Abstract
One of the most sharply defined sexual conflicts arises when the act of mating is accompanied by an inflated risk of death. Several reports have documented an increased death rate of female Drosophila as a result of recurrent mating. Transgenic and mutation experiments have further identified components of seminal fluid that are at least in part responsible for this toxicity. Variation among males in their tendency for matings to be toxic to their partners has also been documented, but here for the first time we identify polymorphism within particular genes conferring differential post-mating female mortality. Such polymorphism is important, as it raises the challenge of whether sexual conflict models can provide means for maintenance of polymorphism. Using a set of second chromosome extraction lines, we scored differences in post-mating female fecundity and longevity subsequent to mating, and identified significant among-line differences. Seventy polymorphisms in ten male reproductive genes were scored and permutation tests were used to identify significant associations between genotype and phenotype. One polymorphism upstream of PEBII and an amino acid substitution in CG17331 were both associated with male-induced female mortality. The same allele of CG17331 that is toxic to females also induces greater refractoriness to remating in the females, providing an example of an allele-specific sexual conflict. Postcopulatory sexual selection could lead to sexual conflict by favouring males that prevent their mates from mating, even when there is a viability cost to those females.
Keywords: cost of reproduction, sexual selection, sexual conflict, accessory gland proteins, sexually antagonistic coevolution
1. Introduction
Sexual conflict can arise when selection favours the evolution of a trait in one sex that consequently reduces the fitness of members of the opposite sex (Parker 1979). Sexual conflict can be divided into two major categories, intralocus and interlocus sexual conflict (see Chapman et al. 2003a). Intralocus sexual conflict occurs when a trait is expressed in both males and females, but the optimal fitness value for that trait differs between the sexes (see Rice & Holland 1997; Rice & Chippindale 2001). This conflict may impede evolution unless sex limited expression develops (see Parker & Partridge 1998; Rice & Chippindale 2001). There is substantial evidence for intralocus sexual conflict using Drosophila melanogaster as a model system (Rice 1992, 1998; Chippindale et al. 2001; Gibson et al. 2002).
In comparison, interlocus sexual conflict can arise when the reproductive interests of males and females differ (Rice & Holland 1997). Reproductive interests may include conflicts over remating rate, sperm utilization, offspring provisioning, and female refractoriness to remating. Such conditions may lead to opportunities for sexually antagonistic coevolution (Chapman & Partridge 1996; Rice 2000). For example, Rice (2000) proposed a hypothetical case, where a mutation at a male specific locus causes a gain in male fitness at a cost to his mate. This allele could fix in the population despite its cost to females. Females could counter-adapt if a female specific mutation arises that abates the cost of the original male benefit mutation, and the cycle could repeat leading to rapid evolution and ‘chase away’ sexual selection (Holland & Rice 1998).
Perhaps the best studied aspect of sexually antagonistic coevolution is the phenomenon of male-induced postcopulatory increase in female mortality, often referred to simply as ‘cost of mating’. In many species, the act of mating is costly to females in that it reduces her lifespan or reproductive success (Fowler & Partridge 1989; Yanagi & Miyatake 2003; Kemp & Rutowski 2004). This cost can be inflicted in a variety of different ways. Increased mortality could be the result of trade-offs in resource allocation; fecundity and lifespan are often negatively correlated (see Partridge et al. 2005). Mating can also increase the risk of predation (Magnhagen 1991) or the risk of sexually transmitted diseases or parasites (Knell & Webberley 2004). Costs can also result from damage incurred during the act of mating. For example, male bean weevils Callosobruchus maculatus have spines on their intromittent organ that can damage the female genitalia during copulation (Crudgington & Siva-Jothy 2000). Potentially damaging copulation is taken to the extreme in bedbugs, where males physically pierce the body wall to inseminate females but females have evolved a specialized organ to reduce the damage caused by traumatic insemination (Morrow & Arnqvist 2003; Reinhardt et al. 2003). Males can also induce harm to females through chemical rather than physical means.
In Drosophila, seminal fluid proteins that are transferred to the female during copulation are responsible for at least a portion of the male induced cost of mating (Chapman et al. 1995). Recently, sex peptide (Acp70A) was shown to be a major contributor to the cost of mating (Wigby & Chapman 2005). Sex peptide is known to benefit males by decreasing female receptivity to further matings and increasing egg laying rate in females (Chen et al. 1988; Chapman et al. 2003b; Liu & Kubli 2003) and thus represents an excellent candidate gene to be involved in sexual conflict. Civetta & Clark (2000) demonstrated that natural variation in sperm competitive ability was positively correlated with male induced cost of mating but did not attempt to identify the underlying genetic basis. Furthermore, Pitnick & Garcia-Gonzalez (2002) demonstrated that larger males imposed a greater cost to females in D. melanogaster. Such findings are consistent with the cost of mating being a pleiotropic effect of selection favouring alleles conferring advantages to males in sexual selection (see Morrow et al. 2003). The goal of this study is to investigate the genetic basis of natural variation in male induced cost of mating through association tests. The advantage of this approach is that it can establish particular alleles at particular loci in their role in the cost of mating phenomenon. Data of this sort are needed to fully model and understand the kinds of antagonistic trade-offs that occur in experiments designed to quantify sexual conflict.
2. Material and methods
(a) Drosophila lines
One hundred and one chromosome 2 substitution lines (Lazzaro et al. 2004; Fiumera et al. 2005) derived from a natural population in central Pennsylvania were used in this study. These lines are homozygous across the genome and differ from each other only at their second chromosomes. Each chromosome 2 substitution line contains a unique second chromosome that was recovered from the natural population. The tester males and females were a standard stock centre cn bw strain. Lines were reared at 24 °C on 12 h light/dark cycle using standard agar–dextrose–yeast medium.
(b) Measuring the cost of mating
Male-induced cost of mating was estimated for each of the chromosome 2 substitution lines and was scored during the ‘defence’ sperm competition experiments (see Fiumera et al. 2005). Cost of mating was calculated as the proportion of females that died within 10 days after mating to an experimental male and then a tester male. To estimate the cost of mating, virgin males and females were collected over CO2 and housed separately in vials until 4–7 days old. For each experimental line, two replicates of 10 experimental males and 10 cn bw females each were mass mated on the evening of day 0. On the morning of day 1, females were transferred to individual vials (vial 1) and the males were discarded. On the evening of day 3, two virgin tester cn bw males were added to each vial. On the morning of day 4, the females were transferred to new vials (vial 2) and the tester males were discarded. Females were transferred to fresh vials (vial 3) on day 7 and then discarded on day 14. Eye colour was used to determine paternity and only those females that mated to both the experimental male and the tester male were used in this experiment. Cost of mating was calculated as the proportion of females that died in vials 2 and 3 after having mated to both males. The exact day of death was not recorded, limiting our ability to calculate either female longevity or mortality rate (Civetta & Clark 2000). Cost of mating was measured across two blocks that represented separate generations of experimental and tester flies. Permutation tests based on χ2 statistics were used to test for significant line effects on male-induced female mortality. In brief, the observed χ2 statistic was calculated and compared to the distribution of χ2 statistics calculated from 5000 random permutations of the data. This approach combined the block effect with the error and thus represents a conservative measure of significance.
(c) Genotyping
A total of 70 polymorphic nucleotide positions were scored in 10 male reproductive genes (Acp26Aa, CG8137, Acp29Ab, CG31872, Acp32CD, Acp33A, CG17331, Acp36DE, Acp53Ea, PEBII) in the chromosome 2 substitution lines (Fiumera et al. 2005). We scored one additional polymorphism not presented in Fiumera et al. (2005), an aspartic acid versus asparagine amino acid polymorphism at position 74 in CG17331. This polymorphism was scored using Pyrosequencing (Biotage) according to manufacturer's protocols and the following primers (forward biotinylated amplification primer, 5′-/5Bio/TGTACAAGATGCGCAATGGCT-3′; reverse amplification primer, 5′-TCCTTACCCGGCCACAAA-3′; reverse sequencing primer, 5′-ATTCACGTGGACTTAAAT-3′; dispensation order during sequence analysis: ATCAGTAGC).
(d) Association testing
Simple linear models with significance assessed by 5000 permutations (Churchill & Doerge 1994; Fiumera et al. 2005) were conducted in MATLAB to infer associations between particular alleles of the candidate genes and cost of mating. As a control, tests were conducted to identify associations with polymorphisms in immunity genes, which had already been genotyped in these same lines. A total of 128 immunity polymorphisms were scored in the chromosome 2 substitution lines (Lazzaro et al. 2004), and significance tests were performed in the same manner.
3. Results
Cost of mating was successfully scored in 95 out of the 101 chromosome 2 substitution lines. We scored cost of mating in only 95 of the 101 lines because developmental time differences between the lines resulted in some flies not emerging in time to restrict the analysis to 4–7 days old virgin males. A total of 1584 females from the 95 lines were analysed. There were significant line effects (p=0.035) for male-induced female mortality in the chromosome 2 substitution lines (figure 1) indicating there is a genetic component to the cost of mating. The 95 lines presented a wide range of variation in other sperm competition and female response phenotypes. There were highly significant line effects for the proportion of offspring sired by the first male to mate (P1′), female fecundity and male-induced female refractoriness (Fiumera et al. 2005). Cost of mating was not correlated with any of these other phenotypes (table 1). The lack of correlation with female fecundity suggests that female mortality was not due to increases in egg production. For this reason, we did not need to correct for variation in the physiological stress of egg laying in the estimates of female mortality. In addition, the lines revealed substantial polymorphism in all male reproductive genes, providing an initial indication that the study was adequately powered for the association tests (see Fiumera et al. (2005) for data on original polymorphisms). A total of 91 of the lines was successfully genotyped for the polymorphism at amino acid position 74 in CG17331 that was not previously scored. The common allele, coding for aspartic acid, was at a frequency of 0.66 at this site. Most assays were successfully genotyped in more than 90 of the lines. Failed genotypes could represent lines with a third segregating nucleotide, potential cases where the locus failed to homogenize during chromosomal extraction (ca 1% of failed genotypes) or, more likely, cases with an unidentified polymorphism under a primer. Such failures will probably decrease our power to detect significant associations only slightly, and should in no way lead to spurious associations (see Fiumera et al. 2005).
Figure 1.

Histogram of line means for male induced cost of mating. Cost of mating is the proportion of females that died after mating to both an experimental and tester male.
Table 1.
Correlations among line means for cost of mating and sperm competition phenotypes.
| P1′ | female refractoriness | female fecundity | |
|---|---|---|---|
| correlation coefficient (r) | 0.067 | 0.169 | −0.065 |
| p-value | 0.52 | 0.10 | 0.53 |
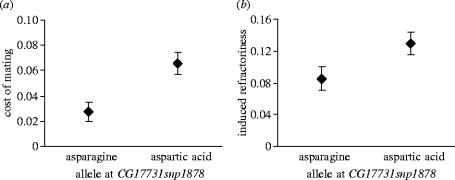
Two significant associations were identified at the markerwise p<0.01. The FDR for these associations was 0.22 and thus fewer than 1 should be a false discovery (Storey & Tibshirani 2003). A nine base pair insertion–deletion upstream of PEBII and an amino acid polymorphism at position 74 of CG17331 both associated with cost of mating. Males carrying the nine base pair deletion upstream of PEBII inflict three times higher mortality levels on females compared to males with the nine base pair insertion (insertion, 0.02±0.008 s.e. versus deletion, 0.07±0.009 s.e.). Expression level of PEBII measured in virgin males (Fiumera et al. 2005) was not associated with cost of mating (p=0.28), but since Acps are known to be upregulated post-mating (DiBenedetto et al. 1990; Betram et al. 1992), it would be valuable to investigate the relationship between cost of mating and expression of Acps in non-virgin males. We also did not identify any polymorphisms in the nearby mating plug gene, PEBme, that were associated with female mortality (data not shown). In the second association, females that mated to males homozygous for aspartic acid at position 74 in CG17331 were more than twice as likely to die as females mated to males that had an asparagine at that site (figure 2). Interestingly, this polymorphism is also weakly associated with male-induced female refractoriness (p=0.058; figure 2). The allele that results in higher mortality in females also reduces the probability that females will remate. For the significant associations in PEBII and CG17331, the allele which induced higher mortality in females was ancestral and also at higher frequency in the scored lines. There were no significant associations (markerwise p<0.01) between cost of mating and any of the 128 polymorphisms in immunity genes, suggesting that neither long distance linkage disequilibrium nor population subdivision was the cause of the associations (see Fiumera et al. 2005).
Figure 2.
Sexual conflict at an amino acid polymorphism in CG17331. (a) Mean cost of mating and standard errors for females mating to males with alternative alleles at amino acid position 74 in CG17331. (b) Mean refractoriness and standard errors for females mating to males with alternative alleles at amino acid position 74 in CG17331.
4. Discussion
Here, we provide strong evidence for allele-specific cost of mating at two segregating polymorphisms in different male reproductive genes. In addition, sexual conflict acting between male control of female remating rate and male induced female mortality appears to be operating. A single amino acid change at position 74 of CG17331 was strongly associated with our measure of cost of mating and weakly associated with male induced female refractoriness. The allele that was beneficial to males reduced the probability that a female remated but induced higher mortality in the mated females. The benefit to the males thus came at a cost to females, presenting a clear case of an allele-specific sexual conflict.
Explanations for the evolution of harmful male adaptations have taken two distinct approaches (see Morrow et al. 2003). The ‘pleiotropic harm hypothesis’ proposed by Parker (1979) suggests that harm is a negative pleiotropic consequence of a trait that is beneficial to males. Alternative ‘adaptive harm hypotheses’ suggest that the induced harm to females is beneficial to males in that it either decreases the probability that the female will remate (Johnstone & Keller 2000) or alter her resource allocation towards current rather than future reproduction (Michiels 1998). In our experiment, female fecundity was not correlated with cost of mating and thus our results are inconsistent with the hypothesis that harm is adaptive because it biases female resource allocation in favour of current reproduction. We are not able to differentiate between the pleiotropic harm hypothesis and the hypothesis that the harm itself reduces the probability that the female will remate. Other evidence, however, provides support in favour of the pleiotropic harm hypothesis. For example, Morrow et al. (2003) found no evidence that physical harm to females caused them to resist remating or lay more eggs. In addition, Civetta & Clark (2000) identified a positive correlation between a measure of sperm competitive ability and cost of mating. Finally, pleiotropic effects on multiple phenotypes appear common in many male reproductive genes. For example, sex peptide is known to affect female remating rate, egg laying rate (Chen et al. 1988; Chapman et al. 2003b; Liu & Kubli 2003), and also cost of mating (Wigby & Chapman 2005). Fiumera et al. (2005), using an association approach, identified two male reproductive genes that had pleiotropic effects on different phenotypes affecting sperm competitive ability. In one case, alternative alleles acted antagonistically on different phenotypes. While these experiments are not perfect tests of the alternative hypotheses, their accumulated findings do favour the evolution of harmful male adaptations through a form of pleiotropy. An implicit assumption in the models of evolution of sexual conflict is that mutations arise that influence both reproductive success and harm in a sex-specific way. A crucial test of this idea is to find genes that harbour allelic differences in the magnitude of sexual conflict. Our results, while they do succeed in achieving a demonstration that genetic variation in conflict is present, do not fully explain the maintenance of that polymorphism.
CG17331 has been shown to have pleiotropic effects on several phenotypes affecting sperm competition. Fiumera et al. (2005) identified associations between natural variation in CG17331 and the proportion of offspring sired by the second male to mate (P2′), male-induced female refractoriness, the proportion of offspring sired by the first male to mate (P1′), and with expression of another seminal fluid protein, Acp29Ab. In this study, we find associations between natural variation in CG17331 and both male-induced cost of mating and male-induced female refractoriness. CG17331 is a component of the 20S core particle of the 26S proteasome (Ma et al. 2002) and is thus a key player in the ubiquitin/proteasome pathway controlling regulated proteolysis in cells (Baumeister et al. 1998). In Drosophila, several subunits of the 20S proteasome have male-specific isoforms expressed in the male germ line (Ma et al. 2002). The exact role for CG17331 is yet to be determined, but variation in CG17331 might be affecting critical steps in the regulation of protein degradation within the accessory gland and such a role would be consistent with our observation of pleiotropic effects on multiple components of postcopulatory sexual selection and sexual conflict. The critical test requires phenotyping null or RNAi knockdown mutants and we hope to pursue this avenue in the future.
Sexual conflict has also been proposed to lead to sexually antagonistic coevolution and rapid evolution of the characters involved (Chapman & Partridge 1996; Rice 2000) including seminal fluid proteins (Swanson & Vacquier 2002). Empirical support for sexually antagonistic coevolution is mixed. Studies in natural populations of water striders conclude that sexually antagonistic coevolution is an important selective force (Arnqvist & Rowe 2002; Rowe & Arnqvist 2002) as do experimental studies in laboratory populations of dung flies (Martin & Hosken 2004) and fruit flies (Rice 1996). Comparative studies using spiders and insects, however, conclude that signatures of sexually antagonistic coevolution are weak or non-existent (Eberhard 2004a,b).
Is CG17331 undergoing adaptive evolution due to sexual conflict? Perhaps, but full sequence data, including both polymorphism and divergence from multiple species will be required to test that hypothesis. Based on our current knowledge, the allele imparting a high cost to females and high benefit to males appears to be ancestral, as indicated by its presence in Drosophila simulans. Thus, the most parsimonious explanation is that the low fitness allele is a derived deleterious recessive that has reached appreciable frequency through genetic drift. If this hypothesis is true, then it does not appear that sexual conflict is promoting rapid molecular evolution at this polymorphic site, at least not since the divergence of D. simulans and D. melanogaster. A more complicated, but feasible, alternative hypothesis is that simultaneous selection on females has led to adaptive evolution via sexually antagonistic coevolution. Under this scenario, the aspartic acid allele in D. melanogaster (current high fitness/high cost) would be secondarily derived and favoured in the current D. melanogaster female genotype. In the past, sexual conflict would have favoured the asparagine allele (current low fitness/low cost) in an ancestral female genotype driving it to fixation and the current aspartic acid allele is a back mutation in the process of increasing in frequency. These alternative hypotheses are testable, but a thorough comparative analysis of the molecular evolution of CG17331 (and other male reproductive genes) will be required to understand how selection (possibly sexually antagonistic) has shaped patterns of genotypic diversity since their origination.
(a) Future prospects
Although male-induced female mortality contributes to sexual conflict and potentially sexually antagonistic coevolution, it is critical that such phenomena be evaluated within a framework incorporating both costs and benefits to males and females (see Pizzari & Snook 2003, 2004; Arnqvist 2004). Even though females pay a cost in terms of reduced longevity by mating with males of a given genotype they may gain indirect benefits. For example, if cost of mating is positively correlated with a trait that is beneficial to male reproductive success as predicted under the pleiotropic harm hypothesis (Parker 1979) then females can gain indirect benefits by having sons with the advantageous male phenotype. To fully understand the evolution of such traits it is important then to investigate both the direct costs and indirect benefits that may be contributing to sexual conflict and sexual selection (see Eberhard 2005). A well motivated experiment is to quantify indirect benefits for females mating to males with the alternative alleles we identified in this study. Such information, combined with estimates of dominance for the alternative alleles (see below), will hopefully allow for quantitative predictions of evolutionary trajectories.
The field of sexual conflict is dominated by quantitative genetic approaches, and it is instructive to contrast that approach to attempts to find segregating factors in single Mendelian genes that are relevant to conflict. Quantitative genetics allows one to work with a much more diverse array of organisms, and it is only in this way that the biological generality of principles can be tested. In addition, it is possible to arrive at satisfying answers about the heritability and genetic correlation of traits much faster than one could having to first identify and map Mendelian factors. A compromise approach is to map quantitative trait loci (QTL), and Civetta et al. (2005) illustrate the power of this approach by mapping factors responsible for the differences between D. simulans and D. sechellia in post-mating female mortality. QTL mapping provides the first step toward identifying individual genes associated with a complex character, but the magnitude of the effort between first identifying a QTL and subsequently isolating the single gene (or cluster of genes) responsible for the phenotype can range widely.
Where single gene associations have been successful, investigators had already identified excellent candidate genes to begin with, such as accessory gland protein-coding genes (Chapman et al. 1995, 2003b; Wigby & Chapman 2005). But it is still important to identify allele-specific associations with reproductive traits, as it is only in this way that we can start to formulate mechanistic models that account for specific allelic changes and their effects. This highlights the contrast between quantitative genetic modelling and models that include the genetic details like dominance, pleiotropy and recombination. Quantitative genetic models have been essential in formulating theories for how sexual conflict might arise and evolve, and no doubt there will continue to be creative and insightful applications of this approach. At some level, however, we need to understand the genetic details as well, and it is only by explicit population genetic models with allelic effects at individual genes that we can get there. For example, such models will yield insights regarding the prospects for pleiotropy maintaining polymorphism for sexual conflicts within populations.
In addition to these theoretical problems, there are several aspects of the Mendelian genetics of sexual conflict that remain to be investigated. For example, the role of dominance in sexual conflict theories has been neglected. Our experiments use homozygous lines and therefore we have not yet estimated dominance of the alternative alleles, but it is clear that such a study would be a simple matter of generating the heterozygotes by crossing the lines. Now that we have identified CG17331 as an excellent candidate for a sexually antagonistic gene we need to investigate its phenotypic effects in null mutations or RNAi knockdown experiments. If a null mutation can be created, it would be extremely informative to reconstruct the alternative alleles we have identified here thus genetically controlling for any confounding effects of the genetic background. This will allow us to specifically test if the amino acid substitution at position 74 is the causative site and also refine our estimates of the phenotypic effects. Furthermore, we know very little about the response of females to male induced harm and toxic seminal fluids. Linder & Rice (2005) have demonstrated that there is genetic variation for female resistance to harm, much of which is mediated by variation in female remating rate. Understanding the genes involved in the female side of sexual conflict will allow for exciting tests of molecular and phenotypic coevolution. Finally, population genetic models that explicitly pose effects on specific alleles are very strongly motivated by our results, and they will likely suggest paths for fruitful experimental work as well.
5. Summary
A variety of mechanisms have been proposed whereby sexual conflicts might arise in a population. Most of these efforts have made use of artificial selection experiments, showing that reduction of conflict through forced monogamy has a correlated response in altered female resistance or other attributes (e.g. Martin & Hosken 2004). Other efforts have established that the protein products of individual genes, generally seminal proteins, have toxic effects, but these studies have relied on knock-out and knock-down experiments, rather than on naturally occurring polymorphism. The idea of seeking allele-specific associations with mortality is to establish that natural polymorphisms at these loci might be maintained through the operation of extant sexual conflicts. Here, we present not only cases showing allele-specific differences in cost of mating, but also pleiotropic effects with male reproductive genes. Altogether, seventy sequence variants in ten male reproductive genes were scored and statistical tests identified significant associations between candidate gene alleles and female reproductive phenotypes. One polymorphism upstream of PEBII and one amino acid change in CG17331 were both associated with male-induced female mortality. The toxic allele of CG17331 also resulted in elevated refractoriness to remating in the females (after mating with a male carrier), providing the first case of an allele-specific sexual conflict.
Acknowledgements
We would like to thank B. Haerum (Hughes Undergraduate Scholar), S. Galasinski and B. Sceurman for their assistance with DNA sequencing and sperm competition scoring. C. Aquadro, B. Lazzaro, L. McGraw, K. McKean, K. Montooth, T. Schlenke T. Wittkopp, J. Walters, M. Wolfner, and A. Wong provided suggestions during the course of this study. This work was supported by a National Science Foundation grant (DEB-0242987 to A.G.C.), and a National Institutes of Health Ruth L. Kirschstein Postdoctoral fellowship (NGA 1 F32 GM70300-01 to A.C.F.).
Footnotes
One contribution of 13 to a Discussion Meeting Issue ‘Sexual conflict: a new paradigm?’.
References
- Arnqvist G. Sexual conflict and sexual selection: lost in the chase. Evolution. 2004;58:1383–1388. doi: 10.1111/j.0014-3820.2004.tb01716.x. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92:367–380. doi: 10.1016/s0092-8674(00)80929-0. 10.1016/S0092-8674(00)80929-0 [DOI] [PubMed] [Google Scholar]
- Bertram M.J, Akerkar G.A, Ard R.L, Gonzalez C, Wolfner M.F. Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mech. Dev. 1992;38:33–40. doi: 10.1016/0925-4773(92)90036-j. 10.1016/0925-4773(92)90036-J [DOI] [PubMed] [Google Scholar]
- Chapman T, Partridge L. Sexual conflict as fuel for evolution. Nature. 1996;381:189–190. doi: 10.1038/381189a0. 10.1038/381189a0 [DOI] [PubMed] [Google Scholar]
- Chapman T, Liddle L.F, Kalb J.M, Wolfner M.F, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. 10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003a;18:41–47. 10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner M.F, Smith H.K, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl Acad. Sci. USA. 2003b;100:9923–9928. doi: 10.1073/pnas.1631635100. 10.1073/pnas.1631635100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.S, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Bohlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. 10.1016/0092-8674(88)90192-4 [DOI] [PubMed] [Google Scholar]
- Chippindale A.K, Gibson J.R, Rice W.R. Negative genetic correlation for adult fitness between sexes reveals ontogenetic conflict in Drosophila. Proc. Natl Acad. Sci. USA. 2001;98:1671–1675. doi: 10.1073/pnas.041378098. 10.1073/pnas.041378098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill G.A, Doerge R.W. Empirical threshold values for quantitative trait mapping. Genetics. 1994;138:963–971. doi: 10.1093/genetics/138.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta A, Clark A.G. Correlated effects of sperm competition and postmating female mortality. Proc. Natl Acad. Sci. USA. 2000;97:13162–13165. doi: 10.1073/pnas.230305397. 10.1073/pnas.230305397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta A, Montooth K.L, Mendelson M. Quantitative trait loci and interaction effects responsible for variation in female postmating mortality in Drosophila simulans and D. sechellia introgression lines. Heredity. 2005;94:94–100. doi: 10.1038/sj.hdy.6800570. 10.1038/sj.hdy.6800570 [DOI] [PubMed] [Google Scholar]
- Crudgington H.S, Siva-Jothy M.T. Genital damage, kicking and early death—the battle of the sexes takes a sinister turn in the bean weevil. Nature. 2000;407:855–856. doi: 10.1038/35038154. 10.1038/35038154 [DOI] [PubMed] [Google Scholar]
- DiBenedetto A.J, Harada H.A, Wolfner M.F. Structure, cell-specific expression, and mating-induced regulation of a Drosophila melanogaster male accessory-gland gene. Dev. Biol. 1990;139:134–148. doi: 10.1016/0012-1606(90)90284-p. 10.1016/0012-1606(90)90284-P [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Male–female conflict and genitalia: failure to confirm predictions in insects and spiders. Biol. Rev. Camb. Phil. Soc. 2004a;79:121–186. doi: 10.1017/s1464793103006237. 10.1017/S1464793103006237 [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Rapid divergent evolution of sexual morphology: comparative tests of antagonistic coevolution and traditional female choice. Evolution. 2004b;58:1947–1970. doi: 10.1554/04-143. [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Evolutionary conflicts of interest: are female sexual decisions different? Am. Nat. 2005;165:S19–S25. doi: 10.1086/429348. 10.1086/429348 [DOI] [PubMed] [Google Scholar]
- Fiumera A.C, Dumont B.L, Clark A.G. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics. 2005;169:243–257. doi: 10.1534/genetics.104.032870. 10.1534/genetics.104.032870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler K, Partridge L. A cost of mating in female fruit-flies. Nature. 1989;338:760–761. 10.1038/338760a0 [Google Scholar]
- Gibson J.R, Chippindale A.K, Rice W.R. The X chromosome is a hot spot for sexually antagonistic fitness variation. Proc. R. Soc. B. 2002;269:499–505. doi: 10.1098/rspb.2001.1863. 10.1098/rspb.2001.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Perspective: chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Johnstone R.A, Keller L. How males can gain by harming their mates: sexual conflict, seminal toxins, and the cost of mating. Am. Nat. 2000;156:368–377. doi: 10.1086/303392. 10.1086/303392 [DOI] [PubMed] [Google Scholar]
- Kemp D.J, Rutowski R.L. A survival cost to mating in a polyandrous butterfly, Colias eurytheme. Oikos. 2004;105:65–70. 10.1111/j.0030-1299.2004.12874.x [Google Scholar]
- Knell R.J, Webberley M.K. Sexually transmitted diseases of insects: distribution, evolution, ecology and host behaviour. Biol. Rev. 2004;79:557–581. doi: 10.1017/s1464793103006365. 10.1017/S1464793103006365 [DOI] [PubMed] [Google Scholar]
- Lazzaro B.P, Sceurman B.K, Clark A.G. Genetic basis of natural variation in D. melanogaster antibacterial immunity. Science. 2004;303:1873–1876. doi: 10.1126/science.1092447. 10.1126/science.1092447 [DOI] [PubMed] [Google Scholar]
- Linder J.E, Rice W.R. Natural selection and genetic variation for female resistance to harm from males. J. Evol. Biol. 2005;18:568–575. doi: 10.1111/j.1420-9101.2004.00872.x. 10.1111/j.1420-9101.2004.00872.x [DOI] [PubMed] [Google Scholar]
- Liu H.F, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. 10.1073/pnas.1631700100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Katz E, Belote J.M. Expression of proteasome subunit isoforms during spermatogenesis in Drosophila melanogaster. Insect Mol. Biol. 2002;11:627–639. doi: 10.1046/j.1365-2583.2002.00374.x. 10.1046/j.1365-2583.2002.00374.x [DOI] [PubMed] [Google Scholar]
- Magnhagen C. Predation risk as a cost of reproduction. Trends Ecol. Evol. 1991;6:183–185. doi: 10.1016/0169-5347(91)90210-O. 10.1016/0169-5347(91)90210-O [DOI] [PubMed] [Google Scholar]
- Martin O.Y, Hosken D.J. Reproductive consequences of population divergence through sexual conflict. Curr. Biol. 2004;14:906–910. doi: 10.1016/j.cub.2004.04.043. 10.1016/j.cub.2004.04.043 [DOI] [PubMed] [Google Scholar]
- Michiels N.K. Mating conflicts and sperm competition in simultaneous hermaphrodites. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 219–254. [Google Scholar]
- Morrow E.H, Arnqvist G. Costly traumatic insemination and a female counter-adaptation in bed bugs. Proc. R. Soc. B. 2003;270:2377–2381. doi: 10.1098/rspb.2003.2514. 10.1098/rspb.2003.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow E.H, Arnqvist G, Pitnick S. Adaptation versus pleiotropy: why do males harm their mates? Behav. Ecol. 2003;14:802–806. 10.1093/beheco/arg073 [Google Scholar]
- Parker G.A. Sexual selection and sexual conflict. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; New York: 1979. pp. 123–166. [Google Scholar]
- Parker G.A, Partridge L. Sexual conflict and speciation. Phil. Trans. R. Soc. B. 1998;353:261–274. doi: 10.1098/rstb.1998.0208. 10.1098/rstb.1998.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers D.J. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. 10.1016/j.cell.2005.01.026 [DOI] [PubMed] [Google Scholar]
- Pitnick S, Garcia-Gonzalez F. Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. B. 2002;269:1821–1828. doi: 10.1098/rspb.2002.2090. 10.1098/rspb.2002.2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Snook R.R. Perspective: sexual conflict and sexual selection: chasing away paradigm shifts. Evolution. 2003;57:1223–1236. doi: 10.1111/j.0014-3820.2003.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Pizzari T, Snook R.R. Sexual conflict and sexual selection: measuring antagonistic coevolution. Evolution. 2004;58:1389–1393. [Google Scholar]
- Reinhardt K, Naylor R, Siva-Jothy M.T. Reducing a cost of traumatic insemination: female bedbugs evolve a unique organ. Proc. R. Soc. B. 2003;270:2371–2375. doi: 10.1098/rspb.2003.2515. 10.1098/rspb.2003.2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R. Sexually antagonistic genes: experimental evidence. Science. 1992;256:1436–1439. doi: 10.1126/science.1604317. [DOI] [PubMed] [Google Scholar]
- Rice W.R. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. 10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Rice W.R. Male fitness increases when females are eliminated from gene pool: implications for the Y chromosome. Proc. Natl Acad. Sci. USA. 1998;95:6217–6221. doi: 10.1073/pnas.95.11.6217. 10.1073/pnas.95.11.6217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R. Dangerous liaisons. Proc. Natl Acad. Sci. USA. 2000;97:12953–12955. doi: 10.1073/pnas.97.24.12953. 10.1073/pnas.97.24.12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R, Chippindale A.K. Intersexual ontogenetic conflict. J. Evol. Biol. 2001;14:685–693. 10.1046/j.1420-9101.2001.00319.x [Google Scholar]
- Rice W.R, Holland B. The enemies within: intergenomic conflict, interlocus contest evolution (ICE), and the intraspecific Red Queen. Behav. Ecol. Sociobiol. 1997;41:1–10. 10.1007/s002650050357 [Google Scholar]
- Rowe L, Arnqvist G. Sexually antagonistic coevolution in a mating system: combining experimental and comparative approaches to address evolutionary processes. Evolution. 2002;56:754–767. doi: 10.1111/j.0014-3820.2002.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Storey J.D, Tibshirani R. Statistical significance for genomewide studies. Proc. Natl Acad. Sci. USA. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson W.J, Vacquier V.D. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 2002;3:137–144. doi: 10.1038/nrg733. 10.1038/nrg733 [DOI] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. 10.1016/j.cub.2005.01.051 [DOI] [PubMed] [Google Scholar]
- Yanagi S, Miyatake T. Costs of mating and egg production in female Callosobruchus chinensis. J. Insect Physiol. 2003;49:823–827. doi: 10.1016/S0022-1910(03)00119-7. 10.1016/S0022-1910(03)00119-7 [DOI] [PubMed] [Google Scholar]