Abstract
Inter-locus sexual conflict occurs by definition when there is sexually antagonistic selection on a trait so that the optimal trait value differs between the sexes. As a result, there is selection on each sex to manipulate the trait towards its own optimum and resist such manipulation by the other sex. Sexual conflict often leads additionally to the evolution of harmful behaviour and to self-reinforcing and even perpetual sexually antagonistic coevolution. In an attempt to understand the determinants of these different outcomes, I compare two groups of traits—those related to parental investment (PI) and to mating—over which there is sexual conflict, but which have to date been explored by largely separate research traditions. A brief review suggests that sexual conflict over PI, particularly over PI per offspring, leads less frequently to the evolution of manipulative behaviour, and rarely to the evolution of harmful behaviour or to the rapid evolutionary changes which may be symptomatic of sexually antagonistic coevolution. The chief determinants of the evolutionary outcome of sexual conflict are the benefits of manipulation and resistance, the costs of manipulation and resistance, and the feasibility of manipulation. All three of these appear to contribute to the differences in the evolutionary outcome of conflicts over PI and mating. A detailed dissection of the evolutionary changes following from sexual conflict exposes greater complexity than a simple adaptation–counter-adaptation cycle and clarifies the role of harm. Not all of the evolutionary changes that follow from sexual conflict are sexually antagonistic, and harm is not necessary for sexually antagonistic coevolution to occur. In particular, whereas selection on the trait over which there is conflict is by definition sexually antagonistic, collateral harm is usually in the interest of neither sex. This creates the opportunity for palliative adaptations which reduce collateral harm. Failure to recognize that such adaptations are in the interest of both sexes can hinder our understanding of the evolutionary outcome of sexual conflict.
Keywords: sexual conflict, sexually antagonistic selection, sexually antagonistic coevolution, parental investment, fecundity, mating
1. Introduction
The expression ‘sexual conflict’ encapsulates the capacity of individuals of one sex to inflict damage on individuals of the other sex. In the original definition by Parker (1979)—‘a conflict between the evolutionary interests of individuals of the two sexes’—and ongoing use by evolutionary biologists, this damage is in terms of genetic fitness, so that all instances of sexual conflict are by definition underlain by sexually antagonistic selection. Not all traits involved in interaction between the sexes are subject to antagonistic selection, but when a trait is, the antagonistic selection favours the evolution of other ‘manipulative’ traits in each sex that cause the trait over which there is conflict (the ‘conflict trait’) to evolve towards their own optimum. Such evolutionary responses to inter-locus sexual conflict often seem to beget further conflict: first, the manipulative traits themselves sometimes have a negative impact on individuals of the other sex—in other words, harmful behaviour evolves. (‘Behaviour’ is used here in a broad sense in the same way as ‘strategy’ is used in game theory (Maynard Smith 1982) to include morphological and physiological traits); second, sexual conflict by definition implies reciprocal selection pressures, and in some cases this appears to lead to ongoing antagonistic coevolution in which each evolutionary gain by one sex provokes a counteracting evolutionary response in the other (Parker 1979; Rice & Holland 1997; Rice 1998, 2000). Much of the recent work on sexual conflict has focused on these two features and emphasized their generality as outcomes of sexually antagonistic selection (Rice & Holland 1997; Rice 1998, 2000; Chapman et al. 2003a). However, not all sexual conflict leads to the evolution of harmful behaviour or ongoing antagonistic coevolution, and an equally interesting question is why that should be so: what are the characteristics of particular instances or types of sexual conflict that lead to these kinds of evolutionary outcome?
This paper represents an initial attempt at addressing this question, using a comparison of sexual conflict over parental investment (PI) and over mating as a framework for thought. Sexual conflict potentially occurs over a wide range of traits from sex ratios to whether to accept helpers-at-the-nest (Lessells 1999), but the overwhelming majority of research has centred on conflicts over either PI or mating. Moreover, research in these two areas has developed relatively independently: the PI ‘tradition’ relies heavily on game theoretical models lacking manipulative or harmful behaviour and seeking evolutionarily stable strategies (Maynard Smith 1977; Grafen & Sibly 1978; Houston & Davies 1985; Houston et al. 2005). In contrast, the mating conflict ‘tradition’ tends to emphasize the lack of long-term stable evolutionary outcomes and the widespread occurrence of harmful behaviour. Motivated by these differences, this paper asks two main questions: first, is it indeed true that conflicts over PI lead to more stable and less manipulative and harmful outcomes than conflicts over mating? Second, if this is true, what are the features of conflict over PI and mating that result in these divergent evolutionary outcomes? In so doing, the paper may serve two purposes: first, in empirical studies, to stimulate alertness to the possibility of manipulative and harmful behaviour in the resolution of conflicts over PI and, more importantly, to stimulate comparative studies where meaningful comparisons can be made of the frequency of different kinds of evolutionary outcome in response to different kinds of conflict, and second, on a theoretical level, to encourage further thought about the factors that are responsible for different kinds of evolutionary outcome.
2. The subjects of sexual conflict
(a) Parental investment
PI is defined as ‘any investment by the parent in an individual offspring that increases the offspring's chance of surviving (and hence of reproductive success) at the cost of the parent's ability to invest in other offspring’ (Trivers 1972). PI therefore consists of investment in gametes (Parker et al. 1972; Parker 1978) and parental care of offspring (Clutton-Brock 1991). In both of these cases, the cost of PI by each parent is generally paid by that parent alone, while the benefit in terms of fitness gained through the (eventual) offspring is accrued by both parents. The inevitable consequence is that there is conflict over PI: for each parent's investment, that parent's optimum is lower than the other parent's. The only time that this is not the case is when there is ‘true’ monogamy, in other words monogamy is complete and lifelong, with neither partner able to remate after the death of their mate (but see below for comments regarding PI per offspring and fecundity). True monogamy appears to exist only as a theoretical reference point rather than a practical eventuality (at least outside experimental laboratory systems; Holland & Rice 1999; Pitnick et al. 2001; Martin & Hosken 2003). This implies that, whatever the evolutionary resolution of conflict over PI, the sexual conflict itself (i.e. sexually antagonistic selection) continues to exist. One point to bear in mind is that in any reproductive attempt, sexual conflict over PI concerns two traits: PI by the female and PI by the male. The argument above that sexual conflict over PI is a logical consequence of sexual reproduction applies equally to PI by the male and PI by the female.
The total PI of a parent depends on the PI per offspring and fecundity, and for reasons given above sexual conflict must occur over one or both of these components. When there is no parental care in any sense (including, for example, searching for suitable oviposition sites), the total fitness accrued through offspring will increase linearly with fecundity. In this case, females maximize their fitness by maximizing the number of eggs that they lay over their entire lifetime. However, if females mate multiply, males gain fitness only through the eggs that they fertilize, and this proportion will generally drop when the female with whom they have mated remates. As a result, a male's fitness will be maximized by a higher oviposition rate in the immediate future than would maximize the female's fitness (Simmons 2001) and there will always be sexual conflict over fecundity.
In contrast, when a parent provides care, its optimum clutch size is generally intermediate (between minimum and maximum limits) and depends on how benefits vary with PI per offspring and their costs vary with their total PI. As a result, if both parents provide care, and they suffer the same fitness costs, their optimal clutch size is identical and there is no conflict over fecundity. However, when their costs differ there will be conflict over clutch size, even when there are no costs to egg production per se (Houston & Davies 1985; Winkler 1987). Whether or not there is conflict over clutch size, there will always be conflict over the amount of parental care.
(b) Mating
Unlike sexual conflict over PI, sexual conflict over mating is not an inevitable consequence of sexual reproduction. There will be no mating conflict when both potential mates in a given interaction are selected to mate, or both selected not to mate (Parker 1979; Parker & Partridge 1998). When there are no costs to mating to either sex, both mates will be selected to mate (assuming some benefit to mating) so mating conflict will not occur. Mating costs are, therefore, a logical necessity for mating conflict. Costs may arise in one of two general ways: first mating may be costly because it has an effect on the female's survival or future fecundity. (When there is conflict, it is generally females that are selected not to mate (Trivers 1972; Parker 1979; Parker & Partridge 1998), and for the sake of brevity this is the case that is assumed here.) For example, matings may be costly in terms of energy expenditure, increased risk of predation or infection, or damage caused by the male. As a result, except in species where nuptial gifts compensate for these costs, high mating rates will be selected against. In addition, low mating rates may reduce a female's fertility, so intermediate mating rates are often optimal (Arnqvist & Nilsson 2000). In contrast, the fitness of males usually increases with their mating rate (Bateman 1948; Trivers 1972), so that a male encountering a female is generally selected to mate, while the female may not be. Mating conflict generated in this way does not depend on variation in the value of males as mates. Similar arguments explain conflict over the rate of fusion between gametes, because of the effects of fusion rate on the risk of polyspermy (Rice & Holland 1997; Partridge & Hurst 1998).
The second general way in which costs of mating for females can arise is when males vary in their value to females as mates. If mating with a low-quality mate deprives the female of the chance of having her eggs fertilized by a high-quality mate, that mating carries an ‘opportunity cost’ equivalent to the difference in the fitness gain she would have received from mating with the two types of male. When this opportunity cost is large enough, females will be selected not to mate with males of low mate value (Parker 1979; Parker & Partridge 1998).
These two explanations involve selection on males to gain paternity (Parker 1970; Simmons 2001; referred to as ‘offence’ behaviour by Rice & Holland 1997). In addition to behaviour related to obtaining matings, offence behaviour will also include the replacement of any sperm from previous matings that the female contains. There will also be selection for males to avoid losing paternity as a result of females later remating with a different male (Parker 1970; Simmons 2001; referred to as ‘defence’ behaviour by Rice & Holland 1997). While remating may be beneficial to the female, males almost inevitably lose fitness when a female with whom they have mated remates, so there can be conflict over remating. Defence behaviour may also include behaviour that reduces the probability of a male's sperm being replaced if remating does occur.
3. Evolutionary outcomes in relation to the subject of conflict
This section briefly reviews the occurrence of different evolutionary outcomes in sexual conflicts (table 1). The aim of this section is not simply to document that these kinds of outcome occur, but rather to try and associate these with different subjects of conflict: PI per offspring, fecundity, mating (and offence phenotypes) and remating (and defence phenotypes).
Table 1.
A review of the evolutionary outcomes of sexual conflict in relation to the subject of conflict.
| PI per offspring | fecundity | mating probability | remating interval | |
|---|---|---|---|---|
| (a) manipulative behaviour | ||||
| direct manipulation | genomic imprinting | Acps | Acps | Acps |
| other chemicals | sensory exploitation | anti-aphrodisiacs | ||
| ?genomic imprinting | apyrene sperm | |||
| mating plugs etc. | ||||
| coercion | prevention of polygyny | armaments | punishment | |
| wars of attrition | ||||
| punishment | ||||
| infanticide | ||||
| deception | concealment of non-paternity/state of breeding attempt | concealment of mating status | ||
| (b) harmful behaviour | prevention of polygyny | Acp70A | punishment | Acp70A |
| infanticide | punishment | |||
| traumatic insemination | ||||
| (c) rapid evolutionary change | Acp70A | Acp36DE | Acp70A | |
| Acp26Aa | fertilization proteins | Acp36DE | ||
| external genitalia | ||||
| armaments |
(a) Manipulative behaviour
Sexual conflict by definition involves sexually antagonistic selection, so while conflict is ongoing, there is selection in at least one sex for the evolution of manipulative behaviour that changes the trait over which there is conflict in that sex's favour. Manipulative traits fall into a number of different categories (table 1a).
First, individuals of one sex can directly manipulate the trait over which there is conflict, usually by exploiting some internal or external signalling system. The most renowned examples of this are the accessory gland proteins (Acps) in the seminal fluid of Drosophila species (Chapman 2001; Wolfner 2002; Kubli 2003; Fiumera et al. 2006). There are over 80 different seminal peptides, some of which are known to modify female reproductive behaviour, in some cases by mimicking female hormones. Acps have effects on female fecundity (Herndon & Wolfner 1995; Heifitz et al. 2000; Saudan et al. 2002; Chapman et al. 2003b; Liu & Kubli 2003), sperm storage (Neubaum & Wolfner 1999; Tram & Wolfner 1999), success in sperm competition (Clark et al. 1995) and future female receptivity (Chapman et al. 2003b; Liu & Kubli 2003). Acps have not been reported to influence PI by the female in individual eggs, although the possibility has never been systematically investigated. Another important kind of direct manipulation is sensory exploitation in male mating signals (Ryan et al. 1990), in which males exploit pre-existing sensory biases to stimulate females to mate at a rate that is super-optimal for the females. Direct manipulation of PI per offspring (and possibly also fecundity; Hager & Johnstone 2003) occurs by genomic imprinting (Haig 1992). Inter-locus sexual conflict is normally waged between genes expressed in the parents, but in the case of genomic imprinting, conflict is mediated via maternally and paternally derived alleles expressed in the offspring. A modification of the DNA during gametogenesis reveals the parental origin of the allele, and when the imprinted locus affects PI by the female (for example, when it codes for a growth factor; Haig & Graham 1991), there will be selection for differential expression of maternally and paternally derived alleles (Haig 2000). Effectively, genes carried by the male manipulate the female into making greater PI. An analogous manipulation is possible in birds, where maternally derived yolk hormones can have effects on offspring growth via begging behaviour (Schwabl 1996; Groothuis et al. 2005). Females could thereby manipulate PI by males, but in species where both sexes provision the young, females would need to make a corresponding decrease in their own response to begging. Whether maternal yolk hormones do play a role in sexual conflict is unknown. A final example of manipulative behaviour is the transfer of anti-aphrodisiacs (Andersson et al. 2000) and other means of impeding remating behaviour, such as copulatory plugs, by males to females during mating.
Coercion is a second category of manipulative behaviour. The best known examples occur in relation to mating behaviour and involve the use of armaments, participation in wars of attrition in which the individual that persists longest wins, and punishment by males of females that refuse to cooperate in mating (Parker 1979, 1983; Clutton-Brock & Parker 1995a,b; Parker & Partridge 1998). Infanticide of the offspring of previous matings with other males, which makes the female available for mating, can also be regarded as coercion (e.g. Schneider & Lubin 1996). Coercion can also be involved in preventing a reduction in parental care. For example, in some polygynous species, males provide some parental care, but the amount of care given by the male to an existing female mate's offspring is reduced if the male acquires additional mates. In these species, females may prevent their mate from remating by aggression towards other females (Sandell & Smith 1996; Sandell 1998) or destroying their young (Veiga 1993; Hansson et al. 1997). Another instance seems to be the punishment by males of unfaithfulness by their mate (Valera et al. 2003).
Deception is a third category of manipulative behaviour. Deceit can be involved in manipulating other individuals into mating when they would otherwise not do so by concealing the fact that the first individual is already mated (Haartman 1969; Alatalo et al. 1981). Deceit may be involved in manipulating PI by the other parent by concealing the fact that they lack paternity (or maternity) of offspring or the state of advancement of a breeding attempt thus allowing preemptive desertion leaving the other parent ‘holding the baby’ (Valera et al. 1997).
(b) Harmful behaviour
Sexual conflict involves sexually antagonistic selection by definition, so any manipulative trait reduces the fitness of the individual of the other sex because it moves the value of the conflict trait away from the other individual's optimum. I will refer to this reduction in the fitness of the other sex caused by the change in the value of the conflict trait resulting from manipulation as ‘conflict load’. As a hypothetical example, a male might produce a pheromone (the manipulative trait) which caused his mate to increase the amount of PI that she makes (the conflict trait) above her optimal value. This change in the value of the conflict trait imposes a reduction in fitness—conflict load—on the female. (See §4a and figure 1 for further explanation of conflict load.) All manipulative traits involved in sexual conflict by definition entail conflict load, but some manipulative traits have additional deleterious effects. In the example above, the pheromone that manipulated female PI might also have a direct toxic effect on the female. Such a reduction in the other sex's fitness by manipulative behaviour over and above the conflict load is referred to as ‘harm’ in the sexual conflict literature (Johnstone & Keller 2000; Morrow et al. 2003; Lessells 2005). Not all manipulative behaviour need be harmful in this specialized sense: in the pheromone example, the pheromone might, or might not, have toxic effects, but if it manipulates a conflict trait (PI in this case) it will always impose conflict load. Conflict load and harm both reduce the fitness of the manipulated individual, so at first sight there seems little point in distinguishing these two deleterious effects. However, conflict load and harm often have opposing effects on the fitness of the individual carrying out the manipulation. The manipulation increases this individual's fitness by reducing its own conflict load (and incidentally imposing conflict load on the other sex), whereas harm will often affect its fitness negatively. For this reason, it is important to conceptually partition the fitness consequence of any given manipulative behaviour into conflict load and harm (see also §5).
Figure 1.
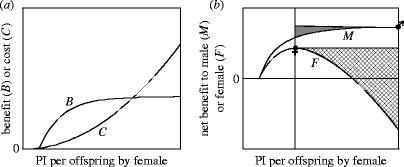
Conflict load in sexual conflict over PI per offspring by the female when there is uniparental maternal care of a brood containing a single offspring. (a) As the amount of PI by the female increases, the fitness benefit, B, through the offspring increases, but with diminishing returns, while the female also pays a fitness cost, C, in reduced survival or future reproduction which increases at an accelerating rate at higher levels of PI. (Here, it is assumed that all costs are paid after the offspring becomes independent; cf. §4c and figure 3.) (b) The net fitness gain to the female, F, is the fitness benefit minus the fitness cost, and peaks at an intermediate value of PI. This is the female's optimum for the amount of PI that she makes and is indicated by a filled female symbol. The male receives the same fitness benefit through the offspring as the female, but pays no cost, so his net benefit, M, is the benefit through the offspring. This peaks where the benefit through the offspring reaches an asymptote (or the maximum level of PI of which the female is capable, whichever is lower). This is the male's optimum for the amount of PI by the female and is indicated by a filled male symbol. Conflict load for each sex (cross-hatching for females; shading for males) is the reduction in fitness experienced because the trait value is not at the respective sex's optimum. Conflict load is not shown below the female's optimum value for her PI because selection is not sexually antagonistic in this region.
Harmful behaviour seems surprising because it often has a negative effect on the indvidual carrying out the manipulation, as well as on the manipulated individual. There are two types of explanation for harmful behaviour: in the first, harm is a deleterious side-effect of the manipulative behaviour (‘collateral harm’ is a better term than ‘pleiotropic harm’ suggested by Morrow et al. 2003, because under the alternative hypothesis manipulation of the conflict trait and harm are also pleiotropic). In the second, the reduction in survival caused by the harmful behaviour is the primary effect and invokes changes in the conflict trait (‘adaptive harm’). In the case of mating damage by males to females, these have been suggested to include an increase in remating interval and oviposition rate (Constantz 1984; Michiels 1998; Lessells 1999; Johnstone & Keller 2000). Mathematical models show that adaptive harm can in principle evolve in this way (Johnstone & Keller 2000; Lessells 2005). At present, however, there is no unequivocal empirical evidence for adaptive harm (Morrow et al. 2003), although some cases of physical damage are hard to explain in other ways (e.g. Michiels 1998).
The best known examples of harmful behaviour are damage caused by males to females during mating by seminal fluid proteins in Drosophila melanogaster (Chapman et al. 1995) and Caenorhabditis elegans (Gems & Riddle 1996), and by physical damage in several insect species (Crudgington & Siva-Jothy 2000; Blackenhorn et al. 2002). None of these studies identified the trait over which there was sexual conflict, but a limited number of other studies have done this. For Acps, a significant association between harm (female survival) and a male's defence phenotype, but not offence phenotype, has been shown in D. melanogaster (Civetta & Clark 2000), and the sex peptide (Acp70A) in the same species, which is known to influence a female's fecundity and remating propensity, has been shown to be a major contributor to the reduction in female survival caused by Acps (Wigby & Chapman 2005). Thus, Acps manipulating fecundity and defence phenotype, but not offence phenotype, have been shown to harm females. Traumatic insemination in bedbugs causes a reduction in female survival, and presumably evolved in relation to mating conflict (Stutt & Siva-Jothy 2001; Siva-Jothy 2006). Punishment of females to induce mating compliance and act as a disincentive for unfaithfulness, and infanticide to induce willingness to mate and to prevent the loss of parental care by an existing mate are other examples of harmful behaviour where the trait over which there is sexual conflict can be identified.
Although this brief review is far from complete (table 1b), harmful behaviour seems to be rare for sexual conflict over PI per offspring. One potential harmful trait that could be used to manipulate PI but is not found is punishment analogous to that meted out by males to females who do not cooperate in mating. Breeding individuals use this kind of behaviour on potential helpers within the social group in several species with cooperative breeding (Mulder & Langmore 1993; Clutton-Brock & Parker 1995b), but have not been reported using the same tactic on their mates in these or other species with biparental care.
(c) Rapid evolution
Rice and colleagues (Rice & Holland 1997; Rice 1998, 2000) have stressed that sexual conflict can lead to self-reinforcing and even perpetual antagonistic coevolution of males and females. One line of evidence that this occurs is the rapid rate of evolution of traits that may be, or are known to be, manipulative in sexual conflicts (Rice & Holland 1997; Rice 1998, 2000). For example, proteins in the reproductive tracts and gonads of males and females evolve at high rates compared to other proteins (Civetta & Singh 1995, 1998; True et al. 1996; Swanson & Vacquier 2002), although there are other reasons than sexual conflict why these proteins might evolve rapidly (Swanson & Vacquier 2002). Acps also tend to diverge rapidly and be highly polymorphic (Thomas & Singh 1992; Cirera & Aguadé 1997; Wolfner 1997; Aguadé 1998; Tsaur et al. 1998). However, although these studies show that rapid evolution occurs in traits that are probably involved in sexual conflict, there are relatively few studies in which there is evidence of rapid evolution and the trait over which there is conflict has been identified (table 1c). This has been done for some Acps, including Acp26Aa which stimulates egg laying (Aguadé et al. 1992; Herndon & Wolfner 1995; Heifitz et al. 2000), and Acp36DE which is involved in sperm storage and competition (Neubaum & Wolfner 1999; Begun et al. 2000; Chapman et al. 2000). Moreover, among seven polymorphic Acp loci, none were related to a male's offence phenotype (his ability to remate or remove sperm), but four were related to his defence phenotype (his ability to reduce remating by the female; Clark et al. 1995). Some traits other than Acps that show rapid evolution are related to fertilization or mating in a way that suggests that they are involved in manipulating mating if they are involved at all in sexual conflict. These include fertilization proteins (Swanson et al. 2001) and genital morphology (Eberhard 1985, although Eberhard (2005) argues against a link with sexual conflict). Armament in water striders also shows rapid evolutionary change, and in this case there is good evidence that this trait is involved in mating conflict (Arnqvist & Rowe 2002a). No traits involved in mediating conflict over PI per offspring have been reported as evolving rapidly. In particular, imprinted genes do not evolve rapidly (McVean & Hurst 1997).
4. Determinants of evolutionary outcomes
The previous section briefly reviews the occurrence of different evolutionary outcomes of sexual conflict. While inevitably incomplete and unsystematic, the review suggests that the evolutionary outcome of conflict may be related to the subject of conflict, with harmful behaviour and antagonistic coevolution being rarer as outcomes of conflict over PI than conflict over mating. This section attempts to understand why that might be, by considering the main determinants of different evolutionary outcomes and how these vary with the subject of conflict (table 2).
Table 2.
A summary of the determinants of the evolutionary outcome of sexual conflict in relation to the subject of conflict. (‘—’indicates no clear prediction.)
| PI per offspring | fecundity | mating probability | remating interval | |
|---|---|---|---|---|
| benefit of manipulation to male | small (and non-existent under the assumptions of Smith & Fretwell's (1974) model) | may be large | may be large | may be large |
| fitness obtained by male at female's optimal value of conflict trait | large (also as a proportion of fitness obtained by the male at his optimum) | some | none | — |
| as value of conflict trait moves from female's to male's optimum: | ||||
| selection gradient on manipulation by male | decreases | is constant | is constant | probably decreases |
| selection gradient on resistance by female | increases | increases | is constant | — |
| benefit of resistance to female | large | large | may be small | may be small |
| costs of manipulation | opportunity costs | — | no opportunity costs | — |
| strategy set for manipulation: | ||||
| exploitation of internal signalling system | difficult | possible | possible | possible |
| exploitation of external signalling system | difficult | difficult | possible | — |
(a) Benefits of manipulation and resistance
The first factor determining the evolutionary outcome of sexual conflict is the fitness benefit of manipulation of the conflict trait by one sex, and resistance to manipulation by the other sex. Sexual conflict by definition involves sexually antagonistic selection: in other words the optimal value of the trait differs for males and females. It is therefore impossible for both sexes to be at their optimal value of the trait, and one or both sexes must experience a loss in fitness as a result. This loss of fitness can be termed ‘conflict load’ in the same way that the loss of fitness due to mutations is referred to as ‘mutation load’. Figure 1 illustrates conflict load for sexual conflict over PI per offspring. Conflict load implies that each sex would benefit by manipulating the trait towards its own optimum, because this removes part of the conflict load. Any such manipulation would also at the same time impose conflict load on the other sex, and there is thus a benefit to resisting manipulation. The relative size of these two selection pressures—the benefit of manipulation and the benefit of resistance—has been called the ‘value of winning’ by Parker (2006), who points out that this will be a major determinant of the evolutionary resolution of sexual conflict, with the sex that has more to gain being more likely to ‘win’ the conflict.
The benefits of manipulation and resistance will vary in different ways depending on the subject of conflict, and the following considers conflict over PI per offspring, fecundity, mating and remating (figure 2). For ease of comparison of these four cases, I consider PI per offspring when there is uniparental maternal care in a brood of a single offspring, fecundity when there is no parental care, mating probability when males are selected to mate and females not to mate, and the propensity to remate represented by remating interval. This aids comparison because the female's optimal trait value is then numerically lower than the male's for each of the four traits. Moreover, I consider the case in which the traits are initially at the female's optimum, so that males are selected to manipulate the trait and females to resist. (This means that the benefit of resistance is equal to the female's conflict load (cross-hatched areas in figures 1b and 2), while the benefit of manipulation is the complement of the male's conflict load (vertically hatched area in figure 2 and shaded area in figure 1b, respectively). However, ‘male’ and ‘female’ are to a large extent used here as convenient arbitrary labels: it should be remembered that sexual conflict can also occur in the reverse direction (e.g. females selected to mate and males to resist mating) or may occur reciprocally over the same trait in the two sexes (as in the case of PI, where there is sexual conflict over both PI by the male and PI by the female, with each sex selected to manipulate the other into making the lion's share of investment), and that, in terms of selection, manipulation and resistance are two sides of the same coin.
Figure 2.
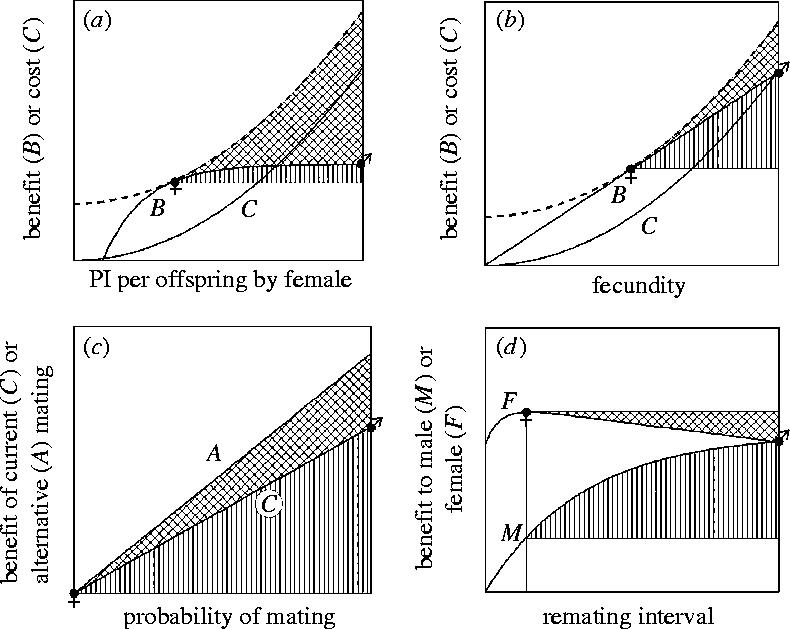
Benefits of manipulation and resistance in sexual conflict over (a) PI per offspring, (b) fecundity, (c) mating probability and (d) remating interval. Filled male and female symbols indicate the male's and female's optima, respectively. The vertically hatched areas are the benefit of manipulation to the male, and cross-hatched areas the benefit of resistance to the female. The following assumptions are made in the models: (i) females care for broods of a single offspring in a species with uniparental care; (ii) there is no parental care, so the fitness of each offspring is independent of fecundity and (iii) the only cost of mating to either sex is an opportunity cost to females of forgoing alternative matings with males of higher mate value. In (a) and (b), the benefit curve is for fitness gained through the offspring, and the cost curve is the cost to the female of her PI. The optimal PI is found where the cost curve is a tangent to the benefit curve. In (c), both of the curves are the fitness gained through a mating of the kind indicated. In (d), the curves are the fitness gains to each sex through the offspring produced by the female over the remainder of her lifespan.
The first trait to be considered here is PI per offspring. Figure 2a shows how selection acts on the amount of PI by the female when there is uniparental maternal care in broods of a single offspring. In modelling PI, it is generally assumed that, as the amount of PI per offspring increases, the benefit through the offspring increases, but with diminishing returns at higher levels of investment, and that the cost to the parent making the investment (in this case the female) increases at an accelerating rate. The net fitness gain to the female is the difference between the benefit and cost, so the female's optimum PI per offspring is where the vertical separation of the benefit and cost curves is maximal, which is also graphically where a curve parallel to the cost curve is a tangent to the benefit curve (and is indicated on figure 2a by a filled female sign). Because the male gains the same benefit as the female, but does not pay any cost, his net benefit is simply the benefit through the offspring, and the male's optimal PI per offspring by the female occurs where the benefit through the offspring reaches an asymptote (or the maximum level of PI of which the female is capable, whichever is smaller). This graphical method of finding the optima also shows the benefits of manipulation and resistance. The benefit of manipulation (to the male) is the increase in his net benefit of PI above what he would receive if the female made her optimal amount of PI. This is the vertically hatched area in figure 2a. Similarly the benefit of resistance (to the female) is the cross-hatched area in figure 2a. Three conclusions follow from the general assumptions given above about the benefit and cost curves: first, the benefit of manipulation is generally small, especially as a proportionate gain of the benefit that the male would have received without manipulation; second, the benefit of manipulation increases at a decreasing rate the closer the amount of PI by the female is to the male's optimum (i.e. the selection gradient on manipulation decreases); third, the benefit of resistance increases at an increasing rate the closer the amount of PI by the female is to the male's optimum (i.e. the selection gradient on resistance increases). In summary, selection for manipulation is weak and the value of winning (benefit of manipulation/benefit of resistance) decreases strongly as the amount of manipulation increases.
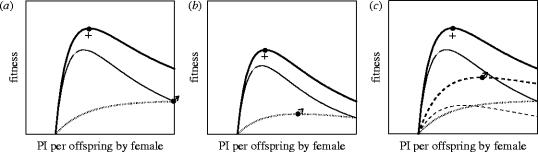
Several factors decrease selection for manipulation of PI per offspring still further. First, the above model assumes that any costs paid by the female are paid after the offspring reaches independence. If, however, there are ‘current costs’ (Lazarus & Inglis 1986)—for example, raised levels of PI have an immediate impact on the female's survival, and the offspring also perish if the female dies—the offspring benefit curve may not continue to rise monotonically with increasing PI, but will peak at an intermediate value of PI beyond which the increasing risk that the female will die before the offspring are independent more than offsets any advantages that the offspring gain through greater investment (figure 3b). The male's optimal level of PI by the female is then reduced to this intermediate value of PI. Second, the above model assumes that the costs paid by the female do not have any impact on the male's fitness through future reproduction. However, if the male pays ‘future costs’—for example, if reproductive success increases with the amount of previous breeding experience with a particular mate—the male's optimal level of PI by the female is also reduced below the maximum possible (figure 3c).
Figure 3.
Sexual conflict over parental investment by the female in a species with uniparental maternal care when there are (a) no current costs of investment, and no future costs for the male, (b) current costs of investment, but no future costs for the male and (c) no current costs of investment, but future costs for the male. Filled male and female symbols indicate the optimal investment by females for the male and female, respectively. Curves shown are the expected fitness from the current brood (dotted line; which in (a) and (b) is also the male's expected remaining-lifetime fitness with the female), the expected future fitness of the female (thin solid line) or male (thin dashed line), and the expected total remaining-lifetime fitness of the female (thick solid line) or male (thick dashed line in (c)). Values and functions assumed in the figures are: , and p=0.7. Model: the curves shown are based on a model in which a female produces one brood per year and invests x in the brood. As a consequence of this investment, the female survives with a probability of s(x) to the start of the following breeding season, and, provided that she survives until the young are independent, b(x) young from the brood survive to maturity. Females are assumed not to senesce, so that the same functions b(x) and s(x) govern the female's productivity and survival throughout her lifespan. This also means that her optimal investment is independent of her age. If she invests the same each year throughout her life, at the start of each season the expected further number of seasons in which she attempts to breed (including the current one) is 1/(1−s(x)). (a) If the survival costs of reproduction are paid after the current brood is independent, the fitness that she accrues from the current brood is b(x) and her expected fitness from future broods is , summing to a total fitness over her remaining lifespan of . Her optimal investment, , is found by setting the partial derivative of her total remaining-lifetime fitness equal to zero and solving for , and is given by , where primes denote the partial derivate with respect to x. If pairs breed together in only one year, a male's expected lifetime fitness with the current female is b(x), and his optimal investment by the female, , then occurs at the maximum of this function. If b(x) increases monotonically with x, then his optimum is the maximum value of x of which the female is capable. (b) If the survival costs of reproduction are paid entirely before the current brood is independent, the fitness from the current brood is b(x)s(x), the female's expected fitness from future broods is , and her total expected remaining-lifetime fitness . Her optimal investment, , is then given by . If pairs breed together in only 1 year, the male's optimal investment by the female, , is that which gives maximum fitness from the current brood, and is given by . (c) If the survival costs of reproduction are paid (as in (a)) after the current brood is independent, but either of the pair benefit from the survival of the mate, the optimal investment of that sex may change. Here, I assume somewhat implausibly, but for the purposes of illustration, that pairs breed together provided that they are still alive, that the female can remate costlessly if her mate dies, and that males are polygynous and the rate at which they acquire additional mates and the breeding success of all their mates is unaffected by their harem size. Under these conditions the fitness and optimal investment for the female is unchanged. However, the expected remaining-lifetime fitness of a male with a female is now , where p is his annual survival and is independent of the female's investment, and the female's investment is the same each year. His optimal investment by the female, is now given by .
Third, the conclusion that selection for manipulation is weak depends only on the general assumptions of diminishing returns and accelerating costs of increased PI, but the precise strength of selection will depend on the exact shape of the cost and benefit curves. The benefit curve is the outcome of the offspring's developmental strategy. Detailed theoretical consideration of how the benefit curve should evolve is outside the scope of this paper, but when parental care is reduced the developmental strategy of offspring should prioritize the uses of care which have the biggest impact on the offspring's fitness (cf. Collins 1980). If this is the case, the benefit curve would evolve so that it asymptotes rather sharply, and this would in turn result in smaller benefits to the male of manipulation. A similar argument can be applied to the shape of the parental cost curve.
Fourth, the arguments given regarding sexual conflict over PI per offspring consider this trait in isolation. However, when there is a trade-off between PI per offspring and fecundity, there may no longer be sexual conflict over PI per offspring (although conflict over fecundity, and hence the total amount of PI, remains). The optimal behaviour for the female when there is a trade-off between PI per offspring and fecundity has been previously explored by Smith & Fretwell (1974). They showed that when this trade-off is generated by the division of a limited amount of resource over the eggs produced, and the fitness of an individual offspring depends only on ‘egg size’ (the amount of resources devoted to the individual offspring) per se and not on fecundity, there is a constant optimal egg size irrespective of the amount of resource to be divided. In essence, this is the egg size that gives the best conversion rate of resource into fitness through offspring. If the male's fitness is governed by the same trade-off between egg size and fecundity (his ‘fecundity’ is the number of eggs that he fertilizes), the male will have the same optimal egg size: a male that could (costlessly) manipulate the female would maximize the amount of resource she invested in egg laying but leave egg size unchanged (assuming that the female is initially producing her optimal egg size). In other words, there will be no sexual conflict over PI per offspring. Does this mean that there is not usually sexual conflict over PI? The answer is no, because optimal fecundity may not be governed solely by the trade-off between egg size and fecundity. First, when there is some form of parental care, fecundity per se will generally affect the fitness of individual offspring, so the argument will not usually apply when there is parental care. Second, the male may not experience the same trade-off as the female: for example, in a promiscuous species of insect without parental care, the female's reproductive physiology may result in the number of eggs that will become available for the male to fertilize already being fixed at the time of mating, whereas the amount of resources devoted to each egg may not be. In this case, the male does not face a trade-off between egg size and his fecundity, and there is sexual conflict over egg size (as in figures 1 and 2a). However, the extension of Smith & Fretwell's (1974) model to males does caution that there may be cases in which there is no sexual conflict over PI per offspring.
The second trait to be considered in terms of the benefits of manipulation and resistance is fecundity. When there is parental care, there may or may not be conflict over fecundity (see §2a), so here I consider the case without parental care (figure 2b). Without parental care, the total fitness gain through offspring will increase in direct proportion to fecundity. As in the case of PI per offspring, it is assumed that the cost to the female will increase at an accelerating rate with increased fecundity. The female's and male's optimal fecundity, and the benefits of manipulation (vertically hatched area in figure 2b) and resistance (cross-hatched area in figure 2b), can be found graphically in the same way as they were for PI per offspring. Three conclusions follow from the general assumptions made about the benefit and cost curves: first, the benefit of manipulation can be larger for fecundity than for PI per offspring (because fitness through offspring increases in proportion with fecundity, but shows diminishing returns for PI per offspring), but as for PI per offspring, males do gain some fitness at the female's optimum level of fecundity; second, the benefit of manipulation increases at a constant rate as fecundity increases above the female's optimum (i.e. the selection gradient on the male is constant); third, the benefit of resistance increases at an increasing rate as fecundity increases above the female's optimum (i.e. the selection gradient on the female increases). In summary, selection for manipulation will tend to be stronger than for PI per offspring, and the value of winning (benefit of manipulation/benefit of resistance) decreases as the amount of manipulation increases. As in the case of PI per offspring, the amount of sexual conflict will be reduced when there are current and future costs to the male of the female's investment. Evolution of the female's cost curve will leave the benefits of manipulation unchanged, but may increase the sharpness of the acceleration in the cost curve, and hence increase the benefits of resistance.
The third conflict trait to be considered is mating probability. As argued in §2b, there must be mating costs for there to be sexual conflict over mating. Here I consider the case where sexual conflict arises solely because of variation between males in their value as mates, so that the only cost of mating is an opportunity cost for females of not mating with an alternative mate of higher value (figure 2c). This case is considered because the benefits of manipulation and resistance are easy to show graphically, but the general conclusions are the same when mating costs are paid in reduced survival or future fecundity. The expected fitness benefit of mating to a female who encounters a male rises linearly from zero when the probability of mating is zero, to some value that is determined by the mate value of the male when the probability of mating is one. The current male always gains by mating, so his optimal mating probability is one. The female's optimal mating probability depends on the opportunity costs and hence on the mate value of alternative mates. When the value of alternative mates is higher than that of the current male, she should not mate with the current male, and her optimal mating probability is zero. When the female is selected not to mate, the benefit to the male of manipulating the probability of mating is the vertically hatched area in figure 2c. The benefit of resistance to the female is the opportunity cost—the difference in the benefit she would gain with the current male and an alternative mate (the cross-hatched area in figure 2c). The relative benefits of manipulation and resistance will depend on the amount of variation in mate value. In general, it is argued that in conflicts over mating, the benefits of manipulation will be larger than the benefits of resistance (Parker 1979, 1983; Parker & Partridge 1998). (This asymmetry will reverse if the female can expect an alternative mate with at least twice the quality of the present mate.) In addition, the following general conclusions can be made: first, a male with whom a female is in conflict over mating gains no fitness if the female achieves her optimum mating probability (i.e. they do not mate), unlike sexual conflict over PI per offspring and fecundity; second, the benefit of manipulation increases at a constant rate as the probability of mating increases (i.e. the selection gradient for manipulation is constant); third, the benefit of resistance increases at a constant rate as the probability of mating increases (i.e. the selection gradient for resistance is constant). In summary, males do not gain fitness without manipulation, and the value of winning (benefit of manipulation/benefit of resistance) remains constant as the amount of manipulation increases.
The last of the four traits to be considered here is remating interval (figure 2d). In promiscuous mating systems, the fitness that a female accrues over the remainder of her lifespan is likely to have a maximum at an intermediate value of remating interval (Arnqvist & Nilsson 2000): the fitness benefits of remating, including those resulting from the acquisition of sperm or resources, are likely to show diminishing returns with increased remating rate, while the fitness costs, including those resulting from time or energy costs, risk of predation or male mating harm, are likely to accelerate with increasing mating rate. In contrast, the fitness of the male that currently mates with a female will usually increase monotonically with the remating interval up to a value equal to the female's fitness accrued over remaining lifespan if the female does not remate. Beyond the conclusion that the female's optimum remating interval will often be intermediate, resulting in sexual conflict over remating interval (Arnqvist & Nilsson 2000), there is little in the way of general conclusions that can be made: the benefit of manipulation is likely to increase at a decreasing rate as the remating interval increases above the female's optimum (i.e. the selection gradient on manipulation decreases), and the value of winning (benefit of manipulation/benefit of resistance) to decrease as the remating interval changes in the same way. However, the relative benefits of manipulation and resistance, even in broad qualitative terms, are dependent on the specific biological details.
(b) Costs of manipulation and resistance
When there is sexual conflict over a trait, manipulation and resistance to manipulation of that trait bring fitness benefits by reducing conflict load or avoiding its imposition. However, the traits that are used to manipulate or resist manipulation will usually be costly to produce, and the extent to which manipulation and resistance evolve will depend on both the benefits and costs. The relative size of the cost of manipulation and the cost of resistance has been called ‘power’ by Parker (2006), who points out that that it will be a second major determinant of the outcome of sexual conflict.
Compared to the benefits of manipulation and resistance, it is relatively hard to make general statements about the costs of manipulation and resistance (Parker 1979). Parker (1979, 1983; Parker & Partridge 1998) has argued plausibly that, in mating conflicts, the costs to a female of resisting a mating may be much lower than those to a male of imposing a mating. Two other conjectures can be made regarding costs of manipulation or resistance: first, the opportunity costs of manipulation may be different in conflicts over PI and mating. These opportunity costs arise from the other activities that the individual might pursue that would contribute to its fitness using the time and effort that it spent on manipulation. In a promiscuous mating system, obtaining matings is essentially the only way that a male can accrue fitness. Thus, attempting to manipulate a female into mating only carries an opportunity cost if there are other females available that would mate more readily. On the other hand, attempting to manipulate a mate into making greater PI may carry an opportunity cost, because the time and effort could be spent in attempting to obtain additional matings. Moreover, if the attempt to manipulate PI occurs during the period of parental care (as opposed to before this period, as in the case of genomic imprinting), necessitating proximity to the mate and therefore brood, the manipulating individual could easily instead invest itself in the brood. Forgoing this investment is an opportunity cost. This is especially the case when there is biparental care, when increasing investment itself may be a better option for an individual in terms of maximizing its fitness than attempting to manipulate its mate into increasing investment.
A second conjecture regarding costs is that the way in which the traits involved in manipulation and resistance interact is likely to influence how costs vary in relation to the amount of manipulation or resistance. The most obvious way in which these traits might interact is in a contest in which the amount or probability that the conflict trait is moved towards an individual's sex's optimum increases with the costs that the individual pays (see especially Parker 1979, 1983). In this case, evolutionary changes in the traits involved in manipulation and resistance that give an advantage in the conflict will involve ever increasing costs. Alternatively, the traits involved in manipulation and resistance may interact in the same way as a lock-and-key, with changes in the manipulative trait (‘key’) being favoured if they increase complementarity (the goodness of fit of the key to the lock), and changes in the resistance trait (‘lock’) being favoured if they decrease complementarity. In this case successive evolutionary changes in the traits involved in manipulation and resistance need not involve any systematic increase in costs if the changes are equivalent to changing the lock or key (although there may be costs to the initial evolution of a ‘key’). One example of such a system might be manipulation of a molecular signalling system in the other sex.
(c) Feasibility of manipulation
The third important determinant of the evolutionary outcome of conflict is whether there is a means available of manipulating the conflict trait. Several authors have emphasized that there may simply be nothing (more) that one of the two sexes can do to further their own interests (Parker 1979; Partridge & Hurst 1998; Parker et al. 2002). In comparing the feasibility of manipulating different kinds of trait over which there is conflict, one important point is that manipulation often exploits an internal or external signalling system. Manipulation of PI per offspring, and in particular parental care is relatively difficult by both these means. Internal fertilization offers a notable opportunity for males to transfer chemicals that exploit the female's internal signalling system (Rice 1998; Lessells 1999), but if chemicals transferred at mating are to manipulate parental care their effect must be long-lasting and this may be relatively difficult to achieve. One way of accomplishing this would be to transfer or modify genetic material (Rice 1998), and it is significant that the only known example of direct manipulation of parental care—genomic imprinting—does just that. Turning to external signalling systems, males can only manipulate females by exploiting such a system if the female processes signals from the male in relation to that activity. In the case of mating decisions it is more or less essential for her to process signals from the male in order to direct mating behaviour to an appropriate partner, and this opens a channel by which he can manipulate her—for example, by sensory exploitation. However, there is no need to process signals from the male in order to direct parental care to appropriate recipients (although females may respond to signals of male quality in the ‘differential allocation’ of PI; Sheldon 2000; Limbourg et al. 2004).
5. Discussion
(a) The evolution of manipulative behaviour
Resistance to manipulation of traits under sexual conflict will only evolve once manipulative behaviour has evolved. Thus, the initial evolution of manipulation depends only on the benefits and costs of manipulation, and on the feasibility of manipulation, and not on the benefits and costs of resistance. An explanation for the relative rarity of manipulative behaviour in sexual conflict over PI per offspring (table 1) must therefore be sought in differences in the costs, benefits and feasibility of manipulation for PI per offspring compared with the other traits over which there is sexual conflict that have been considered. All three of these factors appear to contribute (table 2): the benefits of manipulation are small, especially as a proportion of what the individual would gain without manipulation; opportunity costs are probably larger for manipulation of PI per offspring, at least compared with those for manipulation of mating probability; and manipulation of PI per offspring may simply be difficult or impossible. The contribution of the first and last of these is supported by the known cases of manipulation of PI per offspring: first, the goal of most examples of manipulative behaviour for PI per offspring (prevention of polygyny, concealment of non-paternity or mating status) seems to be preventing the mate from withdrawing its parental care altogether, rather than increasing its level of PI above its optimum when it makes parental care. Withdrawal of PI by the other parent would have a much larger impact on fitness than the small gain to be made by manipulating that individual into increasing its PI, suggesting that the small benefits of manipulating PI per offspring upwards are at least partially responsible for the failure of such manipulation to evolve. Second, the only example of direct manipulation of PI per offspring (genomic imprinting) involves the transfer of genetic material, thus solving the problem of achieving a long-lasting manipulation using chemicals transferred during insemination. This suggests the need to achieve a long-lasting effect may be an important constraint on the evolution of manipulation of PI per offspring, and particularly parental care.
(b) The evolution of harmful behaviour
Sexual conflicts over PI per offspring are not only characterized by the relative rarity of manipulative behaviour, but also by the almost complete absence of harmful behaviour. Harmful behaviour is manipulative behaviour that reduces the other sex's fitness over and above the reduction caused by the imposition of conflict load. In many cases, the effects of harm will also be at least partially felt by the individual inflicting the harm (Parker 1979). For example, mating harm by the male may cause a reduction in female survival within the period after mating when the male is still gaining paternity of at least some of the eggs that the female lays. By reducing the female's survival the male therefore reduces his own fitness gain to some extent. The evolution of harmful behaviour can only be beneficial to the male because the harmful trait also manipulates the value of a trait over which there is conflict towards the male's optimum, reducing the male's conflict load. It is important to note that the harm done by the male potentially affects not only this extra fitness that he gains through the removal of conflict load, but also the fitness that he would have gained without manipulating the female. The scope for harmful behaviour, therefore, depends on the proportional increase in fitness that manipulation of the conflict trait yields: when this is small, as it is in the case of PI per offspring, even a small percentage reduction through harm to the male's fitness will more than offset the benefit of manipulation. In contrast, in sexual conflict over mating probability, the male gains no fitness if he does not manipulate the female: harm that causes anything short of a 100% reduction in the male's fitness through mating with the female will then be of selective advantage. The single example of harmful behaviour in relation to sexual conflict over PI per offspring (the prevention by females of polygynous mating of their mates by aggression towards or infanticide of the offspring of other females) supports the argument that harmful behaviour is usually rare in sexual conflict over PI per offspring because the small proportional benefit of manipulation in these cases gives little scope for harmful behaviour. This is because it is one of the rare cases where the harm does not have repercussions on the fitness of the individual that is inflicting the harm.
(c) Sexually antagonistic coevolution
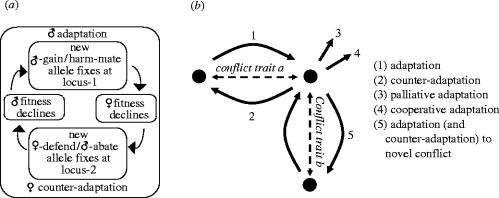
The propensity for sexual conflict to lead to what Parker (1979) refers to as ‘unresolvable evolutionary chases’ in which each evolutionary change by one sex is met by further change in the other sex without the warring sexes ever settling to a stable resolution was recognized early in the development of the study of sexual conflict (Dawkins 1976 elaborated by Dawkins 1989; Parker 1979). More recently, Rice and colleagues (Rice & Holland 1997; Rice 1998, 2000) have emphasized the capacity of the sexually antagonistic selection that defines sexual conflict to generate self-reinforcing and even perpetual sexually antagonistic coevolution. Their verbal and graphical models are framed around a simple adaptation–counter-adaptation cycle in which harm is given equal status to male-gain as a factor (see figure 4a). This conceptualization undoubtedly captures the essential feature of the interaction—that the reciprocal selection pressures may provoke unending evolutionary change—but going beyond this fundamental insight may be worthwhile in improving our understanding of which components create the impulse for ongoing coevolution and in particular help in clarifying the role of harm. This section attempts to do this by dissecting the different kinds of evolutionary change that are confounded in the general model.
Figure 4.
Evolutionary outcome of sexual conflict. (a) Rice and colleagues (Rice & Holland 1997; Rice 1998, 2000) have stressed the reciprocal nature of selection in males and females and given equal status to male gain and harm in sexually antagonistic selection. (Reproduced with permission from Rice (2000). Copyright (2000) National Academy of Sciences, USA.) (b) A dissection of the evolutionary changes in response to sexual conflict. Sexual conflict over a trait (trait a) can lead to (1) adaptations and (2) counter-adaptations that manipulate the value of that trait, (3) palliative adaptations to collateral harm, (4) cooperative adaptations enabled by novel trait states, and (5) adaptation (and counter-adaptation) to novel sexual conflicts.
The simplest starting situation is a single trait, a, over which there is sexual conflict. This is represented by the two-headed dashed arrow in the upper left corner of figure 4b, the whole of which represents a multi-dimensional trait space that has been squashed flat onto a two-dimensional surface. The arrow represents the axis along which a varies, and because there is sexual conflict over the trait, the optimal values of a for each of the two sexes differ. These two optima are indicated by the filled circles at either end of the arrow. If the initial evolutionary state is at the left hand of these two optima, the other sex is experiencing conflict load and is selected to evolve manipulative traits that move the value of a towards its own optimum (‘adaptation’; figure 4b). Section 5a discusses the factors determining whether this initial adaptation will occur. If they do, the next question is whether the new evolutionary state will involve complete manipulation of a to the other optimum, or only partial manipulation. This depends on how the benefits and costs of manipulation vary along the axis. Manipulation is more likely to be complete in sexual conflict over fecundity (in cases when there is no parental care) or mating probability (because the selection gradient is constant; table 2), than over PI per offspring and remating interval (because the selection gradient decreases). Manipulation is also more likely to be complete when costs increase linearly (or not at all) with the amount of manipulation, rather than accelerating.
As trait a evolves away from the optimum where it began, conflict load is imposed on that sex, and it begins to be selected to resist manipulation or manipulate a back towards its own optimum (both ‘manipulation’ in the broad sense; ‘counter-adaptation’; figure 4b). The obvious question is now whether one sex wins, the male and female reach a stable compromise at a value of a intermediate between the two optima, or whether the value of a cycles backwards and forwards along the conflict trait axis. Dawkins (1976, 1989) and Parker (1979) both provide examples that reveal the propensity for the selective pressures involved in sexual conflict to lead to cyclical dynamics. However, whether they do so will also depend on the genetic determination of the traits involved, and models that incorporate this have been developed relatively recently (Gavrilets 2000; Gavrilets et al. 2001; Gavrilets & Waxman 2002; Rowe et al. 2005). A number of variables potentially affect the outcome: the costs and benefits of manipulation, how the manipulative traits interact to determine the value of the conflict trait (i.e. whether there is a ‘contest’ or whether success depends instead on complementarity, or lack of it, between the traits (‘lock-and-key’)), and how the traits are genetically determined including whether new values of the traits can arise by mutation. Given the number of combinations of these factors, the relatively small number of models that have been produced to date cover only a small proportion of the possibilities. In particular, all of these models concern conflict over mating rate, and assume that female fitness depends on the overall rate at which she is mated, and hence on the distribution of male phenotypes in the whole population instead of only the phenotype of the male(s) with whom she mates. The situation is likely to be different in conflict over other traits, in that only the phenotype of males who mate will determine female fitness. For example, if males manipulate fecundity using chemicals transmitted to the female in seminal fluid, a female's fecundity will depend on the phenotype of the males with whom she has mated. Moreover, in many cases, mating rate and the details of sperm competition will mean that it is largely the phenotype of the male(s) who fertilize her offspring that determines her fitness.
Although the models of sexual conflict referred to in the previous paragraph are not comprehensive, they do give some important insights: first, and not surprisingly, continuous evolutionary chases often result when there are no costs to the manipulative traits. In this case, there is nothing to hinder continued exaggeration of both the male and female traits. The conclusion that the lack of costs of manipulation and resistance leads to continuous evolutionary chases applies equally when the interaction between traits is equivalent to a contest (Gavrilets et al. 2001) or depends on complementarity between the manipulative traits (Gavrilets 2000). However, this does not mean that the two types of interaction are empirically equally likely to give rise to evolutionary chases, because the two types of traits are not equally likely to invoke costs. As argued in §4b, evolutionary change in manipulative traits whose interaction depends on complementarity may well be costless, while this is unlikely to be the case for manipulative traits whose interaction is a contest. This would support Rice & Holland's (1997; Rice 1998) claim that that gene-product/gene-product interactions are likely to lead to perpetual arms races, but suggest that this is for a different reason than the one that they propose (that they can change in a mutation/countermutation fashion).
When continuous evolutionary chases are predicted by models, it is important to distinguish between evolutionary change in the trait over which there is conflict, and in the manipulative traits of the males and females. It is possible for the latter to be changing evolutionarily while leaving the value of the former unchanged. Gavrilets' (2000) model generates such an outcome: the male and female phenotypes which determine compatibility in mating, and hence overall mating rate, evolve in a continuous evolutionary chase, but the average compatibility, and hence mating rate—the trait over which there is conflict—remains constant. Waterstrider armaments appear to provide an empirical example of this phenomenon, with a comparative analysis revealing rapid evolution of the armaments of males and females involved in sexual conflict over mating, but correlated evolutionary changes in males and females of a species leaving the balance of armaments, and hence the mating rate, unchanged (Arnqvist & Rowe 2002a,b).
Theoretical models in which there are no costs to manipulative traits do not always lead to evolutionary chases. When the genetic determination of the manipulative traits assumed in the models allows the manipulative traits to evolve to have multi-modal distributions, the evolutionary outcome can instead be diversification of the female, and sometimes also the male, traits (Gavrilets & Waxman 2002). One can speculate that the kind of evolutionary outcome predicted by Gavrilets & Waxman's model might select for duplication of the locus for the male's manipulative trait and genetic diversification between the loci, giving the male a bunch of keys rather than a single key. In this respect, it is noteworthy that the Acp70A gene is duplicated in Drosophila subobscura (Cirera & Aguadé 1998).
Some of the above theoretical models have also investigated the evolutionary outcome when the manipulative traits are costly (Gavrilets et al. 2001; Rowe et al. 2005). In this case, the outcome can be an equilibrium (of both the manipulative traits and the trait over which there is conflict) or stable limit cycle (of the manipulative traits; it is not apparent from the reported results whether this also resulted in cyclical variation in the conflict trait). However, it is not yet clear how often these two types of outcome are expected to occur. In general, as mentioned above, many of the combinations of factors that might affect the evolutionary outcome of these kinds of models have not yet been investigated, and further and more systematic modelling would undoubtedly add to our understanding.
The models above consider possible evolutionary change in the values of existing traits, and with the exception of Gavrilets & Waxman's model do not allow for mutation limitation. If mutations were strongly limiting one could imagine a situation in which favourable mutations in one of the manipulative traits swept to equilibrium or fixation before the next favourable mutation arose, leading to oscillations in the value of the conflict trait. The models also consider evolution of a single pair of manipulative traits, but the evolution of new manipulative traits will also contribute to continuing adaptation and counter-adaptation.
So far, the role of harmful behaviour has not been considered. The selection pressure that is responsible for the manipulative adaptations and counter-adaptations that have been discussed is the conflict load that individuals of each sex experience whenever the value of the trait over which there is conflict is not at their sex's optimum. Moreover, none of the models referred to earlier in this section incorporate harm, so it is clear that the evolutionary chases and limit cycles that these models sometimes predict is not predicated on adaptations or counter-adaptations to sexual conflict being harmful. However, manipulative behaviour may involve collateral or adaptive harm. In the case of adaptive harm, the most efficient counter-adaptation may involve preventing or repairing the harm. In contrast, collateral harm is an unfortunate side-effect that reduces the fitness of the individual that is being manipulated, and usually also of the individual that is doing the manipulation. If this is so, then whereas reversal of the manipulation is only in one sex's interest, reversal of the harm is to the advantage of both sexes. This opens the door to palliative adaptations (figure 4b) which reduce the harm without influencing the trait over which there is conflict. (This means that the evolutionary change takes place in a different trait dimension than adaptation and counter-adaptation, although this is difficult to represent convincingly in two-dimensions; figure 4b.) Because palliative adaptations are in the interests of both sexes, they may involve coordinated traits in the two sexes. One example is the evolution of the spermalege in bedbugs, a specialized organ that has evolved in females at the site used by males for traumatic insemination, and which appears to reduce the risk of infection to the female (Morrow & Arnqvist 2003; Reinhardt et al. 2003; Siva-Jothy 2006). The fact that males use this area when inseminating the females suggests that it is also in their interest to do so. Palliative adaptations are not sexually antagonistic and do not contribute to sexually antagonistic coevolution.
The new state created by changes in the adaptation–counter-adaptation cycle could create other opportunities for evolutionary changes that are in the interest of both sexes (cooperative adaptation; figure 4b). Good examples do not spring to mind, but if, for example, the original conflict led to the evolution of biparental care, both sexes might then gain through the evolution of role specialization. Like palliative adaptation, cooperative adaptation takes place in a different trait dimension than adaptation and counter-adaptation, and does not contribute to sexually antagonistic coevolution.
Lastly, changes that take place in the original adaptation–counter-adaptation cycle may create new sexual conflict; that is, sexual conflict over new traits. This might occur either because adaptation creates a new state—for example, a conflict over mating (with the female unwilling to mate) might be resolved by the provision of nuptial gifts, but provoke a new conflict over remating (with the male attempting to prevent the female from doing so)—or because adaptation might involve collateral harm—for example, collateral harm caused by Acps manipulating fecundity might provoke a new conflict over mating. In either of these cases, there can be adaptation and counter-adaptation to the new conflict (adaptation to novel conflict; figure 4b), and continuing evolutionary change for the same reasons as in the original sexual conflict.
This dissection of the evolutionary changes that follow from sexual conflict reveals greater complexity than the simple model shown in figure 4a. In particular, a number of different kinds of evolutionary change are involved: first, adaptation and counter-adaptation provoked by the conflict load implicit in sexual conflict can lead to continuous evolutionary change for three reasons: evolutionary chases (including cyclical dynamics) involving existing variants of traits, evolution of novel variants of traits (new alleles at existing loci), and evolution of novel manipulative traits (new loci); second, adaptation and counter-adaptation can generate sexual conflict over new traits which will then elicit the same kinds of sexually antagonistic coevolution; third, sexually antagonistic coevolution may create trait combinations selecting for mutualistic evolutionary change (including palliative adaptations).
The dissection of the evolutionary changes that follow from sexual conflict also clarifies the role of harm. In general, harmful behaviour is not necessary for sexually antagonistic coevolution to occur: manipulative behaviour need not be harmful, and evolutionary chases and limit cycles can occur without harmful behaviour. Moreover, in the case of collateral harm, the manipulation to which it is a side-effect is subject to sexually antagonistic selection, while the harm itself is not. The palliative adaptations that result are not sexually antagonistic. The one way in which harm does contribute positively to sexually antagonistic coevolution is by creating novel sexual conflicts. It is an interesting, important and entirely open question whether harm is essential in perpetuating sexually antagonistic coevolution by ‘winding up’ conflict systems that would otherwise ‘run down’.
(d) Concluding remarks
One of the main goals of this paper was to attempt to bring two groups of traits—those related to PI and mating—over which there is sexual conflict, but which are normally treated separately, into the same conceptual framework. The aim of this was to examine the extent to which the evolutionary outcomes of sexual conflict depend on the subject of conflict, and thus whether sexual conflict lives up to its billing as ‘a new paradigm’. A preliminary and highly provisional review suggests that this is not the case and that conflicts over PI are not so strongly associated with harmful behaviour and rapid evolutionary change (which may be symptomatic of sexually antagonistic coevolution) as conflicts over mating. To some extent these differences are understandable in terms of qualitative differences in the way that selection acts on PI and mating, and on the feasibility of manipulating PI. However, the analysis presented also points to other factors that cut across the classification in terms of the trait over which there is conflict having important effects on the evolutionary outcome. In particular, the way that manipulative traits interact in determining the value of the conflict trait may be influential: traits that interact like a lock-and-key may be particularly likely to exhibit continuing antagonistic coevolution because of the lack of costs associated with evolutionary changes.
The review of evolutionary outcomes in relation to the subject of conflict (table 1) also emphasizes the need for further empirical work. The apparent rarity of manipulative and harmful behaviour may be driven by expectations based on the assumptions of theoretical models in this area, rather than the other way around, and I hope that this paper will encourage empiricists to be alert to the possibility of such behaviour. More importantly, more studies are needed where we can make meaningful quantitative comparisons of the relative frequency of manipulation, the frequency and extent of harmful behaviour, and the rates of evolutionary change of manipulative traits in relation to the subject of conflict. Acps seem to offer a particularly promising group for study in this respect.
Lastly, conflict load and harm often seem to be confused in arguments concerning sexual conflict, probably because they both lead to a loss in fitness for the manipulated individual. However, whereas conflict load is what drives sexually antagonistic coevolution, collateral harm generally reduces the fitness of both sexes. As a result, the expected evolutionary response is different, and keeping the distinction between conflict load and harm distinct is, therefore, important. In particular, failure to recognize the potential for palliative adaptation can hinder understanding. Without the insight that palliative adaptations to collateral harm are in the interest of both sexes, the complicity of the manipulative sex (such as the use of the spermalege as the site of traumatic insemination by male bedbugs) can seem paradoxical. Palliative adaptations that involve changes in female traits may also give rise to a reduction of female fitness in inter-population crosses, and thus add to existing explanations (see Long et al. 2006) for such decreases. In conclusion, while harm is often a pervasive feature of sexual conflict, its evolutionary consequences can be very different from those of the conflict load which defines conflict and which provides the selective scope for the evolution of harmful behaviour.
Acknowledgments
I wish to thank Tracey Chapman, Tom Tregenza and Nina Wedell for organizing the Royal Society discussion meeting on Sexual conflict: a new paradigm? and inviting me to give the talk on which this paper is based, Nick Colegrave for suggesting the expression ‘conflict load’, Göran Arnqvist, Hanna Kokko and Locke Rowe for other comments and suggestions that have helped to remove ambiguities and errors from this paper, and Nick Colegrave, Geoff Parker and Dave Shuker for comments on an earlier draft of the paper.
Footnotes
One contribution of 13 to a Discussion Meeting Issue ‘Sexual conflict: a new paradigm?’.
References
- Aguadé M. Different forces drive the evolution of the Acp26Aa and Acp26Ab accessory gland genes in the Drosophila melanogaster species complex. Genetics. 1998;150:1079–1089. doi: 10.1093/genetics/150.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguadé M, Miyashita N, Langley C.H. Polymorphism and divergence in the Mst26A male accessory gland gene region in Drosophila. Genetics. 1992;132:755–770. doi: 10.1093/genetics/132.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatalo R.V, Carlson A, Lundberg A, Ulfstrand S. The conflict between male polygamy and female monogamy: the case of the pied flycatcher Ficedula hypoleuca. Am. Nat. 1981;117:738–753. 10.1086/283756 [Google Scholar]
- Andersson J, Borg-Karlson A.K, Wiklund C. Sexual cooperation and conflict in butterflies: a male-transferred anti-aphrodisiac reduces harassment of recently mated females. Proc. R. Soc. B. 2000;267:1271–1275. doi: 10.1098/rspb.2000.1138. 10.1098/rspb.2000.1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist G, Nilsson T. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 2000;60:145–164. doi: 10.1006/anbe.2000.1446. 10.1006/anbe.2000.1446 [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Correlated evolution of male and female morphologies in water striders. Evolution. 2002a;56:936–947. doi: 10.1111/j.0014-3820.2002.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002b;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- Bateman A.J. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Begun D.J, Whitley P, Todd B.L, Waldrip-Dail H.M, Clark A.G. Molecular population genetics of male accessory gland proteins in Drosophila. Genetics. 2000;156:1879–1888. doi: 10.1093/genetics/156.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackenhorn W.U, Hosken D.J, Martin O.Y, Reim C, Teuschl Y, Ward P.I. The costs of copulating in the dung fly Sepsis cynipsea. Behav. Ecol. 2002;13:353–358. 10.1093/beheco/13.3.353 [Google Scholar]
- Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. 10.1046/j.1365-2540.2001.00961.x [DOI] [PubMed] [Google Scholar]
- Chapman T, Lindsay F, Liddle F, Kalb J.M, Wolfner M.F, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by accessory gland proteins. Nature. 1995;373:241–244. doi: 10.1038/373241a0. 10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Chapman T, Neubaum D.M, Wolfner M.F, Partridge L. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc. R. Soc. B. 2000;267:1097–1105. doi: 10.1098/rspb.2000.1114. 10.1098/rspb.2000.1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003a;18:41–47. 10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Chapman T, Bangham J, Vinti G, Siegfried B, Lung O, Wolfner M.F, Smith H.K, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc. Natl Acad. Sci. USA. 2003b;100:9923–9928. doi: 10.1073/pnas.1631635100. 10.1073/pnas.1631635100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirera S, Aguadé M. Evolutionary history of the sex-peptide (Acp70a) gene region in Drosophila melanogaster. Genetics. 1997;147:189–197. doi: 10.1093/genetics/147.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirera S, Aguadé M. The sex-peptide gene (Acp70a) is duplicated in Drosophila subobscura. Gene. 1998;210:247–254. doi: 10.1016/s0378-1119(98)00069-9. 10.1016/S0378-1119(98)00069-9 [DOI] [PubMed] [Google Scholar]
- Civetta A, Clark A.G. Correlated effects of sperm competition and postmating female mortality. Proc. Natl Acad. Sci. USA. 2000;97:13 162–13 165. doi: 10.1073/pnas.230305397. 10.1073/pnas.230305397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civetta A, Singh R.S. High divergence of reproductive tract proteins and their association with post-zygotic reproductive isolation in Drosophila melanogaster and Drosophila viridis group species. J. Mol. Evol. 1995;41:1085–1095. doi: 10.1007/BF00173190. 10.1007/BF00173190 [DOI] [PubMed] [Google Scholar]
- Civetta A, Singh R.S. Sex-related genes, directional sexual selection, and speciation. Mol. Biol. Evol. 1998;15:901–909. doi: 10.1093/oxfordjournals.molbev.a025994. [DOI] [PubMed] [Google Scholar]
- Clark A, Aguadé G.M, Prout T, Harshman L, Langley C.H. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Princeton University Press; Princeton, NJ: 1991. The evolution of parental care. [Google Scholar]
- Clutton-Brock T.H, Parker G.A. Sexual coercion in animal societies. Anim. Behav. 1995a;49:1345–1365. 10.1006/anbe.1995.0166 [Google Scholar]
- Clutton-Brock T.H, Parker G.A. Punishment in animal societies. Nature. 1995b;373:209–216. doi: 10.1038/373209a0. 10.1038/373209a0 [DOI] [PubMed] [Google Scholar]
- Collins N.S. Developmental responses to food limitation as indicators of environmental conditions for Ephydra cinerea Jones (Diptera) Ecology. 1980;61:650–661. [Google Scholar]
- Constantz G.D. Sperm competition in poeciliid fishes. In: Smith R.L, editor. Sperm competition and the evolution of animal mating systems. Academic Press; London: 1984. pp. 465–485. [Google Scholar]
- Crudgington H.S, Siva-Jothy M.T. Genital damage, kicking and early death. Nature. 2000;47:855–856. doi: 10.1038/35038154. [DOI] [PubMed] [Google Scholar]
- Dawkins R. Oxford University Press; Oxford: 1976. The selfish gene. [Google Scholar]
- Dawkins R. Oxford University Press; Oxford: 1989. The selfish gene—new edition. [Google Scholar]
- Eberhard W.G. Harvard University Press; Cambridge, MA: 1985. Sexual selection and animal genitalia. [Google Scholar]
- Eberhard W.G. Evolutionary conflicts of interest: are female sexual decisions different? Am. Nat. 2005;165:519–525. doi: 10.1086/429348. 10.1086/429348 [DOI] [PubMed] [Google Scholar]
- Fiumera A.C, Dumont B.L, Clark A.G. Natural variation in male-induced ‘cost of mating’ and allele-specific association with male reproductive genes in Drosophila melanogaster. Phil. Trans. R. Soc. B. 2006;361:355–361. doi: 10.1098/rstb.2005.1791. 10.1098/rstb.2005.1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S. Rapid evolution of reproductive barriers driven by sexual conflict. Nature. 2000;403:886–889. doi: 10.1038/35002564. 10.1038/35002564 [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Waxman D. Sympatric speciation by sexual conflict. Proc. Natl Acad. Sci. USA. 2002;99:10 533–10 538. doi: 10.1073/pnas.152011499. 10.1073/pnas.152011499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S, Arnqvist G, Friberg U. The evolution of mate choice by sexual conflict. Proc. R. Soc. B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. 10.1098/rspb.2000.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D, Riddle D.L. Longevity in Caenorhabditis elegans reduced by mating but not gamete production. Nature. 1996;379:723–725. doi: 10.1038/379723a0. 10.1038/379723a0 [DOI] [PubMed] [Google Scholar]
- Grafen A, Sibly R. A model of mate desertion. Anim. Behav. 1978;26:645–652. 10.1016/0003-3472(78)90131-8 [Google Scholar]
- Groothuis T.G.G, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Hager R, Johnstone R.A. The genetic basis of family conflict resolution in mice. Nature. 2003;421:533–535. doi: 10.1038/nature01239. 10.1038/nature01239 [DOI] [PubMed] [Google Scholar]
- Haig D. Genomic imprinting and the theory of parent–offspring conflict. Semin. Dev. Biol. 1992;3:153–160. [Google Scholar]
- Haig D. The kinship theory of imprinting. Annu. Rev. Ecol. Syst. 2000;31:9–32. 10.1146/annurev.ecolsys.31.1.9 [Google Scholar]
- Haig D, Graham C. Genomic imprinting and the strange case of the insulin-like growth factor II receptor. Cell. 1991;64:1045–1046. doi: 10.1016/0092-8674(91)90256-x. 10.1016/0092-8674(91)90256-X [DOI] [PubMed] [Google Scholar]
- Hansson B, Bensch S, Hasselquist D. Infanticide in great reed warblers: secondary females destroy eggs of primary females. Anim. Behav. 1997;54:297–304. doi: 10.1006/anbe.1996.0484. 10.1006/anbe.1996.0484 [DOI] [PubMed] [Google Scholar]
- Heifitz Y, Lung O, Frongillo E.A, Wolfner M.F. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 2000;10:99–102. doi: 10.1016/s0960-9822(00)00288-8. 10.1016/S0960-9822(00)00288-8 [DOI] [PubMed] [Google Scholar]
- Herndon L.A, Wolfner M.F. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc. Natl Acad. Sci. USA. 1995;92:10 114–10 118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc. Natl Acad. Sci. USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. 10.1073/pnas.96.9.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston A.I, Davies N.B. The evolution of cooperation and life history in the dunnock. In: Sibly R.M, Smith R.H, editors. Behavioural ecology. Blackwell Scientific Publications; Oxford: 1985. pp. 471–487. [Google Scholar]
- Houston A.I, Székely T, McNamara J.M. Conflict between parents over care. Trends Ecol. Evol. 2005;20:33–38. doi: 10.1016/j.tree.2004.10.008. 10.1016/j.tree.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Johnstone R.A, Keller L. How males can gain by harming their mates: sexual conflict, seminal toxins, and the cost of mating. Am. Nat. 2000;156:368–377. doi: 10.1086/303392. 10.1086/303392 [DOI] [PubMed] [Google Scholar]
- Kubli E. Sex-peptides: seminal peptides of the Drosophila male. Cell. Mol. Life Sci. 2003;60:1689–1704. doi: 10.1007/s00018-003-3052. 10.1007/s00018-003-3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus J, Inglis I.R. Shared and unshared parental investment, parent–offspring conflict, and brood size. Anim. Behav. 1986;34:1791–1804. [Google Scholar]
- Lessells C.M. Sexual conflict in animals. In: Keller L, editor. Levels of selection in evolution. Princeton University Press; Princeton, NJ: 1999. pp. 75–99. [Google Scholar]
- Lessells C.M. Why are males bad for females? Models for the evolution of damaging male mating behaviour. Am. Nat. 2005;165:S46–S63. doi: 10.1086/429356. 10.1086/429356 [DOI] [PubMed] [Google Scholar]
- Limbourg T, Mateman A.C, Andersson S, Lessells C.M. Female blue tits adjust parental effort to manipulated male UV attractiveness. Proc. R. Soc. B. 2004;271:1903–1908. doi: 10.1098/rspb.2004.2825. 10.1098/rspb.2004.2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. 10.1073/pnas.1631700100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long T.A.F, Montgomerie R, Chippindale A.K. Quantifying the gender load: can population crosses reveal interlocus sexual conflict? Phil. Trans. R. Soc. B. 2006;361:363–374. doi: 10.1098/rstb.2005.1786. 10.1098/rstb.2005.1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin O.Y, Hosken D.J. Costs and benefits of evolving under experimentally enforced polyandry or monogamy. Evolution. 2003;57:2765–2772. doi: 10.1111/j.0014-3820.2003.tb01518.x. [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. Parental investment: a prospective analysis. Anim. Behav. 1977;25:1–9. 10.1016/0003-3472(77)90062-8 [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge, UK: 1982. Evolution and the theory of games. [Google Scholar]
- McVean G.T, Hurst L.D. Molecular evolution of imprinted genes: no evidence for antagonistic coevolution. Proc. R. Soc. B. 1997;264:739–746. doi: 10.1098/rspb.1997.0105. 10.1098/rspb.1997.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels N.K. Mating conflicts and sperm competition in simultaneous hermaphrodites. In: Birkhead T.R, Møller A.P, editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 219–254. [Google Scholar]
- Morrow E.H, Arnqvist G. Costly traumatic insemination and a female counter-adaptation in bed bugs. Proc. R. Soc. B. 2003;270:2377–2381. doi: 10.1098/rspb.2003.2514. 10.1098/rspb.2003.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow E.H, Arnqvist G, Pitnick S. Adaptation versus pleiotropy: why do males harm their mates? Behav. Ecol. 2003;6:802–806. 10.1093/beheco/arg073 [Google Scholar]
- Mulder R.A, Langmore N.E. Dominant males punish helpers for temporary defection in superb fairy-wrens. Anim. Behav. 1993;45:830–833. 10.1006/anbe.1993.1100 [Google Scholar]
- Neubaum D.M, Wolfner M.F. Mated female Drosophila melanogaster require a seminal fluid protein Acp36DE, to store sperm efficiently. Genetics. 1999;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970;45:525–568. [Google Scholar]
- Parker G.A. Selection on non-random fusion of gametes during the evolution of anisogamy. J. Theor. Biol. 1978;73:1–28. doi: 10.1016/0022-5193(78)90177-7. 10.1016/0022-5193(78)90177-7 [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sexual selection and sexual conflict. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; London: 1979. pp. 123–166. [Google Scholar]
- Parker G.A. Mate quality and mating decisions. In: Bateson P.P.G, editor. Mate choice. Cambridge University Press; Cambridge, UK: 1983. pp. 141–166. [Google Scholar]
- Parker G.A. Sexual conflict over mating and fertilization: an overview. Phil. Trans. R. Soc. B. 2006;361:235–259. doi: 10.1098/rstb.2005.1785. 10.1098/rstb.2005.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A, Partridge L. Sexual conflict and speciation. Phil. Trans. R. Soc. B. 1998;353:261–274. doi: 10.1098/rstb.1998.0208. 10.1098/rstb.1998.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A, Baker R.R, Smith V.G.F. The origin and evolution of gamete dimorphism and the male–female phenomenon. J. Theor. Biol. 1972;36:529–553. doi: 10.1016/0022-5193(72)90007-0. 10.1016/0022-5193(72)90007-0 [DOI] [PubMed] [Google Scholar]
- Parker G.A, Royle N.J, Hartley I.R. Interfamilial conflict and parental investment: a synthesis. Phil. Trans. R. Soc. B. 2002;357:295–307. doi: 10.1098/rstb.2001.0950. 10.1098/rstb.2001.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Hurst L.D. Sex and conflict. Science. 1998;281:2003–2008. doi: 10.1126/science.281.5385.2003. 10.1126/science.281.5385.2003 [DOI] [PubMed] [Google Scholar]
- Pitnick S, Miller G.T, Reagan J, Holland B. Males' evolutionary responses to experimental removal of sexual selection. Proc. R. Soc. B. 2001;268:1071–1080. doi: 10.1098/rspb.2001.1621. 10.1098/rspb.2001.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt K, Naylor R, Siva-Jothy M.T. Reducing a cost of traumatic insemination: female bedbugs evolve a unique organ. Proc. R. Soc. B. 2003;270:2371–2375. doi: 10.1098/rspb.2003.2515. 10.1098/rspb.2003.2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R. Intergenomic conflict, interlocus antagonistic coevolution, and the evolution of reproductive isolation. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University Press; Oxford: 1998. pp. 261–270. [Google Scholar]
- Rice W.R. Dangerous liaisons. Proc. Natl Acad. Sci. USA. 2000;97:12 953–12 955. doi: 10.1073/pnas.97.24.12953. 10.1073/pnas.97.24.12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R, Holland B. The enemies within: intergenomic conflict, interlocus contest evolution (ICE), and the intraspecific Red Queen. Behav. Ecol. Sociobiol. 1997;41:1–10. 10.1007/s002650050357 [Google Scholar]
- Rowe L, Cameron E, Day T. Escalation retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. Am. Nat. 2005;165:S5–S18. doi: 10.1086/429395. 10.1086/429395 [DOI] [PubMed] [Google Scholar]
- Ryan M.J, Fox J.H, Wilczynski W, Rand A.S. Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature. 1990;343:66–67. doi: 10.1038/343066a0. 10.1038/343066a0 [DOI] [PubMed] [Google Scholar]
- Sandell M. Female aggression and the maintenance of monogamy: female behaviour predicts male mating status in European starlings. Proc. R. Soc. B. 1998;265:1307–1311. 10.1098/rspb.1998.0434 [Google Scholar]
- Sandell M.I, Smith H.G. Already mated females constrain male mating success in the European starling. Proc. R. Soc. B. 1996;263:743–747. [Google Scholar]
- Saudan P, et al. Ductus ejactulatorius peptide 99B (DUP99B), a novel Drosophila melanogaster sex-peptide pheromone. Eur. J. Biochem. 2002;269:989–997. doi: 10.1046/j.0014-2956.2001.02733.x. 10.1046/j.0014-2956.2001.02733.x [DOI] [PubMed] [Google Scholar]
- Schneider J.M, Lubin Y. Infanticidal male eresid spiders. Nature. 1996;381:655–656. 10.1038/381655a0 [Google Scholar]
- Schwabl H. Maternal testosterone in the egg enhances postnatal growth. Comp. Biochem. Physiol. 1996;114:271–276. doi: 10.1016/0300-9629(96)00009-6. 10.1016/0300-9629(96)00009-6 [DOI] [PubMed] [Google Scholar]
- Sheldon B.C. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 2000;15:397–402. doi: 10.1016/s0169-5347(00)01953-4. 10.1016/S0169-5347(00)01953-4 [DOI] [PubMed] [Google Scholar]
- Simmons L.W. Princeton University Press; Princeton, NJ: 2001. Sperm competition and its evolutionary consequences in the insects. [Google Scholar]
- Siva-Jothy M.T. Trauma, disease and collateral damage: conflict in cimicids. Phil. Trans. R. Soc. B. 2006;361:269–275. doi: 10.1098/rstb.2005.1789. 10.1098/rstb.2005.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.C, Fretwell S.D. The optimal balance between size and number of offspring. Am. Nat. 1974;108:499–506. 10.1086/282929 [Google Scholar]
- Stutt A.D, Siva-Jothy M.T. Traumatic insemination and sexual conflict in the bedbug Cimex lectularius. Proc. Natl Acad. Sci. USA. 2001;98:5683–5687. doi: 10.1073/pnas.101440698. 10.1073/pnas.101440698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson W.J, Vacquier V.D. Reproductive protein evolution. Annu. Rev. Ecol. Syst. 2002;33:161–179. 10.1146/annurev.ecolsys.33.010802.150439 [Google Scholar]
- Swanson W.J, Clark A.G, Waldrip-Dail H.M, Wolfner M.F, Aquadro C.F. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc. Natl Acad. Sci. USA. 2001;98:7375–7379. doi: 10.1073/pnas.131568198. 10.1073/pnas.131568198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Singh R.S. A comprehensive study of genetic variation in natural populations of Drosophila melanogaster. VII. Varying rates of genetic divergence as revealed by two-dimensional electrophoresis. Mol. Biol. Evol. 1992;9:507–525. doi: 10.1093/oxfordjournals.molbev.a040738. [DOI] [PubMed] [Google Scholar]
- Tram U, Wolfner M.F. Male seminal fluid proteins are essential for sperm storage in Drosophila melanogaster. Genetics. 1999;153:837–844. doi: 10.1093/genetics/153.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man, 1871–1971. Aldine Atherton; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- True J.R, Weir B.S, Laurie C.C. A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritanica chromosomes into Drosophila simulans. Genetics. 1996;142:819–837. doi: 10.1093/genetics/142.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaur S.-C, Ting C.-T, Wu C.-I. Positive selection during the evolution of a gene of male reproduction, Acp26Aa, of Drosophila: II. Divergence versus polymorphism. Mol. Biol. Evol. 1998;15:1040–1046. doi: 10.1093/oxfordjournals.molbev.a026002. [DOI] [PubMed] [Google Scholar]
- Valera F, Hoi H, Schleicher B. Egg burial in penduline tits, Remiz pendulinus: its role in mate desertion and female polyandry. Behav. Ecol. 1997;8:20–27. [Google Scholar]
- Valera F, Hoi H, Kristin A. Male shrikes punish unfaithful females. Behav. Ecol. 2003;14:403–408. 10.1093/beheco/14.3.403 [Google Scholar]
- Veiga J.P. Prospective infanticide and ovulation retardation in free-living house sparrows. Anim. Behav. 1993;45:43–46. 10.1006/anbe.1993.1005 [Google Scholar]
- Vox Haartman L. Nest-site and the evolution of polygamy in European passerine birds. Ornis Fenn. 1969;46:1–12. [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. 10.1016/j.cub.2005.01.051 [DOI] [PubMed] [Google Scholar]
- Winkler D. A general model for parental care. Am. Nat. 1987;130:526–543. 10.1086/284729 [Google Scholar]
- Wolfner M.F. Tokens of love: function and regulation of Drosophila male accessory gland proteins. Insect Biochem. Mol. Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. 10.1016/S0965-1748(96)00084-7 [DOI] [PubMed] [Google Scholar]
- Wolfner M.F. The gifts that keep on giving: physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity. 2002;88:85–93. doi: 10.1038/sj.hdy.6800017. 10.1038/sj.hdy.6800017 [DOI] [PubMed] [Google Scholar]