Abstract
We consider mathematical models describing the evolutionary consequences of antagonistic interactions between male offence, male defence and female reproductive tract and physiology in controlling female mating rate. Overall, the models support previous verbal arguments about the possibility of continuous coevolutionary chase between the sexes driven by two-way (e.g. between male offence and female traits) and three-way (e.g. between male offence, male defence and female traits) inter-sexual antagonistic interactions. At the same time, the models clarify these arguments by identifying various additional potential evolutionary dynamics and important parameters (e.g. genetic variances, female optimum mating rates, strength of selection in females and the relative contributions of first and second males into offspring) and emphasizing the importance of initial conditions. Models also show that sexual conflict can result in the evolution of monandry in an initially polyandrous species and in the evolution of random mating in a population initially exhibiting non-random mating.
Keywords: sexual conflict, mating rate, remating suppression, monandry, random mating, mathematical models
1. Introduction
The available data are still fragmentary but they are consistent with the hypothesis that there is a three-way tug-of-war between [male] offence, [male] defence, the female reproductive tract and physiology. This three-way conflict generates many opportunities for both two-way and three-way interlocus antagonistic coevolution.
(Rice 1998, p. 264; see also Rice & Holland 1997; Holland & Rice 1998)
A graphical illustration of this three-way tug-of-war is given in figure 1. An example used by Rice to introduce this hypothesis considers a female that mates with two males. The traits of the first male (e.g. the properties of his seminal fluids) are selected for ‘defence’, that is, the capacity to reduce remating in the female and, if it fails, to prevent his sperm from being displaced by the second male. The traits of the second male are selected for ‘offence’, that is, the ability to persuade the female to remate with him and then to displace the sperm of the first male. From the female's perspective, there can be some benefits of remating, such as additional nutrients (if a nuptial gift is provided), the insurance against infertility or low sperm levels of some males, help in caring for offspring, the ability to gain resources without male harassment, etc. (Arnqvist & Nilsson 2000; Jennions & Petrie 2000). However, at some level of remating its costs will probably outweigh its benefits. Therefore, the optimum remating probability for females is likely to be intermediate between 0 and 1.
Figure 1.

A three-way tug-of-war (after Rice 1998).
Recent work stimulated by a surge of interest in sexual conflict and its evolutionary consequences (reviewed in Stockley 1997; Chapman et al. 2003; Pizzari & Snook 2003; Arnqvist & Rowe 2005; see also other articles in this issue and in the special issues of American Naturalist (Hosken & Snook 2005) and Evolutionary Ecology (Härdling & Smith 2005)) has largely supported Rice's hypothesis. The existence of different two- and three-way intra- and inter-sexual conflicts is well appreciated by now. The purpose of this paper is to explore potential evolutionary consequences of these conflicts using a series of simple, yet general, mathematical models. A major reason for using mathematical models is the complexity of predicted evolutionary outcomes which results in the limitations of biological intuition and reasoning based on generalization from data to identify most important and plausible patterns of evolutionary change and factors driving it. The models to be studied here are intended to help train our intuition. The hope is that once one has relatively clear ideas about expected dynamics, one can come up with some falsifiable hypotheses and better theoretical guidance for empirical work.
2. General modelling framework
Here, we will consider conflict over mating rate. Let f(x) be the distribution of a female trait x (‘resistance’ to the male strategy) and g(y, z) be the distribution of a pair of male traits y and z (‘stimulus to mate’ and ‘remating suppression’, respectively). The traits are assumed to be controlled by different loci. For definiteness, we will assume that x, y and z can take any value between −∞ and ∞. Let , and be the corresponding mean values and Gx, Gy and Gz the corresponding additive genetic variances.
In models, two functions will control the between-sexes interactions. The first function, which we denote as ψ(x, y), is the probability that a virgin female x accepts male y (0≤ψ≤1). This function is analogous to the so-called ‘preference function’ common in models of sexual selection (e.g. Lande 1981; Sved 1981a,b; Gavrilets 2004). The second function, which we denote as ϕ(x, y, z), is the probability that a female x that has already mated with a male with remating suppression trait z mates again with a male with stimulus trait y (0≤ϕ≤1). Between-sexes interactions will translate into fitness components: wf and wm for female and male fitness, respectively. Sexual conflict will be incorporated into the model by assuming that female fitness wf is maximized at a mating rate Popt the value of which is different from the mating rate optimum for males.
In analysing the models, we will use a quantitative genetics approach (e.g. Lande 1976, 1979; Iwasa et al. 1991; Abrams et al. 1993; Gavrilets 1997). This approach represents a valuable alternative to the game-theory approaches (e.g. Parker 1979; Parker & Partridge 1998; Härdling et al. 1999, 2001; Smith & Härdling 2000; Ball & Parker 2003; Lessells 2005) and standard population genetic approaches (e.g. Prout & Clark 1996; Clark et al. 2000; Gavrilets & Waxman 2002; Haygood 2004), which have been extensively used for studying sexual conflict. Our approximations will imply that selection resulting from between- and within-sexes interactions is weak and that genetic variances are constant (see Gavrilets 2000; Gavrilets et al. 2001 for an explanation of the mathematical techniques used). Note that within the realm of our approximations the degree of linkage between different loci is irrelevant. We neglect microenvironmental effects on the traits values because within our approximations, these effects merely weaken selection. To clearly identify the effects of different forces involved in between-sexes interactions during reproduction on the evolutionary dynamics, we neglect direct natural selection on the traits considered.
Whenever possible, we will be using a Kolmogorov-type approach (Kolmogorov 1936; translated in Oliveira-Pinto & Conolly 1982), specifying only the general properties of important functions rather than their exact form. This approach greatly increases the generality of theoretical results.
3. Two-way conflict over mating
We start with a model formulated in terms of only two traits: male mating stimulus trait y and female resistance to mating trait x while neglecting male remating suppression trait z. This is a model of a two-way tug-of-war between male offence and female reproductive tract and physiology (see figure 2). Within this framework, any effects of previous matings on remating probability are neglected so that function ψ(x, y) describes mating behaviour of both virgin and previously mated females.
Figure 2.
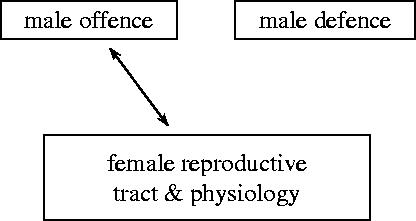
A two-way tug-of-war between male offence and female reproductive tract and physiology.
We will assume that the preference function ψ(x, y) depends on x and y only through their difference: ψ(x, y)=ψ(u), where u=y−x and that ψ is a symmetric, monotonically increasing S-shaped function, such that ψ(−∞)=0, ψ(0)=1/2, ψ(+∞)=1 such as in figure 3a. This implies that large values of y and small values of x promote mating, whereas small y and large x make mating less probable. We assume that each male mating with a given female fathers an equal proportion of her offspring.
Figure 3.
The probabilities of mating and remating. (a) The probability of mating, ψ(u). (b) The probability of remating, ϕ(v).
Let g(y) be the distribution of trait y among males. The average preference of female x for males in the population is
Note that in this model 0≤P(x)≤1. Variable P(x) can be viewed as a proxy for female mating rate (Gavrilets 2000; Gavrilets et al. 2001). Therefore, we will assume that female fitness is a unimodal function of P=P(x):
with a maximum at P=Popt (0≤Popt≤1). (A unimodal female fitness function wf(P) will arise if the costs of remating to females keep increasing with P while the corresponding benefits approach a limit.) This implies that for P<Popt and for P>Popt. Note that the average fitness of females is approximately . By our assumptions, a male y that mates with a female x fathers a (small) fraction of her offspring proportional to 1/P(x). Therefore, male fitness, i.e. the average contribution to offspring, is
The average fitness of males is 1. Under this model, the change in the average trait values per generation can be approximated as
| (3.1a) |
| (3.1b) |
where P, ψ and ψ′ are evaluated at and the coefficient 1/2 reflects the fact that each trait is subject to selection only in half of the whole population. This shows that while males always evolve to increase their trait (i.e. always), females always evolve to achieve their optimum mating rate (so that if P<Popt and if P>Popt).
The change in per generation can be written as
| (3.2) |
The dynamics of u and, consequently, the dynamics of the average mating rate P will depend on the relative positions of the graphs of functions h1=Gy/Gx and . In particular, if the graphs do not intersect, which happens if h1>h2 for all 0≤P≤1, then the average trait values and increase at a (quickly) decreasing rate, their difference quickly becomes large so that females mate at the maximum rate (i.e. P→1). This is a ‘males win’ scenario. Because in this scenario, , and, thus, ψ→1, one can also interpret this regime as resulting in the evolution of random mating. If the graphs of h1 and h2 intersect once, the system will approach a dynamic state in which both male and female traits keep increasing at a constant rate while their difference approaches a constant value and the mating rate P approaches a constant value P* which is larger than Popt (i.e. , ψ→const.<1, P→const.<Popt). This is a ‘dynamic compromise between the sexes’ scenario. In this regime, ψ<1 and, thus, the population maintains non-random mating.
Example 1. Let the female fitness function be
| (3.3) |
where V is a parameter measuring the strength of selection in females. Then h2=P(P−Popt)/V. Note that h2>0 only for P>Popt. Now if
| (3.4) |
then h1>h2 always. In this case, males eventually evolve trait values that force females to mate at a maximum rate (i.e. , ψ→1, P→1; see figure 4a–c). This outcome is promoted by increasing male variance Gy, decreasing female variance Gx, increasing the optimum female mating rate Popt, and decreasing the strength of selection (i.e. increasing V). If inequality (3.4) is not satisfied, then the population approaches a dynamic state at which female mating rate is
| (3.5) |
and is larger than Popt but smaller than 1 (see figure 4d–f). This outcome is promoted by decreasing male variance Gy, increasing female variance Gx, decreasing the optimum mating rate Popt, and increasing the strength of selection in females (i.e. decreasing V).
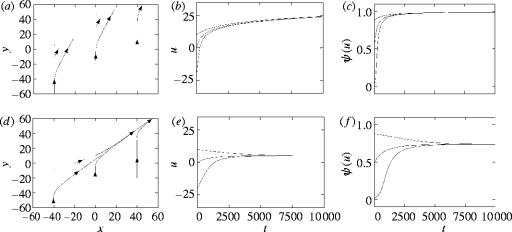
Figure 4.
The dynamics in Example 1. (a–c) Evolution towards the maximum female mating rate (‘males win’ scenario; condition (3.4) is satisfied). (d–f) Evolution towards an intermediate female mating rate (‘dynamic compromise between the sexes’ scenario; condition (3.4) is not satisfied). (a, d) The dynamics on the phase-plane. (b, e) The dynamics of in time. (c, f) The dynamics of the average mating rate ψ(u) in time. The trajectories for different initial conditions are shown.
These results represent a generalization of those in Gavrilets et al. (2001; see also Gavrilets & Waxman 2002; Rowe et al. 2003 and a review of similar models in Gavrilets & Hayashi 2005) for the case of more general functions ψ and wf. Other generalizations have also been explored. In particular, Gavrilets et al. (2001) have incorporated stabilizing natural selection on x and y which is neglected here. As expected, the addition of stabilizing selection prevents runaway evolution towards infinite trait values. Stabilizing selection can also result in the appearance of cyclic behaviour, similar to that in models of sexual selection (Iwasa & Pomiankowski 1995), and in the existence of multiple, simultaneously stable equilibria. In the latter case, the eventual outcome of the evolutionary dynamics depends on initial conditions. Gavrilets & Waxman (2002) and Gavrilets & Hayashi (2005) have explicitly incorporated multiallelic genes into this framework. Their results (see also Frank 2000; Haygood 2004) have demonstrated the possibility of the maintenance of extensive genetic variation in female loci only or in both male and female loci. Numerical individual-based simulations of Gavrilets & Hayashi (2005) explicitly accounting for random genetic drift confirmed the generality of the conclusions based on simple analytical approximations.
Generalizing from the simple model considered above and more complex models studied elsewhere, it appears that in models of two-way tug over mating between male offence and female traits there are at least six different dynamic regimes: (1) continuous coevolutionary chase between the sexes, (2) evolution towards an equilibrium, (3) cyclic evolution, (4) evolution towards a line of equilibria with subsequent random drift along this line, (5) ‘Buridan's Ass’ regime (Gavrilets & Waxman 2002) involving extensive diversification in female alleles without comparable diversification in male alleles and (6) extensive diversification in both male and female alleles. Models show that different dynamic regimes can be observed with the same set of parameter values but under different initial conditions. It is also possible that the same population switches from one regime to another as a result of stochastic perturbations due to random genetic drift. Moreover, different sets of loci controlling mating and fertilization in the same species can follow different dynamic regimes. Some of these findings have important implications for the quantitative theory of speciation (Gavrilets 2004). The most relevant in this regard are regime (1), which can result in allopatric speciation as a by-product (Gavrilets 2000), and regime (6), which can result in sympatric speciation (Gavrilets & Waxman 2002).
4. Two-way conflict over remating
Next, we consider male remating suppression trait z and female resistance to remating suppression trait x but neglect the effects of male trait y. This is a model of a two-way tug-of-war between male defence and female reproductive tract and physiology (see figure 5). We assume that mating is random.
Figure 5.
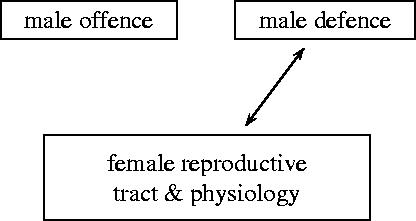
A two-way tug-of-war between male defence and female reproductive tract and physiology.
Let ϕ(x, z) be the probability that female x remates after mating with a male z. For simplicity, we posit that ϕ depends on x and z only through their difference: ϕ(x, z)=ϕ(v), where v=z−x, and that ϕ is a symmetric, monotonically decreasing S-shaped function, such that ϕ(−∞)=1, ϕ(0)=1/2, ϕ(+∞)=0 such as in figure 3b. This implies that large values of z and small values of x promote remating suppression, whereas with small z and large x remating suppression is ineffective. We assume that the last male to mate with a female fertilizes all her eggs.
Consider a previously mated female x. The overall probability that this female remates upon encountering a male is
where g(z) is the distribution of z among males. The probability that female x stops remating after the kth mating can be approximated as
The average number of matings per female x is , which simplifies to
| (4.1) |
Note that in this model the meaning of P is different from that in the previous model and that P≥1 (because each female mates at least once). The average remating probability of females is
Now consider a male z. The probability that he suppresses remating of a randomly chosen female is
The probability that he suppresses remating of a female that has mated k times overall is
Therefore, the average number of females fertilized by male z is , which simplifies to
| (4.2) |
Let wf be the female fitness function which we write as a function of P(x): wf=wf(P). As before, we assume that this function has a single maximum at P=Popt, so that for P<Popt and for P>Popt. Although, by our assumption, a single mating is sufficient to fertilize all eggs, Popt can be larger than 1 if there are some additional benefits of mating for females (Arnqvist & Nilsson 2000; Jennions & Petrie 2000). Note that the average fitness of females is approximately . Male fitness is simply the number of females fertilized: wm(z)=Q(z), and the average fitness of males is approximately 1.
The changes in the mean trait values per generation are
| (3.3a) |
| (4.3b) |
where both ϕ′ and P are evaluated at . Note that whereas always (i.e. males always evolve to suppress remating), is positive or negative depending on whether the average number of matings per female P is larger or smaller than Popt (i.e. females always evolve to achieve an optimum mating rate Popt).
The change in per generation can be written as
| (4.4) |
This equation is very similar to equation (3.2). The dynamics of , and consequently the dynamics of the average mating rate P, will depend on the relative positions of the graphs of functions h1=Gz/Gx and . In particular, if the graphs do not intersect, which happens if h1>h2 for all P≥1, then the average trait values and increase at a (quickly) decreasing rate, their difference quickly becomes very large, and males completely suppress remating (i.e. , ϕ→0, P→1). This is a ‘males win’ scenario. If the graphs of h1 and h2 intersect once, the system will approach a dynamic state in which both male and female traits keep increasing at a constant rate while their difference approaches a constant value and the mating rate P approaches a constant value which is smaller than Popt (i.e. , ϕ→const.<1, P→const.<Popt). This is a ‘dynamic compromise between the sexes’ scenario.
Example 2. Let the female fitness function be given by equation (3.3) as in example 1. Now h2=P(Popt−P)/V. Note that h2>0 only for P>Popt. Assume for simplicity that Popt≥2. Now if
| (4.5a) |
then functions h1 and h2 do not intersect and complete remating suppression evolves (i.e. , ϕ→0, P→1; see figure 6a–c). This outcome is promoted by increasing male variance Gz, decreasing female variance Gx, decreasing the optimum mating rate Popt, and decreasing the strength of selection (i.e. increasing V). If
| (4.5b) |
then functions h1 and h2 intersect once and the population approaches a dynamic state at which P is between Popt−1 and Popt (see figure 6d–f). Exactly, P=P**, where
This outcome is promoted by decreasing male variance Gz, increasing female variance Gx, increasing the optimum mating rate Popt, and increasing the strength of selection (i.e. decreasing V). If
| (4.5c) |
then functions h1 and h2 intersect twice and the dynamics depend on the initial value of P (see figure 6g–i). If P is small, exactly if P<P**, complete remating suppression evolves (, ϕ→0, P→1). Otherwise, the population approaches a dynamic state at which P is smaller than Popt (exactly, P=P**).
Figure 6.
The dynamics in Example 2. (a–c) Evolution towards zero female remating rate (‘males win’ scenario; condition (4.5a) is satisfied). (d–f) Evolution towards an intermediate female remating rate (‘dynamic compromise between the sexes’ scenario; condition (4.5b) is satisfied). (g–i) The outcome depends on initial conditions (condition (4.5c) is satisfied). (a, d, g) The dynamics on the phase-plane. (b, e, h) The dynamics of in time. (c, f, i) The dynamics of the average remating rate ϕ(v) in time. The trajectories for different initial conditions are shown.
5. Three-way conflict over mating
Finally, we consider all three traits x, y and z simultaneously. This is a model of a three-way tug-of-war between male offence, male defence and female reproductive tract and physiology (see figure 1). As a first step of building a theory of remating, we will assume that each female is subject to two mating attempts by randomly chosen males (cf. Johnston & Keller 2000; Ball & Parker 2003).
Let ψ(x, y) be the probability that a virgin female x accepts male y (0≤ψ≤1). We will assume that ψ increases with y but decreases with x (so that , ). The overall probability that a virgin female x mates upon encountering a randomly chosen male is
| (5.1) |
The overall proportion of virgin females x that reject the first male they encounter is 1−M(x).
Let ϕ(x, y, z) be the probability that a female x that has already mated with a male with remating suppression trait z mates again with a male with stimulus trait y (0≤ϕ≤1). We will assume that ϕ increases with y but decreases with x and z (so that , , ). The overall probability that a previously mated female x mates for the second time is
| (5.2) |
where y1, z1 and y2, z2 are the corresponding trait values of the first and second males. Then the proportions of females x that reject both males they encounter, accept only the second male, accept only the first male and mating twice are
| (5.3a) |
| (5.3b) |
| (5.3c) |
| (5.3d) |
respectively. Let the overall normalized fertility (which we treat as the lifetime fitness) of females mating once and twice be 1 and 1+s, respectively (s>−1). If s>0, females get fitness advantage from multiple matings; if s<0, second matings reduce female overall fitness. In terms of the previous model, s<0 corresponds to Popt=1, whereas s>0 corresponds to Popt=2. The female fitness function can be written as
| (5.4) |
Consider a male (y, z). The probability that he is accepted as a first mate by a female that does not mate anymore or by a female that did not mate on the first attempt is
The probability that he is accepted as a first mate by a female that later remates is
| (5.5a) |
The probability that this male is accepted as a second mate is
| (5.5b) |
Note that this probability does not depend on z. Assume that the second male fathers a proportion ρ of the female's offspring. Then male fitness can be written as
| (5.6) |
Then, the equations for the change in , and per generation take form
| (5.7a) |
| (5.7b) |
| (5.7c) |
where both functions ψ and ϕ and their derivatives (with respect to the variables given by the subscripts) are evaluated at .
Three general observations follow immediately. First, the male remating suppression trait z keeps continuously increasing (although at a quickly decreasing rate) if
| (5.8) |
that is, if female fitness advantage from the second mating is smaller than the relative advantage of the second male over the first male in fertilizing the female's eggs. Note that this condition is always satisfied if the second mating does not bring any benefits to females (i.e. if s<0). If condition (5.8) is not satisfied, the male remating suppression trait z keeps continuously decreasing. Second, the male stimulus y keeps continuously increasing: . (The proof follows from the fact that 1+(s−ρ−ρs)ϕ≥0 for all possible values of s, including s=−1.) How these two dynamics affect the evolution of remating probability depends on the dynamics of and . Third, if s≈0 (i.e. the benefits of remating are small) or ψ≈0 (i.e. virgin females are very reluctant to mate), then (female resistance decreases). If ψ≈1 (virgin females are eager to mate), then the sign of is opposite of that of s. In particular, female resistance trait will increase if s<0 (i.e. if the second mating decreases female fitness).
As before, we assume that the preference function ψ(y, x) depends on x and y only through their difference: ψ(y, x)=ψ(y−x), where ψ satisfies the same conditions as before (see figure 3a). We consider two models for remating suppression which both will be different from the one used in the two-way tug-of-war considered above. In the first model, the probability that a female x that has already mated with a male with remating suppression trait z mates again with a male with stimulus trait y is
| (5.9a) |
where r(z) is a monotonically decreasing function measuring the degree of mating suppression caused by male z (0≤r(z)≤1, r′(z)≤0). In this model, the effects of the two male traits interact in a multiplicative way. In the second model, remating suppression is incorporated by assuming that the male mating with a female effectively increases her trait value for the next mating. Specifically, the probability that a female x that has already mated with a male with remating suppression trait z mates again with a male with stimulus trait y is set to
| (5.9b) |
that is, we measure all three traits on the same scale and avoid introducing an additional function. In this model, the effects of the two male traits interact in a non-multiplicative way.
Under the first model, the dynamic equations simplify to
| (5.10a) |
| (5.10b) |
| (5.10c) |
where . Note that ψ′(u)>0. In this model, males completely suppress remating , if condition (5.8) is satisfied. Otherwise males completely loose remating suppression ability . Considering the difference , it is straightforward to show that in both cases (i.e. both for r=0 and r=1), u keeps increasing and, thus, mating rate ψ approaches one.
Summarizing, if remating benefits for females are relatively low (i.e. condition (5.8) is satisfied), the population evolves to a state with random mating of virgin females (ψ=1) and no subsequent remating (r=0, ϕ=0). If remating benefits for females are sufficiently high (i.e. condition (5.8) is not satisfied), then remating suppression disappears completely (r=1, ϕ=ψ) and the population evolves random mating (i.e. ψ=1). In either case, the average trait values evolve at (quickly) decreasing rates. Note that these conclusions do not depend on genetic variances.
Under the second model, the dynamic equations simplify to
| (5.11a) |
| (5.11b) |
| (5.11c) |
where and . Note that ψ′(u), ψ′(v)>0.
To analyse this model, we consider the equations for and . From these equations, it is straightforward to show that there are always two equilibria: (i) ψ(u)=ϕ(u)=1, describing a state with random mating and no remating suppression and (ii) ψ(u)=1, ϕ(u)=0, describing a state with random mating and complete suppression of remating. If the second mating is strongly deleterious, specifically if
| (5.12a) |
there is an additional equilibrium with ψ(u)=ψ*<1 (non-random mating of virgin females) and ψ(v)=1 (no remating suppression). Here,
| (5.12b) |
This equilibrium is a saddle point and is never stable. Furthermore, one can show that the system evolves to a state with random mating and no remating suppression (ψ(u)=ψ(v)=1) if
| (5.13) |
that is, if remating benefits to females are sufficiently large (assuming s>0) or remating losses are not too big (assuming s<0). This outcome is promoted when genetic variation in male offence (Gy) is larger than that in male defence (Gz). Otherwise, complete suppression of remating evolves (i.e. v→−∞, ψ(v)→0). This can happen even when females gain from the second mating (i.e. s>0), provided the genetic variation in male defence (Gz) is larger than that in male offence (Gy). Note that in contrast to the previous model, here genetic variances matter a lot.
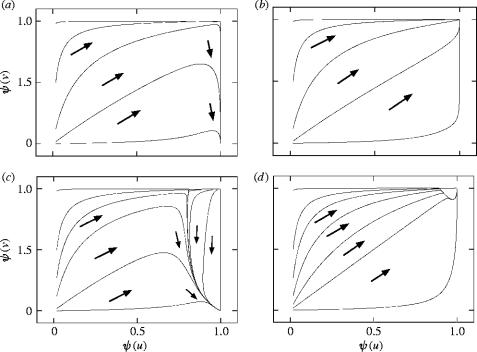
Summarizing, if condition (5.13) is satisfied, the system evolves to a state with ψ(u)=ψ(v)=1 (random mating, no remating suppression). If condition (5.13) is not satisfied, the system evolves to a state with ψ(u)=1, ψ(v)=0 (random mating, complete remating suppression). Note that during these dynamics, the male trait z continuously increases or decreases depending on whether condition (5.8) is satisfied or not. Possible dynamics are illustrated in figures 7 and 8 in terms of the probabilities of mating (ψ(u)) and remating (ψ(v)) for average phenotypes.
Figure 7.
The dynamics in the second model of the three-way conflict on a (ψ(u), ψ(v))-plane. (a, c) Evolution towards a state with random mating and complete suppression of remating (condition (5.13) is not satisfied). (b, d) Evolution towards a state with random mating and no remating suppression (condition (5.13) is satisfied). The equilibrium ψ(u)=ψ*, ψ(v)=1 does not exist in (a) and (b) (condition (5.13) is not satisfied). The equilibrium ψ(u)=ψ*, ψ(v)=1 exists in (c) and (d) (condition (5.13) is not satisfied). The trajectories for different initial conditions are shown.
Figure 8.

The blow-up of the upper right corner of figure 7d. The trajectories eventually approach the state with ψ(u)=ψ(v)=1 (random mating, no remating suppression).
6. Discussion
Here, we have analysed three related mathematical models of sexual conflict. The first model considers a two-way conflict between male offence and female reproductive tract and physiology. The role of male offence is played by a male trait stimulating females to mate, whereas the female trait is interpreted as ‘resistance to mate’. We assumed that all males that have mated with a given female father an equal proportion of her offspring. In this conflict, males are evolving to maximize female mating rate, whereas females are evolving to maintaining an optimum mating rate Popt. We showed that there are two possible dynamic regimes. In the first regime, males manage to maximize female mating rate. This is a ‘males win’ scenario. In this regime, females do not exhibit any mating preferences so that random mating evolves in a population that initially mates non-randomly. In the second regime, both sexes are locked in coevolutionary chase, where both male ‘stimulus’ trait and female resistance trait keep continuously increasing while female mating rate is at a constant value that is intermediate between female and male optimums (and is larger than Popt). This is a ‘dynamic compromise between the sexes’ scenario. The first regime is promoted by large genetic variance in males, small genetic variance in females, higher values of Popt and weaker selection in females. The second regime is promoted by smaller genetic variance in males, larger genetic variance in females, lower values of Popt and stronger selection in females.
The second model considers a two-way conflict between male defence and female reproductive tract and physiology. The role of male defence is played by the male's remating suppression ability, whereas the female trait is interpreted as ‘resistance to remating suppression.’ We assumed that the male who manages to suppress female remating fathers all of her offspring. In this conflict males are evolving to completely suppress female remating, whereas females are evolving to maintain an optimum remating rate Popt. The behaviour of this model is similar to that of the first one. In particular, we showed that there are two possible dynamic regimes. In the first regime, males manage to completely suppress female remating so that monandry evolves in an initially polyandrous population. This is a ‘males win’ scenario. In the second regime, both sexes are locked in coevolutionary chase, where both male and female traits keep continuously increasing while female remating rate is at a constant value that is intermediate between female and male optimums (and is smaller than Popt). This is the ‘dynamic compromise between the sexes’ scenario. The first regime is promoted by large genetic variance in males, small genetic variance in females, smaller values of Popt and weaker selection in females. The second regime is promoted by smaller genetic variance in males, larger genetic variance in females, larger values of Popt and stronger selection in females. Under some parameter combinations, which of the two regimes is realized depends not only on parameter values but also on initial conditions.
The third model considers a three-way conflict between male offence, male defence and female reproductive tract and physiology. In this conflict, males are evolving to stimulate females into mating and then to completely suppress female remating, whereas females are evolving to maintain an optimum number of matings. Two important limitations of this model are that (i) each female mates no more than twice, and that (ii) the first and second males father fixed proportions of her offspring. We analysed two versions of this model differing in the way the interaction of male offence and defence was defined. The overall conclusions are qualitatively similar in both models. Specifically, if the benefits of remating for females are sufficiently high, random mating evolves and remating suppression disappears completely. Otherwise, complete remating suppression can evolve even if females gain fitness advantage from remating. Interpreting these outcomes in terms of winning and losing sides is not straightforward (and, probably, not productive) because of the complexity of three-way interactions and the fact that with no more than two matings, the female's interest coincides with that of either male offence (if Popt=2) or male defence (if Popt=1).
Overall, these models support previous verbal arguments about the possibility of continuous coevolutionary chase between the sexes driven by two- and three-way inter-sexual antagonistic interactions. At the same time, the models clarify these arguments by identifying various additional evolutionary dynamics and important parameters (e.g. genetic variances, optimum mating rates, strength of selection in females and the relative contributions of first and second males into offspring) and emphasizing the importance of initial conditions. The models highlight the complexity of expected evolutionary dynamics and the difficulties in making clear-cut conclusions and predictions. In some cases, the models have been able to identify simple and intuitive conditions in terms of measurable parameters for observing one outcome or another (e.g. condition (5.8), which tells one that males completely suppress remating if female fitness advantage from the second mating is smaller than the relative advantage of the second male over the first male in fertilizing the female's eggs). Obtaining empirically-based information on these parameters would increase the predictive power of the models.
The models also show that sexual conflict can result in the evolution of monandry in an initially polyandrous species and in the evolution of random mating in a population initially exhibiting non-random mating. These outcomes are driven by selection related to the costs and benefits of mating and remating for females.
An encouraging feature of the models studied here, which strongly supports the generality of theoretical conclusions made, is that similar outcomes are observed in different models under a minimum number of assumptions. At the same time, the models do have certain limitations. The most crucial appears to be the assumptions that genetic variances are constant and that direct natural selection on the traits is absent. Removing these limitations is a necessary next step.
Acknowledgments
We thank Tracey Chapman, Nina Wedell and Tom Tregenza for organizing the Royal Society meeting and Carrie Eaton, Rick Dilling and reviewers for comments on the manuscript. Supported by National Institutes of Health grant GM56693 and by the National Science Foundation grant DEB-0111613.
Footnotes
One contribution of 13 to a Discussion Meeting Issue ‘Sexual conflict: a new paradigm?’.
References
- Abrams P.A, Harada Y, Matsuda H. On the relationship between quantitative genetic and ESS models. Evolution. 1993;47:982–985. doi: 10.1111/j.1558-5646.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Nilsson T. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 2000;60:145–164. doi: 10.1006/anbe.2000.1446. doi:10.1006/anbe.2000.1446 [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Rowe L. Sexual conflict. Princeton University Press; Princeton, NJ: 2005. [Google Scholar]
- Ball M.A, Parker G.A. Sperm competition games: sperm selection by females. J. Theor. Biol. 2003;224:27–42. doi: 10.1016/s0022-5193(03)00118-8. doi:10.1016/S0022-5193(03)00118-8 [DOI] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. doi:10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Clark A.G, Dermitzakis E.T, Civetta A. Nontransitivity of sperm precedence in Drosophila. Evolution. 2000;54:1030–1035. doi: 10.1111/j.0014-3820.2000.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Frank S.A. Sperm competition and female avoidance of polyspermy mediated by sperm–egg biochemistry. Evol. Ecol. Res. 2000;2:613–625. [Google Scholar]
- Gavrilets S. Coevolutionary chase in exploiter–victim systems with polygenic characters. J. Theor. Biol. 1997;186:527–534. doi: 10.1006/jtbi.1997.0426. doi:10.1006/jtbi.1997.0426 [DOI] [PubMed] [Google Scholar]
- Gavrilets S. Rapid evolution of reproductive isolation driven by sexual conflict. Nature. 2000;403:886–889. doi: 10.1038/35002564. doi:10.1038/35002564 [DOI] [PubMed] [Google Scholar]
- Gavrilets S. Fitness landscapes and the origin of species. Princeton University Press; Princeton, NJ: 2004. [Google Scholar]
- Gavrilets S, Hayashi T.I. Speciation and sexual conflict. Evol. Ecol. 2005;19:167–198. [Google Scholar]
- Gavrilets S, Waxman D. Sympatric speciation by sexual conflict. Proc. Natl Acad. Sci. USA. 2002;99:10 533–10 538. doi: 10.1073/pnas.152011499. doi:10.1073/pnas.152011499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilets S, Arnqvist G, Friberg U. The evolution of female mate choice by sexual conflict. Proc. R. Soc. B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. doi:10.1098/rspb.2000.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härdling R, Smith H.G. Introduction: evolutionary processes in sexual conflicts. Evol. Ecol. 2005;19:109–110. [Google Scholar]
- Härdling R, Jormalainen V, Tuomi J. Fighting costs stabilize aggressive behavior in intersexual conflicts. Evol. Ecol. 1999;13:245–265. doi:10.1023/A:1006667103497 [Google Scholar]
- Härdling R, Smith H.G, Jormalainen V, Tuomi J. Resolution of evolutionary conflicts: costly behaviours enforce the evolution of cost-free competition. Evol. Ecol. Res. 2001;3:829–844. [Google Scholar]
- Haygood R. Sexual conflict and protein polymorphism. Evolution. 2004;58:1414–1423. doi: 10.1111/j.0014-3820.2004.tb01723.x. [DOI] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Hosken D, Snook R. How important is sexual conflict? Am. Nat. 2005;165:S1–S4. doi:10.1086/429355 [Google Scholar]
- Iwasa Y, Pomiankowski A. Continual change in mate preferences. Nature. 1995;377:420–422. doi: 10.1038/377420a0. doi:10.1038/377420a0 [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Pomiankowski A, Nee S. The evolution of costly mate preferences. II. The “handicap” principle. Evolution. 1991;45:1431–1442. doi: 10.1111/j.1558-5646.1991.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol. Rev. 2000;75:21–64. doi: 10.1017/s0006323199005423. doi:10.1017/S0006323199005423 [DOI] [PubMed] [Google Scholar]
- Johnston R.A, Keller L. How males can gain by harming their mates: sexual conflict, seminal toxins, and the costs of mating. Am. Nat. 2000;156:368–377. doi: 10.1086/303392. doi:10.1086/303392 [DOI] [PubMed] [Google Scholar]
- Kolmogorov A. Sulla teoria di Volterra della Lotka per l'esistenza. Giornale Instituto Italiano degli Attuari. 1936;7:74–80. [Google Scholar]
- Lande R. Natural selection and random genetic drift in phenotypic evolution. Evolution. 1976;30:314–334. doi: 10.1111/j.1558-5646.1976.tb00911.x. [DOI] [PubMed] [Google Scholar]
- Lande R. Effective deme size during long-term evolution estimated from rates of chromosomal rearrangement. Evolution. 1979;33:234–251. doi: 10.1111/j.1558-5646.1979.tb04678.x. [DOI] [PubMed] [Google Scholar]
- Lande R. Models of speciation by sexual selection on polygenic characters. Proc. Natl Acad. Sci. USA. 1981;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessells C.M. Why are males bad for females? Models for the evolution of damaging male mating behavior. Am. Nat. 2005;165:S46–S63. doi: 10.1086/429356. doi:10.1086/429356 [DOI] [PubMed] [Google Scholar]
- Oliveira-Pinto F, Conolly B.W. Applicable mathematics of non-physical phenomena. Ellis Horwood; Chichester: 1982. [Google Scholar]
- Parker G.A. Sexual selection and sexual conflict. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; New York: 1979. pp. 123–166. [Google Scholar]
- Parker G.A, Partridge L. Sexual conflict and speciation. Phil. Trans. R. Soc. B. 1998;353:261–274. doi: 10.1098/rstb.1998.0208. doi:10.1098/rstb.1998.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzari T, Snook R.R. Perspective: sexual conflict and sexual selection: chasing away paradigm shifts. Evolution. 2003;57:1223–1236. doi: 10.1111/j.0014-3820.2003.tb00331.x. [DOI] [PubMed] [Google Scholar]
- Prout T, Clark A.G. Polymorphism in genes that influence sperm displacement. Genetics. 1996;144:401–408. doi: 10.1093/genetics/144.1.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R. Intergenomic conflict, interlocus antagonistic coevolution, and the evolution of reproductive isolation. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University Press; New York: 1998. pp. 261–270. [Google Scholar]
- Rice W.R, Holland B. The enemies within: intergenomic conflict, interlocus contest evolution (ICE), and intraspecific Red Queen. Behav. Ecol. Sociobiol. 1997;41:1–10. doi:10.1007/s002650050357 [Google Scholar]
- Rowe L, Cameron E, Day T. Detecting sexually antagonistic coevolution with population crosses. Proc. R. Soc. B. 2003;270:2009–2016. doi: 10.1098/rspb.2003.2453. doi:10.1098/rspb.2003.2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.G, Härdling R. Clutch size evolution under sexual conflict enhances the stability of mating systems. Proc. R. Soc. B. 2000;267:2163–2170. doi: 10.1098/rspb.2000.1264. doi:10.1098/rspb.2000.1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P. Sexual conflict resulting from adaptations to sperm competition. Trends Ecol. Evol. 1997;12:154–159. doi: 10.1016/s0169-5347(97)01000-8. doi:10.1016/S0169-5347(97)01000-8 [DOI] [PubMed] [Google Scholar]
- Sved J.A. A two-sex polygenic model for the evolution of premating isolation. I. Deterministic theory for natural populations. Genetics. 1981a;97:197–215. doi: 10.1093/genetics/97.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved J.A. A two-sex polygenic model for the evolution of premating isolation. II. Computer simulation of experimental selection procedures. Genetics. 1981b;97:217–235. doi: 10.1093/genetics/97.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]