Abstract
Chronic heart failure, secondary to left ventricular hypertrophy or myocardial infarction, is a condition with increasing morbidity and mortality. Although the mechanisms underlying the development and progression of this condition remain a subject of intense interest, there is now growing evidence that redox-sensitive pathways play an important role. This article focuses on the involvement of reactive oxygen species derived from a family of superoxide-generating enzymes, termed NADPH oxidases (NOXs), in the pathophysiology of ventricular hypertrophy, the accompanying interstitial fibrosis and subsequent heart failure. In particular, the apparent ability of the different NADPH oxidase isoforms to define the response of a cell to a range of physiological and pathophysiological stimuli is reviewed. If confirmed, these data would suggest that independently targeting different members of the NOX family may hold the potential for therapeutic intervention in the treatment of cardiac disease.
Keywords: NADPH oxidase, heart, hypertrophy, heart failure, oxidative stress, reactive oxygen species
1. Introduction
Chronic heart failure (CHF) affects up to 2% of the adult population in the Western world and is a condition with increasing incidence and substantial morbidity and mortality despite medical treatment. CHF occurs typically in response to sustained increases in cardiac workload, most commonly secondary to hypertension or following myocardial infarction (Swynghedauw 1999). In the former setting, the heart initially adapts in a process termed left ventricular hypertrophy (LVH), which involves alterations in cardiac myocyte, extracellular matrix and coronary vessel structure and function that are driven largely by changes in gene expression. The increase in LV wall thickness and mass and changes in contractile properties that occur with LVH may initially be adaptive by normalizing wall stress. However, progressive LVH leads to contractile depression, ventricular dilatation and the development of CHF. This process is also accompanied by significant interstitial cardiac fibrosis. CHF secondary to myocardial infarction involves a process known as adverse cardiac remodelling which involves changes in cardiac muscle properties analogous to those found in LVH, together with substantial alterations in the shape and volume of the heart which are driven by profound remodelling of the extracellular matrix. Like LVH, the process of remodelling post-myocardial infarction may initially be compensatory by normalizing cardiac work, but it eventually leads to CHF as the ventricle dilates and becomes impaired.
The mechanisms responsible for the development of LVH and subsequent transition to CHF as well as those underlying adverse remodelling post-myocardial infarction are the subject of intense investigation (Hunter & Chien 1999). In this article, we consider the role of increases in oxidative stress, particularly that deriving from the NADPH oxidase (NOX) family of enzymes.
2. Effects of reactive oxygen species in the heart
Reactive oxygen species (ROS) could, in principle, have several different effects in the heart. Traditionally, free radicals have been recognized to be capable of inducing oxidation and damage of macromolecules, membranes and DNA and thereby contributing to cellular damage and acceleration of cell death through apoptosis and necrosis. This mechanism may be especially important in advanced heart failure (Suematsu et al. 2003). In addition, ROS can profoundly impair cellular energetics through actions on mitochondrial enzymes. More recently, it has been appreciated that oxidative stress may also exert more subtle modulatory effects. First, inactivation of the signalling molecule nitric oxide (NO) by ROS is recognized to be a key mechanism underlying reduced NO bioavailability, which may also be an important contributor to disease pathophysiology (Hua & Harrison 2000). Second, the tightly regulated production of ROS can modulate the activity of diverse intracellular molecules and signalling pathways (a mechanism commonly termed ‘redox signalling’), with the potential to induce highly specific acute and chronic changes in cell phenotype (Finkel 1999).
There is growing evidence that redox-sensitive signalling pathways play important roles in the processes underlying LVH and LV remodelling. For example, the hypertrophy of isolated cardiomyocytes induced by α-adrenergic agonists, angiotensin II (AngII), endothelin-1, tumour necrosis factor-α (TNF-α), or cyclic stretch has been shown to involve increased ROS production (Nakamura et al. 1998; Pimentel et al. 2001; Hirotani et al. 2002). Inhibition of the antioxidant Cu–Zn superoxide dismutase, which leads to increased intracellular ROS levels, also induces hypertrophy of isolated cardiomyocytes. The development of pressure-overload LVH in mice as well as the transition from compensated pressure-overload LVH to heart failure in guinea-pigs are inhibited by antioxidants, supporting a role for ROS production in cardiac hypertrophy in vivo (Dhalla et al. 1996; Date et al. 2002). Increased ROS production is also implicated in LV remodelling following experimental myocardial infarction, which can be inhibited by ROS scavengers (Kinugawa et al. 2000; Sia et al. 2002). In the clinical setting, there is good evidence of increased oxidative stress in CHF patients, which has been correlated with myocardial dysfunction and the overall severity of heart failure (McMurray et al. 1993; Hornig et al. 1998; Mallat et al. 1998; Ellis et al. 2000).
3. Potential sources of ROS
Potential sources of ROS in LVH and CHF include xanthine oxidase, mitochondria, uncoupled nitric oxide synthases (NOSs) and infiltrating inflammatory cells. Excessive mitochondrial-derived ROS generation has been demonstrated in cardiomyocytes from experimental models of both rapid pacing-induced heart failure and myocardial infarction and was shown to be associated with LV contractile dysfunction (Ide et al. 2000, 2001). Elevated xanthine oxidase expression and activity has previously been reported in both human end-stage heart failure (Cappola et al. 2001) and canine rapid pacing-induced heart failure (Ekelund et al. 1999); the latter study also showing that LV contractile function and myocardial efficiency were improved by the xanthine oxidase inhibitor, allopurinol. These sources may be especially relevant in advanced heart failure. Any setting in which there is significant oxidative stress can also potentially lead to further ROS production by uncoupled NOSs secondary to the oxidation of the essential NOS cofactor BH4 (Landmesser et al. 2003).
Despite the presence of these multiple ROS sources, studies in the last decade have indicated that a major source of ROS involved in redox signalling is a family of NADPH oxidases. These superoxide-generating enzymes are major cardiovascular sources of ROS, particularly in the vasculature, where they have been shown to play important roles in the disease pathophysiology (Griendling et al. 2000a,b). Compelling data implicates ROS generated by NADPH oxidases in the pathophysiology of AngII-dependent and low renin hypertension, vascular smooth muscle (VSM) proliferation, atherosclerosis, angiogenesis, and in endothelial dysfunction associated with hypercholesterolaemia, diabetes and aging.
4. NADPH oxidases in cardiovascular cells
The classical NADPH oxidase complex comprises a membrane-bound cytochrome b558 (composed of one gp91phox and one p22phox subunit) which forms the catalytic core of the enzyme, and four cytosolic regulatory subunits (p47phox, p67phox, p40phox and Rac) which translocate to the cytochrome b558 to activate the enzyme (Lassegue & Clempus 2003)—figure 1. In the last few years, several groups including our own have reported the presence of NADPH oxidases in cardiovascular cells including endothelial cells (EC), adventitial fibroblasts, VSM and cardiomyocytes. Unlike the neutrophil oxidase, the enzyme in cardiovascular cells continuously generates intracellular ROS at a low level even in the absence of cell stimulation. However, NADPH oxidase activity may be significantly enhanced by several different stimuli, many of which are relevant to LVH and heart failure, e.g. cyclic stretch, AngII, α-adrenergic agonists, endothelin-1 and TNF-α (Ushio-Fukai et al. 1996; De Keulenaer et al. 1998a,b; Li et al. 2002b). NADPH oxidase activation by these stimuli involves both transcriptional upregulation of component oxidase subunits and acute activation through post-translational modification of oxidase regulatory subunits.
Figure 1.

Schematic showing the structure of the classical NADPH oxidase of neutrophils. The cytosolic subunits p47phox, p67phox, p40phox and Rac translocate to the NOX2–p22phox complex to activate the enzyme. Several NOX isoforms have been described but whether they all require these subunits for activation remains uncertain. NOX1-containing oxidases have been shown to associate with isoforms of p47phox and p67phox termed NOXO1 and NOXA1, respectively (Lambeth 2004).
Several isoforms of the gp91phox catalytic subunit of NADPH oxidase have been described in the last 4 years, each encoded by separate genes. These isoforms are now termed NOXs, and comprise NOX1–5 and Duox1 and 2; NOX2 is the new name for gp91phox (Lambeth 2004). Cardiovascular cells exhibit specific patterns of NOX expression, with several cell types expressing more than one isoform. NOX2 is abundantly expressed in ECs, fibroblasts and cardiomyocytes. NOX1 was initially identified in colon carcinoma cells, and is highly expressed in cultured VSM, but is not found to any significant level in cardiomyocytes or ECs. NOX4 was first identified in the kidney and appears to be quite widely expressed. Interestingly, cardiomyocytes and ECs coexpress NOX4 and NOX2 (Byrne et al. 2003; Ago et al. 2004). The cardiomyocyte NOX2 : NOX4 mRNA expression ratio by real-time PCR is ca 1 : 2 (Byrne et al. 2003).
5. Involvement of NADPH oxidases in LVH and heart failure
Recent studies have begun to implicate NADPH oxidases in LVH and heart failure in vivo. In experimental pressure-overload LVH induced by aortic banding in guinea-pigs, we reported that NADPH oxidase subunit expression as well as activity were increased in parallel with activation of mitogen-activated protein kinases (MAPKs); oxidase expression was documented in both cardiomyocytes and ECs (Li et al. 2002a). In this model, ROS derived from the oxidase also appeared to contribute to the inactivation of endothelium-derived nitric oxide and the consequent LV diastolic dysfunction (MacCarthy et al. 2001). Similar NADPH oxidase activation is also observed in pressure-overload LVH in mice (Byrne et al. 2003). An increased cardiac expression of the NADPH oxidase subunits, NOX2 and p22phox, was also reported after myocardial infarction both in an animal model (Fukui et al. 2001) and in human myocardium (Krijnen et al. 2003). Recently, our group and others confirmed that myocardium from end-stage human CHF patients demonstrates increased NADPH oxidase activity (Heymes et al. 2003; Maack et al. 2003).
Recently, the role of the NOX2-containing NADPH oxidase in LVH has been specifically addressed in studies in NOX2-deficient mice, which were initially developed as a model of neutrophil oxidase deficiency (Pollock et al. 1995). We first studied AngII-induced in vivo cardiac hypertrophy, using a model of 7–14 day subpressor AngII infusion by osmotic minipump (Bendall et al. 2002). AngII caused a dose-dependent increase in LV NADPH oxidase activity in wild-type but not NOX2−/− mice. In parallel, in vivo hypertrophy (assessed by heart/body weight ratio or myocyte area) and increases in atrial natriuretic factor mRNA expression in response to AngII infusion were markedly inhibited in NOX2−/− mice. These data provide the first definitive evidence for an essential role of the NOX2 oxidase in AngII-induced cardiac hypertrophy in vivo.
Although AngII plays a role in the development of in vivo pressure-overload LVH, several other stimuli including the rise in intracavitary pressure per se (i.e. mechanical forces) and additional neurohumoral factors are involved. We therefore studied the response to aortic banding in NOX2−/− mice and matched wild-type controls (Byrne et al. 2003). Banding of the abdominal aorta induced a similar haemodynamic load in both groups of mice. In contrast to the AngII infusion studies, morphological LVH and the associated rises in ANF mRNA in response to aortic banding were similar in NOX2−/− and wild-type mice (Byrne et al. 2003). Similar results have also been reported by an independent laboratory (Maytin et al. 2004). However, we found that aortic banding significantly increased LV NADPH oxidase activity and in situ production not only in wild-type but also NOX2−/− mice, which was probably the result of increased expression of NOX4 in the banded NOX2−/− animals since NOX4 mRNA and protein were both found to be elevated. Furthermore, chronic oral treatment of banded NOX2−/− mice with the antioxidant N-acetylcysteine significantly reduced the extent of LVH, consistent with ROS generation contributing to in vivo LVH (Byrne et al. 2003). These results suggest that whereas NOX2 is pivotally involved in the development of AngII-induced LVH, that induced by pressure overload may be more dependent on NOX4. However, further studies indicate that NOX2 nevertheless plays an important role in other aspects of pressure-overload LVH. When LV contractile function was compared in banded and control wild-type and NOX2−/− mice using state-of-the-art pressure–volume analysis in ejecting hearts, we found that banded NOX2−/− mice were significantly protected against the LV systolic and diastolic contractile dysfunction that occurred with banding in wild-type animals (Grieve et al. 2004). Furthermore, a similar preservation of contractile function in banded NOX2−/− versus banded wild-type mice was also found in cardiomyocytes isolated from these animals.
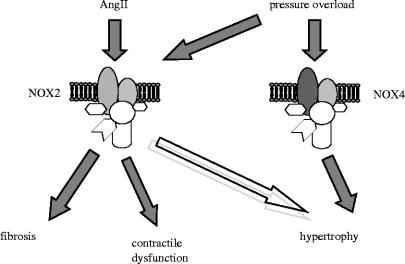
Taken together, these results suggest distinct roles for NOX2 versus NOX4 in different components of the overall hypertrophic response to pressure overload (figure 2). While NOX2 is not essential for the development of pressure-overload LVH per se (where NOX4 may play a role), it appears to be critically involved in the contractile dysfunction caused by pressure overload, at least in the early phases of LVH. These data also suggest that the downstream effects of ROS generated by NADPH oxidases may depend upon the specific isoform activated (possibly due to different subcellular compartmentation), and also indicate a clear dissociation between the development of contractile dysfunction versus hypertrophy per se.
Figure 2.
Potential roles of the NADPH oxidase isoforms NOX2 and NOX4 in the cardiac responses to pressure overload and to angiotensin II (AngII).
6. NADPH oxidase and interstitial cardiac fibrosis
Multiple lines of evidence suggest that the intracellular redox balance is an important determinant of pro-fibrotic processes. Oxidative stress has been shown to be a key regulator of fibrosis in many organs such as the liver, lungs, kidney and vasculature (Poli & Parola 1997; Park & Schiffrin 2002). Often, this is associated with inflammatory infiltration, with both the infiltrating cells and resident tissue cells contributing to ROS generation. The proliferation of fibroblasts is modulated by superoxide (Irani et al. 1997), which contributes to the activation of quiescent fibroblasts into the myofibroblast phenotype which produces large amounts of extracellular matrix. A functional NADPH oxidase is known to be present in fibroblasts (Meier et al. 1991), and both NOX2 (Pagano et al. 1997) and NOX4 are expressed in this cell type (Chamseddine & Miller 2003). In the vasculature, NADPH oxidase-derived ROS have been suggested to be involved in extracellular matrix deposition and vascular remodelling that occur following AngII infusion (Wang et al. 2001; Virdis et al. 2004). Furthermore, NADPH oxidase had been shown to be involved in the activation of matrix metalloproteinases (MMP) in response to mechanical stretch. Grote et al. (2003) found that stretch-induced MMP-2 activation in VSM cells was associated with increased membrane translocation of p47phox and was abolished in VSM cells from p47phox−/− mice. AngII also failed to increase MMP-2 activity in these mice (Luchtefeld et al. 2005).
There are, therefore, good reasons to postulate that NADPH oxidase-derived ROS may also play a role in cardiac fibrosis. This question has recently been addressed by our laboratory and others. We investigated interstitial cardiac fibrosis in NOX2−/− mice infused with AngII for up to two weeks. In addition to demonstrating reduced cardiac hypertrophy in these animals, interstitial cardiac fibrosis was also almost completely abolished (Bendall et al. 2002; Johar et al. 2004). In addition, the mRNA expression of procollagen I and III as well as the activation of MMP-2 were found to be suppressed in NOX2−/− mice infused with AngII compared with wild-type animals (Johar et al. 2004). Interestingly, NOX2 also appears to have a pro-fibrotic role in pressure-overload LVH since we found that NOX2-deficient mice subjected to aortic banding had reduced interstitial fibrosis compared to banded wild-type animals. In an experimental model of aldosterone-driven interstitial cardiac fibrosis, Sun et al. (2002) reported evidence of increased oxidative stress together with increased expression of NOX2 in the ventricular myocardium although a cause–effect relationship between these observations was not established in this study.
The above results suggest a significant role for NADPH oxidase in the processes leading to interstitial cardiac fibrosis. Indeed, these enzymes seem to be important for fibrosis in many organs including the vasculature (Pu et al. 2003), liver (Bataller et al. 2003) and lungs (Manoury et al. 2005).
7. Mechanisms underlying NADPH oxidase-induced pro-hypertrophic and pro-fibrotic actions
While the in vivo studies discussed above provide increasing evidence for the importance of NADPH oxidase activation in LVH, fibrosis and remodelling, the underlying subcellular processes that are involved remain poorly studied to date. The major ROS species generated by NADPH oxidases is , but modulation of signal transduction pathways may often involve H2O2 (produced by dismutation of ) or reaction with NO.
At a cellular level, a wide range of G-protein-coupled receptor agonists (e.g. AngII, Bendall et al. 2002; phenylephrine, Amin et al. 2001; endothelin-1, Duerrschmidt et al. 2000), growth factors, cytokines (e.g. TNF-α), tyrosine kinase-dependent agonists (e.g. insulin; Mahadev et al. 2004) and mechanical stimuli are capable of activating NADPH oxidase. However, the responses to different agonists may vary among cell types with relatively little information available for cardiomyocytes. NADPH oxidases have been implicated in AngII-dependent signalling and norepinephrine-induced hypertrophy in isolated cardiomyocytes (Wenzel et al. 2001; Xiao et al. 2002), while the small GTP-binding protein Rac1 (which plays a role in NADPH oxidase activation) is involved in endothelin-1, AngII- and phenylephrine-induced cardiomyocyte hypertrophy (Pracyk et al. 1998; Takemoto et al. 2001; Higuchi et al. 2003). Emerging evidence suggests that different NOX isoforms may be activated in an agonist-specific manner and this also may be dependent upon the cell type. For example, NOX1 is implicated in platelet-derived growth factor and AngII signalling in VSM cells (Lassegue et al. 2001), while in cardiomyocytes NOX2 appears to be critical in AngII responses (Bendall et al. 2002).
The redox-sensitive signalling pathways that are influenced by NADPH oxidase activation in the heart remain poorly characterized. Potential redox-sensitive downstream targets include Ras, c-Src, the MAPKs (p38MAPK, ERK1/2, JNK), p90RSK, the PI3 kinase (PI3K)/protein kinase B/Akt pathway, AP-1, NF-κB, HIF-1 and others (Lassegue et al. 2001; Xiao et al. 2001; Herkert et al. 2002; Gorin et al. 2003; Adachi et al. 2004; Djordjevic et al. 2005; Kuster et al. 2005). The involvement of NADPH oxidase in activating these pathways has been mostly studied in vascular cells with only a few studies in cardiomyocytes. Several recent studies suggest that the small GTPase RAS is a redox-sensitive signalling switch in many cell types. Kuster et al. (2005) demonstrated that α-adrenergic receptor hypertrophic signalling in adult rat ventricular myocytes is dependent upon a thioredoxin-1 sensitive post-translational oxidative modification of thiols on RAS. Similarly, Xiao et al. (2001) reported a greater than sixfold acute activation of RAS in cardiomyocytes; downstream of RAS, there was phosphorylation of MEK1/2, ERK1/2 and p90SRK within 5 min. In VSM cells, Adachi et al. (2004) demonstrated that S-glutathiolation of RAS plays a critical role in AngII-induced hypertrophic signalling and importantly that overexpression of dominant negative p47phox prevented p38MAPK and Akt phosphorylation. RAS may also directly mediate NADPH oxidase activation to generate intracellular ROS (Irani et al. 1997). Thus, NIH-3T3 fibroblasts stably transformed with a constitutively active isoform of p21Ras, H-Rasv12, produced large amounts of superoxide which was inhibited by dominant negative Ras or Rac1 (Irani et al. 1997).
c-Src is a ubiquitously expressed 60 kD kinase of the Src kinase family known to be activated by G-protein-coupled receptors. Seshiah et al. (2002) proposed a model whereby c-Src was activated by H2O2 derived from a rapid protein kinase C-dependent activation of NADPH oxidase by AngII in VSM cells. Activation of c-Src in turn transactivated the epidermal growth factor receptor which is required for subsequent Rac activation and a more sustained activation of NADPH oxidase. In agreement with this, our unpublished data in cardiomyocytes demonstrate that c-Src inhibition prevented hypertrophic responses to both phenylephrine and endothelin-1 without affecting ROS production—implying that c-Src may be downstream of NADPH oxidase activation. In contrast, however, Touyz et al. (2003) reported evidence in rat aortic VSM cells that c-Src was upstream of NADPH oxidase in AngII-stimulated H2O2 production.
Many studies have shown that activation of several members of the MAPK family is redox sensitive (Li et al. 1998; Griendling et al. 2000a,b; Xiao et al. 2001; Herkert et al. 2002; Kimura et al. 2005; Kuster et al. 2005). Of these, an involvement of NAPDH oxidase has convincingly been demonstrated for p38MAPK (Ushio-Fukai et al. 1998; Viedt et al. 2000; Herkert et al. 2002; Touyz et al. 2005) and JNK activation (Fei et al. 2000; Viedt et al. 2000) in VSM cells through the use of specific molecular approaches (e.g. p22phox or p47phox antisense). Results for ERK1/2 activation are more controversial with studies demonstrating both positive (Gorin et al. 2004; Djordjevic et al. 2005) and negative (Viedt et al. 2000; Touyz et al. 2005) findings. However, in ECs, AngII- and TNF-α-dependent ERK1/2 activation are clearly NADPH oxidase dependent (Li & Shah 2003; Li et al. 2005). Similarly, in glomerular mesangial cells, AngII-dependent ERK1/2 activation involves NADPH oxidase (Gorin et al. 2004). Several studies have also consistently reported an involvement of NADPH oxidase-derived ROS in activating PI3K/Akt (Ushio-Fukai et al. 1999; Herkert et al. 2002; Seshiah et al. 2002; Gorin et al. 2003; Djordjevic et al. 2005; Touyz et al. 2005). In mesangial cells, NOX4 antisense oligonucleotides or dominant negative Rac1 abolished AngII-induced activation of Akt, while in human pulmonary artery smooth muscle cells, p22phox or NOX4 antisense prevented urotensin II-induced ROS production and Akt activation (Djordjevic et al. 2005). Likewise, in rat aortic VSM cells, an NADPH oxidase inhibitor significantly inhibited AngII-induced Akt activation.
Taken together, the above data suggest several potential redox-sensitive signalling pathways that may be modulated by NADPH oxidase in various cell types. However, which precise pathways are involved in NADPH oxidase-dependent effects on cardiac hypertrophy remains the subject of ongoing investigation.
8. Conclusions
A large body of evidence suggests a role for increased ROS production in the pathophysiology of LVH and heart failure. While several different sources of ROS may be involved in CHF, the NADPH oxidases may be especially important in modulating redox-sensitive signalling pathways that underlie the development of cardiomyocyte hypertrophy, interstitial cardiac fibrosis and ventricular remodelling. NADPH oxidases are specifically activated by several stimuli known to be important in these processes, e.g. AngII, cytokines and mechanical forces. Studies in gene-modified mice lacking the NADPH oxidase isoform NOX2 clearly demonstrate an important role for this enzyme in mediating AngII-dependent cardiomyocyte hypertrophy and interstitial fibrosis as well as LV contractile dysfunction associated with pressure-overload LVH. On the other hand, cardiomyocyte hypertrophy in response to pressure overload may involve the NOX4 isoform. These results suggest not only an isoform-specific activation of NOX isoforms in cardiac disease, but also distinct downstream targets for these isoforms. Thus, it is likely that each NOX protein subserves distinct biological functions in cardiac hypertrophy and remodelling. Independently targeting different members of the NOX family may hold the potential for therapeutic intervention in the treatment of cardiac disease.
Acknowledgments
The authors' work is supported by the British Heart Foundation (BHF). A.M.S. holds the BHF Chair of Cardiology. S.J. was supported by a BHF Clinical Ph.D. Fellowship. D.G. was funded by BHF Programme grant RG/03/008 and M.Z. by BHF project grant PG/01/168.
Footnotes
One contribution of 18 to a Theme Issue ‘Reactive oxygen species in health and disease’.
References
- Adachi T, Pimentel D.R, Heibeck T, Hou X, Lee Y.J, Jiang B, Ido Y, Cohen R.A. S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 2004;279:29 857–29 862. doi: 10.1074/jbc.M313320200. 10.1074/jbc.M313320200 [DOI] [PubMed] [Google Scholar]
- Ago T, et al. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109:227–233. doi: 10.1161/01.CIR.0000105680.92873.70. 10.1161/01.CIR.0000105680.92873.70 [DOI] [PubMed] [Google Scholar]
- Amin J.K, Xiao L, Pimental D.R, Pagano P.J, Singh K, Sawyer D.B, Colucci W.S. Reactive oxygen species mediate alpha-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J. Mol. Cell. Cardiol. 2001;33:131–139. doi: 10.1006/jmcc.2000.1285. 10.1006/jmcc.2000.1285 [DOI] [PubMed] [Google Scholar]
- Bataller R, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J. Clin. Invest. 2003;112:1383–1394. doi: 10.1172/JCI18212. 10.1172/JCI200318212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall J.K, Cave A.C, Heymes C, Gall N, Shah A.M. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. 10.1161/hc0302.103712 [DOI] [PubMed] [Google Scholar]
- Byrne J.A, Grieve D.J, Bendall J.K, Li J.M, Gove C, Lambeth J.D, Cave A.C, Shah A.M. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ. Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. 10.1161/01.RES.0000099504.30207.F5 [DOI] [PubMed] [Google Scholar]
- Cappola T.P, Kass D.A, Nelson G.S, Berger R.D, Rosas G.O, Kobeissi Z.A, Marban E, Hare J.M. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- Chamseddine A.H, Miller F.J., Jr gp91phox contributes to NADPH oxidase activity in aortic fibroblasts, but not smooth muscle cells. Am. J. Physiol. 2003;285:H2284–H2289. doi: 10.1152/ajpheart.00459.2003. [DOI] [PubMed] [Google Scholar]
- Date M.O, et al. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J. Am. Coll. Cardiol. 2002;39:907–912. doi: 10.1016/s0735-1097(01)01826-5. 10.1016/S0735-1097(01)01826-5 [DOI] [PubMed] [Google Scholar]
- De Keulenaer G.W, Alexander R.W, Ushio-Fukai M, Ishizaka N, Griendling K.K. Tumor necrosis factor α activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem. J. 1998a;329:653–657. doi: 10.1042/bj3290653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keulenaer G.W, Chappell D.C, Ishizaka N, Nerem R.M, Alexander R.W, Griendling K.K. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state. Role of a superoxide-producing NADH oxidase. Circ. Res. 1998b;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- Dhalla A.K, Hill M.F, Singal P.K. Role of oxidative stress in transition of hypertrophy to heart failure. J. Am. Coll. Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. 10.1016/0735-1097(96)00140-4 [DOI] [PubMed] [Google Scholar]
- Djordjevic T, BelAiba R.S, Bonello S, Pfeilschifter J, Hess J, Gorlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2005;25:519–525. doi: 10.1161/01.ATV.0000154279.98244.eb. 10.1161/01.ATV.0000154279.98244.eb [DOI] [PubMed] [Google Scholar]
- Duerrschmidt N, Wippich N, Goettsch W, Broemme H.J, Morawietz H. Endothelin-1 induces NAD(P)H oxidase in human endothelial cells. Biochem. Biophys. Res. Commun. 2000;269:713–717. doi: 10.1006/bbrc.2000.2354. 10.1006/bbrc.2000.2354 [DOI] [PubMed] [Google Scholar]
- Ekelund U.E, Harrison R.W, Shokek O, Thakkar R.N, Tunin R.S, Senzaki H, Kass D.A, Marban E, Hare J.M. Intravenous allopurinol decreases myocardial oxygen consumption and increases mechanical efficiency in dogs with pacing-induced heart failure. Circ. Res. 1999;85:437–445. doi: 10.1161/01.res.85.5.437. [DOI] [PubMed] [Google Scholar]
- Ellis G.R, et al. Neutrophil superoxide anion-generating capacity, endothelial function and oxidative stress in chronic heart failure: effects of short- and long-term vitamin C therapy. J. Am. Coll. Cardiol. 2000;36:1474–1482. doi: 10.1016/s0735-1097(00)00916-5. 10.1016/S0735-1097(00)00916-5 [DOI] [PubMed] [Google Scholar]
- Fei J, Viedt C, Soto U, Elsing C, Jahn L, Kreuzer J. Endothelin-1 and smooth muscle cells: induction of jun amino-terminal kinase through an oxygen radical-sensitive mechanism. Arterioscler. Thromb. Vasc. Biol. 2000;20:1244–1249. doi: 10.1161/01.atv.20.5.1244. [DOI] [PubMed] [Google Scholar]
- Finkel T. Signal transduction by reactive oxygen species in non-phagocytic cells. J. Leukoc. Biol. 1999;65:337–340. doi: 10.1002/jlb.65.3.337. [DOI] [PubMed] [Google Scholar]
- Fukui T, Yoshiyama M, Hanatani A, Omura T, Yoshikawa J, Abe Y. Expression of p22-phox and gp91-phox, essential components of NADPH oxidase, increases after myocardial infarction. Biochem. Biophys. Res. Commun. 2001;281:1200–1206. doi: 10.1006/bbrc.2001.4493. 10.1006/bbrc.2001.4493 [DOI] [PubMed] [Google Scholar]
- Gorin Y, Ricono J.M, Kim N.H, Bhandari B, Choudhury G.G, Abboud H.E. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am. J. Physiol. 2003;285:F219–F229. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- Gorin Y, Ricono J.M, Wagner B, Kim N.-H, Bhandari B, Choudhury G.G, Abboud H.E. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem. J. 2004;381:231–239. doi: 10.1042/BJ20031614. 10.1042/BJ20031614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling K.K, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler. Thromb. Vasc. Biol. 2000a;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- Griendling K.K, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase. Role in cardiovascular biology and disease. Circ. Res. 2000b;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Grieve D.J, Siva A, Byrne J.A, Cave A.C, Shah A.M. Divergent effects of a Nox2-containing NADPH oxidase on cardiac contractile function and hypertrophy after imposition of chronic pressure overload. Circulation. 2004;110:III-133–III-134. [Google Scholar]
- Grote K, Flach I, Luchtefeld M, Akin E, Holland S.M, Drexler H, Schieffer B. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ. Res. 2003;92:80e–86e. doi: 10.1161/01.RES.0000077044.60138.7C. 10.1161/01.RES.0000077044.60138.7C [DOI] [PubMed] [Google Scholar]
- Herkert O, Diebold I, Brandes R.P, Hess J, Busse R, Gorlach A. NADPH oxidase mediates tissue factor-dependent surface procoagulant activity by thrombin in human vascular smooth muscle cells. Circulation. 2002;105:2030–2036. doi: 10.1161/01.cir.0000014611.28864.1e. 10.1161/01.CIR.0000014611.28864.1E [DOI] [PubMed] [Google Scholar]
- Heymes C, Bendall J.K, Ratajczak P, Cave A.C, Samuel J.L, Hasenfuss G, Shah A.M. Increased myocardial NADPH oxidase activity in human heart failure. J. Am. Coll. Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. 10.1016/S0735-1097(03)00471-6 [DOI] [PubMed] [Google Scholar]
- Higuchi Y, et al. The small GTP-binding protein Rac1 induces cardiac myocyte hypertrophy through the activation of apoptosis signal-regulating kinase 1 and nuclear factor-kappa B. J. Biol. Chem. 2003;278:20 770–20 777. doi: 10.1074/jbc.M213203200. 10.1074/jbc.M213203200 [DOI] [PubMed] [Google Scholar]
- Hirotani S, et al. Involvement of nuclear factor-κB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation. 2002;105:509–515. doi: 10.1161/hc0402.102863. 10.1161/hc0402.102863 [DOI] [PubMed] [Google Scholar]
- Hornig B, Arakawa N, Kholer C, Drexler H. Vitamin C improves endothelial function of conduit arteries in patients with chronic heart failure. Circulation. 1998;97:363–368. doi: 10.1161/01.cir.97.4.363. [DOI] [PubMed] [Google Scholar]
- Hua C, Harrison D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Hunter J.J, Chien K.R. Signalling pathways for cardiac hypertrophy and failure. N. Engl. J. Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. 10.1056/NEJM199910213411706 [DOI] [PubMed] [Google Scholar]
- Ide T, et al. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ. Res. 2000;86:152–157. doi: 10.1161/01.res.86.2.152. [DOI] [PubMed] [Google Scholar]
- Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier J.L, Sollott S.J, Der C.J, Fearon E.R, Sundaresan M, Finkel T, Goldschmidt-Clermont P.J. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. 10.1126/science.275.5306.1649 [DOI] [PubMed] [Google Scholar]
- Johar S, Cave A.C, Grieve D.J, Shah A.M. A critical role for a gp91phox-containing NADPH oxidase in interstitial cardiac fibrosis. Circulation. 2004;110:III-188. [Google Scholar]
- Kimura S, Zhang G.X, Nishiyama A, Shokoji T, Yao L, Fan Y.Y, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. 10.1161/01.HYP.0000157169.27818.ae [DOI] [PubMed] [Google Scholar]
- Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodelling and failure after experimental myocardial infarction in mice: role of oxidative stress. Circ. Res. 2000;87:392–398. doi: 10.1161/01.res.87.5.392. [DOI] [PubMed] [Google Scholar]
- Krijnen P.A, Meischl C, Hack C.E, Meijer C.J, Visser C.A, Roos D, Niessen H.W. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J. Clin. Pathol. 2003;56:194–199. doi: 10.1136/jcp.56.3.194. 10.1136/jcp.56.3.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster G.M, Pimentel D.R, Adachi T, Ido Y, Brenner D.A, Cohen R.A, Liao R, Siwik D.A, Colucci W.S. α-Adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes is mediated via thioredoxin-1-sensitive oxidative modification of thiols on Ras. Circulation. 2005;111:1192–1198. doi: 10.1161/01.CIR.0000157148.59308.F5. 10.1161/01.CIR.0000157148.59308.F5 [DOI] [PubMed] [Google Scholar]
- Lambeth J.D. Nox enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. 10.1038/nri1312 [DOI] [PubMed] [Google Scholar]
- Landmesser U, Dikalov S, Price S.R, McCann L, Fukai T, Holland S.M, Mitch W.E, Harrison D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. 10.1172/JCI200314172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassegue B, Clempus R.E. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am. J. Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant S.L, Lambeth J.D, Griendling K.K. Novel gp91phox homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- Li J.-M, Shah A.M. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II: role of the p47phox subunit. J. Biol. Chem. 2003;278:12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- Li X, Lee J.W, Graves L.M, Earp H.S. Angiotensin II stimulates ERK via two pathways in epithelial cells: protein kinase C suppresses a G-protein coupled receptor-EGF receptor transactivation pathway. EMBO J. 1998;17:2574–2583. doi: 10.1093/emboj/17.9.2574. 10.1093/emboj/17.9.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.-M, Gall N.P, Grieve D.G, Chen M, Shah A.M. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002a;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. 10.1161/01.HYP.0000032031.30374.32 [DOI] [PubMed] [Google Scholar]
- Li L, Fink G.D, Watts S.W, Northcott C.A, Galligan J.J, Pagano P.J, Chen A.F. Endothelin-1 increases vascular superoxide via endothelinA-NADPH oxidase pathway in low-renin hypertension. Circulation. 2002b;107:1053–1058. doi: 10.1161/01.cir.0000051459.74466.46. 10.1161/01.CIR.0000051459.74466.46 [DOI] [PubMed] [Google Scholar]
- Li J.-M, Fan L.M, Christie M.R, Shah A.M. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol. Cell. Biol. 2005;25:2320–2330. doi: 10.1128/MCB.25.6.2320-2330.2005. 10.1128/MCB.25.6.2320-2330.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchtefeld M, et al. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem. Biophys. Res. Commun. 2005;328:183–188. doi: 10.1016/j.bbrc.2004.12.152. 10.1016/j.bbrc.2004.12.152 [DOI] [PubMed] [Google Scholar]
- Maack C, Kartes T, Kilter H, Schafers H.J, Nickenig G, Bohm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of Rac1-GTPase and represents a target for statin therapy. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. 10.1161/01.CIR.0000091084.46500.BB [DOI] [PubMed] [Google Scholar]
- MacCarthy P.A, Grieve D.J, Li J.-M, Dunster C, Kelly F.J, Shah A.M. Impaired endothelial regulation of ventricular relaxation in cardiac hypertrophy: role of reactive oxygen species and NADPH oxidase. Circulation. 2001;104:2967–2974. doi: 10.1161/hc4901.100382. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Motoshima H, Wu X, Ruddy J.M, Arnold R.S, Cheng G, Lambeth J.D, Goldstein B.J. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell. Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. 10.1128/MCB.24.5.1844-1854.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin F2α in pericardial fluid of patients with heart failure—a potential role for in vivo oxidant stress in ventricular dilatation and progression in heart failure. Circulation. 1998;97:1536–1539. doi: 10.1161/01.cir.97.16.1536. [DOI] [PubMed] [Google Scholar]
- Manoury B, Nenan S, Leclerc O, Guenon I, Boichot E, Planquois J.M, Bertrand C.P, Lagente V. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir. Res. 2005;6:11. doi: 10.1186/1465-9921-6-11. 10.1186/1465-9921-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maytin M, Siwik D.A, Ito M, Xiao L, Sawyer D.B, Liao R, Colucci W.S. Pressure overload-induced myocardial hypertrophy in mice does not require gp91phox. Circulation. 2004;109:1168–1171. doi: 10.1161/01.CIR.0000117229.60628.2F. 10.1161/01.CIR.0000117229.60628.2F [DOI] [PubMed] [Google Scholar]
- McMurray J, Chopra M, Abdullah I, Smith W.E, Dargie H.J. Evidence of oxidative stress in chronic heart failure in humans. Eur. Heart J. 1993;14:1493–1498. doi: 10.1093/eurheartj/14.11.1493. [DOI] [PubMed] [Google Scholar]
- Meier B, Cross A.R, Hancock J.T, Kaup F.J, Jones O.T. Identification of a superoxide-generating NADPH oxidase system in human fibroblasts. Biochem. J. 1991;275:241–245. doi: 10.1042/bj2750241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-α and angiotensin II. Circulation. 1998;98:794–799. doi: 10.1161/01.cir.98.8.794. [DOI] [PubMed] [Google Scholar]
- Pagano P.J, Clark J.K, Cifuentes-Pagano M.E, Clark S.M, Callis G.M, Quinn M.T. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc. Natl Acad. Sci. USA. 1997;94:14 483–14 488. doi: 10.1073/pnas.94.26.14483. 10.1073/pnas.94.26.14483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.B, Schiffrin E.L. Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: role of endothelin-1. Am. J. Hypertens. 2002;15:164–169. doi: 10.1016/s0895-7061(01)02291-9. 10.1016/S0895-7061(01)02291-9 [DOI] [PubMed] [Google Scholar]
- Pimentel D.R, et al. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ. Res. 2001;89:453–460. doi: 10.1161/hh1701.096615. [DOI] [PubMed] [Google Scholar]
- Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic. Biol. Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. 10.1016/S0891-5849(96)00327-9 [DOI] [PubMed] [Google Scholar]
- Pollock J.D, Williams D.A, Gifford M.A, Li L.L, Du X, Fisherman J, Orkin S.H, Doerschuk C.M, Dinauer M.C. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. 10.1038/ng0295-202 [DOI] [PubMed] [Google Scholar]
- Pracyk J.B, et al. A requirement for the rac1 GTPase in the signal transduction pathway leading to cardiac myocyte hypertrophy. J. Clin. Invest. 1998;102:929–937. doi: 10.1172/JCI2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Q, Neves M.F, Virdis A, Touyz R.M, Schriffrin E.L. Endothelin antagonism on aldosterone-induced oxidative stress and vascular remodeling. Hypertension. 2003;42:49–55. doi: 10.1161/01.HYP.0000078357.92682.EC. 10.1161/01.HYP.0000078357.92682.EC [DOI] [PubMed] [Google Scholar]
- Seshiah P.N, Weber D.S, Rocic P, Valppu L, Taniyama Y, Griendling K.K. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ. Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. 10.1161/01.RES.0000033523.08033.16 [DOI] [PubMed] [Google Scholar]
- Sia Y.T, et al. Beneficial effects of long-term use of the antioxidant probucol in heart failure in the rat. Circulation. 2002;105:2549–2555. doi: 10.1161/01.cir.0000016721.84535.00. 10.1161/01.CIR.0000016721.84535.00 [DOI] [PubMed] [Google Scholar]
- Suematsu N, et al. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. 10.1161/01.CIR.0000055318.09997.1F [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang J, Lu L, Chen S.S, Quinn M.T, Weber K.T. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am. J. Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swynghedauw B. Molecular mechanisms of myocardial remodelling. Physiol. Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Node K, Nakagami H, Liao Y, Grimm M, Takemoto Y, Kitakaze M, Liao J.K. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J. Clin. Invest. 2001;108:1429–1437. doi: 10.1172/JCI13350. 10.1172/JCI200113350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touyz R.M, Yao G, Schiffrin E.L. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. 10.1161/01.ATV.0000069236.27911.68 [DOI] [PubMed] [Google Scholar]
- Touyz R.M, Yao G, Quinn M.T, Pagano P.J, Schiffrin E.L. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2005;25:512–518. doi: 10.1161/01.ATV.0000154141.66879.98. 10.1161/01.ATV.0000154141.66879.98 [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Zafari A.M, Fukui T, Ishizaka N, Griendling K.K. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J. Biol. Chem. 1996;271:23 317–23 321. doi: 10.1074/jbc.271.38.23317. 10.1074/jbc.271.38.23317 [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander R.W, Akers M, Griendling K.K. p38 mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J. Biol. Chem. 1998;273:15 022–15 029. doi: 10.1074/jbc.273.24.15022. 10.1074/jbc.273.24.15022 [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander R.W, Akers M, Yin Q, Fujio Y, Walsh K, Griendling K.K. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 1999;274:22 699–22 704. doi: 10.1074/jbc.274.32.22699. 10.1074/jbc.274.32.22699 [DOI] [PubMed] [Google Scholar]
- Viedt C, Soto U, Krieger-Brauer H.I, Fei J, Elsing C, Kubler W, Kreuzer J. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler. Thromb. Vasc. Biol. 2000;20:940–948. doi: 10.1161/01.atv.20.4.940. [DOI] [PubMed] [Google Scholar]
- Virdis A, Neves M.F, Amiri F, Touyz R.M, Schiffrin E.L. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J. Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. 10.1097/00004872-200403000-00016 [DOI] [PubMed] [Google Scholar]
- Wang H.D, Xu S, Johns D.G, Du Y, Quinn M.T, Cayatte A.J, Cohen R.A. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- Wenzel S, Taimor G, Piper H.M, Schluter K.D. Redox-sensitive intermediates mediate angiotensin II-induced p38 MAP kinase activation, AP-1 binding activity, and TGF-β expression in adult ventricular cardiomyocytes. FASEB J. 2001;15:2291–2293. doi: 10.1096/fj.00-0827fje. [DOI] [PubMed] [Google Scholar]
- Xiao L, Pimental D.R, Amin J.K, Singh K, Sawyer D.B, Colucci W.S. MEK1/2-ERK1/2 mediates α1-adrenergic receptor-stimulated hypertrophy in adult rat ventricular myocytes. J. Mol. Cell. Cardiol. 2001;33:779–787. doi: 10.1006/jmcc.2001.1348. 10.1006/jmcc.2001.1348 [DOI] [PubMed] [Google Scholar]
- Xiao L, Pimentel D.R, Wang J, Singh K, Colucci W.S, Sawyer D.B. Role of reactive oxygen species and NAD(P)H oxidase in α1-adrenoceptor signaling in adult rat cardiac myocytes. Am. J. Physiol. 2002;282:C926–C934. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]