Abstract
Health effects stemming from depleted uranium (DU) exposure in a cohort of Gulf War veterans who were in or on US Army vehicles hit by friendly fire involving DU munitions are being carefully monitored through the Baltimore Veterans Affairs (VA) DU Follow-Up Program initiated in 1993. DU exposure in this cohort has been directly measured using inductively coupled plasma-mass spectrometer (ICP-MS) isotopic analysis for DU in urine specimens. Soldiers with embedded DU fragments continue to excrete elevated concentrations of U in their urine, documenting ongoing systemic exposure to U released from their fragments. Biennial surveillance visits provide a detailed health assessment that includes basic clinical measures and surveillance for early changes in kidney function, an expected target organ for U. Tests also include measurements of genotoxicity and neuroendocrine, neurocognitive and reproductive function. With the exception of the elevated urine U excretion, no clinically significant expected U-related health effects have been identified to date. Subtle changes in renal function and genotoxicity markers in veterans with urine U concentrations greater than 0.1 μg−1 creatinine, however, indicate the need for continued surveillance of these DU-exposed veterans.
Keywords: depleted uranium, clinical surveillance, renal toxicity, genotoxicity, urine uranium
1. Introduction
While the duration of actual combat engagement during the first Gulf War in February 1991 was relatively short, measured in days, the legacy of adverse health effects presumed related to it has been disproportionately lengthy. The constellation of symptom complaints in returned troops termed ‘Gulf War Syndrome’ or more generally, ‘unexplained illness’, has received the bulk of both scientific and public attention. However, a small collection of ‘explained’ adverse health outcomes have also been reported over the 15 years since the War's end. One of the best-characterized examples in this category involves the cluster of DU ‘friendly fire’ incidents and the DU-related health effects accrued to those soldiers who were its victims.
During one 48 h period of the Gulf War in February 1991, approximately 115 US tank crew members in 20 US vehicles (six Abrams tanks and 14 Bradley fighting vehicles) were mistakenly fired upon by other US forces using DU penetrators (OSAGWI 2000). DU is a high-density metal, lending it armour-piercing capability. At the time a DU penetrator strikes a target, its ‘pyrophoric’ character and the high temperatures generated by the impact promote small particles of DU to ignite and form a DU oxide dust that deposits on and within the targeted vehicle. Small shards of DU metal are also produced when the penetrator pierces the target's armour (AEPI 1995; Toohey 2003; Parkhurst et al. 2005).
Eleven fatalities resulted from these friendly fire incidents and approximately 50 casualties required medical attention (OSAGWI 2000). Traumatic injuries were the primary consequence, although the potential for significant wound contamination, inhalation and ingestion exposures to DU dust also occurred to both tank crew members and rescuers (Parkhurst et al. 2005), and some soldiers were hit by DU metal fragments that remain embedded in soft tissues. Each soldier's level of exposure to DU dust and/or shrapnel by these potential exposure routes would have differed depending on his location and movements subsequent to the impact of the munitions. Thus, exposure assessment has been an important component of the Baltimore VA DU Follow-Up Surveillance Program.
2. History of the depleted uranium surveillance program
In 1993, the US Department of VA in conjunction with the Department of Defense initiated a medical surveillance programme to assess the health of the Gulf War veterans involved in the friendly fire incidents described above. The cohort defined as ‘DU-exposed’ in this programme is composed of soldiers with the highest potential for significant DU exposure, i.e. those who were in or on the Army vehicles when they were hit. In addition to assessing health, the programme was charged with evaluating and developing methods to measure uranium body burdens under different exposure scenarios, including the novel exposure mode of metal released from fragments present in soft tissue. As well, the surgical management of embedded fragments and inherent medical consequences were to be evaluated.
The surgical management of shrapnel in place in 1991 for these affected servicemen was based on a standardized protocol that considered metal fragment size and location in the body as the primary determinants for removal. Additionally the surgical morbidity expected from extensive fragment removal was also calculated. Many soldiers possessed multiple fragments, from 10 to more than 30, comprised of dimensions ranging from 1 mm to much larger, approximately 20 mm in diameter (Toohey 2003). Easily accessible fragments were removed, but because of the extensive traumatic injuries sustained by many of these soldiers, the smaller and more difficult to reach surgically were left in place by the surgeons who first cared for these soldiers in Iraq, Germany and during their initial care at Army Medical Centers in the US.
A biennial schedule of surveillance commenced in late 1993 for approximately 100 survivors of the Gulf War friendly fire events. To date, 74 of the soldiers in the ‘DU-exposed’ cohort have been evaluated, out of the approximate 90 for whom contact information is available. Although all expenses for the evaluation are paid for, including a reimbursement for lost wages during the 3-day, in-patient assessment, a number of victims of the original friendly fire incidents have not felt the need to participate in health evaluations. The number of participants involved in each of the biennial visits to date is shown in table 1. Many of the ‘DU-exposed’ cohort have participated in all of the visits, providing the ability to examine changes in clinical parameters over time. In 1997, using an enhanced protocol, the health status of the soldiers in the ‘DU-exposed’ cohort was reassessed and compared with Gulf War deployed soldiers with no known potential for DU exposure as judged by their answers to questions on a DU exposure questionnaire which provided information on their location and activities during the Gulf War (McDiarmid et al. 2000). Subsequent analysis of DU-exposure status of this ‘Non-DU-exposed’ cohort was confirmed by whole body radiation counting (McDiarmid et al. 2000) and urine U isotopic analysis (Gwiazda et al. 2004).
Table 1.
Summary of surveillance visits for Gulf War soldiers (74 unique DU-exposed cases have been evaluated).
| year | DU-exposed | non-exposed | total |
|---|---|---|---|
| 1993–1994 | 33 | 33 | |
| 1997 | 29 | 38 | 67 |
| 1999 | 21+29 new | 50 | |
| 2001 | 31+8 new | 39 | |
| 2003 | 32 | 32 | |
| 2005 | 30+4 new | 34 |
The enhanced health surveillance protocol conducted in 1997 was based on the known and presumed toxicology of uranium and the unique nature of the on-going absorption of U from embedded shrapnel via dissolution of the metal fragments. With minor modifications, this enhanced protocol continues as the basis for the DU Program biennial surveillance visits.
3. Natural and depleted uranium toxicity
DU poses both radiological and chemical hazards to human health (The Royal Society 2001, 2002). Created as a by-product of the uranium enrichment process, which involves removal of natural U's more radioactive isotopes: U235 and U234, DU is weakly radioactive with a radioactivity approximately 40% lower than that of natural U (AEPI 1995; table 2). Assessments of radiation doses from DU conducted by The Royal Society (2002) indicate that high energy, poorly penetrating alpha emissions from DU contribute the most to tissue dose rates, with effects primarily on tissue in immediate contact with the metal. Beta particles released from the daughter product 234mPa which is in secular equilibrium with 238U will penetrate further into tissues, but dose rates are over tenfold lower than those from the alpha particle emissions (The Royal Society 2002).
Table 2.
Comparison of the radioactivity of natural and depleted uranium.
| radioactivity | natural uranium | depleted uranium | |
|---|---|---|---|
| isotope | Ci g−1 | isotope ratio (%) | isotope ratio (%) |
| 234U | 6200 | 0.01 | 0.00 |
| 235U | 2.2 | 0.72 | 0.20 |
| 238U | 0.33 | 99.28 | 99.80 |
| relative radioactivity | 1 | 0.6 |
Radiation dose estimates for Gulf War soldiers in or on vehicles hit by munitions containing DU penetrators have also been calculated using DU oxide air concentration data generated by the Capstone DU Aerosols: Generation and Characterization Study conducted by the US Army Heavy Metals Office (Parkhurst et al. 2005). Based on estimated inhalation exposure doses to mixtures of DU oxides of different solubilities, the most likely and upper bound exposure scenarios yielded maximum calculated 50 year committed effective doses [E(50)] of 6.0 and 8.7 rem, respectively, which exceed the US annual occupational radiation exposure dose limit of 5 rem total effective dose equivalent, but are less than the Nuclear Regulatory Commission (NRC) planned special exposure limit of 10 rem (10 CFR 20). Estimated 50 year committed effective doses to tissues [HT(50)] were highest for the lung by a factor of at least 10 compared to other tissues, including the bone surface, kidney, red marrow and liver, which is consistent with risk assessments developed for likely Gulf War exposures by The Royal Society (2002). The most likely and upper bound HT(50)s for lung reported using data from the Capstone report were 44 and 61 rem, respectively (Parkhurst et al. 2005).
The health effects of exposure to depleted, natural and enriched forms of U have been extensively investigated using animals studies (Leggett 1989; ATSDR 1999; The Royal Society 2001). Work that started in the 1940s as part of the Manhattan Project provided a strong database for establishing ‘safe’ exposure levels for workers in uranium industries (Voegtlin & Hodge 1949, 1953; Tannenbaum 1951; Hodge et al. 1973). As a whole, these studies have shown that health risks from exposure to natural U are highly dependent on the solubility of the U compounds involved and the timing and route of exposure. Acute and chronic inhalation exposure to insoluble U compounds can cause radiation-induced lung tissue damage; for acute inhalation and oral exposures to soluble U compounds, the kidneys are the primary target organ due to uranium's chemical toxicity (ATSDR 1999; The Royal Society Report 2001). Recent studies involving chronic exposure scenarios have also demonstrated the sensitivity of the kidney to U. Both animal experiments (Gilman et al. 1998a–c) and studies of occupationally (Saccomanno 1982; Thun et al. 1985) and environmentally (Zamora et al. 1998, 2002; Kurttio et al. 2002) exposed populations have reported renal dysfunction following chronic inhalation and oral U exposures.
Although in vitro genotoxicity (Lin et al. 1993; Miller et al. 1998a,b, 2002a,b, 2003; Yazzie et al. 2003; Stearns et al. in press) and in vivo animal carcinogenicity studies (Hueper et al. 1952; Leach et al. 1973; Cross et al. 1981; Mitchel et al. 1999; Hahn et al. 2002) using natural U and DU raise some concern regarding cancer risks in DU exposed veterans, studies of occupationally exposed cohorts do not support the extrapolation of the results of these studies to humans. There is poor evidence for an increased risk of lung cancer in uranium-exposed cohorts, except for uranium miners whose risk is attributed to their co-exposure to radon, a more intensely radioactive constituent than natural uranium by a factor of 10 000 (Kathren & Moore 1986; Samet 1989; Kathren et al. 1989; Samet et al. 1989). In addition, although bone is known to be an important long-term storage depot for uranium (Kathren et al. 1989), excesses in bone cancer or leukaemia have not been reported in exposed cohorts (IOM 2000). Although questions remain regarding the similarity between occupational exposures and those experienced by Gulf War soldiers, these results are consistent with the low risk of increased cancer predicted by The Royal Society (2001, 2002) for Gulf War exposures.
Based on the extensive literature available on U and DU toxicity, health concerns in DU-exposed veterans have focused on the chemical effects of this metal as well as its radiological toxicity. In particular, effects of DU on the kidney and other target organs known to be affected by other heavy metals have been examined. The expanded surveillance protocol developed for the Gulf War DU cohort for the 1997 visit included neuroendocrine, immunologic and reproductive parameters, in addition to renal markers and measures of genotoxicity (McDiarmid et al. 2000, 2001, 2004a,b, 2006).
4. Methods for measuring uranium body burden and systemic exposure to uranium
Quantitative exposure assessment is a critical component of surveillance programmes designed to identify health effects related to chemical exposures. In the case of DU, sensitive methods needed to be developed to accurately determine systemic exposure to DU. In addition, although the radioactivity of DU is lower than that of natural uranium, the concern that biological effects of low-level radiation could lead to health consequences established the need to quantify radiation as well as chemical exposure.
(a) Whole body radiation counting and estimated radiation doses
Whole body radioactivity measurements in the Baltimore VA Gulf War friendly fire cohort were conducted at the Boston VA using whole body counting chambers with low background radiation levels achieved by their construction with 6–12 in. thick pre-World War II battleship steel. DU gamma-ray emissions were detected using NaI(T1) scintillation crystals as described in McDiarmid et al. (2000). Two NaI(T1) detectors (one above and one below a reclining subject) were used to conduct whole body scans by counting seven consecutive body segments for 10 min each. K40 measurements were used to correct for body weight.
Despite the low background level of this whole body counting chamber, only nine of the 27 ‘DU-exposed’ Gulf War veterans in the Baltimore VA friendly fire cohort exceeded the detection limit for this technique (figure 1). All of the veterans with whole body radiation scores above background were known to have embedded fragments. None of the ‘Non-DU-exposed’ veterans seen in 1997 gave results above the detection limit. For soldiers with embedded fragments, body scan data also provided information on the distribution of DU fragments in the body and direct radiation counting of small areas known to have fragments provided a means of verifying whether the fragments were composed of DU or non-DU metal for many of the veterans.
Figure 1.
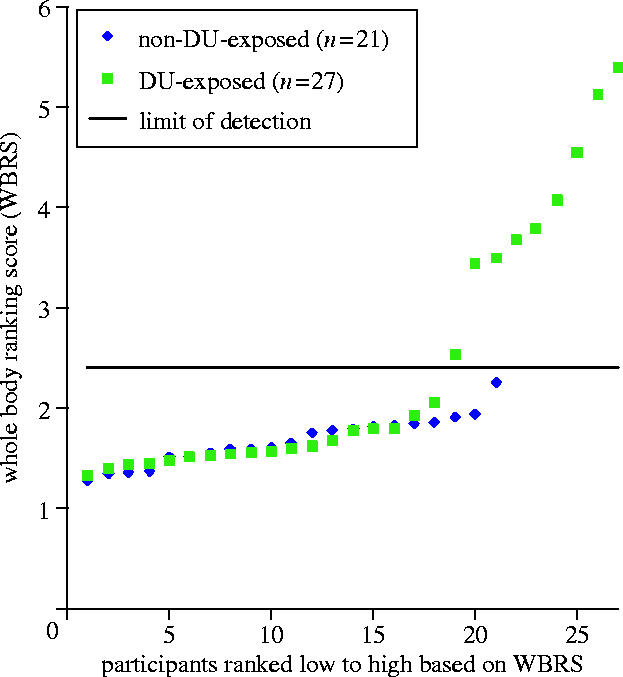
Whole body radiation counting results for ‘DU-exposed’ and ‘Non-DU-exposed’ Gulf War veterans participating in the Baltimore VA DU Follow-Up Program. Participants are ranked from low to high along the x-axis based on their whole body ranking scores (WBCS; McDiarmid et al. 2000). Measurements from only nine of the 27 veterans in the ‘DU-exposed’ cohort were above the limit of detection.
Radiation dose estimates have also been determined using urine U excretion data from veterans with shrapnel and the ICRP (1995) biokinetic model for U. Shown by Leggett & Pelmar (2003) to be applicable for estimating the time-dependent rates of release of U from embedded fragments from urinary excretion rates, the ICRP biokinetic model was used with urine U excretion data collected from veterans for up to 10 years post-exposure to predict the rate of accumulation of U in tissues from back-calculated rates of release of DU from shrapnel (Squibb et al. 2005). The maximum upper estimate of the lifetime (50 years) effective dose determined using this method was 0.06 Sv, which is slightly above the NCRP (1993) public lifetime limit of 0.05 Sv but below the occupational limit of 1 Sv. The tissue with the highest upper estimated lifetime committed effective dose was the bone surface (0.9 Sv), followed by the kidney (0.3 Sv), liver (0.1 Sv) and red marrow (0.1 Sv). These are all well below the NCRP (1993) occupational lifetime tissue committed effective dose of 25 Sv. It is important to note that these radiation dose estimates are only for DU released from embedded shrapnel and do not include radiation doses resulting from inhalation, ingestion or wound contamination by DU oxides generated during the friendly fire incidents.
(b) Chemical analysis of urine uranium excretion
Early measurements of total uranium in urine specimens using kinetic phosphorescence analysis (KPA; Brina & Miller 1992; Hedaya et al. 1997) indicated that urine excretion of U was significantly higher in the 15 Gulf War soldiers with embedded shrapnel (Hooper et al. 1999) than in those exposed to DU oxides only by inhalation, ingestion or wound contamination. This and subsequent work (McDiarmid et al. 2000, 2001, 2004a,b, 2006) confirmed that urine U excretion is a good measure for quantifying systemic exposure to U in this cohort, as it serves as an indication of the extent to which U was being released to blood from embedded fragments and/or tissue stores of previously released, inhaled or ingested DU.
To more accurately assess low-level exposures to DU and specifically quantify DU versus natural U exposure, a more sensitive analytical method than KPA was developed using an inductively coupled plasma dynamic reaction cell mass spectrometer (ICP-DRC-MS) (Ejnik et al. 2005). The detection limit for quantifying total U in urine using the ICP-DRC-MS technique is 0.1 ng l−1, an improvement in sensitivity of 200-fold compared to KPA analysis. Accurate isotopic analysis for calculating the U235/U238 ratio in urine specimens is also possible using this quadrapole ICP-DRC-MS technique for samples with U concentrations as low as 10 ng l−1 (Ejnik et al. 2005). With this capability, it is possible to calculate the amount of DU in a urine sample that also contains natural U at total U concentrations of 10 ng l−1 and above, thus providing direct evidence of DU exposure. The use of the dynamic reaction cell in this methodology provided a simple means by which to remove a polyatomic interference at m/z 234.8 present in some urine samples (Gwiazda et al. 2004), which artificially elevates the U235/U238 ratio and can falsely indicate the presence of enriched U. Other techniques developed for solving this problem involve magnetic sector high-resolution ICP-MS instruments or more extensive sample preparation to first remove the uranium from matrix constituents (Gwiazda et al. 2004). A QA/QC comparative study involving multiple laboratories using either the ICP-DRC-MS or high-resolution magnetic sector instruments (Gwiazda et al. 2004; Ejnik et al. 2005) showed excellent correlations between these techniques for total U uranium and U235/U238 ratio results at urine U concentrations as low as 10 ng l−1.
5. Uranium exposure assessment
Since whole body counting was not a sensitive enough method for quantifying DU exposure in the Gulf War friendly fire cohort, urinary excretion of U was established as a measure of systemic U exposure arising from the in situ release of DU from embedded fragments and/or tissue stores.
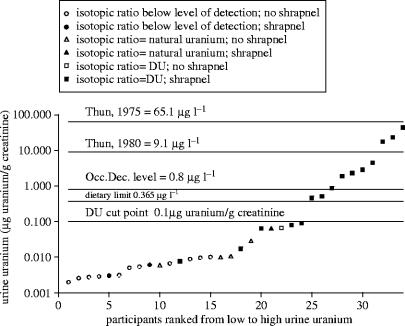
The results of 24 h total urine uranium analysis for the sub-cohort of Gulf War ‘DU-exposed’ veterans seen in the 2005 surveillance visit are shown in figure 2. Uranium concentrations for this group ranged from 0.003 to 44.1 μg g−1 creatinine. All values equal to or over the upper limit for dietary exposure to U for the general population from drinking water (0.365 μg l−1, ICRP 1974) were from participants with known retained shrapnel fragments and U isotopic analysis results indicated the presence of DU in their urine samples. Five individuals with a history of shrapnel had urine U concentrations below the 0.365 μg l−1 value, but still had detectable DU in their urine. The urine U concentration of the one veteran with no evidence of shrapnel but with measurable DU in his urine was low (less than 0.1 μg g−1 creatinine), which is consistent with a wound contamination, ingestion and/or inhalation only exposure to DU. One individual with known shrapnel injuries had no evidence of DU based on isotopic ratio results, suggesting that either his shrapnel is not composed of DU or that release of DU from the shrapnel is so low that it is not detectable. Others in this cohort have total urine U concentrations below the limit of detection for DU analysis.
Figure 2.
Uranium concentrations in 24 h urine specimens distributed from low to high for Gulf War veterans participating in the DU Follow-Up Program surveillance visit in 2005. The top two lines (65.1and 9.1 μg l−1) represent the mean total urine uranium found in a sub-cohort of uranium fabrication workers in 1975 and 1980, as reported in a study by Thun et al. (1985). The alternating dot/dash line (0.8 μg l−1) depicts an occupational exposure decision level used at the Department of Energy's Fernald Environmental Management Project (Fernald Environmental Management Project 1997; McDiarmid et al. 2000) as a trigger for investigating work areas for sources of elevated uranium exposure. The dotted line (0.365 μg l−1) is an upper limit for the dietary contribution of uranium in urine for the general population from drinking water (ICRP 1974; McDiarmid et al. 2000). This value was calculated by dividing the upper limit for 24 h uranium excretion for ‘reference man’ by 1.4 l 24h−1. Studies have shown that corrections per gram creatinine and per litre urine are generally equal for ‘reference man’ and for this group of veterans with normal renal function (Ting 1999; NHANES 2003). The bold solid line (0.01 μg g−1 creatinine) indicates the cut point established by the DU Follow-Up Program to identify low versus high urine uranium concentrations (McDiarmid et al. 2000). Designation of natural versus depleted uranium is based on isotopic ratio results.
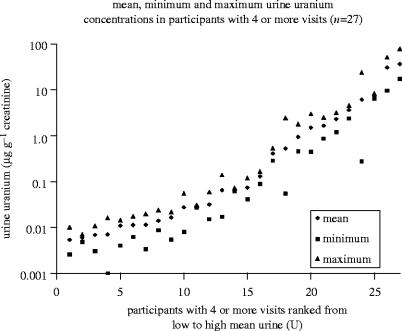
The concentration of uranium in a 24 h collection of urine, expressed as micrograms per gram of creatinine, has been used in this surveillance programme as a measure by which to establish two groups (high versus low urine U) for comparing differences in clinical parameters for their relationship to urine U excretion. ‘High’ has been defined as urine uranium concentrations greater than 0.10 μg g−1 creatinine. While there is no generally accepted standard normal urine uranium value, we chose a value intermediate between, at the low end, several estimates of mean urine uranium concentration in non-exposed populations in the literature (6–22 ng l−1, Dang et al. 1992; Medley et al. 1994; Ting et al. 1999; NHANES 2003); and at the high end, upper dietary limits due to natural uranium in soil and ground water (up to 0.365 μg l−1) (ICRP 1974). Over the past 12 years, mean urine U concentrations in the high and low-exposure groups of Gulf War veterans participating in each of the six surveillance visits have been consistent (figure 3). Urine U concentrations have not dropped significantly in the veterans with embedded DU fragments, demonstrating that these individuals are experiencing chronic U exposure due to release of U from their fragments.
Figure 3.
Mean, maximum and minimum urine uranium concentrations for 27 Gulf War veterans with at least four urine samples submitted between 1993 and 2005. Results for individual veterans are distributed from low to high based on their mean urine U concentration. Urine uranium excretion has remained elevated over the 14 years since the Gulf War for most veterans with above normal urine uranium concentrations (greater than 0.10 μg g−1 creatinine).
6. DU health effects: surveillance results
(a) Clinical assessment
The clinical assessments performed in veterans during their biennial visits to the Baltimore Veterans Affairs Medical Centre (BVAMC) have included a detailed medical history, including an extensive exposure history, a thorough physical examination, laboratory studies and radiologic surveys for retained DU fragments. The laboratory studies included haematologic and blood clinical chemistry measures, as well as neuroendocrine, immunologic and genotoxicity parameters. Semen quality was also evaluated. Urine samples were obtained for measurement of total uranium excretion and clinical chemistry parameters related to renal function. Participants also underwent a battery of neurocognitive tests and all participants were clinically evaluated during their first visit for post-traumatic stress disorder.
(b) Clinical findings
As we have reported over these past years of follow-up (Hooper et al. 1999; McDiarmid et al. 2000, 2001, 2004a,b, 2006), we have yet to identify any consistent, clinically significant differences in any laboratory parameters between the low and high-exposed uranium groups. The benefit of longitudinal follow-up is seen in table 3, where multi-year surveillance results for clinical parameters are summarized. None of the inevitable, chance-related statistical findings observed in a single year have been sustained over serial assessments. The overview provided in table 3 also allows a quick comparison of trends observed, by indicating the direction of an excursion, i.e. greater than or less than, between the two groups of veterans stratified by their urine U concentration. Specific results for each outcome for each of the past surveillance visits can be found in the references listed above which provide detailed reports of each visit.
Table 3.
Summary of significant observations in clinical results for five surveillance visits from 1994 to 2003 (L, Low urine uranium group (U<0.1 μg g−1 creatinine); H, High urine uranium group (U≥0.1 μg g−1 creatinine); ns, no significant differences between groups).
| clinical parameter | evaluation year | ||||
|---|---|---|---|---|---|
| 1994 | 1997 | 1999 | 2001 | 2003 | |
| renal function | |||||
| urine creatinine | ns | ns | 1>ha (p=0.07) | ns | ns |
| urine calcium | ns | ns | |||
| urine PO4 | ns | ns | |||
| urine β-2 microglobulin | ns | ns | ns | ns | ns |
| urine intestinal alkaline phosphatase (IAP) | ns | ns | ns | ||
| urine N-acetyl-ß-glucosaminidase (NAG) | ns | ns | ns | ||
| urine total protein | ns | ns | ns | h>1 (p=0.06) | 1>h (p=0.21) |
| urine microalbumin | ns | ||||
| retinol-binding protein (RBP) | ns | ns | h>1 (p=0.06) | 1>h (p=ns) | |
| serum creatinine | ns | ns | ns | L>Hb | ns |
| serum calcium | ns | ns | H>L | ns | ns |
| serum PO4 | ns | ns | ns | ns | H>L |
| serum uric acid | ns | ns | ns | ns | ns |
| other serum values | |||||
| GPT | ns | ns | ns | ns | ns |
| GOT | ns | ns | ns | ns | 1>h (p=0.1) |
| CPK | ns | ns | |||
| LDH | ns | ns | L>H | L>H | ns |
| alkaline phosphate | ns | ns | ns | ns | ns |
| semen characteristics | |||||
| days abstinence (2–5) | ns | ns | ns | ns | |
| semen volume (2–5 ml) | ns | ns | ns | ns | |
| sperm concentration (greater than 20 million ml−1) | H>L | h>1 p=0.09 | h>l ns | h>1 ns | |
| total sperm count (greater than 40 million) | ns | H>L | h>1 p=0.06 | h>1 ns | |
| percent motile sperm (greater than 50%) | ns | ns | ns | ns | |
| total progressive sperm (greater than 20 million) | ns | H>L | ns | ns | |
| per cent progressive sperm (greater than 50%) | ns | ns | ns | ns | |
| total rapid progressive sperm (greater than 10 million) | ns | H>L | ns | ns | |
| per cent rapid progressive sperm (greater than 25%) | ns | ns | ns | ns | |
| neuroendocrine hormones | |||||
| FSH | ns | ns | ns | ns | |
| LH | ns | ns | ns | ns | |
| prolactin | ns | ns | l>h p=0.06 | ns | |
| testosterone | ns | ns | ns | ns | |
| TSH | ns | ns | ns | ||
| free thyroxine | ns | L>H | ns | ||
| haematologic function | |||||
| white blood cells | H>L | ns | ns | ns | ns |
| haematocrit | ns | ns | ns | L>H | ns |
| haemoglobin | ns | ns | ns | L>H | ns |
| platelets | ns | ns | ns | ns | ns |
| lymphocytes | L>H | ns | L>H | ns | ns |
| neutrophils | H>L | ns | H>L | ns | ns |
| basophils | ns | ns | ns | ns | ns |
| eosinophils | ns | H>L | ns | ns | ns |
| monocytes | L>H | ns | L>H | ns | ns |
lower case letters indicate non-significant findings.
upper case letters indicate significant findings (p<0.05).
(c) Renal function parameters
Overall, test results measuring renal function have shown no statistically significant (p<0.05) differences between the low and high urine U groups, with an occasional exception, i.e. serum phosphate concentration in the 2003 surveillance visit was higher in the high U group, but was not outside the normal range. Urine excretion of phosphate was not different, however, suggesting that the elevated serum level was not a result of impaired renal function.
The mean retinol-binding protein (RBP) urine concentration has been higher in the high U group in the two most recent evaluations, though the difference between the groups has not reached statistical significance at the p<0.05 level. This observation of a higher excretion of this low molecular weight protein is biologically plausible, as U can exert an inhibitory effect on protein reabsorption by renal proximal tubule cells. We are following this RBP observation carefully, as a potential ‘sentinel’ marker of proximal tubular effects from U (McDiarmid et al. 2006; Squibb et al. 2005).
(d) Haematologic parameters
These parameters have been the most variable among the clinical outcome measures we have followed. This is understandable due to the large number of factors that can affect these parameters, the large number of observations made and the non-independent nature of the differential white blood cell count, where, if one subclass is elevated, another is inevitably depressed, by definition. Nonetheless, most measures of each blood element over time have been within normal limits, regardless of the urine U excretion levels. As well, there have been no consistent differences observed in any parameter between the high versus low urine U groups.
(e) Blood chemistries
Typical blood chemistry and electrolyte panels have also been included in the surveillance protocol. Again, we have seen few abnormalities associated with the high U group and no consistent trends over time. Lactate dehydrogenase (LDH) mean serum concentrations were decreased in the high U group in the 1999 and 2001 visits, but were not different between the low and high U groups in 2003. A difference in a liver function result, serum glutamic–oxaloacetic transaminase (SGOT), was observed in 2003, with a p value of 0.1 (table 3).
(f) Neuroendocrine function
With one exception, no statistically significant differences between U groups have been observed in mean prolactin, FSH, LH, testosterone, free thyroxine or TSH values. The single exception was a significantly lower mean free thyroxine seen in the high urine U group in the 2001 visit, however this was not observed in 2003. A lower prolactin level in the high U group in 2001 approached significance, but also was not observed in the 2003 visit. There have been occasional individual excursions in laboratory results outside the normal range, but these observations have not been consistent, and upon follow-up have not been driven by the veteran's U exposure measured by urine U excretion.
(g) Semen characteristics
Semen quality, as classified by WHO 1987 norms, has not been observed to be significantly different between U groups over the course of this longitudinal surveillance programme. If anything, semen quality in the high uranium group appears better than that in the low U group based on sperm concentrations and total sperm counts in the ejaculate.
(h) Genotoxicity
We have employed several measures of genotoxicity over the course of this surveillance to examine evidence for effects from long-term exposure to U as a potential genotoxic compound via either a chemically or radiologically mediated mechanism. Sister chromatid exchange measurements in lymphocytes were included to measure point mutations. Chromosomal aberrations were counted as a measure of clastogenic insult and, more recently, the hypoxanthine–guanine phosphoribosyl transferase (HPRT) mutation assay, the most widely used measure of somatic gene mutations in humans (Cole & Skopek 1994; Robinson et al. 1994; Albertini & Hayes 1997; Albertini 2001), was added to our test battery.
While there is good biologic plausibility for U to be genotoxic, based on animal data (Hueper et al. 1952; Leach et al. 1973; Cross et al. 1981; Mitchell et al. 1999; Hahn et al. 2002), some epidemiologic data in exposed workers (Martin et al. 1991) and, more recently, the evidence reported from in vitro studies (Lin et al. 1993; Miller et al. 1998b, 2002a,b, 2003; Yazzie et al. 2003; Stearns et al. 2005), the results of our studies are mixed. The HPRT frequency results, when analysed using the U low/high stratified grouping, are not significantly different, although mean mutation frequency (MF) was higher in the high U group in both 2001 and 2003 (McDiarmid et al. 2004a,b, 2006). There appears to be an inflection point in the mean MF distribution when MF data are plotted against the natural log of urine U concentrations. This inflection is driven by a small number of veterans with higher urine U concentrations, suggesting that there may be an exposure threshold for the increase in MF. We have not, however, observed consistent changes in sister chromatid exchange (SCE) or chromosomal aberrations (CA) (table 4). A more descriptive level of analysis of the types of mutations observed in the HPRT assay are underway to assist in interpreting our findings. In the 2005 assessment, we added fluorescent in situ hybridization (FISH) assessment of peripheral blood lymphocyte DNA to examine specific chromosomal ‘hot spots’ for genotoxic injury resulting from U exposure. These results will be helpful in assessing the in vivo genotoxicity of DU in humans.
Table 4.
Summary of genotoxicity parameters (L, Low urine uranium group (U<0.1 μg g−1 creatinine); H, High urine uranium group (U≥0.1 μg g−1 creatinine); ns, no significant differences between groups).
| genotoxicity parameter | evaluation year | ||||
|---|---|---|---|---|---|
| 1994 | 1997 | 1999 | 2001 | 2003 | |
| sister chromatid exchange (SCE) | 1>ha ns | H>L | 1>h ns | ns | |
| chromosomal aberrations (CA) | ns | ns | H>L | ns | |
| hypoxanthine–guanine phosphoribosyl transferase (HPRT) mutation frequency | h>1 (p=0.1) | h>1 ns | |||
lower case letters indicate non-significant findings.
(i) Neurocognitive parameters
Since surveillance began on this cohort, responses assessed by neurocognitive testing have generally been within normal ranges, regardless of U exposure stratification. Additionally, there has been a consistent lack of any statistically significant difference between outcomes in the high/low U exposure groups. We have remained attentive to trends that have not reached statistical significance and have observed a difference in accuracy scores derived from a computerized test battery (lower scores indicative of decreased accuracy in the high U group) over time. This observed difference, however, is being driven by only two veterans with severely complex co-morbid conditions, making attribution to U doubtful (McDiarmid et al. 2004a,b, 2006). However, we again benefit from the longitudinal nature of this surveillance activity and will gain insight from repeated assessments.
7. Summary
The Baltimore VA DU Follow-up Program continues to conduct health surveillance in a cohort of Gulf War soldiers exposed to DU when they were in or on vehicles hit by friendly fire involving munitions with DU penetrators. Urine U concentrations remain elevated in veterans with embedded DU fragments, demonstrating a chronic systemic exposure to U in this group of soldiers. Not all soldiers in the ‘DU-exposed’ Gulf War friendly fire cohort have embedded DU fragments; thus potential health effects from DU exposure by inhalation, ingestion and wound contamination alone are also being monitored through this programme. With the exception of the elevated urine U excretion, no clinically significant, expected U-related health effects have yet been identified in veterans with or without embedded fragments, though subtle changes in renal function and genotoxicity markers in soldiers with urine U concentrations greater than 0.1 μg−1 creatinine have been observed.
Footnotes
One contribution of 17 to a Theme Issue ‘The health of Gulf War veterans’.
References
- Albertini R.J. HPRT mutation in humans: biomarkers for mechanistic studies. Mutat. Res. 2001;489:1–16. doi: 10.1016/s1383-5742(01)00064-3. doi:10.1016/S1383-5742(01)00064-3 [DOI] [PubMed] [Google Scholar]
- Albertini R.J, Hayes R.B. Somatic mutations in cancer epidemiology. In: Toniolo P, Boffetta P, Shuker D, Rothman N, Hulka B, Pearce N, editors. IARC Scientific Publication No. 142. International Agency for Research on Cancer (IARC); Lyon, France: 1997. pp. 159–184. [PubMed] [Google Scholar]
- Army Environmental Policy Institute (AEPI). 1995 Health and environmental consequences of depleted uranium use in the US Army Technical Report, Atlanta, GA: AEPI.
- ATSDR (Agency for Toxic Substances and Disease Registry) US Department of Health and Human Services; Washington, DC: 1999. Toxicological profile for uranium. [Google Scholar]
- Brina R, Miller A.G. Direct detection of trace levels of uranium by laser-induced phosphorimetry. Anal. Chem. 1992;64:1413–1418. [Google Scholar]
- Cole J, Skopek T.R. International commission for protection against environmental mutagens and carcinogens Working paper 3: somatic mutant frequency, mutation rates and mutational spectra in the human population in vivo. Mutat. Res. 1994;304:33–106. doi: 10.1016/0027-5107(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Cross F.T, Palmer R.F, Busch R.H, Filipy R.E, Stuart B.O. Development of lesions in Syrian Golden hamsters following exposure to radon daughters and uranium ore dust. Health Phys. 1981;41:135–153. doi: 10.1097/00004032-198107000-00012. [DOI] [PubMed] [Google Scholar]
- Dang H.S, Pullat V.R, Pillai K.C. Determining the normal concentration of uranium in urine and application of the data to its biokinetics. Health Phys. 1992;62:562–566. doi: 10.1097/00004032-199206000-00010. [DOI] [PubMed] [Google Scholar]
- Ejnik J.W, Todorov T.I, Mullick F.G, Squibb K, McDiarmid M.A, Centeno J.A. Uranium analysis in urine by inductively coupled plasma dynamic reaction cell mass spectrometry. Anal. Bioanal. Chem. 2005;382:73–79. doi: 10.1007/s00216-005-3173-9. doi:10.1007/s00216-005-3173-9 [DOI] [PubMed] [Google Scholar]
- Fernald Environmental Management Project (FEMP). 1997 Technical basis for internal dosimetry at the Fernald Environmental Management Project Revision 3 dated December 23, 1997. Fernald, Ohio: FEMP Publication SD 2008.
- Gilman A.P, Villeneuv D.C, Secours V.E, Yagminas A.P, Tracy B.L, Quinn J.M, Valli V.E, Willes R.J, Moss M.A. Uranyl nitrate: 28-day and 91-day toxicity studies in the Sprague–Dawley rat. Toxicol. Sci. 1998;41:117–128. doi: 10.1006/toxs.1997.2367. doi:10.1006/toxs.1997.2367 [DOI] [PubMed] [Google Scholar]
- Gilman A.P, Villeneuv D.C, Secours V.E, Yagminas A.P, Tracy B.L, Quinn J.M, Valli V.E, Moss M.A. Uranyl nitrate: 28-day and 91-day toxicity studies in the New Zealand white rabbit. Toxicol. Sci. 1998;41:129–137. doi: 10.1006/toxs.1997.2368. doi:10.1006/toxs.1997.2368 [DOI] [PubMed] [Google Scholar]
- Gilman A.P, Villeneuv D.C, Secours V.E, Yagminas A.P, Tracy B.L, Quinn J.M, Valli V.E, Moss M.A. Uranyl nitrate: 91-day exposure and recovery studies in the male New Zealand white rabbit. Toxicol. Sci. 1998c;41:138–151. doi: 10.1006/toxs.1997.2369. doi:10.1006/toxs.1997.2369 [DOI] [PubMed] [Google Scholar]
- Gwiazda R.H, Squibb K, McDiarmid M, Smith D. Detection of depleted uranium in urine of veterans from the 1991 Gulf War. Health Phys. 2004;86:12–18. doi: 10.1097/00004032-200401000-00004. doi:10.1097/00004032-200401000-00004 [DOI] [PubMed] [Google Scholar]
- Hahn F.F, Guilmette R.A, Hoover M.D. Implanted depleted uranium fragments cause soft tissue sarcomas in the muscles of rats. Environ. Health Perspect. 2002;110:51–59. doi: 10.1289/ehp.0211051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedaya M.S, Birkenfeld H.P, Kathren R.L. A sensitive method for the determination of uranium in biological samples utilizing kinetic phosphorescence analysis. J. Pharm. Biomed. Anal. 1997;15:1157–1165. doi: 10.1016/s0731-7085(96)01957-7. doi:10.1016/S0731-7085(96)01957-7 [DOI] [PubMed] [Google Scholar]
- Hodge H.C, Stannard N, Hursh J.B, editors. Uranium, plutonium, transplutonic elements. Handbook of experimental pharmacology. vol. 36. Springer; New York, NY: 1973. [Google Scholar]
- Hooper F.J, Squibb K.S, Siegel E.L, McPhaul K, Keogh J.P. Elevated urine uranium excretion by soldiers with retained uranium shrapnel. Health Phys. 1999;77:512–519. doi: 10.1097/00004032-199911000-00004. [DOI] [PubMed] [Google Scholar]
- Hueper W.C, Zuefle J.H, Link A.M, Johnson M.G. Experimental studies in metal cancerigenesis II. Experimental uranium cancers in rats. J. Natl Cancer Inst. 1952;13:291–305. [PubMed] [Google Scholar]
- ICRP (International Commission on Radiological Protection) Report of the task groups on reference man. vol. 23. Pergamon Press; Elmsford, NY: 1974. [Google Scholar]
- ICRP (International Commission on Radiological Protection) Ingestion dose coefficients. Pergamon Press; Oxford, UK: 1995. Age-dependent doses to members of the public from intake of radionuclides, Part 3. ICRP Publication 69. [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Gulf War and health. In: Fulco C.E, Liverman C.T, Sox H.C, editors. Depleted uranium, pyridostigmine bromide, sarin, vaccines. vol. 1. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- Kathren R.L, Moore R.H. Acute accidental inhalation of U: a 38-year follow-up. Health Phys. 1986;51:609–619. doi: 10.1097/00004032-198611000-00004. [DOI] [PubMed] [Google Scholar]
- Kathren R.L, McInroy J.F, Moore R.H, Dietert S.E. Uranium in the tissues of an occupationally exposed individual. Health Phys. 1989;57:17–21. doi: 10.1097/00004032-198907000-00002. [DOI] [PubMed] [Google Scholar]
- Kurttio P, Auvinen A, Salonen L, Saha H, Pekkannen J, Makelainen I, Vaisanen S.B, Penttila I.M, Komulainen H. Renal effects of uranium in drinking water. Environ. Health Perspect. 2002;110:337–342. doi: 10.1289/ehp.02110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach L.J, Yuile C.L, Hodge G.C, Sylvester G.E, Wilson H.B. A five-year inhalation study with natural uranium dioxide (UO2) Dust-II. Postexposure retention and biologic effect in the monkey, dog and rat. Health Phys. 1973;25:239–258. doi: 10.1097/00004032-197309000-00003. [DOI] [PubMed] [Google Scholar]
- Leggett R.W. The behavior and chemical toxicity of U in the kidney: a reassessment. Health Phys. 1989;57:365–383. doi: 10.1097/00004032-198909000-00001. [DOI] [PubMed] [Google Scholar]
- Leggett R.W, Pellmar T.C. The biokinetics of uranium migrating from embedded DU fragments. J. Environ. Radioactivity. 2003;64:205–225. doi: 10.1016/s0265-931x(02)00050-4. doi:10.1016/S0265-931X(02)00050-4 [DOI] [PubMed] [Google Scholar]
- Lin R.H, Wu L.J, Lee C.H, Lin-Shiau S.Y. Cytogenetic toxicity of uranyl nitrate in Chinese hamster ovary cells. Mutat. Res. 1993;319:197–203. doi: 10.1016/0165-1218(93)90079-s. doi:10.1016/0165-1218(93)90079-S [DOI] [PubMed] [Google Scholar]
- Martin F, Earl R, Tawn E.J. A cytogenetic study of men occupationally exposed to uranium. Br. J. Ind. Med. 1991;48:98–102. doi: 10.1136/oem.48.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDiarmid M.A, et al. Health effects of depleted uranium on exposed Gulf War veterans. Environ. Res. 2000;82:168–180. doi: 10.1006/enrs.1999.4012. doi:10.1006/enrs.1999.4012 [DOI] [PubMed] [Google Scholar]
- McDiarmid M.A, et al. Surveillance of depleted uranium exposed Gulf War veterans: health effects observed in an enlarged ‘friendly fire’ cohort. J. Occup. Environ. Med. 2001;43:991–1000. doi: 10.1097/00043764-200112000-00001. [DOI] [PubMed] [Google Scholar]
- McDiarmid M.A, et al. Health effects of depleted uranium on exposed Gulf War veterans: a ten-year follow-up. J. Toxicol. Environ. Health. A. 2004a;67:277–296. doi: 10.1080/15287390490273541. [DOI] [PubMed] [Google Scholar]
- McDiarmid M.A, Squibb K, Engelhardt S.M. Biologic monitoring for urinary uranium in Gulf War I veterans. Health Phys. 2004b;87:51–56. doi: 10.1097/00004032-200407000-00006. doi:10.1097/00004032-200407000-00006 [DOI] [PubMed] [Google Scholar]
- McDiarmid M.A, et al. Biological monitoring and surveillance results of Gulf War I veterans exposed to depleted uranium. Int. Arch. Occup. Environ. Health. 2006;79:11–21. doi: 10.1007/s00420-005-0006-2. [DOI] [PubMed] [Google Scholar]
- Medley D.W, Kathren R.L, Miller A.G. Diurnal urinary volume and uranium output in uranium workers and unexposed controls. Health Phys. 1994;67:122–130. doi: 10.1097/00004032-199408000-00002. [DOI] [PubMed] [Google Scholar]
- Miller A.C, Fuciarelli A.F, Jackson W.E, Ejnik J, Edmond C, Strocko S, Hogan J, Page N, Pellmar T. Urinary and serum mutagenicity studies with rats implanted with depleted uranium or tantalum pellets. Mutagenesis. 1998a;13:643–648. doi: 10.1093/mutage/13.6.643. [DOI] [PubMed] [Google Scholar]
- Miller A.C, et al. Transformation of human osteoblast cells to the tumorigenic phenotype by depleted uranium–uranyl chloride. Environ. Health Perspect. 1998b;106:465–471. doi: 10.1289/ehp.98106465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.C, Stewart M, Brooks K, Shi L, Page N. Depleted uranium-catalyzed oxidative DNA damage: absence of significant alpha particle decay. J. Inorg. Biochem. 2002;91:246–252. doi: 10.1016/s0162-0134(02)00391-4. doi:10.1016/S0162-0134(02)00391-4 [DOI] [PubMed] [Google Scholar]
- Miller A.C, Xu J, Stewart M, Brooks K, Hodge S, Shi L, Page N, McClain D. Observation of radiation-specific damage in human cells exposed to depleted uranium: dicentric frequency and neoplastic transformation as endpoints. Radiat. Prot. Dosimetry. 2002b;99:275–278. doi: 10.1093/oxfordjournals.rpd.a006783. [DOI] [PubMed] [Google Scholar]
- Miller A.C, Brooks K, Stewart M, Anderson B, Shi L, McLain D, Page L. Genomic instability in human osteoblast cells after exposure to depleted uranium: delayed lethality and micronuclei formation. J. Environ. Radiol. 2003;64:247–259. doi: 10.1016/s0265-931x(02)00053-x. doi:10.1016/S0265-931X(02)00053-X [DOI] [PubMed] [Google Scholar]
- Mitchell R.E.J, Jackson J.S, Heinmiller B. Inhaled uranium ore dust and lung cancer risk in rats. Health Phys. 1999;76:145–155. doi: 10.1097/00004032-199902000-00006. [DOI] [PubMed] [Google Scholar]
- NCRP (National Council on Radiation Protection and Measurements) National council on radiation protection and measurements; Bethesda, MD: 1993. Limitation of exposure to ionizing radiation. Report no. 116. [Google Scholar]
- NHANES (National Health and Nutrition Examination Survey) Centers for Disease Control and Prevention; Atlanta, GA: 2003. Second national report on human exposure to environmental chemicals. NCEH Publication No. 02-0716. [Google Scholar]
- OSAGWI (Office of the Special Assistant for Gulf War Illness). 2000 Environmental exposure report—Depleted uranium in the Gulf (II), Interim Report, Washington, DC: US Department of Defense. http://www.deploymentlink.osd.mil/du_library/du_ii/
- Parkhurst M.A, Daxon E.G, Lodde G.M, Szrom F, Guilmette R.A, Roszell L.E, Falo G.A, McKee C.B. Summary of U.S. assessments. Battelle Press; Columbus, OH: 2005. Depleted uranium aerosol doses and risks. [Google Scholar]
- Robinson D, et al. An analysis of in vivo HPRT mutant frequency in circulating T lymphocytes in the normal human population: a comparison of four databases. Mutat. Res. 1994;313:227–247. doi: 10.1016/0165-1161(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Saccomanno G. The contribution of uranium miners to lung cancer histogenesis. In: Band P.R, editor. Early detection and localization of lung tumors in high risk groups. Springer; New York, NY: 1982. pp. 43–52. [DOI] [PubMed] [Google Scholar]
- Samet J.M. Radon and lung cancer. J. Natl Cancer Inst. 1989;8:745–757. doi: 10.1093/jnci/81.10.745. [DOI] [PubMed] [Google Scholar]
- Samet J.M, Pathak D.R, Morgan M.V, Marbury M.C, Key C.R, Valdivia A.A. Radon progeny exposure and lung cancer risk in New Mexico U miners: a case-control study. Health Phys. 1989;56:415–421. doi: 10.1097/00004032-198904000-00002. [DOI] [PubMed] [Google Scholar]
- Squibb K.S, et al. Prediction of renal concentrations of depleted uranium and radiation dose in Gulf War veterans with embedded shrapnel. Health Phys. 2005;89:267–273. doi: 10.1097/01.hp.0000165451.80061.7e. doi:10.1097/01.HP.0000165451.80061.7e [DOI] [PubMed] [Google Scholar]
- Stearns D, Yazzie M, Bradley A.S, Coryell V.H, Shelley T, Ashby A, Asplund C.S, Lantz R.C. Uranyl acetate induces hprt mutations and uranium—DNA adducts in Chinese hamster ovary EM9 cells. Mutagenesis. 2005;20:417–423. doi: 10.1093/mutage/gei056. [DOI] [PubMed] [Google Scholar]
- Tannenbaum A, editor. Toxicology of uranium. McGraw-Hill, Inc.; New York, NY: 1951. [Google Scholar]
- The Royal Society . Royal Society; London, UK: 2001. The health hazards of depleted uranium munitions part I. Policy document 6/01. Report available at: www.royalsociety.ac.uk. [Google Scholar]
- The Royal Society . Royal Society; London, UK: 2002. The health effects of depleted uranium munitions. Summary. Policy document 6/02. Report available at: www.royalsociety.ac.uk. [Google Scholar]
- Thun M.J, Baker D.B, Steenland K, Smith A.B, Halperin W, Berl T. Renal toxicity in uranium mill workers. Scand. J. Work Environ. Health. 1985;11:83–90. doi: 10.5271/sjweh.2249. [DOI] [PubMed] [Google Scholar]
- Ting B.G, Pashal D.C, Jarrett J.M, Pirkly J.L, Jackson R.J, Sampson E.J, Miller D.T, Caudill S.P. Uranium and thorium in urine of United States residents: reference range concentrations. Environ. Res. 1999;81:45–51. doi: 10.1006/enrs.1998.3951. doi:10.1006/enrs.1998.3951 [DOI] [PubMed] [Google Scholar]
- Toohey R.E. Excretion of depleted uranium by Gulf War veteran. Radiat. Prot. Dosimetry. 2003;105:171–174. doi: 10.1093/oxfordjournals.rpd.a006217. [DOI] [PubMed] [Google Scholar]
- Voegtlin I.C, Hodge H.C, editors. Pharmacology and toxicology of uranium compounds. National nuclear energy series (VI) McGraw-Hill; New York, NY: 1949. [Google Scholar]
- Voegtlin C, Hodge H.C, editors. Pharmacology and toxicology of uranium compounds. McGraw–Hill; New York, NY: 1953. [Google Scholar]
- Yazzie M, Gamble S.L, Civitello E.R, Stearns D.M. Uranyl acetate causes DNA single strand breaks in vitro in the presence of ascorbate (vitamin C) Chem. Res. Toxicol. 2003;16:524–530. doi: 10.1021/tx025685q. doi:10.1021/tx025685q [DOI] [PubMed] [Google Scholar]
- Zamora M.L, Tracy B.L, Zielinski J.M, Meyerhof D.P, Moss M.A. Chronic ingestion of uranium in drinking water: a study of kidney bioeffects in humans. Toxicol. Sci. 1998;43:68–77. doi: 10.1006/toxs.1998.2426. doi:10.1006/toxs.1998.2426 [DOI] [PubMed] [Google Scholar]
- Zamora M.L, Zielinski J.M, Meyerhof D.P, Tracy B.L. Gastrointestinal absorption of uranium in humans. Health Phys. 2002;83:35–45. doi: 10.1097/00004032-200207000-00004. doi:10.1097/00004032-200207000-00004 [DOI] [PubMed] [Google Scholar]