Abstract
Drugs currently known as calcium channel blockers (CCB) were initially called calcium antagonists because of their ability to inhibit calcium-evoked contractions in depolarized smooth muscles. Blocking the entry of calcium reduces the active tone of vascular smooth muscle and produces vasodilatation. This pharmacological property has been the basis for the use of CCBs in the management of hypertension and coronary heart disease. A major question is whether drugs reducing blood pressure have other effects that help prevent the main complications of hypertension, such as atherosclerosis, stroke, peripheral arterial disease, heart failure and end-state renal disease. Experimental studies that focus on this question are reviewed in the present paper.
Keywords: calcium channel blockers, calcium antagonists, hypertension, atherosclerosis, antioxidant effects
1. Introduction
The best-identified targets of calcium channel blockers (CCB) are Ca channels located at the level of the plasma membrane and operated by cell depolarization. Those are named voltage-operated calcium channels (VOC). In resting cells those channels are usually closed; opening those channels with various stimuli allows the entry of calcium ions (Ca2+) according to their electrochemical gradient. Prototypes of those agents, initially named calcium antagonists, were shown to inhibit external calcium-evoked contractions in depolarized arteries, producing dose–effect curves similar to those observed in studies on the inhibition of neurotransmitter action by specific antagonists (Godfraind & Kaba 1969a). This pharmacological property has allowed the identification of a class of agents with different chemical structures (figure 1). For the management of hypertension, three chemical types represent this class of agents: phenylalkylamines (PAA; e.g. verapamil), benzothiazepines (BTZ; e.g. diltiazem) and dihydropyridines (DHP; e.g. nifedipine). Drugs belonging to the diphenylpiperazine group are used for treating neurological disorders such as migraines, neuropathic pain, dementia and subarachnoid haemorrhage; this group will not be considered in this review that deals with the effects on hypertension and atherosclerosis.
Figure 1.
Chemical structures of the major families of calcium channel blockers as represented by their lead compound.
2. Early steps to the identification of calcium channel blockers
Ringer reported the need for calcium in the maintenance of cellular activity as early as 1883. Some years later, Stiles observed that calcium activates smooth muscle contraction (Ringer 1883; Stiles 1901). The key role of calcium in intracellular functions was not perceived until 70 years later by Kamada in Japan (Kamada & Kinosita 1943) and Heilbrunn in the United States (Heilbrunn & Wiercinski 1947).
Nowadays, it is recognized that calcium is responsible for regulating a wide range of cellular processes and is generally considered the ubiquitous second messenger. At the beginning of life, it mediates the process of fertilization. As cells differentiate to perform specific functions, Ca2+ is regulating processes as diverse as muscle contraction, exocytosis, energy metabolism, chemotaxis, neurotransmission and synaptic plasticity during learning and memory. It is also involved in pathological processes such as cardiac hypertrophy and vascular hypertrophy. Irreversible damage that occurs during cardiac or cerebral ischaemia results from prolonged elevations of [Ca2+]i; the intracellular level of Ca2+ in resting cells is normally held within a narrow range of 20–100 nmol l−1. It is believed that dysregulation of this homoeostasis has pathological consequences in cardiovascular diseases, such as hypertension, atherosclerosis, coronary insufficiency and cardiac failure. The mechanisms involved are multiple and complex and, therefore, the therapeutic mechanism of action of drugs used to manage those diseases might involve various targets.
Investigations into the pharmacology of calcium have only begun in the last 40 years. Examination of the literature from the end of the 1950s and the early 1960s reveals that the role of calcium in physiological processes was initially quantified in smooth muscle studies (Evans et al. 1958; Durbin & Jenkinson 1961). Edman & Schild (1961, 1962, 1963), for example, showed that depolarization of a rat uterus rendered the membrane of the smooth muscle cell permeable to extracellular calcium and that the force of the contraction developed by the preparation was proportional to the concentration of calcium in the extracellular fluid. In addition, they provided evidence that acetylcholine-evoked contractions of smooth muscles were dependent on both intracellular and extracellular calcium. Similar observations were made with serotonin, histamine and oxytocin (Yukisada & Ebashi 1961). Since this period, great advances have been made in understanding how smooth muscle contraction is regulated by calcium and this has extensively been reviewed (Ebashi 1980, 1993; Somlyo & Somlyo 1994; Karaki et al. 1997; Karaki 2004).
Studies on the influence of the extracellular concentration of Ca2+ on the action of various pharmacological agents, both stimulatory and inhibitory, have been extended to drugs classically termed antispasmodics because of their capacity to reduce smooth muscle contraction evoked by various spasmogens. The mechanism of action of antispasmodics could not be accounted for by the receptor theory, which implied that agonists and antagonists compete for the same binding site and that a given concentration of a specific antagonist could inhibit the response to only one given agonist. In order to take into account the action of such drugs that are blocked at the same concentration as the contractile response to various spasmogens, it was proposed that they could interfere with a transmembrane mechanism involving Ca2+ translocation activated by the interaction of the various agonists with their own receptors. This hypothesis implied that the targets of the blockers were membranous structures, which, when activated by depolarization, allowed the translocation of Ca2+ from outside to inside the cell. An initial experimental demonstration of this hypothesis was provided by the action of the diphenylpiperazines lidoflazine or cinnarizine which similarly inhibited the contractile response of isolated arteries to various agonists, indicating that they could interfere with the putative mechanism activated by various vasoconstrictors (Godfraind et al. 1966, 1968). According to the indirect evidence available at that time, the calcium activating the contractile machinery subsequent to agonist stimulation could have been translocated from either the outside to the inside of the cell or within the cell from an intracellular store. To determine if the antagonism was related to one or the other of those possible mechanisms, the action of diphenylpiperazines was examined in an adrenaline-evoked contraction of arterial smooth muscle in the presence and absence of calcium. The action of the diphenylpiperazines, which was obvious in the presence of a physiological concentration of Ca2+, was not perceived in the reduced contraction evoked in the absence of extracellular Ca2+. The blocking of this contraction in the presence of extracellular Ca2+ supported the hypothesis that blocking calcium entry is a mechanism of a drug's action (Godfraind & Kaba 1969a,b). For a concentration down to 10−9 M, a dose of cinnarizine dependently displaced the calcium dose–effect curves obtained from depolarized mesenteric arteries to the right, indicating a role for depolarization (figure 2). Cinnarizine also relaxed depolarized smooth muscle that contracted in the presence of Ca2+. To characterize the effect of diphenylpiperazines, Godfraind and colleagues (Godfraind et al. 1966; Godfraind & Polster 1968; Godfraind et al. 1968; Godfraind & Kaba 1969a,b) used the term ‘antagoniste du calcium’ (calcium antagonist), a term coincidentally used by Fleckenstein et al. in their study of the role of Ca2+ in cardiac muscle during use of high energy phosphates in relation to muscle contraction and oxygen consumption (Fleckenstein et al. 1969).
Figure 2.
The effect of cinnarizine on contractions evoked by Ca2+ in K+-depolarized rabbit mesenteric arteries. Arterial preparations were pre-incubated in Ca2+-free physiological solution depolarized in a Ca2+-free, KCl-rich solution and then further incubated with increasing Ca2+ concentrations. Cumulative concentration–effect curves were obtained before (circle) and after (triangle) addition of cinnarizine (C) at the concentrations indicated. Responses are expressed as the percentage of maximal contraction evoked before the addition of cinnarizine. The inhibitory effect of cinnarizine is observed at concentrations as low as 1 nM and resembles the action of antagonists in receptor studies. In view of this similarity, the term ‘calcium antagonist’ was suggested to describe the action of cinnarizine. From Godfraind & Kaba (1969a).
This mode of activation seemed particularly suitable for the radiochemical study of Ca2+ fluxes and the interaction of CCBs with calcium channels in various controlled conditions because depolarization-induced contraction is primarily maintained by a sustained increased in intracellular Ca2+. Noradrenaline or KCl stimulations evoke a 20-fold increase in the rate of calcium influx through channels sensitive to calcium antagonists. This stimulated influx is blocked by calcium antagonists at concentrations reducing the contractile response. The inhibition of contraction may be related to the blocking of voltage-operated Ca2+ channels, since dose–effect curves relating the degree of Ca influx inhibition and contraction to the concentration of the Ca antagonist are superimposed, as has been shown for nifedipine (Godfraind 1983; Godfraind & Miller 1983).
With the various dihydropyridines studied so far, there is a close similarity between IC50 values obtained on intact tissue and Ki values measured in binding studies performed on microsomal preparations. This shows that the pharmacological action of calcium antagonists may be related to specific binding sites. Furthermore, the use of enantiomeric pairs of dihydropyridines has demonstrated the stereoselectivity of the binding, confirming its specificity (Godfraind et al. 1986; Salomone et al. 1991). Using the digitonin shift method to allow the identification of activities associated with the plasma membrane, it was observed that dihydropyridine-specific binding sites are associated with the plasmalemmal membrane of the smooth muscle cell, as shown by the higher amount of specific binding sites in the microsomal fraction. Binding sites have also been identified in mitochondria. Ca2+ enhances this low affinity binding at high ionic strength. This is at variance with the binding sites found on plasmalemma membranes. Furthermore, these mitochondrial sites do not exhibit stereoselectivity and are not involved in the regulation of smooth muscle tone (Salomone et al. 1991).
Tissue depolarization induces a conformational change in the calcium channels, resulting in an increased affinity for dihydropyridines to the level found in isolated membranes. As was shown with isradipine, the Kd value obtained in depolarized arteries is similar to that reported in membrane preparations and is close to the IC50 measured after 35 min of depolarization. Both pharmacological effect and specific binding to intact tissue may show stereospecificity and voltage-dependency, depending on the molecular structure of the blocker (Morel & Godfraind 1987, 1988). The modulated receptor model may help to describe this interaction, assuming that channels can exist in three convertible states: the resting state (when the channel is closed but available for opening) which predominates in polarized cells, the open or activated state (which is promoted by depolarization pulses beyond a certain threshold) and the inactivated state (when the channel is closed but unavailable for opening). The inactivated state is favoured by prolonged depolarization. Most 1,4-dihydropyridines preferentially bind to the inactivated state. If KL and KH are dissociation constants for low-affinity and high-affinity states, respectively, and L is the fraction of channels in the resting state in the absence of the drug, the model predicts that, at any given holding potential, the concentration dependence of drug binding and, thus, of calcium current inhibition follows a simple adsorption isotherm with an apparent dissociation constant that is given by:
Therefore, depolarizing holding potentials that increase the proportion of inactivated channels but fail to open them enhance the pharmacological potency of CCBs. This might account for their selective efficacy in some disease states when the ionic environment of the vasculature is altered, resulting in a tissue-selective vasorelaxation (Morel & Godfraind 1988, 1989).
3. Calcium channel blockers and calcium channels
Calcium channels have been identified by combined electrophysiological and pharmacological techniques and biochemical approaches. The use of the patch-clamp technique by Richard Tsien and his colleagues is a major step in the delineation of the various Ca2+ currents. Richard Tsien and colleagues (Nowycky et al. 1985; Hess et al. 1986; Fox et al. 1987a,b; Tsien & Tsien 1990) identified different calcium currents, which they called L, T and N. Together with molecular studies (Catterall 2000; Catterall et al. 2002), this paved the way to an actual understanding of the role of calcium channels in biology.
There are three major pathways through which extracellular Ca2+ can enter animal cells: receptor-activated Ca2+ channels, ligand-gated Ca2+ channels and VOC. In addition, there is also evidence for more targeted sensory mechanisms of Ca2+ entry: mechanically activated Ca2+ channels, pH-activated Ca channels and temperature-activated Ca channels.
VOCs are activated by a conformational modification of the protein structure which is induced by a change in membrane potential. These channels can vary considerably in their electrophysiological properties, such as voltage-dependencies, activation/inactivation kinetics, sensitivity to extracellular and intracellular ligands, ion selectivity and single channel properties. The different electrophysiological and pharmacological properties of native VOCs led to the description of a number of distinct voltage-operated Ca2+ currents, which have been classified as L-, N-, T-, P-, Q- and R-types according to their sensitivity to membrane potential variation and the time required to reach inactivation (Sher et al. 1991). They are complex proteins composed of four or five distinct subunits, which are encoded by multiple genes. They belong to a gene superfamily of transmembrane proteins, which also includes voltage-operated K+ and Na+ channels. The current classification of VOCs (table 1) is derived from the degree of sequence homology/similarity between the genes coding for the various Ca2+ channels. The channel protein is composed of multiple subunits: the obligatory α1-subunit comprises the Ca2+ pathway. The α1-subunit is the largest constituent of the final protein and incorporates the conduction pore, the voltage sensor and many modulator sites. Thus, the diversity of voltage-operated Ca2+ currents primarily arises from the existence of multiple forms of α1-subunits. High homology between the various cloned α1-subunits suggests that they evolved from a single ancestral gene. The β-, α2δ- and γ-accessory subunits play modulator roles. The α1-subunit is constituted of four homologous domains (I–IV), each of which contains six α-helical transmembrane segments (S1–S6). The S4 segment is rich in positively charged amino acids and it is believed to serve as the voltage sensor. The peptide chain between S5 and S6 (H5 or P-loop) lines the channel pore and constitutes the Ca2+ selectivity filter. The binding sites for dihydropyridines (DHP), phenylalkylamines (PAA), benzothiazepines (BTZ), gating-modifier toxins (T1) and pore-blocking toxins (T2) have been localized at specific sites of the channel protein.
Table 1.
Nomenclatures of voltage-operated calcium channels (Ertel et al. 2000; Catterall et al. 2002).
| type | α1-subunit | splice | current |
|---|---|---|---|
| Cav 1.1 | α1 1.1 | L | |
| Cav 1.2 | α1 1.2 | Cav 1.2a | L |
| Cav 1.2b | L | ||
| Cav 1.2c | L | ||
| Cav 1.3 | α1 1.3 | L | |
| Cav 1.4 | α1 1.4 | L | |
| Cav 2.1 | α1 2.1 | Cav 2.1a | P/Q |
| Cav 2.1b | |||
| Cav 2.2 | α1 2.2 | Cav 2.2a | N |
| Cav 2.2b | N | ||
| Cav 2.3 | α1 2.3 | Cav 2.3a | R |
| Cav 2.3b | R | ||
| Cav 3.1 | α1 3.1 | T | |
| Cav 3.2 | α1 3.2 | T | |
| Cav 3.3 | α1 3.3 | T |
It is worth noting that there is no specific blocker of the various Ca currents but that the various CCBs show variable selectivity when the Ca2+ current blocking ratio is analysed.
4. The management of hypertension: a challenge for experimentalists
Blocking calcium entry reduces the active tone of vascular smooth muscle and produces vasodilatation. This pharmacological property has been the basis for the use of CCBs in the management of hypertension. Hemodynamic observations in both humans and experimental animals show that CCBs have an activity profile that is different from classical arteriolar vasodilators. For instance, in humans, therapeutic regimens of CCBs evoke a reduction in blood pressure that is more pronounced in hypertensive than normotensive subjects (Leonetti et al. 1982). On the other hand, Knorr & Garthoff (1984) have compared the activity of nitrendipine and hydralazine on the blood pressure of SHR and WKY and observed that the vasodilator hydralazine was equipotent in both strains but that nitrendipine evoked a much smaller reduction in blood pressure in WKY than in SHR. Such a differential response between hypertensivity and normotensivity is not due to the tissue concentration of CCBs, as shown in table 2, but to a differential sensitivity of the target vessel according to its origin. It is generally accepted that abnormalities of resistance arteries may play a role in the pathogenesis and pathophysiology of hypertension in experimental animals and humans. Vascular tone, which results from the contractile activity of vascular smooth muscle cells in the walls of small arteries and arterioles, is the major determinant of the resistance to blood flow through the circulation. It plays an important role in the regulation of blood pressure and the distribution of blood flow between and within the tissues and organs of the body. The increase of reactivity to vasoconstrictors observed in hypertension (Asano et al. 1993, 1995; Morel & Godfraind 1994; Liu et al. 1997; Jackson 2000; Cox & Rusch 2002) might be related to both the upregulation of Ca channels and the loss of KV channels. Pratt et al. (2002) have shown that arteries from SHR expressed higher levels of α1C-subunit mRNA and protein than WKY arteries. Furthermore, arteries of SHR are depolarized when compared with arteries from WKY (Morel & Godfraind 1994). Therefore, not only the number of channels but also the proportion of inactivated channels is increased in hypertensive vessels, accounting for a higher specific binding of CCBs (figure 3). Those experimental data provide a rationale for explaining why CCB-evoked reduction of blood pressure is more pronounced in hypertensive than normotensive subjects. This suggests that CCBs may be considered specific antihypertensive agents rather than simple vasodilator agents, a hypothesis that is reinforced when examining their long-term effects.
Table 2.
Dihydropyridine-free tissue concentration in hypertensive and normotensive rats after chronic treatment by nisoldipine (80 mg kg−1 a day p.o.) or amlodipine (10 mg kg−1 a day p.o.).
| drug | nisoldipine | amlodipine | ||
|---|---|---|---|---|
| rat strain | decrease in SBP (mmHg) | free tissue concentration | decrease in SBP (mmHg) | free tissue concentration |
| SHR | 79±5.8 | 15–75 pMa | 35±6.9 | 4–6 nMb |
| WKY | n.s. | 15–75 pMa | n.s. | 3–5 nMb |
Data recalculated from Godfraind et al. (1991) and Morel & Godfraind (1994).
Estimated from the inhibition of the specific binding of 3H(+) isradipine in heart membrane (nisoldipine).
Estimated from the displacement of concentration–contraction curve of Bay K8644 in aortic rings.
Figure 3.

Specific binding of +isradipine (+PN 200-110) in an intact aorta isolated from a WKY (circle) and a SHR (triangle) bathed in physiological solutions at various KCl concentrations shown on the abcissa. Note the higher binding in SHR arteries that illustrates the higher affinity of the hypertensive arteries' binding sites compared with normotensive ones. Data from Morel & Godfraind (1994).
A major feature of the long-term action of CCBs is the prevention of the structural changes induced by hypertension in heart and arteries. It has been shown that salt is an important factor in the pathogenesis of essential hypertension. High salt intake directly affects complications of hypertension, particularly hypertensive renal disease, cerebro-vascular disease and compliance of large arteries (Messerli et al. 1997). Another major complication of hypertension is atherosclerosis causing coronary disease that is related to the severity of hypertension. Increased peripheral intima-medial thickening and enhanced oxidation-specific low-density lipoproteins (ox-LDL) are related to blood pressure elevation. Clinical observations of Toikka et al. (2000) suggest that these changes occur early in hypertension. Oxidation of LDL resulting from increased oxidative stress is an important early event in the pathogenesis of atherosclerosis. ox-LDL has been implicated in the increased formation of fatty streaks in the arterial intima, which represent the earliest form of atherosclerotic lesion. Several randomized control clinical trials, including the international nifedipine trial on antiatherosclerotic therapy, the verapamil in hypertension and atherosclerosis study, the prospective randomized evaluation of the vascular effects of norvasc trial with amlodipine and the European lacidipine study of atherosclerosis have substantiated the inhibitory effect of CCBs on the progression of atherosclerosis associated with hypertension (for reference see Godfraind 2004).
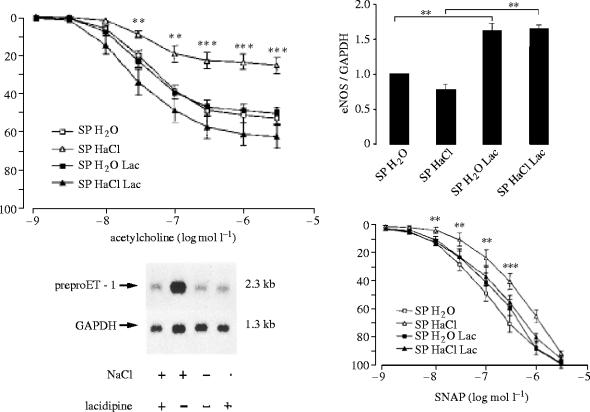
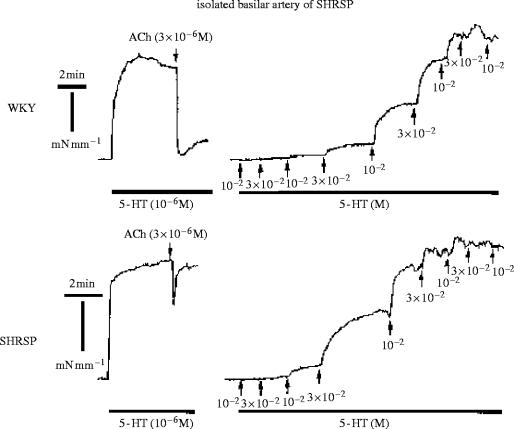
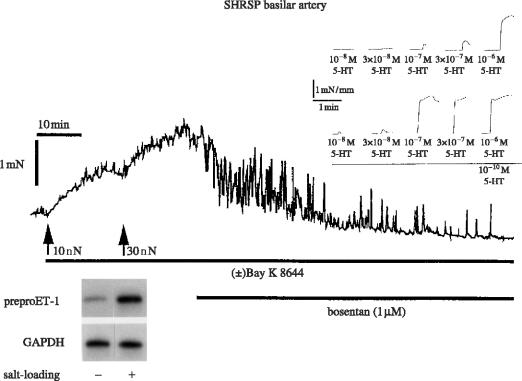
Experimental analysis of the therapeutic action of CCBs has been performed in long-term studies with spontaneously hypertensive stroke-prone rats (SHRSP), a salt-sensitive model of hypertension developing stroke followed by death when exposed to a high salt diet for about 10 weeks. The salt diet induces hypertrophy of the cardiovascular organs, renal failure associated with a high plasma renin activity (PRA), cerebral oedema and disorganization of the structure of cerebral arteries. Treatment with 1,4-DHP CCBs prevents SHRSP mortality. Salt-load induced cardiac and vascular hypertrophy accompany various genes' expression in the cardiovascular system: the levels of preproET-1 gene transcripts in the heart are significantly augmented by salt loading and a similar pattern is observed for TGF-β1 mRNA and the abundance of mRNA of β- and β-myosin-heavy high chain and skeletal and cardiac α-actin. One of the features of cardiac hypertrophy in hypertension is the extension of myocardial fibrosis. The mRNA level of type 1 collagen is also increased by salt loading. Those changes are prevented by the DHP lacidipine (Kyselovic et al. 1998, 2001) which also prevents the overexpression of the ET-1 gene in the vasculature and increases the vascular levels of eNOS (figure 4; Krenek et al. 2001). Salt-loading in a SHRSP induces structural and functional alterations in cerebral arteries: the normal orientation of smooth muscle cells in the media of arteries from rats exposed to a high salt diet is modified. Furthermore, there is a change in the cellular distribution within the vessel wall, exemplified by an increase in cell numbers in the adventia (Arribas et al. 1999). Morphological and biochemical alterations are associated with functional changes. A basilar artery from an SHRSP exhibits reduced endothelium-dependent vasorelaxation and increased threshold sensitivity to the action of vasoconstrictors such as serotonin (the most important regulator of cerebral vessel tone), as illustrated in figure 5. Such functional changes are amplified by a salt diet (Godfraind et al. 1997). High sensitivity to vasoconstrictors is probably due to the increased activation of VOCs involving ET-1 action. Indeed, as illustrated by figure 6, the contractile response recorded after salt-load is reduced in vitro by treating the vessel with bosentan, an endothelin antagonist (Salomone et al. 1996). It is associated with an increased abundance in the mRNA of preproET-1, leading to high production of a functionally active level of endothelin in this vessel. The upper inset of figure 6 shows how the contractile response of basilar artery to serotonin is augmented in a vessel exposed to a low dose of ET-1. This increased level of endothelin after salt-load is prevented by treatment with lacidipine. Lacidipine also stimulates the activation of the endothelial nitric oxide synthase (eNOS) gene, the enzyme producing the vasorelaxant agent nitric oxide (NO; Krenek et al. 2001). Simultaneously, the weak vasorelaxation in response to acetylcholine that is observed in a SHRSP exposed to a high salt diet is greatly augmented in arteries isolated from a SHRSP exposed to a high salt diet and treated with lacidipine (figure 4). In hypertension, this is believed to account for the improvement of reduced organ perfusion.
Figure 4.
Left upper panel and right lower panel: Effects of salt diet without and with lacidipine (Lac) on endothelium-dependent (acetylcholine) and endothelium-independent (SNAP) NO-vasorelaxation of arteries isolated from SHRSPs exposed or not exposed to a salt diet with or without lacidipine. Left lower panel: note that salt-evoked over-expression of ET-1 mRNA is prevented by lacidipine. Right upper panel: note increased levels of eNOS mRNA in vessels isolated from SHRSPs treated with lacidipine. Data from Krenek et al. (2001).
Figure 5.
Response of rat isolated basilar artery to acetylcholine (ACh) and serotonin (5-HT). Upper panel: basilar artery isolated from normotensive WKY rat. Left: artery pre-contracted by 5-HT and relaxed by ACh. Right: artery exposed to increasing concentrations of 5-HT. Lower panel: basilar artery isolated from hypertensive SHRSP rat. Left: artery pre-contracted by 5-HT and relaxed by ACh. Right: artery exposed to increasing concentrations of 5-HT. Note the difference in responses between the WKY and the SHRSP, indicating a reduced production of NO in the SHRSP. Data from Salomone et al. (1997).
Figure 6.
The response of the basilar artery, isolated from a SHRSP exposed to a salt diet, to the calcium channel activator Bay K8644 is reversed by bosentan, an endothelin antagonist. Lower panel left: note ET-1 mRNA over-expression in the vessel wall showing that functional ET-1 was produced locally. Higher panel right: note the increased response to 5HT in the basilar artery isolated from WKY and bathed with ET-1 (10−9 M). Data from Salomone et al. (1996) and unpublished experiments.
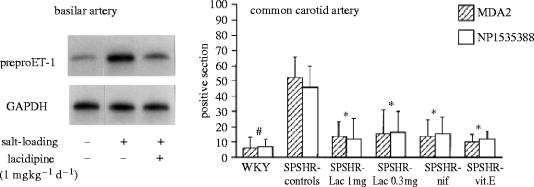
A SHRSP exposed to salt-load not only presents endothelial dysfunction but also shows a notable augmentation of oxidative products when compared with a WKY exposed to the same diet. This is observed in paraffin-embedded serial sections of arteries immunostained and assessed for the intimal presence of ox-LDL epitopes and native apolipoprotein B (Napoli et al. 1999). Oxidation-specific epitopes do not occur in normal arteries but small intimal lesions and vascular dysfunction are frequently found in SPSHR arteries. As shown in figure 7, the carotid artery in the SHRSP control group that was exposed to only a salt diet contains significantly more intimal apolipoprotein B and ox-LDL epitopes than those of the groups treated with CCBs or vitamin E. The fact that not only the ox-LDL but also the native LDL decreased in treated animals is in agreement with the qualitative observation of fewer small intimal lesions in these groups.
Figure 7.
Left panel: salt-evoked over-expression of ET-1 mRNA in a basilar artery from a SHRSP is prevented by lacidipine treatment. Right panel: salt-evoked accumulation of LDL and ox-LDL in the cerebral arteries of a SHRSP is prevented by CCBs and vitamin E. Data from Napoli et al. (1999) and unpublished experiments.
LDL peroxidation is also observed in SHRSP plasma. Lipid oxidation may be initiated by any primary free radical that has sufficient reactivity to extract a hydrogen atom from a reactive methylene group of a polyunsaturated fatty acid. Formation of the initiating species is accompanied by the rearrangement of bonds into diene conjugates. The lipid radical then takes up oxygen to form the peroxyl radical. These radicals are also capable of abstracting hydrogen from fatty acid side chains and thus perpetuating the peroxidative chain reactions. Hence, a single initiation event can result in the conversion of hundreds of fatty acid side chains into lipid monohydroperoxides or cycloperoxides. These are fairly stable molecules under physiological conditions and the cleavage of the carbon bonds during peroxidation results in the formation of alkanals such as MDA. Total plasma lipoperoxide levels were approximately 0.87 mmol l−1 in SHRSP controls but decreased down to approximately 0.63 mmol l−1 in the group treated with 1 mg lacidipine and approximately 0.68 mmol l−1 in nifedipine-treated animals (p<0.05 versus control SHRSP for all values). Vitamin E significantly reduced plasma peroxidation in SHRSP (approx. 0.58 mmol l−1; p<0.05 versus control SHRSP). Normotensive WKY had lower levels of plasma peroxidation than control SHRSP (approx. 0.73 mmol l−1; p<0.05). These results demonstrate that both 1,4-DHP CCBs and vitamin E reduce the total amount of lipid oxidative compounds in the bloodstream. In general, all measures of lipid oxidation are lower in normotensive WKY than in control SHRSP. Proteins are particularly susceptible to direct attack from oxygen radicals and peroxidative intermediates such as alkoxyl and peroxyl radicals. In some experiments by Napoli et al. (1999), LDL was oxidized and its relative electrophoretic mobility in agarose gel was evaluated in order to investigate whether the presence of CCBs could also protect apolipoprotein B from oxygen radical-induced damage. The agarose gel mobility of LDL from a WKY subjected to X/XO oxidation is reduced compared with LDL from control SHRSP. The mobility of LDL from a SHRSP treated with 1,4-DHP CCBs or vitamin E is significantly reduced down to the level of LDL from a WKY. The degree of modification of protein amino groups inducible by X/XO was tested using trinitrobenzene sulphonate (TNBS). When control LDL of a SHRSP was incubated with oxygen radicals, about 20% of TNBS reactivity was lost. The loss of TNBS reactivity was significantly smaller in animals treated with 1,4-DHP CCBs or vitamin E compared with both control groups, indicating that fewer lysine residues of apolipoprotein B were oxidatively modified. Moreover, lacidipine and vitamin E were more effective than nifedipine in the prevention of apolipoprotein B modifications. Thus, treatment with CCBs conveys significant protection against plasma and LDL oxidation (both lipid and protein components) and formation of oxidation-specific epitopes in the arterial wall. Treatment of SHRSP with vitamin E had a similar effect on the development of oxidation products but it reduced mortality to a lower extent than CCBs did.
ApoE-deficient mice develop spontaneous atheromatous lesions that are morphologically similar to those found in humans. Lesions form in a reproducible manner and the transition from a lipid-laden lesion to fibrous plaque can be evaluated. Oxidative modification of LDL has been implicated in atherogenesis. Evidence consistent with this hypothesis includes the presence of oxidized lipids in atherosclerotic lesions and the acceleration of atherogenesis by in vivo delivery of the gene for 15-lipoxygenase, an oxidizing enzyme present in atherosclerotic lesions. Pratico et al. (1998) have reported that oxidative stress is increased in the ApoE−/− mouse and can be suppressed by oral administration of vitamin E, reducing the evolution of atherosclerosis without reducing elevated cholesterol. Feeding animals dietary fat and cholesterol may accelerate the progression of lesion development. In atherosclerosis, lesion formation depends upon calcium-regulated cellular processes such as chemotaxis, adhesion, migration, proliferation, lipid uptake and necrosis. As was reported by Henry (1990), interventions acting on cell calcium uptake including treatment with calcium chelating agents, lanthanum trichloride and calcium antagonists may retard atherogenesis in fat-fed animals in the absence of hypolipidaemic effects. Spontaneous hypercholesterolaemia and atherosclerotic lesions of ApoE-deficient mice are augmented by a lipid-rich Western-type diet (WD) (Nakashima et al. 1994). Moreover, even on a normal diet (ND) these mice exhibit several functional alterations of the cardiovascular system, including endothelial dysfunction (Plump et al. 1992; Bonthu et al. 1997; Barton et al. 1998; d'Uscio et al. 2001) that has been proposed to trigger initial molecular and cellular events in atherogenesis (Libby & Galis 1995; Libby et al. 1995; Barton & Haudenschild 2001). This dysfunction may be evidenced by reduced endothelial nitric oxide-mediated vasorelaxation which influences vascular tone and hemodynamics (d'Uscio et al. 2001). Impairment of endothelial function in ApoE−/− mice has been attributed to a reduction of endothelial NO-synthase activity and an increased production of causing inactivation of NO (d'Uscio et al. 2001). In this animal model, the development of atherogenesis is associated with increased peroxidation of plasma lipids, LDL and VLDL, as well as increased susceptibility of lipoproteins to be oxidized ex vivo (Hayek et al. 1994). Experimental studies in isolated mouse vessels have shown that ox-LDL and superoxide impaired endothelial NO function (Jiang et al. 2001; Lu & Kassab 2004).
In recent experiments from our group (Kyselovic et al. 2004, 2005), ApoE−/− mice (six weeks old) were exposed to either a ND or a WD (adjusted calories diet containing 42% fat) for eight weeks. Vascular reactivity was examined in isolated aorta arch rings pre-contracted by noradrenaline and relaxed in the presence of acetylcholine or SNAP after blocking NO-synthase. The WD induced a reduction of NO-mediated, endothelium-dependent relaxation to acetylcholine (max. relaxation %=55.8±2.7 for ND and 46.6±2.0 for WD, n=8, p<0.001). Dose-relaxation curves to SNAP, a NO donor, were also significantly shifted to the right (n=7, p<0.01) in WD compared with ND arteries. Chronic treatment of WD mice with lacidipine (1 mg kg−1 a day) significantly increased the acetylcholine-evoked relaxation (to 76.6±3.4%, n=10, ANOVA, p<0.001) and prevented the loss of responsiveness to SNAP in ApoE KO mice exposed to a WD. Thiobarbituric acid-reactive substances (TBARS), markers of oxidative stress, were estimated according to Ohkawa et al. (1979). TBARS were significantly lower in the kidneys of ApoE KO mice treated with lacidipine than in untreated ones (p<0.001).
Lacidipine, which prevents endothelial dysfunction, also prevents the development of atherosclerosis in ApoE KO mice as shown in figure 8. This effect might involve several mechanisms, including a reduction in reactive oxygen species (ROS) effects—an action occurring without high cholesterol reduction. Indeed, as reported above, CCBs including lacidipine reduce plasma and LDL oxidation as well as the formation of oxidation-specific epitopes in the arteries of SHRSPs (Napoli et al. 1999). More recently it has been reported that the resistance of mouse plasma LDL to undergoing lipid peroxidation was significantly increased in ApoE-deficient mice treated with lacidipine (Cristofori et al. 2004). Lacidipine-induced reduction of TBARS levels, indicating decreased oxidative stress in ApoE-deficient mice exposed to a WD, is consistent with observations of SHRSPs exposed to a salt-diet (as reported above) that have illustrated both the in vivo and ex vivo antioxidant effects of lacidipine (table 3). Antioxidants improve NO-dependent relaxation (d'Uscio et al. 2001; Sato et al. 2002). Therefore, it is likely that lacidipine prevented the reduction of endothelium-dependent vasorelaxation and the loss in sensitivity to the NO donor SNAP observed in aortic arches isolated from mice exposed to a WD by reducing oxidative stress. ApoE−/− mice have been used to test the effects of various drugs which do not act on plasma cholesterol levels but which might interfere with the oxidative stress within the arterial wall on atherosclerosis. Such studies have provided indirect evidence that the renin–angiotensin system could be involved in the pathogenesis of atherosclerosis in ApoE−/− mice, since either inhibiting angiotensin converting enzyme activity or blocking angiotensin type 1 receptors resulted in a decrease in the development of atherosclerotic lesions (Keidar et al. 2000; Prasad et al. 2000). Direct evidence has been provided for the proatherogenic role of angiotensin II in ApoE−/− mice. It was shown that for the same increase in blood pressure, angiotensin II administered via osmotic minipumps for eight weeks evoked a significantly larger atherosclerosis than did norepinephrine (Weiss et al. 2001). Our studies demonstrate that PRA was increased in SHRSPs exposed to a salt diet and in ApoE−/− mice exposed to a WD (Kyselovic et al. 2003). According to Muller et al. (1998) the uptake of circulating renin by cardiac and vascular tissue allows local production of functionally active angiotensin II. Angiotensin II may play a role in the development of vascular alterations leading to stroke or atherosclerosis, probably via its effect on activation of oxidative stress (leading to increased oxidized LDL incorporation into the vascular wall) and its proinflammatory action (Schiffrin 2002). Furthermore, angiotensin II increases vascular ET-1 levels (Krenek et al. 1999; Hsu et al. 2004). This cascade appeared to operate in vivo. In SHRSPs exposed to a salt diet, we observed that ET-1 plasma levels accompanied PRA levels. In ApoE-deficient mice, a WD evokes an increase in ET-1 plasma levels (Kyselovic et al. 2003) and a significant correlation has been reported between plasma ET-1 values and the extent of atherosclerotic lesions (Cristofori et al. 2000). Furthermore, endothelin antagonists may reduce the development of atherosclerosis, indicating a role for this peptide in the pathogenesis of this process (Barton et al. 1998).
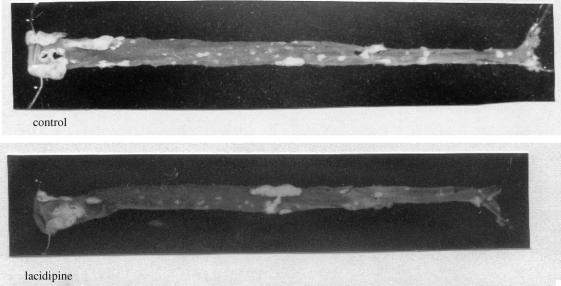
Figure 8.
Gross appearance of the atherosclerotic lesions in the aorta of apoE-deficient mouse exposed to a Western-type diet for 20 weeks without (upper panel) or with lacidipine (lower panel). Whole aortas (from heart to iliac bifurcation) were placed into cold (4 °C) physiological solution. They were photographed against a black background in a standard fashion, allowing plaques and even minor fatty streaks to appear whitish in contrast to the darker, opaquely transparent normal tissue. Note the reduced number of fatty streaks in an aorta from a mouse treated with lacidipine.
Table 3.
Ex vivo peroxidation of LDL prepared from the plasma of rats exposed to various treatments.
| rat treatment for five weeks | rate: nmol (min mg LDL)−1 |
|---|---|
| WKY (control) | 9.9±1.5* |
| SHRSP exposed to salt diet | 12.4±1.5 |
| +nifedipine (80 mg kg−1 a day) | 9.1±1.4* |
| +lacidipine (1 mg kg−1 a day) | 8.8±1.5* |
| +lacidipine (0.3 mg kg−1 a day) | 9.0±1.5* |
| +vitamin E (100 IU kg−1 a day) | 8.4±1.6* |
Plasma samples were taken and LDL was isolated by ultracentrifugation. LDL was then exposed to oxygen radicals generated by a xanthine/xanthine oxidase reaction (2 mmol l−1 xanthine+100 mU ml−1 xanthine oxidase) and the rate of oxidation was determined. *denotes results that are statistically different from SHRSPs exposed to a salt diet only. Data from Napoli et al. (1999).
Experimental observations in both SHRSP and ApoE-deficient mice show that CCBs may reduce the consequences of oxidative stress. Potential mechanisms involved are considered in the next section.
5. Antioxidant effects and protective action of calcium channel blockers
Antioxidants are defined as substances that, when present at a low concentration relative to an oxidizable substrate, significantly delay or prevent oxidation of that substrate. Living organisms have evolved a number of antioxidant defences to maintain their survival against oxidative stress. Antioxidant mechanisms can be divided into two major classes based on their mode of action: antioxidant enzymes and non-enzymatic antioxidants (scavengers). The class of antioxidant enzymes consists of superoxide dismutase (SOD), catalase and glutathione peroxidase. Dismutation of by SOD produces H2O2 (a more stable ROS) which, in turn, is converted to H2O by catalase and glutathione peroxidase. Non-enzymatic scavengers comprise vitamins (a/o C and E), sulphydryl groups (glutathione, etc.), flavonoids, albumin and other proteins. An antioxidant effect may result from either activation or mimicry of antioxidant defences. Alternatively, it may be due to interaction with factors involved in the activation of oxidative stress. Oxidative stress describes the injury caused to cells by the oxidizing of macromolecules resulting from increased formation of ROS and/or decreased antioxidant reserve. Oxidant stress contributes to vascular diseases by promoting vascular smooth muscle proliferation, monocyte/macrophage infiltration, vascular tone alteration and matrix metalloproteinases activation. A membrane-bound NADH/NADPH oxidase, the major source of ROS in blood vessels, is activated in rats with induced hypertension via prolonged angiotensin II infusion (Zalba et al. 2001). This increase in superoxide anion production contributes to impaired endothelium-dependent relaxation, the hypertension being ameliorated by treatment with membrane-targeted forms of SOD (which is one of the major cellular defences against superoxide anion). Hypertrophy of vascular smooth muscle cells caused by angiotensin II is mediated by ROS derived mainly from the membrane-bound NADH/NADPH oxidase. Similarly, a recombinant heparin-binding SOD acutely lowers blood pressure in spontaneously hypertensive rats (Nakazono et al. 1991). Taken together, these findings indicate that oxidant stress critically contributes to the pathogenesis of hypertension and its related vascular disease (Griendling et al. 2000). Enhanced production of ROS contributes to the dysregulation of physiological processes, which leads to structural and functional alterations observed in hypertension and atherosclerosis. Enzymatic sources of ROS in the vascular wall that play a functional role in hypertension are NAD(P)H oxidase, NO synthase (NOS), xanthine oxidase and cyclooxygenase. Several experimental observations have shown an enhanced superoxide generation as a result of the activation of vascular NAD(P)H oxidase with shear stress (Hwang et al. 2003).
Several authors have described the antioxidant properties of CCBs as being due to either a direct scavenging effect or the preservation of the SOD activity. Observations reported above indicate that CCBs may also act by reducing the production of angiotensin and endothelin. Under controlled experimental conditions, they may inhibit lipid peroxide formation at concentrations present in plasma. This antioxidant activity is found with high lipophilic CCBs when their chemical structure facilitates proton-donating and resonance-stabilization mechanisms that quench the free radical reaction. Inserted in a location in the membrane near polyunsaturated fatty acids at relatively high concentrations, dihydropyridines are capable of donating protons to lipid peroxide molecules, thereby blocking the peroxidation process. The remaining unpaired free electron associated with the CCB molecule can be stabilized in well-defined resonance structures associated with the dihydropyridine ring (Mason 2002). The reaction that describes the antioxidant effects of a dihydropyridine (DHP) CCB is as follows (in which LOO* represents a lipid peroxide molecule):
The antioxidant capacity of any chemical compound can be measured in vitro. According to Ursini (1997), for all dihydropyridines, the constant rate for the interaction with peroxyl radicals is three orders of magnitude lower than that of the vitamin E derivative and, therefore, all these molecules must be considered rather weak antioxidants. However, it is worth considering the binding to specific structures and the partition coefficient of membranes which provides a high local concentration.
The antioxidant action of CCBs has been demonstrated in other experimental setups. Berkels et al. (2001) have reported that treatment of the endothelium with dihydropyridine calcium antagonists resulted in an increased release of NO that is not due to a modulation of L-type calcium channels because macrovascular endothelial cells do not express this channel. In their study, Berkels et al. investigated how long-term (48 h) treatment of porcine endothelial cell cultures with nifedipine resulted in enhanced NO liberation. Regarding the underlying mechanism, they examined whether nifedipine changed the mRNA and protein levels of the constitutive endothelial NOS in endothelial cell cultures or whether it exerted an NO protective effect via its antioxidative properties, as revealed in a cell culture model and with native endothelium from porcine coronary arteries. They observed that nifedipine induced a significant time- and concentration-dependent increase in the basal NO liberation that was not due to elevated eNOS mRNA and protein levels. However, at the same concentration, nifedipine significantly reduced the basal and glucose-stimulated formation of ROS, indicating that nifedipine might protect released NO from oxidation by ROS. They concluded that this increased NO availability may cause part of the vasodilation and might contribute to the antithrombotic, antiproliferative and antiatherosclerotic effects of dihydropyridine calcium antagonists.
In SHRSPs exposed to salt load and treated with CCBs or vitamin E, Napoli et al. (1999) have observed a marked reduction in the presence of oxidation-specific epitopes in carotid and middle cerebral arteries (figure 7). This reduction was unrelated to the degree of decrease in elevated blood pressure and was similar to the action of vitamin E that was without effect on blood pressure. Like vitamin E, nifedipine and lacidipine reduced the rate of LDL oxidation measured in the blood ex vivo (table 3). In contrast to lacidipine, vitamin E did not lower cardiac weight in salt-loaded SHRSPs, although it did convey strong antioxidant protection. It proved far less effective than CCBs for mortality reduction. In an ApoE knockout study by Thomas et al. (2001), supplementation with 0.2% Vitamin E alone had a moderate antiatherogenic effect in the aortic root only, whereas Pratico et al. observed am approximately 60% decrease in the aortic lesion area. Several differences between the two studies may explain the apparent discrepancy. Thomas et al. used a high-fat diet which resulted in a total plasma cholesterol of approximately 845 mg dl−1 whereas Pratico et al. used a ND and reported plasma total cholesterol of approximately 500 mg dl−1. In SHRSPs exposed to salt diet, the vitamin E treatment gave strong antioxidant protection. However, it proved far less effective than CCBs on mortality, suggesting that the antioxidant effects of these compounds may be responsible for only part of the beneficial effect and that reduction of blood pressure plays an important role. This conclusion is valid for both hypertension and atherosclerosis.
Acknowledgments
Most of the experimental work of the author and his coworkers was supported by grants from the Fonds National de la Recherche Scientifique, the Fonds de la Recherche Scientifique Médicale, the Communauté française de Belgique, the Belgian Government, the Centre for Biomedical Research and Development (Belgian non-profit-making organization) and the Fondation Professeur Lucien Dautrebande.
Footnotes
One contribution of 18 to a Theme Issue ‘Reactive oxygen species in health and disease’.
References
- Arribas S.M, Costa R, Salomone S, Morel N, Godfraind T, McGrath J.C. Functional reduction and associated cellular rearrangement in SHRSP rat basilar arteries are affected by salt load and calcium antagonist treatment. J. Cereb. Blood Flow Metab. 1999;19:517–527. doi: 10.1097/00004647-199905000-00006. 10.1097/00004647-199905000-00006 [DOI] [PubMed] [Google Scholar]
- Asano M, Matsuda T, Hayakawa M, Ito K.M, Ito K. Increased resting Ca2+ maintains the myogenic tone and activates K+ channels in arteries from young spontaneously hypertensive rats. Eur. J. Pharmacol. 1993;247:295–304. doi: 10.1016/0922-4106(93)90198-i. 10.1016/0922-4106(93)90198-I [DOI] [PubMed] [Google Scholar]
- Asano M, Nomura Y, Ito K, Uyama Y, Imaizumi Y, Watanabe M. Increased function of voltage-dependent Ca++ channels and Ca++-activated K+ channels in resting state of femoral arteries from spontaneously hypertensive rats at prehypertensive stage. J. Pharmacol. Exp. Ther. 1995;275:775–783. [PubMed] [Google Scholar]
- Barton M, Haudenschild C.C. Endothelium and atherogenesis: endothelial therapy revisited. J. Cardiovasc. Pharmacol. 2001;38(Suppl. 2):S23–S25. doi: 10.1097/00005344-200111002-00007. [DOI] [PubMed] [Google Scholar]
- Barton M, Haudenschild C.C, d'Uscio L.V, Shaw S, Munter K, Luscher T.F. Endothelin ETA receptor blockade restores NO-mediated endothelial function and inhibits atherosclerosis in apolipoprotein E-deficient mice. Proc. Natl Acad. Sci. USA. 1998;95:14 367–14 372. doi: 10.1073/pnas.95.24.14367. 10.1073/pnas.95.24.14367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkels R, Egink G, Marsen T.A, Bartels H, Roesen R, Klaus W. Nifedipine increases endothelial nitric oxide bioavailability by antioxidative mechanisms. Hypertension. 2001;37:240–245. doi: 10.1161/01.hyp.37.2.240. [DOI] [PubMed] [Google Scholar]
- Bonthu S, Heistad D.D, Chappell D.A, Lamping K.G, Faraci F.M. Atherosclerosis, vascular remodeling, and impairment of endothelium-dependent relaxation in genetically altered hyperlipidemic mice. Arterioscler. Thromb. Vasc. Biol. 1997;17:2333–2340. doi: 10.1161/01.atv.17.11.2333. [DOI] [PubMed] [Google Scholar]
- Catterall W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. 10.1146/annurev.cellbio.16.1.521 [DOI] [PubMed] [Google Scholar]
- Catterall W.A, Chandy K.G, Gutman G.A. IUPHAR Media; Leeds: 2002. The IUPHAR Compendium of voltage-gated ion channels. [Google Scholar]
- Cox R.H, Rusch N.J. New expression profiles of voltage-gated ion channels in arteries exposed to high blood pressure. Microcirculation. 2002;9:243–257. doi: 10.1038/sj.mn.7800140. 10.1038/sj.mn.7800140 [DOI] [PubMed] [Google Scholar]
- Cristofori P, Lanzoni A, Quartaroli M, Pastorino A.M, Zancanaro C, Cominacini L, Gaviraghi G, Turton J. The calcium-channel blocker lacidipine reduces the development of atherosclerotic lesions in the apoE-deficient mouse. J. Hypertens. 2000;18:1429–1436. doi: 10.1097/00004872-200018100-00010. 10.1097/00004872-200018100-00010 [DOI] [PubMed] [Google Scholar]
- Cristofori P, et al. Reduced progression of atherosclerosis in apolipoprotein E-deficient mice treated with lacidipine is associated with a decreased susceptibility of low-density lipoprotein to oxidation. Int. J. Exp. Pathol. 2004;85:105–114. doi: 10.1111/j.0959-9673.2004.00375.x. 10.1111/j.0959-9673.2004.00375.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin R.P, Jenkinson D.H. The calcium-dependence of tension development in depolarized smooth muscle. J. Physiol. 1961;161:90–96. doi: 10.1113/jphysiol.1961.sp006707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Uscio L.V, Baker T.A, Mantilla C.B, Smith L, Weiler D, Sieck G.C, Katusic Z.S. Mechanism of endothelial dysfunction in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:1017–1022. doi: 10.1161/01.atv.21.6.1017. [DOI] [PubMed] [Google Scholar]
- Ebashi S. Regulation of muscle contraction. Proc. R. Soc. B. 1980;207:259–286. doi: 10.1098/rspb.1980.0024. [DOI] [PubMed] [Google Scholar]
- Ebashi S. From the relaxing factor to troponin. Biomed. Res. 1993;14:1–7. [Google Scholar]
- Edman K.A.P, Schild H.O. Interaction of acetylcholine, calcium and depolarization in the contraction of smooth muscle. Nature. 1961;190:350–352. doi: 10.1038/190350b0. [DOI] [PubMed] [Google Scholar]
- Edman K.A.P, Schild H.O. The need for calcium in the contractile responses induced by acetylcholine and potassium in the rat uterus. J. Physiol. 1962;161:424–441. doi: 10.1113/jphysiol.1962.sp006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K.A.P, Schild H.O. Calcium and the stimulant and inhibitory effects of adrenaline in depolarized smooth muscle. J. Physiol. 1963:404–411. doi: 10.1113/jphysiol.1963.sp007265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel E.A, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. 10.1016/S0896-6273(00)81057-0 [DOI] [PubMed] [Google Scholar]
- Evans D.H.L, Schild H.O, Thesleff S. Effect of drugs on depolarized plain muscle. J. Physiol. 1958;143:474–485. doi: 10.1113/jphysiol.1958.sp006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A, Tritthart H, Flackenstein B, Herbst A, Grun G. A new group of competitive divalent Ca-antagonists (iproveratril, D 600, prenylamine) with potent inhibitory effects on electromechanical coupling in mammalian myocardium. Pflugers Arch. 1969;307:R25. [PubMed] [Google Scholar]
- Fox A.P, Nowycky M.C, Tsien R.W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J. Physiol. 1987a;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A.P, Nowycky M.C, Tsien R.W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J. Physiol. 1987b;394:173–200. doi: 10.1113/jphysiol.1987.sp016865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T. Actions of nifedipine on calcium fluxes and contraction in isolated rat arteries. J. Pharmacol. Exp. Ther. 1983;224:443–450. [PubMed] [Google Scholar]
- Godfraind, T. 2004 Calcium channel blockers Basel–Boston–Berlin: Birkhaüser.
- Godfraind T, Kaba A. Blockade or reversal of the contraction induced by calcium and adrenaline in depolarized arterial smooth muscle. Br. J. Pharmacol. 1969a;36:549–560. doi: 10.1111/j.1476-5381.1969.tb08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T, Kaba A. Inhibition by cinnarizine and chlorpromazine of the contraction induced by calcium and adrenaline in vascular smooth muscle. Br. J. Pharmacol. 1969b;35:P354–P355. [PMC free article] [PubMed] [Google Scholar]
- Godfraind T, Miller R.C. Specificity of action of Ca++ entry blockers. A comparison of their actions in rat arteries and in human coronary arteries. Circ. Res. 1983;52:I81–I91. [PubMed] [Google Scholar]
- Godfraind T, Polster P. Comparative study of drugs inhibiting the contractile response of isolated vessels of human and animal origin. Therapie. 1968;23:1209–1220. [PubMed] [Google Scholar]
- Godfraind T, Kaba A, Polster P. Specific antagonism to the direct and indirect action of angiotensin on the isolated guinea-pig ileum. Br. J. Pharmacol. Chemother. 1966;28:93–104. doi: 10.1111/j.1476-5381.1966.tb01877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T, Kaba A, Polster P. Differences in sensitivity of arterial smooth muscles to inhibition of their contractile response to depolarization by potassium. Arch. Int. Pharmacodyn. Ther. 1968;172:235–239. [PubMed] [Google Scholar]
- Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacol. Rev. 1986;38:321–416. [PubMed] [Google Scholar]
- Godfraind T, Kazda S, Wibo M. Effects of a chronic treatment by nisoldipine, a calcium antagonistic dihydropyridine, on arteries of spontaneously hypertensive rats. Circ. Res. 1991;68:674–682. doi: 10.1161/01.res.68.3.674. [DOI] [PubMed] [Google Scholar]
- Godfraind T, Salomone S, Morel N, Ghisdal P. Preclinical study of the action of a calcium channel blocker during salt load. Bull. Acad. Natl Med. 1997;181:289–298. discussion 299–300. [PubMed] [Google Scholar]
- Griendling K.K, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Hayek T, Oiknine J, Brook J.G, Aviram M. Increased plasma and lipoprotein lipid peroxidation in apo E-deficient mice. Biochem. Biophys. Res. Commun. 1994;201:1567–1574. doi: 10.1006/bbrc.1994.1883. 10.1006/bbrc.1994.1883 [DOI] [PubMed] [Google Scholar]
- Heilbrunn L.V, Wiercinski F.J. The action of various cations on muscle protoplasm. J. Cell Comp. Physiol. 1947;29:15–32. doi: 10.1002/jcp.1030290103. 10.1002/jcp.1030290103 [DOI] [PubMed] [Google Scholar]
- Henry P.D. Atherogenesis, calcium and calcium antagonists. Am. J. Cardiol. 1990;66:3I–6I. doi: 10.1016/0002-9149(90)91256-6. 10.1016/0002-9149(90)91256-6 [DOI] [PubMed] [Google Scholar]
- Hess P, Lansman J.B, Tsien R.W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J. Gen. Physiol. 1986;88:293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.H, Chen J.J, Chang N.C, Chen C.H, Liu J.C, Chen T.H, Jeng C.J, Chao H.H, Cheng T.H. Role of reactive oxygen species-sensitive extracellular signal-regulated kinase pathway in angiotensin II-induced endothelin-1 gene expression in vascular endothelial cells. J. Vasc. Res. 2004;41:64–74. doi: 10.1159/000076247. 10.1159/000076247 [DOI] [PubMed] [Google Scholar]
- Hwang J, Ing M.H, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai T.K. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ. Res. 2003;93:1225–1232. doi: 10.1161/01.RES.0000104087.29395.66. 10.1161/01.RES.0000104087.29395.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson W.F. Ion channels and vascular tone. Hypertension. 2000;35:173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Gibson A.P, Dusting G.J. Endothelial dysfunction induced by oxidized low-density lipoproteins in isolated mouse aorta: a comparison with apolipoprotein-E deficient mice. Eur. J. Pharmacol. 2001;424:141–149. doi: 10.1016/s0014-2999(01)01140-2. 10.1016/S0014-2999(01)01140-2 [DOI] [PubMed] [Google Scholar]
- Kamada T, Kinosita H. Disturbances initiated from naked surface of muscle protoplasm. Jpn J. Zool. 1943;10:469–493. [Google Scholar]
- Karaki H. Historical techniques: cytosolic Ca2+ and contraction in smooth muscle. Trends Pharmacol. Sci. 2004;25:388–393. doi: 10.1016/j.tips.2004.05.008. 10.1016/j.tips.2004.05.008 [DOI] [PubMed] [Google Scholar]
- Karaki H, et al. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- Keidar S, Attias J, Coleman R, Wirth K, Scholkens B, Hayek T. Attenuation of atherosclerosis in apolipoprotein E-deficient mice by ramipril is dissociated from its antihypertensive effect and from potentiation of bradykinin. J. Cardiovasc. Pharmacol. 2000;35:64–72. doi: 10.1097/00005344-200001000-00008. 10.1097/00005344-200001000-00008 [DOI] [PubMed] [Google Scholar]
- Knorr A, Garthoff B. Differential influence of the calcium antagonist nitrendipine and the vasodilator hydralazine on normal and elevated blood pressure. Arch. Int. Pharmacodyn. Ther. 1984;269:316–322. [PubMed] [Google Scholar]
- Krenek P, Kyselovic J, Morel N, Wibo M, Godfraind T. Angiotensin-induced endothelin expression in isolated rat aorta, functional consequence and inhibition by calcium antagonist. Br. J. Pharmacol. 1999;126:129P. [Google Scholar]
- Krenek P, Salomone S, Kyselovic J, Wibo M, Morel N, Godfraind T. Lacidipine prevents endothelial dysfunction in salt-loaded stroke-prone hypertensive rats. Hypertension. 2001;37:1124–1128. doi: 10.1161/01.hyp.37.4.1124. [DOI] [PubMed] [Google Scholar]
- Kyselovic J, Morel N, Wibo M, Godfraind T. Prevention of salt-dependent cardiac remodeling and enhanced gene expression in stroke-prone hypertensive rats by the long-acting calcium channel blocker lacidipine. J. Hypertens. 1998;16:1515–1522. doi: 10.1097/00004872-199816100-00017. 10.1097/00004872-199816100-00017 [DOI] [PubMed] [Google Scholar]
- Kyselovic J, Krenek P, Wibo M, Godfraind T. Effects of amlodipine and lacidipine on cardiac remodelling and renin production in salt-loaded stroke-prone hypertensive rats. Br. J. Pharmacol. 2001;134:1516–1522. doi: 10.1038/sj.bjp.0704398. 10.1038/sj.bjp.0704398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyselovic J, Krenek P, Klimas J, Godfraind T. Renin production in ApoE-deficient mice exposed to Western-type diet. Pflugers Arch. 2003;445:R3. [Google Scholar]
- Kyselovic J, Martinka P, Batova S, Godfraind T. Action of calcium channel blocker on endothelial dysfunction in ApoE KO mice. Fundam. Clin. Pharmacol. 2004;18:75. [Google Scholar]
- Kyselovic J, Martinka P, Batova Z, Gazova A, Godfraind T. Calcium channel blocker inhibits western-type diet-evoked atherosclerosis development in ApoE-deficient mice. J. Pharmacol. Exp. Ther. 2005;315:320–328. doi: 10.1124/jpet.105.089847. [DOI] [PubMed] [Google Scholar]
- Leonetti G, Cuspidi C, Sampieri L, Terzoli L, Zanchetti A. Comparison of cardiovacular, renal, and humoral effects of acute administration of two calcium channel blockers in normotensive and hypertensive subjects. J. Cardiovasc. Pharmacol. 1982;4(Suppl. 3):S319–S324. [PubMed] [Google Scholar]
- Libby P, Galis Z.S. Cytokines regulate genes involved in atherogenesis. Ann. NY Acad. Sci. 1995;748:158–168. doi: 10.1111/j.1749-6632.1994.tb17315.x. discussion 168–170. [DOI] [PubMed] [Google Scholar]
- Libby P, Sukhova G, Lee R.T, Galis Z.S. Cytokines regulate vascular functions related to stability of the atherosclerotic plaque. J. Cardiovasc. Pharmacol. 1995;25(Suppl. 2):S9–S12. doi: 10.1097/00005344-199500252-00003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pleyte K, Knaus H.G, Rusch N.J. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- Lu X, Kassab G.S. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J. Physiol. 2004;561:575–582. doi: 10.1113/jphysiol.2004.075218. 10.1113/jphysiol.2004.075218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R.P. Mechanisms of plaque stabilization for the dihydropyridine calcium channel blocker amlodipine: review of the evidence. Atherosclerosis. 2002;165:191–199. doi: 10.1016/s0021-9150(01)00729-8. 10.1016/S0021-9150(01)00729-8 [DOI] [PubMed] [Google Scholar]
- Messerli F, Schmieder R.E, Weir M.R. Salt. A perpetrator of hypertensive target organ disease? Arch. Intern. Med. 1997;157:2449–2452. doi: 10.1001/archinte.157.21.2449. 10.1001/archinte.157.21.2449 [DOI] [PubMed] [Google Scholar]
- Morel N, Godfraind T. Prolonged depolarization increases the pharmacological effect of dihydropyridines and their binding affinity for calcium channels of vascular smooth muscle. J. Pharmacol. Exp. Ther. 1987;243:711–715. [PubMed] [Google Scholar]
- Morel N, Godfraind T. Selective modulation by membrane potential of the interaction of some calcium entry blockers with calcium channels in rat mesenteric artery. Br. J. Pharmacol. 1988;95:252–258. doi: 10.1111/j.1476-5381.1988.tb16571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel N, Godfraind T. Pharmacological properties of voltage-dependent calcium channels in functional microvessels isolated from rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 1989;340:442–451. doi: 10.1007/BF00167047. 10.1007/BF00167047 [DOI] [PubMed] [Google Scholar]
- Morel N, Godfraind T. Selective interaction of the calcium antagonist amlodipine with calcium channels in arteries of spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 1994;24:524–533. doi: 10.1097/00005344-199410000-00002. [DOI] [PubMed] [Google Scholar]
- Muller D.N, Fischli W, Clozel J.P, Hilgers K.F, Bohlender J, Menard J, Busjahn A, Ganten D, Luft F.C. Local angiotensin II generation in the rat heart: role of renin uptake. Circ. Res. 1998;82:13–20. doi: 10.1161/01.res.82.1.13. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Plump A.S, Raines E.W, Breslow J.L, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Nakazono K, Watanabe N, Matsuno K, Sasaki J, Sato T, Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc. Natl Acad. Sci. USA. 1991;88:10 045–10 048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C, et al. 1,4-Dihydropyridine calcium channel blockers inhibit plasma and LDL oxidation and formation of oxidation-specific epitopes in the arterial wall and prolong survival in stroke-prone spontaneously hypertensive rats. Stroke. 1999;30:1907–1915. doi: 10.1161/01.str.30.9.1907. [DOI] [PubMed] [Google Scholar]
- Nowycky M.C, Fox A.P, Tsien R.W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- Plump A.S, Smith J.D, Hayek T, Aalto-Setala K, Walsh A, Verstuyft J.G, Rubin E.M, Breslow J.L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. 10.1016/0092-8674(92)90362-G [DOI] [PubMed] [Google Scholar]
- Prasad A, Tupas-Habib T, Schenke W.H, Mincemoyer R, Panza J.A, Waclawin M.A, Ellahham S, Quyyumi A.A. Acute and chronic angiotensin-1 receptor antagonism reverses endothelial dysfunction in atherosclerosis. Circulation. 2000;101:2349–2354. doi: 10.1161/01.cir.101.20.2349. [DOI] [PubMed] [Google Scholar]
- Pratico D, Tangirala R.K, Rader D.J, Rokach J, FitzGerald G.A. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat. Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- Pratt P.F, Bonnet S, Ludwig L.M, Bonnet P, Rusch N.J. Upregulation of L-type Ca2+ channels in mesenteric and skeletal arteries of SHR. Hypertension. 2002;40:214–219. doi: 10.1161/01.hyp.0000025877.23309.36. 10.1161/01.HYP.0000025877.23309.36 [DOI] [PubMed] [Google Scholar]
- Ringer S. A further contribution regarding the influence of the blood on the contraction of the heart. J. Physiol. 1883;4:29–42. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomone S, Wibo M, Morel N, Godfraind T. Binding sites for 1,4-dihydropyridine Ca2+-channel modulators in rat intestinal smooth muscle. Naunyn Schmiedebergs Arch. Pharmacol. 1991;344:698–705. doi: 10.1007/BF00174754. 10.1007/BF00174754 [DOI] [PubMed] [Google Scholar]
- Salomone S, Dessy C, Morel N, Godfraind T. Inhibition by bosentan, an endothelin antagonist, of the hypersensitivity to Ca2+ channel activator evoked by salt-loading in basilar artery of stroke-prone spontaneously hypertensive rats. Life Sci. 1996;59:PL247–PL253. doi: 10.1016/0024-3205(96)00463-8. 10.1016/0024-3205(96)00463-8 [DOI] [PubMed] [Google Scholar]
- Salomone S, Morel N, Godfraind T. Role of nitric oxide in the contractile response to 5-hydroxytryptamine of the basilar artery from Wistar Kyoto and stroke-prone rats. Br. J. Pharmacol. 1997;121:1051–1058. doi: 10.1038/sj.bjp.0701227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato J, O'Brien T, Katusic Z.S, Fu A, Nygren J, Singh R, Nair K.S. Dietary antioxidants preserve endothelium dependent vasorelaxation in overfed rats. Atherosclerosis. 2002;161:327–333. doi: 10.1016/s0021-9150(01)00649-9. 10.1016/S0021-9150(01)00649-9 [DOI] [PubMed] [Google Scholar]
- Schiffrin E.L. Beyond blood pressure: the endothelium and atherosclerosis progression. Am. J. Hypertens. 2002;15:115S–122S. doi: 10.1016/s0895-7061(02)03006-6. 10.1016/S0895-7061(02)03006-6 [DOI] [PubMed] [Google Scholar]
- Sher E, Biancardi E, Passafaro M, Clementi F. Physiopathology of neuronal voltage-operated calcium channels. FASEB J. 1991;5:2677–2683. doi: 10.1096/fasebj.5.12.1655547. [DOI] [PubMed] [Google Scholar]
- Somlyo A.P, Somlyo A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. 10.1038/372231a0 [DOI] [PubMed] [Google Scholar]
- Stiles P.G. On the rhythmic activity of the oesophagus and the influence upon it of various media. Am. J. Physiol. 1901;5:338–357. [Google Scholar]
- Thomas S.R, Leichtweis S.B, Pettersson K, Croft K.D, Mori T.A, Brown A.J, Stocker R. Dietary cosupplementation with vitamin E and coenzyme Q(10) inhibits atherosclerosis in apolipoprotein E gene knockout mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:585–593. doi: 10.1161/01.atv.21.4.585. [DOI] [PubMed] [Google Scholar]
- Toikka J.O, Laine H, Ahotupa M, Haapanen A, Viikari J.S, Hartiala J.J, Raitakari O.T. Increased arterial intima-media thickness and in vivo LDL oxidation in young men with borderline hypertension. Hypertension. 2000;36:929–933. doi: 10.1161/01.hyp.36.6.929. [DOI] [PubMed] [Google Scholar]
- Tsien R.W, Tsien R.Y. Calcium channels, stores, and oscillations. Annu. Rev. Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Ursini F. Tissue protection by lacidipine: insight from redox behavior. J. Cardiovasc. Pharmacol. 1997;30:S28–S30. [Google Scholar]
- Weiss D, Kools J.J, Taylor W.R. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103:448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- Yukisada N, Ebashi F. Role of calcium in drug action on smooth muscle. Jpn J. Pharmacol. 1961;11:46–53. doi: 10.1254/jjp.11.46. [DOI] [PubMed] [Google Scholar]
- Zalba G, San Jose G, Moreno M.U, Fortuno M.A, Fortuno A, Beaumont F.J, Diez J. Oxidative stress in arterial hypertension: role of NAD(P)H oxidase. Hypertension. 2001;38:1395–1399. doi: 10.1161/hy1201.099611. [DOI] [PubMed] [Google Scholar]