Abstract
Reactive oxygen and nitrogen species (ROS and RNS) play an important role in signal transduction and cell injury processes. Nitric oxide synthase (NOS)—the key enzyme producing nitric oxide (NO)—is found in neuronal structures, vascular endothelium and, possibly, in acinar and ductal epithelial cells in the pancreas. NO is known to regulate cell homeostasis, and its effects on the acinar cells are reviewed here. ROS are implicated in the early events within the acinar cells, leading to the development of acute pancreatitis. The available data on ROS/RNS involvement in the apoptotic and necrotic death of pancreatic acinar cells will be discussed.
Keywords: nitric oxide, reactive oxygen species, reactive nitrogen species, pancreatic acinar cells, pancreatitis
1. Introduction
Reactive oxygen and nitrogen species (ROS and RNS) are groups of related molecules with individually distinct chemical and biological properties. ROS refers to all oxidation and excitation states of molecular oxygen that arise in physiological conditions, including superoxide, singlet oxygen, hydroxyl radical, hydrogen peroxide, other peroxides and hypohalites. RNS refers to nitric oxide (NO) and its intermediate oxidation/reduction products that arise in physiological settings including nitroxyl, nitrous oxide, nitrosonium, nitric dioxide, and some unstable adducts of NO with biomolecules such as S-nitrosothiols and dinitrosyl iron complexes. ROS/RNS are integral parts of intracellular signalling, but become damaging at high levels of production.
Acute pancreatitis (AP) is an often-fatal human disease in which the pancreas digests itself. The disease process is triggered by activation of proteases inside the dominant acinar cells and there is a substantial body of evidence demonstrating that ROS/RNS contribute to the acinar cell damage during the early phases of pancreatitis (Sanfey et al. 1984; Tsai et al. 1998; Schulz et al. 1999). However, if the generation of NO and ROS in physiological and pathological conditions is firmly established at the level of the whole pancreas as well as in many non-acinar cell types, the data from the acinar cells are more ambiguous. Here we try to review the existing information on RNS/ROS production inside pancreatic acinar cells and the downstream effects of these species on the cells.
2. Localization of nitric oxide synthase in pancreas
Nitric oxide (NO) is a messenger molecule that is involved in many signalling processes, via the cellular cyclic guanosine-3′,5′-monophosphate (cGMP) pathway and through the nitrosylation of regulatory thiols (Blaise et al. 2005). It is produced de novo by a family of enzymes named nitric oxide synthase (NOS), of which neuronal and endothelial isoforms (nNOS and eNOS) are expressed constitutively, regulated by calmodulin and requiring a [Ca2+]i elevation above the basal level. These constitutive NOS isoforms are responsible for regulated low-level NO generation. eNOS can also be activated via phosphorylation by various protein kinases (Fleming & Busse 2003). An inducible isoform (iNOS) is expressed in response to immunological stimuli. iNOS is Ca-insensitive and can produce large amounts of NO as long as it has enough substrate (l-arginine). Mitochondria may contain their own constitutive NOS (Ghafourifar & Richter 1997; Giulivi et al. 1998; Lacza et al. 2003), although it is not entirely clear whether this isoform is of neuronal, endothelial or a new type. In addition to de novo generation from l-arginine by NOS, NO can be recycled back from nitrite, a stable end-product of NO metabolism, by enzymes such as nitrite reductase (Kozlov et al. 1999) and xanthine oxidase (Millar et al. 1998). Superoxide dismutase and copper-containing enzymes can also produce NO by processing hydroxyurea (Sato et al. 1997).
The main sources of constitutive NO generation in the pancreas are neurons in intrapancreatic ganglia, intra- and extrapancreatic nerve endings (Kirchgessner et al. 1994) and vascular endothelium (Konturek et al. 1993), expressing high levels of neuronal and endothelial type of NOS, respectively. The potential targets of these nitrergic axons are acini, ducts and blood vessels (Kirchgessner et al. 1994; Umehara et al. 1997). Lower levels of constitutive NOS were found in the islets of Langerhans (Burrell et al. 1996; Salehi et al. 1996; Umehara et al. 1997; Nakada et al. 2003). The data on the presence of NOS in the pancreatic acinar and ductal epithelia are ambiguous and species specific. Some investigations show absence of constitutive NOS in pancreatic acini (Worl et al. 1994; Umehara et al. 1997; Al Mufti et al. 1998; Ember et al. 2000), while others report detectable levels of NOS mRNA (Jaworek et al. 2000) and protein (Xu et al. 1997; Nam et al. 1998; DiMagno et al. 2004b). The work of DiMagno and collaborators (2004b) shows that 30% of the eNOS in the mouse pancreas is of acinar origin, but this proportion is less (9%) for nNOS (which the authors attributed to possible contamination from other cell types). In contrast, work of Xu et al. (1997) points to predominant nNOS expression in rat acinar cells. In the above-mentioned studies, intrapancreatic NOS was detected by means of Western blotting, immunocytochemistry (using nNOS and eNOS selective antibodies) and histochemistry (NADPH diaphorase activity). Apart from this, nuclei in the exocrine pancreas, including acinar cells, showed immunoreactivity against a new eNOS-interacting protein (Konig et al. 2002).
NOS presence in the pancreatic acinar cells was also confirmed in functional studies. NOS activity in isolated cell/acini in response to secretagogues was detected using arginine to citrulline conversion (Gukovskaya & Pandol 1994; Wrenn et al. 1994; Ahn et al. 1998), by evaluating nitrite concentration (Wrenn et al. 1994; Ahn et al. 1998; Jaworek et al. 2000), or cGMP production (Haymovits & Scheele 1976; Gardner & Rottman 1980; Pandol & Schoeffield-Payne 1990; Wrenn et al. 1994; Gilon et al. 1995; Gukovskaya & Pandol 1995; Xu et al. 1997; Yoshida et al. 1997; Ahn et al. 1998). Leptin receptor (Jaworek et al. 2002a) and protease-activated receptor-2 stimulation (Kawabata et al. 2002) was also able to generate NO in acinar cells. Additional circumstantial evidence for the ability of pancreatic acinar cells to produce NO during stimulation with agonists came from the observation that NOS blockade can modulate cell-to-cell coupling through gap junctions in isolated acini (Chanson et al. 1999).
Thus, there seems to be a substantial body of evidence that pancreatic acinar cells are capable of producing NO by constitutive NOS, though in much lower quantities than many neighbouring cells.
NO lifetime in physiological solutions allows for free diffusion up to several cell size distances from the production site (Beckman & Tsai 1994). Thus, the specificity of NO as a messenger depends on the spatial NO concentration profile determined by NO sources and sinks. In this respect, pancreatic acinar cells appear to be weak NO releasers, while they can be effective free radical sinks/buffers (Niederau et al. 1996). It is interesting to note that pancreatic beta cells are both NO releasers and NO targets. Beta cells from different islets may synchronize their Ca2+ oscillations in response to the ambient glucose concentration, via diffusion of NO (Grapengiesser et al. 2001) and carbon monoxide (CO; Lundquist et al. 2003) and subsequent activation of soluble guanylate cyclase. In this situation, acinar cells should probably serve as a buffering medium, limiting NO/CO communication between the islets. It is, however, conceivable that these diffusible messengers could also coordinate the activity of beta cells and surrounding acinar cells.
3. NO and pancreatic acinar cell function
The major function of the acinar cells in the pancreas is to synthesize and secrete digestive enzymes. The reported effects of NO on the regulation of pancreatic exocrine secretion are controversial. Studies in vivo suggest a positive influence of NO upon pancreatic secretion. Inhibition of endogenous NO synthesis decreased stimulated secretion of pancreatic enzymes in rats (Konturek et al. 1994; Molero et al. 1995; Vaquero et al. 1998; Jyotheeswaran et al. 2000; Zoucas et al. 2001), mice (DiMagno et al. 2004a), pigs (Holst et al. 1994), humans (Konturek et al. 1997), cats (Patel et al. 1995) and dogs (Konturek et al. 1993). NOS blockade was also shown to suppress basal zymogen secretion in fasting dogs (Maczka et al. 1994) and rats (Vaquero et al. 1998), although no effect was observed in mice (DiMagno et al. 2004a) and fed dogs (Konturek et al. 1993). The effects of NOS inhibition on in vitro secretion are variable. NOS inhibition was reported to reduce CCK-8-stimulated amylase secretion from isolated rat pancreatic acinar cells (Ahn et al. 1998), carbachol-stimulated amylase secretion from isolated rat pancreatic acini (Wrenn et al. 1994) and carbachol and CCK-stimulated secretion in guinea-pig acini (Gardner & Rottman 1980), while cGMP analogues caused amylase secretion in guinea-pig pancreatic lobules (Haymovits & Scheele 1976). In contrast, another study reported reduction of electric field-stimulated amylase secretion by the NO donor SNP and 8-Br-cGMP (Yago et al. 2002). Other studies showed no effect of NOS inhibition on CCK-8 or carbachol-stimulated or basal secretion in isolated rat pancreatic acini (Konturek et al. 1994; Molero et al. 1995; Yoshida et al. 1997), lobules (Kirchgessner et al. 1994) and isolated guinea-pig (Gunther & Jamieson 1979) and canine pancreatic acini (Konturek et al. 1993). Given such discrepancy between in vivo and in vitro data, NO effects on zymogen secretion in the acinar cells should be indirect and mediated (at least partly) by NO production and action at non-acinar cell types. This may include vasculature, nervous, endocrine cells and other NO producers/targets. The effects of NO were shown to be influenced by the site of NO generation, the amount of NO produced, and depended on the specific NOS isoforms involved in NO synthesis (Vaquero et al. 1998; Zoucas et al. 2001; DiMagno et al. 2004a).
The digestive enzyme secretion by acinar cells is controlled by intracellular Ca2+ signalling. The relationship between NO and calcium signalling has been studied at the level of isolated acini and cells. The reported effects of NO on [Ca2+]i regulation are contradictory: studies from two groups showed that NO has a stimulatory effect on calcium oscillations and calcium influx (Pandol & Schoeffield-Payne 1990; Bahnson et al. 1993; Gukovskaya & Pandol 1994; Xu et al. 1994, 1997; Chanson et al. 1999), while other investigations described little or no effects on Ca2+ signals (Gilon et al. 1995; Yoshida et al. 1997; Yago et al. 2002), concluding that the NO/cGMP system is not significantly involved in the mediation of Ca2+ signalling. The NO-stimulated Ca2+ influx in acinar cells observed by the laboratories of Muallem & Pandol was dependent on cGMP. This is different from the Ca2+ influx regulation by direct S-nitrosylation (independent of cGMP) of the influx channel expressed in HEK-293 cells reported by Gill's group (van Rossum et al. 2000). The latter may be a possible NO regulating mechanism of Ca2+ entry in parotid acinar cells, which is also cGMP independent (Watson et al. 1999). The reports of NO influence on Ca2+ homeostasis in pancreatic acinar cells contrast with data from their structural and morphological analogues—acinar cells from salivary glands, which display more complex patterns of NO—Ca2+ interplay. In acinar cells isolated from salivary glands, NO was shown to influence Ca2+ release from intracellular stores via cGMP by increasing IP3 production and simultaneously affecting ryanodine-sensitive Ca2+ stores (Looms et al. 2002).
Overall the data on the direct role of NO in fast pancreatic acinar cell reactions such as Ca2+ signalling and enzyme secretion are controversial. It was proposed that the homeostatic role of ROS and RNS ‘may be to link the behavioural and differentiative commitments of a cell to its metabolic budget’ (Nathan 2003). In this respect, the main effects of endogenous NO may be regulation of metabolism and maintenance of pancreatic tissue.
4. NO links to pancreatic acinar cell homeostasis and pathology
Endogenous NO may be implicated in regulation of pancreatic acinar cell homeostasis in terms of control of gene expression, protein synthesis, cytoskeletal organization and energy metabolism, growth and apoptosis. NO was shown to exert a tonic inhibition of apoptosis in the pancreas during both basal conditions and growth stimulated by CCK-8, thus regulating the balance between proliferation and apoptosis (Trulsson et al. 2002). In rat pancreatic acini, exogenous NO was found to increase tyrosine phosphorylation of the focal adhesion non-receptor tyrosine kinase p125FAK and the cytoskeleton-associated protein paxillin (Garcia-Benito et al. 2000), which may both be implicated in cell growth, migration and mitogenesis. In this cell type, p125FAK and paxillin may be important mediators of EGF action (Tapia et al. 1999). Another regulatory role of NO comes from its ability, as well as that of some ROS, to stimulate TALK-1 and TALK-2 K+ channels, which are highly and specifically expressed in pancreatic acinar cells and important in setting the membrane potential (Duprat et al. 2005).
Non-selective blockade of NOS generally leads to a decrease in the pancreatic blood flow, increase of pancreatic weight, pancreatic enzyme elevation and pancreatic acinar cell degeneration in vivo (Konturek et al. 1994; Kolaja et al. 2004). However, these pancreatic changes appear to be only transient, suggesting a progressive adaptation to NOS inhibition (Kolaja et al. 2004). Administration of high doses of l-arginine causes oxidative and nitrosative stress in the pancreas (Czako et al. 1998) and leads to pancreatic tissue damage (Mizunuma et al. 1984; Hegyi et al. 2004).
Evidence suggests that NO is implicated in certain pathological processes in the pancreas. It was shown that patients with AP have reduced levels of serum l-arginine and l-citrulline (Sandstrom et al. 2003). This indicates the potential significance of arginine metabolism and NO in the pathogenesis of this disease. The role of NO produced by either constitutive or inducible NOS in experimental models of AP is controversial. Generally, NO generated by iNOS, which during AP is induced in infiltrating neutrophils and macrophages (Satoh et al. 1998) and also in the other cells of pancreatic tissue (Al Mufti et al. 1998; Rau et al. 2001; Vaquero et al. 2001; Qader et al. 2003; Um et al. 2003), has injurious effects (Lomis et al. 1995; Ayub et al. 2001; Simsek et al. 2001; Cuzzocrea et al. 2002; Ozturk et al. 2003; Um et al. 2003; Chen et al. 2004; Isik et al. 2004; Sandstrom et al. 2005). The inducible form of cyclooxygenase, producing CO in a manner similar to NO production by iNOS, also exacerbates the severity of pancreatitis (Song et al. 2002). A beneficial effect of iNOS on the course of pancreatitis was, however, shown in the model of AP caused by coxsackievirus B4 infection (Zaragoza et al. 1999; Flodstrom et al. 2001) and in one iNOS knockout mice study (Qui et al. 2001). The effect of constitutive NOS during AP is complex. First, eNOS, but not nNOS, was shown to be protective in the initiation of caerulein-induced AP in rats (DiMagno et al. 2004b). Meanwhile, another study on the same model of AP implied that neuronal secretion of NO may cause significant protection (Dembinski et al. 1996). It is also worth noting that, unlike iNOS, constitutive NOS activity becomes progressively depleted during AP (Takacs et al. 2002; Qader et al. 2003). This may explain adaptation to non-selective NOS inhibition over time (Kolaja et al. 2004).
The pancreas is highly susceptible to ischaemic damage. There is increasing evidence that pancreatic ischaemia plays an important role in initiating and aggravating AP. NO appears to reduce pancreatic ischaemia/reperfusion injury (Tanaka et al. 1996; Benz et al. 2002; Obermaier et al. 2004a,b; Yuan et al. 2004). However, while the overall protective effect of NO supplementation was firmly established, the effect of NO synthesis inhibition is not always equally clear (Hotter et al. 1995; Tanaka et al. 1996; Benz et al. 2002; Yuan et al. 2004). One study suggests that NOS can be induced following ischaemia and reperfusion of the pancreas (Viola et al. 2000), contributing to local and systemic damage.
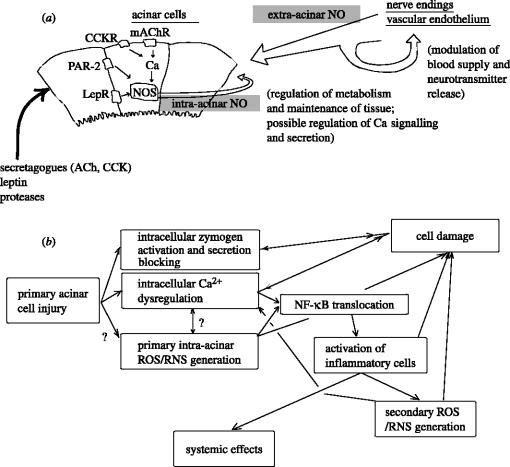
The protective action of NO on pancreatic acinar tissue occurs mainly at the extra-acinar level, as seen from comparison of studies in vitro and in vivo (Werner et al. 1997, 1998; DiMagno et al. 2004b), and includes pancreatic blood flow enhancement (Konturek et al. 1993; DiMagno et al. 2004b; Obermaier et al. 2004a) and leukocyte adherence inhibition (Dembinski et al. 1996; Obermaier et al. 2004b). NO also contributes to prostanoid biosynthesis in the earlier stages of AP (Closa et al. 1994b), which may play a role in cellular defence mechanisms (Closa et al. 1994c). However, the amelioration of pancreatitis by leptin (Jaworek et al. 2002b; Konturek et al. 2002; Warzecha et al. 2002) and PAR-2 activation (Namkung et al. 2004; Sharma et al. 2005), which both involve NO (Jaworek et al. 2002a; Kawabata et al. 2002; Konturek et al. 2002), may at least partly result from the primary effects on the acinar cells. The beneficial effects of leptin appear to be dependent on the increase in pancreatic cell growth and the limitation of pro-inflammatory interleukin-1beta synthesis, whereas the PAR-2 effect is possibly mediated by inhibiting ERK1/2 translocation to the nucleus in the acinar cells. NO increases non-apoptotic cell death and can contribute to hypoplasia caused by repetitive injections of high concentrations of CCK-8 (Trulsson et al. 2001, 2004). On the other hand, NO suppresses apoptosis and facilitates pancreatic hyperplasia induced by slow infusion of CCK-8 (Andrzejewska et al. 2002; Trulsson et al. 2002). Some of these effects may be due to universal mechanisms of cell metabolism regulation by NO such as tonic modulation of cell respiration and inhibition of caspases. NO was also reported to inhibit cathepsin B (Stamler et al. 1992), which may mediate intrapancreatic trypsinogen activation (Greenbaum et al. 1959; Halangk & Lerch 2004) and lead to AP. The stimulatory effect of NO on exocytosis may also exert a beneficial action on the cells, since inhibition of secretion is associated with pancreatic acinar cell pathology (Lerch & Adler 1994; figure 1).
Figure 1.
(a) Sources of NO in the pancreas and NO effects on the pancreatic acini. (b) Role of ROS/RNS in cell pathology during acute pancreatitis.
In contrast to the protective action of low levels of NO, excess of NO causes injurious effects by potentiating oxidative stress (Dabrowski & Gabryelewicz 1994; Al Mufti et al. 1998).
5. Mechanisms of ROS and RNS induced damage in pancreatic acinar cells
There are numerous indications that ROS/RNS play a significant role in various forms of pancreatic pathology including chronic (Acheson et al. 1985; Schoenberg et al. 1995) and acute pancreatitis (Sanfey et al. 1984).
AP is a rapidly developing systemic disease. According to the current view, the primary injury of the acinar cells leads to intracellular trypsinogen activation and inhibition of acinar cell secretion. There are data suggesting that the acinar cell damage is followed within minutes by the production of ROS. The primary free radical production may exacerbate the cell damage, causing lesions of cells membranes and cytoskeleton, impairing functions of intracellular proteins, damaging DNA, evoking lipid peroxidation, decreasing the level of antioxidants and activating NF-κB. The translocation of NF-κB into the nucleus results in intra-acinar transcription of chemokines and cytokines. These molecules lead to invasion and activation of inflammatory cells, which produce more ROS/RNS and are responsible for acinar necrosis and amplification of the inflammation in the pancreas.
Oxidative stress was documented in pancreatic tissue by detecting generation of ROS with chemiluminescence (Gough et al. 1990) or fluorescence (Urunuela et al. 2002) and accumulation of products of ROS-mediated lipid peroxidation (Nonaka et al. 1989b, 1990; Guyan et al. 1990; Schoenberg et al. 1991, 1995; Tsai et al. 1998; Rau et al. 2000) and protein modification (Reinheckel et al. 1998), with concomitant depletion of low molecular weight antioxidants (Neuschwander-Tetri et al. 1994; Luthen et al. 1995; Tsai et al. 1998; Schulz et al. 1999; Kruse et al. 2001; Rau et al. 2001; Rahman et al. 2004). It was also confirmed by ESR spectroscopy using spin traps (Nonaka et al. 1989a). In one case of human AP, an in situ histological study using confocal microscopy showed ROS-derived cerium perhydroxide depositions in pancreatic acini and neighbouring capillaries, indicating strong local oxidative stress (Telek et al. 2001).
However, lipid peroxidation may perhaps be a consequence rather than the cause of pancreatic inflammation. Moreover, it was shown that artificial ROS production or intracellular glutathione depletion, leading to oxidative stress similar to that observed in models of AP, could not bring about equal acinar cell damage and necrosis (Fu et al. 1997; Rau et al. 2000). Thus, ROS/RNS may play a secondary role in the pathogenesis of AP. In addition, ROS-induced lipid peroxidation could not be proven in haemorrhagic necrotizing pancreatitis in humans (Schoenberg et al. 1995). Only limited clinical studies have indicated the therapeutic efficacy of antioxidants against pancreatic necrosis (Schulz et al. 1999). Nevertheless, evidence from clinical trials suggests the importance of free radicals in the genesis of oedema in AP (Schulz et al. 1999). Moreover, the level of ROS production during pancreatitis can be strongly dependent on the experimental type of AP model (Nordback & Cameron 1993).
Among four models of AP in dogs used by Nordback et al. (intra-arterial infusion of oleic acid that mimics hyperlipemic pancreatitis, partial obstruction of the pancreatic duct that mimics gallstone pancreatitis, a 2 h period of ischaemia before perfusion that mimics shock pancreatitis, and the infusion of caerulein at supramaximal stimulatory doses, which lacks an obvious clinical counterpart), all but caerulein pancreatitis exhibited the presence of toxic oxygen metabolites, highlighting the importance of ROS in the early pathogenesis of AP (Nordback & Cameron 1993). In that study, an antioxidant, dithiothreitol, ameliorated all except the caerulein form of pancreatitis. ROS scavenger therapy was also found effective in taurocholate- (Gough et al. 1990; Schoenberg et al. 1991, 1994), caerulein- (Guice et al. 1986; Wisner et al. 1988; Schoenberg et al. 1994) and pancreatic duct obstruction (Sevillano et al. 2003) induced AP in rats, as well as in caerulein- and choline-deficient/ethionine-enriched diet induced AP in mice (Nonaka et al. 1991, 1992; Cuzzocrea et al. 2004). However, in other studies of caerulein and choline-deficient/ethionine-enriched diet models of pancreatitis in rodents, administration of ROS scavengers and suppression of ROS production was unable to reduce the degree of the acinar cell injury (Steer et al. 1991; Schulz et al. 2001). Antioxidants also inhibited caerulein-induced NF-κB activation in isolated rat pancreatic acinar cells (Yu et al. 2002). Interestingly, the synthetic capacity of the pancreas for the endogenous intracellular antioxidant glutathione is substantially less than that of the kidney or liver (Neuschwander-Tetri et al. 1997), which may explain the susceptibility of pancreatic acinar cells and the pancreas as a whole to oxidative damage.
At the molecular level, the main cellular sources of ROS include the mitochondrial electron transport chain, NADPH oxidase, xanthine oxidase and cytochrome 450 isoenzymes. NADH oxidase is strongly expressed in inflammatory cells and is capable of producing vast amounts of superoxide. Neutrophil NADPH oxidase was found to potentiate caerulein-induced trypsin activation (Gukovskaya et al. 2002). This enzyme seems to be the main source of ROS after development of pancreatic inflammation during AP. However, so far, none of its isoforms have been found in pancreatic acinar cells (Gukovskaya et al. 2002). Xanthine oxidoreductase enzyme usually (under physiological conditions) functions as xanthine dehydrogenase and uses NAD+ as electron acceptor. However, under certain circumstances it may reversibly or irreversibly convert to xanthine oxidase, which transfers electrons to molecular oxygen, giving rise to superoxide and hydrogen peroxide. Limited proteolytic cleavage switches the enzyme to oxidase irreversibly, and such conversion in experimental AP models has been reported (Closa et al. 1994a). In contrast, reversible conversion of the enzyme via sulphydryl group oxidation was suggested in another study (Nordback & Cameron 1993). Xanthine oxidase was proven to be a source of ROS contributing to both local and systemic effects of AP, studied in vivo and in situ (Nonaka et al. 1989b; Cassone et al. 1991; Telek et al. 2001). Furthermore, xanthine oxidase has been shown to generate reactive oxygen metabolites in the caerulein-stimulated isolated acinar cells (Suzuki et al. 1993). While xanthine oxidase is the main suspected source of primary ROS during early stages of AP, cytochrome P-450 isoenzymes, i.e. phase I xenobiotic-metabolizing enzymes, are incriminated in ROS production in chronic pancreatitis (CP). Induction of cytochrome P-450 was reported during CP (Acheson et al. 1985; Foster et al. 1993; Wacke et al. 1998) as well as in other forms of exocrine pancreatic disease such as AP (Acheson et al. 1985) and pancreatic cancer (Acheson et al. 1985; Foster et al. 1993). The increased oxidant load from long-term cytochrome P-450 induction can eventually lead to depletion of the antioxidant defence in pancreatic acinar cells and to cell injury (Braganza 1998), and may be a significant factor in pathogenesis of CP. This central role of ROS production, by phase I metabolizing enzymes, and glutathione depletion, by ensuing ROS and phase II conjugating enzymes (glutathione S-transferase pi), in the evolution of CP, however, was questioned in several studies (Niederau et al. 1991; Wallig 1998).
In vitro, exposure of isolated pancreatic acinar cells to oxidative stress caused rapid cell damage and death. Oxidizing agents induce Ca2+ oscillations, which occur before other functional and morphological alterations (Thorn et al. 1992; Niederau et al. 1996; Klonowski-Stumpe et al. 1997; Gerasimenko et al. 2002). These oscillations were mediated by Ca2+ release from both thapsigargin sensitive and insensitive intracellular stores (Klonowski-Stumpe et al. 1997; Gonzalez et al. 2002). Interestingly, ROS-induced Ca2+ oscillations produce no protein secretion (Klonowski-Stumpe et al. 1997; Sweiry et al. 1999). It is worth noting, however, that xanthine oxidase—used together with the substrate hypoxanthine as a standard ROS production system in many studies in vitro—gives rise to Ca2+ signals itself in a substrate independent way. The proteinase activity in commercial xanthine oxidase has been reported (Ager et al. 1984). Therefore, commercial preparations of xanthine oxidase may activate PAR-2 receptors and, consequently, bring about Ca2+ oscillations (Namkung et al. 2004), and contribute to some effects attributed to ROS.
Microsomes, lysosomes and mitochondria were found to be the organelles most susceptible to oxidative damage in caerulein-induced AP (Sanchez-Bernal et al. 2004). Another study showed that isolated zymogen granules were markedly more sensitive to oxidative stress than intact acinar cells (Niederau et al. 1996). Early changes induced by oxidative stress in the acinar cells also included vacuolization and structural alterations of mitochondria, endoplasmic reticulum and nucleus (Han et al. 2000). Treatment of the pancreatic AR4-2J cell line with hydrogen peroxide resulted in selective mitochondrial DNA damage with nuclear DNA unaffected (Ehlers et al. 1999). In mitochondria, ROS evoked a decrease in the dehydrogenase activity (Weber et al. 1998), inner membrane depolarization, opening of the permeability transition pore and cytochrome c release, which results in activation of programmed cell death (Ehlers et al. 1999; Gerasimenko et al. 2002). ROS was shown to affect actin filament polymerization in mouse pancreatic acinar cells, and this effect depended on Ca2+ mobilization, but not on protein kinase C. Interestingly, in this study H2O2 almost completely inhibited CCK-8-induced amylase release (Rosado et al. 2002). The action of ROS on actin filaments might be the mechanism of free radical-induced injury of pancreatic acinar cells (Jungermann et al. 1995). A link between ROS and MAPK, JNK/SAPK and p38 MAPK signalling cascades has been established in pancreatic acinar cells (Dabrowski et al. 2000). It was found that hydrogen peroxide and menadione strongly activated these pathways in the isolated cells. It has been hypothesized that this activation may be one of the mechanisms of ROS-induced dysregulation of the cytoskeleton (in addition to the more direct ROS-induced disruption of the actin network; Dabrowski et al. 2000; Dabrowski 2003).
NO overproduction by iNOS in the pancreas is implicated in the exacerbation of cell injury during AP (Lomis et al. 1995; Viola et al. 2000; Ayub et al. 2001; Simsek et al. 2001; Cuzzocrea et al. 2002; Ozturk et al. 2003; Um et al. 2003; Chen et al. 2004; Isik et al. 2004; Sandstrom et al. 2005). One possible mechanism of NO-mediated damage is potentiation of oxidative stress by NO. First, inhibition of cell respiration by NO may favour generation of superoxide (Moncada & Erusalimsky 2002). Second, peroxynitrite, the product of the reaction of NO with superoxide anion, is a more aggressive radical than superoxide or NO and can participate is some specific cell-damaging chemical reactions such as irreversible tyrosine nitration (Ischiropoulos 2003). Peroxynitrite may also be produced inside the mitochondria as a result of superoxide generation by the mitochondrial electron transport chain and NO production by mitochondrial NOS activated by mitochondrial Ca2+ uptake (Bringold et al. 2000). Peroxynitrite formed inside mitochondria may cause cytochrome c release (Ghafourifar et al. 1999) and lead to cell apoptosis.
6. Conclusions
Mounting evidence suggests that NO may participate in the regulation of pancreatic acinar cell homeostasis at the level of energy supply, Ca2+ signalling, enzyme secretion, cell growth and death. This regulation occurs partly at the intra-acinar level. Pancreatic acinar cells appear to possess some relatively weak NOS activity, but many other cell types of the pancreas were shown to be potent NO releasers. The acinar cells probably serve as both targets and scavengers of free radicals (both RNS and ROS). Free radicals may play an important role in the pathogenesis of AP and CP and may mediate cell injury following pancreatic ischaemia/reperfusion. The importance of ROS/RNS as mediators of cell damage strongly depends on the experimental model of pancreatitis. In several models of AP, ROS/RNS were shown to be involved in the amplification of local and systemic inflammation. The source of free radicals can be external to the acinar cells; nevertheless, some studies also suggest that acinar cells, when exposed to noxious stimuli, may produce ROS themselves. Further research in this field may yield important information about the general mechanisms of regulation and pancreatic pathology.
Acknowledgments
We thank Mark Houghton for his expert technical assistance. The work of the laboratory is sponsored by a Medical Research Council (MRC) program grant (G880/575).
Footnotes
One contribution of 18 to a Theme Issue ‘Reactive oxygen species in health and disease’.
References
- Acheson D.W, Rose P, Houston J.B, Braganza J.M. Induction of cytochromes P-450 in pancreatic disease: consequence, coincidence or cause? Clin. Chim. Acta. 1985;153:73–84. doi: 10.1016/0009-8981(85)90158-5. 10.1016/0009-8981(85)90158-5 [DOI] [PubMed] [Google Scholar]
- Ager A, Wenham D.J, Gordon J.L. Stimulation of endothelial cells by protease activity in commercial preparations of xanthine oxidase. Thromb. Res. 1984;35:43–52. doi: 10.1016/0049-3848(84)90311-6. 10.1016/0049-3848(84)90311-6 [DOI] [PubMed] [Google Scholar]
- Ahn S.H, Seo D.W, Ko Y.K, Sung D.S, Bae G.U, Yoon J.W, Hong S.Y, Han J.W, Lee H.W. NO/cGMP pathway is involved in exocrine secretion from rat pancreatic acinar cells. Arch. Pharm. Res. 1998;21:657–663. doi: 10.1007/BF02976753. [DOI] [PubMed] [Google Scholar]
- Al Mufti R.A, Williamson R.C, Mathie R.T. Increased nitric oxide activity in a rat model of acute pancreatitis. Gut. 1998;43:564–570. doi: 10.1136/gut.43.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrzejewska A, Jurkowska G, Augustynowicz A. The influence of nitric oxide synthesis modulation on the pancreatic acinar cells in caerulein-induced acute pancreatitis. An ultrastructural and morphometric study. Pol. J. Pathol. 2002;53:215–221. [PubMed] [Google Scholar]
- Ayub K, Serracino-Inglott F, Williamson R.C, Mathie R.T. Expression of inducible nitric oxide synthase contributes to the development of pancreatitis following pancreatic ischaemia and reperfusion. Br. J. Surg. 2001;88:1189–1193. doi: 10.1046/j.0007-1323.2001.01841.x. 10.1046/j.0007-1323.2001.01841.x [DOI] [PubMed] [Google Scholar]
- Bahnson T.D, Pandol S.J, Dionne V.E. Cyclic GMP modulates depletion-activated Ca2+ entry in pancreatic acinar cells. J. Biol. Chem. 1993;268:10 808–10 812. [PubMed] [Google Scholar]
- Beckman J, Tsai H.-H. Reactions and diffusion of nitric oxide and peroxynitrite. Biochemist. 1994;16:8–10. [Google Scholar]
- Benz S, et al. Effect of nitric oxide in ischemia/reperfusion of the pancreas. J. Surg. Res. 2002;106:46–53. doi: 10.1006/jsre.2002.6457. 10.1006/jsre.2002.6457 [DOI] [PubMed] [Google Scholar]
- Blaise G.A, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005;208:177–192. doi: 10.1016/j.tox.2004.11.032. 10.1016/j.tox.2004.11.032 [DOI] [PubMed] [Google Scholar]
- Braganza J.M. A framework for the aetiogenesis of chronic pancreatitis. Digestion. 1998;59(Suppl. 4):1–12. doi: 10.1159/000051438. 10.1159/000051438 [DOI] [PubMed] [Google Scholar]
- Bringold U, Ghafourifar P, Richter C. Peroxynitrite formed by mitochondrial NO synthase promotes mitochondrial Ca2+ release. Free Radic. Biol. Med. 2000;29:343–348. doi: 10.1016/s0891-5849(00)00318-x. 10.1016/S0891-5849(00)00318-X [DOI] [PubMed] [Google Scholar]
- Burrell M.A, Montuenga L.M, Garcia M, Villaro A.C. Detection of nitric oxide synthase (NOS) in somatostatin-producing cells of human and murine stomach and pancreas. J. Histochem. Cytochem. 1996;44:339–346. doi: 10.1177/44.4.8601693. [DOI] [PubMed] [Google Scholar]
- Cassone E, Maneschi E.M, Faccas J.G. Effects of allopurinol on ischemic experimental pancreatitis. Int. J. Pancreatol. 1991;8:227–234. doi: 10.1007/BF02924541. [DOI] [PubMed] [Google Scholar]
- Chanson M, Mollard P, Meda P, Suter S, Jongsma H.J. Modulation of pancreatic acinar cell to cell coupling during ACh-evoked changes in cytosolic Ca2+ J. Biol. Chem. 1999;274:282–287. doi: 10.1074/jbc.274.1.282. 10.1074/jbc.274.1.282 [DOI] [PubMed] [Google Scholar]
- Chen C.C, Wang S.S, Tsay S.H, Lee F.Y, Lu R.H, Chang F.Y, Lee S.D. Effects of nitric oxide synthase inhibitors on retrograde bile salt-induced pancreatitis rats. J. Chin. Med. Assoc. 2004;67:9–14. [PubMed] [Google Scholar]
- Closa D, Bulbena O, Hotter G, Rosello-Catafau J, Fernandez-Cruz L, Gelpi E. Xanthine oxidase activation in cerulein- and taurocholate-induced acute pancreatitis in rats. Arch. Int. Physiol. Biochim. Biophys. 1994a;102:167–170. doi: 10.3109/13813459409007532. [DOI] [PubMed] [Google Scholar]
- Closa D, Hotter G, Prats N, Bulbena O, Rosello-Catafau J, Fernandez-Cruz L, Gelpi E. Prostanoid generation in early stages of acute pancreatitis: a role for nitric oxide. Inflammation. 1994b;18:469–480. doi: 10.1007/BF01560694. 10.1007/BF01560694 [DOI] [PubMed] [Google Scholar]
- Closa D, Hotter G, Rosello-Catafau J, Bulbena O, Fernandez-Cruz L, Gelpi E. Prostanoids and oxygen free radicals in early stages of experimental acute pancreatitis. Dig. Dis. Sci. 1994c;39:1537–1543. doi: 10.1007/BF02088061. 10.1007/BF02088061 [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, et al. Inducible nitric oxide synthase-deficient mice exhibit resistance to the acute pancreatitis induced by cerulein. Shock. 2002;17:416–422. doi: 10.1097/00024382-200205000-00013. 10.1097/00024382-200205000-00013 [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, Di Paola R, Muia C, Britti D, Salvemini D. Reduction in the development of cerulein-induced acute pancreatitis by treatment with M40401, a new selective superoxide dismutase mimetic. Shock. 2004;22:254–261. doi: 10.1097/01.shk.0000132490.79498.11. 10.1097/01.shk.0000132490.79498.11 [DOI] [PubMed] [Google Scholar]
- Czako L, Takacs T, Varga I.S, Tiszlavicz L, Hai D.Q, Hegyi P, Matkovics B, Lonovics J. Involvement of oxygen-derived free radicals in l-arginine-induced acute pancreatitis. Dig. Dis. Sci. 1998;43:1770–1777. doi: 10.1023/a:1018839821176. 10.1023/A:1018839821176 [DOI] [PubMed] [Google Scholar]
- Dabrowski A. Exocrine pancreas; molecular basis for intracellular signaling, damage and protection—Polish experience. J. Physiol. Pharmacol. 2003;54(Suppl. 3):167–181. [PubMed] [Google Scholar]
- Dabrowski A, Gabryelewicz A. Nitric oxide contributes to multiorgan oxidative stress in acute experimental pancreatitis. Scand. J. Gastroenterol. 1994;29:943–948. doi: 10.3109/00365529409094868. [DOI] [PubMed] [Google Scholar]
- Dabrowski A, Boguslowicz C, Dabrowska M, Tribillo I, Gabryelewicz A. Reactive oxygen species activate mitogen-activated protein kinases in pancreatic acinar cells. Pancreas. 2000;21:376–384. doi: 10.1097/00006676-200011000-00008. 10.1097/00006676-200011000-00008 [DOI] [PubMed] [Google Scholar]
- Dembinski A, Warzecha Z, Konturek P.J, Ceranowicz P, Konturek S.J. Influence of capsaicin-sensitive afferent neurons and nitric oxide (NO) on cerulein-induced pancreatitis in rats. Int. J. Pancreatol. 1996;19:179–189. doi: 10.1007/BF02787366. [DOI] [PubMed] [Google Scholar]
- DiMagno M.J, Hao Y, Tsunoda Y, Williams J.A, Owyang C. Secretagogue-stimulated pancreatic secretion is differentially regulated by constitutive nitric oxide synthase isoforms in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2004a;286:G428–G436. doi: 10.1152/ajpgi.00368.2003. 10.1152/ajpgi.00368.2003 [DOI] [PubMed] [Google Scholar]
- DiMagno M.J, Williams J.A, Hao Y, Ernst S.A, Owyang C. Endothelial nitric oxide synthase is protective in the initiation of caerulein-induced acute pancreatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2004b;287:G80–G87. doi: 10.1152/ajpgi.00525.2003. 10.1152/ajpgi.00525.2003 [DOI] [PubMed] [Google Scholar]
- Duprat F, Girard C, Jarretou G, Lazdunski M. Pancreatic two P domain K+ channels TALK-1 and TALK-2 are activated by nitric oxide and reactive oxygen species. J. Physiol. 2005;562:235–244. doi: 10.1113/jphysiol.2004.071266. 10.1113/jphysiol.2004.071266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers R.A, Hernandez A, Bloemendal L.S, Ethridge R.T, Farrow B, Evers B.M. Mitochondrial DNA damage and altered membrane potential (delta psi) in pancreatic acinar cells induced by reactive oxygen species. Surgery. 1999;126:148–155. 10.1067/msy.1999.98725 [PubMed] [Google Scholar]
- Ember Z, Reti A, Feher E. Enzyme- and immunohistochemical localization of nitric oxide synthase in nerves of the porcine pancreas. Neurosci. Lett. 2000;292:163–166. doi: 10.1016/s0304-3940(00)01455-5. 10.1016/S0304-3940(00)01455-5 [DOI] [PubMed] [Google Scholar]
- Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;284:R1–12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- Flodstrom M, Horwitz M.S, Maday A, Balakrishna D, Rodriguez E, Sarvetnick N. A critical role for inducible nitric oxide synthase in host survival following coxsackievirus B4 infection. Virology. 2001;281:205–215. doi: 10.1006/viro.2000.0801. 10.1006/viro.2000.0801 [DOI] [PubMed] [Google Scholar]
- Foster J.R, Idle J.R, Hardwick J.P, Bars R, Scott P, Braganza J.M. Induction of drug-metabolizing enzymes in human pancreatic cancer and chronic pancreatitis. J. Pathol. 1993;169:457–463. doi: 10.1002/path.1711690412. 10.1002/path.1711690412 [DOI] [PubMed] [Google Scholar]
- Fu K, Sarras M.P, Jr, De Lisle R.C, Andrews G.K. Expression of oxidative stress-responsive genes and cytokine genes during caerulein-induced acute pancreatitis. Am. J. Physiol. 1997;273:G696–G705. doi: 10.1152/ajpgi.1997.273.3.G696. [DOI] [PubMed] [Google Scholar]
- Garcia-Benito M, San Roman J.I, Lopez M.A, Garcia-Marin L.J, Calvo J.J. Nitric oxide stimulates tyrosine phosphorylation of p125(FAK) and paxillin in rat pancreatic acini. Biochem. Biophys. Res. Commun. 2000;274:635–640. doi: 10.1006/bbrc.2000.3192. 10.1006/bbrc.2000.3192 [DOI] [PubMed] [Google Scholar]
- Gardner J.D, Rottman A.J. Evidence against cyclic GMP as a mediator of the actions of secretagogues on amylase release from guinea-pig pancreas. Biochim. Biophys. Acta. 1980;627:230–243. doi: 10.1016/0304-4165(80)90452-3. [DOI] [PubMed] [Google Scholar]
- Gerasimenko J.V, Gerasimenko O.V, Palejwala A, Tepikin A.V, Petersen O.H, Watson A.J. Menadione-induced apoptosis: roles of cytosolic Ca(2+) elevations and the mitochondrial permeability transition pore. J. Cell Sci. 2002;115:485–497. doi: 10.1242/jcs.115.3.485. [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–296. doi: 10.1016/s0014-5793(97)01397-5. 10.1016/S0014-5793(97)01397-5 [DOI] [PubMed] [Google Scholar]
- Ghafourifar P, Schenk U, Klein S.D, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J. Biol. Chem. 1999;274:31 185–31 188. doi: 10.1074/jbc.274.44.31185. 10.1074/jbc.274.44.31185 [DOI] [PubMed] [Google Scholar]
- Gilon P, Obie J.F, Bian X, Bird G.S, Putney J.W., Jr Role of cyclic GMP in the control of capacitative Ca2+ entry in rat pancreatic acinar cells. Biochem. J. 1995;311:649–656. doi: 10.1042/bj3110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulivi C, Poderoso J.J, Boveris A. Production of nitric oxide by mitochondria. J. Biol. Chem. 1998;273:11 038–11 043. doi: 10.1074/jbc.273.18.11038. 10.1074/jbc.273.18.11038 [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Schmid A, Salido G.M, Camello P.J, Pariente J.A. XOD-catalyzed ROS generation mobilizes calcium from intracellular stores in mouse pancreatic acinar cells. Cell. Signal. 2002;14:153–159. doi: 10.1016/s0898-6568(01)00247-9. 10.1016/S0898-6568(01)00247-9 [DOI] [PubMed] [Google Scholar]
- Gough D.B, Boyle B, Joyce W.P, Delaney C.P, McGeeney K.F, Gorey T.F, Fitzpatrick J.M. Free radical inhibition and serial chemiluminescence in evolving experimental pancreatitis. Br. J. Surg. 1990;77:1256–1259. doi: 10.1002/bjs.1800771119. [DOI] [PubMed] [Google Scholar]
- Grapengiesser E, Gylfe E, Dansk H, Hellman B. Nitric oxide induces synchronous Ca2+ transients in pancreatic beta cells lacking contact. Pancreas. 2001;23:387–392. doi: 10.1097/00006676-200111000-00009. 10.1097/00006676-200111000-00009 [DOI] [PubMed] [Google Scholar]
- Greenbaum L.M, Hirshkowitz A, Shoichet I. The activation of trypsinogen by cathepsin B. J. Biol. Chem. 1959;234:2885–2890. [PubMed] [Google Scholar]
- Guice K.S, Miller D.E, Oldham K.T, Townsend C.M, Jr, Thompson J.C. Superoxide dismutase and catalase: a possible role in established pancreatitis. Am. J. Surg. 1986;151:163–169. doi: 10.1016/0002-9610(86)90027-9. 10.1016/0002-9610(86)90027-9 [DOI] [PubMed] [Google Scholar]
- Gukovskaya A.S, Pandol S.J. Nitric oxide production regulates cGMP formation and calcium influx in pancreatic acinar cells. Am. J. Physiol. 1994;266:G350–G356. doi: 10.1152/ajpgi.1994.266.3.G350. [DOI] [PubMed] [Google Scholar]
- Gukovskaya A.S, Pandol S.J. Dual regulation of cGMP formation by calcium in pancreatic acinar cells. Am. J. Physiol. 1995;268:G900–G907. doi: 10.1152/ajpgi.1995.268.6.G900. [DOI] [PubMed] [Google Scholar]
- Gukovskaya A.S, Vaquero E, Zaninovic V, Gorelick F.S, Lusis A.J, Brennan M.L, Holland S, Pandol S.J. Neutrophils ADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974–984. doi: 10.1053/gast.2002.32409. 10.1053/gast.2002.32409 [DOI] [PubMed] [Google Scholar]
- Gunther G.R, Jamieson J.D. Increased intracellular cyclic GMP does not correlate with protein discharge from pancreatic acinar cells. Nature. 1979;280:318–320. doi: 10.1038/280318a0. 10.1038/280318a0 [DOI] [PubMed] [Google Scholar]
- Guyan P.M, Uden S, Braganza J.M. Heightened free radical activity in pancreatitis. Free Radic. Biol. Med. 1990;8:347–354. doi: 10.1016/0891-5849(90)90100-w. 10.1016/0891-5849(90)90100-W [DOI] [PubMed] [Google Scholar]
- Halangk W, Lerch M.M. Early events in acute pancreatitis. Gastroenterol. Clin. North Am. 2004;33:717–731. doi: 10.1016/j.gtc.2004.07.009. 10.1016/j.gtc.2004.07.009 [DOI] [PubMed] [Google Scholar]
- Han B, Klonowski-Stumpe H, Luthen R, Schreiber R, Haussinger D, Niederau C. Menadione-induced oxidative stress inhibits cholecystokinin-stimulated secretion of pancreatic acini by cell dehydration. Pancreas. 2000;21:191–202. doi: 10.1097/00006676-200008000-00013. 10.1097/00006676-200008000-00013 [DOI] [PubMed] [Google Scholar]
- Haymovits A, Scheele G.A. Cellular cyclic nucleotides and enzyme secretion in the pancreatic acinar cell. Proc. Natl Acad. Sci. USA. 1976;73:156–160. doi: 10.1073/pnas.73.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegyi P, Rakonczay Z, Jr, Sari R, Gog C, Lonovics J, Takacs T, Czako L. l-Arginine-induced experimental pancreatitis. World J. Gastroenterol. 2004;10:2003–2009. doi: 10.3748/wjg.v10.i14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J.J, Rasmussen T.N, Schmidt P. Role of nitric oxide in neurally induced pancreatic exocrine secretion in pigs. Am. J. Physiol. 1994;266:G206–G213. doi: 10.1152/ajpgi.1994.266.2.G206. [DOI] [PubMed] [Google Scholar]
- Hotter G, Closa D, Pi F, Prats N, Fernandez-Cruz L, Bulbena O, Gelpi E, Rosello-Catafau J. Nitric oxide and arachidonate metabolism in ischemia–reperfusion associated with pancreas transplantation. Transplantation. 1995;59:417–421. [PubMed] [Google Scholar]
- Ischiropoulos H. Biological selectivity and functional aspects of protein tyrosine nitration. Biochem. Biophys. Res. Commun. 2003;305:776–783. doi: 10.1016/s0006-291x(03)00814-3. 10.1016/S0006-291X(03)00814-3 [DOI] [PubMed] [Google Scholar]
- Isik A.T, et al. The effect of combination therapy of hyperbaric oxygen, meropenem, and selective nitric oxide synthase inhibitor in experimental acute pancreatitis. Pancreas. 2004;28:53–57. doi: 10.1097/00006676-200401000-00008. 10.1097/00006676-200401000-00008 [DOI] [PubMed] [Google Scholar]
- Jaworek J, Jachimczak B, Tomaszewska R, Konturek P.C, Pawlik W.W, Sendur R, Hahn E.G, Stachura J, Konturek S.J. Protective action of lipopolysaccharides in rat caerulein-induced pancreatitis: role of nitric oxide. Digestion. 2000;62:1–13. doi: 10.1159/000007771. 10.1159/000007771 [DOI] [PubMed] [Google Scholar]
- Jaworek J, Bonio J, Leja-Szpa A, Nawrot K, Tomaszewska M.R, Stachura J, Pawlik W.W, Konturek S.J. Sensory nerves in central and peripheral control of pancreatic integrity by leptin and melatonin. J. Physiol. Pharmacol. 2002a;53:51–74. [PubMed] [Google Scholar]
- Jaworek J, et al. Leptin protects the pancreas from damage induced by caerulein overstimulation by modulating cytokine production. Pancreatology. 2002b;2:89–99. doi: 10.1159/000055897. 10.1159/000055897 [DOI] [PubMed] [Google Scholar]
- Jungermann J, Lerch M.M, Weidenbach H, Lutz M.P, Kruger B, Adler G. Disassembly of rat pancreatic acinar cell cytoskeleton during supramaximal secretagogue stimulation. Am. J. Physiol. 1995;268:G328–G338. doi: 10.1152/ajpgi.1995.268.2.G328. [DOI] [PubMed] [Google Scholar]
- Jyotheeswaran S, Li P, Chang T.M, Chey W.Y. Endogenous nitric oxide mediates pancreatic exocrine secretion stimulated by secretin and cholecystokinin in rats. Pancreas. 2000;20:401–407. doi: 10.1097/00006676-200005000-00011. 10.1097/00006676-200005000-00011 [DOI] [PubMed] [Google Scholar]
- Kawabata A, Kuroda R, Nishida M, Nagata N, Sakaguchi Y, Kawao N, Nishikawa H, Arizono N, Kawai K. Protease-activated receptor-2 (PAR-2) in the pancreas and parotid gland: immunolocalization and involvement of nitric oxide in the evoked amylase secretion. Life Sci. 2002;71:2435–2446. doi: 10.1016/s0024-3205(02)02044-1. 10.1016/S0024-3205(02)02044-1 [DOI] [PubMed] [Google Scholar]
- Kirchgessner A.L, Liu M.T, Gershon M.D. NADPH diaphorase (nitric oxide synthase)-containing nerves in the enteropancreatic innervation: sources, co-stored neuropeptides, and pancreatic function. J. Comp. Neurol. 1994;342:115–130. doi: 10.1002/cne.903420111. 10.1002/cne.903420111 [DOI] [PubMed] [Google Scholar]
- Klonowski-Stumpe H, Schreiber R, Grolik M, Schulz H.U, Haussinger D, Niederau C. Effect of oxidative stress on cellular functions and cytosolic free calcium of rat pancreatic acinar cells. Am. J. Physiol. 1997;272:G1489–G1498. doi: 10.1152/ajpgi.1997.272.6.G1489. [DOI] [PubMed] [Google Scholar]
- Kolaja K.L, Bell R.R, Janssen D, Manning P.T, Schlosser M.J, Khan K.N. Evaluation of the long-term pancreatic effects of constitutive nitric oxide synthase inhibition in dogs. Inflammopharmacology. 2004;12:33–45. doi: 10.1163/156856004773121356. 10.1163/156856004773121356 [DOI] [PubMed] [Google Scholar]
- Konig P, Dedio J, Muller-Esterl W, Kummer W. Distribution of the novel eNOS-interacting protein NOSIP in the liver, pancreas, and gastrointestinal tract of the rat. Gastroenterology. 2002;123:314–324. doi: 10.1053/gast.2002.34212. 10.1053/gast.2002.34212 [DOI] [PubMed] [Google Scholar]
- Konturek S.J, Bilski J, Konturek P.K, Cieszkowski M, Pawlik W. Role of endogenous nitric oxide in the control of canine pancreatic secretion and blood flow. Gastroenterology. 1993;104:896–902. doi: 10.1016/0016-5085(93)91028-g. [DOI] [PubMed] [Google Scholar]
- Konturek S.J, Szlachcic A, Dembinski A, Warzecha Z, Jaworek J, Stachura J. Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int. J. Pancreatol. 1994;15:19–28. doi: 10.1007/BF02924384. [DOI] [PubMed] [Google Scholar]
- Konturek J.W, Hengst K, Kulesza E, Gabryelewicz A, Konturek S.J, Domschke W. Role of endogenous nitric oxide in the control of exocrine and endocrine pancreatic secretion in humans. Gut. 1997;40:86–91. doi: 10.1136/gut.40.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek P.C, Jaworek J, Maniatoglou A, Bonior J, Meixner H, Konturek S.J, Hahn E.G. Leptin modulates the inflammatory response in acute pancreatitis. Digestion. 2002;65:149–160. doi: 10.1159/000064935. 10.1159/000064935 [DOI] [PubMed] [Google Scholar]
- Kozlov A.V, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. 10.1016/S0014-5793(99)00788-7 [DOI] [PubMed] [Google Scholar]
- Kruse P, Anderson M.E, Loft S. Minor role of oxidative stress during intermediate phase of acute pancreatitis in rats. Free Radic. Biol. Med. 2001;30:309–317. doi: 10.1016/s0891-5849(00)00472-x. 10.1016/S0891-5849(00)00472-X [DOI] [PubMed] [Google Scholar]
- Lacza Z, Snipes J.A, Zhang J, Horvath E.M, Figueroa J.P, Szabo C, Busija D.W. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic. Biol. Med. 2003;35:1217–1228. doi: 10.1016/s0891-5849(03)00510-0. 10.1016/S0891-5849(03)00510-0 [DOI] [PubMed] [Google Scholar]
- Lerch M.M, Adler G. Experimental animal models of acute pancreatitis. Int. J. Pancreatol. 1994;15:159–170. [PubMed] [Google Scholar]
- Lomis T.J, et al. First place winner of the Conrad Jobst Award in the gold medal paper competition. Nitric oxide synthase inhibitors N-monomethylarginine and aminoguanidine prevent the progressive and severe hypotension associated with a rat model of pancreatitis. Am. Surg. 1995;61:7–10. [PubMed] [Google Scholar]
- Looms D, Tritsaris K, Pedersen A.M, Nauntofte B, Dissing S. Nitric oxide signalling in salivary glands. J. Oral Pathol. Med. 2002;31:569–584. doi: 10.1034/j.1600-0714.2002.00047.x. 10.1034/j.1600-0714.2002.00047.x [DOI] [PubMed] [Google Scholar]
- Lundquist I, Alm P, Salehi A, Henningsson R, Grapengiesser E, Hellman B. Carbon monoxide stimulates insulin release and propagates Ca2+ signals between pancreatic beta-cells. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1055–E1063. doi: 10.1152/ajpendo.00498.2002. [DOI] [PubMed] [Google Scholar]
- Luthen R, Niederau C, Grendell J.H. Intrapancreatic zymogen activation and levels of ATP and glutathione during caerulein pancreatitis in rats. Am. J. Physiol. 1995;268:G592–G604. doi: 10.1152/ajpgi.1995.268.4.G592. [DOI] [PubMed] [Google Scholar]
- Maczka M, Thor P, Bilski J, Konturek S.J. Nitric oxide and the interrelation between intestinal motility and pancreatic secretion in fasted and fed dogs. J. Physiol. Pharmacol. 1994;45:285–298. [PubMed] [Google Scholar]
- Millar T.M, Stevens C.R, Benjamin N, Eisenthal R, Harrison R, Blake D.R. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. 10.1016/S0014-5793(98)00430-X [DOI] [PubMed] [Google Scholar]
- Mizunuma T, Kawamura S, Kishino Y. Effects of injecting excess arginine on rat pancreas. J. Nutr. 1984;114:467–471. doi: 10.1093/jn/114.3.467. [DOI] [PubMed] [Google Scholar]
- Molero X, Guarner F, Salas A, Mourelle M, Puig V, Malagelada J.R. Nitric oxide modulates pancreatic basal secretion and response to cerulein in the rat: effects in acute pancreatitis. Gastroenterology. 1995;108:1855–1862. doi: 10.1016/0016-5085(95)90150-7. 10.1016/0016-5085(95)90150-7 [DOI] [PubMed] [Google Scholar]
- Moncada S, Erusalimsky J.D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. 10.1038/nrm762 [DOI] [PubMed] [Google Scholar]
- Nakada S, Ishikawa T, Yamamoto Y, Kaneko Y, Nakayama K. Constitutive nitric oxide synthases in rat pancreatic islets: direct imaging of glucose-induced nitric oxide production in beta-cells. Pflugers Arch. 2003;447:305–311. doi: 10.1007/s00424-003-1176-y. 10.1007/s00424-003-1176-y [DOI] [PubMed] [Google Scholar]
- Nam S.W, Seo D.W, Sung D.S, Han J.W, Hong S.Y, Lee H.W. Nitric oxide synthase from bovine pancreas: purification and characterization. Arch. Pharm. Res. 1998;21:128–134. doi: 10.1007/BF02974016. [DOI] [PubMed] [Google Scholar]
- Namkung W, Han W, Luo X, Muallem S, Cho K.H, Kim K.H, Lee M.G. Protease-activated receptor 2 exerts local protection and mediates some systemic complications in acute pancreatitis. Gastroenterology. 2004;126:1844–1859. doi: 10.1053/j.gastro.2004.03.019. 10.1053/j.gastro.2004.03.019 [DOI] [PubMed] [Google Scholar]
- Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Invest. 2003;111:769–778. doi: 10.1172/JCI18174. 10.1172/JCI200318174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuschwander-Tetri B.A, Barnidge M, Janney C.G. Cerulein-induced pancreatic cysteine depletion: prevention does not diminish acute pancreatitis in the mouse. Gastroenterology. 1994;107:824–830. doi: 10.1016/0016-5085(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri B.A, Presti M.E, Wells L.D. Glutathione synthesis in the exocrine pancreas. Pancreas. 1997;14:342–349. doi: 10.1097/00006676-199705000-00003. [DOI] [PubMed] [Google Scholar]
- Niederau C, Schultz H.U, Letko G. Involvement of free radicals in the pathophysiology of chronic pancreatitis: potential of treatment with antioxidant and scavenger substances. Klin. Wochenschr. 1991;69:1018–1024. doi: 10.1007/BF01645151. 10.1007/BF01645151 [DOI] [PubMed] [Google Scholar]
- Niederau C, Klonowski H, Schulz H.U, Sarbia M, Luthen R, Haussinger D. Oxidative injury to isolated rat pancreatic acinar cells vs. isolated zymogen granules. Free Radic. Biol. Med. 1996;20:877–886. doi: 10.1016/0891-5849(95)02153-1. 10.1016/0891-5849(95)02153-1 [DOI] [PubMed] [Google Scholar]
- Nonaka A, Manabe T, Asano N, Kyogoku T, Imanishi K, Tamura K, Tobe T, Sugiura Y, Makino K. Direct ESR measurement of free radicals in mouse pancreatic lesions. Int. J. Pancreatol. 1989a;5:203–211. doi: 10.1007/BF02924420. [DOI] [PubMed] [Google Scholar]
- Nonaka A, Manabe T, Tamura K, Asano N, Imanishi K, Tobe T. Changes of xanthine oxidase, lipid peroxide and superoxide dismutase in mouse acute pancreatitis. Digestion. 1989b;43:41–46. doi: 10.1159/000199859. [DOI] [PubMed] [Google Scholar]
- Nonaka A, Manabe T, Kyogoku T, Tamura K, Tobe T. Changes in lipid peroxide and oxygen radical scavengers in cerulein-induced acute pancreatitis. Imbalance between the offense and defense systems. Digestion. 1990;47:130–137. doi: 10.1159/000200487. [DOI] [PubMed] [Google Scholar]
- Nonaka A, Manabe T, Tobe T. Effect of a new synthetic ascorbic acid derivative as a free radical scavenger on the development of acute pancreatitis in mice. Gut. 1991;32:528–532. doi: 10.1136/gut.32.5.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka A, Manabe T, Kyogoku T, Tamura K, Tobe T. Evidence for a role of free radicals by synthesized scavenger, 2-octadecylascorbic acid, in cerulein-induced mouse acute pancreatitis. Dig. Dis. Sci. 1992;37:274–279. doi: 10.1007/BF01308183. 10.1007/BF01308183 [DOI] [PubMed] [Google Scholar]
- Nordback I.H, Cameron J.L. The mechanism of conversion of xanthine dehydrogenase to xanthine oxidase in acute pancreatitis in the canine isolated pancreas preparation. Surgery. 1993;113:90–97. [PubMed] [Google Scholar]
- Obermaier R, Von Dobschuetz E, Benthues A, Ansorge N, Schareck W, Hopt U.T, Benz S. Exogenous and endogenous nitric oxide donors improve post-ischemic tissue oxygenation in early pancreatic ischemia/reperfusion injury in the rat. Eur. Surg. Res. 2004a;36:219–225. doi: 10.1159/000078856. 10.1159/000078856 [DOI] [PubMed] [Google Scholar]
- Obermaier R, Von Dobschuetz E, Muhs O, Keck T, Drognitz O, Jonas L, Schareck W, Hopt U.T, Benz S. Influence of nitric oxide on microcirculation in pancreatic ischemia/reperfusion injury: an intravital microscopic study. Transpl. Int. 2004b;17:208–214. doi: 10.1007/s00147-004-0702-y. 10.1111/j.1432-2277.2004.tb00430.x [DOI] [PubMed] [Google Scholar]
- Ozturk M, et al. The role of inducible nitric oxide synthase inhibitor, meropenem, and taurine in experimental acute necrotizing pancreatitis. Pancreas. 2003;26:357–362. doi: 10.1097/00006676-200305000-00008. 10.1097/00006676-200305000-00008 [DOI] [PubMed] [Google Scholar]
- Pandol S.J, Schoeffield-Payne M.S. Cyclic GMP mediates the agonist-stimulated increase in plasma membrane calcium entry in the pancreatic acinar cell. J. Biol. Chem. 1990;265:12 846–12 853. [PubMed] [Google Scholar]
- Patel A.G, Toyama M.T, Nguyen T.N, Cohen G.A, Ignarro L.J, Reber H.A, Ashley S.W. Role of nitric oxide in the relationship of pancreatic blood flow and exocrine secretion in cats. Gastroenterology. 1995;108:1215–1220. doi: 10.1016/0016-5085(95)90222-8. 10.1016/0016-5085(95)90222-8 [DOI] [PubMed] [Google Scholar]
- Qader S.S, Ekelund M, Andersson R, Obermuller S, Salehi A. Acute pancreatitis, expression of inducible nitric oxide synthase and defective insulin secretion. Cell Tissue Res. 2003;313:271–279. doi: 10.1007/s00441-003-0764-7. 10.1007/s00441-003-0764-7 [DOI] [PubMed] [Google Scholar]
- Qui B, Mei Q.B, Ma J.J, Korsten M.A. Susceptibility to cerulein-induced pancreatitis in inducible nitric oxide synthase-deficient mice. Pancreas. 2001;23:89–93. doi: 10.1097/00006676-200107000-00013. 10.1097/00006676-200107000-00013 [DOI] [PubMed] [Google Scholar]
- Rahman S.H, Ibrahim K, Larvin M, Kingsnorth A, McMahon M.J. Association of antioxidant enzyme gene polymorphisms and glutathione status with severe acute pancreatitis. Gastroenterology. 2004;126:1312–1322. doi: 10.1053/j.gastro.2004.02.002. 10.1053/j.gastro.2004.02.002 [DOI] [PubMed] [Google Scholar]
- Rau B, Poch B, Gansauge F, Bauer A, Nussler A.K, Nevalainen T, Schoenberg M.H, Beger H.G. Pathophysiologic role of oxygen free radicals in acute pancreatitis: initiating event or mediator of tissue damage? Ann. Surg. 2000;231:352–360. doi: 10.1097/00000658-200003000-00008. 10.1097/00000658-200003000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau B, Bauer A, Wang A, Gansauge F, Weidenbach H, Nevalainen T, Poch B, Beger H.G, Nussler A.K. Modulation of endogenous nitric oxide synthase in experimental acute pancreatitis: role of anti-ICAM-1 and oxygen free radical scavengers. Ann. Surg. 2001;233:195–203. doi: 10.1097/00000658-200102000-00008. 10.1097/00000658-200102000-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinheckel T, Nedelev B, Prause J, Augustin W, Schulz H.U, Lippert H, Halangk W. Occurrence of oxidatively modified proteins: an early event in experimental acute pancreatitis. Free Radic. Biol. Med. 1998;24:393–400. doi: 10.1016/s0891-5849(97)00271-2. 10.1016/S0891-5849(97)00271-2 [DOI] [PubMed] [Google Scholar]
- Rosado J.A, Gonzalez A, Salido G.M, Pariente J.A. Effects of reactive oxygen species on actin filament polymerisation and amylase secretion in mouse pancreatic acinar cells. Cell. Signal. 2002;14:547–556. doi: 10.1016/s0898-6568(01)00273-x. 10.1016/S0898-6568(01)00273-X [DOI] [PubMed] [Google Scholar]
- Salehi A, Carlberg M, Henningson R, Lundquist I. Islet constitutive nitric oxide synthase: biochemical determination and regulatory function. Am. J. Physiol. 1996;270:C1634–C1641. doi: 10.1152/ajpcell.1996.270.6.C1634. [DOI] [PubMed] [Google Scholar]
- Sanchez-Bernal C, Garcia-Morales O.H, Dominguez C, Martin-Gallan P, Calvo J.J, Ferreira L, Perez-Gonzalez N. Nitric oxide protects against pancreatic subcellular damage in acute pancreatitis. Pancreas. 2004;28:e9–e15. doi: 10.1097/00006676-200401000-00021. 10.1097/00006676-200401000-00021 [DOI] [PubMed] [Google Scholar]
- Sandstrom P, Gasslander T, Sundqvist T, Franke J, Svanvik J. Depletion of serum l-arginine in patients with acute pancreatitis. Pancreas. 2003;27:261–266. doi: 10.1097/00006676-200310000-00012. 10.1097/00006676-200310000-00012 [DOI] [PubMed] [Google Scholar]
- Sandstrom P, Brooke-Smith M.E, Thomas A.C, Grivell M.B, Saccone G.T, Toouli J, Svanvik J. Highly selective inhibition of inducible nitric oxide synthase ameliorates experimental acute pancreatitis. Pancreas. 2005;30:e10–e15. [PubMed] [Google Scholar]
- Sanfey H, Bulkley G.B, Cameron J.L. The role of oxygen-derived free radicals in the pathogenesis of acute pancreatitis. Ann. Surg. 1984;200:405–413. doi: 10.1097/00000658-198410000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Akaike T, Sawa T, Miyamoto Y, Suga M, Ando M, Maeda H. Nitric oxide generation from hydroxyurea via copper-catalyzed peroxidation and implications for pharmacological actions of hydroxyurea. Jpn. J. Cancer Res. 1997;88:1199–1204. doi: 10.1111/j.1349-7006.1997.tb00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Shimosegawa T, Kimura K, Moriizumi S, Masamune A, Koizumi M, Toyota T. Nitric oxide is overproduced by peritoneal macrophages in rat taurocholate pancreatitis: the mechanism of inducible nitric oxide synthase expression. Pancreas. 1998;17:402–411. doi: 10.1097/00006676-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Schoenberg M.H, Buchler M, Baczako K, Bultmann B, Younes M, Gasper M, Kirchmayr R, Beger H.G. The involvement of oxygen radicals in acute pancreatitis. Klin. Wochenschr. 1991;69:1025–1031. doi: 10.1007/BF01645152. 10.1007/BF01645152 [DOI] [PubMed] [Google Scholar]
- Schoenberg M.H, Buchler M, Younes M, Kirchmayr R, Bruckner U.B, Beger H.G. Effect of antioxidant treatment in rats with acute hemorrhagic pancreatitis. Dig. Dis. Sci. 1994;39:1034–1040. doi: 10.1007/BF02087555. 10.1007/BF02087555 [DOI] [PubMed] [Google Scholar]
- Schoenberg M.H, Buchler M, Pietrzyk C, Uhl W, Birk D, Eisele S, Marzinzig M, Beger H.G. Lipid peroxidation and glutathione metabolism in chronic pancreatitis. Pancreas. 1995;10:36–43. doi: 10.1097/00006676-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Schulz H.U, Niederau C, Klonowski-Stumpe H, Halangk W, Luthen R, Lippert H. Oxidative stress in acute pancreatitis. Hepatogastroenterology. 1999;46:2736–2750. [PubMed] [Google Scholar]
- Schulz H.U, et al. Randomized, placebo-controlled trial of lazaroid effects on severe acute pancreatitis in rats. Crit. Care Med. 2001;29:861–869. doi: 10.1097/00003246-200104000-00035. 10.1097/00003246-200104000-00035 [DOI] [PubMed] [Google Scholar]
- Sevillano S, De la Mano A.M, De Dios I, Ramudo L, Manso M.A. Major pathological mechanisms of acute pancreatitis are prevented by N-acetylcysteine. Digestion. 2003;68:34–40. doi: 10.1159/000073223. 10.1159/000073223 [DOI] [PubMed] [Google Scholar]
- Sharma A, Tao X, Gopal A, Ligon B, Andrade-Gordon P, Steer M.L, Perides G. Protection against acute pancreatitis by activation of protease-activated receptor-2. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G388–G395. doi: 10.1152/ajpgi.00341.2004. 10.1152/ajpgi.00341.2004 [DOI] [PubMed] [Google Scholar]
- Simsek I, et al. Inhibition of inducible nitric oxide synthase reduces bacterial translocation in a rat model of acute pancreatitis. Pancreas. 2001;23:296–301. doi: 10.1097/00006676-200110000-00011. 10.1097/00006676-200110000-00011 [DOI] [PubMed] [Google Scholar]
- Song A.M, Bhagat L, Singh V.P, Van Acker G.G, Steer M.L, Saluja A.K. Inhibition of cyclooxygenase-2 ameliorates the severity of pancreatitis and associated lung injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:G1166–G1174. doi: 10.1152/ajpgi.00370.2001. [DOI] [PubMed] [Google Scholar]
- Stamler J.S, Simon D.I, Osborne J.A, Mullins M, Jaraki O, Michel T, Singel D, Loscalzo J. Exposure of sulfhydryl containing proteins to nitric oxide and endothelium-derived relaxing factor confers novel bioactivity and modulates their intrinsic functional properties. In: Moncada S, Marletta M, Hibbs J, Higgs A, editors. The biology of nitric oxide. Portland Press; London: 1992. pp. 20–23. [Google Scholar]
- Steer M.L, Rutledge P.L, Powers R.E, Saluja M, Saluja A.K. The role of oxygen-derived free radicals in two models of experimental acute pancreatitis: effects of catalase, superoxide dismutase, dimethylsulfoxide, and allopurinol. Klin. Wochenschr. 1991;69:1012–1017. doi: 10.1007/BF01645149. 10.1007/BF01645149 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Suematsu M, Miura S, Asako H, Kurose I, Ishii H, Houzawa S, Tsuchiya M. Xanthine oxidase-mediated intracellular oxidative stress in response to cerulein in rat pancreatic acinar cells. Pancreas. 1993;8:465–470. doi: 10.1097/00006676-199307000-00010. [DOI] [PubMed] [Google Scholar]
- Sweiry J.H, Shibuya I, Asada N, Niwa K, Doolabh K, Habara Y, Kanno T, Mann G.E. Acute oxidative stress modulates secretion and repetitive Ca2+ spiking in rat exocrine pancreas. Biochim. Biophys. Acta. 1999;1454:19–30. doi: 10.1016/s0925-4439(99)00021-6. [DOI] [PubMed] [Google Scholar]
- Takacs T, Czako L, Morschl E, Laszlo F, Tiszlavicz L, Rakonczay Z, Jr, Lonovics J. The role of nitric oxide in edema formation in l-arginine-induced acute pancreatitis. Pancreas. 2002;25:277–282. doi: 10.1097/00006676-200210000-00010. 10.1097/00006676-200210000-00010 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kamiike W, Kosaka H, Ito T, Kumura E, Shiga T, Matsuda H. Detection of nitric oxide production and its role in pancreatic ischemia–reperfusion in rats. Am. J. Physiol. 1996;271:G405–G409. doi: 10.1152/ajpgi.1996.271.3.G405. [DOI] [PubMed] [Google Scholar]
- Tapia J.A, Camello C, Jensen R.T, Garcia L.J. EGF stimulates tyrosine phosphorylation of focal adhesion kinase (p125FAK) and paxillin in rat pancreatic acini by a phospholipase C-independent process that depends on phosphatidylinositol 3-kinase, the small GTP-binding protein, p21rho, and the integrity of the actin cytoskeleton. Biochim. Biophys. Acta. 1999;1448:486–499. doi: 10.1016/s0167-4889(98)00157-8. 10.1016/S0167-4889(98)00157-8 [DOI] [PubMed] [Google Scholar]
- Telek G, Regoly-Merei J, Kovacs G.C, Simon L, Nagy Z, Hamar J, Jakab F. The first histological demonstration of pancreatic oxidative stress in human acute pancreatitis. Hepatogastroenterology. 2001;48:1252–1258. [PubMed] [Google Scholar]
- Thorn P, Brady P, Llopis J, Gallacher D.V, Petersen O.H. Cytosolic Ca2+ spikes evoked by the thiol reagent thimerosal in both intact and internally perfused single pancreatic acinar cells. Pflugers Arch. 1992;422:173–178. doi: 10.1007/BF00370417. 10.1007/BF00370417 [DOI] [PubMed] [Google Scholar]
- Trulsson L.M, Svanvik J, Permert J, Gasslander T. Cholecystokinin octapeptide induces both proliferation and apoptosis in the rat pancreas. Regul. Pept. 2001;98:41–48. doi: 10.1016/s0167-0115(00)00223-8. 10.1016/S0167-0115(00)00223-8 [DOI] [PubMed] [Google Scholar]
- Trulsson L.M, Gasslander T, Sundqvist T, Svanvik J. The influence of nitric oxide on basal and cholecystokinin-8-induced proliferation and apoptosis in the rat pancreas. Regul. Pept. 2002;106:97–104. doi: 10.1016/s0167-0115(02)00056-3. 10.1016/S0167-0115(02)00056-3 [DOI] [PubMed] [Google Scholar]
- Trulsson L.M, Gasslander T, Svanvik J. Cholecystokinin-8-induced hypoplasia of the rat pancreas: influence of nitric oxide on cell proliferation and programmed cell death. Basic Clin. Pharmacol. Toxicol. 2004;95:183–190. doi: 10.1111/j.1742-7843.2004.pto_950406.x. [DOI] [PubMed] [Google Scholar]
- Tsai K, Wang S.S, Chen T.S, Kong C.W, Chang F.Y, Lee S.D, Lu F.J. Oxidative stress: an important phenomenon with pathogenetic significance in the progression of acute pancreatitis. Gut. 1998;42:850–855. doi: 10.1136/gut.42.6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um S.H, et al. The role of nitric oxide in experimental cerulein induced pancreatitis. J. Korean Med. Sci. 2003;18:520–526. doi: 10.3346/jkms.2003.18.4.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara K, Kataoka K, Ogura T, Esumi H, Kashima K, Ibata Y, Okamura H. Comparative distribution of nitric oxide synthase (NOS) in pancreas of the dog and rat: immunocytochemistry of neuronal type NOS and histochemistry of NADPH-diaphorase. Brain Res. Bull. 1997;42:469–478. doi: 10.1016/s0361-9230(96)00374-7. 10.1016/S0361-9230(96)00374-7 [DOI] [PubMed] [Google Scholar]
- Urunuela A, Sevillano S, De la Mano A.M, Manso M.A, Orfao A, De Dios I. Time-course of oxygen free radical production in acinar cells during acute pancreatitis induced by pancreatic duct obstruction. Biochim. Biophys. Acta. 2002;1588:159–164. doi: 10.1016/s0925-4439(02)00160-6. [DOI] [PubMed] [Google Scholar]
- van Rossum D.B, Patterson R.L, Ma H.T, Gill D.L. Ca2+ entry mediated by store depletion, S-nitrosylation, and TRP3 channels. Comparison of coupling and function. J. Biol. Chem. 2000;275:28 562–28 568. doi: 10.1074/jbc.M003147200. 10.1074/jbc.M003147200 [DOI] [PubMed] [Google Scholar]
- Vaquero E, Molero X, Puig-Divi V, Malagelada J.R. Contrasting effects of circulating nitric oxide and nitrergic transmission on exocrine pancreatic secretion in rats. Gut. 1998;43:684–691. doi: 10.1136/gut.43.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero E, Gukovsky I, Zaninovic V, Gukovskaya A.S, Pandol S.J. Localized pancreatic NF-kappaB activation and inflammatory response in taurocholate-induced pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G1197–G1208. doi: 10.1152/ajpgi.2001.280.6.G1197. [DOI] [PubMed] [Google Scholar]
- Viola G, Al Mufti R.A, Sohail M, Williamson R.C, Mathie R.T. Nitric oxide induction in a rat model of selective pancreatic ischemia and reperfusion. Hepatogastroenterology. 2000;47:1250–1255. [PubMed] [Google Scholar]
- Wacke R, et al. Up-regulation of cytochrome P450 1A2, 2C9, and 2E1 in chronic pancreatitis. Pancreas. 1998;16:521–528. doi: 10.1097/00006676-199805000-00011. [DOI] [PubMed] [Google Scholar]
- Wallig M.A. Xenobiotic metabolism, oxidant stress and chronic pancreatitis. Focus on glutathione. Digestion. 1998;59(Suppl. 4):13–24. doi: 10.1159/000051439. 10.1159/000051439 [DOI] [PubMed] [Google Scholar]
- Warzecha Z, Dembinski A, Ceranowicz P, Jaworek J, Konturek P.C, Dembinski M, Bilskl J, Konturek S.J. Influence of leptin administration on the course of acute ischemic pancreatitis. J. Physiol. Pharmacol. 2002;53:775–790. [PubMed] [Google Scholar]
- Watson E.L, Jacobson K.L, Singh J.C, Ott S.M. Nitric oxide acts independently of cGMP to modulate capacitative Ca(2+) entry in mouse parotid acini. Am. J. Physiol. 1999;277:C262–C270. doi: 10.1152/ajpcell.1999.277.2.C262. [DOI] [PubMed] [Google Scholar]
- Weber H, et al. Increased cytosolic Ca2+ amplifies oxygen radical-induced alterations of the ultrastructure and the energy metabolism of isolated rat pancreatic acinar cells. Digestion. 1998;59:175–185. doi: 10.1159/000007486. 10.1159/000007486 [DOI] [PubMed] [Google Scholar]
- Werner J, Rivera J, Fernandez-del Castillo C, Lewandrowski K, Adrie C, Rattner D.W, Warshaw A.L. Differing roles of nitric oxide in the pathogenesis of acute edematous versus necrotizing pancreatitis. Surgery. 1997;121:23–30. doi: 10.1016/s0039-6060(97)90178-1. 10.1016/S0039-6060(97)90178-1 [DOI] [PubMed] [Google Scholar]
- Werner J, Fernandez-del Castillo C, Rivera J.A, Kollias N, Lewandrowski K.B, Rattner D.W, Warshaw A.L. On the protective mechanisms of nitric oxide in acute pancreatitis. Gut. 1998;43:401–407. doi: 10.1136/gut.43.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner J, Green D, Ferrell L, Renner I. Evidence for a role of oxygen derived free radicals in the pathogenesis of caerulein induced acute pancreatitis in rats. Gut. 1988;29:1516–1523. doi: 10.1136/gut.29.11.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worl J, Wiesand M, Mayer B, Greskotter K.R, Neuhuber W.L. Neuronal and endothelial nitric oxide synthase immunoreactivity and NADPH-diaphorase staining in rat and human pancreas: influence of fixation. Histochemistry. 1994;102:353–364. doi: 10.1007/BF00268906. [DOI] [PubMed] [Google Scholar]
- Wrenn R.W, Currie M.G, Herman L.E. Nitric oxide participates in the regulation of pancreatic acinar cell secretion. Life Sci. 1994;55:511–518. doi: 10.1016/0024-3205(94)00743-8. 10.1016/0024-3205(94)00743-8 [DOI] [PubMed] [Google Scholar]
- Xu X, Star R.A, Tortorici G, Muallem S. Depletion of intracellular Ca2+ stores activates nitric-oxide synthase to generate cGMP and regulate Ca2+ influx. J. Biol. Chem. 1994;269:12 645–12 653. [PubMed] [Google Scholar]
- Xu X, Zeng W, Diaz J, Lau K.S, Gukovskaya A.C, Brown R.J, Pandol S.J, Muallem S. nNOS and Ca2+ influx in rat pancreatic acinar and submandibular salivary gland cells. Cell Calcium. 1997;22:217–228. doi: 10.1016/s0143-4160(97)90015-4. 10.1016/S0143-4160(97)90015-4 [DOI] [PubMed] [Google Scholar]
- Yago M.D, Tapia J.A, Salido G.M, Adeghate E, Juma L.M, Martinez-Victoria E, Manas M, Singh J. Effect of sodium nitroprusside and 8-bromo cyclic GMP on nerve-mediated and acetylcholine-evoked secretory responses in the rat pancreas. Br. J. Pharmacol. 2002;136:49–56. doi: 10.1038/sj.bjp.0704693. 10.1038/sj.bjp.0704693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Tsunoda Y, Owyang C. Effect of uncoupling NO/cGMP pathways on carbachol- and CCK-stimulated Ca2+ entry and amylase secretion from the rat pancreas. Pflugers Arch. 1997;434:25–37. doi: 10.1007/s004240050359. 10.1007/s004240050359 [DOI] [PubMed] [Google Scholar]
- Yu J.H, Lim J.W, Namkung W, Kim H, Kim K.H. Suppression of cerulein-induced cytokine expression by antioxidants in pancreatic acinar cells. Lab. Invest. 2002;82:1359–1368. doi: 10.1097/01.lab.0000032377.09626.c7. [DOI] [PubMed] [Google Scholar]
- Yuan C.H, Liu Y.F, Cheng Y, Zhao N, Li G.C, Liang J, He S.G. Protective effects of l-arginine on reperfusion injury after pancreaticoduodenal transplantation in rats. Hepatobiliary Pancreat. Dis. Int. 2004;3:349–354. [PubMed] [Google Scholar]
- Zaragoza C, Ocampo C.J, Saura M, Bao C, Leppo M, Lafond-Walker A, Thiemann D.R, Hruban R, Lowenstein C.J. Inducible nitric oxide synthase protection against coxsackievirus pancreatitis. J. Immunol. 1999;163:5497–5504. [PubMed] [Google Scholar]
- Zoucas E, Nilsson C, Ihse I. Differential roles of endogenous nitric oxide on neural regulation of basal exocrine pancreatic secretion in intact and denervated pancreas. Pancreatology. 2001;1:96–101. doi: 10.1159/000055800. 10.1159/000055800 [DOI] [PubMed] [Google Scholar]