Abstract
Much of the literature on male–female coevolution concerns the processes by which male traits and female preferences for these can coevolve and be maintained by selection. There has been less explicit focus on the origin of male traits and female preferences. Here, I argue that it is important to distinguish origin from subsequent coevolution and that insights into the origin can help us appreciate the relative roles of various coevolutionary processes for the evolution of diversity in sexual dimorphism. I delineate four distinct scenarios for the origin of male traits and female preferences that build on past contributions, two of which are based on pre-existing variation in quality indicators among males and two on exploitation of pre-existing sensory biases among females. Recent empirical research, and theoretical models, suggest that origin by sensory exploitation has been widespread. I argue that this points to a key, but perhaps transient, role for sexually antagonistic coevolution (SAC) in the subsequent evolutionary elaboration of sexual traits, because (i) sensory exploitation is often likely to be initially costly for individuals of the exploited sex and (ii) the subsequent evolution of resistance to sensory exploitation should often be associated with costs due to selective constraints. A review of a few case studies is used to illustrate these points. Empirical data directly relevant to the costs of being sensory exploited and the costs of evolving resistance is largely lacking, and I stress that such data would help determining the general importance of sexual conflict and SAC for the evolution of sexual dimorphism.
Keywords: evolutionary constraints, female choice, mate choice, sexual selection, speciation
1. Introduction
No traits generally exhibit such high degrees of diversity across related animal taxa as do sex-limited traits. This holds true across widely different types of traits, such as genitalia in internally fertilizing animals (Eberhard 1985; Arnqvist 1998), dichromatism in birds and fish (Andersson 1994), pheromones in many vertebrates and insects (Johnston et al. 1999; Greenfield 2002), gonad proteins and ejaculatory substances in insects (Arnqvist & Rowe 2005) and proteins mediating interactions between gametes (Swanson & Vacquier 2002). The common themes among such traits is that they clearly evolve rapidly, differ between closely related taxa and are believed to have evolved by some type of coevolution between sets of genes with sex-limited expression. The fact that male–female coevolution may also play a key role in speciation (Panhuis et al. 2001; Kirkpatrick & Ravigné 2002; Coyne & Orr 2004) has boosted recent interest in understanding the processes that have generated this form of trait diversity.
During the past 25 years, a large number of theoretical models for the evolution of female choice have been published, most of which delineate the circumstances under which female mate preferences can evolve (see Andersson 1994; Kokko et al. 2003, Arnqvist & Rowe 2005 for reviews). The majority of these models ultimately try to account for the evolutionary maintenance of female preferences for secondary sexual traits in males. There are good reasons for this focus: the most problematic issue from a theoretical point of view is to understand selection on female traits that bias mating, or fertilization, success among males. Without positive selection on female choice traits in cases where they are at all costly, they would rapidly be lost by selection thus nullifying or halting male–female coevolution. Understanding the nature of this positive selection for the maintenance of female choice remains a major task for empirical and theoretical research in this domain (Kokko et al. 2003; Arnqvist & Rowe 2005). Yet, if our goal is to understand the diversity in sex-limited traits that we observe, this ambition is insufficient. The reason is simply that, before they can coevolve, male and female traits must first originate, and this evolutionary origin is at the root of the observed diversity (Ryan & Rand 1993). Furthermore, the processes responsible for origin of female choice are, in part, distinct from those invoked to explain subsequent evolutionary elaboration and/or maintenance. Here, I will argue that we can gain important insights into the relative role of various modes of male–female coevolution by better understanding the origin of male and female traits.
2. Models for the origin of female preferences
Darwin (1871) did not elaborate much on the origin of female choice but later work has discussed at least four distinct and general scenarios for the origin of female choice by selection. Because the simultaneous origin of both a male trait and a female preference for this trait is highly unlikely, all assume that one pre-dates the other. Two assume pre-existing variation among males. First, it is a rarely acknowledged fact that Fisher (1930) outlined a distinct mechanism for the origin of female choice (see Maynard-Smith 1991). He assumed pre-existing phenotypic variance in a male trait that is correlated with males' breeding value for fitness in a particular environment (i.e. a male quality indicator; sensu Hunt et al. 2004). Although, he did not detail how such pre-existing variance could be maintained, which is an obvious problem under this scenario, he did suggest that geographic variation in which populations are ‘differentially adapted to different parts of the range’ offers one possibility. In any case, given pre-existing variance in a quality indicator among males, a novel mutation expressed in females that led to a preference for high quality males could become established in a population because bearers of this mutation would produce more fit offspring (Arnqvist & Rowe 2005). Note that this assumes that no, or at least relatively low, costs are associated with the novel female preference mutation. In essence, thus, female preference originates by exploitation of a pre-existing variation in genetic quality among males (Berglund et al. 1996). I will refer to this here as Fisherian origin. In a better known part of his treatment of sexual selection, Fisher (1930) then went on to discuss how this situation would lead to a genetic correlation between the male trait and the female preference that fuels a self-reinforcing process that is now known as Fisher's runaway process.
Second, instead of assuming pre-existing phenotypic variance in genetic quality indicators among males, as Fisher (1930) did, one could instead envision pre-existing phenotypic variance among males in a trait that is correlated with males' ability to provide direct (i.e. non-genetic) benefits to their mates. This is essentially what Darwin (1871) did, indirectly, when outlining a manner in which female preference could evolve even under monogamy in taxa where males provide paternal care, and others have expanded on this possibility (e.g. Kirkpatrick 1985; Kirkpatrick et al. 1990; Price et al. 1993). Under this scenario, female preference can be said to originate by exploitation of a pre-existing variation among males in the direct effects they have on female fitness. I will refer to this here as a Darwinian origin. This is a less problematic hypothesis than that suggested by Fisher (1930), since it does not rely on standing genetic variation in the breeding value for fitness among males. For example, it is easy to see how female preference for large males could become established if large males were in better phenotypic condition and thus also better able to provide paternal care of offspring.
Two other scenarios instead rely on pre-existing characteristics of females' sensory system that dictate which male traits are likely to evolve (see Ryan 1990; Endler & Basolo 1998 for reviews). The idea is simply that novel male traits that exploit pre-existing sensory biases in females are more likely to become established (referred to here as sensory exploitation; but see Endler & Basolo 1998). The role of such pre-existing sensory biases, defined broadly to include biases in how females detect, perceive, assess and act upon any novel male ‘signal’ that they are exposed to, have been discussed for a long time and by authors from different fields of biology. This has produced somewhat diverse views in the literature (Endler & Basolo 1998). For the purposes of this article, I distinguish two types of pre-existing sensory biases.
First, most female response repertoires have no doubt become established as the result of strong selection. Females are thus adapted to respond in particular ways to a range of stimuli in order to, for example, successfully find food, avoid predators and breed at optimal rates, times and places. Such multi-dimensional response repertoires form a virtually infinite number of pre-existing sensory biases that are potential targets for novel male traits. I will refer to these here as adaptive sensory biases. Ethologists and sensory physiologists recognized early on that male courtship traits seem to match female sensory capabilities across taxa (see, e.g. Hinde 1966) and some even suggested that males may have exploited female responses (Wickler 1968). It was, however, West-Eberhard (1979, 1984) that first elaborated on the possibility that males may evolve novel traits that capitalize on pre-existing female response repertoires that are adaptive in other contexts and that males, by doing so, manipulate female behaviour or physiology in their own favour. Females would thus be caught in a ‘sensory trap’. Although most subsequent discussions of sensory traps have stressed sensory biases that result from natural selection (e.g. Christy 1995), such biases can in theory result from any form of selection including sexual selection by female choice for other male traits (Ryan 1990; Ryan & Rand 1993; Endler & Basolo 1998).
Second, pre-existing sensory biases need not be the direct result of selection. In theory, they can simply be incidental and selectively neutral consequences of how organisms are built (Ryan 1990; Endler & Basolo 1998). For example, artificial neural network models have shown that networks trained to recognize a certain stimuli seem to generally produce various sensory biases for novel stimuli as a by-product (Enquist & Arak 1993, 1994; Arak & Enquist 1993; Johnstone 1994). Similarly, research in ‘receiver psychology’ (Guilford & Dawkins 1991, 1993; Rowe 1999; Ghirlanda & Enquist 2003; Rowe & Skelhorn 2004) have also suggested that higher brain processes may incidentally produce pre-existing sensory biases for particular male traits. Following Arak & Enquist (1993), I will refer to such sensory biases as hidden preferences. These, then, can be seen as side-effects or contingencies of how the sensory system, defined in its widest sense, of the receiver is constructed.
3. Why bother?
There are at least two reasons for why we might not want to bother much about the origin of female preferences. The first is that the processes that have led to the elaboration of male traits and the maintenance of female preferences for such traits may be distinct from those responsible for their origin. The processes responsible for the evolutionary origin may rapidly become disassociated from those driving subsequent evolution (see Williams 1966), and we may be interested primarily in the latter. The second reason is that questions about the mechanisms involved in the origin of female preferences are historical (Ryan 1990; Ryan & Rand 1993), and as such are inherently difficult to study (Martins 2000). Even if modern comparative phylogenetic methods offer many useful tools for addressing these questions (Shaw 1995), and even though integration of experimental and comparative approaches can help (Autumn et al. 2002; Rowe & Arnqvist 2002), it is difficult to make solid inferences about the origin of female preferences (Basolo 1995; Basolo & Endler 1995; Christy & Backwell 1995; Sherman & Wolfenbarger 1995a,b; Ryan 1996).
There are, however, also two good reasons for why we should care more. First, no matter what the coevolutionary process might be that have led to the elaboration of male traits and/or the maintenance of female preferences, it is the processes responsible for the evolutionary origin of male and female traits that dictates which traits will be involved in subsequent coevolution. Thus, if we wish to understand why certain traits are sexually dimorphic and others are not, we need to better understand the processes involved in the origin of sexual dimorphism (Ryan & Rand 1993). This problem is exacerbated if different processes are commonly responsible for the origin and the maintenance of female choice and male traits. If this is true, then the body of experimental studies of extant species upon which we base our understanding is likely to yield a false view of the relative roles that different processes have had for the evolution of sexually dimorphic traits.
Further, the different models for the origin of preferences differ with regards to how likely they are to spark various types of coevolutionary processes immediately following the origin (see table 1 and §5). Thus, information relating to the origin of preferences is important, at least to some extent, when trying to understand which coevolutionary processes are likely to have generated subsequent elaboration and exaggeration of female preferences and male traits.
Table 1.
Summary of the four scenarios for the origin of male sexual traits and female preferences discussed here.
| assumptions and predictions | Fisherian origin | Darwinian origin | adaptive sensory bias | hidden preferences |
|---|---|---|---|---|
| relies on | pre-existing indicator of male genetic quality | pre-existing indicator of direct benefits to females | pre-existing sensory bias shaped by selection | pre-existing incidental sensory bias |
| type of selection at the time of origin | indirect (among females) | direct (among females) | direct (among males) | direct (among males) |
| historical pattern | male trait pre-dates female preference | male trait pre-dates female preference | female preference pre-dates male trait | female preference pre-dates male trait |
| male traits preferred | reliable indicators of genetic quality (armament, dominance rank, etc.) | indicators of paternal investment (overall body size, territory, health and vigour, etc.) | traits that exploit female sensory capabilities used in other contexts (see table 2) | arbitrary traits that do not match female sensory capabilities used in other contexts (tail length in birds, symmetry, etc.) |
| number of male traits preferred in any one taxa | one or a few | one or a few | few or many | few or many |
| type of coevolution likely to follow after origin | good genes processes, Fisher's runaway process | direct benefits processes, Fisher's runaway process | sexually antagonistic coevolution, direct benefits processes, Fisher's runaway process | none, sexually antagonistic coevolution, Fisher's runaway process |
4. How can we distinguish between different scenarios?
The different scenarios for the origin of female choice rely on different assumptions (see §2), and make different predictions about the historical order of events and of the types of male traits that should be preferred by females (table 1). At least six different types of empirical information have been, or could be, used to provide information regarding the likelihood of each scenario in particular cases.
(a) Historical inferences
The fact that female preferences should pre-date male exploitation of sensory biases in females under sensory exploitation scenarios has been exploited in several comparative studies, combining experimental quantifications of female preferences in extant taxa with phylogenetic reconstructions (e.g. Ryan 1990; Proctor 1992; Ryan & Rand 1993; Basolo 1995; Shaw 1995; McClintock & Uetz 1996; Endler & Basolo 1998). In short, if basal taxa show female preference but not male traits while terminal taxa exhibit both, this is taken as evidence for female preferences pre-dating male traits. The utility and problems with using phylogenetic comparative methods to distinguish between these scenarios have been discussed quite extensively (see references in §3), and so will not be dealt with further here. It is, however, worth noting that this empirical strategy has almost exclusively been used to support scenarios based on sensory exploitation. In principle, the other scenarios (the Fisherian or Darwinian origin hypotheses) could be tested using the same logic by assessing whether variation in male genetic or phenotypic quality indicators seem to pre-date female preferences (Ryan & Rand 1993; Cotton et al. 2004). By analogy, observations of genetic or phenotypic quality indicators but no female preference for these in basal taxa but existence of both in terminal taxa would provide some support for these scenarios (see Cotton et al. 2004 for an example). Comparative tests such as these are, however, fairly weak (Shaw 1995). The main reason is that if there is selection on male traits and/or female preferences/biases, then the probability that the origin of the two traits is separated in time (thus occurring on different nodes) is low. The sequence of separate origins is thus unlikely to be preserved in the topology of the phylogeny of extant taxa.
(b) Associative data
Phylogenetic patterns that show an apparent association between types of male secondary sexual characteristic and female sensory capabilities used in contexts other than mate choice have been seen as support for adaptive sensory biases. For example, across larger groups of spiders, males tend to employ (i) visual courtship signals in taxa where females hunt using eye-sight (e.g. jumping spiders), (ii) drumming and/or vibrational signals in taxa where the ground dwelling females identify prey by surface vibrations (e.g. wolf spiders) and (iii) vibratory signals transmitted through the web in groups where females sense and capture prey in webs (e.g. Witt & Rovner 1982). Patterns such as this can be observed in many groups of animals, but constitute weak evidence at best in the absence of information on the order of events (Shaw 1995). The reason is simply that under all forms of origin, a female preference that falls within her cognitive capabilities is more likely to become established (Schluter & Price 1993). For example, in an imaginary taxon showing two alternative pre-existing male quality indicators, females are more likely to evolve to exploit the one that they can perceive (all else being equal, Berglund et al. 1996). To put things bluntly, we can learn little from the fact that females have not evolved a preference for colourful males in blind taxa.
(c) The nature of traits
Detailed information on the function of male traits and the nature of female mating biases can sometimes provide insights into the likely origin of female preferences. For example, it is somewhat difficult to envision situations in which even embryos of many extant traits considered to have originated as male quality indicators (i.e. ‘handicaps’) could have predated female preference for these. Why would males in an ancestral bird species evolve colourful plumage that is costly to produce and attracts predators if there is no female preference for such plumage? There are, however, ways out of this dilemma. Borgia (1979) and Berglund et al. (1996) have argued that female preferences for male traits that are used in overt male–male competition (e.g. armaments) is consistent with a Fisherian origin and Price et al. (1993) noted that it is consistent with a Darwinian origin. The main point is, in essence, that traits under intrasexual selection are more likely than other traits to become pre-existing indicators of male genetic or phenotypic quality in the absence of female preferences. Similarly, a close match between a male trait and a particular female sensory bias (i.e. male traits that are mimics) is often seen as evidence for origin by adaptive sensory bias. For example, in the swordtail characin (Corynopoma riisei), males are equipped with a long and slender extension of the gill cover, the tip of which is enlarged, flattened and provided with a dark chromatophore. This ‘paddle’ is normally inconspicuously placed along the body, but when females are courted males erect the paddle at a right angle from the body and twitch and shake the tip in front of the female. The tip of the paddle looks like and is moved like a small prey organism, and the fact that females are attracted to, attack and bite at the tip of the paddle strongly suggest that this peculiar male trait has evolved to exploit female foraging behaviour (Nelson 1964; Wickler 1968). Remarkably enough, there appear to be three independent origins of such ‘lures’ in males within the Glandulocaudinae tetras (see Arnqvist & Rowe 2005).
(d) Novel traits
Several experimental studies have documented female preferences for novel artificial traits in males, such as coloured leg bands or feathers in birds (Burley et al. 1982; Johnson et al. 1993; Fiske & Amundsen 1997; Burley & Symanski 1998; Witte & Curio 1999), and this has been seen as evidence for sensory exploitation scenarios. While such experiments do reveal the existence of pre-existing sensory biases, they are very indirect evidence for origin by sensory exploitation simply because the male traits involved in these studies have, in fact, not evolved. In fact, observations such as these show that female preferences for particular male traits need not cause evolution of those traits in males.
(e) Theoretical considerations
Both the Fisherian and the Darwinian origin scenarios have theoretical difficulties explaining the evolution of female preferences for multiple male traits (e.g. Andersson 1994). Put simply, once a reliable indicator of male quality and female preference for this trait are established, costly additional preferences do not easily invade even for male traits that are more accurate indicators of male quality (Pomiankowski & Iwasa 1993; Schluter & Price 1993; Iwasa & Pomiankowski 1994; Johnstone 1995). In contrast, because females may exhibit a very large number of pre-existing biases, each of which is potentially exploitable, sensory exploitation scenarios can more easily explain the origin of female preferences for multiple male traits. The fact that female preferences for multiple male traits appears common (see Candolin 2003) has been seen as indicating that origin by sensory exploitation has been common (Arak & Enquist 1993, 1995; Ryan & Rand 1993; Arnqvist & Rowe 2005).
It is also worth noting that Fisherian origin relies on indirect selection among females, a form of selection that is often considered a weak force especially when facing direct selection (e.g. Kirkpatrick & Barton 1997). Further, both Fisherian and Darwinian origin scenarios relies on selection that is rooted in variation in reproductive success among females. Since variance in reproductive success is typically larger among males than females, the selection assumed under the sensory exploitation scenarios (table 1) should often be a more potent evolutionary force.
(f) Artificial selection
In theory at least, artificial selection experiments could be used to shed light on the origin of mate choice, although this has to my knowledge never been attempted. This would involve studying whether female preferences for male indicator traits arise de novo in selection treatments in which variation in male phenotypic and/or genetic quality has been experimentally induced or elevated. Conversely, in selection treatments where female sensory repertoires are experimentally manipulated, males would be expected to evolve traits that exploit any novel sensory bias that is introduced. Admittedly, the feasibility of these sorts of experiments is restricted, due to practical problems and the limited number of taxa amenable to artificial selection experiments.
5. Subsequent coevolution
Given that a female preference and a male trait has originated, different male–female coevolutionary processes can enter and drive elaboration, modification, divergence between taxa and exaggeration. Discussions in this field have by tradition been fairly polarized (Bradbury & Andersson 1989; Andersson 1994), but most recent contributions have noted that the different modes of coevolution are not mutually exclusive and have pointed to the possibility that they commonly interact with one another, each reinforcing or countering others (Rowe & Houle 1996; Kirkpatrick & Barton 1997; Kokko et al. 2003; Chapman et al. 2003), and that their relative importance may vary during the coevolutionary course of events (Arnqvist & Rowe 2005). The main processes are referred to here as the Fisher's runaway process, good genes mechanisms, direct benefits and sexually antagonistic coevolution (SAC; see Andersson 1994; Kokko et al. 2003; Arnqvist & Rowe 2005 for definitions and reviews), and there is empirical support for all. The issue, thus, relates more to their relative importance in the evolution of the diversity we see in male sexual traits and in female preferences for these.
The different scenarios for the origin of female choice and male traits do differ with regards to what is likely to follow the origin (table 1). All scenarios predict that male traits and female preferences can to some extent become statistically associated through linkage disequilibrium, so the self-reinforcing effect known as Fisher's runaway process can contribute to subsequent coevolution to varying degrees. Under a Fisherian origin, however, alleles for female preference will also become associated with high fitness genes (i.e. indirect selection) and this association might be expected to drive the evolution of elaborated indicators of genetic quality among males and more stringent preferences among females (Berglund et al. 1996).
Under a Darwinian origin, traits in males that signal direct benefits to females should become elaborated as female preference for such traits increases in magnitude (Kirkpatrick 1985; Price et al. 1993), although such scenarios may be particularly sensitive to invasion of male traits that are dishonest (i.e. cheating) because of phenotypic trade-offs in males between allocation to signals of direct benefits and the direct benefits themselves (Schluter & Price 1993).
Origin of male traits via exploitation of pre-existing sensory biases in females can, in theory, have three subsequent evolutionary results. First, once established, the evolution of an exploitative trait in males may not feed back into any form of selection at all in females. If this is true, there will (by definition) be no male–female coevolution, but the male trait may, of course, nevertheless evolve to track evolutionary changes in female sensory biases that are due to natural selection unrelated to mate choice or to genetic drift (i.e. sensory drive; see Endler & Basolo 1998). Second, females that are more responsive to male exploitation may directly benefit from their sensory bias. Dawkins & Guilford (1996) have argued that this may be the case in taxa where females experience costs of searching for mates. Whenever this is true, female preference for the male trait may increase in magnitude and should coevolve with exaggeration of the exploitative male trait. This positive coevolution will, then, be driven by direct benefits to females. Third, females that are more responsive to male exploitation may suffer direct costs from their sensory bias. Although this possibility is implicit in many of the early contributions in this field (Wickler 1968; West-Eberhard 1979, 1984; Ryan 1990) and in the terminology itself (e.g. exploitation, manipulation, deception, capitalizing), it was Rowe et al. (1994) and in particular Holland & Rice (1998) that first pointed out that this will lead to sexual conflict. Whenever this is true, we expect to see bursts of SAC. Females will be selected to minimize the costs of male exploitation (i.e. evolve resistance) and males to more efficiently exploit female sensory biases.
Below, I will argue that there are reasons to believe that SAC should be a common outcome of origin by sensory exploitation, in particular under adaptive sensory bias scenarios. Before doing so, however, it should be stressed that the relative importance of various modes of male–female coevolution can be expected to vary during the origin, evolution, and maintenance of female preferences for male traits (Maynard-Smith 1991). For example, all theoretical models assume or predict that male traits and female preferences become genetically correlated, so the self-reinforcing Fisher's runaway process may quite generally contribute to coevolutionary elaboration. All models of female choice also predict that at equilibrium, further evolution of the male trait is held back by some form of natural selection (i.e. trait expression is costly). Under such situations, it is reasonable to expect that variation across males in their ability to express the preferred trait to some extent reflects phenotypic condition (Price et al. 1993) and even genetic quality (Rowe & Houle 1996). Thus, no matter what processes brought female preference for a particular male trait to its equilibrium, direct and/or indirect benefits to females may contribute to the maintenance of the system (Arnqvist & Rowe 2005). There is some data suggesting that systems that have originated as sensory traps, and possibly evolved by SAC, can reach a stage where the exploitative trait has evolved into an honest indicator of quality (Backwell et al. 1995; LeBas et al. 2003; Garcia & Ramirez 2005).
6. The costs of being exploited
It is important to recognize that the foundation for all sensory exploitation scenarios, in contrast to the other scenarios for the origin of female choice (table 1), is direct reproductive competition among members of the sex with the highest potential reproductive rate. It is such competition that makes novel exploitative traits beneficial to their bearers. Although, males are portrayed as ‘exploiters’ and females as ‘exploited’ here, these roles are not inherent in the sexes but merely reflect the relative reproductive investment made by males and females in most animal taxa. This point is beautifully illustrated by certain dance flies, where sex role reversal has resulted in female exploitation of sensory biases in males (see Funk & Tallamy 2000).
The last few years have seen an increased interest in sensory exploitation scenarios, and origin by sensory exploitation has received some support even for male traits that have been regarded as indicator traits in the past (e.g. Pryke & Andersson 2002; Rodd et al. 2002; Madden & Tanner 2003; Smith et al. 2004). The fact that origin by sensory exploitation may be much more common than previously believed indicates that the question of what should follow such exploitation is an important one. Dawkins & Guilford (1996) vividly argued that females should often ‘benefit from being exploited’ because this reduces the direct costs of searching for conspecific males. While valid in theory, this suggestion is somewhat difficult to evaluate simply because we know little about the actual net costs of mate searching for females (Jennions & Petrie 1997) especially when balanced against the possible negative pleiotropic direct effects of suboptimal mating (see below). More importantly, the generality of this argument is limited since it does not apply in animals where males, rather than females, search actively for mates and does not apply for male traits that are not involved in mate searching or mate attraction.
In contrast, I suggest that females should generally suffer direct net costs as a result of the invasion of an exploitative trait in males (see also Holland & Rice 1998). If we assume that females are in some sense adapted to mate optimally (i.e. at an appropriate rate, in environments and at times/ages that are favourable, with a given number of males, etc.), then the invasion of exploitative traits in males should often result in suboptimal mating among females. Faced with novel traits that exploit their sensory biases, females with more pronounced sensory biases may suffer direct costs from, for example, mating too frequently (e.g. Arnqvist & Nilsson 2000; Byrne & Roberts 2000), engaging in matings that are costly (e.g. Arnqvist & Kirkpatrick 2005), mating at suboptimal times (e.g. Markow 2000) or in environments that are suboptimal (e.g. Byrne & Roberts 1999). I note that costs may, in theory, be associated with all forms of sensory exploitation, also those that are unrelated with mating per se (see §7). A more general statement would be that if we assume that females are adapted to regulate their own reproduction, then novel male traits that interfere with this regulation are likely to often bring about non-adaptive reproductive behaviours or decisions in females. This, in turn, will select for female resistance.
Note that exploitative traits that are costly for females will only be favoured among males if the benefits in terms of increased reproductive success for the bearers of these traits are larger than the costs they experience by lowering the fitness of their mates (Parker 1979; Arnqvist & Rowe 2005).
To my knowledge, we lack empirical data directly relevant to this question. It should certainly be possible to experimentally assess the fitness costs and benefits to females that are associated with the introduction of a novel male trait that capitalize on a pre-existing female sensory bias. Empirical studies addressing the costs and benefits of ‘being exploited’ would provide very valuable information.
7. The costs of evolving resistance
If male exploitation of a female sensory bias carries direct costs to females, females are predicted to evolve resistance to the male stimuli. In the simplest of cases, females may efficiently achieve resistance by evolving ‘emancipation from exploitation’ (Bradbury & Verencamp 2000) by essentially eliminating the sensory bias. This could be achieved either by evolving insensitivity, or indifference, to the sensory capability that males are exploiting (Rowe et al. 2005) or by evolving increased discriminatory ability allowing females to disentangle reproductive responses from other types of responses (Garcia & Ramirez 2005). This is then expected to lead to the loss of costly male traits (Rosenthal & Servedio 1999). Bradbury & Vehrencamp (2000) have suggested that this should be common and should act as an evolutionary ‘filter’: only a fraction of the male traits that originate by sensory exploitation may in fact be maintained over evolutionary time. Female reproductive responses to male traits certainly do evolve (Ritchie 1996; Rowe et al. 2005) and hidden preferences are perhaps particularly likely to be lost in the face of direct selection against them simply because there is, by definition, no selection maintaining such sensory biases.
An alternative possibility is that females are constrained from evolving resistance to exploitative male traits (Rodriguez & Snedden 2004), either in the form of genetic or selective constraints (Arnold 1992). Although the general importance of evolutionary constraints is debated, it is certainly possible that the genetic architecture of many sensory biases is such that they cannot easily be purged by selection (Enquist et al. 2002). Yet, I suggest that a far more influential type of constraints should occur under male exploitation of adaptive sensory biases. Adaptive sensory biases are in place because they are favoured by natural selection in another context, and such biases often involve critical and adaptive responses to food, prey or predators (see table 2). Because the evolution of resistance (no matter form it takes) is often likely to compromise such adaptive responses, females may truly be caught in sensory traps (West-Eberhard 1984). When this is true, the evolution of female resistance to costly male exploitation of their sensory biases will itself be costly.
Table 2.
Examples of male traits exploiting biases that are advantageous to females outside the context of mate choice (i.e. sensory traps) and the possible costs and benefits to females following the origin.
| taxon | male sensory trap (trait/stimuli) | natural selection on female receiver bias | possible initial direct costs to females | possible initial direct benefits to females | references |
|---|---|---|---|---|---|
| bowerbirds | colourful bower decorations | foraging (fruit) | interference with foraging, results in suboptimal mating | decreased mate search costs | Madden & Tanner (2003) |
| water mites | vibratory courtship signals | foraging (copepod prey) | interference with foraging, results in suboptimal mating | decreased mate search costs | Proctor (1991, 1992) |
| lesser wax moths | ultrasound signals | antipredatory (bat avoidance) | lead to increased predation risk, results in suboptimal mating | decreased mate search costs | Greenfield & Weber (2000), Greenfield (2002) |
| guppies | orange spots | foraging (fruit) | interference with foraging, results in suboptimal mating | decreased mate search costs | Rodd et al. (2002) |
| three-spined sticklebacks | red coloration, red nest decoration | foraging (crustacean prey) | interference with foraging, results in suboptimal mating | decreased mate search costs | Östlund-nilsson & Holmlund (2003), Smith et al. (2004) |
| many insects | nuptial gift/feeding | foraging (generalized) | suboptimal mating rate or duration | nutritional | Wickler (1968), Sakaluk (2000) |
| nursery web spiders | wrapped nuptial gift | maternal care (egg sac recognition) | suboptimal mating | nutritional | Stålhandske (2002) |
| fiddler crabs | constructs sand/mud hoods | antipredatory (hiding) | suboptimal mating | reduced predation risk | Christy (1988, 1995), Christy et al. (2003a,b) |
| oriental fruit moths | pheromones | foraging (fermented fruit) | interference with foraging, results in suboptimal mating | decreased mate search costs | Nishida et al. (1985), Löfstedt et al. (1989) |
| bush crickets | genitalia | fertilization (release of sperm from storage) | shortage of sperm | increased sperm viability | Von helversen & Von helversen (1991) |
| swordtail characins | opercular paddle | foraging (cladoceran prey) | interference with foraging, results in suboptimal mating | decreased mate search costs | Nelson (1964), Wickler (1968) |
| garter snakes | physical interference with female respiration | antipredatory (cloacal gaping) | hypoxic stress | none? | Shine et al. (2003) |
| butterflies | non-fertilizing sperm | reproductive (sufficient sperm supplies) | shortage of sperm | none? | Cook & Wedell (1999) |
| many internally fertilizing animals | seminal gonadotropins | reproductive (regulation of oogenesis) | superoptimal egg production rate | none? | Arnqvist & Rowe (2005) |
| many insects | seminal myotropins | reproductive (regulation of ovulation) | superoptimal ovulation rate | none? | Arnqvist & Rowe (2005) |
| orchid bees | flower fragrances | foraging (nectar) | interference with foraging, results in suboptimal mating | decreased mate search costs | Dressler (1982), Eltz et al. (1999), Peruquetti (2000), Cameron (2004) |
| goodeinae fish | tail fin pattern | foraging (invertebrate prey) | interference with foraging, results in suboptimal mating | decreased mate search costs | Garcia & Ramirez (2005) |
| dance fliesa | inflatable abdomen/leg scales | male mate choice | lower reproductive output | none? | Funk & Tallamy (2000) |
Sex role reversal.
Consider the following hypothetical cases. In a taxon where females forage for red berries, males evolve red spots that mimic such berries (same size and colour). Female are attracted to males bearing such spots and as a result suffer direct costs from suboptimal mating. To resist this, females could in various ways evolve to lower their general response to red objects (Gavrilets et al. 2001; Rowe et al. 2005). However, this is likely to simultaneously reduce their attraction to, and foraging success when searching for, red berries. Alternatively, female could evolve resistance by improving their ability to discriminate between red berries and male spots, thus reinforcing selection in males for realistic berry mimics. However, such discrimination is likely to be associated with recognition errors, which again is likely to lead to reduced foraging success when searching for red berries. The general point is that, in both cases, the evolution of resistance will be associated with negative pleiotropic side-effects and thus be costly. Gavrilets et al. (2001) showed that scenarios such as these can serve as illustrations of SAC, and that they can result in the evolution of costly female choice for exploitative male traits that are also costly to their bearers.
Whether SAC should follow male exploitation of adaptive sensory biases is contingent upon the idea that there are selective constraints on the evolution of female resistance. I note that while many of the potential empirical examples of adaptive sensory biases involve female responses that may well be selectively constrained (see table 2 and §8), I know of no data that directly address this important point. Next, I will discuss a few illuminating and intriguing case studies.
8. Case studies
A brief account of a few empirical examples can be used to illustrate the point that invasion of traits by sensory exploitation can be associated with costs for individuals of the exploited sex. Shine et al. (2003) recently described an intriguing case in the garter snake Thamnophis sirtalis. Here, males exploit a female antipredatory stress response to achieve copulations. Males crawl up on top of females and exhibit rhythmic pulsating waves of muscular contraction that interferes with female respiration. The female hypoxic stress response that results from this ‘courtship’ involves cloacal gaping, which functions in other contexts to repel predators by extruding faeces and musk. However, cloacal gaping also permits intromission and males have evolved to take advantage of this fact. That hypoxic stress should be costly is confirmed by the fact that many females die of suffocation during the mating season (Shine et al. 2001). Here, sensory exploitation must clearly be costly to the exploited sex.
The male ejaculate of internally fertilizing taxa contains a suite of substances, besides sperm, that are transferred to females at mating. Common among these are ‘mimics’ of substances that females produce endogenously to regulate their own reproduction (see Arnqvist & Rowe 2005; and references therein). For example, mammalian semen contains female pituitary gland hormones (notably luteinizing hormone and follicle-stimulating hormone) and males of many insects transfer both gonadotropic peptides, that stimulate egg maturation, and myotropic peptides that cause muscle contractions in the ovaries and the oviduct thus increasing the rate of egg laying. It is worthwhile considering this fact in the context of life history theory (see Nilsson et al. 2002). Research in this field, both theoretically and empirically, has shown that females of iteroparous species exhibit some optimal reproductive rate, representing trade-offs between the costs and benefits of current and future reproduction. The internal machinery that females employ for regulating reproductive rate includes a large number of receptors, representing adaptive sensory biases, that could potentially be exploited by males in their own interests—to elevate reproductive rate of their mates after copulation. If we accept the notion that an intermediate reproductive rate is optimal for females, then the invasion by sensory exploitation of a novel male seminal substance that elevates reproductive rate in their mates will result in suboptimal reproduction and so be associated with direct costs to females (figure 1). Again, sensory exploitation will be costly to the exploited sex.
Figure 1.
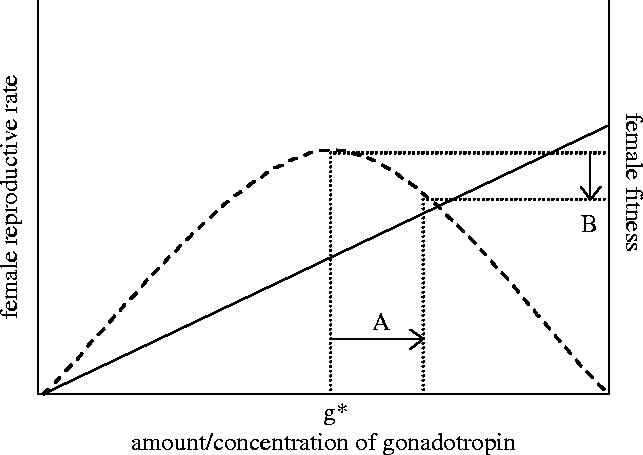
In internally fertilizing species, females regulate their reproductive rate by endogenously produced gonadotropic substances and there is typically a positive dose dependent reproductive rate response of the concentration of such substances (solid line). Because of life history trade-offs, however, an intermediate amount of gonadotropins (g*) is expected to maximize female fitness (dashed line). If males capitalize on female sensory responses by evolving an ability to provide an additional dose (A) of gonadotropins to females in their seminal fluid, which will benefit males in many polyandrous species given their limited genetic interest in the future offspring of their mate, this will cause a depression (B) of the fitness of their mates. Note that this logic applies to any means by which males manipulate females by providing stimuli that females use to regulate their current reproductive effort (Arnqvist & Rowe 2005).
Another interesting example of sensory exploitation occurs in the dance fly Rhamphomyia longicauda (Empididae; Funk & Tallamy 2000; Hockham & Ritchie 2000; LeBas et al. 2003). In these flies, a male carrying arthropod prey items enters a swarm of females, appears to choose a female, donates his prey item and the pair fly off to copulate while the female feeds on the ‘nuptial gift’. In R. longicauda, females are equipped with inflatable abdominal sacs and conspicuous leg scales that exaggerate the apparent size of the female. Assuming that males prefer to pair with large females because they are more fecund, Funk & Tallamy (2000) suggested that females have evolved secondary sexual traits which exploit adaptive sensory biases in males: male preference for large females. If true, the invasion of exploitative female traits should have manipulated males into allocating their paternal effort in a suboptimal manner. Although males are the exploited sex in this case, sensory exploitation is likely to have been associated with costs to the exploited sex. Seemingly deceptive female ornaments are also found in other dance flies with similar mating system (e.g. R. marginata, R. tarsata and Empis borealis; Svensson & Peterson 1987; Cumming 1994; Svensson 1997; LeBas et al. 2003).
The swordtail characin (C. riisei) discussed in §4c has internal fertilization and females store sperm for at least several months but are exposed to courting males throughout the year. Males employ their ‘paddles’ to lure females into proximity, followed by a lightning-fast movement during which sperm is transferred to the female. Remarkably enough, after mating, females become cataleptic and/or suffer convulsions for several minutes (Nelson 1964). The reasons for these stress responses are unknown, but they should be associated with, or indicative of, costs to females. Thus, the natural history and behaviour of this species suggests that females suffer direct costs from sensory exploitation in terms of suboptimal mating.
Another set of examples illustrate the possibility that the evolution of resistance to exploitation may be under selective constraints and thus be associated with costs. One case is the classic example of the water mite Neumania papillator studied by Proctor (1991, 1992). These mites are sit-and-wait predators of copepods, and they orient towards and clutch at vibrations caused by swimming prey. Males search for mates. When a male encounters a female, he trembles his legs near the female thus producing vibrations at a rate than match those produced by swimming prey. Females respond as they do to prey, by turning towards the source of vibrations and clasping the male which increases the probability of successful fertilization. Although, the costs of sensory exploitation in this system are probably low, it seems likely that there are significant selective constraints on the evolution of female resistance: depressing the response to male vibrations would likely extend to a lower response to prey as well. The fact that female responses to males and prey are phenotypically integrated is supported by the observation that hungry females orient towards and clutch trembling males more often than do well-fed females.
The neotropical orchid bees (Apidae: Euglossini) feed on nectar and pollen from various tubular flowers. Male orchid bees have evolved the amazing habit of making ‘perfumes’ out of fragrant substances collected from flowers, primarily orchids, that they then use to attract females (see Dressler 1982; Eltz et al. 1999; Peruquetti 2000; Bembe 2004; Cameron 2004). The fragrances are absorbed or scraped up with stiff specialized brushes on the forelegs and are then transferred into complex internal chambers in the greatly enlarged hind tibia of males that are filled with a spongy tissue with branched hairs, known as the tibial organ. This is where fragrances are stored and blended. Males also further ‘process’ the fragrances by applying labial gland lipids to the tibial organ. By vibrating their wings they then spray off aerosol clouds of fragrance bouquets, or perfumes, that attract females that use fragrances to locate flowers for foraging. The evolutionary origin of these remarkable adaptations certainly must have involved sensory exploitation, and it seems very probable that female resistance to male perfumes might be under selective constraints: reduced attraction to fragrant males would most likely also lead to reduced attraction to food resources.
Moths exhibit striking negative reactions to pulses of ultrasound. They become immobile, dropping to the ground if flying and stopping if moving on a substrate. This behaviour is considered an important adaptation against predation by bats, since bats use ultrasound to locate moving objects. Remarkably enough, males of the pyralid moth Achroia grisella produce bursts of ultrasonic signals as a part of their courtship (see Greenfield & Weber 2000). Greenfield (2002) suggested that this may have originated by males capitalizing on female anti-predatory responses. By essentially triggering an immobility reflex in females, males may increase the probability of successful copulation. A similar argument has also been invoked to explain the origin of a form of male behaviour common in beetles: tapping or rubbing encountered females with antennae or legs (West-Eberhard 1984). Immobility is a common response when being touched in beetles, presumably representing an anti-predatory adaptation, and courting males may have evolved an ability to capitalize upon this reflex. In both of these examples, it is likely that the evolution of a reduced response to male signals would also lead to an increased risk of predation for females. Here, it is again easy to envision how the evolution of female resistance might be costly.
9. Conclusions
When striving to understand the amazing diversity in sexual dimorphism that we can observe and the processes of female choice that are responsible for this diversity, it is important to make the distinction between (i) origin, (ii) subsequent evolution and (iii) maintenance of male traits and female preferences (Fisher 1930). Yet, there is some confusion in the literature that is due to a lack of making this distinction. Further, most discussions and models concern the second and third point. I have suggested that we would benefit from a better understanding of the origin of male traits and female preferences (see also Ryan & Rand 1993; Berglund et al. 1996), and I have tried to explicitly delineate a set of alternative scenarios for such origin.
There are theoretical reasons for why origin by sensory exploitation of pre-existing sensory biases should be more common than previously believed, and recent empirical research in this area seems to support this. I have argued that such exploitation should often initially bring about costs to individuals of the exploited sex, and that the evolution of resistance to exploitation may be associated with costs due to evolutionary constraints. If this is true, then SAC is a much underappreciated process in terms of generating diversity in sexual dimorphism (Holland & Rice 1998), in part because the number of potentially exploitable pre-existing sensory biases is so large (Arak & Enquist 1993). Following origin by sensory exploitation, bursts of relatively rapid evolution by SAC may then have been replaced with phases of maintenance, or slower evolution, dominated by other processes and even indirect selection. In part, it is this dynamic dilemma that makes sexual conflict and SAC a difficult field to study experimentally.
To some extent, how likely we consider this scenario will depend on the importance we place on constraints on adaptive evolution in general. Our current understanding is hampered by a lack good empirical data, particularly relating to two key questions. What are the initial costs and benefits of sensory exploitation for individuals of the exploited sex? Is sensory exploitation commonly followed by the evolution of resistance against such exploitation, and is resistance associated with costs for individuals of the resisting sex when it occurs? These questions are, admittedly, very difficult indeed to address empirically. Comparative studies combining historical and experimental approaches can help (Arnqvist & Rowe 2002; Autumn et al. 2002; Rowe & Arnqvist 2002), but innovative experimental studies introducing novel traits or sensory biases in extant species should also be helpful.
Acknowledgements
I am grateful to two anonymous referees for comments on an earlier version of this paper, to Locke Rowe for discussions and to Tracey Chapman, Tom Tregenza and Nina Wedell for inviting me to write this paper. This work was funded by the Swedish Research Council.
Footnotes
One contribution of 13 to a Discussion Meeting Issue ‘Sexual conflict: a new paradigm?’.
References
- Andersson M.Sexual selection1994Princeton University Press; Princeton, NJ [Google Scholar]
- Arak A, Enquist M. Hidden preferences and the evolution of signals. Phil. Trans. R. Soc. B. 1993;340:207–213. [Google Scholar]
- Arnold S.J. Constraints on phenotypic evolution. Am. Nat. 1992;140:S85–S107. doi: 10.1086/285398. 10.1086/285398 [DOI] [PubMed] [Google Scholar]
- Arnqvist G. Comparative evidence for the evolution of genitalia by sexual selection. Nature. 1998;393:784–786. 10.1038/31689 [Google Scholar]
- Arnqvist G, Rowe L.Sexual conflict2005Princeton University Press; Princeton, NJ [Google Scholar]
- Arnqvist G, Rowe L. Antagonistic coevolution between the sexes in a group of insects. Nature. 2002;415:787–789. doi: 10.1038/415787a. [DOI] [PubMed] [Google Scholar]
- Autumn K, Ryan M.J, Wake D.B. Integrating historical and mechanistic biology enhances the study of adaptation. Q. Rev. Biol. 2002;77:383–408. doi: 10.1086/344413. 10.1086/344413 [DOI] [PubMed] [Google Scholar]
- Backwell P.R.Y, Jennions M.D, Christy J.H, Schober U. Pillar building in the fiddler crab Uca beebei—evidence for a condition dependent ornament. Behav. Ecol. Sociobiol. 1995;36:185–192. 10.1007/s002650050139 [Google Scholar]
- Basolo A.L. Phylogenetic evidence for the role of a preexisting bias in sexual selection. Proc. R. Soc. B. 1995;259:307–311. doi: 10.1098/rspb.1995.0045. [DOI] [PubMed] [Google Scholar]
- Basolo A.L, Endler J.A. Sensory biases and the evolution of sensory systems. Trends Ecol. Evol. 1995;10:489–489. doi: 10.1016/s0169-5347(00)89196-x. 10.1016/S0169-5347(00)89196-X [DOI] [PubMed] [Google Scholar]
- Bembe B. Functional morphology in male euglossine bees and their ability to spray fragrances (Hymenoptera, Apidae, Euglossini) Apidologie. 2004;35:283–291. [Google Scholar]
- Berglund A, Bisazza A, Pilastro A. Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 1996;58:385–399. 10.1006/bijl.1996.0043 [Google Scholar]
- Borgia G. Sexual selection and the evolution of mating systems. In: Blum M.S, Blum N.A, editors. Sexual selection and reproductive competition in insects. Academic Press; New York: 1979. pp. 19–80. [Google Scholar]
- Bradbury J.W, Vehrencamp S.L. Economic models of animal communication. Anim. Behav. 2000;59:259–268. doi: 10.1006/anbe.1999.1330. 10.1006/anbe.1999.1330 [DOI] [PubMed] [Google Scholar]
- Burley N, Symanski R. ‘A taste for the beautiful’: latent aesthetic mate preferences for white crests in two species of Australian grasfinches. Am. Nat. 1998;152:792–802. doi: 10.1086/286209. 10.1086/286209 [DOI] [PubMed] [Google Scholar]
- Burley N, Krantzberg G, Radman P. Influence of colour-banding on the conspecific preferences of zebra finches. Anim. Behav. 1982;30:444–455. [Google Scholar]
- Byrne P.G, Roberts J.D. Simultaneous mating with multiple males reduces fertilization success in the myobatrachid frog Crinia georgiana. Proc. R. Soc. B. 1999;266:717–721. 10.1098/rspb.1999.0695 [Google Scholar]
- Byrne P.G, Roberts J.D. Does multiple paternity improve fitness of the frog Crinia georgiana? Evolution. 2000;54:968–973. doi: 10.1111/j.0014-3820.2000.tb00096.x. [DOI] [PubMed] [Google Scholar]
- Cameron S.A. Phylogeny and biology of neotropical orchid bees (Euglossini) Ann. Rev. Entomol. 2004;49:377–404. doi: 10.1146/annurev.ento.49.072103.115855. 10.1146/annurev.ento.49.072103.115855 [DOI] [PubMed] [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol. Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. 10.1017/S1464793103006158 [DOI] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. 10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Christy J.H. Attractiveness, mate choice and a sensory trap in the fiddler crab Uca beebei. Am. Zool. 1988;28:A133–A133. [Google Scholar]
- Christy J.H. Mimicry, mate choice and the sensory trap hypothesis. Am. Nat. 1995;146:171–181. 10.1086/285793 [Google Scholar]
- Christy J.H, Backwell P.R.Y. The sensory exploitation hypothesis. Trends Ecol. Evol. 1995;10:417–417. doi: 10.1016/s0169-5347(00)89161-2. 10.1016/S0169-5347(00)89161-2 [DOI] [PubMed] [Google Scholar]
- Christy J.H, Baum J.K, Backwell P.R.Y. Attractiveness of sand hoods built by courting male fiddler crabs. Uca musica: test of a sensory trap hypothesis. Anim. Behav. 2003a;66:89–94. 10.1006/anbe.2003.2196 [Google Scholar]
- Christy J.H, Backwell P.R.Y, Schober U. Interspecific attractiveness of structures built by courting male fiddler crabs: experimental evidence of a sensory trap. Behav. Ecol. Sociobiol. 2003b;53:84–91. [Google Scholar]
- Cook P.A, Wedell N. Non-fertile sperm delay female remating. Nature. 1999;397:486. 10.1038/17257 [Google Scholar]
- Cotton S, Fowler K, Pomiankowski A. Heightened condition dependence is not a general feature of male eyespan in stalk-eyed flies (Diptera: Diopsidae) J. Evol. Biol. 2004;17:1310–1316. doi: 10.1111/j.1420-9101.2004.00754.x. 10.1111/j.1420-9101.2004.00754.x [DOI] [PubMed] [Google Scholar]
- Coyne J.A, Orr H.A. Speciation. Sinauer Ass; Sunderland, MA: 2004. [Google Scholar]
- Cumming J.M. Sexual selection and the evolution of dance fly mating systems (Diptera: Empididae: Empidinae) Can. Entomol. 1994;126:907–920. [Google Scholar]
- Darwin C. The descent of man and selection in relation to sex. J. Murray; London: 1871. [Google Scholar]
- Dawkins M.S, Guilford T. Sensory bias and the adaptiveness of female choice. Am. Nat. 1996;148:937–942. 10.1086/285964 [Google Scholar]
- Dressler R.L. Biology of the orchid bees (Euglossini) Ann. Rev. Ecol. Syst. 1982;13:373–394. 10.1146/annurev.es.13.110182.002105 [Google Scholar]
- Eberhard W.G. Sexual selection and animal genitalia. Harvard University Press; Cambridge, Mass: 1985. [Google Scholar]
- Eltz T, Whitten W.M, Roubik D.W, Linsenmair K.E. Fragrance collection, storage, and accumulation by individual male orchid bees. J. Chem. Ecol. 1999;25:157–176. 10.1023/A:1020897302355 [Google Scholar]
- Endler J.A, Basolo A.L. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- Enquist M, Arak A. Selection of exaggerated male traits by female aesthetic senses. Nature. 1993;361:446–448. doi: 10.1038/361446a0. 10.1038/361446a0 [DOI] [PubMed] [Google Scholar]
- Enquist M, Arak A. Symmetry, beauty and evolution. Nature. 1994;372:169–172. doi: 10.1038/372169a0. 10.1038/372169a0 [DOI] [PubMed] [Google Scholar]
- Enquist M, Arak A, Ghirlanda S, Wachtmeister C.A. Spectacular phenomena and limits to rationality in genetic and cultural evolution. Phil. Trans. R. Soc. B. 2002;357:1585–1594. doi: 10.1098/rstb.2002.1067. 10.1098/rstb.2002.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.A. The genetical theory of natural selection. The Clarendon Press; Oxford: 1930. [Google Scholar]
- Fiske P, Amundsen T. Female bluethroats prefer males with symmetric colour bands. Anim. Behav. 1997;54:81–87. doi: 10.1006/anbe.1996.0436. 10.1006/anbe.1996.0436 [DOI] [PubMed] [Google Scholar]
- Funk D.H, Tallamy D.W. Courtship role reversal and deceptive signals in the long-tailed dance fly, Rhamphomyia longicuada. Anim. Behav. 2000;59:411–421. doi: 10.1006/anbe.1999.1310. 10.1006/anbe.1999.1310 [DOI] [PubMed] [Google Scholar]
- Garcia C.M, Ramirez E. Evidence that sensory traps can evolve into honest signals. Nature. 2005;434:501–505. doi: 10.1038/nature03363. 10.1038/nature03363 [DOI] [PubMed] [Google Scholar]
- Gavrilets S, Arnqvist G, Friberg U. The evolution of female mate choice by sexual conflict. Proc. R. Soc. B. 2001;268:531–539. doi: 10.1098/rspb.2000.1382. 10.1098/rspb.2000.1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirlanda S, Enquist M.A. A century of generalization. Anim. Behav. 2003;66:15–36. 10.1006/anbe.2003.2174 [Google Scholar]
- Greenfield M.D. Signalers and receivers: mechanisms and evolution of arthropod communication. Oxford University Press; New York: 2002. [Google Scholar]
- Greenfield M.D, Weber T. Evolution of ultrasonic signalling in wax moths: discrimination of ultrasonic mating calls from bat echolocation signals and the exploitation of an anti-predator receiver bias by sexual advertisement. Ethol. Ecol. Evol. 2000;12:259–279. [Google Scholar]
- Guilford T, Dawkins M.S. Receiver phychology and the evolution of animal signals. Anim. Behav. 1991;42:1–14. [Google Scholar]
- Guilford T, Dawkins M.S. Receiver phychology and the design of animal signals. Trends Neurosci. 1993;16:430–436. doi: 10.1016/0166-2236(93)90068-w. [DOI] [PubMed] [Google Scholar]
- Hinde R.A. Animal behaviour: a synthesis of ethology and comparative psychology. McGraw-Hill; New York: 1966. [Google Scholar]
- Hockham L.R, Ritchie M.G. Female secondary sexual characteristics: appearances might be deceptive. Trends Ecol. Evol. 2000;15:436–438. doi: 10.1016/s0169-5347(00)01963-7. 10.1016/S0169-5347(00)01963-7 [DOI] [PubMed] [Google Scholar]
- Holland B, Rice W.R. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Hunt J, Bussiere L.F, Jennions M.D, Brooks R. What is genetic quality? Trends Ecol. Evol. 2004;19:329–333. doi: 10.1016/j.tree.2004.03.035. 10.1016/j.tree.2004.03.035 [DOI] [PubMed] [Google Scholar]
- Iwasa Y, Pomiankowski A. The evolution of mate preferences for multiple sexual ornaments. Evolution. 1994;48:853–867. doi: 10.1111/j.1558-5646.1994.tb01367.x. [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. 10.1017/S0006323196005014 [DOI] [PubMed] [Google Scholar]
- Johnson K, Dalton R, Burley N. Preferences of female American goldfinches (Carduelis tristis) for natural and artificial male traits. Behav. Ecol. 1993;4:138–143. [Google Scholar]
- Johnston R.E, Müller-Schwarze D, Sorenson P.W, editors. Advances in chemical signals in vertebrates. Kluwer; New York: 1999. [Google Scholar]
- Johnstone R.A. Female preference for symmetrical males as a by-product of selection for mate recognition. Nature. 1994;372:172–175. doi: 10.1038/372172a0. 10.1038/372172a0 [DOI] [PubMed] [Google Scholar]
- Johnstone R.A. Honest advertisement of multiple qualities using multiple signals. J. Theor. Biol. 1995;177:87–94. [Google Scholar]
- Kirkpatrick M. Evolution of female choice and male parental investment in polygynous species: the demise of the ‘sexy son’. Am. Nat. 1985;125:788–810. 10.1086/284380 [Google Scholar]
- Kirkpatrick M, Barton N.H. The strength of indirect selection on female mating preferences. Proc. Natl Acad. Sci. USA. 1997;94:1282–1286. doi: 10.1073/pnas.94.4.1282. 10.1073/pnas.94.4.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Ravigné V. Speciation by natural and sexual selection: models and experiments. Am. Nat. 2002;159:S22–S35. doi: 10.1086/338370. 10.1086/338370 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Price T, Arnold S.J. The Darwin–Fisher theory of sexual selection in monogamous birds. Evolution. 1990;44:180–193. doi: 10.1111/j.1558-5646.1990.tb04288.x. [DOI] [PubMed] [Google Scholar]
- Kokko H, Brooks R, Jennions M.D, Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. 10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBas N.R, Hockham L.R, Ritchie M.G. Nonlinear and correlational sexual selection on ’honest’ female ornamentation. Proc. R. Soc. B. 2003;270:2159–2165. doi: 10.1098/rspb.2003.2482. 10.1098/rspb.2003.2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfstedt C, Vickers N.J, Roelofs W.L, Baker T.C. Diet related courtship success in the oriental fruit moth, Grapholita molets (Tortricidae) Oikos. 1989;55:402–408. [Google Scholar]
- Madden J.R, Tanner K. Preferences for coloured bower decorations can be explained in a nonsexual context. Anim. Behav. 2003;65:1077–1083. 10.1006/anbe.2003.2126 [Google Scholar]
- Markow T.A. Forced matings in natural populations of Drosophila. Am. Nat. 2000;156:100–103. doi: 10.1086/303368. 10.1086/303368 [DOI] [PubMed] [Google Scholar]
- Martins E.P. Adaptation and the comparative method. Trends Ecol. Evol. 2000;15:296–299. doi: 10.1016/s0169-5347(00)01880-2. 10.1016/S0169-5347(00)01880-2 [DOI] [PubMed] [Google Scholar]
- Maynard-Smith J. Theoreies of sexual selection. Trends Ecol. Evol. 1991;6:146–151. doi: 10.1016/0169-5347(91)90055-3. 10.1016/0169-5347(91)90055-3 [DOI] [PubMed] [Google Scholar]
- McClintock W.J, Uetz G.W. Female choice and pre-existing bias: visual cues during courtship in two Schizocosa wolf spiders (Araneae: Lycosidae) Anim. Behav. 1996;52:167–181. 10.1006/anbe.1996.0162 [Google Scholar]
- Nelson K. Behavior and morphology in the glandulocaudinae fishes (Ostariophysi, Characidae) Univ. California Publ. Zool. 1964;75:59–152. [Google Scholar]
- Nilsson T, Fricke C, Arnqvist G. Patterns of divergence in the effects of mating on female reproductive performance in flour beetles. Evolution. 2002;56:111–120. doi: 10.1111/j.0014-3820.2002.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Nishida R, Fukami H, Baker T.C, Roelofs W.L, Acree T.E. Oriental fruit moth pheromone: attraction of females by an herbal essence. In: Acree T.E, Soderlund D.M, editors. Semiochemistry: flavors and pheromones. Walter de Gruyter & Co; Berlin: 1985. pp. 47–63. [Google Scholar]
- Östlund-Nilsson S, Holmlund M. The artistic three-spined stickleback (Gasterosteous aculeatus) Behav. Ecol. Sociobiol. 2003;53:214–220. 10.1007/s00265-002-0574-z [Google Scholar]
- Panhuis T.M, Butlin R, Zuk M, Tregenza T. Sexual selection and speciation. Trends Ecol. Evol. 2001;16:364–371. doi: 10.1016/s0169-5347(01)02160-7. 10.1016/S0169-5347(01)02160-7 [DOI] [PubMed] [Google Scholar]
- Peruquetti R.C. Function of fragrances collected by Euglossini males (Hymenoptera: Apidae) Entomol. Gen. 2000;25:33–37. [Google Scholar]
- Pomiankowski A, Iwasa Y. Evolution of multiple sexual preferences by Fisher runaway process of sexual selection. Proc. R. Soc. B. 1993;253:173–181. [Google Scholar]
- Price T, Schluter D, Heckman N.E. Sexual selection when the female directly benefits. Biol. J. Linn. Soc. 1993;48:187–211. [Google Scholar]
- Proctor H.C. Courtship in the water mite Neumania papillator—males capitalize on female adaptations for predation. Anim. Behav. 1991;42:589–598. [Google Scholar]
- Proctor H.C. Sensory exploitation and the evolution of male mating behaviour—a cladistic test using water mites (Acari, Parasitengona) Anim. Behav. 1992;44:745–752. [Google Scholar]
- Pryke S.R, Andersson S. A generalized female bias for long tails in a short-tailed widowbird. Proc. R. Soc. B. 2002;269:2141–2146. doi: 10.1098/rspb.2002.2131. 10.1098/rspb.2002.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M.G. The shape of female mating preferences. Proc. Natl Acad. Sci. USA. 1996;93:14628–14631. doi: 10.1073/pnas.93.25.14628. 10.1073/pnas.93.25.14628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd F.H, Hughes K.A, Grether G.F, Baril C.T. A possible non-sexual origin of mate preference: are male guppies mimicking fruit? Proc. R. Soc. B. 2002;269:475–481. doi: 10.1098/rspb.2001.1891. 10.1098/rspb.2001.1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R.L, Snedden W.A. On the functional design of mate preferences and receiver biases. Anim. Behav. 2004;68:427–432. 10.1016/j.anbehav.2003.08.031 [Google Scholar]
- Rosenthal G.G, Servedio M.R. Chase-away sexual selection: resistance to ‘resistance’. Evolution. 1999;53:296–299. doi: 10.1111/j.1558-5646.1999.tb05356.x. [DOI] [PubMed] [Google Scholar]
- Rowe C. Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 1999;58:921–931. doi: 10.1006/anbe.1999.1242. 10.1006/anbe.1999.1242 [DOI] [PubMed] [Google Scholar]
- Rowe L, Arnqvist G. Sexually antagonistic coevolution in a mating system: combining experimental and comparative approaches to address evolutionary processes. Evolution. 2002;56:754–767. doi: 10.1111/j.0014-3820.2002.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. B. 1996;263:1415–1421. [Google Scholar]
- Rowe C, Skelhorn J. Avian psychology and communication. Proc. R. Soc. B. 2004;271:1435–1442. doi: 10.1098/rspb.2004.2753. 10.1098/rspb.2003.2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L, Arnqvist G, Sih A, Krupa J.J. Sexual conflict and the evolutionary ecology of mating patterns: water striders as a model system. Trends Ecol. Evol. 1994;9:289–293. doi: 10.1016/0169-5347(94)90032-9. 10.1016/0169-5347(94)90032-9 [DOI] [PubMed] [Google Scholar]
- Rowe L, Cameron E, Day T. Escalation, retreat, and female indifference as alternative outcomes of sexually antagonistic coevolution. Am. Nat. 2005;165:S5–S18. doi: 10.1086/429395. 10.1086/429395 [DOI] [PubMed] [Google Scholar]
- Ryan M.J. Sexual selection, sensory systems, and sensory exploitation. Oxford Surv. Evol. Biol. 1990;7:157–195. [Google Scholar]
- Ryan M.J. Phylogenetics and behavior: some cautions and expectations. In: Martins E, editor. Phylogenies and the comparative method in animal behavior. Oxford University Press; Oxford: 1996. pp. 1–21. [Google Scholar]
- Ryan M.J, Rand A.S. Sexual selection and signal evolution—the ghost of biases past. Phil. Trans. R. Soc. B. 1993;340:187–195. [Google Scholar]
- Sakaluk S.K. Sensory exploitation as an evolutionary origin to nuptial food gifts in insects. Proc. R. Soc. B. 2000;267:339–343. doi: 10.1098/rspb.2000.1006. 10.1098/rspb.2000.1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D, Price T. Honesty, perception and population divergence in sexually selected traits. Proc. R. Soc. B. 1993;253:117–122. doi: 10.1098/rspb.1993.0089. [DOI] [PubMed] [Google Scholar]
- Shaw K. Phylogenetic tests of the sensory exploitation model of sexual selection. Trends Ecol. Evol. 1995;10:117–120. doi: 10.1016/s0169-5347(00)89005-9. 10.1016/S0169-5347(00)89005-9 [DOI] [PubMed] [Google Scholar]
- Sherman P.W, Wolfenbarger L.L. Genetic correlations as tests for sensory exploitation. Trends Ecol. Evol. 1995;10:246–247. doi: 10.1016/S0169-5347(00)89079-5. 10.1016/S0169-5347(00)89079-5 [DOI] [PubMed] [Google Scholar]
- Sherman P.W, Wolfenbarger L.L. Sensory biases and the evolution of sensory systems. Trends Ecol. Evol. 1995b;10:489–489. doi: 10.1016/s0169-5347(00)89196-x. 10.1016/S0169-5347(00)89197-1 [DOI] [PubMed] [Google Scholar]
- Shine R, LeMaster M.P, Moore I.T, Olsson M.M, Mason R.T. Bumpus in the snake den: effects of sex, size and body condition on mortality in redsided garter snakes. Evolution. 2001;55:598–604. doi: 10.1554/0014-3820(2001)055[0598:bitsde]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Shine R, Langkilde T, Mason R.T. Cryptic forcible insemination: male snakes exploit female physiology, anatomy, and behavior to obtain coercive matings. Am. Nat. 2003;162:653–667. doi: 10.1086/378749. 10.1086/378749 [DOI] [PubMed] [Google Scholar]
- Smith C, Barber L, Wootton R.J, Chittka L. A receiver bias in the origin of three-spined stickleback mate choice. Proc. R. Soc. B. 2004;271:949–955. doi: 10.1098/rspb.2004.2690. 10.1098/rspb.2004.2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stålhandske P. Nuptial gifts of male spiders function as sensory traps. Proc. R. Soc. B. 2002;269:905–908. doi: 10.1098/rspb.2001.1917. 10.1098/rspb.2001.1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson B.G. Swarming behavior, sexual dimorphism, and female reproductive status in the sex role-reversed dance fly species Rhamphomyia marginata. J. Insect Behav. 1997;10:783–804. 10.1023/B:JOIR.0000010413.20596.28 [Google Scholar]
- Svensson B.G, Petersson E. Sex role reversed courtship behavior, sexual dimorphism and nuptial gifts in the dance fly, Empis borealis (L) Ann. Zool. Fennici. 1987;24:323–334. [Google Scholar]
- Swanson W.J, Vacquier V.D. The rapid evolution of reproductive proteins. Nat. Rev. Gen. 2002;3:137–144. doi: 10.1038/nrg733. 10.1038/nrg733 [DOI] [PubMed] [Google Scholar]
- Von Helversen D, Von Helversen O. Pre-mating sperm removal in the bush-cricket Metaplastes ornatus Ramme 1931 (Orthoptera: Tettigonoidea: Phaneropteridae) Behav. Ecol. Sociobiol. 1991;28:391–396. 10.1007/BF00164120 [Google Scholar]
- West-Eberhard M.J. Sexual selection, social competition, and evolution. Proc. Am. Phil. Soc. 1979;123:222–234. [Google Scholar]
- West-Eberhard M.J. Sexual selection, competitive communication and species-specific signals in insects. In: Lewis T, editor. Insect communication. Academic Press; New York: 1984. pp. 283–342. [Google Scholar]
- Wickler W. Mimicry in plants and animals. McGraw-Hill; New York: 1968. [Google Scholar]
- Williams G.C. Princeton University Press; Princeton, NJ: 1966. Adaptation and natural selection. [Google Scholar]
- Witt P.W, Rovner J.S, editors. Spider communication: mechanisms and ecological significance. Princeton University Press; Princeton, NJ: 1982. [Google Scholar]
- Witte K, Curio E. Sexes of a monomorphic species differ in preference for mates with a novel trait. Behav. Ecol. 1999;10:15–21. 10.1093/beheco/10.1.15 [Google Scholar]


