Abstract
Six sister populations of Drosophila melanogaster kept under identical environmental conditions for greater than 600 generations were reciprocally crossed to investigate the incidence of population divergence in allopatry. Population crosses directly influenced fitness, mating frequency, and sperm competition patterns. Changes in both female remating rate and the outcome of male sperm competition (P1, P2) in response to foreign males were consistent with intersexual coevolution. Moreover, seven of the 30 crosses between foreign mates resulted in significant reductions in female fitness, whereas two resulted in significant increases, compared to local matings. This tendency for foreign males to reduce female fitness may be interpreted as evidence for either sexually antagonistic coevolution or the disruption of mutualistic interactions. However, instances in which female fitness improved via cohabitation with foreign males may better reveal sexual conflict, signalling release from the cost of interacting with locally adapted males. By this metric, female reproduction in D. melanogaster is strongly constrained by local adaptation by males, a situation that would promote antagonistic coevolution between the sexes. We conclude that sexual selection can promote population differentiation in allopatry and that sexual conflict is likely to have played a role in population differentiation in this study system.
Keywords: sexual conflict, sperm competition, gender load, intersexual coevolution, speciation, reproductive divergence, Drosophila melanogaster
1. Introduction
Males and females often evolve different fitness-maximizing strategies (Bateman 1948; Trivers 1972), creating the potential for conflict and, under some circumstances, antagonistic coevolution (Parker 1979; Rice 1996; Holland & Rice 1998). Recently, an intraspecific Red Queen process of adaptation and counter-adaptation between the sexes has been implicated in accelerated gene sequence change for reproductive traits in several animal species (Rice & Holland 1997; Rice 1998; Swanson & Vacquier 2002). If intersexual coevolution follows idiosyncratic pathways in allopatric populations, then rapid population differentiation may result, even in the absence of changes in the ecological setting (Rice 1998; Arnqvist et al. 2000). Indeed, several recent verbal (Rice 1996; Parker & Partridge 1998) and mathematical (Gavrilets 2000; Gavrilets et al. 2001) models have been developed that support the hypothesis that sexual conflict is potentially ‘the strongest driver of speciation’ (Martin & Hosken 2003).
Despite the growing view that coevolution resulting from sexual conflict is an important force that may be taxonomically widespread, most of the compelling data to date have come from laboratory evolution experiments with insects. These experiments have either limited selection to one sex (Rice 1996, 1998) or have manipulated the potential level of sexual conflict (e.g. Promislow et al. 1998; Holland & Rice 1999; Martin & Hosken 2003; Wigby & Chapman 2004). Many of the rapid changes in pre- and post-mating interactions between the sexes that have been observed are broadly consistent with sexually antagonistic coevolution. For example, when gene-expression was limited to males in Drosophila melanogaster, with females drawn from a separate ‘target’ population each generation, male fitness increased relative to controls through both increased mating success and post-copulatory sperm competition (Rice 1996).
A potentially more tractable approach to testing coevolutionary hypotheses is to perform interpopulation crosses by reciprocally trading mates between different allopatric populations of the same species (e.g. Andrés & Arnqvist 2001). Because of stochastic processes such as genetic drift and mutation in finite populations, intersexual coevolution is expected to take different pathways in allopatric populations of the same species (Arak & Enquist 1993, 1995; Holland & Rice 1998; Parker & Partridge 1998). As populations diverge, one would expect each sex to become better adapted, on average, to the signals of within-population (local) mates than to those of between-population (foreign) mates.
It has been suggested, or at least implied, that if intersexual coevolution has been predominantly antagonistic, foreign males will be more harming to females than coevolved local males (e.g. Holland & Rice 1998; Parker & Partridge 1998, but see Rowe et al. 2003). This idea is predicated on the assumption that male signals exploit vulnerabilities in the female receiver system for short-term reproductive gain, and that females evolve specialized defences to those males that improve their own lifetime reproductive success. By this argument, females should be well defended against, or most resistant to, the males they coevolved with and poorly defended against the signals of foreign males. Despite the appeal of this verbal model, experiments involving interpopulation crosses have yielded inconsistent results. Statistical interactions between populations are commonly observed, but the direction of change in fitness has varied, both between and within studies (reviewed by Chapman et al. 2003).
One possible explanation for the inconsistency of this literature is that most studies employing interpopulation crosses have made use of natural populations tested under common garden conditions in the lab. Lack of control over the evolutionary history of the study populations creates several potential artefacts, including genotype-by-environment interactions for performance under novel test conditions (see Rose et al. 1996). The evolutionary distance between the populations may also be critical to the outcome of the cross (Parker & Partridge 1998)—incipient species are certain to behave differently than recently isolated allopatric populations. Finally, researchers may not be able to discern which components of the life history are most relevant to fitness, the currency of conflict, for a given species. Under these conditions, it will be difficult to distinguish between adaptive coevolution, genetic drift, and other sources of evolutionary change.
With well-characterized laboratory evolution systems, it may be possible to avoid many of the artefacts associated with the common garden approach in looking for the footprints of intersexual coevolution. The general idea is to use laboratory phylogenies consisting of identical replicate populations (same ancestor, same conditions of subsequent evolution) to factor out the role of natural selection, as well as isolating the effects of drift and sexual selection, which interact to drive rapid but undirected evolutionary change. With a well-known life cycle, it may be possible to measure fitness and avoid genotype-by-environment interactions and other potential confounds in designing test conditions. Two recent studies, which we describe below, have employed this approach (Attia & Tregenza 2004; Fricke & Arnqvist 2004).
Fricke & Arnqvist (2004) examined the response of several female fitness characters to interpopulation crosses in two distinct laboratory strains of the bean weevil, Callosobruchus maculatus. Over several decades, these two strains had both been established in the same three laboratories at various times, creating three replicates of each original population ranging from 60 to 312 generations apart. Each laboratory had a somewhat different maintenance protocol, and the amount of time each population had to adapt to it varied. Nonetheless, the authors assumed that natural selection would promote adaptation to environmental conditions the same way in each strain. When crosses were performed, the effect of foreign males on female lifespan and reproduction was markedly different for each strain, even when they were taken from the same lab population, suggesting that divergence between laboratory populations had occurred in a non-parallel fashion between strains. Fricke & Arnqvist (2004) argued that this result was consistent with coevolution driven by sexual selection because natural selection would produce similar outcomes, while sexual selection would proceed along more arbitrary trajectories. While this reasoning is compelling, their experiment incorporated some of the undesirable features of the common garden approach. For example, differences in the standing genetic variation between the two original strains of C. maculatus (one collected in Brazil, one in India) may have influenced their potential for convergent evolution. Differences in laboratory culture conditions (temperature, diet, and humidity) also led to compromises, in that standardized test conditions were employed that may have favoured some populations over others. These concerns aside, Fricke & Arnqvist's (2004) data suggest a role for sexual selection in population divergence, but provide little support for any particular model of intersexual coevolution.
Attia & Tregenza (2004) performed reciprocal crosses between six strains of the beetle, Tribolium castaneum, which had been maintained under similar environmental and culture conditions for many generations. Crosses between foreign and local mates did not yield differences in the rate of successful matings, but females produced the fewest offspring when mated to males from their own population. Whether this effect was due to F1 heterosis, to sexually antagonistic male stimulation of female oviposition, or to a byproduct of female adaptations to preferentially use sperm from foreign males so that they could avoid inbreeding, could not be disentangled. Attia & Tregenza (2004) favoured the last explanation, particularly because T. casteneum females reject spermatophores at a high rate. However, another study (Pai & Yan 2002) showed that female T. castaneum display no preference for foreign males or their sperm in pre- or post-copulatory assays. Moreover, both small sample sizes for local matings (10 females/cross) and lack of control over the origin, genetic background and effective population sizes of the marked ‘tester’ strains (R. Beeman 2001, personal communication) make it difficult to interpret Attia & Tregenza's results.
Both of these studies suffer from the same problem: lack of control over the origin and selection history of the source populations. To refine this approach, we took advantage of an unusually well replicated laboratory phylogeny. Michael Rose's laboratory has maintained six large sister populations of D. melanogaster for over two decades (Rose 1984). These populations had an outbred, laboratory-adapted ancestor, and had experienced identical handling since being isolated more than 600 generations before the work we report on here began (see figure 1, §2). Thus, within this synthetic phylogeny, there has been little potential for differential natural selection among populations, but ample opportunity for intersexual coevolution within them.
Figure 1.
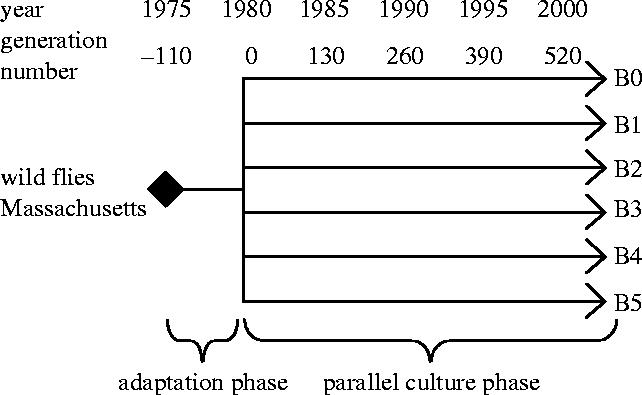
Phylogeny of experimental Drosophila melanogaster populations used in this study. The ancestral population (Ives) underwent a period of adaptation to laboratory culture before being split into six replicate populations (B0–B5) in 1980 by Dr M. R. Rose (Rose 1984) and have been maintained under similar culture conditions ever since.
We generated all possible (six within- and 30 between-population) combinations of males and females from the different populations and experimentally assayed each combination for male mating success with previously mated females, remating defence, sperm offence and defence, and net fitness. We also measured components of fitness in the F1 hybrid progeny to check for lineage effects, such as differential inbreeding depression. This manifestation of genetic drift was not expected to be important because of repeated checks over the years and substantial (over 103) population sizes. However, drift and mutation may still have taken populations along different trajectories, with sexual selection amplifying their effects. Thus we set out to see if population crosses would reveal meaningful insights into the tempo and mode of evolutionary change through sexual selection.
2. Material and methods
(a) Population history and maintenance
The experimentally evolved populations used in this study were derived from a single ancestral D. melanogaster population (Ives) that was collected in South Amherst, MA, USA in 1975 (Rose 1984). The Ives population underwent ca 110 generations of adaptation to laboratory culture before being used to create five additional replicate ‘Baseline’ populations (B1–B5) in 1980 by Rose (1984). These six populations were all handled in the same way except that the Ives population was kept at a 40% larger population size (2800±700 adults per generation in Ives versus 2000±500 adults in each B population) in the Rose Lab. All populations were cultured in parallel on a 14 day, discrete-generation schedule under identical conditions (i.e. 25 °C, 24 h light, controlled moderate density, and banana/agar/killed-yeast medium). When we obtained these populations from Dr Rose in October 2002, we applied the same culture protocol, with the exception of a 12L : 12D diurnal light cycle and standardized population sizes of 2000–2500 adults per generation for all six populations. For simplicity in reporting, and to emphasize its near-identity to the B populations, we also renamed the Ives population ‘B0’ for this paper. The populations were kept in our lab for 40 generations before population crosses were performed, by which time the total divergence between these populations was estimated at 637 generations.
(b) Experimental matings
D. melanogaster cultures were initiated (day 0) in 25×95 mm vials containing 10 ml (±1 ml) of banana/agar/killed-yeast media at a constant density of 100 eggs per vial for each population. The larvae pupated on acetate inserts on the walls of the vials, and on day 8 the pupae were removed on those inserts to holding vials provisioned with medium. We saved the natal culture vials for later use as described below. Adult flies of both sexes were collected as virgins under light CO2 anaesthesia as they eclosed from pupae on day 9. All possible crosses (six local, 30 foreign) between males and females of each population were made by combining 20 virgin flies of each sex in the females' natal vials (10 and 5 replicates per combination for local and foreign population crosses, respectively). Using this method, we were able to move males among populations with minimal interruption of the normal culture cycle.
Adults were allowed to mate and freely interact in the females' natal vials for the next five days, until the normal culture day (day 14) when all adult flies from each vial were separated under light CO2 anaesthesia into two groups (to simplify later egg counting). Both male and female flies were then transferred onto new medium, and the females allowed to oviposit for 2 h. This procedure closely mimics the normal culture regime experienced by these populations, where only the eggs laid in a single 2 h period are used to found the next generation. This assay thus provides a precise estimate of fitness under the conditions of the culture protocol that the flies had experienced for hundreds of generations.
The entire experiment was replicated on two consecutive days (i.e. two independent blocks) to control for random environmental and handling effects. Blocks were created by randomly splitting each population one generation prior to assay. The crosses within each block were handled in a random order each day and were raised in separate incubators. All eggs laid by a total of 8015 females during the experiment were counted by a single observer (TAFL). All eggs laid in each vial during a 2 h period were counted then divided by the number of females in the vial to calculate the mean number of eggs laid per female, for use in all analyses of female fecundity.
(c) Remating and sperm competition
In the generation immediately following the assessment of female fecundity, we conducted experiments on remating and post-copulatory reproductive behaviour. To do this, eggs were obtained from each of the six stock B-populations at a constant density of 100 eggs per vial, and virgin adult male and female flies were collected as they eclosed from their pupae on day 9. We also collected adult males in a similar fashion from an unrelated tester stock carrying a dominant, autosomal marker (bwD) that produces a brown-eyed phenotype. Adult flies were placed into groups of 12 in single-sex vials containing media.
In the ‘defence’ assay, virgin B-population females were first mated to B-population males in all 36 possible population crosses, replicated four times per combination (i.e. four vials of 12 pairs per group). The sexes were allowed to interact for 2 h, during which time they were watched to ensure all females had mated once. The B-population males were then removed and replaced with bwD males and the vials were put into an incubator. In the ‘offence’ assay, virgin B-females were first mated to bwD males before being introduced to B-population males following the same procedure as in the defence assay.
In both the defence and offence assays, vials were retrieved from the incubator after 18 h, and 10 females from each vial were placed into individual 13×100 mm test tubes that contained 2 ml (±0.5 ml) of media. Because single females were used and we needed sufficient progeny to score a ratio, these females were allowed to oviposit for 24 h. Egg densities in the test tubes were still low, thus minimizing any potential differences in larval competitiveness that might otherwise have confounded estimates of sperm precedence (Gilchrist & Partridge 1997).
All offspring that emerged from the test tubes were counted and scored for eye colour. Because the brown-eyed male's genotype was dominant, the presence of red-eyed offspring in the offence assay indicated that the female had remated, while in the defence assay, brown-eyed progeny indicated remating. In D. melanogaster, the most recent male to mate usually fathers the majority of the subsequent offspring (Boorman & Parker 1976; Gromko et al. 1984), which makes it very unlikely that we missed any actual cases of remating by females as both the genotype and the order of males is known. Sperm precedence statistics were defined as the proportion of red-eyed offspring in the progeny of remated females (i.e. P1 calculated in the defence assay and P2 in the offence assay).
(d) Survival and growth
We examined pre-adult viability to assess the degree to which our measure of fecundity reliably estimated overall fitness. At the same time as the remating and sperm competition assays, we conducted another mate-switching experiment following the same experimental matings protocol described above. On day 14, all flies were transferred to 200 ml containers where they were allowed to oviposit onto 35 mm diameter food plates. After 2 h, exactly 100 eggs were counted into each of five vials containing media, for each of the 36 B-population crosses. These vials were incubated for 48 h at which time they were scored for unhatched eggs before being returned to the incubator. Twelve days later all adult flies were removed and counted. We used the number of unhatched eggs and emerged adults to compute egg hatchability and egg-to-adult survivorship rates.
We also measured the body size of the adult flies that emerged from the 180 survivorship-assay vials. Males and females were each collected in groups of five, placed in a drying oven at 60 °C overnight and weighed on a Cahn C-33 microbalance to the nearest 0.001 mg. Average dry body mass per fly was calculated for each sex in each of the 36 population crosses.
(e) Statistical analyses
Analysis of variance (ANOVA) was used to test for the effects of block, male population, female population, and their interactions on female fecundity. Residuals from the full model were not significantly different from normal (Shapiro–Wilks test, p=0.23, n=426), so analyses were performed on untransformed data. Dunnett's tests were used for post hoc comparisons of mean female fecundity to compare the fecundity from mating foreign males to that from mating with local males (control), with Bonferroni adjustment of the experiment-wide significance levels when multiple tests were performed.
For those analyses involving proportional data (i.e. hatchability, egg-to-adult survivorship and post-copulatory sperm precedence), we constructed generalized linear models (GLMs) using the statistical package GLMSTAT X v. 6.0 (available at http://www.glmstat.com). In each GLM analysis, we used a logit link function and binomial error distributions, as is appropriate for dichotomous data (see Fricke & Arnqvist 2004), with scale estimated from the deviance and the degrees of freedom to compensate for overdispersion of data. We checked the validity of all GLMs by visually inspecting the residuals but no outliers were detected. We used and report log-likelihood ratio (LLR) χ2 values to test each main effect and interaction, comparing the deviance of each model including all factors with a model excluding the effect being tested (e.g. Andrés & Arnqvist 2001).
For GLMs involving P1 and P2 estimates from twice-mated females, we used the number of red-eyed progeny in each brood as the response variable, and the total number of offspring produced per female as the denominator. For analyses involving hatchability and egg-to-adult survivorship, we used the number of surviving individuals (to 48 h and 14 days, respectively) per vial as the response variables and the initial number of eggs (100) per vial as the denominator. Models were initially constructed with female population of origin, type of experimental cross (foreign or local), and the interaction between these two variables as independent factors. Non-significant interaction terms were removed from the model. For the analysis of remating propensity, we constructed logistic regression models to predict whether a female would remate (1) or not (0). Non-significant interaction terms were removed from each model to create the final model in each case.
3. Results
(a) Fitness assay
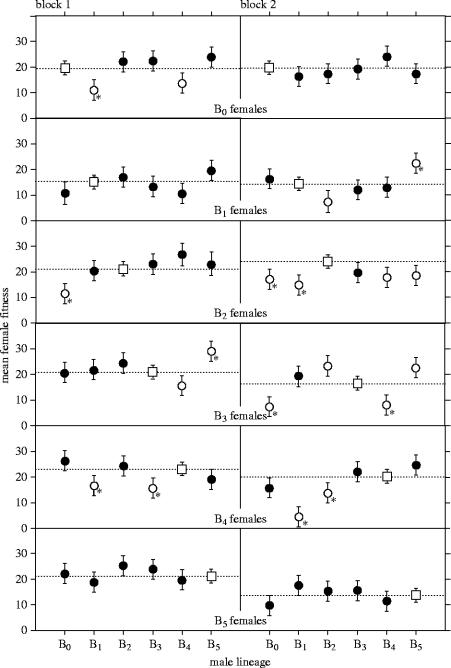
With a few notable exceptions, the net fitness of females (as measured by the number of eggs laid in a 2 h period) was similar between populations and blocks (days) when they consorted with males from their own population (i.e. local mates; figure 2). There were no significant differences among populations B0, B2, and B4 in either experimental block (p>0.05, Tukey's post hoc tests), but B1 females had significantly lower fitness than those lineages in both blocks, and both B3 and B5 females had significantly lower fitness than B2 females in the second block (figure 2). Mean fecundity also declined by approximately 10% from the first day to the second, contributing to a significant effect of block in the analysis (table 1).
Figure 2.
Female fitness (mean eggs laid in 2 h period) in each population when mated to males from each of the six experimental D. melanogaster populations studied. Data are shown for each block separately because of the significant interactions with block (table 1). Plotted here are means±95% CL (calculated from the pooled estimate of error variance). Fitnesses from matings with local males are shown as white squares and dotted lines; fitnesses from matings with foreign males are shown with circles that are either not significantly different (filled) or significantly different (open; p<0.05, Dunnett's tests) from fitnesses when mating with local males. Comparisons that remained significant after Bonferroni correction are indicated with an asterisk.
Table 1.
Analysis of variance (F=7.2, d.f.=71, 354, p<0.0001, r2=0.59) of female fitness in experimental combinations of males and females from different B-populations.
| source of variation | MS | d.f. | F | p |
|---|---|---|---|---|
| male population | 278.8 | 5 | 14.1 | <0.0001 |
| female population | 247.7 | 5 | 12.5 | <0.0001 |
| block | 1107.6 | 1 | 55.9 | <0.0001 |
| male population×female population | 124.3 | 25 | 6.3 | <0.0001 |
| male population×block | 25.4 | 5 | 1.3 | 0.27 |
| female population×block | 178.3 | 5 | 9.0 | <0.0001 |
| male population×female population×block | 99.3 | 25 | 5.0 | <0.0001 |
Mating with foreign males increased the variance among populations in female fecundity, with the coefficient of variation among mean population fitnesses increasing from 18.1 (local crosses; n=12, with six in each block) to 30.6 (foreign crosses; n=60, with 30 in each block). Over all crosses, the interaction between female and male lineages was significantly different between blocks (table 1). This three-way interaction term makes the interpretation of lower order interactions and main effects problematic. To alleviate this problem, we performed an ANOVA on the data within blocks separately (figure 2) to look for differences in female fitness when mated to local versus foreign males.
Over both experimental blocks, mating with foreign males induced changes in female fitness in both directions, ranging from −77 to +57%, relative to their fitness in local matings (figure 2). Negative outcomes to foreign crosses were slightly more frequent than positive ones (33 negative versus 27 positive), but this difference was not significant (binomial test, p=0.48). However, overall, the fitness of females mated to local males (least squares mean fitness=19.2 eggs per female in 2 h) was significantly higher than that of females mated to foreign males (17.9 eggs per female; F=4.2, p=0.04, ANOVA controlling for female, block and female×block interactions, all of which were also significant). Thus females suffered, on average, a 7% decline in fitness when mated to foreign males. These results together show that, when female fitness changed in response to cohabitation with foreign males, negative changes were greater in magnitude than were positive changes, on average.
The preponderance of significant male lineage effects on female fitness were also negative. Fifteen of the 60 foreign crosses (30 foreign crosses per block) resulted in significantly reduced female fitness (p<0.05, Dunnett's tests) compared to their fitness when housed with local males (all greater than 20% reduction in mean fitness), while 10 more had moderate, but not significant, negative effect sizes (greater than 10% reduction in mean fitness; figure 2). Correcting for multiple comparisons reduces the number of significant negative outcomes from interaction with foreign males to 10 (figure 2), involving seven different foreign male×female combinations (B1× B0, B0×B2, B1×B2, B0×B3, B4×B3, B1×B4, and B3×B4, where the male lineage is given first). In three of these foreign crosses (B0×B2, B4×B3, and B1×B4), the reduction in female fitness was significant in both blocks, and in six of the seven crosses there was a reduction in fitness in both blocks, relative to local matings.
On the other hand, four of the 60 foreign crosses (30 per block) resulted in large (all greater than 35% increases in mean fitness) and significant positive changes in female fitness, with 17 more having moderate, but not significant positive effect sizes (greater than 10% increases in mean fitness; figure 2) relative to local crosses for each population. Correcting for multiple comparisons reduces the number of significant increases in fitness to two, involving two different foreign crosses (B5×B1 and B5×B3).
(b) Defence assays
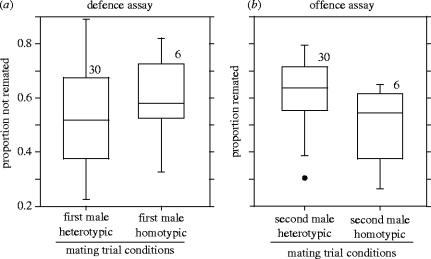
Of the 1440 females used to assay remating propensity and sperm usage in the defence assay (i.e. focal males were the first to mate), 51 (3.5%) did not lay any eggs, and were excluded from subsequent analyses. Of the 1389 females that did produce offspring, 634 showed evidence of remating (i.e. some fraction of their progeny had brown eyes), while the remaining 755 females produced only red-eyed offspring. There was no significant difference in the likelihood that a female would remate if she had initially copulated with either a local (40.9% remated, n=235) or a foreign male (46.6% remated, n=1154; figure 3a; logistic regression, Wald χ12=2.64, p=0.10, controlling for female population, interaction term not significant, p=0.15). Nonetheless, the propensity of females to remate varied significantly among populations in this model (logistic regression, Wald χ52=8.13, p<0.0001).
Figure 3.
(a) Proportion of once-mated D. melanogaster females that did not remate following mating to either a foreign or local male in the remating defence assay. (b) Proportion of once-mated D. melanogaster females that did remate to either a foreign or local male in the remating offence assay. Box plots show the 10th, 25th, 50th, 75th and 90th percentiles and all data outside this range; sample sizes are shown at the top of each box.
Looking at the propensity to remate relative to both male and female population origin in the defence assay, we found significant interaction between male and female populations (logistic regression, Wald χ252=60, p=0.0007) as well as among male (Wald χ52=12, p=0.04) and female (Wald χ52=128, p=<0.0001) populations.
(c) Offence assays
Of the 1440 females used to assay remating propensity and sperm usage in the offence assay, 68 (4.7%) did not lay any eggs, and were excluded from subsequent analyses. Of the 1372 females that did produce offspring, 827 showed evidence of remating (i.e. some fraction of their progeny had red eyes), while the remaining 545 females produced only brown-eyed offspring. Individual females were significantly less likely to remate (49.8% remated, n=233) if the second males that they had the opportunity to mate with were from their local population than if those second males were from a foreign population (62.4% remated, n=1139)(figure 3b; logistic regression, Wald χ12=13, p=0.0003, controlling for female population, interaction term not significant, p=0.64). As in the defence assay, the propensity of females to remate varied significantly among populations in this model (logistic regression, Wald χ52=36, p<0.0001).
Looking at the propensity to remate relative to both male and female population origin in the offence assay, we found significant interaction between male and female populations (logistic regression, Wald χ252=39, p=0.04) as well as among female (Wald χ52=49, p=<0.0001) but not male (Wald χ52=2.6, p=0.76) populations. Overall, the proportion of females that remated in the 36 experimental combinations was significantly correlated between the offence and defence remating assays (r=0.68, p<0.001, n=36). The frequency of remating among females varied among populations (figure 4) in both the defence (GLM: whole model: LLR χ52=141.4, p<0.0001) and offence (LLR χ52=49.7, p<0.0001) assays. There was no correlation between the estimates of first-male sperm precedence (P1) obtained from the defence assay and estimates of second-male sperm precedence (P2) from the offence assay (r=−0.08, p=0.64, n=36).
Figure 4.
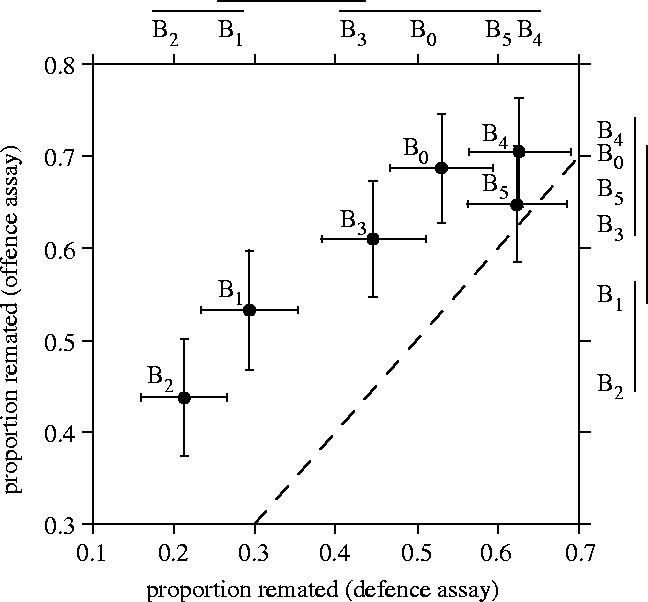
Relation between proportions (mean±95% CL) of once-mated females that remated in offence and defence assays involving six experimental D. melanogaster populations. The dashed line indicates equal proportions in both Defence and Offence assays. Means not connected by a solid line above and to the right of the graph are significantly different according to Tukey–Kramer HSD tests.
(d) Survival and body size assays
Overall, egg hatchability was very high (mean=98%, range 91–100%, n=180 vials), but differed among mating combinations (whole model: LLR χ62=23.3, p=0.003, with non-significant interaction term removed from model). There was no difference between the proportion of eggs that hatched in local versus foreign crosses (LLR χ12=1.21, p=0.31). There was, however, significant variation in egg hatchability among the maternal populations (LLR χ52=22.1, p=0.002). Similarly, the pattern for egg-to-adult survivorship also differed among mating combinations (whole model; LLR χ62=41.8, p=0.02, with non-significant interaction term removed from model), but there was no difference between the survivorship of offspring produced through local and foreign crosses (LLR χ12=5.9, p=0.14). Again there was a significant maternal effect reflecting variation in survivorship based on population of origin of the female parent (LLR χ52=36.0, p=0.02).
Incorporating egg-to-adult survivorship into our previous estimates of female fitness based solely on egg-laying rates did not appreciably change any results (analyses not shown), presumably because egg-to-adult survivorship was not correlated with average female fecundity across all crosses (r=0.24, p=0.14, n=36 crosses). As a result, the correlation between fitness based on fecundity alone and fitness based on fecundity and survival was near unity (r=0.99, p<0.0001, n=36).
The masses of F1 adult males from crosses between local versus foreign mates were not significantly different, nor was there a significant difference based on maternal population (table 2). When the interaction term was removed from the model, the mass of adult males varied significantly among maternal populations (F=2.3, d.f.=5, 173, p=0.05) but not between local and foreign crosses (F=0.33, d.f.=1, 173, p=0.57).
Table 2.
Analyses of variance testing the effects of type of experimental mating (foreign versus local) and female population origin on adult male (F=1.91, d.f.=11, 168, p=0.04) and adult female (F=3.5, d.f.=11, 168, p=0.0002) masses of offspring produced by those crosses in D. melanogaster.
| variable | source of variation | SS | d.f. | F | p |
|---|---|---|---|---|---|
| adult male mass | foreign versus local mating | 1.2×10−3 | 1 | 0.33 | 0.56 |
| female population | 7.1×10−5 | 5 | 1.14 | 0.34 | |
| foreign versus local mating×female population | 1.9×10−3 | 5 | 1.79 | 0.12 | |
| adult female mass | foreign versus local mating | 4.8×10−3 | 1 | 7.38 | 0.007 |
| female population | 7.1×10−3 | 5 | 2.19 | 0.06 | |
| foreign versus local mating×female population | 10.7×10−3 | 5 | 3.27 | 0.008 |
The analysis of F1 adult female mass revealed a significant interaction between the effects of female population origin and type of cross (foreign versus local). When females from all B-populations, except B4, were mated to foreign males, they produced daughters that were, on average, significantly heavier than those produced from matings with local males (means±s.e. mass of females produced from: foreign crosses=0.330 mg±0.005, n=150 sample means; local crosses=0.316 mg±0.002, n=30 sample means; t-test, t=1.97, p=0.05).
Differences in body size can be correlated with the amount of harm that males inflict on their mates (Pitnick & Garcia-Gonzalez 2002). Thus, some line effects may be pleiotropic effects if the replicate B-populations had diverged in size while in allopatry for greater than 600 generations. However, we found no difference in the average mass of purebred adults obtained from these six populations for either males (F=0.98, d.f.=5, 24, p=0.45) or females (F=1.4, d.f.=5, 24, p=0.25), suggesting that there has been no divergence in body size among populations.
4. Discussion
The B-populations we used in this study have shown considerable homogeneity for the several life history and morphological traits that have been measured (see papers compiled in Leroi et al. 1994; Rose et al. 2004; A. K. Chippindale 1994, unpublished analyses of longevity) over the past 23 years they have been studied. On the surface, then, it appears that these populations have evolved in parallel, or reached an evolutionary equilibrium point for the environmental and demographic conditions of their routine culture. However, it is possible that these populations have diverged through sexual selection or genetic drift influencing characters that have not been measured. In particular, behavioural and post-copulatory biochemical traits (e.g. accessory peptides) may have diverged rapidly if the sexes are coevolving antagonistically. We, therefore, performed crosses among the B-populations to break up any coevolved relationships that may have developed over the 637 generations of experimentally enforced allopatry.
With the exception of the B1 population, our data show that female fitness was similar for most of the replicate populations when they cohabited with males from their own population. However, several instances of strong inter-population interaction emerged when populations were crossed. This result would be expected if rapid coevolution had occurred, but a variety of higher-order interactions and block effects complicate the interpretation of this result. We, therefore, also examined ancillary fitness traits related to male mating success and sperm competition. These traits showed clearer patterns associated with outcrossing, suggesting local adaptation of one or both sexes to the other in pre- and post-copulatory components of fitness.
(a) Fitness, interactions, and the diagnosis of sexual conflict from crosses
Performing a reciprocal cross experiment, as we have done, presents a number of analytical challenges. Foremost among these is giving up the population replication normally used in experimental evolution studies by creating an array of unique crosses. While B1 and B2, etc. are replicates of an experimental treatment, we have only one B1×B2 cross in the experimental matrix. We approached this problem by executing the entire fitness assay in two independent blocks. It is common to see significant block effects, even when flies are handled the same way on successive days, but we also encountered significant interactions between female population and block, and a significant three-way factor (male×female×block) that all make the full factorial ANOVA model difficult to interpret. The three-way interaction is particularly troublesome because it suggests that the consequences of intersexual interactions depended upon both the specific populations that were mixed and any subtle handling differences between days in the assay. In fact, about half (16/30) of the foreign population matings changed fitness between blocks from positive to negative (or vice versa) relative to the local mate treatment for that female lineage in that block.
Some aspects of this three-way interaction may have originated in handling differences—despite large sample sizes, some features of the fitness assay may have emphasized experimental error. In particular, the egg-laying window for our fitness estimate was brief (2 h), and followed shortly after sorting under CO2 anaesthesia. Alternatively, this sensitivity may be genetically mediated and of interest, reflecting instability in the response of females to novel partners. Thus, even small differences in handling from one generation to the next may have contributed to the maintenance of genetic variation in these populations. Since complete replication of an experiment like this is rare in the literature on population crosses, it is difficult to know how widespread the sensitivity that we uncovered might be. But, if common, it may help to explain the inconsistent outcomes of past studies.
Despite a low signal-to-noise ratio in these fitness data, we still see a net trend towards negative outcomes. Thus, the majority of significant changes in fitness were negative (15 (10) decreases, seven of which were unique combinations of mates, versus four (2) increases; values in parentheses corrected for multiple comparisons), and there was a significant overall decline in female fitness when they were mated to foreign males. Given that B1 had the lowest fitness, it is interesting to note that B1 males accounted for four of the ten significant Bonferroni-corrected negative outcomes in foreign crosses. B0 males accounted for three of the other significant negative outcomes, while B5 males were involved in the two significant increases. If general ‘armament level’ varied and coevolution had occurred (e.g. as suggested by Arnqvist & Rowe 2002 for water striders), then one might expect B1 and B0 females to show the highest defence levels, and therefore the greatest increase in fitness in response to foreign males, with B5 females showing the opposite pattern. The fact that this did not occur suggests that, if antagonistic coevolution has taken place, it has not done so through investment in some general trait, like body size or armament level. Our body mass analysis also supports this conclusion as there was little variation in adult body mass among populations.
The idea that foreign males should have a deleterious impact on female fitness is built on the assumption that males either over-stimulate females at an inopportune time (e.g. force higher immediate fertility, thereby compromising later fertility) or cause direct harm to females as an incidental byproduct of such manipulations. However, depressed female fitness in interpopulation crosses is not unequivocally diagnostic of evolutionary conflict between the sexes (Panhuis et al. 2001). For example, reduction of fitness in a cross could also result from the breakdown of coevolved cooperation between mates if, say, foreign males fail to stimulate beneficial reproductive responses, or do so at the wrong time (Pizzari & Snook 2003, Pitnick et al. 2003). Mutualistic interaction may even be more likely to evolve when trade-offs between current and future reproduction are less significant, as may be the case for flies with a brief adult lifespan like most lab Drosophila. Therefore, we side with Pizzari & Snook (2003) and Rowe et al. (2003) in interpreting reduced fitness in interpopulation crosses with caution. At the same time, these data do suggest that some of the B-populations have diverged due to sexual selection.
But what, if anything, can be made of positive outcomes to interpopulation crosses? In theory, increases in female fecundity in response to foreign males may reveal part of the gender load (Rice & Chippindale 2002) imposed by sexually antagonistic coevolution within populations. In other words, these increases in fitness reflect instances in which females were fortuitously adapted to resist novel male antagonistic traits, allowing them to escape the load established during evolution with their own, locally coadapted mates. There is good evidence that Drosophila males can and do adapt to the specific attributes of females they interact with, and that this reflects antagonistic coevolution between the sexes (Rice 1996, 1998). However this does not mean that males ‘lead’ in the coevolutionary arms race. If females are relatively well defended, and there are few and specific points of vulnerability available that males can exploit, then males should be locally adapted and the predictions are reversed—males from foreign populations should, on average, have ineffective offensive strategies. The increases in fitness seen in some of our crosses could, therefore, be similar to the increase in vigour that is sometimes seen when invasive species escape from the predators and parasites in their native range.
If positive outcomes to crosses represent this kind of escape from antagonistic adaptations carried by mates, then a wide variety of mate combinations will help to establish the magnitude of harm. The differential between the highest female fitness achieved with a foreign male and the fitness achieved with local males by the same female line in this study suggests that female fitness may be depressed by as much as 57% due to male specialization. Alternatively, independent neutral allelic substitutions may have accumulated by chance in different lines, and have beneficial fitness effects realized only in combination. We consider this possibility unlikely for two reasons. First, the observed effects are direct and restricted to female fecundity (i.e. they do not appear to result from hybrid vigour or dysgenesis). Second, there will be constant selection on females to capitalize on fecundity-enhancing traits carried by their mates. Because selection in the laboratory life cycle is uncomplicated by trade-offs between current and future reproduction after about four days of adult age, females should do everything possible to maximize fertility at that age, even if the outcome is an effectively semelparous life-cycle.
Another possibility is the kind of adaptive inbreeding avoidance suggested by Attia & Tregenza (2004), involving discrimination against local males to accrue benefits from hybrid vigour. We have some weak evidence (i.e. slightly heavier F1 females, but not males, in most crosses between populations; see Kidwell & Kidwell (1966)), but for the same reasons noted above, and due to the history of large controlled population sizes in the B-populations, we do not suspect that inbreeding has played a major role in these populations. Nonetheless we cannot exclude the possibility that genetic drift in general, or inbreeding specifically, has contributed measurably to population divergence. These are not exclusive of coevolutionary explanations and may account for some of the bidirectionality of outcomes and noise associated with assaying fitness in the population crosses.
(b) Mechanisms of population interaction: mating
The ability to acquire copulations with non-virgin females is critical to male fitness because (i) females mate shortly after maturing, (ii) they store enough sperm to lay eggs for an extended period of time, and (iii) strong sperm precedence means that the last male sires the majority of the offspring (Gromko et al. 1984). In our remating offence assay, females who had mated once with the dominantly marked (bwD) tester males remated significantly more frequently when housed with foreign males than with local males. Rather than physical coercion, Drosophila males use pheromonal cues, displays and persistent harassment to increase their mating success (e.g. Greenspan & Ferveur 2000). Thus it seems most likely that foreign males, or their courtship signals, were in some way more attractive to females than were local males, rather than that males expended more energy in courtship when they encountered unfamiliar females. Selection in isolated populations should not favour males that show restraint in pursuing local mates. Overall, the finding that females mated more readily with foreign males is in accord with models of sexually antagonistic coevolution that envision males evolving traits to ‘antagonistically seduce’ females (Rice & Holland 1997).
The remating defence assay, in which females had the opportunity to remate with tester-males following copulation with males from one of the B-populations, failed to reveal significant differences between local and foreign males. Female remating propensity was substantially lower than observed in the offence assay (see figure 3), presumably because males expressing bwD are less fit than wild-type males. Either their lack of courtship vigour or discrimination against them by wild-type (B-population) females would have made them less stringent competitors in the experiment, perhaps weakening this test. Furthermore, because the bwD tester population is more foreign to females than any of the B-populations, its use highlights important questions for experimental design—are males from this stock equivalent in their effects on all B-population females, or are they more like one population or another by chance? The probability that the tester-population was similar to one or more B-populations clearly depends upon the number and complexity of traits involved in mating interactions.
Overall, one of the more surprising results from our experiments was the extensive variation in female mating rate that was independent of male type. Whether females encountered local or foreign B-population, or foreign tester males as second suitors, remating propensity varied by 2–3-fold based on the population of origin of the female and was consistent between assays (see figure 4). Interpreting the presence of heritable variation for polyandry presents a challenge. If there are sizeable fitness costs to females associated with high mating rate, as has been suggested in D. melanogaster (Fowler & Partridge 1989; Chapman et al. 1995; Linder & Rice 2005) then one would not expect the observed level of variation among populations. There was also no suggestion that females from high-remating populations (i.e. B0, B4, B5) differed in overall fitness or in the outcome of interaction with foreign mates compared to females from relatively low-remating populations (figure 2). In other words, these results are surprising because there appears to be considerable variation in the opportunity for sexual conflict that is not expressed in simple female lineage effects. It may be that our assays, which looked at the probability of remating over the 18 h subsequent to a female's first copulation, do not reflect mating dynamics over the 4–5 days that the sexes normally interact before a new generation is cultivated in the lab. For now, the variation observed among females in remating propensity is an observation awaiting explanation.
(c) Mechanisms of population interaction: sperm competition
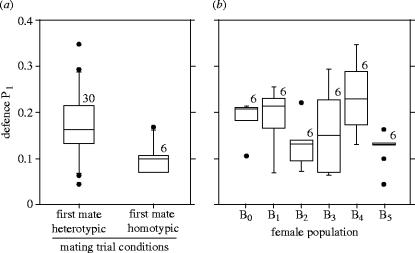
In the sperm defence assays (figures 5 and 6), the amount of first male sperm that was retained by the female depended upon whether the male was local or foreign, and upon the female's population of origin. Thus, as shown by Clark & Begun (1998) for inbred stocks, female genotype can influence the outcome of sperm competition at the population level. Moreover, there was a strong and consistent advantage to foreign males. Foreign males were 80% more successful than local males, on average, at maintaining sperm in the female's reproductive tract following remating by a female with a tester-male. This finding suggests better sperm binding in the female reproductive tract by foreign males, conferring resistance to either (i) the challenge of second male sperm and accessory proteins or (ii) lower sperm dumping (Snook & Hosken 2004) by females during remating. If females who remate do so to discharge aged or otherwise unwanted sperm, then this result would suggest more effective manipulation by foreign males, and thus sexual conflict. However, females may also have biased this measure of P1 because they find foreign males more attractive (as suggested by our remating results), or the sperm from these males may be more resistant to displacement for other reasons (e.g. superior survival or vigour).
Figure 5.
Proportion of offspring fathered by the first (wild-type) male to mate with doubly mated D. melanogaster females (defence P1). (a) Defence P1 means from broods when the first male was either foreign or local. (b) Defence P1 means separated according to female population of origin. Box plots as in figure 3.
Figure 6.
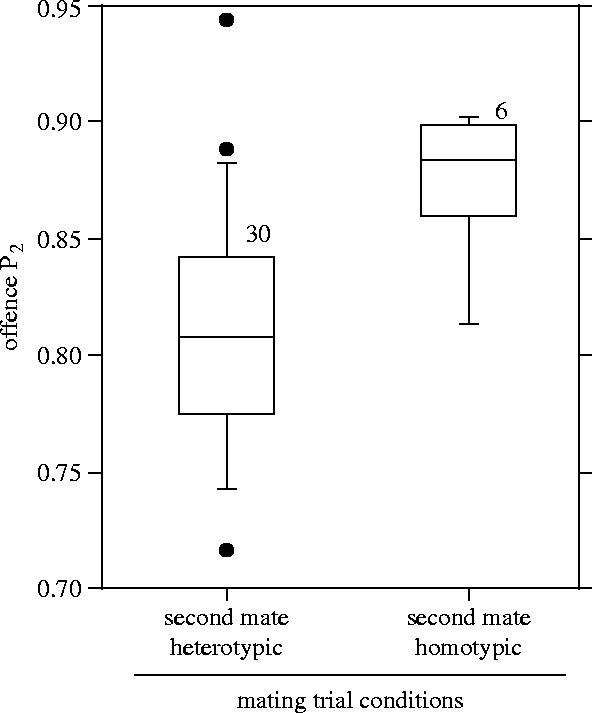
Proportion of offspring fathered by the second (wild-type) male to mate with doubly mated D. melanogaster females (offence P2) when the second male was either foreign or local. Box plots as in figure 3.
In the sperm offence assay, local males were more successful than foreign males in the P2 role. Although these results are consistent with many documented cases of conspecific sperm precedence (reviewed in Howard 1999), they contrast with Hosken et al. (2002) who found that foreign yellow dung flies males were more successful (greater P2) in post-copulatory competition than were local males. In that species, where females cryptically sort sperm (Ward 2000), it may be that the optimal level of sperm displacement for females is fairly low. If similar manipulation of sperm occurs in Drosophila, then this finding may reflect the release of more sperm from storage by females following copulation with a local second male than with a foreign male (i.e. evolved cooperation between mates). Alternatively, the reduced P2 of foreign males reported here may reflect a mismatch between the sperm, or other components of the ejaculate, and the female reproductive tract, having nothing to do with female intervention. For this reason, divergence occurring due to genetic drift or male–male intrasexual competition could also explain these results.
5. Conclusions
Our most exciting finding is that sexual selection appears to have driven substantial population differentiation without noticeable phenotypic differences accruing among these same populations. We emphasize adaptive processes as potential drivers of this divergence because the data from our experimental crosses revealed little evidence of F1 hybrid vigour, and large population sizes have been kept up throughout the evolution of the B-populations. Furthermore, in contrast to the net fitness data, the consistent outcomes of the remating and sperm competition assays suggest some form of local adaptation of one sex to the other, or coadaptation between mates, rather than a purely random process.
The lack of obvious genetic correlations among traits related to mating (courtship) success and sperm competition in males may help to explain why so many different fitness outcomes were observed in interpopulation crosses. The agents of male manipulation—presumably courtship and mating behaviours, pheromones and seminal fluid peptides—are known to have measurable and often harming effects on components of female fitness. If these male traits have evolved independently, then each population of males presents a potentially complex and different set of locally adapted signals. In crosses with foreign females, some of these signals may work while others do not, assuming the female's receiver system has comparable complexity. This complexity may tend to average the impact of males via the cancelling among multiple effects, and perhaps explain why fitness was so sensitive to block effects. Thus, while sexual selection may have been important in shaping each of the male fitness traits that we measured, their amalgamation in a complete phenotype may have variously muted or exaggerated the direction and magnitude of their influence on female fitness. These are speculations that deserve more detailed investigation through, for example, simultaneous molecular genetic analyses of both male and female transcriptomes.
Given the difficulties encountered in interpreting the results from (what we thought was) an ideal system, it would be tempting to dismiss the population-cross approach altogether. Rather, we suggest that crosses may be poor tools for establishing the mechanisms of evolutionary change, but can provide a useful first step towards identifying population divergence. Whether or not we can define the specific mechanisms involved, it is clear that 637 generations of allopatric evolution in Drosophila were sufficient to generate substantial divergence in reproductive characters. Laboratory phylogenies like the one we used hold the potential to resolve questions about the tempo of evolutionary change that cannot be addressed by an arbitrary snapshot taken during the process of evolution. Given the importance of recreating the context under which fitness has evolved, and interpreting specific responses in that light, laboratory systems may present the most powerful application for population crosses in diagnosing the nature of intersexual coevolution. This conclusion is ironic because one promise of population crosses has been their simplicity, and the ease with which they can be applied to a variety of species.
Finally, this work suggests a novel application for population crosses in diagnosing sexual conflict. Positive effects of population crosses on female fitness may reveal the load placed on those same females by local males. Just as isolating females from males can increase their lifetime fitness (e.g. Chapman et al. 1995, Linder & Rice 2005), replacing their normal mates with poorly coadapted males may also reveal the cost of interlocus sexual conflict. Curiously, establishing the reasons for increased fitness in crosses between populations may be important in determining the sources of conflict within them. At the same time, intralocus sexual conflict (e.g. Rice & Chippindale 2002), in which alleles favoured in one sex are selected despite being disfavoured in the other, will further add to this load. Thus, the accumulating evidence from Drosophila suggests that gender load substantially reduces female, and therefore, population fitness. This adds to the catalogue of known costs to males, and to the cost of sexual reproduction in general.
Acknowledgements
We thank H. Castillo, T. Day, C. Eckert, S. Lougheed, and W. Rice for their extensive comments and discussions as well as the hard-working members of the Chippindale lab for their fly pushing. M. Rose generously provided us with the B-population flies. T. Tregenza and two anonymous reviewers are thanked for providing insightful comments on drafts of the manuscript. This research was funded though grants to A.C. and R.M. from the Natural Sciences and Engineering Research Council of Canada, to R.M. from a Killiam Research Fellowship; and the Canada Foundation for Innovation and the Canada Research Chairs program to A.C. T.A.F.L. was supported by a NSERC PGS-B Scholarship.
Footnotes
One contribution of 13 to a Discussion Meeting Issue ‘Sexual conflict: a new paradigm?’.
References
- Andrés J.A, Arnqvist G. Genetic divergence of the seminal signal-receptor system in houseflies: the footprints of sexually antagonistic coevolution? Proc. R. Soc. B. 2001;268:399–405. doi: 10.1098/rspb.2000.1392. 10.1098/rspb.200.1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arak A, Enquist M. Hidden preferences and the evolution of signals. Phil. Trans. R. Soc. B. 1993;340:207–213. [Google Scholar]
- Arak A, Enquist M. Conflict, receiver bias and the evolution of signal form. Phil. Trans. R. Soc. B. 1993;349:337–334. doi: 10.1098/rstb.1995.0122. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Edvardsson M, Friberg U, Nilsson T. Sexual conflict promotes speciation in insects. Proc. Natl Acad. Sci. USA. 2000;97:10 460–10 464. doi: 10.1073/pnas.97.19.10460. 10.1073/pnas.97.19.10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia F.A, Tregenza T. Divergence revealed by population crosses in the red flour beetle Tribolium castaneum. Evol. Ecol. Res. 2004;6:927–935. [Google Scholar]
- Bateman A.J. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Boorman E, Parker G.A. Sperm (ejaculate) competition in Drosophila melanogaster, and the reproductive value of females in relation to female age and mating status. Ecol. Entomol. 1976;1:145–155. [Google Scholar]
- Chapman T, Liddle L.F, Kalb J.B, Wolfner M.F, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. 10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. 10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Clark A.G, Begun D.J. Female genotypes affect sperm displacement in Drosophila. Genetics. 1998;149:1487–1493. doi: 10.1093/genetics/149.3.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler G.L, Partridge L. A cost of mating in female fruit flies. Nature. 1989;338:760–761. 10.1038/338760a0 [Google Scholar]
- Fricke C, Arnqvist G. Divergence in replicated phylogenies: the evolution of partial post-mating prezygotic isolation in bean weevils. J. Evol. Biol. 2004;17:1345–1354. doi: 10.1111/j.1420-9101.2004.00757.x. 10.1111/j.1420-9101.2004.00757.x [DOI] [PubMed] [Google Scholar]
- Gavrilets S. Rapid evolution of reproductive barriers driven by sexual conflict. Nature. 2000;403:888–889. doi: 10.1038/35002564. 10.1038/35002564 [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Arnqvist G, Friberg U. The evolution of female mate choice by sexual conflict. Proc. R. Soc. B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. 10.1098/rspb.2000.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist A.S, Partridge L. Heritability of pre-adult viability differences can explain apparent heritability of sperm displacement ability in Drosophila melanogaster. Proc. R. Soc. B. 1997;264:1271–1275. doi: 10.1098/rspb.1997.0175. 10.1098/rspb.1997.0175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan R.J, Ferveur J.-F. Courtship in Drosophila. Annu. Rev. Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. 10.1146/annurev.genet.34.1.205 [DOI] [PubMed] [Google Scholar]
- Gromko M.H, Gilbert D.G, Richmond R.C. Sperm transfer and use in the multiple mating system of Drosophila melanogaster. In: Smith R.L, editor. Sperm competition and the evolution of animal mating systems. London; Academic Press: 1984. pp. 371–426. [Google Scholar]
- Holland B, Rice W.R. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. 10.1073/pnas.96.9.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken D.J, Blanckenhorn W.U, Garner T.W.J. Heteropopulation males have a fertilization advantage during sperm competition in the yellow dung fly (Scathophaga stercoraria) Proc. R. Soc. B. 2002;269:1701–1707. doi: 10.1098/rspb.2002.2094. 10.1098/rspb.2002.2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D.J. Conspecific sperm and pollen precedence and speciation. Ann. Rev. Ecol. Syst. 1999;30:109–132. 10.1146/annurev.ecolsys.30.1.109 [Google Scholar]
- Kidwell J.F, Kidwell M.M. The effects of inbreeding on body weight and abdominal chaeta number in Drosophila melanogaster. Can. J. Genet. Cytol. 1966;8:207–215. doi: 10.1139/g66-026. [DOI] [PubMed] [Google Scholar]
- Leroi A.M, Chippindale A.K, Rose M.R. Long-term laboratory evolution of a genetic life-history trade-off in Drosophila melanogaster. 1. The role of genotype-by-environment interaction. Evolution. 1994;48:122–1257. doi: 10.1111/j.1558-5646.1994.tb05309.x. [DOI] [PubMed] [Google Scholar]
- Linder J.E, Rice W.R. Natural selection and genetic variation for female resistance to harm from males. J. Evol. Biol. 2005;18:568–575. doi: 10.1111/j.1420-9101.2004.00872.x. 10.1111/j.1420-9101.2004.00872.x [DOI] [PubMed] [Google Scholar]
- Martin O.Y, Hosken D.J. The evolution of reproductive isolation through sexual conflict. Nature. 2003;423:979–982. doi: 10.1038/nature01752. 10.1038/nature01752 [DOI] [PubMed] [Google Scholar]
- Pai A, Yan G. Female choice in relation to heterozygosity in Tribolium castaneum. J. Evol. Biol. 2002;15:1076–1082. 10.1046/j.1420-9101.2002.00456.x [Google Scholar]
- Panhuis T.M, Butlin R, Zuk M, Tregenza T. Sexual selection and speciation. Trends Ecol. Evol. 2001;16:364–371. doi: 10.1016/s0169-5347(01)02160-7. 10.1016/S0169-5347(01)02160-7 [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sexual selection and sexual conflict. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; New York: 1979. pp. 123–163. [Google Scholar]
- Parker G.A, Partridge L. Sexual conflict and speciation. Phil. Trans. R. Soc. B. 1998;353:261–274. doi: 10.1098/rstb.1998.0208. 10.1098/rstb.1998.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S, García-Gonzàlez F. Harm to females increases with male body size in Drosophila melanogaster. Proc. R. Soc. B. 2002;269:1821–1828. doi: 10.1098/rspb.2002.2090. 10.1098/rspb.2002.2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S, Miller G.T, Schneider K, Markow T.A. Ejaculate-female coevolution in Drosophila mojavensis. Proc. R. Soc. B. 2003;270:1507–1512. doi: 10.1098/rspb.2003.2382. 10.1098/rspb.2003.2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Snook R. Sexual conflict and sexual selection: chasing away paradigm shifts. Evolution. 2003;57:1223–1236. doi: 10.1111/j.0014-3820.2003.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Promislow D.E.L, Smith E.A, Pearse L. Adult fitness consequences of sexual selection in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 1998;95:10 687–10 692. doi: 10.1073/pnas.95.18.10687. 10.1073/pnas.95.18.10687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. 10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Rice W.R. Intergenomic conflict, interlocus antagonistic coevolution and the evolution of reproductive isolation. In: Howard D.J, Berlocher S.H, editors. Endless forms—species and speciation. Oxford University Press; New York: 1998. pp. 261–270. [Google Scholar]
- Rice W.R, Chippindale A.K. The evolution of hybrid infertility: perpetual coevolution between gender-specific and sexually antagonistic genes. Genetica. 2002;116:179–188. 10.1023/A:1021205130926 [PubMed] [Google Scholar]
- Rice W.R, Holland B. The enemies within: intergenomic conflict, interlocus contest evolution (ICE) and the intraspecific Red Queen. Behav. Evol. Sociobiol. 1997;41:1–10. 10.1007/s002650050357 [Google Scholar]
- Rose M.R. Laboratory evolution of postponed senescence in Drosophila melanogaster. Evolution. 1984;38:1004–1010. doi: 10.1111/j.1558-5646.1984.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Rose M.R, Nusbaum T.J, Chippindale A.K. The experimental Wonderland and the Cheshire cat syndrome. In: Rose M.R, Lauder G.V, editors. Adaptation. Academic Press; New York: 1996. pp. 221–241. [Google Scholar]
- Rose M.R, Passananti H.B, Matos M, editors. Methuselah flies: a case study in the evolution of ageing. World Scientific; Singapore: 2004. [Google Scholar]
- Rowe L, Cameron E, Day T. Detecting sexually antagonistic coevolution with population crosses. Proc. R. Soc. B. 2003;270:2009–2016. doi: 10.1098/rspb.2003.2453. 10.1098/rspb.2003.2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook R.R, Hosken D.J. Sperm death and dumping in Drosophila. Nature. 2004;428:939–941. doi: 10.1038/nature02455. 10.1038/nature02455 [DOI] [PubMed] [Google Scholar]
- Swanson W.J, Vacquier V.D. The rapid evolution of reproductive proteins. Nat. Rev. Gen. 2002;3:137–144. doi: 10.1038/nrg733. 10.1038/nrg/733 [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man, 1871–1971. Aldine-Atherton; Chicago: 1972. pp. 136–179. [Google Scholar]
- Ward P.I. Cryptic female choice in the yellow dung fly Scathophaga stercoraria. Evolution. 2000;54:1680–1686. doi: 10.1111/j.0014-3820.2000.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Female resistance to male harm evolves in response to manipulation of sexual conflict. Evolution. 2004;58:1028–1037. doi: 10.1111/j.0014-3820.2004.tb00436.x. [DOI] [PubMed] [Google Scholar]