Abstract
Reactive oxygen species (ROS) have an important role in various physiological processes including host defence, mitogenesis, hormone biosynthesis, apoptosis and fertilization. Currently, the most characterized ROS-producing system operates in phagocytic cells, where ROS generated during phagocytosis act in host defence. Recently, several novel homologues of the phagocytic oxidase have been discovered and this protein family is now designated as the NOX/DUOX family of NADPH oxidases. NOX/DUOX enzymes function in a variety of tissues, including colon, kidney, thyroid gland, testis, salivary glands, airways and lymphoid organs. Importantly, members of the enzyme family are also found in non-mammalian species, including Caenorhabditis elegans and sea urchin. The physiological functions of novel NADPH oxidase enzymes are currently largely unknown. This review focuses on our current knowledge about dual oxidases.
Keywords: dual oxidases, DUOX, NOX, NADPH oxidase, reactive oxygen species, hydrogen peroxide
1. Introduction
When phagocytic cells, including neutrophil granulocytes, meet foreign pathogens they produce large quantities of reactive oxygen, which contributes to the killing of the invading micro-organisms. In phagocytic cells, the reactive oxygen species (ROS) precursor, superoxide, is produced by the NADPH oxidase enzyme complex (Quinn & Gauss 2004). NADPH oxidase catalyses the transfer of one electron from NADPH to molecular oxygen, resulting in the formation of superoxide. The phagocytic NADPH oxidase (phox) is an enzyme complex composed of the membrane-bound cytochrome b558, several cytosolic factors (p47phox, p67phox, p40phox) and the small GTPase Rac2. During the activation of the enzyme, cytosolic components translocate to the membrane initiating the production of superoxide. Over the years several details were revealed about the molecular architecture of the phagocytic oxidase (Takeya et al. 2003; Quinn & Gauss 2004). It was also known for years that non-phagocytic cells can also produce reactive oxygen, although the detected levels of ROS were usually much lower when compared to the output of the phagocytic oxidase. ROS are now considered important components of cellular signalling pathways and they also have a well-established role in thyroid hormone biosynthesis and fertilization (Lambeth 2004). The enzymatic basis of non-phagocytic ROS production was poorly understood for a long time; however, with the recent expansion of genomic databases this situation has changed rapidly. In the past few years, beginning in 1999, several groups identified homologues of gp91phox and this family of proteins is now designated as the NOX/DUOX family of NADPH oxidases. It is highly likely that the dedicated function of this protein family is ROS production in various tissues (Geiszt & Leto 2004; Lambeth 2004). The family now has seven members, including NOX1, NOX2 (formerly known as gp91phox), NOX3, NOX4, NOX5, DUOX1 and DUOX2. Recent reviews provide detailed characterization of this protein family (Bokoch & Knaus 2003; Geiszt & Leto 2004; Lambeth 2004), therefore this review focuses solely on our current understanding of the structure and function of dual oxidases.
2. Structural features of dual oxidases (DUOX1 and DUOX2)
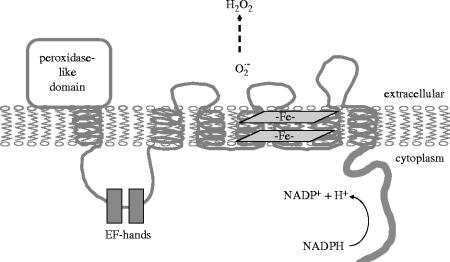
Dual oxidases were originally described as thyroid oxidases because they were first described in thyroid gland (Dupuy et al. 1999; De Deken et al. 2000). Edens et al. (2001), who reported the cloning of homologous sequences from Caenorhabditis elegans, suggested the DUOX (DUal OXidase) nomenclature, based on the structural features of the proteins. The genes for DUOX1 and DUOX2 are located on chromosome 15, where they are arranged in a head-to-head configuration, separated by a 16 kb region (Pachucki et al. 2004). The DUOX1 gene is approximately 36 kb containing 35 exons, while the DUOX2 gene is 22 kb and contains 34 exons. Human DUOX1 and DUOX2 proteins contain 1551 and 1548 amino acids, respectively, and show 83% sequence similarity. The NADPH oxidase portion of the protein shows 47% similarity to gp91phox (NOX2). Besides their NADPH oxidase portions, these large proteins also have N-terminal peroxidase-homology domains, which show high homology to other peroxidases (figure 1). Peroxidases are heme-containing proteins; however, the peroxidase-like domains of DUOX proteins probably do not bind heme, because they lack key amino acid residues essential for heme binding that are present in highly conserved positions in all other peroxidases, including thyroperoxidase (figure 2). Between the peroxidase homology and the NADPH oxidase domains are two EF-hand motifs, suggesting that calcium ions regulate their enzymatic activity. The presence of EF-hands explains the stimulatory effect of calcium ions on ROS production in thyroid and airway epithelial cells (Dupuy et al. 1988; Geiszt et al. 2003). The presence of a long intracellular loop, linking the first transmembrane domain to the NADPH oxidase portion of the protein, suggests that in addition to calcium binding, other functions are likely associated with this part of DUOX.
Figure 1.
Membrane topology and domain structure of dual oxidases.
Figure 2.
Comparison of amino acid sequences of thyroperoxidase (TPO) and the peroxidase-like domain of human DUOX1. Positions of conserved histidines in the TPO sequence are marked by arrows.
3. Dual oxidases in the thyroid gland
In the thyroid gland, DUOX proteins produce hydrogen peroxide, which is then utilized in the thyroperoxidase-mediated oxidation of iodide into reactive compounds. DUOX proteins are localized in the apical poles of thyroid cells exposed to the colloid of thyroid follicles, where they co-localize with thyroperoxidase (De Deken et al. 2000). This localization is consistent with their suggested role in hormone biosynthesis. Both DUOX isoforms are present in the thyroid, although we currently do not understand the functional significance of the presence of two highly homologous isoforms in the same tissue. Beside the stimulatory effect of calcium, very little is known about the regulation of DUOX enzymes. A major obstacle in the investigation of DUOX function is the absence of a cell model where the activity of heterologously expressed DUOX enzymes could be studied. When DUOX enzymes are expressed in various mammalian cell lines, they remain within the endoplasmic reticulum (ER) and do not express enzymatic activity (De Deken et al. 2002). Morand et al. (2004) used truncated DUOX mutants to examine the mechanism of ER retention. By constructing proteins that contained the peroxidase-like domain and various portions of the first intracellular loop, they identified retention signals between amino acids 596–685 in the human DUOX protein and in the first intracellular loop of the pig DUOX protein. They also found several cysteine residues in the peroxidase-like domain and in the intracellular loop, which were necessary for the maturation of DUOX2. It is likely that additional protein partners, present only in differentiated epithelial cells, are necessary for the proper maturation and transport of DUOX proteins to the plasma membrane. NOX proteins, including NOX2, NOX1 and NOX4, were shown to interact with p22phox, and in the case of NOX2, complex formation is essential for the NADPH oxidase activity (Ambasta et al. 2004; Quinn & Gauss 2004). In co-immunoprecipitation experiments, Wang et al. (2005) showed that DUOX proteins also interact with p22phox; however, the functional consequence of this interaction is unclear. It is noteworthy that lower organisms, including C. elegans and Drosophila melanogaster, also express DUOX homologues, but their genomes do not appear to encode homologues of p22phox (M. Geiszt et al., unpublished observation 2005). This observation suggests that DUOX proteins function in the absence of p22phox. Using yeast two-hybrid screen for DUOX-interacting proteins, Wang et al. (2005) has isolated EFP1, a thioredoxin-related protein. The authors also demonstrated interaction between DUOX and EFP1 in transfected cell models, but it is still unknown whether the two proteins interact in thyroid cells. In the absence of a cell model where heterologously expressed DUOX is active, it is unclear whether this interaction has any effect on ROS output or could regulate intracellular trafficking of DUOX. Rac proteins have an essential role in the regulation of NOX2 activity and probably also regulate NOX1 (Takeya et al. 2003). Fortemaison et al. (2005) have used Clostridium difficile toxin B to inhibit Rac function in a thyroid cell line, although this treatment had no effect on carbachol-induced H2O2 production, excluding a regulatory role of Rac in this process. However, this study did not directly address the question of whether the source of carbachol-induced H2O2 production was solely DUOX.
While an agonist-induced increase in intracellular calcium concentration can acutely stimulate the enzymatic activity of DUOX, changes in gene expression levels probably also modify the ROS output of DUOX-expressing cells. In dog thyrocytes, increasing the level of cAMP by forskolin stimulated the expression of DUOX enzymes, but human thyrocytes did not respond to this agonist (De Deken et al. 2000). Functional studies on human DUOX1 and DUOX2 promoters showed both promoters to be active in differentiated thyroid cell lines (PC-Cl3 and FRTL5); however, in contrast to the promoters of other thyroid-specific genes, such as thyroglobulin, the DUOX promoters were also active in Hela cells and Rat-2 fibroblasts (Pachucki et al. 2004).
The essential role for DUOX2 in thyroid hormone biosynthesis was recently proven by the identification of patients who suffer from hypothyroidism due to mutations in the DUOX2 gene. Moreno et al. (2002) have identified one patient who had severe deficiency in thyroid hormone biosynthesis explained by a homozygous nonsense mutation in the DUOX2 gene, deleting all functional domains of the protein. Three other patients had milder form of hypothyroidism caused by heterozygous mutations in the DUOX2 gene. The fact that DUOX2 deficiencies result in hypothyroidism suggests that DUOX1 and DUOX2 each have unique roles in the process of hormone formation. Besides chronic granulomatous disease, which is caused by deficiency of the phagocytic oxidase, DUOX2-deficient hypothyroidism is the only known disease that is caused by the absence of a specific NOX/DUOX enzyme.
4. Dual oxidases in extracellular matrix modification
Hydrogen peroxide also has a role in cross-linking proteins during the formation of the extracellular matrix. Recent papers suggest that at least in lower organisms, such as C. elegans and sea urchin, DUOX enzymes provide the oxidative basis of this process. Edens et al. (2001) identified genes encoding two DUOX isozymes in the genome of C. elegans. They have cloned the cDNA for one of the homologues and named it Ce-DUOX1. Ce-DUOX1 encodes a 1497 amino acid protein, which shows close homology to its human orthologues. They also identified a second transcript predicting a 1313 amino acid protein that lacks domains considered essential for NADPH oxidase function. According to gene annotation data published on www.wormbase.org, the Ce-DUOX2 gene also encodes a full protein comprising 1503 amino acids (M. Geiszt et al., unpublished observation). What could be the function of DUOX proteins in an organism that lacks thyroid hormone synthesis? Edens et al. used RNA interference to address this question. This technique involved injection of double-stranded, gene-specific RNA (dsRNA) into gonads of C. elegans. Worms injected with DUOX-specific double-stranded RNA displayed phenotypes including blisters and other morphological defects resulting from defective cuticle biosynthesis. Similar phenotypes in C. elegans have been identified in association with mutations in the collagen biosynthetic pathway. In accordance with the observed defects, the authors could detect DUOX expression in the hypodermal cells of the worms. During a systemic functional analysis of the C. elegans genome by RNAi feeding, Kamath et al. (2003) observed similar phenotypes. Since the two C. elegans DUOX isoforms are highly similar at the mRNA level, RNA interference would not discriminate between them and additional experiments are required to identify which isoform is critical in cuticle biosynthesis.
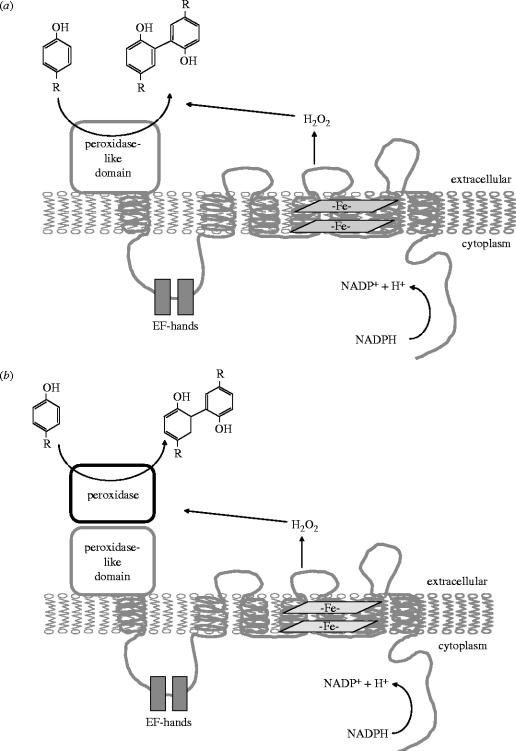
Cross-linking of proteins during cuticle biosynthesis occurs through the formation of di- and trityrosine linkages. Peroxidases, including the sea urchin ovoperoxidases and mammalian peroxidases (thyroperoxidase, myeloperoxidase and lactoperoxidase), have been described as enzymes capable of catalysing this reaction in the presence of hydrogen peroxide. When the peroxidase-like domains of Ce-DUOX1 and human DUOX1 were expressed in Escherichia coli, they both catalysed the formation of di- and trityrosine residues. This is an interesting observation, since these domains lack residues considered critical for heme binding, thus, they cannot be considered ‘classical’ peroxidases. Their observed activity seems to challenge the classical view of peroxidase chemistry, in which heme binding is essential for the enzymatic activity. Thus, future experiments should compare the enzymatic activity of DUOX peroxidase-like domains with other heme-containing peroxidases. According to the model suggested by Edens et al., in a coupled reaction the NADPH oxidase portion of dual oxidases generates hydrogen peroxide for the cross-linking reaction, which is then catalysed by the peroxidase-like portion of the molecule (figure 3a). To demonstrate the important role for DUOX enzymes in dityrosine formation, the cuticles of DUOX-deficient worms contained significantly less di- and trityrosines when compared the normal, untreated animals (Edens et al. 2001). However, this phenotype is also consistent with a model in which the DUOX enzymes are responsible solely for H2O2 production and a distinct peroxidase would catalyse the cross-linking (coupling) reaction (figure 3b).
Figure 3.
Alternative models for the role of DUOX proteins in protein cross-linking reactions.
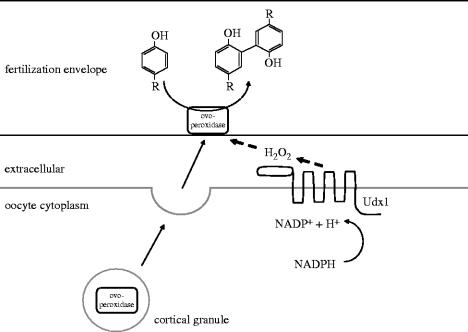
Another interesting example of the extracellular matrix-modifying activity of DUOX enzymes is the process of sea urchin fertilization. Fertilization envelope formation in echinoderm eggs is initiated by an increase in intracellular Ca2+ that induces the exocytosis of secretory granules. The content of cortical granules associates with the vitelline membrane, forming the soft fertilization envelope. Among the secreted proteins there is ovoperoxidase, which uses hydrogen peroxide to cross-link proteins through dityrosine formation, thus inducing hardening of the envelop (Heinecke & Shapiro 1992). A hardened, stable fertilization envelope is important not only to prevent polyspermy, but also for the protection of the developing embryo. An increased oxygen consumption that parallels hydrogen peroxide production was first described almost a century ago; however, the biochemical basis of hydrogen peroxide formation was unknown for a long time. Early experiments have suggested that hydrogen peroxide generated during fertilization does not originate from the mitochondria, but a separate, calcium-dependent NADPH oxidase enzyme exists, which would be similar to the enzyme complex of neutrophil granulocytes (Heinecke & Shapiro 1992).
This assumption was recently proven by Wong et al. (2004) who identified urchin dual oxidase (Udx1), a DUOX isozyme in sea urchins Strongylocentrotus purpuratus and Lytechinus variegatus. Udx1 mRNA accumulates in developing oocytes and the protein is present on the egg surface. Interestingly, the majority of the Udx1 protein pool is present within intracellular yolk-enriched fractions. Microinjection of an antibody raised against the NADPH-binding region of Udx1, suppressed calcium ionophore-induced hydrogen peroxide production, suggesting that Udx1 was responsible for the respiratory burst of fertilization. Similar to its C. elegans and mammalian homologues, the peroxidase-like domain of Udx1 lacks conserved histidines that would be necessary for heme binding, therefore, it seems likely that the primary function of this enzyme is the production of hydrogen peroxide, which is then utilized in the ovoperoxidase-catalysed cross-linking reaction (figure 4). Wong et al. suggested that the peroxidase-like domain might act as a catalase-like protein, thus shielding the egg from the toxic effect of hydrogen peroxide. In the absence of apparent sequence homology between the peroxidase-like domain and catalase, this idea requires further investigation.
Figure 4.
Sea urchin dual oxidase (Udx1) produces hydrogen peroxide during sea urchin fertilization.
5. Dual oxidases on mucosal surfaces. A potential role in host defence
During thyroid hormone biosynthesis, iodide is oxidized by thyroperoxidase into reactive iodide species. In a similar host defence system present in saliva, tears and milk, lactoperoxidase (LPO) oxidizes thiocyanate (SCN−) and iodide (I−) into reactive compounds with potent antimicrobial activity. The lactoperoxidase system was identified many years ago (Reite & Oram 1967), but the H2O2 source in this system had not been known. Since there are functional similarities between the activities of thyroperoxidase and lactoperoxidase, we examined the roles of DUOX1 and DUOX2 as potential H2O2 sources for LPO.
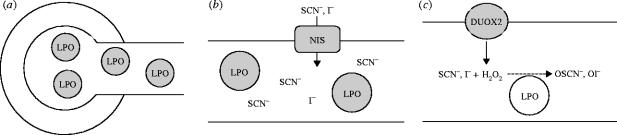
We studied DUOX expression in salivary glands by Northern blotting and by in situ hybridization (Geiszt et al. 2003). We could detect DUOX2 mRNA in human and rat salivary glands. In situ hybridization on rat submandibular salivary glands revealed that DUOX2 is specifically expressed in terminal portions of the salivary duct system, within epithelial cells of intralobal, interlobal and main excretory ducts. This expression pattern suggests that H2O2 is secreted into saliva during the final steps of saliva formation. We also detected DUOX2 in the human parotid gland by in situ hybridization, where we found that the DUOX2 transcript is present in terminal ducts, similar to results seen in rat salivary gland. The mRNA of lactoperoxidase was detected in the serous acini of the gland. The sodium–iodide symporter (NIS), which also transports thiocyanate, was previously detected in this gland (Riedel et al. 2001), although the mechanisms of I− and SCN− transport into saliva is still poorly characterized. Our in situ hybridization results suggested that this transport occurs within smaller, intercalated ducts, where the highest NIS mRNA levels were detected. Based on the observed expression patterns and the enzymatic activity of LPO and DUOX, we suggested the following model for the DUOX–LPO system: LPO is secreted in the serous acini of the gland and thiocyanate is added during the early stage of saliva formation. LPO oxidizes thiocyanate into hypothiocyanate only in late stages of saliva formation, as it encounters hydrogen peroxide just prior to delivery into the oral cavity (figure 5). We also studied DUOX2 levels in several other human gastrointestinal tissues and detected DUOX2 expression in the caecum, ascending colon and rectum (Geiszt et al. 2003). In situ hybridization experiments with the rat rectum showed DUOX2 expression in epithelial cells predominantly within the lower half of the glands. El Hassani et al. (2005) recently studied DUOX2 expression along the entire gastrointestinal tract and found particularly high DUOX2 expression in the caecum and sigmoidal colon. Immunohistochemical experiments revealed that DUOX2 was localized predominantly at apical poles of the cells, similar to that observed in the thyroid gland. Recent work has documented high LPO expression in sheep airways, where it comprises as much as 1% of soluble secreted protein (Gerson et al. 2000). Using peroxidase inhibitors, they showed that inhibition of airway LPO reduced the antibacterial activity of treated airways. We used in situ hybridization to identify the site of LPO expression and showed high LPO expression within the submucosal glands, where it was localized to the serous acini of the glands.
Figure 5.
A model of the expression and assembly of the DUOX–lactoperoxidase system in salivary glands. LPO is synthesized in early stages of saliva formation in serous acini (a), anionic LPO substrates are transported by the sodium iodide symporter in intermediate stages (b), and the system is completed in late stages, where DUOX provides H2O2 within terminal ducts (c).
We were interested in whether DUOX enzymes were also expressed in the trachea and the bronchium. We detected high DUOX1 mRNA expression within the pseudostratified epithelium of trachea and bronchi, suggesting that DUOX1 is a likely H2O2 source for the LPO system in these tissues. Using a DUOX-specific antibody, Schwarzer et al. (2004) later localized DUOX expression to the apical pole of the cells and stereocilia of the cells were also stained positive for DUOX. We tested cultured normal human bronchial epithelial (NHBE) cells for H2O2 release in response to the calcium ionophore ionomycin. We applied this stimulus because thyroid gland H2O2 production is stimulated by calcium (consistent with the presence of calcium-binding, EF-hands in both DUOX isozymes). NHBE cells produced significant H2O2 in response to ionomycin, which was shown to be diphenylene iodonium sensitive. Treatment of these cells with the DUOX1-specific antisense oligonucleotides significantly reduced the H2O2 output of the cells. Forteza et al. (2005) used ATP as a stimulus and observed H2O2 production, which was comparable to the output stimulated by calcium ionophores.
6. Conclusion
Dual oxidases appear to be ancient players in the story of reactive oxygen. In lower species such as C. elegans and different sea urchin species, their primary function appears to be in the stabilization of extracellular matrix through oxidative cross-linking. The fact that C. elegans expresses two highly similar isoforms of DUOX suggests that DUOX enzymes have some other, hitherto unknown, functions in this organism. In mammals, the best-understood function of DUOX enzymes is in providing the oxidative basis of thyroid hormone biosynthesis. It is interesting that coupling of tyrosine residues also occurs during thyroid hormone synthesis, and this reaction is probably catalysed by thyroperoxidase, an enzyme also responsible for the formation of reactive iodide species. It is noteworthy that expression of DUOX enzymes in mammals is not restricted to the thyroid gland, but also in epithelial cells of exocrine glands and mucosal surfaces, including the gastrointestinal and respiratory tracts. Mucosal surface hydrogen peroxide production by DUOX enzymes might have a role in stabilization of the epithelial barrier through reactions similar to those observed in the cuticle of C. elegans. However, these enzymes can supply hydrogen peroxide to LPO, which has an important role in antimicrobial host defence in several exocrine secretions. The innate oropharyngeal and airway defence systems have important roles in maintaining sterility in the respiratory tract. We believe that dual oxidases provide one more microbicidal arm to the arsenal of mucosal defences, with properties uniquely adapted to this environment. Unlike in phagocytes, the reactive oxidants generated by mucosal epithelia (in this case H2O2) are not contained, but are released into an extracellular space. The abundant LPO in these secretions generates less potent antimicrobial agents, primarily hypothiocyanite, rather than hypochlorite, which are less toxic to host tissues.
Overproduction of ROS also has a role in the pathogenesis of several pulmonary diseases, such as asthma and respiratory distress syndromes, although the exact molecular mechanisms underlying these processes are poorly understood. To date, most work in these areas has focused on the roles of neutrophil and eosinophil granulocytes or environmental agents as the sources of toxic ROS. The discovery of DUOX enzymes in the bronchial epithelium provides novel mediators of these disease processes.
Acknowledgments
Experimental work in the authors' laboratory was financially supported by grants from the Hungarian Research Fund (OTKA 042573) and the Cystic Fibrosis Foundation. M.G. is recipient of a Wellcome Trust International Senior Fellowship.
Footnotes
One contribution of 18 to a Theme Issue ‘Reactive oxygen species in health and disease’.
References
- Ambasta R.K, Kumar P, Griendling K.K, Schmidt H.H, Busse R, Brandes R.P. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J. Biol. Chem. 2004;279:45 935–45 941. doi: 10.1074/jbc.M406486200. 10.1074/jbc.M406486200 [DOI] [PubMed] [Google Scholar]
- Bokoch G.M, Knaus U.G. NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. 10.1016/S0968-0004(03)00194-4 [DOI] [PubMed] [Google Scholar]
- De Deken X, Wang D, Many M.C, Costagliola S, Libert F, Vassart G, Dumont J.E, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J. Biol. Chem. 2000;275:23 227–23 233. doi: 10.1074/jbc.M000916200. 10.1074/jbc.M000916200 [DOI] [PubMed] [Google Scholar]
- De Deken X, Wang D, Dumont J.E, Miot F. Characterization of ThOX proteins as components of the thyroid H(2)O(2)-generating system. Exp. Cell Res. 2002;273:187–196. doi: 10.1006/excr.2001.5444. 10.1006/excr.2001.5444 [DOI] [PubMed] [Google Scholar]
- Dupuy C, Deme D, Kaniewski J, Pommier J, Virion A. Ca2+ regulation of thyroid NADPH-dependent H2O2 generation. FEBS Lett. 1988;233:74–78. doi: 10.1016/0014-5793(88)81358-9. 10.1016/0014-5793(88)81358-9 [DOI] [PubMed] [Google Scholar]
- Dupuy C, Ohayon R, Valent A, Noel-Hudson M.S, Deme D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cDNAs. J. Biol. Chem. 1999;274:37 265–37 269. doi: 10.1074/jbc.274.52.37265. 10.1074/jbc.274.52.37265 [DOI] [PubMed] [Google Scholar]
- Edens W.A, et al. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell. Biol. 2001;154:879–891. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hassani R.A, et al. Dual oxidase2 is expressed all along the digestive tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G933–G942. doi: 10.1152/ajpgi.00198.2004. 10.1152/ajpgi.00198.2004 [DOI] [PubMed] [Google Scholar]
- Fortemaison N, Miot F, Dumont J.E, Dremier S. Regulation of H2O2 generation in thyroid cells does not involve Rac1 activation. Eur. J. Endocrinol. 2005;152:127–133. doi: 10.1530/eje.1.01815. 10.1530/eje.1.01815 [DOI] [PubMed] [Google Scholar]
- Forteza R, Salathe M, Miot F, Forteza R, Conner G.E. Regulated hydrogen peroxide production by duox in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. 10.1165/rcmb.2004-0302OC [DOI] [PubMed] [Google Scholar]
- Geiszt M, Leto T.L. The Nox family of NAD(P)H oxidases: host defense and beyond. J. Biol. Chem. 2004;279:51 715–51 718. doi: 10.1074/jbc.R400024200. 10.1074/jbc.R400024200 [DOI] [PubMed] [Google Scholar]
- Geiszt M, Witta J, Baffi J, Lekstrom K, Leto T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- Gerson C, et al. The lactoperoxidase system functions in bacterial clearance of airways. Am. J. Respir. Cell Mol. Biol. 2000;22:665–671. doi: 10.1165/ajrcmb.22.6.3980. [DOI] [PubMed] [Google Scholar]
- Heinecke J.W, Shapiro B.M. The respiratory burst oxidase of fertilization. A physiological target for regulation by protein kinase C. J. Biol. Chem. 1992;267:7959–7962. [PubMed] [Google Scholar]
- Kamath R.S, et al. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. 10.1038/nature01278 [DOI] [PubMed] [Google Scholar]
- Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- Morand S, et al. Targeting of the dual oxidase 2 N-terminal region to the plasma membrane. J. Biol. Chem. 2004;279:30 244–30 251. doi: 10.1074/jbc.M405406200. 10.1074/jbc.M405406200 [DOI] [PubMed] [Google Scholar]
- Moreno J.C, Bikker H, Kempers M.J, van Trotsenburg A.S, Baas F, de Vijlder J.J, Vulsma T, Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N. Engl. J. Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. 10.1056/NEJMoa012752 [DOI] [PubMed] [Google Scholar]
- Pachucki J, Wang D, Christophe D, Miot F. Structural and functional characterization of the two human ThOX/Duox genes and their 5′-flanking regions. Mol. Cell. Endocrinol. 2004;214:53–62. doi: 10.1016/j.mce.2003.11.026. 10.1016/j.mce.2003.11.026 [DOI] [PubMed] [Google Scholar]
- Quinn M.T, Gauss K.A. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J. Leukoc. Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. 10.1189/jlb.0404216 [DOI] [PubMed] [Google Scholar]
- Reite B, Oram J.D. Bacterial inhibitors in milk and other biological fluids. Nature. 1967;216:328–330. doi: 10.1038/216328a0. [DOI] [PubMed] [Google Scholar]
- Riedel C, Dohan O, De la Vieja A, Ginter C.S, Carrasco N. Journey of the iodide transporter NIS: from its molecular identification to its clinical role in cancer. Trends Biochem. Sci. 2001;26:490–496. doi: 10.1016/s0968-0004(01)01904-1. 10.1016/S0968-0004(01)01904-1 [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Machen T.E, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J. Biol. Chem. 2004;279:36 454–36 461. doi: 10.1074/jbc.M404983200. 10.1074/jbc.M404983200 [DOI] [PubMed] [Google Scholar]
- Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 2003;278:25 234–25 246. doi: 10.1074/jbc.M212856200. 10.1074/jbc.M212856200 [DOI] [PubMed] [Google Scholar]
- Wang D, De D.X, Milenkovic M, Song Y, Pirson I, Dumont J.E, Miot F. Identification of a novel partner of duox: EFP1, a thioredoxin-related protein. J. Biol. Chem. 2005;280:3096–3103. doi: 10.1074/jbc.M407709200. 10.1074/jbc.M407709200 [DOI] [PubMed] [Google Scholar]
- Wong J.L, Creton R, Wessel G.M. The oxidative burst at fertilization is dependent upon activation of the dual oxidase Udx1. Dev. Cell. 2004;7:801–814. doi: 10.1016/j.devcel.2004.10.014. 10.1016/j.devcel.2004.10.014 [DOI] [PubMed] [Google Scholar]