Abstract
Predictions of yield for the globe's major grain and legume arable crops suggest that, with a moderate temperature increase, production may increase in the temperate zone, but decline in the tropics. In total, global food supply may show little change. This security comes from inclusion of the direct effect of rising carbon dioxide (CO2) concentration, [CO2], which significantly stimulates yield by decreasing photorespiration in C3 crops and transpiration in all crops. Evidence for a large response to [CO2] is largely based on studies made within chambers at small scales, which would be considered unacceptable for standard agronomic trials of new cultivars or agrochemicals. Yet, predictions of the globe's future food security are based on such inadequate information. Free-Air Concentration Enrichment (FACE) technology now allows investigation of the effects of rising [CO2] and ozone on field crops under fully open-air conditions at an agronomic scale. Experiments with rice, wheat, maize and soybean show smaller increases in yield than anticipated from studies in chambers. Experiments with increased ozone show large yield losses (20%), which are not accounted for in projections of global food security. These findings suggest that current projections of global food security are overoptimistic. The fertilization effect of CO2 is less than that used in many models, while rising ozone will cause large yield losses in the Northern Hemisphere. Unfortunately, FACE studies have been limited in geographical extent and interactive effects of CO2, ozone and temperature have yet to be studied. Without more extensive study of the effects of these changes at an agronomic scale in the open air, our ever-more sophisticated models will continue to have feet of clay.
Keywords: global change, atmospheric change, crop production, food security, harvest index
1. Introduction
Ever more refined world and regional maps estimating crop production and global food supply under Intergovernmental Panel on Climate Change (IPCC) climate change scenarios continue to be developed (Rosenzweig & Iglesias 1998; Parry et al. 2004; Thomson et al. 2005). These suggest that in the absence of a direct fertilization effect of rising CO2, crop production by 2050 and 2080 will decline across the globe. When CO2 fertilization is included, crop production in temperate zones is increased while production in the arid and sub-humid tropics declines. In sum, global food supply would remain similar to today. Estimations of temporal and spatial variation in future food production are linked into further models to estimate economic impacts (Parry et al. 2004). These efforts depend on sound data on the responses of the major crops to the key variables of climatic and atmospheric change, singly and in combination, and in different locations. Records of spatial and temporal variation in yields at the field-scale provide powerful datasets for prediction of the responses of crops to rising temperature and altered precipitation (Gitay et al. 2001). Tropospheric carbon dioxide ([CO2]) and ozone ([O3]) concentrations are predicted to increase 50 and 20% by 2050 (Prather et al. 2001; Prentice et al. 2001). Both gases have strong direct effects on photosynthesis and crop production. Knowledge of crop responses to both gases is predominantly from small plot trials using laboratory-controlled environments, greenhouses and closed- and open-topped transparent field chambers (Kimball 1983; Cure & Acock 1986; Ainsworth et al. 2002; Morgan et al. 2003). While use of these facilities was pragmatic, and often for comparative questions, major limitations in making quantitative yield predictions are well recognized. No agrochemical company would base its decision on whether to market a new product on tests in such facilities without field testing at an acceptable agronomic test scale, yet estimates of the ability of the globe to feed itself are almost entirely dependent on data gained in such facilities.
This paper will show that failure to examine the impacts of these gases on our major crops in open-air field trials could have led to a serious overestimation of future global food production. It will show that the fertilization effect of [CO2] has probably been overestimated, while omission of [O3] effects from most models could have led to a 20% overestimation of future crop production in the Northern Hemisphere.
2. Why might chamber studies be insufficient?
Thousands of experimental studies have evaluated the response of crops to the increases in atmospheric [CO2] expected to occur this century (reviewed in Kimball 1983; Drake et al. 1997; Amthor 2001; Ainsworth et al. 2002; Jablonski et al. 2002; Kimball et al. 2002). Most information about crop responses to elevated [CO2] has been derived from experimental studies that have used greenhouses, artificially illuminated controlled environmental chambers, transparent field enclosures or open-top chambers (OTCs). While all of these methods provide an atmosphere with enriched [CO2], they also significantly alter other aspects of the environment surrounding the plant. Many of these studies, including some field studies, used plants grown in pots. Arp (1991) showed that rooting volume altered the response of plants to elevated [CO2], and further experiments have reported a strong feedback when roots encounter a physical barrier (Masle et al. 1990; Thomas & Strain 1991).
Most field studies used OTCs, transparent walled chambers, of up to 2 m diameter. Despite the fact that the top of the chamber is open to the atmosphere, there are environmental differences between even the best-engineered OTCs and the adjacent unclosed crop. The effect of the OTC itself may exceed that of elevation of [CO2] or [O3] (Drake et al. 1989; Day et al. 1996). Whitehead et al. (1995) compared microclimatic conditions within and outside OTCs. When the outside photon flux was 1600 μmol m−2 s−1 (about three-quarters of full sunlight in summer at mid-latitudes), air temperature within the chamber was 4.3 °C higher and vapour pressure deficit 0.8 kPa higher. The transmission of total solar irradiance into the chambers was lower and the ratio of diffuse to total solar irradiance in the chambers was altered. Further, OTCs reduce airflow and intercept rainfall. Also, lower canopy leaves, which are poorly coupled to the atmosphere under natural conditions, are as well coupled as upper canopy leaves within an OTC. This is because circulation within the OTC will ensure turbulent transfer of gases to the upper and lower leaves alike, while in the open frictional drag will result in the lower leaves being more poorly coupled to the atmosphere. This artificial coupling potentially exaggerates the effect of decreased stomatal conductance, and overestimates improved soil water status and crop yield (Long et al. 2004). The migration of pathogens and pests is also restricted by enclosures. It may be argued that the OTCs in which [CO2] is maintained at current atmospheric level provide sufficient experimental control. However, temperature, humidity and light modify the response of plants to elevated [CO2] and [O3] (Curtis 1996; Curtis & Wang 1998; Ainsworth et al. 2002). Therefore, even if chamber effects do not change the direction of a response, they will probably alter its magnitude.
Additionally, small isolated plots in agronomic trials and ecological experiments often overestimate treatment effects on biomass, production and yields (Roberts et al. 1993). Gaps caused by sampling within a small area exacerbate this problem. Increased radiation interception at the edges of small plots can exaggerate the effect of a treatment. The maximum practical size of OTCs limits each plot to a ground surface area of less than 3.1 m2. Therefore, in a 2 m diameter chamber, more than 50% of the vegetation is less than 30 cm from the chamber wall and 75% is within 50 cm of the wall. The recommended border or buffer area for agricultural trials is typically twice the vegetation height (Roberts et al. 1993). Therefore, even a 50 cm high semidwarf wheat crop would require a 1 m buffer zone, and thus no area within an OTC would be free from edge effects (Long et al. 2004). Consequently, knowledge of crop responses to elevated [CO2] and [O3] is currently derived from experiments that are considered unacceptable in standard agronomic trials (McLeod & Long 1999).
3. Free-air concentration enrichment
A free-air concentration enrichment (FACE) apparatus is a circular or octagonal system of pipes that release treatment gas, or air enriched with the treatment gas, just above the top of the crop canopy, and for tall canopies (greater than 1 m) at one or two additional heights below the canopy. Wind direction, wind velocity and [CO2] or [O3] are measured at the centre of each plot and this information is used by a computer-controlled system to adjust gas flow rate, controlled by a massflow control valve, to maintain the target elevated [CO2] or [O3]. Only pipes on the upwind side of the plots release gas, unless wind velocity is less than 0.4 m s−1 when it is released alternately from adjacent release points (McLeod & Long 1999). Quantities of released gas decrease with depth into the canopy to reflect the profile of wind speed. The fast feedback proportional integral differential algorithms avoid large overshoots in response to fluctuations in [CO2] or [O3] and provide a stable elevation of concentration. The first large-scale FACE systems diluted CO2 or ozone with air, which was pumped into the ring (Hendrey et al. 1993; Lewin et al. 1994). More recent designs have either pumped out of the ring to baffle upwind of the outlets (Karnosky et al. 1999) or released pure CO2 or a high concentration of ozone at supersonic velocity into the wind. At this speed the exiting air is immediately turbulent, entraining and mixing with the surrounding air (Miglietta et al. 2001). Although several FACE experiments have been conducted with crops, only five locations have used large rings or plots (greater than or equal to 8 m diameter) with fully replicated (n≥3) designs in each year of the experiment (Long et al. 2004). Mini-FACE systems as small as 1 m diameter have been developed (Miglietta et al. 1996), but they do not escape many of the problems of enclosures outlined above. For example, substantial differences in the photosynthetic response of wheat to elevated [CO2] were observed in a mini-FACE (Miglietta et al. 1996) versus a full-size FACE system (Nie et al. 1995a,b; Wall et al. 2000). This review is therefore limited to full-size FACE systems of more than 8 m diameter plots and with the five major food crops at the global scale; i.e. wheat, rice, maize, sorghum and soybean.
FACE is not without limitations (McLeod & Long 1999). Long-term continuous records of [CO2] within FACE rings show that 1 min averages of actual [CO2] are typically within ±10% of the target concentration for about 90% of the time in low stature vegetation, including most arable crops (McLeod & Long 1999). On shorter time-scales (i.e. less than 1 min), as in OTCs, there are larger fluctuations around the target elevated [CO2] (Nagy et al. 1992; Hendrey et al. 1999). An important issue is whether these fluctuations are perceived by the plant, and in particular whether they affect net CO2 exchange. The response of photosynthesis to [CO2] is nonlinear, so if [CO2] fluctuates, the measured rate of photosynthesis at a given [CO2] will decrease as the amplitude of variation around the mean [CO2] increases (Long et al. 2004). In an experiment to address this issue, Hendrey et al. (1997) found that oscillating [CO2] by 225 μmol mol−1 around a mean of 575 μmol mol−1 had no effect on whole-chain electron transport through photosystem II when the oscillation frequency was 1 min or less, but lower frequency oscillations resulted in progressively greater decreases in electron transport. Given that 1 min averages are usually within 10% of the target [CO2] in FACE, these results suggest that the low frequency oscillations in [CO2], necessary to decrease the response of photosynthesis to elevated [CO2], are uncommon.
The advantage of using the wind as the carrier gas, as in FACE, is that the perturbation of the natural microclimate is minimal in contrast to enclosure methods. The disadvantages are that a dilution gradient is generated across the treatment plot and the system is dependent on continuous air movement. So, although the centre of the plot is maintained close to the target, the upwind site may be 100 μmol mol−1 above and the downwind 100 μmol mol−1 below the target. With a strong prevailing wind, a gradient effect would occur across each plot. However, analysis of isotopic composition across FACE plots shows a remarkable uniformity. This suggests that although transient gradients occur, when averaged over growing seasons, these gradients are not detectable (Leavitt et al. 1996).
A further potential disadvantage of FACE is its dependence on continuous air movement. During daylight hours the continual flux of solar radiation and resulting convective currents ensure that still periods are rare, except around dawn. However, at night, still conditions commonly occur. Some FACE systems mix pure CO2 into an airstream which is then pumped into the plots at the release points. This flow of CO2-enriched air moves air into the plot under still conditions. These systems can therefore enrich the atmosphere under still conditions. However, still conditions also result in a climatic inversion, where cold air forms at the surface overlain by warm air. Pumping air into the plot brings the warm air to the surface thus disrupting the inversion (Pinter et al. 2000). Enrichment can be achieved under still conditions, but only by significantly altering the microclimate. The system described by Miglietta et al. (2001) does not predilute CO2 but releases pure CO2 at supersonic velocity through minute nozzles into the wind. The energy of these turbulent jets generates a predilution of the CO2 before the wind carries it back over the treatment plot. This system depends completely on some air movement and cannot operate under perfectly still conditions.
4. Carbon dioxide
Plants can only perceive a change in atmospheric [CO2] through tissues that are exposed to the open air. The protective cuticle of higher-plant leaves and other photosynthetic organs means that only the inner surfaces of the guard cells of stomata and the mesophyll can directly sense a change in atmospheric [CO2]. While many steps in metabolism use or respond to CO2, the only sites where there is convincing evidence for a response in the concentration range of relevance (240–1000 μmol mol−1) are ribulose 1 : 5 bisphosphate carboxylase/oxygenase (Rubisco) and a yet undefined metabolic step affecting stomatal aperture, that may also involve Rubisco (Buckley et al. 2003; Long et al. 2004).
The direct increase in C3 photosynthesis due to elevation of [CO2] results from two properties of Rubisco. (i) The enzyme is not saturated by present atmospheric [CO2], and so elevated [CO2] will increase the velocity of carboxylation and net photosynthesis. (ii) CO2 is a competitive inhibitor of the oxygenation reaction which leads to photorespiration. Photorespiration typically releases 20–40% of recent photosynthate as CO2. This significantly reduces net photosynthesis of C3 crops, and will be suppressed in favour of greater carbon gain by rising [CO2]. Because the kinetic properties of Rubisco are highly conserved across C3 crops, the improvement in photosynthetic gain with rising [CO2] can be calculated for all C3 crops with some confidence. An increase in atmospheric [CO2] from today's 372 to 550 μmol mol−1 would increase net leaf photosynthesis by 12–36%, while elevation to 700 μmol mol−1 would generate a stimulation of 18–63%, for a leaf temperature of 25 °C. The lower end of these ranges represents light-limited photosynthesis, while the greatest stimulation occurs under light-saturated conditions, when the amount of Rubisco is assumed to be limiting. Since crop canopies in the field gain their carbon in roughly equal quantities from light-limited and light-saturated photosynthesis, actual gains are likely to be in the middle of these ranges (Long 1991; Long et al. 2004). Also, this theoretical stimulation increases with temperature because photorespiration increases as a proportion of photosynthesis with temperature. Actual increases are likely to be lower than theoretically possible given feedbacks both within the plant and at the ecosystem level. This will be further complicated by decreased transpiration, which may provide an additional gain due to improved plant water status (reviewed in Long et al. 2004).
How large are the actual yield increases due to [CO2]? Kimball (1983) showed from an exhaustive survey of over 1000 chamber studies that elevation of [CO2] to 660 μmol mol−1 caused an average 33% increase in yields for C3 crops, with 99% confidence limits of 24–43%. This review will consider the effect of elevation to the estimated 2050 [CO2] of ca 560 μmol mol−1, which approximates the level used in large-scale FACE experiments with food crops. The 33% increase observed at 660 μmol mol−1 equates to 23% at 560 μmol mol−1 assuming a linear response, or 25% if a more realistic hyperbolic response with saturation of yield response at 2000 μmol mol−1 is assumed (table 1). Most of the models and studies that have fed into recent assessments, such as the UN–IPCC third assessment report of future staple crop production, derive their ‘CO2-fertilization’ effect from Kimball (1983) (Gitay et al. 2001). The models used to predict future crop yields mathematically approximate carbon gain from either radiation use efficiency or net photosynthesis (e.g. CERES-wheat, Ritchie & Otter 1985; Godwin et al. 1989; CERES-rice, Singh et al. 1989; CERES-maize, Jones & Kiniry 1986; Ritchie et al. 1989; SOYGRO, Jones et al. 1989; EPIC, Stockle et al. 1992). To estimate the effects of greater [CO2] on yield in the future, a CO2-fertilization factor is applied to reflect the direct physiological stimulation of these processes by elevated [CO2]. The CO2-fertilization factor applied in most CERES or SOYGRO modelling exercises (e.g. Parry et al. 1999) is based on the methods of Peart et al. (1989), which used the ratio of photosynthetic carbon gain under elevated [CO2] compared with ambient [CO2] from early literature reviews (Kimball 1983; Cure & Acock 1986; Allen et al. 1987). For example, the direct stimulation of photosynthesis at ca 550 ppm has been assumed to be +21% for soybean, +17% for wheat, +17% for rice and +6% for maize (Rosenzweig & Iglesias 1998), +29% for soybean, +21% for wheat and +8% for maize (Adams et al. 1990) and +10% for maize (Dhakwa et al. 1997). In most EPIC applications (e.g. Brown & Rosenberg 1999; Izaurralde et al. 2003; Thomson et al. 2005), the CO2-fertilization factor for radiation use efficiency uses the method of Stockle et al. (1992), which parameterized a CO2-response function to reproduce the mean yield stimulations reported for elevated [CO2] by Kimball (1983). This corresponds to a yield stimulation at ca 550 ppm of +20% for soybean, +28% for wheat and +8% for maize.
Table 1.
Comparison of chamber and FACE findings on percentage increase in yield with elevation of [CO2] to 550–575 μmol mol−1.
| crop | lineara | hyperbolicb | FACE | some recent model projectionsc |
|---|---|---|---|---|
| wheat | 22 | 25 | 8d | 19e, 23f, 33g |
| rice | 16 | 18 | 10h | 26i |
| maize | 6 | 7 | n.s.j | 10f |
| sorghum | 23 | 34 | n.s.k | |
| soybean | 17 | 19 | 15l | 16f |
| mean (C3) | 23 | 25 | 11 | 19m−27n |
n.s., not significantly different from zero.
Mean projected increase in yield reported linearly extrapolated to 550–575 μmol mol−1 from the averages provided by Kimball (1983) for 660 μmol mol−1 and for wheat from the average observed in open-top chambers across western Europe in the ‘ESPACE-wheat’ project (Bender et al. 1999).
Mean projected increase assuming a hyperbolic response of yield to increasing [CO2], saturating at 2000 μmol mol−1, e.g. Amthor (2001).
Effect of elevation to 550 μmol mol−1 extracted by comparison of projections with and without a direct effect of [CO2].
Irrigated spring wheat, Arizona (Kimball et al. 1995).
Europe (Ewert et al. 2005).
USA (Thomson et al. 2005).
USA & Canada (Rosenzweig & Iglesias 1998).
Rice, Honshu Island, average of 3 years and three nitrogen treatments, reported in fig. 4 of Kim et al. (2003).
ORYZA1 model projection (Matthews et al. 1997).
Rainfed maize, central Illinois (A. D. B. Leakey, M. Uribelarrea, E. A. Ainsworth, S. L. Naidu, A. Rogers, D. R. Ort & S. P. Long, unpublished data).
Irrigated sorghum, Arizona (Ottman et al. 2001).
Rainfed soybean, central Illinois, average of 3 years (Morgan et al. 2005).
Mean of all major crops in Europe, including C4.
USA (Darwin & Kennedy 2000).
The extensive survey of Kimball (1983) suggests that with an increase in [CO2] to 550 μmol mol−1 C3 yield should increase by 25% with a 99% lower confidence interval of 18%. Yet, not one of the three major C3 crops showed an actual yield increase that reached this lower confidence limit of 18% when tested under large scale replicated open-air elevation of [CO2] in FACE. For wheat actual values were a third of that observed in chambers, for rice two-thirds and soybean four-fifths. For the two major C4 crops, there was no significant increase in yield at all in FACE compared to the chamber-predicted increase of 7% and modelled increase of 10% (table 1). Evidence from chamber experiments has been contradictory regarding direct effects of elevated [CO2] on C4 photosynthesis (reviewed in Ghannoum et al. 2000). As described above, models for C4 crops currently assume a direct increase in photosynthesis or radiation use efficiency in elevated [CO2] in addition to reduced stomatal conductance and water-use efficiency. However, FACE experiments with maize under optimal growing conditions indicate no direct response of C4 enzyme activity, photosynthetic flux or yield to elevated [CO2] (A. D. B. Leakey, M. Uribelarrea, E. A. Ainsworth, S. L. Naidu, A. Rogers, D. R. Ort & S. P. Long, unpublished data). Removing a direct CO2-fertilization effect on C4 photosynthesis and radiation use efficiency from the models may correct for this overestimate by basing yield estimates under elevated [CO2] solely on improved water-use efficiency.
Averaged across the FACE experiments of table 1, the yield increase is 11% for C3 crops and 7% for all five major food crops, which is one-third to one-quarter of the direct effect of [CO2] modelled in the recent assessment for Europe and the USA by Darwin & Kennedy (2000) (table 1). Overall, these FACE results suggest that the fertilization effect of [CO2] used in current models of global food production is seriously overestimated. However, this is based on just five fully replicated full-scale FACE experiments (see d, h, j, k, l in table 1). With such a small sample there is the possibility that these results are unrepresentative, but the fact that they all fall below the lower 99% confidence interval established from chamber studies, suggests that this is unlikely. While it might be argued that spring wheat in Arizona is unrepresentative of the major wheat growing areas, sorghum in Arizona, rice in Japan and soybean and maize in Illinois are all in near-ideal climate zones for the respective crops, and yet yield stimulations by elevated [CO2] all fall below expectations. This conclusion suggests a larger difference between FACE and chamber studies, than noted in the earlier reviews by Amthor (2001) and Kimball et al. (2002). What might explain this difference? Amthor (2001) reviewed all previously published studies of wheat grown throughout its life cycle in elevated [CO2]. In common with this study, the yield increase in FACE was less than in chamber studies. It indicates an overestimation in chamber studies relative to FACE, although the difference is considerably less than indicated by table 1. However, most chamber studies used [CO2] of ca 680–700 ppm and Amthor (2001) assumed a linear increase in yield with increase in [CO2] from 350 to 700 ppm. This would underestimate the yield increase that would be observed in chambers at 550 ppm if the response is curvilinear. For example, Fangmeier et al. (1996) observed an increase in yield for spring wheat of 28% when growth [CO2] in OTCs was increased from 360 to 540 ppm, but only observed a further 4% increase in yield when [CO2] was increased from 540 to 650 ppm, suggesting the response to be strongly curvilinear. Kimball et al. (2002) reviewed all prior FACE crop studies, and noted that while the response was less than in chamber studies the difference was not significant. Long et al. (2004) were able to use the larger database of FACE studies that had become available with a further 2 years of primary publications and applied statistical meta-analysis to show a significantly lower seed yield for the major crops, as in table 1.
Despite further publications, unfortunately the current FACE experiments are not adequate to re-parameterize the existing models. They cover far too small an area of the ranges of these crops. FACE experiments have shown increased response of wheat to elevated [CO2] in drought, and of both wheat and rice under low N conditions, as anticipated (reviewed in Kimball et al. 2002; Ainsworth & Long 2005). A major omission is any information on how temperature interacts with elevated [CO2] under the fully open-air conditions of FACE. Based on the kinetics of Rubisco a much larger increase in dry matter production should occur at higher temperatures and a reduced response at low temperatures. This interaction is either not represented or poorly represented in the major models of crop production. It may be significant that cotton grown during the summer at the FACE site in Arizona showed a 42% increase in yield compared to 8% for wheat grown over the winter and spring (reviewed in Kimball et al. 2002; Ainsworth & Long 2005).
5. Ozone
Although elevated tropospheric ozone concentrations ([O3]) have been recognized as a factor lowering the yields of the major food crops since the 1970s and 1980s (reviewed in Ashmore 2002), the major current projections of global food production under atmospheric change scenarios (Gitay et al. 2001; Parry et al. 2004) do not account for the damaging effect of rising [O3]. While elevated background levels are often insufficient to produce the visible lesions apparent after acute exposures, these elevations will lower photosynthetic rate, accelerate leaf senescence and decrease ovule fertilization (McKee et al. 1997). In industrialized countries of the Northern Hemisphere, tropospheric [O3] has risen by 1–2% per year (Chameides et al. 1994). Nearly one-quarter of the Earth's surface is currently at risk from tropospheric ozone in excess of 60 nmol mol−1 during mid-summer, with even greater concentrations occurring locally (Fowler et al. 1999a,b). The croplands of western Europe, the midwest and eastern US and eastern China are being exposed to some of the highest background [O3] (Prather et al. 2001). Although the risks of acute ozone exposure around large cities are well known, it is often not appreciated that background [O3] has been rising in rural areas, distant from centres of industrialization. Tropospheric [O3] forms as a result of the action of sunlight on polluted air masses containing nitrogen oxides, hydrocarbons and carbon monoxide. These polluted air masses can be transported thousands of miles both across and between continents (Prather et al. 2001). Increasing fossil fuel consumption is predicted to raise the production of nitrogen oxides, while increased temperature and hydrocarbon concentrations are expected to cause further increases in tropospheric [O3] (Pritchard & Amthor 2005). The IPCC Third Assessment Report projected an increase in surface [O3] across the globe, with a 2100 July mean increase of ca 40 nmol mol−1 in the Northern Hemisphere and ca 30 nmol mol−1 in the tropics (Prather et al. 2001). These projections also suggest an increase in tropospheric [O3] of 20–25% by 2050, with greater regional increases projected for midwest US, China, Arabia and western Europe. Currently, these areas experience a mean daytime growing season concentration of ca 50–60 nmol mol−1. Extensive trials in the USA and western Europe using standardized designs of OTCs have established yield reduction equations for the major crops, except sorghum (reviewed in Ashmore 2002; figure 1). Yield reduction can begin at concentrations as low as 20 nmol mol−1. At 50–60 nmol mol−1, yield losses of 7, 8, 18 and 22% are projected for maize, rice, wheat and soybean, respectively. The reality of such losses is clearly demonstrated when OTCs with charcoal filters to remove ozone are placed in the field next to control OTCs which blow the unfiltered ambient air into the chamber (Ashmore 2002). The 20% increase in surface [O3] by 2050 would result in yield losses relative to today's yields of 5, 4, 9 and 12% for maize, rice, wheat and soybean, respectively (figure 1), and approximately double these losses by the end of the century. Using similar data to that shown in figure 1, Wang & Mauzerall (2004) project that with the very large increases in surface [O3] projected for east-central China, crop losses for maize, rice and soybean will each exceed 30% by 2020.
Figure 1.
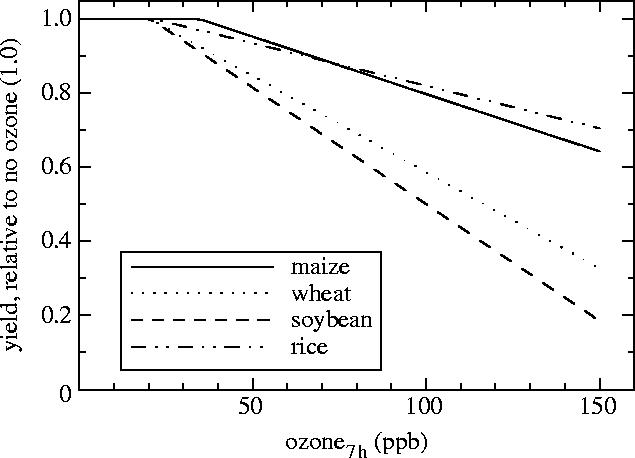
Yield declines due to tropospheric ozone [O3] for the staple crops maize (Zea mays; n=20), rice (Oryza sativa; n=26), soybean (Glycine max; n=41) and wheat (Triticum aestivum; n=33). Estimates were constructed from the equations of Kobayashi et al. (1995), Mills et al. (2000), Ashmore (2002) and Wang & Mauzerall (2004). The number of independent treatments on which the above authors developed each line is indicated after species name. Ozone concentration is based on exposure at the stated average for the highest 7 h of each day.
Again these responses have been established in small chambers that differ substantially from the outside, natural field conditions. Some environmental differences between the chamber and the open air could ameliorate the effect of elevated [O3], while others could exacerbate the effect (reviewed in Morgan et al. 2003). Recognition of this limitation led to the development of FACE systems that elevated pollutants rather than CO2, almost two decades ago (McLeod & Long 1999), but the systems were never deployed with crops until now. Morgan (2004) used a FACE system adapted to elevate [O3] by 20% to examine whether the decreases in yield for soybean projected from chamber experiments occurred in the open air. In 2002, the ambient (control) growing season 8 h average [O3] was 62 nmol mol−1 and the treatment was 75 nmol mol−1, a 21% elevation. In 2003, the ambient growing season 8 h average was 50 nmol mol−1 and the treatment 63 nmol mol−1, a 25% elevation. Seed yield was significantly decreased by 15% in 2002 and by 25% in 2003. How does this compare to expectations established from chamber studies? Based on the equations of Mills et al. (2000) and Ashmore (2002), the expected decreases were 12 and 9% for 2002 and 2003, respectively. Therefore, on average the observed decrease in the open air (20%) was substantially greater than expected. This single and only FACE study of the effect of elevated [O3] suggests that, at least in the case of soybean, not only will the substantial decreases found in chamber studies be realized, but they may be even greater under fully open-air exposure. Should this extrapolate across all major food crops, then even the alarming future yield losses projected by Wang & Mauzerall (2004) may be underestimates.
6. Conclusions
In the absence of any physiological effect of elevated [CO2], global climate change is expected to decrease yields of the major grain crops across the globe (Gitay et al. 2001; Parry et al. 2004). Elevated [CO2] is projected to rescue this situation to the extent that temperate North and South America, western Europe, Australia and China would all see yield increases by 2050 and 2080. As has been noted in such projections, the direct effect of elevated [CO2] on crop yields is one of the largest uncertainties (Parry et al. 2004; Thomson et al. 2005), which is the reason for calculating future yields with and without a physiological effect of elevated [CO2]. Unfortunately, the inclusion makes a world of difference in a very literal sense, a world with sufficient food versus one perhaps without. Here, we show that the database of chamber studies, which has been the mechanistic basis for crop yield models, overestimates the yield gain that is observed under fully open-air conditions in the field. Worse however, current projections of world food supply (e.g. Gitay et al. 2001; Parry et al. 2004) do not account for the damaging effect of rising surface [O3] concentrations. Chamber studies suggest that with the estimated [O3] increase for the Northern Hemisphere, yield loss due to [O3] would numerically offset any increase due to rising [CO2]. There has only been one replicated large-scale fully open-air study of the effects of season-long elevation of [O3] on a grain crop, and this revealed a loss substantially greater than even the large losses recorded in chamber studies. Should this be representative of other major crops and growing areas, it suggests that the yield losses due to rising [O3] will outweigh any gains due to rising [CO2]. This is especially relevant to the projected increase in future crop production in eastern China (Parry et al. 2004), where these gains would almost certainly be reversed if the effects of rising [O3] as projected by Wang & Mauzerall (2004) are included.
Chamber experiments have shown that elevated [CO2] may provide some protection against elevated [O3]. Elevated [CO2] decreases stomatal conductance and therefore decreases uptake of O3 into the leaf. Will this operate in the open? The only FACE experiment to report elevated [O3] and [CO2] effects within a factorial design has been an investigation of deciduous trees in North Wisconsin (Karnosky et al. 2005). Here, it was found that although elevated [CO2] decreased stomatal conductance, the relative decrease in dry matter production and photosynthesis caused by elevated [O3] was the same at ambient and at elevated [CO2]. Since less O3 will have been assimilated, the result suggests that elevated [CO2] grown tissue was metabolically less tolerant of O3 (Karnosky et al. 2005). McKee et al. (1995, 1997) found that with wheat grown in chambers, elevated [O3] had less affect on dry matter production and photosynthesis at elevated [CO2], but yield was similarly depressed because of a direct effect of elevated [O3] on ovule fertilization. Consequently, if we are to have any confidence in projections of future global food security, whether elevated [CO2] provides any protection against rising [O3] in the major food crops under fully open-air conditions requires urgent investigation.
While chamber and glasshouse experiments are important qualitative guides, it is well established both for trials of agrochemicals and transgenic plants that chamber performance is an unreliable quantitative, and sometimes qualitative, predictor of field performance. New agrochemicals apparently effective in a chamber may be ineffective in the field (Anand et al. 2003; Black 2004) and disease resistance apparent in transgenes in the greenhouse may be absent in the field (e.g. Anand et al. 2003). Based on this long-standing agronomic experience it should be no surprise that chamber versus fully open-air treatments on a large scale do not agree. No agrochemical or biotechnology company would base its business plan on chamber studies alone, yet the UN–IPCC (Gitay et al. 2001) bases its projections of future food supply for the whole globe on such potentially flawed data.
Unfortunately, our existing FACE studies of the major crops are too few to provide any real basis for correction. There is good reason to expect significant interactions between [CO2], [O3], temperature and soil moisture, requiring improved technologies for open-air treatment. FACE experiments may have been avoided for cost, but a design based on that of Miglietta et al. (2001) is likely to cost under 180 000€ in components for a four replicate facility. CO2 would be the major recurrent cost, with 20 m plots requiring about 1 t of CO2 each per day. This cost can be greatly decreased if a facility is sited near a natural or industrial source of CO2. A network of such facilities are required first to obtain reliable estimates of the [CO2] and [O3] effects on our major food crops, and then as a means for adapting our crops to these changes. Whatever the cost, it is small compared to the cost of uncertainty. For four midwestern states, losses of $2.03 billion with climate change are projected without a CO2-fertilization effect and gains of $645 million with CO2 fertilization (Crosson 1993). Without these facilities coupled with improved mechanistic understanding of crops responses to rising [CO2] and [O3], our ever-more sophisticated models for projecting future food production and vegetation–atmosphere interactions will continue to rest on feet of clay.
Footnotes
One contribution of 17 to a Discussion Meeting Issue ‘Food crops in a changing climate’.
Present address: UDSA-Agricultural Research Service, Air Quality Research Unit, 3908 Inwood Road, Raleigh, NC 27603, USA.
References
- Adams R.M, et al. Global climate change and US agriculture. Nature. 1990;345:219–224. doi:10.1038/345219a0 [Google Scholar]
- Ainsworth E.A, Long S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. doi:10.1111/j.1469-8137.2004.01224.x [DOI] [PubMed] [Google Scholar]
- Ainsworth E.A, et al. A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Global Change Biol. 2002;8:695–709. doi:10.1046/j.1365-2486.2002.00498.x [Google Scholar]
- Allen L.H, Boote K.J, Jones J.W, Jones P.H, Valle R.R, Acock B, Rogers H.H, Dahlman R.C. Response of vegetation to rising carbon dioxide: photosynthesis, biomass and seed yield of soybean. Global Biogeochem. Cycles. 1987;1:1–14. [Google Scholar]
- Amthor J.S. Effects of atmospheric CO2 concentration on wheat yield: review of results from experiments using various approaches to control CO2 concentration. Field Crops Res. 2001;73:1–34. doi:10.1016/S0378-4290(01)00179-4 [Google Scholar]
- Anand A, Zhou T, Trick H.N, Gill B.S, Bockus W.W, Muthukrishnan S. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J. Exp. Bot. 2003;54:1101–1111. doi: 10.1093/jxb/erg110. doi:10.1093/jxb/erg110 [DOI] [PubMed] [Google Scholar]
- Arp W.J. Effects of source–sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 1991;14:869–875. [Google Scholar]
- Ashmore M.R. Effects of oxidants at the whole plant and community level. In: Bell J.N.B, Treshow M, editors. Air pollution and plant life. Wiley; Chichester, UK: 2002. pp. 89–118. [Google Scholar]
- Bender J, Hertstein U, Black C.R. Growth and yield responses of spring wheat to increasing carbon dioxide, ozone and physiological stresses: a statistical analysis ‘ESPACE-wheat’ results. Eur. J. Agron. 1999;10:185–195. doi:10.1016/S1161-0301(99)00009-X [Google Scholar]
- Black B. Panel discussion: challenges in pesticide translation from lab to greenhouse to field. Abstr. Pap. Am. Chem. Soc. 2004;228:U84. [Google Scholar]
- Brown R.A, Rosenberg N.J. Climate change impacts on the potential productivity of corn and winter wheat in their primary United States growing regions. Climatic Change. 1999;41:73–107. doi:10.1023/A:1005449132633 [Google Scholar]
- Buckley T.N, Mott K.A, Farquhar G.D. A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ. 2003;26:1767–1785. doi:10.1046/j.1365-3040.2003.01094.x [Google Scholar]
- Chameides W.L, Kasibhatla P.S, Yienger J, Levy H. Growth of continental-scale metro-agro-plexes, regional ozone pollution, and world food-production. Science. 1994;264:74–77. doi: 10.1126/science.264.5155.74. [DOI] [PubMed] [Google Scholar]
- Crosson P. Impacts of climate change on the agriculture and economy of the Missouri, Iowa, Nebraska, Kansas (MINK) region. In: Kaiser H.M, Drennen T.E, editors. Agricultural dimensions of global climate change. St Lucie Press; Delray Beach, FL: 1993. pp. 117–135. [Google Scholar]
- Cure J.D, Acock B. Crop responses to carbon-dioxide doubling—a literature survey. Agric. Forest Meteorol. 1986;38:127–145. doi:10.1016/0168-1923(86)90054-7 [Google Scholar]
- Curtis P.S. A meta-analysis of leaf gas exchange and nitrogen in trees grown under elevated carbon dioxide. Plant Cell Environ. 1996;19:127–137. [Google Scholar]
- Curtis P.S, Wang X.Z. A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia. 1998;113:299–313. doi: 10.1007/s004420050381. doi:10.1007/s004420050381 [DOI] [PubMed] [Google Scholar]
- Darwin R, Kennedy D. Economic effects of CO2 fertilization of crops: transforming changes in yield into changes in supply. Environ. Model. Assess. 2000;5:157–168. doi:10.1023/A:1019013712133 [Google Scholar]
- Day F.P, Weber E.P, Hinkle C.R, Drake B.G. Effects of elevated atmospheric CO2 on fine root length and distribution in an oak-palmetto scrub ecosystem in central Florida. Global Change Biol. 1996;2:143–148. [Google Scholar]
- Dhakhwa G.B, Campbell C.L, LeDuc S.K, Cooter E.J. Maize growth: assessing the effects of global warming and CO2 fertilization with crop models. Agric. Forest Meteorol. 1997;87:253–272. doi:10.1016/S0168-1923(97)00030-0 [Google Scholar]
- Drake B.G, Leadley P.W, Arp W.J, Nassiry D, Curtis P.S. An open top chamber for field studies of elevated atmospheric CO2 concentration on saltmarsh vegetation. Funct. Ecol. 1989;3:363–371. [Google Scholar]
- Drake B.G, GonzalezMeler M.A, Long S.P. More efficient plants: a consequence of rising atmospheric CO2? Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997;48:609–639. doi: 10.1146/annurev.arplant.48.1.609. doi:10.1146/annurev.arplant.48.1.609 [DOI] [PubMed] [Google Scholar]
- Ewert F, Rounsevell M.D.A, Reginster I, Metzger M.J, Leemans R. Future scenarios of European agricultural land use I. Estimating changes in crop productivity. Agric. Ecosyst. Environ. 2005;107:101–116. doi:10.1016/j.agee.2004.12.003 [Google Scholar]
- Fangmeier A, Gruters U, Hertstein U, Sandhage-Hofmann A, Vermehren B, Jiger H.J. Effects of elevated CO2, nitrogen supply and tropospheric ozone on spring wheat. 1. Growth and yield. Environ. Pollut. 1996;91:381–390. doi: 10.1016/0269-7491(95)00042-9. doi:10.1016/0269-7491(95)00042-9 [DOI] [PubMed] [Google Scholar]
- Fowler D, Cape J.N, Coyle M, Flechard C, Kuylenstierna J, Hicks K, Derwent D, Johnson C, Stevenson D. The global exposure of forests to air pollutants. Water Air Soil Pollut. 1999a;116:5–32. doi:10.1023/A:1005249231882 [Google Scholar]
- Fowler D, Cape J.N, Coyle M, Smith R.I, Hjellbrekke A.G, Simpson D, Derwent R.G, Johnson C.E. Modelling photochemical oxidant formation, transport, deposition and exposure of terrestrial ecosystems. Environ. Pollut. 1999b;100:43–55. doi: 10.1016/s0269-7491(99)00087-1. doi:10.1016/S0269-7491(99)00087-1 [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Von Caemmerer S, Ziska L.H, Conroy J.P. The growth response of C4 plants to rising atmospheric CO2 partial pressure: a reassessment. Plant Cell Environ. 2000;23:931–942. doi:10.1046/j.1365-3040.2000.00609.x [Google Scholar]
- Gitay H, Brown S, Easterling W, Jallow B. Ecosystems and their goods and services. In: McCarthy J.J, Canziani O.F, Leary N.A, Dokken D.J, White K.S, editors. Climate change 2001: impacts, adaptation, and vulnerability. IPCC/Cambridge University Press; Cambridge, UK: 2001. pp. 237–342. [Google Scholar]
- Godwin D, Ritchie J.T, Singh U, Hunt L. International Fertilizer Development Center; Muscle Shoals, AL: 1989. A user's guide to CERES-wheat v2.10. [Google Scholar]
- Hendrey G.R, Lewin K.F, Nagy J. Free air carbon-dioxide enrichment—development, progress, results. Vegetatio. 1993;104:17–31. doi:10.1007/BF00048142 [Google Scholar]
- Hendrey G.R, Long S.P, McKee I.F, Baker N.R. Can photosynthesis respond to short-term fluctuations in atmospheric carbon dioxide? Photosynth. Res. 1997;51:179–184. doi:10.1023/A:1005804203928 [Google Scholar]
- Hendrey G.R, Ellsworth D.S, Lewin K.F, Nagy J. A free-air enrichment system for exposing tall forest vegetation to elevated atmospheric CO2. Global Change Biol. 1999;5:293–309. doi:10.1046/j.1365-2486.1999.00228.x [Google Scholar]
- Izaurralde R.C, Rosenberg N.J, Brown R.A, Thomson A.M. Integrated assessment of Hadley Center (HadCM2) climate-change impacts on agricultural productivity and irrigation water supply in the conterminous United States—Part II. Regional agricultural production in 2030 and 2095. Agric. Forest Meteorol. 2003;117:97–122. doi:10.1016/S0168-1923(03)00024-8 [Google Scholar]
- Jablonski L.M, Wang X.Z, Curtis P.S. Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol. 2002;156:9–26. doi:10.1046/j.1469-8137.2002.00494.x [Google Scholar]
- Jones C.A, Kiniry J.R. Texas A&M Press; College Station, TX: 1986. CERES-maize: a simulation model of maize growth and development. [Google Scholar]
- Jones J.W, Boote K.J, Hoogenboom G, Jagtap S.S, Wilkerson G.G. Department of Agricultural Engineering and Department of Agronomy, University of Florida; Gainsville, FL: 1989. SOYGRO v5.42: soybean crop growth simulation model. Users Guide. [Google Scholar]
- Karnosky D.F, et al. Effects of tropospheric O3 on trembling aspen and interaction with CO2: results from an O3-gradient and a face experiment. Water Air Soil Pollut. 1999;116:311–322. doi:10.1023/A:1005276824459 [Google Scholar]
- Karnosky D.F, Pregitzer K.S, Zak D.R, Kubiske M.E, Hendrey G.R, Weinstein D, Nosal M, Percy K.E. Scaling ozone responses of forest trees to the ecosystem level in a changing climate. Plant Cell Environ. 2005;28:965–981. [Google Scholar]
- Kim H.Y, Lieffering M, Kobayashi K, Okada M, Miura S. Seasonal changes in the effects of elevated CO2 on rice at three levels of nitrogen supply: a free air CO2 enrichment (FACE) experiment. Global Change Biol. 2003;9:826–837. doi:10.1046/j.1365-2486.2003.00641.x [Google Scholar]
- Kimball B.A. Carbon dioxide and agricultural yields: an assemblage and analysis of 430 prior observations. Agron. J. 1983;75:779–788. [Google Scholar]
- Kimball B.A, Pinter P.J, Garcia R.L, LaMorte R.L, Wall G.W, Hunsaker D.J, Wechsung G, Wechsung F, Kartschall T. Productivity and water use of wheat under free-air CO2 enrichment. Global Change Biol. 1995;1:429–442. [Google Scholar]
- Kimball B.A, Kobayashi K, Bindi M. Responses of agricultural crops to free-air CO2 enrichment. Adv. Agron. 2002;77:293–368. [PubMed] [Google Scholar]
- Kobayashi K, Okada M, Nouchi I. Effects of ozone on dry-matter partitioning and yield of Japanese cultivars of rice (Oryza sativa L.) Agric. Ecosyst. Environ. 1995;53:109–122. doi:10.1016/0167-8809(94)00564-U [Google Scholar]
- Leavitt S.W, Paul E.A, Galadima A, Nakayama F.S, Danzer S.R, Johnson H, Kimball B.A. Carbon isotopes and carbon turnover in cotton and wheat FACE experiments. Plant Soil. 1996;187:147–155. doi:10.1007/BF00017087 [Google Scholar]
- Lewin K.F, Hendrey G.R, Nagy J, Lamorte R.L. Design and application of a free-air carbon-dioxide enrichment facility. Agric. Forest Meteorol. 1994;70:15–29. doi:10.1016/0168-1923(94)90045-0 [Google Scholar]
- Long S.P. Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations—has its importance been underestimated? Plant Cell Environ. 1991;14:729–739. [Google Scholar]
- Long S.P, Ainsworth E.A, Rogers A, Ort D.R. Rising atmospheric carbon dioxide: plants face the future. Annu. Rev. Plant Biol. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. doi:10.1146/annurev.arplant.55.031903.141610 [DOI] [PubMed] [Google Scholar]
- Masle J, Farquhar G.D, Gifford R.M. Growth and carbon economy of wheat seedlings as affected by soil resistance to penetration and ambient partial-pressure of CO2. Aust. J. Plant Physiol. 1990;17:465–487. [Google Scholar]
- Matthews R.B, Kropff M.J, Horie T, Bachelet D. Simulating the impact of climate change on rice production in Asia and evaluating options for adaptation. Agric. Syst. 1997;54:399–425. doi:10.1016/S0308-521X(95)00060-I [Google Scholar]
- McKee I.F, Farage P.K, Long S.P. The interactive effects of elevated CO2 and O3 concentration on photosynthesis in spring wheat. Photosynth. Res. 1995;45:111–119. doi: 10.1007/BF00032582. doi:10.1007/BF00032582 [DOI] [PubMed] [Google Scholar]
- McKee I.F, Bullimore J.F, Long S.P. Will elevated CO2 concentrations protect the yield of wheat from O3 damage? Plant Cell Environ. 1997;20:77–84. doi:10.1046/j.1365-3040.1997.d01-1.x [Google Scholar]
- McLeod A.R, Long S.P. Free air carbon dioxide enrichment (FACE) in global change research: a review. Adv. Ecol. Res. 1999;28:1–55. [Google Scholar]
- Miglietta F, Giuntoli A, Bindi M. The effect of free air carbon dioxide enrichment (FACE) and soil nitrogen availability on the photosynthetic capacity of wheat. Photosynth. Res. 1996;47:281–290. doi: 10.1007/BF02184288. doi:10.1007/BF02184288 [DOI] [PubMed] [Google Scholar]
- Miglietta F, Peressotti A, Vaccari F.P, Zaldei A, deAngelis P, Scarascia-Mugnozza G. Free-air CO2 enrichment (FACE) of a poplar plantation: the POPFACE fumigation system. New Phytol. 2001;150:465–476. doi:10.1046/j.1469-8137.2001.00115.x [Google Scholar]
- Mills, G., Hayes, F., Buse, A. & Reynolds, B. 2000 Air pollution and vegetation. Annual report 1999/2000 of UN/ECE ICP vegetation. Bangor, UK: Centre for Ecology and Hydrology.
- Morgan, P. B. 2004 Soybean's future: photosynthesis, sucrose transport, dry mass accumulation and yield in a changing atmosphere. Ph.D. thesis, University of Illinois at Urbana-Champaign, USA, 191 pp.
- Morgan P.B, Ainsworth E.A, Long S.P. How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant Cell Environ. 2003;26:1317–1328. doi:10.1046/j.0016-8025.2003.01056.x [Google Scholar]
- Morgan P.B, Bollero G.A, Nelson R.L, Dohleman F.G, Long S.P. Smaller than predicted increase in above-ground net primary production and yield of field-grown soybean was found when [CO2] is elevated in fully open-air. Global Change Biol. 2005;11:1856–1865. doi:10.1111/j.1365-2486.2005.001017.x [Google Scholar]
- Nagy J, Lewin K.F, Hendrey G.R, Lipfert F.W, Daum M.L. Face facility engineering performance in 1989. Crit. Rev. Plant Sci. 1992;11:165–185. [Google Scholar]
- Nie G.Y, Hendrix D.L, Webber A.N, Kimball B.A, Long S.P. Increased accumulation of carbohydrates and decreased photosynthetic gene transcript levels in wheat grown at an elevated CO2 concentration in the field. Plant Physiol. 1995a;108:975–983. doi: 10.1104/pp.108.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie G.Y, Long S.P, Garcia R.L, Kimball B.A, Lamorte R.L, Pinter P.J, Wall G.W, Webber A.N. Effects of free-air CO2 enrichment on the development of the photosynthetic apparatus in wheat, as indicated by changes in leaf proteins. Plant Cell Environ. 1995b;18:855–864. [Google Scholar]
- Ottman M.J, Kimball B.A, Pinter P.J, Wall G.W, Vanderlip R.L, Leavitt S.W, LaMorte R.L, Matthias A.D, Brooks T.J. Elevated CO2 increases sorghum biomass under drought conditions. New Phytol. 2001;150:261–273. doi:10.1046/j.1469-8137.2001.00110.x [Google Scholar]
- Parry M, Rosenzweig C, Iglesias A, Fischer G, Livermore M. Climate change and world food security: a new assessment. Global Environ. Change—Hum. Policy Dimens. 1999;9:S51–S67. doi:10.1016/S0959-3780(99)00018-7 [Google Scholar]
- Parry M.L, Rosenzweig C, Iglesias A, Livermore M, Fischer G. Effects of climate change on global food production under SRES emissions and socio-economic scenarios. Global Environ. Change—Hum. Policy Dimens. 2004;14:53–67. doi:10.1016/j.gloenvcha.2003.10.008 [Google Scholar]
- Peart R.M, Jones J.W, Curry R.B, Boote K.J, Allen L.H. Impact of climate change on crop yield in the southeastern USA. In: Smith J.B, Tirpak D.A, editors. The potential effects of global climate change on the United States, Appendix C. Report to Congress. US Environmental Protection Agency; Washington, DC: 1989. EPA-230-05-89-050. [Google Scholar]
- Pinter P.J, et al. Free-air CO2 enrichment (FACE): blower effects on wheat canopy microclimate and plant development. Agric. Forest Meteorol. 2000;103:319–333. doi:10.1016/S0168-1923(00)00150-7 [Google Scholar]
- Prather M, et al. Atmospheric chemistry and greenhouse gases. In: Houghton J.T, Ding Y, Griggs D.J, Noguer M, Van der Linder P.J, Dai X, Maskell K, Johnson C.A, editors. Climate change 2001: the scientific basis Contributions of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge University Press; Cambridge, UK: 2001. pp. 239–287. [Google Scholar]
- Prentice I.C, et al. The carbon cycle and atmospheric carbon dioxide. In: Houghton J.T, Ding Y, Griggs D.J, Noguer M, Van der Linder P.J, Dai X, Maskell K, Johnson C.A, editors. Climate change 2001: the scientific basis Contributions of working group I to the third assessment report of the intergovernmental panel on climate change. Cambridge University Press; Cambridge, UK: 2001. pp. 183–238. [Google Scholar]
- Pritchard S.G, Amthor J.S. Food Products Press; New York: 2005. Crops and environmental change: an introduction to effects of global warming, increasing atmospheric CO2 and O3 concentrations, and soil salinization on crop physiology and yield. [Google Scholar]
- Ritchie J.T, Otter S. Description and performance of CERES-wheat: a user oriented wheat yield model. In: Willis O.W, editor. ARS wheat yield project. Agricultural Research Service, Department of Agriculture; Washington, DC: 1985. pp. 159–175. [Google Scholar]
- Ritchie J.T, Singh U, Godwin D, Hunt L. International Fertilizer Development Centre; Muscle Shoals, AL: 1989. A users guide to CERES-maize v2.10. [Google Scholar]
- Roberts M.J, Long S.P, Tieszen L.L, Beadle C.L. Measurement of plant biomass and net primary production of herbaceous vegetation. In: Hall D.O, Scurlock J.M.O, Bolhar-Nordenkampf H.R, Leegood R.C, Long S.P, editors. Photosynthesis and production in a changing environment: a field and laboratory manual. Chapman & Hall; London: 1993. pp. 1–21. [Google Scholar]
- Rosenzweig C, Iglesias A. The use of crop models for international climate change impact assessment. In: Tsuji G.Y, Hoogenboom G, Thornton P.K, editors. Understanding options for agricultural production. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1998. pp. 267–292. [Google Scholar]
- Singh U, Ritchie J.T, Godwin D. International Fertilizer Development Center; Muscle Shoals, AL: 1989. A users guide to CERES-rice. [Google Scholar]
- Stockle C.O, Williams J.R, Rosenberg N.J, Jones C.A. A method for estimating the direct and climatic effects of rising atmospheric carbon dioxide on growth and yield of crops. Part 1—modification of the EPIC model for climate change analysis. Agric. Syst. 1992;38:225–238. doi:10.1016/0308-521X(92)90067-X [Google Scholar]
- Thomas R.B, Strain B.R. Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon-dioxide. Plant Physiol. 1991;96:627–634. doi: 10.1104/pp.96.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A.M, Brown R.A, Rosenberg N.J, Izaurralde R.C, Benson V. Climate change impacts for the conterminous USA: an integrated assessment—Part 3. Dryland production of grain and forage crops. Climatic Change. 2005;69:43–65. doi:10.1007/s10584-005-3612-9 [Google Scholar]
- Wall G.W, et al. Acclimation response of spring wheat in a free-air CO2 enrichment (FACE) atmosphere with variable soil nitrogen regimes. 2. Net assimilation and stomatal conductance of leaves. Photosynth. Res. 2000;66:79–95. doi: 10.1023/A:1010646225929. doi:10.1023/A:1010646225929 [DOI] [PubMed] [Google Scholar]
- Wang X.P, Mauzerall D.L. Characterizing distributions of surface ozone and its impact on grain production in China, Japan and South Korea: 1990 and 2020. Atmos. Environ. 2004;38:4383–4402. doi:10.1016/j.atmosenv.2004.03.067 [Google Scholar]
- Whitehead D, et al. Performance of large open-top chambers for long-term field investigations of tree response to elevated carbon dioxide concentration. J. Biogeogr. 1995;22:307–313. [Google Scholar]


