Abstract
Normal ageing is associated with a degree of decline in a number of cognitive functions. Apart from the issues raised by the current attempts to expand the lifespan, understanding the mechanisms and the detailed metabolic interactions involved in the process of normal neuronal ageing continues to be a challenge. One model, supported by a significant amount of experimental evidence, views the cellular ageing as a metabolic state characterized by an altered function of the metabolic triad: mitochondria–reactive oxygen species (ROS)–intracellular Ca2+. The perturbation in the relationship between the members of this metabolic triad generate a state of decreased homeostatic reserve, in which the aged neurons could maintain adequate function during normal activity, as demonstrated by the fact that normal ageing is not associated with widespread neuronal loss, but become increasingly vulnerable to the effects of excessive metabolic loads, usually associated with trauma, ischaemia or neurodegenerative processes. This review will concentrate on some of the evidence showing altered mitochondrial function with ageing and also discuss some of the functional consequences that would result from such events, such as alterations in mitochondrial Ca2+ homeostasis, ATP production and generation of ROS.
Keywords: ageing, neurons, mitochondria, Ca2+ homeostasis, neuronal vulnerability, homeostatic reserve
There is always a murmur of expectation and a frisson of curiosity when the discussion reaches the issue of supercentenarians. To date, the human being with the oldest certified lifespan is Madame Jeanne Calment who, at the time of her death in August 1997, was 122 years and 164 days old, according to the Guiness World Book or Records. According to the other important reference source, The Bible, apart from the described instances of immortality, almost the whole of the Old Testament is full of supercentenarians. Adam lived 930 years and the record pertains to Methuselah who lived to 969 years. After the biblical flood, the situation on Earth deteriorated significantly, such that Abraham lived only to the age of 275, and Joseph died at a more realistic 110.
Historical antiquity artefacts record a relatively short lifespan, estimated in pre-Roman Italy at between 28 and 42 years (Capasso et al. 2003), and the increase in lifespan had been rather slow. However, as stressed in a number of recent reports, an analysis of life expectancy from 1840 to present shows a linear, steady-pace increase of almost three months per year, standing currently, at least for women, at 85 years (the men's life expectancy also increased, but at a slower rate, determining a slowly increasing gender gap; Oeppen & Vaupel 2002). Clearly, life expectancy is different in concept from the maximal age at death, keenly recorded by the books of records, but the ‘case’ of Mme. Calment raises an important question to which there is not yet an agreed answer. Was she the exception, a living example of the maximal life span of the human beings, as would be sustained by the gerontologists such as S. J. Olshansky (Carnes et al. 2003), or was she just a pioneer on the road to an ever-expanding life-expectancy, as would be argued by gerontologists such as Vaupel and Carey (Vaupel et al. 1998), with further prospects of immortality in the shape of various strategies for engineering negligible senescence, as coined by deGrey (de Grey et al. 2002)?
Disentangling reality from wishfulness is sometimes difficult, especially in the case of processes as multi-factorial and multi-layered as ageing. As a result, sometime significant paradigm shifts takes place. In the process of understanding the mechanisms that regulate ageing in the brain, an organ in which the principal cells are post-mitotic and thus outside the rules regulating replicative senescence, such an important paradigm shift resulted from the development of better morphological methods for volumetric counting of neurons. As presented in a seminal review in 1997 (Morrison & Hof 1997), normal brain ageing is not, as previously dogmatically thought, associated or explained by a decrease in neuronal numbers. Many studies since then (reviewed in Hof & Morrison 2004) have confirmed this fact and few brain regions show significant neuronal losses. Since normal ageing is, nevertheless, associated with a small decline in cognitive and memory functions (Rosenzweig & Barnes 2003), the substrate for such dysfunctions must be functional, at the level of synaptic activity.
1. Mitochondrial status
All these comments point to the fact that valid explanations require proper understanding of the mechanisms involved, in a bottom-up fashion, from the cellular/sub-cellular level to the network level. At cellular level, particularly for post-mitotic cells, an important theory that is able to explain a variety of the experimental observations is the ‘mitochondrial theory of ageing’ (MTA). A role for the mitochondria was mentioned initially in 1972 by Harman, within the context of his ‘free radicals theory of ageing’ (Harman 1972), but the MTA had been formally introduced in 1980 having as its central plank the view that ageing is, essentially, a consequence of mitochondrial DNA damage by mutation, inactivation or loss (Miquel et al. 1980). Even now, after more than 20 years, normal neuronal ageing can be seen, from a cellular physiology stand-point, as a metabolic state, characterized by the status of mitochondria.
(a) Polarization status
Several lines of experimental evidence show that in the aged tissues the mitochondria are chronically depolarized. In some studies, FACS analysis of the distribution of rhodamine-123 (R123) labelled mitochondria obtained from aged liver showed decreased labelling (Hagen et al. 1997). Other studies have demonstrated quantitatively that the proton leakage of the respiratory chain (resulting in a mitochondrial depolarization) is increased and ATP synthesis is decreased with age in the liver mitochondria of the mouse (Harper et al. 1998). Using a protonophore to release the rhodamine-123 accumulated in the mitochondria of cerebellar neurons in brain slices, we have shown more recently (Xiong et al. 2002) that in the aged neurons there is a significant age-dependent decrease in the amount of mitochondrial dye accumulated in the aged neurons, consistent with the concept of age-induced mitochondrial depolarization. Using a ratiometric mitochondrial dye (JC-1) under confocal microscopy imaging, a similar result was reported for acutely dissociated basal forebrain neurons (Murchison et al. 2004). One possible confounding factor in interpreting the data from such experiments is that ageing can induce also a decrease in the number of mitochondria. Thus, studies in both humans (Miquel 1992) and rats (Bertoni-Freddari et al. 1994; Sastre et al. 1998) show that in young cells there are a relatively large number of small mitochondria. In aged rats and humans, however, there are a smaller number of larger mitochondria; however, the total volume of mitochondria (up to 20% of cell volume) remains roughly the same in young and old rats/humans. These larger, mega-mitochondria are not as bio-energetically efficient as the small mitochondria (Sastre et al. 1998; Wakabayashi 2002).
2. Functional consequences
From the observation that mitochondria in the aged tissues are chronically depolarized, a number of functional consequences must follow. Mitochondrial chronic depolarization should affect the Ca2+ gradient across the mitochondrial membrane system and thus either reduce the effectiveness of the mitochondrial Ca2+ stores during normal Ca2+ signalling or increase the threshold required for the activation of the mitochondrial Ca2+ uptake. Also, mitochondrial depolarization should affect their capacity to produce ATP, as well as reducing their production of free radicals (Nicholls 2004). All together, such changes in the metabolic status would decrease the homeostatic reserve of the neurons and increase their susceptibility to injury.
(a) Calcium uptake and mitochondrial Ca2+ stores
During neuronal activity that involves increases of cytosolic Ca2+, mitochondria can take up significant loads of Ca2+ (Nicholls & Budd 2000), that can play either a beneficial role of coupling increased metabolic demands with increased oxidative phosphorylation activity (McCormack & Denton 1994) or a deleterious one, activating processes that lead ultimately to cell death (Duchen 1999; Nicholls & Budd 2000). Until recently, the nature of this mitochondrial Ca uniporter was not well established, apart from being defined by a relatively low Ca2+ affinity (in the low micromolar range) and a high capacity (Gunter et al. 2000). Although the initial estimations of the apparent affinity were in the range of 200–300 nM Ca2+ (Gunter & Gunter 1994), and thus precluding an intervention of this Ca2+ transport system at resting concentrations of cytosolic Ca2+ (between 50 and 100 nM Ca2+), other studies, using mitochondrially targeted Ca2+-sensitive dyes, showed rapid Ca2+ transients that do not require large cytoplasmic Ca2+ increases (Duchen 1999; Rizzuto et al. 1993). Very little is known about the effects of age on this Ca2+ uniporter. By use of simultaneous recordings of both cytosolic Ca2+ and mitochondrial depolarization, we were able to show that with ageing, either in brain slices or in a model of ageing in primary neuronal cell cultures, there is an increase in the threshold required to activate mitochondrial Ca2+ uptake, as measured by the level of cytosolic Ca2+ at which mitochondrial depolarization is initiated (396±33 in young and 563±43 in old neurons; Xiong et al. 2002, 2004). Recently, by use of direct patch clamp electrophysiological measurements on mitoplasts formed from the inner mitochondrial membranes, a highly specific Ca2+ channel on the inner mitochondrial membrane, with a high Ca2+ affinity (in the nanomolar range) and displaying the pharmacological properties earlier established for the Ca2+ uniporter has been described (Kirichok et al. 2004). A high affinity, coupled with the rather small Ca2+-induced inactivation and together with the fact that the significant electrochemical gradient across the mitochondrial membranes is the driving force and modulator of this transport mode, would support our view that the age-dependent increase in the threshold level for mitochondrial Ca2+ uptake is a result of a decreased electrochemical gradient for Ca2+ secondary to the chronic mitochondrial depolarization, rather than due to a change in the Ca2+ affinity of the transporter.
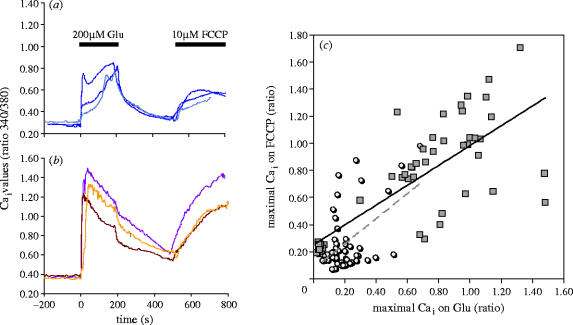
However, the increase of the Ca2+ threshold for the activation of the mitochondrial Ca2+ uptake does not imply a decrease in the effectiveness of the mitochondrial Ca2+ store. The participation of the mitochondrial Ca2+ stores can be assessed at two levels: (i) effects on the rates of cytosolic [Ca2+]i increase that follow stimulation and (ii) amount of Ca2+ taken into the mitochondrial stores. When measured at [Ca2+]i values above the mitochondrial threshold for Ca2+ uptake, mitochondria decreased the rate of cytosolic [Ca2+]i increase, an effect that was not affected by ageing (Murchison et al. 2004; Toescu 2000). Some reports suggest that in the aged neurons the mitochondrial Ca2+ handling properties are less important in shaping the depolarization-induced Ca2+ signals (i.e. when the buffering role of the mitochondria is eliminated by use of a protonophore, the Ca2+ signal evoked by depolarization is almost doubled in the young neurons, and increased only by 30% in the aged neurons; (Murchison et al. 2004). Using again a protonophore to assess the size of the mitochondrial Ca2+ pool following neuronal stimulation and activation of the mitochondrial Ca2+ uptake, we showed that upon glutamatergic stimulation the major determinant controlling the size of the releasable mitochondrial Ca2+ pool is the size of the Ca2+ load to which a particular neuron has been exposed to, independent of age (figure 1). This is despite the fact that, as described above, mitochondria in the aged neurons are chronically depolarized and that there is an increase of the threshold value of activation of Ca2+ uptake, but is in agreement with the fact that an important determinant of the size of the mitochondrial Ca2+ store is the amount of phosphate available in the mitochondrial matrix (Nicholls & Budd 2000).
Figure 1.
Assessment of mitochondrial Ca2+ stores. Cerebellar granule neurons were prepared and maintained as previously described (Xiong et al. 2004). The protocol for assessing the size of the mitochondrial Ca2+ pools on neuronal activation involved a 3 min stimulation of the neurons by bath perifusion with 200 μM glutamate (in the presence of 10 μM glycine and in the absence of added Mg2+). After removal of the stimulus and a standard period of recovery (5 min) the neurons were perifused with 10 μM CCCP, a protonophore that dissipates the mitochondrial membrane potential and activates the release of the mitochondrial Ca2+. Intracellular free Ca2+ was measured using fura-2AM as the Ca2+-sensitive fluorescent dye and the methodologies and technologies described previously (Xiong et al. 2002, 2004). Panels (a) and (b) show average traces from individual experiments, performed either on ‘young’ neurons (Panel (a), neurons at 7–10 DIV) or on ‘old’ neurons (Panel (b), neurons at >28 DIV). In the older cultures, the amplitude of the Ca2+ signal evoked by 200 μM was significantly larger. Panel (c) describes the correlation between the value of the maximal [Ca2+]i on Glu stimulation (on the abscissa, and expressed in 340/380 nm ratio units) against the 5 min values of [Ca2+]i recorded after the addition of the protonophore. The light, shadowed circles represent the correlation values obtained for the young neurons, while the darker squares represent the values of the older neurons. For both populations (‘young’ and ‘old’) there is a highly significant correlation between the two [Ca2+]i parameters, indicating that the level of cytosolic Ca2+ load is a very important factor in regulating the size of the mitochondrial Ca2+ stores. Furthermore, the slopes of the linear fit for the two populations are very similar (‘young’: 0.978 and ‘old’: 0.834), despite the fact that, as discussed in the text, there are significant difference in the level of mitochondrial polarization in the two populations.
(b) ATP production
Mitochondrial dysfunction should also result in possible changes in ATP production or, more importantly, in the ATP/ADP ratio. Albeit extremely important, these issues are, particularly in the context of ageing research, rather under-studied and most of the conclusions are inferred from the study of mitochondrial dysfunction rather than from direct measurements of ATP production. The danger in these conditions is that the multi-factorial nature of the coupling between the oxidative cycles of the respiratory chain and the adenosine disphosphate phosphorylation may lead to significant miscalculations. Thus, Davey et al. showed that in rat brain synaptosomes, differential levels of inhibition of the complexes I, III and IV were required to induce significant decreases of ATP levels: while only 20% inhibition of the complex I was sufficient for the inhibition of ATP production, the complex III was much more resilient, and required a drastic inhibition of up to 80% to achieve the same block of ATP production (Davey et al. 1998). It is also important to note that neurons should have a certain hierarchy of ATP-consuming processes, quite possibly similar to that described for thymocytes (Buttgereit & Brand 1995), where the synthetic processes (protein and nucleic acids synthesis) take priority and use about 50% of the resting ATP production. Another important feature is that neuronal stimulation activates a massive increase in energetic demand, based mainly on the requirements of the Na2+/K2+ ATPase for restoring the ionic balance in the wake of action potentials (Attwell & Laughlin 2001). Despite these significant energetic requirements of neuronal activity, studies on cerebellar granule neurons in primary cultures suggest that ATP supply is not the triggering factor in initiating the delayed Ca2+ dysregulation (DCD) associated with glutamate excitotoxicity (Castilho et al. 1998; Nicholls & Budd 2000). Furthermore, for primary culture models of excitotoxicity a certain degree of mitochondrial depolarization is protective against neuronal death, through preventing Ca2+ overload of the mitochondria (Castilho et al. 1998; Stout et al. 1998). Whether these considerations are applicable to the metabolic state of the aged neurons is not yet clear, and only few direct data are available. Recent direct luminometric measurements of the ATP content and rate of ATP production in resting conditions using mitochondria acutely obtained from Fischer 344 rats showed no difference between adult (12 months) and old (24 months) animals (Drew & Leeuwenburgh 2003). In measuring ATP from whole brain slices (Xiong & Toescu unpublished results) we observed little difference in ATP content between young and old cerebellar slices, but following neuronal activity (45 min after a 5 min pulse of 75 mM KCl) the ATP content in the old slices (from 20 to 23 months old animals) was about 50% less than in the younger slices (12 months old), indicating a decreased functional reserve.
(c) Free radical production
Mitochondria are also a major site of free radical production, and the relationship between mitochondrial functional status and free radical production is a complex and subtle one. Although mitochondria have a high rate of oxygen consumption, careful studies of mitochondria isolated from brain or other organs showed that only a small proportion of this oxygen (less than 3%) generate free radicals (aka, reactive oxygen species, ROS; e.g. Floyd & Hensley 2002; Sastre et al. 2003). For a long period of time, stemming from the two important functional theories discussed above aiming at explaining the process of cellular ageing through a functional/metabolic perspective (the ‘free radicals’ and the ‘mitochondrial’ theories of ageing), a commonly held paradigmatic view was that of ‘live fast, die young’. This was based on the observation of the inverse relationship between mitochondrial ROS production and longevity in mammal species (Ku et al. 1993), and implicit in it was the fact that an activation of the respiratory chain activity will inherently increase the rate of electrons slippage at complex III (predominantly, although a similar event can take place at complex I (Nicholls & Budd 2000)). However, as demonstrated experimentally (Korshunov et al. 1997) and modelled theoretically (Nicholls 2004), the relationship is inversely proportional and at fast mitochondrial respiratory rates (state 3, high substrates levels, high ADP concentration, low ATP/ADP ratio) fewer free radicals are produced than in state 4 (‘resting’ state, with low ADP and high ATP/ADP ratio; Nicholls 2004). However, there are a variety of other reasons for which the role of oxidative stress in the ageing brain is of relevance: a high content of unsaturated fatty acids, that are more liable to peroxidation, a high content of pro-oxidant iron ions and a low reserve of antioxidant defences (Floyd & Hensley 2002). If it is still unclear whether the resting or stimulated rate of free radicals production is affected by the ageing process, what is clear and demonstrated by numerous studies is that ageing is associated with a significant accumulation of markers of oxidative stress. Once generated, free radicals can oxidize proteins, and accumulation of oxidized proteins in many tissues is considered a hallmark of ageing (Stadtman 1992). Other targets of free radicals' action are the lipids, and peroxidation of biological lipids yields a large number of compounds. Amongst them, 4-hydroxynonenal (4-HNE), resulting from the peroxidation of omega-6-conjugated fatty acids (e.g. arachidonic and linoleic acids; Esterbauer et al. 1991), is one of the most studied aldehydes. HNE is very active in biological systems and can react with many types of biological molecules: amino acids in proteins, bases in DNA and other lipid amino groups (Esterbauer et al. 1991), explaining its widespread range of actions from effects on synaptic function (Keller et al. 1997) to mediating excitotoxicity and neuronal death (Mark et al. 1997). Increased levels of lipid peroxidation and particularly HNE adduct products have been demonstrated in the brain of human subjects with neurodegenerative diseases (Yoritaka et al. 1996; Markesbery & Lovell 1998). Another important and recognized target of free radicals attack is the DNA. Many oxidatively damaged DNA bases have been identified, but one of the most widely studied is the 8-OHdG (8-hydroxy-2′-deoxyguanosine; aka, 8-oxo-2-deoxiguanosine, oxo8dG; Floyd et al. 1990). A recent study (Hamilton et al. 2001) showed significant age-dependent increases in 8-OHdG in various tissues in the rat and mouse that resulted not from a decrease in the antioxidant defences, but from an increased sensitivity of the DNA oxidation process.
(d) Increased vulnerability
Thus, with the accumulation of functional defects at different levels, underlined by the activity of the triad Ca2+–mitochondria–ROS, the issue of cellular and neuronal vulnerability and susceptibility to damage as a function of age becomes important. Taking a lead from the physical model of ageing, characterized by frailty, it is a common sense assertion that age brings vulnerability also in the domain of cellular physiology. Indeed, using the traumatic brain injury as an experimental model in rodents, it has been long demonstrated that age is associated with a significantly increased mortality and, for smaller levels of injury, with a greater level of acute neurological deficits (Hamm et al. 1991), with comparable equivalent results in human studies (Susman et al. 2002). However, the issue is fraught with some difficulties of interpretation and assessment, since there is a certain developmentally controlled maturation of vulnerability to excitotoxic insults. Thus, in short-term cultures of hippocampal neurons, an N-methyl-d-aspartate (NMDA)-induced excitotoxic lesion resulted in a minimal degree of neuronal death in neurons derived from 3 day old animals, whereas more that 90% of the neurons obtained from 21 day old animals died (Marks et al. 2000), reproducing in vitro results obtained in vivo in rodents (Liu et al. 1996). This dramatic difference was not due to differences in [Ca2+]i signalling, since both populations of neurons gave similar values, but to significant differences in the mitochondrial depolarization response—much larger in the older population (Marks et al. 2000). Another study, using a similar experimental model and looking at the NMDA-evoked electrophysiological and [Ca2+]i responses, showed that with ageing (26 months old rodents) there was a significant increase in responsiveness when compared with the ‘middle-aged’ (nine months old) neurons (Cady et al. 2001). Interestingly, during attempts to develop an ‘ageing in the dish’ experimental model, it was reported that several of the functional features of the aged neurons as recorded in acute brain slice preparations from the aged animals (either hippocampal or cerebellar) developed during longer term primary neuronal cultures (Blalock et al. 1999; Vergun et al. 1999; Xiong et al. 2004).
It is important to understand that increased vulnerability, as that associated with ageing, does not mean increased neuronal death. Many times the word and the concept of ‘ageing’ are used in the same context as used for ‘neurodegeneration’, and attempts to explain the mechanisms involved in various neurodegenerative diseases are extrapolated to the explanation of the normal process of ageing. Furthermore, there are some who suggest that neurodegeneration and one of its resulting dysfunctions, dementia, are inevitable and will follow inexorably from the advanced age (Terry & Katzman 2001). To counteract these views, others are pointing out significant morphological and functional differences between the aged brain and that afflicted by neurodegeneration (Morrison & Hof 1997; Morrison 2001). Another argument, from the realm of basic neurophysiology, is the observation, confirmed by more and more labs, that the values of resting [Ca2+]i are not affected by age (Thibault et al. 2001; Xiong et al. 2002; Murchison et al. 2004). Within the context of the ‘Ca2+ hypothesis of ageing’, the dysfunction of the aged brain was seen as a result of alterations in one or another of the Ca2+ homeostatic processes (Khachaturian 1994). The value of the resting [Ca2+]i represents a steady-state balance between the Ca2+ entry and Ca2+ extrusion mechanisms (Toescu & Verkhratsky 2000). Alterations, even minimal, in one or another of these Ca2+ flux processes should affect, especially if expressed over a long period of time, the value of the resting [Ca2+]i in the aged neurons. The observation of the stability of the resting [Ca2+]i values across the lifespan is important not only in the context of the older ‘Ca2+ hypothesis of ageing’ (Verkhratsky & Toescu 1998; Toescu et al. 2004) but also in the wider context of the significant deleterious effects of increased cytosolic Ca2+ (Duchen 1999; Nicotera et al. 1999; Nicholls & Budd 2000).
3. Ageing as physiological state of decreased homeostatic reserve
What all this data indicate is that ageing means a decrease in the homeostatic reserve (or, according to other nomenclature, allostatic load (McEwen 2000)). This state can be defined as a decrease in the capacity to oppose the damaging effects of strong, excessive stressors and is entirely compatible with functioning at normal levels of functional load. An example of this process is the response of neurons to different levels of stimulation. One of the most consistent proofs of age-dependent dysregulation of [Ca2+]i homeostasis is the delayed recovery of the resting [Ca2+]i values following large stimulation-evoked Ca2+ signals (Toescu & Verkhratsky 2000; Xiong et al. 2002). The decrease in the [Ca2+]i clearance rate could be explained either by metabolic limitations, as discussed above, or by functional or biological changes in the properties of the Ca2+ removal systems. Evidence for age-dependent alterations in the function of the plasma membrane Ca2+ATPase (PMCA) has been presented, and might involve changes in the phosphorylation properties or calmodulin-binding properties (Zaidi et al. 1998). Another Ca2+ removal system of significance in neuronal physiology is the PM Na+/Ca2+ exchanger (Hoyt et al. 1998; Kiedrowski 1999). Recent exciting data revealed a new mechanism through which the activity of the Na+/Ca2+ exchanger can be modulated, involving a Ca2+-dependent activation of proteolysis and resulting in a loss of function (Bano et al. 2005). This mechanism has been proposed to explain the DCD associated with late, post-ischaemic lesions; whether similar, but subtler, changes in either the types of Na+/Ca2+ exchanger present or in the sensitivity of the proteolytic mechanism to Ca2+ are taking place during the ageing process is not at all clear. The issue of whether the delayed recovery of [Ca2+]i in the aged neurons is due to a functional or an irreversible change is illuminated by the fact that when the cytosolic Ca2+ load is reduced by decreasing the level of stimulation, the rate of recovery in the aged neurons is significantly improved and approaches the values recorded in the young neurons (Toescu & Xiong 2004). Similarly, when maintained in in vitro conditions, for the first 2–3 h, there are no significant differences in the number of compromised neurons between slices obtained from young or old mice; however, by 5 h a significantly higher number of non-viable neurons was recorded in the aged slices (Xiong et al. 2002), reflecting an increased susceptibility of the aged neurons to the metabolically demanding conditions of in vitro maintenance.
In conclusion, it is our view that normal neuronal ageing is a metabolic state characterized by a decreased homeostatic reserve, defined as the capacity of the cells (neurons) to oppose the destabilizing effects of metabolic stressors. Implicit in this definition, and in keeping with the wealth of experimental evidence, is that in the aged neurons normal metabolic activity is maintained at rest or during moderate levels of activity. Functional deficits of the aged neurons are expressed, in a use- and level-dependent manner, only at higher levels of stimulation, usually associated with clinical or subclinical instances of trauma, ischaemia or other excitotoxic events (figure 2). Results obtained in a number of laboratories including ours indicate that a major factor underlying the decreased homeostatic reserve is a change in the mitochondrial status in the aged neurons, resulting in a number of down-stream functional effects. Understanding the intimate relationship that links the members of the functional triad Ca2+–mitochondria–ROS is an important challenge, but should provide new avenues not only for a better understanding of the ageing process, but also for allowing more successful therapeutic interventions in a variety of pathological instances associated with ageing.
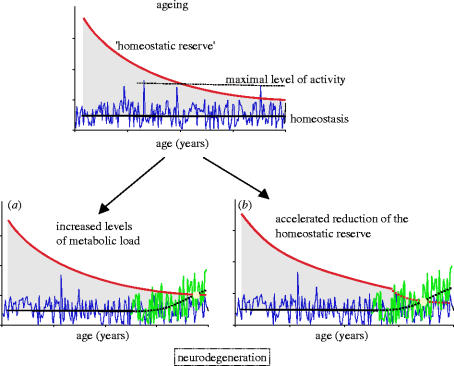
Figure 2.
Relationship between ageing and neurodegeneration. The process of ageing (top panel) can be seen as a continuous decrease of the homeostatic reserve, defined as the capacity of cells to fight various metabolic stressors and maintain the cells/organ on the steady-state level of homeostasis. With age, acute surges of metabolic activity become more dangerous as they reach the limits of homeostatic reserve defences, a process that underlies the age-dependent increased in vulnerability. When the homeostasis line intersects the homeostatic reserve line, the biological system becomes unstable and severe dysfunction/death ensues. The process of neurodegeneration, characterized by extensive neuronal death, becomes mostly manifest at the older ages, on the background of decreased homeostatic reserve, and could result either from (a) an increased level of metabolic load or from (b) an accelerated reduction of the homeostatic reserve or from a combination of both.
Acknowledgments
The author wishes to acknowledge the BBSRC, which provided, through the SAGE Initiative, a significant part of the financial support for the work performed in the author's laboratory and to Dr Jie Xiong for her hard work and involvement. I am also grateful to Professor A. Verkhratsky for discussions on various aspects of the work reported here as I am also to Johann Sebastian Bach.
Footnotes
One contribution of 18 to a Theme Issue ‘Reactive oxygen species in health and disease’.
References
- Attwell D, Laughlin S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. 10.1097/00004647-200110000-00001 [DOI] [PubMed] [Google Scholar]
- Bano D, Young K.W, Guerin C.J, Lefeuvre R, Rothwell N.J, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na2+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. 10.1016/j.cell.2004.11.049 [DOI] [PubMed] [Google Scholar]
- Bertoni-Freddari C, Fattoretti P, Casoli T, Spagna C, Meier-Ruge W. Morphological alterations of synaptic mitochondria during aging. The effect of hydergine treatment. Ann. NY Acad. Sci. 1994;717:137–149. doi: 10.1111/j.1749-6632.1994.tb12081.x. [DOI] [PubMed] [Google Scholar]
- Blalock E, Porter N, Landfield P. Decreased G-protein-mediated regulation and shift in calcium channel types with age in hippocampal cultures. J. Neurosci. 1999;19:8674–8684. doi: 10.1523/JNEUROSCI.19-19-08674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttgereit F, Brand M.D. A hierarchy of ATP-consuming processes in mammalian cells. Biochem. J. 1995;312:163–167. doi: 10.1042/bj3120163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady C, Evans M.S, Brewer G.J. Age-related differences in NMDA responses in cultured rat hippocampal neurons. Brain Res. 2001;921:1–11. doi: 10.1016/s0006-8993(01)03063-3. 10.1016/S0006-8993(01)03063-3 [DOI] [PubMed] [Google Scholar]
- Capasso L, D'Anastasio R, Pierfelice L, Di Fabrizio A, Gallenga P.E. Roman conquest, lifespan, and diseases in ancient Italy. Lancet. 2003;362:668. doi: 10.1016/S0140-6736(03)14175-X. 10.1016/S0140-6736(03)14175-X [DOI] [PubMed] [Google Scholar]
- Carnes B.A, Olshansky S.J, Grahn D. Biological evidence for limits to the duration of life. Biogerontology. 2003;4:31–45. doi: 10.1023/a:1022425317536. 10.1023/A:1022425317536 [DOI] [PubMed] [Google Scholar]
- Castilho R.F, Hansson O, Ward M.M, Budd S.L, Nicholls D.G. Mitochondrial control of acute glutamate excitotoxicity in cultured cerebellar granule cells. J. Neurosci. 1998;18:10 277–10 286. doi: 10.1523/JNEUROSCI.18-24-10277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey G.P, Peuchen S, Clark J.B. Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J. Biol. Chem. 1998;273:12 753–12 757. doi: 10.1074/jbc.273.21.12753. 10.1074/jbc.273.21.12753 [DOI] [PubMed] [Google Scholar]
- de Grey A.D, Ames B.N, Andersen J.K, Bartke A, Campisi J, Heward C.B, McCarter R.J, Stock G. Time to talk SENS: critiquing the immutability of human aging. Ann. NY Acad. Sci. 2002;959:452–462. doi: 10.1111/j.1749-6632.2002.tb02115.x. discussion 463-5. [DOI] [PubMed] [Google Scholar]
- Drew B, Leeuwenburgh C. Method for measuring ATP production in isolated mitochondria: ATP production in brain and liver mitochondria of Fischer-344 rats with age and caloric restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1259–R1267. doi: 10.1152/ajpregu.00264.2003. [DOI] [PubMed] [Google Scholar]
- Duchen M. Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death. J. Physiol. 1999;516:1–17. doi: 10.1111/j.1469-7793.1999.001aa.x. 10.1111/j.1469-7793.1999.001aa.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur R.J, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. 10.1016/0891-5849(91)90192-6 [DOI] [PubMed] [Google Scholar]
- Floyd R.A, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging. 2002;23:795. doi: 10.1016/s0197-4580(02)00019-2. 10.1016/S0197-4580(02)00019-2 [DOI] [PubMed] [Google Scholar]
- Floyd R.A, West M.S, Eneff K.L, Schneider J.E, Wong P.K, Tingey D.T, Hogsett W.E. Conditions influencing yield and analysis of 8-hydroxy-2′-deoxyguanosine in oxidatively damaged DNA. Anal. Biochem. 1990;188:155–158. doi: 10.1016/0003-2697(90)90544-j. 10.1016/0003-2697(90)90544-J [DOI] [PubMed] [Google Scholar]
- Gunter K, Gunter T. Transport of calcium by mitochondria. J. Bioenerg. Biomembr. 1994;26:471–485. doi: 10.1007/BF00762732. 10.1007/BF00762732 [DOI] [PubMed] [Google Scholar]
- Gunter T, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. doi: 10.1054/ceca.2000.0168. 10.1054/ceca.2000.0168 [DOI] [PubMed] [Google Scholar]
- Hagen T, Yowe D, Bartholomew J, Wehr C, Do K, Park J, Ames B. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc. Natl Acad. Sci. USA. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. 10.1073/pnas.94.7.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M.L, Van Remmen H, Drake J.A, Yang H, Guo Z.M, Kewitt K, Walter C.A, Richardson A. Does oxidative damage to DNA increase with age? Proc. Natl Acad. Sci. USA. 2001;98:10 469–10 474. doi: 10.1073/pnas.171202698. 10.1073/pnas.171202698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm R.J, Jenkins L.W, Lyeth B.G, White-Gbadebo D.M, Hayes R.L. The effect of age on outcome following traumatic brain injury in rats. J. Neurosurg. 1991;75:916–921. doi: 10.3171/jns.1991.75.6.0916. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging: dietary implications. Am. J. Clin. Nutr. 1972;25:839–843. doi: 10.1093/ajcn/25.8.839. [DOI] [PubMed] [Google Scholar]
- Harper M.E, Monemdjou S, Ramsey J.J, Weindruch R. Age-related increase in mitochondrial proton leak and decrease in ATP turnover reactions in mouse hepatocytes. Am. J. Physiol. 1998;275:E197–E206. doi: 10.1152/ajpendo.1998.275.2.E197. [DOI] [PubMed] [Google Scholar]
- Hof P.R, Morrison J.H. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. 10.1016/j.tins.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Hoyt K.R, Stout A.K, Cardman J.M, Reynolds I.J. The role of intracellular Na2+ and mitochondria in buffering of kainate-induced intracellular free Ca2+ changes in rat forebrain neurones. J. Physiol. 1998;509:103–116. doi: 10.1111/j.1469-7793.1998.103bo.x. 10.1111/j.1469-7793.1998.103bo.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J.N, Pang Z, Geddes J.W, Begley J.G, Germeyer A, Waeg G, Mattson M.P. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J. Neurochem. 1997;69:273–284. doi: 10.1046/j.1471-4159.1997.69010273.x. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z.S. Calcium hypothesis of Alzheimer's disease and brain aging. Ann. NY Acad. Sci. 1994;747:1–11. doi: 10.1111/j.1749-6632.1994.tb44398.x. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L. N-methyl-d-aspartate excitotoxicity: relationships among plasma membrane potential, Na+/Ca2+ exchange, mitochondrial Ca2+ overload, and cytoplasmic concentrations of Ca2+, H+, and K+ Mol. Pharmacol. 1999;56:619–632. doi: 10.1124/mol.56.3.619. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Krapivinsky G, Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. 10.1038/nature02246 [DOI] [PubMed] [Google Scholar]
- Korshunov S.S, Skulachev V.P, Starkov A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. 10.1016/S0014-5793(97)01159-9 [DOI] [PubMed] [Google Scholar]
- Ku H.H, Brunk U.T, Sohal R.S. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic. Biol. Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-q. 10.1016/0891-5849(93)90165-Q [DOI] [PubMed] [Google Scholar]
- Liu Z, Stafstrom C.E, Sarkisian M, Tandon P, Yang Y, Hori A, Holmes G.L. Age-dependent effects of glutamate toxicity in the hippocampus. Brain Res. Dev. Brain Res. 1996;97:178–184. doi: 10.1016/s0165-3806(96)00141-1. 10.1016/S0165-3806(96)00141-1 [DOI] [PubMed] [Google Scholar]
- Mark R.J, Lovell M.A, Markesbery W.R, Uchida K, Mattson M.P. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J. Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Markesbery W.R, Lovell M.A. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol. Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. 10.1016/S0197-4580(98)00009-8 [DOI] [PubMed] [Google Scholar]
- Marks J.D, Bindokas V.P, Zhang X.M. Maturation of vulnerability to excitotoxicity: intracellular mechanisms in cultured postnatal hippocampal neurons. Brain Res. Dev. Brain Res. 2000;124:101–116. doi: 10.1016/s0165-3806(00)00096-1. 10.1016/S0165-3806(00)00096-1 [DOI] [PubMed] [Google Scholar]
- McCormack J, Denton R. Signal transduction by intramitochondrial Ca2+ in mammalian energy metabolism. NIPS. 1994;9:71–76. [Google Scholar]
- McEwen B.S. Allostasis, allostatic load, and the aging nervous system: role of excitatory amino acids and excitotoxicity. Neurochem. Res. 2000;25:1219–1231. doi: 10.1023/a:1007687911139. 10.1023/A:1007687911139 [DOI] [PubMed] [Google Scholar]
- Miquel J. An update on the mitochondrial-DNA mutation hypothesis of cell aging. Mutat. Res. 1992;275:209–216. doi: 10.1016/0921-8734(92)90024-j. [DOI] [PubMed] [Google Scholar]
- Miquel J, Economos A.C, Fleming J, Johnson J.E., Jr Mitochondrial role in cell aging. Exp. Gerontol. 1980;15:575–591. doi: 10.1016/0531-5565(80)90010-8. 10.1016/0531-5565(80)90010-8 [DOI] [PubMed] [Google Scholar]
- Morrison J.H. Which synapses are affected in aging and what is the nature of their vulnerability? A commentary on “life span and synapses: will there be a primary senile dementia?”. Neurobiol. Aging. 2001;22:349–350. doi: 10.1016/s0197-4580(00)00244-x. 10.1016/S0197-4580(00)00244-X [DOI] [PubMed] [Google Scholar]
- Morrison J, Hof P. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. 10.1126/science.278.5337.412 [DOI] [PubMed] [Google Scholar]
- Murchison D, Zawieja D.C, Griffith W.H. Reduced mitochondrial buffering of voltage-gated calcium influx in aged rat basal forebrain neurons. Cell Calcium. 2004;36:61–75. doi: 10.1016/j.ceca.2003.11.010. 10.1016/j.ceca.2003.11.010 [DOI] [PubMed] [Google Scholar]
- Nicholls D.G. Mitochondrial membrane potential and aging. Aging Cell. 2004;3:35–40. doi: 10.1111/j.1474-9728.2003.00079.x. 10.1111/j.1474-9728.2003.00079.x [DOI] [PubMed] [Google Scholar]
- Nicholls D, Budd S. Mitochondria and neuronal survival. Physiol. Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Leist M, Manzo L. Neuronal cell death: a demise with different shapes. Trends Pharmacol. Sci. 1999;20:46–51. doi: 10.1016/s0165-6147(99)01304-8. 10.1016/S0165-6147(99)01304-8 [DOI] [PubMed] [Google Scholar]
- Oeppen J, Vaupel J.W. Demography. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. 10.1126/science.1069675 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rosenzweig E.S, Barnes C.A. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. 10.1016/S0301-0082(02)00126-0 [DOI] [PubMed] [Google Scholar]
- Sastre J, et al. A Ginkgo biloba extract (EGb 761) prevents mitochondrial aging by protecting against oxidative stress. Free Radic. Biol. Med. 1998;24:298–304. doi: 10.1016/s0891-5849(97)00228-1. 10.1016/S0891-5849(97)00228-1 [DOI] [PubMed] [Google Scholar]
- Sastre J, Pallardo F.V, Vina J. The role of mitochondrial oxidative stress in aging. Free Radic. Biol. Med. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. 10.1016/S0891-5849(03)00184-9 [DOI] [PubMed] [Google Scholar]
- Stadtman E.R. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Stout A.K, Raphael H.M, Kanterewicz B.I, Klann E, Reynolds I.J. Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat. Neurosci. 1998;1:366–373. doi: 10.1038/1577. 10.1038/1577 [DOI] [PubMed] [Google Scholar]
- Susman M, DiRusso S.M, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J. Trauma. 2002;53:219–223. doi: 10.1097/00005373-200208000-00004. [discussion 223-224]. [DOI] [PubMed] [Google Scholar]
- Terry R.D, Katzman R. Life span and synapses: will there be a primary senile dementia? Neurobiol. Aging. 2001;22:347–348. doi: 10.1016/s0197-4580(00)00250-5. 10.1016/S0197-4580(00)00250-5 [discussion 353-354]. [DOI] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield P.W. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J. Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E.C. Mitochondria and Ca2+ signaling. J. Cell. Mol. Med. 2000;4:164–175. doi: 10.1111/j.1582-4934.2000.tb00114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E.C, Verkhratsky A. Parameters of calcium homeostasis in normal neuronal ageing. J. Anat. 2000;197:563–569. doi: 10.1046/j.1469-7580.2000.19740563.x. 10.1046/j.1469-7580.2000.19740563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toescu E.C, Xiong J. Metabolic substrates of neuronal aging. Ann. NY Acad. Sci. 2004;1019:19–23. doi: 10.1196/annals.1297.004. 10.1196/annals.1297.004 [DOI] [PubMed] [Google Scholar]
- Toescu E.C, Verkhratsky A, Landfield P.W. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. 10.1016/j.tins.2004.07.010 [DOI] [PubMed] [Google Scholar]
- Vaupel J.W, et al. Biodemographic trajectories of longevity. Science. 1998;280:855–860. doi: 10.1126/science.280.5365.855. 10.1126/science.280.5365.855 [DOI] [PubMed] [Google Scholar]
- Vergun O, Keelan J, Khodorov B.I, Duchen M.R. Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J. Physiol. 1999;519:451–466. doi: 10.1111/j.1469-7793.1999.0451m.x. 10.1111/j.1469-7793.1999.0451m.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Toescu E. Calcium and neuronal ageing. Trends Neurosci. 1998;21:2–7. doi: 10.1016/s0166-2236(97)01156-9. 10.1016/S0166-2236(97)01156-9 [DOI] [PubMed] [Google Scholar]
- Wakabayashi T. Megamitochondria formation—physiology and pathology. J. Cell. Mol. Med. 2002;6:497–538. doi: 10.1111/j.1582-4934.2002.tb00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Verkhratsky A, Toescu E.C. Changes in mitochondrial status associated with altered Ca2+ homeostasis in aged cerebellar granule neurones in brain slices. J. Neurosci. 2002;22:10 761–10 771. doi: 10.1523/JNEUROSCI.22-24-10761.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Camello P.J, Verkhratsky A, Toescu E.C. Mitochondrial polarisation status and [Ca2+]i signalling in rat cerebellar granule neurones aged in vitro. Neurobiol. Aging. 2004;25:349–359. doi: 10.1016/S0197-4580(03)00123-4. 10.1016/S0197-4580(03)00123-4 [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Hattori N, Uchida K, Tanaka M, Stadtman E.R, Mizuno Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl Acad. Sci. USA. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. 10.1073/pnas.93.7.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A, Gao J, Squier T.C, Michaelis M.L. Age-related decrease in brain synaptic membrane Ca2+-ATPase in F344/BNF1 rats. Neurobiol. Aging. 1998;19:487–495. doi: 10.1016/s0197-4580(98)00078-5. 10.1016/S0197-4580(98)00078-5 [DOI] [PubMed] [Google Scholar]