Abstract
We describe a graphical model of interlocus coevolution used to distinguish between the interlocus sexual conflict that leads to sexually antagonistic coevolution, and the intrinsic conflict over mating rate that is an integral part of traditional models of sexual selection. We next distinguish the ‘laboratory island’ approach from the study of both inbred lines and laboratory populations that are newly derived from nature, discuss why we consider it to be one of the most fitting forms of laboratory analysis to study interlocus sexual conflict, and then describe four experiments using this approach with Drosophila melanogaster. The first experiment evaluates the efficacy of the laboratory model system to study interlocus sexual conflict by comparing remating rates of females when they are, or are not, provided with a spatial refuge from persistent male courtship. The second experiment tests for a lag-load in males that is due to adaptations that have accumulated in females, which diminish male-induced harm while simultaneously interfering with a male's ability to compete in the context of sexual selection. The third and fourth experiments test for a lag-load in females owing to direct costs from their interactions with males, and for the capacity for indirect benefits to compensate for these direct costs.
Keywords: sexually antagonistic coevolution, interlocus sexual conflict, interlocus contest evolution, indirect benefits
1. Introduction
Sexual conflict has three fundamentally different forms. Intralocus conflict occurs when there is a negative correlation between the selection coefficients of the same allele when expressed in males and females (Parsons 1961; Mandel 1971; Charlesworth & Charlesworth 1980; Bull 1983; Rice 1984; Rice & Chippindale 2001). In this case, sex-specific selection at a locus in one sex interferes with adaptation at the same locus by the other sex. Interlocus sexual conflict occurs when: (i) adaptive allelic replacement at a locus that influences a male's success in sexual selection also reduces the lifetime fecundity of females that interact with him, and (ii) there is counter-adaptation at a locus that influences a female's lifetime fecundity, because it protects her from male-induced harm but also interferes with her mates' success in sexual selection. This coupling of selection at the interacting loci perpetuates sexually antagonistic coevolution between these same loci. Lastly, there is an intrinsic conflict over mating rate when the sexes have different levels of parental investment in offspring (Bateman 1948; Trivers 1972). This intrinsic conflict is an integral part of the process of sexual selection, but it is distinct from interlocus sexual conflict, unless adaptations by males to fertilize a female's eggs also harm the female by reducing her lifetime fecundity.
This paper concerns interlocus sexual conflict and has two parts. In the first, we develop a graphical model for coevolution between (i) gene loci that are expressed in males that influence their success during the process of mating and sperm competition, and (ii) other gene loci that are expressed in females that interact with the gene products from the male-expressed loci. We use the graphical model to distinguish interlocus sexually antagonistic coevolution from both interlocus mutualistic coevolution and traditional models of sexual selection. We next develop a set of empirical predictions that can be used to test for the operation of interlocus sexual conflict. In the second part of the paper, we contrast ‘laboratory island analysis’ with other types of laboratory studies, and then use this form of empirical analysis to test for the presence of interlocus sexual conflict in the Drosophila melanogaster laboratory model system. Lastly, we discuss the extrapolation of results from studies on laboratory populations to the process of evolution in natural populations.
2. Basic terms and concepts
Interlocus sexual conflict can occur when the sexes are separate or when they are combined in hermaphrodites. For simplicity, here we will assume separate sexes, but the same logic can be applied to hermaphrodites.
Interlocus sexual conflict is a simple, predictable consequence of two biological attributes that apply to most species.
One sex (usually females, and assumed to be so here) has higher parental investment in offspring than the other, and this asymmetry generates selection in the lower-investing sex to gain access to this resource (Trivers 1972).
Unless there is random mating and lifelong monogamy, the correlation between the fitness of an individual and of his or her sexual partners is less than unity, so that adaptations can evolve that benefit one sexual partner at the expense of the other (modified from Trivers 1972 in Rice 2000).
Both Dawkins (1976) and Parker (1979) first recognized that these attributes could lead to a coevolutionary arms race between the sexes. Although it is convenient to describe such antagonistic coevolution in the context of males and females, except for the mitochondria and the non-recombining portion of the Y chromosome (when it is present in a species), males and females are not distinct genetic entities. As a consequence, antagonistic coevolution occurs between genes that are expressed in males and different genes that are expressed in females. Also, while it is convenient to think of these genes as sex-limited in their expression, this is not a requisite condition for sexually antagonistic coevolution (Rice & Holland 1997). Here we will use the term ‘male-expressed gene’ or ‘female-expressed gene’ to mean a gene expressed in males and females, respectively, but not necessarily exclusively in one sex. If a gene contributes to interlocus sexual conflict and selection is acting in opposite directions in the sexes, then both intra- and interlocus sexual conflict will occur simultaneously. Here we will not consider this complication, and implicitly assume that any associated intralocus sexual conflict is weak and can be ignored.
To distinguish antagonistic versus mutualistic interlocus coevolution between the sexes we first define two broad classes of genetically controlled phenotypes.
Male sexual selection stimulus. These gene products are expressed in males, directly influence a male's competitive ability during mating and fertilization, and indirectly (i.e. through pleiotropy) influence the fitness of his prospective mates.
Female sexual selection receptor. These gene products are expressed in females, directly influence female fecundity, and indirectly (i.e. through pleiotropy) influence the success of males attempting to fertilize her eggs (e.g. a gene reducing a female's attraction to a male's courtship signal would influence both her fecundity (less remating would reduce any harmful influence of seminal fluid proteins on her fecundity) and the male's mating success).
For brevity below, we will abridge these terms to male sex-sel-stimulus and female sex-sel-receptor.
3. Graphical model of sexual conflict
The hallmark of coevolving loci is that they evolve in response to each other, i.e. there is a coupling in which the fitness of the allele(s) present at one locus depends upon the phenotype produced by the allele(s) present at the interacting locus. For example, the efficacy of the product from a hormone-receptor gene depends on the specific structure of the product produced by the corresponding hormone-producing gene, and vice versa, because structural congruence between the two gene products is necessary for the function of each. As a consequence of this coupling, evolution at one locus influences the degree of adaptation at the other locus. Put more quantitatively, the lag-load (i.e. the difference between the mean fitness at a locus and its maximal value) at one locus depends on the phenotype produced by the other locus.
The concept of lag-load (Maynard Smith 1976) will be used extensively below. Lag-load occurs when the mean trait of a population ‘lags behind’ its optimal value (trait-lag) because the environment (physical, biotic, or intragenomic) is changing. Trait-lag generates directional selection to move the mean toward its optimum when the requisite phenotypic variation is present. Lag-load reduces the absolute fitness of a population but leads to evolutionary change only when heritable genetic variation (extant or subsequently generated by mutation) creates differences in the relative fitness of individual genotypes.
The qualitative dynamics of coevolution between pairs of sexually interacting loci can be graphically modelled as shown in figure 1. The attributes of each locus shown in the model are the distribution of phenotypes coded by the currently established alleles at the male sex-sel-stimulus and female sex-sel-receptor loci, and the phenotypic optimum at each locus. The interaction between the loci is depicted by the coupling-line (dashed line and loop, with the arrows indicating the direction of push or pull on the loop) that causes the phenotypic optimum of one trait to be moved in response to changes in the phenotypic mean of the other trait. The coupling-line illustrates only the direction of this interaction, so that the quantitative details are not specified in this qualitative model.
Figure 1.
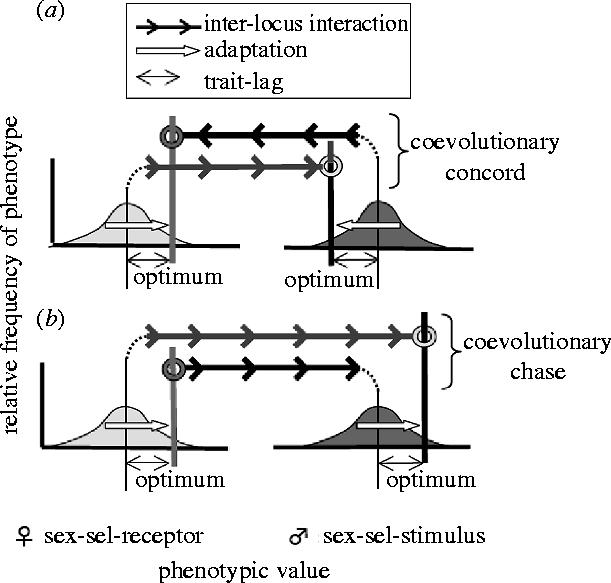
The graphical model of inter-locus coevolution between the sexes. The sex-sel-stimulus locus is expressed in males and influences both male success in sexual selection and the lifetime fecundity of females that interact with them. The sex-sel-receptor locus is expressed in females and influences both female lifetime fecundity and the success in sexual selection of their prospective mates. The further the mean trait (narrow lines in the centre of the phenotypic distributions) is from its optimum (i.e. increasing trait-lag), the greater the lag-load (reduced fitness compared to that which can potentially evolve), and hence the greater the potential for new adaptation. The coupling-line (arrowed line and loop, connected to a phenotypic mean by a dotted line; arrows indicates direction of push or pull on loop due to adaptive evolution) causes the phenotypic optimum of one trait to be moved in response to changes in the mean phenotype of the other trait. (a) Coevolutionary concord: mutualistic inter-locus coevolution reciprocally reduces trait-lag at both loci, and this diminishes the scope for future coevolution. (b) Coevolutionary chase: antagonistic inter-locus coevolution reciprocally increases trait-lag at each locus, and this perpetuates future coevolution.
In the case of mutualistic coevolution between the loci, adaptive evolution at one locus moves the optimum at the other locus closer to its phenotypic mean, i.e. reduces the trait-lag (the distance between the average trait value and its optimum), and hence the lag-load at the interacting locus (figure 1a). For example, if a hormone evolved to better fit its receptor, the trait-lag (and hence the lag-load) at the receptor locus would be reduced. This interaction is expected to dampen future evolutionary change because adaptive evolution at one locus reduces the lag-load at the other locus, and thereby reduces the scope of future evolution.
However, in the case of antagonistic coevolution, adaptive evolution at one locus moves the optimum at the other locus further from the mean of the population, and thereby increases its lag-load (figure 1b). To illustrate the dynamics of this form of interaction between loci, again consider the coevolution between a hormone and its receptor, but suppose that genetic drift has overpowered selection and caused a harmful mutation to evolve that modified the structure of a hormone so that it fits less well with its receptor. In this case, the evolutionary change at the hormone-producing locus would cause the trait-lag (and hence the lag-load) to increase at the hormone-receptor locus, since its optimum depends upon the molecule with which it interacts. The hormone-receptor locus would now be selected to ‘counter-evolve’ to increase its fit to the new structure of the hormone.
In the specific case of antagonistically interacting loci, the lag-load created at one locus due to adaptation at the other is expected to perpetuate evolutionary change at both loci, although this process may eventually stall due to selective constraints on further change at one or both loci (e.g. Rice & Holland 1999), or enter a stable limit cycle (Gavrilets 2000; Gavrilets et al. 2000). Because the mean fitness of males and females is the same, the mean fitness of females is the mean fitness of the population as a whole (see Arnqvist 2004 for discussion). As a consequence, antagonistic coevolution necessarily implies that while evolutionary advance at the male sex-sel-stimulus locus increases the relative fitness of males, this same adaptive evolution reduces the absolute mean fitness of the population (i.e. it reduces the lifetime fecundity of females), and that evolutionary advance at the female sex-sel-receptor locus increases absolute mean fitness of a population. Mutualistic coevolution produces a positive correlation between the change in lag-load (Δlag-load) at the female sex-sel-receptor and male sex-sel-stimulus loci, whereas antagonistic coevolution produces a negative correlation (figure 2). Stated more simply, when adaptive evolution at one locus (male sex-sel-stimulus or female sex-sel-receptor) increases lag-load at the other locus, there is evidence for sexual conflict.
Figure 2.
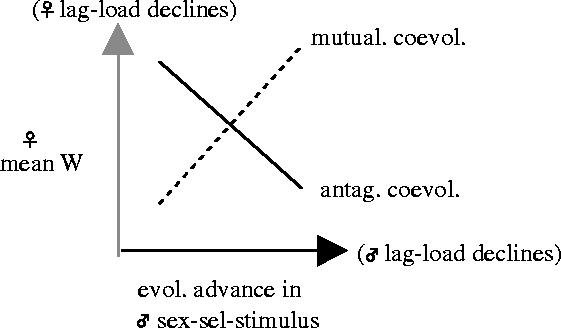
Mutualistic inter-locus coevolution leads to a positive correlation between the change in lag-load at the male and female loci, whereas antagonistic coevolution leads to a negative correlation.
Because the parental investment of females is larger, it is expected that adaptive change at loci influencing male access to female parental investment (i.e. at male sex-sel-stimulus loci) will evolve first, and that loci capable of protecting females from any harmful effects of these male adaptations (i.e. female sex-sel-receptor loci) will evolve secondarily, in response to male-induced harm to females. Because female sex-sel-receptor loci cannot ‘anticipate’ future forms of male-induced harm that will evolve, males are expected to ‘lead’ in the ‘antagonistic dance’ between the sexes (Rice 1996, 1998). Therefore, the cycle of adaptation and counter-adaptation occurs between:
sexually antagonistic male adaptations. Traits coded by male sex-sel-stimulus loci that improve competitive ability in the context of sexual selection, but that simultaneously reduce the lifetime fecundity of a male's sexual partners as a pleiotropic byproduct. These adaptations increase male relative fitness but reduce mean population fitness (which equals mean female fitness) and thereby create a lag-load in females, and
sexually antagonistic female adaptations. Traits coded by female sex-sel-receptor loci that reduce harm from her mates but simultaneously (i.e. through pleiotropy) reduce the efficacy of male adaptations influencing sexual selection. These female adaptations generate a lag-load in males and increase mean population fitness (figures 2 and 3).
Figure 3.
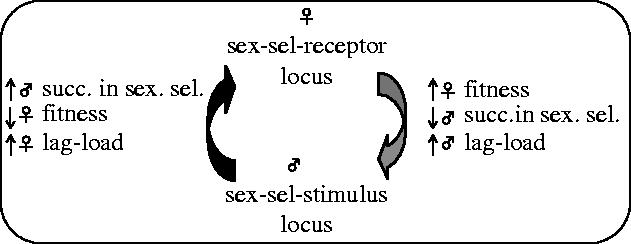
Sexually antagonistic coevolution occurs when adaptations at male-expressed locus influencing sexual selection (male sex-sel-stimulus) reduces female lifetime fecundity, and counter-adaptations at a female-expressed locus (female sex-sel-receptor) interfere with male adaptations influencing sexual selection.
The cycle of adaptation and counter-adaptation between the sexes generates three empirical predictions.
At least some adaptations at male sex-sel-stimulus loci reduce the fitness of a male's sexual partners and thereby reduce mean population fitness, creating a lag-load in females.
Male-induced harm to females is not fully compensated by indirect benefits (increased grand-offspring production; Parker 1979; Arnqvist 1992; Andrés & Morrow 2003; Cordero & Eberhard 2003), and
At least some adaptations at female sex-sel-receptor loci that protect them from male-induced harm also reduce (through pleiotropy) the efficacy of traits in their sexual partners which influence their success in sexual selection, and thereby create a lag-load in males.
Testing these predictions is complicated by the fact that the genome is expected to be a mosaic of sexually interacting loci, only some of which will be evolving antagonistically (for discussion see, Rice & Holland 1997, 1999; Holland & Rice 1998). When antagonistic interactions are collectively stronger than mutualistic interactions, then testing for interlocus sexual conflict is made simpler because one can use males and females as the experimental units to test the above predictions. In the currency of males and females, the predictions become: (i) the net effect of all male interactions with females (beyond the minimum necessary to accomplish fertilization) reduces female mean fitness (=mean population fitness), (ii) this harm is not fully compensated for by indirect benefits, and (iii) reductions in female resistance to male-induced harm increase male fertilization success. When antagonistic interactions are not collectively stronger than mutualistic interactions, the three predictions must be tested on individual pairs of interacting loci (or pairs of interacting traits if a quantitative genetics approach is used) and the operation of interlocus sexual conflict at least some interacting loci would be confirmed if any pair of loci was found to display interlocus sexual conflict. However, for pragmatic purposes, the male/female approach is the simplest starting point in the search for evidence for interlocus sexual conflict.
The three empirical predictions will be tested in the D. melanogaster laboratory model system in §4. However, before we move on to §4, we use the graphical model to contrast sexually antagonistic coevolution with traditional models of sexual selection.
4. Distinguishing interlocus sexual conflict from traditional models of sexual selection
Conflict over mating rate is an integral part of traditional models of sexual selection because males are selected to secure larger numbers of mates than females (Bateman 1948; Trivers 1972). However, as pointed out above, the interlocus sexual conflict that leads to sexually antagonistic coevolution is distinct from this intrinsic conflict between the sexes. The distinction occurs because the male phenotypes coded by the male sex-sel-stimulus loci are assumed, by definition, to directly harm females by reducing their lifetime fecundity, and hence reducing mean population fitness. This is not the case for traditional models of sexual selection, as can be seen by applying the graphical model of sexual conflict and coevolution (figure 2) to them. The major models of sexual selection are:
material-benefits. The male sex-sel-stimulus is an honest indicator of the capacity of a male to provide parental investment;
good-genes. The male sex-sel-stimulus is an honest indicator of the capacity of a male to provide high quality genes that improve offspring fitness outside the context of sexual selection;
Fisher run-away. The male sex-sel-stimulus is a heritable trait that influences female preference (also a heritable trait) in the context of sexual selection (but see Day (2000) for a discussion of the theoretical limitations of this process);
sensory exploitation. The male sex-sel-stimulus is a trait that is preferred by females due to a previously evolved phenotype in females that incidentally predisposes them to prefer a male trait.
To contrast these models, we will trace how change in the mean fitness of females (Δlag-load) is associated with the evolutionary advance (reduced lag-load) of the male sex-sel-stimulus phenotype (figure 4). First, consider the material-benefits model. As the male trait evolves, females are better able to evaluate potential mates and reject males that provide lower parental investment. As a consequence, mean female fitness increases (directly, through increased progeny production and/or indirectly, through increased grand-offspring production when male parental investment results in increased offspring quality) as the male sex-sel-stimulus evolves (figure 4b). The opposite pattern is seen for interlocus sexual conflict (figure 4a). The same pattern as the material-benefits model is seen in the good-genes model. In this latter case, however, as the male sex-sel-stimulus evolves females are able to better evaluate the genetic quality of prospective mates, and this enhanced capacity is expected to increase female mean fitness indirectly via increased grand-offspring production (because a female's offspring have inherited ‘good-genes’, figure 4c). In the sensory exploitation model, the male sex-sel-stimulus has no association with fitness outside the context of sexual selection. As a consequence, average lifetime fecundity of females is unaffected by the evolution at the male sex-sel-stimulus (figure 4d). The same relationship as seen in the sensory exploitation also applies to the Fisher-runaway model, but in this case females are predicted to evolve increased preference for the male display trait (figure 4e). In this way, the Fisher runaway model is superficially like the sexual conflict model, because adaptation at male sex-sel-stimulus loci induces a lag-load in female sex-sel-receptor loci, and vice versa. Also, like the interlocus sexual conflict, female lifetime fecundity would be predicted to decline in response to male–female coevolution, although only when increased female preference has a cost in the currency of her lifetime fecundity (see Day (2000) for a discussion of the theoretical feasibility of this case).
Figure 4.
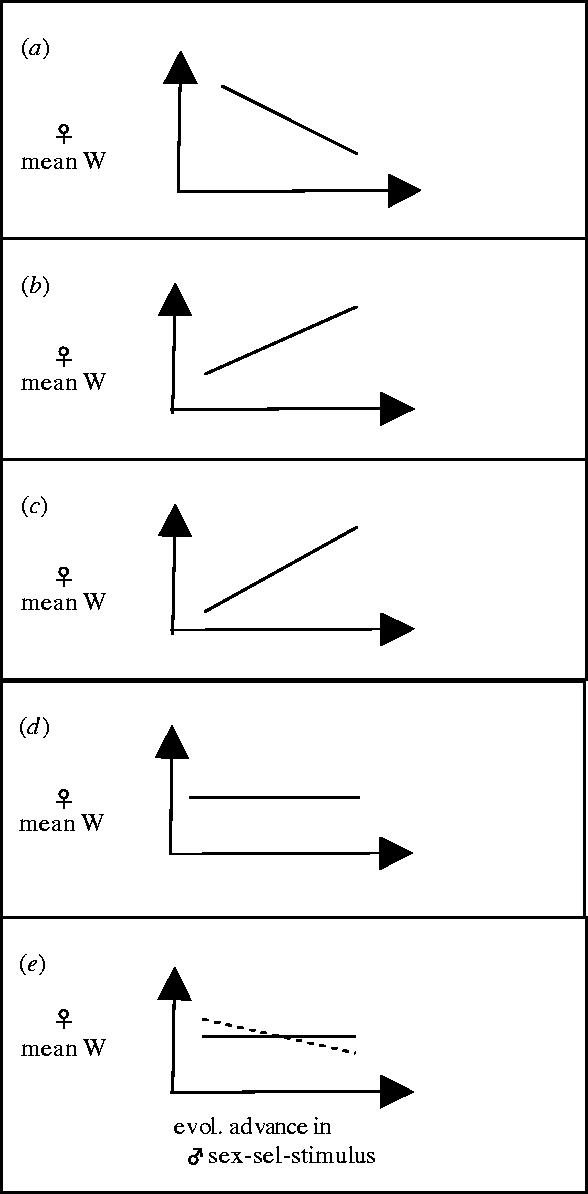
Evolutionary advance at a male sex-sel-stimulus locus causes mean female fitness (which is mean population fitness) to decline (due to direct male harm to females) in the case of sexual conflict but not in any of the traditional models of sexual selection. In the Fisher run-away model, however female fitness will decline indirectly in response to male adaptive evolution when female preference is costly (dotted line). In this latter case, the males do not directly reduce female lifetime fecundity (as is the case with interlocus sexual conflict), but instead females evolve increased preference for the male sex-sel-stimulus that is costly, and this evolution leads to a reduction in female fitness. (a) Interlocus sexual conflict, (b) material benefits, (c) good genes, (d) sensory exploitation, and (e) fisher run-away.
However, there is a fundamental difference between Fisher's run-away model and the interlocus conflict model. The decline in female fitness that is expected in the Fisher's run-away model is indirect (due to concomitant female evolution) and is not caused by direct, male-induced harm to a female's reproductive output, as is the case in the interlocus sexual conflict model. Instead, in the Fisher's run-away model the reduction in female fitness evolves in response to a lag-load generated in females by evolutionary advance of a male sex-sel-stimulus that does not directly reduce the females' lifetime reproductive output.
5. Empirical evaluation of sexual conflict
Here, we test the three predictions of interlocus sexual conflict described above, specifically in the context of the D. melanogaster laboratory model system. For pragmatic reasons, we will focus on the whole-organism interactions between males and females rather than identifying interactions between specific gene loci. We first discuss our use of the laboratory island analysis approach. We next describe the specific protocol used to propagate our laboratory population because this protocol is relevant to the interpretation of our experiments. We then describe a set of four experiments that test for inter-sexual conflict in our laboratory model system.
(a) Laboratory island analysis
D. melanogaster is one of the major model systems used to study sexual conflict, and the predominant model system when genetic analysis is used (see citations in Rice & Holland 1997; Wolfner 1997; Chapman 2001; Chapman et al. 2003). There are two major limitations to this model system: (i) many studies have used inbred strains or populations that are recently derived from nature, and (ii) most laboratory populations are maintained at high density in spatially uniform environments (vials, bottles, or small population cages) in which females lack a spatial refuge from persistent male courtship.
Inbred strains, such as Oregon-R and Canton-S, have been repeatedly bottlenecked, which causes this normally highly heterozygous species to become homozygous due to inbreeding, and to fix unconditionally harmful mutations. As a consequence, some male–female interactions observed in these populations may be artefacts of inbreeding, rather than being due to adaptive coevolution. Therefore, observed male–female interactions in inbred populations are difficult to extrapolate to natural populations. Alternatively, populations that are newly derived from nature have had little time to adapt to the laboratory environment, and as a consequence, sexual interactions observed in the laboratory may be maladaptive transients that do not represent coevolved interactions between the sexes. Therefore, studies of these populations are difficult to interpret (Sgrò & Partridge 2000).
The alternative used by our laboratory and others (e.g. the laboratories of T. Chapman, B. Charlesworth, A. Chippindale, K. Fowler, L. Mueller, L. Partridge, M. Rose, and P. Service) is to take a large founding population from nature, allow it to adapt to laboratory conditions for hundreds of generations at a persistently large effective size, and then assay evolutionarily interesting phenotypes under these same (or very similar) conditions. We refer to this paradigm as laboratory island analysis and its logical foundation is described in detail in Rice et al. (2005).
Island populations have played a pre-eminent role in the study of both evolution and ecology. We think that much of this prevalence derives from the fact that island populations are simpler, in the context of both their biotic and abiotic environments, and therefore easier to understand. Laboratory populations that have adapted for hundreds of generations (continuously at large size, to a competitive laboratory environment) are similar in many ways to natural island populations. These natural populations are descendants of continental populations that have colonized, and adapted to, an isolated island environment. However, just as Darwin used his study of island populations to make inferences about general principles of evolution, rather than trying to use these populations to directly extrapolate to the evolution of the specific continental populations from which they were derived, studies of laboratory island populations do not attempt to directly extrapolate to the natural populations from which they were derived. Because the laboratory environment is so different from nature, it is impossible to directly extrapolate from studies of laboratory-adapted populations to natural populations. However, these populations can be studied in their own right to deduce general principles of evolution. It is these general principles that can be applied to natural populations. We think that it is from this philosophical perspective that studies of long-established outbred populations should be viewed. In §5b, we briefly summarize the protocol used to propagate our laboratory base population (LHM). All of the experiments that we describe below closely match these conditions during the assays of male and female performance.
(b) Propagation of laboratory population
Our LHM base population has been maintained at high population size (greater than 1700 breeding adults) for over 350 generations. It cycles through three sets of vials each generation that we refer to as: juvenile-competition vials, adult-competition vials, and oviposition vials. To begin each generation, on day 1 of the 14-day generation cycle, approximately 10 000 eggs are distributed among 56 juvenile-competition vials at a density of 150–200 per vial. The larval, pupal, and early adult stages occur in these vials during days 1–12.25, with the moderate larval density leading to an egg-to-adult mortality rate of approximately 10%. Next, the flies are mixed among vials and a sample of 1792 adults is placed into a group of 56 adult-competition vials at a density of only 16 pairs per vial. These vials are placed on their sides to give the adults more horizontal space in which to interact, and 10 mg of live yeast is applied to the surface of the 10 ml of killed yeast medium that is located at the end of each vial. There is a steep linear relationship between consumption of live yeast and female fecundity, and the small amount of yeast in the adult competition vials causes intense competition among the females to secure this limiting resource (see description in Linder & Rice 2005). Although almost all females have mated before entering the adult-competition vials, most remate at least once while in these vials (Orteiza et al. 2005). This high level of remating produces strong sexual selection among males during this phase of the life history. Also, because in the adult-competition vials (i) most females remate, (ii) most offspring are derived from the last male to mate a female, and (iii) female fecundity is determined predominantly by the amount of live yeast they obtain during scramble competition for this limiting resource, adult reproductive performance is largely determined by how well an adult competes during the adult competition stage of their life history. Finally, on day 14.25 (i.e. one quarter of the way through day 14) the flies are transferred to ‘oviposition vials’ for 18 h and then discarded. Only eggs laid in the oviposition vials are used to start the next generation (reduced to 150–200 per vial), so fecundity during this time represents a female's lifetime fecundity. A detailed description of the base population and its propagation can be found in Rice et al. (2005).
(c) Lack of a spatial refuge from persistent male courtship in laboratory populations
Even when flies are outbred and adapted to a competitive laboratory environment, another concern, in the context of interlocus sexual conflict, is that males and females are confined at high density to small, spatially uniform environments, and, therefore, females cannot escape persistent male courtship. In nature, male D. melanogaster patrol high quality sites used by females for feeding and oviposition (Markow 1988). As a consequence, females must contend with persistent male courtship when they feed and reproduce, but they can escape persistent male courtship simply by leaving these high quality sites. To partially alleviate this unnatural aspect of the laboratory propagation of flies, we have, as described in §5b, maintained our laboratory population at relatively low adult density during the adult-competition phase of their life cycle. To assess the possible artefact of females having no spatial refuge from persistent male courtship in our in our low-adult-density population, we focused on the remating rate of females as an index of the influence of a lack of a spatial refuge in the laboratory. There is strong evidence that male-induced harm to females increases with the amount of remating (Kuijper et al. submitted) and seminal fluid that they receive (Chapman et al. 1995), so we tested to determine whether females in our population would remate at appreciably lower rates when, as occurs in nature, they had a spatial refuge.
We made a spatial refuge by bisecting culture vials longitudinally with a screen mesh during the adult-competition phase of the life cycle (Byrne et al. submitted). Females were phenocopied to small body size (25.8% smaller than normal) by rearing them on a lower volume of food (1 ml instead of the normal 10 ml). In contrast, males were reared at reduced density (66% lower) in normal food vials. While this male treatment had little effect on the average body size of males (only a 1% increase) it did reduced the variance in body size by 70%, so that males of very small size were eliminated. These treatments produced females that could freely move between the two compartments of a vial, but males that were constrained to remain on the side where they were initially placed at the beginning of the adult-competition stage of their life cycle. The full results from this study will be published elsewhere, but here we briefly report the results of two treatments. In each treatment males and females were reared under the normal culturing protocol of the LHM base population with the exception of the phenocopy procedure used to control body size body size, and that they were collected as virgin soon after eclosion and held in separate, same-sex vials until the beginning of the adult-competition stage of their life cycle (see above), at which point they were placed at normal density in the bisected culturing vials. Females were mated immediately before being placed in the adult-competition vials (by placing them for 90 min with a 50% excess of males) and remating in the adult-competition vials was measured using genetic markers (see Friberg et al. 2005; Linder & Rice 2005; Orteiza et al. 2005, for a description of the genetic marker system). The two treatments were: (i) a low-resource refuge (as occurs in nature): males were confined to the side of the vial containing the live yeast needed for high female fecundity, and (ii) a wide-mesh control in which both males and females could pass through the mesh. Treatments and remating rates are shown in figure 5. There was a 20% reduction in the remating rate between the no-refuge treatment (similar to standard laboratory conditions) and the low-resource refuge (more similar to natural conditions). These results suggest that the presence of a low-quality refuge does reduce remating rate, but only by 20%, so the refuge does not markedly protect females from the costs of remating. The similarity in remating with and without a spatial refuge from persistent male courtship supports the conclusion that lack of such a refuge in the laboratory model system does not appreciably reduce the usefulness of this model system to study sexual conflict.
Figure 5.
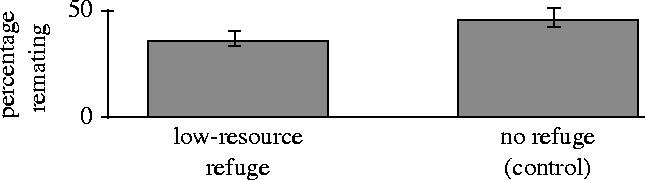
Female remating rate is reduced by about 20% when females are provided with a spatial refuge from persistent male courtship. Error bars are standard errors.
(d) Has past evolution by female sex-sel-receptor loci created a lag-load at male sex-sel-stimulus loci?
To anyone who has spent time watching interactions between adult male and female D. melanogaster in the laboratory, or in the field, it is readily apparent that males persistently court non-virgin females, but these females rarely mate with males (e.g. see Spieth 1952, Markow 1988). Therefore, there seems little doubt that the female resistance to persistent male courtship that has evolved in the past is currently creating a lag-load in males, since nearly all mating attempts by males are rebuffed. We nonetheless decided to carry out a small experiment to confirm this supposition in our LHM base population by showing that the reproductive potential of males is sufficiently large that they would have substantially higher reproductive output if they could overcome the resistance to remating by non-virgin females. This was done by placing a single male into a vial containing 16 virgin females during the 2-day adult competition phase of the life cycle. This treatment gave males a nearly inexhaustible supply of females with minimal resistance to their persistent courtship (because virgin females were sperm-limited and only had one male with which to mate) while all other conditions (age of the flies, amount of the limiting resource of live yeast, etc.) were very similar to the normal propagation of the LHM base population. Remating rate by non-virgin females in the adult competition vials has varied substantially in past experiments in our laboratory (40–100% of females remate at least once). However, a liberal upper-bound for this parameter can be obtained from a recent study in our laboratory that used multiple genetic markers (Morrow et al. in press). This study estimated female remating rate to be just over one remating per female (mean±s.e.=1.24±0.02) under conditions designed to maximize female remating (i.e. females were transferred to fresh vials with live yeast on both days during the adult-competition phase of the life cycle). In sharp contrast, when males were provided with a large number of virgin females, which have greatly reduced resistance to male mating attempts, males mated an average of 9.0±1.0 (mean±s.e.) females and this high rate of mating was associated with markedly increased total offspring production (figure 6; data collected by A. M. Hannes and A. D. Stewart). These data indicate that past evolution at female sex-sel-receptor loci has led to a substantial lag-load at male sex-sel-stimulus loci because males are obtaining a 1/(9.0/1.24)≈7-fold reduction in potential reproductive output compared to the case of nominal female resistance to male persistent courtship. Stated more simply, if males were to evolve a new adaptation that caused them to overcome female resistance to remating, male reproductive capacity is sufficient for such a mutation to have a very large selective advantage.
Figure 6.
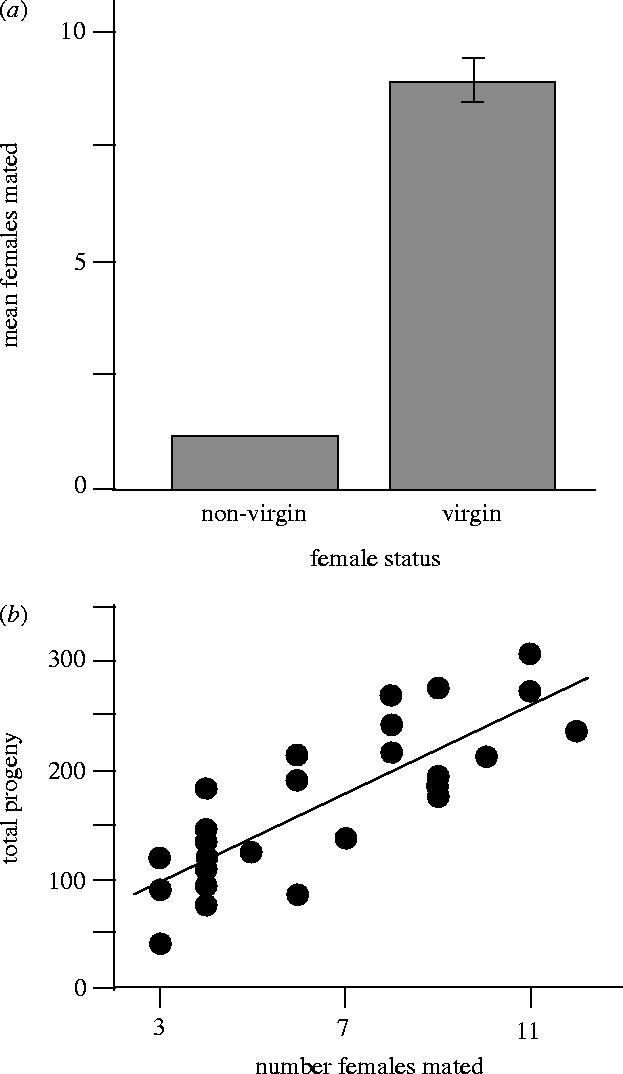
(a) Compared to the case with non-virgin females, male mating rate during the adult-competition phase of the life cycle increases sevenfold when females resistance to mating is reduced by housing males with virgin females at a 1 male : 16 female sex ratio. (b) The increased mating rate is associated with increased progeny production. Error bars are standard errors.
We need to make it clear that the simple experiment described above is not without limitations. For example, when males mate non-virgin females they only obtain about 80% of the fertilizations (i.e. sperm replacement by a second male to mate a female averages about 80% in out LHM base population in matings that occur during the adult competition phase of the life cycle; Morrow et al. in press) so the gain to male fitness reported above would need to be reduced by 20%. Also, males that mate repeatedly over two days may have reduced success in sperm competition, so this factor could further reduce the advantage that was reported above for a mutation causing males to overcome a female's resistance to remating. Nonetheless, despite these factors, it seems clear that male reproductive potential is sufficient that past adaptations which restrict the remating rate of non-virgin females (which has been established to be harmful to them, Chapman et al. 1995; Kuijper et al. submitted) has produced a substantial lag-load at male sex-sel-stimulus loci.
(e) Has past evolution by male sex-sel-stimulus loci created a lag-load at female sex-sel-receptor loci?
To address this issue, we have carried out many experiments in which female lifetime fecundity was measured under two different conditions: (i) a treatment that followed the normal protocol of the LHM base population with the exception that females were collected as virgins, mated by brief exposure (90 min) to a surplus of males at the start of the adult competition phase of their life cycle, and then competed with genetically marked females for the limiting resource of live yeast in the presence of males, and (ii) this same treatment except that the males were removed from the adult competition vials after the initial mating. These protocols are described in detail in Linder & Rice (2005) and Orteiza et al. (2005). By comparing the lifetime fecundity of females from the two experimental treatments, we were able to estimate the net effect of persistent male courtship during the adult-competition phase of the life cycle on the lifetime fitness of the female with which they interacted. Females need to mate with males at least once to obtain the requisite sperm for reproduction. However, in both the laboratory and the in the field, males persistently court non-virgin females, yet females infrequently remate in response to this persistent courtship. Previous studies, especially by Partridge and colleagues, have shown that both persistent male courtship and remating reduces female survival (but not daily fecundity) in the context of a population with overlapping generations (Partridge et al. 1987; Fowler & Partridge 1989; Partridge and Fowler 1987, 1990; Chapman et al. 1995). Because flies in our LHM base population live at most 5 days as adults, and because there is virtually no adult mortality during this time, we needed to test for a cost to female lifetime fecundity due to persistent male courtship of non-virgin females. In two recently published studies from our laboratory, we found that the presence of males in the adult competition vials reduces female fecundity by about 16% (Linder & Rice 2005; Orteiza et al. 2005) and combining this data with 22 additional unpublished studies from our laboratory (figure 7) the average reduction in fecundity is estimated to be 21.6±1.4% (mean±s.e.) with a 95% confidence interval of (18.8%, 24.4%). These data provide evidence that in our LHM base population past adaptations at male sex-sel-stimulus loci are currently producing a substantial lag-load at female sex-sel-receptor loci.
Figure 7.
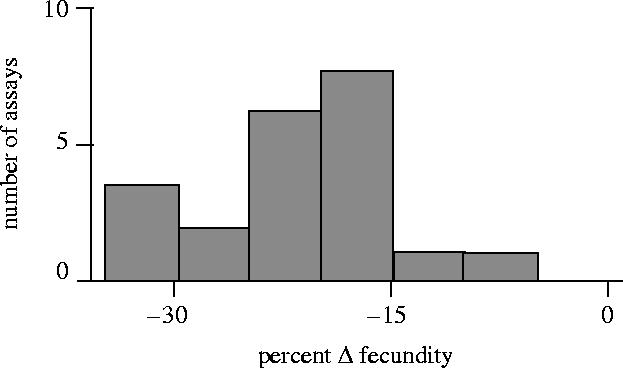
The percentage change in fecundity of the females that were continuously exposed to males, relative to females given minimal exposure to males, during the adult-competition phase of their life cycle. The figure summarizes results from 24 assays.
The study by Linder & Rice (2005) also permitted the selection gradient on female remating rate alone to be estimated. The observed negative slope between female remating rate and female lifetime fecundity demonstrated that selection favours female that are more resistant to persistent male courtship. Another similar study by Friberg et al. (2005) focusing on selection in males demonstrated that the selection gradient on male remating rate, not surprisingly, is positive. Taken together, these two studies demonstrate that males are currently being selected to remate females at higher rate, and females to remate at lower rate, so that evolutionary advance by males (i.e. at male sex-sel-stimulus loci) would be expected to reduce the fitness of females, and thereby increase lag-load at female sex-sel-receptor loci, and that evolutionary advance by females (i.e. at female sex-sel-receptor loci) would similarly create a lag-load at males sex-sel-stimulus loci.
(f) Do indirect benefits compensate for the direct cost of male-induced harm?
The experiments described above demonstrate that, in our laboratory LHM population, the interactions between non-virgin females and males during the adult-competition phase of their life cycle substantially reduces their fecundity. This finding supports the conclusion that past adaptations at male sex-sel-stimulus loci have created a lag-load in at female sex-sel-receptor loci. Studies of other laboratory populations of D. melanogaster corroborate the generality of this finding (Partridge et al. 1987; Fowler & Partridge 1989; Partridge and Fowler 1987, 1990; Chapman et al. 1995). Nevertheless, there remains the possibility that this direct harm to females from persistent male courtship and remating is compensated for by indirect benefits (i.e. increased offspring quality leading to increased grand-offspring). This possibility is an important limitation to the use of past studies of direct male-induced harm to females to infer sexual conflict because, when females gain a net benefit from their interactions with males, in the currency of grand-offspring, then there will not be selection for female resistance and sexually antagonistic coevolution would not be expected to ensue (Parker 1979; Arnqvist 1992; Andrés & Morrow 2003; Cordero & Eberhard 2003).
To address this important confounding factor, we have evaluated the potential for indirect benefits to compensate for direct harm to females in two experiments. We focused on selection acting on a female resistance gene that (i) made females completely, or nearly completely, resistant to male-induced harm during the adult-competition phase of their life cycle, but that also (ii) markedly reduced the females' ability to obtain indirect benefits from remating. In the first experiment, we measured the selective costs and benefits of such a mutation, whereas in the second experiment we used experimental evolution to create such a mutation and then traced its fate over time. If the female resistance allele accumulates across multiple generations, then this provides evidence that indirect benefits do not compensate for the direct harm that males produce during their interactions with non-virgin females, and hence that sexual conflict is operating because it is not compensated by indirect benefits.
As an illustration of the kind of female resistance mutation that we sought to evaluate, consider the following example. Suppose that a new mutation in a gene coding for a male-specific pheromone causes this gene to become activated in females (i.e. it is no longer male-limited in its expression) immediately after mating, and thereby masks females expressing the mutation and caused males to ignore them. As a consequence, once-mated females expressing the mutation would be rarely courted and therefore protected from the harm due to persistent male courtship. However, these females would be simultaneously deprived, to the same degree, of any benefits associated with persistent male courtship that accrue through the indirect benefits associated with good-genes or sexy-sons that are derived from remating.
(i) Quantifying the costs and benefits of male interactions with non-virgin females
The first half of this analysis was the set of experiments described in §5e. These experiments established that male interactions with non-virgin females during the adult-competition phase of their life cycle reduced female lifetime fecundity by an average of 21.6%, with a conservative 95% lower bound for this value of 19.3% (figure 7).
The second half of the experiment estimated the potential indirect benefits to females from these interactions. Females could potentially recoup the direct costs of interacting with males by remating and trading up with males of superior genetic quality. Three past experiments, carried out in two different laboratories, indicated that the good genes route to indirect benefits is not operating in our LHM base population (Holland 2002; Brown et al. 2004; Byrne & Rice 2005). However, past studies by our laboratory have demonstrated considerable heritable genetic variation for male fertilization success in the LHM base population (Rice & Chippindale 2001; Chippindale et al. 2001; Gibson et al. 2002), so there is clearly the potential for indirect benefits through the production of ‘sexy sons,’ which would be more competitive in the context of mating success and/or sperm competition.
To test whether the sexy-sons route to indirect benefits was operating in our LHM base population, we mated virgin females at the beginning of the adult-competition phase of their life cycle (as described in §5b) and then gave them the opportunity to remate with males during the 2-day duration of this stage (Orteiza et al. 2005). By using genetic markers, we were able to determine which females had remated and which sons were derived from primary vs secondary sires. We next competed the two types of son (those from primary or secondary sires) with males from the LHM base population and measured their fertilization success, as described in detail in Orteiza et al. (2005). We found no significant difference in the fertilization success of sons derived from primary vs secondary sires, and hence no apparent evidence that indirect benefits through sexy sons were compensating for the direct costs of male interactions with non-virgin females.
However, the fact that we had previously established that there is substantial heritable genetic variation for male fertilization success makes this finding tentative, because a small benefit may have been missed owing to insufficient statistical power. To address this issue, we calculated the 95% upper-bound for any potential increased fitness of sons derived from secondary sires. This value was 6.13%, meaning we can be 95% confident that an indirect benefit to females from sexy-sons is no larger than this value. Because the sexy-sons route to indirect benefits is only manifest through sons, whereas the direct cost accrues through a reduction in the number of both sons and daughter, the mean benefit of remating, averaged across sons and daughters, was (6.13%+0%)/2=3.07%.
Combing the minimal value from the 95% lower bound for the direct costs (−19.3%) and the maximal value from the 95% upper-bound for the indirect benefits from remating (+3.1%), we find no evidence that non-virgin females recoup the direct costs of persistent male courtship via indirect benefits through sexy sons (figure 8).
Figure 8.
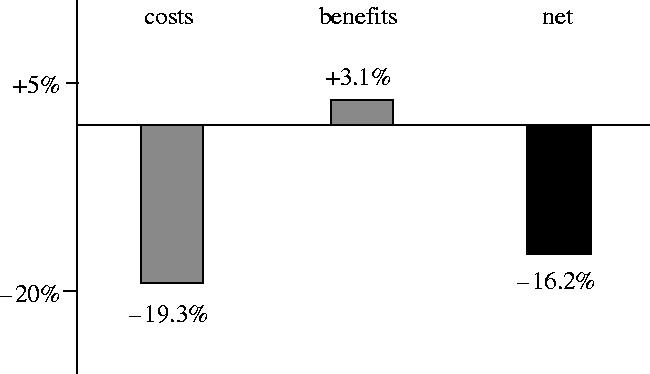
Comparison between the conservative estimate (95% lower bound) for the direct costs to non-virgin females of persistent male courtship, and the liberal estimate (95% upper bound) for the indirect benefits through sexy sons. The net difference between these two bounds is also shown.
(ii) Using experimental evolution to trace the fate of a new female resistance gene
In these experiments, we first back-crossed (23 times) the dominant red-eye allele bw+ into a replica of our LHM base population that was fixed for a recessive allele causing brown eye colour (bw). We next used experimental evolution to make the red-eye allele emulate a new mutation that made females resistant to most of the harm from males (such as the male-specific pheromone previously illustrated), while simultaneously, and to the same degree, depriving them of the opportunity to obtain (via remating) indirect benefits through both the sexy-sons and good-genes routes. The fitness advantage or disadvantage of the resistance allele depends upon the balance between (i) direct costs to non-virgin females due to their interactions with males, and (ii) the indirect benefits obtained when remating leads to offspring of higher genetic quality (via good-genes and/or sexy sons). Therefore, tracing the resistance allele's frequency over multiple generations allows for a test of whether or not indirect benefits from remating compensated for the direct costs incurred by non-virgin females due to all of their interactions with males, since the resistance allele would increase when this is true and decrease when it is false.
To make the arbitrary eye colour marker allele behave as if it were a female resistance allele, we used experimental evolution to link the expression of the red-eye colour allele with a phenotype that emulated the expression of a male-specific pheromone in a female immediately after she mated. More specifically, five replicate populations were started in which the frequency of the red eye colour allele (the bw+ allele, hereafter referred to as the resistance allele) was 7.5%. The populations were reared under the normal protocol for the LHM base population with the following changes. Females were collected as virgins on day-9 and stored in same-sex vials until the beginning of the adult-competition stage of their life cycle. At this point in time, all females were mated to a random sample of males from the same replicate population by brief exposure to males (90 min with a 50% excess of males per female). Next, the social environment that the non-virgin females experienced depended on whether or not they expressed the dominant resistance allele. Females expressing the resistance allele (red-eyed) were placed in adult-competition vials containing only two males randomly taken from the population (the density of females was the normal value for the LHM population, 16 per vial). Females not expressing the resistance allele (brown eyed) were placed in adult-competition vials with the normal 16 males : 16 females sex ratio.
This experimental protocol caused females expressing the resistance allele to experience much less persistent male courtship, but also reduced to the same degree their opportunity to obtain indirect benefits through remating (since the pool of available males with which to remate was reduced by the same factor as the pool of males producing persistent courtship). At the oviposition stage of the life cycle, the females were mixed between the two treatments and transferred to the ‘oviposition vials’ at normal density, but to prevent any remating by females expressing the male resistance allele, males were excluded from these vials. In addition to the five replicates of the experimental treatment, there were five control replicates in which the protocol was identical to the experimental treatment except that all females, irrespective of eye colour, were transferred to adult-competition vials with a 16 males : 16 females sex ratio. In the controls, there was natural selection acting on the red-eye (bw+) allele but no experimental selection causing it to emulate a female resistance allele.
In all five replicates, the female resistance allele increased in frequency in both the experimental and control populations. However, the increase was substantially and significantly higher in the experimental populations compared to the controls (figure 9; Stewart et al. 2005). These results indicate that while there was pleiotropic selection favouring the marker allele, there also was also additional selection favouring the resistance phenotype produced by this allele in the experimental populations. This experiment provides direct, multi-generation evidence that a new mutation at a female sex-sel-receptor locus would evolve in response to the lag-load (harm to female fecundity) produced by extant alleles at male sex-sel-stimulus loci, and thereby perpetuate sexually antagonistic coevolution fuelled by interlocus sexual conflict. The experiments also show that such antagonistic coevolution is not prevented by potential indirect benefits that compensate for direct male-induced harm to females.
Figure 9.
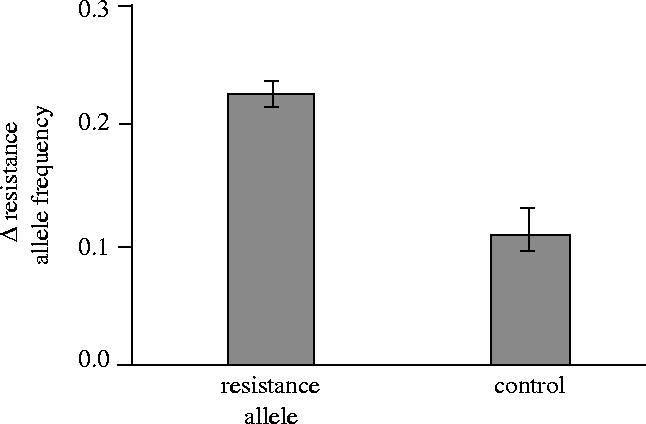
Comparison of the change in frequency of the female resistance allele after five generations in the five experimental populations (resistance allele) and the comparable change in frequency of the marker allele in the five control populations. Error bars are standard errors (p<0.003).
6. Discussion
Coevolution between genes located within the genome of a single species can be mutualistic or antagonistic. We think that the fundamental dynamics of this process are captured by the simple graphical model described in figures 1 and 2. With mutualistic coevolution, adaptation at one locus causes the lag-load at the other, interacting locus to decline, and as a consequence, the rate of evolutionary change is reciprocally dampened at each locus as adaptive allelic replacement proceeds. In contrast, with antagonistic coevolution, adaptation at one locus causes the lag-load at the other, interacting locus to increase, and hence a perpetual cycle of adaptation and counter adaptation ensues due to an evolutionary chase-away process. Interlocus sexually antagonistic coevolution is a special form of this process (Dawkins 1976; Parker 1979; Rowe et al. 1994; Rice & Holland 1997; Holland & Rice 1998; Rice 1998; Chapman et al. 2003).
Interlocus sexual conflict, and the interlocus sexually antagonistic coevolution that it perpetuates, is a phenomenon that is distinct from traditional models of sexual selection. Although inter-sexual conflict over mating rate is an implicit, integral part of traditional models of sexual selection, it is the combination of (i) male adaptation that increases male competitive fertilization success at the expense of the lifetime fecundity of their sexual partners, followed by (ii) female counter-adaptation that recoups female fecundity, but interferes with male competitive fertilization success, that distinguishes the sexual conflict model from traditional models of sexual selection.
The empirical paradigm used in our laboratory to study sexual conflict is laboratory island analysis. This approach does not seek to mimic the natural conditions experienced by a specific wild population in the laboratory. As a consequence, the studies we report here cannot be used to directly extrapolate the characteristics of a specific natural population of D. melanogaster. Instead, the laboratory populations themselves are the focus of interest and they, like the island populations studied by Darwin, are used to infer general principles of evolution rather than to recapitulate the characteristics of the specific natural population from which they were derived. The utility of laboratory island analysis depends on the correspondence between the principles that govern evolution in these microcosms and those operating in nature. If the microcosm is so artificial that the evolutionary principles observed are not representative of what transpires in nature, then the results will have little or no relevance to the natural world. As a consequence, the construction of laboratory island populations must capture the gestalt of natural populations, while simultaneously being suitably simple to be an effective tool to deduce general evolutionary principles. When the laboratory environment and nature differ in key attributes, such as the lack of a spatial refuge for females in laboratory populations, then experiments need to be done to explore the consequences of these differences.
Using evolutionary island analysis, we have looked for evidence of sexual conflict by assessing the net phenotypic interactions between the sexes, rather than studying individual pairs of interacting loci. Because all of the three predictions for the operation of sexual conflict were fulfilled, we concluded that interlocus sexual conflict was operating in our LHM base population. First, we found that the present phenotypes of females (high levels of resistance by non-virgin females to remating, that was presumably built up by past selection in this persistently large, outbred population) produce a substantial lag-load at male sex-sel-stimulus loci. Our evidence in support of this condition was the substantially increased reproductive potential of males (approx. sevenfold) that was manifest when female resistance to remating was reduced by placing individual males with large numbers of virgin females. Second, we found that the present phenotype of males (persistent male courtship and remating of non-virgin females) reduced mean population fitness, and thereby generated a lag-load at female sex-sel-receptor loci. Our evidence supporting this conclusion was the 20% increase in the lifetime fecundity of females when their exposure to persistent male courtship was minimized, i.e. when females were exposed to males just sufficiently long to fertilize their eggs. Third, we found that indirect benefits to females do not compensate for the direct, male-induced harm to them in our LHM base population. Our evidence in support of this conclusion was twofold: (i) the cost-benefit analysis found that direct costs to non-virgin females due to persistent courtship and remating by males far outweighed any indirect benefits obtained from sexy sons (cost benefit ratio is minimally 6.2), and (ii) the study demonstrating that multi-generation selection built-up, rather than drove-down, the frequency of an experimentally produced female resistance allele that reduced male-induced harm from persistent male courtship and remating, but that also reduced indirect benefits (through either good-genes or sexy-sons) to the same degree. Both of these studies supported the hypothesis that indirect benefits do not recoup the direct costs of male-induced harm to females. Moreover, the latter experiment also provides direct evidence for sexually antagonistic coevolution by showing that a female resistance trait will, in fact, counter-evolve in response to extant male-induced harm to females.
Given what we have learned about sexual conflict in our laboratory island population, what can we infer about sexual conflict in nature? The detailed measurements that we have made in the laboratory could not feasibly be made in natural populations (especially the measurement of female lifetime fecundity and the tracing of selection on a female resistance allele across multiple generations that is needed to evaluate indirect benefits), so we think that the sort of direct experimental evidence for sexual conflict that we have described is presently only possible in the context of laboratory island populations. We see no logical reason why the form of sexual conflict that we observed in our laboratory population would not also occur in natural populations. Observations from natural populations demonstrates that males persistently court and try to copulate with females as they forage for food and lay eggs (Markow 1988), and this supports the conclusion that the same qualitative forms of interlocus sexual conflict that we study in laboratory island populations are also occurring in nature. Given that the addition of a spatial refuge from persistent male courtship in our laboratory population did not substantially reduce female remating rate, and that high levels of female remating are not uncommon in nature (Harshman & Clark 1998; Imhof et al. 1998; Jones & Clark 2003) we think that the male–female interaction present in our laboratory population provide a useful surrogate to the study of natural populations. Overall, we conclude that laboratory island analysis captures the gestalt of evolution in natural populations and thereby provides a powerful window into the evolutionary principles that influence male–female coevolution in nature.
Acknowledgements
We thank A. Hannes for help with measuring male mating potential with virgin females, E. Cunningham for her contribution to the collection of 24 assays measuring male-induced harm to females, and U. Friberg and T. Lew for their contribution to our reported results concerning the selection gradient on male remating rate. We also thank the two referees for their helpful suggestions. This work was supported by two grants from the National Science Foundation to W.R.R. (DEB-0128780 and DEB-0410112).
Footnotes
One contribution of 13 to a Discussion Meeting Issue ‘Sexual conflict: a new paradigm’.
References
- Andrés J.A, Morrow E.H. The origin of interlocus sexual conflict: is sex-linkage important? J. Evol. Biol. 2003;16:219–223. doi: 10.1046/j.1420-9101.2003.00525.x. 10.1046/j.1420-9101.2003.00525.x [DOI] [PubMed] [Google Scholar]
- Arnqvist G. Precopulatory fighting in a waterstrider: inter-sexual conflict or mate assessment? Anim. Behav. 1992;43:559–567. 10.1016/0003-3472(92)90079-O [Google Scholar]
- Arnqvist G. Sexual conflict and sexual selection: lost in the chase. Evolution. 2004;58:1383–1388. doi: 10.1111/j.0014-3820.2004.tb01716.x. [DOI] [PubMed] [Google Scholar]
- Bateman A.J. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Brown W.D, Björk A, Schneider K, Pitnick S. No evidence that polyandry benefits females in Drosophila melanogaster. Evolution. 2004;58:1242–1250. doi: 10.1111/j.0014-3820.2004.tb01703.x. [DOI] [PubMed] [Google Scholar]
- Bull J.J. Evolution of sex determining mechanisms. Benjamin/Cummings; Melno park, CA: 1983. [Google Scholar]
- Byrne P.G, Rice W.R. Remating in Drosophila melanogaster: an examination of the trading-up and intrinsic male-quality hypotheses. J. Evol. Biol. 2005;18:1324–1331. doi: 10.1111/j.1420-9101.2005.00918.x. 10.1111/j.1420-9101.2005.00918.x [DOI] [PubMed] [Google Scholar]
- Byrne, P. G., Rice, G. R. & Rice, W. R. Submitted. Influence on female fitness of a refuge from persistent male courtship in the Drosophila melanogaster laboratory environment. Evol. Ecol. Res. [DOI] [PubMed]
- Chapman T. Seminal-fluid mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. 10.1046/j.1365-2540.2001.00961.x [DOI] [PubMed] [Google Scholar]
- Chapman T, Liddle L.F, Kalib J.M, Wolfner M.F, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory glad products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. 10.1038/373241a0 [DOI] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends. Ecol. Evol. 2003;18:41–47. 10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Charlesworth D, Charlesworth B. Sex-differences in fitness and selection for centric fusions between sex-chromosomes and autosomes. Gen. Res. 1980;35:205–214. doi: 10.1017/s0016672300014051. [DOI] [PubMed] [Google Scholar]
- Cordero C, Eberhard W.G. Female choice of antagonistic male adaptations: a critical review of current research. J. Evol. Biol. 2003;16:1–6. doi: 10.1046/j.1420-9101.2003.00506.x. 10.1046/j.1420-9101.2003.00506.x [DOI] [PubMed] [Google Scholar]
- Dawkins R. The selfish gene. Oxford University Press; Oxford: 1976. [Google Scholar]
- Day T. Sexual selection and the evolution of costly female preferences: spatial effects. Evolution. 2000;54:715–730. doi: 10.1111/j.0014-3820.2000.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Fowler K, Partridge L. A cost of mating in female fruit flies. Nature. 1989;338:760–761. 10.1038/338760a0 [Google Scholar]
- Friberg U.T, Lew T.A, Byrne P.G, Rice W.R. Assessing the potential for an ongoing arms race within and between the sexes: selection and heritable variation. Evolution. 2005;59:1540–1551. [PubMed] [Google Scholar]
- Gavrilets S. Rapid evolution of reproductive barriers driven by sexual conflict. Nature. 2000;403:886–889. doi: 10.1038/35002564. 10.1038/35002564 [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Arnqvist G, Friberg U. The evolution of female mate choice by sexual conflict. Proc. R. Soc. B. 2000;268:531–539. doi: 10.1098/rspb.2000.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J.R, Chippindale A.K, Rice W.R. The X chromosome is a hot spot for sexually antagonistic fitness variation. Proc. R. Soc. B. 2002;269:499–505. doi: 10.1098/rspb.2001.1863. 10.1098/rspb.2001.1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harshman L.G, Clark A.G. Inference of sperm competition from broods of field-caught Drosophila. Evolution. 1998;52:1334–1341. doi: 10.1111/j.1558-5646.1998.tb02015.x. [DOI] [PubMed] [Google Scholar]
- Holland B. Sexual selection fails to promote adaptation to a new environment. Evolution. 2002;56:721–730. doi: 10.1111/j.0014-3820.2002.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Perspective: chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Imhof M, Harr B, Brem G, Sclotterer C. Multiple mating in wild Drosophila melanogaster revisited by microsatellite analysis. Mol. Ecol. 1998;7:915–917. doi: 10.1046/j.1365-294x.1998.00382.x. 10.1046/j.1365-294x.1998.00382.x [DOI] [PubMed] [Google Scholar]
- Jones B, Clark A.G. Bayesian sperm competition estimates. Genetics. 2003;163:1193–1199. doi: 10.1093/genetics/163.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijper, B., Stewart, A. D. & Rice, W. R. Submitted. Cost of multiple mating in Drosophila melanogaster Proc. Natl Acad. Sci. USA
- Linder J.E, Rice W.R. Natural selection and genetic variation for female resistance to harm from males. J. Evol. Biol. 2005;18:568–575. doi: 10.1111/j.1420-9101.2004.00872.x. 10.1111/j.1420-9101.2004.00872.x [DOI] [PubMed] [Google Scholar]
- Mandel S.P.H. Owen's model of a genetic system with differential viability between the sexes. Heredity. 1971;26:49–63. doi: 10.1038/hdy.1971.5. [DOI] [PubMed] [Google Scholar]
- Markow T.A. Reproductive behavior of Drosophila in the laboratory and in the field. J. Comp. Psychol. 1988;102:169–173. doi: 10.1037/0735-7036.102.2.169. 10.1037/0735-7036.102.2.169 [DOI] [PubMed] [Google Scholar]
- Maynard Smith J. What determines the rate of evolution? Am. Nat. 1976;110:331–338. 10.1086/283071 [Google Scholar]
- Morrow, E. H., Stewart, A. D. & Rice, W. R. In press. Patterns of sperm precedence are not affected by female mating history in Drosophila melanogaster Evolution. [PubMed]
- Orteiza N, Linder J.E, Rice W.R. Sexy sons from re-mating do not recoup the direct costs of harmful male interactions in the Drosophila melanogaster laboratory model system. J. Evol. Biol. 2005;18:1315–1323. doi: 10.1111/j.1420-9101.2005.00923.x. 10.1111/j.1420-9101.2005.00923.x [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sexual selection and sexual conflict. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; London: 1979. pp. 123–166. [Google Scholar]
- Partridge L, Fowler K. Nonmating costs of exposure to males in female Drosophila melanogaster. J. Insect Physiol. 1990;36:419–425. 10.1016/0022-1910(90)90059-O [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. J. Insect. Physiol. 1987;33:746–749. 10.1016/0022-1910(87)90060-6 [Google Scholar]
- Parsons P.A. The initial progress of new genes with viability differences between the sexes and sex linkage. Heredity. 1961;16:103–107. [Google Scholar]
- Rice W.R. Sex chromosomes and the evolution of sexual dimorphism. Evolution. 1984;38:735–742. doi: 10.1111/j.1558-5646.1984.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Rice W.R. On the instability of polygenic sex determination: the effect of sex-specific selection. Evolution. 1986;40:633–639. doi: 10.1111/j.1558-5646.1986.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Rice W.R. The accumulation of sexually antagonistic genes as a selective agent promoting the evolution of reduced recombination between primitive sex chromosomes. Evolution. 1987;41:911–914. doi: 10.1111/j.1558-5646.1987.tb05864.x. [DOI] [PubMed] [Google Scholar]
- Rice W.R. Sexually anatagonistic male adaptations triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. 10.1038/381232a0 [DOI] [PubMed] [Google Scholar]
- Rice W.R. Intergenomic conflict, interlocus antagonistic coevolution, and the evolution of reproductive isolation. In: Howard D.J, Berlocher S.H, editors. Endless forms: species and speciation. Oxford University Press; Oxford: 1998. pp. 261–270. [Google Scholar]
- Rice W.R. Dangerous liaisons. Proc. Natl Acad. Sci. USA. 2000;97:12953–12955. doi: 10.1073/pnas.97.24.12953. 10.1073/pnas.97.24.12953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R, Holland B. The enemies within: intergenomic conflict, interlocus contest evolution (ICE), and the intraspecific Red Queen. Behav. Ecol. Sociobiol. 1997;41:1–10. 10.1007/s002650050357 [Google Scholar]
- Rice W.R, Holland B. Reply to comments on the chase-away model of sexual selection. Evolution. 1999;53:302–306. doi: 10.1111/j.1558-5646.1999.tb05358.x. [DOI] [PubMed] [Google Scholar]
- Rice W.R, Chippindale A.K. Sexual recombination and the power of natural selection. Science. 2001;294:555–559. doi: 10.1126/science.1061380. 10.1126/science.1061380 [DOI] [PubMed] [Google Scholar]
- Rice W.R, Linder J.E, Friberg U, Lew T.A, Morrow E.H, Stewart A.D. Inter-locus antagonistic coevolution as an engine of speciation: assessment with hemiclonal analysis. Proc. Natl Acad. Sci. USA. 2005;102:6527–6534. doi: 10.1073/pnas.0501889102. 10.1073/pnas.0501889102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L, Arnqvist G, Krupa J, Sih A. Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol. Evol. 1994;9:289–293. doi: 10.1016/0169-5347(94)90032-9. 10.1016/0169-5347(94)90032-9 [DOI] [PubMed] [Google Scholar]
- Sgrò C.M, Partridge L. Evolutionary responses of the life history of wild-caught Drosophila melanogaster to two standard methods of laboratory culture. Am. Nat. 2000;156:341–353. [Google Scholar]
- Spieth H.T. Mating behavior within the genus Drosophila (Diptera) Bull. Am. Mus. Nat. Hist. 1952;99:399–474. [Google Scholar]
- Stewart A.D, Morrow E.H, Rice W.R. Assessing putative interlocus sexual conflict in Drosophila melanogaster using experimental evolution. Proc. R. Soc. B. 2005;272:2029–2035. doi: 10.1098/rspb.2005.3182. 10.1098/rspb.2005.3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers R.L. Paternal investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Wolfner M.F. Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem. Mol. Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. 10.1016/S0965-1748(96)00084-7 [DOI] [PubMed] [Google Scholar]


