuring QI.
All statistical analyses were performed with the SPSS statistical package (version 12.0; SPSS Inc, Chicago, IL). The a;thpriori alpha level was P ≤ .05 for all statistical tests.
RESULTS
We noted no group × time interaction for QI (F 2,26 = 1.68, P = .20, η 2 = 0.03, 1 − β = .35). However, QI increased significantly after the first ( P < .001) and second ( P < .001) exercise sets compared with baseline (F 2,96 = 122.25, P < .001, η 2 = 0.72) (Table). On average, groups did not exhibit different QI (F 1,48 = 0.25, P = .62, η 2 = 0.01, 1 − β = .08). Finally, no significant change in quadriceps MedF was seen over time as a result of multiple MVIC efforts while we measured QI with the superimposed burst technique (F 2,98 = 0.17, P = .84, η 2 = 0.003, 1 − β = .08).
Quadriceps Inhibition and Changes in Lumbar Paraspinal Muscle Median Frequency.
DISCUSSION
In both groups, the quadriceps muscle became more inhibited after fatiguing isometric lumbar paraspinal exercise. These changes in QI occurred without a significant change in quadriceps MedF during MVICs, indicating that subjects were not experiencing quadriceps fatigue due to multiple MVIC efforts. Increased QI after lumbar extension exercise is in agreement with previous findings. 14 However, we previously found that persons with HxLBP exhibited, on average, greater quadriceps activation (ie, less inhibition). It is important to note that substantially fewer subjects were included in the previous study, 14 that the inclusion criteria for the HxLBP group were more liberal, and that 3 exercise sets were performed. In the current study, we used considerably more subjects, who were included only if they had previously experienced at least 3 episodes of LBP. These methodologic differences may account for the conflicting results between groups.
In the current study, the quadriceps became about 3% more inhibited after the first exercise set and about 6% more inhibited after the second set. A person who experiences a 6% increase in QI may be considered to have incomplete quadriceps activation, despite possibly complete activation before the fatiguing exercise sets. In this study, 83% (19/23) of the subjects who exhibited complete activation before exercise (ie, <5% QI) 4, 19, 20 showed more than 5% QI after the fatiguing exercise sets, indicating a change from complete to incomplete quadriceps activation that was not due to knee injury or quadriceps fatigue. This is concerning for persons who have healthy knees and experience QI. Higher amounts of QI are correlated with lower quadriceps strength 5 and may therefore be detrimental to force attenuation during gait. Excessive QI during prolonged exercise may expose the knee joint surfaces to abnormal stresses during gait, possibly resulting in degenerative processes and injury. 4, 8–10 Although the relationship between QI and injury risk remains unclear, we present preliminary data that describe a potential risk to lower extremity joints, a risk that needs to be explored further in a functional setting.
The low back may be exposed to excessive forces that are unabsorbed by weak muscles and transferred through the lower extremities during activity. 21 Persons with HxLBP commonly suffer from hamstring tightness, 22–24 poor spinal flexibility, 23, 24 and reduced lumbar lordosis, 23 in addition to weakness and imbalance of the hip musculature. 21, 25, 26 The results of our current study and previous work 14 show that the quadriceps seem to become more inhibited as the lumbar paraspinal muscles become more fatigued. However, we do not know from these data whether this relationship exists in a functional setting. Quadriceps inhibition may be a necessary adaptation to preserve function during prolonged exercise in persons who have highly fatigable lumbar paraspinal muscles, and it may cause further lower extremity neuromuscular reorganization during exercise or athletic maneuvers that involve a high demand on the quadriceps.
Quadriceps inhibition may result from muscle or joint damage or a disruption or change in afferent information from the knee joint capsule, 27–31 leading to progressive or degenerative conditions of the knee, if mismanaged. The relationship between the knee extensors and the spine is logical to clinicians, as it is prudent to evaluate the hips, pelvis, and spine during a routine physical examination of the knee. However, the neuromuscular relationship between the lumbar paraspinal and knee extensor muscles has not been clearly defined. This relationship was supported by Suter et al, 32 who reported reduced QI in persons with anterior knee pain after sacroiliac joint manipulations. This relationship may be mediated by a change in afferent information due to activation of the sacroiliac joint mechanoreceptors and proprioceptors through joint manipulation. 32 Therefore, lower extremity motor neuron excitability changes may result from a change in afferent information from the sacroiliac joint capsule. Similarly, changes in afferent information from the muscle and joint mechanoreceptors and proprioceptors in the lumbar spine due to prolonged, intense fatiguing exercise may affect quadriceps motor neuron excitability. Previously, intramuscular pain induced by hypertonic saline injection into the longissimus muscle at the L4 level caused delayed and reduced transverse abdominis muscle activity during controlled arm movements. 33 This finding indicates altered motor control of healthy muscles through altered input from lumbar muscle afferents. The observed change in quadriceps motor neuron excitability in the current study makes sense anatomically because afferent nerve fibers from the mid-lumbar vertebrae, muscles, and joints would enter the spinal cord through the same mixed nerve roots that contribute to the femoral nerve from the lumbar plexus. Information from lumbar muscle or joint afferents may synapse with quadriceps efferents or other interneurons in the spinal cord that control the quadriceps motor neuron pool. The mechanism of this relationship may arise from a combination of muscle and joint afferents; however, the origin of altered afferent information is speculative.
It is also possible that an indirect relationship between the lumbar paraspinal and quadriceps muscles explains the results of the current study. Quadriceps inhibition after prone, isometric lumbar extension may be a secondary effect of fatigue in the extensors of the knee, hip, and spine: namely, the lumbar paraspinals, gluteals, and hamstrings. Hamstring and gluteal muscle fatigue contribute to task failure during continuous isometric lumbar extension 34, 35; however, if hamstring fatigue had been excessive, the quadriceps motor neuron pool should have become facilitated as a result of the agonist-antagonist relationships among muscles. Because the posterior muscles were likely active during isometric lumbar extension exercise, fatigue experienced in those muscles may have contributed to the observed QI. Another possibility is that the quadriceps become inhibited in response to posterior fatigue-related muscle inhibition, as a mechanism of maintaining the anterior-posterior muscular symmetry necessary to preserve normal function during activity or gait. Regardless of the mechanism, it is important to learn more about how this adaptation affects neuromuscular function in the lower extremity and spine during prolonged functional and athletic activities.
In conclusion, QI increases after fatiguing lumbar extension exercise. These data describe a potential source of QI that may be an adaptive response to maintain symmetry during fatiguing exercise. The observed increase in QI may expose lower extremity joints to injury or degeneration during functional activity, but this possibility requires further research.
REFERENCES
- Latash ML. Neurophysiological Basis of Movement. Champaign, IL: Human Kinetics; 1998.
- Walton DM, Kuchinad RA, Ivanova TD, Garland SJ. Reflex inhibition during muscle fatigue in endurance-trained and sedentary individuals. Eur J Appl Physiol. 2002;87:462–468. doi: 10.1007/s00421-002-0670-9. [DOI] [PubMed] [Google Scholar]
- Hurley MV, Jones DW, Newham DJ. Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin Sci Lond. 1994;86:305–310. doi: 10.1042/cs0860305. [DOI] [PubMed] [Google Scholar]
- Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004;22:110–115. doi: 10.1016/S0736-0266(03)00154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizner RL, Stevens JE, Snyder-Mackler L. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83:359–365. [PubMed] [Google Scholar]
- Snyder-Mackler L, De Luca PF, Williams PR, Eastlack ME, Bartolozzi AR., III. Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1994;76:555–560. doi: 10.2106/00004623-199404000-00010. [DOI] [PubMed] [Google Scholar]
- Suter E, Herzog W, Bray RC. Quadriceps inhibition following arthroscopy in patients with anterior knee pain. Clin Biomech (Bristol, Avon) 1998;13:314–319. doi: 10.1016/s0268-0033(98)00098-9. [DOI] [PubMed] [Google Scholar]
- Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon) 2002;17:56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- Hurley MV, Newham DJ. The influence of arthrogenous muscle inhibition on quadriceps rehabilitation of patients with early, unilateral osteoarthritic knees. Br J Rheumatol. 1993;32:127–131. doi: 10.1093/rheumatology/32.2.127. [DOI] [PubMed] [Google Scholar]
- Radin EL, Burr DB, Caterson B, Fyhrie D, Brown TD, Boyd RD. Mechanical determinants of osteoarthrosis. Semin Arthritis Rheum. 1991;21:12–21. doi: 10.1016/0049-0172(91)90036-y. (3 suppl 2) [DOI] [PubMed] [Google Scholar]
- Bayramoglu M, Akman MN, Kilinc S, Cetin N, Yavuz N, Ozker R. Isokinetic measurement of trunk muscle strength in women with chronic low-back pain. Am J Phys Med Rehabil. 2001;80:650–655. doi: 10.1097/00002060-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Lee JH, Hoshino Y, Nakamura K, Kariya Y, Saita K, Ito K. Trunk muscle weakness as a risk factor for low back pain: a 5-year prospective study. Spine. 1999;24:54–57. doi: 10.1097/00007632-199901010-00013. [DOI] [PubMed] [Google Scholar]
- Kankaanpaa M, Taimela S, Laaksonen D, Hanninen O, Airaksinen O. Back and hip extensor fatigability in chronic low back pain patients and controls. Arch Phys Med Rehabil. 1998;79:412–417. doi: 10.1016/s0003-9993(98)90142-3. [DOI] [PubMed] [Google Scholar]
- Hart JM, Fritz JM, Kerrigan DC, Saliba EN, Gansneder BM, Ingersoll CD. Reduced quadriceps activation after lumbar paraspinal fatiguing exercise. J Athl Train. 2006;41:79–86. [PMC free article] [PubMed] [Google Scholar]
- Paillard T, Noe F, Passelergue P, Dupui P. Electrical stimulation superimposed onto voluntary muscular contraction. Sports Med. 2005;35:951–966. doi: 10.2165/00007256-200535110-00003. [DOI] [PubMed] [Google Scholar]
- Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21:775–779. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Behm D, Power K, Drinkwater E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve. 2001;24:925–934. doi: 10.1002/mus.1090. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996;19:861–869. doi: 10.1002/(SICI)1097-4598(199607)19:7<861::AID-MUS8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Stackhouse SK, Stevens JE, Lee SC, Pearce KM, Snyder-Mackler L, Binder-Macleod SA. Maximum voluntary activation in nonfatigued and fatigued muscle of young and elderly individuals. Phys Ther. 2001;81:1102–1109. [PubMed] [Google Scholar]
- Stevens JE, Binder-Macleod S, Snyder-Mackler L. Characterization of the human quadriceps muscle in active elders. Arch Phys Med Rehabil. 2001;82:973–978. doi: 10.1053/apmr.2001.23995. [DOI] [PubMed] [Google Scholar]
- Nadler SF, Malanga GA, DePrince M, Stitik TP, Feinberg JH. The relationship between lower extremity injury, low back pain, and hip muscle strength in male and female collegiate athletes. Clin J Sport Med. 2000;10:89–97. doi: 10.1097/00042752-200004000-00002. [DOI] [PubMed] [Google Scholar]
- McClure PW, Esola M, Schreier R, Siegler S. Kinematic analysis of lumbar and hip motion while rising from a forward, flexed position in patients with and without a history of low back pain. Spine. 1997;22:552–558. doi: 10.1097/00007632-199703010-00019. [DOI] [PubMed] [Google Scholar]
- Hultman G, Saraste H, Ohlsen H. Anthropometry, spinal canal width, and flexibility of the spine and hamstring muscles in 45–55-year-old men with and without low back pain. J Spinal Disord. 1992;5:245–253. doi: 10.1097/00002517-199209000-00001. [DOI] [PubMed] [Google Scholar]
- Jones MA, Stratton G, Reilly T, Unnithan VB. Biological risk indicators for recurrent non-specific low back pain in adolescents. Br J Sports Med. 2005;39:137–140. doi: 10.1136/bjsm.2003.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler SF, Malanga GA, Feinberg JH, Prybicien M, Stitik TP, DePrince M. Relationship between hip muscle imbalance and occurrence of low back pain in collegiate athletes: a prospective study. Am J Phys Med Rehabil. 2001;80:572–577. doi: 10.1097/00002060-200108000-00005. [DOI] [PubMed] [Google Scholar]
- Nadler SF, Malanga GA, Bartoli LA, Feinberg JH, Prybicien M, DePrince M. Hip muscle imbalance and low back pain in athletes: influence of core strengthening. Med Sci Sports Exerc. 2002;34:9–16. doi: 10.1097/00005768-200201000-00003. [DOI] [PubMed] [Google Scholar]
- Hopkins JT, Ingersoll CD. Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000;9:135–159. [Google Scholar]
- Palmieri RM, Weltman A, Tom JA. An experimental knee joint effusion does not affect plasma catecholamine concentration in humans. Neurosci Lett. 2004;366:76–79. doi: 10.1016/j.neulet.2004.05.016. et al. [DOI] [PubMed] [Google Scholar]
- Palmieri RM, Ingersoll CD, Edwards JE. Arthrogenic muscle inhibition is not present in the limb contralateral to a simulated knee joint effusion. Am J Phys Med Rehabil. 2003;82:910–916. doi: 10.1097/01.PHM.0000098045.04883.02. et al. [DOI] [PubMed] [Google Scholar]
- Palmieri RM, Tom JA, Edwards JE. Arthrogenic muscle response induced by an experimental knee joint effusion is mediated by pre- and post-synaptic spinal mechanisms. J Electromyogr Kinesiol. 2004;14:631–640. doi: 10.1016/j.jelekin.2004.06.002. et al. [DOI] [PubMed] [Google Scholar]
- Spencer JD, Hayes KC, Alexander IJ. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehabil. 1984;65:171–177. [PubMed] [Google Scholar]
- Suter E, McMorland G, Herzog W, Bray R. Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain. J Manipulative Physiol Ther. 1999;22:149–153. doi: 10.1016/S0161-4754(99)70128-4. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp Brain Res. 2003;151:262–271. doi: 10.1007/s00221-003-1457-x. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM, Mayer JM, Ploutz-Snyder LL, Graves JE. Electromyographic activity of the lumbar and hip extensors during dynamic trunk extension exercise. Arch Phys Med Rehabil. 2002;83:1547–1552. doi: 10.1053/apmr.2002.34828. [DOI] [PubMed] [Google Scholar]
- Plamondon A, Trimble K, Lariviere C, Desjardins P. Back muscle fatigue during intermittent prone back extension exercise. Scand J Med Sci Sports. 2004;14:221–230. doi: 10.1111/j.1600-0838.2004.00363.x. [DOI] [PubMed] [Google Scholar]