Abstract
A randomised, double-blind, parallel, placebo-controlled, 12-week study was carried out to evaluate the efficacy and safety of 20-mg tadalafil taken ‘as needed’ in a population of men with erectile dysfunction (ED) from Egypt and Turkey. One hundred and thirty-two patients were randomised in this study. Tadalafil was superior to placebo on all three co-primary efficacy end points. The mean change from baseline for the erectile function domain of the International Index of Erectile Function was 9.3 ± 0.8 for the tadalafil group and 2.3 ± 1.6 for the placebo group. Tadalafil-treated patients reported a significantly greater improvement in the mean percentage of successful penetrations (tadalafil: 34.5 ± 4.1; placebo: −4.6 ± 8.1) and successful intercourse attempts (tadalafil: 52.2 ± 3.8; placebo: 16.8 ± 7.8) than placebo-treated patients as measured by the Sexual Encounter Profile. Tadalafil was generally well tolerated with 82% of adverse events being mild in severity. Tadalafil 20-mg taken ‘as needed’ significantly improved the erectile function in Egyptian and Turkish men with ED.
Keywords: Tadalafil, erectile dysfunction, randomised controlled trial
Introduction
The effects of erectile dysfunction (ED), defined as the consistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance (1) can be devastating and include decreased overall quality of life (2,3).
Erectile dysfunction is a condition that will affect one in ten men over the age of 16 years at some time in their lives and becomes more prevalent as men get older (4). The Massachusetts Male Aging study found that 52% of men between the ages of 40 and 70 had some degree of ED with the incidence rate of complete impotence tripling from 5% to 15% between men in the same age group (5). Similar findings were observed in studies conducted in South Australia, Finland, France and England (6). Contributing factors that may cause ED include vascular insufficiency, interruption of neural pathways (7), diabetes mellitus, hormonal derangement, psychogenic factors and side effects of concomitant pharmacotherapy.
Treatment of ED aims to gain a sustained improvement in erectile function. Several pharmacotherapies have been used to achieve this goal. The results with injectable treatments were promising for many patients but because of their invasive nature most men prefer less invasive agents (8). Thus, the current climate in sexual medicine has revealed an increased readiness to expand the indications of oral vasoactive pharmacotherapy for the treatment of ED (9). Out of these, it was the selective phosphodiesterase (PDE) inhibitors that gained widespread interest for treating ED (10). Eleven families of PDE isoenzymes are well recognised and of these PDE type 5 (PDE5) was found to be the isoenzyme with the greatest pharmacological relevance for erectile function.(11). From this aspect PDE5 inhibitors started to gain high demand over the invasive means in ED treatment as they stimulate natural erectile responses by activation of biochemical mechanisms, producing the physiological relaxation of erectile tissue (12). Tadalafil (Cialis®; Lilly ICOS LLC Bothell, WA, USA) is one of the most recent PDE5 inhibitors introduced into the market. It differs from other PDE5 inhibitors in the fact that it has an extended period of responsiveness (up to 36 h) due to a plasma half-life of 17.5 h and the lack of pharmacokinetic interaction with food or beverage (10).
Tadalafil has been approved for the treatment of ED in Egypt and Turkey as well as in the USA, European countries, and in other countries around the world. This study was undertaken to evaluate the efficacy and safety of 20-mg tadalafil taken ‘as needed’ in improving erectile function over 12 weeks, in a population of men with ED from Egypt and Turkey.
Materials and Methods
Study Design
This randomised, double-blind, parallel, placebo-controlled study was conducted at seven sites in two countries. The ethical review board for each investigative site approved the protocol and informed consent document. The study was conducted in accordance with the protocol and ethical principles stated in the Declaration of Helsinki 2002 and applicable local laws of each country.
The study objectives were to evaluate the efficacy and safety of ‘as needed’ dosing of 20 mg tadalafil administered for 12 weeks to men with ED. ‘As needed’ dosing in this study refers to the administration of tadalafil prior to the potential for sexual activity, but at a maximum frequency of one dose per day. Thirty doses, to be available ‘as needed’, were dispensed to patients at visits.
The study consisted of two periods. The first period was the screening period of approximately 4 weeks’ duration starting from time when the eligible patients signed the informed consent document till the end of the fourth week at visit 2. Screened patients were stratified at randomisation by the International Index of Erectile Function (IIEF) Erectile Function domain scores in the following category: mild ED = a score higher or equal to 17, moderate ED = 11–16, and severe ED = 1–10. Patients were randomly assigned to treatment groups in a 1:3 (placebo:20 mg tadalafil) fashion. This was followed by a treatment period that lasted approximately 12 weeks.
The International Index of Erectile Function and the Sexual Encounter Profile (SEP) diary were administered through the treatment period. Global Assessment Questions (GAQ) were asked at the end of treatment period or at the early discontinuation visit.
Patient Population
Study participants were male patients at least 18 years of age who anticipated having the same female sexual partner during the study and had symptoms of ED (defined as the consistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance) with any kind of severity and any aetiological reasons for at least 3 months. Major exclusion criteria were: ED caused by premature ejaculation or untreated endocrine disease; failure to achieve erection after pelvic surgery including radical prostatectomy (except bilateral nerve-sparing) or patients receiving nitrates, anti-androgens or chemotherapy; significant penile deformity or penile implant; clinically significant renal or hepatic insufficiency; poorly controlled diabetes (haemoglobin A1c >13%); a recent history of stroke or spinal cord trauma; unstable cardiovascular diseases (e.g. unstable angina, recent myocardial infarction or coronary intervention, history of sudden cardiac arrest despite medical or device therapy, evidence of congestive heart failure, new significant conduction defect or uncontrolled hypertension). Patients with prior ineffective treatment with sildenafil citrate, in the opinion of the investigator, could have been excluded from the study. Concomitant use of other therapies for ED was not allowed during the study. Although the study was originally planned to be performed in three countries with 189 patients, 139 patients from Egypt and Turkey participated in the study. The study remained sufficiently (>90%) powered for all co-primary end point analyses.
Efficacy Measures
The first co-primary efficacy measure was the IIEF Erectile Function domain, defined as the sum of questions 1–5 and 15 of the IIEF. The IIEF was administered at the end of the treatment-free run-in period, and at the end of 4, 8 and 12 weeks of treatment. Further co-primary efficacy measures were the mean per patient percent of ‘yes’ responses to SEP question 2 ]SEP2: Were you able to insert your penis into your partner's vagina? (yes/no)[ and SEP3 ]Did your erection last long enough for you to have successful intercourse? (yes/no)[.
Secondary efficacy measures of the study include: IIEF Intercourse Satisfaction and Overall Satisfaction Domain scores, IIEF questions 3 and 4, mean per patient percent of ‘yes’ responses to SEP4 (Were you satisfied with the hardness of your erection?) and SEP5 (Were you satisfied overall with this sexual experience?) and responses to two GAQ questions (GAQ1-Has the treatment you have been taking during this study improved your erections? GAQ2-If yes, has the treatment improved your ability to engage in sexual activity?).
Safety
Safety was assessed by evaluating the incidence of treatment-emergent adverse events and any changes in laboratory results or vital signs. At the screening and last study visit a full physical examination and clinical laboratory tests were performed. A 12-lead ECG was also performed at the first visit. Study personnel recorded concomitant medication use, blood pressure and pulse at every visit. The date of onset or resolution of any adverse event was recorded at each study visit. Treatment-emergent adverse events (events that first occurred or worsened after baseline) were summarised by the Medical Dictionary for Regulatory Activities (MedDRA) version 5.0, and severity and possible relationship to study drug were noted.
Statistical Analysis
The co-primary efficacy variables were mean change from baseline to end point in the IIEF EF domain score and mean changes from baseline to the post-baseline period in the mean per patient percent of ‘yes’ responses to SEP2 and SEP3.
The null hypothesis was that tadalafil and placebo did not differ on each of the three co-primary efficacy end points. To reject the null hypothesis a significance of 0.05 was required (two-tailed). The study had 90% power to detect a significant treatment effect in each of the three co-primary efficacy variables at the 0.05 alpha level.
The efficacy analyses were conducted on an intent-to-treat basis. Efficacy analyses included all patients who had a baseline measurement and at least one post-baseline measurement. The scores on the IIEF domains and questions were analysed using the last observation carried forward convention. For each SEP question the baseline and post-baseline scores were analysed as the mean per patient percent of ‘yes’ responses (number of yes responses relative to the number of sexual encounters during the run-in and treatment periods respectively).
The efficacy variables were analysed using analysis of covariance (ancova) models, including terms for baseline efficacy value, treatment group, pooled site and baseline-by-treatment group interaction (if p < 0.10). Logistic regression was performed to evaluate the GAQ using the same terms as in the ancova model except that the baseline IIEF EF domain value was used in the model as a covariate. Only patients who responded to the GAQ were included in the model. An additional analysis of the percentage of patients who returned to normal erectile function (as defined by an IIEF Erectile Function Domain score of ≥26) by baseline severity after treatment was performed.
Safety analyses included all randomised patients. The incidence of treatment-emergent adverse events between treatment groups was compared. The statistical analyses in this study were conducted using the SAS statistical package version 8.2 for windows (SAS Institute, Cary, NC, USA).
Results
Patient Demographics and Patient Disposition
Of the 139 patients who entered the study, 132 were randomised to treatment (31 to placebo, 101 to tadalafil). Sixty patients were randomised in Turkey and 72 in Egypt. Most of the baseline characteristics including ED severity and ED aetiology were balanced between the two treatment groups (Table 1). A larger proportion in the placebo group (22.6%) had hypertension than in the tadalafil group (9.9%) while a larger proportion in the tadalafil group (75.2%) had an ED duration ≥1 year than in the placebo group (64.5%). All patients in this study were Caucasian.
Table 1.
Patients demographics and baseline clinical characteristics
| Characteristic | Placebo (n = 31) | Tadalafil (n = 101) |
|---|---|---|
| Mean age, years (range) | 51.7 (27.8–66.1) | 48.7 (26.6–67.7) |
| Mean weight ± SD (kg) | 83.97 ± 14.91 | 80.73 ± 11.68 |
| Mean height ± SD (cm) | 170.84 ± 5.53 | 172.79 ± 7.79 |
| Aetiology, n (%) | ||
| Psychogenic | 2 (6.5) | 11 (10.9) |
| Organic | 9 (29.0) | 31 (30.7) |
| Mixed | 20 (64.5) | 59 (58.4) |
| Erectile dysfunction severity measured by IIEF EF score, n (%) | ||
| Mild (17–30) | 11 (35.5) | 35 (34.7) |
| Moderate (11–16) | 12 (38.7) | 38 (37.6) |
| Severe (1–10) | 8 (25.8) | 28 (27.7) |
| ED duration, n (%) | ||
| ≥3 to < 6 months | 4 (12.9) | 15 (14.9) |
| ≥6 months to 1 year | 7 (22.6) | 10 (9.9) |
| ≥1 year | 20 (64.5) | 76 (75.2) |
| Smokers | 10 (32.3) | 36 (35.6) |
| Consumes alcohol | 2 (6.5) | 7 (6.9) |
| Mean weekly consumed alcohol units* | 0.13 | 0.12 |
| Pre-existing conditions, n (%)† | ||
| Patients with ≥1 condition | 18 (58.1) | 41 (40.6) |
| Diabetes mellitus | 8 (25.8) | 21 (20.8) |
| Hypertension | 7 (22.6) | 10 (9.9) |
| Benign prostatic hyperplasia | 2 (6.5) | 4 (4.0) |
| Hypercholesterolaemia | 1 (3.2) | 3 (3.0) |
| Hyperlipidaemia | 2 (6.5) | 1 (1.0) |
| Urinary tract infection | 2 (6.5) | 1 (1.0) |
Patients were included based on a history of erectile dysfunction. Subsequent assessment of erectile function by IIEF at baseline revealed that two (2%) tadalafil-treated patients had an EF domain score in the normal range (26–30).
Mean alcohol unit for randomised patients.
Conditions that are more frequent than 5% in any of the treatment group are shown in this table.
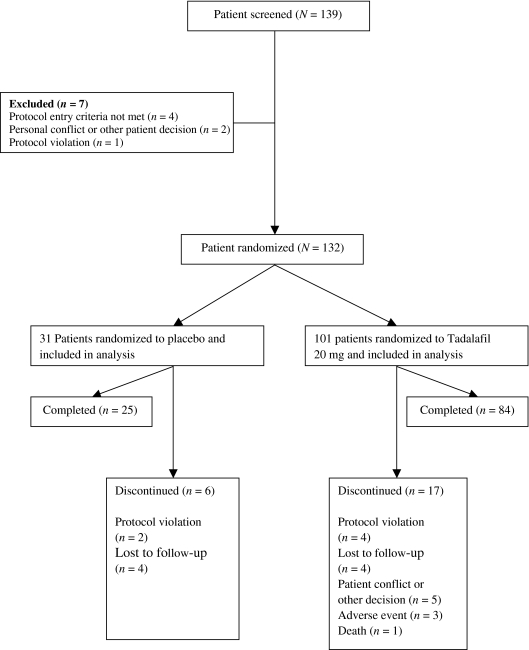
Eighty-one per cent (25/31) of placebo-treated patients and 83% (81/101) of tadalafil-treated patients completed the study. Of the 23 discontinuations, three tadalafil and zero placebo patients discontinued due to an adverse event. Other reasons for discontinuation including patient decision, lost to follow-up, protocol violation and death are summarised in Figure 1.
Figure 1.
Study flow diagram
There were no statistically significant differences between the two treatment groups with respect to the mean number of days on therapy (placebo 105.1 days and tadalafil 98.7 days), the mean of total doses taken during the study (29.5 for placebo and 39.2 for tadalafil) or the mean number of doses taken per week (2.1 for placebo and 2.8 for tadalafil).
Efficacy Results
Tadalafil was superior to placebo on all three primary efficacy end points with p < 0.001 (Table 2). The mean change (± standard errors) from baseline on the IIEF EF domain was 2.3 ± 1.6 for the placebo and 9.3 ± 0.8 for the tadalafil group. Tadalafil-treated patients reported a significantly greater improvement in the mean per patient percent of yes on SEP 2 (successful penetration) (tadalafil: 34.5 ± 4.1%; placebo: −4.6 ± 8.1%) and SEP 3 (successful intercourse) (tadalafil: 52.2 ± 3.8%; placebo: 16.8 ± 7.8%) than placebo-treated patients.
Table 2.
Primary and secondary efficacy results
| Placebo (n = 31) | Tadalafil 20 mg (n = 101) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | End point | Change (mean ± SE) | Baseline | End point | Change (mean ± SE) | p-value | |
| Primary end points | |||||||
| IIEF EF domain | 14.5 | 16.8 | 2.3 ± 1.6 | 14.5 | 23.8 | 9.3 ± 0.8 | <0.001 |
| SEP2 (successful penetration) (mean per patient % yes) | 55.6 | 51.0 | −4.6 ± 8.1 | 44.4 | 78.9 | 34.5 ± 4.1 | <0.001 |
| SEP3 (successful intercourse) (mean per patient % yes) | 17.9 | 34.7 | 16.8 ± 7.8 | 14.0 | 66.1 | 52.2 ± 3.8 | <0.001 |
| Secondary end points | |||||||
| IIEF questions | |||||||
| IIEF Q3. Penetration ability | 2.7 | 2.9 | 0.2 ± 0.3 | 2.6 | 4.1 | 1.5 ± 0.2 | <0.001 |
| IIEF Q4. Maintenance ability | 2.0 | 2.8 | 0.8 ± 0.3 | 1.9 | 3.9 | 2.0 ± 0.2 | <0.001 |
| IIEF domains | |||||||
| Intercourse satisfaction | 7.1 | 9.4 | 2.3 ± 0.7 | 6.4 | 11.2 | 4.8 ± 0.3 | 0.001 |
| Overall satisfaction | 4.6 | 5.7 | 1.1 ± 0.6 | 4.0 | 7.6 | 3.6 ± 0.3 | <0.001 |
| Patient SEP questions (mean per patient % yes) | |||||||
| SEP4 (satisfied with hardness) | 8.7 | 30.3 | 21.7 ± 6.8 | 8.6 | 61.4 | 52.8 ± 3.8 | <0.001 |
| SEP5 (overall satisfaction) | 8.7 | 29.2 | 20.5 ± 6.8 | 8.1 | 57.3 | 49.1 ± 3.9 | <0.001 |
| GAQ (% of patients with ‘yes’ response) | |||||||
| GAQ1. Improved erections | 41.9 | 81.2 | <0.001 | ||||
| GAQ2. Improved sexual activity | 41.9 | 76.2 | <0.001 | ||||
IIEF, International Index of Erectile Function; SEP, Sexual Encounter Profile; GAQ, Global Assessment Question.
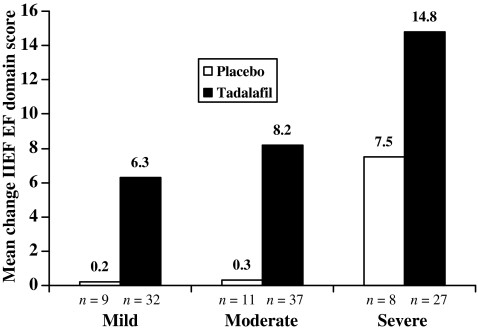
In addition, the mean change from baseline on the IIEF EF domain score was greater among the tadalafil-treated patients than among the placebo-treated patients across all ED severity groups (Figure 2).
Figure 2.
Mean change from baseline in International Index of Erectile Function (IIEF) erectile function domain score, by baseline erectile dysfunction severity
Tadalafil significantly improved erectile function and was superior to placebo on all secondary efficacy variables. Tadalafil treatment significantly improved IIEF Intercourse Satisfaction and Overall Satisfaction domain scores from baseline and resulted in higher end point scores on these two IIEF domains when compared with placebo (Table 2). Responses to IIEF questions 4 and 5 assessing penetration ability and erection maintenance indicated that the improvement from baseline with tadalafil was significantly greater than with placebo (p < 0.001, Table 2). Tadalafil-treated patients had a significantly greater change from baseline compared with placebo-treated patients for SEP4 (satisfaction with erection hardness) and SEP5 (overall satisfaction with sexual experience) (p < 0.001 for both, Table 2). Significantly more tadalafil-treated patients reported an improvement in their erections (‘yes’ response to GAQ1, p < 0.001) and in the ability to engage in sexual activity (‘yes’ response to GAQ2, p < 0.001) compared with placebo-treated patients (Table 2).
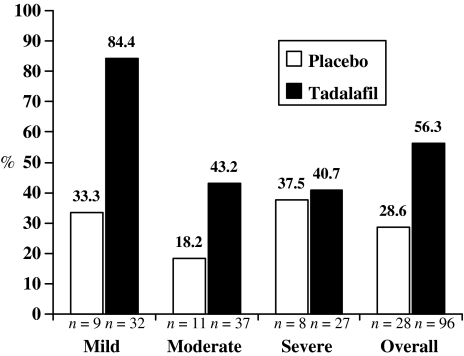
Across all ED severity categories, tadalafil was associated with a higher proportion of patients achieving normal erectile function at end point as measured by the IIEF EF domain (score of ≥26) (13). Overall, 56.3% of patients reported erectile function within the normal range at end point in the tadalafil group and 28.6% in the placebo group (Figure 3).
Figure 3.
Percentage of patients attaining normal International Index of Erectile Function (IIEF) erectile function (EF) domain score (≥26 at end point) by baseline erectile dysfunction severity n = patients with normal IIEF EF domain score (EF = 26) at end point who did not have normal IIEF EF domain score at baseline
Safety
The most commonly reported treatment-emergent adverse events (≥5%) were headache (tadalafil 16.8 %, placebo 9.7 %), back pain (tadalafil 6.9%, placebo 0%) and dyspepsia (2 % tadalafil, 6.5% placebo). Most (82%) of the adverse events reported by tadalafil-treated patients were mild in severity and generally well tolerated. Three patients (3%) in the tadalafil group discontinued due to adverse events that were considered by the investigators as possibly related to study drug (Figure 1). One patient (55 years old) in the tadalafil group died of cardiac arrest. This patient had multiple cardiovascular risk factors, including obesity, smoking, diabetes mellitus and hypertension. Because there was no medical follow-up related to the event, the investigator could not exclude a relationship to study drug or protocol procedures. No other serious adverse events or clinically significant findings related to vital signs and laboratory analytes were reported.
Discussion
Tadalafil at the 20 mg dose taken ‘as needed’ significantly improved erectile function in men with mild, moderate or severe ED compared with placebo in this study population from Egypt and Turkey. This is the first multi-centre trial conducted to assess the efficacy of tadalafil in men with ED in this region of the world. Patients taking tadalafil reported significant improvement (p < 0.001) on each of the co-primary efficacy end points compared with placebo (Figures 3 and Table 2). As an example, an increase of 52% from baseline in the mean per patient percent of ‘yes’ responses to SEP3 (successful intercourse) and a mean increase of 9.3 from baseline in IIEF EF domain score was reported by men with ED treated with tadalafil. These findings are consistent with previous studies assessing the efficacy of tadalafil in the treatment of ED relative to placebo (14–19).
A significant number of tadalafil-treated patients across all ED severity categories (41–84%) achieved a normal IIEF EF domain score (between 26 and 30) at end point (13). This is consistent with the findings of recent tadalafil studies evaluating the efficacy of tadalafil treatment in European populations (19,20). The placebo responses in this study across all severity groups are somewhat higher than in the integrated studies (15), especially the rate for the severe group. It might be associated with the random variation due to the sparseness of data in the placebo group. Overall, in this study of men with ED from Egypt and Turkey, more than half of the patients (56%) had IIEF EF domain scores in the normal range (≥26) by the end of 12 weeks of treatment.
Tadalafil also showed similar efficacy results on all secondary efficacy measurements (Table 2). Tadalafil improved patients’ erections, erection hardness, penetration ability, maintenance of erection, intercourse satisfaction and overall satisfaction. At the end of the study, 76% of patients reported improvement in the ability to engage in sexual activity. These results together with the primary efficacy findings of the study provide further support for the efficacy of tadalafil in the treatment of ED. Our results confirm those reported previously across various geographies (20–23) and extend the results to men from Egypt and Turkey.
In prior studies with PDE 5 inhibitors, headache and dyspepsia were the common adverse events (16,24). Headache, back pain and dyspepsia were the most common adverse events reported in our study during the 12 week treatment period and the majority of these events were mild in severity. Likewise, Carson et al. found in his integrated analysis that most of the events were classified as mild in severity (15). Tadalafil was generally well tolerated in this population, with 3% of patients receiving tadalafil discontinuing due to adverse events.
One limitation of the study may arise from the selection of the patient population. A recent sex survey conducted with Turkish men aged between 40 and 65 years (25) showed that the admission rate to a medical professional for ED is low (7%). Therefore, patients enrolled in this study may represent a highly motivated sample of men with ED seeking medical advice rather than the overall male ED population.
Conclusions
In this randomised, double-blind, placebo-controlled study conducted in Egypt and Turkey, 20 mg ‘as needed’ tadalafil without restrictions on food and beverage intake significantly improved patients’ erectile function. In this study, 56% of patients treated with tadalafil achieved normal IIEF EF domain scores (≥26) and tadalafil was well tolerated.
Acknowledgments
Funding for this study was provided by Lilly ICOS LLC. The authors would like to acknowledge Erkan Tetik, MSc (Eli Lilly and Company) and Waleed Abdelfattah (Eli Lilly and Company) for their assistance in preparation of this manuscript. The authors thank the study participants and study investigators (Bulent Semerci, Erdal Apaydin, Guven Aslan, Ihab Othman, Kadri Anafarta, Kamal Shaeer, Mohamed El Galad, Onder Yaman, Oner Sanli, Tareq Hussein, Tayfun Oktar, Zafer Tokatli).
References
- 1.NIH Consensus Conference Impotence (1993) NIH consensus development panel on impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 2.Wagner TH, Patrick DL, McKenna SP, Froese PS. Cross-cultural development of a quality of life measure for men with erection difficulties. Qual Life Res. 1996;5:443–9. doi: 10.1007/BF00449919. [DOI] [PubMed] [Google Scholar]
- 3.Gheorghiu S, Godschalk M, Gentili A, Mulligan T. Quality of life in patients using self-administered intravenous injections of prostaglandin E1 for erectile dysfunction. J Urol. 1996;156:80–1. [PubMed] [Google Scholar]
- 4.Shabsigh R, Perelman MA, Lockhart DC, Lue TF, Broderick GA. Health issues of men: prevalence and correlates of erectile dysfunction. J Urol. 2005;174:662–7. doi: 10.1097/01.ju.0000165389.73148.d1. [DOI] [PubMed] [Google Scholar]
- 5.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 6.Hoesl CE, Woll EM, Burkart M, Altwein JE. Erectile dysfunction (ed) is prevalent, bothersome and underdiagnosed in patients consulting urologists for benign prostatic syndrome (BPS) Eur Urol. 2005;47:511–7. doi: 10.1016/j.eururo.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Lue TF. Erectile dysfunction. New Engl J Med. 2000;342:1802–13. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 8.Jarow JP, Nana-Sinkam P, Sabbagh M, Eskew A. Outcome analysis of goal directed therapy for impotence. J Urol. 1996;155:1609–12. [PubMed] [Google Scholar]
- 9.Burnett AL. Vasoactive pharmacotherapy to cure erectile dysfunction: fact or fiction. Urology. 2005;65:224–30. doi: 10.1016/j.urology.2004.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Stief CG, Uckert S, Jonas U. Strategies in the oral pharmacotherapy of male erectile dysfunction viewed from bench and bedside (Part I) JMHG. 2005;2:87–94. [Google Scholar]
- 11.Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol. 1998;159:2164–71. doi: 10.1016/S0022-5347(01)63299-3. [DOI] [PubMed] [Google Scholar]
- 12.Rowland DL, Burnett AL. Pharmacotherapy in the treatment of male sexual dysfunction. J Sex Res. 2000;37:226–43. [Google Scholar]
- 13.Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54:346–51. doi: 10.1016/s0090-4295(99)00099-0. [DOI] [PubMed] [Google Scholar]
- 14.Brock GB, McMahon CG, Chen KK, et al. Efficacy and safety of tadalafil for the treatment of erectile dysfunction: results of integrated analyses. J Urol. 2002;168:1332–6. doi: 10.1016/S0022-5347(05)64442-4. [DOI] [PubMed] [Google Scholar]
- 15.Carson CC, Rajfer J, Eardley I, et al. The efficacy and safety of tadalafil: an update. BJU Int. 2004;93:1276–81. doi: 10.1111/j.1464-410X.2004.04819.x. [DOI] [PubMed] [Google Scholar]
- 16.McMahon CG, Samali R, Johnson H. Efficacy, safety and patient acceptance of sildenafil citrate as treatment for erectile dysfunction. J Urol. 2000;164:1192–6. [PubMed] [Google Scholar]
- 17.Padma-Nathan H, McMurray JG, Pullman WE, et al. for the IC351 on-demand dosing study group On-demand IC351 (Cialis™ sans) enhances erectile function in patients with erectile dysfunction. Int J Impot Res. 2001;13:2–9. doi: 10.1038/sj.ijir.3900631. [DOI] [PubMed] [Google Scholar]
- 18.Saenz de Tajada I, Knight JR, Anglin G, Emmick JT. Effects of tadalafil on rectile dysfunction in men with diabetes. Diabetes Care. 2002;25:2159–64. doi: 10.2337/diacare.25.12.2159. [DOI] [PubMed] [Google Scholar]
- 19.Skoumal R, Chen J, Kula K, et al. Efficacy and treatment satisfaction with on-demand tadalafil (Cialis) in men with erectile dysfunction. Eur Urol. 2004;46:362–9. doi: 10.1016/j.eururo.2004.04.026. discussion 369. [DOI] [PubMed] [Google Scholar]
- 20.Eardley I, Gentile V, Austoni E, et al. Efficacy and safety of tadalafil in a Western European population of men with erectile dysfunction. BJU Int. 2004;94:871–7. doi: 10.1111/j.1464-410X.2004.05049.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen KK, Jiann BP, Lin JS, et al. Efficacy and safety of on-demand oral tadalafil in the treatment of men with erectile dysfunction in Taiwan: a randomized, double-blind, parallel, placebo-controlled clinical study. J Sex Med. 2004;1:201–8. doi: 10.1111/j.1743-6109.2004.04029.x. [DOI] [PubMed] [Google Scholar]
- 22.McMahon CG, Stuckey BGA, Lording GA, et al. A 6-month study of the efficacy and safety of tadalafil in the treatment of erectile dysfunction: a randomised, double-blind, parallel-group, placebo-controlled study in Australian men. Int J Clin Pract. 2005;59:143–9. doi: 10.1111/j.1742-1241.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 23.Serge C, Brock GB, Pommerville PJ, et al. Efficacy and safety of oral tadalafil in the treatment of men in Canada with erectile dysfunction: a randomized, double-blind, parallel, placebo-controlled clinical trial. J Sex Med. 2005;2:685–98. doi: 10.1111/j.1743-6109.2005.00097.x. [DOI] [PubMed] [Google Scholar]
- 24.Carson CC, Hatzichristou DG, Carrier S, et al. Patient Response with Vardenafil in Slidenafil Non-Responders (PROVEN) Study Group Erectile response with vardenafil in sildenafil nonresponders: a multicentre, double-blind, 12-week, flexible-dose, placebo-controlled erectile dysfunction clinical trial. BJU Int. 2004;94:1301–9. doi: 10.1111/j.1464-410X.2004.05161.x. [DOI] [PubMed] [Google Scholar]
- 25.Alev L, Bulut Ö. Turkey sexuality survey. Literatur. 2004;253(Suppl.):1–8. [Google Scholar]