Abstract
Land plants possess a multicellular diploid stage (sporophyte) that begins development while attached to a multicellular haploid progenitor (gametophyte). Although the closest algal relatives of land plants lack a multicellular sporophyte, they do produce a zygote that grows while attached to the maternal gametophyte. The diploid offspring shares one haploid set of genes with the haploid mother that supplies it with resources and a paternal haploid complement that is not shared with the mother. Sexual conflict can arise within the diploid offspring because the offspring's maternal genome will be transmitted in its entirety to all other sexual and asexual offspring that the mother may produce, but the offspring's paternally derived genes may be absent from these other offspring. Thus, the selective forces favouring the evolution of genomic imprinting may have been present from the origin of modern land plants. In bryophytes, where gametophytes are long-lived and capable of multiple bouts of asexual and sexual reproduction, we predict strong sexual conflict over allocation to sporophytes. Female gametophytes of pteridophytes produce a single sporophyte and often lack means of asexual reproduction. Therefore, sexual conflict is predicted to be attenuated. Finally, we explore similarities among models of mate choice, offspring choice and segregation distortion.
Keywords: alternation of generations, genomic imprinting, developmental selection, Coleochaete, bryophytes, pteridophytes
1. Introduction
In the evolutionary lineage of land plants, ‘maleness’ and ‘femaleness’ are properties of haploid, rather than diploid, individuals. In this kind of life cycle, haploid individuals, known as gametophytes (‘gamete-plants’), produce genetically identical gametes by mitosis (figure 1a). A distinctive feature of land plants is that eggs are fertilized while still attached to the maternal gametophyte and the resulting zygote undergoes a series of mitotic divisions to produce a diploid embryo. Hence, members of the clade that includes modern land plants are known as embryophytes (Kenrick & Crane 1997). The diploid plant that develops from the zygote (via the embryo) produces haploid spores by meiosis and is known as a sporophyte (‘spore-plant’). In a genetic sense, the sporophyte is an intimate union of the gametophytes that produced the egg and sperm that fused to form the zygote. Thus, conflicts between male and female haploid ‘interests’ may be played out both before and after gamete fusion.
Figure 1.
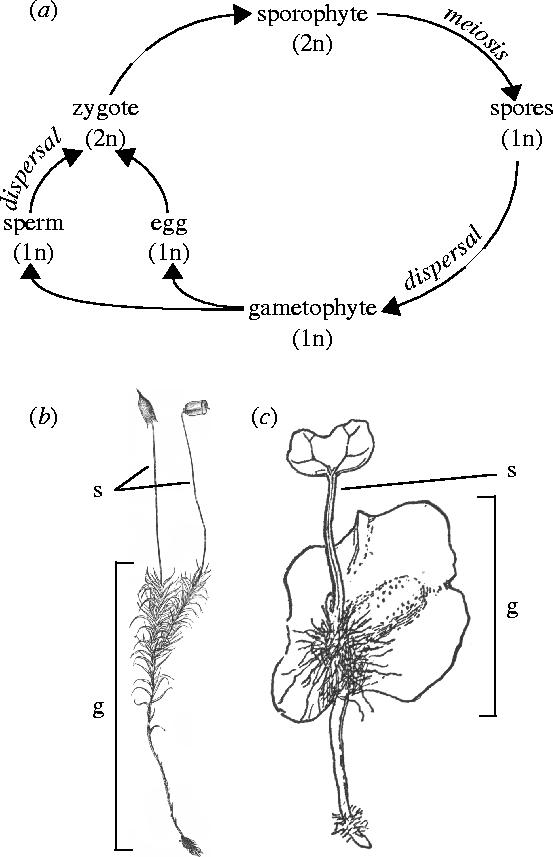
Alternation of generations in plants. (a) Schematic of the stages in the life cycle of plants showing the alternation between haploid and diploid forms. (b) The haploid gametophyte of the moss Polytrichum commune, a bryophyte, with two dependent diploid sporophytes. (c) The haploid gametophyte of the fern Osmunda claytonia, a pteridophyte, showing its attached sporophytic offspring that has rooted and begun its transition to an independent existence. g, gametophyte; s, sporophyte. ((b) after Goebel (1905) and (c) after Campbell (1905).)
Embryophytes are derived from freshwater charophycean algae. Among the extant members of this assemblage, the charalean algae and embryophytes appear to be each other's closest relatives, with Coleochaete and its relatives as a sister-group to the charalean/embryophyte clade (Karol et al. 2001). An implication of this phylogeny is that embryophytes were derived from algal ancestors with a multicellular haploid phase but without a multicellular diploid phase. If so, sporophytes must have originated early in the evolution of land plants by the interpolation of mitotic divisions between syngamy and meiosis to produce a multicellular diploid individual. We do not have details of ancestral life cycles but we can look at modern forms, with similar life cycles, to make inferences about selective forces, particularly those arising from sexual conflict, that may have been operating during the appearance of the earliest sporophytes.
Among extant embryophytes, three broad classes of life cycle can be recognized. These correspond to the traditional botanical groupings of bryophytes, pteridophytes, and seed plants. Of these groupings, only seed plants are monophyletic. Pteridophytes are derived from within bryophytes and seed plants are derived from within pteridophytes (Kenrick & Crane 1997). We will address sexual conflicts in the life cycles of Coleochaete (chosen as an outgroup), bryophytes and pteridophytes. There is a great diversity of life history traits within each of these groups. To pick just one example, among mosses (a major group of bryophytes), a mature sporophyte of Archidium alternifolium releases 16 spores, each 200 μm in diameter, whereas a mature sporophyte of Dawsonia lativaginata releases about 50 million spores, each 8 μm in diameter (Kreulen 1972). We do not have the space to review this fascinating diversity so our discussion will be limited to broad generalizations, with the understanding that for every generalization there are many exceptions.
2. Coleochaete
The multicellular haploid phase of the Coleochaete life cycle is initiated when a flagellated cell (zoospore) settles on a suitable substrate and commences mitotic divisions to form a gametophyte. There is variation among Coleochaete species with regard to sex expression. For example, gametophytes of Coleochaete scutata are dioicous; eggs and sperm are produced by different haploid individuals (Wesley 1930). By contrast, gametophytes of Coleochaete nitellarum are monoicous; a single individual produces both eggs and sperm (Lewis 1907). In either case, eggs remain attached to the maternal gametophyte. (Dioicy and monoicy are used to describe sex expression of haploid individuals in contrast to dioecy and monoecy, which are properties of diploid individuals. Thus, all angiosperm gametophytes are dioicous but angiosperm sporophytes may be monoecious or dioecious (Zander 1985).)
A zygote of Coleochaete is formed when an egg is fertilized by an antherozoid (sperm) released by a paternal gametophyte. Zygotes undergo substantial post-fertilization growth as they accumulate nutrients supplied by the maternal gametophyte. In C. scutata and its relatives, cells of the maternal gametophyte grow around and completely surround the zygote to produce a corticated zygospore that exhibits substantial post-fertilization growth (Graham & Wilcox 1983; Delwiche et al. 2002). Multiple zygospores may be present simultaneously on a female gametophyte, but not all eggs produce mature zygospores. Wesley (1930) reported that disintegrating ‘eggs’ were common on gametophytes of C. scutata (she assumed that fertilization had not occurred although it is possible that these were aborted zygotes). After a period of dormancy, the zygospore undergoes a series of divisions to produce 8–32 cells, enclosed within the zygospore wall that is still attached to the maternal gametophyte. All of these cells are released as zoospores that disperse to initiate new gametophytes.
The nature of the divisions of the zygospore is not fully understood. Allen (1905) observed pronounced differences in the compaction of chromosomes between the first and second divisions of C. scutata zygospores and identified these as the heterotypic and homotypic divisions of chromosome reduction (i.e. meioses I and II). According to this interpretation, zygotic meiosis is followed by one or more mitotic divisions to produce 8–32 zoospores. Hopkins & McBride (1976) undertook a photometric analysis of DNA content in C. scutata. Gametophytic nuclei contained either the 1C or 2C amount of DNA (by reference to the 1C amount of sperm); however, zygotic nuclei contained from the 2C to 8C amount of DNA. These data are compatible with a 2C zygote undergoing two rounds of DNA replication without cell division, followed by reduction from 8C to 1C over the course of three divisions without DNA replication. Whatever the nature of the postzygotic divisions, the 8–32-celled ‘organism’ that forms within the Coleochaete zygospore does not appear to be homologous to the diploid sporophyte of embryophytes.
Sexual reproduction is not the only option available to Coleochaete gametophytes. Vegetative cells can escape their cell walls, develop flagellae, and become zoospores that disperse to establish new gametophytes. In some cases, almost all cells of a gametophyte can transform into zoospores, leaving a skeleton of empty cell walls (Wesley 1928). Thus, maternal gametophytes can produce dispersing propagules through either sexual or asexual means. This life cycle clearly illustrates the ‘twofold cost of sex’ for the genes of a maternal gametophyte. Each gene of the maternal gametophyte is present in only half of the zoospores released from the zygospore wall (sexual progeny) but is present in every zoospore released from a vegetative cell wall (asexual progeny). Thus, asexual reproduction is twice as efficient as sexual reproduction at producing zoospores carrying maternal genes under the assumption that the two kinds of zoospore are equally costly.
Female gametophytes of Coleochaete are faced with the adaptive problem of how much to invest in vegetative growth and asexual reproduction versus sexual reproduction. After fertilization, the additional question arises of how to divide maternal care among multiple zygospores. Male gametophytes are faced with the somewhat simpler problem of allocating resources among asexual reproduction, sexual reproduction (sperm), and vegetative growth. What then is the potential for sexual conflict in the Coleochaete life cycle? If male and female gametophytes are not direct competitors for substrate-space, then their direct interactions are limited to fertilization of eggs by sperm. Females could perhaps exhibit some selectivity in which sperm fertilize their eggs. If so, non-favoured males might be selected to circumvent female barriers to fertilization. However, the major potential for sexual conflict is likely to occur after fertilization in relations between zygospores and maternal gametophytes.
In a genetic sense, the zygospore nucleus contains an intimate union of the haploid genomes of a male and female gametophyte. The genetic interests of the maternal genome are identical with those of the female gametophyte that is providing nutrients to the zygospore. Therefore, maternal genes of a zygospore should favour that distribution of maternal resources among vegetative growth, asexual reproduction, and investment in multiple zygospores that maximizes fitness of the maternal gametophyte. By contrast, the paternal genome of the zygospore will usually be unrelated to the maternal gametophyte (in dioicous species) and may also be unrelated to the paternal genomes of competing zygospores. If so, paternal genomes should favour maximizing the reproductive return from a single fertilization event (their own).
There is potential for sexual conflict at two stages during zygospore maturation. The first of these occurs when a decision is made whether or not to provision a particular zygote. Maternal gametophytes can practice postzygotic ‘mate choice’ by the selective abortion of zygotes (Willson & Burley 1983; Queller 1994). Paternal genomes of zygotes clearly have an interest in avoiding abortion, whereas maternal genomes would concur with the choice of the maternal gametophyte. At this stage, the interests of maternal and paternal genomes coincide if their zygote is chosen, but not if their zygote is aborted. The second stage at which there is potential for sexual conflict is during provisioning of the zygote. The paternal genome of the zygote will favour a higher level of provisioning than the maternal genome because it has less genetic interest in alternative uses of resources by the maternal gametophyte, whether these be investment in vegetative growth, asexual reproduction, or in other zygotes (with an identical maternal genome but, in some cases at least, with different paternal genomes). The level of postzygotic provisioning has clear consequences for paternal fitness: small mature zygospores of Coleochaete pulvinata gave rise to only eight zoospores, whereas larger zygospores produced up to 32 zoospores (Oltmanns 1898).
The production of zygospores and of asexual zoospores compete for limited maternal resources. Therefore, conflict between the maternal and paternal genomes of zygospores requires multiple haploid paternity of the diploid offspring provisioned by a haploid maternal genotype but does not require multiple haploid paternity of the zygospores growing on a particular haploid mother (although this would accentuate the conflict). That is, conflict requires multiple paternity at the level of the genet but not at the level of the ramet. In species with extensive asexual reproduction, this is not a trivial distinction. Conflict between maternal and paternal genomes of zygospores may be possible even when there is low genetic variation at the small spatial scale available for mate sampling by any single haploid ramet.
3. Bryophytes
The ‘bryophytic’ life cycles of liverworts, mosses and hornworts resemble that of Coleochaete in that the dominant multicellular phase is the haploid gametophyte and there is postzygotic provisioning of diploid offspring. Fertilization in bryophytes is effected by motile sperm swimming from paternal gametophytes to eggs retained on maternal gametophytes, and typically takes place over small distances (usually millimetres to centimetres; Korpelainen et al. 2005). Unlike Coleochaete, the zygote undergoes several rounds of mitotic divisions to produce a diploid sporophyte that is nutritionally dependent on the maternal gametophyte. The sporophyte produces a single sporangium that is raised above the substrate. Meiosis takes place within the sporangium to produce large numbers of haploid spores that are (in most cases) aerially dispersed. The sporophyte dies after releasing its spores, but maternal gametophytes usually survive the death of their diploid offspring and may continue to reproduce both sexually and asexually (see figure 1b). Spores germinate, either immediately or after variable periods of dormancy, to form new gametophytes.
The adaptive problem facing bryophyte gametophytes is the optimal allocation of resources among vegetative growth, asexual reproduction, and sexual reproduction. Gametophytes of a substantial minority of bryophyte species produce gemmae, specialized structures for asexual dispersal. Other species undergo asexual dispersal by fragmentation. A female gametophyte may provision multiple sporophytes over the course of her life, but not all sporophytes are provisioned and abortion rates may be high (e.g. Stark & Stephenson 1983; Stark et al. 2000; Stark 2001, 2002).
The basic features of sexual conflict in this life cycle have already been delineated in our previous discussion of Coleochaete. The diploid sporophyte is nutritionally dependent on a maternal gametophyte that is genetically identical to the maternal haploid genome of the sporophyte (figure 1a,b). By contrast, the paternal haploid genome of an outcrossed sporophyte will be genetically unrelated to the maternal gametophyte. Therefore, paternal alleles will have been strongly selected to avoid abortion and to increase the nutrients acquired from the maternal gametophyte, even when these actions do not maximize maternal fitness.
Somewhat more than half of all bryophytes are dioicous, with eggs and sperm produced on separate gametophytes, the remainder are monoicous (eggs and sperm produced on the same gametophyte) or reproduce exclusively by asexual means (Longton & Schuster 1983). The mechanism of sex determination of dioicous gametophytes (when known) is genetic: two spores in each meiotic tetrad germinate to produce female gametophytes and two spores germinate to produce male gametophytes. Sex chromosomes have been reported from several dioicous species: female gametophytes carry an X chromosome; male gametophytes carry a Y chromosome; all sporophytes have an XY karyotype (Smith 1978; Ramsay & Berrie 1982).
X chromosomes are restricted to female gametophytes and are always maternally derived in sporophytes. Conversely, Y chromosomes are restricted to male gametophytes and are always paternally derived in sporophytes. Therefore, sporophytically expressed genes on the differential region of the X chromosome are predicted to promote maternal interests, whereas sporophytically expressed genes on the differential region of the Y chromosome are predicted to promote paternal interests. By contrast, autosomal genes (including pseudoautosomal genes from the recombining portion of X and Y chromosomes, if such exist) have spent half of their haploid history in female gametophytes and half in male gametophytes, and have spent half of their diploid history as maternally derived genes and half as paternally derived genes. If autosomal genes of different parental origin are to express conflicting interests in sporophytes, this would require genomic imprinting.
Sporophyte production is often rare in dioicous bryophytes because of spatial segregation of the sexes (e.g. Gemmell 1950). That is, male and female gametophytes do not occur together within the limited fertilization range of sperm. Spatial segregation is probably, in large part, a consequence of the population dynamics of sessile clonally reproducing organisms. As one clone competitively excludes other clones from a local area, it also excludes potential mates (McLetchie et al. 2002). The rarity of sporophytes in some dioicous species suggests that, when sporophytes are produced, the local mating population may often be small. If all available sperm are produced by a single male gametophyte, all sporophytes formed on a female gametophyte will have identical genotypes. If the local mating population contains two haploid males, a haploid female's diploid offspring will have at most two genotypes, and so on. Sporophytes are considerably more common on monoicous gametophytes than on dioicous gametophytes, suggesting that the predominant mode of sexual reproduction in monoicous species is by gametophytic self-fertilization (Gemmell 1950). The diploid products of haploid self-fertilization are homozygous at all loci, excepting new mutations, and produce genetically uniform haploid products after meiosis. The twofold cost of sex is absent, but so too are most of the putative advantages of sexual recombination.
Monoicous species are often derived from dioicous species via polyploidy (Smith 1978). Although it has been assumed that these are autopolyploids, more recent data suggest that many, perhaps all, polyploid bryophytes are allopolyploids (Boisselier-Dubayle & Bischler 1998). A scenario suggests itself for the origin of monoicous allopolyploids. If the dioicous haploid species had sex chromosomes and produced XY sporophytes, then a first-generation hybrid sporophyte that underwent chromosome doubling would have two X chromosomes from one species and two Y chromosomes from the other. If the X chromosomes paired during meiosis, as did the Y chromosomes, then segregation of an X and Y to each spore would result in diploid XY gametophytes that expressed characters of both sexes. This predicted pattern of sex chromosome segregation in allopolyploids could explain both the transition from dioicy to monoicy upon chromosome doubling as well as the lack of male (YY) and female (XX) diploid gametophytes within the newly formed polyploid population (cf. Smith 1978 who assumed monoicous species were autopolyploids).
A major difference between the life cycle of Coleochaete and that of bryophytes is the intercalation of several mitotic divisions between syngamy and reduction to haploidy, resulting in a multicellular diploid sporophyte. The effect is to amplify the products of a single fertilization event. A Coleochaete zygospore gives rise to up to 32 zoospores and perhaps eight recombinant haploid genotypes (assuming reduction from the 8C level in three divisions). The sporophyte of a moss typically produces 100 000 or more spores and (in dioicous species) many haploid recombinant genotypes. In both groups, the paternal haploid genome of diploid offspring would be selected to make the most of each fertilization event, so the (four orders of magnitude) difference in output can be conjectured to be due to different degrees of acquiescence in diploid expansion by maternal haploid genomes.
An appealing explanation for the elaboration of a sporophyte by land plants is that this was an evolutionary response to the relative rarity of fertilization in terrestrial environments (Searles 1980) and to a relatively small number of haploid males in local mating populations when fertilization did occur. (If a mating population contains multiple haploid males, a female gametophyte would produce a greater diversity of recombinant haploid offspring by spreading her reproductive investment over multiple products of fertilization, rather than investing the same amount in a single sporophyte.)
4. Pteridophytes
The ‘pteridophytic’ life cycles of lycophytes, psilophytes, horsetails, and ferns are characterized by a sporophyte that is initially dependent on the maternal gametophyte but that then attains nutritional independence (figure 1c). Sporophytes are branched structures that produce large numbers of spores in multiple sporangia. These spores are typically larger than spores of most bryophytes (D. Haig 1989, unpublished work). Sporophytes are often long-lived perennials that, in some species, are able to undergo extensive clonal spread. In a reversal of ‘bryophytic’ life cycles, it is the sporophyte that usually outlives its maternal gametophyte. Competition for space largely occurs in the sporophytic rather than in the gametophytic generation.
Genetic determination of gametophytic sex is unknown in pteridophytes. Gametophytes of homosporous species have labile sex determination and may produce eggs or sperm (or both) depending on environmental conditions (Haig & Westoby 1988a). Such gametophytes could be described as potentially monoicous but often functionally dioicous. Heterosporous species produce large megaspores that develop into female gametophytes and small microspores that develop into male gametophytes. Although these gametophytes are strictly dioicous, sex determination is not genotypic because the two kinds of spores are produced from different sporangia of a single sporophyte (Haig & Westoby 1988b). Our focus will be on the majority of species with homosporous life cycles.
Mature sporophytes are usually much larger, much taller, and longer-lived than gametophytes. Moreover, there are likely to be strong priority effects in competition for space among sporophytes. That is, once a region of space has been occupied by a sporophyte, that individual is able to exclude younger (and smaller) sporophytes from its space. As a consequence, gametophytes compete in small local populations, in temporarily available space, to become the haploid parents of a successful sporophyte (Haig & Westoby 1988a). By contrast, gametophytes are the long-term occupiers of space for most bryophytes.
Competition among gametophytes to be the haploid parents of a sporophyte that then dominates a local area may help to explain the absence of (genetically) dioicous gametophytes in pteridophytes. Within a local population, one gametophyte is going to produce the egg that gives rise to a successful sporophyte and one gametophyte (sometimes the same gametophyte) is going to produce the sperm. The successful maternal gametophyte is likely to be the gametophyte with the most resources to invest in a sporophyte either because its spore arrived first, and it had a head-start in growth, or because its spore germinated in a particularly favourable microhabitat. Success as a paternal gametophyte means being in the right place at the right time to produce the sperm that fertilizes a successful egg. Labile sex determination allows gametophytes to keep their sexual options open, to reproduce as a male or female depending on local conditions. If a gametophyte has a head-start in growth over its local competitors, its best option is to attempt to be a maternal parent. Other gametophytes should take their chances in the fertilization lottery (Haig & Westoby 1988a).
Chemical signals are produced by ‘female’ gametophytes of some ferns that signal their presence and induce nearby gametophytes to develop as ‘males’ (reviewed by Haig & Westoby 1988a). The evolution of such signalling systems raises some interesting evolutionary questions that remain largely unexplored. What keeps the signalling system honest? Could a gametophyte who aspires to be the mother of the sporophyte that eventually occupies a site ‘bluff’ her potential competitors into reproducing as males? Whether such games are considered an example of sexual conflict, or of something else, is a question of semantics.
Maternal gametophytes typically invest in a single sporophyte, even though more than one egg may be fertilized (Buchholz 1922). Considering the size and longevity of the pteridophyte sporophyte, this strategy makes sense from an evolutionary perspective. Only one sporophyte is likely to become established at a site and a haploid mother may be in competition with other haploid mothers to produce this sporophyte. If a female were to invest in more than one sporophyte, her diploid offspring would compete with each other for resources and would run the risk of losing the race for local dominance with the offspring of another female that invested everything in a single offspring (Haig & Westoby 1988a). Once a maternal gametophyte commits herself to investment in a particular sporophyte, her interests are served by maximizing the growth of this sporophyte and these interests are identical with those of the paternal genome of the sporophyte. Sexual conflict largely disappears. The potential for postzygotic conflict between maternal and paternal genomes in pteridophytes may be limited to the choice of which sporophyte to provision if multiple eggs are fertilized.
Most pteridophytes lack mechanisms of asexual reproduction by gametophytes. This may be an indirect consequence of sporophytes being more effective than gametophytes as colonizers of new space. About 10% of pteridophyte species, however, have gametophytes that reproduce vegetatively via gemmae (Farrar 1974). In these taxa, maternal gametophytes are faced with a question of how much to invest in asexual versus sexual reproduction. Thus, the maternal and paternal genomes of sporophytes may ‘disagree’ over maternal investment in asexual reproduction. Gemma-producing species sometimes exist as populations that are dominated by gametophytes rather than sporophytes (e.g. Dassler & Farrar 1997) and have on multiple occasions given rise to gametophyte-only populations that reproduce clonally (Farrar 1967, 1990).
5. Genomic imprinting in plant life cycles
The conventional assumption in evolutionary biology has been that maternal and paternal haploid genomes have identical expression in diploid individuals. If this were the case, genes would evolve levels of expression (and phenotypic effects) that are a compromise between maternal and paternal interests. Thus, genetic expression in a zygospore of Coleochaete or the sporophyte of a moss would be selected to favour greater uptake of resources than the maternal gametophyte would be selected to supply. This could be considered an expression of conflict between the haploid parent and its diploid offspring. However, it is possible that a gene could show different patterns of expression in the diploid offspring depending on its parental origin (see Willson & Burley 1983, pp. 19-20, for a prescient discussion of this possibility). In this case, differential gene expression within a diploid offspring could be considered an extension of conflict between its male and female haploid parents. Parent-specific gene expression (genomic imprinting) has been described in the endosperm of flowering plants (Haig & Westoby 1989, 1991; Gehring et al. 2004), but the forces favouring imprinting are also present in any life cycle in which diploid offspring are nourished by a haploid mother. Therefore, we conjecture that genomic imprinting may already have been expressed in zygotes of the ancestors of land plants.
An intriguing, although difficult-to-test, possibility is that imprinting played a role in the origin of sporophytes, entailing (as this did) the intercalation of extra mitotic divisions between syngamy and meiosis. The paternal genome of a zygote would clearly have benefited from increasing the number of sexual propagules produced from a single fertilization event, particularly if this increased the amount of resources committed to the diploid offspring by the maternal gametophyte, whereas the maternal genome would have had competing interests in the allocation of resources to other zygotes and to asexual propagules. Thus, paternally expressed genes might have been responsible for the initiation of post-fertilization mitosis (for a similar argument see Trivers & Burt 1999). From a macroevolutionary perspective, however, the evolutionary success of bryophytic life cycles on land implies an evolutionary advantage to lineages in which maternal haploid genomes acquiesced in the elaboration of a multicellular sporophyte. As we have argued above, the important selective factor may have been the relative rarity of opportunities for fertilization in terrestrial environments.
Imprinted genes employ a conditional strategy, exhibiting one pattern of sporophytic expression when derived from a female gametophyte and a different pattern when derived from a male gametophyte. Therefore, genomic imprinting in bryophytes (if present) would be an autosomal phenomenon because X-linked genes are never present in male gametophytes and Y-linked genes are never present in female gametophytes. All genes in pteridophytes are autosomal, but in these plants the occurrence of genomic imprinting may be limited by an absence of sexual conflict during the provisioning of a sporophyte, because both haploid genomes are committed to the production of a single diploid offspring (see above). If imprinting is to be found in pteridophytes, theory would suggest that this either would affect early embryonic development when maternal gametophytes ‘choose’ which sporophyte to provision or would occur in species with well-developed asexual reproduction by gametophytes.
6. Investment choices in plant life cycles
We have identified two stages at which there is potential for postzygotic sexual conflict during sporophyte development. The first occurs when a choice is made whether or not to provision a sporophyte. The second occurs after this choice is made and concerns how much to invest in the sporophyte. The nature of conflict at each of these stages has distinctive features.
Maternal gametophytes regularly produce more zygotes/embryos than ever develop into mature sporophytes. The selective culling of surplus embryos could, therefore, provide an opportunity for improving the average quality of the subset of diploid offspring that are provisioned (Buchholz 1922; Haig 1987; Stearns 1987). The idea is attractive, but it raises the questions of how the process of culling could be designed to select for superior quality offspring (good genes); of whether the selective process is vulnerable to genotypes that are merely good at being chosen, independent of their intrinsic quality (manipulation); and of whether genes in mothers would benefit from being associated with genes that are merely good at being chosen (run-away). The similarity between mate choice and offspring choice has been noted before (Willson & Burley 1983; Queller 1994). In both situations, genes in females are choosing which set of male-derived genes to recombine with at meiosis in the next diploid generation.
Non-random ‘choice’ of males can result in either sexual conflict (in which one sex presumably benefits at the expense of the other) or benefits to both sexes, and the relative importance of these two processes is the centre of an ongoing debate (e.g. Pizzari & Snook 2003; Eberhard 2005). We have found an analogy from everyday experience to be useful in thinking about this question. In situations of choice, the relationship between the sexes (or between maternal and paternal genomes in sporophytes) is analogous to that between examiner and examinee in a competitive examination or between employer and potential employee in a job interview (Haig 1987). The challenge for the examiner is to design a test that provides useful information about the qualities of examinees. The challenge for examinees is to perform as well as possible, regardless of their intrinsic quality. So, is the relationship between examiner and examinee one of conflict or one of mutual interest? It depends on the qualities of the examinee. If the examinee is the ‘best person for the job’, both parties have a mutual interest in the test providing an accurate measure of quality, but otherwise the interests of examiners and examinees diverge (cheating in exams is not unknown).
One of the risks of any testing process is that one merely selects for individuals who are good at taking exams. Another problem arises if some members of an examining committee have a self-interest (not shared by all members of the committee) in favouring one candidate over another. Fisherian models of sexual selection depend on genetic covariance between genes for male traits (examinees) and genes for female preferences (examiners) but, if there are multiple loci for preference, alleles at different loci will have different degrees of linkage disequilibrium with a trait locus and may, therefore, experience different costs and benefits from the expression of a preference. Testing entails ‘discrimination’ and discrimination is a word with both positive (in the sense of choosing the best) and negative connotations (in the sense of choosing on some basis other than quality). Models of mate choice share many similarities with models of segregation distortion, and these analogies are further explored in the Appendix.
Once a candidate is chosen, even if the candidate is the best for the job, conflict may reappear in negotiations over salary. The analogy here is to how much a female gametophyte should invest in a sporophyte once it is chosen for provisioning. Among bryophytes, a female gametophyte usually has multiple investment options in addition to investment in a particular sporophyte. As a consequence, maternal and paternal genomes of a sporophyte may disagree over how resources should be allocated. By contrast, female gametophytes of many pteridophytes invest all in a single sporophyte. In these cases, the maternal and paternal genomes of the sporophyte are predicted to have entered into a full, and equal, partnership in which what is good for one is also good for the other.
7. Conclusion
In the life cycles of bryophytes and pteridophytes, multicellular haploid ‘females’ nourish diploid offspring. We have argued that the maternal and paternal genomes of the diploid offspring may have conflicting interests because the maternal genome of the offspring is shared, in its entirety, with the haploid mother but the paternal genome of the offspring may be genetically unrelated to the mother. These conflicts should be particularly pronounced in bryophytes, because haploid females may invest in multiple offspring produced both sexually and asexually, but conflict should be attenuated in pteridophytes, because haploid females typically invest in a single diploid offspring.
As yet, there are relatively few data available to directly test these predictions. In the future, two areas of research should be particularly fruitful in understanding postzygotic sexual conflicts in these plants. The first would be a search for imprinted gene expression in sporophytes of bryophytes. If imprinted expression is detected, this should be strong prima facie evidence for the existence of sexual conflicts. The second would be the study of the anatomy and physiology of the gametophyte–sporophyte junction, across which resources are transferred from haploid mother to diploid offspring. Systematists have already noted a great variety of cellular organizations at this junction (Ligrone et al. 1993). We suggest that much of this variation may be explained by variation among taxa in the intensity of parent–offspring (or maternal–paternal) conflict. For example, Ligrone et al. (1993) report the presence of dead and collapsed cells at the gametophyte–sporophyte junction in bryophytes but not in pteridophytes. This observation is concordant with our prediction of greater sexual conflict in bryophytes. However, definitive tests of these ideas will come from correlating variation at the gametophyte–sporophyte junction with variation in life cycles within these broad groups.
Acknowledgments
The manuscript benefited from two anonymous reviews and the helpful comments of Yaniv Brandvain and Jon Seger.
Appendix
Consider a large panmictic population in which haploid males are paired at random and then one is chosen to be the father of a zygote. ‘Choice’ in this model refers to any non-random process by which one of the males is selected to be a father. It could be the expression of a female preference, of different abilities of males to coerce females, or of pre-mating competition between males.
Let there be two alleles A and a with frequencies (1−q) and q before mate choice and zygotic selection. Suppose that an A male is always chosen if a pair of males contains an A male. There will be p2 A/A pairs, 2pq A/a pairs and q2 a/a pairs. The frequency of A fathers will be 1−q2, and the frequency of a fathers q2. Note that the ‘choice’ of haploid male can be conceptualized as segregation distortion in a diploid Aa male.
| AA | Aa | aa | |
|---|---|---|---|
| zygotic frequencies | (1−q)(1−q2) | (1−q)q2+q(1−q2) | q3 |
| zygotic fitnesses | 1 | 1 | w |
If w<1, allele A has higher fitness than a in both males and females and there is no sexual conflict. A fathers confer higher fitness on zygotes than a fathers. Therefore, the process of choice enhances female fitness. However, allele A would also go rapidly to fixation in a simpler model with random choice of fathers.
If w>1, the process of choice reduces average female fitness. That is, a females have higher fitness than A females because only the former can produce high fitness aa zygotes. However, this advantage becomes vanishingly small as a becomes rare. Sexual conflict is present because a enhances female fitness but decreases male fitness. If 2>w>1, A males have higher fitness than a males at all gene frequencies. If w>2, A males have higher fitness than a males above some threshold frequency of allele A.
A trivial calculation shows that the change in allele frequency from one generation to the next is given by
This system has a non-trivial equilibrium at
The equilibrium is unstable and exists in the interval 0<q<1 if w>1.5. If w<1.5, A alleles (if present) will go to fixation. If w>1.5, A alleles cannot invade a population fixed for a alleles, but neither can a alleles invade a population fixed for A alleles. (Seger (1985) presents a more sophisticated two-locus model in which females choose one of n haploid males, with a female's allele at the second locus determining whether she chooses males with a particular allele (if available) or mates at random.)
The principal purpose of this appendix is to emphasize the analogy between models of mate choice and models of segregation distortion. Haig (1996) identified a similar analogy between segregation distortion and parent–offspring conflict. In all these models, there is a departure from a random distribution of genetic goods and the potential for intragenomic conflict because, given meiotic segregation, not all genes share equally in benefits. Segregation distortion can either enhance or reduce population mean fitness at genetic equilibrium, depending on details of the model (Úbeda & Haig 2004), and the same is true of models of mate choice. Our haploid model is simple, but the analogy can be extended to more complex models: a choice between two diploid males can be interpreted as segregation distortion in the gametic output of a ‘tetraploid male’, and so on. From this perspective, preference alleles are analogous to modifiers of segregation distortion. The fates of preference alleles and modifiers of distortion become coupled through linkage disequilibrium to alleles at the locus they modify. Interlocus conflict within the genome is possible because modifiers with different degrees of linkage to the primary locus are subject to different selective forces (i.e. the genetic covariance between preference and trait depends on the recombination fraction).
The analogy between mate choice and meiotic drive is, of course, not exact. In particular, the effect of female preference alleles on the ‘segregation ratio’ of alleles at a male trait locus is less direct than the effect of distorter alleles on the segregation of alleles at a responder locus in systems of meiotic drive. A distorter allele usually acts within a meiotic cell to create an advantage after segregation for gametes that inherit a resistant allele at a responder locus. The distorter allele shares in the advantage of the resistant allele by hitch-hiking, because linkage disequilibrium ensures that it is preferentially transmitted to gametes carrying the resistant allele (Haig & Grafen 1991). When a distorter+resistant haplotype is rare, the frequency of segregation distortion is proportional to the haplotype frequency. In Fisherian models of mate choice, preference alleles expressed in females favour male gametes carrying particular trait alleles. The hitch-hiking effect requires that a preference+trait haplotype in females confers an advantage on a similar preference+trait haplotype in males. If the haplotype were rare and the population well mixed, such interactions would occur in proportion to the square of the haplotype frequency.
Footnotes
One contribution of 13 to a Discussion Meeting Issue ‘Sexual conflict: a new paradigm?’.
References
- Allen C.E. Die Keimung der Zygote bei Coleochaete. Berichte der Deutschen botanischen Gesellschaft. 1905;23:285–292. [Google Scholar]
- Boisselier-Dubayle M.-C, Bischler H. Allopolyploidy in the thalloid liverwort Corsinia (Marchantiales) Bot. Acta. 1998;111:490–496. [Google Scholar]
- Buchholz J.T. Developmental selection in vascular plants. Bot. Gaz. 1922;73:249–286. 10.1086/332991 [Google Scholar]
- Campbell D.H. Macmillan Company; London: 1905. The structure and development of mosses and ferns. [Google Scholar]
- Dassler C.L, Farrar D.R. Significance of form in fern gametophytes: clonal gemmiferous gametophytes of Callistopteris baueriana (Hymenophyllaceae) Int. J. Plant Sci. 1997;158:622–639. 10.1086/297476 [Google Scholar]
- Delwiche C.F, Karol K.G, Cimino M.T, Sytsma K.J. Phylogeny of the genus Coleochaete (Coleochaetales, Charophyta) and related taxa inferred by analysis of the chloroplast gene rbcL. J. Phycol. 2002;38:394–403. 10.1046/j.1529-8817.2002.01174.x [Google Scholar]
- Eberhard W.G. Evolutionary conflicts of interest: are female sexual decisions different? Am. Nat. 2005;165:S19–S25. doi: 10.1086/429348. 10.1086/429348 [DOI] [PubMed] [Google Scholar]
- Farrar D.R. Gametophytes of four tropical fern genera reproducing independently of their sporophytes in the southern Appalachians. Science. 1967;155:1266–1267. doi: 10.1126/science.155.3767.1266. [DOI] [PubMed] [Google Scholar]
- Farrar D.R. Gemmiferous fern gametophytes—Vittariaceae. Am. J. Bot. 1974;61:146–155. [Google Scholar]
- Farrar D.R. Species and evolution in asexually reproducing independent fern gametophytes. Syst. Bot. 1990;15:98–111. [Google Scholar]
- Gehring M, Choi Y, Fischer R.L. Imprinting and seed development. Plant Cell. 2004;16:S203–S213. doi: 10.1105/tpc.017988. 10.1105/tpc.017988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmell A.R. Studies in the Bryophyta. I. The influence of sexual mechanism on varietal production and distribution of British Musci. New Phytol. 1950;49:64–71. [Google Scholar]
- Goebel K. Clarendon Press; Oxford: 1905. Organography of plants, especially of the Archegoniatae and Spermatophyta. (trans. I. B. Balfour) [Google Scholar]
- Graham L.E, Wilcox L.W. The occurrence and phylogenetic significance of putative placental transfer cells in the green alga Coleochaete. Am. J. Bot. 1983;70:113–120. doi: 10.1002/j.1537-2197.1983.tb12439.x. [DOI] [PubMed] [Google Scholar]
- Haig D. Kin conflict in seed plants. Trends Ecol. Evol. 1987;2:337–340. doi: 10.1016/0169-5347(87)90110-8. 10.1016/0169-5347(87)90110-8 [DOI] [PubMed] [Google Scholar]
- Haig D. Gestational drive and the green-bearded placenta. Proc. Natl Acad. Sci. USA. 1996;93:6547–6551. doi: 10.1073/pnas.93.13.6547. 10.1073/pnas.93.13.6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D, Grafen A. Genetic scrambling as a defence against meiotic drive. J. Theor. Biol. 1991;153:531–558. doi: 10.1016/s0022-5193(05)80155-9. [DOI] [PubMed] [Google Scholar]
- Haig D, Westoby M. Sex expression in homosporous ferns: an evolutionary perspective. Evol. Trends Plants. 1988a;2:111–119. [Google Scholar]
- Haig D, Westoby M. A model for the origin of heterospory. J. Theor. Biol. 1988b;134:257–272. [Google Scholar]
- Haig D, Westoby M. Parent-specific gene expression and the triploid endosperm. Am. Nat. 1989;134:147–155. 10.1086/284971 [Google Scholar]
- Haig D, Westoby M. Genomic imprinting in endosperm: its effects on seed development in crosses between species and between different ploidies of the same species, and its implications for the evolution of apomixis. Phil. Trans. R. Soc. B. 1991;333:1–13. [Google Scholar]
- Hopkins A.W, McBride G.E. The life-history of Coleochaete scutata (Chlorophyceae) studied by a Feulgen microspectrophotometric analysis of the DNA cycle. J. Phycol. 1976;12:29–35. [Google Scholar]
- Karol K.G, McCourt R.M, Cimino M.T, Delwiche C.F. The closest living relatives of land plants. Science. 2001;294:2351–2353. doi: 10.1126/science.1065156. 10.1126/science.1065156 [DOI] [PubMed] [Google Scholar]
- Kenrick P, Crane P.R. Smithsonian Institution Press; Washington: 1997. The origin and early diversification of land plants. [Google Scholar]
- Korpelainen H, Pohjamo M, Laaka-Lindberg S. How efficiently does bryophyte dispersal lead to gene flow? J. Hattori Bot. Lab. 2005;97:195–205. [Google Scholar]
- Kreulen D.J.W. Spore output of moss capsules in relation to ontogeny of archesporial tissue. J. Bryol. 1972;7:61–74. [Google Scholar]
- Lewis, I. F. 1907 Notes on the morphology of Coleochaete nitellarum Johns Hopkins University Circular 26 (Whole number 195), 29-30.
- Ligrone R, Duckett J.G, Renzaglia K.S. The gametophyte–sporophyte junction in land plants. Adv. Bot. Res. 1993;19:231–317. [Google Scholar]
- Longton R.E, Schuster R.M. Reproductive biology. In: Schuster R.M, editor. New manual of bryology. Hattori Botanical Laboratory; Nichinan, Miyazaki: 1983. pp. 386–462. [Google Scholar]
- McLetchie D.N, García-Ramos G, Puterbaugh M.N. Local sex ratio dynamics: a model for the dioecious liverwort Marchantia inflexa. Evol. Ecol. 2002;15:231–254. 10.1023/A:1016000613291 [Google Scholar]
- Oltmanns F. Die Entwickelung der Sexualorgane bei Coleochaete pulvinata. Flora. 1898;85:1–14. [Google Scholar]
- Pizzari T, Snook R.R. Sexual conflict and sexual selection: chasing away paradigm shifts. Evolution. 2003;57:1123–1236. doi: 10.1111/j.0014-3820.2003.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Queller D.C. Male–female conflict and parent–offspring conflict. Am. Nat. 1994;144(Suppl.):S84–S99. 10.1086/285654 [Google Scholar]
- Ramsay H.P, Berrie G.K. Sex determination in bryophytes. J. Hattori Bot. Lab. 1982;52:255–274. [Google Scholar]
- Searles R.B. The strategy of the red algal life history. Am. Nat. 1980;115:113–120. 10.1086/283548 [Google Scholar]
- Seger J. Unifying genetic models for the evolution of female choice. Evolution. 1985;39:1185–1193. doi: 10.1111/j.1558-5646.1985.tb05685.x. [DOI] [PubMed] [Google Scholar]
- Smith A.J.E. Cytogenetics, biosystematics and evolution in the Bryophyta. Adv. Bot. Res. 1978;6:195–276. [Google Scholar]
- Stark L.R. Widespread sporophyte abortion following summer rains in Mojave desert populations of Grimmia orbicularis. Bryologist. 2001;104:115–125. [Google Scholar]
- Stark L.R. Skipped reproductive cycles and extensive sporophyte abortion in the desert moss Tortula inermis correspond to unusual rainfall patterns. Can. J. Bot. 2002;80:533–542. 10.1139/b02-053 [Google Scholar]
- Stark L.R, Stephenson A.G. Reproductive biology of Entodon cladorrhizans (Bryopsida, Entodontaceae). II Resource-limited reproduction and sporophyte abortion. Syst. Bot. 1983;8:389–394. [Google Scholar]
- Stark L.R, Mishler B.D, McLetchie D.N. The cost of realized sexual reproduction: assessing patterns of reproductive allocation and sporophyte abortion in a desert moss. Am. J. Bot. 2000;87:1599–1608. [PubMed] [Google Scholar]
- Stearns S.C. The selection-arena hypothesis. In: Stearns S.C, editor. The evolution of sex and its consequence. Birkhäuser Verlag; Basel: 1987. pp. 337–380. [Google Scholar]
- Trivers R, Burt A. Kinship and genomic imprinting. In: Ohlsson R, editor. Genomic imprinting: an interdisciplinary approach. Springer; Berlin: 1999. pp. 1–21. [Google Scholar]
- Úbeda F, Haig D. Sex-specific meiotic drive and selection at an imprinted locus. Genetics. 2004;167:2083–2095. doi: 10.1534/genetics.103.021303. 10.1534/genetics.103.021303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley O.C. Asexual reproduction in Coleochaete. Bot. Gaz. 1928;86:1–31. 10.1086/333869 [Google Scholar]
- Wesley O.C. Spermatogenesis in Coleochaete scutata. Bot. Gaz. 1930;89:180–191. 10.1086/334044 [Google Scholar]
- Willson M.F, Burley N. Princeton University Press; Princeton, NJ: 1983. Mate choice in plants. [Google Scholar]
- Zander R.H. Bryophyte sexual systems: -oicous versus -oecious. Bryologische Beiträge. 1985;3:46–51. [Google Scholar]


