Abstract
We begin by providing an operational definition of sexual conflict that applies to both inter- and intralocus conflict. Using this definition, we examine a series of simple coevolutionary models to elucidate fruitful approaches for detecting interlocus sexual conflict and resultant sexually antagonistic coevolution. We then use published empirical examples to illustrate the utility of these approaches. Three relevant attributes emerge. First, the dynamics of sexually antagonistic coevolution may obscure the conflict itself. Second, competing models of inter-sexual coevolution may yield similar population patterns near equilibria. Third, a variety of evolutionary forces underlying competing models may be acting simultaneously near equilibria. One main conclusion is that studies of emergent patterns in extant populations (e.g. studies of population and/or female fitness) are unlikely to allow us to distinguish among competing coevolutionary models. Instead, we need more research aimed at identifying the forces of selection acting on shared traits and sexually antagonistic traits. More specifically, we need a greater number of functional studies of female traits as well as studies of the consequences of both male and female traits for female fitness. A mix of selection and manipulative studies on these is likely the most promising route.
Keywords: theory, experiments, conflicting selection, population fitness
1. Introduction
There has been considerable controversy over the role of sexual conflict in shaping the evolution of both sexual and non-sexual traits (e.g. Getty 1999; Rice & Holland1999; Rosenthal & Servedio 1999; Cameron et al. 2003; Chapman et al. 2003a,b; Cordero & Eberhard 2003; Eberhard & Cordero 2003; Pizzari & Snook 2003, 2004; Arnqvist 2004). To detect sexual conflict and sexually antagonistic coevolution, it is first required that we define these terms in some way that is amenable to measurement. Parker's (1979) original definition was ‘a conflict between the evolutionary interests of individuals of the two sexes’. This definition has the advantage of being both intuitive and encompassing. Among the wide array of interactions that occur between individual males and females, it is easy to imagine cases in which the interaction will affect the fitness of the male and the female in different and potentially opposite ways. For example, in the case of the biparental care of a diploid offspring, it will often increase the fitness of each parent to reduce the amount of care that they provide, if there is compensation by the interacting parent (Trivers 1972). Likewise, it is easy to imagine that the fitness of a male will increase by inseminating a given female, but that this female's fitness might decrease as a result (Bateman 1948). In short, ‘selection can act in opposing directions on the two sexes’ (Parker 1979), or selection can be sexually antagonistic.
Under Parker's definition, detecting sexual conflict requires measuring the evolutionary interests of individuals of both sexes, and then determining whether they are in evolutionary conflict. In practice, this can sometimes be done in ways that are specific to the study system of interest, but ultimately we require an approach that is broad enough, both conceptually and operationally, that can be applied across a diverse array of taxa, traits and contexts. Only then might we eventually obtain an appreciation for the extent and importance of sexual conflict as causal factor of evolutionary change.
The effects of sexually antagonistic selection at the genetic level can be described in two distinct ways; as inter- or intralocus conflict, depending on whether the target of selection is determined by alleles at different interacting loci (interlocus) in the two sexes, or alleles at one locus expressed in both sexes (intralocus; Holland & Rice 1998; Partridge & Hurst 1998). For example, if mating rate is determined by different loci in the two sexes, then the potential exists for interlocus conflict over mating rate. In this case, we could assay sexually antagonistic selection on mating rate as a measure of sexual conflict. The situation is slightly different in some examples of intralocus conflict, however, because the antagonism does not involve the interaction of a male and a female. Consider, for example, a morphological trait that comes under sexual selection (e.g. tail length in males). It is easy to imagine that, prior to sexual selection, tail length is determined by the same loci in males and females, and that it is at its natural selection optimum. Once sexual selection is introduced, there will then be a new optimum for males, and hence there will be sexually antagonistic selection on a trait that does not involve a male–female interaction. Examples of intralocus conflict that do involve male–female interactions can also be imagined, however, as can examples of interlocus conflict that do not involve such interactions.
There is some evidence that intralocus conflict is occurring in extant populations. Data include measures of sexually antagonistic selection in a variety of birds (e.g. Price & Burley 1994; Merilä et al. 1997; Björklund & Senar 2001), and in Drosophila (Vieira et al. 2000; Mackay 2002). Nevertheless, the existence of widespread sexual dimorphism in many taxa suggests that there is often ample scope for evolutionary divergence of the two sexes, and thus that the constraint of intralocus sexual conflict might be, to some extent, transient. More data on intralocus conflict are required before its power to constrain evolution can be judged. The extent to which intralocus conflict limits adaptive evolution depends on the ease with which sex limited expression of these traits can evolve, and this is debated (e.g. Rice 1984; Halliday & Arnold 1987; Lande 1987; Partridge & Hurst 1998; Rice & Chippindale 2001). Nevertheless, even if the constraint is transient, it is interesting to consider the extent to which the genetic architecture of traits subject to intralocus conflict has been shaped by this conflict. Recent discussions of these issues are given in Rice & Chippindale (2001) and Bonduriansky & Rowe (2005).
There are a considerable amount of data suggesting that there is sexually antagonistic selection on shared traits that appear to be determined by distinct loci in the two sexes (Clutton-Brock & Parker 1995; Lessells 1999; Chapman et al. 2003a; Arnqvist & Rowe 2005). Some of these traits may have been previously constrained by intralocus conflict, as most involve sexually dimorphic traits. These include a range of reproductive traits in both males and females that affect mating rate, time to remating, reproductive rate, and offspring provisioning. Most of these data come from manipulative studies aimed at understanding the costs and benefits of different traits in males and females. For example, there are dozens of studies demonstrating that mating can be costly for female insects, and that mating rate optima differ between the two sexes (Arnqvist & Nilsson 2000). Thus, any traits in males or females that affect these outcomes will be sexually antagonistic.
In this paper, we will focus predominately on interlocus sexual conflict. Ultimately, we are interested in considering how one might infer the existence and importance of sexually antagonistic coevolution in different taxa, and to do so we must first confront several related issues. To begin, we must specify a general and operational definition of sexual conflict, and then use this definition to specify how sexual conflict might be detected. Then we must consider the potential evolutionary outcomes of such sexual conflict as a result of male–female coevolution. Finally, once some understanding of the potential coevolutionary outcomes are in hand, we can then use all of these results to consider how the existence (or lack thereof) of sexually antagonistic coevolution might be inferred from data collected in real populations.
2. Sexual conflict and sexually antagonistic traits
It is unlikely that a completely general yet practical definition of sexual conflict can be found that will appeal to everyone, but we believe that some attempts in this direction are necessary to make further progress in studying sexual conflict. In particular, some specification of sexual conflict is required for tests. Any definition of sexual conflict must first make reference to the object over which conflict is occurring. To this end, it is useful to define shared traits between males and females. A shared trait can be any phenotypic characteristic of an individual, or a phenotypic characteristic that emerges through the interaction of one or more individuals. For example, shared traits would encompass those traits that are part of the phenotype of both sexes (e.g. simple morphological traits like tail length) as well as traits such as the probability of mating that are the result of male–female interactions. They might even be potentially complex phenotypic attributes resulting from the interaction of multiple individuals. For example, the probability of mating between a particular male and female might be affected, not only by the interaction between these two individuals, but also by an interaction with other males (e.g. if there is male–male competition during mating). In any of these cases, the key feature is that the shared trait is the object over which conflict might occur. It is also worth noting that the definition of shared traits has the potential to encompass both intra- and interlocus conflict.
Given a shared trait of interest, we might then define sexual conflict as the occurrence of sexually antagonistic selection on the shared trait. Thus, the presence (and degree) of sexual conflict can be assayed by measuring the degree of sexually antagonistic selection on the shared trait, across the range of variation present in the population (figure 1). In the example of mating interactions, sexual conflict would appear as selection for those males that induce a higher probability of mating, and selection for those females that induce a lower probability of mating. This emphasis on sexually antagonistic selection as the detectable metric of sexual conflict is congruent with Parker's (1979) observation that selection can act in opposing directions in the two sexes and with more recent treatments of the subjects (Shuster & Wade 2003; Arnqvist & Rowe 2005).
Figure 1.
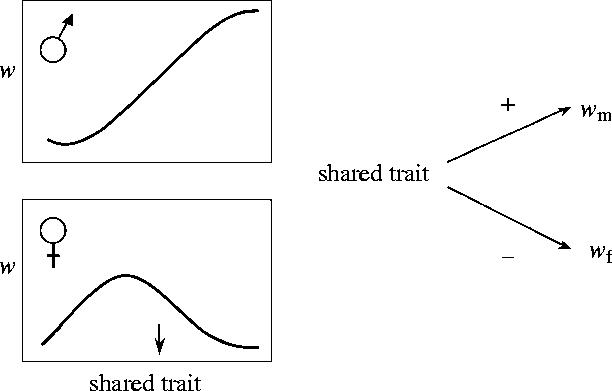
A path diagram (right) illustrating the sign of the correlation between the shared trait and male (wm) and female (wf) relative fitness for patterns of selection depicted on the left. In this case, the mean value of the shared trait in the population (indicated by the arrow in the bottom left figure is such that there is selection for increased values in males (top left) and for reduced values in females (bottom left).
The qualifier that the antagonism should be measured across the range of variation in a population is an important one, because the nature of the variation in the shared trait can affect whether or not sexually antagonistic selection occurs. This is best illustrated using an example. Consider a monogamous species in which there is direct male–male competition for access to females. Once a mating event begins, however, females retain that mate for the duration of their life. Now suppose that the shared trait of interest is ‘aggressiveness during mating’ and that variation in this shared trait is due to variation in male behaviour as well as variation in females' responses to male behaviour. High values of the shared trait are directly detrimental to both female and male fitness because it results in harm to the female, and male reproductive success is completely tied to that of the female. Thus, if variation in male success during male–male competition is unrelated to variation in the shared trait, then no antagonism over the shared trait exists. On the other hand, if aggression is beneficial in male–male competition, and if variation in aggression at this stage of the reproductive cycle is positively associated with variation in aggression during mating, then sexually antagonistic selection over the shared trait will occur.
Thus, the existence of sexually antagonistic selection can depend on how variation in the shared trait arises. It can also be transient if, for example, aggression during competition and aggression during mating eventually evolve to become genetically dissociated in males. This illustrates an important point that sexually antagonistic selection is context specific; it is a function of the current variation that is present in the population. One can readily imagine populations in which sexually antagonistic selection is occurring, but where there are genotypes that, once abundant in the population, would ameliorate any conflict over the shared trait.
Given that an evolutionary conflict exists between males and females over a shared trait, we might then expect some degree of evolutionary change in this trait to occur. The shared trait will often be one that arises from an interaction between males and females, and thus its evolution will be affected by evolutionary change in both males and females. To further understand the nature of sexual conflict it is, therefore, helpful to focus on the evolution of sexually antagonistic traits; these are traits in males and females that function to affect the shared trait that is under sexually antagonistic selection (figure 2). For example, if we assume there is conflict over female remating rate, then a male signal that induces females to remate at a higher rate is a sexually antagonistic trait. Likewise, a female response to this signal is also a sexually antagonistic trait. It is through the interaction of sexually antagonistic traits that the value of the shared trait emerges, and thus sexually antagonistic coevolution will involve the coevolution of male and female sexually antagonistic traits (figure 2).
Figure 2.
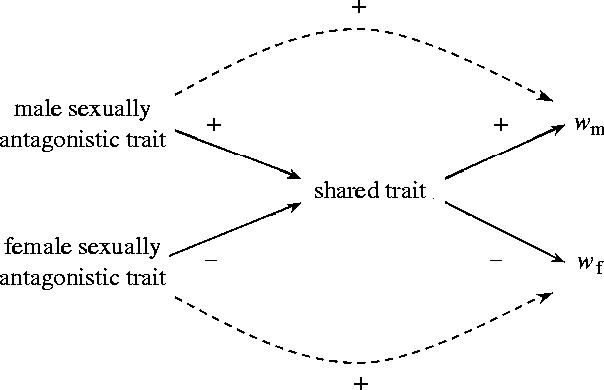
A path diagram illustrating the minimum number of correlations required to demonstrate that male and female traits are sexually antagonistic. These are the sign of correlations (solid lines) between male and female sexually antagonistic traits and the shared trait, and between the shared trait and male and female relative fitness (as in figure 1). There are also correlations (dashed lines) between the male and female sexually antagonistic traits and their relative fitnesses. These lines are dashed to indicate that the correlation is not causal, but exists because these traits affect the value of the shared trait and thereby fitness.
To identify a sexually antagonistic trait requires determining the sign of all those arrows downstream of the trait in figure 2. For example, a potentially antagonistic trait in males should be shown to affect a shared trait known to be under sexually antagonistic selection in such a way that the relative fitness of its bearer is increased. There are relatively few examples of these (reviews in Chapman et al. 2003a; Arnqvist & Rowe 2005). Grasping traits (male) and antigrasping traits (females) in water striders have been shown to be favoured because of their effects on mating rate, which is under sexually antagonistic selection (Rowe et al. 1994; Arnqvist 1997). Similarly, in Drosophila melanogaster, elements of male seminal fluids have been shown to increase relative male fitness through their effects on reproductive traits in females that are under sexually antagonistic selection (Chapman et al. 1995; Wigby & Chapman 2005).
One can easily imagine an escalating arms race as selection within each sex, mediated through an effect on the shared trait, leads to the exaggeration of sexually antagonistic traits in each sex (Parker 1979; Holland & Rice 1998; Gavrilets et al. 2001; Rowe et al. 2005). Nevertheless, direct evidence of sexually antagonistic coevolution among these traits is rare. We are aware of only one case where male and female traits, known to be sexually antagonistic, have been shown to coevolve in natural populations (Arnqvist & Rowe 2002a,b). Several other coevolutionary patterns are suggestive, but these typically lack conclusive evidence that the coevolving traits are indeed sexually antagonistic (e.g. Pitnick et al. 1999; Presgraves et al. 1999; Morrow & Gage 2000; Bergsten et al. 2001; Koene & Schulenburg 2005). It would certainly pay to conduct more studies aimed at identifying sexually antagonistic traits in both sexes, and to determine whether they are, in fact, coevolving.
3. Potential evolutionary outcomes of sexually antagonistic coevolution
Most studies aimed at identifying sexually antagonistic coevolution have been rather indirect, testing emergent predictions of verbal coevolutionary theory. For example, some such theory has suggested that patterns in reproductive traits resulting from within and between population crosses allow one to identify sexually antagonistic coevolution (Clark et al. 1999; Andrés & Arnqvist 2001; Hosken et al. 2002; Nilsson et al. 2002; Long et al. 2006). However, analyses of formal models suggest that patterns yielded from population crosses cannot be used to distinguish sexually antagonistic coevolution from alternative models of male female coevolution (Parker & Partridge 1998; Rowe et al. 2003). Likewise, formal models of speciation suggest that the patterns of speciation arising from assumptions of sexually antagonistic coevolution are the same as those arising from alternative models of male female coevolution (Gavrilets 2000; Gavrilets & Waxman 2002).
The difficulties apparent in distinguishing sexually antagonistic coevolution and alternative coevolutionary processes suggest that we should redouble our efforts to directly identify sexually antagonistic traits (as envisioned in figure 2). However, the frequency of sexually antagonistic selection, and sexually antagonistic traits in extant populations may greatly underestimate the role of sexual conflict in generating the diversity we see today. Several authors have pointed out that antagonistic coevolution in the past has the potential to mask the extent of sexual conflict in extant populations (e.g. Chapman & Partridge 1996; Rice 1998; Härdling et al. 2001; Rowe & Arnqvist 2002).
To address these concerns an alternative approach has been to study the effects of sexual conflict in experimental populations, where the potential for sexually antagonistic coevolution can be manipulated by altering the potential for sexual conflict within evolving populations (Promislow et al. 1998; Holland & Rice 1999; Holland 2002; Martin & Hosken 2003; Wigby & Chapman 2004; Crudgington et al. 2005). Broadly, an ancestral population in which sexual conflict can occur is subdivided into two sets of descendent populations. In one subpopulation, conditions allowing for interlocus sexual conflict continue (there is the potential for sexual selection), in the other set, conditions are such that there can be no interlocus sexual conflict (there is no potential for sexual selection). These conditions are created by allowing polyandry in the first set of lines, and monogamy with random mating of males with respect to female phenotype in the second. At the end of a period of independent evolution, mean female fitness of the two sets of populations can be assessed, divergent evolution can be assayed by exposing males and females from the two sets to each other, and determining the effect on fitness. These results are then compared to verbal theories of sexual conflict. Next, we use a formal model of sexually antagonistic coevolution to interpret these experiments and suggest additional tests.
(a) A model
We assume that there is sexual conflict in the form of sexually antagonistic selection on mating rate (the shared trait). There are two potentially sexually antagonistic traits in males and females, and mating rate is determined by their interaction. The female trait is analogous to a pre-existing mating bias, and the male trait evolves because it can exploit this bias. Exaggeration of the male trait increases female mating rate, and thereby leads to selection on the female trait to evolve in such a way that mating rate is reduced. This is sexually antagonistic coevolution. We also vary the degree of natural selection on male and female traits, so that their evolution comes at some degree of cost. Mean female fitness is determined by the mean rate of mating in the population and the degree to which the female trait has evolved off its optimum. At the point of the initial exaggeration of the exploitative male trait, both male trait and the female trait are at their natural selection optima. We then follow the evolution of mean female fitness, mating rate, and the two sexually antagonistic traits in these hypothetical cases.
There is a single sexually antagonistic male trait, P, and two elements of the sexually antagonistic female trait, T and S. The trait, T, represents the threshold of a function that describes the relationship between the mean male trait in the population and female mating rate (figure 3a); as T increases, mating rate declines. The trait S describes the sensitivity (or slope) of female mating rate function to a change in the mean male trait (figure 3b). A decrease in S leads to decreased variation in the effect of different males traits on mating rate. We allow only one of these female traits to evolve in each case by imposing very strong natural selection on the other female trait. We have chosen to consider both kinds of female trait because early work demonstrated that they lead to very different dynamics of sexually antagonistic coevolution. Further details and analyses of the model can be found in Rowe et al. (2005). Here, we use these models to inform interpretation of past experiments and to suggest future experiments.
Figure 3.
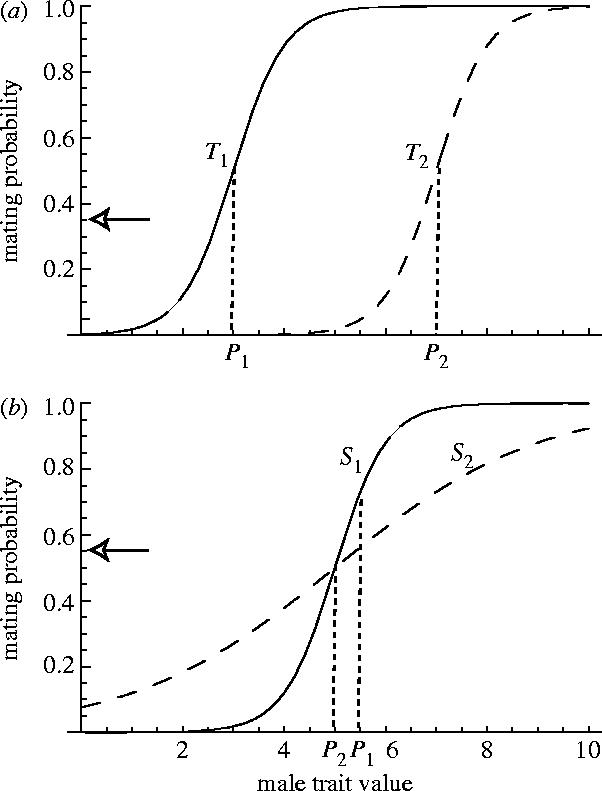
An illustration of the threshold and sensitivity of the female preference function and their coevolution with male sexually antagonistic traits. (a) Solid curve is meant to represent the state of the preference function T1 and the male trait P1 early in their coevolution. There is sexual conflict over mating rate, and T and P are sexually antagonistic traits. The preference function is constrained so that only its threshold can evolve. The optimal mating rate is indicated by the arrow, and it is below the rate achieved at T1 and P1. Therefore, we expect T1 to evolve towards T2. Because the preference exerts selection for exaggeration of P, we expect P1 to evolve towards P2. T and P are expected to continue this evolutionary trajectory until natural selection on either of them halts their joint evolution. (b) This is a similar representation as (a) except in this case the slope (sensitivity) of the preference function is allowed to evolve. At S1, P1, females are mating above their optimum (indicated by the arrow). Therefore, we expect females to reduce their sensitivity to the trait towards S2. This reduces the strength of selection on P and, therefore, we might expect evolution of P towards P2 if there is natural selection against large values of P2.
(b) Model results
To address the potential of sexually antagonistic coevolution for driving mean female fitness downward, we compare cases where either sensitivity or the threshold can evolve, and for each, when the cost of evolution of the female trait is low or high. Figure 4 depicts the cases, where the cost of antagonistic traits is low (natural selection on P and T or S is weak). Examination of the figure illustrates that, regardless of whether the female trait acts like a threshold (figure 4a) or a sensitivity (figure 4b), mean female fitness eventually declines following invasion of the male trait. These data match both verbal and earlier formal theories of sexually antagonistic coevolution (Holland & Rice 1998; Gavrilets et al. 2001). Mean female fitness declines in both cases because exaggeration of the male trait has moved mating rate further from the female optimum, and counter evolution of the female antagonistic trait has moved it off of its natural selection optimum. At equilibrium, females are bearing the costs of increased mating rate, and the costs of their sexually antagonistic trait not being at its natural selection optimum.
Figure 4.
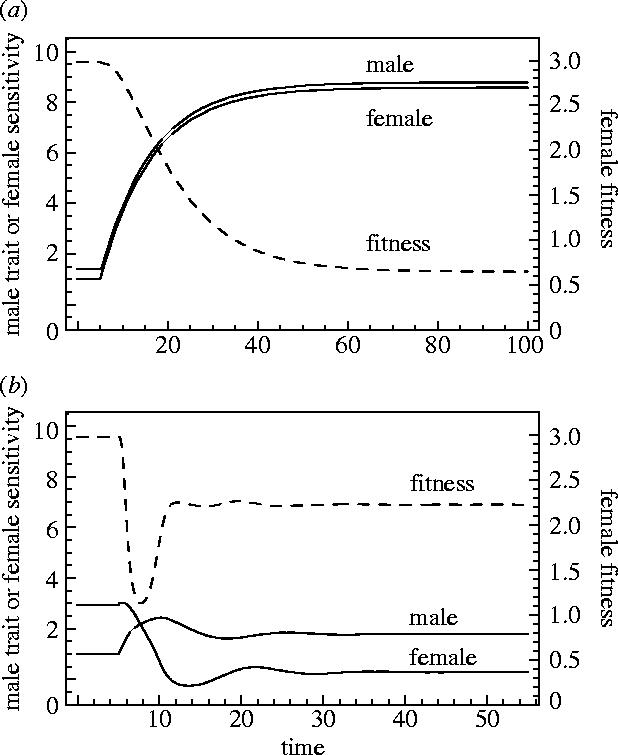
These panels represent coevolutionary trajectories described in §3a. In these cases, there is sexual conflict over mating rate and there is weak natural selection on male and female sexually antagonistic traits. Solid lines represent the trajectories of male and female sexually antagonistic traits, and the dashed line represents mean female fitness. In panel (a) the female sexually antagonistic trait is the threshold of the preference function (see figure 3a) and in (b) the female trait is the sensitivity of the preference function (see figure 3b). In panel (a), there is substantial exaggeration of male and female traits until they are halted by natural selection against further exaggeration. Female fitness is greatly reduced due to the costs of mating above their optimum and the costs of an exaggerated sexually antagonistic trait. Panel (b) shows a run of the model that is similar in starting conditions, except that the female trait is the sensitivity of the preference function. In contrast to (a), sexually antagonistic coevolution results in considerably less exaggeration of the sexually antagonistic traits, and reduction in mean female fitness.
There is, however, an important difference between the two cases in figure 4. In the case of an evolving threshold, the depression of female fitness is much greater than in the case of an evolving sensitivity. The reason for this is that, in the case of an evolving threshold, sexual selection on male antagonistic traits remains strong and can consequently exaggerate the trait a great deal before natural selection on the male trait halts its evolution. In contrast, when the female trait can be described as a sensitivity, evolution of the sensitivity (reduced slope) tends to decrease the strength of sexual selection on the male trait, and hence its evolutionary exaggeration.
In figure 5 we show runs of the model for exactly the same initial trait values and degree of sexually antagonistic selection on mating rate, but differing in having stronger natural selection on male and female antagonistic traits. Here, mean female fitness declines substantially whether the female trait acts like a threshold or sensitivity. The message from these analyses is that, in the presence of sexually antagonistic traits, the cost of sexual conflict to female fitness depends on both the nature of the female trait, and on the strength of selection on both male and female traits, and the shared trait.
Figure 5.
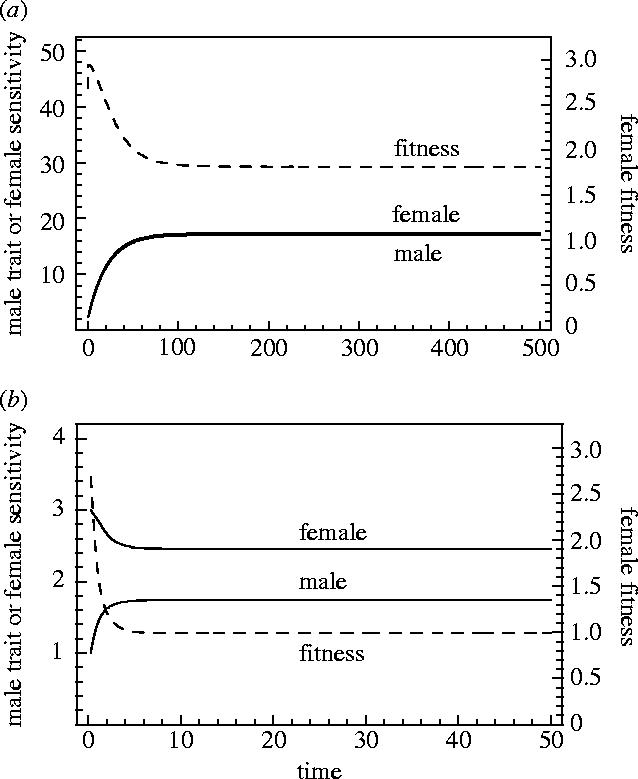
These panels represent coevolutionary trajectories described in §3a. In panel (a) the female antagonistic trait is the threshold of the preference function and in (b) it is the sensitivity of that function. In contrast to figure 4, in these cases natural selection on the sexually antagonistic traits is strong, and consequently there is less exaggeration of these traits. In both cases, there are substantial declines in female fitness.
These results suggest that tests aimed at detecting sexual conflict by contrasting mean fitness in lines evolved with and without the potential for sexual conflict may often fail in spite of the fact that sexual conflict is present in one of the lines. Indeed, laboratory experiments of this sort have yielded mixed results (Promislow et al. 1998; Holland & Rice 1999; Holland 2002; Martin & Hosken 2003; Crudgington et al. 2005). The extent to which conflict depresses fitness depends upon details of the nature of female antagonistic traits (threshold or sensitivity) and the extent of natural selection on them. Therefore, a greater understanding of female traits is required before a null result in such experiments can be interpreted as indicating the absence of sexual conflict in the ancestral lines.
It is also interesting to imagine conducting the experiments we advocated earlier to assay sexual conflict and sexually antagonistic traits at the equilibria of these four scenarios (figures 4 and 5). In all four cases, at equilibrium there is sexually antagonistic selection on mating rate—there is selection for increased mating rate in males and decreased mating rate in females. Therefore, in all cases, sexual conflict is detectable with the sorts of experiments discussed above. Likewise, in all four cases, manipulation of the female trait would reveal it as being a sexually antagonistic trait. Specifically, in two cases (figures 4a and 5a), increasing the threshold will lower mating rate and thereby increase female fitness. In two cases (figures 4b and 5b), decreasing the sensitivity (slope) will have a similar effect. However, the picture is somewhat more complex when testing for male sexually antagonistic traits. In the cases (figures 4a and 5a), where the female trait acts as a threshold, manipulations that increase the male trait will substantially increase mating frequency and thereby increase male fitness. Thus, the male trait will easily be identified as sexually antagonistic. However, in the cases where the female trait acts as a sensitivity (figures 4b and 5b), manipulation of the male trait may have very little effect on male fitness. This is because female sensitivity has evolved towards insensitivity, and here incremental changes in the male trait will have very little effect on mating rate. Notably, these conclusions apply to studies of natural populations as long as female antagonistic traits can take the forms outlined here.
In at least two of the contrasts of populations evolving in polyandry and monogamy, experimenters have gone on to measure the effect of males from contrasting lines on the fitness of females (Holland & Rice 1999; Martin & Hosken 2003). The experiments are aimed at testing the premise that, in the polyandrous lines, males and females should evolve exaggerated sexually antagonistic traits, and in the monogamous lines these traits should be reduced or lost. The data show some support for the premise. These experiments can be viewed as analogues of the sort of initial invasions of sexually antagonistic traits as depicted in figures 4 and 5. Males from the polyandrous lines possess a sexually antagonistic trait that can exploit a bias in females from the monogamous lines. This is the situation depicted at the early stages of figures 4 and 5. We expect these polyandrous males to reduce the fitness of females from monogamous lines simply because these females do not possess sexually antagonistic traits to resist the sexually antagonistic traits in males.
Although these studies are useful for revealing the existence of sexually antagonistic coevolution, they are less useful for evaluating the impact of sexual antagonism on female fitness. In particular, because they are essentially ‘invasion’ studies, they might greatly overestimate the costs of sexual conflict to females at equilibrium. This point is illustrated by inspection of figure 4b. When the male trait invades (analogous to the experiment), female fitness is substantially depressed, but counteradaptation by females eventually reduces this fitness cost. The same caution would apply if you could construct the same experiment in natural lines. Results from these empirical studies do demonstrate two important points about females. They suggest that females have traits that function like pre-existing sensory biases that can be exploited by males. Moreover, the fact that the bias evolves when sexual conflict is removed suggests that there is natural selection on the bias and sexual conflict moves the bias off its natural selection optima. These elements of the female validate key assumptions of our model and previous models (Gavrilets et al. 2001).
4. Discussion
A central message from this overview is that patterns emerging from a process of sexually antagonistic coevolution are unlikely to provide useful information for distinguishing this process from the variety of alternative male–female coevolutionary processes. Instead, we suggest that the defining processes, sexually antagonistic selection and sexually antagonistic traits in the system of interest, need to be studied first. This is not a new message to research in the broader field of male–female coevolution. For some time, it has been clear that distinguishing among more traditional processes of sexual selection (e.g. indirect and direct benefits) requires studies of selection on the preference itself (e.g. Kirkpatrick 1987; Kirkpatrick & Ryan 1991). Analyses presented here and elsewhere (Cameron et al. 2003; Rowe et al. 2003) suggest that these same studies are required to distinguish between these traditional models of sexual selection and sexual conflict.
There is some consensus that the outcome of a coevolutionary process driven by interlocus sexual conflict can result in the depression of female fitness and thereby population fitness (e.g. Rice 1998; Gavrilets et al. 2001; Cameron et al. 2003; Kokko & Brooks 2003; Pizzari & Snook 2003). This prediction has motivated a series of comparative studies of extant diversity when the potential for sexual conflict over evolutionary history is thought to be either high or low (e.g. Gage et al. 2002; Morrow & Pitcher 2003; Morrow et al. 2003), as well as a series of laboratory evolution studies comparing mean female fitness when the potential for sexual conflict is either high or low (Rice 1992; Promislow et al. 1998; Holland & Rice 1999; Holland 2002; Martin & Hosken 2003; Wigby & Chapman 2004; Crudgington et al. 2005). The comparative evidence does not support the hypothesis, and the laboratory evolution studies are mixed in support. Our analysis here suggests that depressions in mean fitness may be weak and transitory, and therefore, they will often be difficult to detect once an equilibrium is reached. Therefore, the absence of a detectable effect of sexual conflict on mean fitness does not provide a strong rationale for rejecting the hypothesis that sexual conflict has been an important factor during the evolutionary history of a lineage.
The difficulty in detecting depressions in mean fitness may be compounded when one includes other potentially co-occurring forces of selection. For example, earlier theory suggests that direct selection on male traits by female preference is likely to lead to the evolution of condition dependence of male traits near equilibrium, and therefore, to the potential for a good genes effect on the preference (Rowe & Houle 1996). This theory applies to female preference, whatever its origin, and therefore, also applies in the models discussed above. Earlier theory has also shown that the good genes process of sexual selection can lead to an evolutionary increase in female fitness (e.g. Agrawal 2001; Siller 2001; Lorch et al. 2003). In those cases where the costs to mean fitness of sexually antagonistic are modest (figure 4b), these good gene effects may obscure any cost to female fitness at equilibrium.
Although the lack of a substantial decrease in mean fitness is not necessarily indicative of the lack of sexual conflict, one might hope that a clear decrease in mean female fitness in the presence of sexual selection might, at least, be held as strong evidence for sexually antagonistic evolution. Unfortunately, however, this is not the case either. In fact, any process that leads to sexual selection on a trait expressed in both males and females has the potential to lead to reduced mean fitness ( Lande 1980, 1987). The reason for this is that, if there is an intersexual genetic correlation for the trait, then exaggeration in males due to sexual selection will lead to costly exaggeration in females. In fact, even models where direct benefits drive the evolution of female preference have shown that mean fitness can some times be depressed (Price et al. 1993). Therefore, we conclude that without additional studies first identifying sexual conflict and sexually antagonistic traits, changes in mean fitness are unlikely to allow us to distinguish among competing models of male–female coevolution.
Our results suggest that the most direct way to detect sexual conflict remains the study of the natural history of males and females. By this we mean studies aimed at determining whether sexually antagonistic selection on a shared trait is present, and whether sexually antagonistic traits exist in the population. Recent reviews suggest that there is considerable evidence of sexual conflict in nature, but less evidence for sexually antagonistic traits, particularly in females (Clutton-Brock & Parker 1995; Lessells 1999; Chapman et al. 2003a; Arnqvist & Rowe 2005). Studies of potentially sexually antagonistic traits could take two forms: correlative studies of selection and manipulative studies. Selection studies have the advantage of assessing the strength and sign of selection across trait variation that is expressed in the population. However, they also suffer from being correlative in nature, which makes conclusions about the true target of selection difficult. Manipulative studies that work within the range of natural variation address this problem, because they allow assessment of selection on the trait, or simply its function, independent of potentially correlated features of the phenotype.
In summary, our analyses of the effects of sexually antagonistic coevolution on mean female fitness suggest that accurate inference about the process of male–female coevolution is not possible from an evaluation of fitness patterns alone. This adds to a series of papers suggesting that patterns emerging during male–female coevolution do not indicate underlying process (e.g. Parker & Partridge 1998; Gavrilets 2000; Gavrilets & Waxman 2002; Rowe et al. 2003). Instead, we suggest that more research emphasis should be placed on the natural history of sexual conflict and sexually antagonistic traits. For both shared traits and sexually antagonistic traits, correlative studies of selection and manipulative studies of function are required.
Acknowledgments
We thank T. Chapman, T. Tregenza and N. Wedell for the opportunity to participate in the Royal Society of London meeting that led to this manuscript. We are grateful to Erin Cameron for much discussion. L.R. and T.D. were both supported by Natural Sciences and Engineering Research Council (NSERC) grants, Premiers Research Excellence Awards, and the Canada Research Chairs program.
Footnotes
One contribution of 13 to a Discussion Meeting Issue ‘Sexual conflict: a new paradigm?’.
References
- Agrawal A.F. Sexual selection and the maintenance of sexual reproduction. Nature. 2001;411:692–695. doi: 10.1038/35079590. 10.1038/35079590 [DOI] [PubMed] [Google Scholar]
- Andrés J.A, Arnqvist G. Genetic divergence of the seminal signal-receptor system in houseflies: the footprints of sexually antagonistic coevolution? Proc. R. Soc. B. 2001;268:399–405. doi: 10.1098/rspb.2000.1392. 10.1098/rspb.2000.1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist G. The evolution of water strider mating systems: causes and consequences of sexual conflicts. In: Choe J.C, Crespi B.J, editors. The Evolution of mating systems in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 146–163. [Google Scholar]
- Arnqvist G. Sexual conflict and sexual selection: lost in the chase. Evolution. 2004;58:1383–1388. doi: 10.1111/j.0014-3820.2004.tb01716.x. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Nilsson T. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 2000;60:145–164. doi: 10.1006/anbe.2000.1446. 10.1006/anbe.2000.1446 [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Princeton University Press; Princeton, NJ: 2005. Sexual conflict. [Google Scholar]
- Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002a;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Correlated evolution of male and female morphologies in water striders. Evolution. 2002b;56:936–947. doi: 10.1111/j.0014-3820.2002.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Bateman A.J. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Bergsten J, Töyrä A, Nilsson A. Intraspecific variation and intersexual correlation in secondary sexual characters of three diving beetles (Coleoptera: Dytiscidae) Biol. J. Linn. Soc. 2001;73:221–232. 10.1006/bijl.2001.0540 [Google Scholar]
- Björklund M, Senar J.C. Sex differences in survival selection in the serin, Serinus serinus. J. Evol. Biol. 2001;14:841–849. 10.1046/j.1420-9101.2001.00334.x [Google Scholar]
- Bonduriansky R, Rowe L. Intralocus sexual conflict and the genetic architecture of sexually dimorphic traits in Prochyliza xanthostoma (Diptera: Piophilidae) Evolution. 2005;59:1965–1975. [PubMed] [Google Scholar]
- Cameron E, Day T, Rowe L. Sexual conflict and indirect benefits. J. Evol. Biol. 2003;16:1055–1060. doi: 10.1046/j.1420-9101.2003.00584.x. 10.1046/j.1420-9101.2003.00584.x [DOI] [PubMed] [Google Scholar]
- Chapman T, Partridge L. Sexual conflict as fuel for evolution. Nature. 1996;381:189–190. doi: 10.1038/381189a0. 10.1038/381189a0 [DOI] [PubMed] [Google Scholar]
- Chapman T, Liddle L.F, Kalb J.M, Wolfner M.F, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. 10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003a;18:41–47. 10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Response to Eberhard and Cordero, and Córdoba-Aguilar and Contreras-Garduńo: sexual conflict and female choice. Trends Ecol. Evol. 2003b;18:440–441. 10.1016/S0169-5347(03)00179-4 [Google Scholar]
- Clark A.G, Begun D.J, Prout T. Female×male interactions in Drosophila sperm competition. Science. 1999;283:217–220. doi: 10.1126/science.283.5399.217. 10.1126/science.283.5399.217 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H, Parker G.A. Sexual coercion in animal societies. Anim. Behav. 1995;49:1345–1365. 10.1006/anbe.1995.0166 [Google Scholar]
- Cordero C, Eberhard W.G. Female choice of sexually antagonistic male adaptations: a critical review of some current research. J. Evol. Biol. 2003;16:1–6. doi: 10.1046/j.1420-9101.2003.00506.x. 10.1046/j.1420-9101.2003.00506.x [DOI] [PubMed] [Google Scholar]
- Crudgington H.S, Beckerman A.P, Brustle L, Green K, Snook R.R. Experimental removal and elevation of sexual selection: does sexual selection generate manipulative males and resistant females? Am. Nat. 2005;165:S72–S87. doi: 10.1086/429353. 10.1086/429353 [DOI] [PubMed] [Google Scholar]
- Eberhard W.G, Cordero C. Sexual conflict and female choice. Trends Ecol. Evol. 2003;18:438–439. doi: 10.1016/s0169-5347(00)89205-8. 10.1016/S0169-5347(03)00180-0 [DOI] [PubMed] [Google Scholar]
- Gage M.J.G, Parker G.A, Nylin S, Wiklund C. Sexual selection and speciation in mammals, butterflies and spiders. Proc. R. Soc. B. 2002;269:2309–2316. doi: 10.1098/rspb.2002.2154. 10.1098/rspb.2002.2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S. Rapid evolution of reproductive barriers driven by sexual conflict. Nature. 2000;403:886–889. doi: 10.1038/35002564. 10.1038/35002564 [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Waxman D. Sympatric speciation by sexual conflict. Proc. Natl Acad. Sci. USA. 2002;99:10 533–10 538. doi: 10.1073/pnas.152011499. 10.1073/pnas.152011499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S, Arnqvist G, Friberg U. The evolution of female mate choice by sexual conflict. Proc. R. Soc. B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. 10.1098/rspb.2000.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getty T. Chase-away sexual selection as noisy reliable signaling. Evolution. 1999;53:299–302. doi: 10.1111/j.1558-5646.1999.tb05357.x. [DOI] [PubMed] [Google Scholar]
- Halliday T, Arnold S.J. Multiple mating by females: a perspective from quantitative genetics. Anim. Behav. 1987;35:939–941. [Google Scholar]
- Härdling R, Smith H.G, Jormalainen V, Tuomi J. Resolution of evolutionary conflicts: costly behaviours enforce the evolution of cost-free competition. Evol. Ecol. Res. 2001;3:829–844. [Google Scholar]
- Holland B. Sexual selection fails to promote adaptation to a new environment. Evolution. 2002;56:721–730. doi: 10.1111/j.0014-3820.2002.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. 10.1073/pnas.96.9.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken D.J, Blanckenhorn W.U, Garner T.W.J. Heteropopulation males have a fertilization advantage during sperm competition in the yellow dung fly (Scathophaga stercoraria) Proc. R. Soc. B. 2002;269:1701–1707. doi: 10.1098/rspb.2002.2094. 10.1098/rspb.2002.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M. The evolutionary forces acting on female mating preferences in polygynous animals. In: Bradbury J.W, Andersson M.B, editors. Sexual selection: testing the alternatives. Wiley; Chichester, NY: 1987. pp. 67–82. [Google Scholar]
- Kirkpatrick M, Ryan M.J. The evolution of mating preferences and the paradox of the lek. Nature. 1991;350:33–38. 10.1038/350033a0 [Google Scholar]
- Koene J.M, Schulenburg H. Shooting darts: co-evolution and counter-adaptation in hermaphroditic snails. BMC Evol. Biol. 2005;5:25. doi: 10.1186/1471-2148-5-25. 10.1186/1471-2148-5-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H, Brooks R. Sexy to die for? Sexual selection and the risk of extinction. Ann. Zool. Fenn. 2003;40:207–219. [Google Scholar]
- Lande R. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution. 1980;34:292–305. doi: 10.1111/j.1558-5646.1980.tb04817.x. [DOI] [PubMed] [Google Scholar]
- Lande R. Genetic correlations between the sexes in the evolution of sexual dimorphism and mating preferences. In: Bradbury J.W, Andersson M.B, editors. Sexual selection: testing the alternatives. Wiley; Chichester, NY: 1987. pp. 83–94. [Google Scholar]
- Lessells C.M. Sexual conflict in animals. In: Keller L, editor. Levels of selection in evolution. Princeton University Press; Princeton, NJ: 1999. pp. 75–99. [Google Scholar]
- Long T.A.F, Montgomerie R, Chippindale A. Quantifying the gender load: can population crosses reveal interlocus sexual conflict? Phil. Trans. R. Soc. B. 2006;361 doi: 10.1098/rstb.2005.1786. 10.1098/rstb.2005.1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch P.D, Proulx S, Rowe L, Day T. Condition-dependent sexual selection can accelerate adaptation. Evol. Ecol. Res. 2003;5:867–881. [Google Scholar]
- Mackay T.F.C. The nature of quantitative genetic variation for Drosophila longevity. Mech. Ageing Dev. 2002;123:95–104. doi: 10.1016/s0047-6374(01)00330-x. 10.1016/S0047-6374(01)00330-X [DOI] [PubMed] [Google Scholar]
- Martin O.Y, Hosken D.J. Costs and benefits of evolving under experimentally enforced polyandry or monogamy. Evolution. 2003;57:2765–2772. doi: 10.1111/j.0014-3820.2003.tb01518.x. [DOI] [PubMed] [Google Scholar]
- Merilä J, Sheldon B.C, Ellegren H. Antagonistic natural selection revealed by molecular sex identification of nestling collared flycatchers. Mol. Ecol. 1997;6:1167–1175. 10.1046/j.1365-294X.1997.00295.x [Google Scholar]
- Morrow E.H, Gage M.J.G. The evolution of sperm length in moths. Proc. R. Soc. B. 2000;267:307–313. doi: 10.1098/rspb.2000.1001. 10.1098/rspb.2000.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow E.H, Pitcher T.E. Sexual selection and the risk of extinction in birds. Proc. R. Soc. B. 2003;270:1793–1799. doi: 10.1098/rspb.2003.2441. 10.1098/rspb.2003.2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow E.H, Pitcher T.E, Arnqvist G. No evidence that sexual selection is an ‘engine of speciation’ in birds. Ecol. Lett. 2003;6:228–234. 10.1046/j.1461-0248.2003.00418.x [Google Scholar]
- Nilsson T, Fricke C, Arnqvist G. Patterns of divergence in the effects of mating on female reproductive performance in flour beetles. Evolution. 2002;56:111–120. doi: 10.1111/j.0014-3820.2002.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sexual selection and sexual conflict. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; New York: 1979. pp. 123–166. [Google Scholar]
- Parker G.A, Partridge L. Sexual conflict and speciation. Phil. Trans. R. Soc. B. 1998;353:261–274. doi: 10.1098/rstb.1998.0208. 10.1098/rstb.1998.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Hurst L.D. Sexual conflict. Science. 1998;281:2003–2008. doi: 10.1126/science.281.5385.2003. 10.1126/science.281.5385.2003 [DOI] [PubMed] [Google Scholar]
- Pitnick S, Markow T, Spicer G.S. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution. 1999;53:1804–1822. doi: 10.1111/j.1558-5646.1999.tb04564.x. [DOI] [PubMed] [Google Scholar]
- Pizzari T, Snook R.R. Perspective: sexual conflict and sexual selection: chasing away paradigm shifts. Evolution. 2003;57:1223–1236. doi: 10.1111/j.0014-3820.2003.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Pizzari T, Snook R.R. Sexual conflict and sexual selection: measuring antagonistic coevolution. Evolution. 2004;58:1389–1393. [Google Scholar]
- Presgraves D.C, Baker R.H, Wilkinson G.S. Coevolution of sperm and female reproductive tract morphology in stalk-eyed flies. Proc. R. Soc. B. 1999;266:1041–1047. 10.1098/rspb.1999.0741 [Google Scholar]
- Price D.K, Burley N.T. Constraints on the evolution of attractive traits: selection in male and female zebra finches. Am. Nat. 1994;144:908–934. 10.1086/285718 [Google Scholar]
- Price T, Schluter D, Heckman N.E. Sexual selection when the female directly benefits. Biol. J. Linn. Soc. 1993;48:187–211. 10.1006/bijl.1993.1014 [Google Scholar]
- Promislow D.E.L, Smith E.A, Pearse L. Adult fitness consequences of sexual selection in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 1998;95:10 687–10 692. doi: 10.1073/pnas.95.18.10687. 10.1073/pnas.95.18.10687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R. Sex-chromosomes and the evolution of sexual dimorphism. Evolution. 1984;38:735–742. doi: 10.1111/j.1558-5646.1984.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Rice W.R. Sexually antagonistic genes: experimental evidence. Science. 1992;256:1436–1439. doi: 10.1126/science.1604317. [DOI] [PubMed] [Google Scholar]
- Rice W.R. Intergenomic conflict, interlocus antagonistic coevolution, and the evolution of reproductive isolation. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University Press; New York: 1998. pp. 261–270. [Google Scholar]
- Rice W.R, Chippindale A.K. Intersexual ontogenetic conflict. J. Evol. Biol. 2001;14:685–693. 10.1046/j.1420-9101.2001.00319.x [Google Scholar]
- Rice W.R, Holland B. Reply to comments on the chase-away model of sexual selection. Evolution. 1999;53:302–306. doi: 10.1111/j.1558-5646.1999.tb05358.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal G.G, Servedio M.R. Chase-away sexual selection: resistance to ‘resistance’. Evolution. 1999;53:296–299. doi: 10.1111/j.1558-5646.1999.tb05356.x. [DOI] [PubMed] [Google Scholar]
- Rowe L, Arnqvist G. Sexually antagonistic coevolution in a mating system: combining experimental and comparative approaches to address evolutionary processes. Evolution. 2002;56:754–767. doi: 10.1111/j.0014-3820.2002.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. B. 1996;263:1415–1421. [Google Scholar]
- Rowe L, Arnqvist G, Sih A, Krupa J. Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol. Evol. 1994;9:289–293. doi: 10.1016/0169-5347(94)90032-9. 10.1016/0169-5347(94)90032-9 [DOI] [PubMed] [Google Scholar]
- Rowe L, Cameron E, Day T. On detecting sexually antagonistic coevolution with population crosses. Proc. R. Soc. B. 2003;270:2009–2016. doi: 10.1098/rspb.2003.2453. 10.1098/rspb.2003.2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L, Cameron E, Day T. Escalation and retreat during sexually antagonistic coevolution. Am. Nat. 2005;165(S5):5–18. doi: 10.1086/429395. 10.1086/429395 [DOI] [PubMed] [Google Scholar]
- Shuster S.M, Wade M.J. Princeton University Press; Princeton, NJ: 2003. Mating systems and strategies. [Google Scholar]
- Siller S. Sexual selection and the maintenance of sex. Nature. 2001;411:689–692. doi: 10.1038/35079578. 10.1038/35079578 [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aladine; Chicago: 1972. pp. 136–179. [Google Scholar]
- Vieira C, Pasyukova E.G, Zeng Z.B, Hackett J.B, Lyman R.F, Mackay T.F.C. Genotype–environment interaction for quantitative trait loci affecting life span in Drosophila melanogaster. Genetics. 2000;154:213–227. doi: 10.1093/genetics/154.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Female resistance to male harm evolves in response to manipulation of sexual conflict. Evolution. 2004;58:1028–1037. doi: 10.1111/j.0014-3820.2004.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. 10.1016/j.cub.2005.01.051 [DOI] [PubMed] [Google Scholar]


