Abstract
The NADPH oxidase is the main weapon of phagocytic white blood cells that are the first line of defence of our body against invading pathogens, and patients lacking a functional oxidase suffer from severe and recurrent infections. The oxidase is a multisubunit enzyme complex that transports electrons from cytoplasmic NADPH to molecular oxygen in order to generate superoxide free radicals. Electron transport across the plasma membrane is electrogenic and is associated with the flux of protons through voltage-activated proton channels. Both proton and electron currents can be recorded with the patch-clamp technique, but whether the oxidase is a proton channel or a proton channel modulator remains controversial. Recently, we have used the inside–out configuration of the patch-clamp technique to record proton and electron currents in excised patches. This approach allows us to measure the oxidase activity under very controlled conditions, and has provided new information about the enzymatic activity of the oxidase and its coupling to proton channels. In this chapter I will discuss how the unique characteristics of the electron and proton currents associated with the redox activity of the NADPH oxidase have extended our knowledge about the thermodynamics and the physiological regulation of this remarkable enzyme.
Keywords: NADPH oxidases, ion channels, patch-clamp, eosinophils, proton channels
Introduction
NADPH oxidases (NOX) are a growing family of plasma membrane enzymes whose function is to generate superoxide by transferring electrons from cytosolic NADPH to extracellular O2. The founding father of the NOX family is the phagocytic NADPH oxidases (phox), which is expressed in phagocytic white blood cells such as neutrophils, eosinophils and macrophages (Babior et al. 2002; Quinn & Gauss 2004). The oxidase of phagocytes was identified early, because biochemical studies showed that activation of neutrophils results in a massive increase in non-mitochondrial consumption of oxygen, a process termed the ‘respiratory burst’ (Baldridge & Gerard 1933; Klebanoff 1971; Babior et al. 1975). Patients with a defective respiratory burst suffer from chronic granulomatous disease (CGD), a predominantly X-linked disease associated with severe recurring bacterial and fungal infections (Segal et al. 1978; Segal 1987). This genetic linkage highlighted the importance of the phagocytic oxidase in the defence of our body against microbial infections, and led to the early cloning of the CYBB gene coding for the large transmembrane subunit of the phagocytic oxidase, the glycoprotein gp91phox (Royer-Pokora et al. 1986; Dinauer et al. 1987). The most frequent forms of CGD are due to mutations that result in an absent or non-functional gp91phox, but the disease can also be caused by defects in one of the other oxidase components (non-X-linked forms; Roos et al. 1996). The oxidase is a multi-component enzyme, which contains both catalytic transmembrane proteins and regulatory cytosolic proteins. The enzymatic core of the NADPH oxidase consists of the two integral membrane proteins gp91phox and p22phox, which together form the flavocytochrome b558, named from its absorbance peak at 558 nm (Segal 1987). The regulatory subunits comprise the cytosolic proteins p47, p67 and p40, as well as the small GTPase Rac (either Rac1 or Rac2). Upon cellular activation, the regulatory subunits relocate from the cytosol to the plasma membrane or to the phagosomal membrane and induce enzymatic activity (Clark et al. 1990). Biochemical analysis established that the cytosolic proteins p47, p67 and Rac were sufficient to reconstitute superoxide production in a cell-free system containing purified flavocytochrome b558, provided that an anionic amphiphile was used as an activator (Bromberg & Pick 1984; Clark et al. 1987). Further studies indicated that the activation of the oxidase in the cell-free system absolutely required p67phox and Rac but not p47phox, suggesting that p67phox functions as ‘activator’ to initiate electron flow across gp91phox, while p47phox and Rac act as ‘organizers’ of the p67phox–gp91phox interaction (Nisimoto et al. 1999). Several non-phagocytic oxidase homologues also require cytosolic subunits for assembly and activation, and a similar model has been proposed for the regulation of NOX1 by the organizer and activator proteins NOXO1 and NOXA1 (Lambeth 2004). All the NOX enzymes transfer electrons across the plasma membrane, using NADPH as electron donor and molecular oxygen as electron acceptor. For this purpose, the active enzymes have an unusually low redox potential which enables the direct reduction of molecular oxygen to superoxide (; Cross et al. 1981). The superoxide anions might be subsequently converted into hydrogen peroxide (H2O2), hypochlorous acid (HOCl), and other reactive oxygen species (ROS). In phagocytes, ROS are used for the killing of phagocytosed micro-organisms, while in non-phagocytic cells ROS generated by NOX enzymes might be used for signal transduction, or for remodelling of the extracellular matrix (Lambeth 2004).
The transfer of electrons across biological membranes is an electrogenic process, which generates currents as charges are separated and moved across the lipid bilayer. The amplitude of the electron current carried by the phagocyte NADPH oxidase can be calculated from the amount of superoxide generated extracellularly by the cells, which is classically measured by absorbance measurements as the superoxide dismutase-dependent reduction of cytochrome c (Babior et al. 1975). Using this assay, human neutrophils typically generate 10 nmoles of O2− per minute per million of cells (Shult et al. 1985). This translates into a flux of 108 electrons per second per cell, which is equivalent to an ‘electronic’ current of 16 pA. A current of this magnitude, if not compensated, is expected to produce a large depolarization of the plasma membrane. Indeed, the first direct evidence that the oxidase is electrogenic came from membrane potential measurements (Henderson et al. 1987). These measurements indicated that the activation of the oxidase depolarized cells to voltages exceeding 0 mV, the upper limit of the measurements. The same logic can be applied to calculate the amount of protons generated inside cells by the enzymatic activity of oxidase, which separates NADPH into NADP+ and H+ in the cytosol. Because one proton is released in the cytosol for each electron translocated, 108 protons are generated every second in an activated neutrophil (Van Zwieten et al. 1981). Given the buffering power of the cytosol, this acid production, if not compensated, will cause the intracellular pH to drop by 1 unit per minute. Indeed, a cytosolic acidification of this magnitude can be measured in cells with an activated oxidase when all plasma membrane H+ transporters are inhibited (Demaurex et al. 1996). The acidification is exacerbated during continuous activation of the oxidase, because the regeneration of NADPH by the hexose monophosphate shunt required to sustain the oxidase activity generates additional acid equivalents (Borregaard et al. 1984; Grinstein & Furuya 1986).
Because the oxidase both depolarizes and acidifies cells, it was realized very early that the most efficient charge compensating mechanism would be to extrude H+ ions from the cells through an electrogenic pathway (Henderson et al. 1988a,b). A similar process occurs in mitochondria, where the flux of electrons across mitochondrial cytochromes is used to move protons across the inner mitochondrial membrane (Mitchell & Moyle 1967). The proton and electron transport pathways are often contained within the same protein, the best-studied system being the cytochrome c oxidase which functions as a redox-driven proton pump (Wikstrom 1977). Although the mechanism of proton translocation across the cytochrome c oxidase is not yet fully understood at the molecular level, the fluxes of H+ and e− are clearly coupled and involve histidine residues within the cytochrome (Wikstrom 1989, 2004). As discussed below, a similar mechanism has been proposed for electron and proton transport by NADPH oxidases, based essentially on functional evidence. Unlike mitochondrial cytochromes, NADPH oxidases are expressed at the plasma membrane and the fluxes of electrons and protons associated with their activity can be recorded directly with electrophysiological approaches. Using the patch-clamp technique, one can thus measure several parameters that directly or indirectly reflect the activity of the NADPH oxidase, namely: (i) the plasma membrane voltage, (ii) proton currents and (iii) electron currents. The data obtained by whole-cell patch-clamp recordings of cells expressing a phagocytic NADPH oxidase will be discussed in the following sections.
1. Proton currents in phagocytes
Based on the thermodynamic considerations discussed above and on the functional analogy with mitochondrial cytochrome, attempts were made early to test whether a proton channel is contained within the phagocytic oxidase. Studies with fluorescent dyes revealed that the activity of the oxidase is coupled to the efflux of protons through a voltage, pH and Zn2+-sensitive pathway (Henderson et al. 1987). At the same time, electrophysiological studies reported voltage, pH and Zn2+-sensitive proton currents in snail neurons and rat lung epithelia (Thomas & Meech 1982; Byerly et al. 1984; Decoursey 1991). Our report of proton currents in the phagocytic cell line HL-60 cells (Demaurex et al. 1993) followed by the report of similar currents in neutrophils, macrophages and eosinophils (Decoursey & Cherny 1993; Kapus et al. 1993; Schrenzel et al. 1996) indicated that a proton conducting pathway was expressed in phagocytes. The currents had all the features of previously described proton currents: they were activated by depolarizing voltages and by a cytosolic acidification, were highly selective for H+ over other ions, were blocked by micromolar concentrations of Zn2+ and were associated with a large alkalinization of the cytosol (figure 1; Demaurex et al. 1993). The activation of outward proton current by intracellular acidification and membrane depolarization functionally coupled proton extrusion to oxidase activation. This suggested that the flux of protons was coupled to the flux of electrons through the same protein, gp91phox. Contrary to this prediction, proton currents were observed shortly after in neutrophils from CGD patients (Nanda et al. 1994) and subsequently in eosinophils from CGD patients (Banfi et al. 1999) and in CGD cell lines (Decoursey et al. 2001b). This observation indicated without ambiguity that a protein other than the transmembrane oxidase subunit gp91phox was acting as a proton channel (figure 2; Banfi et al. 1999). Because the amplitude and kinetics of the outward proton currents recorded in phagocytes from CGD patients were undistinguishable from the currents recorded in control cells, it was also concluded that gp91phox contributed only a minor fraction, if any, of the proton conductance. However, this conclusion was premature because the conditions that allowed the recording of electron currents had not yet been established, and at that time proton current was recorded in conditions that prevented the activity of the oxidase.
Figure 1.
Proton currents in HL-60 cells. (a) Depolarization increases cytosolic pH and activates outward currents in HL-60 cells. Cells were loaded with the pH-sensitive indicator carboxy-SNARF-1 and voltage clamped at −60 mV in the whole-cell configuration using KCl-based solutions buffered to pH 6.5 with 10 mM pipes. Where indicated, voltage was changed first to 0 mV, then to +60 mV for 30 s. The voltage protocol is shown in the lower trace. (b) Time-course of the voltage-activated currents and block by divalent cations. Top: families of whole-cell currents elicited by depolarizing pulses lasting 2 s (inset). Currents were recorded in bilateral CsAsp solutions, bath pH 7.5; pipette pH 5.5. Bottom: the current elicited by a depolarization to +20 mV is reversibly blocked by the addition of 3 mM Cd2+ to the external solution. Reproduced from Demaurex et al., ‘Proton currents in human granulocytes: regulation by membrane potential and intracellular pH’. The Journal of Physiology, 1993, vol. 466, pp. 329–344, by copyright permission of Blackwell Publishing Ltd.
Figure 2.
Proton currents in resting eosinophils from CGD patients. Proton currents recorded in eosinophils from a healthy donor (a) or from a 6-year-old Caucasian boy with X-linked CGD, who completely lacked the gp91phox subunit (b, X91°). The currents were elicited by 5 s depolarizing steps ranging from −60 to 0 mV, in conditions preventing the activation of the NADPH oxidase (pHi 6.1, 0.2 mM EGTA, no GTPγS). Reproduced from The Journal of Experimental Medicine, 1999, vol. 190, pp. 183–194, by copyright permission of the Rockefeller University Press.
2. Electron currents in phagocytes
As noted above, the predicted flux of electrons carried by the oxidase is in the picoamperes range, well within the reach of patch-clamp amplifiers. Despite the theoretical feasibility of electron current measurements however, most patch-clamp studies in phagocytes focused initially on the description of proton currents and of other ion channels. One reason was that the currents carried by proton and other ions are two orders of magnitude larger than electron currents. Typical proton current amplitudes can be several hundreds of picoamperes in neutrophils and eosinophils, and these currents are easy to record. Another trivial reason is that most experimenters, including us, did not include the oxidase substrate NADPH in their pipette solution during whole-cell patch-clamp recordings. Under whole-cell conditions the cytosolic pool of NADPH is rapidly depleted and electron currents are absent because the oxidase cannot catalyse electron transport in the absence of substrate. Electron currents are immediately apparent however, if one simply includes the enzyme substrate together with known activators of the oxidase in the patch pipette, the most potent being the non-hydrolysable GTP analogue GTPγS (Schrenzel et al. 1998). The slowly activating inward currents were generated by the flow of electrons across the oxidase, because (i) the currents were associated with the formation of formazan precipitates in the cell cytosol, indicating that superoxide was generated, (ii) the currents were blocked by diphenylene iodonium (DPI), a well-known oxidase inhibitor, (iii) the currents were absent in cells from CGD patients and (iv) the currents were not observed in the absence of oxygen.
3. Interactions between proton and electron currents
Once the conditions to record electron currents were established, it became immediately apparent that the properties of the proton currents were altered in cells with an active oxidase. In cells perfused with NADPH and GTPγS to elicit electron currents, proton currents activated at much lower voltages, enabling the inward flux of H+ into the cell (Banfi et al. 1999). This was very surprising, because only outward proton currents had been recorded so far, and the underlying proton channel had been touted as a uniquely designed ‘one way only’ proton extruder. In all conditions studied so far, proton currents activated 20 mV above the equilibrium potential for H+, EH+, such that the driving force for H+ was always directed outward. In contrast, in cells with an active oxidase the voltage sensitivity of the proton channel was shifted by 40 mV to more negative voltages, such that the channel activated below EH+. Other anomalies were noted in the ‘activated’ proton current phenotype, including a much faster kinetics of activation, an increased sensitivity to inhibition by Zn2+ and a slower kinetics of deactivation. A shift in the voltage sensitivity of proton currents had been previously reported in phagocytes stimulated with arachidonic acid (AA), which promotes the activation of the oxidase in the cell-free system (Kapus et al. 1994; Schrenzel et al. 1996). However, the shifts in the voltage-sensitivity of the proton channel observed in response to AA were of much smaller magnitude, not large enough to induce current reversal. Furthermore, both the activation and deactivation kinetics of proton currents became faster in cells stimulated with AA (Kapus et al. 1994), while proton currents in cells dialysed with NADPH and GTPγS had a faster kinetics of activation, but a slower kinetics of deactivation. The observation that proton currents in cells with an active oxidase had distinct characteristics prompted us to re-examine the role of the oxidase in proton transport.
We thus studied eosinophils from CGD patients, who lack a functional oxidase but retain a proton conductance, and checked whether proton currents in CGD cells were altered by the intracellular application of NADPH and GTPγS. As shown in figure 3, the shift in the voltage activation of proton currents was not observed in activated eosinophils from patients lacking either the gp91phox or the p47phox subunit (Banfi et al. 1999). This observation indicated that a functional oxidase was required for the shift in voltage activation of the proton channel. Importantly, oxidase assembly and activation, but not its redox function, was required for the changes in proton currents because low threshold proton currents were also observed in cells perfused with NADPH and GTPγS in the absence of oxygen or in the presence of DPI (Banfi et al. 1999). From these observations, we concluded that the NADPH oxidase or a closely associated protein provides a new type of proton conductance during phagocyte activation (Banfi et al. 1999). In subsequent studies, similar alterations in proton currents were observed in neutrophils and eosinophils using the permeabilized-patch configuration to prevent NADPH washout through the patch pipette and phorbol myristate acetate (PMA) or AA to activate the oxidase (Decoursey et al. 2000, 2001a; Cherny et al. 2001). Oxidase activation could be documented under these conditions and was also associated with a negative shift in voltage sensitivity, a faster activation and a slower deactivation of proton currents. Interestingly, AA did not consistently slow the deactivation of proton currents and could enhance proton currents at concentrations that did not elicit detectable electron currents (Cherny et al. 2001). Contrary to us, these authors concluded that the changes in proton current phenotype reflected the modulation of pre-existing channels, because the amplitudes of proton and electron currents activated by PMA were not correlated and because activation of H+ currents by AA could be dissociated from its activation of the oxidase. Indeed, because a proton conductance is present in CGD cells the alterations in proton current observed during oxidase activation are compatible either with the oxidase acting as a modulator of pre-existing channels or with the oxidase acting as a H+ channel during electron transfer.
Figure 3.
Proton currents in activated eosinophils from CGD patients. Proton currents recorded in eosinophils from a healthy donor (a) or from a 6-year-old Caucasian boy with X-linked CGD, who completely lacked the gp91phox subunit (b, X91°). The currents were elicited by 5 s depolarizing steps ranging from 0 mV (CGD) or −20 mV (control) to +50 mV, in conditions favouring the activation of the NADPH oxidase (pHi=7.6, 25 μM GTPγS). Reproduced from The Journal of Experimental Medicine, 1999, vol. 190, pp. 183–194, by copyright permission of the Rockefeller University Press.
The observation that the activation of the oxidase modulated proton currents prompted us to take a closer look at the structure of the gp91phox cytochrome. The predicted topology of the gp91phox protein is shown in figure 4. The protein contains six transmembrane domains and two heme groups buried within the plasma membrane. The heme groups are coordinated by histidine residues located in the third and fifth transmembrane domains (Biberstine-Kinkade et al. 2001), and additional histidine residues are present along the third transmembrane domain and predicted to be aligned along the axis of the alpha-helix. This string of histidine residues caught our attention, because a similar motif, engineered into the voltage-sensing domain of the Shaker potassium channel by the group of Francisco Bezanilla, had been shown to be sufficient to turn a non-conductive K+ channel into a voltage-gated proton channel (Starace et al. 1997). Using this motif for an expressed sequence tags (EST) database screen we identified the first gp91phox homologue, NOH-1, together with the group of Karl-Heinz Krause (Banfi et al. 2000). The group of J. David Lambeth had independently cloned the same protein, termed Mox1 for mitogenic oxidase because it promoted cells growth when expressed in NIH-3T3 cells (Suh et al. 1999). This first homologue of the catalytic subunit of the superoxide-generating NADPH oxidase of phagocytes has now been renamed NOX1, and several new homologues have since been identified (reviewed in Lambeth 2004). Expression in HEK-293 cells of the cDNA coding for the full-length NOX1 protein was associated with voltage and pH activated, Zn2+ sensitive, H+-selective currents (Banfi et al. 2000). Expression in HEK-293 cells of gp91phox (now renamed NOX2) generated nearly identical proton currents (Maturana et al. 2001), while the expression of a truncated NOX1 protein containing only the first four transmembrane domains, but retaining the predicted H+ channel motif, was sufficient to induce H+ currents (Banfi et al. 2000). Moreover, expression of the Ca2+-activated oxidase NOX5, which contains four EF-hand domains in its N-terminus and is activated directly by Ca2+, generated H+ currents that were activated by an increase in the cytosolic free Ca2+ concentration (Banfi et al. 2001). This indicated that specific domains within NOX proteins contained all the information required to induce proton currents or to modulate proton currents in an isoform-specific manner when expressed in HEK-293 cells.
Figure 4.
Predicted topology of the gp91phox cytochrome. The gp91phox cytochrome contains two heme groups buried within the plasma membrane that are coordinated by histidine residues located on the third and fifth transmembrane domains. Two additional histidine residues are located on the third transmembrane domain around the H115 residue and predicted to be aligned along the axis of the alpha-helix. This histidine motif can function as a proton wire, and all NOX isoforms that contain this string of histidines generate proton currents when expressed in HEK-293 cells or CHO cells. Reproduced from The Journal of General Physiology, 2002, vol. 120, pp. 781–786, by copyright permission of the Rockefeller University Press.
Further evidence for the role of NOX in proton transport came from mutagenesis studies, performed by our group and by the group of Lydia Henderson. When proton currents where recorded in CHO cells expressing mutated versions of NOX2, histidine mutagenesis revealed that His residues at positions 111 and 119 contributed to voltage gating, while the His residue at position 115 was critical for H+ selectivity (Henderson & Meech 1999). In HEK-293 cells, mutation of His-115 generated a protein that was expressed at the plasma membrane but was devoid of heme, and proton currents were absent (Maturana et al. 2001). All this evidence suggested that a proton pathway was contained within the gp91phox protein, as originally proposed by the group of Lydia Henderson (Henderson et al. 1995, 1997). The critical role of the His-115 residue further suggested that protonation and deprotonation of this residue was involved in H+ transport, and that de-ligation of the heme molecule increased the mobility of this histidine residue during electron flow. This ‘histidine’ model of proton transport is shown in figure 5 (Maturana et al. 2002). Heme de-ligation might facilitate H+ transport by increasing the mobility of His-115, allowing the protonation and deprotonation of this histidine residue as it is exposed alternatively to the interior and exterior faces of the cell membrane. This model is attractive because it nicely explains the coupling of electron and proton transport.
Figure 5.
Mechanism of proton and electron transport by NADPH oxidases. We propose that protons are carried along a hydrogen-bonded chain comprising histidine residues within the catalytic domain of NADPH oxidases. The proton flow is limited by the mobility of the central His-115 residue, which acts as heme ligand. During electron flow, de-ligation of the heme molecule increases the mobility of His-115 and facilitates proton transport. The bi-functional His-115 residue at the ‘core’ of the proton wire functionally couples electron and proton transport. Reproduced from The Journal of General Physiology, 2002, vol. 120, pp. 781–786, by copyright permission of the Rockefeller University Press.
Despite this body of evidence however, the role of NOX as proton channels still remains controversial, for several reasons. While all NOX isoforms tested so far, except the histidine mutants, generate H+ currents in HEK-293 and CHO cells, NOX expression is usually not associated with proton currents when the protein is expressed in COS-7 cells (Morgan et al. 2002, but see Murillo & Henderson 2005). More problematic, COS-7 cells expressing all the oxidase subunits (Cos-phox) generate superoxide, but lack H+ currents (Morgan et al. 2002). The different interpretations of these findings and the controversy regarding the channel nature of NOX proteins have been discussed in detail in recent reviews (Decoursey et al. 2002; Henderson & Meech 2002; Maturana et al. 2002; Touret & Grinstein 2002), and will not be repeated here. The only consensus between the different groups is that, to account for the different observations, NOX proteins must be either proton channels or proton channel modulators.
Because studies in expression systems were inconclusive, we decided to use a different experimental approach to study the mechanism of proton and electron transport by NADPH oxidases. For this purpose, we developed strategies to record both electron and proton currents in patches excised from human eosinophils. The data obtained with this approach will be discussed in the following sections.
4. Proton and electron currents in excised patches from human eosinophils
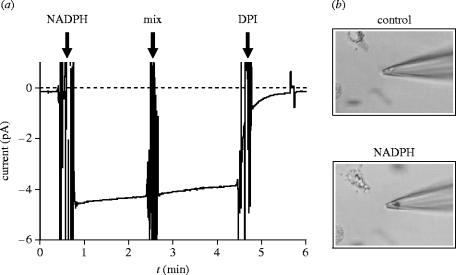
We initially recorded proton currents in patches excised from resting eosinophils, i.e. cells that were not activated with chemical stimuli but only by their adhesion to the glass cover-slip. Only outward proton currents were recorded under these conditions, and the currents had all the features of the ‘classical’ proton currents previously reported: activation by voltage and pH, inhibition by Zn2+, and high selectivity for H+ ions (Petheö et al. 2003). We then proceeded to check whether the modulation of proton channels observed under whole-cell conditions following the activation of the oxidase persisted in excised membrane patches. Indeed, as shown in figure 6, very different proton currents were recorded when the patches were excised from eosinophils that had been pre-treated with PMA to activate the oxidase. In keeping with our findings from whole-cells recordings, proton currents activated at much lower voltages and allowed the influx of protons, the inward H+ currents often being as large as the outward H+ currents at an equal driving force (Petheö et al. 2003). Proton currents also activated much faster upon depolarization in cells pre-treated with PMA, as observed in the whole-cell configuration. However, while proton currents in resting cells were stable for several minutes, the low-threshold proton currents recorded in activated cells ran down within minutes, the residual currents resembling those observed in non-activated cells. We then verified that the oxidase was active in these cells by recording electron currents in excised patches (Petheö et al. 2003). The addition of NADPH to a patch excised from a PMA-stimulated eosinophil elicited instantaneous inward currents whose amplitude ranged from 0.2 to 4 pA. The currents were fully inhibited by the oxidase inhibitor DPI and were associated with the formation of black insoluble formazan precipitates when the patch pipette contained nitro blue tetrazolium (NBT) (figure 7). This indicated that superoxide was produced in the patch pipette when the inward currents were evoked by the addition of NADPH to the cytosolic side of the patch. On the basis of their NADPH requirement, DPI sensitivity and association with superoxide generation, the inward currents were identified as electron currents. Similar to the low-threshold proton currents observed under these conditions, the electron currents recorded in excised patches ran down within minutes. The run-down could be largely prevented by the addition of ATP and GTPγS to the cytsolic side of the patch.
Figure 6.
Proton currents in patches excised from resting and activated eosinophils. Patches were excised from human eosinophils that were either not stimulated (a) or stimulated with PMA to activate the oxidase (b). (a) Currents elicited by voltage steps ranging from −60 to 80 mV in a patch excised from a resting eosinophil at pHi/o 7.5/7.5. (b) Inward and outward H+ currents elicited by pulses to 0 and 60 mV in a patch excised from a PMA-stimulated eosinophil at pHi/o 7.5/7.0. The bath solution contained 5 mM ATP and 25 μM GTPγS. The driving forces for H+ current at 0 and 60 mV were around −30 and 30 mV, respectively. Reproduced from The Journal of General Physiology, 2003, vol. 122, pp. 713–726, by copyright permission of the Rockefeller University Press.
Figure 7.
Electron currents and superoxide production in patches excised from human eosinophils. (a) Electron current evoked by bath application of 1.6 mM NADPH to an inside–out patch excised from a PMA-stimulated eosinophil. Electron currents were continuously recorded at −40 mV, and the pipette solution contained 3 mM Zn2+ to minimize proton channel activity. The current was fully inhibited by 12 μM DPI. (b) Superoxide production in a patch excised with a pipette containing 0.5 mg ml−1 NBT was photographed shortly before (a) and 15 min after (b) bath application of 0.8 mM NADPH. Note the formation of a dark formazan precipitate due to production near the pipette tip. Reproduced from The Journal of General Physiology, 2003, vol. 122, pp. 713–726, by copyright permission of the Rockefeller University Press.
The ability to reconstitute the oxidase-associated proton and electron currents in membrane patches allowed quantification of the two transport process under well-controlled conditions. The amplitude of proton and electron currents recorded in excised patches correlated very well, indicating that the underlying transport proteins co-segregated in the plasma membrane (Petheö et al. 2003). Moreover, the run-down of proton and electron currents was inhibited equally well by the inclusion of ATP and GTPγS to the cytosolic side of the patch. A detailed analysis of this run-down process, which we believe reflects the inactivation of the oxidase and of its associated proton channel, is now under way in our laboratory. We expect that this will yield new information regarding the inactivation of the oxidase, a process that is difficult to study with classical biochemical approaches. Preliminary observations indicate that the run-down of both electron and proton currents is hampered by the inclusion of specific phosphoinositides in the bath solution (G. L. Petheö & N. Demaurex 2005, unpublished data). Interestingly, factors that promote the stability of electron currents in excised patches also increase the stability of proton currents, raising the possibility that the inactivation of the oxidase and of the proton channel is coupled.
5. Voltage and NADPH dependence of electron currents
The possibility to record electron currents in excised patches allowed us to probe several key aspects of the enzymatic activity of the oxidase in intact membranes under voltage-clamp conditions (Petheö & Demaurex 2005). The inside–out configuration provided a unique advantage compared to biochemical approaches or to other patch-clamp configurations, because the cytosolic side of the plasma membrane which contains all the oxidase regulatory subunits is exposed to the bath solution whose composition can be manipulated selectively. Furthermore, after optimizing the recording conditions the electron currents evoked by the addition of NADPH to the cytosolic side of the patch were stable for several minutes, allowing quantitative measurements of the enzyme activity under physiological conditions. We took advantage of this feature to measure the activity of the oxidase at different NADPH concentrations. As shown in figure 8, the addition of a low dose of NADPH elicited small electron currents, while the subsequent addition of a higher dose of NADPH elicited larger currents in the same patch. This procedure allowed us to perform a dose–response of the oxidase activity by normalizing the currents recorded at intermediate concentrations to the currents recorded with a maximal dose of NADPH in the same patch. The amplitude of electron currents was minimal at 8 μM NADPH, increased steadily in the submillimolar range, and was maximal above 1 mM NADPH (Petheö & Demaurex 2005). Using this approach, we could establish the Km (concentration required for half-maximal activity) of the enzyme in intact membranes, which averaged 110±11 μM at the holding voltage of −60 mV.
Figure 8.
Voltage and NADPH-dependence of electron currents. Electron currents were evoked by bath application of increasing concentrations of NADPH, and voltage ramps ranging from −120 to 200 mV were applied to establish the current to voltage relationship of the electron current. (a) Steady-state amplitude of electron current at −40 mV. (b) Ramp currents recorded at intermediate and saturating concentrations of NADPH. At low NADPH concentration, the electron currents become voltage independent at negative voltages. Reproduced from Petheö & Demaurex, ‘Voltage- and NADPH-dependence of electron currents generated by the phagocytic NADPH oxidase’, The Biochemical Journal, 2005, vol. 388, pp. 485–491, by copyright permission of Portland Press Ltd.
The excised patch approach also enabled us to determine the voltage dependence of electron currents. For this purpose, we applied voltage ramps ranging from −120 to +200 mV before and after the addition of NADPH. Using a simple ramp subtraction protocol, the ‘steady-state’ current to voltage relationship of the electron currents (Ie–V) could be obtained, at different concentrations of NADPH. A similar approach had been applied in perforated whole-cell recordings, but in this case the concentration of NADPH could not be controlled and the currents were defined by their sensitivity to the oxidase inhibitor DPI (Decoursey et al. 2003). In our inside–out patches, we could determine the voltage dependence of the oxidase in the absence of DPI and, more importantly, at different concentrations of NADPH. Contrary to the whole-cell perforated patch study, the amplitude of electron currents decreased steeply with voltage when saturating concentrations of NADPH were used, with no apparent rectification (Petheö & Demaurex 2005). In perforated patch recordings, electron currents were nearly voltage independent at negative voltages, and the shape of the Ie–V was very variable between different cells. In excised patches, the electron current amplitude varied linearly with voltage in the physiological voltage range (−60 to +60 mV), and the shape of the Ie–V was nearly identical between individual patches when a saturating concentration of NADPH was used. When lower concentrations of NADPH were used, however, electron currents became voltage independent at negative voltages and the shape of the Ie–V resembled those reported in the whole-cell study. These data indicated that the apparent voltage dependence of the oxidase was strongly influenced by the concentration of substrate. To quantify more precisely this phenomenon, we determined the Km of the enzyme at different voltages by systematically recording Ie–V at different concentrations of NADPH. These experiments revealed that the apparent Km of the NADPH oxidase gradually decreased by 40% with membrane depolarization from −60 to +60 mV. These data indicate that the voltage independence of the electron currents reported in the perforated whole-cell study was due to substrate limitation and is not an intrinsic property of the oxidase. As can be expected for an electrogenic transporter, the enzyme becomes voltage independent when the availability of the substrate is limiting, such that the rate-limiting step is not the electron translocation across the membrane but the delivery of electrons from NADPH. Surprisingly, this limitation occurred within the physiological concentration range of NADPH, which has been estimated to be around 170–220 μM (Olsen et al. 2003). Because the average cytosolic concentration of NAPDH is below the saturating level of the oxidase, our data indicate that under physiological conditions the activity of the enzyme is mainly determined by the availability of substrate. Accordingly, imaging studies have shown that the concentration of NADPH oscillates in neutrophils, and that the cytosolic waves of NADPH correlate both spatially and temporally with the release of superoxide at the lammellipodium of the cells (Kindzelskii & Petty 2002). It is tempting to speculate that the activity of the other NADPH oxidase isoforms is also regulated in space and time by changes in substrate concentration. So far, however, fundamental biochemical and biophysical characterization of the novel NADPH oxidase isoforms is lacking.
6. Membrane potential changes
The Ie–Vs obtained in excised patches also allowed us to assess the capacity of the oxidase to depolarize the plasma membrane. In most cases, the amplitude of electron currents declined asymptotically to zero at voltages exceeding +200 mV. These observations were confirmed by recording the changes in plasma membrane voltage during activation of electron currents in excised patches. In the current clamp mode, the addition of NADPH induced a depolarization to +180 mV when charge compensation due to the activity of proton channels was blocked by Zn2+ (G. L. Petheö & N. Demaurex, unpublished data). In early studies, plasma membrane depolarizations ranging from −40 to 0 mV had been reported in intact phagocytes using voltage-sensitive dyes, and the depolarization was absent in patients lacking a functional oxidase (Seligmann et al. 1980). More recently, a depolarization to voltages as high as +58 mV was reported in activated neutrophils using a more accurate method based on the rates of Mn2+ influx through endogenous Ca2+ channels (Jankowski & Grinstein 1999). In whole-cell recordings, activation of the oxidase induced a depolarization to +80 mV in the presence of Zn2+ (Banfi et al. 1999). Our I–Vs indicate that the oxidase is able to depolarize the plasma membrane to voltages close to 200 mV. This value probably represents a maximal limit, which is not reached in vivo due to the activation of charge compensating mechanisms. Both H+ channels (Henderson et al. 1987; Banfi et al. 1999) and large conductance calcium-activated K+ channels (Ahluwalia et al. 2004) are involved in charge compensation, but their relative contribution remains to be determined (Decoursey 2004). K+ flux appears to compensate for a small fraction of electron transport (Reeves et al. 2002; Rada et al. 2004) mainly during the initial phase of superoxide production (Rada et al. 2004). Therefore, H+ channels might be required to prevent deleterious membrane depolarization, as at membrane potentials exceeding 100 mV the electrical field generated across the thin layer of the plasma membrane (ca 105 V cm−1) will cause most insulators to break down irreversibly (the axon guide, www.axon.com/mr_Axon_Guide.html). A close coupling between the oxidase and proton channel is, thus, primordial not only to sustain oxidase activity, but also to prevent membrane damage.
Acknowledgments
This research is supported by the operating grant no. 31-068317.02 from the Swiss National Science Foundation.
Footnotes
One contribution of 18 to a Theme Issue ‘Reactive oxygen species in health and disease’.
References
- Ahluwalia J, Tinker A, Clapp L.H, Duchen M.R, Abramov A.Y, Pope S, Nobles M, Segal A.W. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature. 2004;427:853–858. doi: 10.1038/nature02356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Babior B.M, Curnutte J.T, Kipnes B.S. Pyridine nucleotide-dependent superoxide production by a cell-free system from human granulocytes. J. Clin. Invest. 1975;56:1035–1042. doi: 10.1172/JCI108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B.M, Lambeth J.D, Nauseef W. The neutrophil NADPH oxidase. Arch. Biochem. Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- Baldridge C.W, Gerard R.W. The extra respiration of phagocytosis. Am. J. Physiol. 1933;103:235–236. [Google Scholar]
- Banfi B, Schrenzel J, Nusse O, Lew D.P, Ligeti E, Krause K.H, Demaurex N. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J. Exp. Med. 1999;190:183–194. doi: 10.1084/jem.190.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi B, Maturana A, Jaconi S, Arnaudeau S, Laforge T, Sinha B, Ligeti E, Demaurex N, Krause K.H. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–142. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause K.H. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001;276:37 594–37 601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- Biberstine-Kinkade K.J, Deleo F.R, Epstein R.I, Leroy B.A, Nauseef W.M, Dinauer M.C. Heme-ligating histidines in flavocytochrome b(558): identification of specific histidines in gp91(phox) J. Biol. Chem. 2001;276:31 105–31 112. doi: 10.1074/jbc.M103327200. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Schwartz J.H, Tauber A.I. Proton secretion by stimulated neutrophils. Significance of hexose monophosphate shunt activity as source of electrons and protons for the respiratory burst. J. Clin. Invest. 1984;74:455–459. doi: 10.1172/JCI111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg Y, Pick E. Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cell. Immunol. 1984;88:213–221. doi: 10.1016/0008-8749(84)90066-2. [DOI] [PubMed] [Google Scholar]
- Byerly L, Meech R, Moody W., Jr Rapidly activating hydrogen ion currents in perfused neurones of the snail, Lymnaea stagnalis. J. Physiol. 1984;351:199–216. doi: 10.1113/jphysiol.1984.sp015241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny V.V, Henderson L.M, Xu W, Thomas L.L, Decoursey T.E. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. J. Physiol. 2001;535:783–794. doi: 10.1111/j.1469-7793.2001.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.A, Leidal K.G, Pearson D.W, Nauseef W.M. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J. Biol. Chem. 1987;262:4065–4074. [PubMed] [Google Scholar]
- Clark R.A, Volpp B.D, Leidal K.G, Nauseef W.M. Two cytosolic components of the human neutrophil respiratory burst oxidase translocate to the plasma membrane during cell activation. J. Clin. Invest. 1990;85:714–721. doi: 10.1172/JCI114496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A.R, Jones O.T, Harper A.M, Segal A.W. Oxidation–reduction properties of the cytochrome b found in the plasma-membrane fraction of human neutrophils. A possible oxidase in the respiratory burst. Biochem. J. 1981;194:599–606. doi: 10.1042/bj1940599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoursey T.E. Hydrogen ion currents in rat alveolar epithelial cells. Biophys. J. 1991;60:1243–1253. doi: 10.1016/S0006-3495(91)82158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoursey, T. E. 2004 During the respiratory burst, do phagocytes need proton channels or potassium channels, or both? Sci. STKE, pe21. ( 10.1126/stke.2332004pe21.) [DOI] [PubMed]
- Decoursey T.E, Cherny V.V. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys. J. 1993;65:1590–1598. doi: 10.1016/S0006-3495(93)81198-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoursey T.E, Cherny V.V, Zhou W, Thomas L.L. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc. Natl Acad. Sci. USA. 2000;97:6885–6889. doi: 10.1073/pnas.100047297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoursey T.E, Cherny V.V, Decoursey A.G, Xu W, Thomas L.L. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J. Physiol. 2001a;535:767–781. doi: 10.1111/j.1469-7793.2001.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoursey T.E, Cherny V.V, Morgan D, Katz B.Z, Dinauer M.C. The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps. J. Biol. Chem. 2001b;276:36 063–36 066. doi: 10.1074/jbc.C100352200. [DOI] [PubMed] [Google Scholar]
- Decoursey T.E, Morgan D, Cherny V.V. The gp91phox component of NADPH oxidase is not a voltage-gated proton channel. J. Gen. Physiol. 2002;120:773–779. doi: 10.1085/jgp.20028704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoursey T.E, Morgan D, Cherny V.V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Grinstein S, Jaconi M, Schlegel W, Lew D.P, Krause K.H. Proton currents in human granulocytes: regulation by membrane potential and intracellular pH. J. Physiol. 1993;466:329–344. [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Downey G.P, Waddell T.K, Grinstein S. Intracellular pH regulation during spreading of human neutrophils. J. Cell Biol. 1996;133:1391–1402. doi: 10.1083/jcb.133.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinauer M.C, Orkin S.H, Brown R, Jesaitis A.J, Parkos C.A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature. 1987;327:717–720. doi: 10.1038/327717a0. [DOI] [PubMed] [Google Scholar]
- Grinstein S, Furuya W. Cytoplasmic pH regulation in phorbol ester-activated human neutrophils. Am. J. Physiol. 1986;251:C55–C65. doi: 10.1152/ajpcell.1986.251.1.C55. [DOI] [PubMed] [Google Scholar]
- Henderson L.M, Meech R.W. Evidence that the product of the human X-linked CGD gene, gp91-phox, is a voltage-gated H+ pathway. J. Gen. Physiol. 1999;114:771–786. doi: 10.1085/jgp.114.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.M, Meech R.W. Proton conduction through gp91phox. J. Gen. Physiol. 2002;120:759–765. doi: 10.1085/jgp.20028708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.M, Chappell J.B, Jones O.T. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.M, Chappell J.B, Jones O.T. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils. Further evidence for the presence of an H+ conducting channel. Biochem. J. 1988a;251:563–567. doi: 10.1042/bj2510563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L.M, Chappell J.B, Jones O.T. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem. J. 1988b;255:285–290. [PMC free article] [PubMed] [Google Scholar]
- Henderson L.M, Banting G, Chappell J.B. The arachidonate-activable, NADPH oxidase-associated H+ channel. Evidence that gp91-phox functions as an essential part of the channel. J. Biol. Chem. 1995;270:5909–5916. doi: 10.1074/jbc.270.11.5909. [DOI] [PubMed] [Google Scholar]
- Henderson L.M, Thomas S, Banting G, Chappell J.B. The arachidonate-activatable, NADPH oxidase-associated H+ channel is contained within the multi-membrane-spanning N-terminal region of gp91-phox. Biochem. J. 1997;325:701–705. doi: 10.1042/bj3250701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski A, Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential. Quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J. Biol. Chem. 1999;274:26 098–26 104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- Kapus A, Romanek R, Qu A.Y, Rotstein O.D, Grinstein S. A pH-sensitive and voltage-dependent proton conductance in the plasma membrane of macrophages. J. Gen. Physiol. 1993;102:729–760. doi: 10.1085/jgp.102.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapus A, Romanek R, Grinstein S. Arachidonic acid stimulates the plasma membrane H+ conductance of macrophages. J. Biol. Chem. 1994;269:4736–4745. [PubMed] [Google Scholar]
- Kindzelskii A.L, Petty H.R. Apparent role of traveling metabolic waves in oxidant release by living neutrophils. Proc. Natl Acad. Sci. USA. 2002;99:9207–9212. doi: 10.1073/pnas.132630999. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Klebanoff S.J. Intraleukocytic microbicidal defects. Annu. Rev. Med. 1971;22:39–62. doi: 10.1146/annurev.me.22.020171.000351. [DOI] [PubMed] [Google Scholar]
- Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Maturana A, Arnaudeau S, Ryser S, Banfi B, Hossle J.P, Schlegel W, Krause K.H, Demaurex N. Heme histidine ligands within gp91(phox) modulate proton conduction by the phagocyte NADPH oxidase. J. Biol. Chem. 2001;276:30 277–30 284. doi: 10.1074/jbc.M010438200. [DOI] [PubMed] [Google Scholar]
- Maturana A, Krause K.H, Demaurex N. NOX family NADPH oxidases: do they have built-in proton channels? J. Gen. Physiol. 2002;120:781–786. doi: 10.1085/jgp.20028713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P, Moyle J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature. 1967;213:137–139. doi: 10.1038/213137a0. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cherny V.V, Price M.O, Dinauer M.C, Decoursey T.E. Absence of proton channels in COS-7 cells expressing functional NADPH oxidase components. J. Gen. Physiol. 2002;119:571–580. doi: 10.1085/jgp.20018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo I, Henderson L.M. Expression of gp91phox/Nox2 in COS-7 cells: cellular localization of the protein and the detection of outward proton currents. Biochem. J. 2005;385:649–657. doi: 10.1042/BJ20040829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A, Romanek R, Curnutte J.T, Grinstein S. Assessment of the contribution of the cytochrome b moiety of the NADPH oxidase to the transmembrane H+ conductance of leukocytes. J. Biol. Chem. 1994;269:27 280–27 285. [PubMed] [Google Scholar]
- Nisimoto Y, Motalebi S, Han C.H, Lambeth J.D. The p67(phox) activation domain regulates electron flow from NADPH to flavin in flavocytochrome b(558) J. Biol. Chem. 1999;274:22 999–23 005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- Olsen L.F, Kummer U, Kindzelskii A.L, Petty H.R. A model of the oscillatory metabolism of activated neutrophils. Biophys. J. 2003;84:69–81. doi: 10.1016/S0006-3495(03)74833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petheö G.L, Demaurex N. Voltage- and NADPH-dependence of electron currents generated by the phagocytic NADPH oxidase. Biochem. J. 2005;388:485–491. doi: 10.1042/BJ20041889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petheö G.L, Maturana A, Spat A, Demaurex N. Interactions between electron and proton currents in excised patches from human eosinophils. J. Gen. Physiol. 2003;122:713–726. doi: 10.1085/jgp.200308891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M.T, Gauss K.A. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J. Leukoc. Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- Rada B.K, Geiszt M, Kaldi K, Timar C, Ligeti E. Dual role of phagocytic NADPH oxidase in bacterial killing. Blood. 2004;104:2947–2953. doi: 10.1182/blood-2004-03-1005. [DOI] [PubMed] [Google Scholar]
- Reeves E.P, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Roos D, et al. Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood. 1996;87:1663–1681. [PubMed] [Google Scholar]
- Royer-Pokora B, Kunkel L.M, Monaco A.P, Goff S.C, Newburger P.E, Baehner R.L, Cole F.S, Curnutte J.T, Orkin S.H. Cloning the gene for an inherited human disorder—chronic granulomatous disease—on the basis of its chromosomal location. Nature. 1986;322:32–38. doi: 10.1038/322032a0. [DOI] [PubMed] [Google Scholar]
- Schrenzel J, Lew D.P, Krause K.H. Proton currents in human eosinophils. Am. J. Physiol. 1996;271:C1861–C1871. doi: 10.1152/ajpcell.1996.271.6.C1861. [DOI] [PubMed] [Google Scholar]
- Schrenzel J, Serrander L, Banfi B, Nusse O, Fouyouzi R, Lew D.P, Demaurex N, Krause K.H. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 1998;392:734–737. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- Segal A.W. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987;326:88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- Segal A.W, Jones O.T, Webster D, Allison A.C. Absence of a newly described cytochrome b from neutrophils of patients with chronic granulomatous disease. Lancet. 1978;2:446–449. doi: 10.1016/S0140-6736(78)91445-9. [DOI] [PubMed] [Google Scholar]
- Seligmann B.E, Gallin E.K, Martin D.L, Shain W, Gallin J.I. Interaction of chemotactic factors with human polymorphonuclear leukocytes: studies using a membrane potential-sensitive cyanine dye. J. Membr. Biol. 1980;52:257–272. doi: 10.1007/BF01869194. [DOI] [PubMed] [Google Scholar]
- Shult P.A, Graziano F.M, Wallow I.H, Busse W.W. Comparison of superoxide generation and luminol-dependent chemiluminescence with eosinophils and neutrophils from normal individuals. J. Lab. Clin. Med. 1985;106:638–645. [PubMed] [Google Scholar]
- Starace D.M, Stefani E, Bezanilla F. Voltage-dependent proton transport by the voltage sensor of the Shaker K+ channel. Neuron. 1997;19:1319–1327. doi: 10.1016/S0896-6273(00)80422-5. [DOI] [PubMed] [Google Scholar]
- Suh Y.A, Arnold R.S, Lassegue B, Shi J, Xu X, Sorescu D, Chung A.B, Griendling K.K, Lambeth J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- Thomas R.C, Meech R.W. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982;299:826–828. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- Touret N, Grinstein S. Voltage-gated proton “channels”: a spectator's viewpoint. J. Gen. Physiol. 2002;120:767–771. doi: 10.1085/jgp.20028706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zwieten R, Wever R, Hamers M.N, Weening R.S, Roos D. Extracellular proton release by stimulated neutrophils. J. Clin. Invest. 1981;68:310–313. doi: 10.1172/JCI110250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom M.K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977;266:271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Wikstrom M. Identification of the electron transfers in cytochrome oxidase that are coupled to proton-pumping. Nature. 1989;338:776–778. doi: 10.1038/338776a0. [DOI] [PubMed] [Google Scholar]
- Wikstrom M. Cytochrome c oxidase: 25 years of the elusive proton pump. Biochim. Biophys. Acta. 2004;1655:241–247. doi: 10.1016/j.bbabio.2003.07.013. [DOI] [PubMed] [Google Scholar]